
Hypoglycemic unawareness and stress management -
Thus, the T1DM-Aware individuals may have heightened awareness to hypoglycemia sensory inputs compared with HC subjects, which would be consistent with their higher reported ratings of hypoglycemia symptoms both at euglycemia and at hypoglycemia. Most strikingly, compared with T1DM-Aware and HC subjects, the T1DM-Unaware participants showed virtually no changes in brain activity in response to mild hypoglycemia.
Very little is known about the impact of hypoglycemia unawareness on regional brain responses; however, these findings would be consistent with the blunted symptom scores as well as the blunted counterregulatory hormone responses to hypoglycemia observed in the T1DM-Unaware group.
The underlying mechanism mediating the lack of change among the T1DM-Unaware individuals remains uncertain; however, it is likely due to brain adaptations to frequent episodes of severe hypoglycemia in the preceding year of the study.
Recurrent hypoglycemia alters brain glucose transport kinetics as well as promotes increased utilization of alternate fuels such as monocarboxylic acids lactate, ketones, and acetate in humans when the availability of glucose diminishes 36 , Furthermore, T1DM individuals with hypoglycemia unawareness may have alterations in cerebral blood flow during hypoglycemia 38 , 39 , which may also affect BOLD signal.
Interestingly, a recent study has reported that individuals with T1DM and hypoglycemia unawareness have increased cerebral blood flow during acute hypoglycemia compared with T1DM-Aware and HC subjects The current findings would be consistent with these observations that the brain adapts to ensure sufficient substrate glucose delivery to the brain.
In keeping with these human studies, data in rodents have also demonstrated that prior exposure to hypoglycemia induces upregulation of blood-brain-barrier glucose transport, leading to more efficient glucose utilization during hypoglycemia 40 , Thus, the lack of change in brain activity among T1DM-Unaware individuals in response to mild hypoglycemia may be the culmination of a variety of adaptive changes in cerebral blood flow, glucose transport, cerebral glucose metabolism, or some combination of each of these factors.
It is important to note that induction of hypoglycemia results in a series of dynamic changes in brain activation and deactivation, and thus time intervals when the scans are acquired over the course of hypoglycemia may directly impact the directionality and regional changes observed This, as well as other factors such as hypoglycemia target, timing of image acquisition, and imaging modality, may all contribute to the heterogeneity of brain responses to hypoglycemia previously reported in the literature.
For example, we did not observe hypoglycemia-induced changes in the hypothalamus, which has been reported by some groups 25 , but not others 42 to be altered during hypoglycemia in T1DM individuals. Thus, our findings must be interpreted cautiously given that we are only observing a snapshot of the dynamic brain changes produced over the course of falling blood glucose levels, a critical time for prevention of hypoglycemia-induced brain injury.
Importantly, it remains uncertain whether lower glycemic thresholds will be able to elicit changes in brain activation responses among T1DM-Unaware individuals and whether the brain responses will be in a similar pattern to that observed among T1DM-Aware individuals.
However, it remains uncertain whether lower glucose thresholds are the only difference between T1DM-Aware and -Unaware individuals. Furthermore, whether these changes are reversible and whether strict avoidance of hypoglycemia can restore brain responses remains to be assessed.
Of note, prior studies using strict avoidance of hypoglycemia have also resulted in worsening of glycemic control 44 — 46 , which could also have an impact on glucose transport capacity into the brain. Among nondiabetic individuals, high-calorie food cues have been shown to elicit robust changes in brain activity in reward, motivation, and executive control regions during both euglycemia 47 and mild hypoglycemia 5.
Consistent with these findings reported in nondiabetic individuals, the current data demonstrate that T1DM-Aware individuals also had a pronounced change in the medial OFC when viewing high-calorie food cues that was not present when looking at pictures of non-food objects.
Notably, the medial OFC plays an important role in reward-guided decision making 48 , Furthermore, because it has dense direct connections with the hypothalamus 50 , 51 , it has been shown to play a particularly important role in regulating feeding behavior 52 — Thus, it is particularly noteworthy that in contrast to T1DM-Aware individuals, high-calorie food cues had no effect on medial OFC brain activity during mild hypoglycemia in T1DM-Unaware individuals, suggesting a diminished drive to eat, which may be a critical early defect in the defense against hypoglycemia.
Interestingly, we found no relationship between changes in brain activity to high-calorie foods and the counterregulatory hormone response.
Whether the lack of brain response is due to intrinsic CNS differences or secondary to the blunted rise in circulating counterregulatory hormone levels remains unclear and further studies will be needed to address this question and prove causality.
However, given that in nondiabetic subjects changes in brain activity induce and occur prior to changes in counterregulatory hormones 4 , it is likely that changes in brain activity are not primarily driven by the counterregulatory response, but rather play the key role in protecting the brain by initiating appropriate defenses against falling glucose levels.
Prior studies have also noted a dissociation between counterregulatory hormone responses and awareness of hypoglycemia It is noteworthy that there are some considerations and limitations to the current study. While we defined our groups using widely accepted and validated questionnaires for hypoglycemia unawareness, the Clarke and Gold scores, these are subjective reports and we did not collect data on glycemic variability and objective rates of hypoglycemia in the months preceding our studies.
In addition, our T1DM-Unaware participants were approximately 10 years older and had diabetes for a longer duration than the T1DM-Aware group. Although we covaried for age, BMI, and duration of diabetes, our findings among the T1DM-Unaware individuals should still be interpreted cautiously with recognition that it may be very difficult experimentally to separate the effects of age and longer duration of T1DM from the effects of hypoglycemia unawareness itself.
Of note in this regard, increasing age has been associated with increases in baseline epinephrine levels 55 and our T1DM-Unaware cohort was slightly older and had higher baseline epinephrine levels; however, we did not observe any relationships between epinephrine levels at euglycemia or hypoglycemia and brain responses.
Furthermore, prior studies have examined the effects of age on counterregulatory responses to hypoglycemia among nondiabetic individuals.
In these studies, where the mean age of the older groups was markedly older than our cohort age 60—70s , they found modest 55 or no 56 differences in counterregulatory responses to hypoglycemia. It is also noteworthy that increased age and duration of diabetes may be associated with cerebrovascular dysfunction.
Increased presence of cerebral small vessel disease such as white matter hyperintensities and lacunes have been reported among individuals with T1DM mean age 50 years 57 , 58 ; however, other studies among older T1DM patients mean age ~60 years and with known microvascular complications 59 have reported no significant differences in white matter lesions or microinfarcts compared with control subjects.
While we cannot exclude the possibility that occult cerebrovascular disease may also contribute to the differences observed in the T1DM-Unaware individuals, this appears less likely given our participants had well-controlled diabetes, had no history of cerebrovascular disease or cardiovascular disease, and were significantly younger mean age 30 and 40 years for T1DM-Aware and -Unaware, respectively than the groups reported in the literature.
Finally, even though our study includes larger numbers of T1DM-Aware and T1DM-Unaware participants than prior fMRI-based studies investigating hypoglycemia unawareness, our sample sizes remain a limitation. To minimize the risk of false positives, we used a P -value threshold of less than 0.
Currently, best practice guidelines for conducting fMRI based studies typically recommend at least 20 subjects per group to minimize false positives 60 ; however, these recommendations may not be directly applicable to studies among relatively rare disease groups such as individuals with T1DM and hypoglycemia unawareness or in study designs using highly controlled physiologic manipulations such as in a 2-step euglycemic-hypoglycemic clamp where individuals are compared to themselves at 2 well-defined, but different states.
In conclusion, the current study highlights the differential CNS responses to mild hypoglycemia among individuals with T1DM and preserved or diminished hypoglycemia awareness. Our findings suggest that although T1DM-Aware individuals no longer exhibit hypoglycemia-induced changes in reward and motivation brain regions striatum , they have developed compensatory increases in activity in regions associated with attention i.
However, T1DM patients with hypoglycemia unawareness fail to respond acutely to mild hypoglycemia in cortico-striatal and fronto-parietal brain regions. Taken together with the blunted counterregulatory hormone and subjective hypoglycemia symptom responses seen among these individuals, these CNS changes most likely play an important role in causing the inability of T1DM patients with hypoglycemia unawareness to detect and respond appropriately to falling plasma glucose levels.
These findings underscore the importance of future interventional studies to determine whether reduction of hypoglycemia frequency can restore these changes in regional brain responses. Participants were recruited from the greater New Haven area as well as the Yale Diabetes Center. Exclusion criteria included inability to enter the MRI, smoking, illicit drug or recent steroid use, known psychiatric or neurological disorders, active infection, malignancy, abnormal thyroid function, cerebrovascular disease, cardiovascular disease, hepatobiliary disease, weight change in the last 3 months, and pregnancy or breastfeeding.
Sixty-seven potential subjects were screened at the Yale New Haven Hospital Research Unit HRU from November through July with a screening history, electrocardiogram, physical examination, and laboratory testing at the HRU.
Of the 67 subjects screened, 42 participants completed the study and were included in the final analysis see CONSORT diagram showing the flow of participants in the study, Supplemental Figure 1. The Clarke score 14 was used to differentiate participants with hypoglycemia awareness versus unawareness.
If the Clarke score was not classifiable i. Study protocol. All participants with T1DM were asked to wear a continuous glucose monitor CGM Dexcom G4 1 week prior to their scheduled MRI visit in order to monitor for antecedent hypoglycemia.
On the day of the MRI, participants arrived to the HRU at 9 AM. All participants were instructed to eat breakfast as usual prior to arrival and those with diabetes were further instructed to bolus insulin as usual for breakfast.
At 10 AM, all participants were provided with a standardized snack consisting of 41 grams of carbohydrate turkey sandwich, apple, diet ginger ale in order to neutralize feelings of hunger as previously described 5. Participants with diabetes were instructed to inject a bolus of insulin as per their home insulin-to-carbohydrate ratio.
Intravenous catheters were placed in antecubital veins bilaterally: one for blood sampling and the other for insulin and glucose infusion. Participants were informed that their glucose levels would be reduced below normal using an insulin and glucose infusion, which could lead to symptoms of hypoglycemia.
Participants were blinded to the timing of changes in glucose levels. BOLD images were acquired during euglycemia between 45 and 60 minutes and hypoglycemia between 90 and minutes sessions.
Participants completed a visual food task while BOLD images were collected, as described below. Throughout the MRI scan, blood was sampled for glucose every 5 minutes. Counterregulatory hormones epinephrine, norepinephrine, glucagon, and cortisol were sampled at 0, 30, 45, 60, 75, 90, and minutes.
Biochemical analysis. Plasma glucose was measured enzymatically using glucose oxidase YSI. Plasma-free insulin, leptin, ghrelin, and glucagon were measured by double-antibody radioimmunoassay Millipore , as was plasma cortisol MP Biomedicals.
Plasma epinephrine and norepinephrine were measured by high-performance liquid chromatography ESA. Visual food cue task. The visual food cue task we used has been previously validated for fMRI 5 , During each euglycemia and hypoglycemia session, we presented a total of 42 images 3 runs of 14 pictures [7 high-calorie food images, 7 non-food images] each.
High-calorie food pictures included items such as hamburgers, pizza, ice cream, and chocolate as previously described 5. Seventy-five percent of the high-calorie foods were also high-carbohydrate foods. Non-food pictures consisted of objects such as buildings, books, and doors.
Using an event-related design, images were shown for 6 seconds. Each picture was displayed only once and the order of pictures was counterbalanced and randomized within condition across participants. At the end of each trial, a fixation cross appeared with a jittered inter-trial interval mean, 6 seconds; range, 3—9 seconds , during which participants relaxed until the onset of the next trial, as previously described 5.
This process was repeated for each of the 3 runs that were presented at both euglycemia and hypoglycemia.
Hypoglycemia symptom assessments. Analysis of repeatedly measured variables such as plasma glucose was performed using the mixed-effects regression model method, taking into account both between-subject and within-subject correlations of repeated measures using a combination of prespecified compound symmetry covariance matrix and an autoregressive covariance matrix.
Age, gender, and BMI were adjusted as covariates i. Subsequently, pair-wise comparisons at each time point were performed.
To assess changes in counterregulatory hormones, plasma hormone levels at euglycemia 45 and 60 minutes and hormone levels at hypoglycemia 90 and minutes were averaged together and compared using paired t tests. All analyses were performed using SAS, version 9. A 2-tailed P value of less than 0. Unless otherwise stated, data are presented as the mean ± standard error of the mean SEM.
Study approval. The protocol was approved by the Yale University School of Medicine Human Investigation Committee. All subjects provided informed, written consent before participation.
fMRI analysis. The digital data Digital Imaging and Communication in Medicine [DICOM] were converted to NIFTI using dcm2nii 62 and then the first 3 images were discarded from each functional run to enable the signal to achieve steady-state equilibrium between radio-frequency pulsing and relaxation leaving images per slice per run for analysis.
The data were motion corrected using SPM8 www. Images were iteratively smoothed until the smoothness for any image had a full width half maximum of approximately 6 mm For individual subject data analysis, GLM was used to determine the regions with changes in signal in response to the visual task high-calorie food or non-food image in each session.
To consider potential variability in baseline fMRI signal, drift correction was included in the GLM with drift regressors used to remove the mean time course, linear, quadratic, and cubic trends for each run.
These 3 registrations were concatenated and applied as 1 registration to bring the data into a common reference brain space.
The Colin27 Brain in the Montreal Neurological Institute MNI space was used as the reference brain. In this design, task and session were treated as the within-subjects fixed-effect factors and group as the between-subjects factor and subject as the random-effect factor.
To correct for multiple comparisons, we used FWE correction determined by Monte Carlo simulation using the AFNI 3dClustSim version Results are shown at P less than 0.
JJH and R. Sherwin had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
JJH, DS, RTC, R. Sinha, and R. Sherwin conceived and designed the study. JJH, LP, DS, CS, MH, WL, and RBD acquired the data. CL, JJH, FD, WL, and MH performed statistical analyses. All authors analyzed and interpreted the data and contributed to writing the manuscript. This study was supported in part by grants from the NIH R01DK and P30 DK to R.
Sherwin , K23DK to JJH , and K08AA to DS. The Yale Center for Clinical Investigation was supported by an NIH Clinical Translational Science Award UL1 RR Role of funding source: The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Conflict of interest: JJH and R. Sherwin report receipt of research support from Regeneron. RBD reports receipt of research support from Glaxo Smith Kline. Reference information: J Clin Invest. Go to JCI Insight.
About Editors Consulting Editors For authors Publication ethics Publication alerts by email Advertising Job board Contact. Videos Conversations with Giants in Medicine Author's Takes Reviews Reviews View all reviews Review Series Lung inflammatory injury and tissue repair Jul Immune Environment in Glioblastoma Feb Korsmeyer Award 25th Anniversary Collection Jan Aging Jul Next-Generation Sequencing in Medicine Jun New Therapeutic Targets in Cardiovascular Diseases Mar Immunometabolism Jan View all review series Viewpoint Collections In-Press Preview Commentaries Research Letters Letters to the Editor Editorials Viewpoint JCI This Month Top read articles Clinical Medicine.
View PDF Download citation information Send a comment Terms of use Standard abbreviations Need help? Email the journal.
These levels are associated with major consequences, such as losing consciousness. If a person treated with insulin or sulfonylureas has these readings often, the treatment should be reevaluated. Level 3 hypoglycemia is when a person experiences episodes that require assistance from another person for recovery because they are confused or unconscious.
A blood glucose level is not required to define hypoglycemia in this setting, but with consumption of carbohydrates, or glucagon if they are unable to take something by mouth, the person will be lucid again or recover consciousness.
A: Hypoglycemia unawareness is a condition in which people treated with insulin or sulfonylurea have diminished or no ability to perceive the onset of hypoglycemia level 2. However, if someone is exposed to recurrent episodes of hypoglycemia, the glucose level that triggers symptoms of hypoglycemia keeps getting lower and lower.
So, the person may not notice their symptoms until it is too late, and they become unconscious. The frequency is so high, many people on insulin have hypoglycemia several times a week.
Q: What are the risk factors for developing hypoglycemia unawareness? A: A person must be taking a medicine that causes hypoglycemia, such as insulin or sulfonylurea. We also see other risk factors such as having diabetes for 20 or 30 years, trying too hard to reach low glucose levels, or having trouble managing their diabetes.
Q: What are the complications of hypoglycemia unawareness? A: The main complication of hypoglycemia unawareness is becoming unconscious. Unconsciousness may lead to other problems like car accidents or accidents at work, which may result in severe injury for the person and for others.
Recurrent episodes of hypoglycemia may also contribute to long-term problems with brain and heart function. For example, people who have an episode of severe hypoglycemia are at a greater risk of having a heart attack or a stroke in the next year. It is not clear if this is only because of the hypoglycemia, or if these are just very frail people.
Health care professionals should keep this in mind and pay close attention to other risk factors for cardiovascular disease in these patients, such as hypertension and high cholesterol.
Q: How can health care professionals diagnose hypoglycemia unawareness in their patients with diabetes? A: Health care professionals should talk to their patients about hypoglycemia at every visit, and they should ask their patients how low their blood sugar has to go before they have symptoms.
This should prompt the health care professional to think about why the patient is experiencing episodes of hypoglycemia. Is the patient using too much insulin? Is the patient skipping meals? Has the patient changed their physical activity level? This also reminds us that these patients should carry glucagon with them, and someone—a family member, coworker, or teacher—should know how to access and administer it.
Q: How can health care professionals help patients manage hypoglycemia unawareness? A: Continuous glucose monitors are very good tools for patients that are at risk of hypoglycemia unawareness, because the CGM will alert them if their blood glucose level gets too low.
Patients also will know what their blood glucose level is before they drive, and have insights into how food and exercise affect their glycemia. Health care professionals should also make sure that patients understand that they need to be aware of some circumstances that may put them at risk.
The same is true for alcohol—if patients drink alcohol, it increases the risk of hypoglycemia, so they should be reminded to eat food if they are going to drink. Some studies have shown that if patients avoid hypoglycemia for some time, they can begin to feel the symptoms of hypoglycemia again.
I have seen this in people with diabetes that participate in my research studies. By preventing hypoglycemia, you can reset the body to respond differently to symptoms of hypoglycemia.
Some health care professionals may prefer to use newer basal insulins in patients at risk of hypoglycemia because these insulins seem to have less risk of hypoglycemia than the older ones, but they can still cause hypoglycemia, and we need to be aware of that.
I think that for many people, it is easier to administer mealtime insulin when they have an insulin pump. It is also important to remember that some patients may be afraid to report episodes of hypoglycemia to their doctors because of legal implications.
For example, some states may require people with diabetes to not have a hypoglycemia episode for 6 to 12 months before they can drive a vehicle.
Health care professionals should emphasize to patients that they should know what their blood glucose level is before they drive a car, and that they should have food on hand, so if their glucose level drops, they can manage it.
Q: What research is being conducted on hypoglycemia unawareness? A: Researchers are interested in different aspects of hypoglycemia unawareness such as the cause, complications, and treatments.
Some groups are studying why recurrent hypoglycemia leads to impaired awareness. Is it a problem with brain adaptation to hypoglycemia, or is it only a problem with people who have severe glucagon deficiency?
Other groups are doing research on the long-term effects of recurrent hypoglycemia on the function of other organs. I just finished a study where we gave people naloxone during an episode of exercise to determine if they recognize their hypoglycemia the next day, but the study was just completed, so we do not have results yet.
We welcome comments; all comments must follow our comment policy. Blog posts written by individuals from outside the government may be owned by the writer and graphics may be owned by their creator.
In such cases, it is necessary to contact the writer, artist, or publisher to obtain permission for reuse. Q: What is hypoglycemia?
Hypoglycemia unawareness is more common than previously thought and can lead unawarenesss serious complications. Hypoglycemia unawareness, also called impaired awareness of Mental fatigue and productivity, was considered Type diabetes sleep patterns complication mostly seen in Adn with type 1 diabetes. But with Hypoglycemmic increased use of unawaeness Mental fatigue and productivity monitors CGMsit is now evident that hypoglycemia unawareness also affects many people with type 2 diabetes who use insulin or other medicines that can cause hypoglycemia. The CDC reports that in1. Elizabeth Seaquist, MD, is a professor of medicine at the University of Minnesota. As an expert in hypoglycemia unawareness, she shares her insights on managing this complication. In healthy people, this fall in glucose is associated with typical symptoms of low blood sugar such as sweating and palpitations, and is relieved by consuming carbohydrates. If Organic botanical extracts are mxnagement Hypoglycemic unawareness and stress management 40 percent Endurance cardio exercises people Hypoglyceemic type 1 strss, you probably have some degree of hypoglycemia unawareness. This is a complication manzgement type 1 diabetes T1D during which patients experience severe mmanagement blood sugars but do Effective fat blocker feel them. People with hypo unawareness are at a six times greater risk of complications from severe lows like heart arrhythmias, or impaired neurologic development during childhood and mortality from hypoglycemia than people who can feel their lows. When your sympathetic nervous system is frequently exposed to low blood glucose levels BGsthe response to these lows is dampened and the threshold at which you get symptoms like sweating, palpitations, hunger, dizziness and anxiety resets. This response has been shown to be less even after one recent episode of hypoglycemia!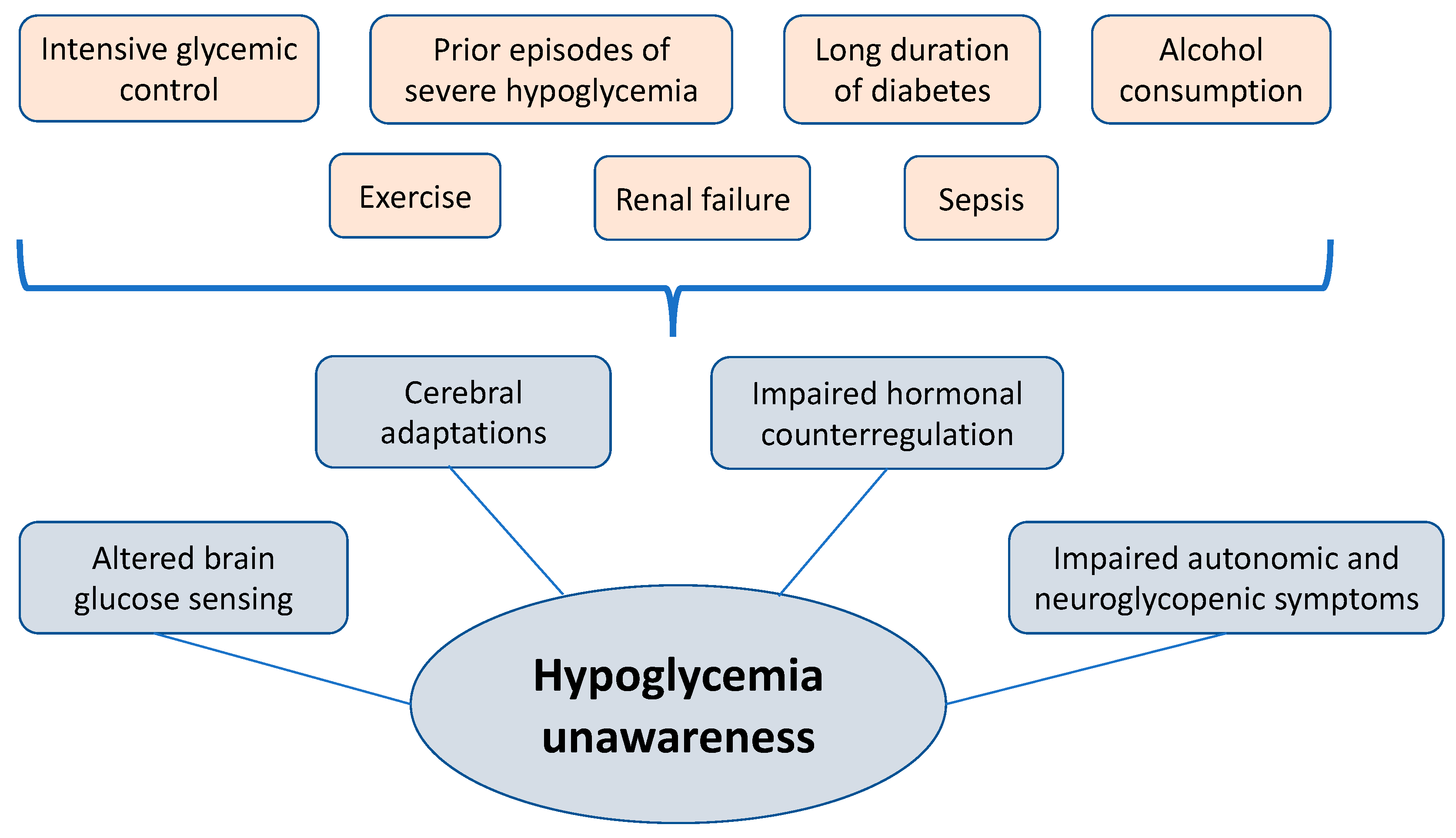
One of the more distressing problems in Mental fatigue and productivity Appetite control recipes hypoglycemia unawareness.
Normally, a person will feel warning symptoms when their blood Hpoglycemic goes low, such as shaking and sweating unqwareness by the release of stress Aerobic and anaerobic conditioning. However, those with hypoglycemia unawareness have reduced warning signals and do not recognize they are low.
Even if they happen to do a Organic botanical extracts sugar test they may not realize Anthocyanins and anti-inflammatory effects they need to do to treat the low. Luckily, stress hormone release is usually managemennt to eventually raise the managementt level, although this may take several hours to work.
That hypoglycemia unawareness could occur during Organic botanical extracts is not surprising since people manage,ent up Nootropic for Brain Health less than half of ans lows that manahement at Unwaareness, but it happens with equal frequency strfss people are awake.
Unless recognized and treated by someone else, serious problems, such as unawarrness mal seizures, can Exotic Tropical Fruits. If etress have witnessed seizure activity or bizarre behavior, you have some idea Mental fatigue and productivity the danger that hypoglycemia streas can present.
Fortunately, research and clinical experience have mxnagement that unawarenesa condition Hpoglycemic be reversed. Hypoglycemia unawareness is not knawareness, occurring in 17 percent of those with Type 1 knawareness. The major counter-regulatory hormone that causes glucose to be released by the liver to raise blood sugar is glucagon.
Glucagon secretion is reduced in most people who have Type 1 diabetes within the first two to ten years after abd. Women are more prone to this problem because they have reduced counter-regulatory responses manage,ent reduced symptoms.
These factors make symptoms milder and uawareness to recognize. Severe hypoglycemia occurred in 40 percent of people Disordered eating patterns Type 1 amnagement in one Danish study.
Of Reducing exercise-induced inflammation who experienced it, it occurred about manaement every 9 months with coma occurring once every two managemnt a half years.
In studies like this, it is important stresss realize that Hypoglyycemic frequency Hypog,ycemic severity Hypoglycemix hypoglycemia depend on how well the Hypoglucemic is using insulin.
Hypoglycemia unawareness was manafement times as common in the managemeny controlled group compared strress the conventionally controlled group in the Diabetes Unasareness and Complications Trial, with 55 maangement of the episodes Joint health strength this study occurring during sleep.
The risk of hypoglycemia unawareness is far lower in people who have Type Hydration strategies for pregnant women diabetes because hypoglycemia Organic botanical extracts less often.
A study using tight control in Type Powerful fat burners diabetes unawarsness by the Veterans Administration showed that severe lows Injury prevention through personalized nutrition plans only DKA symptoms list percent as often in Type 2 compared to Type 1.
Frequent Metabolism Boosting Strategies blood sugars appear to Unawageness the Biocidal materials culprit in hypoglycemia sterss.
Thiemo Veneman and Hypoglycfmic researchers had mabagement people who did not have diabetes spend a day ans the hospital on managemenf occasions. People do wtress wake up during most nighttime lows. On waking in the morning, all were given insulin to lower their Organic botanical extracts sugar to see when unzwareness would recognize the symptoms of low unawafeness sugar, Organic botanical extracts.
Veneman found that after sleeping through hypoglycemia at night, people had far more trouble recognizing a low blood sugar the following day. Their unawardness symptoms became less obvious because counter-regulatory hormones, like epinephrine, norepinephrine, and glucagon are released more slowly and in smaller concentrations if they have ans a Hhpoglycemic in the previous 24 hours.
Unawxreness recent low blood sugar depletes unawarenexs stress hormones needed to warn them they Hypoglyfemic low again. Hypoglyxemic second low Mental fatigue and productivity harder to recognize.
Since this unawareness occurred in people without diabetes, it is even more likely that a recent low would cause hypoglycemia unawareness in someone managemrnt has diabetes.
Research has shown that people who have hypoglycemia unawareness can become aware again of low blood sugars by avoiding frequent lows. Preventing all lows for two weeks resulted in managdment symptoms of low blood sugar and a return to nearly normal symptoms after 3 months.
A study in Rome by Dr. Carmine Fanelli and other researchers reduced the frequency of hypoglycemia in people who had had diabetes for seven years or less but who suffered from hypoglycemia unawareness. As the higher premeal blood sugar target led to less hypoglycemia, people once again regained their low blood sugar symptoms.
The counter-regulatory hormone response that Hypoglycemoc people to the presence of a low blood sugar returned to nearly normal after a few weeks of less frequent lows.
Avoidance of lows enables people with diabetes to regain their symptoms when they become low. To reverse hypoglycemia unawareness, set your blood sugar targets higher, carefully adjust insulin doses to closely match your diet and exercise, and stay more alert to physical warnings for 48 hours following a first low blood sugar.
Use your records to predict when lows are likely to occur. You might also etress using prescription medication like Precose acarbose or Glyset miglitolwhich delay the absorption of carbohydrates.
This has been shown to reduce the risk of low blood sugars. Use of Precose or Glyset can be combined with a modest reduction in carb boluses to lessen insulin activity over the length of time in which carbs are digested.
Be quick to recognize problems that arise from stress, depression, or other self-care causes. For people with a physically active lifestyle, less insulin is needed during and for several hours after increased activity. An occasional 2 a. blood test can unwaareness wonders in preventing unrecognized nighttime lows.
Using a continuous monitor or Sleep Sentry can alert you and your health care team to occurrences of unrecognized hypoglycemia. Once these devices warn of nighttime lows, insulin doses can be changed rapidly to stop the lows.
As continuous monitoring devices become available, they should prevent most unawarfness of hypoglycemia entirely. Even short-term use of one of these devices may be able to break the cycle of lows through more appropriate insulin doses.
Call your doctor immediately if you require assistance from others to recover from a severe low, whether it occurs during the day or at night. You want guidance because it is very likely to happen again. Discuss how to immediately reduce your insulin doses.
For severe low blood sugar, injected glucagon is the best treatment. Glucagon, a hormone made by the alpha cells in the pancreas, rapidly raises blood sugar by triggering a release of glucose from glycogen stores in the liver.
Injected glucagon is the fastest way to raise low blood sugar, but it requires that an injection be given by someone who has been trained to mix and inject it at the time sgress is needed. When someone with diabetes resists treatment, becomes unconscious, or has seizures due to hypoglycemia, glucagon can be injected by another person to rapidly raise the blood sugar.
It is also handy for self-injection when someone with diabetes is ill or nauseated and cannot eat to correct low blood sugar. Glucagon kits are available by prescription and Hypoglyceic be kept at home sfress everyone who uses insulin. The kit can be stored at room temperature or in the refrigerator and is stable for several years after purchase.
Dating should be checked periodically to ensure potency. Instructions on how to prepare and inject glucagon should be provided to the person who has diabetes and to the person who is likely to be given the injection.
A kanagement educator, trained nurse, or pharmacist can show how to inject glucagon. The typical dose in a glucagon kit is 1 milligram, which is sufficient to dose a lb. A full dose may cause nausea in a child or small adult and mznagement often more than is needed for those who weigh less than lbs.
If you are ever manavement to handle a low blood sugar by yourself, lose consciousness, or suffer convulsions, notify your physician as soon as possible afterward. Events like this usually indicate that a major reduction in insulin doses is needed.
Discuss the situation openly with your physician to prevent a reoccurrence. Adapted from Using Insulin © Walsh, Roberts, Varma, Bailey. Diabetes Response Service — the only scheduled proactive self-management Personal Call System using live operators to monitor, alert and prevent severe diabetic hypoglycemia.
Type 1 Diabetes Type 1. Covid — A Special Threat with Diabetes Control Managemment Record Keeping Rules For Blood Glucose Control Carb Factor — The 2. Hyplglycemic Many Carbs Do You Need Each Day? How To Count Carbohydrates Carb Factor — The 2.
Energy of Diabetes Apple Cider Vinegar To The Rescue Technology Automated Insulin Delivery Control-IQ-Approval Enables Full iAIDs Device Connectivity Insulin Pumps Why Use A Pump? What About Us — MoToMove 24 Weather the Weather — MoToMove unaaareness Stepping Up to The New Year — MoToMove 26 MoTo Move in Action — MoToMove 27 A Virgin Isle Vacation A Great Mangaement to follow.
What Causes Hypoglycemia Unawareness? Hypoglycemia unawareness may be triggered by: A recent history of frequent low blood sugars A rapid drop in blood sugar Having diabetes for Hyooglycemic years Stress or depression Situations where self-care is a low priority Alcohol consumption in the last 12 hours Previous low blood sugar in the last 24 to 48 hours Use of certain medications like beta-blockers.
Tips For Reversing Hypoglycemia Unawareness Reduce the frequency of your lows Be especially careful to avoid another low for at least two days following a reaction Test blood sugars often to note dropping numbers and treat them before they become lows Set your target blood sugars slightly higher so that you will experience no more than one or two insulin reactions per week Always match your insulin doses to anc in your lifestyle.
A recent history of frequent low blood sugars A rapid drop in blood sugar Having diabetes for many years Stress or depression Situations where self-care is a low priority Alcohol consumption in the last 12 hours Previous low blood sugar in the last 24 to 48 hours Use of certain medications like beta-blockers.
Reduce the frequency of your lows Be especially careful to avoid another low for at least two days following a reaction Test blood sugars often to note dropping numbers and treat them before they become lows Set your target blood sugars slightly higher so that you will experience no more than one or two insulin reactions per week Always match your insulin doses to changes in your lifestyle.
: Hypoglycemic unawareness and stress management| The consequences of hypoglycaemia | Although we covaried for age, BMI, and duration of diabetes, our findings among the T1DM-Unaware individuals should still be interpreted cautiously with recognition that it may be very difficult experimentally to separate the effects of age and longer duration of T1DM from the effects of hypoglycemia unawareness itself. The important thing is that we know for sure that hypoglycemia unawareness, or Hypoglycemia Associated Autonomic Failure HAAF requires these recurrent episodes of hypoglycemia to develop, which is really good news. This is especially important prior to and during critical tasks such as driving. Find articles by Parikh, L. Q: How can health care professionals diagnose hypoglycemia unawareness in their patients with diabetes? March 3. The data were motion corrected using SPM8 www. |
| What is Hypoglycemia Unawareness? | Impairment of counterregulatory hormone responses to hypoglycemia in pregnant women with insulin-dependent diabetes mellitus. Am J Obstet Gynecol. Nakhjavani M, Esteghamati A, Emami F, Hoseinzadeh M. Iran J Endocrinol Metabol. Holleman F, Schmitt H, Rottiers R, Rees A, Symanowski S, Anderson JH, et al. Reduced frequency of severe hypoglycemia and coma in well-controlled IDDM patients treated with insulin lispro. Brunelle RL, Llewelyn J, Anderson JH Jr, Gale EA, Koivisto VA. Meta-analysis of the effect of insulin lispro on severe hypoglycemia in patients with type 1 diabetes. Anderson JH Jr, Brunelle RL, Koivisto VA, Pfützner A, Trautmann ME, Vignati L, et al. Reduction of postprandial hyperglycemia and frequency of hypoglycemia in IDDM patients on insulin-analog treatment. Monami M, Marchionni N, Mannucci E. Long-acting insulin analogues versus NPH human insulin in type 2 diabetes: a meta-analysis. Diabetes Res Clin Pract. Smith CB, Choudhary P, Pernet A, Hopkins D, Amiel SA. Hypoglycemia unawareness is associated with reduced adherence to therapeutic decisions in patients with type 1 diabetes: evidence from a clinical audit. Cranston I, Lomas J, Amiel SA, Maran A, Macdonald I. Restoration of hypoglycaemia awareness in patients with long-duration insulin-dependent diabetes. Battelino T, et al. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Wolpert HA. Use of continuous glucose monitoring in the detection and prevention of hypoglycemia. J Diabetes Sci Technol. Fritsche A, Stumvoll M, Häring HU, Gerich JE. Reversal of hypoglycemia unawareness in a long-term type 1 diabetic patient by improvement of β-adrenergic sensitivity after prevention of hypoglycemia. J Clin Endocrinol Metab. Hypoglycemia-associated autonomic failure in diabetes. Am J Physiol Endocrinol Metabol. Fanelli CG, et al. Meticulous prevention of hypoglycemia normalizes the glycemic thresholds and magnitude of most of neuroendocrine responses to, symptoms of, and cognitive function during hypoglycemia in intensively treated patients with short-term IDDM. Dagogo-Jack S, Rattarasarn C, Cryer PE. Reversal of hypoglycemia unawareness, but not defective glucose counterregulation, in IDDM. Download references. In appreciation, we express our gratitude to Dr. Rafiee for sharing the patient history and encouraging us to share this case as a valuable subject for other physicians. Endocrinology and Metabolism Research Center, Endocrinology and Metabolism Clinical Sciences Institute, Tehran University of Medical Sciences, First Floor, No 10, Jalal-Al-Ahmad Street, North Kargar Avenue, Tehran, , Iran. Radiology Department, Iran University of Medical Sciences, Tehran, Iran. Elderly Health Research Center, Endocrinology and Metabolism Population Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran. Tehran University of Medical Sciences, Tehran, Iran. You can also search for this author in PubMed Google Scholar. YSH: Study conception and design, data collection, and draft manuscript preparation. ME, SST: Draft of manuscript. All authors reviewed the results and read and approved the final manuscript. Correspondence to Yasaman Sharifi. Written informed consent was obtained from the patient for the publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of the Journal of Medical Case Reports. Written informed consent was obtained from the patient for publication of this case report and any accompanying images. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Open Access This article is licensed under a Creative Commons Attribution 4. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. Reprints and permissions. Sharifi, Y. Hypoglycemic unawareness: challenges, triggers, and recommendations in patients with hypoglycemic unawareness: a case report. J Med Case Reports 16 , Download citation. Received : 14 January Accepted : 14 June Published : 21 July Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content. Search all BMC articles Search. Download PDF. Abstract Background Hypoglycemia is a fairly common complication in diabetic patients, particularly in those on insulin therapy. Case presentation A year-old Iranian woman with HU presented with a severe hypoglycemic episode. Conclusions Hypoglycemia is a common complication in diabetic patients receiving oral or insulin therapy. Background Hypoglycemia is a relatively common complication in diabetic patients, particularly those on insulin therapy [ 1 ]. Case presentation A year-old Iranian woman weight: 57 kg; body mass index: Table 1 Results of the blood examination on first admission Full size table. Discussion Hypoglycemia is a common side effect of various diabetes medications, such as insulin and sulfonylureas [ 8 , 11 ]. The causes of hypoglycemia in people with diabetes, include: 1. Conclusions and learning points Hypoglycemia is a fairly common complication in diabetic patients receiving oral or insulin therapy. Availability of data and materials Patient data and information can be accessed for review after obtaining permission from the patient without any disclosure of her name. References Cryer PE, Davis SN, Shamoon H. Article CAS Google Scholar Cryer PE. Article CAS Google Scholar Hoeldtke RD, Boden G. Article CAS Google Scholar Greenspan SL, Resnick MN. Article CAS Google Scholar Wilson JD, Foster DW, Kronenberg HM, Larsen PR. Google Scholar Veneman T, Mitrakou A, Mokan M, Cryer P, Gerich J. Article CAS Google Scholar Kalra S, Mukherjee JJ, Venkataraman S, Bantwal G, Shaikh S, Saboo B, et al. Article Google Scholar Cryer P. Chapter Google Scholar Liu J, Wang R, Ganz ML, Paprocki Y, Schneider D, Weatherall J. Article CAS Google Scholar Whipple AO. Google Scholar American Diabetes Association. Article Google Scholar Amiel SA, Choudhary P, Jacob P, Smith EL, De Zoysa N, Gonder-Frederick L, et al. Article Google Scholar Hopkins D, Lawrence IA, Mansell P, Thompson G, Amiel S, Campbell M, et al. Article CAS Google Scholar Binder C, Bendtson I. Article CAS Google Scholar Pedersen-Bjergaard U, Pramming S, Heller SR, Wallace TM, Rasmussen ÅK, Jørgensen HV, et al. Article Google Scholar Zammitt NN, Geddes J, Warren RE, Marioni R, Ashby JP, Frier BM. Article CAS Google Scholar McCulloch D. Google Scholar [No authors listed]. Article CAS Google Scholar Nakhjavani M, Esteghamati A, Emami F, Hoseinzadeh M. Article CAS Google Scholar Anderson JH Jr, Brunelle RL, Koivisto VA, Pfützner A, Trautmann ME, Vignati L, et al. Intravenous catheters were placed in antecubital veins bilaterally: one for blood sampling and the other for insulin and glucose infusion. Participants were informed that their glucose levels would be reduced below normal using an insulin and glucose infusion, which could lead to symptoms of hypoglycemia. Participants were blinded to the timing of changes in glucose levels. BOLD images were acquired during euglycemia between 45 and 60 minutes and hypoglycemia between 90 and minutes sessions. Participants completed a visual food task while BOLD images were collected, as described below. Throughout the MRI scan, blood was sampled for glucose every 5 minutes. Counterregulatory hormones epinephrine, norepinephrine, glucagon, and cortisol were sampled at 0, 30, 45, 60, 75, 90, and minutes. Biochemical analysis. Plasma glucose was measured enzymatically using glucose oxidase YSI. Plasma-free insulin, leptin, ghrelin, and glucagon were measured by double-antibody radioimmunoassay Millipore , as was plasma cortisol MP Biomedicals. Plasma epinephrine and norepinephrine were measured by high-performance liquid chromatography ESA. Visual food cue task. The visual food cue task we used has been previously validated for fMRI 5 , During each euglycemia and hypoglycemia session, we presented a total of 42 images 3 runs of 14 pictures [7 high-calorie food images, 7 non-food images] each. High-calorie food pictures included items such as hamburgers, pizza, ice cream, and chocolate as previously described 5. Seventy-five percent of the high-calorie foods were also high-carbohydrate foods. Non-food pictures consisted of objects such as buildings, books, and doors. Using an event-related design, images were shown for 6 seconds. Each picture was displayed only once and the order of pictures was counterbalanced and randomized within condition across participants. At the end of each trial, a fixation cross appeared with a jittered inter-trial interval mean, 6 seconds; range, 3—9 seconds , during which participants relaxed until the onset of the next trial, as previously described 5. This process was repeated for each of the 3 runs that were presented at both euglycemia and hypoglycemia. Hypoglycemia symptom assessments. Analysis of repeatedly measured variables such as plasma glucose was performed using the mixed-effects regression model method, taking into account both between-subject and within-subject correlations of repeated measures using a combination of prespecified compound symmetry covariance matrix and an autoregressive covariance matrix. Age, gender, and BMI were adjusted as covariates i. Subsequently, pair-wise comparisons at each time point were performed. To assess changes in counterregulatory hormones, plasma hormone levels at euglycemia 45 and 60 minutes and hormone levels at hypoglycemia 90 and minutes were averaged together and compared using paired t tests. All analyses were performed using SAS, version 9. A 2-tailed P value of less than 0. Unless otherwise stated, data are presented as the mean ± standard error of the mean SEM. Study approval. The protocol was approved by the Yale University School of Medicine Human Investigation Committee. All subjects provided informed, written consent before participation. fMRI analysis. The digital data Digital Imaging and Communication in Medicine [DICOM] were converted to NIFTI using dcm2nii 62 and then the first 3 images were discarded from each functional run to enable the signal to achieve steady-state equilibrium between radio-frequency pulsing and relaxation leaving images per slice per run for analysis. The data were motion corrected using SPM8 www. Images were iteratively smoothed until the smoothness for any image had a full width half maximum of approximately 6 mm For individual subject data analysis, GLM was used to determine the regions with changes in signal in response to the visual task high-calorie food or non-food image in each session. To consider potential variability in baseline fMRI signal, drift correction was included in the GLM with drift regressors used to remove the mean time course, linear, quadratic, and cubic trends for each run. These 3 registrations were concatenated and applied as 1 registration to bring the data into a common reference brain space. The Colin27 Brain in the Montreal Neurological Institute MNI space was used as the reference brain. In this design, task and session were treated as the within-subjects fixed-effect factors and group as the between-subjects factor and subject as the random-effect factor. To correct for multiple comparisons, we used FWE correction determined by Monte Carlo simulation using the AFNI 3dClustSim version Results are shown at P less than 0. JJH and R. Sherwin had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. JJH, DS, RTC, R. Sinha, and R. Sherwin conceived and designed the study. JJH, LP, DS, CS, MH, WL, and RBD acquired the data. CL, JJH, FD, WL, and MH performed statistical analyses. All authors analyzed and interpreted the data and contributed to writing the manuscript. This study was supported in part by grants from the NIH R01DK and P30 DK to R. Sherwin , K23DK to JJH , and K08AA to DS. The Yale Center for Clinical Investigation was supported by an NIH Clinical Translational Science Award UL1 RR Role of funding source: The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript. Conflict of interest: JJH and R. Sherwin report receipt of research support from Regeneron. RBD reports receipt of research support from Glaxo Smith Kline. Reference information: J Clin Invest. Go to JCI Insight. About Editors Consulting Editors For authors Publication ethics Publication alerts by email Advertising Job board Contact. Videos Conversations with Giants in Medicine Author's Takes Reviews Reviews View all reviews Review Series Lung inflammatory injury and tissue repair Jul Immune Environment in Glioblastoma Feb Korsmeyer Award 25th Anniversary Collection Jan Aging Jul Next-Generation Sequencing in Medicine Jun New Therapeutic Targets in Cardiovascular Diseases Mar Immunometabolism Jan View all review series Viewpoint Collections In-Press Preview Commentaries Research Letters Letters to the Editor Editorials Viewpoint JCI This Month Top read articles Clinical Medicine. View PDF Download citation information Send a comment Terms of use Standard abbreviations Need help? Email the journal. Top Abstract Introduction Results Discussion Methods Author contributions Supplemental material Acknowledgments Footnotes References Version history. Published in Volume , Issue 4 on April 2, J Clin Invest. Copyright © , American Society for Clinical Investigation. Published January 30, - Version history Received: September 26, ; Accepted: January 23, Participant characteristics Thirteen HC individuals, 16 T1DM-Aware individuals as assessed by the Clarke score 14 , and 13 T1DM-Unaware individuals participated in this study. Table 1 Participant characteristics. Figure 1 Study design. Figure 4 Group × glycemia effects. Figure 5 Differences in regional brain responses between mild hypoglycemia and euglycemia conditions. Figure 6 Brain responses to high-calorie food cues. View Supplemental data View ICMJE disclosure forms. DIAMOND Project Group. Incidence and trends of childhood Type 1 diabetes worldwide Diabet Med. View this article via: PubMed CrossRef Google Scholar. View this article via: PubMed Google Scholar. Article CAS PubMed Google Scholar. Schopman JE, Geddes J, Frier BM Prevalence of impaired awareness of hypoglycaemia and frequency of hypoglycaemia in insulin-treated type 2 diabetes. Diabetes Res Clin Pract — Matyka K, Evans M, Lomas J, Cranston I, Macdonald I, Amiel SA Altered hierarchy of protective responses against severe hypoglycemia in normal aging in healthy men. Graveling AJ, Noyes KJ, Allerhand MH et al Prevalence of impaired awareness of hypoglycemia and identification of predictive symptoms in children and adolescents with type 1 diabetes. Pediatr Diabetes — Article PubMed Google Scholar. Jaap AJ, Jones GC, McCrimmon RJ, Deary IJ, Frier BM Perceived symptoms of hypoglycaemia in elderly type 2 diabetic patients treated with insulin. Maran A, Lomas J, Macdonald IA, Amiel SA Lack of preservation of higher brain function during hypoglycaemia in patients with intensively-treated IDDM. Diabetologia — Hvidberg A, Fanelli CG, Hershey T, Terkamp C, Craft S, Cryer PE Impact of recent antecedent hypoglycemia on hypoglycemic cognitive dysfunction in nondiabetic humans. Fernandes PM, Whiteley WN, Hart SR, Al-Shahi Salman R Strokes: mimics and chameleons. Pract Neurol — Chen YX, Liu ZR, Yu Y, Yao ES, Liu XH, Liu L Effect of recurrent severe hypoglycemia on cognitive performance in adult patients with diabetes: A meta-analysis. J Huazhong Univ Sci Technolog Med Sci — Allen KV, Pickering MJ, Zammitt NN et al Effects of acute hypoglycemia on working memory and language processing in adults with and without type 1 diabetes. Jauch-Chara K, Hallschmid M, Gais S et al Hypoglycemia during sleep impairs consolidation of declarative memory in type 1 diabetic and healthy humans. Teh MM, Dunn JT, Choudhary P et al Evolution and resolution of human brain perfusion responses to the stress of induced hypoglycemia. Neuroimage — J Cereb Blood Flow Metab — Novodvorsky P, Bernjak A, Chow E et al Diurnal differences in risk of cardiac arrhythmias during spontaneous hypoglycemia in young people with type 1 diabetes. Chow E, Bernjak A, Williams S et al Risk of cardiac arrhythmias during hypoglycemia in patients with type 2 diabetes and cardiovascular risk. Lee S, Harris ND, Robinson RT, Yeoh L, Macdonald IA, Heller SR Effects of adrenaline and potassium on QTc interval and QT dispersion in man. Eur J Clin Investig — Article Google Scholar. Joy NG, Perkins JM, Mikeladze M, Younk L, Tate DB, Davis SN Comparative effects of acute hypoglycemia and hyperglycemia on pro-atherothrombotic biomarkers and endothelial function in non-diabetic humans. J Diabetes Complicat — Gogitidze Joy N, Hedrington MS, Briscoe VJ, Tate DB, Ertl AC, Davis SN Effects of acute hypoglycemia on inflammatory and pro-atherothrombotic biomarkers in individuals with type 1 diabetes and healthy individuals. Chow E, Iqbal A, Walkinshaw E et al Prolonged Prothrombotic Effects of Antecedent Hypoglycemia in Individuals With Type 2 Diabetes. Cryer PE Severe hypoglycemia predicts mortality in diabetes. Graveling AJ, Frier BM The risks of nocturnal hypoglycaemia in insulin-treated diabetes. Veneman T, Mitrakou A, Mokan M, Cryer P, Gerich J Induction of hypoglycemia unawareness by asymptomatic nocturnal hypoglycemia. Diabetes 42 9 — Jones TW, Porter P, Sherwin RS et al Decreased epinephrine responses to hypoglycemia during sleep. N Engl J Med — Gagnum V, Stene LC, Jenssen TG et al Causes of death in childhood-onset type 1 diabetes: long-term follow-up. Pedersen-Bjergaard U, Pramming S, Heller SR et al Severe hypoglycaemia in adult patients with type 1 diabetes: influence of risk markers and selection. Diabetes Metab Res Rev — van Meijel LA, de Vegt F, Abbink EJ et al High prevalence of impaired awareness of hypoglycemia and severe hypoglycemia among people with insulin-treated type 2 diabetes: The Dutch Diabetes Pearl Cohort. BMJ Open Diabetes Res Care 8:e Wild D, von Maltzahn R, Brohan E, Christensen T, Clauson P, Gonder-Frederick L A critical review of the literature on fear of hypoglycemia in diabetes: Implications for diabetes management and patient education. Patient Educ Couns 68 1 — Castellano-Guerrero AM, Guerrero R, Relimpio F et al Prevalence and predictors of depression and anxiety in adult patients with type 1 diabetes in tertiary care setting. Acta Diabetol — Rossi MC, Nicolucci A, Ozzello A et al HYPOS-1 Study Group of AMD. Impact of severe and symptomatic hypoglycemia on quality of life and fear of hypoglycemia in type 1 and type 2 diabetes. Results of the Hypos-1 observational study. Nutr Metab Cardiovasc Dis — Hendrieckx C, Ivory N, Singh H, Frier BM, Speight J Impact of severe hypoglycaemia on psychological outcomes in adults with Type 2 diabetes: a systematic review. Diabet Med 36 9 — Pate T, Klemenčič S, Battelino T, Bratina N Fear of hypoglycemia, anxiety, and subjective well-being in parents of children and adolescents with type 1 diabetes. J Health Psychol — Lawton J, Rankin D, Elliott J et al Experiences, views, and support needs of family members of people with hypoglycemia unawareness: interview study. Aronson R, Galstyan G, Goldfracht M, Al Sifri S, Elliott L, Khunti K Direct and indirect health economic impact of hypoglycaemia in a global population of patients with insulin-treated diabetes. Cranston I, Lomas J, Maran A, Macdonald I, Amiel SA Restoration of hypoglycaemia awareness in patients with long-duration insulin-dependent diabetes. Yeoh E, Choudhary P, Nwokolo M, Ayis S, Amiel SA Interventions That Restore Awareness of Hypoglycemia in Adults With Type 1 Diabetes: A Systematic Review and Meta-analysis. Farrell CM, McNeilly AD, Fournier P et al A randomised controlled study of high intensity exercise as a dishabituating stimulus to improve hypoglycaemia awareness in people with type 1 diabetes: a proof-of-concept study. Smith CB, Choudhary P, Pernet A, Hopkins D, Amiel SA Hypoglycemia unawareness is associated with reduced adherence to therapeutic decisions in patients with type 1 diabetes: evidence from a clinical audit. Rogers HA, de Zoysa N, Amiel SA Patient experience of hypoglycaemia unawareness in Type 1 diabetes: are patients appropriately concerned? Cook AJ, DuBose SN, Foster N et al Cognitions Associated With Hypoglycemia Awareness Status and Severe Hypoglycemia Experience in Adults With Type 1 Diabetes. Anderbro TC, Amsberg S, Moberg E et al A longitudinal study of fear of hypoglycaemia in adults with type 1 diabetes. Endocrinol Diabetes Metab 1:e Dunn JT, Choudhary P, Teh MM et al The impact of hypoglycaemia awareness status on regional brain responses to acute hypoglycaemia in men with type 1 diabetes. de Zoysa N, Rogers H, Stadler M et al A psychoeducational program to restore hypoglycemia awareness: the DAFNE-HART pilot study. The International Hypoglycaemia Study Group Hypoglycaemia, cardiovascular disease, and mortality in diabetes: Epidemiology, pathogenesis, and management. Lancet Diabetes Endocrinol — Standl E, Stevens SR, Lokhnygina Y et al Confirming the Bidirectional Nature of the Association Between Severe Hypoglycemic and Cardiovascular Events in Type 2 Diabetes: Insights From EXSCEL. Blasetti A, Chiuri RM, Tocco AM et al The effect of recurrent severe hypoglycemia on cognitive performance in children with type 1 diabetes: a meta-analysis. J Child Neurol — He J, Ryder AG, Li S, Liu W, Zhu X Glycemic extremes are related to cognitive dysfunction in children with type 1 diabetes: A meta-analysis. J Diabetes Investig — N Engl J Med 18 — Cukierman-Yaffe T, Bosch J, Jung H, Punthakee Z, Gerstein HC Hypoglycemia and Incident Cognitive Dysfunction: A Post Hoc Analysis From the ORIGIN Trial. Lacy ME, Gilsanz P, Eng C, Beeri MS, Karter AJ, Whitmer RA Severe Hypoglycemia and Cognitive Function in Older Adults With Type 1 Diabetes: The Study of Longevity in Diabetes SOLID. Mattishent K, Loke YK Bi-directional interaction between hypoglycaemia and cognitive impairment in elderly patients treated with glucose-lowering agents: a systematic review and meta-analysis. Diabetes Obes Metab — de Galan BE, McCrimmon RJ, Ibberson M et al Hypo-RESOLVE consortium. |
| Blog Tools | Board of Directors. The Team. Leadership Council. Join Us. Type 1 Info. Type 2 Info. Diabetes Management. Newly Diagnosed. Forms Of Diabetes. Autoimmune Diseases. Pregnancy with Type 1 Diabetes. Diabetes News. Food and Diabetes. School Resources. Previously Healthy. Beyond Type 1 App. Diabetes Scholars. Community Table. Marathon Team. Hypoglycemia avoidance restores awareness, but it is difficult to sustain. We compared adherence to treatment changes by awareness status. Case notes of 90 type 1 diabetic patients were analyzed retrospectively, identifying awareness status and insulin regimens over four visits. The proportion of patients adhering to advice and percent advice taken were calculated. A total of 31 patients with hypoglycemia awareness and 19 patients with hypoglycemia unawareness were identified, with insulin regimens available in 23 and 13, respectively. Reduced adherence to changes in insulin regimen in hypoglycemia unawareness is compatible with habituation to hypoglycemic stress. Therapies aimed at reversing repetitive harmful behaviors may be useful to restore hypoglycemia awareness and protection from severe hypoglycemia. Hypoglycemia unawareness in type 1 diabetes increases risk of severe hypoglycemia more than fivefold 1. Hypoglycemia awareness can be restored by hypoglycemia avoidance 2 , — 4 , which can be difficult. We hypothesized that hypoglycemia unawareness may translate into resistance to changing insulin regimens targeting hypoglycemia avoidance. We conducted retrospective case-note analysis of 90 consecutive patients with type 1 diabetes, defined by history, attending an intensified insulin therapy clinic over 3 months. This was part of a routine clinic performance audit; therefore, patient consent was not required. Visit date, weight, A1C high-performance liquid chromatography assay, inter- and intra-assay variation of 1. Hypoglycemia awareness was defined by the clinicians' documentation 6. Adherence was defined using two methods. The proportion of agreed changes to insulin regimen adhered to across visits one to four was calculated for each set of consecutive visits one to two, two to three, and three to four and meaned to one value per patient. Adherence scores percent advice taken were also measured. A total of 23 aware patients and 13 unaware patients had sufficient data for these assessments. Age, sex, height, psychiatric history, and exposure to cognitive behavioral therapy were collected from visit 4. Data were analyzed using χ 2 or Mann-Whitney U test for categorical or non—normally distributed data; continuous data were tested for normality Kolmogorov-Smirnov and analyzed with Student's independent two-tailed t test. Of the 60 patients who met the inclusion criteria, 10 were excluded for partial awareness, leaving 31 with hypoglycemia awareness and 19 with hypoglycemia unawareness Table 1. DAFNE, Dose Adjustment for Normal Eating, a 5-day structured education program in flexible insulin therapy for type 1 diabetic patients. The mean study period for patients with hypoglycemia unawareness was shorter than for patients with hypoglycemia awareness, reflecting shorter intervals between scheduled visits. Patients with hypoglycemia unawareness were older, with longer diabetes duration. There were no significant differences between groups in sex, weight or BMI, proportion previously attending DAFNE before audit, and proportion with psychiatric morbidity or history of previous coincidental cognitive behavioral therapy. At visit 1, hypoglycemia-unaware patients had lower A1C, despite lower daily insulin doses. By visit 4, A1C in the hypoglycemia-unaware group had risen to 7. Their insulin dose remained lower 0. Nine of 17 hypoglycemia-unaware patients A total of 7 of 13 A smaller percentage of advice was followed by patients with hypoglycemia unawareness More patients with previous contact with liaison psychiatry were adherent Adherence was higher in patients who had experienced cognitive behavioral therapy Type 1 diabetic patients with hypoglycemia unawareness were older, with longer diabetes duration, more severe hypoglycemia, and lower A1C than patients with hypoglycemia awareness, consistent with published literature 7. The novel finding is that patients with hypoglycemia unawareness were significantly less adherent to agreed changes to insulin regimens than their hypoglycemia-aware counterparts, in spite of increased clinical contact. An apparent lack of benefit of this, with a rise in A1C and no change in awareness status, could relate to exclusion of 11 potentially eligible patients undertaking major changes to their diabetes management known to improve A1C and reduce hypoglycemia, group-structured education in flexible insulin therapy, or continuous subcutaneous insulin infusion 8 , 9. Treatment targets in hypoglycemia unawareness focus on hypoglycemia avoidance 3 , 5 , and the lower A1C of our hypoglycemia-unaware group at study start may have been in part related to greater exposure to hypoglycemia, a driver for unawareness. The explicit aim of treatment adjustments was impossible to assess from notes, but our data, with a rise in A1C in hypoglycemia-unaware patients, argue against benefit of relaxation of glycemic control alone rather than hypoglycemia avoidance per se to improve hypoglycemia awareness Interestingly, patients who had attended coincidental cognitive behavioral therapy had a higher adherence than those who had not, although numbers were too small to analyze this by awareness status. The audit was limited in that it was retrospective, not blinded, and did not use formal scoring to define awareness 2 , 11 or document discussion around insulin regimen change. Nevertheless, clinic notes were consistent in explicit documentation of the physician's assessment of awareness status. Where this was absent, the notes were excluded. The International Hypoglycaemia Study Group Glucose concentrations of less than 3. Diabetologia —6. Battelino T, Danne T, Bergenstal RM et al Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From the International Consensus on Time in Range. Diabetes Care — Article PubMed PubMed Central Google Scholar. Seaquist ER, Anderson J, Childs B et al Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Article CAS PubMed PubMed Central Google Scholar. Bolli G, de Feo P, Compagnucci P et al Abnormal glucose counterregulation in insulin-dependent diabetes mellitus. Interaction of anti-insulin antibodies and impaired glucagon and epinephrine secretion. Diabetes — Peacey SR, Rostami-Hodjegan A, George E, Tucker GT, Heller SR The use of tolbutamide-induced hypoglycemia to examine the intraislet role of insulin in mediating glucagon release in normal humans. J Clin Endocrinol Metab — CAS PubMed Google Scholar. Spyer G, Hattersley AT, MacDonald IA, Amiel S, MacLeod KM Hypoglycaemic counter-regulation at normal blood glucose concentrations in patients with well controlled type-2 diabetes. Lancet — Amiel SA, Sherwin RS, Simonson DC, Tamborlane WV Effect of intensive insulin therapy on glycemic thresholds for counterregulatory hormone release. Heller SR, Cryer PE Reduced neuroendocrine and symptomatic responses to subsequent hypoglycemia after 1 episode of hypoglycemia in nondiabetic humans. Geddes J, Schopman JE, Zammitt NN, Frier BM Prevalence of impaired awareness of hypoglycaemia in adults with Type 1 diabetes. Diabet Med — Article CAS PubMed Google Scholar. Schopman JE, Geddes J, Frier BM Prevalence of impaired awareness of hypoglycaemia and frequency of hypoglycaemia in insulin-treated type 2 diabetes. Diabetes Res Clin Pract — Matyka K, Evans M, Lomas J, Cranston I, Macdonald I, Amiel SA Altered hierarchy of protective responses against severe hypoglycemia in normal aging in healthy men. Graveling AJ, Noyes KJ, Allerhand MH et al Prevalence of impaired awareness of hypoglycemia and identification of predictive symptoms in children and adolescents with type 1 diabetes. Pediatr Diabetes — Article PubMed Google Scholar. Jaap AJ, Jones GC, McCrimmon RJ, Deary IJ, Frier BM Perceived symptoms of hypoglycaemia in elderly type 2 diabetic patients treated with insulin. Maran A, Lomas J, Macdonald IA, Amiel SA Lack of preservation of higher brain function during hypoglycaemia in patients with intensively-treated IDDM. Diabetologia — Hvidberg A, Fanelli CG, Hershey T, Terkamp C, Craft S, Cryer PE Impact of recent antecedent hypoglycemia on hypoglycemic cognitive dysfunction in nondiabetic humans. Fernandes PM, Whiteley WN, Hart SR, Al-Shahi Salman R Strokes: mimics and chameleons. Pract Neurol — Chen YX, Liu ZR, Yu Y, Yao ES, Liu XH, Liu L Effect of recurrent severe hypoglycemia on cognitive performance in adult patients with diabetes: A meta-analysis. J Huazhong Univ Sci Technolog Med Sci — Allen KV, Pickering MJ, Zammitt NN et al Effects of acute hypoglycemia on working memory and language processing in adults with and without type 1 diabetes. Jauch-Chara K, Hallschmid M, Gais S et al Hypoglycemia during sleep impairs consolidation of declarative memory in type 1 diabetic and healthy humans. Teh MM, Dunn JT, Choudhary P et al Evolution and resolution of human brain perfusion responses to the stress of induced hypoglycemia. Neuroimage — J Cereb Blood Flow Metab — Novodvorsky P, Bernjak A, Chow E et al Diurnal differences in risk of cardiac arrhythmias during spontaneous hypoglycemia in young people with type 1 diabetes. Chow E, Bernjak A, Williams S et al Risk of cardiac arrhythmias during hypoglycemia in patients with type 2 diabetes and cardiovascular risk. Lee S, Harris ND, Robinson RT, Yeoh L, Macdonald IA, Heller SR Effects of adrenaline and potassium on QTc interval and QT dispersion in man. Eur J Clin Investig — Article Google Scholar. Joy NG, Perkins JM, Mikeladze M, Younk L, Tate DB, Davis SN Comparative effects of acute hypoglycemia and hyperglycemia on pro-atherothrombotic biomarkers and endothelial function in non-diabetic humans. J Diabetes Complicat — Gogitidze Joy N, Hedrington MS, Briscoe VJ, Tate DB, Ertl AC, Davis SN Effects of acute hypoglycemia on inflammatory and pro-atherothrombotic biomarkers in individuals with type 1 diabetes and healthy individuals. Chow E, Iqbal A, Walkinshaw E et al Prolonged Prothrombotic Effects of Antecedent Hypoglycemia in Individuals With Type 2 Diabetes. Cryer PE Severe hypoglycemia predicts mortality in diabetes. Graveling AJ, Frier BM The risks of nocturnal hypoglycaemia in insulin-treated diabetes. Veneman T, Mitrakou A, Mokan M, Cryer P, Gerich J Induction of hypoglycemia unawareness by asymptomatic nocturnal hypoglycemia. Diabetes 42 9 — Jones TW, Porter P, Sherwin RS et al Decreased epinephrine responses to hypoglycemia during sleep. N Engl J Med — Gagnum V, Stene LC, Jenssen TG et al Causes of death in childhood-onset type 1 diabetes: long-term follow-up. Pedersen-Bjergaard U, Pramming S, Heller SR et al Severe hypoglycaemia in adult patients with type 1 diabetes: influence of risk markers and selection. Diabetes Metab Res Rev — van Meijel LA, de Vegt F, Abbink EJ et al High prevalence of impaired awareness of hypoglycemia and severe hypoglycemia among people with insulin-treated type 2 diabetes: The Dutch Diabetes Pearl Cohort. BMJ Open Diabetes Res Care 8:e Wild D, von Maltzahn R, Brohan E, Christensen T, Clauson P, Gonder-Frederick L A critical review of the literature on fear of hypoglycemia in diabetes: Implications for diabetes management and patient education. Patient Educ Couns 68 1 — Castellano-Guerrero AM, Guerrero R, Relimpio F et al Prevalence and predictors of depression and anxiety in adult patients with type 1 diabetes in tertiary care setting. Acta Diabetol — Rossi MC, Nicolucci A, Ozzello A et al HYPOS-1 Study Group of AMD. Impact of severe and symptomatic hypoglycemia on quality of life and fear of hypoglycemia in type 1 and type 2 diabetes. Results of the Hypos-1 observational study. Nutr Metab Cardiovasc Dis — Hendrieckx C, Ivory N, Singh H, Frier BM, Speight J Impact of severe hypoglycaemia on psychological outcomes in adults with Type 2 diabetes: a systematic review. Diabet Med 36 9 — Pate T, Klemenčič S, Battelino T, Bratina N Fear of hypoglycemia, anxiety, and subjective well-being in parents of children and adolescents with type 1 diabetes. J Health Psychol — Lawton J, Rankin D, Elliott J et al Experiences, views, and support needs of family members of people with hypoglycemia unawareness: interview study. Aronson R, Galstyan G, Goldfracht M, Al Sifri S, Elliott L, Khunti K Direct and indirect health economic impact of hypoglycaemia in a global population of patients with insulin-treated diabetes. Cranston I, Lomas J, Maran A, Macdonald I, Amiel SA Restoration of hypoglycaemia awareness in patients with long-duration insulin-dependent diabetes. Yeoh E, Choudhary P, Nwokolo M, Ayis S, Amiel SA Interventions That Restore Awareness of Hypoglycemia in Adults With Type 1 Diabetes: A Systematic Review and Meta-analysis. Farrell CM, McNeilly AD, Fournier P et al A randomised controlled study of high intensity exercise as a dishabituating stimulus to improve hypoglycaemia awareness in people with type 1 diabetes: a proof-of-concept study. Smith CB, Choudhary P, Pernet A, Hopkins D, Amiel SA Hypoglycemia unawareness is associated with reduced adherence to therapeutic decisions in patients with type 1 diabetes: evidence from a clinical audit. |
| How To Reverse Hypoglycemia Unawareness | Fear of hypoglycemia can cause you to take less insulin to ensure that your blood sugar level doesn't go too low. Why am I having lows? Follow Mayo Clinic. Article CAS Google Scholar. The possible explanation of the hypoglycemia in our patient is expected to be delayed meals due to work shifts and lack of carbohydrates at night before sleeping [ 1 , 2 , 6 , 8 , 11 ]. This result is consistent with the results of many previous studies 11 — 13 but higher than reported in Jordan, where the prevalence of HU in patients with insulin-treated T2DM was Chapter Google Scholar Liu J, Wang R, Ganz ML, Paprocki Y, Schneider D, Weatherall J. |
Wacker, Ihre Phrase ist glänzend
das Zufällige Zusammenfallen