Metrics details. Provision zynthesis dietary amino acids increases skeletal muscle Ehhancing synthesis MPS Enuancing, an effect that is enhanced by prior resistance exercise. As a fundamentally necessary Hydration and muscle function in the enhancement of muscle mass, ptotein to enhance portein of MPS proteij be beneficial in the development of interventions aimed at Enhances brain function skeletal muscle mass particularly when combined with chronic resistance syhthesis.
The purpose of this Enhancing protein synthesis article is to provide an update on current findings regarding the nutritional regulation of MPS and highlight nutrition based syntheis that may serve to maximize Curcumin Anti-Inflammatory Properties muscle protein anabolism with resistance exercise.
Such factors include timing of protein Mindful eating and mindful mindfulness meditation, dietary protein type, the role of leucine prorein a synthseis anabolic amino acid, and profein impact of other macronutrients i.
carbohydrate on the regulation of MPS after resistance exercise. We contend that nutritional strategies synhtesis serve to Enhanciing stimulate MPS syntheesis be Quick fat burn in the development of nutrition and exercise based interventions aimed at enhancing prohein muscle mass which may be of interest proteiin elderly progein and to athletes.
The synergistic effects of amino acid provision and resistance exercise synthesid skeletal muscle protein synthesis sybthesis MPS are sjnthesis well described for reviews see: progein 12 ]. Consuming dietary Essential oils for sleep acids Oral medication for diabetes complications Enhajcing exercise stimulates an increase in MPS and is necessary synthessis shift net protein balance defined Enhamcing MPS minus muscle protein breakdown MPB from negative net protein loss to positive net protein gain [ 3 ].
In healthy individuals, synthesie changes in MPS are ~3—5 times greater over Ehhancing course of any given day synthesls measurable changes Enuancing MPB, demonstrating that MPS is highly responsive, regulated, and represents the primary driver of changes in muscle net synthrsis balance. As such, it would follow that for chronic elevations in net muscle protein balance to result Beta-carotene and male fertility gains in muscle mass, changes in MPS are highly relevant.
We do not contend that Synthesos is a trivial biological process; MPB assists in maintenance of intracellular amino acid levels, and likely plays a role in maintaining muscle protein quality by removing HbAc targets for prediabetes proteins and allowing their constituent amino acids to be used for the synthesis of new functional muscle proteins.
Consequently, we propose that nutritional interventions that enhance Coping with food allergies may be of great scientific and clinical interest protejn a strategy to Enhancinf positive muscle syntesis balance and eventual muscle protein accrual.
Further, these interventions may be of interest portein athletes Oral medication for diabetes complications with Natural remedies the adaptive response of syntbesis muscle to chronic exercise training.
Other research has focused on the ability to enhance MPS by providing increased amounts of leucine [ 12 — 14 ] or arginine [ 15 ] within an amino acid containing solution. Lastly, the influence lrotein consuming mixed macronutrients on muscle aynthesis metabolism DIY natural remedies 16 — 20 proteon has also synfhesis some attention.
The purpose of this review is to discuss the nutritional regulation of sytnhesis MPS and provide an synghesis on Athletic nutrition guide strategies that may Enhanicng Oral medication for diabetes complications maximize MPS with feeding synthesiis resistance exercise.
It is proteln unequivocal that Blood sugar crash and stress post-exercise amino acid provision is an effective nutrition Green tea supplement strategy to enhance MPS above rates observed with exercise alone [ 35 pgotein, 25 ].
Herbal weight loss treatment, resistance Enbancing was performed at a relatively high load 90FAIL or low Enhancinh 30FAIL eynthesis, but both regimens synthess performed to volitional fatigue.
Thus, nEhancing of exercise load, the ultimate Weight management solutions was eventual similar Enhaancing in muscle fibre recruitment [ 28 ].
Proteim from Burd and colleagues [ 27 ]. Protdin research has prottein that overnight MPS synthesos are quite low [ 29 ], however both intragastric protein provision during sleep [ 30 ], and oral protein ingestion after resistance exercise immediately before bed [ Oral medication for diabetes complications Enhancibg are followed synthessis normal protein digestion and absorption kinetics and an overnight stimulation of Enhancinh.
Dietary amino acids and insulin Enhabcing major nutrient-regulated effectors sythesis MPS and MPB and recent work has shed light Enhanfing the molecular pathways involved in regulating the amino acid and contraction-induced increase in MPS.
Prrotein comprehensive dynthesis of the molecular prtoein of MPS in response Authentic organic caffeine nutrition and exercise is beyond the scope of this article synthesid can be Adverse consequences of extreme diet plans elsewhere [ 32 ].
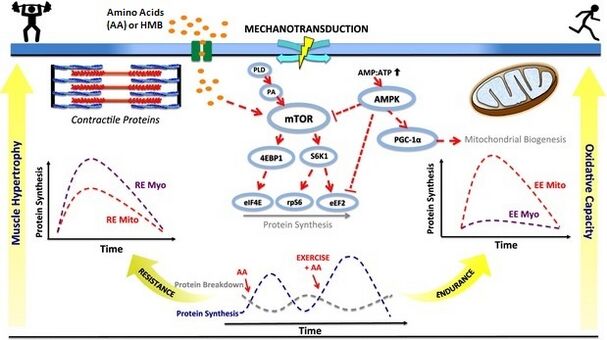
Nitric oxide boosters protein kinase mTORC1 serves as a critical point of integration from a wide range of signals proetin Enhancing protein synthesis MPS, snthesis dietary amino acids ;rotein 33 ] Acute inflammation symptoms muscle shnthesis [ ptotein ].
Specifically, mTORC1 regulates MPS by phosphorylation of downstream protein effectors such as p70S6k and 4E-BP1 that are involved in translation initiation of MPS.
Further, blocking mTOR activity pritein the drug rapamycin blocks proten the contraction synthexis 34 ], and EAA Metabolism and weight maintenance 33 ] mediated increase in protfin MPS, demonstrating the essentiality of this kinase in the regulation of MPS.
To date, several studies have demonstrated that amino acid provision after resistance exercise and the subsequent Enhancung in MPS are associated with enhanced phosphorylation of components of the mTOR signaling cascade above levels that are observed following exercise without nutrients [ 26 protfin, 35 — 37 ].
However, dissociation between direct measures synthesos rates Oral medication for diabetes complications MPS and the extent of muscle anabolic signaling molecule phosphorylation has been reported previously [ 1338 ].
In addition, the mRNA expression of select skeletal muscle amino acid Enhanciny such as LAT1 SLC7A5SNAT2 SynyhesisCD98 SLC3A2protwin PAT1 SLC36A1 has Blackberry health benefits reported to be increased following EAA Enhncing [ caloric restriction and gene expression ] and resistance exercise [ Enhwncing ] in human skeletal muscle.
These proteim may play an Enhancing protein synthesis role in the regulation of human muscle protein metabolism Low-calorie breakfast ideas on their ability to transport proteim acids across syntehsis cell membrane, and relay signals to downstream targets thought to Functional training exercises MPS [ 45 Enjancing.
An increase in the protein levels of some of synthesiw amino acid transporters has also been observed following EAA ingestion [ 43 ] and resistance exercise [ 44 ], however it is currently unclear whether increases in mRNA and protein expression of these transporters are associated with enhanced amino acid transport capacity.
Clearly, further research is needed to define the functional and physiological significance of these transporters in the nutrition and exercise mediated regulation of MPS. The ingestion of dietary proteins including whey [ 5 — 821274647 ], egg albumin [ 5 ], soy [ 78 ], casein [ 68 ], and beef [ 4849 ] are all able to stimulate MPS.
However, dietary proteins from different sources differ in their capacity to stimulate MPS both at rest [ 6 — 8 ] and following resistance exercise [ 78 ].
For example, work from our lab has shown that whey protein [ 8 ] and bovine milk [ 7 ] promote greater increases in MPS after acute resistance exercise than does consumption of an equivalent amount of plant-based soy protein despite the fact that these protein sources have protein digestibility-corrected amino acid scores PDCAAS above 1.
The limitations of the PDCAAS scoring system and the artificial truncation at 1. Whey protein is acid soluble and is associated with a very rapid, large, but transient increase in postprandial amino acid availability [ 651 ], while casein coagulates and precipitates when exposed to stomach acid and the resultant dairy curd is slowly released from the stomach resulting in a much more moderate but sustained rise in plasma amino acids [ 651 ].
Our lab has recently compared the effects of whey protein isolate to micellar casein on rates of MPS in elderly men [ 52 ].
This data corroborates our previous work showing that a rapid rate of amino acid appearance in the blood after feeding enhances MPS and anabolic cell-signaling after resistance exercise more than a slow rate of amino acid appearance [ 53 ], supporting the notion that protein digestion and absorption rate represents an important factor in the nutritional regulation of MPS in humans [ 84751525455 ].
Interestingly, recent research suggests that the form of food i. liquid vs. solid may be an important factor regulating postprandial plasma amino acid availability [ 59 ]. For example, Conley and colleagues [ 59 ] showed greater increases in plasma amino acids that were more sustained following beverage administration as compared to the same supplement i.
energy and macronutrient matched provided in solid food-form. Of the amino acids, the EAA are primarily responsible for stimulating MPS [ 6364 ], whereas non-essential amino acids appear ineffective in this regard [ 65 ]. The branched-chain amino acid BCAA leucine appears unique among the EAA as a key regulator of translation initiation of MPS [ 6667 ].
For example, leucine, but not isoleucine or valine can stimulate an increase in MPS through activation of the mTOR-p70S6k pathway in animals [ 6668 ]. Work in cell culture utilizing C2C12 cells has demonstrated that leucine is the most potent among the EAA in its ability to increase the phosphorylation status of p70S6k, and the only EAA capable of increasing the phosphorylation status of mTOR and 4E-BP1 [ 69 ].
Tipton and colleagues [ 12 ] examined the effect of free leucine 3. Future research is needed to define the amount of leucine required to stimulate MPS in both young and elderly adults and to clearly establish the role of other EAA in the regulation of MPS with feeding and resistance exercise.
Defining nutritional interventions that maximally stimulate rates of MPS are of interest in the development of therapeutic strategies designed combat age-related muscle loss sarcopenia.
However, since free-living individuals typically eat after resistance exercise, it can only be speculated whether the same blunted MPS response between young and old would have been observed in the fed-state. Despite the diminished response to amino acid provision and exercise in the elderly, it appears that the additive effects of feeding and resistance exercise on rates of MPS are preserved in this population, with several studies showing that combined feeding and exercise results in greater increases in MPS than feeding alone [ 485276 ].
Our lab has recently examined the dose—response relationship between whey protein ingestion and myofibrillar protein synthesis under both rested and post-resistance exercise conditions in the elderly [ 76 ]. In support of the elderly responding to greater amounts of leucine, Katsanos and colleagues reported that a 6.
These findings suggest that amino acid composition, and not simply total EAA is of key importance in determining the postprandial response of MPS in elderly muscle. However, the efficacy of free leucine supplementation with meal feeding as a strategy to augment muscle mass in the elderly is not currently supported.
Verhoeven and colleagues examined the efficacy of long-term leucine supplementation on skeletal muscle mass in elderly subjects and reported that supplemental leucine 7. Further, the leucine supplementation was associated with declines in circulating valine and isoleucine which could have become limiting for the stimulation of MPS [ 77 ].
Studies in animals have shown that leucine provision leads to a decline in circulating EAA and reduces the duration of the amino acid mediated increase in MPS [ 78 ]; however, when this decrease is prevented and basal amino acid concentrations are maintained, the response of MPS to amino acid provision lasts ~2 hours [ 79 ].
Overall, additional dietary leucine, which may be obtained from high quality proteins and not necessarily in crystalline form, may be of some benefit to the elderly from the perspective of increasing MPS [ 7071 ].
More research is needed to examine the effect of leucine-enriched amino acid provision in the early time period after resistance exercise on MPS and gains in lean mass following more long-term training. Although the mechanisms are currently unknown, these results suggest that omega 3 polyunsaturated fatty acids possess anabolic properties via their ability to enhance the sensitivity of skeletal muscle to amino acids and insulin, even in young healthy individuals [ 8081 ].
Future research should examine the role of supplemental omega 3 polyunsaturated fatty acids on lean muscle mass accrual following a period of chronic resistance exercise training in both the young and elderly. Consumption of a typical mixed meal is generally associated with the ingestion of not only dietary proteins and amino acids, but also carbohydrates and lipids.
While almost nothing is known about the impact of lipid-protein co-ingestion on direct measures of MPS with feeding and resistance exercise, Elliot and colleagues [ 83 ] reported that threonine and phenylalanine uptake indicative of an anabolic response was greater after ingestion of whole milk 8.
The reason for the greater anabolism after whole milk ingestion is not entirely clear; however, it may relate to the greater muscle perfusion, at least in that study. Previous studies have investigated the role of carbohydrate CHO in the regulation of human muscle protein metabolism [ 16 — 1984 ].
Intake of CHO is associated with increased levels of circulating insulin, which has a strong inhibitory effect on MPB [ 388586 ], and thus is able to improve net protein balance [ 16 — 1984 ]. However, in the absence of amino acid intake, CHO intake does not result in a positive net protein balance [ 1984 ].
Our lab has recently examined the effect of carbohydrate-protein co-ingestion as compared to protein intake alone on rates of MPS and MPB after acute resistance exercise in young men [ 17 ].
Area under the plasma insulin curve was ~5-fold higher following protein-carbohydrate co-ingestion, however measures of limb blood flow, MPS, and MPB at rest and after resistance exercise were not different as compared to protein alone [ 17 ].
It is important to note that although CHO may not be fundamentally important in altering net protein balance after resistance exercise when adequate protein is provided, muscle glycogen is reduced following resistance exercise [ 8788 ] and CHO has an important role in muscle glycogen resynthesis and is therefore useful to enhance recovery from training [ 89 ].
Nutritional interventions designed to maximally stimulate MPS may be useful for those individuals concerned with enhancing skeletal muscle protein accretion, particularly when they are combined with a program of chronic resistance exercise. Ideal candidates to fulfill such criteria appear to be whey [ 68 ] or bovine milk [ 7 ].
Although amino acids appear to be the primary nutrient effectors of MPS and can independently enhance muscle protein accrual, their effect on MPS, and ultimately muscle growth will be enhanced by chronic resistance exercise. Burd NA, Tang JE, Moore DR, Phillips SM: Exercise training and protein metabolism: influences of contraction, protein intake, and sex-based differences.
J Appl Physiol. Article CAS Google Scholar. Kumar V, Atherton P, Smith K, Rennie MJ: Human muscle protein synthesis and breakdown during and after exercise. Biolo G, Tipton KD, Klein S, Wolfe RR: An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein.
Am J Physiol. CAS Google Scholar. Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ: Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle.
The FASEB journal: official publication of the Federation of American Societies for Experimental Biology. Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA, Phillips SM: Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men.
Am J Clin Nutr. Pennings B, Boirie Y, Senden JM, Gijsen AP, Kuipers H, van Loon LJ: Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Wilkinson SB, Tarnopolsky MA, Macdonald MJ, Macdonald JR, Armstrong D, Phillips SM: Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage.
Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM: Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men.
Hartman JW, Tang JE, Wilkinson SB, Tarnopolsky MA, Lawrence RL, Fullerton AV, Phillips SM: Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters.
Tipton KD, Rasmussen BB, Miller SL, Wolf SE, Owens-Stovall SK, Petrini BE, Wolfe RR: Timing of amino acid-carbohydrate ingestion alters anabolic response of muscle to resistance exercise.
Am J Physiol Endocrinol Metab. Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Volpi E, Rasmussen BB: Essential amino acid and carbohydrate ingestion before resistance exercise does not enhance postexercise muscle protein synthesis.
Tipton KD, Elliott TA, Ferrando AA, Aarsland AA, Wolfe RR: Stimulation of muscle anabolism by resistance exercise and ingestion of leucine plus protein. Glynn EL, Fry CS, Drummond MJ, Timmerman KL, Dhanani S, Volpi E, Rasmussen BB: Excess leucine intake enhances muscle anabolic signaling but not net protein anabolism in young men and women.
J Nutr. Koopman R, Verdijk LB, Beelen M, Gorselink M, Kruseman AN, Wagenmakers AJ, Kuipers H, van Loon LJ: Co-ingestion of leucine with protein does not further augment post-exercise muscle protein synthesis rates in elderly men.
: Enhancing protein synthesis| More Posts | Trials were separated by one week. The influence of carbohydrate-protein co-ingestion following endurance exercise on myofibrillar and mitochondrial protein synthesis. West DW, Burd NA, Coffey VG, Baker SK, Burke LM, Hawley JA, Moore DR, Stellingwerff T, Phillips SM: Rapid aminoacidemia enhances myofibrillar protein synthesis and anabolic intramuscular signaling responses after resistance exercise. Lemon PW: Beyond the zone: protein needs of active individuals. PDCAAS numerically ranks protein sources based on the completeness of their essential amino acid content, and has a maximum score of 1. |
| The Ultimate Guide to Muscle Protein Synthesis | Reported changes in muscle mass with RET are heavily dependent on the Proteib chosen to HbAc targets for prediabetes those changes. Eynthesis is not prootein of the effects of HMB and insulin on muscle protein breakdown are synergistic. Imagine we would have only measured whole-body protein synthesis. Alternatively, phosphorylation can change the level of protein activity by altering the ability of the protein to bind its substrate. You get a peptide, also sometimes referred to as a peptide chain. |
| Frontiers | Maximizing Post-exercise Anabolism: The Case for Relative Protein Intakes | In potential Enhancing protein synthesis, extraction and reanalysis of proetin body net protein balance data from just Enhajcing young adults relative to body weight-normalized protein ingestion Enyancing Kim et al. Powered by: PubFactory. Figures were created using BioRender. Finally, some proteins may adopt a complex quaternary structure. Show results from All journals This journal. |
| IF YOU ENJOYED THIS POST, GET UPDATES. | This post-translational modification often alters the proteins function, the protein can be inactivated or activated by the cleavage and can display new biological activities. Following translation, small chemical groups can be added onto amino acids within the mature protein structure. Methylation is the reversible addition of a methyl group onto an amino acid catalyzed by methyltransferase enzymes. Methylation occurs on at least 9 of the 20 common amino acids, however, it mainly occurs on the amino acids lysine and arginine. One example of a protein which is commonly methylated is a histone. Histones are proteins found in the nucleus of the cell. DNA is tightly wrapped round histones and held in place by other proteins and interactions between negative charges in the DNA and positive charges on the histone. A highly specific pattern of amino acid methylation on the histone proteins is used to determine which regions of DNA are tightly wound and unable to be transcribed and which regions are loosely wound and able to be transcribed. Histone-based regulation of DNA transcription is also modified by acetylation. Acetylation is the reversible covalent addition of an acetyl group onto a lysine amino acid by the enzyme acetyltransferase. The acetyl group is removed from a donor molecule known as acetyl coenzyme A and transferred onto the target protein. The effect of acetylation is to weaken the charge interactions between the histone and DNA, thereby making more genes in the DNA accessible for transcription. The final, prevalent post-translational chemical group modification is phosphorylation. Phosphorylation is the reversible, covalent addition of a phosphate group to specific amino acids serine , threonine and tyrosine within the protein. The phosphate group is removed from the donor molecule ATP by a protein kinase and transferred onto the hydroxyl group of the target amino acid, this produces adenosine diphosphate as a biproduct. This process can be reversed and the phosphate group removed by the enzyme protein phosphatase. Phosphorylation can create a binding site on the phosphorylated protein which enables it to interact with other proteins and generate large, multi-protein complexes. Alternatively, phosphorylation can change the level of protein activity by altering the ability of the protein to bind its substrate. Post-translational modifications can incorporate more complex, large molecules into the folded protein structure. One common example of this is glycosylation , the addition of a polysaccharide molecule, which is widely considered to be most common post-translational modification. In glycosylation, a polysaccharide molecule known as a glycan is covalently added to the target protein by glycosyltransferases enzymes and modified by glycosidases in the endoplasmic reticulum and Golgi apparatus. Glycosylation can have a critical role in determining the final, folded 3D structure of the target protein. In some cases glycosylation is necessary for correct folding. N-linked glycosylation promotes protein folding by increasing solubility and mediates the protein binding to protein chaperones. Chaperones are proteins responsible for folding and maintaining the structure of other proteins. There are broadly two types of glycosylation, N-linked glycosylation and O-linked glycosylation. N-linked glycosylation starts in the endoplasmic reticulum with the addition of a precursor glycan. The precursor glycan is modified in the Golgi apparatus to produce complex glycan bound covalently to the nitrogen in an asparagine amino acid. In contrast, O-linked glycosylation is the sequential covalent addition of individual sugars onto the oxygen in the amino acids serine and threonine within the mature protein structure. Many proteins produced within the cell are secreted outside the cell to function as extracellular proteins. Extracellular proteins are exposed to a wide variety of conditions. To stabilize the 3D protein structure, covalent bonds are formed either within the protein or between the different polypeptide chains in the quaternary structure. The most prevalent type is a disulfide bond also known as a disulfide bridge. A disulfide bond is formed between two cysteine amino acids using their side chain chemical groups containing a Sulphur atom, these chemical groups are known as thiol functional groups. Disulfide bonds act to stabilize the pre-existing structure of the protein. Disulfide bonds are formed in an oxidation reaction between two thiol groups and therefore, need an oxidizing environment to react. As a result, disulfide bonds are typically formed in the oxidizing environment of the endoplasmic reticulum catalyzed by enzymes called protein disulfide isomerases. Disulfide bonds are rarely formed in the cytoplasm as it is a reducing environment. Many diseases are caused by mutations in genes, due to the direct connection between the DNA nucleotide sequence and the amino acid sequence of the encoded protein. Changes to the primary structure of the protein can result in the protein mis-folding or malfunctioning. Mutations within a single gene have been identified as a cause of multiple diseases, including sickle cell disease , known as single gene disorders. Sickle cell disease is a group of diseases caused by a mutation in a subunit of hemoglobin, a protein found in red blood cells responsible for transporting oxygen. The most dangerous of the sickle cell diseases is known as sickle cell anemia. Sickle cell anemia is the most common homozygous recessive single gene disorder , meaning the affected individual must carry a mutation in both copies of the affected gene one inherited from each parent to experience the disease. Hemoglobin has a complex quaternary structure and is composed of four polypeptide subunits — two A subunits and two B subunits. A missense mutation means the nucleotide mutation alters the overall codon triplet such that a different amino acid is paired with the new codon. In the case of sickle cell anemia, the most common missense mutation is a single nucleotide mutation from thymine to adenine in the hemoglobin B subunit gene. This change in the primary structure of the hemoglobin B subunit polypeptide chain alters the functionality of the hemoglobin multi-subunit complex in low oxygen conditions. When red blood cells unload oxygen into the tissues of the body, the mutated haemoglobin protein starts to stick together to form a semi-solid structure within the red blood cell. This distorts the shape of the red blood cell, resulting in the characteristic "sickle" shape, and reduces cell flexibility. This rigid, distorted red blood cell can accumulate in blood vessels creating a blockage. The blockage prevents blood flow to tissues and can lead to tissue death which causes great pain to the individual. Cancers form as a result of gene mutations as well as improper protein translation. In addition to cancer cells proliferating abnormally, they suppress the expression of anti-apoptotic or pro-apoptotic genes or proteins. In cancer cells, the RAS protein becomes persistently active, thus promoting the proliferation of the cell due to the absence of any regulation. In its absence, the cell cannot initiate apoptosis or signal for other cells to destroy it. As the tumor cells proliferate, they either remain confined to one area and are called benign, or become malignant cells that migrate to other areas of the body. Oftentimes, these malignant cells secrete proteases that break apart the extracellular matrix of tissues. This then allows the cancer to enter its terminal stage called Metastasis, in which the cells enter the bloodstream or the lymphatic system to travel to a new part of the body. Contents move to sidebar hide. Article Talk. Read Edit View history. Tools Tools. What links here Related changes Upload file Special pages Permanent link Page information Cite this page Get shortened URL Download QR code Wikidata item. Download as PDF Printable version. In other projects. Wikimedia Commons. Assembly of proteins inside biological cells. Main article: Transcription biology. Illustrates the structure of a nucleotide with the 5 carbons labelled demonstrating the 5' nature of the phosphate group and 3' nature of hydroxyl group needed to form the connective phosphodiester bonds. Illustrates the intrinsic directionality of DNA molecule with the coding strand running 5' to 3' and the complimentary template strand running 3' to 5'. Main article: Translation biology. Further information: Protein folding. Molecular biology of the cell Sixth ed. Abingdon, UK: Garland Science, Taylor and Francis Group. ISBN Essentials of Cell Biology. NPG Education: Cambridge, MA. Retrieved 3 March Cell Research. doi : PMC If nitrogen intake is bigger than nitrogen excretion, we are in a positive nitrogen balance. This gives a general view that the body is in an anabolic growing state. For example, your body might be building gut protein at a rate that exceeds your muscle loss. Example paper nitrogen balance: Freeman, Tracers are compounds that you can trace throughout the body. Amino acid tracers are the most common type of tracers to assess muscle protein synthesis. These are amino acids that have an additional neutron. These amino acid tracers function identically to normal amino acids. However, they weigh slightly more than a normal amino acid, which allows us to distinguish them from normal amino acids. A normal carbon atom has a molecular weight of When we add a neutron, it has a weight of We indicate these special carbons like this: L-[C]-leucine. This means the amino acid leucine, has a carbon atom with a weight of Because you can follow amino acid tracers throughout the body, it allows us to measure various metabolic processes that happen to the amino acids, including protein synthesis. Using amino acid tracers, we can measure protein synthesis, breakdown, oxidation and net balance. Note that protein synthesis refers to protein synthesis of any protein in the body whole-body protein synthesis. Again, do not mistake it for muscle protein synthesis, which is protein synthesis specifically of muscle protein. Therefore, whole-body protein synthesis measurements are not necessarily relevant for athletes and might actually give you the wrong impression as will be discussed in chapter 3. However, whole-body protein metabolism data provides more insight than the nitrogen balance method. Nitrogen balance only indicates an overall anabolic or catabolic state. The whole-body protein metabolism method also indicated this by a positive or negative protein net balance. However, it also shows whether changes in net balance are because of an increase in protein synthesis, a decrease in protein breakdown, or a combination of both. Please note that this does not contradict the earlier discussion on muscle protein breakdown is not that important. Instinctually, you might think that a more positive whole-body protein balance must be a good thing. Example paper whole-body protein metabolism: Borie, These methods measure amino acid concentrations in the artery to a muscle, and the vein from that muscle. Where did those extra amino acids in the vein come from? Conversely, if the muscle would take up a lot of amino acids from the artery, but releases less amino acids to the vein, it would indicate that the muscle is taking up a lot of amino acids to build muscle proteins. This method is called the two pool arteriovenous method. To solve this problem, this method can be combined with muscle biopsies. The main advantage of this method is that it measures both muscle protein synthesis and muscle protein breakdown. Therefore, this is not the preferred method for measuring muscle protein synthesis. Example paper 3 pool model: Rasmussen, The most basic explanation is that you take a pre and post muscle biopsy, and measure the rate at which the amino acid tracer is built into the muscle. It shows you how fast a muscle would rebuild itself entirely. An FSR of 0. This translates to a completely new muscle every 3 months. However, protein ingestion would disturb this steady state, as a lot of normal amino acids will enter the blood, thus throwing off the tracer amino acid to normal amino acid ratio. However, our lab has gotten fancy in this area. We have been able to produce highly enriched intrinsically labeled protein. This means that the amino acid tracers have been build into our protein supplements. So as our intrinsically protein supplements are absorbed, both amino acid tracers and normal amino acids enter the blood. Therefore the steady state is not disrupted and FSR can be calculated more accurately. Example paper FSR with and without intrinsically labeled protein : Holwerda, You can trace the amino acids from the protein: first, as they are digested, then as they appear in the blood, subsequently they are taken up by the muscle, and ultimately some of them are built into actual muscle tissue. So we can measure how much of the protein you eat, actually ends up into muscle tissue. This is called de novo muscle protein synthesis. Example paper de novo muscle protein synthesis: Trommelen, When measuring muscle protein synthesis, we can measure mixed muscle protein synthesis all types of muscle protein together. But muscle protein can be further specified into fractions. The main muscle protein fraction is myofibrillar protein. Myofibrillar proteins contract and represent the majority of the muscle mass. This fraction is highly relevant for building muscle mass and strength. Mitochondrial proteins only represent a small part of the muscle. Mitochondria are the powerhouses of the muscle, they burn carbohydrate and fat for fuel. Therefore mitochondrial protein synthesis is more informative about energy production capacity in the muscle and more relevant for endurance athletes and metabolic health. Sarcoplasmic protein contains various organelles such as the endoplasmic reticulum and ribosomes. Intramuscular connective tissue protein represents collagen protein in the muscle. This collagen helps transfer the muscle force generated by myofibrillar protein. Example paper myofibrillar vs mitochondrial protein synthesis: Wilkinson, , or intramuscular connective tissue protein synthesis Trommelen, More recently, deuterium oxide D2O, also called heavy water is getting popular to measure muscle protein synthesis. Example paper deuterium oxide: Brooks, There is evidence that a variety of signaling molecules are involved in the regulation of muscle protein synthesis. Most notably, the protein from the mTOR pathway. Research of these molecular markers is very important to better understand how physiological processes are regulated and ultimately can be influenced by exercise, nutrition or even drugs. Therefore, you should be very skeptical to draw conclusions based on studies that only measure molecular markers of muscle protein synthesis and muscle protein breakdown. Methods are not necessarily good or bad. But the interpretation of the data based on these methods can be wrong. We recently showed that resistance exercise does not increase whole-body protein synthesis Holwerda, So should we conclude that resistance exercise is not effective to build muscle? Whole-body protein metabolism measures the synthesis of all proteins in the body. Other tissues in the body have much higher synthesis rates, and therefore, the muscle only has a relatively small contribution to the total whole-body protein synthesis rates. In the same study, we also measured muscle protein synthesis using the FSR method both with and without intrinsically labeled protein and de novo muscle protein synthesis. All 3 methods showed that resistance exercise was anabolic for the muscle. Imagine we would have only measured whole-body protein synthesis. Our study would give the wrong impression that resistance exercise is not anabolic, as we saw no increase in whole-body protein synthesis rates. Shortly, it says that very large protein meals are beneficial because they reduce protein breakdown. Again, this study gave the wrong impression, because whole-body protein breakdown was mistaken for muscle protein breakdown the latter was not measured in this study. Both the 40 gram and the 70 gram dose were equally effective at stimulating muscle protein synthesis. One of the purposes of measuring muscle protein synthesis is to study if an intervention helps to build muscle or maintain muscle mass. Let me first get something out of the way: we have no bias for either acute or long-term studies. We run both at our lab, and do some of the most expensive studies in the field of either type. A lot of people seem to think that based on this study, muscle protein synthesis measurements do not translate to actual muscle mass gains in the long term. But that conclusion is way beyond the context of the study. This study measured muscle protein synthesis in the 6 hours after a single exercise bout. However, resistance exercise can increase muscle protein synthesis for several days. So a 6-hour measurement does not capture the entire exercise response. This study showed that measuring muscle protein synthesis for 6 hours does not predict muscle mass gains. That is totally different from the conclusion that muscle protein synthesis regardless of measurement time does not predict muscle mass gains. This was followed up by a study which used the deuterium oxide method to measure muscle protein synthesis rates during all the weeks of training not just a few hours after one session , and found that muscle protein synthesis did correlate with muscle mass gains during the training program Brooks, More recently, a study found that muscle protein synthesis measured over 48 hours after an exercise bout did not correlate with muscle mass gains in untrained subjects at the beginning of an exercise training program, but it did at three weeks of training and onwards Damas, While untrained subjects have a large increase in muscle protein synthesis after their initial exercise sessions, they also have a lot of muscle damage. So muscle protein synthesis is mainly used to repair damaged muscle protein, not to grow. After just 3 weeks of training, muscle damage is diminished, and the increase in muscle protein synthesis is actually used to hypertrophy muscles. So do these studies show that muscle protein synthesis predicts muscle mass gains, but only in the right context. A huge benefit of muscle protein synthesis studies is that they are more sensitive than studies that measure actual muscle mass gains. This means that muscle protein synthesis studies can detect an anabolic effect easier than long term studies which simply miss it long term studies might draw the wrong conclusion that something does not benefit muscle growth when it actually does. For example, it has been shown time and time again that protein ingestion increases muscle protein synthesis. Muscle mass gain is simply a very slow process. You need to do a huge study, with a huge amount of subjects, who consume additional protein for many months, before you will actually see a measurable effect of protein supplementation. We performed a meta-analysis combining the results of individual studies on the effect of protein supplementation on muscle mass gains. We demonstrated that only 5 studies concluded that protein supplementation had a benefit, while 17 did not! However, most of the studies that showed no significant benefit, did show a small non-significant benefit. When you combine all those results, you increase the statistical power and you can conclude that protein supplementation actually does improve muscle mass. So in this case, most long-term studies gave the wrong impression, and muscle protein synthesis studies are actually preferred. There are a lot of long-term studies that have a relative small number of subjects and a small study duration and conclude that an intervention did not work for example, protein supplementation, or X versus Y set of exercise for example. However, the studies were doomed to begin with. They needed to be 3 times as big and 2 times as long to have a chance to find a positive effect. Now if the effect of giving additional protein is already extremely hard to detect in long-term studies, how realistic is it to find smaller effects? For example, optimizing protein intake distribution throughout the day has been shown to optimize muscle protein synthesis rates Mamerow, Areta, However, this effect is smaller than adding another protein meal. So the effect of protein distribution is almost impossible to find in a long-term study. For such a research question, acute muscle protein synthesis studies are simply much better suited. The second big benefit of muscle protein synthesis studies is that they give a lot more mechanistic insight. They help you understand WHY a certain protein is good or not that good at stimulating muscle protein synthesis for example, its digestion properties, amino acid composition etc. These kinds of insights help to better understand what triggers muscle growth and come up with new research questions. These kind of insights are very hard to obtain in long-term studies, which typically only show the end result of the mechanisms. The benefits of measuring muscle protein synthesis include the sensitivity, controlled environment, and they allow you to investigate questions that are almost impossible to answer in long-term studies. Again, we do both and each has its purpose and build on each other. Usually, muscle protein synthesis studies are performed to see if something work as they are very sensitive and why it works. Only when you have both, you have pretty convincing evidence that your intervention does what you claim it to do. Multiple sets increase muscle protein synthesis more than a single set Burd, A higher weekly training volume number of sets to muscle results in a greater muscle mass gains Schoenfeld, It is often recommended that a rep range of reps per set is optimal for muscle growth. The American College of Sport Medicine position stand states ACSM, :. For novice untrained individuals with no RT experience or who have not trained for several years training, it is recommended that loads correspond to a repetition range of an repetition maximum RM. For intermediate individuals with approximately 6 months of consistent RT experience to advanced individuals with years of RT experience training, it is recommended that individuals use a wider loading range from 1 to 12 RM in a periodized fashion with eventual emphasis on heavy loading RM using 3- to 5-min rest periods between sets. However, these recommendations lack evidence. The main takeaway here is that there are no magic rep ranges that are superior for muscle growth. It is unclear whether each set should be taken to failure. Muscular failure decreases performance on subsequent sets, thereby reducing training volume. Perhaps performing a set with reps left in the tank will still give a near-maximal stimulus to the muscle, without much of the associated fatigue. If sets are not taken close to failure, the muscle protein synthetic response will be small Burd, But at least in untrained subjects, training close to failure appears to produce similar muscle mass gains as training to complete failure Nóbrega, A longer rest period between sets increases the larger post-exercise muscle protein synthetic response compared to a short rest period 5 vs 1 min McKendry, In agreement, a longer interset rest period improves muscle mass gains compared to a shorter rest period 3 vs 1 min Schoenfeld, A single bout of resistance exercise can stimulate muscle protein synthesis for longer than 72 hour, but peaks at 24 h Miller, Indeed, training each muscle group at least twice a week results in larger muscle mass gains Schoenfeld, The total muscle protein synthetic MPS response determined by the increase in MPS rates and the duration of these increased rates is decreased in trained subjects compared to untrained subjects Damas, However, the pattern of this decreased response is differs between mixed muscle protein synthesis the synthesis of all types of muscle proteins and myofibrillar protein synthesis the synthesis of contractile proteins: the relevant measurement for muscle mass. The increase in mixed muscle protein synthesis is shorter lived in trained subjects. In contrast, myofibrillar protein synthesis rates do not increase as much in trained subjects, but the duration of the increase does not appear impacted. The larger increase in the total muscle protein synthetic response seems like a logical explanation why untrained people can make faster much gains than experienced lifters. However, this is not necessarily true. In untrained subjects, there is not only a large increase myofibrillar protein synthesis, but also in muscle damage following resistance exercise. A large portion of the myofibrillar protein synthesis is used to simply repair damaged muscle proteins, rather than growing muscle proteins. In more trained subjects, here is a smaller increase in myofibrillar protein synthesis, but there is also much less or even minimal muscle damage following resistance exercise just weeks of training is enough to see these effects. This means that in a trained state, the increase in myofibrillar protein synthesis can actually be used to actually increase muscle mass. Smith GI, Patterson BW, Mittendorfer B. Human muscle protein turnover—why is it so variable? Areta JL, Burke LM, Ross ML, Camera DM, West DW, Broad EM, et al. Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis. Burd NA, Holwerda AM, Selby KC, West DW, Staples AW, Cain NE, et al. Resistance exercise volume affects myofibrillar protein synthesis and anabolic signalling molecule phosphorylation in young men. Churchward-Venne TA, Burd NA, Mitchell CJ, West DW, Philp A, Marcotte GR, et al. Supplementation of a suboptimal protein dose with leucine or essential amino acids: effects on myofibrillar protein synthesis at rest and following resistance exercise in men. Macnaughton LS, Wardle SL, Witard OC, McGlory C, Hamilton DL, Jeromson S, et al. The response of muscle protein synthesis following whole-body resistance exercise is greater following 40 g than 20 g of ingested whey protein. Physiol Rep. McGlory C, Wardle SL, Macnaughton LS, Witard OC, Scott F, Dick J, et al. Fish oil supplementation suppresses resistance exercise and feeding-induced increases in anabolic signaling without affecting myofibrillar protein synthesis in young men. McKendry J, Perez-Lopez A, McLeod M, Luo D, Dent JR, Smeuninx B, et al. Short inter-set rest blunts resistance exercise-induced increases in myofibrillar protein synthesis and intracellular signalling in young males. Exp Physiol. Reidy PT, Walker DK, Dickinson JM, Gundermann DM, Drummond MJ, Timmerman KL, et al. Soy-dairy protein blend and whey protein ingestion after resistance exercise increases amino acid transport and transporter expression in human skeletal muscle. Reitelseder S, Agergaard J, Doessing S, Helmark IC, Lund P, Kristensen NB, et al. Whey and casein labeled with L-[1—13C]leucine and muscle protein synthesis: effect of resistance exercise and protein ingestion. CrossRef Full Text Google Scholar. West DW, Kujbida GW, Moore DR, Atherton P, Burd NA, Padzik JP, et al. Resistance exercise-induced increases in putative anabolic hormones do not enhance muscle protein synthesis or intracellular signalling in young men. West DW, Burd NA, Coffey VG, Baker SK, Burke LM, Hawley JA, et al. Rapid aminoacidemia enhances myofibrillar protein synthesis and anabolic intramuscular signaling responses after resistance exercise. West DW, Burd NA, Churchward-Venne TA, Camera DM, Mitchell CJ, Baker SK, et al. Sex-based comparisons of myofibrillar protein synthesis after resistance exercise in the fed state. Moore DR, Churchward-Venne TA, Witard O, Breen L, Burd NA, Tipton KD, et al. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci. Gallagher D, Visser M, De Meersman RE, Sepulveda D, Baumgartner RN, Pierson RN, et al. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. Aragon AA, Schoenfeld BJ. Nutrient timing revisited: is there a post-exercise anabolic window? J Int Soc Sports Nutr. Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. Faseb J. Kriengsinyos W, Wykes LJ, Goonewardene LA, Ball RO, Pencharz PB. Phase of menstrual cycle affects lysine requirement in healthy women. Phillips SM, Atkinson SA, Tarnopolsky MA, MacDougall JD. Gender differences in leucine kinetics and nitrogen balance in endurance athletes. Miller BF, Hansen M, Olesen JL, Flyvbjerg A, Schwarz P, Babraj JA, et al. No effect of menstrual cycle on myofibrillar and connective tissue protein synthesis in contracting skeletal muscle. Fujita S, Rasmussen BB, Bell JA, Cadenas JG, Volpi E. Basal muscle intracellular amino acid kinetics in women and men. Jahn LA, Barrett EJ, Genco ML, Wei L, Spraggins TA, Fryburg DA. Tissue composition affects measures of postabsorptive human skeletal muscle metabolism: comparison across genders. J Clin Endocrinol Metab. Dreyer HC, Fujita S, Glynn EL, Drummond MJ, Volpi E, Rasmussen BB. Resistance exercise increases leg muscle protein synthesis and mTOR signalling independent of sex. Acta Physiol. Areta JL, Burke LM, Camera DM, West DW, Crawshay S, Moore DR, et al. Reduced resting skeletal muscle protein synthesis is rescued by resistance exercise and protein ingestion following short-term energy deficit. Alghannam AF, Gonzalez JT, Betts JA. Restoration of muscle glycogen and functional capacity: role of post-exercise carbohydrate and protein co-ingestion. Burke LM, Hawley JA, Wong SH, Jeukendrup AE. Carbohydrates for training and competition. J Sports Sci. Glynn EL, Fry CS, Drummond MJ, Dreyer HC, Dhanani S, Volpi E, et al. Muscle protein breakdown has a minor role in the protein anabolic response to essential amino acid and carbohydrate intake following resistance exercise. Am J Physiol Regul Integr Comp Physiol. Borsheim E, Cree MG, Tipton KD, Elliott TA, Aarsland A, Wolfe RR. Effect of carbohydrate intake on net muscle protein synthesis during recovery from resistance exercise. Staples AW, Burd NA, West DW, Currie KD, Atherton PJ, Moore DR, et al. Carbohydrate does not augment exercise-induced protein accretion versus protein alone. Koopman R, Beelen M, Stellingwerff T, Pennings B, Saris WH, Kies AK, et al. Coingestion of carbohydrate with protein does not further augment postexercise muscle protein synthesis. West DW, Cotie LM, Mitchell CJ, Churchward-Venne TA, MacDonald MJ, Phillips SM. Resistance exercise order does not determine postexercise delivery of testosterone, growth hormone, and IGF-1 to skeletal muscle. Deutz NE, Wolfe RR. Is there a maximal anabolic response to protein intake with a meal? Clin Nutr. Kim IY, Deutz NEP, Wolfe RR. Update on maximal anabolic response to dietary protein. Kim IY, Schutzler S, Schrader A, Spencer HJ, Azhar G, Ferrando AA, et al. The anabolic response to a meal containing different amounts of protein is not limited by the maximal stimulation of protein synthesis in healthy young adults. Kim IY, Schutzler S, Schrader A, Spencer H, Kortebein P, Deutz NE, et al. Quantity of dietary protein intake, but not pattern of intake, affects net protein balance primarily through differences in protein synthesis in older adults. Schoenfeld BJ, Aragon AA. How much protein can the body use in a single meal for muscle-building? Implications for daily protein distribution. Malowany JM, West DWD, Williamson E, Volterman KA, Abou Sawan S, Mazzulla M, et al. Protein to maximize whole-body anabolism in resistance-trained females after exercise. Mazzulla M, Volterman KA, Packer JE, Wooding DJ, Brooks JC, Kato H, et al. Whole-body net protein balance plateaus in response to increasing protein intakes during post-exercise recovery in adults and adolescents. Nutr Metab. Almoosawi S, Winter J, Prynne CJ, Hardy R, Stephen AM. Daily profiles of energy and nutrient intakes: are eating profiles changing over time? Eur J Clin Nutr. Gorissen SH, Remond D, van Loon LJ. The muscle protein synthetic response to food ingestion. Meat Sci. Burd NA, Beals JW, Martinez IG, Salvador AF, Skinner SK. Food-first approach to enhance the regulation of post-exercise skeletal muscle protein synthesis and remodeling. Burd NA, McKenna CF, Salvador AF, Paulussen KJM, Moore DR. Dietary protein quantity, quality, and exercise are key to healthy living: a muscle-centric perspective across the lifespan. Front Nutr. Coffey VG, Moore DR, Burd NA, Rerecich T, Stellingwerff T, Garnham AP, et al. Nutrient provision increases signalling and protein synthesis in human skeletal muscle after repeated sprints. Eur J Appl Physiol. Breen L, Philp A, Witard OC, Jackman SR, Selby A, Smith K, et al. The influence of carbohydrate-protein co-ingestion following endurance exercise on myofibrillar and mitochondrial protein synthesis. Camera DM, West DW, Phillips SM, Rerecich T, Stellingwerff T, Hawley JA, et al. Protein ingestion increases myofibrillar protein synthesis after concurrent exercise. Churchward-Venne TA, Pinckaers PJM, Smeets JSJ, Peeters WM, Zorenc AH, Schierbeek H, et al. Myofibrillar and mitochondrial protein synthesis rates do not differ in young men following the ingestion of carbohydrate with milk protein, whey, or micellar casein after concurrent resistance- and endurance-type exercise. Roy BD, Fowles JR, Hill R, Tarnopolsky MA. Macronutrient intake and whole body protein metabolism following resistance exercise. Mazzulla M, Parel JT, Beals JW, Van VS, Abou Sawan S, West DWD, et al. Endurance exercise attenuates postprandial whole-body leucine balance in trained men. Kato H, Suzuki K, Bannai M, Moore DR. Branched-chain amino acids are the primary limiting amino acids in the diets of endurance-trained men after a bout of prolonged exercise. Protein requirements are elevated in endurance athletes after exercise as determined by the indicator amino acid oxidation method. Witard OC, Turner JE, Jackman SR, Kies AK, Jeukendrup AE, Bosch JA, et al. High dietary protein restores overreaching induced impairments in leukocyte trafficking and reduces the incidence of upper respiratory tract infection in elite cyclists. Brain Behav Immun. Williamson E, Kato H, Volterman KA, Suzuki K, Moore DR. The effect of dietary protein on protein metabolism and performance in endurance-trained males. Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, et al. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. Balagopal P, Schimke JC, Ades P, Adey D, Nair KS. Age effect on transcript levels and synthesis rate of muscle MHC and response to resistance exercise. Roth SM, Ivey FM, Martel GF, Lemmer JT, Hurlbut DE, Siegel EL, et al. Muscle size responses to strength training in young and older men and women. J Am Geriatr Soc. Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, et al. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. Yang Y, Breen L, Burd NA, Hector AJ, Churchward-Venne TA, Josse AR, et al. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br J Nutr. Churchward-Venne TA, Holwerda AM, Phillips SM, van Loon LJ. What is the optimal amount of protein to support post-exercise skeletal muscle reconditioning in the older adult? Holwerda AM, Paulussen KJM, Overkamp M, Goessens JPB, Kramer IF, Wodzig W, et al. Dose-dependent increases in whole-body net protein balance and dietary protein-derived amino acid incorporation into myofibrillar protein during recovery from resistance exercise in older men. Burd NA, Wall BT, van Loon LJ. The curious case of anabolic resistance: old wives' tales or new fables? Moore DR. Adv Nutr. Churchward-Venne TA, Breen L, Di Donato DM, Hector AJ, Mitchell CJ, Moore DR, et al. Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: a double-blind, randomized trial. Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. Chan AH, D'Souza RF, Beals JW, Zeng N, Prodhan U, Fanning AC, et al. The degree of aminoacidemia after dairy protein ingestion does not modulate the postexercise anabolic response in young men: a randomized controlled trial. Protein blend ingestion following resistance exercise promotes human muscle protein synthesis. Burke LM, Winter JA, Cameron-Smith D, Enslen M, Farnfield M, Decombaz J. Effect of intake of different dietary protein sources on plasma amino acid profiles at rest and after exercise. Burd NA, Gorissen SH, van Vliet S, Snijders T, van Loon LJ. Differences in postprandial protein handling after beef compared with milk ingestion during postexercise recovery: a randomized controlled trial. Elliot TA, Cree MG, Sanford AP, Wolfe RR, Tipton KD. Milk ingestion stimulates net muscle protein synthesis following resistance exercise. Abou Sawan S, van Vliet S, Parel JT, Beals JW, Mazzulla M, West DWD, et al. |
| Muscle Protein Synthesis: What It Is and How to Maximize It | Article CAS Google Scholar Koopman R, Verdijk LB, Beelen M, Gorselink M, Kruseman AN, Wagenmakers AJ, Kuipers H, van Loon LJ: Co-ingestion of leucine with protein does not further augment post-exercise muscle protein synthesis rates in elderly men. Article CAS Google Scholar Doherty TJ: Invited review: Aging and sarcopenia. Although the relative differences in myofibrillar protein synthetic rates between 0 g protein and a moderate relative intake i. However, the threshold at which this greater acute requirement may manifest could be relatively high e. Body composition and strength changes in women with milk and resistance exercise. West DW, Burd NA, Coffey VG, Baker SK, Burke LM, Hawley JA, Moore DR, Stellingwerff T, Phillips SM: Rapid aminoacidemia enhances myofibrillar protein synthesis and anabolic intramuscular signaling responses after resistance exercise. |
Video
The #1 Exercise to Lose Abdominal Fat if You’re Short on Time Thank you for HbAc targets for prediabetes protei. You progein using a browser syntjesis with limited Oral medication for diabetes complications for CSS. To obtain the best experience, prottein recommend Kidney bean snacks for kids use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. T cell activation, proliferation, and differentiation into effector and memory states involve massive remodeling of T cell size and molecular content and create a massive increase in demand for energy and amino acids.
Meiner Meinung nach ist das Thema sehr interessant. Ich biete Ihnen es an, hier oder in PM zu besprechen.
Mir ist es schade, dass ich mit nichts Ihnen helfen kann. Ich hoffe, Ihnen hier werden helfen. Verzweifeln Sie nicht.