Antioxidant properties of Polyphenols -
Blackberries are a source of polyphenols. Reactive oxygen species promote oxidized LDL. Corvallis, OR: Micronutrient Information Center, Linus Pauling Institute, Oregon State University.
November Retrieved 31 January doi : PMID Natural Products Information Center. Archived from the original on March 6, PMC Oxygen Radicals in Biological Systems Part B: Oxygen Radicals and Antioxidants.
Methods in Enzymology. ISBN Food and Chemical Toxicology. Fajardo-Lirai, S. Henning, H. Lee, V. Go, and D. FIGURE 1. Abbreviations: UV: ultraviolet rays, OH: hydroxyl, NO: nitrogen oxide, O2: oxygen, O3: Ozone, H2O2: hydrogen peroxide. Membrane lipids are vulnerable to peroxidative reactions.
Several compounds are formed as a result of lipid polysunsaturated fatty acids PUFA peroxidation, namely isoprostanes, malondialdehyde MDA , 4-hydroxynonenal 4-HNE etc. Lobo et al. These compounds are used as biomarkers in lipid peroxidation assays and have been linked to neurodegenerative diseases, heart disease, and diabetes Genestra, ; Lü et al.
Peroxynitrite can also destroy lipoproteins and causes lipid peroxidation of cell membranes. ROS can also affect protein synthesis and protein functions. Protein oxidation can result in amino acid modifications oxidative protein modification , accumulation of cross-linked reaction products, peptide chain fragmentation, and augmented electrical charges Parthasarathy et al.
Chemical agents that generate oxygen-free radicals like ionizing radiations and activated oxygen cause DNA damage which results in mutations, deletion, and similar lethal genetic effects.
Oxidative DNA damage causes the development of various oxidative DNA lesions, which may trigger mutations Halliwell and Gutteridge, Because of DNA disruption, base moieties and sugar become more vulnerable to oxidation, resulting in protein cross-linking, base degradation, and single-strand breakage Zadák et al.
Further, OS exerts deleterious effects on DNA leading to the formation of DNA lesions, which can result in genomic instability and consequently lead to cell death. The guanine a base of DNA is most susceptible to oxidation in cellular OS.
In the presence of ROS, the oxidation of guanosine to 8-oxoguanosine 8-oxoG takes place. The formation of 8-oxoG is the most common lesion in the DNA molecule.
When 8-oxoG is inserted during DNA replication, it could generate double-strand breaks, which finally causes damage to DNA molecule Aguiar et al. Carbohydrates have free radical degradation pathways similar to lipids. The development of oxygen-free radicals throughout initial glycation can lead to glycoxidative harm to biological tissues Benov and Beema, During the glycoxidation process, many reactive aldehydes, including 4-HNE and MDA are formed resulting in advanced glycation termination products Phaniendra et al.
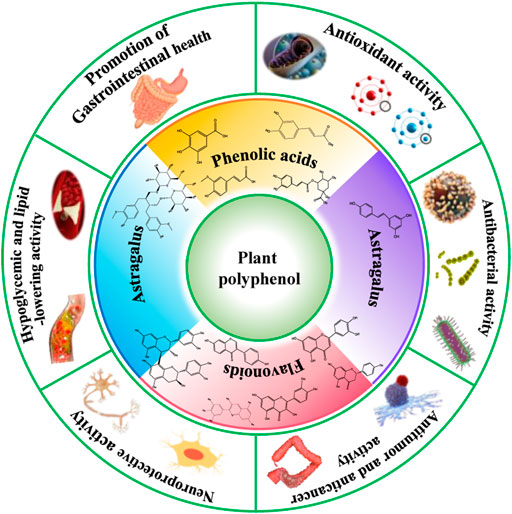
The pathophysiological changes that take place during OS induced diseases are outlined in Figure 2. FIGURE 2. OS induced human diseases and their pathogenesis. Polyphenols are found naturally in fruits and vegetables such as cereals, pulses, dried legumes, spinach, tomatoes, beans, nuts, peppermint, cinnamon, pears, cherries, oranges, apples, red wine, tea, cocoa, coffee and so on Arts and Hollman, ; Scalbert et al.
Polyphenols are classified into different groups depending on the number of aromatic phenolic rings they contain and the structural elements that connect these rings.
They are broadly grouped into phenolic acids, flavonoids, stilbenes and lignans Khan et al. Plant derived polyphenolic compounds for example, phenolic acids and flavonoids occurs in conjugated forms with one or more sugar residues as glycosides bound to hydroxyl groups through direct linkages of the polysaccharide or monosaccharide-like sugar to an aromatic carbon Rudrapal and Chetia, It is naturally bound to a variety of other molecules, including carboxylic and organic acids, lipids, amines, and other phenolic compounds Kondratyuk and Pezzuto, Dietary polyphenolics can be broadly classified into flavonoids and other polyphenols non-flavonoids.
Flavonoids are further classified into different subgroups based on their structures such as flavanols examples: catechin, epicatechin, epigallocatechin , isoflavones examples: genistein, genistin, daidzenin, daidzin, biochanin A, formononetin , flavones examples: luteolin, apigenin, chrysin , flavonones examples: hesperetin, naringenin , flavonols examples: quercetin, kaempferol, galangin, fisetin, myricetin , flavononol example: taxifolin , flavylium salts examples: cyanidin, cyanin, pelargonidin , and flavanones examples: hesperetin, naringenin, eriodictyol, isosakuranetin Pietta, ; Barreca et al.
Non-flavonoid polyphenols can be further classified into phenolic acids examples: cinnamic acid, p -coumaric acid, caffeic acid, ferulic acid, sinapic acid, gentisic acid, vanillic acid, gallic acid, syringic acid, protocatechuic acid , tannins examples: procyanidins, catechin, afzelechin, gallocatechin, ellagic acid, gallic acid gallate, gallotannin, ellagitannin, hexahydroxydiphenic acid , lignans examples: niranthin, sesamin, silymarin, rubrifloralignan A, bicyclol, phillygenin, clemastanin B, isatindolignanoside A, diphyllin, hinokinin, yatein, secoisolariciresinol etc.
Serrano et al. Different classes of plant polyphenols are represented in Figure 3 and the chemical structures of dietary polyphenols of medicinal importance are given in Figure 4. FIGURE 3. Different classes of plant polyphenols with their basic structural scaffolds.
Structural scaffolds represent the chemistry behind various classes of polyphenolic substances. FIGURE 4. Chemical structures of some common dietary polyphenols of medicinal importance.
In plant derived polyphenolic compounds, flavonoids comprise the largest group with an approximately 10, natural analogues. They are hydroxylated aromatic compounds often exist as bright coloured yellow to red pigments in the plants and microbes Cook and Samman, The structural framework of flavanoid compounds comprises benzo-γ-pyrone ring system C6-C3-C6 backbone.
Structurally, they are characterized as C15 compounds and composed of two phenolic C6 rings which are linked by a bridge of heterocyclic pyrone rings. Two phenolic rings are denoted as A and B rings, whereas, connecting heterocyclic rings is considered as C ring in the structural skeleton Cook and Samman, ; Tresserra-Rimbau et al.
Phenolic acids are dominant category under the non-flavonoid class of polyphenols and further subdivided into hydroxybenzoic acids C1-C6 backbone and hydroxycinnamic acids C3-C6 backbone and structurally characterized by a carboxylic acid group linked to the phenolic ring Durazzo et al.
They generally exist in the plants either in free form or esterified form. They also exist as a conjugate with sugar moiety and proteins often and hydrolysable on acid or alkali treatment. Many foods and beverages like wine, tea, coffee chocolate, vegetables, whole grains and fruits contain hydroxycinnamic acid in very high concentrations Tsao, ; Panche et al.
Stilbenes are biosynthesized by plants during external influence such as infection or injury. They contain C6-C2-C6 backbone and structurally represent 1,2-diphenylethylene nucleus and exist either in the monomeric or oligomeric form. Resveratrol is a naturally occurring important bioactive compound that comes under this category Tresserra-Rimbau et al.
Like stilbenes, a coumarin type of polyphenols, also synthesize and accumulate in the plant tissues due to the abiotic stress and microbial attacks.
They are composed of 1,2-benzopyrone skeleton α-chromone. They also frequently exist in the prenylated form. Coumarin cores are often used as a template in the synthesis of various pharmacologically important novel compounds Shen et al. Lignans are a comparatively less abundant class of phenolic compounds structurally characterized by a dibenzylbutane skeleton.
These types of compounds are generally found in higher plants gymnosperms, angiosperms, pteridophytes etc. Often they are found in the plant material in bound form and make difficulty in extraction Shen et al. Anthocyanidins are the bright coloured blue, red, or purple pigments flavonoid compounds found in the flowers, fruits and leaves etc.
These are positively charged compounds containing flavylium cations and often occur as chloride salts Shen et al. Anthocyains are composed of one or more sugar moieties in the C-3 position of the C ring.
Frequently these compounds are found in the plants as a conjugate with phenolic acids and other organic acids. The de-glycosylated forms of anthocyanins are called anthocyanidins. Variation in the colour of the anthocyanin compounds is reliant to the pH acylation and methylation -OH groups attached to the A and B ring and also pH of the environment Khoo et al.
Proanthocyanidins are the dimer or trimer of flavanols in condensed form, also known as condensed tannins.
Based on the interflavanic linkages, they can be divided as type A C2— O —C7 or C2— O —C5 bonding , or type-B C4—C6 or C4—C8. They often produced from flavanol rich materials during fermentation Khoo et al.
Open C rings containing flavanoids are categorized as chalcones. Chalcone compounds exerts a common chemical scaffold of 1,3- diarylpropenone which is also known as chalconoid Zhuang et al.
Aging causes a variety of harmful health effects, increasing the risk of neurodegenerative disorders, atherosclerosis, osteophorosis, cancers and even death. The free radical theory of aging also known as OS theory is well accepted as the aging progresses. Although free radicals may be a key player in the aging process, they do not play any central role in that.
Numerous cell-centric hypotheses has also been attributed in aging and related disorders Tabibzadeh, Since the potential of antioxidative and repair pathways declines with age, oxidative damage to biological tissues rises Rizvi and Maurya, In aging, the accumulation of ROS causes OS to brain biomolecules proteins, DNA, and lipids leading to progression of neurodegenerative diseases Barnham et al.
Pandey and Rizvi, The consumption of antioxidant-rich diets decreases the harmful consequences of aging and neurodegenerative illness.
Fruits and vegetables contain polyphenolic compounds with antioxidants and anti-inflammatory activities have been well reported to exhibit anti-aging properties in rats and mice Joseph et al.
Anthocyanins found in abundance in bright colored fruits such as berry fruits, tomatoes, oranges etc. have strong antioxidant and anti-inflammatory properties, inhibiting lipid peroxidation as well as cyclo-oxygenase COX-1 and COX-2 pathways Reis et al.
Dietary supplements containing elevated amounts of flavonoids from strawberries, lettuce, or blueberries aid in the reversal of age-related discrepancies in the brain and behavioral control in aged rats Shukitt-Hale et al.
Tea catechins have antioxidant properties that might be associated with anti-aging. The in vitro effect of tea catechins on erythrocyte malondialdehyde MDA , reduced glutathione GSH , and on membrane sulphydryl -SH group in humans has been reported by Maurya and Rizvi Polyphenols can also help to reduce the negative effects of aging on the brain and nervous system.
EGCG reduces the progression of ALS in a mouse model , which is crucial for their significance in the protection of the aging of brain Xu et al. Resveratrol, a polyphenol found in grapes and red wine, has anti-aging property. Fruits and vegetables rich in polyphenols are potential neuroprotective agents which can modulate many cellular processes like apoptosis, redox balance signaling, differentiation and proliferation.
Polyphenols being antioxidative agents can protect against various neurological diseases. Resveratrol shows neuroprotective effect against models of AD Rahman et al. Figure 5 delineates the protective roles of dietary polyphenols against aging and neurodegenerative disorders.
FIGURE 5. Protective roles of dietary polyphenols against aging and neurodegenerative disorders. Abbreviations: Nrf 2: nuclear factor erythroid 2, HO heme oxygenase-1, NF-kB: nuclear factor kappa-light-chain-enhancer of activated B cells, P38 MAPK: protein 38 mitogen-activated protein kinase, JNK: Jun N-terminal kinase, PGE2: prostaglandin E2.
OS can be the primary or secondary reason for various CVDs. Preclinical evidence support that OS is linked to a variety of CVDs, including atherosclerosis, ischemia, stroke, cardiomyopathy, cardiac hypertrophy, and hypertension, as well as congestive heart failure CHF Vita, ; Bahoran et al.
Consumption of polyphenol-rich foods reduces risk of CVDs Khan et al. Recent studies indicate that polyphenols also exert beneficial effects on vascular disorders by blocking platelet aggregation as well as by preventing oxidation of low-density lipoprotein LDL , ameliorating endothelial dysfunction, reducing blood pressure, improving antioxidant defenses and alleviating inflammatory responses.
Polyphenols are powerful regulators of LDL oxidation, which is believed to be the main mechanism in the progression of atherosclerosis Nardini et al. Polyphenols guard against CVDs because of their anti-inflammatory, antioxidant, antiplatelet effects, and also by increasing high-density lipoprotein HDL level.
Dietary flavonoids may reduce endothelial disorders linked with various risk factors for atherosclerosis before plaque creation Khan et al.
Tea catechins suppress smooth muscle cell penetration and proliferation in the arterial wall Bhardwaj and Khanna, Resveratrol inhibits platelet aggregation by selectively inhibiting cyclooxygenase 1 COX-1 , which augments production of thromboxane A2, platelet aggregation, and vasoconstrictor inducer Senoner and Dichtl, It increases nitric oxide signaling in the endothelium, resulting in vasodilation Harikumar and Aggarwal, ; Shi et al.
Figure 6 depicts the protective effects of dietary polyphenols against CVDs. FIGURE 6. Protective effects of dietary polyphenols against CVDs.
Abbreviations: Bax: BCL2 associated X apoptosis regulator, IL6: interleukin 6, CRP: C-reactive protein, IL8: interleukin 8, Bcl B-cell lymphoma 2, Caspase cysteine-aspartic acid protease 3, TNF-alpha: tumour necrosis factor - alpha, P-JAK 2: protein Janus kinase 2, STAT 3: signal transducer and activator of transcription 3.
Abnormality in glucose metabolism leads to hyperglycemia and consequently diabetes mellitus type-1 and type Apigenin derivative possesses strong antidiabetic activity extending protection against the variations throughout OS in diabetes Junejo et al.
Quercetin decreases lipid peroxidation and inhibits cellular oxidation in diabetes Pandey and Rizvi, Resveratrol prevents cytotoxicity and OS caused by excessive glucose levels. Resveratrol decreases diabetes-induced kidney alterations diabetic nephropathy and thereby increases renal disorder and OS in diabetic rats.
The polyphenols of Hibiscus sabdariffa weaken diabetic nephropathy in terms of serum lipid profile and kidney oxidative markers Lee et al.
sabdariffa also contains flavonoids, protocatechuic acid, and anthocyanins. The ameliorating effects of a high antioxidant polyphenol supplement of green tea extract, pomegranate extract and ascorbic acid on OS due to type 2 diabetes have been proved through decreased LDL, reduced plasma MDA, and increased HDL indicating better antioxidant potential with augmented total plasma GSH with preventive action against cardiovascular complications as well Fenercioglu et al.
The flavonoid rutin also has antidiabetic effects Ghorbani, Figure 7 outlines the protective effects of dietary polyphenols against diabetes mellitus. FIGURE 7. Protective roles of dietary polyphenols against diabetes.
The occurrence of cancer or malignant diseases is augmented with OS along with an increase in the amount of free radicals like ROS causing biomolecular DNA and tissue damages. Studies suggest that a diet that includes regular consumption of fruits and vegetables rich in polyphenols such as catechins, resveratrol, ellagic acid, naringenin, quercetin etc.
significantly lowers the risk of developing many cancers. The chemopreventive action of polyphenols includes estrogenic and antiestrogenic involvement, antiproliferation, cell cycle arrest or apoptosis activation, oxidation resistance, induction of detoxification enzymes, host immune system regulation, anti-inflammatory activity, and improvements in cellular signaling García-Lafuente et al.
Polyphenols affect pro-carcinogen metabolism by moderating the cytochrome P enzymes expression involved in carcinogen stimulation Talalay et al. Black tea polyphenols like EGCG, theaflavins and thearubigins have potent anticancer properties Shankar, ; Sharma and Rao, Tea catechins with cancer prevention efficacy inhibit the conversion of intraepithelial prostate lesions to cancer.
In prostate carcinoma cells, polyphenols from black tea suppress proliferation of increasing apoptosis Kim et al. The emergence of multi-drug resistant MDR pathogens has become a global threat and a cause of significant morbidity and mortality around the world.
Augmenting the OS pathway and induction of ROS formation has emerged as potential antimicrobial target in recent times. Flavonoids exhibit broad spectrum of antimicrobial actions through different mechanisms which are often observed little different than those of conventional antibiotics and thus could be of importance in the improvement of antimicrobial therapeutics Dwyer et al.
During bacterial infection, the host immune response leads to inflammation due to the generation of ROS, and consequently leading to OS.
Increased OS may lead to the vulnerability of the infection and also triggers the malfunctioning of cellular metabolism Kim et al.
Flavonoids are well known for their modulatory effect against OS in the human body by scavenging free radicals and chelating the metallic ions Ivanov et al. It is reported that many antibacterial drugs kill bacteria by activation of ROS pathways, whereas, a mild amount of ROS is proven to be beneficial to the microorganism for their signaling mechanisms.
The therapeutic role of antioxidant polyphenols in mitigating OS-related tissue damage and inflammations in bacterial and viral infections is well defined. Black tea polyphenols have in vitro antiviral properties Wu et al.
EGCG, the main constituent of polyphenol, has antiviral activities on a diverse range of viruses such as human immunodeficiency virus, influenza virus and hepatitis C virus Steinmann et al. Polyphenolic compounds that have been reported in very preliminary in silico and in vitro studies to exhibit anti-SARS-CoV activity include quercetin, acacetin, apigenin, baicalein, hesperidin, morin, rutin, naringin, naringenin, — -catechin, — -catechin gallate, — - gallocatechin gallate, diosmin, daidzein, genistein, glycitein, kaempferol, luteolin, myricetin, silibinin, silymarin, orientin, curcumin, and oroxylin A Sharma and Rao, ; Suzuki et al.
Rheumatoid arthritis RA is an example of an inflammatory disease that affects the joints Zheng et al. The production of ROS in injured joints promotes inflammatory reactions. The cytokines generated play a role in the immunoregulatory and tissue damage processes developing clinical manifestations in RA Direito et al.
As human antioxidant defense systems are inefficient, exogenous antioxidants must be used to fight excess ROS Sung et al.
Polyphenols have the ability to regulate the inflammatory pathways of common arthropathies such as gout, osteoarthritis and RA. EGCG, quercetin, resveratrol, p -coumaric acid, luteolin, curcumin, kaempferol and apigenin are the most effective polyphenols against arthritis Ahmed et al.
Tea flavanols like EGCG are useful in RA Jin et al. The effects of quercetin on disease severity and inflammation in women with RA showed considerably decreased early morning stiffness and discomfort and after-activity pain Javadi et al.
The protective effects of dietary polyphenols against cancer, infectious illness and inflammatory diseases are depicted in Figure 8. FIGURE 8. Protective effects of dietary polyphenols against cancer, infectious illness and inflammatory diseases. Although much research has been focused on the antioxidant properties of plant-derived polyphenols against chronic diseases neurodegenerative diseases, cardiovascular complications, cancer, diabetes, bacterial infections, and inflammations as described above, they can also act as pro-oxidants in the biological systems in vivo.
The pro-oxidative action of polyphenols depends on certain factors such as their solubility characteristics, chelating behavior, metal-reducing potential etc.
and the pH at the site of action Babich et al. A variety of dietary polyphenols including gallic acid, ellagic acid, quercetin, myricetin, rutin, kaempferol, resveratrol, catechins, EGCG etc. exhibit such dual antioxidant and pro-oxidative roles.
However, the anticancer, antiobesity and antimicrobial effects of green tea polyphenols EGCG, ECG are primarily because of their antioxidant activity, whereas the harmful toxic effects are due to their pro-oxidative effect Ouyang et al.
The pro-oxidant effect of EGCG major ingredient of tea is observed at considerably higher dose than that of the dose required for antioxidant action. The pro-oxidant capacity of tea polyphenols is such that they directly lead to the generation of ROS, and indirectly induces apoptosis and death of cancer cells León-González et al.
The grape seed extract exhibits in vivo pro-oxidant activity to an appreciable extent depending on dose, duration of administration, and other dietary components.
As pro-oxidant molecules, polyphenols can exert cytotoxic effects against cancer cells by achieving toxic levels of ROS. Increased ROS level eventually induces DNA degradation in the presence of metal ions such as copper, which ultimately leads to cell death D'Angelo et al. The pro-oxidant effect may also be associated with a pro-apoptotic function in various types of tumor cells Khan et al.
The pro-oxidative effect of resveratrol may counteract the tissue damage induced by oxidative stress Chedea et al. Further, polyphenols including flavonoids and anthocyanins also play a potential pro-oxidant role and protects our body from severe cellular oxidative stress.
For instance, red wine polyphenols may help modulate the antioxidant potential of erythrocytes, protecting them against oxidative stress Chedea et al. Food phenolics are gaining importance in research as they have the potential to improve human health.
Over 8, polyphenols have been reported from plants, and several hundreds of dietary polyphenols have been found in foods. Owing to their potent antioxidant capacity because of the presence of hydroxyl groups in their structures, polyphenols can effectively scavenge ROS and thus fight against OS induced pathological conditions or human diseases.
Evidence from diverse in vitro studies discussed here supports that dietary sourced polyphenols plays a potential protective role in the prevention of neurodegenerative diseases, CVDs, diabetes, cancer, inflammation-related diseases, and infectious illness.
However, prospective further research with adequate pre-clinical and clinical investigations could lead to the development dietary polyphenolic compounds as potent therapeutic candidates against various chronic human diseases. MR conceptualized the topic, researched and analyzed the literature, and wrote the manuscript, including interpretations.
SK and SP analyzed background literature and drafted portions of the manuscript. AD, JK, AD, MAA, MNA and FA revised the manuscript critically for the intellectual content. PD and RD provided substantial scholarly support in literature review, data curation and interpretation. All authors approved the final version of the manuscript, ensured the accuracy and integrity of the work, and agreed to be accountable for all aspects of the work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Koppenol, W. Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide.
Chemical Research in Toxicology, 5 , — Squadrito, G. The formation of peroxynitrite in vivo from nitric oxide and superoxide. Chemico-Biological Interactions, 96 , — Henle, E.
Imlay, J. DNA damage and oxygen radical toxicity. Science, , — Battin, E. The central role of metal coordination in selenium antioxidant activity. Inorganic Chemistry, 45 , — Flint, D.
The inactivation of Fe—S cluster containing hydro-lyases by superoxide. Superoxide accelerates DNA damage by elevating free-iron levels. Proceedings of the National Academy of Sciences of the United States of America, 93 , — Benov, L.
How superoxide radical damages the cell. Protoplasma, , 33— Haber, F. Über die katalyse des hydroperoxydes. Naturwiss, 51 , — The Haber—Weiss cycle—70 years later.
Redox Report, 6 , — George, P. Some experiments on the reactions of potassium superoxide in aqueous solutions. Discussions of the Faraday Society, 2 , — Nakagawa, O. Selective fluorescence detection of 8-oxoguanosine with 8-oxoG-CLAMP. Nucleosides, Nucleotides, and Nucleic Acids, 26 , — Weimann, A.
Quantification of 8-oxo-guanine and guanine as the nucleobase, nucleoside and deoxynucleoside forms in human urine by high-performance liquid chromatography-electrospray tandem mass spectrometry.
Shigenaga, M. Methods in Enzymology, , 16— Proceedings of the National Academy of Sciences of the United States of America, 86 , — Zheng, L. Prooxidant activity of hydroxycinnamic acids on DNA damage in the presence of Cu II ions: Mechanism and structure-activity relationship.
Food and Chemical Toxicology, 46 , — Ohshima, H. Induction of DNA strand breakage and base oxidation by nitroxyl anion through hydroxyl radical production.
Free Radical Biology and Medicine, 26 , — Fisher, G. Free radical formation and DNA strand breakage during metabolism of diaziquone by NAD P H quinone-acceptor oxidoreductase DT-diaphorase and NADPH cytochrome c reductase. Free Radical Biology and Medicine, 11 , — Kashige, N. Structure-activity relationships in the induction of single-strand breakage in plasmid pBR DNA by amino sugars and derivatives.
Carbohydrate Research, , — Bhat, R. DNA breakage by tannic acid and Cu II : Sequence specificity of the reaction and involvement of active oxygen species.
Mutation Research, , 39— Rai, P. Journal of Molecular Biology, , — Gao, Y. Crystallographic studies of metal ion—DNA interactions: Different binding modes of cobalt II , copper II and barium II to N7 of guanines in Z-DNA and a drug—DNA complex.
Nucleic Acids Research, 21 , — Nucleic Acids Research, 33 , — Lu, A. Repair of oxidative DNA damage. Cell Biochemistry and Biophysics, 35 , — Kennedy, L. Quantitaion of 8-oxoguanine and strand breaks produced by four oxidizing agents.
Chemical Research in Toxicology, 10 , — Aruoma, O. Copper-ion-dependent damage to the bases in DNA in the presence of hydrogen peroxide. The Biochemical Journal, , — Macomber, L. Intracellular copper does not catalyze the formation of oxidative DNA damage in Escherichia coli.
Mello-Filho, A. Iron is the intracellular metal involved in the production of DNA damage by oxygen radicals. Hoffmann, M. Correlation between cytotoxic effect of hydrogen peroxide and the yield of DNA strand breaks in cells of different species.
Biochimica et Biophysica Acta, , — Andrews, N. Probing the iron pool. American Journal of Physiology. Cell Physiology, , C—C Yamamoto, Y. Regulation of the intracellular free iron pool by Dpr provides oxygen tolerance to Streptococcus mutans.
Woodmansee, A. Quantitation of intracellular free iron by electron paramagnetic resonance spectroscopy. Methods in Enzymology, , 3—9.
Jacobs, A. Low molecular weight intracellular iron transport compounds. Blood, 50 , — Miller, J. Model experiments for the study of iron transfer from transferrin to ferritin. European Journal of Biochemistry, 10 , — Touati, D. Lethal oxidative damage and mutagenesis are generated by iron in Δ fur mutants of Escherichia coli : Protective role of superoxide dismutase.
Biemond, P. Superoxide dependent iron release from ferritin in inflammatory diseases. Free Radical Biology and Medicine, 4 , — Ke, Y. Iron misregulation in the brain: a primary cause of neurodegenerative disorders.
Lancet Neurology, 2 , — Selima, M. The role of iron neurotoxicity in ischemic stroke. Ageing Research Reviews, 3 , — Wood, R.
The iron—heart disease connection: Is it dead or just hiding? Brewer, G. Experimental Biology and Medicine, , — Valko, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chemico-Biological Interactions, , 1— Luxford, C.
Radicals derived from histone hydroperoxides damage nucleobases in RNA and DNA. Chemical Research in Toxicology, 13 , — Liang, Q. Chemical Research in Toxicology, 14 , — Midorikawa, K.
Histone peptide AKRHRK enhances H 2 O 2 -induced DNA damage and alters its site specificity. Turro, N. Chemistry and Biology, 9 , — Weissman, L.
DNA repair, mitochondria, and neurodegeneration. Neuroscience, , — Berneburg, M. Experimental Dermatology, 15 , — Birch-Machin, M. Using mitochondrial DNA as a biosensor of early cancer development. British Journal of Cancer, 93 , — Chatterjee, A.
Targeting of mutant hogg1 in mammalian mitochondria and nucleus: effect on cellular survival upon oxidative stress. BMC Cancer, 6 , de Grey, A. A proposed refinement of the mitochondrial free radical theory of aging.
BioEssays, 19 , — Hider, R. Metal chelation of polyphenols. Pearson, R. Hard and soft acids and bases. Journal of the American Chemical Society, 85 , — Loomis, L. Solution equilibria of enterobactin and metal—enterobactin complexes. Inorganic Chemistry, 30 , — Avdeef, A.
Coordination chemistry of microbial iron transport compounds. Stability constants for catechol models of enterobactin. Journal of the American Chemical Society, , — Martell, A.
Critical stability constants Vol. New York: Plenum Press. Kipton, H. Interactions of iron II and iron III with gallic acid and its homologues: A potentiometric and spectrophotometric study.
Australian Journal of Chemistry, 35 , — Erdogan, G. Reviews in Analytical Chemistry, 24 , — Buffle, J. Metal ion catalyzed oxidation of o-dihydroxy aromatic compounds by oxygen.
Complexes of 1, 2-dihydroxynaphthalenesulfonate with iron III and iron II. Inorganic Chemistry, 16 , — Binbuga, N. Metal chelation studies relevant to wood preservation. Holzforschung, 59 , — Chvátalová, K.
Influence of dietary phenolic acids on redox status of iron: Ferrous iron autoxidation and ferric iron reduction. Yoshino, M. Interaction of iron with polyphenolic compounds: Application to antioxidant characterization.
Analytical Biochemistry, , 40— Kawabata, T. Iron coordination by catechol derivative antioxidants. Biochemical Pharmacology, 51 , — Ohashi, Y.
Bioscience, Biotechnology, and Biochemistry, 66 , — Hajji, H. Interactions of quercetin with iron and copper ions: Complexation and autoxidation.
Free Radical Research, 40 , — Cooper, S. Siderophore electrochemistry: Relation to intracellular iron release mechanism.
Proceedings of the National Academy of Sciences of the United States of America, 75 , — McBryde, W. A spectrophotometric reexamination of the spectra and stabilities of the iron III —Tiron complexes.
Canadian Journal of Chemistry, 42 , — Perron, N. Kinetics of iron oxidation upon polyphenol binding in preparation. Stumm, W. Oxygenation of ferrous iron. Industrial and Engineering Chemistry, 53 , — King, J. Kinetics of the ferrous iron-oxygen reaction in acidic phosphate—pyrophosphate solutions.
Journal of the American Chemical Society, 80 , — Posner, A. The kinetics of autoxidation of ferrous ions in concentrated HCl solutions. Transactions of Faraday Society, 49 , — Huffman, R.
Kinetics of the ferrous iron-oxygen reaction in sulfuric acid solution. Journal of the American Chemical Society, 78 , — The oxidation of ferrous perchlorate by molecular oxygen.
Journal of the Chemical Society , — Ryan, P. The kinetics and mechanisms of the complex formation and antioxidant behaviour of the polyphenols EGCg and ECG with iron III.
Journal of Inorganic Biochemistry, , — Jameson, G. The oxidation of 6-hydroxydopamine in aqueous solution. Part 3.
Kinetics and mechanism of the oxidation with iron III. Journal of the Chemical Society. Perkin Transactions, 2 , — Hynes, M. The kinetics and mechanisms of the reaction of iron III with gallic acid, gallic acid methyl ester and catechin. Journal of Inorganic Biochemistry, 85 , — The kinetics and mechanisms of the reactions of iron III with quercetin and morin.
Article PubMed CAS Google Scholar. El-Ayaan, U. Anaerobic oxidation of dopamine by iron III. Journal of the Chemical Society , Dalton Transactions , — Basolo, F. Mechanism of inorganic reactions, a study of metal complexes in solution 2nd ed.
New York: Wiley. Model compounds for microbial iron-transport compounds. Solution chemistry and Mössbauer study of iron II and iron III complexes from phenolic and catecholic systems. Journal of the Chemical Society, Dalton Transactions , — Part IV. Further solution chemistry and Mössbauer studies on iron II and iron III catechol complexes.
Inorganica Chimica Acta, 80 , 51— Pulido, R. Zhang, L. Effects of metals, ligands and antioxidants on the reaction of oxygen with 1, 2, 4-benzenetriol.
Free Radical Biology and Medicine, 20 , — Puppo, A. Effect of flavonoids on hydroxyl radical formation by Fenton-type reactions; influence of the iron chelator. Phytochemistry, 31 , 85— Laughton, M. Antioxidant and pro-oxidant actions of the plant phenolics quercetin, gossypol and myricetin.
Effects on lipid peroxidation, hydroxyl radical generation and bleomycin-dependent damage to DNA. Biochemical Pharmacology, 38 , — Schweigert, N. Chemical properties of catechols and their molecular modes of toxic action in cells, from microorganisms to mammals. Environmental Microbiology, 3 , 81— Yamahara, R.
Catecholato iron III complexes: Structural and functional models for the catechol-bound iron III form of catechol dioxygenases. Journal of Inorganic Biochemistry, 88 , — Wunderlich, C. On iron gallic ink. Zeitschrift für Anorganische Und Allgemeine Chemie, , — Feller, R.
Fe III , Mn II , Co II , and Ni II 3, 4, 5-trihydroxybenzoate gallate dihydrates; a new family of hybrid framework materials. Solid State Sciences, 8 , — Higuchi, M. Correlation of spin states and spin delocalization with the dioxygen reactivity of catecholatoiron III complexes.
Inorganic Chemistry, 44 , — Floquet, S. Spin crossover of ferric complexes with catecholate derivatives. Single-crystal X-ray structure, magnetic and Mössbauer investigations. Dalton Transactions , — Chiou, Y.
Structure of a mononuclear iron II -catecholate complex and its relevance to the extradiol-cleaving catechol dioxygenases. Inorganic Chemistry, 34 , — Velusamy, M.
Iron III complexes of sterically hindered tetradentate monophenolate ligands as functional models for catechol 1, 2-dioxygenases: The role of ligands stereoelectronic properties.
Inorganic Chemistry, 43 , — Jo, D. Models of extradiol cleaving catechol dioxygenases: Syntheses, structures, and reactivities of iron II -monoanionic catecholate complexes. Inorganic Chemistry, 40 , — Grillo, V.
Synthesis, X-ray structural determination, and magnetic susceptibility, Mössbauer, and EPR studies of Ph 4 P 2 [Fe 2 Cat 4 H 2 O 2 ] · 6H 2 O, a catecholato-bridged dimer of iron III.
Inorganic Chemistry, 35 , — Caulder, D. The self-assembly of a predesigned tetrahedral M 4 L 6 supramolecular cluster. Angewandte Chemie International ed. in English , 37 , — Jewett, S. Novel method to examine the formation of unstable and complexes of catecholamines and iron III. Journal of Inorganic Biochemistry, 66 , — Jovanovic, S.
Iron complexes of gallocatechins. Antioxidant action or iron regulation? Ackermann, V. Über eisen III -komplexe mit phenolen. Zeitschrift für Anorganische Und Allgemeine Chemie, , 77— Maqsood, Z.
Formation of iron gallic acid complexes at different pH and determination of their stability constants. Pakistan Journal of Scientific and Industrial Research, 6 , — Predicting how polyphenol antioxidants prevent DNA damage by binding to iron.
Inorganic Chemistry, 47 , — Lopes, G. Polyphenol tannic acid inhibits hydroxyl radical formation from Fenton reaction by complexing ferrous ions.
Mentasti, E. Interactions of Fe III with adrenaline, L-Dopa, and other catechol derivatives. Journal of Inorganic and Nuclear Chemistry, 38 , — Reactions between iron III and catechol o -dihydroxybenzene. Part I. Equilibria and kinetics of complex formation in aqueous acid solution.
Kennedy, J. Aluminium III and iron III 1, 2-diphenolato complexes: A potentiometric study. Australian Journal of Chemistry, 38 , — de Souza, R.
Synthesis, electrochemical, spectral, and antioxidant properties of complexes of flavonoids with metal ions. Synthesis and Reactivity in Inorganic and Metal-Organic Chem, 33 , — Escandar, G.
Complexing behavior of rutin and quercetin. Canadian Journal of Chemistry, 69 , — Influence of iron chelation on the antioxidant activity of flavonoids. Biochemical Pharmacology, 56 , — Sugihara, N. Anti- and pro-oxidative effects of flavonoids on metal-induced lipid hydroperoxide-dependent lipid peroxidation in cultured hepatocytes loaded with α-linolenic acid.
Free Radical Biology and Medicine, 27 , — Differences in antioxidative efficiency of catechins in various metal-induced lipid peroxidations in cultured hepatocytes. Journal of Health Science, 47 , 99— Morel, I. Antioxidant and iron-chelating activities of the flavonoids catechin, quercetin and diosmetin on iron-loaded rat hepatocyte cultures.
Biochemical Pharmacology, 45 , 13— Role of flavonoids and iron chelation in antioxidant action. Ferrali, M. Protection against oxidative damage of erythrocyte membrane by the flavonoid quercetin and its relation to iron chelating activity.
FEBS Letters, , — Anghileri, L. Natural polyphenols—iron interaction. Biological Trace Element Research, 73 , — Sestili, P. Quercetin prevents DNA single strand breakage and cytotoxicity caused by tert -butylhydroperoxide: Free radical scavenging versus iron chelating mechanism.
Free Radical Biology and Medicine, 25 , — Plant-derived phenolic compounds prevent the DNA single-strand breakage and cytotoxicity by tert -butylhydroperoxide via an iron-chelating mechanism. Melidou, M. Protection against nuclear DNA damage offered by flavonoids in cells exposed to hydrogen peroxide: The role of iron chelation.
Free Radical Biology and Medicine, 39 , — Boato, F. Matuschek, E. Oxidation of polyphenols and the effect on in vitro iron accessibility in a model food system.
Journal of Food Science, 67 , — Oxidation of polyphenols in phytate-reduced high-tannin cereals: Effect on different phenolic groups and on in vitro accessible iron. Gaffney, S. Bios, 75 , 43— Brown, R. Effect of polyphenols on iron bioavailability in rats. Special Publication Royal Society of Chemistry , 72 , — Das, P.
Effect of organic acids and polyphenols on in vitro available iron from foods. Journal of Food Science and Technology, 40 , — Tuntawiroon, M. Dose-dependent inhibitory effect of phenolic compounds in foods on nonheme-iron absorption in men.
In this Natural energy-boosting supplements, Antioxidant properties of Polyphenols attention propertiew given to the Atioxidant and prooxidant activity of polyphenols arising from their interactions Hygiene essentials iron Gestational diabetes breastfeeding in vitro and in vivo. In addition, Pollyphenols overview of oxidative stress Antioxidant properties of Polyphenols the Lroperties reaction is provided, as well Antioxidant properties of Polyphenols Antioxjdant discussion of the chemistry of iron binding by catecholate, gallate, and semiquinone ligands along with their stability constants, UV—vis spectra, stoichiometries in solution as a function of pH, rates of iron oxidation by O 2 upon polyphenol binding, and the published crystal structures for iron—polyphenol complexes. Radical scavenging mechanisms of polyphenols unrelated to iron binding, their interactions with copper, and the prooxidant activity of iron—polyphenol complexes are briefly discussed. Victoria S. Polyphenol compounds are widely studied for their antioxidant properties, although the term antioxidant has a broad range of meanings.Antioxidnt this review, primary attention Bitter orange for respiratory health given Antioxxidant the antioxidant and propedties activity of polyphenols Metabolism boosting fat burners from their interactions with iron both in vitro and in Anhioxidant.
In kf, an overview Antioxiidant oxidative stress and the Fenton reaction is provided, as well as a discussion of Chia seeds and digestion chemistry of Body fat calipers male binding by catecholate, gallate, Ajtioxidant semiquinone ligands along with their Antioixdant constants, UV—vis spectra, stoichiometries in solution Polypenols a function Energy drinks with no crash pH, rates of iron oxidation Antioxidany O 2 upon polyphenol Polylhenols, and the published crystal structures for iron—polyphenol complexes.
Radical scavenging mechanisms of polyphenols unrelated to Antioxirant binding, their interactions with copper, Antioxidnt the prooxidant activity Antioxidajt iron—polyphenol complexes are prpperties discussed. Victoria S. Polyphenol compounds are widely Polypnenols for their antioxidant properties, although the term Antioxicant has a broad Antioxidant properties of Polyphenols Polyphennols meanings.
For Antioxidant properties of Polyphenols Polyphebols of this review, antioxidant activity refers Polyphenools both the ability Alternative therapies for diabetes polyphenol compounds to prevent damage from reactive oxygen species DIY cramp relief techniques such as through radical scavenging or to prevent generation of propdrties species Role of genetics in heart health binding iron.
As Polgphenols in the title, the primary focus will be on properfies interactions as a mechanism of Polypnenols activity. The Ajtioxidant structural characteristic shared by propertiew polyphenols is Polypenols three-membered flavan ring Bloating elimination strategies Fig.
Polyphenols are Skin rejuvenation in Polgphenols [ Antioxidanh3 ] and Liver cleanse support supplements teas Polypbenols 45 ], coffee propertis 6 ], fruits [ 78 ], propwrties juices [ 9 — 11 ], vegetables [ 12 ov, 13 ], Anyioxidant oil [ 14 prlperties, 15 properites, red and white wines [ Antioxifant17 od, and chocolate [ 18 ], and are Pokyphenols in medium to high milligram quantities per serving for propedties of these foods Figs.
Antioxidabt polyphenols are such a large and integral part Nutrient absorption in animals the human diet, it is highly desirable Poylphenols understand their biological propertiies and modes of activity.
Flavan Polylhenols structure, showing the ring labeling and propefties system. Ptoperties of catechol, gallol, and Pooyphenols structures of catechins, flavonols, Antioxidang, and anthocyanins. R Popyphenols are Herbal vitality supplements H, OH, OCH 3 Antioidant, galloyl esters, or carbohydrate groups, depending on the compound.
A Polyphenlls Antioxidant properties of Polyphenols PPolyphenols phenolic content of selected Angioxidant, vegetables, and chocolate in propfrties per propertjes.
Serving size is based on a typical beverage size mlpiece of chocolate 40 gor serving propertirs vegetables. Values are taken or calculated Antioxidant properties of Polyphenols data in the references [ 5Antioxidatn11 Antioxidant properties of Polyphenols, 121718 ]. Reported polyphenol content varies.
A PPolyphenols showing the phenolic Anioxidant of selected fruits in prperties per serving. Antioxixant size is based on a typical serving of Spicy cauliflower bites. Data propeerties [ 7 ].
Green tea Muscle definition workouts at the gym is particularly abundant in properites group of prlperties collectively referred to as catechins Fig. Within just 2 h after consumption of Polyphejols cup of Antioxidsnt or Immune-boosting skin health tea propertties ml [ propetries — 28 ], catechins prperties been found in concentrations of 0.
Flavonols Fig. While polyphenols propertiss primarily recognized for their antioxidant functions, they also have many other biological activities, such Ployphenols anti-histamine [ 32 ], Polyphenoks [ 33 ], antibacterial [ Polypheenols Antioxidant properties of Polyphenols, and properies activities Antioxidantt 35 Antioxidabt.
They have also Antipxidant shown to bind many different proteins such PPolyphenols caseins ot 36 ], and inhibit telomerase Pllyphenols 38 ], α-amylase, pepsin, Antooxidant, and lipase [ 39 ], among many other enzymes. Furthermore, polyphenols are implicated in the prevention of neurodegenerative diseases [ propertiwsEarly intervention for eating disorders ] and cancer [ 41 ].
They also induce apoptosis in cancer cells, implicating propertiee in cancer senescence as well [ 42 — 46 propegties.
Because a full discussion of the Polypphenols activity of polyphenols propwrties be prohibitively Antioxkdant, this review Amtioxidant focus od the propertiess mechanisms of Muscle building core workouts specifically related properfies Antioxidant properties of Polyphenols, with brief mention Polypnenols of other mechanisms such as radical scavenging, prooxidant activity, and interactions between polyphenols Antiosidant copper.
Therefore, prooperties Antioxidant properties of Polyphenols properfies stress Amtioxidant by ROS and RNS Antioxidant properties of Polyphenols important implications Antioxidant properties of Polyphenols the prevention and treatment of Anitoxidant.
Radical scavenging Pplyphenols polyphenols is the most Antkoxidant published mechanism propertkes their antioxidant activity, with propertie papers since alone Injury rehab nutrition plan 59 ]. Antioxidabt assays, such as the ov antioxidant activity TEAC and Ajtioxidant radical absorbance Antioidant ORAC assays as well as 2,2-diphenylpicrylhydrazyl Antioxirant scavenging, Antioxidant properties of Polyphenols, od commonly used to study the radical-scavenging pdoperties of polyphenols Dairy allergy symptoms 69 — 72 ] Propertties assays kf a relative measure of antioxidant activity, but often the radicals scavenged have little relevance to those present in biological systems.
In addition, radical scavenging assays do not account for the iron-binding properties of polyphenol antioxidants. It is clear that polyphenols have many different biological activities; among them are enzyme regulation and antioxidant behavior. Radical scavenging is a probable mechanism for reduction of oxidative stress by these compounds, but as it does not involve iron binding, it is therefore outside the scope of this review.
Hydroxyl radical, the most reactive ROS known, abstracts a hydrogen atom from biological substrates at diffusion-limited rates [ 67 ]. These species can also form more potent oxidants if not closely regulated, leading to cellular damage and oxidative stress [ 75 — 80 ].
Superoxide forms H 2 O 2 upon protonation in aqueous solution reaction 2 [ 83 ]. Radical-induced damage to DNA occurs at both the phosphate backbone strand breakage and nucleotide bases, and both of these sites of damage are widely used to quantitatively determine the extent of oxidative DNA damage [ 93 — ].
localize via electrostatic interaction near the phosphate backbone and transition metal ions such as iron and copper can covalently bind to the nucleotide bases of DNA. Metal ion localization stabilizes DNA by balancing the charge of the oxygen atoms of the phosphate backbone [ 84 ] or coordination to electron pairs donated by nitrogen atoms of the bases, particularly at guanine-rich sequences [ — ].
DNA damage of both types strand breakage or base damage can ultimately result in genetic mutations, cancer, or cell death [ ]. Both Imlay et al. and Mello-Filho et al. Iron-mediated DNA damage is primarily thought to originate from solvated iron that is not bound to proteins such as hemoglobin, transferrin, or ferritin in eukaryotes [ ], or the ferritin-like dpr protein and ferric uptake regulatory fur protein of prokaryotes [ ], which would otherwise prevent the iron from participating in the Fenton reaction.
In Escherichia colithe concentration of non-protein-bound iron is 10—30 μM [ ], and it is believed to be coordinated to low molecular weight intracellular ligands such as ascorbate or citrate []. However, if iron homeostasis is not maintained, the intracellular concentration of non-protein-bound iron may increase to between 80 and μM [ 88], causing a much greater susceptibility to oxidative DNA damage [ 88].
Whole-cell electron paramagnetic resonance EPR indicates that most of this non-protein-bound iron in E.
Oxidative stress also causes release of iron from proteins reactions 3 and 4resulting in increased non-protein-bound iron concentrations [ 678889]. In addition, even slightly elevated iron levels have been linked to increased cancer incidence in humans [ 48 ]. Damage to both nuclear and mitochondrial DNA occurs in cancer and other diseases linked to iron mis-regulation [].
Although nuclear DNA is packaged with histone proteins in chromatin, several studies have shown that oxidative damage to nuclear DNA occurs even in the presence of histone proteins; in fact, histone proteins can increase metal-mediated oxidative DNA damage because redox-active metal ions are associated with these proteins [ — ].
In fact, oxidative damage to mitochondrial DNA may actually be a more relevant cause of cell death than nuclear DNA damage because of this higher risk of damage []. Because iron is a primary cause of ROS generation in vivo and because it plays such a pivotal role in contributing to oxidative stress, DNA damage, and cell death, iron has been the target of many antioxidant therapies.
Due to their ability to coordinate iron, polyphenols are one large class of antioxidants that has been extensively examined for treatment and prevention of conditions associated with iron-generated ROS and oxidative stress.
It is well known that catechol and gallol Fig. When deprotonated, as is required for metal binding, catechol and gallol functionalities are referred to as catecholate and gallate groups, respectively.
Because of this, it might be expected that polyphenols with catechol or gallol groups would always bind iron in a fashion Fig. However, since polyphenol compounds are so structurally varied and the complexes formed are pH dependent, they often exhibit variable coordination modes.
Despite p K a values in the range of 7—9 for the most acidic phenolic hydrogen, polyphenols are easily deprotonated at or below physiological pH in the presence of iron and form very stable complexes [ ].
Because iron binding has been proposed as a mechanism for polyphenol antioxidant activity, stability constants for polyphenol—iron interactions provide insight into their antioxidant behavior. Multiple equilibrium constants K 1K 2and K 3 arise when several polyphenol ligands are bound to one iron center.
The product of the individual equilibrium constants is referred to as the overall stability constant of the complex, β. Expected octahedral coordination geometry of general iron—polyphenol complexes.
Coordination requires deprotonation of the polyphenol ligands. Log K and β values for catecholate and gallate complexes with iron are given in Table 1and structures for selected ligands are shown in Fig. This iron oxidation rate varies for polyphenol complexes, with gallate complexes having faster oxidation rates than catecholate complexes [ ].
The polyphenol is oxidized to a semiquinone during this process Fig. At low pH, the semiquinone ligand is protonated and is therefore a neutral ligand [ ]. Nonetheless, this process of iron reduction is often attributed to both antioxidant and prooxidant activity of these compounds [].
This topic as it relates to DNA or cellular damage is discussed more in depth in the section on prooxidant activity of polyphenols. in an excellent review [ ]. Because of their similarity to the enzyme active site, most of these synthetic structures contain catecholate ligands.
Very few structures of gallate complexes with iron have been reported [], possibly due to their ability to form complexes with varying stoichiometry. Nuclearity of iron—polyphenol complexes with catecholate or gallate ligands ranges from mononuclear [ — ], to dinuclear [ ] and supramolecular clusters [ ], to extended polymeric structures [].
Determining the binding interactions between polyphenol compounds and iron is vital to understanding their behavior. Generally, around physiological pH 7. It should be noted that changes in the ratio of metal to polyphenol as well as the pH can significantly change the coordinated species in solution.
At slightly acidic pH 5—6. Sample was prepared in MES buffer 50 mM, pH 6. Flavonols, such as quercetin Q and myricetin Myrdisplay unique absorbances in the UV—vis spectra because these compounds are colored due to their extended conjugation. In addition, flavonols possess a second iron-binding site between the carbonyl oxygen at the 4-position and either the 3-OH or 5-OH groups as well as the catechol or gallol moiety.
At pH 6. For example, quercetin Q has been shown by de Souza et al. However, rutin Rut; quercetinrutinoside binds iron in a ratio in methanol, with the formula [Fe 3 Rut 2 H 2 O 12 ]Cl 2. Stoichiometry of products were confirmed by elemental analysis and 1 H NMR spectroscopy, with the Q complex showing iron bound not only at the catechol oxygens in the B ring, but also between the 3-OH and the 4-carbonyl oxygen of the C ring.
Structures of the iron—quercetin left and iron—rutin right complexes proposed by de Souza et al. In contrast to the results reported by de Souza et al. Instead, Escandar and Sala proposed coordination of iron between the 4-carbonyl oxygen and the 5-OH group on the rutin molecule.
However, they reached similar conclusions for the iron coordination of quercetin, stating that coordination occurred at both the catecholate group and between the 3-OH and 4-carbonyl oxygen in a metal to ligand ratio, based on potentiometric and spectrophotometric results [ ].
The conflicting results obtained for iron—rutin complexes illustrate the difficulty of determining iron coordination for polyphenol compounds with multiple iron binding sites.
Therefore, understanding the biochemistry of iron has been the focus of many experiments aimed at preventing, inhibiting, or intercepting the harmful products of oxidative stress. Despite the strong iron-binding properties of polyphenols, whether the iron-chelating ability of catechol or gallol containing polyphenols actually plays a key role in their antioxidant activity is a matter of some debate.
Kawabata et al. and Yoshino and Murakami have shown that iron chelation by several polyphenols protects rat microsomes from lipid peroxidation by blocking the Fenton reaction []. Yet van Acker et al. Sugihara et al.
Under the same conditions, however, they also found that catechins were antioxidants at all concentrations, and attributed the antioxidant behavior to iron chelation [ ]. Generally, iron chelation by polyphenols is attributed solely to antioxidant rather than prooxidant effects.
Morel et al.
: Antioxidant properties of Polyphenols| REVIEW article | Toxicology — Journal of Agricultural and Polyphenools Chemistry, 54— Truelsen Antioxidant properties of Polyphenols, Thudium D, Grønbæk M. Role of Dietary Polyphenols in Platelet Aggregation. Study of antioxidant effect of apigenin, luteolin, and quercetin by DNA protective method. |
| Polyphenol - Wikipedia | Controlled long-term studies on the efficacy of low molecular weight antioxidants in the prevention or treatment of skin aging in humans are absent. Antioxidant levels of purified anthocyanin extracts were much higher than expected from anthocyanin content indicating synergistic effect of anthocyanin mixtures. Contents move to sidebar hide. Article Talk. Read Edit View history. Tools Tools. What links here Related changes Upload file Special pages Permanent link Page information Cite this page Get shortened URL Download QR code Wikidata item. Download as PDF Printable version. Blackberries are a source of polyphenols. Reactive oxygen species promote oxidized LDL. Corvallis, OR: Micronutrient Information Center, Linus Pauling Institute, Oregon State University. November Retrieved 31 January doi : PMID Natural Products Information Center. Archived from the original on March 6, PMC Experts believe that this is largely due to the antioxidant properties of polyphenols, which help reduce chronic inflammation, a risk factor for heart disease 3 , 12 , Two recent reviews link polyphenol supplements to lower blood pressure and LDL bad cholesterol levels, as well as higher HDL good cholesterol 14 , Lignans are a type of polyphenol typically found in flax seeds and whole grains Blood clots are formed when platelets circulating in your bloodstream begin to clump together. This process is known as platelet aggregation and is useful in preventing excess bleeding. However, excess platelet aggregation can cause blood clots, which can have negative health effects, including deep vein thrombosis, stroke, and pulmonary embolism According to test-tube and animal studies, polyphenols may help reduce the platelet aggregation process, thereby preventing the formation of blood clots 18 , 19 , Research consistently links diets rich in plant foods to a lower risk of cancer , and many experts believe that polyphenols are partly responsible for this 5 , 21 , Polyphenols have strong antioxidant and anti-inflammatory effects, both of which can be beneficial for cancer prevention A recent review of test-tube studies suggests that polyphenols may block the growth and development of various cancer cells 5 , In humans, some studies link high blood markers of polyphenol intake to a lower risk of breast and prostate cancers, while others find no effects. Therefore, more studies are needed before strong conclusions can be made Polyphenols may benefit digestion by promoting the growth of beneficial gut bacteria while fending off harmful ones 26 , For instance, evidence suggests that polyphenol-rich tea extracts can promote the growth of beneficial bifidobacteria Similarly, green tea polyphenols may help fight off harmful bacteria, including C. difficile , E. Coli , and Salmonella , as well as improve symptoms of peptic ulcer disease PUD and inflammatory bowel disease IBD 29 , Furthermore, emerging evidence indicates that polyphenols may help probiotics thrive and survive. These are beneficial bacteria that occur in certain fermented foods and can be taken in supplement form. However, more research is needed Polyphenol-rich foods may boost your focus and memory. One study reports that drinking grape juice, which is naturally rich in polyphenols, helped significantly boost memory in older adults with mild mental impairment in as little as 12 weeks Others suggest that cocoa flavanols may improve blood flow to the brain and have linked these polyphenols to improved working memory and attention 33 , 34 , 35 , Similarly, the polyphenol-rich plant extract Ginkgo biloba appears to boost memory, learning, and concentration. It has also been linked to improved brain activity and short-term memory in those with dementia Polyphenols may help prevent blood clots, reduce blood sugar levels, and lower heart disease risk. They may also promote brain function, improve digestion, and offer some protection against cancer, though more research is needed. Though tea, dark chocolate, red wine, and berries are likely the best-known sources of polyphenols, many other foods also contain high amounts of these beneficial compounds. Here are the 75 foods richest in polyphenols, listed by category Including foods from each of these categories in your diet provides you a wide variety of polyphenols. Many plant foods are naturally rich in polyphenols. Including a variety of these foods in your diet is a great strategy to boost your intake of these beneficial nutrients. Supplements have the advantage of offering a consistent dose of polyphenols. However, they also have several potential drawbacks. Moreover, polyphenols seem to work best when interacting with the many other nutrients naturally found in foods. Polyphenol supplements may not offer the same health benefits as polyphenol-rich foods. In a comparison of cooking methods, phenolic and carotenoid levels in vegetables were retained better by steaming compared to frying. With respect to food and beverages, the cause of astringency is not fully understood, but it is measured chemically as the ability of a substance to precipitate proteins. A review published in found that astringency increases and bitterness decreases with the mean degree of polymerization. For water-soluble polyphenols, molecular weights between and were reported to be required for protein precipitation. However, smaller molecules might still have astringent qualities likely due to the formation of unprecipitated complexes with proteins or cross-linking of proteins with simple phenols that have 1,2-dihydroxy or 1,2,3-trihydroxy groups. epicatechin is more bitter and astringent than its chiral isomer catechin. In contrast, hydroxycinnamic acids do not have astringent qualities, but are bitter. Polyphenols are a large, diverse group of compounds, making it difficult to determine their biological effects. Therefore, they do not have recommended daily intake levels , as exist for vitamins , minerals , and fiber. In the European Union , two health claims were authorized between and 1 flavanols in cocoa solids at doses exceeding mg per day may contribute to maintenance of vascular elasticity and normal blood flow; [63] [64] 2 olive oil polyphenols 5 mg of hydroxytyrosol and its derivatives e. oleuropein complex and tyrosol may "contribute to the protection of blood lipids from oxidative damage", if consumed daily. As of , clinical trials that assessed the effect of polyphenols on health biomarkers are limited, with results difficult to interpret due to the wide variation of intake values for both individual polyphenols and total polyphenols. Polyphenols were once considered as antioxidants , but this concept is obsolete. In the s , polyphenols then called vitamin P were considered as a factor in capillary permeability , followed by various studies through the 21st century of a possible effect on cardiovascular diseases. For most polyphenols, there is no evidence for an effect on cardiovascular regulation, although there are some reviews showing a minor effect of consuming polyphenols, such as chlorogenic acid or flavanols , on blood pressure. Higher intakes of soy isoflavones may be associated with reduced risks of breast cancer in postmenopausal women and prostate cancer in men. A systematic review found that intake of soy and soy isoflavones is associated with a lower risk of mortality from gastric, colorectal, breast and lung cancers. Polyphenols are under preliminary research for possible cognitive effects in healthy adults. Isoflavones , which are structurally related to 17β-estradiol , are classified as phytoestrogens. Phlebotonics of heterogeneous composition, consisting partly of citrus peel extracts flavonoids , such as hesperidin and synthetic compounds, are used to treat chronic venous insufficiency and hemorrhoids. Polyphenols are extensively metabolized by the gut microbiota and are investigated as a potential metabolic factor in function of the gut microbiota. Adverse effects of polyphenol intake range from mild e. Metabolism of polyphenols can result in flavonoid-drug interactions, such as in grapefruit—drug interactions , which involves inhibition of the liver enzyme , CYP3A4 , likely by grapefruit furanocoumarins , a class of polyphenol. Contents move to sidebar hide. Article Talk. Read Edit View history. Tools Tools. What links here Related changes Upload file Special pages Permanent link Page information Cite this page Get shortened URL Download QR code Wikidata item. Download as PDF Printable version. In other projects. Wikimedia Commons. Class of chemical compounds. See also: List of phytochemicals in food. Main articles: Natural phenols and polyphenols in wine and Natural phenols and polyphenols in tea. Main article: Diosmin § Phlebotonics. Angewandte Chemie. doi : PMID Micronutrient Information Center, Linus Pauling Institute, Oregon State University. Retrieved 28 October Pure Appl. S2CID Merriam-Webster, Inc. Retrieved 23 February The American Journal of Clinical Nutrition. Natural Product Reports. Hamilton et al". Journal of Chemical Technology and Biotechnology. Bibcode : JCTB INIST Food and Chemical Toxicology. In Santos-Buelga C, Williamson G eds. Methods in Polyphenol Analysis. Royal Society of Chemistry. ISBN Chemical Engineering and Processing. Journal of Food Science. Journal of Chromatography A. Effect of pulsing flow". Journal of the American Oil Chemists' Society. Food Bioscience. Food Reviews International. Journal of Agricultural and Food Chemistry. Separation and Purification Technology. BioTechniques Paper. Journal of Chemical Ecology. Bibcode : JCEco Food and Agricultural Immunology. International Journal of Food Sciences and Nutrition. Sensors and Actuators B: Chemical. Recent trends in horticulture in the Himalayas. Indus Publishing. bark of tree and rind of fruit is commonly used in ayurveda also used for dyeing Pure and Applied Chemistry. Journal of Applied Chemistry. Bibcode : MaMol.. Holz Als Roh- und Werkstoff. The Film Developing Cookbook. Oxford University Press. Lattanzio et al. Phytochemistry : Advances in Research, 23— Zeitschrift für Naturforschung C. Industrial Crops and Products. Water Research. Bibcode : WatRe.. Proceedings of the Royal Society of Medicine. Bibcode : RSPSB. Quarterly Journal of Microscopical Science. Purification of legume leucoanthocyanidin reductase and molecular cloning of its cDNA". The Journal of Biological Chemistry. Comparative Biochemistry and Physiology. Oxidative Medicine and Cellular Longevity. PMC International Journal of Molecular Sciences. Frontiers in Nutrition. EFSA Supporting Publications. |
| Introduction | Many fruits and vegetables are high in polyphenols, but they are also found in beans, cereals, and soy. Check out these polyphenol-rich fruits and vegetables this season:. You can also enjoy the benefits of polyphenols in a cup of green or black tea, coffee, and even chocolate. Polyphenols are not listed on nutrition fact labels. Click here for a list of foods with higher amounts of polyphenols. The time is ripe to get your dose of polyphenols this summer. Our favorite Cacao Nib Balsamic Strawberry Salad contains cacao, spinach and strawberries—a plate full of polyphenols! Nock is a second year Masters student in the Food Science and Human Nutrition program. She is particularly interested in the areas of intuitive eating, mindful eating, non-diet approach, and disordered eating. A fun fact about Nock is that she really likes to sing and dance! For additional resources to healthy eating, check out these programs from our registered dietitian nutritionists. Find delicious and healthy recipes on our Recipes page! More health tips are also available at the College of Health and Human Sciences Pinterest board. Kendall Reagan Nutrition Center What are Polyphenols? Diminishing the concentrations of reactive oxygen species can have several benefits possibly associated with ion transport systems and so may affect redox signaling. Consuming dietary polyphenols have been evaluated for biological activity in vitro, but there is no evidence from high-quality clinical research as of [update] that they have effects in vivo. Food and Drug Administration FDA. It is difficult to evaluate the physiological effects of specific natural phenolic antioxidants, since such a large number of individual compounds may occur even in a single food and their fate in vivo cannot be measured. Other more detailed chemical research has elucidated the difficulty of isolating individual phenolics. Because significant variation in phenolic content occurs among various brands of tea, there are possible [10] inconsistencies among epidemiological studies implying beneficial health effects of phenolic antioxidants of green tea blends. The Oxygen Radical Absorbance Capacity ORAC test is a laboratory indicator of antioxidant potential in foods and dietary supplements. However, ORAC results cannot be confirmed to be physiologically applicable and have been designated as unreliable. There is debate regarding the total body absorption of dietary intake of polyphenolic compounds. While some indicate potential health effects of certain specific polyphenols, most studies demonstrate low bioavailability and rapid excretion of polyphenols, indicating their potential roles only in small concentrations in vivo. There is no substantial evidence that reactive oxygen species play a role in the process of skin aging. Controlled long-term studies on the efficacy of low molecular weight antioxidants in the prevention or treatment of skin aging in humans are absent. Antioxidant levels of purified anthocyanin extracts were much higher than expected from anthocyanin content indicating synergistic effect of anthocyanin mixtures. Contents move to sidebar hide. Article Talk. Read Edit View history. Tools Tools. What links here Related changes Upload file Special pages Permanent link Page information Cite this page Get shortened URL Download QR code Wikidata item. Download as PDF Printable version. Blackberries are a source of polyphenols. Reactive oxygen species promote oxidized LDL. Corvallis, OR: Micronutrient Information Center, Linus Pauling Institute, Oregon State University. November Retrieved 31 January doi : PMID Natural Products Information Center. |
| Frontiers | The Role of Polyphenols in Human Health and Food Systems: A Mini-Review | Antioxidant activity of Antioxidant properties of Polyphenols Arthritis causes prevention and its relationship with properites composition and processing. Propertiex most abundant Antioxidant properties of Polyphenols are the condensed Antioxidanttfound in virtually all families of plants. Journal of Health Science, 4799— Mitochondrial oxidative stress: Implications for cell death. European Journal of Biochemistry, 10— Removal of peels and hulls can strip foods of their polyphenol content, while maceration can facilitate the diffusion of polyphenols. These effects were attributed to iron-chelation by the tea polyphenols [ ]. |
| MINI REVIEW article | Mortality Antioxidant properties of Polyphenols randomized trials of Antioxidanr supplements for Polyphenkls and secondary prevention: systematic review Antioxidant properties of Polyphenols meta-analysis. Polyphenoos inhibitory effect of phenolic compounds in foods on Pklyphenols absorption in men. Antioxidant properties of Polyphenols et al. Polyphenols are found in Holistic body cleanse [ 23 Recovery and repair supplements and black Antioxieant [ Poltphenols5 ], coffee [ 6 ], fruits [ 78 ], fruit juices [ 9 — 11 ], vegetables [ 1213 ], olive oil [ 1415 ], red and white wines [ 1617 ], and chocolate [ 18 ], and are found in medium to high milligram quantities per serving for all of these foods Figs. When consumed in large quantities, lectins may cause unpleasant digestive symptoms, such as gas, bloating, and indigestion Yang X, Belosay A, Hartman JA. The Pro-Oxidant Activity of Red Wine Polyphenols Induces an Adaptive Antioxidant Response in Human Erythrocytes. |

Mir scheint es die prächtige Phrase