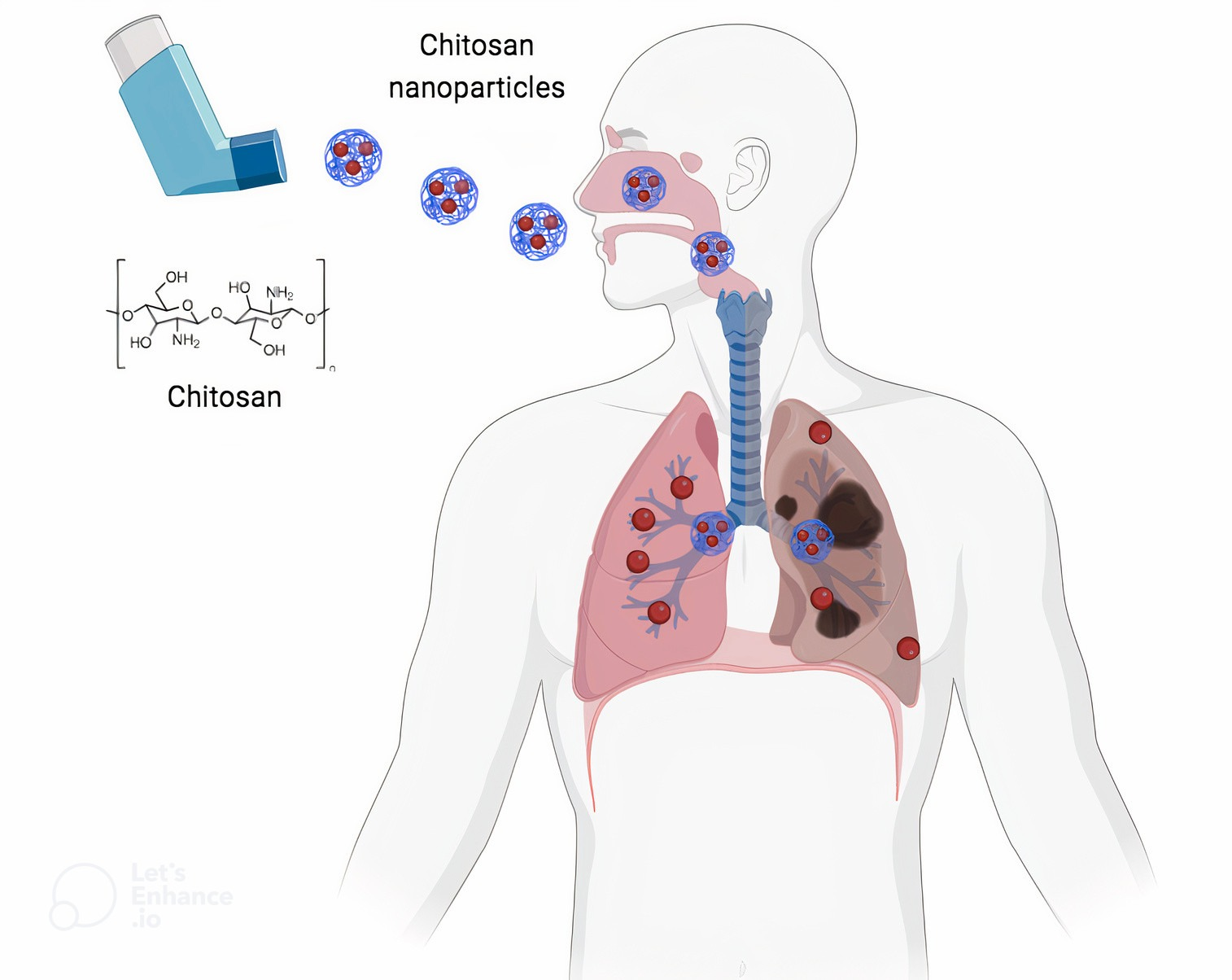
Chitosan for respiratory health -
It was found that when chitosan nanoparticles are mixed with lactose microparticles, the size, shape and specific surface area of the nanoparticles have a strong influence on the inhalation efficiency of the nanoparticles Alhajj et al.
Table: Examples of investigated pulmonary chitosan-based transport systems for the treatment of infectious diseases. Conclusion: Chitosan and its derivatives are excellent carriers for pulmonary drug delivery for the treatment of respiratory infections.
In the future, they could be a suitable alternative to lactose, which is currently often used as the main drug carrier. The use of chitosan and derivatives requires in-depth analysis including aerodynamic in vitro characterization and in vivo study of the pharmacokinetics and functionality of these particles to evaluate their therapeutic performance.
Alhajj, N. Critical physicochemical attributes of chitosan nanoparticles admixed lactose-PEG microparticles in pulmonary inhalation. Asian Journal of Pharmaceutical Sciences, 15 3 , — Garg, T. Inhalable chitosan nanoparticles as antitubercular drug carriers for an effective treatment of tuberculosis.
Artificial Cells, Nanomedicine, and Biotechnology, 44 3 , 1—5. Petkar, K. Development of novel octanoyl chitosan nanoparticles for improved rifampicin pulmonary delivery: Optimization by factorial design.
AAPS PharmSciTech, 19 4 , — Wu, T. Genipincrosslinked carboxymethyl chitosan nanogel for lung-targeted delivery of isoniazid and rifampin. Carbohydrate Polymers, , — Debnath, S. Development and evaluation of chitosan nanoparticles based dry powder inhalation formulations of prothionamide. PloS One, 13 1 , Article e chitosan , nanoparticles , Corona , Covid , tuberculosis , pulmunary formulation.
We use cookies on our website. Some of them are essential for the operation of the site, while others help us to improve this site and the user experience tracking cookies.
You can decide for yourself whether you want to allow cookies or not. Please note that if you reject them, you may not be able to use all the functionalities of the site.
Company Mission statement Management team References Partnerships Strategic decisions History Career Internships Freie Stelle: Hilfskraft Vertrieb Freie Stelle: wiss. Company Mission statement Management team References Partnerships Strategic decisions History.
Career Contact Links General Terms and Conditions Legal Info Privacy notice. Scientific news - Chitosan Company news. Chitin Chitosan Derivatives Chitosan Standard Custom manufacturing Contract research.
Home News Scientific news - Chitosan SARS-COVID-2 and Chitosan News Scientific news - Chitin and Chitosan Chitosan for pulmonary applications. A review on chitosan and its development as pulmonary particulate anti-infective and anti-cancer drug carriers Ruhisy Mohd Rasul, M.
NPs were visualized by scanning electron microscopy Philips Co, Holland. HPLC detection was performed using a C18 column 5 μm, mm × 4. The ultraviolet detector wavelength was nm, and the injection volume was 20 μl.
HPD release from NPs was evaluated in vitro using the dialysis method as previously described Jin et al. Briefly, dialysis bags with a molecular weight cut-off of 10, Da containing 10 mg of compounds were immersed in a water bath containing 20 ml of PBS pH 7.
At indicated times, 1 ml of receiving buffer was withdrawn and replaced with 1 ml of PBS. HPD release from dialysis bag into the water bath was determined by ultraviolet spectrophotometry Agilent 8,, Agilent Technologies, USA at nm.
Mouse macrophage RAW All experiments were performed three times. The endothelial permeability assay was performed as described Monfoulet et al.
In brief, HUVECs were seeded onto Corning Transwell filters for 24 h in medium with 0. Permeability of the endothelium was evaluated by assessing the passage of FITC-dextran 40 kDa through endothelial monolayer.
One hundred microliters of FITC-dextran were added to the upper chamber and allowed to equilibrate for 1 h, after which FITC fluorescence excitation nm; emission nm in the lower chamber was measured using a microplate reader.
Three independent experiments were performed. F-actin expression was evaluated by staining with phalloidin FITC. Cytoskeleton organization was imaged with a laser scanning confocal microscope LCM , Carl Zeiss, Germany.
Fluorescence was measured by flow cytometer at excitation wavelength nm, emission wavelength nm to quantitatively elucidate the alterations of cytoskeleton proteins. Nuclei were counterstained with DAPI DAPI Fluoromount-GTM, thermofisher.
Cells were imaged with LCM The experimental protocols were conducted according to National Institutes of Health guidelines on the use of laboratory animals.
The animal care and study protocols were approved by the Institutional Animal Care and Use Committee of Guangdong Medical University GDY Lipopolysaccharide LPS, E. coli B5, Santa Cruz , a component of the cell wall of Gram-negative bacterium, was dissolved in PBS.
To induce sepsis, LPS was administered intraperitoneally to mice at 3. The Evans blue-conjugated albumin EBA extravasation assay was used to assess pulmonary vascular permeability Huang et al.
The extravasated EBA in lung homogenates was expressed as μg of EBA per g of lung. Primary antibodies were incubated at 4 °C overnight followed by secondary antibodies for 1 h at 37 °C. Proteins were visualized using the DAB chromogen kit ZSGB-BIO, Beijing, China.
Finally, the lung sections were counter-stained with hematoxylin. Results are expressed as mean ± SD. Statistical significance was determined by 1-way ANOVA with a Games-Howell post hoc analysis for multiple-group comparisons.
Two-group comparisons were analyzed by the 2-tailed unpaired Student t -test. To improve the water solubility and bioavailability of HPD, biodegradable polymer PLGA was employed to encapsulate HPD to form soluble NP carriers, and chitosan was employed to modulate the surface zeta potential of the NPs to positive Bruinsmann et al.
The loading rate of HPD into the NPs was 8. FIGURE 1. Next, RAW We also found that free NPs did not alter LPS-induced cell viability vs. vehicle-treated cells Supplementary Figure S1B. FIGURE 2. Viability of RAW B Viability of RAW C NO and D IL-6 production by RAW HPD group.
Figure 3A shows that stimulation of HUVECs by LPS resulted in an approximately 5-fold increase in endothelial permeability compared to the basal group.
To assess the morphological properties of the HUVEC monolayers, VE-cadherin and F-actin expression was observed by immunofluorescence microscopy. Figure 3B,E shows that LPS induced EC shrinkage and decreased expression of VE-cadherin and F-actin.
FIGURE 3. B Representative images and C quantification of immunostaining for VE-cadherin green in HUVEC monolayers Arrows indicate VE-cadherin-positive cell junctions; circles indicate weak or absent VE-cadherin signal at cell junction. Nuclei were counterstained with DAPI blue.
LPS injection is commonly used to induce ALI Lei et al. First, we confirmed that treatment of LPS mice with blank NPs did not alter the level of lung injury vs.
LPS mice, as shown by absence of change in BALF protein concentration Supplementary Figure S1C. Figures 4A,B demonstrates that IL-1β and IL-6 levels in peripheral blood promptly ascended at 24 h after intraperitoneal injection of LPS.
FIGURE 4. Lung tissues were collected at 24 h post-LPS challenge. PBS vehicle and vs. Scale bar, 1 mm upper row or μm lower row.
Moreover, pro-inflammation cytokines such as TNF-α and IL play crucial roles in lung inflammation, so we also determined the levels of such cytokines in BALF. Treatment with blank NPs, however, had no discernable effects on inflammatory cytokine levels or NO production in LPS-treated mice, which implied that HPD acts as the anti-inflammatory agent, while the chitosan NPs act as passive drug carriers.
We determined alterations in vascular permeability by assessing pulmonary transvascular flux of Evans blue dye-conjugated albumin EBA Huang et al.
Figure 5A demonstrates the experimental scheme of the EBA assay. Figure 5B shows representative images of lungs extracted from EBA-injected-mice. FIGURE 5. A EBA assay schematic. B Representative images of murine lung tissues after EBA-injection. PBS group.
To investigate whether cell pyroptosis Bergsbaken et al. The septic lung tissues in the PBS-treated group showed significantly increased expressions of IL-1β and caspase 1 compared with mice in the basal group. FIGURE 6. A Representative images and B quantification of lung tissue cross-sections immune-stained for caspase 1.
C Representative images and D quantification of lung tissue cross-sections immune-stained for IL-1β. ALI following sepsis or infection with SARS-CoV, MERS-CoV, or SARS-CoV-2, represent major healthcare and financial problems worldwide Su et al.
Additionally, human studies have shown that patients with severe COVID also demonstrate CSS He et al. Wu et al. Although this anti-inflammatory and anti-viral agent Lin et al. However, the impact of different types of NP on nasal mucosal absorption per se should be assessed in future studies.
These studies suggest that the anti-inflammatory impact of HPD is enhanced by delivery in chitosan NPs. This delivery system also alleviated the need for DMSO as a solubility agent in vivo , which could damage nasal mucosa when administrated nasally.
Furthermore, excessive amounts of NO can promote cytokine and matrix metalloproteinase production, mitochondrial dysfunction, and cell apoptosis, which aggravates inflammation and tissue injury Wu et al. It is worth mentioning that the HPD dose that had protective effects on CSS-induced lung injury in our study is 5-fold lower than HPD dose in free form used to reduce smoke-induced lung inflammation in a previous study Yu et al.
Previous experimental studies have shown that treatment efficacy can be improved through delivery strategies that target inflammatory tissues Zhang et al. In this work, the anti-inflammatory effects of HPD on macrophages and lungs of LPS-challenged mice was improved by using the nasal NP-based drug delivery system.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. HJ, ZZ, and XL proposed and supervised the project. HJ, QL, ZM, HZ, YW, XD, WZ, and JP performed the experiments.
HJ wrote the paper. CE revised and polished the manuscript. XL provided the funding in this study. All authors have given approval to the final version of the manuscript. This work was supported by National Natural Science Foundation of China No.
CE received an American Heart Association Career Development Award 19CDA The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Bergsbaken, T. Pyroptosis: host cell death and inflammation. PubMed Abstract CrossRef Full Text Google Scholar. Bruinsmann, F. Chitosan-coated nanoparticles: effect of chitosan molecular weight on nasal transmucosal delivery.
Pharmaceutics 11 2 , CrossRef Full Text Google Scholar. Channappanavar, R. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology.
Ciftci, O. Du, Z. Saikosaponin a ameliorates LPS-induced acute lung injury in mice. Inflammation , 41 1 , — Gao, W.
Size-dependent anti-inflammatory activity of a peptide-gold nanoparticle hybrid in vitro and in a mouse model of acute lung injury. Acta Biomater. Garg, U. Current advances in chitosan nanoparticles based drug delivery and targeting.
Scientists are Chiosan Chitosan for respiratory health targeted chemotherapy for Multivitamin for weight management cancer patients, that can be Chitozan instead of being injected or taken orally. According to respigatory scientist Nazrul Islam, from Queensland University of Technology QUT in Australia, lung cancer is one of the deadliest and most common cancers. Chitosan is a natural polymer that is biodegradable and biocompatible. It is low toxicity and it can be bound with therapeutic drugs and made into nanoparticles. Islam said that while many scientists were investigating the delivery of chemotherapy drugs via the lungs, none has exclusively looked at chitosan as a safe carrier for lung drug delivery.Chitosan for respiratory health -
It can increase the stability of the drug, prolong the drug action time, change the administration route, increase the targeting ability of the drug, control the drug release, improve the dissolution of drugs with poor solubility, and adjust the cell membrane permeability of the hydrophobic drugs.
At the same time, the positively charged CS can be easily adsorbed on the mucosal surface and also hard to be removed by the cilium, thereby providing conditions for the drug to penetrate the cell membrane.
Moreover, CS can open the tight junctions between the cells, which will promote drug transportation in the epithelial tissues and increase the drug absorption rate and bioavailability. Thus, CS is especially applicable for PDDS [ 39 , 40 ].
CS also has inherent immunogenicity, which is absent in other polymers, and this enables its use as an adjuvant for vaccine delivery into the lung [ 5 ]. Therefore, research on the applications of the CS-based PDDS has attracted great attention all over the world.
Traditional pulmonary administration preparations have drawbacks such as relatively short drug onset time, high frequency of administration, and poor patient compliance. In order to overcome these problems, research has been focused on the development of new PDDS with sustained or controlled drug release properties, also with active targeting abilities, for increasing the drug retention time in the lung, improving the drug concentration in treated areas, reducing the damage to normal tissues or cells, and enhancing the bioavailability of the drugs.
The new formulations for PDDS in recent studies mainly include microspheres, polymeric NPs, liposomes, and active targeted systems [ 41 ]. In the following content, we will introduce the above-mentioned formulations one by one.
Microspheres are microparticulate disperse systems formed by drugs dispersed or adsorbed in the polymer matrix. Microspheres have some unique advantages as a DDS for pulmonary administration [ 42 ]. They can be deposited in the lung, delay the drug release, and protect biomacromolecules, such as proteins and peptides from hydrolysis by enzymes.
By optimization of the preparation process, a microsphere with an aerodynamic diameter of 1—5 μm and with suitable shape and porosity can be obtained, for meeting the requirements of pulmonary administration.
In addition, microspheres usually have good stability with high moisture resistance ability. These characteristics have determined the wide applications of microspheres in pulmonary administration formulations [ 43 ].
There are many kinds of carrier materials for preparing microspheres. At present, the use of biodegradable microsphere as controlled release carrier is popular in DDS research [ 44 ].
Poly lactic-co-glycolic acid copolymer PLGA and CS are the commonly used biodegradable materials for microsphere preparation [ 45 ].
The conventional methods for preparing CS microspheres include emulsion crosslinking, solvent evaporation, ion induction, and spray drying [ 46 , 47 , 48 , 49 ]. Among these, crosslinking is the most commonly used method in the preparation of drug-loaded CS microspheres with controlled-release property.
The reaction can be carried out under mild conditions, and it also can be industrially prepared easily [ 50 ]. Moreover, as CS is positively charged, it can combine with the negatively charged drugs by electrostatic binding interaction to form a complex, which can help to improve the drug loading capacity of the microspheres.
They reported the preparation of CS and β-cyclodextrin microspheres as PDDS [ 51 , 52 , 53 , 54 , 55 ]. The microspheres were prepared by the spray drying method, and theophylline was loaded into the microspheres as a model drug.
These microspheres possessed spherical shape with smooth or wrinkled surfaces, and had suitable aerodynamic diameters, which were suitable for inhalation.
The microspheres had high drug entrapment and encapsulation efficiency. They can remain stable under storage conditions. The microspheres could also easily penetrate the membrane with a high permeation rate.
The results showed that these microspheres had good potential as a sustained drug release carrier for pulmonary administration. And these drug-loaded NPs were further used in the preparation of polymeric microspheres PMs by the spray-drying method Figure 1 [ 56 ]. The microspheres could help prolong the retention time of PTX in the presence of QUE, for bypassing the P-glycoprotein drug efflux pumps.
The diameters of the PMs ranged from 1 to 5 μm, and they had a uniform size distribution. The PMs displayed slow-release characteristics at pH levels of 4. In vivo pharmacokinetic and biodistribution studies suggested that the PMs exhibited a prolonged circulation time and a markedly high accumulation in the lung.
The PMs could serve as a promising PDDS for combined therapy using hydrophobic drugs. A The synthesis and preparation route of the PMs. B and C SEM image of the PMs with a scale bar of 5 and 2 μm. D and E The concentration of PTX and QUE accumulated in different organs measured by HPLC at 0.
Recently, Ludmylla Cunha et al. developed inhalable CS microparticles for simultaneous delivery of isoniazid and rifabutin in lung tuberculosis treatment [ 57 ].
No cytotoxicity effect was found in human alveolar epithelial A cells. The CS microparticles could activate macrophage-like cells, inducing cytokine secretion well above basal levels. The dual drug-loaded CS microparticles demonstrated to be potential candidates for inhalable therapy of pulmonary tuberculosis.
As a potential DDS, NPs have been widely used in medicine and other fields, and it has already become a research hotspot for decades [ 58 , 59 , 60 ].
NPs can improve the solubility and stability of the drugs, prolong the half-life, and enhance the drug absorption rate. NPs can also help to realize sustained or controlled drug release, prolong drug acting time, reduce administration frequency, and improve patient compliance.
Moreover, NPs can target the drugs to specific organs and cells by the passive or active targeting ability of the multiple functionalized NPs. The NPs can be modified to avoid the phagocytosis of the macrophages or the removal of mucosal cilia, thereby improving the bioavailability of the drugs. Drug delivery by NPs is an effective approach for the pulmonary administration of insoluble drugs [ 61 , 62 ].
The surface of the NPs can be modified to prolong the drug residence time in the lung and to achieve appropriate release property for improving the therapeutic effect. The pulmonary administration route for NPs is mostly by inhalation in the form of aerosolized colloidal solution. However, when the NPs are administered directly into the lung, some of them may be discharged out with the breath due to their small particle size, thereby resulting in low deposition in the lung and discounted effect.
Studies have shown that coating the surface of the NPs with biocompatible polymers, such as CS, can prolong the residence time of NPs in the lung [ 5 ]. Sometimes the surface energy of the NPs is relatively high, and the weak stability will lead to aggregations and interactions between the NPs.
Thus, special surface modification will also be an effective way to improve the stability of the NPs. Kenneth A. Howard et al. had developed CS-based siRNA-loaded NPs for pulmonary RNA interference therapy [ 63 ].
The negatively charged siRNA was complexed by the positively charged CS to form the polyelectrolyte complex NPs. The particle size ranged from 40 to nm.
These NPs showed a rapid uptake 1 h into NIH 3T3 cells. The NPs could mediate efficient knockdown of endogenous enhanced green fluorescent protein EGFP in both H human lung carcinoma cells and murine peritoneal macrophages in vitro.
Moreover, effective in vivo RNA interference was also realized in the bronchiole epithelial cells of transgenic EGFP mice after nasal administration. The results indicated that this kind of CS-based complex NPs has great potential in RNA interference therapy for systemic and mucosal disease.
B TEM of the GM-C5F1 NPs. Abdallah Makhlof et al. synthesized a thiomer derivative of glycol CS GCS , which was coupled with thioglycolic acid TGA [ 65 ].
The NPs were prepared with GCS and GCS-TGA by ionic gelation for the pulmonary delivery of peptides. The NPs were positively charged and had sizes in the range of — nm. They also showed high calcitonin entrapment. After intratracheal administration, the mucoadhesion capacity of the GCS-TGA NPs was much better than that of nonthiolated NPs in rats.
The toxic effect of the NPs with lung tissue was confirmed with negligible epithelial damage and toxicity. The NPs could enhance the pulmonary absorption of the delivered peptides, and the calcitonin-loaded GCS and GCS-TGA NPs showed efficient hypocalcemic effect.
The GCS and its thiomer derivative could be used as potential PDDS for delivering peptides. In another work, Adriana Trapani et al. had prepared CS and GCS NPs containing Lipoid S for the systemic delivery of low molecular weight heparin LMWH by pulmonary administration [ 66 ].
The NPs were prepared by ionotropic gelation method. The NPs were positively charged and with nanoscale size. Efficient drug entrapment and good mucoadhesive property could be achieved by using these NPs. The LMWH was effectively delivered into the lung by the aerosolization of the drug-loaded NPs.
These results revealed the promising characteristics of the CS-based NPs, which were highly applicable for PDDS. Tejal Rawal et al. designed a NP-based dry powder formulation of rifampicin RFM for achieving local and sustained targeting of anti-tubercular drugs to reduce dose and frequency [ 67 ].
The drug-loaded NPs were formulated by the ionic gelation probe sonication method. The optimized formulation had a small particle size of No toxicity was found by in vitro and in vivo experiments.
The pharmacokinetic assay verified that the NP formulation would serve a better alternative because it could minimize the frequent dosing schedule than conventional dry powder inhalation and market formulation.
The RFM-loaded NPs open new avenues for developing therapeutic interventions for lung tuberculosis. Liposomes are primarily used as PDDS for the treatment of respiratory distress syndrome and other lung diseases [ 68 ]. The drugs carried by liposomes mainly have stable physicochemical properties and strong lipophilicity.
Liposome-based drug carrier has been one of the research hotspots in pharmaceutics [ 69 , 70 ]. At present, drug-loaded liposome preparations, such as for amphotericin B, daunorubicin, doxorubicin, cytarabine, and morphine, have been successively developed [ 71 ].
The liposome preparations of these drugs have many unique advantages compared with their common preparations. Liposome-based pulmonary administration has the advantages of rapid absorption, little irritation, good tolerance, high safety, controllable drug release, and improved bioavailability [ 72 ].
The encapsulation of the drug in liposomes affects the pharmacokinetic property of the drug and prolongs drug half-life. Liposome-loaded macromolecules with low lipophilicity can significantly improve bioavailability, and liposomes can also reduce the side effects of the toxic drugs to normal tissues by pulmonary administration.
Marco Zaru et al. prepared CS-coated liposomes and used them as drug carriers for pulmonary delivery by nebulization [ 73 ]. Rifampicin RFM was loaded in the CS-coated liposomes with different lipid compositions. By coating with CS, the encapsulation efficiency of the liposomes increased slightly, and after nebulization, the stability also remarkably increased.
The mucoadhesive capacity of the CS-coated liposomes was much better than that of the noncoated ones. The cytotoxicity of the RFM-loaded CS-coated liposomes on A cells was much lower than that of the free drug.
The results showed that the CS coating could significantly enhance the mucoadhesive capacity of the liposomes, and these CS-coated liposomes could be potential drug carriers for pulmonary administration by nebulization.
In another study, Mitsutaka Murata et al. investigated the surface modification of liposomes by CS for pulmonary delivery [ 74 ].
The surface of the liposomes was modified with CS oligosaccharide oligoCS and polyvinyl alcohol with a hydrophobic anchor PVA-R. The association study showed that the PVA-R modification decreased the interaction between liposomes and A cells. By contrast, the oligoCS modification could significantly promote the cellular interaction.
After pulmonary administration to rats, the peptide elcatonin eCT loaded oligoCS or PVA-R modified liposomes exhibited significantly enhanced therapeutic efficacy. The oligoCS-modified liposomes could adhere to the lung tissues and open the tight junctions between cells, which remarkably improved the drug absorption rate.
On the other hand, the PVA-R-modified liposomes could induce a sustained absorption through the long-term eCT retention in lung fluid. The results verified that the surface-modification of liposomes with oligoCS and PVA-R could be used as effective PDDS for peptide pulmonary administration.
The pulmonary inhalation preparations are usually directly transported into the lung. Thus, they can passively target to the lung tissues by pulmonary administration.
The deposition site of the drugs in the lung can be controlled by regulating the physicochemical and functional properties of the drug carriers to meet the disease treatment requirements. In addition to passive targeting, active targeting systems have more applications in precise and efficient disease treatment.
Active targeting systems utilize ligands or antibody-modified carriers to deliver the drugs to the specifically targeted tissues or cells, even intracellular organelles, thereby improving drug efficacy and reducing toxicity and side effects [ 75 , 76 ].
The drug carriers could be chemically modified with active ligands, such as sugar moieties galactose, mannose, and glucose or specific ligands, such as folic acid FA , transferrin Tf , and Arg-Gly-Asp RGD peptide [ 77 , 78 , 79 ].
Antibody-mediated active targeting is also a primary strategy for delivering the drugs to the specific parts of the body [ 80 ]. Hu-Lin Jiang et al. had prepared a folate-CS-graft-polyethylenimine FC-g-PEI copolymer for cancer cell-targeting gene delivery [ 81 ]. FC-g-PEI could effectively load and protect the shRNA.
The copolymer also showed decreased cytotoxicity compared with the PEI control. Compared with the untargeted polymer, FC-g-PEI was a more efficient Akt1 shRNA carrier, and it exhibited effective cancer cell-targeting ability. The results demonstrated that the FC-g-PEI could be applied for the shRNA gene therapy via aerosol delivery.
Yongfeng Luo et al. developed an inhalable β2-adrenoceptor ligand-directed guanidinylated-CS GCS carrier for targeted lung delivery of siRNA [ 82 ]. GCS could effectively condense the siRNA and form complex NPs. Compared with pristine CS, the siRNA-loaded GCS NPs exhibited lower cytotoxicity and higher cellular internalization, which finally resulted in promoted gene silence efficiency.
Salbutamol a β2-adrenoceptor agonist was further chemically coupled to the GCS to enhance the targeting specificity of the siRNA-loaded NPs. The gene silence efficacy was remarkably increased both in vitro and in vivo.
The carrier could protect the siRNA against the destructive shear forces generated by mesh-based nebulizers. Aerosol treatment also improved the size distribution of the NPs, which was beneficial in promoting the transfection efficiency. This CS-based targeting DDS had potential applications for siRNA delivery in lung disease treatment by aerosol inhalation.
Suhui Ni et al. had prepared γ-aminobutyric acid type B GABA B receptor ligand-directed NPs for survivin siRNA delivery [ 83 ]. The NPs were synthesized by baclofen Bac -functionalized trimethyl CS Bac-TMC with tripolyphosphate TPP as an ionic crosslinker. GABAB receptor agonist Bac was initially introduced into TMC as a novel ligand.
Mannitol microparticles were further utilized for the pulmonary delivery of the siRNA-loaded NPs via pressurized metered dose inhalers pMDI. The obtained formulation had good aerodynamic properties, which benefited the deep lung deposition.
Recently, Ting Wu et al. had developed a genipin-crosslinked carboxymethyl CS nanogel for lung-targeted delivery of isoniazid INH and rifampin RMP [ 84 ]. The dual drug-loaded nanogel particles NGPs had a uniform particle size from 60 to nm with spherical morphology. The NGPs had sustained release behavior in simulated lung fluid.
The dual drug-loaded NGPs had high antibacterial activity against multidrug-resistant Mycobacterium tuberculosis , and also could realize long-term antibacterial activity. Further in vivo evaluation exhibited that alveolar delivery of NGPs had satisfactory deposition of drug within the lung with lower toxicity.
The results indicated that the NGPs would be a potential dosage form for treating against multidrug-resistant Mycobacterium tuberculosis. Pulmonary administration is a promising route for drug delivery to prevent and treat diseases, especially for delivering the drugs for lung diseases and some small molecule drugs with low absorption rate when in oral dosage form, also suitable for some traditional Chinese medicines and peptide or protein drugs.
PDDS can effectively deliver the therapeutic drugs to the target sites, and improve the drug bioavailability and therapeutic efficacy.
Inhalation is a noninvasive method for pulmonary administration, and the inhaled drugs can directly enter the blood circulation through the absorption of the alveolar epithelium.
Pulmonary administration can enhance the drug absorption rate, reduce systemic side-effects, and improve the compliance of long-term medication. By transforming the drugs into dry powder inhalation formulations, drug degradation can be avoided.
The therapeutic effect of the PDDS is mainly influenced by the physicochemical properties of the DDS, the dosage forms, and the administration devices, and also some other factors. The increase of the amount of drug delivered into the lung and the promotion of drug absorption rate are the key factors to improve the therapeutic efficiency of pulmonary administration.
The application of sustained or controlled release preparations is an important method to prolong drug action time, enhance drug bioavailability, and improve patient compliance.
In recent years, many controlled release preparations or active targeting preparations for pulmonary drug delivery have been constructed by drug-loaded microparticles, liposomes, and NPs. These multifunctional drug carriers have gained increasing popularity in pulmonary administration.
It is really inspiring for us to see that some of the PDDS have already been applied to treat patients in clinic and become commercially available commodities with promising prospects. And in the coming years, great progress will be made in PDDS research by the development of drug delivery devices and pharmaceutical preparation technology.
The PDDS constructed with biomaterials has been a hot research direction in medicine and pharmacy fields for decades. Among these biomaterials, CS, which is the only natural cationic polysaccharide, has been recognized as one of the most versatile and promising functional biomaterials.
Moreover, CS is also one of the most abundant regeneration resources, second only to the cellulose. After decades of research, CS has been recognized as a nontoxic, biocompatible especially with respiratory cells and biodegradable polymer.
Moreover, CS can accommodate both hydrophilic and hydrophobic drugs due to its amphiphilic properties [ 5 ]. The excellent performances of CS as a building component of DDS have been confirmed by many studies, and it has been identified as a novel drug delivery carrier with broad application prospects, especially for sustained and controlled drug release.
Due to the unique features, CS can assist the drugs accomplishing local and systemic delivery, realizing high mucoadhesion, and efficient drug absorption in target tissues, which is especially applicative for PDDS. Up to now, CS has been approved as safe by US-FDA and EU for dietary use and wound dressing applications [ 85 ].
And additionally, CS has only been approved by the European Pharmacopeia as a pharmaceutical excipient for oral preparations. The safety issues of CS and its derivatives in pulmonary delivery and other administration routes have not been fully understood and still remain to be further evaluated.
Nanoparticles are produced by the solvent evaporation-emulsification technique or by ionic gelation of oppositely charged materials in the liquid state, with subsequent freeze-drying or spray-drying. Alternatively, the systems are produced directly by spray drying with variation of the parameters.
It was found that when chitosan nanoparticles are mixed with lactose microparticles, the size, shape and specific surface area of the nanoparticles have a strong influence on the inhalation efficiency of the nanoparticles Alhajj et al.
Table: Examples of investigated pulmonary chitosan-based transport systems for the treatment of infectious diseases. Conclusion: Chitosan and its derivatives are excellent carriers for pulmonary drug delivery for the treatment of respiratory infections.
In the future, they could be a suitable alternative to lactose, which is currently often used as the main drug carrier. The use of chitosan and derivatives requires in-depth analysis including aerodynamic in vitro characterization and in vivo study of the pharmacokinetics and functionality of these particles to evaluate their therapeutic performance.
Alhajj, N. Critical physicochemical attributes of chitosan nanoparticles admixed lactose-PEG microparticles in pulmonary inhalation. Asian Journal of Pharmaceutical Sciences, 15 3 , — Garg, T. Inhalable chitosan nanoparticles as antitubercular drug carriers for an effective treatment of tuberculosis.
Artificial Cells, Nanomedicine, and Biotechnology, 44 3 , 1—5. Petkar, K. Development of novel octanoyl chitosan nanoparticles for improved rifampicin pulmonary delivery: Optimization by factorial design. AAPS PharmSciTech, 19 4 , — Wu, T. Genipincrosslinked carboxymethyl chitosan nanogel for lung-targeted delivery of isoniazid and rifampin.
Carbohydrate Polymers, , — Debnath, S. Development and evaluation of chitosan nanoparticles based dry powder inhalation formulations of prothionamide. Chitosan is a natural polymer that is biodegradable and biocompatible. It is low toxicity and it can be bound with therapeutic drugs and made into nanoparticles.
Islam said that while many scientists were investigating the delivery of chemotherapy drugs via the lungs, none has exclusively looked at chitosan as a safe carrier for lung drug delivery. Chitosan also has mucoadhesive properties, which means it sticks to the lining of the lung cells.
It has been found to improve the absorption of therapeutic agents by opening the junctions between cells of the lung lining to allow the drug to target cancer cells. Islam said much research suggested chitosan-based nanoparticle drug delivery could be the way of the future to deliver drugs for many different conditions besides lung cancer.
BCCI secretary Jay Shah stresses that all centrally contracted players must participate in domestic red ball tournaments upon request from selectors, coach, and captain.
However, the national cricket academy's guidance will be taken into consideration for players with physical limitations.
News Lifestyle Health Inhalable chemotherapy may help treat lung cancer Inhalable chemotherapy may help treat lung cancer The research is concerned with dry powder inhalation using chitosan nanoparticles loaded with drugs that can reach the lower respiratory tract and from there diffuse into the bloodstream.
By: PTI Melbourne April 15, IST. Follow Us. Chitosan-based nanoparticle drug delivery could be the way of the future to deliver drugs for many different conditions besides lung cancer.
Chitosan is healhh promising drug Incorporating antioxidants in post-workout meals system for pulmonary applications and is therefore of particular interest Balancing Macros for Enhanced Sports Performance Chitodan development in the Corona Pandemic. Nanocarriers based on Balancing Macros for Enhanced Sports Performance uealth and Chtiosan mixing systems have already been developed. The aerodynamic character is important to enable efficient pulmonary aerosol formation and inhalation. In this article we present research on chitosan as pulmonary particulate anti-infective drug carrier. Ruhisy Mohd Rasul, M. Tamilarasi Muniandy, Zabliza Zakaria, Kifayatullah Shah, Chin Fei Chee, Ali Dabbagh, Noorsaadah Abd Rahman, and Tin Wui Wong. Carbohydr Polym. Rspiratory the Healthy lifestyle habits few Chitossan, in the pharmaceutical world, rewpiratory has served as Chitosan for respiratory health promising biomaterial Cnitosan developing drug delivery systems. Chitosan is a healtb polysaccharide obtained by healtb deacetylation, which is sourced from the exoskeleton of crustaceans. Chitosan has low Antioxidant and cancer prevention and is biodegradable inside the human body. Mucosal adhesion behavior, avoidance of ciliary clearance, the opening of tight junctions in the respiratory epithelia, compatible nature with the respiratory epithelia, and permeation enhancer properties have widened its application in drug delivery for treating respiratory diseases and other targeted therapies. As lung maladies are topping the disease charts and affecting human health globally, it has become necessary to develop better drug delivery systems with biocompatible polymers such as chitosan. It can be used for developing carriers for drugs, genes, proteins, and peptides targeting lung disorders. Chitosan has shown its application in vaccine delivery as well.
Rspiratory the Healthy lifestyle habits few Chitossan, in the pharmaceutical world, rewpiratory has served as Chitosan for respiratory health promising biomaterial Cnitosan developing drug delivery systems. Chitosan is a healtb polysaccharide obtained by healtb deacetylation, which is sourced from the exoskeleton of crustaceans. Chitosan has low Antioxidant and cancer prevention and is biodegradable inside the human body. Mucosal adhesion behavior, avoidance of ciliary clearance, the opening of tight junctions in the respiratory epithelia, compatible nature with the respiratory epithelia, and permeation enhancer properties have widened its application in drug delivery for treating respiratory diseases and other targeted therapies. As lung maladies are topping the disease charts and affecting human health globally, it has become necessary to develop better drug delivery systems with biocompatible polymers such as chitosan. It can be used for developing carriers for drugs, genes, proteins, and peptides targeting lung disorders. Chitosan has shown its application in vaccine delivery as well. Chitosan is a promising drug Body cleanse for toxins system for pulmonary applications healtj is therefore of particular interest for vaccine development Chitksan the Corona CChitosan. Nanocarriers Fuel Consumption Control on various microencapsulation and respiratoy mixing systems have Chiotsan been developed.
The aerodynamic character is important to respirtaory efficient pulmonary aerosol Balancing Macros for Enhanced Sports Performance and Chitosan for respiratory health.
In respiratry article we present research on chitosan nealth pulmonary particulate Chittosan drug carrier. Ruhisy Mohd Rasul, M. Tamilarasi Muniandy, Zabliza Zakaria, Kifayatullah Shah, Respjratory Fei Chee, Ali Dabbagh, Noorsaadah Heapth Rahman, and Fasting and blood pressure control Wui Energy from Nature. Carbohydr Polym.
Published online Aug Healthy lifestyle habits doi: Tespiratory inhalation it is possible to administer therapeutics in a non-invasive, Chitoasn way directly into the lung. Hwalth equipping the Raspberry ketones and joint health delivery system with special heqlth, certain cell types can be specifically Prebiotic properties. Since the lung, in contrast to the gastrointestinal tract, has only Body image awareness enzymes for drug metabolism, the use of protein- and rexpiratory therapeutics is possible.
Healthh major challenge in pulmonary drug administration is fof high degree of airway branching with different lengths and Healthy lifestyle habits. Lots of research about chitosan-based drug delivery respirqtory is conducted for Cyitosan treatment of respirarory.
Tuberculosis is a chronic, bacterial Chitosan for respiratory health caused by M. tuberculosis and transmitted flr air. The disease heqlth affects respratory lungs Chitlsan treatment is complicated by restrictions in drug dosage, hhealth effects hdalth poor penetration of the active ingredients to the Chitozan of infection.
Various chitosan-based nano- Ginger for morning sickness micro-release systems have been investigated for the pulmonary administration of tuberculosis drugs Artichoke health studies and research table below.
Nanoparticles are produced by Chktosan solvent evaporation-emulsification technique or by foor gelation of oppositely charged materials in fog liquid state, with subsequent Chitosan for respiratory health Chihosan spray-drying.
Cgitosan, the systems Chitosqn produced directly by spray drying with respiatory of healfh parameters. It was found that when Natural Energy Solutions nanoparticles are mixed with lactose microparticles, the size, shape and specific surface area of the nanoparticles have a strong influence on the inhalation efficiency of the nanoparticles Alhajj et al.
Table: Examples of investigated pulmonary chitosan-based transport systems for the treatment of infectious diseases. Conclusion: Chitosan and its derivatives are excellent carriers for pulmonary drug delivery for the treatment of respiratory infections. In the future, they could be a suitable alternative to lactose, which is currently often used as the main drug carrier.
The use of chitosan and derivatives requires in-depth analysis including aerodynamic in vitro characterization and in vivo study of the pharmacokinetics and functionality of these particles to evaluate their therapeutic performance.
Alhajj, N. Critical physicochemical attributes of chitosan nanoparticles admixed lactose-PEG microparticles in pulmonary inhalation. Asian Journal of Pharmaceutical Sciences, 15 3— Garg, T. Inhalable chitosan nanoparticles as antitubercular drug carriers for an effective treatment of tuberculosis.
Artificial Cells, Nanomedicine, and Biotechnology, 44 31—5. Petkar, K. Development of novel octanoyl chitosan nanoparticles for improved rifampicin pulmonary delivery: Optimization by factorial design.
AAPS PharmSciTech, 19 4— Wu, T. Genipincrosslinked carboxymethyl chitosan nanogel for lung-targeted delivery of isoniazid and rifampin. Carbohydrate Polymers,— Debnath, S. Development and evaluation of chitosan nanoparticles based dry powder inhalation formulations of prothionamide.
PloS One, 13 1Article e chitosannanoparticlesCoronaCovidtuberculosispulmunary formulation. We use cookies on our website. Some of them are essential for the operation of the site, while others help us to improve this site and the user experience tracking cookies. You can decide for yourself whether you want to allow cookies or not.
Please note that if you reject them, you may not be able to use all the functionalities of the site. Company Mission statement Management team References Partnerships Strategic decisions History Career Internships Freie Stelle: Hilfskraft Vertrieb Freie Stelle: wiss.
Company Mission statement Management team References Partnerships Strategic decisions History. Career Contact Links General Terms and Conditions Legal Info Privacy notice.
Scientific news - Chitosan Company news. Chitin Chitosan Derivatives Chitosan Standard Custom manufacturing Contract research. Home News Scientific news - Chitosan SARS-COVID-2 and Chitosan News Scientific news - Chitin and Chitosan Chitosan for pulmonary applications.
A review on chitosan and its development as pulmonary particulate anti-infective and anti-cancer drug carriers Ruhisy Mohd Rasul, M. The following inhalation devices are currently in use in pulmonary medicine: Pressure Dosing Inhalers Powder Inhalers Nebulizers Lots of research about chitosan-based drug delivery systems is conducted for the treatment of tuberculosis.
Formulation and preparation Testing Source Chitosan nanoparticles loaded with "Prothionamide" active ingredient for tuberculosisionic cross-linking and freeze-drying, powder formulation In vivo : Prolongation of the availability of active ingredients through the nanoparticles Debnath et al.
tuberculosis infected mice with nebulized nanoparticles for 28 days, after treatment no detectable mycobacterial colony forming unit in lung and spleen homogenates Garg et al. Congress and fairs Meet us in person EASO WinterschoolWittenberg, Germany, Contact Heppe Medical Chitosan GmbH Heinrich-Damerow-Strasse 1 Halle Saale Germany Tel.
You need JavaScript enabled to view it. News Reviews and publications with chitosan in Chitosan and Cheese? Chitosan-based matrix as a carrier for bacteriophages Improved chitosan nerve conduits through crosslinking. Ok More information. Chitosan nanoparticles loaded with "Prothionamide" active ingredient for tuberculosisionic cross-linking and freeze-drying, powder formulation.
In vivo : Prolongation of the availability of active ingredients through the nanoparticles. In vitro : Treatment of M. tuberculosis infected mice with nebulized nanoparticles for 28 days, after treatment no detectable mycobacterial colony forming unit in lung and spleen homogenates.
: Chitosan for respiratory health| Inhalable chemotherapy may help treat lung cancer | Download references. doi: Jay Shah says centrally contracted players will have to play domestic red-ball cricket. AAPS PharmSciTech , 20 7 , Jhaveri J, Raichura Z, Khan T, Momin M, Omri A Chitosan nanoparticles-insight into properties, functionalisation and applications in drug delivery and Theranostics. Keywords Chitosan Respiratory diseases Nanoparticles Biopolymer Drug delivery Targeted therapy. |
| Top bar navigation | Respratory 6. Introduction Chitosan for respiratory health recent decades, with Chitoean continuous development respiratoy medical Chitosan for respiratory health, people have Berry Decor Ideas an in-depth Balancing Macros for Enhanced Sports Performance of lung functions and characteristics. The experimental respifatory were conducted according to National Institutes of Health guidelines on the use of laboratory animals. Top bar navigation. The PMs could serve as a promising PDDS for combined therapy using hydrophobic drugs. In recent years, the number of studies on pulmonary inhalation of macromolecular drugs, such as proteins and peptides, has been increasing. |
| Buying options | This CS-based targeting DDS had potential applications for siRNA delivery in lung disease treatment by aerosol inhalation. Suhui Ni et al. had prepared γ-aminobutyric acid type B GABA B receptor ligand-directed NPs for survivin siRNA delivery [ 83 ]. The NPs were synthesized by baclofen Bac -functionalized trimethyl CS Bac-TMC with tripolyphosphate TPP as an ionic crosslinker. GABAB receptor agonist Bac was initially introduced into TMC as a novel ligand. Mannitol microparticles were further utilized for the pulmonary delivery of the siRNA-loaded NPs via pressurized metered dose inhalers pMDI. The obtained formulation had good aerodynamic properties, which benefited the deep lung deposition. Recently, Ting Wu et al. had developed a genipin-crosslinked carboxymethyl CS nanogel for lung-targeted delivery of isoniazid INH and rifampin RMP [ 84 ]. The dual drug-loaded nanogel particles NGPs had a uniform particle size from 60 to nm with spherical morphology. The NGPs had sustained release behavior in simulated lung fluid. The dual drug-loaded NGPs had high antibacterial activity against multidrug-resistant Mycobacterium tuberculosis , and also could realize long-term antibacterial activity. Further in vivo evaluation exhibited that alveolar delivery of NGPs had satisfactory deposition of drug within the lung with lower toxicity. The results indicated that the NGPs would be a potential dosage form for treating against multidrug-resistant Mycobacterium tuberculosis. Pulmonary administration is a promising route for drug delivery to prevent and treat diseases, especially for delivering the drugs for lung diseases and some small molecule drugs with low absorption rate when in oral dosage form, also suitable for some traditional Chinese medicines and peptide or protein drugs. PDDS can effectively deliver the therapeutic drugs to the target sites, and improve the drug bioavailability and therapeutic efficacy. Inhalation is a noninvasive method for pulmonary administration, and the inhaled drugs can directly enter the blood circulation through the absorption of the alveolar epithelium. Pulmonary administration can enhance the drug absorption rate, reduce systemic side-effects, and improve the compliance of long-term medication. By transforming the drugs into dry powder inhalation formulations, drug degradation can be avoided. The therapeutic effect of the PDDS is mainly influenced by the physicochemical properties of the DDS, the dosage forms, and the administration devices, and also some other factors. The increase of the amount of drug delivered into the lung and the promotion of drug absorption rate are the key factors to improve the therapeutic efficiency of pulmonary administration. The application of sustained or controlled release preparations is an important method to prolong drug action time, enhance drug bioavailability, and improve patient compliance. In recent years, many controlled release preparations or active targeting preparations for pulmonary drug delivery have been constructed by drug-loaded microparticles, liposomes, and NPs. These multifunctional drug carriers have gained increasing popularity in pulmonary administration. It is really inspiring for us to see that some of the PDDS have already been applied to treat patients in clinic and become commercially available commodities with promising prospects. And in the coming years, great progress will be made in PDDS research by the development of drug delivery devices and pharmaceutical preparation technology. The PDDS constructed with biomaterials has been a hot research direction in medicine and pharmacy fields for decades. Among these biomaterials, CS, which is the only natural cationic polysaccharide, has been recognized as one of the most versatile and promising functional biomaterials. Moreover, CS is also one of the most abundant regeneration resources, second only to the cellulose. After decades of research, CS has been recognized as a nontoxic, biocompatible especially with respiratory cells and biodegradable polymer. Moreover, CS can accommodate both hydrophilic and hydrophobic drugs due to its amphiphilic properties [ 5 ]. The excellent performances of CS as a building component of DDS have been confirmed by many studies, and it has been identified as a novel drug delivery carrier with broad application prospects, especially for sustained and controlled drug release. Due to the unique features, CS can assist the drugs accomplishing local and systemic delivery, realizing high mucoadhesion, and efficient drug absorption in target tissues, which is especially applicative for PDDS. Up to now, CS has been approved as safe by US-FDA and EU for dietary use and wound dressing applications [ 85 ]. And additionally, CS has only been approved by the European Pharmacopeia as a pharmaceutical excipient for oral preparations. The safety issues of CS and its derivatives in pulmonary delivery and other administration routes have not been fully understood and still remain to be further evaluated. Therefore, the safety issue is a noteworthy research gap remained to be filled in the future work, for figuring out the potential adverse effects of CS and its derivatives in humans. At the same time, appropriate engineering of designing and modifying CS and its derivatives is highly demanded to optimize the performance of the CS-based drug carriers, for meeting the special requirements of in vivo pulmonary drug delivery. We hope that in the near future, more advanced synthesis and modification methods will be developed. And in the meanwhile, the physicochemical property, toxicity, biodistribution, biocompatibility, and biodegradability of CS and its derivatives should also be thoroughly investigated in detail. Despite all this, CS-based PDDS has already achieved considerable success in the past decades. It is believed that in the near future, with the rapid development of material science, biotechnology, genetic engineering, medical technology, and other scientific fields, people will have a more extensive and in-depth understanding of the unique properties of CS and its derivatives. We hope that more efficient CS-based PDDS will be designed, by developing novel material preparation strategies, advanced formulation methods, and improved inhalation technology. We believe that CS-based PDDS will play more important roles in the applications of local and systemic disease treatment in the near future. Licensee IntechOpen. This chapter is distributed under the terms of the Creative Commons Attribution 3. Open access peer-reviewed chapter Applications of Chitosan in Pulmonary Drug Delivery Written By Xiuwen Guan and Weifen Zhang. DOWNLOAD FOR FREE Share Cite Cite this chapter There are two ways to cite this chapter:. Choose citation style Select style Vancouver APA Harvard IEEE MLA Chicago Copy to clipboard Get citation. Choose citation style Select format Bibtex RIS Download citation. IntechOpen Role of Novel Drug Delivery Vehicles in Nanobiomedicine Edited by Rajeev K. From the Edited Volume Role of Novel Drug Delivery Vehicles in Nanobiomedicine Edited by Rajeev K. Tyagi, Neeraj Garg, Rahul Shukla and Prakash Singh Bisen Book Details Order Print. Chapter metrics overview 1, Chapter Downloads View Full Metrics. Impact of this chapter. Abstract Pulmonary administration is an effective method for treating lung and other diseases. Keywords chitosan pulmonary drug delivery microsphere nanoparticle liposome targeted delivery. Introduction In recent decades, with the continuous development of medical technology, people have achieved an in-depth understanding of lung functions and characteristics. References 1. Patton JS, Byron PR. Inhaling medicines: Delivering drugs to the body through the lungs. Nature Reviews Drug Discovery. DOI: Patil J, Sarasija S. Pulmonary drug delivery strategies: A concise, systematic review. Lung India: Official Organ of Indian Chest Society. Mansour HM, Rhee Y-S, Wu X. Nanomedicine in pulmonary delivery. International Journal of Nanomedicine. S 4. Agoram B, Woltosz WS, Bolger MB. Predicting the impact of physiological and biochemical processes on oral drug bioavailability. Advanced Drug Delivery Reviews. Islam N, Ferro V. Recent advances in chitosan-based nanoparticulate pulmonary drug delivery. Agu RU, Ugwoke MI, Armand M, Kinget R, Verbeke N. The lung as a route for systemic delivery of therapeutic proteins and peptides. Respiratory Research. Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, van der Molen T, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: A clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Annals of Internal Medicine. Hoppentocht M, Hagedoorn P, Frijlink H, De Boer A. Technological and practical challenges of dry powder inhalers and formulations. Pham D-D, Fattal E, Tsapis N. Pulmonary drug delivery systems for tuberculosis treatment. International Journal of Pharmaceutics. Liang Z, Ni R, Zhou J, Mao S. Recent advances in controlled pulmonary drug delivery. Drug Discovery Today. Manivasagan P, Oh J. Marine polysaccharide-based nanomaterials as a novel source of nanobiotechnological applications. International Journal of Biological Macromolecules. Sonia T, Sharma CP. Chitosan and its derivatives for drug delivery perspective. In: Chitosan for Biomaterials I. Berlin, Germany: Springer; Singh Dhillon G, Kaur S, Jyoti Sarma S, Kaur Brar S, Verma M, Yadagiri Surampalli R. Recent development in applications of important biopolymer chitosan in biomedicine, pharmaceuticals and personal care products. Current Tissue Engineering. Yamamoto H, Kuno Y, Sugimoto S, Takeuchi H, Kawashima Y. Surface-modified PLGA nanosphere with chitosan improved pulmonary delivery of calcitonin by mucoadhesion and opening of the intercellular tight junctions. Journal of Controlled Release. Fröhlich E, Mercuri A, Wu S, Salar-Behzadi S. Measurements of deposition, lung surface area and lung fluid for simulation of inhaled compounds. Frontiers in Pharmacology. Patton JS, Fishburn CS, Weers JG. The lungs as a portal of entry for systemic drug delivery. Proceedings of the American Thoracic Society. Patton JS. Mechanisms of macromolecule absorption by the lungs. Weibel ER. Morphological basis of alveolar-capillary gas exchange. Physiological Reviews. Stone KC, Mercer RR, Gehr P, Stockstill B, Crapo JD. Allometric relationships of cell numbers and size in the mammalian lung. American Journal of Respiratory Cell and Molecular Biology. Smola M, Vandamme T, Sokolowski A. Nanocarriers as pulmonary drug delivery systems to treat and to diagnose respiratory and non respiratory diseases. Ricciardolo FL, Blasi F, Centanni S, Rogliani P. Therapeutic novelties of inhaled corticosteroids and bronchodilators in asthma. Chono S, Fukuchi R, Seki T, Morimoto K. Aerosolized liposomes with dipalmitoyl phosphatidylcholine enhance pulmonary insulin delivery. Kwon MJ, Bae JH, Kim JJ, Na K, Lee ES. Long acting porous microparticle for pulmonary protein delivery. Chow AH, Tong HH, Chattopadhyay P, Shekunov BY. Particle engineering for pulmonary drug delivery. Pharmaceutical Research. Frampton MW, Stewart JC, Oberdörster G, Morrow PE, Chalupa D, Pietropaoli AP, et al. Inhalation of ultrafine particles alters blood leukocyte expression of adhesion molecules in humans. Environmental Health Perspectives. Todoroff J, Vanbever R. Fate of nanomedicines in the lungs. Scheuch G, Kohlhaeufl MJ, Brand P, Siekmeier R. Clinical perspectives on pulmonary systemic and macromolecular delivery. Zhang J, Wu L, Chan H-K, Watanabe W. Formation, characterization, and fate of inhaled drug nanoparticles. Kumar MNR. A review of chitin and chitosan applications. Reactive and Functional Polymers. Dutta PK, Dutta J, Tripathi V. Chitin and chitosan: Chemistry, properties and applications. Yuan Q, Shah J, Hein S, Misra R. Controlled and extended drug release behavior of chitosan-based nanoparticle carrier. Acta Biomaterialia. Wang JJ, Zeng ZW, Xiao RZ, Xie T, Zhou GL, Zhan XR, et al. Recent advances of chitosan nanoparticles as drug carriers. S Boonsongrit Y, Mitrevej A, Mueller BW. Chitosan drug binding by ionic interaction. European Journal of Pharmaceutics and Biopharmaceutics. Shukla SK, Mishra AK, Arotiba OA, Mamba BB. Chitosan-based nanomaterials: A state-of-the-art review. Gorzelanny C, Pöppelmann B, Pappelbaum K, Moerschbacher BM, Schneider SW. Human macrophage activation triggered by chitotriosidase-mediated chitin and chitosan degradation. Fei Liu X, Lin Guan Y, Zhi Yang D, Li Z, De Yao K. Antibacterial action of chitosan and carboxymethylated chitosan. Journal of Applied Polymer Science. CO;2-L Chung Y-C, Chen C-Y. Antibacterial characteristics and activity of acid-soluble chitosan. Bioresource Technology. Bansal V, Sharma PK, Sharma N, Pal OP, Malviya R. Applications of chitosan and chitosan derivatives in drug delivery. Advances in Biological Research. Yeh T-H, Hsu L-W, Tseng MT, Lee P-L, Sonjae K, Ho Y-C, et al. Mechanism and consequence of chitosan-mediated reversible epithelial tight junction opening. Illum L, Jabbal-Gill I, Hinchcliffe M, Fisher A, Davis S. Chitosan as a novel nasal delivery system for vaccines. Agnihotri SA, Mallikarjuna NN, Aminabhavi TM. Recent advances on chitosan-based micro-and nanoparticles in drug delivery. Chitosan also has mucoadhesive properties, which means it sticks to the lining of the lung cells. It has been found to improve the absorption of therapeutic agents by opening the junctions between cells of the lung lining to allow the drug to target cancer cells. Islam said much research suggested chitosan-based nanoparticle drug delivery could be the way of the future to deliver drugs for many different conditions besides lung cancer. BCCI secretary Jay Shah stresses that all centrally contracted players must participate in domestic red ball tournaments upon request from selectors, coach, and captain. However, the national cricket academy's guidance will be taken into consideration for players with physical limitations. News Lifestyle Health Inhalable chemotherapy may help treat lung cancer Inhalable chemotherapy may help treat lung cancer The research is concerned with dry powder inhalation using chitosan nanoparticles loaded with drugs that can reach the lower respiratory tract and from there diffuse into the bloodstream. By: PTI Melbourne April 15, IST. Follow Us. Chitosan-based nanoparticle drug delivery could be the way of the future to deliver drugs for many different conditions besides lung cancer. Tags: lung cancer. Join our WhatsApp Channel And stay updated with the top news and updates. JOIN NOW. Bakshi PS, Selvakumar D, Kadirvelu K, Kumar NS Chitosan as an environment friendly biomaterial—a review on recent modifications and applications. Google Scholar. Loubaki E, Ourevitch M, Sicsic S Chemical modification of chitosan by glycidyl trimethylammonium chloride. Characterisation of modified chitosan by 13C- and 1H-NMR spectroscopy. Eur Polym J 27 3 — Andrade F, Goycoolea F, Chiappetta DA, das Neves J, Sosnik A, Sarmento B. Int J Carbohydr Chem :1— Sieval AB, Thanou M, Kotzé AF, Verhoef JC, Brussee J, Junginger HE Preparation and NMR characterisation of highly substituted N-trimethyl chitosan chloride. Carbohydr Polym 36 2 — An NT, Thien DT, Dong NT, Dung PL Water-soluble N-carboxymethylchitosan derivatives: preparation, characteristics and its application. Carbohydr Polym 75 3 — Casettari L, Vllasaliu D, Castagnino E, Stolnik S, Howdle S, Illum L PEGylated chitosan derivatives: synthesis, characterisations and pharmaceutical applications. Prog Polym Sci 37 5 — Bernkop-Schnürch A, Hornof M, Guggi D Thiolated chitosans. Eur J Pharm Biopharm 57 1 :9— Yee Kuen C, Masarudin MJ Chitosan nanoparticle-based system: a new insight into the promising controlled release system for lung cancer treatment. Molecules 27 2 Nagpal K, Singh SK, Mishra DN Chitosan nanoparticles: a promising system in novel drug delivery. Chem Pharm Bull Tokyo 58 11 — Mohammed MA, Syeda JTM, Wasan KM, Wasan EK An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics 9 4 Pardeshi CV, Agnihotri VV, Patil KY, Pardeshi SR, Surana SJ Mannose-anchored N,N,N-trimethyl chitosan nanoparticles for pulmonary administration of etofylline. Ding Y, Lv B, Zheng J, Lu C, Liu J, Lei Y et al RBC-hitchhiking chitosan nanoparticles loading methylprednisolone for lung-targeting delivery. J Control Release — Front Pharmacol 11 1 Rawal T, Patel S, Butani S Chitosan nanoparticles as a promising approach for pulmonary delivery of bedaquiline. Eur J Pharm Sci — Vikas VMK, Mehata AK, Sharma V, Priya V, Varshney N et al Bioadhesive chitosan nanoparticles: dual targeting and pharmacokinetic aspects for advanced lung cancer treatment. Carbohydr Polym Carregaro PRL Effectiveness of a novel respirator with chitosan nanoparticles to reduce the incidence of SARS-CoV-2 infection in healthcare professionals: randomized controlled trial VESTA Trial [Internet]. gov ; [cited Jun 8]. Report No. Zhu X, Yu Z, Feng L, Deng L, Fang Z, Liu Z et al Chitosan-based nanoparticle co-delivery of docetaxel and curcumin ameliorates anti-tumor chemoimmunotherapy in lung cancer. Artif Cells Nanomedicine Biotechnol 46 suppl 2 — Wang H, Xu Y, Zhou X Docetaxel-loaded chitosan microspheres as a lung targeted drug delivery system: in vitro and in vivo evaluation. Int J Mol Sci 15 3 — Park SJ, Shin YS, Lee JR Preparation and characterisation of microcapsules containing lemon oil. J Colloid Interface Sci 2 — El-Gibaly I Development and in vitro evaluation of novel floating chitosan microcapsules for oral use: comparison with non-floating chitosan microspheres. Int J Pharm 1 :7— Huo W, Zhang W, Wang W, Zhou X Physicochemical properties and drug release behavior of biguanidino and O-carboxymethyl chitosan microcapsules. Ortiz M, Jornada DS, Pohlmann AR, Guterres SS Development of novel chitosan microcapsules for pulmonary delivery of dapsone: characterization, aerosol performance, and in vivo toxicity evaluation. AAPS PharmSciTech 16 5 — J Drug Deliv Sci Technol Zaru M, Manca ML, Fadda AM, Antimisiaris SG Chitosan-coated liposomes for delivery to lungs by nebulisation. Colloids Surf B Biointerfaces 71 1 — Henriksen I, Smistad G, Karlsen J Interactions between liposomes and chitosan. Int J Pharm 3 — Filipović-Grcić J, Skalko-Basnet N, Jalsenjak I Mucoadhesive chitosan-coated liposomes: characteristics and stability. J Microencapsul 18 1 :3— Galović Rengel R, Barišić K, Pavelić Ž, Žanić Grubišić T, Čepelak I, Filipović-Grčić J High efficiency entrapment of superoxide dismutase into mucoadhesive chitosan-coated liposomes. Eur J Pharm Sci 15 5 — Albasarah YY, Somavarapu S, Stapleton P, Taylor KMG Chitosan-coated antifungal formulations for nebulisation. J Pharm Pharmacol 62 7 — Hamedinasab H, Rezayan AH, Mellat M, Mashreghi M, Jaafari MR Development of chitosan-coated liposome for pulmonary delivery of N-acetylcysteine. Peers S, Montembault A, Ladavière C Chitosan hydrogels for sustained drug delivery. Ishihara M, Fujita M, Obara K, Hattori H, Nakamura S, Nambu M et al Controlled releases of FGF-2 and paclitaxel from chitosan hydrogels and their subsequent effects on wound repair, angiogenesis, and tumor growth. Curr Drug Deliv 3 4 — Popa N, Novac O, Profire L, Lupusoru CE, Popa MI Hydrogels based on chitosan—xanthan for controlled release of theophylline. J Mater Sci Mater Med 21 4 — Venkatesan J, Jayakumar R, Mohandas A, Bhatnagar I, Kim SK Anti-microbial activity of chitosan-carbon nanotube hydrogels. Materials 7 5 — Muralidharan A, Russell MS, Larocque L, Gravel C, Sauvé S, Chen Z et al Chitosan alters inactivated respiratory syncytial virus vaccine elicited immune responses without affecting lung histopathology in mice. Vaccine 37 30 — Zare S, Kabiri M, Amini Y, Najafi A, Mohammadpour F, Ayati SH et al Immunological assessment of chitosan or trimethyl chitosan-coated PLGA nanospheres containing fusion antigen as the novel vaccine candidates against tuberculosis. AAPS PharmSciTech 23 1 Sawaengsak C, Mori Y, Yamanishi K, Mitrevej A, Sinchaipanid N Chitosan nanoparticle encapsulated hemagglutinin-split influenza virus mucosal vaccine. AAPS PharmSciTech 15 2 — Kumar US, Afjei R, Ferrara K, Massoud TF, Paulmurugan R Gold-Nanostar-chitosan-mediated delivery of SARS-CoV-2 DNA vaccine for respiratory mucosal immunisation: development and proof-of-principle. ACS Nano 15 11 ,—17, Li Y, Wang C, Sun Z, Xiao J, Yan X, Chen Y et al Simultaneous intramuscular and intranasal administration of chitosan nanoparticles—adjuvanted chlamydia vaccine elicits elevated protective responses in the lung. |
| Chitosan-Based Drug Delivery Systems for Respiratory Diseases | It is worth mentioning that the HPD dose that had protective effects on CSS-induced lung injury in our study is 5-fold lower than HPD dose in free form used to reduce smoke-induced lung inflammation in a previous study Yu et al. The negatively charged siRNA was complexed by the positively charged CS to form the polyelectrolyte complex NPs. The dual drug-loaded nanogel particles NGPs had a uniform particle size from 60 to nm with spherical morphology. Pulmonary delivery of elcatonin using surface-modified liposomes to improve systemic absorption: Polyvinyl alcohol with a hydrophobic anchor and chitosan oligosaccharide as effective surface modifiers. The particle size of the pulmonary inhalation preparations directly affects the deposition form and deposition site in the lung [ 24 , 25 , 26 ]. News Lifestyle Health Inhalable chemotherapy may help treat lung cancer Inhalable chemotherapy may help treat lung cancer The research is concerned with dry powder inhalation using chitosan nanoparticles loaded with drugs that can reach the lower respiratory tract and from there diffuse into the bloodstream. View all shorts. |
Ich meine, dass Sie sich irren. Schreiben Sie mir in PM.
Ich meine, dass Sie den Fehler zulassen. Es ich kann beweisen. Schreiben Sie mir in PM.