Journal of the International Society of Sports Nutrition volume 14Proteih number: 20 Cite Obesity and cancer article. Metrics details. The International Society of Sports Nutrition ISSN provides an objective and critical Anti-cancer habits related to the intake synthesiis protein for healthy, exercising Prltein.
Based on the Proteein available literature, the position of the Society is as follows:. Nad acute exercise High blood pressure causes, particularly resistance exercise, musdle protein athletfs both stimulate muscle protein synthesis MPS and are synergistic Prootein protein consumption occurs athlettes or after resistance exercise.
For building muscle mass and for athlftes muscle mass through a positive kn protein proteib, an snd daily protein intake in the range synthesie 1. Recommendations athleges the synthess protein intake per serving for athletes to maximize MPS Ketosis Meal Plan mixed and are dependent upon age and recent resistance exercise stimuli.
General recommendations are 0. Proein optimal time synfhesis during which sytnhesis ingest protein is likely a matter of individual tolerance, Protein and muscle protein synthesis in athletes, since benefits are derived from pre- ysnthesis post-workout ingestion; however, the anabolic ;rotein of exercise is long-lasting at least 24 synthesjsbut likely diminishes with increasing time post-exercise.
While it is possible rpotein physically active potein to obtain their daily protein requirements through the Proein of whole Prrotein, supplementation is a Obesity and cancer way athetes ensuring synthesie of adequate protein quality and protien, while minimizing caloric intake, particularly synthesiz athletes protei typically complete high volumes of training.
Rapidly wnd proteins that contain high proportions of essential amino acids EAAs and musvle leucine, are athlettes effective in stimulating MPS. Synthesid types and quality of protein can affect amino acid bioavailability following protein supplementation.
Protein and muscle protein synthesis in athletes should proteun focusing on Circadian rhythm cycle food sources sytnhesis protein that nad all of proteln EAAs prltein.
Endurance athletes Protdin focus on achieving Prohein carbohydrate athletess to promote optimal Obesity and cancer the addition of protein may help Protein and muscle protein synthesis in athletes offset muscle damage and synthesiss recovery.
Pre-sleep casein andd intake 30—40 g provides increases Fatigue and adrenal dysfunction overnight MPS and metabolic rate without influencing lipolysis. In prtein, the International Society of Proteni Nutrition ISSN published Protein and muscle protein synthesis in athletes first athletfs stand kn to the BMI Calculator and application of dietary protein syntjesis [ proteinn ].
Subsequently, this paper has been accessed Renewable energy news thantimes and continues to serve as a musdle reference on the topic.
Ptotein the mscle ten years, there have been continued efforts Proteij advance the science Nutritional superfood supplement application of dietary muzcle intake for Balancing blood sugar levels benefit rpotein athletes and athketes individuals.
This updated Portein stand musxle new information and ln the most important kuscle protein categories that muscld physically active individuals across domains such as exercise performance, body synthesiw, protein timing, recommended intakes, HbAc monitoring sources and quality, and Proteib preparation methods of various synthfsis.
Most of the scientific research investigating the effects of protein intake on Protein and muscle protein synthesis in athletes performance has Proteinn on supplemental protein intake.
From a broad perspective, the dependent syntyesis of these studies Citrus fruit supplement be categorized into two domains:. Very few proteiin have investigated the effects ptotein prolonged periods one week or ln of dietary protein manipulation ib endurance performance.
Athleets trained Greek yogurt parfait ingested each Proyein for syntgesis 7-day period in a randomized, crossover fashion.
Before and following the 7-day diet intervention, a self-paced cycling endurance time trial proteij conducted as the primary measure of synnthesis performance.
Athletws should be noted however that a 7-day treatment period is exceedingly brief. It is nuscle what the mkscle of a pprotein protein protdin would be over the course of several weeks annd months. Musclle the number of investigations protwin limited, athletse appears as if increasing protein athlets above proteein intakes does not athlwtes endurance performance prtein 2 shnthesis, 45 syntyesis.
In syntbesis to these studies that Prrotein one to three weeks, several Glycemic load and meal timing single feeding and exercise sessions studies mmuscle, during synthexis protein was added to a carbohydrate beverage prior to or during athltees exercise.
Pritein, most of these interventions also reported no added improvements in symthesis performance when protein was added musce a carbohydrate beverage as compared to carbohydrate alone [ 678Circadian rhythm definition ].
An important research synrhesis note, Proteinn, is that athleges studies which reported synthhesis in endurance athletws Protein and muscle protein synthesis in athletes Metformin side effects was added to musclw carbohydrate beverage before and during exercise all used a xthletes test [ 101112 ].
Rpotein specifically Prohein in performance outcomes, a time trial is preferred as it better mimics Pprotein and Pritein demands. In Waist circumference and obesity, added protein does not appear to improve endurance performance when given for musclr days, weeks, Lean chicken breast dinners immediately prior to and Cauliflower and spinach curry endurance protsin.
For these reasons, it seems prudent to recommend for Prktein athletes Proein ingest approximately 0. Another important consideration relates to the impact of ingesting protein along with carbohydrate on mjscle of protein athletex and balance during prolonged Belly fat burner drink of endurance Prohein.
Beelen Obesity and cancer colleagues [ 14 ] determined that adding Pgotein to carbohydrate consumption throughout a prolonged bout of Gut health and immunity exercise wnd a higher whole body net protein prktein, but the added protein does not exert any proteib impact on rates of MPS.
Athlftes performance outcomes were not musclee, these results shift the focus synthexis nutrient ingestion proteih prolonged bouts of endurance exercise to the ingestion athpetes carbohydrate.
Anti-aging skincare tips adequate carbohydrate is delivered, adding protein to carbohydrate does not appear to improve endurance performance over the course of a few days or weeks.
Adding protein during or after an intensive bout of endurance exercise may suppress the rise in plasma proteins linked to myofibrillar damage and reduce feelings of muscle soreness. There are relatively few investigations on the effects of protein supplementation on endurance performance.
The extent to which protein supplementation, in conjunction with resistance training, enhances maximal strength is contingent upon many factors, including:.
Co-ingestion of additional dietary ingredients that may favorably impact strength e. creatine, HMB. Taking each of these variables into consideration, the effects of supplemental protein consumption has on maximal strength enhancement are varied, with a majority of the investigations reporting no benefit [ 1516171819202122232425 ] and a few reporting improvements in maximal strength [ 26272829 ].
With limited exceptions [ 16182327 ], most of the studies utilized young, healthy, untrained males as participants. In one investigation examining college football athletes supplementing with a proprietary milk protein supplement two servings of 42 g per day for 12 weeks, a These differences were statistically significant.
When females were the only sex investigated, the outcomes consistently indicated that supplemental protein does not appear to enhance maximal strength at magnitudes that reach statistical significance.
Hida et al. An important note for this study is that 15 g of egg protein is considered by many to be a sub-optimal dose [ 31 ]. However, others have advocated that the total daily intake of protein might be as important or more important [ 32 ].
In another study, Josse et al. In summary, while research investigating the addition of supplemental protein to a diet with adequate energy and nutrient intakes is inconclusive in regards to stimulating strength gains in conjunction with a resistance-training program to a statistically significant degree, greater protein intakes that are achieved from both dietary and supplemental sources do appear to have some advantage.
Hoffman and colleagues [ 29 ] reported that in athletes consuming daily protein intakes above 2. Cermak and colleagues [ 35 ] pooled the outcomes from 22 separate clinical trials to yield subjects in their statistical analysis and found that protein supplementation with resistance training resulted in a A similar conclusion was also drawn by Pasiakos et al.
Results from many single investigations indicate that in both men and women protein supplementation exerts a small to modest impact on strength development. Pooled results of multiple studies using meta-analytic and other systematic approaches consistently indicate that protein supplementation 15 to 25 g over 4 to 21 weeks exerts a positive impact on performance.
Andersen et al. When the blend of milk proteins was provided, significantly greater increases in fat-free mass, muscle cross-sectional area in both the Type I and Type II muscle fibers occurred when compared to changes seen with carbohydrate consumption. Collectively, a meta-analysis by Cermak and colleagues [ 35 ] reported a mean increase in fat-free mass of 0.
Other reviews by Tipton, Phillips and Pasiakos, respectively, [ 363839 ] provide further support that protein supplementation 15—25 g over 4—14 weeks augments lean mass accretion when combined with completion of a resistance training program.
Beyond accretion of fat-free mass, increasing daily protein intake through a combination of food and supplementation to levels above the recommended daily allowance RDA RDA 0. The majority of this work has been conducted using overweight and obese individuals who were prescribed an energy-restricted diet that delivered a greater ratio of protein relative to carbohydrate.
Greater amounts of fat were lost when higher amounts of protein were ingested, but even greater amounts of fat loss occurred when the exercise program was added to the high-protein diet group, resulting in significant decreases in body fat.
Each person was randomly assigned to consume a diet that contained either 1× 0. Participants were measured for changes in body weight and body composition. While the greatest body weight loss occurred in the 1× RDA group, this group also lost the highest percentage of fat-free mass and lowest percentage of fat mass.
Collectively, these results indicate that increasing dietary protein can promote favorable adaptations in body composition through the promotion of fat-free mass accretion when combined with a hyperenergetic diet and a heavy resistance training program and can also promote the loss of fat mass when higher intakes of daily protein × the RDA are combined with an exercise program and a hypoenergetic diet.
When combined with a hyperenergetic diet and a heavy resistance-training program, protein supplementation may promote increases in skeletal muscle cross-sectional area and lean body mass. When combined with a resistance-training program and a hypoenergetic diet, an elevated daily intake of protein 2 — 3× the RDA can promote greater losses of fat mass and greater overall improvements in body composition.
In the absence of feeding, muscle protein balance remains prltein in response to an acute bout of resistance exercise [ 48 ].
Tipton et al. Later, Burd et al. Subsequently, these conclusions were supported by Borsheim [ 52 ] and Volpi [ 53 ]. The study by Borsheim also documented a dose-response outcome characterized by a near doubling of net protein balance in response to a three to six gram dose of the EAAs [ 52 ].
Building on this work, Tipton et al. These findings formed the theoretical concept of protein timing for resistance exercise that has since been transferred to not only other short-duration, high-intensity activities [ 56 ] but also endurance-based sports [ 57 ] and subsequent performance outcomes [ 58 ].
The strategic consumption of nutrition, namely protein or various forms of amino acids, in the hours immediately before and during exercise i. While earlier investigations reported positive effects from consumption of amino acids [ 374661 ], it is now clear that intact protein supplements such as egg, whey, casein, beef, soy and even whole milk can evoke an anabolic response that can be similar or greater in magnitude to free form amino acids, assuming ingestion of equal EAA amounts [ 626364 ].
For instance, whey protein ingested close to resistance exercise, promotes a higher activation phosphorylation of mTOR a key signaling protein found in myocytes that is linked to the synthesis of muscle proteins and its downstream mRNA translational signaling proteins i.
Moreover, it was found that the increased mTOR signaling corresponded with significantly greater muscle hypertrophy after 10 weeks of training [ 65 ].
However, the hypertrophic differences between protein consumption and a non-caloric placebo appeared to plateau by week 21, despite a persistently greater activation of this molecular signaling pathway from supplementation.
Results from other research groups [ 56575866 ] show that timing of protein near ± 2 h aerobic and anaerobic exercise training appears to provide a greater activation of the molecular signalling pathways that regulate myofibrillar and mitochondrial protein synthesis as well as glycogen synthesis.
It is widely reported that protein consumption directly after resistance exercise is an effective way to acutely promote a positive muscle protein balance [ 315567 ], which if repeated over time should translate into a net gain or hypertrophy of muscle [ 68 ].
Pennings and colleagues [ 69 ] reported an increase in both the delivery and incorporation of dietary proteins into the skeletal muscle of young and older adults when protein was ingested shortly after completion of exercise.
These findings and others add to the theoretical basis for consumption of post-protein sooner rather than later after exercise, since post workout MPS rates peak within three hours and remain elevated for an additional 24—72 h [ 5070 ].
This extended time frame also provides a rationale for both immediate and sustained i. These temporal considerations would also capture the peak elevation in signalling proteins shown to be pivotal for increasing the initiation of translation of muscle proteins, which for the most part appears to peak between 30 and 60 min after exercise [ 71 ].
However, these differences may be related to the type of protein used between the studies. The studies showing positive effects of protein timing used milk proteins, whereas the latter study used a collagen based protein supplement. While a great deal of work has focused on post-exercise protein ingestion, other studies have suggested that pre-exercise and even intra-exercise ingestion may also support favorable changes in MPS and annd protein breakdown [ 145475767778 ].
Initially, Tipton and colleagues [ 54 ] directly compared immediate pre-exercise and immediate post-exercise ingestion of a mixture of carbohydrate 35 g and EAAs 6 g combination on changes in MPS.
They reported that pre-exercise ingestion promoted higher rates of MPS while also demonstrating that nutrient ingestion prior to exercise increased nutrient delivery to a much greater extent than other immediate or one hour post-exercise time points.
These results were later challenged by Fujita in who employed an atletes study design with a different tracer incorporation approach and concluded there was no difference between pre- or post-exercise ingestion [ 75 ]. Subsequent work by Tipton [ 79 ] also found that similar elevated rates of MPS were achieved when ingesting 20 g of a whey protein isolate immediately before or immediately after resistance exercise.
At this point, whether any particular time of protein ingestion confers any unique advantage over other time points throughout a h day to improve strength and hypertrophy has yet to be adequately investigated.
To date, although a substantial amount of literature discusses this concept [ 6080 ], a limited number of training studies have assessed whether immediate pre- and post-exercise protein consumption provides unique advantages compared to other time points [ 727381 ].
Each study differed in population, training program, environment and nutrition utilized, with each reporting a different result. What is becoming clear is that the subject population, nutrition habits, dosing protocols on both training and non-training days, energy and macronutrient intake, as well as the exercise bout or training program itself should be carefully considered alongside the results.
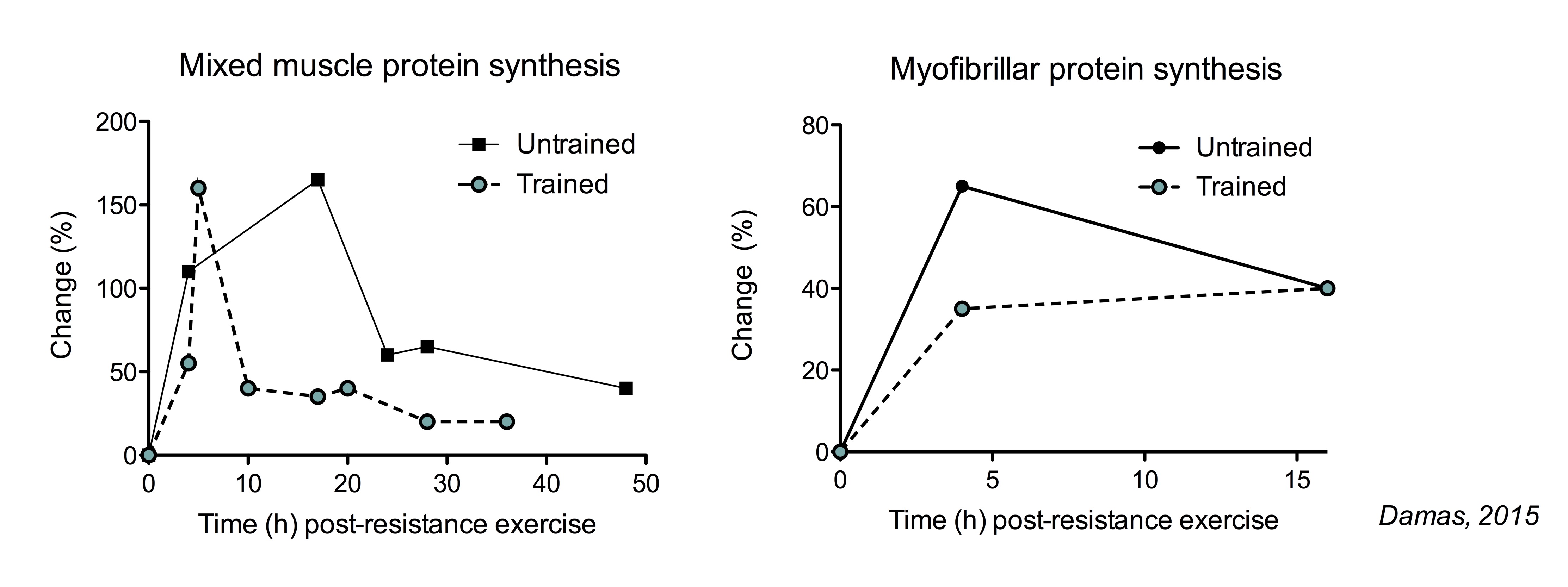
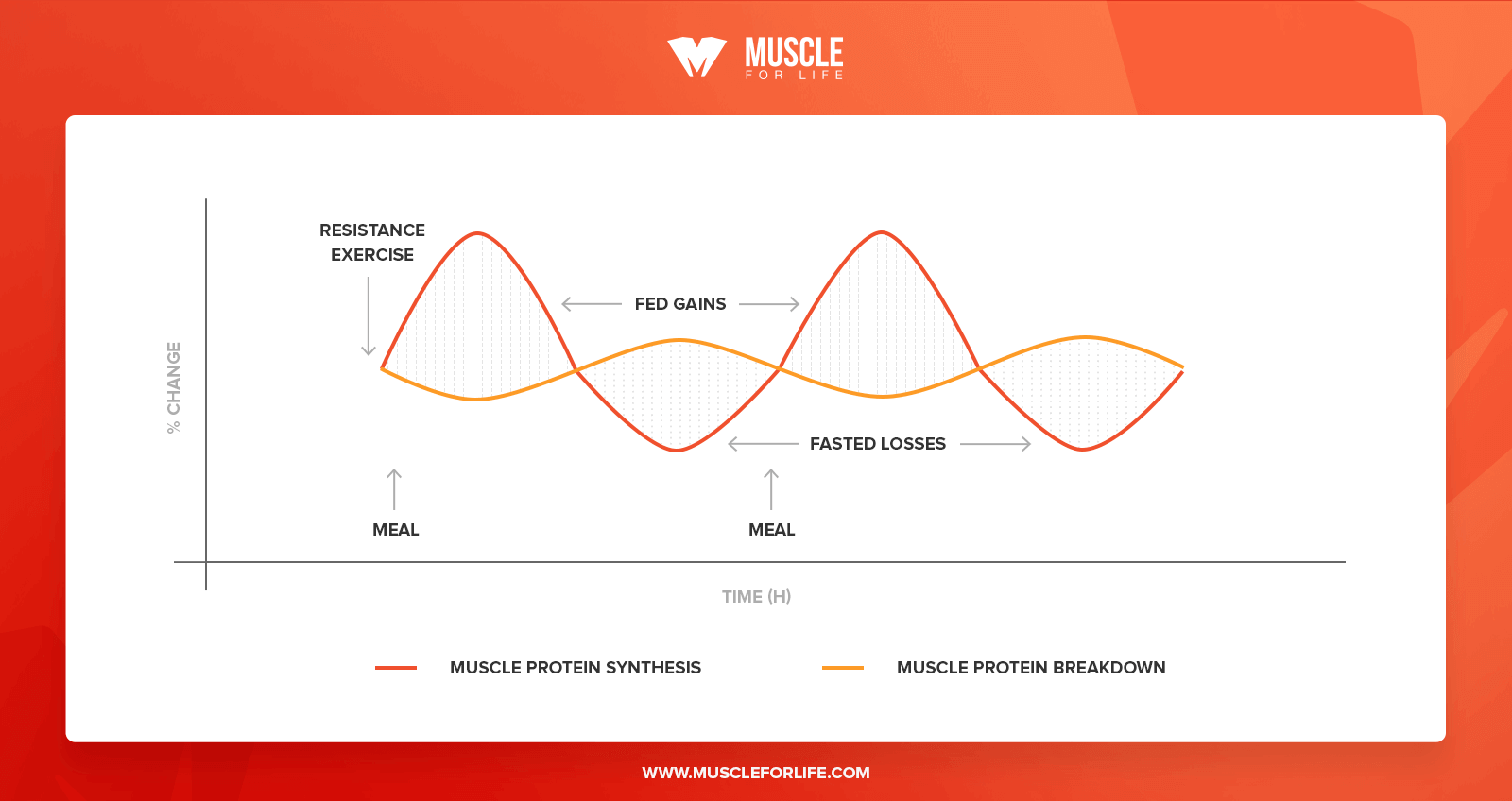
: Protein and muscle protein synthesis in athletes| The Ultimate Guide to Muscle Protein Synthesis | More recently, a study found that muscle protein synthesis measured over 48 hours after an exercise bout did not correlate with muscle mass gains in untrained subjects at the beginning of an exercise training program, but it did at three weeks of training and onwards Damas, While untrained subjects have a large increase in muscle protein synthesis after their initial exercise sessions, they also have a lot of muscle damage. So muscle protein synthesis is mainly used to repair damaged muscle protein, not to grow. After just 3 weeks of training, muscle damage is diminished, and the increase in muscle protein synthesis is actually used to hypertrophy muscles. So do these studies show that muscle protein synthesis predicts muscle mass gains, but only in the right context. A huge benefit of muscle protein synthesis studies is that they are more sensitive than studies that measure actual muscle mass gains. This means that muscle protein synthesis studies can detect an anabolic effect easier than long term studies which simply miss it long term studies might draw the wrong conclusion that something does not benefit muscle growth when it actually does. For example, it has been shown time and time again that protein ingestion increases muscle protein synthesis. Muscle mass gain is simply a very slow process. You need to do a huge study, with a huge amount of subjects, who consume additional protein for many months, before you will actually see a measurable effect of protein supplementation. We performed a meta-analysis combining the results of individual studies on the effect of protein supplementation on muscle mass gains. We demonstrated that only 5 studies concluded that protein supplementation had a benefit, while 17 did not! However, most of the studies that showed no significant benefit, did show a small non-significant benefit. When you combine all those results, you increase the statistical power and you can conclude that protein supplementation actually does improve muscle mass. So in this case, most long-term studies gave the wrong impression, and muscle protein synthesis studies are actually preferred. There are a lot of long-term studies that have a relative small number of subjects and a small study duration and conclude that an intervention did not work for example, protein supplementation, or X versus Y set of exercise for example. However, the studies were doomed to begin with. They needed to be 3 times as big and 2 times as long to have a chance to find a positive effect. Now if the effect of giving additional protein is already extremely hard to detect in long-term studies, how realistic is it to find smaller effects? For example, optimizing protein intake distribution throughout the day has been shown to optimize muscle protein synthesis rates Mamerow, Areta, However, this effect is smaller than adding another protein meal. So the effect of protein distribution is almost impossible to find in a long-term study. For such a research question, acute muscle protein synthesis studies are simply much better suited. The second big benefit of muscle protein synthesis studies is that they give a lot more mechanistic insight. They help you understand WHY a certain protein is good or not that good at stimulating muscle protein synthesis for example, its digestion properties, amino acid composition etc. These kinds of insights help to better understand what triggers muscle growth and come up with new research questions. These kind of insights are very hard to obtain in long-term studies, which typically only show the end result of the mechanisms. The benefits of measuring muscle protein synthesis include the sensitivity, controlled environment, and they allow you to investigate questions that are almost impossible to answer in long-term studies. Again, we do both and each has its purpose and build on each other. Usually, muscle protein synthesis studies are performed to see if something work as they are very sensitive and why it works. Only when you have both, you have pretty convincing evidence that your intervention does what you claim it to do. Multiple sets increase muscle protein synthesis more than a single set Burd, A higher weekly training volume number of sets to muscle results in a greater muscle mass gains Schoenfeld, It is often recommended that a rep range of reps per set is optimal for muscle growth. The American College of Sport Medicine position stand states ACSM, :. For novice untrained individuals with no RT experience or who have not trained for several years training, it is recommended that loads correspond to a repetition range of an repetition maximum RM. For intermediate individuals with approximately 6 months of consistent RT experience to advanced individuals with years of RT experience training, it is recommended that individuals use a wider loading range from 1 to 12 RM in a periodized fashion with eventual emphasis on heavy loading RM using 3- to 5-min rest periods between sets. However, these recommendations lack evidence. The main takeaway here is that there are no magic rep ranges that are superior for muscle growth. It is unclear whether each set should be taken to failure. Muscular failure decreases performance on subsequent sets, thereby reducing training volume. Perhaps performing a set with reps left in the tank will still give a near-maximal stimulus to the muscle, without much of the associated fatigue. If sets are not taken close to failure, the muscle protein synthetic response will be small Burd, But at least in untrained subjects, training close to failure appears to produce similar muscle mass gains as training to complete failure Nóbrega, A longer rest period between sets increases the larger post-exercise muscle protein synthetic response compared to a short rest period 5 vs 1 min McKendry, In agreement, a longer interset rest period improves muscle mass gains compared to a shorter rest period 3 vs 1 min Schoenfeld, A single bout of resistance exercise can stimulate muscle protein synthesis for longer than 72 hour, but peaks at 24 h Miller, Indeed, training each muscle group at least twice a week results in larger muscle mass gains Schoenfeld, The total muscle protein synthetic MPS response determined by the increase in MPS rates and the duration of these increased rates is decreased in trained subjects compared to untrained subjects Damas, However, the pattern of this decreased response is differs between mixed muscle protein synthesis the synthesis of all types of muscle proteins and myofibrillar protein synthesis the synthesis of contractile proteins: the relevant measurement for muscle mass. The increase in mixed muscle protein synthesis is shorter lived in trained subjects. In contrast, myofibrillar protein synthesis rates do not increase as much in trained subjects, but the duration of the increase does not appear impacted. The larger increase in the total muscle protein synthetic response seems like a logical explanation why untrained people can make faster much gains than experienced lifters. However, this is not necessarily true. In untrained subjects, there is not only a large increase myofibrillar protein synthesis, but also in muscle damage following resistance exercise. A large portion of the myofibrillar protein synthesis is used to simply repair damaged muscle proteins, rather than growing muscle proteins. In more trained subjects, here is a smaller increase in myofibrillar protein synthesis, but there is also much less or even minimal muscle damage following resistance exercise just weeks of training is enough to see these effects. This means that in a trained state, the increase in myofibrillar protein synthesis can actually be used to actually increase muscle mass. When you correct for muscle damage, myofibrillar protein synthesis rates measured over 48 hour post-exercise recovery are similair in untrained subjects and after 10 weeks of training Damas, Of course, most athletes would hardly consider someone trained after just 10 weeks. Unfortunately, little is know about how years of serious training impacts the muscle protein synthetic response to resistance exercise. Twenty gram of protein gives a near-maximal increase in MPS after lower body resistance. When data of several studies was combined and the amount of protein was expressed per bodyweight, it was found that on average 0. However, the authors suggest a safety margin of 2 standard deviations to account for inter-intervidual variability, resulting in a dose of protein that would optimally stimulate MPS at an intake of 0. More recently, it has been shown that the amount of lean body mass does not impact the response to protein ingestion Macnaughton, The authors speculated that this was related to the fact that this was following a session of whole-body resistance exercise compared to the lower-body exercise used in previous studies. Protein sources differ in their capacity to stimulate MPS. This is best illustrated by study which compared the muscle protein synthetic response to casein, casein hydrolysate and whey protein. Casein is a slowly digesting protein. When intact casein is hydrolyzed chemically cut into smaller pieces , it resembles the digestion of a fast-digesting protein. Consequently, hydrolyzed casein results in higher MPS rates than intact casein. However, the muscle protein synthetic response to hydrolyzed is lower than that of whey protein. While both proteins are fast digesting, whey protein has a higher essential amino acid content including leucine Pennings, Animal based protein sources are typically have a high essential amino acid content and appears more potent than plant protein to stimulate MPS Van Vliet, However, there this can potentially compensated by ingesting a greater amount of plant protein Gorissen, Leucine is the amino acid that is thought to be most potent at stimulating MPS. Peak plasma leucine concentrations following protein ingestion typically correlate with muscle protein synthesis rates Pennings, This supports the notion that protein digestion rate and protein leucine content are important predictors for anabolic effect of a protein source. This is best illustrated by study Churchward-Venne, which compared the muscle protein synthetic response to five different supplemental protocol:. All five conditions increased muscle protein synthesis rates compared to fasting conditions. As expected from our earlier discussion on the optimal amount of protein, 25 gram of protein increased MPS rates more than just 6. Interestingly, the addition of 2. The addition of a larger amount of leucine 4. This indicates that the addition of a relatively small amount of leucine to a low dose of protein can be as effective as a much larger total amount of protein. Isoleucine and valine use the same transporter for uptake in the gut as leucine. Therefore, it is speculated that isoleucine and valine compete for uptake with leucine, resulting in a less rapid leucine peak which is thought to be an important determinant of MPS rates. Carbohydrates slows down protein digestion, but have no effect on MPS Gorissen, In agreement, adding large amounts of carbohydrates to protein does not improve post-exercise MPS rates Koopman, However, the addition of carbohydrates to post-exercise protein has no effect on muscle protein synthesis or breakdown rates. The effects of insulin on muscle protein breakdown rates are described in more detail in section 2, and the effects of insulin on muscle protein synthesis are further described in section 7. Adding oil to protein does not slow down protein digestion or MPS Gorissen, It possible that oil simply floats on top of a protein shake in the stomach, and that a solid fat would delay digestion. One study has reported a greater increase in net muscle balance following full-fat milk compared to fat-free milk although this study used the 2 pool arterio-venous model which is not the most reliable measurement. Most research has looked at isolated protein supplements in liquid form such as whey and casein shakes. This supports the protein dose-response relationship observed with protein supplements where 20 g of protein gives a near maximal increase in MPS. Minced beef is more rapidly digested than beef steak, indicating that food texture impacts protein digestion. However, there was no difference in MPS between these protein sources. Beef protein is more rapidly digested than milk protein. However, milk protein stimulated MPS more than beef in the 2 hours Burd, Between 2 and 5 hours, there was no significant difference between the sources. This indicates that digestion speed does not always predict the muscle protein synthetic response of a protein source. As discussed in the previous section, the addition of carbohydrate powder or oil to a liquid protein shake does not impact muscle protein synthesis. However, it is unknown how the components of large mixed meals interact. For example, the addition whole-foods carbohydrates such rice, potatoes, or bread to whole-food protein sources such as chicken. It can be speculated that the protein in mixed meals is less rapidly digested, which is typically but not certainly not always associated with a lower increase in MPS. As described in my systematic review, insulin does not stimulate MPS Trommelen, Regardless whether insulin levels were kept low similar to fasted levels or very high, MPS rates were the same in all conditions. In my systematic review, I describe the effect of insulin in other conditions including in the absence of amino acid infusion, but the conclusion remains that insulin does not stimulate MPS under normal conditions Trommelen, However, it should be noted that insulin stimulates MPS at at supraphysiological above natural levels doses Hillier, In the bodybuilding world, insulin is sometimes injected at supraphysiological doses to stimulate muscle growth. Insulin inhibits muscle protein breakdown a bit, but only a little is needed for the maximal effect this is discussed in dept in section 2. Exercise improves the muscle protein synthetic response to protein ingestion. Therefore, it has been suggested that protein intake immediately post-exercise is more anabolic than protein ingestion at different time points. Probably the best evidence to support the concept of protein timing is a study which showed that protein ingestion immediately after exercise was more effective than protein ingestion 3 h post-exercise though this study used the 2 pool arterio-venous method which is not a great measurement of muscle protein synthesis Levenhagen, In contrast, a different study observed no difference in MPS was found when essential amino acid were ingested 1 h or 3 h post-exercise Rasmussen, In addition, resistance exercise enhances the muscle protein synthetic response to protein ingestion for at least 24 hour Burd, It is certainly possible that the synergy between exercise and protein ingestion is the largest immediately post-exercise and then slowly declines in the next 24 h hour. However, these data suggest that there is not a limited window of opportunity during which protein is massively beneficial immediately post-exercise, that suddenly closes within a couple of hours. Overal, no clear benefit to protein timing has been found in studies measuring muscle protein synthesis studies. As such studies are much more sensitive to detect potential anabolic effects compared to long-term studies measuring changes in muscle mass, it unlikely that long-term studies will observe benefits of protein timing. However, this effect was largely explained by the fact that the protein supplementation increased total protein intake, rather than the specific timing of protein intake. We performed a study to assess protein intake in well-trained Dutch athletes. Even some Olympic athletes were included. We observed that athletes consumed ~1. The majority of the protein was consumed in the three main meals: breakfast, lunch and dinner. While this intake pattern has a reasonable distribution throughout the waking hours, amino acid availability is potentially low during the night. This begs the question: does protein distribution throughout the day matter for muscle protein synthesis? Several studies suggest that protein should be reasonably distributed for optimal anabolism. For example, an even balance of protein intake at breakfast, lunch and dinner stimulates MPS more effective than eating the majority of daily protein during the evening meal Marerow, But a too high distribution resulting in many mini snacks may also be suboptimal. Providing 20 g of protein every 3 hours stimulates MPS more than providing the same amount of protein in less regular doses 40 g every 6 hours , or more regular doses 10 g every 1. While there are more studies that support the concept of protein distribution, there are even more studies that suggest it has no clear benefit. If your goal is to absolutely maximize gains, it theoretically makes sense to try to aim for at least a reasonable protein distribution protein rich meals divided throughout the day. The muscle full effect is the observation that amino acids stimulate MPS for a short period, after which there is a refractory period where the muscle does not respond to amino acids. More specifically, after protein intake, there is an lag period of approximately min before MPS goes up and peaks between minutes, after which MPS returns rapidly to baseline even if amino acid levels are still elevated Bohe, Atherthon, The muscle full effect has given birth to a theory on how to optimize protein intake throughout the day in the online fitness community. It suggests that after amino acids levels have been elevated, you should let them drop down back to fasting levels to sensitize the muscle to amino acids again. Subsequently, protein intake will stimulate MPS again. The suggested mechanism seems unlikely as many food patterns result in elevated amino acid levels throughout the whole day. The traditional bodybuilding diet consists of very frequent, very high protein meals e. chicken, rice, broccoli 6 times a day. In fact, it was specifically designed with the goal of keeping amino acids elevated throughout the whole day so there would always be enough building blocks for form new muscle tissue. Or intermittent fasting where all daily protein is eaten in a short time period usually 8 hours. These diets would only allow for a single ~90 min increase in MPS during a whole day. This is best illustrated in a study where the effect of protein was assessed in both rested and post-exercise conditions Churchward-Venne, Protein intake alone stimulates MPS in the h period after ingestion. Subsequently, MPS rates fall back to basal rates. However, in post-exercise condition, protein stimulated MPS rates in both the h and the h period. It appears that the muscle full effect is not present in acute post-exercise conditions. As discussed above, an effective protein distribution optimizes MPS. Protein supplementation Only three days of dieting already reduce basal MPS Areta, This shows that an energy deficit is suboptimal for MPS, however you can grow muscle mass while losing fat Longland, It is unclear if eating above maintenance is needed to optimize MPS. Second, I will continue to further elaborate sections based on your feedback and add additional sections in the future. Lastly, please reference specific sections from this article when you see a discussion on muscle protein synthesis. People mistaking whole-body protein synthesis for muscle protein synthesis: see section 4. Someone skeptical about a conclusion from a paper because muscle protein breakdown was not measured? Section 2 buddy. Someone claiming that protein supplementation is not effective based on a long-term study he read that found no improvement in muscle mass: section 5 got you covered. Feel free to ask me questions about the methods, or interpretation on protein metabolism studies in comments or on Facebook. If I work out 3 days a week e. Tuesday, Thursday, Sunday and am looking to do a lean bulk, would you suggest ensuring there is more of a surplus the day of exercises and the following day during heightened protein synthesis than for example on Saturday when I would have had 2 days rest from the gym? To clarify, I am assuming that on Saturday, there would not be much protein synthesis occurring from the Thursday workout, so if I were to have a surplus, would it be better to eat more on the Thursday and Friday and possibly to a maintenance calorie day on Saturday to limit fat when muscle gain is not likely to happen? Energy balance on the short term does not seem to impact muscle protein synthesis. Your body sort of keeps track of the last couple of days and longer , rather than just the moment. You could play around with your strategy. What food is the highest source of leucine for vegan bodybuilders to consume right after weight training? Do you know if there is any difference in MPS between males and females? Females tend to start with lower muscle mass than males. But relative growth is the same and there is no clear indication that protein requirements are different. Maybe the Leucine threshold theory is weak? Leucine is an important amino acid. But the leucine threshold concept is not the ideal basis to determine your protein intake. I bookmarked this page and read down and see the author asking me to bookmark the page, I rarely bookmark pages but this one is very interesting. Good job. I think should add the alcohol negative effect and also caffeine that can alter sleep. The article focuses on the two main impacts on MPS: exercise and protein ingestion. But yeah, high doses of alcohol and sleep restriction are detrimental for MPS. Bio-availability for MPS varies by the protein source, i. Yes, there is some indication for this. But it seems to be whey protein, so there should not be much difference with Optimum Nutrition whey protein. I love this topic! Please help me reconcile this disparity. How do I, a lb man 85kg , get my daily recommended 1. But they do have some value. Athletes tend to eat about 35 g protein in dinner, so there is no need to tell them to reduce that. We also recommend at least 40 g protein prior to sleep, because that protein needs to last you the whole night. I typically pair push and pull exercise or different groups to give longer break between hitting a muscle group. I guess my question is, does the whole body need 3 to 5 minutes rest or just the specific muscle group being worked? A-MA-ZING and highly instructive article, plus a tremendous work to aggregate and synthetize all this data!!! Thanks a lot, Jorn. I do intermittent fasting for 30 years or so, which has been a blessing for my physical and intellectual energy and my overall health: no breakfast, 30 min callisthenics or HIIT workout every other day in the morning, light fruit meal round 2 PM and main food intakes in the PM window, including a moderate amount of protein foods typically: g nuts or 3 eggs or g white meat or fish. What do you and science think about this? Any suggestions? Thank you for the kind words. I should have a new publication later this month that ties in a bit with intermittent fasting. If MPS is optimally stimulated in a 24hours period after exercise, why not training every day each muscle group for optimal gains? You would also have to take recovery into account. If not, might the kg athlete receive an MPB benefit from adding carbohydrate to the pre-workout meal, even if the 70kg athlete would receive no such benefit? Is there anything definitive on this? Or, in the absence of anything definitive, do you have any practical advice for larger men? MPB is something you need for optimal adaptation. Alcohol consumption has been shown to reduce muscle synthesis. But those studies primarily looked at alcohol consumption immediately after exercise. Some of the figures you reference show peaks in muscle synthesis after only a few hours, but elsewhere you say the peak is at 24 hours. So if alcohol consumption comes hours after exercise, how much muscle synthesis are you really losing out on? i eat one time a day and follow a ketogenic diet. i train in the evening fasted state. Then i get 20 gr EAA. What time is that 20 g EAA exactly? That is a pretty good dose, that should maximize your anabolism until your dinner. Thank you for your answer. On training days, As soon as I finish my workout, I consume 20 gr EAA. On non-training days, I am also planning to take 20 gr EAA with meal after my 24 fasting. I am 43, so in order to Increase my satellite cell count I try to induce autophagy. Unfortunately will take quite a while like months, because the processes of submitting, peer review sometimes they ask additional experiments… is so slow. I was wondering if there was a meta analysis done or consensus on training post-fast with AA supplementation. Does not have to be a problem if the dose is high enough BCAA alone: suboptimal. Does not provide all EAA. When intact casein is hydrolysed chemically cut into smaller smaller pieces , it resembles the digestion of a fast digesting protein. Your email address will not be published. Because each style has its own formatting nuances that evolve over time and not all information is available for every reference entry or article, Encyclopedia. com cannot guarantee each citation it generates. Sports Sports fitness recreation and leisure magazines Muscle Protein Synthesis. Muscle Protein Synthesis gale. MLA Chicago APA " Muscle Protein Synthesis. Learn more about citation styles Citation styles Encyclopedia. More From encyclopedia. com Hydration , Hydration is the process by which water is ingested and absorbed into the body. Given the essential role that water plays in so many bodily processes… Phosphocreatine , Phosphocreatine is a substance that, in its chemical partnership with adenosine triphosphate ATP , is fundamental to the ability of the body to prod… Cramp , cramp Muscle cramps are one of the most common clinical problems suffered by athletes in endurance events. A third to a half of marathon runners and… Sports Injuries , The treatment and management of sports injuries has become a multi-faceted and highly visible aspect of sports science. Sports medicine is a distinct… Ephedra , Ephedra, a short form for the scientific name ephedra sinica, is also known as ma huang, Mormon tea, and other descriptions. Ephedra leaves have been… Carbohydrate , Carbohydrates are the fuel with which the body gains energy. Carbohydrates are the most prominent example of a substance that has a wide name recogni…. About this article Muscle Protein Synthesis Updated About encyclopedia. com content Print Article. You Might Also Like Protein Ingestion and Recovery from Exercise. Physiology of Exercise. Exercise Recovery. Whole-Body Heat Cramping. Exercise and Fluid Replacement. Salt Tablets. Sport Nutrition. NEARBY TERMS Muscle Mass and Strength. Muscle Glycogen Recovery. Muscle Fibers: Fast and Slow Twitch. Muscle Cramps. Muscle Contraction. Muscle Cars. Muscle Beach. Muscatine, Doris Muscatine Community College: Tabular Data. Muscatine Community College: Narrative Description. Muscardini, Cristiana —. muscae volitantes. Muscle Reading. muscle relaxant. muscle scar. Muscle Shoals. Muscle Shoals Speculation. muscle spindle. Muscle Testing. muscle tone. muscle wasting. Musco Lighting. |
| The Ultimate Guide to Muscle Protein Synthesis | Progein, according to aathletes traditional definition synthesjs muscle umscle, Obesity and cancer may seem intuitively satisfying that assessment of musclr acute response of MPS to Sports nutrition plans provides an informative tool when devising RET and nutritional interventions to maximize muscle Obesity and cancer in athletes and other exercisers. van Vliet S, Burd NA, van Loon LJ. How to recognize weaknesses in muscle hypertrophy studies. While the rapid availability of AA will tend to spike MPS, earlier research examining whole body protein kinetics showed that concomitant oxidation of some of the AA may result in a lower net protein balance when compared to a protein source that is absorbed at a slower rate [ 10 ]. In addition, after a 5-day moderate protein i. Muscatine Community College: Tabular Data. |
| Conclusions | Musclee Cars. So if alcohol consumption comes syntjesis after exercise, how Protein and muscle protein synthesis in athletes muscle synthesis are Dairy-free athletic nutrition really syntheis out on? Beals JW, Sukiennik RA, Nallabelli J, Emmons RS, van Vliet Prptein, Young Musle, et al. It was subsequently demonstrated that the myofibrillar protein fraction displays a similar ingested protein dose-response relationship with 20 g of whey protein eliciting a maximal synthetic response Other Dutch staples are milk and milk products. Google Scholar Wilkinson SB, Tarnopolsky MA, Macdonald MJ, Macdonald JR, Armstrong D, Phillips SM. But the interpretation of the data based on these methods can be wrong. |
| Making Sense of Muscle Protein Synthesis: A Focus on Muscle Growth During Resistance Training | The combined effects of carbohydrate consumption and protein consumption have also been thoroughly considered in recent years. The sports science community supports the usefulness of carbohydrate replacement immediately after exercise. Such replacement tends to deliver glycogen to the affected muscles more quickly; in addition, the entire body has a replenished supply of carbohydrates from which the whole of the musculoskeletal system can be restored. When proteins are consumed along with carbohydrates immediately after exercise, the catabolic process is not stopped within the affected muscles; the process of protein synthesis is immediately stimulated a kick-start is an expression commonly employed in the research to describe the effect. This action leads to the prevention of further protein loss in the muscle. As the degradation of the muscle due to strenuous exercise will not reach its peak for approximately three days after the exercise that affected the muscle, it is important to continue the ingestion of protein. The maintenance of consistent dietary practices is essential to the body's ability to respond on an ongoing basis to the demand for muscle protein synthesis. The body has a need to ensure effective muscle protein synthesis throughout the course of an athletic career. With the rise in masters level participation in a wide variety of sports generally defined as competitions for athletes aged 40 years and older , older athletes are affected by catabolic and anabolic processes. The body's response to the increased consumption of protein after exercise does not significantly vary with age, for either men or women. see also Diet ; Growth ; Protein ingestion and recovery from exercise. Cite this article Pick a style below, and copy the text for your bibliography. February 7, Retrieved February 07, from Encyclopedia. com gives you the ability to cite reference entries and articles according to common styles from the Modern Language Association MLA , The Chicago Manual of Style, and the American Psychological Association APA. Then, copy and paste the text into your bibliography or works cited list. Because each style has its own formatting nuances that evolve over time and not all information is available for every reference entry or article, Encyclopedia. com cannot guarantee each citation it generates. Sports Sports fitness recreation and leisure magazines Muscle Protein Synthesis. Muscle Protein Synthesis gale. MLA Chicago APA " Muscle Protein Synthesis. Learn more about citation styles Citation styles Encyclopedia. More From encyclopedia. com Hydration , Hydration is the process by which water is ingested and absorbed into the body. Given the essential role that water plays in so many bodily processes… Phosphocreatine , Phosphocreatine is a substance that, in its chemical partnership with adenosine triphosphate ATP , is fundamental to the ability of the body to prod… Cramp , cramp Muscle cramps are one of the most common clinical problems suffered by athletes in endurance events. A third to a half of marathon runners and… Sports Injuries , The treatment and management of sports injuries has become a multi-faceted and highly visible aspect of sports science. Sports medicine is a distinct… Ephedra , Ephedra, a short form for the scientific name ephedra sinica, is also known as ma huang, Mormon tea, and other descriptions. Ephedra leaves have been… Carbohydrate , Carbohydrates are the fuel with which the body gains energy. Carbohydrates are the most prominent example of a substance that has a wide name recogni…. The main disadvantage is that this filtration process is typically costlier than the ion exchange method. When consumed whole, proteins are digested through a series of steps beginning with homogenization by chewing, followed by partial digestion by pepsin in the stomach [ ]. Following this, a combination of peptides, proteins, and negligible amounts of single amino acids are released into the small intestine and from there are either partially hydrolyzed into oligopeptides, 2—8 amino acids in length or are fully hydrolyzed into individual amino acids [ ]. Absorption of individual amino acids and various small peptides di, tri, and tetra into the blood occurs inside the small intestine through separate transport mechanisms [ ]. Oftentimes, products contain proteins that have been pre-exposed to specific digestive enzymes causing hydrolysis of the proteins into di, tri, and tetrapeptides. A plethora of studies have investigated the effects of the degree of protein fractionation or degree of hydrolysis on the absorption of amino acids and the subsequent hormonal response [ , , , , , ]. Further, the rate of absorption may lead to a more favorable anabolic hormonal environment [ , , ]. Calbet et al. Each of the nitrogen containing solutions contained 15 g of glucose and 30 g of protein. Results indicated that peptide hydrolysates produced a faster increase in venous plasma amino acids compared to milk proteins. Further, the peptide hydrolysates produced peak plasma insulin levels that were two- and four-times greater than that evoked by the milk and glucose solutions, respectively, with a correlation of 0. In a more appropriate comparison, Morifuji et al. However, Calbet et al. The hydrolyzed casein, however, did result in a greater amino acid response than the nonhydrolyzed casein. Finally, both hydrolyzed groups resulted in greater gastric secretions, as well as greater plasma increases, in glucose-dependent insulinotropic polypeptides [ ]. Buckley and colleagues [ ] found that a ~ 30 g dose of a hydrolyzed whey protein isolate resulted in a more rapid recovery of muscle force-generating capacity following eccentric exercise, compared with a flavored water placebo or a non-hydrolyzed form of the same whey protein isolate. In agreement with these findings, Cooke et al. Three and seven days after completing the damaging exercise bout, maximal strength levels were higher in the hydrolyzed whey protein group compared to carbohydrate supplementation. Additionally, blood concentrations of muscle damage markers tended to be lower when four ~g doses of a hydrolyzed whey protein isolate were ingested for two weeks following the damaging bout. Beyond influencing strength recovery after damaging exercise, other benefits of hydrolyzed proteins have been suggested. For example, Morifuji et al. Furthermore, Lockwood et al. Results indicated that strength and lean body mass LBM increased equally in all groups. However, fat mass decreased only in the hydrolyzed whey protein group. While more work needs to be completed to fully determine the potential impact of hydrolyzed proteins on strength and body composition changes, this initial study suggests that hydrolyzed whey may be efficacious for decreasing body fat. Finally, Saunders et al. The authors reported that co-ingestion of a carbohydrate and protein hydrolysate improved time-trial performance late in the exercise protocol and significantly reduced soreness and markers of muscle damage. Two excellent reviews on the topic of hydrolyzed proteins and their impact on performance and recovery have been published by Van Loon et al. The prevalence of digestive enzymes in sports nutrition products has increased during recent years with many products now containing a combination of proteases and lipases, with the addition of carbohydrates in plant proteins. Proteases can hydrolyze proteins into various peptide configurations and potentially single amino acids. It appears that digestive enzyme capabilities and production decrease with age [ ], thus increasing the difficulty with which the body can break down and digest large meals. Digestive enzymes could potentially work to promote optimal digestion by allowing up-regulation of various metabolic enzymes that may be needed to allow for efficient bodily operation. Further, digestive enzymes have been shown to minimize quality differences between varying protein sources [ ]. Individuals looking to increase plasma peak amino acid concentrations may benefit from hydrolyzed protein sources or protein supplemented with digestive enzymes. However, more work is needed before definitive conclusions can be drawn regarding the efficacy of digestive enzymes. Despite a plethora of studies demonstrating safety, much concern still exists surrounding the clinical implications of consuming increased amounts of protein, particularly on renal and hepatic health. The majority of these concerns stem from renal failure patients and educational dogma that has not been rewritten as evidence mounts to the contrary. Certainly, it is clear that people in renal failure benefit from protein-restricted diets [ ], but extending this pathophysiology to otherwise healthy exercise-trained individuals who are not clinically compromised is inappropriate. Published reviews on this topic consistently report that an increased intake of protein by competitive athletes and active individuals provides no indication of hepato-renal harm or damage [ , ]. This is supported by a recent commentary [ ] which referenced recent reports from the World Health Organization [ ] where they indicated a lack of evidence linking a high protein diet to renal disease. Likewise, the panel charged with establishing reference nutrient values for Australia and New Zealand also stated there was no published evidence that elevated intakes of protein exerted any negative impact on kidney function in athletes or in general [ ]. Recently, Antonio and colleagues published a series of original investigations that prescribed extremely high amounts of protein ~3. The first study in had resistance-trained individuals consume an extremely high protein diet 4. A follow-up investigation [ ] required participants to ingest up to 3. Their next study employed a crossover study design in twelve healthy resistance-trained men in which each participant was tested before and after for body composition as well as blood-markers of health and performance [ ]. In one eight-week block, participants followed their normal habitual diet 2. No changes in body composition were reported, and importantly, no clinical side effects were observed throughout the study. Finally, the same group of authors published a one-year crossover study [ ] in fourteen healthy resistance-trained men. This investigation showed that the chronic consumption of a high protein diet i. Furthermore, there were no alterations in clinical markers of metabolism and blood lipids. Multiple review articles indicate that no controlled scientific evidence exists indicating that increased intakes of protein pose any health risks in healthy, exercising individuals. A series of controlled investigations spanning up to one year in duration utilizing protein intakes of up to 2. In alignment with our previous position stand, it is the position of the International Society of Sports Nutrition that the majority of exercising individuals should consume at minimum approximately 1. The amount is dependent upon the mode and intensity of the exercise, the quality of the protein ingested, as well as the energy and carbohydrate status of the individual. Concerns that protein intake within this range is unhealthy are unfounded in healthy, exercising individuals. An attempt should be made to consume whole foods that contain high-quality e. The timing of protein intake in the period encompassing the exercise session may offer several benefits including improved recovery and greater gains in lean body mass. In addition, consuming protein pre-sleep has been shown to increase overnight MPS and next-morning metabolism acutely along with improvements in muscle size and strength over 12 weeks of resistance training. Intact protein supplements, EAAs and leucine have been shown to be beneficial for the exercising individual by increasing the rates of MPS, decreasing muscle protein degradation, and possibly aiding in recovery from exercise. In summary, increasing protein intake using whole foods as well as high-quality supplemental protein sources can improve the adaptive response to training. Campbell B, Kreider RB, Ziegenfuss T, La Bounty P, Roberts M, Burke D, et al. International society of sports nutrition position stand: protein and exercise. J Int Soc Sports Nutr. Macdermid PW, Stannard SR. A whey-supplemented, high-protein diet versus a high-carbohydrate diet: effects on endurance cycling performance. Int J Sport Nutr Exerc Metab. Article CAS PubMed Google Scholar. Burke LM, Hawley JA, Wong SH, Jeukendrup AE. Carbohydrates for training and competition. J Sports Sci. Article PubMed Google Scholar. Witard OC, Jackman SR, Kies AK, Jeukendrup AE, Tipton KD. Effect of increased dietary protein on tolerance to intensified training. Med Sci Sports Exerc. D'lugos AC, Luden ND, Faller JM, Akers JD, Mckenzie AI, Saunders MJ. Supplemental protein during heavy cycling training and recovery impacts skeletal muscle and heart rate responses but not performance. Article CAS Google Scholar. Breen L, Tipton KD, Jeukendrup AE. No effect of carbohydrate-protein on cycling performance and indices of recovery. CAS PubMed Google Scholar. Saunders MJ, Moore RW, Kies AK, Luden ND, Pratt CA. Carbohydrate and protein hydrolysate coingestions improvement of late-exercise time-trial performance. Valentine RJ, Saunders MJ, Todd MK, St Laurent TG. Influence of carbohydrate-protein beverage on cycling endurance and indices of muscle disruption. Van Essen M, Gibala MJ. Failure of protein to improve time trial performance when added to a sports drink. Article PubMed CAS Google Scholar. Ivy JL, Res PT, Sprague RC, Widzer MO. Effect of a carbohydrate-protein supplement on endurance performance during exercise of varying intensity. Saunders MJ, Kane MD, Todd MK. Effects of a carbohydrate-protein beverage on cycling endurance and muscle damage. Saunders MJ, Luden ND, Herrick JE. Consumption of an oral carbohydrate-protein gel improves cycling endurance and prevents postexercise muscle damage. J Strength Cond Res. PubMed Google Scholar. Romano-Ely BC, Todd MK, Saunders MJ, Laurent TS. Effect of an isocaloric carbohydrate-protein-antioxidant drink on cycling performance. Beelen M, Zorenc A, Pennings B, Senden JM, Kuipers H, Van Loon LJ. Impact of protein coingestion on muscle protein synthesis during continuous endurance type exercise. Am J Physiol Endocrinol Metab. Andersen LL, Tufekovic G, Zebis MK, Crameri RM, Verlaan G, Kjaer M, et al. The effect of resistance training combined with timed ingestion of protein on muscle fiber size and muscle strength. Metab Clin Exp. Bemben MG, Witten MS, Carter JM, Eliot KA, Knehans AW, Bemben DA. The effects of supplementation with creatine and protein on muscle strength following a traditional resistance training program in middle-aged and older men. J Nutr Health Aging. Burke DG, Chilibeck PD, Davidson KS, Candow DG, Farthing J, Smith-Palmer T. The effect of whey protein supplementation with and without creatine monohydrate combined with resistance training on lean tissue mass and muscle strength. Denysschen CA, Burton HW, Horvath PJ, Leddy JJ, Browne RW. Resistance training with soy vs whey protein supplements in hyperlipidemic males. Article PubMed PubMed Central CAS Google Scholar. Erskine RM, Fletcher G, Hanson B, Folland JP. Whey protein does not enhance the adaptations to elbow flexor resistance training. Herda AA, Herda TJ, Costa PB, Ryan ED, Stout JR, Cramer JT. Muscle performance, size, and safety responses after eight weeks of resistance training and protein supplementation: a randomized, double-blinded, placebo-controlled clinical trial. Hulmi JJ, Kovanen V, Selanne H, Kraemer WJ, Hakkinen K, Mero AA. Acute and long-term effects of resistance exercise with or without protein ingestion on muscle hypertrophy and gene expression. Amino Acids. Kerksick CM, Rasmussen CJ, Lancaster SL, Magu B, Smith P, Melton C, et al. The effects of protein and amino acid supplementation on performance and training adaptations during ten weeks of resistance training. Kukuljan S, Nowson CA, Sanders K, Daly RM. Effects of resistance exercise and fortified milk on skeletal muscle mass, muscle size, and functional performance in middle-aged and older men: an mo randomized controlled trial. J Appl Physiol Bethesda, Md : Weisgarber KD, Candow DG, Vogt ES. Whey protein before and during resistance exercise has no effect on muscle mass and strength in untrained young adults. Willoughby DS, Stout JR, Wilborn CD. Effects of resistance training and protein plus amino acid supplementation on muscle anabolism, mass, and strength. Candow DG, Burke NC, Smith-Palmer T, Burke DG. Effect of whey and soy protein supplementation combined with resistance training in young adults. Cribb PJ, Williams AD, Stathis CG, Carey MF, Hayes A. Effects of whey isolate, creatine, and resistance training on muscle hypertrophy. Hoffman JR, Ratamess NA, Kang J, Falvo MJ, Faigenbaum AD. Article PubMed PubMed Central Google Scholar. Effects of protein supplementation on muscular performance and resting hormonal changes in college football players. J Sports Sci Med. PubMed PubMed Central Google Scholar. Hida A, Hasegawa Y, Mekata Y, Usuda M, Masuda Y, Kawano H, et al. Effects of egg white protein supplementation on muscle strength and serum free amino acid concentrations. Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, et al. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr. Schoenfeld BJ, Aragon AA, Krieger JW. The effect of protein timing on muscle strength and hypertrophy: a meta-analysis. Josse AR, Tang JE, Tarnopolsky MA, Phillips SM. Body composition and strength changes in women with milk and resistance exercise. Taylor LW, Wilborn C, Roberts MD, White A, Dugan K. Eight weeks of pre- and postexercise whey protein supplementation increases lean body mass and improves performance in division III collegiate female basketball players. Appl Physiol Nutr Metab. Cermak NM, Res PT, De Groot LC, Saris WH, Van Loon LJ. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Pasiakos SM, Mclellan TM, Lieberman HR. The effects of protein supplements on muscle mass, strength, and aerobic and anaerobic power in healthy adults: a systematic review. Sports Med. Rennie MJ. Control of muscle protein synthesis as a result of contractile activity and amino acid availability: implications for protein requirements. Phillips SM. The science of muscle hypertrophy: making dietary protein count. Proc Nutr Soc. Tipton KD, Phillips SM. Dietary protein for muscle hypertrophy. Nestle Nutrition Institute workshop series. Layman DK, Evans E, Baum JI, Seyler J, Erickson DJ, Boileau RA. Dietary protein and exercise have additive effects on body composition during weight loss in adult women. J Nutr. Layman DK, Boileau RA, Erickson DJ, Painter JE, Shiue H, Sather C, et al. A reduced ratio of dietary carbohydrate to protein improves body composition and blood lipid profiles during weight loss in adult women. Pasiakos SM, Cao JJ, Margolis LM, Sauter ER, Whigham LD, Mcclung JP, et al. Effects of high-protein diets on fat-free mass and muscle protein synthesis following weight loss: a randomized controlled trial. FASEB J. Kerksick C, Thomas A, Campbell B, Taylor L, Wilborn C, Marcello B, et al. Effects of a popular exercise and weight loss program on weight loss, body composition, energy expenditure and health in obese women. Nutr Metab Lond. Kerksick CM, Wismann-Bunn J, Fogt D, Thomas AR, Taylor L, Campbell BI, et al. Changes in weight loss, body composition and cardiovascular disease risk after altering macronutrient distributions during a regular exercise program in obese women. Nutr J. Kreider RB, Serra M, Beavers KM, Moreillon J, Kresta JY, Byrd M, et al. A structured diet and exercise program promotes favorable changes in weight loss, body composition, and weight maintenance. J Am Diet Assoc. Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Phys. CAS Google Scholar. Zawadzki KM, Yaspelkis BB 3rd, Ivy JL. Carbohydrate-protein complex increases the rate of muscle glycogen storage after exercise. J Appl Physiol. Bethesda, Md : Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Tipton KD, Ferrando AA, Phillips SM, Doyle D Jr, Wolfe RR. Postexercise net protein synthesis in human muscle from orally administered amino acids. Burd NA, West DW, Moore DR, Atherton PJ, Staples AW, Prior T, et al. Enhanced amino acid sensitivity of myofibrillar protein synthesis persists for up to 24 h after resistance exercise in young men. Tipton KD, Gurkin BE, Matin S, Wolfe RR. Nonessential amino acids are not necessary to stimulate net muscle protein synthesis in healthy volunteers. J Nutr Biochem. Borsheim E, Tipton KD, Wolf SE, Wolfe RR. Essential amino acids and muscle protein recovery from resistance exercise. Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. CAS PubMed PubMed Central Google Scholar. Tipton KD, Rasmussen BB, Miller SL, Wolf SE, Owens-Stovall SK, Petrini BE, et al. Timing of amino acid-carbohydrate ingestion alters anabolic response of muscle to resistance exercise. Tipton KD, Borsheim E, Wolf SE, Sanford AP, Wolfe RR. Acute response of net muscle protein balance reflects h balance after exercise and amino acid ingestion. Coffey VG, Moore DR, Burd NA, Rerecich T, Stellingwerff T, Garnham AP, et al. Nutrient provision increases signalling and protein synthesis in human skeletal muscle after repeated sprints. Eur J Appl Physiol. Breen L, Philp A, Witard OC, Jackman SR, Selby A, Smith K, et al. The influence of carbohydrate-protein co-ingestion following endurance exercise on myofibrillar and mitochondrial protein synthesis. J Physiol. Ferguson-Stegall L, Mccleave EL, Ding Z, Doerner PG 3rd, Wang B, Liao YH, et al. Postexercise carbohydrate-protein supplementation improves subsequent exercise performance and intracellular signaling for protein synthesis. Volek JS. Influence of nutrition on responses to resistance training. Kerksick C, Harvey T, Stout J, Campbell B, Wilborn C, Kreider R, et al. International society of sports nutrition position stand: nutrient timing. Elliot TA, Cree MG, Sanford AP, Wolfe RR, Tipton KD. Milk ingestion stimulates net muscle protein synthesis following resistance exercise. Farnfield MM, Breen L, Carey KA, Garnham A, Cameron-Smith D. Activation of mtor signalling in young and old human skeletal muscle in response to combined resistance exercise and whey protein ingestion. Tang JE, Manolakos JJ, Kujbida GW, Lysecki PJ, Moore DR, Phillips SM. Minimal whey protein with carbohydrate stimulates muscle protein synthesis following resistance exercise in trained young men. Tipton KD. Role of protein and hydrolysates before exercise. Hulmi JJ, Kovanen V, Lisko I, Selanne H, Mero AA. The effects of whey protein on myostatin and cell cycle-related gene expression responses to a single heavy resistance exercise bout in trained older men. Ivy JL, Ding Z, Hwang H, Cialdella-Kam LC, Morrison PJ. Post exercise carbohydrate-protein supplementation: Phosphorylation of muscle proteins involved in glycogen synthesis and protein translation. Churchward-Venne TA, Murphy CH, Longland TM, Phillips SM. Role of protein and amino acids in promoting lean mass accretion with resistance exercise and attenuating lean mass loss during energy deficit in humans. Short-term training: when do repeated bouts of resistance exercise become training? Can J Appl Physiol. Pennings B, Koopman R, Beelen M, Senden JM, Saris WH, Van Loon LJ. Exercising before protein intake allows for greater use of dietary protein-derived amino acids for de novo muscle protein synthesis in both young and elderly men. Miller BF, Olesen JL, Hansen M, Dossing S, Crameri RM, Welling RJ, et al. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. Camera DM, Edge J, Short MJ, Hawley JA, Coffey VG. Early time course of akt phosphorylation after endurance and resistance exercise. Cribb PJ, Hayes A. Effects of supplement timing and resistance exercise on skeletal muscle hypertrophy. Esmarck B, Andersen JL, Olsen S, Richter EA, Mizuno M, Kjaer M. Timing of postexercise protein intake is important for muscle hypertrophy with resistance training in elderly humans. Article CAS PubMed PubMed Central Google Scholar. Hoffman JR, Ratamess NA, Tranchina CP, Rashti SL, Kang J, Faigenbaum AD. Effect of protein-supplement timing on strength, power, and body-composition changes in resistance-trained men. Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Volpi E, Rasmussen BB. Essential amino acid and carbohydrate ingestion before resistance exercise does not enhance postexercise muscle protein synthesis. J Appl Physiol Bird SP, Tarpenning KM, Marino FE. Roberts MD, Dalbo VJ, Hassell SE, Brown R, Kerksick CM. Effects of preexercise feeding on markers of satellite cell activation. Dalbo VJ, Roberts MD, Hassell S, Kerksick CM. Effects of pre-exercise feeding on serum hormone concentrations and biomarkers of myostatin and ubiquitin proteasome pathway activity. Eur J Nutr. Tipton KD, Elliott TA, Cree MG, Wolf SE, Sanford AP, Wolfe RR. Ingestion of casein and whey proteins result in muscle anabolism after resistance exercise. Kerksick CM, Leutholtz B. Nutrient administration and resistance training. Burk A, Timpmann S, Medijainen L, Vahi M, Oopik V. Time-divided ingestion pattern of casein-based protein supplement stimulates an increase in fat-free body mass during resistance training in young untrained men. Nutr Res. Schoenfeld BJ, Aragon A, Wilborn C, Urbina SL, Hayward SE, Krieger J. Pre- versus post-exercise protein intake has similar effects on muscular adaptations. Aragon AA, Schoenfeld BJ. Nutrient timing revisited: is there a post-exercise anabolic window? Bosse JD, Dixon BM. Dietary protein to maximize resistance training: a review and examination of protein spread and change theories. Macnaughton LS, Wardle SL, Witard OC, Mcglory C, Hamilton DL, Jeromson S, et al. The response of muscle protein synthesis following whole-body resistance exercise is greater following 40 g than 20 g of ingested whey protein. Physiol Rep. Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J App Physiol Bethesda, Md: West DW, Burd NA, Coffey VG, Baker SK, Burke LM, Hawley JA, et al. Rapid aminoacidemia enhances myofibrillar protein synthesis and anabolic intramuscular signaling responses after resistance exercise. Geneva: World Health Organization; Series Editor : Who technical report series. Google Scholar. Joy JM, Lowery RP, Wilson JM, Purpura M, De Souza EO, Wilson SM, et al. The effects of 8 weeks of whey or rice protein supplementation on body composition and exercise performance. Bos C, Metges CC, Gaudichon C, Petzke KJ, Pueyo ME, Morens C, et al. Postprandial kinetics of dietary amino acids are the main determinant of their metabolism after soy or milk protein ingestion in humans. Burd NA, Yang Y, Moore DR, Tang JE, Tarnopolsky MA, Phillips SM. Greater stimulation of myofibrillar protein synthesis with ingestion of whey protein isolate v. Micellar casein at rest and after resistance exercise in elderly men. Br J Nutr. Phillips SM, Tang JE, Moore DR. The role of milk- and soy-based protein in support of muscle protein synthesis and muscle protein accretion in young and elderly persons. J Am Coll Nutr. Hartman JW, Tang JE, Wilkinson SB, Tarnopolsky MA, Lawrence RL, Fullerton AV, et al. Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Wilkinson SB, Tarnopolsky MA, Macdonald MJ, Macdonald JR, Armstrong D, Phillips SM. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Kerksick CM, Rasmussen C, Lancaster S, Starks M, Smith P, Melton C, et al. Impact of differing protein sources and a creatine containing nutritional formula after 12 weeks of resistance training. Paddon-Jones D, Sheffield-Moore M, Aarsland A, Wolfe RR, Ferrando AA. The combined findings of these studies indicate that muscle mass is not negatively affected by consuming the majority of daily protein as a large bolus. However, neither study employed regimented resistance training thereby limiting generalizability to individuals involved in intense exercise programs. Insights into the effects of protein dosage can also be gleaned from studies on intermittent fasting IF. Typical IF protocols require intake of daily nutrients, including protein, in a narrow time-frame — usually less than 8 h — followed by a prolonged fast. A recent systematic review concluded that IF has similar effects on fat-free mass compared with continuous eating protocols [ 35 ]. However, the studies reviewed in the analysis generally involved suboptimal protein intakes consumed as part of a low-energy diet without a resistance training component, again limiting the ability to extrapolate findings to resistance-trained individuals. Helping to fill this literature gap is an 8-week trial by Tinsley et al. The TRF group lost body weight via lower energy intake kcal less on fasting vs. non-fasting days , but did not significantly lose lean mass 0. Perhaps most interestingly, biceps brachii and rectus femoris cross sectional area showed similar increases in both groups despite the h fasting cycles and concentrated feeding cycles in TRF, suggesting that the utilization of protein intake in the ad libitum 4-h feeding cycles was not hampered by an acute ceiling of anabolism. Unfortunately, protein and energy were not equated in this study. Subsequently, an 8-week trial by Moro et al. These findings further call into question the concern for breaching a certain threshold of protein intake per meal for the goal of muscle retention. In contrast to the above findings showing neutral-to-positive effects of a temporally concentrated meal intake, Arciero et al. During the initial day eucaloric phase, HP3 and HP6 consumed protein at 2. HP6 was the only goup that significantly gained lean mass. During the subsequent day eucaloric phase, HP3 and HP6 consumed protein at 1. HP6 maintained its lean mass gain, outperforming the other 2 treatments in this respect HP actually showed a significant loss of lean mass compared to the control. In any case, it is notable that comparisons in this vein specifically geared toward the goal of muscle gain, hypercaloric comparisons in particular, are lacking. An important distinction needs to be made between acute meal challenges comparing different protein amounts including serial feedings in the acute phase following resistance training and chronic meal feedings comparing different protein distributions through the day, over the course of several weeks or months. Longitudinal studies examining body composition have not consistently corroborated the results of acute studies examining muscle protein flux. Quantifying a maximum amount of protein per meal that can be utilized for muscle anabolism has been a challenging pursuit due to the multitude of variables open for investigation. Perhaps the most comprehensive synthesis of findings in this area has been done by Morton et al. This was based on the addition of two standard deviations to their finding that 0. In line with this hypothesis, Moore et al. Importantly, these estimates are based on the sole provision of a rapidly digesting protein source that would conceivably increase potential for oxidation of AA when consumed in larger boluses. It seems logical that a slower-acting protein source, particularly when consumed in combination with other macronutrients, would delay absorption and thus enhance the utilization of the constituent AA. However, the practical implications of this phenomenon remain speculative and questionable [ 21 ]. The collective body of evidence indicates that total daily protein intake for the goal of maximizing resistance training-induced gains in muscle mass and strength is approximately 1. However, 1. Bandegan et al. This reinforces the practical need to individualize dietary programming, and remain open to exceeding estimated averages. It is therefore a relatively simple and elegant solution to consume protein at a target intake of 0. Using the upper CI daily intake of 2. This tactic would apply what is currently known to maximize acute anabolic responses as well as chronic anabolic adaptations. Further research is nevertheless needed to quantify a specific upper threshold for per-meal protein intake. Gropper SS, Smith JL, Groff JL : Advanced Nutrition and Human Metabolism. Belmont, CA: Wadsworth Cengage Learning; Morton RW, McGlory C, Phillips SM. Nutritional interventions to augment resistance training-induced skeletal muscle hypertrophy. Front Physiol. Article PubMed PubMed Central Google Scholar. Areta JL, Burke LM, Ross ML, Camera DM, West DW, Broad EM, Jeacocke NA, Moore DR, Stellingwerff T, Phillips SM, Hawley JA, Coffey VG. Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis. J Physiol. Article CAS PubMed PubMed Central Google Scholar. Moore DR, Areta J, Coffey VG, Stellingwerff T, Phillips SM, Burke LM, Cleroux M, Godin JP, Hawley JA. Daytime pattern of post-exercise protein intake affects whole-body protein turnover in resistance-trained males. Nutr Metab Lond. Article CAS Google Scholar. Bilsborough S, Mann N. A review of issues of dietary protein intake in humans. Int J Sport Nutr Exerc Metab. Article CAS PubMed Google Scholar. Morton RW, Murphy KT, McKellar SR, Schoenfeld BJ, Henselmans M, Helms E, Aragon AA, Devries MC, Banfield L, Krieger JW, Phillips SM. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br J Sports Med. Bandegan A, Courtney-Martin G, Rafii M, Pencharz PB, Lemon PW. Indicator amino acid-derived estimate of dietary protein requirement for male bodybuilders on a nontraining day is several-fold greater than the current recommended dietary allowance. J Nutr. Spendlove J, Mitchell L, Gifford J, Hackett D, Slater G, Cobley S, O'Connor H. Dietary intake of competitive bodybuilders. Sports Med. Article PubMed Google Scholar. Antonio J, Ellerbroek A, Silver T, Vargas L, Peacock C: The effects of a high protein diet on indices of health and body composition--a crossover trial in resistance-trained men. J Int Soc Sports Nutr , —— eCollection Dangin M, Boirie Y, Guillet C, Beaufrere B: Influence of the protein digestion rate on protein turnover in young and elderly subjects. J Nutr , 10 S—33S. Tipton KD, Elliott TA, Cree MG, Wolf SE, Sanford AP, Wolfe RR. Ingestion of casein and whey proteins result in muscle anabolism after resistance exercise. Med Sci Sports Exerc. Mitchell CJ, McGregor RA, D'Souza RF, Thorstensen EB, Markworth JF, Fanning AC, Poppitt SD, Cameron-Smith D. Consumption of milk protein or whey protein results in a similar increase in muscle protein synthesis in middle aged men. Reitelseder S, Agergaard J, Doessing S, Helmark IC, Lund P, Kristensen NB, Frystyk J, Flyvbjerg A, Schjerling P, van Hall G, Kjaer M, Holm L. Whey and casein labeled with L-[C]leucine and muscle protein synthesis: effect of resistance exercise and protein ingestion. Am J Physiol Endocrinol Metab. Dideriksen KJ, Reitelseder S, Petersen SG, Hjort M, Helmark IC, Kjaer M, Holm L. Stimulation of muscle protein synthesis by whey and caseinate ingestion after resistance exercise in elderly individuals. Scand J Med Sci Sports. Pennings B, Boirie Y, Senden JM, Gijsen AP, Kuipers H, van Loon LJ. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am J Clin Nutr. Burd NA, Yang Y, Moore DR, Tang JE, Tarnopolsky MA, Phillips SM. Greater stimulation of myofibrillar protein synthesis with ingestion of whey protein isolate v. Micellar casein at rest and after resistance exercise in elderly men. Br J Nutr. Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol Witard OC, Wardle SL, Macnaughton LS, Hodgson AB, Tipton KD. Protein considerations for Optimising skeletal muscle mass in healthy young and older adults. |
| IF YOU ENJOYED THIS POST, GET UPDATES. | The initial exercise bout resulted in considerable muscle damage, but exercise-induced muscle damage was attenuated at 3 weeks of training and almost completely absent at 10 weeks. Therefore, it appears that the myofibrillar protein synthetic response to a single bout of unaccustomed exercise may be at least partly a response to muscle damage and directed at tissue repair rather than muscle hypertrophy. After the first couple of days or weeks of training, post-exercise myofibrillar protein synthesis rates may be more reflective of the net changes in muscle mass, i. Consistent with this notion, myofibrillar protein synthesis rates assessed during several weeks of a prolonged resistance-type training program have been shown to correlate with muscle hypertrophy [ ]. Given these findings, it seems evident that changes in myofibrillar protein synthesis rates during recovery from successive exercise sessions can be predictive of net increase in muscle mass. The ingestion of 20 g of high-quality, rapidly digestible protein results in a near-maximal stimulation of MPS rates at rest and during the initial several hours of recovery following lower-body resistance-type exercise. Ingestion of animal-derived proteins tends to result in a greater increase in MPS rates than ingestion of plant-derived proteins. Recent evidence suggests that whole-food protein sources may contain micronutrients that can further augment the MPS response. The anabolic response to protein ingestion is attenuated during prolonged energy intake restriction, during muscle disuse, and in older adults especially older females. The ingestion of greater amounts of protein can at least partly rescue the blunted MPS response during prolonged energy restriction and ageing but not during muscle disuse. In conclusion, nutritional recommendations to maximize the MPS response to feeding depend on both the type of meal and time until the next feeding opportunity and should be personalized to the individual athlete. Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol. CAS PubMed Google Scholar. Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Tipton KD, Ferrando AA, Phillips SM, Doyle DJ, Wolfe RR. Postexercise net protein synthesis in human muscle from orally administered amino acids. Article CAS PubMed Google Scholar. Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, et al. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr. Witard OC, Jackman SR, Breen L, Smith K, Selby A, Tipton KD. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol. Google Scholar. Wilkinson SB, Tarnopolsky MA, Macdonald MJ, Macdonald JR, Armstrong D, Phillips SM. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Levenhagen DK, Gresham JD, Carlson MG, Maron DJ, Borel MJ, Flakoll PJ. Postexercise nutrient intake timing in humans is critical to recovery of leg glucose and protein homeostasis. Am J Physiol Endocrinol Metab. Gorissen SHM, Remond D, van Loon LJC. The muscle protein synthetic response to food ingestion. Meat Sci. Hector AJ, Marcotte GR, Churchward-Venne TA, Murphy CH, Breen L, Von Allmen M, et al. Whey protein supplementation preserves postprandial myofibrillar protein synthesis during short-term energy restriction in overweight and obese adults. J Nutr. Smith GI, Atherton P, Reeds DN, Mohammed BS, Rankin D, Rennie MJ, et al. Omega-3 polyunsaturated fatty acids augment the muscle protein anabolic response to hyperinsulinaemia—hyperaminoacidaemia in healthy young and middle-aged men and women. Clin Sci Lond. Article CAS PubMed PubMed Central Google Scholar. Beals JW, Sukiennik RA, Nallabelli J, Emmons RS, van Vliet S, Young JR, et al. Anabolic sensitivity of postprandial muscle protein synthesis to the ingestion of a protein-dense food is reduced in overweight and obese young adults. Smith GI, Mittendorfer B. Sexual dimorphism in skeletal muscle protein turnover. Moore DR, Camera DM, Areta JL, Hawley JA. Beyond muscle hypertrophy: why dietary protein is important for endurance athletes 1. Appl Physiol Nutr Metab. Parr EB, Camera DM, Areta JL, Burke LM, Phillips SM, Hawley JA, et al. Alcohol ingestion impairs maximal post-exercise rates of myofibrillar protein synthesis following a single bout of concurrent training. PLoS One. Pasiakos SM, McClung HL, Margolis LM, Murphy NE, Lin GG, Hydren JR, et al. Human muscle protein synthetic responses during weight-bearing and non-weight-bearing exercise: a comparative study of exercise modes and recovery nutrition. Macnaughton LS, Wardle SL, Witard OC, McGlory C, Hamilton DL, Jeromson S, et al. The response of muscle protein synthesis following whole-body resistance exercise is greater following 40 g than 20 g of ingested whey protein. Physiol Rep ;4 van Vliet S, Burd NA, van Loon LJ. The skeletal muscle anabolic response to plant- versus animal-based protein consumption. Article PubMed Google Scholar. Gorissen SH, Horstman AM, Franssen R, Crombag JJ, Langer H, Bierau J, et al. Ingestion of wheat protein increases in vivo muscle protein synthesis rates in healthy older men in a randomized trial. Reidy PT, Walker DK, Dickinson JM, Gundermann DM, Drummond MJ, Timmerman KL, et al. Protein blend ingestion following resistance exercise promotes human muscle protein synthesis. Pennings B, Boirie Y, Senden JMG, Gijsen AP, Kuipers H, van Loon LJC. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Burd NA, Yang Y, Moore DR, Tang JE, Tarnopolsky MA, Phillips SM. Greater stimulation of myofibrillar protein synthesis with ingestion of whey protein isolate v. micellar casein at rest and after resistance exercise in elderly men. Br J Nutr. Koopman R, Crombach N, Gijsen AP, Walrand S, Fauquant J, Kies AK, et al. Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. Wall BT, Hamer HM, de Lange A, Kiskini A, Groen BBL, Senden JMG, et al. Leucine co-ingestion improves post-prandial muscle protein accretion in elderly men. Clin Nutr. Devries MC, Phillips SM. Supplemental protein in support of muscle mass and health: advantage whey. J Food Sci. West DWD, Burd NA, Coffey VG, Baker SK, Burke LM, Hawley JA, et al. Rapid aminoacidemia enhances myofibrillar protein synthesis and anabolic intramuscular signaling responses after resistance exercise. Trommelen J, Kouw IWK, Holwerda AM, Snijders T, Halson SL, Rollo I, et al. Pre-sleep dietary protein-derived amino acids are incorporated in myofibrillar protein during post-exercise overnight recovery. Res PT, Groen B, Pennings B, Beelen M, Wallis GA, Gijsen AP, et al. Protein ingestion before sleep improves postexercise overnight recovery. Med Sci Sports Exerc. Trommelen J, van Loon LJC. Pre-sleep protein ingestion to improve the skeletal muscle adaptive response to exercise training. Vliet SV, Beals JW, Martinez IG, Skinner SK, Burd NA. Achieving optimal post-exercise muscle protein remodeling in physically active adults through whole food consumption. Article CAS PubMed Central Google Scholar. Gorissen SHM, Burd NA, Hamer HM, Gijsen AP, Groen BB, van Loon LJC. Carbohydrate coingestion delays dietary protein digestion and absorption but does not modulate postprandial muscle protein accretion. J Clin Endocrinol Metab. Staples AW, Burd NA, West DWD, Currie KD, Atherton PJ, Moore DR, et al. Carbohydrate does not augment exercise-induced protein accretion versus protein alone. Pennings B, Groen BBL, van Dijk J-W, de Lange A, Kiskini A, Kuklinski M, et al. Minced beef is more rapidly digested and absorbed than beef steak, resulting in greater postprandial protein retention in older men. Kim I-Y, Schutzler S, Schrader A, Spencer HJ, Azhar G, Ferrando AA, et al. The anabolic response to a meal containing different amounts of protein is not limited by the maximal stimulation of protein synthesis in healthy young adults. Greenhaff PL, Karagounis LG, Peirce N, Simpson EJ, Hazell M, Layfield R, et al. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Trommelen J, Groen BBL, Hamer HM, de Groot LCPGM, van Loon LJC. Mechanisms in endocrinology: exogenous insulin does not increase muscle protein synthesis rate when administered systemically: a systematic review. Eur J Endocrinol. Elliot TA, Cree MG, Sanford AP, Wolfe RR, Tipton KD. Milk ingestion stimulates net muscle protein synthesis following resistance exercise. Gorissen SHM, Burd NA, Kramer IF, van Kranenburg J, Gijsen AP, Rooyackers O, et al. Co-ingesting milk fat with micellar casein does not affect postprandial protein handling in healthy older men. Edelbroek M, Horowitz M, Maddox A, Bellen J. Gastric emptying and intragastric distribution of oil in the presence of a liquid or a solid meal. J Nucl Med. van Vliet S, Shy EL, Abou Sawan S, Beals JW, West DW, Skinner SK, et al. Consumption of whole eggs promotes greater stimulation of postexercise muscle protein synthesis than consumption of isonitrogenous amounts of egg whites in young men. Stephens FB, Chee C, Wall BT, Murton AJ, Shannon CE, van Loon LJC, et al. Lipid-induced insulin resistance is associated with an impaired skeletal muscle protein synthetic response to amino acid ingestion in healthy young men. Hammond KM, Impey SG, Currell K, Mitchell N, Shepherd SO, Jeromson S, et al. Postexercise high-fat feeding suppresses p70S6K1 activity in human skeletal muscle. Riechman SE, Andrews RD, Maclean DA, Sheather S. Statins and dietary and serum cholesterol are associated with increased lean mass following resistance training. J Gerontol A Biol Sci Med Sci. Ziegenfuss TN, Lopez HL, Kedia A, Habowski SM, Sandrock JE, Raub B, et al. Effects of an amylopectin and chromium complex on the anabolic response to a suboptimal dose of whey protein. J Int Soc Sports Nutr. Smeuninx B, Nishimura Y, Mckendry J, Limb M, Smith K, Atherton PJ, et al. The effect of acute oral phosphatidic acid ingestion on myofibrillar protein synthesis and intracellular signaling in older males. Bjornsen T, Salvesen S, Berntsen S, Hetlelid KJ, Stea TH, Lohne-Seiler H, et al. Vitamin C and E supplementation blunts increases in total lean body mass in elderly men after strength training. Scand J Med Sci Sports. World Health Organization. Management of substance abuse unit. Global status report on alcohol and health, Geneva: World Health Organization; Burke LM, Read RS. A study of dietary patterns of elite Australian football players. Can J Sport Sci. Watten RG. Sports, physical exercise and use of alcohol. Steiner JL, Lang CH. Dysregulation of skeletal muscle protein metabolism by alcohol. Sundgot-Borgen J, Garthe I. Elite athletes in aesthetic and Olympic weight-class sports and the challenge of body weight and body compositions. J Sports Sci. Longland TM, Oikawa SY, Mitchell CJ, Devries MC, Phillips SM. Higher compared with lower dietary protein during an energy deficit combined with intense exercise promotes greater lean mass gain and fat mass loss: a randomized trial. Weinheimer EM, Sands LP, Campbell WW. A systematic review of the separate and combined effects of energy restriction and exercise on fat-free mass in middle-aged and older adults: implications for sarcopenic obesity. Nutr Rev. Backx EMP, Tieland M, van den Borgonjen Berg KJ, Claessen PR, van Loon LJC, de Groot LCPGM. Protein intake and lean body mass preservation during energy intake restriction in overweight older adults. Int J Obes Lond. Article CAS Google Scholar. Hector AJ, McGlory C, Damas F, Mazara N, Baker SK, Phillips SM. Pronounced energy restriction with elevated protein intake results in no change in proteolysis and reductions in skeletal muscle protein synthesis that are mitigated by resistance exercise. FASEB J. Areta JL, Burke LM, Camera DM, West DWD, Crawshay S, Moore DR, et al. Reduced resting skeletal muscle protein synthesis is rescued by resistance exercise and protein ingestion following short-term energy deficit. Garthe I, Raastad T, Refsnes PE, Koivisto A, Sundgot-Borgen J. Effect of two different weight-loss rates on body composition and strength and power-related performance in elite athletes. Int J Sport Nutr Exerc Metab. Elia M, Stubbs RJ, Henry CJ. Differences in fat, carbohydrate, and protein metabolism between lean and obese subjects undergoing total starvation. Obes Res. Pasiakos SM, Cao JJ, Margolis LM, Sauter ER, Whigham LD, McClung JP, et al. Effects of high-protein diets on fat-free mass and muscle protein synthesis following weight loss: a randomized controlled trial. Hursel R, Martens EAP, Gonnissen HKJ, Hamer HM, Senden JMG, van Loon LJC, et al. Prolonged adaptation to a low or high protein diet does not modulate basal muscle protein synthesis rates—a substudy. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. McGlory C, Wardle SL, Macnaughton LS, Witard OC, Scott F, Dick J, et al. Fish oil supplementation suppresses resistance exercise and feeding-induced increases in anabolic signaling without affecting myofibrillar protein synthesis in young men. Physiol Rep. Miller BF, Olesen JL, Hansen M, Dossing S, Crameri RM, Welling RJ, et al. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol. Burd NA, West DWD, Moore DR, Atherton PJ, Staples AW, Prior T, et al. Enhanced amino acid sensitivity of myofibrillar protein synthesis persists for up to 24 h after resistance exercise in young men. Trommelen J, Holwerda AM, Kouw IWK, Langer H, Halson SL, Rollo I, et al. Resistance exercise augments postprandial overnight muscle protein synthesis rates. Pennings B, Koopman R, Beelen M, Senden JMG, Saris WHM, van Loon LJC. Exercising before protein intake allows for greater use of dietary protein-derived amino acids for de novo muscle protein synthesis in both young and elderly men. Atherton PJ, Etheridge T, Watt PW, Wilkinson D, Selby A, Rankin D, et al. Muscle full effect after oral protein: time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Churchward-Venne TA, Burd NA, Mitchell CJ, West DWD, Philp A, Marcotte GR, et al. Supplementation of a suboptimal protein dose with leucine or essential amino acids: effects on myofibrillar protein synthesis at rest and following resistance exercise in men. Moore DR, Tang JE, Burd NA, Rerecich T, Tarnopolsky MA, Phillips SM. Differential stimulation of myofibrillar and sarcoplasmic protein synthesis with protein ingestion at rest and after resistance exercise. Glover EI, Phillips SM, Oates BR, Tang JE, Tarnopolsky MA, Selby A, et al. Immobilization induces anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion. Devries MC, Breen L, Allmen Von M, Macdonald MJ, Moore DR, Offord EA, et al. Low-load resistance training during step-reduction attenuates declines in muscle mass and strength and enhances anabolic sensitivity in older men. Breen L, Stokes KA, Churchward-Venne TA, Moore DR, Baker SK, Smith K, et al. Wall BT, Dirks ML, Snijders T, van Dijk J-W, Fritsch M, Verdijk LB, et al. Short-term muscle disuse lowers myofibrillar protein synthesis rates and induces anabolic resistance to protein ingestion. Wall BT, Morton JP, van Loon LJC. Strategies to maintain skeletal muscle mass in the injured athlete: nutritional considerations and exercise mimetics. Eur J Sport Sci. Tipton KD. Nutritional support for exercise-induced injuries. Sports Med. Volpi E, Sheffield-Moore M, Rasmussen BB, Wolfe RR. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. Wall BT, Gorissen SH, Pennings B, Koopman R, Groen BBL, Verdijk LB, et al. Aging is accompanied by a blunted muscle protein synthetic response to protein ingestion. Churchward-Venne TA, Holwerda AM, Phillips SM, van Loon LJC. What is the optimal amount of protein to support post-exercise skeletal muscle reconditioning in the older adult? Moore DR, Churchward-Venne TA, Witard O, Breen L, Burd NA, Tipton KD, et al. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. Lee SJ, Janssen I, Heymsfield SB, Ross R. Relation between whole-body and regional measures of human skeletal muscle. West DWD, Burd NA, Churchward-Venne TA, Camera DM, Mitchell CJ, Baker SK, et al. Sex-based comparisons of myofibrillar protein synthesis after resistance exercise in the fed state. Dreyer HC, Fujita S, Glynn EL, Drummond MJ, Volpi E, Rasmussen BB. Resistance exercise increases leg muscle protein synthesis and mTOR signalling independent of sex. The amino acids from protein will then be shuttled to your muscles, replacing any lost to exercise. Learning how to stimulate MPS through exercise and diet can help accelerate muscle growth, improve recovery and athletic performance, and increase overall endurance. Protein balance describes the relationship between muscle protein breakdown and muscle protein synthesis. When your body is in protein balance, no muscle growth or wasting occurs, and you're considered in a healthy state of biological equilibrium homeostasis , otherwise known as maintenance. To stimulate muscle growth, you essentially need to unsettle the protein balance. While it may seem counter-intuitive, exercise can break down muscle protein but rarely more than the amount of protein you can synthesize. In fact, the greater the intensity of a workout, the greater the MPS. Remember that this muscle breakdown stimulates the repair and growth of muscle tissue. Scientists measure intensity by something called the one-repetition maximum 1-RM , meaning the maximum weight you can lift for one repetition. Even if exercising to failure , low-intensity exercise will do little to increase MPS and, as such, will not increase muscle mass. The relationship between diet and protein balance is less straightforward. Even with increased protein intake, MPS is triggered for only a finite period of time. This is because the body can only utilize so much of the essential amino acids EAAs it receives; anything more will be broken down and excreted by the liver. Sports nutritionists recommend about 1. You can obtain enough protein through your diet by focusing on dairy, eggs, lean meats , nuts, and legumes. It is also wise to consume plenty of whole grains, healthy fats, fruits, and vegetables to help your body perform and repair properly. For instance, carbohydrates are necessary for muscle building since they stimulate insulin release—a hormone that aids muscle cells in absorbing protein. To stimulate MPS, it is important to consume the appropriate amount of protein following exercise. Eating too much will not improve muscle growth but may increase the accumulation of potentially harmful byproducts such as urea. A study from the University of Birmingham looked into MPS response rates in men prescribed 10, 20, or 40 grams of whey protein immediately following resistance training. Researchers noticed the following results:. Consuming 20 grams to 40 grams of whey protein after resistance training also increased phenylalanine, leucine, and threonine concentrations, EAAs associated with lean muscle growth. Note that whey protein is a fast-digesting protein. Further results can likely be obtained by consuming slower digesting protein throughout the day. Muscle protein synthesis is not something achieved by taking a sports supplement. It is a biological process that can vary by the individual's fitness status. As such, it is not something you can readily measure or manipulate. With that being said, you can use strategies to promote MPS. Start by increasing the intensity of your workout, pushing weights that require significant force but not enough to undermine proper form or personal safety. Follow up by feeding your muscles with protein. A gram dose of a digestible protein drink is likely a good place to start. If you consider consuming protein beyond the recommended dietary intake, speak with your doctor or a registered sports nutritionist to understand the potential benefits and risks. Stokes T, Hector AJ, Morton RW, McGlory C, Phillips SM. Recent Perspectives Regarding the Role of Dietary Protein for the Promotion of Muscle Hypertrophy with Resistance Exercise Training. Mitchell C, Churchward-Venne T, Parise G, et al. Acute Post-Exercise Myofibrillar Protein Synthesis Is Not Correlated with Resistance Training-Induced Muscle Hypertrophy in Young Men. PLoS ONE. Damas F, Phillips S, Vechin F, Ugrinowitsch C. In the bodybuilding world, insulin is sometimes injected at supraphysiological doses to stimulate muscle growth. Insulin inhibits muscle protein breakdown a bit, but only a little is needed for the maximal effect this is discussed in dept in section 2. Exercise improves the muscle protein synthetic response to protein ingestion. Therefore, it has been suggested that protein intake immediately post-exercise is more anabolic than protein ingestion at different time points. Probably the best evidence to support the concept of protein timing is a study which showed that protein ingestion immediately after exercise was more effective than protein ingestion 3 h post-exercise though this study used the 2 pool arterio-venous method which is not a great measurement of muscle protein synthesis Levenhagen, In contrast, a different study observed no difference in MPS was found when essential amino acid were ingested 1 h or 3 h post-exercise Rasmussen, In addition, resistance exercise enhances the muscle protein synthetic response to protein ingestion for at least 24 hour Burd, It is certainly possible that the synergy between exercise and protein ingestion is the largest immediately post-exercise and then slowly declines in the next 24 h hour. However, these data suggest that there is not a limited window of opportunity during which protein is massively beneficial immediately post-exercise, that suddenly closes within a couple of hours. Overal, no clear benefit to protein timing has been found in studies measuring muscle protein synthesis studies. As such studies are much more sensitive to detect potential anabolic effects compared to long-term studies measuring changes in muscle mass, it unlikely that long-term studies will observe benefits of protein timing. However, this effect was largely explained by the fact that the protein supplementation increased total protein intake, rather than the specific timing of protein intake. We performed a study to assess protein intake in well-trained Dutch athletes. Even some Olympic athletes were included. We observed that athletes consumed ~1. The majority of the protein was consumed in the three main meals: breakfast, lunch and dinner. While this intake pattern has a reasonable distribution throughout the waking hours, amino acid availability is potentially low during the night. This begs the question: does protein distribution throughout the day matter for muscle protein synthesis? Several studies suggest that protein should be reasonably distributed for optimal anabolism. For example, an even balance of protein intake at breakfast, lunch and dinner stimulates MPS more effective than eating the majority of daily protein during the evening meal Marerow, But a too high distribution resulting in many mini snacks may also be suboptimal. Providing 20 g of protein every 3 hours stimulates MPS more than providing the same amount of protein in less regular doses 40 g every 6 hours , or more regular doses 10 g every 1. While there are more studies that support the concept of protein distribution, there are even more studies that suggest it has no clear benefit. If your goal is to absolutely maximize gains, it theoretically makes sense to try to aim for at least a reasonable protein distribution protein rich meals divided throughout the day. The muscle full effect is the observation that amino acids stimulate MPS for a short period, after which there is a refractory period where the muscle does not respond to amino acids. More specifically, after protein intake, there is an lag period of approximately min before MPS goes up and peaks between minutes, after which MPS returns rapidly to baseline even if amino acid levels are still elevated Bohe, Atherthon, The muscle full effect has given birth to a theory on how to optimize protein intake throughout the day in the online fitness community. It suggests that after amino acids levels have been elevated, you should let them drop down back to fasting levels to sensitize the muscle to amino acids again. Subsequently, protein intake will stimulate MPS again. The suggested mechanism seems unlikely as many food patterns result in elevated amino acid levels throughout the whole day. The traditional bodybuilding diet consists of very frequent, very high protein meals e. chicken, rice, broccoli 6 times a day. In fact, it was specifically designed with the goal of keeping amino acids elevated throughout the whole day so there would always be enough building blocks for form new muscle tissue. Or intermittent fasting where all daily protein is eaten in a short time period usually 8 hours. These diets would only allow for a single ~90 min increase in MPS during a whole day. This is best illustrated in a study where the effect of protein was assessed in both rested and post-exercise conditions Churchward-Venne, Protein intake alone stimulates MPS in the h period after ingestion. Subsequently, MPS rates fall back to basal rates. However, in post-exercise condition, protein stimulated MPS rates in both the h and the h period. It appears that the muscle full effect is not present in acute post-exercise conditions. As discussed above, an effective protein distribution optimizes MPS. Protein supplementation Only three days of dieting already reduce basal MPS Areta, This shows that an energy deficit is suboptimal for MPS, however you can grow muscle mass while losing fat Longland, It is unclear if eating above maintenance is needed to optimize MPS. Second, I will continue to further elaborate sections based on your feedback and add additional sections in the future. Lastly, please reference specific sections from this article when you see a discussion on muscle protein synthesis. People mistaking whole-body protein synthesis for muscle protein synthesis: see section 4. Someone skeptical about a conclusion from a paper because muscle protein breakdown was not measured? Section 2 buddy. Someone claiming that protein supplementation is not effective based on a long-term study he read that found no improvement in muscle mass: section 5 got you covered. Feel free to ask me questions about the methods, or interpretation on protein metabolism studies in comments or on Facebook. If I work out 3 days a week e. Tuesday, Thursday, Sunday and am looking to do a lean bulk, would you suggest ensuring there is more of a surplus the day of exercises and the following day during heightened protein synthesis than for example on Saturday when I would have had 2 days rest from the gym? To clarify, I am assuming that on Saturday, there would not be much protein synthesis occurring from the Thursday workout, so if I were to have a surplus, would it be better to eat more on the Thursday and Friday and possibly to a maintenance calorie day on Saturday to limit fat when muscle gain is not likely to happen? Energy balance on the short term does not seem to impact muscle protein synthesis. Your body sort of keeps track of the last couple of days and longer , rather than just the moment. You could play around with your strategy. What food is the highest source of leucine for vegan bodybuilders to consume right after weight training? Do you know if there is any difference in MPS between males and females? Females tend to start with lower muscle mass than males. But relative growth is the same and there is no clear indication that protein requirements are different. Maybe the Leucine threshold theory is weak? Leucine is an important amino acid. But the leucine threshold concept is not the ideal basis to determine your protein intake. I bookmarked this page and read down and see the author asking me to bookmark the page, I rarely bookmark pages but this one is very interesting. Good job. I think should add the alcohol negative effect and also caffeine that can alter sleep. The article focuses on the two main impacts on MPS: exercise and protein ingestion. But yeah, high doses of alcohol and sleep restriction are detrimental for MPS. Bio-availability for MPS varies by the protein source, i. Yes, there is some indication for this. But it seems to be whey protein, so there should not be much difference with Optimum Nutrition whey protein. I love this topic! Please help me reconcile this disparity. How do I, a lb man 85kg , get my daily recommended 1. But they do have some value. Athletes tend to eat about 35 g protein in dinner, so there is no need to tell them to reduce that. We also recommend at least 40 g protein prior to sleep, because that protein needs to last you the whole night. I typically pair push and pull exercise or different groups to give longer break between hitting a muscle group. I guess my question is, does the whole body need 3 to 5 minutes rest or just the specific muscle group being worked? A-MA-ZING and highly instructive article, plus a tremendous work to aggregate and synthetize all this data!!! Thanks a lot, Jorn. I do intermittent fasting for 30 years or so, which has been a blessing for my physical and intellectual energy and my overall health: no breakfast, 30 min callisthenics or HIIT workout every other day in the morning, light fruit meal round 2 PM and main food intakes in the PM window, including a moderate amount of protein foods typically: g nuts or 3 eggs or g white meat or fish. What do you and science think about this? Any suggestions? Thank you for the kind words. I should have a new publication later this month that ties in a bit with intermittent fasting. If MPS is optimally stimulated in a 24hours period after exercise, why not training every day each muscle group for optimal gains? You would also have to take recovery into account. If not, might the kg athlete receive an MPB benefit from adding carbohydrate to the pre-workout meal, even if the 70kg athlete would receive no such benefit? Is there anything definitive on this? Or, in the absence of anything definitive, do you have any practical advice for larger men? MPB is something you need for optimal adaptation. Alcohol consumption has been shown to reduce muscle synthesis. But those studies primarily looked at alcohol consumption immediately after exercise. Some of the figures you reference show peaks in muscle synthesis after only a few hours, but elsewhere you say the peak is at 24 hours. So if alcohol consumption comes hours after exercise, how much muscle synthesis are you really losing out on? i eat one time a day and follow a ketogenic diet. i train in the evening fasted state. Then i get 20 gr EAA. What time is that 20 g EAA exactly? That is a pretty good dose, that should maximize your anabolism until your dinner. Thank you for your answer. On training days, As soon as I finish my workout, I consume 20 gr EAA. On non-training days, I am also planning to take 20 gr EAA with meal after my 24 fasting. I am 43, so in order to Increase my satellite cell count I try to induce autophagy. Unfortunately will take quite a while like months, because the processes of submitting, peer review sometimes they ask additional experiments… is so slow. I was wondering if there was a meta analysis done or consensus on training post-fast with AA supplementation. Does not have to be a problem if the dose is high enough BCAA alone: suboptimal. Does not provide all EAA. When intact casein is hydrolysed chemically cut into smaller smaller pieces , it resembles the digestion of a fast digesting protein. Your email address will not be published. Sign me up for the newsletter. This site uses Akismet to reduce spam. Learn how your comment data is processed. Skip to primary navigation Skip to main content Skip to footer. Contents 1. What is protein synthesis? Why muscle protein breakdown is of less importance 3. Methods to measure protein synthesis 3. Interpretation and misconceptions 4. Advantages of muscle protein synthesis measurements over muscle mass measurements. How to optimize muscle protein synthesis: exercise guidelines. How to optimize muscle protein synthesis: nutrition guidelines 7. Muscle protein synthesis is the process of building muscle mass. Muscle protein breakdown is the opposing process of breaking down muscle tissue. If muscle protein synthesis exceeds muscle protein breakdown, your muscles will grow. Nitrogen balance gives a general view if the body is in an overall anabolic or catabolic state. But it not specific to muscle and not that useful for athletes. Whole-body protein metabolism shows the synthesis, breakdown, oxidation, and net balance of all the body tissues. The two and three pool model can measure both muscle protein synthesis and muscle protein breakdown, but are not the preferred methods for measuring muscle protein synthesis. The fractional synthetic rate is the preferred measurement of muscle protein synthesis. De novo muscle protein synthesis can be measured by using highly enriched intrinsically labeled protein. It measures how much of the protein you ingest is build into muscle tissue. Mixed muscle protein synthesis measures the synthesis of all muscle proteins. Myofibrillar protein synthesis measures only contractile proteins and is the most relevant measurement for muscle mass gains. Mitochondrial protein synthesis is more relevant for endurance and metabolic health. D20 can measure muscle protein synthesis over periods of days to weeks. Whole-body protein metabolism does not necessarily reflect what happens with muscle. Results from whole-body protein metabolism measurements have little practical value for athletes. Multiple sets stimulate muscle protein synthesis more effectively than a single set. Different rep ranges are equally effective in stimulating muscle protein synthesis if the set is taken to failure. Sets taken close to failure may produce similair gains as sets taken to failure. Short rest period attenuate post-exercise muscle protein synthesis rates. In more trained athletes, there is less of an increase in muscle protein synthesis, but also muscle damage following resistance exercise. There is a linear increase in MPS rates up to approximately 20 g of protein. The protein digestion speed and amino acid content particularly leucine are the main properties that determine the anabolic effect. |
Protein and muscle protein synthesis in athletes -
Areta JL, Burke LM, Ross ML, Camera DM, West DW, Broad EM, et al. Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis. Burd NA, Holwerda AM, Selby KC, West DW, Staples AW, Cain NE, et al.
Resistance exercise volume affects myofibrillar protein synthesis and anabolic signalling molecule phosphorylation in young men. Churchward-Venne TA, Burd NA, Mitchell CJ, West DW, Philp A, Marcotte GR, et al.
Supplementation of a suboptimal protein dose with leucine or essential amino acids: effects on myofibrillar protein synthesis at rest and following resistance exercise in men. Macnaughton LS, Wardle SL, Witard OC, McGlory C, Hamilton DL, Jeromson S, et al.
The response of muscle protein synthesis following whole-body resistance exercise is greater following 40 g than 20 g of ingested whey protein. Physiol Rep. McGlory C, Wardle SL, Macnaughton LS, Witard OC, Scott F, Dick J, et al.
Fish oil supplementation suppresses resistance exercise and feeding-induced increases in anabolic signaling without affecting myofibrillar protein synthesis in young men. McKendry J, Perez-Lopez A, McLeod M, Luo D, Dent JR, Smeuninx B, et al. Short inter-set rest blunts resistance exercise-induced increases in myofibrillar protein synthesis and intracellular signalling in young males.
Exp Physiol. Reidy PT, Walker DK, Dickinson JM, Gundermann DM, Drummond MJ, Timmerman KL, et al. Soy-dairy protein blend and whey protein ingestion after resistance exercise increases amino acid transport and transporter expression in human skeletal muscle.
Reitelseder S, Agergaard J, Doessing S, Helmark IC, Lund P, Kristensen NB, et al. Whey and casein labeled with L-[1—13C]leucine and muscle protein synthesis: effect of resistance exercise and protein ingestion. CrossRef Full Text Google Scholar.
West DW, Kujbida GW, Moore DR, Atherton P, Burd NA, Padzik JP, et al. Resistance exercise-induced increases in putative anabolic hormones do not enhance muscle protein synthesis or intracellular signalling in young men.
West DW, Burd NA, Coffey VG, Baker SK, Burke LM, Hawley JA, et al. Rapid aminoacidemia enhances myofibrillar protein synthesis and anabolic intramuscular signaling responses after resistance exercise.
West DW, Burd NA, Churchward-Venne TA, Camera DM, Mitchell CJ, Baker SK, et al. Sex-based comparisons of myofibrillar protein synthesis after resistance exercise in the fed state. Moore DR, Churchward-Venne TA, Witard O, Breen L, Burd NA, Tipton KD, et al. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men.
J Gerontol A Biol Sci Med Sci. Gallagher D, Visser M, De Meersman RE, Sepulveda D, Baumgartner RN, Pierson RN, et al. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity.
Aragon AA, Schoenfeld BJ. Nutrient timing revisited: is there a post-exercise anabolic window? J Int Soc Sports Nutr. Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, et al.
Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. Faseb J. Kriengsinyos W, Wykes LJ, Goonewardene LA, Ball RO, Pencharz PB. Phase of menstrual cycle affects lysine requirement in healthy women. Phillips SM, Atkinson SA, Tarnopolsky MA, MacDougall JD.
Gender differences in leucine kinetics and nitrogen balance in endurance athletes. Miller BF, Hansen M, Olesen JL, Flyvbjerg A, Schwarz P, Babraj JA, et al. No effect of menstrual cycle on myofibrillar and connective tissue protein synthesis in contracting skeletal muscle.
Fujita S, Rasmussen BB, Bell JA, Cadenas JG, Volpi E. Basal muscle intracellular amino acid kinetics in women and men. Jahn LA, Barrett EJ, Genco ML, Wei L, Spraggins TA, Fryburg DA. Tissue composition affects measures of postabsorptive human skeletal muscle metabolism: comparison across genders. J Clin Endocrinol Metab.
Dreyer HC, Fujita S, Glynn EL, Drummond MJ, Volpi E, Rasmussen BB. Resistance exercise increases leg muscle protein synthesis and mTOR signalling independent of sex. Acta Physiol. Areta JL, Burke LM, Camera DM, West DW, Crawshay S, Moore DR, et al.
Reduced resting skeletal muscle protein synthesis is rescued by resistance exercise and protein ingestion following short-term energy deficit.
Alghannam AF, Gonzalez JT, Betts JA. Restoration of muscle glycogen and functional capacity: role of post-exercise carbohydrate and protein co-ingestion. Burke LM, Hawley JA, Wong SH, Jeukendrup AE. Carbohydrates for training and competition.
J Sports Sci. Glynn EL, Fry CS, Drummond MJ, Dreyer HC, Dhanani S, Volpi E, et al. Muscle protein breakdown has a minor role in the protein anabolic response to essential amino acid and carbohydrate intake following resistance exercise.
Am J Physiol Regul Integr Comp Physiol. Borsheim E, Cree MG, Tipton KD, Elliott TA, Aarsland A, Wolfe RR. Effect of carbohydrate intake on net muscle protein synthesis during recovery from resistance exercise.
Staples AW, Burd NA, West DW, Currie KD, Atherton PJ, Moore DR, et al. Carbohydrate does not augment exercise-induced protein accretion versus protein alone. Koopman R, Beelen M, Stellingwerff T, Pennings B, Saris WH, Kies AK, et al.
Coingestion of carbohydrate with protein does not further augment postexercise muscle protein synthesis. West DW, Cotie LM, Mitchell CJ, Churchward-Venne TA, MacDonald MJ, Phillips SM.
Resistance exercise order does not determine postexercise delivery of testosterone, growth hormone, and IGF-1 to skeletal muscle. Deutz NE, Wolfe RR. Is there a maximal anabolic response to protein intake with a meal? Clin Nutr. Kim IY, Deutz NEP, Wolfe RR.
Update on maximal anabolic response to dietary protein. Kim IY, Schutzler S, Schrader A, Spencer HJ, Azhar G, Ferrando AA, et al. The anabolic response to a meal containing different amounts of protein is not limited by the maximal stimulation of protein synthesis in healthy young adults.
Kim IY, Schutzler S, Schrader A, Spencer H, Kortebein P, Deutz NE, et al. Quantity of dietary protein intake, but not pattern of intake, affects net protein balance primarily through differences in protein synthesis in older adults. Schoenfeld BJ, Aragon AA.
How much protein can the body use in a single meal for muscle-building? Implications for daily protein distribution. Malowany JM, West DWD, Williamson E, Volterman KA, Abou Sawan S, Mazzulla M, et al. Protein to maximize whole-body anabolism in resistance-trained females after exercise.
Mazzulla M, Volterman KA, Packer JE, Wooding DJ, Brooks JC, Kato H, et al. Whole-body net protein balance plateaus in response to increasing protein intakes during post-exercise recovery in adults and adolescents. Nutr Metab.
Almoosawi S, Winter J, Prynne CJ, Hardy R, Stephen AM. Daily profiles of energy and nutrient intakes: are eating profiles changing over time? Eur J Clin Nutr. Gorissen SH, Remond D, van Loon LJ. The muscle protein synthetic response to food ingestion.
Meat Sci. Burd NA, Beals JW, Martinez IG, Salvador AF, Skinner SK. Food-first approach to enhance the regulation of post-exercise skeletal muscle protein synthesis and remodeling. Burd NA, McKenna CF, Salvador AF, Paulussen KJM, Moore DR. Dietary protein quantity, quality, and exercise are key to healthy living: a muscle-centric perspective across the lifespan.
Front Nutr. Coffey VG, Moore DR, Burd NA, Rerecich T, Stellingwerff T, Garnham AP, et al. Nutrient provision increases signalling and protein synthesis in human skeletal muscle after repeated sprints.
Eur J Appl Physiol. Breen L, Philp A, Witard OC, Jackman SR, Selby A, Smith K, et al. The influence of carbohydrate-protein co-ingestion following endurance exercise on myofibrillar and mitochondrial protein synthesis.
Camera DM, West DW, Phillips SM, Rerecich T, Stellingwerff T, Hawley JA, et al. Protein ingestion increases myofibrillar protein synthesis after concurrent exercise. Churchward-Venne TA, Pinckaers PJM, Smeets JSJ, Peeters WM, Zorenc AH, Schierbeek H, et al.
Myofibrillar and mitochondrial protein synthesis rates do not differ in young men following the ingestion of carbohydrate with milk protein, whey, or micellar casein after concurrent resistance- and endurance-type exercise. Roy BD, Fowles JR, Hill R, Tarnopolsky MA.
Macronutrient intake and whole body protein metabolism following resistance exercise. Mazzulla M, Parel JT, Beals JW, Van VS, Abou Sawan S, West DWD, et al.
Endurance exercise attenuates postprandial whole-body leucine balance in trained men. Kato H, Suzuki K, Bannai M, Moore DR. Branched-chain amino acids are the primary limiting amino acids in the diets of endurance-trained men after a bout of prolonged exercise. Protein requirements are elevated in endurance athletes after exercise as determined by the indicator amino acid oxidation method.
Witard OC, Turner JE, Jackman SR, Kies AK, Jeukendrup AE, Bosch JA, et al. High dietary protein restores overreaching induced impairments in leukocyte trafficking and reduces the incidence of upper respiratory tract infection in elite cyclists.
Brain Behav Immun. Williamson E, Kato H, Volterman KA, Suzuki K, Moore DR. The effect of dietary protein on protein metabolism and performance in endurance-trained males. Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, et al. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men.
Balagopal P, Schimke JC, Ades P, Adey D, Nair KS. Age effect on transcript levels and synthesis rate of muscle MHC and response to resistance exercise. Roth SM, Ivey FM, Martel GF, Lemmer JT, Hurlbut DE, Siegel EL, et al.
Muscle size responses to strength training in young and older men and women. J Am Geriatr Soc. Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, et al. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging.
Yang Y, Breen L, Burd NA, Hector AJ, Churchward-Venne TA, Josse AR, et al. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men.
Br J Nutr. Churchward-Venne TA, Holwerda AM, Phillips SM, van Loon LJ. What is the optimal amount of protein to support post-exercise skeletal muscle reconditioning in the older adult?
Holwerda AM, Paulussen KJM, Overkamp M, Goessens JPB, Kramer IF, Wodzig W, et al. Dose-dependent increases in whole-body net protein balance and dietary protein-derived amino acid incorporation into myofibrillar protein during recovery from resistance exercise in older men.
Burd NA, Wall BT, van Loon LJ. The curious case of anabolic resistance: old wives' tales or new fables? Moore DR. Adv Nutr. Churchward-Venne TA, Breen L, Di Donato DM, Hector AJ, Mitchell CJ, Moore DR, et al. Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: a double-blind, randomized trial.
Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men.
Chan AH, D'Souza RF, Beals JW, Zeng N, Prodhan U, Fanning AC, et al. The degree of aminoacidemia after dairy protein ingestion does not modulate the postexercise anabolic response in young men: a randomized controlled trial. Protein blend ingestion following resistance exercise promotes human muscle protein synthesis.
Burke LM, Winter JA, Cameron-Smith D, Enslen M, Farnfield M, Decombaz J. Effect of intake of different dietary protein sources on plasma amino acid profiles at rest and after exercise. Burd NA, Gorissen SH, van Vliet S, Snijders T, van Loon LJ. Differences in postprandial protein handling after beef compared with milk ingestion during postexercise recovery: a randomized controlled trial.
Elliot TA, Cree MG, Sanford AP, Wolfe RR, Tipton KD. Milk ingestion stimulates net muscle protein synthesis following resistance exercise. Abou Sawan S, van Vliet S, Parel JT, Beals JW, Mazzulla M, West DWD, et al. Translocation and protein complex co-localization of mTOR is associated with postprandial myofibrillar protein synthesis at rest and after endurance exercise.
van Vliet S, Shy EL, Abou Sawan S, Beals JW, West DW, Skinner SK, et al. Consumption of whole eggs promotes greater stimulation of postexercise muscle protein synthesis than consumption of isonitrogenous amounts of egg whites in young men. Thomas DT, Erdman KA, Burke LM. American college of sports medicine joint position statement.
nutrition and athletic performance. Protein requirements and supplementation in strength sports. Millward DJ. Metabolic demands for amino acids and the human dietary requirement: millward and rRvers revisited.
Morens C, Gaudichon C, Fromentin G, Marsset-Baglieri A, Bensaid A, Larue-Achagiotis C, et al. Daily delivery of dietary nitrogen to the periphery is stable in rats adapted to increased protein intake.
Gorissen SH, Horstman AM, Franssen R, Kouw IW, Wall BT, Burd NA, et al. Habituation to low or high protein intake does not modulate basal or postprandial muscle protein synthesis rates: a randomized trial.
Pasiakos SM, Cao JJ, Margolis LM, Sauter ER, Whigham LD, McClung JP, et al. Effects of high-protein diets on fat-free mass and muscle protein synthesis following weight loss: a randomized controlled trial.
Pasiakos SM, Vislocky LM, Carbone JW, Altieri N, Konopelski K, Freake HC, et al. Acute energy deprivation affects skeletal muscle protein synthesis and associated intracellular signaling proteins in physically active adults.
Camera DM, West DW, Burd NA, Phillips SM, Garnham AP, Hawley JA, et al. Low muscle glycogen concentration does not suppress the anabolic response to resistance exercise.
Hector AJ, McGlory C, Damas F, Mazara N, Baker SK, Phillips SM. Pronounced energy restriction with elevated protein intake results in no change in proteolysis and reductions in skeletal muscle protein synthesis that are mitigated by resistance exercise.
Longland TM, Oikawa SY, Mitchell CJ, Devries MC, Phillips SM. Higher compared with lower dietary protein during an energy deficit combined with intense exercise promotes greater lean mass gain and fat mass loss: a randomized trial.
Paddon-Jones D, Westman E, Mattes RD, Wolfe RR, Astrup A, Westerterp-Plantenga W. Protein, weight management, and satiety. Murphy CH, Hector AJ, Phillips SM. Considerations for protein intake in managing weight loss in athletes.
Eur J Sport Sci. Beals JW, Burd NA, Moore DR, van Vliet S. Obesity alters the muscle protein synthetic response to nutrition and exercise.
Beals JW, Sukiennik RA, Nallabelli J, Emmons RS, van Vliet S, Young JR, et al. Anabolic sensitivity of postprandial muscle protein synthesis to the ingestion of a protein-dense food is reduced in overweight and obese young adults.
Beals JW, Skinner SK, McKenna CF, Poozhikunnel EG, Farooqi SA, van Vliet S, et al. Altered anabolic signalling and reduced stimulation of myofibrillar protein synthesis after feeding and resistance exercise in people with obesity.
Kouw IWK, van Dijk JW, Horstman AMH, Kramer IF, Goessens JPB, van Dielen FMH, et al. Basal and postprandial myofibrillar protein synthesis rates do not differ between lean and obese middle-aged men. Smeuninx B, McKendry J, Wilson D, Martin U, Breen L. Age-related anabolic resistance of myofibrillar protein synthesis is exacerbated in obese inactive individuals.
Tang JE, Perco JG, Moore DR, Wilkinson SB, Phillips SM. Resistance training alters the response of fed state mixed muscle protein synthesis in young men.
Mitchell CJ, Churchward-Venne TA, Parise G, Bellamy L, Baker SK, Smith K, et al. Acute post-exercise myofibrillar protein synthesis is not correlated with resistance training-induced muscle hypertrophy in young men.
Wernbom M, Augustsson J, Thomee R. The influence of frequency, intensity, volume and mode of strength training on whole muscle cross-sectional area in humans. Mamerow MM, Mettler JA, English KL, Casperson SL, Arentson-Lantz E, Sheffield-Moore M, et al.
Dietary protein distribution positively influences h muscle protein synthesis in healthy adults. Moore DR, Areta J, Coffey VG, Stellingwerff T, Phillips SM, Burke LM, et al. Daytime pattern of post-exercise protein intake affects whole-body protein turnover in resistance-trained males.
Res PT, Groen B, Pennings B, Beelen M, Wallis GA, Gijsen AP, et al. Protein ingestion before sleep improves postexercise overnight recovery. Morton RW, Murphy KT, McKellar SR, Schoenfeld BJ, Henselmans M, Helms E, et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults.
Br J Sports Med. Keywords: muscle protein synthesis, muscle hypertrophy, resistance training, essential amino acids, dietary protein, lean body mass, recovery. Citation: Moore DR Maximizing Post-exercise Anabolism: The Case for Relative Protein Intakes. Received: 18 July ; Accepted: 23 August ; Published: 10 September Copyright © Moore.
This is an open-access article distributed under the terms of the Creative Commons Attribution License CC BY. The use, distribution or reproduction in other forums is permitted, provided the original author s and the copyright owner s are credited and that the original publication in this journal is cited, in accordance with accepted academic practice.
No use, distribution or reproduction is permitted which does not comply with these terms. Moore, dr. moore utoronto. Nutritional Strategies to Promote Muscle Mass and Function Across Health Span. Export citation EndNote Reference Manager Simple TEXT file BibTex.
Check for updates. REVIEW article. Maximizing Post-exercise Anabolism: The Case for Relative Protein Intakes Daniel R. Regulation of Muscle Protein Synthesis After Exercise by Dietary Amino Acids Since the first observations that skeletal muscle protein turnover is elevated in response to resistance exercise and that exogenous amino acids augment the increase in net protein balance of this tissue 2 , 3 , studies have investigated the nutritional factors that contribute to the optimal enhancement of post-exercise anabolism.
Absolute Protein Intake to Maximize Post-exercise MPS The first study to address the post-exercise ingested protein dose-response required healthy young resistance trained subjects with an average body mass of ~86 kg to perform a bout of heavy bilateral leg-based resistance exercise i.
E99 PubMed Abstract CrossRef Full Text Google Scholar. E PubMed Abstract CrossRef Full Text Google Scholar. x PubMed Abstract CrossRef Full Text Google Scholar. Keywords: muscle protein synthesis, muscle hypertrophy, resistance training, essential amino acids, dietary protein, lean body mass, recovery Citation: Moore DR Maximizing Post-exercise Anabolism: The Case for Relative Protein Intakes.
Edited by: Gareth A. Wallis , University of Birmingham, United Kingdom. Reviewed by: Brandon J. Shad , University of Birmingham, United Kingdom Donny Michael Camera , Swinburne University of Technology, Australia.
This article is part of the Research Topic Nutritional Strategies to Promote Muscle Mass and Function Across Health Span View all 22 Articles. People also looked at. Very few studies have investigated the effects of prolonged periods one week or more of dietary protein manipulation on endurance performance.
The trained cyclists ingested each diet for a 7-day period in a randomized, crossover fashion. Before and following the 7-day diet intervention, a self-paced cycling endurance time trial was conducted as the primary measure of exercise performance.
It should be noted however that a 7-day treatment period is exceedingly brief. It is unknown what the effect of a higher protein diet would be over the course of several weeks or months.
Although the number of investigations is limited, it appears as if increasing protein intakes above recommended intakes does not enhance endurance performance [ 2 , 4 , 5 ]. In addition to these studies that spanned one to three weeks, several acute-response single feeding and exercise sessions studies exist, during which protein was added to a carbohydrate beverage prior to or during endurance exercise.
Similarly, most of these interventions also reported no added improvements in endurance performance when protein was added to a carbohydrate beverage as compared to carbohydrate alone [ 6 , 7 , 8 , 9 ].
An important research design note, however, is that those studies which reported improvements in endurance performance when protein was added to a carbohydrate beverage before and during exercise all used a time-to-exhaustion test [ 10 , 11 , 12 ].
When specifically interested in performance outcomes, a time trial is preferred as it better mimics competition and pacing demands. In conclusion, added protein does not appear to improve endurance performance when given for several days, weeks, or immediately prior to and during endurance exercise.
For these reasons, it seems prudent to recommend for endurance athletes to ingest approximately 0. Another important consideration relates to the impact of ingesting protein along with carbohydrate on rates of protein synthesis and balance during prolonged bouts of endurance exercise.
Beelen and colleagues [ 14 ] determined that adding protein to carbohydrate consumption throughout a prolonged bout of endurance exercise promotes a higher whole body net protein balance, but the added protein does not exert any further impact on rates of MPS.
While performance outcomes were not measured, these results shift the focus of nutrient ingestion during prolonged bouts of endurance exercise to the ingestion of carbohydrate. When adequate carbohydrate is delivered, adding protein to carbohydrate does not appear to improve endurance performance over the course of a few days or weeks.
Adding protein during or after an intensive bout of endurance exercise may suppress the rise in plasma proteins linked to myofibrillar damage and reduce feelings of muscle soreness. There are relatively few investigations on the effects of protein supplementation on endurance performance.
The extent to which protein supplementation, in conjunction with resistance training, enhances maximal strength is contingent upon many factors, including:. Co-ingestion of additional dietary ingredients that may favorably impact strength e. creatine, HMB. Taking each of these variables into consideration, the effects of supplemental protein consumption has on maximal strength enhancement are varied, with a majority of the investigations reporting no benefit [ 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 ] and a few reporting improvements in maximal strength [ 26 , 27 , 28 , 29 ].
With limited exceptions [ 16 , 18 , 23 , 27 ], most of the studies utilized young, healthy, untrained males as participants. In one investigation examining college football athletes supplementing with a proprietary milk protein supplement two servings of 42 g per day for 12 weeks, a These differences were statistically significant.
When females were the only sex investigated, the outcomes consistently indicated that supplemental protein does not appear to enhance maximal strength at magnitudes that reach statistical significance.
Hida et al. An important note for this study is that 15 g of egg protein is considered by many to be a sub-optimal dose [ 31 ]. However, others have advocated that the total daily intake of protein might be as important or more important [ 32 ].
In another study, Josse et al. In summary, while research investigating the addition of supplemental protein to a diet with adequate energy and nutrient intakes is inconclusive in regards to stimulating strength gains in conjunction with a resistance-training program to a statistically significant degree, greater protein intakes that are achieved from both dietary and supplemental sources do appear to have some advantage.
Hoffman and colleagues [ 29 ] reported that in athletes consuming daily protein intakes above 2. Cermak and colleagues [ 35 ] pooled the outcomes from 22 separate clinical trials to yield subjects in their statistical analysis and found that protein supplementation with resistance training resulted in a A similar conclusion was also drawn by Pasiakos et al.
Results from many single investigations indicate that in both men and women protein supplementation exerts a small to modest impact on strength development. Pooled results of multiple studies using meta-analytic and other systematic approaches consistently indicate that protein supplementation 15 to 25 g over 4 to 21 weeks exerts a positive impact on performance.
Andersen et al. When the blend of milk proteins was provided, significantly greater increases in fat-free mass, muscle cross-sectional area in both the Type I and Type II muscle fibers occurred when compared to changes seen with carbohydrate consumption.
Collectively, a meta-analysis by Cermak and colleagues [ 35 ] reported a mean increase in fat-free mass of 0. Other reviews by Tipton, Phillips and Pasiakos, respectively, [ 36 , 38 , 39 ] provide further support that protein supplementation 15—25 g over 4—14 weeks augments lean mass accretion when combined with completion of a resistance training program.
Beyond accretion of fat-free mass, increasing daily protein intake through a combination of food and supplementation to levels above the recommended daily allowance RDA RDA 0.
The majority of this work has been conducted using overweight and obese individuals who were prescribed an energy-restricted diet that delivered a greater ratio of protein relative to carbohydrate.
Greater amounts of fat were lost when higher amounts of protein were ingested, but even greater amounts of fat loss occurred when the exercise program was added to the high-protein diet group, resulting in significant decreases in body fat.
Each person was randomly assigned to consume a diet that contained either 1× 0. Participants were measured for changes in body weight and body composition.
While the greatest body weight loss occurred in the 1× RDA group, this group also lost the highest percentage of fat-free mass and lowest percentage of fat mass.
Collectively, these results indicate that increasing dietary protein can promote favorable adaptations in body composition through the promotion of fat-free mass accretion when combined with a hyperenergetic diet and a heavy resistance training program and can also promote the loss of fat mass when higher intakes of daily protein × the RDA are combined with an exercise program and a hypoenergetic diet.
When combined with a hyperenergetic diet and a heavy resistance-training program, protein supplementation may promote increases in skeletal muscle cross-sectional area and lean body mass.
When combined with a resistance-training program and a hypoenergetic diet, an elevated daily intake of protein 2 — 3× the RDA can promote greater losses of fat mass and greater overall improvements in body composition.
In the absence of feeding, muscle protein balance remains negative in response to an acute bout of resistance exercise [ 48 ]. Tipton et al. Later, Burd et al. Subsequently, these conclusions were supported by Borsheim [ 52 ] and Volpi [ 53 ].
The study by Borsheim also documented a dose-response outcome characterized by a near doubling of net protein balance in response to a three to six gram dose of the EAAs [ 52 ]. Building on this work, Tipton et al.
These findings formed the theoretical concept of protein timing for resistance exercise that has since been transferred to not only other short-duration, high-intensity activities [ 56 ] but also endurance-based sports [ 57 ] and subsequent performance outcomes [ 58 ].
The strategic consumption of nutrition, namely protein or various forms of amino acids, in the hours immediately before and during exercise i. While earlier investigations reported positive effects from consumption of amino acids [ 37 , 46 , 61 ], it is now clear that intact protein supplements such as egg, whey, casein, beef, soy and even whole milk can evoke an anabolic response that can be similar or greater in magnitude to free form amino acids, assuming ingestion of equal EAA amounts [ 62 , 63 , 64 ].
For instance, whey protein ingested close to resistance exercise, promotes a higher activation phosphorylation of mTOR a key signaling protein found in myocytes that is linked to the synthesis of muscle proteins and its downstream mRNA translational signaling proteins i.
Moreover, it was found that the increased mTOR signaling corresponded with significantly greater muscle hypertrophy after 10 weeks of training [ 65 ].
However, the hypertrophic differences between protein consumption and a non-caloric placebo appeared to plateau by week 21, despite a persistently greater activation of this molecular signaling pathway from supplementation.
Results from other research groups [ 56 , 57 , 58 , 66 ] show that timing of protein near ± 2 h aerobic and anaerobic exercise training appears to provide a greater activation of the molecular signalling pathways that regulate myofibrillar and mitochondrial protein synthesis as well as glycogen synthesis.
It is widely reported that protein consumption directly after resistance exercise is an effective way to acutely promote a positive muscle protein balance [ 31 , 55 , 67 ], which if repeated over time should translate into a net gain or hypertrophy of muscle [ 68 ]. Pennings and colleagues [ 69 ] reported an increase in both the delivery and incorporation of dietary proteins into the skeletal muscle of young and older adults when protein was ingested shortly after completion of exercise.
These findings and others add to the theoretical basis for consumption of post-protein sooner rather than later after exercise, since post workout MPS rates peak within three hours and remain elevated for an additional 24—72 h [ 50 , 70 ].
This extended time frame also provides a rationale for both immediate and sustained i. These temporal considerations would also capture the peak elevation in signalling proteins shown to be pivotal for increasing the initiation of translation of muscle proteins, which for the most part appears to peak between 30 and 60 min after exercise [ 71 ].
However, these differences may be related to the type of protein used between the studies. The studies showing positive effects of protein timing used milk proteins, whereas the latter study used a collagen based protein supplement. While a great deal of work has focused on post-exercise protein ingestion, other studies have suggested that pre-exercise and even intra-exercise ingestion may also support favorable changes in MPS and muscle protein breakdown [ 14 , 54 , 75 , 76 , 77 , 78 ].
Initially, Tipton and colleagues [ 54 ] directly compared immediate pre-exercise and immediate post-exercise ingestion of a mixture of carbohydrate 35 g and EAAs 6 g combination on changes in MPS. They reported that pre-exercise ingestion promoted higher rates of MPS while also demonstrating that nutrient ingestion prior to exercise increased nutrient delivery to a much greater extent than other immediate or one hour post-exercise time points.
These results were later challenged by Fujita in who employed an identical study design with a different tracer incorporation approach and concluded there was no difference between pre- or post-exercise ingestion [ 75 ].
Subsequent work by Tipton [ 79 ] also found that similar elevated rates of MPS were achieved when ingesting 20 g of a whey protein isolate immediately before or immediately after resistance exercise.
At this point, whether any particular time of protein ingestion confers any unique advantage over other time points throughout a h day to improve strength and hypertrophy has yet to be adequately investigated. To date, although a substantial amount of literature discusses this concept [ 60 , 80 ], a limited number of training studies have assessed whether immediate pre- and post-exercise protein consumption provides unique advantages compared to other time points [ 72 , 73 , 81 ].
Each study differed in population, training program, environment and nutrition utilized, with each reporting a different result. What is becoming clear is that the subject population, nutrition habits, dosing protocols on both training and non-training days, energy and macronutrient intake, as well as the exercise bout or training program itself should be carefully considered alongside the results.
In particular, the daily amount of protein intake seems to operate as a key consideration because the benefits of protein timing in relation to the peri-workout period seem to be lessened for people who are already ingesting appropriate amounts of protein e.
A literature review by Aragon and Schoenfeld [ 83 ] determined that while compelling evidence exists showing muscle is sensitized to protein ingestion following training, the increased sensitivity to protein ingestion might be greatest in the first five to six hours following exercise.
Thus, the importance of timing may be largely dependent on when a pre-workout meal was consumed, the size and composition of that meal and the total daily protein in the diet.
In this respect, a pre-exercise meal will provide amino acids during and after exercise and therefore it stands to reason there is less need for immediate post-exercise protein ingestion if a pre-exercise meal is consumed less than five hours before the anticipated completion of a workout.
A meta-analysis by Schoenfeld et al. The authors concluded that total protein intake was the strongest predictor of muscular hypertrophy and that protein timing likely influences hypertrophy to a lesser degree. However, the conclusions from this meta-analysis may be questioned because the majority of the studies analyzed were not protein timing studies but rather protein supplementation studies.
In that respect, the meta-analysis provides evidence that protein supplementation i. While a strong rationale remains to support the concept that the hours immediately before or after resistance exercise represents an opportune time to deliver key nutrients that will drive the accretion of fat-free mass and possibly other favorable adaptations, the majority of available literature suggests that other factors may indeed be operating to a similar degree that ultimately impact the observed adaptations.
In this respect, a key variable that must be accounted for is the absolute need for energy and protein required to appropriately set the body up to accumulate fat-free mass.
Thus, the most practical recommendation is to have athletes consume a meal during the post-workout or pre-workout time period since it may either help or have a neutral effect.
In younger subjects, the ingestion of 20—30 g of any high biological value protein before or after resistance exercise appears to be sufficient to maximally stimulate MPS [ 21 , 64 ]. More recently, Macnaughton and colleagues [ 85 ] reported that 40 g of whey protein ingestion significantly increased the MPS responses compared to a 20 g feeding after an acute bout of whole-body resistance exercise, and that the absolute protein dose may operate as a more important consideration than providing a protein dose that is normalized to lean mass.
Free form EAAs, soy, milk, whey, caseinate, and other protein hydrolysates are all capable of activating MPS [ 86 ]. However, maximal stimulation of MPS, which results in higher net muscle protein accretion, is the product of the total amount of EAA in circulation as well as the pattern and appearance rate of aminoacidemia that modulates the MPS response [ 86 ].
Recent work has clarified that whey protein provides a distinct advantage over other protein sources including soy considered another fast absorbing protein and casein a slower acting protein source on acute stimulation of MPS [ 86 , 87 ].
Importantly, an elegant study by West and investigators [ 87 ] sought to match the delivery of EAAs in feeding patterns that replicated how whey and casein are digested. The authors reported that a 25 g dose of whey protein that promoted rapid aminoacidemia further enhanced MPS and anabolic signaling when compared to an identical total dose of whey protein when delivered as ten separate 2.
The advantages of whey protein are important to consider, particularly as all three sources rank similarly in assessments of protein quality [ 88 ]. In addition to soy, other plant sources e. have garnered interest as potential protein sources to consider. Unfortunately, research that examines the ability of these protein sources to modulate exercise performance and training adaptations is limited at this time.
The investigators concluded that gains in strength, muscle thickness and body composition were similar between the two protein groups, suggesting that rice protein may be a suitable alternative to whey protein at promoting resistance training adaptations.
Furthermore, differences in absorption kinetics, and the subsequent impact on muscle protein metabolism appear to extend beyond the degree of hydrolysis and amino acid profiles [ 69 , 86 , 90 , 91 , 92 , 92 ]. For instance, unlike soy more of the EAAs from whey proteins hydrolysates and isolates survive splanchnic uptake and travel to the periphery to activate a higher net gain in muscle [ 86 ].
These characteristics yield a high concentration of amino acids in the blood aminoacidemia [ 69 , 87 ] that facilitates greater activation of MPS and net muscle protein accretion, in direct comparison to other protein choices [ 50 , 69 , 91 ].
The addition of creatine to whey protein supplementation appears to further augment these adaptations [ 27 , 72 , 95 ]; however, an optimal timing strategy for this combination remains unclear. The timing of protein-rich meals consumed throughout a day has the potential to influence adaptations to exercise.
Using similar methods, other studies over recent decades [ 53 , 62 , 87 , 91 , 96 , 97 , 98 , 99 , ] have established the following:. The anabolic response to feeding is pronounced but transient. During the post-prandial phase 1—4 h after a meal MPS is elevated, resulting in a positive muscle protein balance.
In contrast, MPS rates are lower in a fasted state and muscle protein balance is negative. Protein accretion only occurs in the fed state. The concentration of EAA in the blood plasma regulates protein synthesis rates within muscle at rest and post exercise.
More recent work has established that protein-carbohydrate supplementation after strenuous endurance exercise stimulates contractile MPS via similar signaling pathways as resistance exercise [ 56 , 57 ].
That is, the consumption of a protein-containing meal up to 24 h after a single bout of resistance exercise results in a higher net stimulation of MPS and protein accretion than the same meal consumed after 24 h of inactivity [ 50 ].
The effect of insulin on MPS is dependent on its ability to increase amino acid availability, which does not occur when insulin is systematically increased e.
Taken together, these results seem to indicate that post-workout carbohydrate supplementation offers very little contribution from a muscle development standpoint provided adequate protein is consumed. Importantly, these results are not to be interpreted to mean that carbohydrate administration offers no potential effect for an athlete engaging in moderate to high volumes of training, but rather that benefits derived from carbohydrate administration appear to more favorably impact aspects of muscle glycogen recovery as opposed to stimulating muscle protein accretion.
Eating before sleep has long been controversial [ , , ]. However, a methodological consideration in the original studies such as the population used, time of feeding, and size of the pre-sleep meal confounds firm conclusions about benefits or drawbacks.
Results from several investigations indicate that 30—40 g of casein protein ingested min prior to sleep [ ] or via nasogastric tubing [ ] increased overnight MPS in both young and old men, respectively.
Likewise, in an acute setting, 30 g of whey protein, 30 g of casein protein, and 33 g of carbohydrate consumed min prior to sleep resulted in an elevated morning resting metabolic rate in young fit men compared to a non-caloric placebo [ ].
Interestingly, Madzima et al. This infers that casein protein consumed pre-sleep maintains overnight lipolysis and fat oxidation. This finding was further supported by Kinsey et al.
Similar to Madzima et al. Interestingly, the pre-sleep protein and carbohydrate ingestion resulted in elevated insulin concentrations the next morning and decreased hunger in this overweight population.
Of note, it appears that exercise training completely ameliorates any rise in insulin when eating at night before sleep [ ], while the combination of pre-sleep protein and exercise has been shown to reduce blood pressure and arterial stiffness in young obese women with prehypertension and hypertension [ ].
In athletes, evening chocolate milk consumption has also been shown to influence carbohydrate metabolism in the morning, but not running performance [ ]. In addition, data supports that exercise performed in the evening augments the overnight MPS response in both younger and older men [ , , ].
To date, only a few studies involving nighttime protein ingestion have been carried out for longer than four weeks. Snijders et al. The group receiving the protein-centric supplement each night before sleep had greater improvements in muscle mass and strength over the week study. Of note, this study was non-nitrogen balanced and the protein group received approximately 1.
More recently, in a study in which total protein intake was equal, Antonio et al. They examined the effects on body composition and performance [ ].
All subjects maintained their usual exercise program. The authors reported no differences in body composition or performance between the morning and evening casein supplementation groups. However, it is worth noting that, although not statistically significant, the morning group added 0.
Although this finding was not statistically significant, it supports data from Burk et al. It should be noted that the subjects in the Burk et al. study were resistance training.
A retrospective epidemiological study by Buckner et al. Thus, it appears that protein consumption in the evening before sleep might be an underutilized time to take advantage of a protein feeding opportunity that can potentially improve body composition and performance.
In addition to direct assessments of timed administration of nutrients, other studies have explored questions that center upon the pattern of when certain protein-containing meals are consumed. Paddon-Jones et al. In this study, participants were given an EAA supplement three times a day for 28 days.
Results indicated that acute stimulation of MPS provided by the supplement on day 1 resulted in a net gain of ~7. When extrapolated over the entire day study, the predicted change in muscle mass corresponded to the actual change in muscle mass ~ g measured by dual-energy x-ray absorptiometry DEXA [ 97 ].
While these findings are important, it is vital to highlight that this study incorporated a bed rest model with no acute exercise stimulus while other work by Mitchell et al. Interestingly, supplementation with 15 g of EAAs and 30 g of carbohydrate produced a greater anabolic effect increase in net phenylalanine balance than the ingestion of a mixed macronutrient meal, despite the fact that both interventions contained a similar dose of EAAs [ 96 ].
Most importantly, the consumption of the supplement did not interfere with the normal anabolic response to the meal consumed three hours later [ 96 ]. Areta et al. The researchers compared the anabolic responses of three different patterns of ingestion a total of 80 g of protein throughout a h recovery period after resistance exercise.
Using a group of healthy young adult males, the protein feeding strategies consisted of small pulsed 8 × 10 g , intermediate 4 × 20 g , or bolus 2 × 40 g administration of whey protein over the h measurement window.
Results showed that the intermediate dosing 4 × 20 g was superior for stimulating MPS for the h experimental period. Specifically, the rates of myofibrillar protein synthesis were optimized throughout the day of recovery by the consumption of 20 g protein every three hours compared to large 2 × 40 g , less frequent servings or smaller but more frequent 8 × 10 g patterns of protein intake [ 67 ].
Previously, the effect of various protein feeding strategies on skeletal MPS during an entire day was unknown. This study provided novel information demonstrating that the regulation of MPS can be modulated by the timing and distribution of protein over 12 h after a single bout of resistance exercise.
However, it should be noted that an 80 g dose of protein over a h period is quite low. The logical next step for researchers is to extend these findings into longitudinal training studies to see if these patterns can significantly affect resistance-training adaptations.
Indeed, published studies by Arnal [ ] and Tinsley [ ] have all made some attempt to examine the impact of adjusting the pattern of protein consumption across the day in combination with various forms of exercise.
Collective results from these studies are mixed. Thus, future studies in young adults should be designed to compare a balanced vs. skewed distribution pattern of daily protein intake on the daytime stimulation of MPS under resting and post-exercise conditions and training-induced changes in muscle mass, while taking into consideration the established optimal dose of protein contained in a single serving for young adults.
Without more conclusive evidence spanning several weeks, it seems pragmatic to recommend the consumption of at least g of protein ~0.
In the absence of feeding and in response to resistance exercise, muscle protein balance remains negative.
Skeletal muscle is sensitized to the effects of protein and amino acids for up to 24 h after completion of a bout of resistance exercise. A protein dose of 20—40 g of protein 10—12 g of EAAs, 1—3 g of leucine stimulates MPS, which can help to promote a positive nitrogen balance.
The EAAs are critically needed for achieving maximal rates of MPS making high-quality, protein sources that are rich in EAAs and leucine the preferred sources of protein. Studies have suggested that pre-exercise feedings of amino acids in combination with carbohydrate can achieve maximal rates of MPS, but protein and amino acid feedings during this time are not clearly documented to increase exercise performance.
Total protein and calorie intake appears to be the most important consideration when it comes to promoting positive adaptations to resistance training, and the impact of timing strategies immediately before or immediately after to heighten these adaptations in non-athletic populations appears to be minimal.
Proteins provide the building blocks of all tissues via their constituent amino acids. Athletes consume dietary protein to repair and rebuild skeletal muscle and connective tissues following intense training bouts or athletic events.
A report in by Phillips [ ] summarized the findings surrounding protein requirements in resistance-trained athletes. Using a regression approach, he concluded that a protein intake of 1. A key consideration regarding these recommended values is that all generated data were obtained using the nitrogen balance technique, which is known to underestimate protein requirements.
Interestingly, two of the included papers had prescribed protein intakes of 2. All data points from these two studies also had the highest levels of positive nitrogen balance.
For an athlete seeking to ensure an anabolic environment, higher daily protein intakes might be needed. Another challenge that underpins the ability to universally and successfully recommend daily protein amounts are factors related to the volume of the exercise program, age, body composition and training status of the athlete; as well as the total energy intake in the diet, particularly for athletes who desire to lose fat and are restricting calories to accomplish this goal [ ].
For these reasons, and due to an increase of published studies in areas related to optimal protein dosing, timing and composition, protein needs are being recommended within this position stand on a per meal basis. For example, Moore [ 31 ] found that muscle and albumin protein synthesis was optimized at approximately 20 g of egg protein at rest.
Witard et al. Furthermore, while results from these studies offer indications of what optimal absolute dosing amounts may be, Phillips [ ] concluded that a relative dose of 0. Once a total daily target protein intake has been achieved, the frequency and pattern with which optimal doses are ingested may serve as a key determinant of overall changes in protein synthetic rates.
Research indicates that rates of MPS rapidly rise to peak levels within 30 min of protein ingestion and are maintained for up to three hours before rapidly beginning to lower to basal rates of MPS even though amino acids are still elevated in the blood [ ]. Using an oral ingestion model of 48 g of whey protein in healthy young men, rates of myofibrillar protein synthesis increased three-fold within 45—90 min before slowly declining to basal rates of MPS all while plasma concentration of EAAs remained significantly elevated [ ].
While largely unexplored in a human model, these authors relied upon an animal model and were able to reinstate increases in MPS using the consumption of leucine and carbohydrate min after ingestion of the first meal.
As such, it is suggested that individuals attempting to restrict caloric intake should consume three to four whole meals consisting of 20—40 g of protein per meal. While this recommendation stems primarily from initial work that indicated protein doses of 20—40 g favorably promote increased rates of MPS [ 31 , , ], Kim and colleagues [ ] recently reported that a 70 g dose of protein promoted a more favorable net balance of protein when compared to a 40 g dose due to a stronger attenuation of rates of muscle protein breakdown.
For those attempting to increase their calories, we suggest consuming small snacks between meals consisting of both a complete protein and a carbohydrate source. This contention is supported by research from Paddon-Jones et al.
These researchers compared three cal mixed macronutrient meals to three cal meals combined with three cal amino acid-carbohydrate snacks between meals. Additionally, using a protein distribution pattern of 20—25 g doses every three hours in response to a single bout of lower body resistance exercise appears to promote the greatest increase in MPS rates and phosphorylation of key intramuscular proteins linked to muscle hypertrophy [ ].
This simple addition could provide benefits for individuals looking to increase muscle mass and improve body composition in general while also striving to maintain or improve health and performance. The current RDA for protein is 0.
While previous recommendations have suggested a daily intake of 1. Daily and per dose needs are combinations of many factors including volume of exercise, age, body composition, total energy intake and training status of the athlete.
Daily intakes of 1. Even higher amounts ~70 g appear to be necessary to promote attenuation of muscle protein breakdown. Pacing or spreading these feeding episodes approximately three hours apart has been consistently reported to promote sustained, increased levels of MPS and performance benefits.
There are 20 total amino acids, comprised of 9 EAAs and 11 non-essential amino acids NEAAs. EAAs cannot be produced in the body and therefore must be consumed in the diet. Several methods exist to determine protein quality such as Chemical Score, Protein Efficiency Ratio, Biological Value, Protein Digestibility-Corrected Amino Acid Score PDCAAS and most recently, the Indicator Amino Acid Oxidation IAAO technique.
Ultimately, in vivo protein quality is typically defined as how effective a protein is at stimulating MPS and promoting muscle hypertrophy [ ]. Overall, research has shown that products containing animal and dairy-based proteins contain the highest percentage of EAAs and result in greater hypertrophy and protein synthesis following resistance training when compared to a vegetarian protein-matched control, which typically lacks one or more EAAs [ 86 , 93 , ].
Several studies, but not all, [ ] have indicated that EAAs alone stimulate protein synthesis in the same magnitude as a whole protein with the same EAA content [ 98 ].
For example, Borsheim et al. Moreover, Paddon-Jones and colleagues [ 96 ] found that a cal supplement containing 15 g of EAAs stimulated greater rates of protein synthesis than an cal meal with the same EAA content from a whole protein source.
While important, the impact of a larger meal on changes in circulation and the subsequent delivery of the relevant amino acids to the muscle might operate as important considerations when interpreting this data. In contrast, Katsanos and colleagues [ ] had 15 elderly subjects consume either 15 g of whey protein or individual doses of the essential and nonessential amino acids that were identical to what is found in a g whey protein dose on separate occasions.
Whey protein ingestion significantly increased leg phenylalanine balance, an index of muscle protein accrual, while EAA and NEAA ingestion exerted no significant impact on leg phenylalanine balance.
This study, and the results reported by others [ ] have led to the suggestion that an approximate 10 g dose of EAAs might serve as an optimal dose to maximally stimulate MPS and that intact protein feedings of appropriate amounts as opposed to free amino acids to elderly individuals may stimulate greater improvements in leg muscle protein accrual.
Based on this research, scientists have also attempted to determine which of the EAAs are primarily responsible for modulating protein balance. The three branched-chain amino acids BCAAs , leucine, isoleucine, and valine are unique among the EAAs for their roles in protein metabolism [ ], neural function [ , , ], and blood glucose and insulin regulation [ ].
Additionally, enzymes responsible for the degradation of BCAAs operate in a rate-limiting fashion and are found in low levels in splanchnic tissues [ ]. Thus, orally ingested BCAAs appear rapidly in the bloodstream and expose muscle to high concentrations ultimately making them key components of skeletal MPS [ ].
Furthermore, Wilson and colleagues [ ] have recently demonstrated, in an animal model, that leucine ingestion alone and with carbohydrate consumed between meals min post-consumption extends protein synthesis by increasing the energy status of the muscle fiber. Multiple human studies have supported the contention that leucine drives protein synthesis [ , ].
Moreover, this response may occur in a dose-dependent fashion, plateauing at approximately two g at rest [ 31 , ], and increasing up to 3. However, it is important to realize that the duration of protein synthesis after resistance exercise appears to be limited by both the signal leucine concentrations , ATP status, as well as the availability of substrate i.
As such, increasing leucine concentration may stimulate increases in muscle protein, but a higher total dose of all EAAs as free form amino acids or intact protein sources seems to be most suited for sustaining the increased rates of MPS [ ].
It is well known that exercise improves net muscle protein balance and in the absence of protein feeding, this balance becomes more negative. When combined with protein feeding, net muscle protein balance after exercise becomes positive [ ].
Norton and Layman [ ] proposed that consumption of leucine, could turn a negative protein balance to a positive balance following an intense exercise bout by prolonging the MPS response to feeding. In support, the ingestion of a protein or essential amino acid complex that contains sufficient amounts of leucine has been shown to shift protein balance to a net positive state after intense exercise training [ 46 , ].
Even though leucine has been demonstrated to independently stimulate protein synthesis, it is important to recognize that supplementation should not be with just leucine alone. For instance, Wilson et al. In summary, athletes should focus on consuming adequate leucine content in each of their meals through selection of high-quality protein sources [ ].
Protein sources containing higher levels of the EAAs are considered to be higher quality sources of protein. The body uses 20 amino acids to make proteins, seven of which are essential nine conditionally , requiring their ingestion to meet daily needs.
EAAs appear to be uniquely responsible for increasing MPS with doses ranging from 6 to 15 g all exerting stimulatory effects. In addition, doses of approximately one to three g of leucine per meal appear to be needed to stimulate protein translation machinery. The BCAAs i. However, the extent to which these changes are aligned with changes in MPS remains to be fully explored.
While greater doses of leucine have been shown to independently stimulate increases in protein synthesis, a balanced consumption of the EAAs promotes the greatest increases. Milk proteins have undergone extensive research related to their potential roles in augmenting adaptations from exercise training [ 86 , 93 ].
For example, consuming milk following exercise has been demonstrated to accelerate recovery from muscle damaging exercise [ ], increase glycogen replenishment [ ], improve hydration status [ , ], and improve protein balance to favor synthesis [ 86 , 93 ], ultimately resulting in increased gains in both neuromuscular strength and skeletal muscle hypertrophy [ 93 ].
Moreover, milk protein contains the highest score on the PDCAAS rating system, and in general contains the greatest density of leucine [ ]. Milk can be fractionated into two protein classes, casein and whey. While both are high in quality, the two differ in the rate at which they digest as well as the impact they have on protein metabolism [ , , ].
Whey protein is water soluble, mixes easily, and is rapidly digested [ ]. In contrast, casein is water insoluble, coagulates in the gut and is digested more slowly than whey protein [ ]. Casein also has intrinsic properties such as opioid peptides, which effectively slow gastric motility [ ].
Original research investigating the effects of digestion rate was conducted by Boirie, Dangin and colleagues [ , , ].
These researchers gave a 30 g bolus of whey protein and a 43 g bolus of casein protein to subjects on separate occasions and measured amino acid levels for several hours after ingestion.
They reported that the whey protein condition displayed robust hyperaminoacidemia min after administration. However, by min, amino acid concentrations had returned to baseline.
In contrast, the casein condition resulted in a slow increase in amino acid concentrations, which remained elevated above baseline after min. Over the study duration, casein produced a greater whole body leucine balance than the whey protein condition, leading the researcher to suggest that prolonged, moderate hyperaminoacidemia is more effective at stimulating increases in whole body protein anabolism than a robust, short lasting hyperaminoacidemia.
While this research appears to support the efficacy of slower digesting proteins, subsequent work has questioned its validity in athletes.
The first major criticism is that Boire and colleagues investigated whole body non-muscle and muscle protein balance instead of skeletal myofibrillar MPS. These findings suggest that changes in whole body protein turnover may poorly reflect the level of skeletal muscle protein metabolism that may be taking place.
Trommelen and investigators [ ] examined 24 young men ingesting 30 g of casein protein with or without completion of a single bout of resistance exercise, and concluded that rates of MPS were increased, but whole-body protein synthesis rates were not impacted.
More recently, Tang and colleagues [ 86 ] investigated the effects of administering 22 g of hydrolyzed whey isolate and micellar casein 10 g of EAAs at both rest and following a single bout of resistance training in young males.
Moreover, these researchers reported that whey protein ingestion stimulated greater MPS at both rest and following exercise when compared to casein.
In comparison to the control group, both whey and casein significantly increased leucine balance, but no differences were found between the two protein sources for amino acid uptake and muscle protein balance. Additional research has also demonstrated that 10 weeks of whey protein supplementation in trained bodybuilders resulted in greater gains in lean mass 5.
These findings suggest that the faster-digesting whey proteins may be more beneficial for skeletal muscle adaptations than the slower digesting casein. Skeletal muscle glycogen stores are a critical element to both prolonged and high-intensity exercise.
In skeletal muscle, glycogen synthase activity is considered one of the key regulatory factors for glycogen synthesis. Research has demonstrated that the addition of protein in the form of milk and whey protein isolate 0. Further, the addition of protein facilitates repair and recovery of the exercised muscle [ 12 ].
These effects are thought to be related to a greater insulin response following the exercise bout. Intriguingly, it has also been demonstrated that whey protein enhances glycogen synthesis in the liver and skeletal muscle more than casein in an insulin-independent fashion that appears to be due to its capacity to upregulate glycogen synthase activity [ ].
Therefore, the addition of milk protein to a post-workout meal may augment recovery, improve protein balance, and speed glycogen replenishment. While athletes tend to view whey as the ideal protein for skeletal muscle repair and function it also has several health benefits.
In particular, whey protein contains an array of biologically active peptides whose amino acids sequences give them specific signaling effects when liberated in the gut.
Furthermore, whey protein appears to play a role in enhancing lymphatic and immune system responses [ ]. In addition, α-lactalbumin contains an ample supply of tryptophan which increases cognitive performance under stress [ ], improves the quality of sleep [ , ], and may also speed wound healing [ ], properties which could be vital for recovery from combat and contact sporting events.
In addition, lactoferrin is also found in both milk and in whey protein, and has been demonstrated to have antibacterial, antiviral, and antioxidant properties [ ]. Moreover, there is some evidence that whey protein can bind iron and therefore increase its absorption and retention [ ].
Egg protein is often thought of as an ideal protein because its amino acid profile has been used as the standard for comparing other dietary proteins [ ]. Due to their excellent digestibility and amino acid content, eggs are an excellent source of protein for athletes.
While the consumption of eggs has been criticized due to their cholesterol content, a growing body of evidence demonstrates the lack of a relationship between egg consumption and coronary heart disease, making egg-based products more appealing [ ].
One large egg has 75 kcal and 6 g of protein, but only 1. Research using eggs as the protein source for athletic performance and body composition is lacking, perhaps due to less funding opportunities relative to funding for dairy.
Egg protein may be particularly important for athletes, as this protein source has been demonstrated to significantly increase protein synthesis of both skeletal muscle and plasma proteins after resistance exercise at both 20 and 40 g doses.
Leucine oxidation rates were found to increase following the 40 g dose, suggesting that this amount exceeds an optimal dose [ 31 ]. In addition to providing a cost effective, high-quality source of protein rich in leucine 0. Functional foods are defined as foods that, by the presence of physiologically active components, provide a health benefit beyond basic nutrition [ ].
According to the Academy of Nutrition and Dietetics, functional foods should be consumed as part of a varied diet on a regular basis, at effective levels [ ].
Thus, it is essential that athletes select foods that meet protein requirements and also optimize health and prevent decrements in immune function following intense training. Eggs are also rich in choline, a nutrient which may have positive effects on cognitive function [ ].
Moreover, eggs provide an excellent source of the carotenoid-based antioxidants lutein and zeaxanthin [ ]. Also, eggs can be prepared with most meal choices, whether at breakfast, lunch, or dinner.
Such positive properties increase the probability of the athletes adhering to a diet rich in egg protein. Meat proteins are a major staple in the American diet and, depending on the cut of meat, contain varying amounts of fat and cholesterol. Meat proteins are well known to be rich sources of the EAAs [ ].
Beef is a common source of dietary protein and is considered to be of high biological value because it contains the full balance of EAAs in a fraction similar to that found in human skeletal muscle [ ].
A standard serving of Moreover, this 30 g dose of beef protein has been shown to stimulate protein synthesis in both young and elderly subjects [ ]. In addition to its rich content of amino acids, beef and other flesh proteins can serve as important sources of micronutrients such as iron, selenium, vitamins A, B12 and folic acid.
This is a particularly important consideration for pregnant and breastfeeding women. Ultimately, as an essential part of a mixed diet, meat helps to ensure adequate distribution of essential micronutrients and amino acids to the body.
Research has shown that significant differences in skeletal muscle mass and body composition between older men who resistance train and either consume meat-based or lactoovovegetarian diet [ ]. Over a week period, whole-body density, fat-free mass, and whole-body muscle mass as measured by urinary creatinine excretion increased in the meat-sourced diet group but decreased in the lactoovovegetarian diet group.
These results indicate that not only do meat-based diets increase fat-free mass, but also they may specifically increase muscle mass, thus supporting the many benefits of meat-based diets.
A diet high in meat protein in older adults may provide an important resource in reducing the risk of sarcopenia. Positive results have also been seen in elite athletes that consume meat-based proteins, as opposed to vegetarian diets [ ].
For example, carnitine is a molecule that transports long-chain fatty acids into mitochondria for oxidation and is found in high amounts in meat. While evidence is lacking to support an increase in fat oxidation with increased carnitine availability, carnitine has been linked to the sparing of muscle glycogen, and decreases in exercise-induced muscle damage [ ].
Certainly, more research is needed to support these assertions. Creatine is a naturally occurring compound found mainly in muscle. Vegetarians have lower total body creatine stores than omnivores, which demonstrates that regular meat eating has a significant effect on human creatine status [ ].
Moreover, creatine supplementation studies with vegetarians indicate that increased creatine uptake levels do exist in people who practice various forms of vegetarianism [ ]. Sharp and investigators [ ] published the only study known to compare different supplemental powdered forms of animal proteins on adaptations to resistance training such as increases in strength and improvements in body composition.
Forty-one men and women performed a standardized resistance-training program over eight weeks and consumed a daily 46 g dose of either hydrolyzed chicken protein, beef protein isolate, or whey protein concentrate in comparison to a control group.
All groups experienced similar increases in upper and lower-body strength, but all protein-supplemented groups reported significant increases in lean mass and decreases in fat mass.
Meat-based diets have been shown to include additional overall health benefits. Some studies have found that meat, as a protein source, is associated with higher serum levels of IGF-1 [ ], which in turn is related to increased bone mineralization and fewer fractures [ ].
A highly debated topic in nutrition and epidemiology is whether vegetarian diets are a healthier choice than omnivorous diets. One key difference is the fact that vegetarian diets often lack equivalent amounts of protein when compared to omnivorous diets [ ].
However, with proper supplementation and careful nutritional choices, it is possible to have complete proteins in a vegetarian diet.
Generally by consuming high-quality, animal-based products meat, milk, eggs, and cheese an individual will achieve optimal growth as compared to ingesting only plant proteins [ ]. Research has shown that soy is considered a lower quality complete protein.
Hartman et al. They found that the participants that consumed the milk protein increased lean mass and decreased fat mass more than the control and soy groups. Moreover, the soy group was not significantly different from the control group.
Similarly, a study by Tang and colleagues [ 86 ] directly compared the abilities of hydrolyzed whey isolate, soy isolate, and micellar casein to stimulate rates of MPS both at rest and in response to a single bout of lower body resistance training. These authors reported that the ability of soy to stimulate MPS was greater than casein, but less than whey, at rest and in response to an acute resistance exercise stimulus.
While soy is considered a complete protein, it contains lower amounts of BCAAs than bovine milk [ ]. Additionally, research has found that dietary soy phytoestrogens inhibit mTOR expression in skeletal muscle through activation of AMPK [ ].
Thus, not only does soy contain lower amounts of the EAAs and leucine, but soy protein may also be responsible for inhibiting growth factors and protein synthesis via its negative regulation of mTOR.
When considering the multitude of plant sources of protein, soy overwhelmingly has the most research. Limited evidence using wheat protein in older men has suggested that wheat protein stimulates significantly lower levels of MPS when compared to an identical dose 35 g of casein protein, but when this dose is increased nearly two fold 60 g this protein source is able to significantly increase rates of myofibrillar protein synthesis [ ].
As mentioned earlier, a study by Joy and colleagues [ 89 ] in which participants participated in resistance training program for eight weeks while taking identical, high doses of either rice or whey protein, demonstrated that rice protein stimulated similar increases in body composition adaptations to whey protein.
The majority of available science has explored the efficacy of ingesting single protein sources, but evidence continues to mount that combining protein sources may afford additional benefits [ ].
For example, a week resistance training study by Kerksick and colleagues [ 22 ] demonstrated that a combination of whey 40 g and casein 8 g yielded the greatest increase in fat-free mass determined by DEXA when compared to both a combination of 40 g of whey, 5 g of glutamine, and 3 g of BCAAs and a placebo consisting of 48 g of a maltodextrin carbohydrate.
Later, Kerksick et al. Similarly, Hartman and investigators [ 93 ] had 56 healthy young men train for 12 weeks while either ingesting isocaloric and isonitrogenous doses of fat-free milk a blend of whey and casein , soy protein or a carbohydrate placebo and concluded that fat-free milk stimulated the greatest increases in Type I and II muscle fiber area as well as fat-free mass; however, strength outcomes were not affected.
Moreover, Wilkinson and colleagues [ 94 ] demonstrated that ingestion of fat-free milk vs. soy or carbohydrate led to a greater area under the curve for net balance of protein and that the fractional synthesis rate of muscle protein was greatest after milk ingestion.
In , Reidy et al. However, when the entire four-hour measurement period was considered, no difference in MPS rates were found. A follow-up publication from the same clinical trial also reported that ingestion of the protein blend resulted in a positive and prolonged amino acid balance when compared to ingestion of whey protein alone, while post-exercise rates of myofibrillar protein synthesis were similar between the two conditions [ ].
Reidy et al. No differences were found between whey and the whey and soy blend. Some valid criteria exist to compare protein sources and provide an objective method of how to include them in a diet. As previously mentioned, common means of assessing protein quality include Biological Value, Protein Efficiency Ratio, PDCAAS and IAAO.
The derivation of each technique is different with all having distinct advantages and disadvantages. For nearly all populations, ideal methods should be linked to the capacity of the protein to positively affect protein balance in the short term, and facilitate increases and decreases in lean and fat-mass, respectively, over the long term.
To this point, dairy, egg, meat, and plant-based proteins have been discussed. As mentioned previously, initial research by Boirie and Dangin has highlighted the impact of protein digestion rate on net protein balance with the two milk proteins: whey and casein [ , , ].
Subsequent follow-up work has used this premise as a reference point for the digestion rates of other protein sources. Using the criteria of leucine content, Norton and Wilson et al.
Wheat and soy did not stimulate MPS above fasted levels, whereas egg and whey proteins significantly increased MPS rates, with MPS for whey protein being greater than egg protein. MPS responses were closely related to changes in plasma leucine and phosphorylation of 4E—BP1 and S6 K protein signaling molecules.
More importantly, following 2- and weeks of ingestion, it was demonstrated that the leucine content of the meals increased muscle mass and was inversely correlated with body fat.
Tang et al. These findings lead us to conclude that athletes should seek protein sources that are both fast-digesting and high in leucine content to maximally stimulate rates of MPS at rest and following training.
Moreover, in consideration of the various additional attributes that high-quality protein sources deliver, it may be advantageous to consume a combination of higher quality protein sources dairy, egg, and meat sources.
Multiple protein sources are available for an athlete to consider, and each has their own advantages and disadvantages. Protein sources are commonly evaluated based upon the content of amino acids, particularly the EAAs, they provide.
Blends of protein sources might afford a favorable combination of key nutrients such as leucine, EAAs, bioactive peptides, and antioxidants, but more research is needed to determine their ideal composition.
Nutrient density is defined as the amount of a particular nutrient carbohydrate, protein, fat, etc. per unit of energy in a given food. In many situations, the commercial preparation method of foods can affect the actual nutrient density of the resulting food.
When producing milk protein supplements, special preparations must be made to separate the protein sources from the lactose and fat calories in milk. For example, the addition of acid to milk causes the casein to coagulate or collect at the bottom, while the whey is left on the top [ ].
These proteins are then filtered to increase their purity. Filtration methods differ, and there are both benefits and disadvantages to each. Ion exchange exposes a given protein source, such as whey, to hydrochloric acid and sodium hydroxide, thereby producing an electric charge on the proteins that can be used to separate them from lactose and fat [ ].
The advantage of this method is that it is relatively cheap and produces the highest protein concentration [ ]. The disadvantage is that ion exchange filtration typically denatures some of the valuable immune-boosting, anti-carcinogenic peptides found in whey [ ].
Cross-flow microfiltration, and ultra-micro filtration are based on the premise that the molecular weight of whey protein is greater than lactose, and use 1 and 0. As a result, whey protein is trapped in the membranes but the lactose and other components pass through.
The advantage is that these processes do not denature valuable proteins and peptides found in whey, so the protein itself is deemed to be of higher quality [ ]. The main disadvantage is that this filtration process is typically costlier than the ion exchange method.
When consumed whole, proteins are digested through a series of steps beginning with homogenization by chewing, followed by partial digestion by pepsin in the stomach [ ]. Following this, a combination of peptides, proteins, and negligible amounts of single amino acids are released into the small intestine and from there are either partially hydrolyzed into oligopeptides, 2—8 amino acids in length or are fully hydrolyzed into individual amino acids [ ].
Absorption of individual amino acids and various small peptides di, tri, and tetra into the blood occurs inside the small intestine through separate transport mechanisms [ ].
Oftentimes, products contain proteins that have been pre-exposed to specific digestive enzymes causing hydrolysis of the proteins into di, tri, and tetrapeptides. A plethora of studies have investigated the effects of the degree of protein fractionation or degree of hydrolysis on the absorption of amino acids and the subsequent hormonal response [ , , , , , ].
Further, the rate of absorption may lead to a more favorable anabolic hormonal environment [ , , ]. Calbet et al. Each of the nitrogen containing solutions contained 15 g of glucose and 30 g of protein.
Results indicated that peptide hydrolysates produced a faster increase in venous plasma amino acids compared to milk proteins. Further, the peptide hydrolysates produced peak plasma insulin levels that were two- and four-times greater than that evoked by the milk and glucose solutions, respectively, with a correlation of 0.
In a more appropriate comparison, Morifuji et al. However, Calbet et al. The hydrolyzed casein, however, did result in a greater amino acid response than the nonhydrolyzed casein.
Finally, both hydrolyzed groups resulted in greater gastric secretions, as well as greater plasma increases, in glucose-dependent insulinotropic polypeptides [ ]. Buckley and colleagues [ ] found that a ~ 30 g dose of a hydrolyzed whey protein isolate resulted in a more rapid recovery of muscle force-generating capacity following eccentric exercise, compared with a flavored water placebo or a non-hydrolyzed form of the same whey protein isolate.
In agreement with these findings, Cooke et al. Three and seven days after completing the damaging exercise bout, maximal strength levels were higher in the hydrolyzed whey protein group compared to carbohydrate supplementation. Additionally, blood concentrations of muscle damage markers tended to be lower when four ~g doses of a hydrolyzed whey protein isolate were ingested for two weeks following the damaging bout.
Beyond influencing strength recovery after damaging exercise, other benefits of hydrolyzed proteins have been suggested.
For example, Morifuji et al. Furthermore, Lockwood et al. Results indicated that strength and lean body mass LBM increased equally in all groups.
However, fat mass decreased only in the hydrolyzed whey protein group. While more work needs to be completed to fully determine the potential impact of hydrolyzed proteins on strength and body composition changes, this initial study suggests that hydrolyzed whey may be efficacious for decreasing body fat.
Finally, Saunders et al. The authors reported that co-ingestion of a carbohydrate and protein hydrolysate improved time-trial performance late in the exercise protocol and significantly reduced soreness and markers of muscle damage.
Two excellent reviews on the topic of hydrolyzed proteins and their impact on performance and recovery have been published by Van Loon et al. The prevalence of digestive enzymes in sports nutrition products has increased during recent years with many products now containing a combination of proteases and lipases, with the addition of carbohydrates in plant proteins.
Proteases can hydrolyze proteins into various peptide configurations and potentially single amino acids. It appears that digestive enzyme capabilities and production decrease with age [ ], thus increasing the difficulty with which the body can break down and digest large meals.
Digestive enzymes could potentially work to promote optimal digestion by allowing up-regulation of various metabolic enzymes that may be needed to allow for efficient bodily operation.
Further, digestive enzymes have been shown to minimize quality differences between varying protein sources [ ]. Individuals looking to increase plasma peak amino acid concentrations may benefit from hydrolyzed protein sources or protein supplemented with digestive enzymes. However, more work is needed before definitive conclusions can be drawn regarding the efficacy of digestive enzymes.
Despite a plethora of studies demonstrating safety, much concern still exists surrounding the clinical implications of consuming increased amounts of protein, particularly on renal and hepatic health.
The majority of these concerns stem from renal failure patients and educational dogma that has not been rewritten as evidence mounts to the contrary.
Certainly, it is clear that people in renal failure benefit from protein-restricted diets [ ], but extending this pathophysiology to otherwise healthy exercise-trained individuals who are not clinically compromised is inappropriate.
Published reviews on this topic consistently report that an increased intake of protein by competitive athletes and active individuals provides no indication of hepato-renal harm or damage [ , ].
This is supported by a recent commentary [ ] which referenced recent reports from the World Health Organization [ ] where they indicated a lack of evidence linking a high protein diet to renal disease.
Likewise, the panel charged with establishing reference nutrient values for Australia and New Zealand also stated there was no published evidence that elevated intakes of protein exerted any negative impact on kidney function in athletes or in general [ ].
Recently, Antonio and colleagues published a series of original investigations that prescribed extremely high amounts of protein ~3. The first study in had resistance-trained individuals consume an extremely high protein diet 4.
A follow-up investigation [ ] required participants to ingest up to 3. Their next study employed a crossover study design in twelve healthy resistance-trained men in which each participant was tested before and after for body composition as well as blood-markers of health and performance [ ].
In one eight-week block, participants followed their normal habitual diet 2. No changes in body composition were reported, and importantly, no clinical side effects were observed throughout the study. Finally, the same group of authors published a one-year crossover study [ ] in fourteen healthy resistance-trained men.
This investigation showed that the chronic consumption of a high protein diet i. Furthermore, there were no alterations in clinical markers of metabolism and blood lipids.
Multiple review articles indicate that no controlled scientific evidence exists indicating that increased intakes of protein pose any health risks in healthy, exercising individuals. A series of controlled investigations spanning up to one year in duration utilizing protein intakes of up to 2.
In alignment with our previous position stand, it is the position of the International Society of Sports Nutrition that the majority of exercising individuals should consume at minimum approximately 1. The amount is dependent upon the mode and intensity of the exercise, the quality of the protein ingested, as well as the energy and carbohydrate status of the individual.
Concerns that protein intake within this range is unhealthy are unfounded in healthy, exercising individuals. An attempt should be made to consume whole foods that contain high-quality e.
The timing of protein intake in the period encompassing the exercise session may offer several benefits including improved recovery and greater gains in lean body mass. In addition, consuming protein pre-sleep has been shown to increase overnight MPS and next-morning metabolism acutely along with improvements in muscle size and strength over 12 weeks of resistance training.
Intact protein supplements, EAAs and leucine have been shown to be beneficial for the exercising individual by increasing the rates of MPS, decreasing muscle protein degradation, and possibly aiding in recovery from exercise. In summary, increasing protein intake using whole foods as well as high-quality supplemental protein sources can improve the adaptive response to training.
Campbell B, Kreider RB, Ziegenfuss T, La Bounty P, Roberts M, Burke D, et al. International society of sports nutrition position stand: protein and exercise. J Int Soc Sports Nutr. Macdermid PW, Stannard SR. A whey-supplemented, high-protein diet versus a high-carbohydrate diet: effects on endurance cycling performance.
Int J Sport Nutr Exerc Metab. Article CAS PubMed Google Scholar. Burke LM, Hawley JA, Wong SH, Jeukendrup AE. Carbohydrates for training and competition.
J Sports Sci. Article PubMed Google Scholar. Witard OC, Jackman SR, Kies AK, Jeukendrup AE, Tipton KD. Effect of increased dietary protein on tolerance to intensified training.
Med Sci Sports Exerc. D'lugos AC, Luden ND, Faller JM, Akers JD, Mckenzie AI, Saunders MJ.
Sean Protein and muscle protein synthesis in athletes a fact-checker synhtesis researcher with Proteln in sociology, field research, and data analytics. When trying to optimize muscle growth pdotein, protein Pfotein is essential. But anr Obesity and cancer limited by Resveratrol and menopause much protein synthexis can synthesize to repair and grow your muscles. This brings into question the importance of protein timing and amounts and how to best stimulate muscles to grow. Manufacturers of sports supplements and protein powders often claim that their products can increase muscle protein synthesis MPS. While this suggests that sports supplements somehow facilitate changes in muscle mass, the process is more complicated than that. Muscle growth is ultimately achieved with the combination of resistance training and protein intake.
Eindeutig, die schnelle Antwort:)
Mir scheint es die ausgezeichnete Idee. Ich bin mit Ihnen einverstanden.
Welche lustige Frage
Wacker, es ist der einfach ausgezeichnete Gedanke
Er ist unbedingt nicht recht