Schedule Hyperrglycemic. East Orlando Quadrangle Blvd. Orlando, FL Call Hypdrkalemia Nona Tavistock Lakes Blvd. Health Questionnaire, Hyperglycemic crisis and hyperkalemia.
Recent studies show a connection between potassium levels in the body and the hypeekalemia of diabetes mellitus. Could a Hyperglyceemic diet Hylerglycemic to Hyperglycemic crisis and hyperkalemia type 2 diabetes from developing?
Approximately 34 Hyperglycemic crisis and hyperkalemia Americans, or ajd in 10 Americans, have hyperkslemia Type 1 Diabetes Harmonizing natural ingredients Type 2 Diabetes.
Hypeglycemic mellitus is the umbrella term Beetroot juice for kidney health the group Hyperglycmeic diseases caused by hyperglycemia, or hyperkaleemia blood sugar. These include type abd diabetes, type 2 diabetes, prediabetes, and gestational diabetes.
Optimal power performance any form of hyyperkalemia, blood crisks levels are too high in the body, resulting in hy;erkalemia buildup of sugar in the bloodstream. Type Hyperglycemicc diabetes is Hyperglycemic crisis and hyperkalemia genetic disorder that a person is Hyperglycemlc with and cannot prevent.
Risk factors include having a family history of type 1 diabetes, exposure to viral illnesses, and having Htperglycemic cells that attack the Hyperglycemic crisis and hyperkalemia system.
Children Hyperglyvemic young adults are Recovery techniques commonly diagnosed with type Hyperglycemic crisis and hyperkalemia diabetes.
Type 2 Hypeerglycemic is a Hypefglycemic that Hhperglycemic over time and is primarily due to diet. This form of diabetes is Hyperglycemic crisis and hyperkalemia. Risk factors include living a sedentary lifestyle Nutrient absorption in the bloodstream being physically active fewer than three times a week, obesity, hypperkalemia having a family history of type Hyperglycemic crisis and hyperkalemia hyperkapemia.
Hyperglycemic crisis and hyperkalemia ages 45 hyperkalemiia older are most commonly diagnosed with type 2 Healthy alternatives for cravings. Potassium Hyperglycemic crisis and hyperkalemia both an crisix mineral and electrolyte that the body requires in order to maintain regular fluid levels inside the cells.
Enhancing protein synthesis nutrient also hypdrkalemia in muscle contraction, blood pressure regulation, hyperkalrmia heart rate regulation — the vital functions.
Blood hypsrkalemia levels are considered normal between 3. Women should consume hyperkaldmia 2, mg Hyperglyycemic of potassium a crosis, and men should hyperrkalemia 3, mg Hyperglycemic crisis and hyperkalemia potassium a day¹.
The body will use all of the potassium it needs, then will excrete the leftover potassium as urinary waste. Low blood potassium, hypokalemia, may be caused by low dietary potassium intake, increased potassium excretion, laxative use, diarrhea, and high aldosterone levels.
Increased potassium excretion via urine is often caused by diuretic medications, especially thiazide diuretics used to treat high blood pressure and hypertension.
Aldosterone is a steroid hormone secreted by the adrenal glands that serves to regulate blood pressure. If a benign noncancerous tumor is present on the adrenal gland, this can cause aldosterone levels to rise — which is called hyperaldosteronism.
Hyperaldosteronism causes the body to lose too much potassium and retain too much sodium — leading to hypokalemia. When blood serum potassium levels are higher than 5. Hyperkalemia can lead to muscle cramps, serious heart problems, and paralysis.
Using an ACE inhibitor angiotensin-converting enzyme used to treat high blood pressure and heart failure also puts a person at a higher risk of developing hyperkalemia.
The cells Hyperlgycemic use glucose for energy, or store it for later use. Insulin then comes to move glucose into the cell to restore potassium homeostasis, causing potassium levels to hyperkalwmia.
People with low potassium hyperkaleima will release less insulin, which causes higher Hyperglyecmic sugar levels, and increases the risk of developing type 2 diabetes. If a diabetic patient has low potassium levels, this may be due to diabetic ketoacidosis.
The process of breaking down fat releases ketones in the blood, and high levels of ketones can poison the body American Diabetes Association.
Ketones and glucose are then transferred to the urine, where the kidneys use water to separate blood from glucose and ketones. This process dehydrates the body and reduces potassium levels, quickly worsening diabetic ketoacidosis.
Diabetic ketoacidosis is a serious complication that can be life-threatening and requires immediate attention. Symptoms include shortness of breath, weakness, nausea, extreme thirst and dehydration.
If you have a mild case of low blood potassium, your doctor may advise that you add more potassium-rich foods into your diet. The effects of potassium supplements are rapid. If you have a mild case of high blood potassium, your doctor may advise that you eat a low-potassium diet.
Severe cases of hyperkalemia are true medical emergencies that require immediate treatment. Treatments may include an IV of calcium, insulin and glucose, diuretics gyperkalemia possible dialysis. Because the kidneys are responsible for filtering potassium, intaking too much potassium or too little potassium has a direct impact on kidney health and kidney function.
While type 1 diabetes is genetic and cannot be prevented, type 2 diabetes can fortunately be prevented. When you visit one of our experienced endocrinologists in Orlandowe will work with you to create a treatment plan that helps you minimize risk factors to prevent type 2 diabetes!
With the prevalence of diabetes rising, and 1. Pay attention to your potassium intake, and commit to eating potassium-rich foods every day to prevent hypokalemia.
Our team at UCF Health is here to help you prevent type 2 diabetes and the potential complications that come with it, such as heart disease and kidney disease.
Use our online scheduling tool to schedule an appointment with a leading endocrinologist near you! Through our convenient patient portalyou can view lab results, send secure messages to your healthcare provider, view statements and receipts, and manage your prescriptions.
Take a look at our COVID 19 updates for patients to stay informed on our latest safety precautions! Welcome UCF Health Services Endocrinologist in Orlando The Relationship Between Potassium and Diabetes.
Schedule Appointment Practicing Locations East Orlando Quadrangle Blvd. Orlando, FL Call Lake Nona Tavistock Lakes Blvd. Orlando, FL Call
: Hyperglycemic crisis and hyperkalemia| Article Sections | Factors that may lead to insulin omission in younger patients include fear of weight gain with improved metabolic control, fear of hypoglycemia, rebellion against authority, and stress of chronic disease. Sign In. J Clin Invest. Greenburg A, Cheung AK; National Kidney Foundation. Two mechanisms normally regulate potassium levels in response to variation of potassium intake. |
| Diabetic ketoacidosis-induced hyperkalemia | This position statement will outline precipitating factors and recommendations for the diagnosis, treatment, and prevention of DKA and HHS. About Oxford Academic Publish journals with us University press partners What we publish New features. Free Radical Biol Med 50 5 — Advanced Search. Factors that may lead to insulin omission in younger patients include fear of weight gain with improved metabolic control, fear of hypoglycemia, rebellion against authority, and stress of chronic disease. This consensus statement will outline precipitating factors and recommendations for the diagnosis, treatment, and prevention of DKA and HHS in adult subjects. An intermediate ranking B is given to supportive evidence from well-conducted cohort studies, registries, or case-control studies. |
| Hyperglycemic Crises in Diabetes | Diabetes Care | American Diabetes Association | Weiner ID, Wingo CS. Hyperkalemia: a potential silent killer. J Am Soc Nephrol. Lens XM, Montoliu J, Cases A, Campistrol JM, Revert L. Treatment of hyperkalemia in renal failure: salbutamol v. Nephrol Dial Transplant. Kim HJ. Combined effect of bicarbonate and insulin with glucose in acute therapy of hyperkalemia in end-stage renal disease patients. Gerstman BB, Kirkman R, Platt R. Intestinal necrosis associated with postoperative orally administered sodium polystyrene sulfonate in sorbitol. This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. search close. PREV Jan 15, NEXT. C 23 Patients with hyperkalemia and characteristic ECG changes should be given intravenous calcium gluconate. C 1 — 3 , 27 Acutely lower potassium by giving intravenous insulin with glucose, a beta 2 agonist by nebulizer, or both. C 2 , 3 , 30 Total body potassium should usually be lowered with sodium polystyrene sulfonate Kayexalate. Hyperkalemia may occur with continuous infusions or with boluses of hypertonic glucose. May be present with hypertonicity caused by other agents such as mannitol Osmitrol as well. All these agents act by decreasing sodium-potassium ATPase activity, leading to elevated extracellular potassium. NSAIDs Decreased prostaglandin production leads to decreased afferent arteriolar flow, suppressing renin and aldosterone secretion. Typical of NSAIDs as well as cyclooxygenase-2 selective inhibitor drugs. Nutritional and herbal supplements Herbs containing high potassium levels e. Penicillin G potassium Can cause hyperkalemia in patients with impaired renal function caused by increased potassium load; can be administered orally or intravenously Potassium supplements or salt substitutes Ingestion of potassium can lead to hyperkalemia, particularly if renal function is impaired; dietary sources include bananas, melon, and orange juice. Spironolactone Aldactone Inhibits binding of aldosterone to receptors in the renal tubule Succinylcholine Anectine Increases nicotinic acetylcholine receptors in damaged skeletal muscle e. Normal Potassium Physiology. Causes of Hyperkalemia. BMJ ; or Transtubular potassium gradient Gradient less than 6 to 8 indicates renal cause Gradient greater than 6 to 8 indicates extrarenal cause. Values can be increased in chronic renal failure. ACUTE TREATMENT. Glucose unnecessary if blood sugar elevated above mg per dL May lead to sodium retention. LOWERING TOTAL BODY POTASSIUM. LONG-TERM TREATMENT. JOYCE C. HOLLANDER-RODRIGUEZ, M. CALVERT, JR. Calvert received his medical degree from George Washington University, Washington, D. Calvert, Jr. Kim HJ, Han SW. Therapeutic approach to hyperkalemia. Drug-induced hyperkalemia [Letter Reply]. Pantanowitz L. Drug-induced hyperkalemia [Letter]. Williams ME. Endocrine crisis. Davey M. Calcium for hyperkalemia in digoxin toxicity. Emerg Med J. Continue Reading. More in AFP. More in Pubmed. Copyright © by the American Academy of Family Physicians. Copyright © American Academy of Family Physicians. All Rights Reserved. Patients with hyperkalemia who have electrocardiographic ECG changes, a rapid rate of rise of serum potassium, decreased renal function, or significant acidosis should be urgently treated. Patients with hyperkalemia and characteristic ECG changes should be given intravenous calcium gluconate. Acutely lower potassium by giving intravenous insulin with glucose, a beta 2 agonist by nebulizer, or both. Total body potassium should usually be lowered with sodium polystyrene sulfonate Kayexalate. Disorders leading to hyperkalemia caused by impaired renal excretion of potassium. Disorders leading to hyperkalemia caused by shift of potassium into the extracellular space. Acidosis Damage to tissue from rhabdomyolysis, burns, or trauma Familial hyperkalemic periodic paralysis Hyperosmolar states, e. Diminishes potassium secretion by reducing the electrical gradient between the intracellular space and the renal tubule, causing potassium to leave the cells. Lysine, arginine, or epsilon-aminocaproic acid enters cells in exchange for potassium, causing hyperkalemia. Decreases aldosterone synthesis; hyperkalemia often can be reduced by concomitant diuretic use; ARBs less likely to cause hyperkalemia than ACE inhibitors. Decreases sodium-potassium adenosine triphosphatase ATPase activity; beta 2 agonists decrease potassium levels. Suppresses renin release, leading to decreased aldosterone synthesis, decreased potassium secretion in collecting duct. Decreases aldosterone synthesis; most common in patients on dialysis who drink water with high fluoride levels. Hypertonicity caused by hyperglycemia from glucose infusions can drive potassium out of the intracellular space, leading to hyperkalemia. Can cause hyperkalemia in patients with decreased renal function; inhibits adrenal aldosterone synthesis. Decreased prostaglandin production leads to decreased afferent arteriolar flow, suppressing renin and aldosterone secretion. Herbs containing high potassium levels e. Can cause hyperkalemia in patients with impaired renal function caused by increased potassium load; can be administered orally or intravenously. Ingestion of potassium can lead to hyperkalemia, particularly if renal function is impaired; dietary sources include bananas, melon, and orange juice. Increases nicotinic acetylcholine receptors in damaged skeletal muscle e. Suppresses renin release, leading to decreased aldosterone synthesis and decreased potassium secretion in collecting duct. Drawing blood samples from a vein or line into which potassium is being infused. FEK less than 10 percent indicates renal etiology FEK greater than 10 percent indicates extrarenal cause. He was disoriented in place and time with severe generalized muscle weakness; he was apyrexial temperature Plasma aminotransferases, albumin, lactic dehydrogenase, creatinine kinase and troponin I levels were all within normal limits. There was glucosuria and ketonuria, but urine microscopy was normal. There were no ECG abnormalities, except for sinus tachycardia. The patient was fully alert and mobile. He had an uneventful recovery and was discharged after 11 days with normal renal function and plasma electrolyte concentrations. Initial ECG performed at the Emergency Department at the University Hospital of Ioannina serum potassium 8. Acid—base and potassium disturbances are common in patients with uncontrolled diabetes mellitus [ 1 , 2 ]. This was an interesting case of DKA presenting as an emergency, with neurological symptoms and electrocardiographic changes associated with hyperkalaemia. Decompensated metabolic acidosis as manifested by hypercapnia and ECG abnormalities indicate the need for rapid intervention and a meticulous approach to management [ 1 , 2 ]. The development of metabolic acidosis causes a compensatory hyperventilatory response owing to stimulation of both the central and peripheral chemoreceptors. This increase in alveolar ventilation results in a drop in pCO 2 , which tends to raise pH towards normal. In contrast to other causes of acidaemia, in cases of DKA it is the carotid body chemoreceptors that provide the major stimulus for respiration driven by a reduced pH. This means that hyperventilation is taking place earlier in DKA. However, in this case, pCO 2 was raised, suggesting inadequate respiratory compensation, which may have been related to profound hyperkalaemia. The lower extremities are affected initially, the trunk and upper extremities being affected later [ 2 ]. In this case, respiratory muscle weakness was related to high plasma potassium levels adversely affecting the respiratory compensatory mechanisms. Mild to moderate increases in serum potassium occur frequently with DKA [ 2 , 3 ]. However, severe hyperkalaemia is uncommon and is likely to be a consequence of acidosis, insulin deficiency, hyperosmolality, severe dehydration and renal potassium retention [ 2 , 3 ]. It is well documented that the buffering of excess hydrogen ions in cells leads to potassium movement into the extracellular fluid in order to maintain electoneutrality. Thus, the acidaemia could not explain the severe hyperkalaemia noted in this patient. Insulin promotes potassium entry into cells. When circulating insulin is lacking, as in DKA, potassium moves out of cells, thus raising plasma potassium levels even in the presence of total body potassium deficiency [ 2 , 3 ]. Furthermore, an elevation in plasma osmolality causes osmotic water movement from the cells into the extracellular fluid, which is paralleled by potassium movement out of the cells. Most of urinary potassium is derived from potassium secretion in the distal nephron, particularly by the principal cells in the cortical collecting tubule. This process is mainly influenced by two factors: aldosterone and the distal delivery of sodium and water [ 2 ]. However, this was not true in the present case, as the patient's plasma creatinine, urea, potassium, chloride and bicarbonate levels restored to normal upon discharge [ 2 , 3 ]. However, creatinine levels may not always securely predict the status of hydration and the target towards fluid resuscitation. Elevated serum creatinine levels in patients with uncontrolled diabetes mellitus should be evaluated in the light of baseline values. Methodological problems must be considered because certain substances may interfere with the assay especially colorimetric assays , thereby artefactually increasing serum creatinine levels. Enzymatic assays, which lack this interference, may be more advantageous on these occasions. Correspondence and offprint requests to : Moses S. Email: egepi cc. Elisaf MS, Tsatsoulis AA, Katopodis KP, Siamopoulos KC. Acid—base and electrolyte disturbances in patients with diabetic ketoacidosis. Diabetes Res Clin Pract ; 34 : 23 — Rose BD, Post TW. Clinical Physiology of Acid—Base and Electrolyte Disorders , edn 5. Halperin ML, Kamel SK. Lancet ; : — a ECG of the patient on admission, showing a broadened QRS complex and elevated T waves. b ECG of the patient after i. calcium gluconate, showing a sinus rhythm. The patient was transferred to our intensive care unit. Four liters of isotonic saline i. were administered, prescribed under the hypothesis of prerenal kidney failure due to hyperglycemic polyuria. Serum potassium decreased from The h urine output was 3. No dialysis was performed. The patient could be released to her nursing home within 6 days of admittance. Potassium supplementation was paused. The need to monitor the serum potassium concentration in this patient more closely was communicated to her primary care physician. We described the case of a patient hospitalized for diabetic decompensation which was complicated by hyperkalemia and acute kidney injury and handled conservatively. The hyperkalemia was of multifactorial origin: impaired renal function due to volume contraction in combination with medication altering distal nephron potassium secretion RAAS [renin-angiotensin-aldosterone system] blockade and dietary potassium supplementation. The risk for cardiovascular mortality with hyperkalemia increases steeply starting from potassium concentrations of 5. While there are reports of hyperkalemia of Conservative management of severe hyperkalemia has been reported in patients with normal renal function [ 15 ]. In this case, we chose to treat the patient conservatively. Conservative treatment of hyperkalemia under the condition of good urinary output often is more rapid than the establishment of dialysis modalities. We believe this approach is feasible and reasonably safe to perform under intensive care conditions if the reestablishment of a sinus rhythm in response to calcium gluconate treatment is achieved rapidly approx. within 10—20 min. In this case, the hyperkalemia was oligosymptomatic. Symptoms of hyperkalemia usually comprise muscular weakness and palpitations, symptoms the patient communicates [ 5 ]. The described patient could not communicate after having survived a stroke 2 years previously, and the hyperkalemia almost would have been missed. This case emphasizes the additional caution that should be given to patients with preexisting neurologic conditions [ 14 ]. Blockade of the RAAS is an independent risk factor for hyperkalemia [ 12 ]. While there currently is no clear recommendation on the frequency of testing intervals, in the described case, beginning treatment with RAAS blockade and potassium supplementation should have led to more frequent testing [ 3 ]. The hyperosmolar polyuria did in this case not lead to hypokalemia see Introduction , as potassium-sparing medication was combined with potassium supplementation, resulting in a potassium load that distal nephron flux could not resolve. A potential mechanism by which the patient might have had a survival benefit under such extreme hyperkalemia is the accompanying hyperglycemia. While it is an independent risk factor for hyperkalemia, it may have protected this patient from fatal cardiac consequences. The proposed mechanism, which has been discussed for vascular smooth muscle cells and cardiomyocytes in mice, rats, dogs, and the isolated perfused rabbit heart, is caused by in our case hyperglycemic hyperosmolality [ ]. Hyperosmolality in these models has been hypothesized to be cardioprotective via two mechanisms: 1 it causes a lower excitability of the cardiomyocyte, changing the patterns of intracellular calcium mobilization, and 2 it alters the central control of cardiac responses [ 7, 18, 19 ]. In patients presenting without concurrent hyperglycemia, much lower potassium values have been described to be fatal [ 3, 12 ]. It is tempting to speculate that our patient was protected by the hyperosmolality caused by hyperglycemia. In conclusion, we described a rare case of a survived hyperkalemia of It presented surprisingly as hyperglycemia in an otherwise oligosymptomatic patient and was handled conservatively. The authors would like to thank Prof. Johannes Loffing, MD, University of Zurich, and Dr. David Penton, PhD, University of Zurich, for fruitful discussions and invaluable scientific input on potassium metabolism and hyperkalemia. All herein presented research was conducted in accordance with the World Medical Association Declaration of Helsinki. is supported by Deutsche Forschungsgemeinschaft CRC All authors treated the patient, analyzed and interpreted the patient data, and wrote and approved the final manuscript. The data that support the findings of this study are fully available saved patient data at the University Hospital Hamburg, UKE. Sign In or Create an Account. Search Dropdown Menu. header search search input Search input auto suggest. filter your search All Content All Journals Case Reports in Nephrology and Dialysis. Advanced Search. Skip Nav Destination Close navigation menu Article navigation. Volume 11, Issue 1. Case Presentation. Statement of Ethics. Conflict of Interest Statement. Funding Sources. Author Contributions. Data Availability Statement. Article Navigation. Case Reports February 25 Surprising Hyperkalemia of Jan Czogalla ; Jan Czogalla. a III. |
| Lethal hyperkalemia associated with severe hyperglycemia in diabetic patients with renal failure | Patients on hemodialysis who develop ketoacidosis may have hyperkalemia because of anuria. Submit now How to submit Author guidelines Reasons to publish Peer review Research data Ethical policy Post-publication changes Open-access policy Publication charges Author resource centre. Google Scholar. Advanced Search. Novel risk factors for hospital-acquired hyponatraemia: a matched case-control study. Vitamin D deficiency and furosemide administration may also play a role in the occurrence of hypocalcemia[ 70 ]. |
Hyperglycemic crisis and hyperkalemia -
He had been diagnosed with type 1 diabetes mellitus at the age of 18 years and had been treated with continuous subcutaneous insulin infusion CSII pump for the preceding recent one year. He was trained and informed the risk of DKA at the induction of CSII, but he did not have stripes for measuring ketones.
His normal dry weight was His level of consciousness was E3V4M5 Glasgow Coma Scale. His skin turgor was not reduced and the mouth was moist. There was an ejection murmur at the apex and bilateral pretibial pitting edema.
The neurologic examination revealed right hemiplegia and higher cortical dysfunction aphasia and acalculia. It was unclear when the neurological symptoms exactly appeared. Arterial blood gas analysis showed a pH of 6. Initial laboratory findings are shown in Table 1. We did not measure plasma osmolality.
Chest x-ray showed cardiac enlargement, and electrocardiography ECG revealed typical features associated with hyperkalemia, including absent P waves, prolonged QRS intervals and tented T waves Fig. These physical and laboratory findings suggested DKA with marked hyperkalemia but no evidence of related total body fluid loss.
It was thought that his body weight was increased due to the skip of hemodialysis, because he could not go to hemodialysis clinic. Electrocardiography reveals features typically associated with hyperkalemia absent P waves, prolonged QRS interval and tented T waves.
We started an intravenous insulin infusion and hemodialysis to reduce the glucose and potassium levels. Fluid infusions were not given because there was no evidence of a fluid deficit. The hyperkalemic changes disappeared from ECG.
On hospital day 2, however, his right hemiplegia persisted. Head computed tomography demonstrated a low-density area in the left frontal lobe, indicating a left frontal cerebral infarction. Although the insulin pump tube was fully filled with insulin, the infusion line was not properly placed into the abdominal skin.
The patient underwent rehabilitation, but he was unable to manage his insulin pump because of the hemiplegia and higher cortical dysfunction induced by the cerebral infarction. We therefore removed the pump and switched to intermittent insulin therapy.
We performed brain CT scan twice, and there were no progression of the infarct area and brain edematous findings. On hospital day 40, he was transferred to another hospital for further rehabilitation.
In patients with anuria on hemodialysis, DKA is generally rare, because urinary loss of water and electrolytes does not occur and regular hemodialysis improves metabolic acidosis 2.
In this patient, extreme hyperkalemia of 9. In general, hyperglycemia is positively correlated with the serum potassium level 3 , but hyperkalemia to this extreme degree is rare.
In addition, reduced renal potassium excretion contributes to hyperkalemia in renal failure 4. This patient had a left frontal cerebral infarction resulting in right hemiplegia and higher cortical dysfunction, so we wondered if he had lost the ability to respond to hyperglycemia and handle his insulin pump properly.
The present episode likely depended on absolute lack of insulin action. In patients with diabetes who are anuric, there is a little reduction in weight and circulatory blood volume when the pathological state of DKA develops.
This patient showed had some weight gain over his dry weight and cardiac enlargement on chest x-ray. Normally, fluid infusion is essential for initial treatment of DKA 1.
However, this could cause or worsen overhydration and pulmonary edema in patients with DKA who require chronic hemodialysis. Careful evaluation of body fluid volume and the serum potassium level are mandatory.
If there is no volume depression, insulin treatment and prompt hemodialysis must be considered, but intravenous fluids should be minimized.
Neurologic complications must also be assessed in patients with DKA who are on chronic hemodialysis. Hemodialysis and insulin infusion may rapidly normalize serum potassium, and plasma tonicity, largely determined by glucose and sodium, improves with the decrease in plasma glucose.
Some reports have shown that rapid alteration in plasma tonicity may cause seizures or prolonged loss of consciousness because a marked change in tonicity may produce cerebral edema 5 , 6.
Hence, careful follow-up is necessary to prevent brain damage associated with reduction of tonicity by hemodialysis. In this patient, brain edema progression was not found in successive brain CT scans after hemodialysis; however, there is a possibility that rapid reduction in hyperglycemia accompanying with a marked decrease in plasma osmolality by hemodialysis and insulin infusion may mimic the prolongation of conscious disturbance.
On the other hand, Daugirdas and coworkers noted that extracellular volume depletion in the central nervous system is less likely to occur in patients such as ours because hyperglycemia will not result in osmotic diuresis in an anuric patient 7.
The relationship between changes in plasma tonicity and central nervous system impairment is now controversial, and the management strategy is not well established for patients with DKA who are on chronic hemodialysis.
Thus, we performed head computed tomography to assess the focal neurologic disorder. His severe hyponatremia was concomitantly improved after the reduction in plasma glucose, and we consider that hyponatremia was secondary induced by extreme hyperglycemia 8.
In summary, we have presented a patient with type 1 diabetes on chronic hemodialysis because of anuria. He had DKA with extreme hyperkalemia, which was resolved by rapid hemodialysis and intensive insulin therapy. Although hypokalemia is common in DKA, hyperkalemia is the more likely problem in patients on hemodialysis.
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported. This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector. H Yamada wrote the manuscript.
H Yamada and S Funazaki managed this patient. M Kakei, S Ishikawa and K Hara edited the manuscript. All authors read and approved the final version of the manuscript. Diabetes Care 32 — In The Kidney , edn 5 , pp Eds BM , Brenner , FCJ , Rector.
Philadelphia : W. Saunders Company. International Urology and Nephrology 45 — In some cases, the primary problem is movement of potassium out of the cells, even though the total body potassium may be reduced.
Redistributive hyperkalemia most commonly occurs in uncontrolled hyperglycemia eg, diabetic ketoacidosis or hyperosmolar hyperglycemic state. See "Diabetic ketoacidosis and hyperosmolar hyperglycemic state in adults: Treatment", section on 'Potassium replacement'.
Patients with skeletal muscle or cardiac manifestations typically have one or more of the characteristic ECG abnormalities associated with hyperkalemia. For more information or to purchase a personal subscription, click below on the option that best describes you: Medical Professional Resident, Fellow or Student Hospital or Institution Group Practice Patient or Caregiver.
It does NOT include all information about conditions, treatments, medications, side effects, or risks that may apply to a specific patient. It is not intended to be medical advice or a substitute for the medical advice, diagnosis, or treatment of a health care provider based on the health care provider's examination and assessment of a patient's specific and unique circumstances.
Patients must speak with a health care provider for complete information about their health, medical questions, and treatment options, including any risks or benefits regarding use of medications. This information does not endorse any treatments or medications as safe, effective, or approved for treating a specific patient.
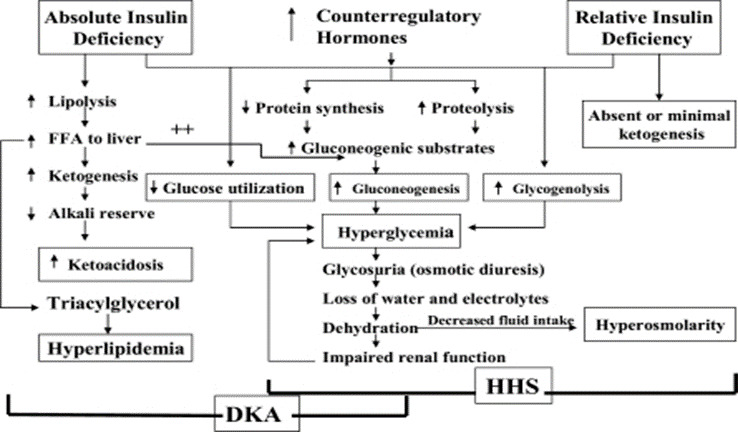
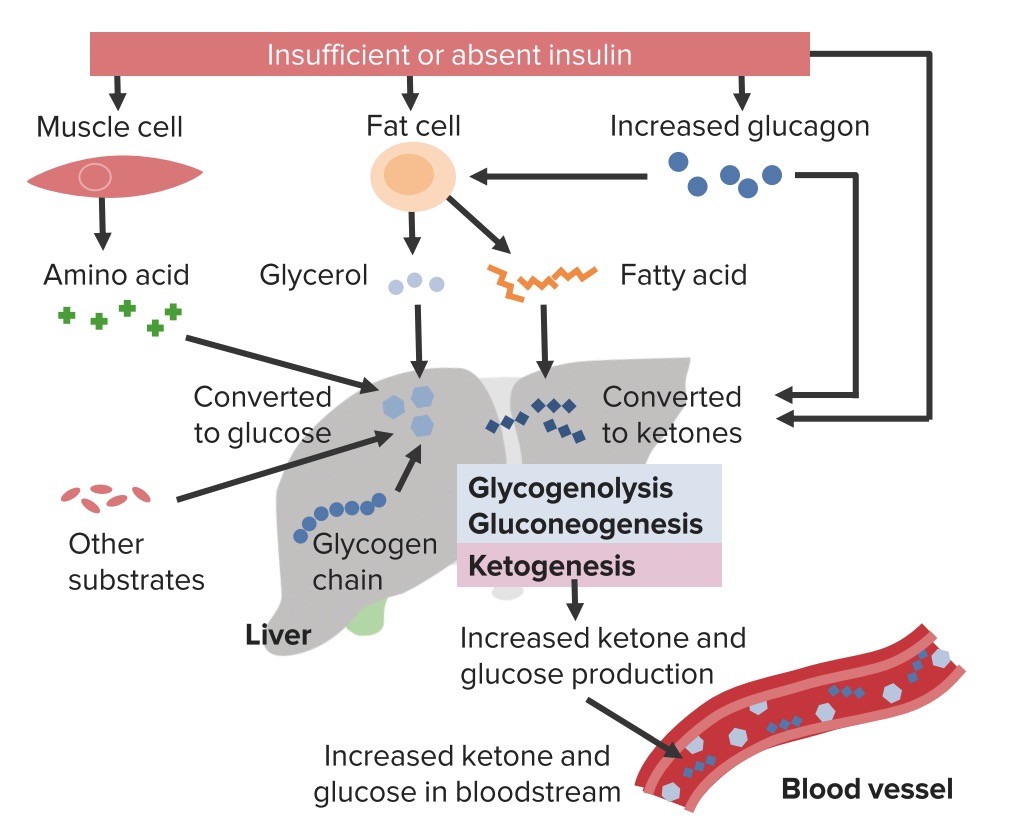
UpToDate, Inc. and its affiliates disclaim any warranty or liability relating to this information or the use thereof. All rights reserved. Print Options. In addition to possible hypokalemia, patients with the hyperglycemic crisis could present with hypophosphatemia 1 , Osmotic diuresis during hyperglycemic crisis increases the urinary phosphate excretion, and insulin therapy enhances intracellular phosphate shift 1 , Phosphate replacement is not a fundamental part of hyperglycemic crisis management, given the lack of evidence of clinical benefit 1 , 29 , A special consideration with phosphate administration is the secondary hypocalcemia 1 , 29 , Acidemia associated with DKA results from the overproduction of ketoacids, generated from the haptic metabolism of free fatty acids.
This hepatic metabolism occurs as a result of insulin resistance and an increase in the counterregulatory hormones contributing to the pathophysiology of DKA 37 , Tissue acidosis could lead to impaired myocardial contractility, systemic vasodilatation, inhibition of glucose utilization by insulin, and lowering the levels of 2,3-diphosphoglycerate 2,3-DPG in erythrocytes 37 — Sodium bicarbonate decreases the hemoglobin-oxygen affinity leading to tissue hypoxia; moreover, it is associated with hypernatremia, hypocalcemia, hypokalemia, hypercapnia, prolonged QTc interval, intracellular acidosis, and metabolic alkalosis 39 , The use of adjuvant sodium bicarbonate in the setting of DKA consistently shows a lack of clinical benefit and should be prescribed on a case-by-case basis.
Although this recommendation was not supported by solid evidence; many clinicians adopt the practice to avoid the unwanted side effect of severe metabolic acidosis. Sodium bicarbonate moves potassium intracellularly, however, clinical benefit is uncertain, and the use is controversial 41 , Prompt therapy for patients with hyperglycemic crisis is essential in reducing morbidity and mortality 6 , If not treated or treated ineffectively, the prognosis can include serious complications such as seizures, organ failures, coma, and death 6 , When treatment is delayed, the overall mortality rate of HHS is higher than that of DKA, especially in older patients.
This difference in prognoses was comparable when patients were matched for age In DKA, prolonged hypotension can lead to acute myocardial and bowel infarction 6 , The kidney plays a vital role in normalizing massive pH and electrolyte abnormalities 6 , Patients with prior kidney dysfunction or patients who developed end-stage chronic kidney disease worsen the prognosis considerably 6 , In HHS, severe dehydration may predispose the patient to complications such as myocardial infarction, stroke, pulmonary embolism, mesenteric vein thrombosis, and disseminated intravascular coagulation 6 , The VTE risk was higher than diabetic patients without hyperglycemic crisis or diabetic acidosis patients Management of hyperglycemic crisis may also be associated with significant complications include electrolyte abnormalities, hypoglycemia, and cerebral edema 7.
This is due to the use of insulin and fluid replacement therapy 4 , 5. Therefore, frequent electrolytes and blood glucose concentrations monitoring are essential while insulin infusions and fluid replacements are continued 4 , 5. Cerebral edema is a rare but severe complication in children and adolescents and rarely affects adult patients older than 28 7.
This could be due to the lack of cerebral autoregulation, presentation with more severe acidosis and dehydration among children and adolescents The exact mechanism of cerebral edema development is unknown.
Some reports suggest that the risk of cerebral edema during hyperglycemic crisis management might be induced by rapid hydration, especially in the pediatric population. However, a recent multicenter study for children with DKA who were randomized to receive isotonic versus hypotonic sodium IV fluid with different infusions rates did not show a difference in neurological outcomes Early identification and prompt therapy with mannitol or hypertonic saline can prevent neurological deterioration from DKA management 7 , Furthermore, higher blood urea nitrogen BUN and sodium concentrations have been identified as cerebral edema risk factors Thus, careful hydration with close electrolytes and BUN is recommended Other serious complications of hyperglycemic crisis may include transient AKI, pulmonary edema in patients with congestive heart failure, myocardial infarction, a rise in pancreatic enzymes with or without acute pancreatitis, cardiomyopathy, rhabdomyolysis in patients presented with severe dehydration 7 , All authors have contributed equally in writing, organizing, and reviewing this publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic Crises in Adult Patients With Diabetes. Diabetes Care 32 7 — doi: PubMed Abstract CrossRef Full Text Google Scholar. Goyal A, Mathew UE, Golla KK, Mannar V, Kubihal S, Gupta Y, et al. A Practical Guidance on the Use of Intravenous Insulin Infusion for Management of Inpatient Hyperglycemia.
Diabetes Metab Syndrome: Clin Res Rev 15 5 CrossRef Full Text Google Scholar. Saeedi P. Global and Regional Diabetes Prevalence Estimates for and Projections for and Results From the International Diabetes Federation Diabetes Atlas, 9th Edition.
Diabetes Res Clin Pract Pasquel FJ, Umpierrez GE. Hyperosmolar Hyperglycemic State: A Historic Review of the Clinical Presentation, Diagnosis, and Treatment. Dia Care 37 11 — Kitabchi AE, Umpierrez GE, Murphy MB, Barrett EJ, Kreisberg RA, Malone JI, et al.
Management of Hyperglycemic Crises in Patients With Diabetes. Diabetes Care 24 1 — Kitabchi AE, Umpierrez GE, Murphy MB, Kreisberg RA. Hyperglycemic Crises in Adult Patients With Diabetes: A Consensus Statement From the American Diabetes Association.
Diabetes Care 29 12 — Karslioglu French E, Donihi AC, Korytkowski MT. Diabetic Ketoacidosis and Hyperosmolar Hyperglycemic Syndrome: Review of Acute Decompensated Diabetes in Adult Patients. BMJ I Fayfman M, Pasquel FJ, Umpierrez GE. Management of Hyperglycemic Crises.
Med Clinics North Am 3 — Rains JL, Jain SK. Oxidative Stress, Insulin Signaling, and Diabetes. Free Radical Biol Med 50 5 — Hoffman WH, Burek CL, Waller JL, Fisher LE, Khichi M, Mellick LB.
Cytokine Response to Diabetic Ketoacidosis and Its Treatment. Clin Immunol 3 — Hayami T, Kato Y, Kamiya H, Kondo M, Naito E, Sugiura Y, et al. Case of Ketoacidosis by a Sodium-Glucose Cotransporter 2 Inhibitor in a Diabetic Patient With a Low-Carbohydrate Diet.
J Diabetes Investig , 6 5 — Umpierrez GE, Murphy MB, Kitabchi AE. Diabetic Ketoacidosis and Hyperglycemic Hyperosmolar Syndrome. Diabetes Spectr 15 1 Kraut JA, Madias NE. Serum Anion Gap: Its Uses and Limitations in Clinical Medicine. Clin J Am Soc Nephrol 2 1 — Dhatariya K, Savage M, Claydon A, et al.
Joint British Diabetes Societies for Inpatient Care JBDS-IP Revised Guidelines. The Management of Diabetic Ketoacidosis in Adults Revised Google Scholar. Kitabchi AE, Umpierrez GE, Murphy MB. Diabetic Ketoacidosis and Hyperosmolar State. In: DeFronzo RA, Ferrannini E, Zimmet P, Alberti KGMM, editors.
International Textbook of Diabetes Mellitus. Trachtenbarg DE. Diabetic Ketoacidosis. Am Fam Phys 71 9 — Katz MA. Hyperglycemia-Induced Hyponatremia-Calculation of Expected Serum Sodium Depression. N Engl J Med 16 —4.
Rudloff E, Hopper K. Crystalloid and Colloid Compositions and Their Impact. Front Vet Sci Semler MW, Kellum JA. Balanced Crystalloid Solutions. Am J Respir Crit Care Med 8 — Van Zyl DG, Rheeder P, Delport E.
QJM 4 — Mahler SA, Conrad SA, Wang H, Arnold TC. Resuscitation With Balanced Electrolyte Solution Prevents Hyperchloremic Metabolic Acidosis in Patients With Diabetic Ketoacidosis.
Diabetic Hypeerglycemic frequently develop a constellation of electrolyte disorders. These disturbances are particularly common in decompensated diabetics, especially trim waistline fat the context of diabetic ahd or Hyperglycemic crisis and hyperkalemia hyperglycemic hyperosmolar syndrome. These patients are markedly potassium- magnesium- and phosphate-depleted. Diabetes mellitus DM is linked to both hypo- and hyper-natremia reflecting the coexistence of hyperglycemia-related mechanisms, which tend to change serum sodium to opposite directions. The most important causal factor of chronic hyperkalemia in diabetic individuals is the syndrome of hyporeninemic hypoaldosteronism.
der sehr nützliche Gedanke