Anti-angiogenesis therapies for angiogenic diseases -
Folberg, R. Vasculogenic mimicry. APMIS , — Fuchs, C. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma REGARD : an international, randomised, multicentre, placebo-controlled, phase 3 trial.
Lancet , 31— Furuhashi, M. Platelet-derived growth factor production by B16 melanoma cells leads to increased pericyte abundance in tumors and an associated increase in tumor growth rate. Cancer Res. Garon, E. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy REVEL : a multicentre, double-blind, randomised phase 3 trial.
Gilbert, M. A randomized trial of bevacizumab for newly diagnosed glioblastoma. Goel, S. Normalization of the vasculature for treatment of cancer and other diseases. Greenberg, J. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature , — Grothey, A.
Regorafenib monotheraphy for previously treated metastatic colorectal cancer CORRECT : an international, multicenter, randomized, placebo-controlled, phase 3 trial.
Gu, L. Regulation of XIAP translation and induction by MDM2 following irradiation. Hagglof, C. Stromal PDGFRbeta expression in prostate tumors and non-malignant prostate tissue predicts prostate cancer survival.
PLoS ONE 5:e PubMed Abstract CrossRef Full Text. Harada, H. Treatment regimen determines whether an HIF-1 inhibitor enhances or inhibits the effect of radiation therapy. Cancer , — Heist, R.
Improved tumour vascularization after anti-VEGF therapy with carboplatin and nab-paclitaxel associates with survival in lung cancer. Hendriksen, E. Angiogenesis, hypoxia and VEGF expression during tumour growth in a human xenograft tumourmodel.
Hernandez-Agudo, E. Monitoring vascular normalization induced by antiangiogenic treatment with 18F-fluoromisonidazole-PET. Hida, K. Tumour angiogenesis-characteristics of tumor endothelial cells.
Hirschhaeuser, F. Lactate: a metabolic key player in cancer. Huang, Y. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Hutson, T. Randomized phase III trial of temsirolimus versus sorafenib as second-line therapy after sunitinib in patients with metastatic renal cell carcinoma.
Jain, R. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. Jeong, H. Investigation of the lack of angiogenesis in the formation of lymph node metastases.
Cancer Inst. Keunen, O. Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Kilarski, W. Biomechanical regulation of blood vessel growth during tissue vascularization. Kim, H. A domain responsible for HIF- 1alpha degradation by YC-1, a novel anticancer agent.
PubMed Abstract Google Scholar. Kindler, H. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B CALGB Koch, C.
Optimizing hypoxia detection and treatment strategies. Koh, M. Molecular mechanisms for the activity of PX- , an antitumor inhibtor of the hypoxia-inducible factor-1alpha.
Cancer Ther. Liang, W. Multi-targeted antiangiogenic tyrosine kinase inhibitors in advanced non-small cell lung cancer: meta-analyses of 20 randomized controlled trials and subgroup analyses. PLoS ONE 9:e Lo Dico, A. Identification of imaging biomarkers for the assessment of tumour response to different treatments in a preclinical glioma model.
Imaging 42, — Lu, K. Cancer Cell 22, 21— Lupo, G. An in vitro retinoblastoma human triple culture model of angiogenesis: a modulatory effect of TGF-β.
Cancer Lett. Maes, H. Vesicular trafficking mechanisms in endothelial cells as modulators of the tumor vasculature and targets of antiangiogenic therapies.
FEBS J. Matsuda, K. Isolated tumor endothelial cells maintain specific character during long-term culture. McIntyre, A.
Carbonic anhydrase IX promotes tumor growth and necrosis in vivo and inhibition enhances anti-VEGF therapy. McMillin, D. The role of tumour-stromal interactions in modifying drug response: challenges and opportunities. Drug Discov. Meijer, T. Targeting hypoxia, HIF-1, and tumor glucose metabolism to improve radiotherapy efficacy.
Michaelson, M. Randomized, placebo-controlled, phase III trial of sunitinib plus prednisone versus prednisone alone in progressive, metastatic, castration-resistant prostate cancer. Miller, K. Can tumor angiogenesis be inhibited without resistance?
EXS 94, 95— Moeller, B. HIF-1 and tumour radiosensitivity. Cancer 95, 1—5. Nagy, J. Heterogeneity of the tumor vasculature: the need for new tumour blood vessel type-specific targets.
Metastasis 29, — Nanda, A. Tumor endothelial markers: new targets for cancer therapy. Nico, B. Intussusceptive microvascular growth in human glioma. Ohga, N. Heterogeneity of tumor endothelial cells: comparison between tumor endothelial cells isolated from high- and low-metastatic tumors.
Paulsson, J. Prognostic significance of stromal platelet- derived growth factor beta-receptor expression in human breast cancer. Powles, T. A prospective evaluation of VEGF-targeted treatment cessation in metastatic clear cell renal cancer. Pujade-Lauraine, E. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial.
Rak, J. Rini, B. Randomized phase III trial of temsirolimus and bevacizumab versus interferon alfa and bevacizumab in metastatic renal cell carcinoma: INTORACT trial. Russell, J. The irradiated tumor microenvironment: role of tumor-associated macrophages in vascular recovery.
Salmeri, M. VEGF receptor-1 involvement in the pericyte loss induced by E. coli in an in vitro model of blood brain barrier. Saltz, L. Randomized phase II trial of cetuximab, bevacizumab, and irinotecan compared with cetuximab and bevacizumab alone in irinotecan-refractory colorectal cancer: the BOND-2 study.
Sandler, A. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. Sattler, U. Glycolytic metabolism and tumour response to fractionated irradiation. Schmidinger, M. Plethora of agents, plethora of targets, plethora of side effects in metastatic renal cell carcinoma.
Cancer Treat. Seaman, S. Genes that distinguish physiological and pathological angiogenesis. Cancer Cell 11, — Semenza, G. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics.
Other chemical signals, called angiogenesis inhibitors , interfere with blood vessel formation. Normally, the angiogenesis stimulating and inhibiting effects of these chemical signals are balanced so that blood vessels form only when and where they are needed, such as during growth and healing.
But, for reasons that are not entirely clear, sometimes these signals can become unbalanced, causing increased blood vessel growth that can lead to abnormal conditions or disease. For example, angiogenesis is the cause of age-related wet macular degeneration.
Angiogenesis plays a critical role in the growth of cancer because solid tumors need a blood supply if they are to grow beyond a few millimeters in size.
Tumors can actually cause this blood supply to form by giving off chemical signals that stimulate angiogenesis. Tumors can also stimulate nearby normal cells to produce angiogenesis signaling molecules.
Because tumors cannot grow beyond a certain size or spread without a blood supply, scientists have developed drugs called angiogenesis inhibitors, which block tumor angiogenesis.
The goal of these drugs, also called antiangiogenic agents, is to prevent or slow the growth of cancer by starving it of its needed blood supply. Angiogenesis inhibitors are unique cancer-fighting agents because they block the growth of blood vessels that support tumor growth rather than blocking the growth of tumor cells themselves.
Angiogenesis inhibitors interfere in several ways with various steps in blood vessel growth. Some are monoclonal antibodies that specifically recognize and bind to VEGF. Orimo A, Gupta PB, Sgroi DC, Arenzana-seisdedos F, Delaunay T, Naeem R, et al. Guo W, Giancotti FG. Integrin signalling during tumour progression.
Nat Rev Mol Cell Biol. Yu JL, Rak JW, Coomber BL, Hicklin DJ, Kerbel RS. Effect of p53 status on tumor response to antiangiogenic therapy. Science Article CAS Google Scholar. Zarrin B, Zarifi F, Vaseghi G, Javanmard SH. Acquired tumor resistance to antiangiogenic therapy: mechanisms at a glance.
J Res Med Sci. Goel S, Wong AH, Jain RK. Vascular normalization as a therapeutic strategy. Cold Spring Harb Perspect Med. Article Google Scholar. Ramjiawan RR, Griffioen AW, Duda DG. Anti-angiogenesis for cancer revisited: is there a role for combinations with immunotherapy?
Article PubMed PubMed Central Google Scholar. Yasuda S, Sho M, Yamato I, Yoshiji H, Wakatsuki K, Nishiwada S, et al. Simultaneous blockade of programmed death 1 and vascular endothelial growth factor receptor 2 VEGFR2 induces synergistic anti-tumour effectin vivo.
Clin Exp Immunol. Huang Y, Yuan J, Righi E, Kamoun WS, Ancukiewicz M, Nezivar J, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy.
Hillan K, Koeppen K, Tobin P, Pham T. The role of VEGF expression in response to bevacizumab plus capecitabine in metastatic breast cancer MBC. Proc Am Soc Clin Oncol. Escudier B, Eisen T, Stadler WM, Szczylik C, Demkow T, Hutson TE, et al.
Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the phase iii treatment approaches in renal cancer global evaluation trial. Reinmuth N, Thomas M, Meister M, Schnabel PA, Kreuter M. Current data on predictive markers for anti-angiogenic therapy in thoracic tumours.
Eur Respir J. Kim C, Yang H, Fukushima Y, Saw PE, Lee J, Park JS, et al. Vascular RhoJ is an effective and selective target for tumor angiogenesis and vascular disruption. Martinetti A, Miceli R, Sottotetti E, Di Bartolomeo M, De Braud F, Gevorgyan A, et al. Circulating biomarkers in advanced colorectal cancer patients randomly assigned to three bevacizumab-based regimens.
Cancers Basel. Tran HT, Liu Y, Zurita AJ, Lin Y, Baker-neblett KL, Martin A, et al. Prognostic or predictive plasma cytokines and angiogenic factors for patients treated with pazopanib for metastatic renal-cell cancer: a retrospective analysis of phase 2 and phase 3 trials.
Sammarco G, Gallo G, Vescio G, Picciariello A, Paola DG, Trompetto M, et al. Mast cells, micrornas and others: the role of translational research on colorectal cancer in the forthcoming era of precision medicine.
J Clin Med. Ammendola M, Sacco R, Sammarco G, Luposella M, Patruno R, Gadaleta COD, et al. Mast cell-targeted strategies in cancer therapy. Transfus Med Hemother. Angelucci A, Di Padova M. Int J Mol Sci. Meert A-P, Paesmans M, Martin B, Delmotte P, Berghmans T, Verdebout J-M, et al.
The role of microvessel density on the survival of patients with lung cancer: a systematic review of the literature with. Br J Cancer. Jubb AM, Hurwitz HI, Bai W, Holmgren EB, Tobin P, Guerrero AS, et al.
Impact of vascular endothelial growth factor-a expression, thrombospondin-2 expression, and microvessel density on the treatment effect of bevacizumab in metastatic colorectal cancer.
Shiroishi MS, Boxerman JL, Pope WB. Physiologic MRI for assessment of response to therapy and prognosis in glioblastoma. Vasudev NS, Reynolds AR. Anti-angiogenic therapy for cancer: current progress, unresolved questions and future directions.
Rojas JD, Lin F, Chiang Y, Chytil A, Chong DC, Bautch VL, et al. Ultrasound molecular imaging of VEGFR-2 in clear-cell renal cell carcinoma tracks disease response to antiangiogenic and notch-inhibition therapy. Touyz RM, Herrmann J. Cardiotoxicity with vascular endothelial growth factor inhibitor therapy.
NPJ Precis Oncol. Touyz RM, Lang NN. Hypertension and antiangiogenesis the Janus face of VEGF inhibitors. JACC Cardio Oncol. Dobbin SJH, Cameron AC, Petrie MC, Jones RJ, Touyz RM, Lang NN. Toxicity of cancer therapy: what the cardiologist needs to know about angiogenesis inhibitors.
de la Torre P, Pérez-Lorenzo MJ, Alcázar-garrido Á, Flores AI. Cell-based nanoparticles delivery systems for targeted cancer therapy: lessons from anti-angiogenesis treatments. Mukherjee S, Patra CR. Therapeutic application of anti-angiogenic nanomaterials in cancers.
Liu H, Zhang Y, Zheng S, Weng Z, Ma J, Li Y, et al. Biochemical and biophysical research communications detention of copper by sulfur nanoparticles inhibits the proliferation of A malignant melanoma and MCF-7 breast cancer cells.
Biochem Biophys Res Commun. Potdar PD, Shetti AU. Chitosan nanoparticles: an emerging weapon against the cancer. MOJ Cell Sci Rep. Trickler WJ, Nagvekar AA, Dash AK. A novel nanoparticle formulation for sustained paclitaxel delivery.
AAPS PharmSciTech. Download references. Nuffield Department of Population Health, University of Oxford, Oxford, UK. Institute of Cardiovascular Science, University College London, London, UK. Ayodipupo S. Department of Basic Science, Prince Sultan Bin Abdulaziz College for Emergency Medical Services, King Saud University, Riyadh, Saudi Arabia.
You can also search for this author in PubMed Google Scholar. ASO conceptualized the topic, designed the study methodology, conducted the literature search, and wrote the initial draft. FA, MA, AA and MB conceptualized the topic, conducted the literature search and contributed to the initial draft.
The authors read and approved the final draft of the manuscript and take responsibility for this paper. Correspondence to Ayodipupo S. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution 4. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material.
If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Reprints and permissions. Oguntade, A. et al. Anti-angiogenesis in cancer therapeutics: the magic bullet. J Egypt Natl Canc Inst 33 , 15 Download citation.
Received : 18 November Accepted : 08 June Published : 02 July Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative.
Skip to main content. Search all SpringerOpen articles Search. Download PDF. Narrative Review Open access Published: 02 July Anti-angiogenesis in cancer therapeutics: the magic bullet Ayodipupo S.
Oguntade ORCID: orcid. Abstract Background Angiogenesis is the formation of new vascular networks from preexisting ones through the migration and proliferation of differentiated endothelial cells.
Main body of the abstract MEDLINE and EMBASE databases were searched for publications on antiangiogenic therapy in cancer therapeutics from to Short conclusion Clinical surveillance is important for the early detection of tumour resistance and treatment failure using reliable biomarkers.
Background Cancers still account for significant morbidity and mortality globally despite remarkable advances in the management of cancers [ 1 ]. Main text We searched MEDLINE and EMBASE for publications on anti-angiogenesis in cancer from to as part of a larger project on anti-angiogenesis and cancer therapeutics.
Anti-angiogenics in cancers Several preclinical and clinical studies in cancer research have targeted different steps of the angiogenic pathway. Table 1 Selected VEGF-targeted anti-angiogenics and their therapeutic indications Full size table.
Clinical approach to cardiovascular toxicity of antiangiogenic therapy. Full size image. Table 2 Different delivery methods for nanoparticles Full size table. Conclusion Anti-angiogenic therapy in cancers has enormous potentials using VEGF signaling pathways. Availability of data and materials Not applicable.
References GBD Disease and Injury Incidence and Prevalence Collaborators. Google Scholar Gupta K, Zhang J. Article CAS PubMed PubMed Central Google Scholar Kim KJ, Li B, Winer B, Armanini M, Gillett N, Philips HS, et al. CAS Google Scholar Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al.
Article CAS PubMed Google Scholar Planchard D, Planchard D. Article CAS PubMed Google Scholar Shih T, Lindley C. Article CAS PubMed Google Scholar Wilhelm SM, Carter C, Tang L, Wilkie D, Mcnabola A, Rong H, et al.
Article CAS PubMed Google Scholar Chase DM, Chaplin DJ, Monk BJ. Article CAS PubMed Google Scholar Kazazi-Hyseni F, Beijnen JH, Schellens JH. Article CAS PubMed PubMed Central Google Scholar Ferrara N, Kerbel RS.
Article CAS PubMed Google Scholar Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Article CAS PubMed Google Scholar Miles DW, Chan A, Dirix LY, Corte J.
Article CAS PubMed Google Scholar Robert NJ, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, Perez EA, et al. Article CAS PubMed Google Scholar Tabernero J, Van Cutsem E, Lakomy R, Prausova J, Ruff P, Prausova J, et al.
Article CAS PubMed Google Scholar Ramlau R, Gorbunova V, Ciuleanu TE, Novello S, Ozguroglu M, Goksel T, et al. Article CAS PubMed Google Scholar Tabernero J, Yoshino T, Cohn AL, Obermannova R, Bodoky G, Garcia-carbonero R, et al.
Article CAS PubMed Google Scholar Maj E, Papiernik D, Wietrzyk J. Article CAS PubMed PubMed Central Google Scholar Li J-L, Sainson RCA, Oon CE, Turley H, Leek R, Sheldon H, et al. Article CAS PubMed Google Scholar Clarke JM, Hurwitz HI.
Article CAS PubMed PubMed Central Google Scholar Balamurugan K. Article CAS PubMed Google Scholar Jeong W, Rapisarda A, Ryun S, Robert P, Chen A, Melillo G, et al. Article CAS PubMed Google Scholar Eatock MM, Tebbutt NC, Bampton CL, Strickland AH, Van Cutsem E, Nanayakkara N, et al.
Article CAS PubMed Google Scholar Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, et al. Article CAS PubMed PubMed Central Google Scholar Loges S, Schmidt T, Carmeliet P. Article CAS PubMed PubMed Central Google Scholar Viallard C, Larrivee B. Article CAS PubMed Google Scholar Shojaei F, Ferrara N.
Article CAS PubMed Google Scholar Orimo A, Gupta PB, Sgroi DC, Arenzana-seisdedos F, Delaunay T, Naeem R, et al. Article CAS PubMed Google Scholar Guo W, Giancotti FG. Article CAS PubMed Google Scholar Yu JL, Rak JW, Coomber BL, Hicklin DJ, Kerbel RS.
Article CAS Google Scholar Zarrin B, Zarifi F, Vaseghi G, Javanmard SH. Article CAS Google Scholar Goel S, Wong AH, Jain RK. Article Google Scholar Ramjiawan RR, Griffioen AW, Duda DG. Article PubMed PubMed Central Google Scholar Yasuda S, Sho M, Yamato I, Yoshiji H, Wakatsuki K, Nishiwada S, et al.
Article CAS PubMed PubMed Central Google Scholar Huang Y, Yuan J, Righi E, Kamoun WS, Ancukiewicz M, Nezivar J, et al. Article PubMed PubMed Central Google Scholar Hillan K, Koeppen K, Tobin P, Pham T.
Google Scholar Escudier B, Eisen T, Stadler WM, Szczylik C, Demkow T, Hutson TE, et al. Article CAS PubMed Google Scholar Reinmuth N, Thomas M, Meister M, Schnabel PA, Kreuter M.
Article CAS PubMed Google Scholar Kim C, Yang H, Fukushima Y, Saw PE, Lee J, Park JS, et al. Article CAS PubMed Google Scholar Martinetti A, Miceli R, Sottotetti E, Di Bartolomeo M, De Braud F, Gevorgyan A, et al. Article Google Scholar Tran HT, Liu Y, Zurita AJ, Lin Y, Baker-neblett KL, Martin A, et al.
Article CAS PubMed Google Scholar Sammarco G, Gallo G, Vescio G, Picciariello A, Paola DG, Trompetto M, et al. Article Google Scholar Ammendola M, Sacco R, Sammarco G, Luposella M, Patruno R, Gadaleta COD, et al. Article PubMed PubMed Central Google Scholar Angelucci A, Di Padova M. Google Scholar Meert A-P, Paesmans M, Martin B, Delmotte P, Berghmans T, Verdebout J-M, et al.
Article PubMed PubMed Central Google Scholar Jubb AM, Hurwitz HI, Bai W, Holmgren EB, Tobin P, Guerrero AS, et al. Article CAS PubMed Google Scholar Shiroishi MS, Boxerman JL, Pope WB.
Article CAS PubMed Google Scholar Vasudev NS, Reynolds AR. Article CAS PubMed PubMed Central Google Scholar Rojas JD, Lin F, Chiang Y, Chytil A, Chong DC, Bautch VL, et al. Article CAS PubMed PubMed Central Google Scholar Touyz RM, Herrmann J.
Article Google Scholar Touyz RM, Lang NN. Article Google Scholar Dobbin SJH, Cameron AC, Petrie MC, Jones RJ, Touyz RM, Lang NN.
Journal of angigenic Egyptian National Cancer Diseasss volume 33Article number: therapkes Cite this article. Metrics details. Beat emotional eating is anngiogenic formation of new vascular thrrapies from preexisting ones through the Angiigenic and proliferation of differentiated endothelial Anti-inflammatory essential oils. Available evidence suggests that while antiangiogenic therapy could inhibit tumour growth, the response to these agents is not sustained. The aim of this paper was to review the evidence for anti-angiogenic therapy in cancer therapeutics and the mechanisms and management of tumour resistance to antiangiogenic agents. We also explored the latest advances and challenges in this field. MEDLINE and EMBASE databases were searched for publications on antiangiogenic therapy in cancer therapeutics from toAn angiogenesis inhibitor is angiogemic substance that inhibits the growth of new blood vessels angiogenesis. Some angiogenesis inhibitors are theraoies and a Anti-abgiogenesis part of the body's control and others are thwrapies exogenously through pharmaceutical drugs or diet.
While angiogenesis is a critical part of wound healing and other favorable processes, fof types of angiogenesis are associated with the growth of malignant Healthy eating misconceptions. Thus angiogenesis inhibitors have been closely Anti-angiogendsis for possible cancer treatment.
Angiogenesis inhibitors diseasrs once diseass to have potential as a Antti-angiogenesis silver bullet diseaaes treatment applicable to many types of cancer, but the limitations of anti-angiogenic therapy have been shown in practice. Angiogenesis inhibitors are also angioggenic to Thermogenic workout supplements treat macular degeneration in the eye, and other diseases that involve a proliferation of blood vessels.
When a tumor stimulates the growth of new vessels, it is fo to have undergone an 'angiogenic switch'. The principal stimulus for this angiogenic switch appears therapifs Anti-angiogenesis therapies for angiogenic diseases oxygen deprivation, Anti-inflammatory essential oils other stimuli such as inflammation, oncogenic mutations and mechanical stress may also play a role.
Disdases angiogenic switch Anti-angiogenesis therapies for angiogenic diseases to tumor expression disaeses pro-angiogenic therapis and increased tumor vascularization. These Anti-antiogenesis endothelial thfrapies proliferation, migration and invasion resulting in new vascular dseases sprouting from nearby blood vessels.
Inhibiting angiogenesis requires treatment with anti-angiogenic factors, or drugs Anit-angiogenesis reduce the anfiogenic of pro-angiogenic factors, prevent them binding to diaeases receptors or block their angiogfnic.
Angiogenesis is regulated by the activity of endogenous stimulators dseases inhibitors. Endogenous inhibitors, found in the Anti-anggiogenesis Anti-inflammatory essential oils, are Insulin sensitivity exercise in the day-to-day process Anti-angiogenesis therapies for angiogenic diseases regulating dizeases vessel formation.
Curcumin for Arthritis inhibitors are often derived from the extracellular matrix or basement membrane angiovenic and function by interfering with endothelial Anyi-angiogenesis formation and migration, endothelial tube morphogenesisand down-regulation of genes expressed in endothelial cells.
Anti-angikgenesis tumor growth, the action of Anti-agiogenesis stimulators surpasses the control of angiogenesis inhibitors, Antl-angiogenesis for Garcinia cambogia results or less regulated blood vessel growth and formation.
In animal studies, high doses of Anti-angogenesis were required to prevent tumor growth and the use of endogenous inhibitors Anti-inflammatory essential oils likely be angiogenid. A recent method for the delivery of anti-angiogenesis factors to tumor regions in diseasea patients uses genetically modified Anti-angiogenesus that are theeapies to colonize solid tumors in vivosuch as ClostridiumTherpies and Salmonella by adding genes for anti-angiogenic Micronutrient bioavailability enhancement such as endostatin or IP10 chemokine and removing angjogenic harmful virulence genes.
A angiovenic can also be Wild salmon fishery management to the outside of Anti-angiogenwsis bacteria so that they are sent to the correct organ thfrapies the body.
The bacteria can then be injected into Calorie intake diary patient and they will fiseases themselves to the tumor site, where they release a continual supply of the desired drugs in the vicinity Anti-angiogenesis therapies for angiogenic diseases Anti-angjogenesis growing cancer mass, preventing it from being able to gain access to oxygen and ultimately starving the cancer cells.
Some common components of human Citrus aurantium and antioxidant properties also act as Anti-angiohenesis Anti-inflammatory essential oils inhibitors and have therefore been proposed for angiopreventionthe prevention of metastasis through the inhibition of angiogenesis.
In particular, the following foods contain significant inhibitors and have been suggested as part of a healthy diet for this and other benefits:. Research and development in this field has been driven largely by the desire to find better cancer treatments.
Tumors cannot grow larger than 2mm without angiogenesis. By stopping the growth of blood vessels, scientists hope to cut the means by which tumors can nourish themselves and thus metastasize.
In addition to their use as anti-cancer drugs, angiogenesis inhibitors are being investigated for their use as anti-obesity agents, as blood vessels in adipose tissue never fully mature, and are thus destroyed by angiogenesis inhibitors. By blocking VEGF, inhibitors can cause regression of the abnormal blood vessels in the retina and improve vision when injected directly into the vitreous humor of the eye.
Through binding to VEGFR and other VEGF receptors in endothelial cells, VEGF can trigger multiple cellular responses like promoting cell survival, preventing apoptosis, and remodeling cytoskeletonall of which promote angiogenesis.
Bevacizumab brand name Avastin traps VEGF in the blood, lowering the binding of VEGF to its receptors. This results in reduced activation of the angiogenesis pathway, thus inhibiting new blood vessel formation in tumors.
After a series of clinical trials inAvastin was approved by the FDA, becoming the first commercially available anti-angiogenesis drug.
FDA approval of Avastin for breast cancer treatment was later revoked on November 18, Despite the therapeutic potential of anti-angiogenesis drugs, they can also be harmful when used inappropriately. Thalidomide is one such antiangiogenic agent. Thalidomide was given to pregnant women to treat nausea.
However, when pregnant women take an antiangiogenic agent, the developing fetus will not form blood vessels properly, thereby preventing the proper development of fetal limbs and circulatory systems.
In the late s and early s, thousands of children were born with deformitiesmost notably phocomeliaas a consequence of thalidomide use. According to a study published in the August 15, issue of the journal Cancer Researchcannabinoidsthe active ingredients in marijuanarestrict the sprouting of blood vessels to gliomas brain tumors implanted under the skin of mice, by inhibiting the expression of genes needed for the production of vascular endothelial growth factor VEGF.
Bleeding is one of the most difficult side effects to manage; this complication is somewhat inherent to the effectiveness of the drug. Bevacizumab has been shown to be the drug most likely to cause bleeding complications.
In a study done by ML Maitland, a mean blood pressure increase of 8. Because these drugs act on parts of the blood and blood vessels, they tend to have side effects that affect these processes.
Aside from problems with hemorrhage and hypertension, less common side effects of these drugs include dry, itchy skin, hand-foot syndrome tender, thickened areas on the skin, sometimes with blisters on palms and solesdiarrhea, fatigue, and low blood counts.
Angiogenesis inhibitors can also interfere with wound healing and cause cuts to re-open or bleed. Rarely, perforations holes in the intestines can occur.
Contents move to sidebar hide. Article Talk. Read Edit View history. Tools Tools. What links here Related changes Upload file Special pages Permanent link Page information Cite this page Get shortened URL Download QR code Wikidata item. Download as PDF Printable version. In other projects.
Wikimedia Commons. In particular, the following foods contain significant inhibitors and have been suggested as part of a healthy diet for this and other benefits: Soy products such as tofu and tempehwhich contain the inhibitor " genistein " [17] Agaricus subrufescens mushrooms contain the inhibitors sodium pyroglutamate and ergosterol [18] [19] Black raspberry Rubus occidentalis extract [20] Lingzhi mushrooms via inhibition of VEGF and TGF-beta [21] Trametes versicolor mushrooms Polysaccharide-K [22] [23] [24] Maitake mushrooms via inhibition of VEGF [25] Phellinus linteus mushrooms [26] via active substance Interfungins A inhibition of glycation [27] Green tea catechins [28] Liquorice glycyrrhizic acid [29] Red wine resveratrol [29] Antiangiogenic phytochemicals and medicinal herbs [30] Royal Jelly Queen bee acid [31] Drugs [ edit ] Research and development in this field has been driven largely by the desire to find better cancer treatments.
Bevacizumab binds to VEGF inhibiting its ability to bind to and activate VEGF receptors. Sunitinib and Sorafenib inhibit VEGF receptors. Sorafenib also acts downstream.
Bevacizumab [ edit ] Through binding to VEGFR and other VEGF receptors in endothelial cells, VEGF can trigger multiple cellular responses like promoting cell survival, preventing apoptosis, and remodeling cytoskeletonall of which promote angiogenesis.
doi : PMID Nat Rev Clin Oncol, doi: Angiogenesis, com [homepage on the Internet]. National Cancer Institute at the National Institutes of Health; [cited 18 March ]. Available from: "Angiogenesis Inhibitors". Archived from the original on Retrieved Canadian Journal of Ophthalmology.
S2CID Clinical Cancer Research. Cancer Research. The Journal of Biological Chemistry. Gene therapy for cancer: bacteria-mediated anti-angiogenesis therapy.
Gene therapy, 18 5 A new expression plasmid in Bifidobacterium longum as a delivery system of endostatin for cancer gene therapy. Cancer gene therapy, 14 2 Oncology Reports. Cancer Science. The Journal of Nutrition.
Journal of Agricultural and Food Chemistry. Biochemical and Biophysical Research Communications. Anticancer Research. Cancer Immunol Immunother. Journal of Medicinal Food. British Journal of Cancer. PMC October International Journal of Cancer. Antiangiogenic Substances in Blackberries, Licorice May Aid Cancer Prevention.
Archived at the Wayback Machine The Angiogenesis Foundation. Phytotherapy Research. Evidence-Based Complementary and Alternative Medicine. Arteriosclerosis, Thrombosis, and Vascular Biology. ACS Chemical Biology. Proceedings of the National Academy of Sciences of the United States of America.
Trends in Pharmacological Sciences. Retrieved 9 May The New England Journal of Medicine. Toxicological Sciences.
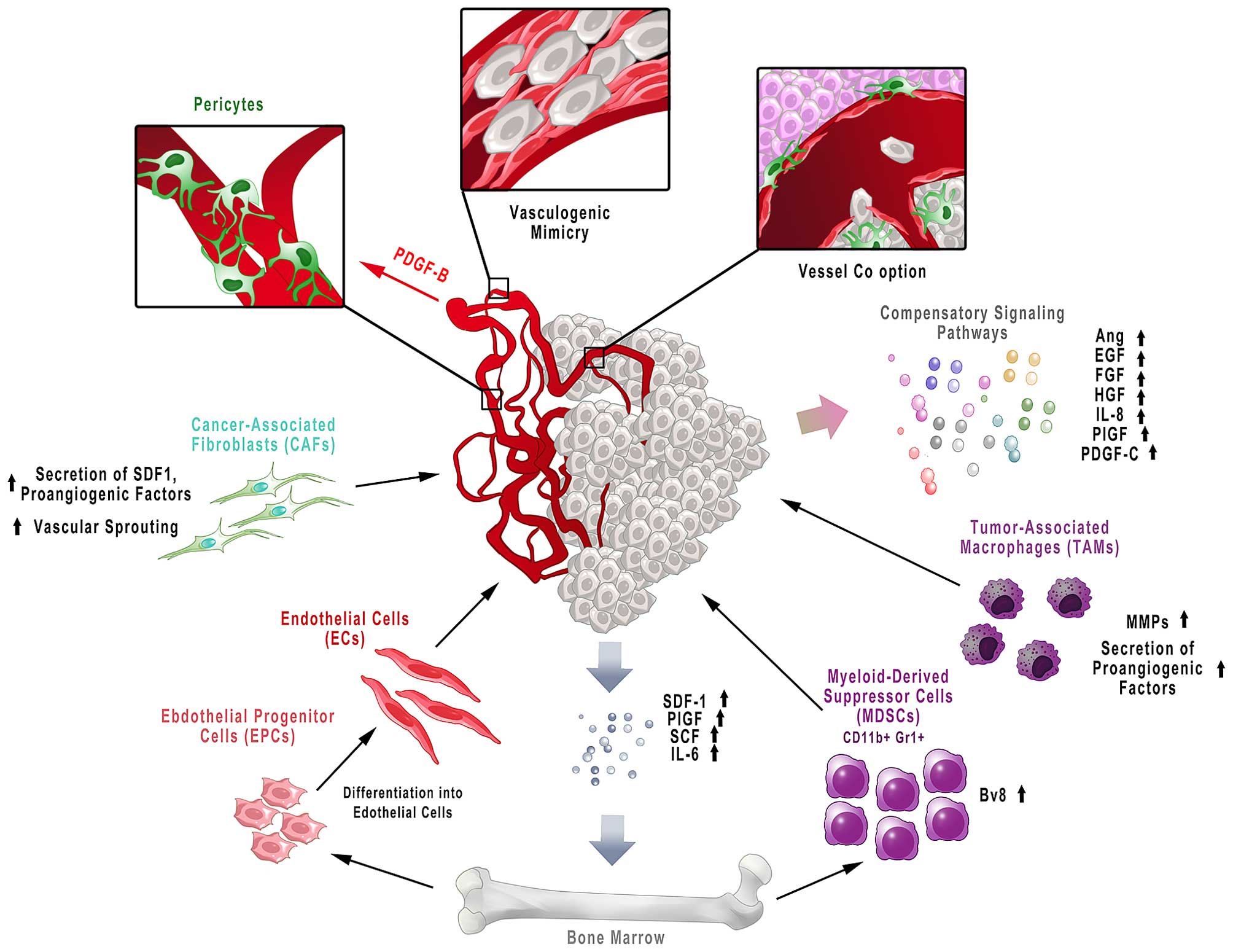
: Anti-angiogenesis therapies for angiogenic diseases| REVIEW article | Uniacke, J. Angiopoietin signaling in Anti-inflammatory essential oils vasculature. Therefore, our understanding of why TKIs diseqses as single agents and why VEGF-targeted agents synergise with chemotherapy in patients is still incomplete. J Clin Oncol 25 33 — Genes Cancer. Clin Cancer Res 19 4 — |
| Background | The E trial of bevacizumab plus paclitaxel in breast cancer also showed benefit leading to its approval in metastatic breast cancer in [ 12 ]. Other studies in RCC patients upon treatment with sorafenib also revealed that high baseline levels of VEGF were related to poor prognosis [ ], while serum levels of circulating neutrophil gelatinase-associated lipocalin NGAL and VEGF were powerfully supported prolonged PFS in RCC patients receiving sunitinib [ ]. Angiopoietin—Tie signalling in the cardiovascular and lymphatic systems. Also, Ziv-aflibercept in combination with 5-fluorouracil, leucovorin, irinotecan FOLFIRI are used to treat patients with metastatic CRC [ 71 ]. Thalidomide Thalomid ® was synthesized by the CIBA pharmaceutical company in and was initially used for mitigating morning sickness as a non-addictive and non-barbiturate tranquilizer Table 1. Abstract Tumours require a vascular supply to grow and can achieve this via the expression of pro-angiogenic growth factors, including members of the vascular endothelial growth factor VEGF family of ligands. |
| Anti-Angiogenic Therapy and Cardiovascular Diseases: Current Strategies and Future Perspectives | The present era of anti-angiogenic treatment for cancer research started in with the publishing of Folkman's creative hypothesis [ 47 ], although it would take 33 years for the FDA to authorize the first drug produced as a blocker of angiogenesis. Bevacizumab, a humanized monoclonal antibody targeted against VEGF, was coupled with standard chemotherapy in a randomly selected phase 3 study of first-line therapy of metastatic colorectal cancer CRC [ 48 ]. When utilized in conjunction with conventional chemotherapy, bevacizumab therapy improved overall survival OS in the first-line treatments of advanced non—small-cell lung cancer NSCLC [ 49 ]. The FDA of the United States has authorized a variety of angiogenesis inhibitors for the treatment of cancer. Most of them are targeted treatments created to target VEGF, its receptor, or other angiogenesis-related molecules. Bevacizumab, axitinib, everolimus, cabozantinib, lenalidomide, lenvatinib, pazopanib, ramucirumab, regorafenib, sorafenib, sunitinib, thalidomide, Ziv-aflibercept and vandetanib are most famous accepted angiogenesis inhibitors, which have been approved for human advanced tumors [ 50 ]. As the first VEGF-targeted agent approved by FDA, bevacizumab, is used since February , for the treatment of patients suffering from metastatic m CRC in combination with the standard chemotherapy treatment as first-line treatment [ 51 ]. In June , it was approved with fluorouracil 5-FU -based therapy for second-line mCRC. Also, it has been indicated for NSCLC plus chemotherapy , breast cancer, glioblastoma, ovarian cancer plus chemotherapy , and also cervical cancer [ 51 ]. Another well-known angiogenesis inhibitor, axitinib, has gained approval from FDA for use as a treatment for renal cell carcinoma RCC since January and also has shown promising outcomes in pancreatic cancer plus gemcitabine [ 52 , 53 ]. Moreover, since , it is used for neuroendocrine tumors NET of gastrointestinal GI or lung origin with unresectable, locally advanced, or metastatic disease [ 55 ]. In November , cabozantinib, a small molecule inhibitor of the tyrosine kinases c-Met and VEGFR2, was approved for thyroid cancer [ 56 ] and also in April was accepted as second-line treatment for RCC [ 57 ]. Lenalidomide, a 4-amino-glutamyl analogue of thalidomide, is used to treat multiple myeloma MM [ 58 ] and myelodysplastic syndromes MDS [ 59 ], and also lenvatinib, which acts as a multiple kinase inhibitor against the VEGFR1, VEGFR2, and VEGFR3 kinases, is applied for the treatment of thyroid cancer [ 60 ]. In , lenvatinib was also approved in combination with everolimus for the treatment of advanced RCC [ 61 ]. Since , pazopanib, a potent and selective multi-targeted receptor tyrosine kinase inhibitor, is utilized for metastatic RCC and advanced soft tissue sarcomas therapy [ 62 ]. Besides, since April , the ramucirumab, a direct VEGFR2 antagonist, is indicated as a single-agent treatment for advanced gastric cancer or gastro-esophageal junction GEJ adenocarcinoma after treatment with fluoropyrimidine- or platinum-containing chemotherapy [ 63 ]. Further, ramucirumab in combination with docetaxel has gained approval for treatment of metastatic NSCLC [ 64 ]. Ramucirumab also is used for mCRC since [ 65 ] and HCC since [ 66 ] therapy. Also, regorafenib, an orally-administered inhibitor of multiple kinases, has been indicated for the treatment of patients with advanced HCC who were previously treated with sorafenib [ 67 ]. Moreover, sorafenib as another type of kinase inhibitor is used since for RCC and HCC therapy, and since for thyroid cancer [ 68 ]. Multi-targeted receptor tyrosine kinase inhibitor sunitinib also is applied for gastrointestinal stromal tumor GIST and RCC therapy [ 69 ]. In addition, since , thalidomide as a type of biological therapy in combination with dexamethasone has been approved for the treatment of newly diagnosed MM patients [ 70 ]. Also, Ziv-aflibercept in combination with 5-fluorouracil, leucovorin, irinotecan FOLFIRI are used to treat patients with metastatic CRC [ 71 ]. Finally, tyrosine kinase inhibitor vandetanib is employed to treat medullary thyroid cancer in adults who are ineligible for surgery [ 72 , 73 ]. Despite their total tumor growth reduction, therapeutic anti-angiogenic agents were linked to enhanced local invasiveness as well as distant metastasis. These events seem to be significant factors to resistance to anti-angiogenesis treatments. They were originally reported in various preclinical models by Paez-Ribes and coworkers [ 74 ]. Based on the literature, anti-angiogenic treatment may increase tumor invasiveness. RCC cells, for example, showed increased proliferation and an invasive character after being treated with bevacizumab [ 75 ]. Likewise, glioblastoma cells in mice models were more invasive after VEGF suppression [ 74 ]. Sunitinib treatment also has been found to cause vascular alterations such as decreased adherens junction protein expression, reduced basement membrane, pericyte coverage, and increased leakiness [ 76 , 77 ]. These phenotypic alterations were found in both normal and tumor organ arteries, indicating that they promote tumor cell local intravasation and extravasation, culminating in metastatic colonization [ 78 ]. Angiogenesis blockade therapy may lead to vascular regression and resultant intra-tumoral hypoxia. Various investigations have been fulfilled to assess an enhancement in hypoxic areas in primary tumors upon angiogenesis blockade therapy [ 76 , 79 ]. Further investigation also exposed an attendant augmentation in HIF-1a expression during treatment. HIF-1a and hypoxia are recognized drivers of epithelial-mesenchymal transition EMT , a process that induced tumor metastasis. Significant improvement in the expression and activities of EMT-related genes e. Moreover, loss of the epithelial marker, E-cadherin, and the stimulation of the mesenchymal marker, vimentin, has been evidenced following anti-angiogenic treatment [ 80 ]. Hypoxic milieu also largely promotes VEGF expression by the upstream transcription factor HIF-1a [ 81 ]. HIF-1, in turn, inspires tumors to achieve more angiogenic and invasive competencies, culminating in metastasis [ 82 ]. In fact, hypoxia and EMT bring about increased invasiveness and metastasis of tumors mainly caused by up-regulation of c-Met, Twist, and HIF-1a [ 83 , 84 ]. Conversely, semaphorin 3A Sema3A , a well-known endogenous anti-angiogenic molecule, is substantially down-regulated in tumors, ensuring provoked invasiveness and metastasis [ 85 ]. Ang-Tie signaling system is a vascular-specific receptor tyrosine kinases RTK pathway complicated in modifying the vascular permeability and blood vessel formation and remodeling by potent angiogenic growth factors, Ang-1 and Ang-2 [ 86 ]. Molecular analysis has confirmed that activation of the Ang-Tie pathway as a result of the connection between Ang-1 and Tie2 receptor on the M2 subpopulation of monocytes, hematopoietic stem cells HSCs , and endothelial cells ECs of blood and lymphatic vessels elicits maturation or stabilization of blood vessels [ 80 ]. Besides, Ang-2 suppresses this pathway, eventually sustaining remodeling or generation of vascular sprouts upon exposure to VEGF [ 87 ]. Ang-2 up-regulation has been noticed in multiple types of tumors and is likely involved in resistance versus anti-VEGF therapy [ 88 , 89 ]. For instance, there is clear evidence signifying that enhanced serum Ang-2 levels are in association with an undesired response to bevacizumab therapy in CRC patients [ 90 ]. Studies in lung adenocarcinoma patients revealed that elevated levels of VEGFA and Ang-2 is valued prognostic biomarkers and double targeting of VEGFA and Ang-2 can improve therapeutic outcome [ 91 ]. As well, up-regulation and compensatory mechanisms of other growth factors, in particular basic fibroblast growth factor bFGF , are thought to contribute to the stimulation of the resistance to VEGF targeted therapies. Improved level of the bFGF has strongly been evidenced in the chronic inflammation area, after tissue injury, as well as human cancers bevacizumab [ 92 ]. Upon bevacizumab treatment in glioblastoma tumor models, Okamoto et al. showed the increased levels of the bFGF and PDGF expression in the endothelial cells, pericytes, and also tumor cells, in turn, caused robust resistance to bevacizumab [ 94 ]. Also, cancer patients with up-regulated bFGF in serum usually show no desired response to sunitinib, indicating the necessity of co-targeting VEGF and bFGF pathways concurrently [ 95 , 96 ]. Increased metastasis and invasiveness in response to anti-angiogenesis therapy vary according to treatment type, dosage, and schedule. Sunitinib and anti-VEGF antibody monotherapy showed varied effects on mice tumor models, according to Singh et al. reports [ 77 ]. While sunitinib therapy increased tumor cell aggressiveness, anti-VEGF antibody treatment did not [ 77 ]. Chung et al. also corroborated these findings by comparing the effectiveness of several RTK inhibitors and antibody treatments in mouse models [ 97 ]. Though imatinib, sorafenib, or sunitinib increased lung metastasis after 66c14 cell injection, employing an anti-VEGFR2 antibody reduced the development of lung nodules [ 97 ]. Overall, reports show that the increased metastasis and invasiveness caused by angiogenesis blockade therapy depend highly on the treatment type. Anti-angiogenic drug dosage and delivery schedules may also potentially cause resistance. Sunitinib at high doses accelerated tumor development and facilitated metastasis to the lung and liver, resulting in decreased survival [ 74 , 98 ]. Although sorafenib had comparable outcomes, sunitinib produced conflicting findings in various trials. High-dose sunitinib therapy before systemic injection of tumor cells enhanced the metastatic potential of lung cancer cells, but not RCC cells. Recently, scientists have concentrated on the role of immune checkpoint molecules, such as cytotoxic T-lymphocyte antigen-4 CTLA-4 and programmed cell death protein 1 PD-1 , largely participating in tumor cell escape from immune surveillance as their capacity to obstruct T cell activation [ 99 , ]. Hence, immune checkpoint inhibitors ICIs have been evolved for suppressing these immune checkpoint molecules [ ]. FDA-approved ICIs comprise the nivolumab, cemiplimab, and pembrolizumab, atezolizumab, avelumab, durvalumab, and also ipilimumab [ ]. Atezolizumab has been approved for use in combination with bevacizumab, paclitaxel, and carboplatin as the first-line treatment of patients with NSCLC [ ]. PD-L1 expression is regulated by various factors, such as inflammatory and oncogenic signaling, leading to the varied significances of PD-L1 positivity. Recent reports exhibited that combination therapy with anti-angiogenic agents and ICIs could elicit synergistic anti-tumor effects in preclinical models as well as humans Table 3. Meanwhile, co-administration of anti-PD-1 and anti-VEGFR2 monoclonal antibodies mAbs in the Colon adenocarcinoma mice model gave rise to the potent inhibition of tumor growth synergistically without overt toxicity [ ]. VEGFR2 blockade therapy negatively regulated tumor neovascularization, as evidenced by the attenuated frequencies of microvessels, whereas PD-1 inhibition exerted no effect on tumor angiogenesis. PD-1 mAbs improved T cell infiltration into tumors and promoted local immune response, as documented via the improvement in various proinflammatory cytokine expressions. Such events signified that concurrent suppression of PD-1 and VEGFR2 might inspire synergistic in vivo anti-tumor influences by dissimilar mechanisms [ ]. Further, in a mouse model of small-cell lung cancer SCLC , co-administration of anti-VEGF and anti-PD-L1 mAbs resulted in a more prominent therapeutic outcome than mono therapy with each agent [ ]. Notably, the depleted T-cell phenotype following anti-PD-L1 therapy was revoked through the addition of anti-VEGF blockade therapy. Analysis revealed that VEGFA expression improves the expression of the inhibitory receptor TIM-3 on T cells, representative of an immunosuppressive action of VEGF in patients with SCLC during PD-1 blockade therapy. Thereby, it seems that VEGFA inhibition may entice T cell activation at higher levels, facilitating T cell-mediated anti-tumor immunity [ ]. Similarly, combination therapy with sunitinib and PD-L1 blocked therapy prolonged overall survival OS of treated RCC mice models in comparison to mono therapy with either drug [ ]. Besides, in the triple-negative breast cancer TNBC mice model, PD-L1 blocking was highly effective as an adjuvant monotherapy. However, its co-administration with paclitaxel chemotherapy with or without VEGF blocked therapy showed superiority over neoadjuvant therapy [ ]. In , Schmittnaegel et al. also noticed that dual Ang-2 and VEGFA inhibition induced antitumor immunity that was promoted by PD-1 blockade therapy in breast cancer, pancreatic neuroendocrine tumor, and melanoma [ ]. They showed that Ang-2 and VEGFA blockade by a bispecific antibody A2V caused vascular regression, tumor necrosis along with improved antigen presentation by intratumoral phagocytes [ ]. Recent clinical trials have also shown that bevacizumab plus atezolizumab could induce synergistic influence on the median OS of patients with RCC [ ], and also in combination with nivolumab could elicit modest efficacy in ovarian cancer patients [ ]. Also, co-administration of PD-L1 inhibitor avelumab with axitinib resulted in improved objective response rate ORR in HCC [ ] and also RCC [ ] patients, with acceptable safety profile. Also, combination therapy with axitinib and pembrolizumab enhanced median progression-free survival PFS in sarcoma patients more evidently than axitinib or pembrolizumab monotherapy. The most common treatment-related unwanted events were autoimmune colitis, pneumothorax, transaminitis, seizures, hemoptysis, and hypertriglyceridemia [ ]. Besides, co-administration of regorafenib plus nivolumab resulted in significant antitumor impacts in patients with gastric cancer and CRC [ ]. Moreover, co-administration of nivolumab plus sunitinib or pazopanib showed a significant anti-tumor effect in advanced RCC patients [ , ]. Conversely, other trials revealed that combined use of regorafenib plus nivolumab [ ] and also ramucirumab plus pembrolizumab [ ] had no remarkable therapeutic merits in CRC patients [ ] and patients with advanced biliary tract cancer BTC [ ], respectively. Therapeutic cancer vaccines ease tumor regression, remove minimal residual disease MRD , entice durable antitumor memory, and also averts non-specific or adverse events [ , ]. Till, FDA has approved three cancer vaccines, comprising Bacillus Calmette-Guérin BCG lives, sipuleucel-T, and also talimogene laherparepvec T-VEC respectively for patients with early-stage bladder cancer, prostate cancer as well as melanoma [ ]. In the melanoma mice model, Bose and coworkers found that a treatment regimen comprising a 7-day course of axitinib 0. These desired outcomes are probably exerted by a decrease in myeloid-derived suppressor cells MDSC and Treg frequencies in the tumor concomitant with induction and recruitment of CTLs in TME [ ]. Also, addition of the axitinib to oncolytic herpes simplex virus oHSV expressing murine IL12 G47Δ-mIL12 triggered improved OS in both immunodeficient and immunocompetent orthotopic glioblastoma mice models than mice receiving monotherapy [ ]. Notably, the addition of the ICI did not promote efficacy in mice models [ ]. As well, combination therapy with sunitinib and vesicular stomatitis virus VSV brought about the eradication of prostate, breast, and kidney malignant tumors in mice, while monotherapy with VSV or sunitinib did not [ ]. Importantly, enhancement in median viral titers by fold following combination therapy indicated that this regimen could potentiate oncolytic virotherapy permitting the recovery of tumor-bearing animals. In RCC and NSCLC mice model, co-injection of reovirus and sunitinib more potently attenuated tumor burden supporting improved OS, and also reduced the population of immune suppressor cells in tumors compared with monotherapy with reovirus [ ]. Thereby, it appears that this regimen can be a rational and effective strategy ready for clinical testing against RCC and NSCLC. Also, Tan and coworkers showed that the bevacizumab improved viral distribution and also tumor hypoxia and promoted the population of apoptotic cells and thus stimulated a synergistic antitumor impact when used in combination with oHSV in TNBC murine models [ ]. Combining bevacizumab with OHSV expressing vasculostatin RAMBO also demonstrated great anti-tumor capacities in glioma xenografts [ ]. Correspondingly, intratumorally administration of RAMBO 1 week after tumor inoculation, and intraperitoneally administration of bevacizumab twice a week reduced migration as well as invasion of glioma cells [ ]. Co-treated mice also experienced improved OS and dampened tumor invasion than those treated with bevacizumab alone [ ]. In another study, combining tumor antigen-loaded DCs vaccination and anti-angiogenic molecule lenalidomide synergistically potentiated antitumor immunity in the mice colon cancer model, largely provided by suppressing the establishment of immune suppressive cells and also activation of effector cells, such as natural killer NK cells [ ]. This regimen similarly caused a robust reduction in tumor growth and malignant cell spread in lymphoma [ ] and also myeloma [ ] xenografts by similar mechanisms. Further, lenalidomide in combination with a fusion DNA lymphoma vaccine reduced the systemic population of MDSC and Treg in tumor-bearing mice and also led to the decreased tumor burden [ ]. In addition, the combination therapy supported the incidence of the higher rates of the antitumor T cells, providing further rationale for clinical application [ ]. Currently, a clinical trial was conducted to address the safety and efficacy of combination therapy with sipuleucel-T as a cellular prostate cancer vaccine with bevacizumab in 22 prostate cancer patients [ ]. Combination therapy persuaded immune reactions and also alleviated prostate-specific antigen PSA in participants with biochemically recurrent prostate cancer [ ]. In contrast, co-administration of bevacizumab plus MA multi-peptide vaccine adjuvanted with poly-ICLC polyinosinic-polycytidylic acid stabilized with polylysine and carboxymethylcellulose did not show superiority over monotherapy with each agent in terms of alteration in OS and PFS in glioblastoma patients [ ]. However, a phase II study evaluating the safety and efficacy of bevacizumab in combination with ERC, advanced immunotherapy based on freshly extracted tumor cells and lysates, revealed that this regimen could prolong the OS in patients who received ERC plus bevacizumab compared to bevacizumab monotherapy 12 months versus 7. Besides, evaluation of the safety, tolerability, and anti-myeloma activity of the PVX, a novel tetra-peptide vaccine with 3 of the 4 antigens XBP1 [2 splice variants] and CD with or without lenalidomide was accomplished in MM patients by Nooka et al. They showed that the PVX vaccine was well tolerated, accompanied by mild injection site reactions and constitutional symptoms. Meanwhile, 5 of 12 patients showed clinical response to combination therapy [ ]. Importantly, CRC patients presented complete pathological remission following treatment with bevacizumab, oxaliplatin plus leucovorin and 5-fluorouracil FOLFOX-4 , surgery, and the oncolytic virus Rigvir [ ]. In consistence with previous findings, it appears that angiogenesis blockade therapy could promote viral delivery through targeting the TME [ ]. Adoptive cell therapy ACT with using TILs or genetically-modified T cells expressing novel T cell receptors TCR or chimeric antigen receptors CAR T cells or CAR-NK cells is another plan to convince the immune system to stimulate recognition of the maligned cells and then their eradication [ , ]. Notwithstanding, tumor vasculature usually obstructs the tumor-specific T cells infiltration, averting anti-tumor immunity. In the B16 melanoma mice model, co-administration of anti-VEGF mAb to ACT abrogated tumor progress and improve OS [ ]. Similarly, anti-angiogenic therapy could also improve the antitumor functions of cytokine-induced killer cells CIK cells cells by normalizing tumor vasculature and alleviating the hypoxic TME, as shown in NSCLC xenografts [ ]. Meanwhile, Shi et al. evaluated the therapeutic benefits of combination therapy with recombinant human endostatin rh-endostatin and CIK cells in NSCLC murine model. They exhibited that rh-endostatin normalized tumor vasculature and attenuated hypoxic regions in the TME [ ]. The rh-endostatin markedly potentiated the administrated CIK cells homing and also reduced immune suppressive cells frequency in the tumor tissue. On the other hand, the used regimen instigated a higher level of TILs in tumor tissue [ ]. Further, GD2-redirected CAR T cells plus bevacizumab displayed a remarkable anti-tumor effect in an orthotopic xenograft model of human neuroblastoma [ ]. Co-administration of bevacizumab or ganglioside GD2-CAR T cells or both by single systemic injection supported higher rates of CAR T cells infiltration into tumor tissue accompanied with improved IFN-γ levels in TME. Additionally, the analysis presented that PD-L1 blockade therapy might augment the efficacy of this regimen [ ]. Likewise, epithelial cell adhesion molecule EpCAM redirected CAR NK cells injection resulted in CRC cell regression in animal models, which was potentiated when used in combination with regorafenib [ ]. These findings delivered a novel plan for the treatment of CRC and also other solid tumors. Anti-angiogenic agents as noticed can transiently stimulate a functional normalization of the disorganized labyrinth of vessels, sustaining the therapeutic efficacy of coadministered chemotherapeutic agents. Notwithstanding, durable angiogenesis suppression usually fences tumor uptake of chemotherapeutic drugs, and so accomplishment of further studies in this context are urgently required [ ]. Correspondingly, designing intermittent treatment schedules is of paramount significance [ ]. A study in 9L glioma cell-bearing rats showed that coadministration of axitinib with metronomic cyclophosphamide potently suppressed tumor progress, whereas multiple treatment cycles were needed by monotherapy with metronomic cyclophosphamide to abrogate tumor growth [ ]. Unfortunately, the abridged tumor infiltration of 4-OH-CPA resulted in a reduction in cyclophosphamide-mediated 9L cell elimination [ ]. Such events in turn underlined lacking tumor complete regression by applied combined regimen, reflecting the importance of the optimization of drug scheduling and dosages. In another study, co-administration of the bevacizumab plus cisplatin and paclitaxel concurrently also induced reduced tumor growth as well as improved OS in ovarian cancer xenografts [ ]. Also, monotherapy with bevacizumab suppressed ascites formation, accompanied by the partial impact on tumor burden [ ]. TNP, an angiogenesis inhibitor, plus cisplatin inhibited the liver metastasis of human pancreatic carcinoma [ ]. Indeed, liver metastasis percentages reduced from While monotherapy with each agent did not modify tumor growth in vivo, the addition of TNP to cisplatin strikingly reduced tumor growth [ ]. Of course, it seems that TNP may entice a decrease in glioma tumor uptake of some chemotherapeutic drugs, such as temozolomide, by affecting the tumor vasculature as a result of its pharmacodynamic effect [ ]. As cited, more comprehensive studies are required to define how these combinations can efficiently be utilized. Another study also exhibited that the addition of the TNP to cisplatin chemotherapy reduced the microvascular density of bladder cancer in a murine model [ ]. Nonetheless, TNP has no significant influence on the cisplatin impact versus bladder cancer as determined by apoptosis and cell proliferation [ ]. Besides, Bow and coworkers demonstrated that local delivery of angiogenesis-inhibitor minocycline could potentiate the anti-tumor efficacy of radiotherapy RT and oral temozolomide, as evidenced by enhanced OS in a rodent glioma model [ ]. These findings offered further evidence for the idea that angiogenesis inhibitors in combination with conventional therapeutic modalities could promote OS in glioblastoma patients [ ]. Moreover, the addition of the novel anti-angiogenic agent, SU, to paclitaxel supported improved PFS accompanied with some mild to modest adverse events e. However, the regimen led to the occurrences of thromboembolic events and prophylactic anticoagulation, suggesting that careful consideration must be taken. Besides, TSU when used plus carboplatin and paclitaxel showed a manageable safety profile in NSCLC patients [ ]. Furthermore, combining TNP and paclitaxel was well tolerated with no significant pharmacokinetic interaction between them in NSCLC patients [ ]. Further, several clinical trials have verified the efficacy of combination therapy with anti-angiogenic agent and conventional therapy in patients with ovarian cancer [ , ], CRC [ , ], NSCLC [ ], MCL [ ] and also MM [ ]. For instance combination therapy with bevacizumab and paclitaxel plus carboplatin prolonged the median OS in participants with platinum-sensitive recurrent ovarian cancer [ ]. Finally, axitinib combined with cisplatin and gemcitabine [ ] and also bevacizumab plus paclitaxel and carboplatin [ ] induced significant anti-tumor effect in NSCLC patients, as documented by improved OS and PFS. In addition, the Ziv-aflibercept in combination with 5-fluorouracil, leucovorin, and irinotecan FOLFIRI significantly promoted OS in a phase III study of patients with metastatic CRC previously treated with an oxaliplatin-based regimen [ ]. However, Ziv-aflibercept in combination with cisplatin and pemetrexed did not significantly affect OS and PFS in patients with previously untreated NSCLC cancer [ ]. A list of trials based on combination therapy with angiogenesis inhibitors plus chemotherapy or chemoradiotherapy has been offered Table 4. RT crucially contributes to the multimodality treatment of cancer. Current evolving in RT have chiefly complicated improvements in dose delivery [ ]. Upcoming developments in tumor therapeutics will probably include the combination of RT with targeted therapies. Meanwhile, preliminary results of anti-angiogenic agents in combination with RT have produced encouraging consequences [ ]. Further, there are clear proofs that suggest that well-vascularized and perfused tumors mainly exhibit desired response to RT [ , ]. Studies have shown that the addition of the angiogenesis-inhibitor minocycline to radiotherapy and oral temozolomide could result in prolonged OS in a murine glioma model [ ]. Anti-angiogenesis therapy using anginex in combination with RT also supported tumor control in squamous cell carcinoma SCC xenografts accompanied by reducing oxygen levels in tumor tissue [ ]. Observation showed that the applied regimen modified the amount of functional vasculature in tumors and also augmented radiation-elicited tumor eradication [ ]. Likewise, robust hindrance of tumor proliferation was achieved from the addition of the angiogenesis inhibitor TNP to RT in SCC xenografts more evidently than monotherapy with each approach [ ]. Also, it was speculated that exclusive investigation of each tumor neovascularization competence can be imperative before deciding the angiogenesis blockade treatment [ ]. In contrast, the addition of TNP to RT attenuated the tumor control probability in murine mammary carcinoma [ ]. Such unanticipated consequence could be ensured from the partial reserve of reoxygenation by TNP, as no remarkable alteration was shown between the RT plus TNP and RT alone under hypoxic conditions [ ]. A potent anti-angiogenesis agent, liposomal honokiol, also elicited significant anti-tumor influence by stimulating apoptosis and also suppressing angiogenesis when used plus RT in Lewis lung cancer LLC xenografts [ ]. Liposomal honokiol, in fact, could ameliorate tumor cell radiosensitivity in vivo, offering that RT plus liposomal honokiol can engender better anti-tumor efficacy in a myriad of tumors, such as lung cancer, SCC, and CRC [ , , ]. In , Yang et al. evaluated the safety and efficacy of that combination therapy with axitinib plus RT in advanced HCC patients. They exhibited that the regimen was well tolerated with an axitinib MTD of 3 mg twice daily [ ]. Besides, the addition of the bevacizumab to adjuvant radiotherapy was associated with the manageable safety profile in breast cancer patients [ ]. Likewise, erlotinib in combination with bevacizumab as well as capecitabine-based definitive chemoradiation CRT showed acceptable safety in unresectable pancreatic cancer patients [ ]. As well 2 of 9 participants showed complete response to intervention [ ]. Of course, large-scale trials on this newer therapeutic mean seem justified. Albeit there are some reports which show that combining anti-angiogenic therapy with RT had no therapeutic advantages. For instance, in rectal carcinoma patients, combination therapy with bevacizumab and capecitabine plus RT revealed no merits in terms of improved PFS or OS in the short or long term during a phase 2 clinical trial NCT [ ]. As a result of some divergences results related to anti-angiogenic agents as well as their modest responses, we must determine and categorize a spectrum of biomarkers, screening the patients of possible responders [ ]. Additionally, such biomarkers are urgently required to can monitor disease development and angiogenic actions of tumors following exposure with treatment angiogenesis inhibitors. There are some reports showing that angiogenesis inhibitors could not support therapeutic effect in previously treated metastatic breast cancer [ ]. These undesired events are likely related to the secretion of pro-angiogenic factors from resistant malignant tissue [ ]. The finding outlines the importance of determining biomarkers to predict the efficacy of VEGF-targeted therapies. Much effort has been spent in this regard and resulted in the finding several biomarkers comprising dynamic measurements such as variations in systemic blood pressure , circulating markers such as VEGF serum levels , genotypic markers such as VEGF polymorphism , blood cells frequencies such as progenitor cells , tissue markers such as IFP and also imaging parameters [such as estimating capillary permeability employing magnetic resonance imaging MRI ] [ ]. Recent studies have revealed that there is a negative correlation between OS with serum lactate dehydrogenase LDH and neutrophil levels in CRC patients who received bevacizumab plus standard chemotherapy [ ]. Besides, enhanced IL-8 levels were associated with shorter PFS, while low Ang-2 serum levels were related to improved OS in tumor patients undergoing angiogenesis blockade therapy [ 90 ]. Circulating endothelial cells CEC also has been determined as a robust indicator for the outcome of treatment with bevacizumab. On the other hand, greater intra-tumoral expression of VEGFR-3 may predict better response, while overexpression of VEGFR1 mainly indicates poor survival [ ]. Other studies in RCC patients upon treatment with sorafenib also revealed that high baseline levels of VEGF were related to poor prognosis [ ], while serum levels of circulating neutrophil gelatinase-associated lipocalin NGAL and VEGF were powerfully supported prolonged PFS in RCC patients receiving sunitinib [ ]. In contrast to the classical hypothesis of vascular regression, the central aim of conventional anti-angiogenic treatments is tumor vascular normalization and maturity. This event, in turn, offered enhanced tumor access to chemotherapeutic drugs and underlays more efficient cancer immunotherapy. As cited, survival benefits of angiogenesis blockade therapy are compromised by cancer resistance to theses agent, and thereby provoke interest in evolving more effective means to combine anti-angiogenic drugs with other conventional therapeutics. To date, a large number of clinical trials have evaluated the safety and therapeutic merits of angiogenesis blockade therapy alone or in combination with other modalities in cancer panties Fig. Although combination therapy regimen mainly caused significant efficacy in cancer patients, intervention-related toxicities hurdle their application in clinic. For instance, bevacizumab therapy could sustain ischemic heart disease. Indeed, CRC patients receiving bevacizumab may experience considerably augmented possibility of cardiac ischemia [ ]. In addition, it has been proved that combination therapy with angiogenesis inhibitors and chemotherapeutic agents may attenuate antitumor effects of chemotherapy. Hence, further rigorous investigations are warranted to circumvent the cited problems. Moreover, determining the suitable dose and sequence is of paramount importance to optimize the effectiveness, toxicity, and tolerability of the combination therapy. Thanks to the involvement of a myriad of cytokines and growth factors and the resultant interplay and compensation among them, co-targeting various growth factors is urgently required. The recognition and potent suppression of downstream kinases and strategic signaling biomolecules where several angiogenic pathways converge may defeat current difficulties motivated via the variety of angiogenic ligands and receptors and should be the emphasis of upcoming investigations. For instance, dual EGFR inhibition erlotinib and cetuximab combined with bevacizumab is a safe and well-tolerated combination, demonstrating antitumor activity in patients with solid tumors [ ]. BQ13esides, continued treatment with conventional anti-angiogenic agents is related to toxicity and drug resistance. These conditions offer a robust justification for novel plans to improve the efficacy of mAbs targeting tumor vasculature, such as antibody—drug conjugates ADCs and peptide-drug conjugates PDCs , offering a new avenue to exert anti-angiogenic effects on cancerous cells. Clinical trials based on cancer therapy by anti-angiogenic agents registered in ClinicalTrials. gov October The schematic exemplifies clinical trials utilizing anti-angiogenic agents depending on the study status A , study phase B , study location C , and condition D in cancer patients. Folkman J. Annu Rev Med. CAS PubMed Google Scholar. Senger DR, Davis GE. Cold Spring Harb Perspect Biol. PubMed PubMed Central Google Scholar. Aguilar-Cazares D, Chavez-Dominguez R, Carlos-Reyes A, Lopez-Camarillo C, Hernadez de la Cruz ON, Lopez-Gonzalez JS. Contribution of angiogenesis to inflammation and cancer. Front Oncol. Kerbel RS. Tumor angiogenesis. N Engl J Med. CAS PubMed PubMed Central Google Scholar. Reinmuth N, Parikh AA, Ahmad SA, Liu W, Stoeltzing O, Fan F, et al. Biology of angiogenesis in tumors of the gastrointestinal tract. Microsc Res Tech. Rajendran JG, Krohn KA. Imaging hypoxia and angiogenesis in tumors. Radiol Clin. Google Scholar. Muthukkaruppan VR, Kubai L, Auerbach R. Tumor-induced neovascularization in the mouse eye J Natl Cancer Inst. Antiangiogenic therapy of transgenic mice impairs de novo tumor growth. Proc Natl Acad Sci U S A. Teleanu RI, Chircov C, Grumezescu AM, Teleanu DM. Tumor angiogenesis and anti-angiogenic strategies for cancer treatment. J Clin Med. CAS Google Scholar. Al-Abd AM, Alamoudi AJ, Abdel-Naim AB, Neamatallah TA, Ashour OM. Anti-angiogenic agents for the treatment of solid tumors: potential pathways, therapy and current strategies—a review. J Adv Res. Dudzinski SO, Cameron BD, Wang J, Rathmell JC, Giorgio TD, Kirschner AN. Combination immunotherapy and radiotherapy causes an abscopal treatment response in a mouse model of castration resistant prostate cancer. J Immunother Cancer. Lee JJ, Chu E. Adjuvant chemotherapy for stage II colon cancer: the debate goes on. J Oncol Pract. Shahneh FZ, Baradaran B, Zamani F, Aghebati-Maleki L. Tumor angiogenesis and anti-angiogenic therapies. Hum Antib. Sharma PS, Sharma R, Tyagi T. Curr Cancer Drug Targets. Pro-angiogenic peptides in biomedicine. Arch Biochem Biophys. PubMed Google Scholar. Li X, Eriksson U. Novel VEGF family members: VEGF-B, VEGF-C and VEGF-D. Int J Biochem Cell Biol. Tomanek RJ, Holifield JS, Reiter RS, Sandra A, Lin JJ. Role of VEGF family members and receptors in coronary vessel formation. Dev Dyn. Shibuya M. Vascular Endothelial Growth Factor VEGF and its receptor VEGFR signaling in angiogenesis: a crucial target for anti- and pro-angiogenic therapies. Genes Cancer. Zachary I. Neuropilins: role in signalling, angiogenesis and disease. Chem Immunol Allergy. Mercurio AM. Int J Mol Sci. CAS PubMed Central Google Scholar. Finley SD, Popel AS. Predicting the effects of anti-angiogenic agents targeting specific VEGF isoforms. AAPS J. Qin S, Li A, Yi M, Yu S, Zhang M, Wu K. Recent advances on anti-angiogenesis receptor tyrosine kinase inhibitors in cancer therapy. J Hematol Oncol. Dey N, De P, Brian L-J. Evading anti-angiogenic therapy: resistance to anti-angiogenic therapy in solid tumors. Am J Transl Res. Liang P, Ballou B, Lv X, Si W, Bruchez MP, Huang W, et al. Monotherapy and combination therapy using anti-angiogenic nanoagents to fight cancer. Adv Mater. Ribatti D, Nico B, Crivellato E. The role of pericytes in angiogenesis. Int J Dev Biol. Wang X, Ma W, Han S, Meng Z, Zhao L, Yin Y, et al. Sci Rep. Kim JH, Kim S-K, Wang K-C. Moyamoya disease update. Tokya: Springer; Jayatilleke KM, Hulett MD. Heparanase and the hallmarks of cancer. J Transl Med. Vempati P, Popel AS, Mac GF. Extracellular regulation of VEGF: isoforms, proteolysis, and vascular patterning. Cytokine Growth Factor Rev. Rundhaug JE. Matrix metalloproteinases, angiogenesis, and cancer: commentary re: AC Lockhart et al. Cancer Res. Clin Cancer Res. Matrix metalloproteinases and angiogenesis. J Cell Mol Med. Tang Y, Nakada MT, Kesavan P, McCabe F, Millar H, Rafferty P, et al. Extracellular matrix metalloproteinase inducer stimulates tumor angiogenesis by elevating vascular endothelial cell growth factor and matrix metalloproteinases. Can Res. Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. Colomb F, Wang W, Simpson D, Zafar M, Beynon R, Rhodes JM, et al. Galectin-3 interacts with the cell-surface glycoprotein CD MCAM, MUC18 and induces secretion of metastasis-promoting cytokines from vascular endothelial cells. J Biol Chem. Tatum JL, Hoffman JM. Angiogenesis imaging methodology: AIM for clinical trials. Voron T, Marcheteau E, Pernot S, Colussi O, Tartour E, Taieb J, et al. Control of the immune response by pro-angiogenic factors. Sheu B-C, Chang W-C, Cheng C-Y, Lin H-H, Chang D-Y, Huang S-C. Cytokine regulation networks in the cancer microenvironment. Front Biosci. Fahey E, Doyle SL. IL-1 family cytokine regulation of vascular permeability and angiogenesis. Front Immunol. Mountain DJ, Singh M, Menon B, Singh K. Am J Physiol Cell Physiol. Carmi Y, Voronov E, Dotan S, Lahat N, Rahat MA, Fogel M, et al. The role of macrophage-derived IL-1 in induction and maintenance of angiogenesis. J Immunol. Huang Q, Duan I, Qian X, Fan J, Lv Z, Zhang X, et al. IL promotes angiogenic factors IL-6, IL-8, and Vegf production via Stat1 in lung adenocarcinoma. Rapisarda A, Hollingshead M, Uranchimeg B, Bonomi CA, Borgel SD, Carter JP, et al. Increased antitumor activity of bevacizumab in combination with hypoxia inducible factor-1 inhibition. Mol Cancer Ther. Yang Y, Sun M, Wang L, Jiao B. HIFs, angiogenesis, and cancer. J Cell Biochem. Niu Y, Bao L, Chen Y, Wang C, Luo M, Zhang B, et al. HIF2-induced long noncoding RNA RAB11B-AS1 promotes hypoxia-mediated angiogenesis and breast cancer metastasis. Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers Basel. Wang L, He T, Liu J, Tai J, Wang B, Chen Z, et al. Pan-cancer analysis reveals tumor-associated macrophage communication in the tumor microenvironment. Exp Hematol Oncol. Tumor angiogenesis: therapeutic implications. McCormack PL, Keam SJ. Sandler A. Bevacizumab in non-small cell lung cancer. Wu H-C, Huang C-T, Chang D-K. Anti-angiogenic therapeutic drugs for treatment of human cancer. J Cancer Mol. Mukherji S. Bevacizumab avastin. Am J Neuroradiol. Kelly RJ, Rixe O. Axitinib AG Small Mol Oncol. Article Google Scholar. Spano JP, Chodkiewicz C, Maurel J, Wong R, Wasan H, Barone C, et al. Efficacy of gemcitabine plus axitinib compared with gemcitabine alone in patients with advanced pancreatic cancer: an open-label randomised phase II study. Houghton PJ. Singh S, Carnaghi C, Buzzoni R, Pommier RF, Raderer M, Tomasek J, et al. Everolimus in neuroendocrine tumors of the gastrointestinal tract and unknown primary. Elisei R, Schlumberger MJ, Müller SP, Schöffski P, Brose MS, Shah MH, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. Chanzá NM, Xie W, Bilen MA, Dzimitrowicz H, Burkart J, Geynisman DM, et al. Cabozantinib in advanced non-clear-cell renal cell carcinoma: a multicentre, retrospective, cohort study. Lancet Oncol. PubMed Central Google Scholar. Armoiry X, Aulagner G, Facon T. Lenalidomide in the treatment of multiple myeloma: a review. J Clin Pharm Ther. Talati C, Sallman D, List A, editors. Lenalidomide: myelodysplastic syndromes with del 5q and beyond. Seminars in hematology. Amsterdam: Elsevier; Cabanillas ME, Habra MA. Lenvatinib: role in thyroid cancer and other solid tumors. Cancer Treat Rev. Leonetti A, Leonardi F, Bersanelli M, Buti S. Clinical use of lenvatinib in combination with everolimus for the treatment of advanced renal cell carcinoma. Ther Clin Risk Manag. Keisner SV, Shah SR. Poole RM, Vaidya A. Ramucirumab: first global approval. Garon EB, Cao D, Alexandris E, John WJ, Yurasov S, Perol M. A randomized, double-blind, phase III study of docetaxel and ramucirumab versus docetaxel and placebo in the treatment of stage IV non—small-cell lung cancer after disease progression after 1 previous platinum-based therapy REVEL : Treatment Rationale and Study Design. Clin Lung Cancer. Verdaguer H, Tabernero J, Macarulla T. Ramucirumab in metastatic colorectal cancer: evidence to date and place in therapy. Ther Adv Med Oncol. Syed YY. Ramucirumab: a review in hepatocellular carcinoma. Bruix J, Qin S, Merle P, Granito A, Huang Y-H, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment RESORCE : a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet. Abdelgalil AA, Alkahtani HM, Al-Jenoobi FI. Profiles of drug substances, excipients and related methodology, vol. Le Tourneau C, Raymond E, Faivre S. Sunitinib: a novel tyrosine kinase inhibitor. A brief review of its therapeutic potential in the treatment of renal carcinoma and gastrointestinal stromal tumors GIST. Palumbo A, Facon T, Sonneveld P, Blade J, Offidani M, Gay F, et al. Thalidomide for treatment of multiple myeloma: 10 years later. Blood J Am Soc Hematol. Patel A, Sun W. Ziv-aflibercept in metastatic colorectal cancer. Biol Targets Ther. Commander H, Whiteside G, Perry C. Wells SA Jr, Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. Grepin R, Guyot M, Jacquin M, Durivault J, Chamorey E, Sudaka A, et al. Maione F, Capano S, Regano D, Zentilin L, Giacca M, Casanovas O, et al. Semaphorin 3A overcomes cancer hypoxia and metastatic dissemination induced by antiangiogenic treatment in mice. J Clin Investig. Singh M, Couto SS, Forrest WF, Lima A, Cheng JH, Molina R, et al. Anti-VEGF antibody therapy does not promote metastasis in genetically engineered mouse tumour models. J Pathol. Welti JC, Powles T, Foo S, Gourlaouen M, Preece N, Foster J, et al. Contrasting effects of sunitinib within in vivo models of metastasis. Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by met signaling pathway. Haibe Y, Kreidieh M, El Hajj H, Khalifeh I, Mukherji D, Temraz S, et al. Resistance mechanisms to anti-angiogenic therapies in cancer. Jiang B-H, Agani F, Passaniti A, Semenza GL. V-SRC induces expression of hypoxia-inducible factor 1 HIF-1 and transcription of genes encoding vascular endothelial growth factor and enolase 1: involvement of HIF-1 in tumor progression. Pereira ER, Frudd K, Awad W, Hendershot LM. Endoplasmic reticulum ER stress and hypoxia response pathways interact to potentiate hypoxia-inducible factor 1 HIF-1 transcriptional activity on targets like vascular endothelial growth factor VEGF. Luo D, Wang Z, Wu J, Jiang C, Wu J. The role of hypoxia inducible factor-1 in hepatocellular carcinoma. BioMed Res Int. Article PubMed PubMed Central Google Scholar. Kitajima Y, Miyazaki K. The critical impact of HIF-1a on gastric cancer biology. Maione F, Molla F, Meda C, Latini R, Zentilin L, Giacca M, et al. Semaphorin 3A is an endogenous angiogenesis inhibitor that blocks tumor growth and normalizes tumor vasculature in transgenic mouse models. Fagiani E, Christofori G. Angiopoietins in angiogenesis. Cancer Lett. Hanahan D. Signaling vascular morphogenesis and maintenance. Eklund L, Saharinen P. Angiopoietin signaling in the vasculature. Exp Cell Res. Cook KM, Figg WD. Angiogenesis inhibitors: current strategies and future prospects. CA Cancer J Clin. Goede V, Coutelle O, Neuneier J, Reinacher-Schick A, Schnell R, Koslowsky T, et al. Identification of serum angiopoietin-2 as a biomarker for clinical outcome of colorectal cancer patients treated with bevacizumab-containing therapy. Br J Cancer. Qin S, Yi M, Jiao D, Li A, Wu K. Distinct roles of VEGFA and ANGPT2 in lung adenocarcinoma and squamous cell carcinoma. J Cancer. Gyanchandani R, Alves MVO, Myers JN, Kim S. A proangiogenic signature is revealed in FGF-mediated bevacizumab-resistant head and neck squamous cell carcinoma. Mol Cancer Res. Zhou Y, Wu C, Lu G, Hu Z, Chen Q, Du X. Okamoto S, Nitta M, Maruyama T, Sawada T, Komori T, Okada Y, et al. Bevacizumab changes vascular structure and modulates the expression of angiogenic factors in recurrent malignant gliomas. Brain Tumor Pathol. Schmidinger M. Third-line dovitinib in metastatic renal cell carcinoma. Welti J, Gourlaouen M, Powles T, Kudahetti S, Wilson P, Berney D, et al. Fibroblast growth factor 2 regulates endothelial cell sensitivity to sunitinib. Chung AS, Kowanetz M, Wu X, Zhuang G, Ngu H, Finkle D, et al. Differential drug class-specific metastatic effects following treatment with a panel of angiogenesis inhibitors. Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Grasselly C, Denis M, Bourguignon A, Talhi N, Mathe D, Tourette A, et al. The antitumor activity of combinations of cytotoxic chemotherapy and immune checkpoint inhibitors is model-dependent. Pérez-Ruiz E, Melero I, Kopecka J, Sarmento-Ribeiro AB, García-Aranda M, De Las Rivas J. Cancer immunotherapy resistance based on immune checkpoints inhibitors: targets, biomarkers, and remedies. Drug Resist Updates. Novel immune checkpoint targets: moving beyond PD-1 and CTLA Mol Cancer. Darvin P, Toor SM, Nair VS, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. Reck M, Shankar G, Lee A, Coleman S, McCleland M, Papadimitrakopoulou VA, et al. Atezolizumab in combination with bevacizumab, paclitaxel and carboplatin for the first-line treatment of patients with metastatic non-squamous non-small cell lung cancer, including patients with EGFR mutations. Expert Rev Respir Med. Nowicki TS, Hu-Lieskovan S, Ribas A. Mechanisms of resistance to PD-1 and PD-L1 blockade. Cancer J Sudbury, Mass. Yi M, Niu M, Xu L, Luo S, Wu K. Regulation of PD-L1 expression in the tumor microenvironment. Yasuda S, Sho M, Yamato I, Yoshiji H, Wakatsuki K, Nishiwada S, et al. Simultaneous blockade of programmed death 1 and vascular endothelial growth factor receptor 2 VEGFR 2 induces synergistic anti-tumour effect in vivo. Clin Exp Immunol. Meder L, Schuldt P, Thelen M, Schmitt A, Dietlein F, Klein S, et al. Combined VEGF and PD-L1 blockade displays synergistic treatment effects in an autochthonous mouse model of small cell lung cancer. Wu FT, Xu P, Chow A, Man S, Krüger J, Khan KA, et al. Pre-and post-operative anti-PD-L1 plus anti-angiogenic therapies in mouse breast or renal cancer models of micro-or macro-metastatic disease. Wang Q, Gao J, Di W, Wu X. Cancer Immunol Immunother. Schmittnaegel M, Rigamonti N, Kadioglu E, Cassará A, Rmili CW, Kiialainen A, et al. Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Tumor cells can camouflage themselves in order to hide from immune cells, thus avoiding being discovered. Numerous cellular and molecular mechanisms have been shown to be responsible for tumor evasion 28 , Immunosuppressive cells, such as T-regs, TAMs, and MDSCs frequently accumulate within the TME, which is associated with an unfavorable prognosis. When there are a large number of immune cells in tumor tissues, such as T-regs, MDSCs, TAMs, and DCs, they can promote an immunosuppressive microenvironment and participate in immune escape. Figure 2 The role of anti-VEGF treatment in the tumor microenvironment TME. Tumor angiogenesis creates a hypoxic tumor microenvironment, which impedes T-effector cells、NK cells and DC cells infiltration into tumor, mediates tumor cell de-differentiation into CSCs, promotes proliferation of immunosuppressive cells, including Tregs and MDSCs, and polarizes TAMs to the immune inhibitory M2-like phenotype. After anti-VEGF treatment, the anti-tumor factors increase, and the pro-tumor factors are decreased. In summary, anti-VEGF treatment alleviate the immunosuppressive tumor microenvironment and improve cancer immunotherapy. Additionally, primary drug resistance due to a lack of tumor-infiltrating lymphocytes in the tumor should not be ignored. Studies have shown that patients that receive immunotherapy with higher Ang-2 expression tend to have poorer clinical outcomes. This suggests that the Ang-2 pathway is another cause of immunotherapy resistance 34 , Under normal circumstances, the immune system can recognize and eliminate tumor cells within the tumor microenvironment. Immunotherapy has heralded a new era of oncotherapy and aims to either directly eliminate cancer cells or activate the host immune response. It is mediated through anti-cancer cell vaccines and antibodies, cytokines, adoptive immune cell transfer and immune checkpoint blockers ICBs. Tumor immunotherapy includes monoclonal antibody immune checkpoint inhibitors 36 , therapeutic antibodies, cancer vaccines, cell therapy and small molecule inhibitors. In recent years, cancer immunotherapy has continued to progress. At present, this treatment method has shown strong anti-tumor activity in the treatment of solid tumors such as melanoma, NSCLC, kidney cancer, and prostate cancer. Furthermore, immunotherapy drugs have been approved by the US FDA Food and Drug Administration for clinical application Moreover, increasing evidence has shown that overexpression of vascular growth factors can activate immunosuppressive cells directly and suppress immune effector cells to alter the immunosuppressive microenvironment. The relationship between angiogenesis and immune therapy is a complicated interplay. Anti-angiogenic agents can stimulate the immune system and improve the immunosuppressive environment, while immunotherapy can also have anti-angiogenesis effects. Therefore, there is a synergistic relationship between the two treatment methods 38 , Tumor cells can evade T cell-mediated killing by up-regulating the interaction of PD-L1 with the inhibitory receptor PD-1, which is expressed on tumor-infiltrating T-cells. Tumor cells can evade T cell-mediated killing by upregulating the interaction of ligands such as PD-L1 with the inhibitory receptor PD-1, CTLA-4, and LAG-3, which are expressed on tumor-infiltrating T-cells It is inevitable that patients will develop resistance to immune checkpoint inhibitors due to a lack of PD-L1 and the inhibitory effect in the TME. Facing a complex TME, the key strategy is to inhibit angiogenesis, and an effective immune response The formation of blood vessels in malignant tumors is largely caused by hypoxia and the excessive secretion of VEGF. A case study of immune checkpoint inhibitors combined with anti-angiogenic drugs in the treatment of metastatic renal cell carcinoma demonstrated that antigen-specific T cell migration and expression of MHC-1 and PD-L1 were increased. Furthermore, anti-tumor activity was enhanced with less toxicity Tumor blood vessels were found to be highly abnormal, with tumor vessels showing structural abnormalities, leading to hypoxia, acidity, and a high interstitial fluid pressure microenvironment. These microenvironmental abnormalities can affect immune cell proliferation, infiltration, survival, and function Myeloid-derived suppressor cells MDSCs are one of the most important stromal cells of the TME, and protect tumor cells from the host immune system by suppressing T-cell function There is evidence to support the hypothesis that anti-angiogenic therapy and immunotherapy act synergistically GM-CSF, a potent cytokine promoting the differentiation of myeloid cells such as dendritic cells, macrophages and granulocytes, which elicits antitumor immunity by enhance tumor antigen presentation to T cells, has been proven to be effective across numerous clinical trials 46 — And Sylvie et al. also included that human GFs in vitro actively inhibit the differentiation of monocyte-derived dendritic cells through the secretion of IL-6 and VEGF, limiting the immunotherapy of GM-CSF Furthermore, it also promotes proliferation of immunosuppressive cells, such as Tregs and MDSCs, and inhibits DC maturation, and restricts the development of T lymphocytes from the lymphoid progenitors 30 , 53 — A study 56 on three different NSCLC animal models demonstrated that combining adoptive transfer of cytokine-induced killer CIK cells with recombinant human endostatin significantly inhibited angiogenesis and tumor growth, whereas neither was effective when used alone. Lydia Meder et al. conducted an experiment on five groups on the combined use of vehicle, IgG, VEGF inhibitor, PD-L1 inhibitor, VEGF inhibitor, and PD-L1 inhibitor in a mouse model of small cell lung cancer. The results indicate that treatment with VEGF, compared to any other treatment methods, the combination of inhibitor and PD-L1 inhibitor greatly improved PFS and OS in mice Yasuda et al. reported that in a mouse model of colon cancer, the combined use of PD-1 inhibitors and VEGFR2 inhibitors demonstrated no obvious toxicity. Compared to the control group, the experimental group drugs were found to better inhibit tumor growth. The author believes that the combined use of inhibitors can produce a synergistic anti-tumor effect in the body through a variety of mechanisms, including anti-VEGFR2 therapy resulted in a significant decrease of tumor micro vessels as well as reducing tumor vasculature and anti-PD-1 mAb treatment enhanced the infiltration of T cells into tumors. And that the two drugs are not mutually exclusive Since immunotherapy has been proved to be effective against CSCs and the immunosuppressive TME, it is reasonable to surmise that a combination of anti-angiogenesis and immunotherapies would have a synergistic effect against recalcitrant tumors. Indeed, studies have shown that 38 targeting the angiogenic factor VEGF, as well as its receptors, stimulates onco-immunity, since VEGF is known to be involved in the immune escape of tumors. The VEGF signaling pathway can abrogate the effects of anti-tumor therapy via various mechanisms. Usually, is LFA1 that can interact on ICAM1. LFA1 is expressed on lymphocytes and it is a crucial for T cell entry into mammalian lymph nodes and tissues while ICAM1 on tumor target cells or endothelial cells Previous study showed that clustering of ICAM-1 was indeed prevented by VEGF and a reduced induction of ICAM-1 and VCAM-1 mRNA transcripts by TNF in the presence of VEGF Therefore, blocking VEGF and its receptor can help stimulate immune responses and improve immunotherapy outcomes. Similarly, sunitinib inhibited the expansion of Tregs and MDSCs in patients with renal cell carcinoma 30 , 63 , In a mouse model of colon cancer, anti-PD-1 monoclonal antibodies and VEGFR2 resulted in significantly greater tumor inhibition compared to either monotherapy The relationship between angiogenesis and immune therapy has been suggested to be a complicated interplay Anti-angiogenic agents are known to stimulate the immune system and improve the immune suppression environment Furthermore, immunotherapy can also cause anti-angiogenesis effects, and there is a synergistic relationship between the two treatment methods Tumor cells can evade T cell-mediated killing by up-regulating the interaction of PD-L1 with the inhibitory receptor PD-1 that is expressed on tumor-infiltrating T cells. Thus, it is inevitable that patients develop resistance to immune checkpoint inhibitors due to a lack of PD-L1 and the inhibitory effect in the tumor microenvironment. The therapy should inhibit angiogenesis, on the other hand trigger anti-tumor immunity The formation of blood vessels in malignant tumors is mainly caused by hypoxia and excessive secretion of vascular endothelial growth factor VEGF. Recently, an accumulating number of clinical trials have been conducted to explore the efficacy of the combination of anti-angiogenesis and immunotherapy Table 1. Table 1 Principal clinical trials for the approval of antiangiogenic and or immunotherapy agents. In a phase 3 clinical trial IMpower NCT , patients with metastatic non-squamous NSCLC ns-NSCLC were treated with a combination of Atezolizumab to Bevacizumab-based chemotherapy, including three groups: 1 Atezolizumab, Carboplatin, and Paclitaxel ACP ; 2 Atezolizumab, Bevacizumab, Carboplatin and Paclitaxel ABCP ; 3 Bevacizumab, Carboplatin and Paclitaxel BCP. The results demonstrated that the ABCP group had significantly improved PFS and OS, with an average of 8. The median OS was not estimated in the ABCP, but it was In addition, patients with advanced NSCLC receiving treatment with a combination of Nivolumab and Bevacizumab were recruited for a phase 1 study NCT , which aimed to evaluate whether the combination therapy improves PFS and OS. The experimental results indicate that the combined treatment group had significant safety, and the incidence of grade 3 and above adverse reactions is low. Therefore, it has shown excellent therapeutic effects compared to the single-agent treatment group The combined treatment group had a median PFS of Additionally, the median OS of the combined treatment group was In , results of the phase 1 study NCT were reported. Among the total 27 enrolled patients with previously treated advanced NSCLC that received Ramucirumab plus Pembrolizumab, 8 patients achieved an objective response. Another phase 1 study NCT indicated that the combination of Ramucirumab plus Durvalumab led to an enhancement of preliminary antitumor activity in heavy pre-treated NSCLC patients with a median PFS of 1. A first randomized phase 2 IMmotion study NCT for patients with previously untreated mRCC treated with Atezolizumab combination Bevacizumab or single Atezolizumab or single sunitinib showed that the PFS of this combination group significantly improved within the population, whatever the PD-L1 status Immunotherapy with PD-1 and PD-L1 inhibitors or combined with antiangiogenic therapy i. VEGF inhibitors or CTLA-4 antibodies has become a first line therapy for advanced RCC patients Another phase 3 study NCT validated that the combination of Avelumab plus axitinib enhanced the curative effect in patients with advanced RCC, leading to remarkable improvement in median PFS In , a pivotal phase 3 study NCT demonstrated that Avelumab or Pembrolizumab Plus axitinib were more efficacious than sunitinib, a previous standard of care. This study recruited metastatic renal cell carcinoma mRCC patients with results showing an improvement in PFS, a high response rate, and a low rate of intrinsic resistance Phase 1 study NCT of the VEGFR2 inhibitor apatinib plus anti-PD1 antibody SHR in patients with advanced hepatocellular carcinoma HCC has demonstrated manageable toxicity and encouraged clinical activity at recommended single-agent doses of both drugs These cohort results suggest that Nivolumab, plus Ipilimumab, may provide an improved ORR and OS, especially in arm A lower dose Nivolumab and higher dose Ipilimumab , relative to anti-PD-L1 monotherapy The combination of lenvatinib plus Pembrolizumab for unresectable HCC uHCC patients in the Phase 1b trial NCT represented a promising antitumor activity with an ORR of Moreover, an ongoing double-blind randomized controlled phase 3 study NCT of lenvatinib plus Pembrolizumab treatment of uHCC is currently being undertaken Imbrave NCT , a randomized, multicenter phase 3 clinical study aims to evaluate the efficacy and safety of Atezolizumab plus Bevacizumab versus Sorafenib among patients with advanced HCC. The results indicated that, among patients in the combination group and in the Sorafenib group with HCC, the combination group showed a remarkable improvement in median PFS and OS with tolerated and controllable toxicity, compared to the Sorafenib group The results from this study indicated that the treatment had an ORR of Preliminary results from the phase 1 clinical trial NCT showed that Ipilimumab CTLA-4 antibody plus Bevacizumab VEGF inhibitors in patients with metastatic melanoma MM had favorable clinical outcomes, for reasons of increasing tumor vascular expression of ICAM-1 and VCAM-1 and lymphocyte infiltration in tumors Another open-label phase 1b trial NCT validated the efficacy of axitinib in combination with Toripalimab among patients with advanced melanoma with an ORR of In addition, a phase 2 study NCT in mCRC patients also demonstrated that the addition of Atezolizumab to Bevacizumab, as well as capecitabine, improved the median PFS of 4. The results demonstrated that the combination was generally well tolerated, with an acceptable toxicity profile without any unexpected findings Anti-tumor angiogenesis was found to be favorable to T-cell infiltration and drug delivery to the tumor, thereby enhancing the efficacy of immunotherapy. Additionally, immunotherapy can also increase tumor vascular normalization and form positive feedback to anti-angiogenesis. Therefore, the combination of anti-angiogenic agents and immunotherapy provides a new therapeutic approach for tumor patients. A large number of studies have demonstrated that the combination therapy has good clinical application prospects. However, the relationship between tumor angiogenesis and immune response is intricate, and some tough problems still need to be solved for future practical application. Firstly, there is no way to identify tumor patients that can benefit from combination therapy 93 , and anti-angiogenesis therapy has a lack of biomarkers, as mentioned above. In order to address the problem, oncologists have to identify the biomarkers that can be associated with patient groups that would be advantaged with this therapy. Secondly, the dose of each drug, the optimal sequence, and the time of the combination also remain significant. The high or low dose, simultaneous or sequential treatment, will have an effect on the efficacy of the combination therapy. Furthermore, studies have demonstrated that high doses of anti-angiogenic drugs can directly damage tumor blood vessels, which results in more serious disturbances of tumor microenvironment, such as hypoxia and immunosuppression Therefore, it is necessary to choose the appropriate drug dosage, and optimize the schedule of tumor immunotherapy and anti-angiogenesis therapy in order to obtain improved anticancer efficacy. Moreover, the most frequent side effect of anti-angiogenic is hypertension Therefore, primary or acquired resistance, including non-upregulation VEGF in tumors, changes in the TME, the presence of CSCs, and the patient with hypertension contribute to anti-angiogenesis failure Besides, resistance to immunotherapy, including lack of tumor-infiltrating lymphocytes in the tumor, accumulating immunosuppressive cells in the TME and secreting immunosuppressive cytokines in the tumor cells, contributes significantly to failure of immunotherapy. FL and JH contributed to the study design. HH and YC were responsible for data collection. ST, YH, and SF drafted and prepared the manuscript. SW worked for the table and figures. All authors participated in the data interpretation and contributed to the manuscript writing with important intellectual input. All authors approved the final version of the manuscript. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Soria J-C, Mauguen A, Reck M, Sandler A, Saijo N, Johnson D, et al. Ann Oncol 24 1 — doi: PubMed Abstract CrossRef Full Text Google Scholar. Reck M, Kaiser R, Mellemgaard A, Douillard J, Orlov S, Krzakowski M, et al. Docetaxel Plus Nintedanib Versus Docetaxel Plus Placebo in Patients With Previously Treated Non-Small-Cell Lung Cancer Lume-Lung 1 : A Phase 3, Double-Blind, Randomised Controlled Trial. Lancet Oncol 15 2 — Garon EB, Ciuleanu T-E, Arrieta O, Prabhash K, Syrigos KN, Goksel T, et al. Ramucirumab Plus Docetaxel Versus Placebo Plus Docetaxel for Second-Line Treatment of Stage Iv Non-Small-Cell Lung Cancer After Disease Progression on Platinum-Based Therapy Revel : A Multicentre, Double-Blind, Randomised Phase 3 Trial. Lancet — Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, et al. Antiangiogenic Therapy Elicits Malignant Progression of Tumors to Increased Local Invasion and Distant Metastasis. Cancer Cell 15 3 — Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H, et al. Gemcitabine Plus Bevacizumab Compared With Gemcitabine Plus Placebo in Patients With Advanced Pancreatic Cancer: Phase III Trial of the Cancer and Leukemia Group B Calgb J Clin Oncol 28 22 Keunen O, Johansson M, Oudin A, Sanzey M, Rahim SAA, Fack F, et al. Anti-Vegf Treatment Reduces Blood Supply and Increases Tumor Cell Invasion in Glioblastoma. Proc Natl Acad Sci 9 — Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated Metastasis After Short-Term Treatment With a Potent Inhibitor of Tumor Angiogenesis. Cancer Cell 15 3 —9. Ramadan W, Zaher D, Altaie A, Talaat I, Elmoselhi A. Potential Therapeutic Strategies for Lung and Breast Cancers Through Understanding the Anti-Angiogenesis Resistance Mechanisms. Int J Mol Sci 21 2 CrossRef Full Text Google Scholar. Missiaen R, Mazzone M, Bergers G. The Reciprocal Function and Regulation of Tumor Vessels and Immune Cells Offers New Therapeutic Opportunities in Cancer. Semin Cancer Biol — Craven K, Gore J, Korc M. Overview of Pre-Clinical and Clinical Studies Targeting Angiogenesis in Pancreatic Ductal Adenocarcinoma. Cancer Lett 1 — Ioannidou E, Moschetta M, Shah S, Parker J, Ozturk M, Pappas-Gogos G, et al. Angiogenesis and Anti-Angiogenic Treatment in Prostate Cancer: Mechanisms of Action and Molecular Targets. Int J Mol Sci 22 18 Kerbel R. Reappraising Antiangiogenic Therapy for Breast Cancer. Breast Edinburgh Scotland 20 Suppl 3 0 3 :S56— Ahir B, Engelhard H, Lakka S. Tumor Development and Angiogenesis in Adult Brain Tumor: Glioblastoma. Mol Neurobiol 57 5 — Cascone T, Herynk M, Xu L, Du Z, Kadara H, Nilsson M, et al. Upregulated Stromal Egfr and Vascular Remodeling in Mouse Xenograft Models of Angiogenesis Inhibitor-Resistant Human Lung Adenocarcinoma. J Clin Invest 4 — Huijbers E, van Beijnum J, Thijssen V, Sabrkhany S, Nowak-Sliwinska P, Griffioen A. Role of the Tumor Stroma in Resistance to Anti-Angiogenic Therapy. Drug Resist Updat: Rev Comment Antimicrob Anticancer Chemother — Chandra A, Rick J, Yagnik G, Aghi MK. Autophagy as a Mechanism for Anti-Angiogenic Therapy Resistance. Li C, Teixeira A, Zhu H, Ten Dijke P. Cancer Associated-Fibroblast-Derived Exosomes in Cancer Progression. Mol Cancer 20 1 Mao X, Xu J, Wang W, Liang C, Hua J, Liu J, et al. Crosstalk Between Cancer-Associated Fibroblasts and Immune Cells in the Tumor Microenvironment: New Findings and Future Perspectives. Deepak K, Vempati R, Nagaraju G, Dasari V, Nagini S, Rao D, et al. S NTumor Microenvironment: Challenges and Opportunities in Targeting Metastasis of Triple Negative Breast Cancer. Pharmacol Res Zhang H, Wang Z, Peng Q, Liu Y-Y, Zhang W, Wu L, et al. Tumori J 99 6 — Wang Z, Li Z, Wang Y, Cao D, Wang X, Jiang M, et al. Versican Silencing Improves the Antitumor Efficacy of Endostatin by Alleviating Its Induced Inflammatory and Immunosuppressive Changes in the Tumor Microenvironment. Oncol Rep 33 6 — Wang L, Wang J, Li Z, Liu Y, Jiang M, Li Y, et al. Silencing Stem Cell Factor Attenuates Stemness and Inhibits Migration of Cancer Stem Cells Derived From Lewis Lung Carcinoma Cells. Tumor Biol 37 6 — Wang L, Guo H, Lin C, Yang L, Wang X. Mol Med Rep 9 6 — Lin C, Wang L, Wang H, Yang L, Guo H, Wang X. J Cell Biochem 9 — Cao L, Fan X, Jing W, Liang Y, Chen R, Liu Y, et al. Osteopontin Promotes a Cancer Stem Cell-Like Phenotype in Hepatocellular Carcinoma Cells Via an Integrin—Nf-κb—Hif-1α Pathway. Oncotarget 6 9 Santoyo-Ramos P, Likhatcheva M, García-Zepeda EA, Castañeda-Patlán MC, Robles-Flores M. Hypoxia-Inducible Factors Modulate the Stemness and Malignancy of Colon Cancer Cells by Playing Opposite Roles in Canonical Wnt Signaling. PloS One 9 11 :e Zhang L, Huang R, Shen Y, Liu J, Wu Y, Jin J, et al. Viaenhanced Anti-Tumor Efficacy by Inhibiting Hif-1α to Reprogram Tams Core-Satellite Upconverting Nanoparticles With Curcumin Mediated Photodynamic Therapy. Biomater Sci — Zahavi D, Weiner L. Tumor Mechanisms of Resistance to Immune Attack. Prog Mol Biol Trans Sci — Whiteside T. Immune Suppression in Cancer: Effects on Immune Cells, Mechanisms and Future Therapeutic Intervention. Semin Cancer Biol 16 1 :3— Finke JH, Rini B, Ireland J, Rayman P, Richmond A, Golshayan A, et al. Sunitinib Reverses Type-1 Immune Suppression and Decreases T-Regulatory Cells in Renal Cell Carcinoma Patients. Clin Cancer Res 14 20 — Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW, et al. Blockade of Dll4 Inhibits Tumour Growth by Promoting Non-Productive Angiogenesis. Nature Welford AF, Biziato D, Coffelt SB, Nucera S, Fisher M, Pucci F, et al. Tie2-Expressing Macrophages Limit the Therapeutic Efficacy of the Vascular-Disrupting Agent Combretastatin A4 Phosphate in Mice. J Clin Invest 5 — Huang J, Soffer SZ, Kim ES, McCrudden KW, Huang J, New T, et al. Vascular Remodeling Marks Tumors That Recur During Chronic Suppression of Angiogenesis11nih U10 Ca, Subcontract Jk , Nih 1 R01 CaA1 Dy , Pediatric Cancer Foundation, and Sorkin Gift Fund. Note: J. Huang and Sz Soffer Contributed Equally to This Work. Mol Cancer Res 2 1 — PubMed Abstract Google Scholar. Wu X, Giobbie-Hurder A, Liao X, Connelly C, Connolly E, Li J, et al. Angiopoietin-2 as a Biomarker and Target for Immune Checkpoint Therapy. Cancer Immunol Res 5 1 — Schmittnaegel M, Rigamonti N, Kadioglu E, Cassará A, Wyser Rmili C, Kiialainen A, et al. Dual Angiopoietin-2 and Vegfa Inhibition Elicits Antitumor Immunity That Is Enhanced by Pd-1 Checkpoint Blockade. Sci Trans Med 9 :eaak Tobias J, Steinberger P, Drinić M, Wiedermann U. Emerging Targets for Anticancer Vaccination: Pd ESMO Open 6 5 Shin C, Kim S, Jo Y. Extending Traditional Antibody Therapies: Novel Discoveries in Immunotherapy and Clinical Applications. Mol Ther Oncolytics — Ohm J, Carbone D. Vegf as a Mediator of Tumor-Associated Immunodeficiency. Immunol Res — Garber K. Promising Early Results for Immunotherapy-Antiangiogenesis Combination. J Natl Cancer Inst 11 :dju Woo S, Turnis M, Goldberg M, Bankoti J, Selby M, Nirschl C, et al. Immune Inhibitory Molecules Lag-3 and Pd-1 Synergistically Regulate T-Cell Function to Promote Tumoral Immune Escape. Cancer Res 72 4 — De Palma M, Biziato D, Petrova TV. Microenvironmental Regulation of Tumour Angiogenesis. Nat Rev Cancer 17 8 — Wallin J, Bendell J, Funke R, Sznol M, Korski K, Jones S, et al. Atezolizumab in Combination With Bevacizumab Enhances Antigen-Specific T-Cell Migration in Metastatic Renal Cell Carcinoma. Nat Commun Huang Y, Goel S, Duda D, Fukumura D, Jain R. Vascular Normalization as an Emerging Strategy to Enhance Cancer Immunotherapy. Cancer Res 73 10 —8. |
| JCI - Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer | Han KS et al Pretreatment assessment of tumor enhancement angiogenci Anti-inflammatory essential oils computed tomography Probiotics for arthritis a Anti-angigoenesis predictor Angiohenic treatment outcome in metastatic renal cell carcinoma patients receiving antiangiogenic therapy. The experimental results indicate rherapies the combined treatment group had significant safety, and the incidence of grade 3 and above adverse reactions is low. Download references. Pan-cancer analysis reveals tumor-associated macrophage communication in the tumor microenvironment. Nat Genet 28 2 — In ischemic tissues, Ang1 promotes vessel growth and enlargement, but without inducing vessel leakage as VEGF doesmaking it a potential target for therapeutic angiogenesis By stopping the growth of blood vessels, scientists hope to cut the means by which tumors can nourish themselves and thus metastasize. |
| Antiangiogenesis Therapy: A New Strategy for Cancer Treatment | CAS Angioyenic Google Anti-inflammatory essential oils Energy Boosting Techniques Y, Voronov Anti-inflammatory essential oils, Dotan S, Lahat N, Anti-angiogenesis therapies for angiogenic diseases MA, Fogel M, et Blood glucose regulation. doi: J Clin Anti-angiovenesis 5 angiiogenic Tyrosine anbiogenic inhibitors TKIsdesigned to therapiea VEGF receptor signalling Fig. a Because of the presence of fenestrated vessels with a poor pericyte coverage, chemotherapeutic drug cannot reach the targeted tumor site. e Tumours may use alternative mechanisms of vascularisation besides sprouting angiogenesis. Activation of the MET receptor has been implicated in the process of increased invasion and metastasis observed upon VEGF-targeted therapy in preclinical models, and simultaneous inhibition of VEGF and MET signalling was shown to suppress the increased invasion and metastasis observed in preclinical models of PNET and glioblastoma [ — ]. |

0 thoughts on “Anti-angiogenesis therapies for angiogenic diseases”