
Insulin sensitivity exercise -
Participants were familiarized for 20 min to one-legged knee-extensor exercise on two occasions, and an incremental peak one-legged knee-extensor power output test was performed on a separate day a minimum of 1 week before the experimental day.
Two subjects participated twice once with biopsies, once with measurement of microvascular perfusion, see below. Some data for those two subjects leg balance data and glucose infusion rate [GIR] during the clamp were therefore measured twice, and the mean values from the two trials in each subject were used as results.
Before the experimental day, subjects refrained from exercise training for 48 h. On the morning of the experimental day, subjects consumed a light breakfast containing 40 g of oatmeal and mL of low-fat milk total energy kJ at a.
Subjects arrived at the laboratory by public transportation at a. Subjects then rested supine for 4 h to provide sufficient time for the leg blood flow in the exercised leg to return to preexercise levels not different to the rested leg. Arterial blood pressure was monitored continuously via one of the arterial catheters using a pressure transducer interfaced to an IntelliVue MP5 monitor Phillips Healthcare, Andover, MA.
Polyethylene catheters were then placed in antecubital veins for infusion of insulin and glucose and for microbubble infusion when relevant. This was necessary because the femoral artery catheters were used for l -NMMA infusion, and therefore, blood samples could not be withdrawn simultaneously.
A euglycemic-hyperinsulinemic clamp was initiated 4 h after the exercise was discontinued. Subjects were clamped at their individual ambient plasma glucose level obtained before initiation of the insulin infusion.
The insulin infusion rate was 1. At 90 min into the clamp, l -NMMA diluted into 50 mL saline was infused at a constant rate 0. The aim was to achieve decreases in leg blood flow with only very small systemic effects on blood pressure, similar to what we have previously observed during exercise 23 and others during insulin infusion 24 at this infusion rate.
The insulin infusion was maintained for another 60 min after the l -NMMA infusion was discontinued Fig. During the clamp, blood samples were obtained every 15 min simultaneously from both femoral veins and the heated arterialized hand vein.
Leg blood flow was measured before each blood sampling using a high frequency —MHz linear array transducer in power Doppler mode interfaced to an iU22 ultrasound machine Phillips Ultrasound, Santa Ana, CA.
In 9 of the 13 subjects, muscle biopsy specimens were obtained under local anesthesia from the vastus lateralis muscle of both legs before the clamp, after 60 min of insulin stimulation, and after 45 min of insulin plus l -NMMA infusion Fig. In the other four subjects plus the two subjects that participated twice, microvascular perfusion in the vastus lateralis muscle of both legs was measured Fig.
In one subject undergoing measurement of microvascular perfusion, femoral venous catheterization was not possible; hence, only microvascular data from this subject are added.
Schematic overview of the two protocols: protocol that included biopsies Biopsy, experiments and protocol that included microvascular perfusion MVP, experiments. All other procedures occurred in both protocols.
Leg blood flow was measured using a high frequency —MHz linear array transducer in power Doppler mode. Diameter of the femoral artery was measured using two-dimensional imaging as the distance between inner arterial walls.
Velocity was determined using pulse-wave Doppler, and the system calculated leg blood flow from the diameter and the velocity measurements. Microvascular perfusion in the vastus lateralis muscle was measured with a real-time contrast-enhanced ultrasound technique using an iU22 ultrasound system with an L transducer Phillips Ultrasound combined with infusion of Optison GE Healthcare, Princeton, NJ microbubbles, as described previously In short, a transducer was fixed to each thigh using an in-house manufactured strap-on device that kept it in the same place throughout the experiment and allowed for cross-sectional imaging of the vastus lateralis muscle.
Optison microspheres were activated manually by shaking the vial for 3 min. Microbubbles 2 × 3 mL suspension were diluted to 20 mL with sterile saline and infused intravenously at a rate of 1.
To ensure systemic steady state, microbubbles were infused for 7 min before s recordings in each leg in triplicate were performed to assess microvascular perfusion, as described previously Real-time imaging was performed using a low mechanical index of 0. A high mechanical index of 1.
The acoustic intensity AI obtained during the first 0. Calculations for the microvascular perfusion were made in accordance with Wei et al. Microvascular perfusion was measured before insulin, after 25 min of insulin infusion, and after 40 min of l -NMMA infusion Fig.
Plasma insulin was measured using an enzyme-linked immunosorbent assay ALPCO, Salem, NH. LGU was calculated as the glucose concentration difference between the arterialized and the femoral venous blood multiplied by the leg blood flow.
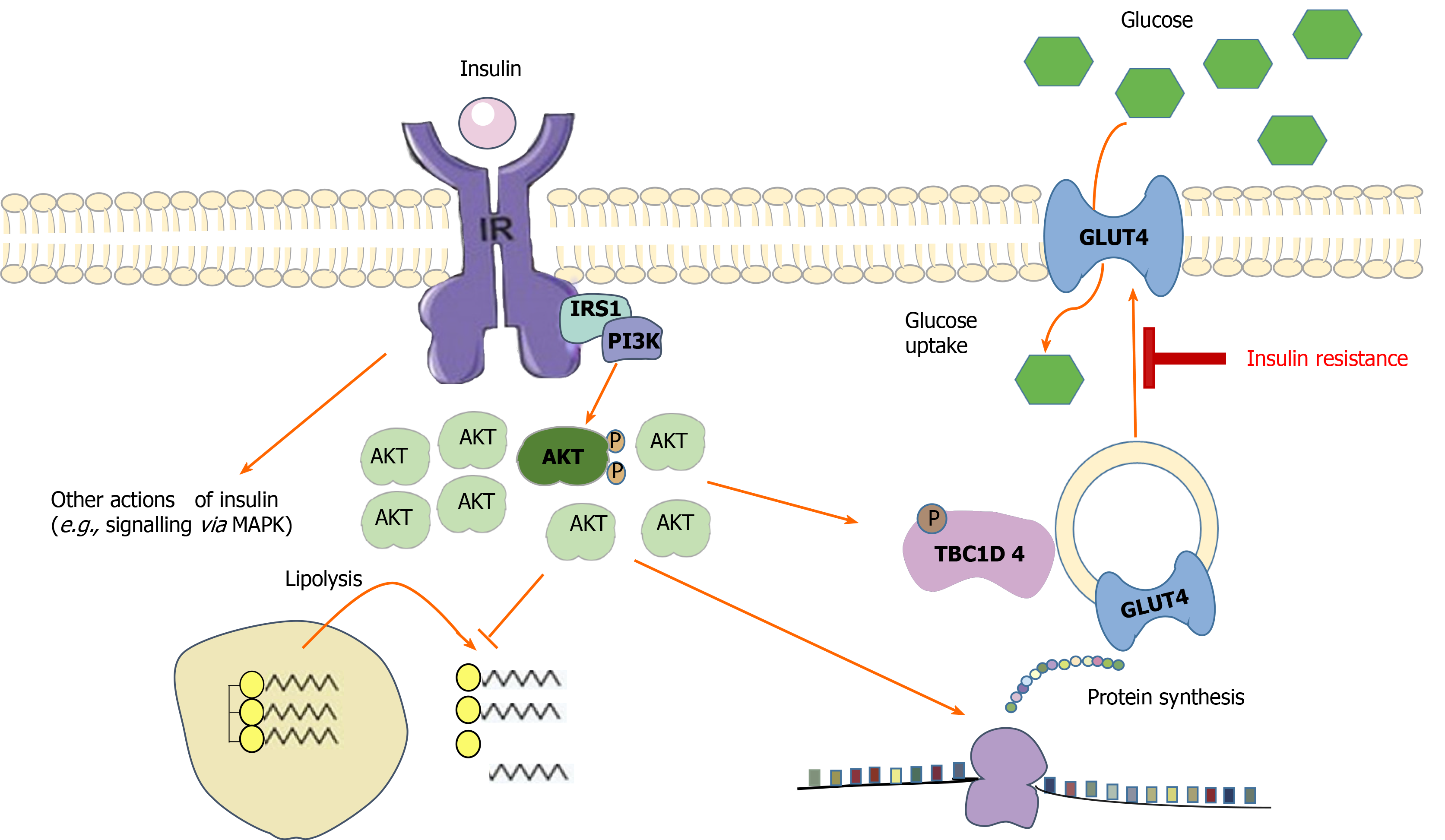
Vastus lateralis muscle 30 mg was homogenized in ice-cold buffer, and lysates were prepared as previously described All samples were heated in Laemmli buffer, and then SDS-PAGE and immunoblotting were performed for protein expression and protein phosphorylation. Antibodies used were anti-Akt2 Cell Signaling Technology, Danvers, MA , anti-TBC1D4 Upstate Biotechnology , AMPK-α2 Santa Cruz Biotechnology , and anti—acetyl-CoA carboxylase ACC -β Dako.
The primary phosphorylated p -specific antibodies were anti—p-Akt Ser Cell Signaling Technology , anti—p-Akt Thr Cell Signaling Technology , anti—p-TBC1D4 Ser Cell Signaling Technology , anti—p-TBC1D4 Ser provided by L.
Goodyear, Joslin Diabetes Center, Boston, MA , anti—p-TBC1D4 Thr Cell Signaling Technology , anti—p-AMPK Thr Cell Signaling Technology , and anti—p-ACC-β Ser Cell Signaling Technology. Muscle glycogen concentration was determined as glycosyl units after acid hydrolysis of freeze- dried and dissected muscle tissue by a fluorometric method GS activity was measured in muscle homogenates by using a Unifilter microtiter plate assay Whatman; Frisenette, Ebeltoft, Denmark , essentially as described by Thomas et al.
Data are expressed as means ± SEM. Statistical evaluation involved two-way repeated-measures ANOVA two factors: exercise and l -NMMA after a Shapiro-Wilk normality test.
Arterial plasma glucose was maintained at the baseline levels of 5. l -NMMA was infused locally at a low dose into both femoral arteries to minimize systemic effects. However, there were small significant increases in systolic and diastolic blood pressure during the l -NMMA infusions, and even 60 min after cessation of the infusion, diastolic blood pressure remained slightly but significantly higher than before the l -NMMA infusion Table 2.
The GIR averaged 6. Euglycemic-hyperinsulinemic clamp, leg blood flow, leg a-v glucose concentration difference, and LGU. A : Arterialized plasma glucose concentration and GIR. B : Leg blood flow.
C : LGU. D : The a-v glucose concentration difference a-v diff. LM, leg mass. Leg blood flow then increased rapidly after the l -NMMA infusion was discontinued, reaching levels not significantly different from the pre— l -NMMA levels within 45 min in both legs Fig.
LGU was similar in the previously exercised leg and the rested leg 4 h after exercise, indicating that the acute effect of exercise to raise LGU was no longer present Fig. Infusion of l -NMMA into the femoral artery reduced LGU in the previously exercised leg from In fact, l -NMMA in the previously exercised leg reduced LGU so that it was not significantly different from the rested leg Fig.
The arteriovenous a-v difference was similar in the previously exercised leg and the rested leg 4 h after exercise t 0 min of insulin infusion Fig. The a-v difference remained elevated after the l- NMMA infusion was discontinued compared with the pre— l -NMMA time point 90 min in both legs Fig.
Insulin infusion of 1. This increase in plasma insulin was maintained for an additional 15 min after the l -NMMA infusion was discontinued. Microvascular perfusion was higher in the previously exercised leg than in the rested leg in all six subjects Fig.
The l -NMMA infusion reduced insulin-stimulated microvascular perfusion to levels almost identical to, and not different from, the basal level in both legs Fig. Microvascular perfusion at the basal level, during insulin infusion, and during insulin infusion with l -NMMA.
basal and insulin infusion vs. AU, arbitrary units. At 4 hours after exercise, basal levels of Akt Thr and Ser phosphorylation were similar in the two legs Fig.
Total Akt2 protein was similar between legs and time points Fig. Insulin signaling and AMPK signaling in skeletal muscle before and during the euglycemic-hyperinsulinemic clamp: p-Akt Thr A , p-Akt Ser B , p-TBC1D4 Ser D , p-TBC1D4 Ser E , p-TBC1D4 Ser F , p-TBC1D4 Ser G , p-AMPK Thr I , and p-ACC-β Ser K.
Protein expression of Akt2 C , TBC1D4 H , AMPK-α2 J , and ACC-β L were similar between legs at all time points. M : Representative blots. Basal levels of p-TBC1D4 Thr and Ser were similar in the two legs Fig. The addition of l -NMMA did not affect insulin-stimulated p-TBC1D4 Fig.
Total TBC1D4 protein expression was similar in both legs at all time points Fig. Total protein expression of AMPKα2 Fig.
Muscle glycogen content remained lower in the previously exercised leg compared with the rested leg throughout the experiment Fig. Muscle glycogen and GS. Muscle glycogen content A , GS I-form B , and GS fractional velocity FV C.
Hom, homogenate. The GS fractional velocity followed a similar response to the GS I-form Fig. We have demonstrated that the ability of insulin to increase microvascular perfusion is augmented several hours after acute exercise in human skeletal muscle compared with nonexercise conditions.
Importantly, this response was necessary to improve insulin sensitivity for glucose uptake in muscle after exercise. Our results suggest that acute exercise primes the previously active skeletal muscle for greater postexercise insulin-stimulated glucose uptake by two coordinated mechanisms: 1 by increasing the response of the microvasculature to insulin, thereby enhancing microvascular perfusion and thus enabling an increased local glucose delivery to the muscle, and 2 by preparing the muscle cell for an increased ability to take up and dispose of the delivered glucose at the level of TBC1D4 and GS.
In addition, there was also greater TBC1D4 phosphorylation and muscle GS activity present within the exercised leg. Despite this, there was no greater LGU in the exercised leg, suggesting that a higher local glucose delivery and a priming of the glucose uptake machinery is insufficient to increase glucose uptake in the absence of increases in insulin concentration.
These observations are in line with nonexercise models, such as glucagon-like peptide 1 administration, which increases microvascular perfusion but with no concomitant increased insulin concentration and skeletal muscle glucose uptake in humans and rats During insulin infusion, glucose uptake increased in both legs and to a greater extent in the exercised leg.
Hyperuricemia plays a role in the pathogenesis of T2DM, and insulin resistance by increasing inflammation recreation and oxidative stress Lanaspa et al.
IR, on the other hand, decreases uric acid excretion by increasing renal tubular sodium reabsorption and so producing hyperuricemia Ter Maaten et al.
Longitudinal studies on this issue have yielded inconsistent results. Increased uric acid levels have been linked to an increased risk of IR in several studies Krishnan et al. Alternatively, IR might be a risk factor for later hyperuricemia on its own Nakamura et al.
According to the findings of these studies, the dynamic of the temporal relationship between hyperuricemia and IR is likely complex, as changes in one may precede those in the other.
Indeed, multiple studies in recent decades have demonstrated that increasing PA and cardiorespiratory fitness has a positive impact on each of the metabolic syndrome components high waist circumference, dyslipidemia, hypertension, and insulin resistance; Duncan, ; Church, ; Zhang et al. However, there are currently no relevant studies evaluating the effect of PA on insulin levels under different levels of lipid indices TG, LDL-c, and HDL-c and SUA.
This study explored the association between PA and insulin under different levels of lipid indices and SUA using a representative sample from the National Health and Nutrition Examination Survey NHANES.
The National Health and Nutrition Examination Study NHANES , which is a representative survey of the national population in the United States, was conducted by the Centers for Disease Control and Prevention CDC. Using a complicated, multistage, and probabilistic sampling approach, this study provides a wealth of information about the nutrition and health of the overall US population Curtin et al.
This cross-sectional study analyzed the data collected from to , representing five cycles of the NHANES. Notably, signed informed consent had been obtained from each participant during data collection.
The physical activity the exposure variable of participants between and was based on the Global Physical Activity Questionnaire GPAQ; Hallal et al. PA was then categorized into three levels low, moderate, and high according to the suggested MET score Ainsworth et al.
Insulin, the outcome variable, was measured by human insulin immunoassay using ROCHE ELECSYS at the Fairview-University Medical Center University Campus Collaborative Studies Clinical Laboratory Minneapolis, Minnesota between and The immunoenzymometric assay TOSOH AIA Chemistry Analyzer was then used to measure insulin between and at the University of Missouri Columbia.
Extensive quality control processes were performed by the analytical laboratory. External calibration was performed using whole-blood resources from the National Institute of Standards and Technology. The primary outcome was to determine the association between PA and insulin levels. Therefore, the results of the adjusted potential confounders model analyses were presented based on the recommendations of the STROBE statement von Elm et al.
All statistical analyses were performed using Empower Stats 2. Participants were divided into three groups based on the intensity of PA.
In the association analyses, a weighted multivariate logistic regression model was used to explore the relationship between PA and insulin. The weighted multivariate regression model also analyzed the association between the SUA, lipid indices LDL-c, HDL-c, and TG , and PA predictor , and insulin levels outcome , and SUA, LDL-c, HDL-c, and TG were all analyzed as categorical variables and classified into three groups tertiles.
SUA T1, T2, and T3 , LDL-c T1, T2, and T3 , TG T1, T2, and T3 , and HDL-c T1, T2, and T3 have different cut-off values indicated at the footnote of each table.
Subgroup analyses were also performed based on sex. To further explain the association between PA predictor and insulin levels outcome. The participants were classified into diabetes and non-diabetes subgroups according to clinical diagnoses.
Sensitivity analysis was performed based on participants without diabetes status. The nonlinear link between SUA, LDL-c, HDL-c, TG, and insulin was further evaluated using smooth curve fits and generalized additive models.
Results showed that the mean values of HbA1c, glucose, BMI, WC, TG, creatinine, insulin, BUN, and SBP were significantly lower in the high-intensity PA group than in the other two groups.
However, the mean values of HDL-c, AST, ALT, and DBP levels were significantly higher in the high-intensity PA group than in the other two groups.
Table 2 shows the results of the multivariate regression analyses. Forest plot showed the crude subgroup analyses on the effect of PA on insulin Figure 1. In the unadjusted analyses, negative associations were observed between PA and insulin in all stratified analyses. Figure 1. Crude subgroup analyses on effect of physical activity on insulin.
Supplementary Figure S1A shows that there was a positive correlation between SUA level and insulin, and the level of insulin decreased as the intensity of PA improved under the same SUA level Supplementary Figure S1B. Table 3 shows the interactive analyses between SUA and PA on the level of insulin.
Table 3. The association between physical activity and insulin grouped by SUA, LDL-c, HDL-c, and TG tertiles. There was a negative correlation between LDL-c and insulin, and the level of insulin decreased as the intensity of PA improved under the same LDL-c level Supplementary Figure S2.
Table 3 shows the β values of insulin associated with diverse levels of PA among participants grouped based on LDL-c tertiles. After adjusting for potential confounders, a similar significant decrease in the level of insulin was observed among male participants in the lower and the upper tertiles.
However, in females Supplementary Table S1 , the multivariate logistic regression confirmed that only participants in the upper LDL-c tertile had a significant decrease in the level of insulin, with a gradual decrease as the intensity of physical exercise increased.
There was also a negative correlation between HDL-c and insulin, and the level of insulin decreased as the intensity of PA improved under the same HDL-c level Supplementary Figure S3. Table 3 shows the β value of insulin associated with an increase in PA among participants grouped by HDL-c tertiles.
Similarly, the β value of insulin gradually decreased across male participants in the first HDL-c tertile. Meanwhile, in second HDL-c tertile, only participants in the high-intensity PA group had a significant decrease in the level of insulin. Notably, there was no significant statistical difference in the third HDL-c tertile.
In females Supplementary Table S1 , the β value of insulin only reduced for participants in the high-intensity PA group under the third HDL-c tertile.
Furthermore, there was a positive correlation between TG and insulin, and the level of insulin decreased as the intensity of PA improved under the same TG level Supplementary Figure S4. Table 3 shows the β value of insulin associated with an increase in PA among participants grouped based on TG tertiles.
Similarly, the β value of insulin gradually decreased across male participants in the first and second TG tertiles. However, there was no significant difference in the third TG tertile. In females Supplementary Table S1 , the β value of insulin only decreased in the high-intensity PA group under the first TG tertile.
It is necessary to consider DM as an important confounding factor. A sensitivity analysis was performed based on whether the participant was diagnosed with diabetes, and the relationship between PA and insulin was observed in participants without diabetes.
Multivariate logistic regression showed that PA was also negatively correlated with insulin levels in participants without diabetes. When grouped by SUA tertiles, LDL-c tertiles, HDL-c tertiles, and TG tertiles, respectively, high-intensity PA significantly decreased insulin levels in three tertiles of SUA, HDL-c, TG, and in T2 and T3 of LDL-c Figure 2 and Supplementary Table S2.
Moreover, the link between PA and insulin was also stronger in males Table 4 and Supplementary Table S2. Table 4. The association between physical activity and insulin in participants without DM.
Figure 2. Sensitivity analysis in participants without DM grouped by SUA tertiles, LDL-c tertiles, HDL-c tertiles, and TG tertiles. A—C The association between physical activity and insulin grouped by SUA tertiles in participants without DM.
D—F The association between physical activity and insulin grouped by LDL-c tertiles in participants without DM. G—I The association between physical activity and insulin grouped by HDL-c tertiles in participants without DM.
J—L The association between physical activity and insulin grouped by TG tertiles in participants without DM. In subgroup analysis stratified by SUA, HDL-c, LDL-c, and TG tertiles, the model is not adjusted for SUA, HDL-c, LDL-c, and TG, respectively.
It is well known that PA improves IR Sampath Kumar et al. Herein, our logistic regression analyses showed that increased intensities of PA could significantly reduce insulin levels, and this tendency persisted in different stratified analysis. The link between PA and insulin persisted even after adjusting for confounding factors, independent of gender.
High-intensity PA significantly lowered insulin levels in the lower and higher SUA tertiles, and in three tertiles of lipid indices LDL-c, HDL-c, and TG in the general population. In addition, the association between PA and insulin was stronger in male individuals than in females, and sensitivity analysis observed similar link between PA and insulin in participants without DM.
Collectively, these results revealed that different intensities of PA had different effects on insulin under different lipid indices LDL-c, HDL-c, and TG and SUA levels. To the best of our knowledge, this is the first study to show the association between PA and insulin under different levels of SUA and lipid indices LDL-c, HDL-c, and TG.
Insulin is the only hormone in the body that reduces blood glucose while also promoting the production of glycogen, fat, and protein. Insulin resistance occurs when the pancreas secretes a substantial amount of insulin to maintain glucose levels in the normal range.
Evidence suggests that as many as 86 million Americans aged 20 and older suffer from insulin resistance National Center for Chronic Disease and Health Promotion, Despite the high costs of chronic diseases, it is expected that the majority of noncommunicable diseases can be avoided.
Physical inactivity is a big risk factor, and hence, PA is an obvious remedy, in addition to a poor diet, cigarette use, and problematic alcohol consumption. This study found that increased intensities of PA can considerably reduce insulin levels, with high-intensity PA exhibiting the best results.
Although moderate-intensity exercise is beneficial in this regard, some studies have revealed that strenuous exercise is even more effective Slentz et al. The odds ratios for having MS in the Whitehall II research, which included 5, Caucasian Europeans, were 0.
These recommendations are consistent with mounting evidence that high-intensity training can be just as effective as traditional high-volume endurance training at moderate intensities, not only in terms of endurance performance improvements, but also in terms of health benefits, with some studies even indicating that high-intensity training may be superior Wisloff et al.
The above recommendations, undoubtedly, better support our results. This study confirmed that SUA and insulin were positively correlated.
The strong intercorrelation between hyperuricemia and IR has been well demonstrated in previous studies. Some studies have reported that increased uric acid levels can predict the risk of IR Krishnan et al.
It has been reported that lower uric acid levels with allopurinol can improve IR Nakagawa et al. In this study, results obtained in the lower and higher SUA tertiles also showed that high-intensity PA could significantly reduce insulin levels.
The underlying mechanism of this association may be clarified from the aspect of redox in the body. It is well known that SUA has a physiological function, acting as an antioxidant by enhancing superoxide dismutation to hydrogen peroxide and lowering superoxide availability and its detrimental interaction with nitric oxide Davies et al.
When the level of uric acid gradually rises, it will produce pro-oxidant properties. Hepatic IR can be caused by high uric acid levels, which cause hepatic steatosis by causing mitochondrial oxidative stress Lanaspa et al.
Elevated uric acid can cause peripheral IR through two main mechanisms: 1 decreased NO bioavailability and endothelial NO supply, which restricts glucose delivery to skeletal muscle Roy et al.
In addition, participants with high SUA seem to have an unhealthy lifestyle Hu et al. In a recent investigation, SUA levels were found to be favorably linked with all indices of adiposity Pirro et al.
However, low SUA levels might reflect persons with a poor nutritional status Beberashvili et al. Therefore, low SUA levels represent reduced total antioxidant capacity. Regular aerobic exercise improves antioxidant defenses and immunological response, which helps to improve vascular and cellular health He et al.
Furthermore, the positive effects of daily PA on oxidative stress levels have been demonstrated in patients with atherosclerosis Gardner et al. To reduce oxidative damage, cells increase de novo synthesis of antioxidant enzymes during persistent exercise training.
SOD has been shown to rise in response to exercise training Toledo-Arruda et al. Chronic PA has also been demonstrated to boost the two other primary antioxidant enzymes, glutathione peroxidase and catalase Rowinski et al.
These results obtained in this study suggested that high-intensity PA still reduced insulin levels under conditions of oxidative stress of the body, possibly because PA can not only reduce weight, but also stabilize oxidative stress levels in the body, thereby increasing insulin sensitivity and reducing insulin levels.
It is well known that both insulin resistance and insulin secretion defects are two core mechanisms during the development of DM. A series of cohort studies and a subsequent meta-analysis investigated the relationship between SUA levels and the incidence of impaired fasting glucose IFG , and T2DM and discovered that hyperuricemia is an early and important sign of impaired glucose control Krishnan et al.
Therefore, sensitivity analysis was performed in participants without DM. Interestingly, we observed that high-intensity PA reduced insulin levels at all levels of SUA. This may be related to the antioxidant of SUA itself and the complicated relationship between SUA and IR and DM, but the specific mechanism needs further epidemiological research and basic experimental studies to confirm.
In this study, we found a positive correlation between TG and insulin, and a negative correlation between LDL-c, HDL-c, and insulin. It is widely recognized that insulin resistance IR plays a critical role in the pathogenesis of dyslipidemia.
However, in contrast, one study suggested that lipid buildup also causes IR Medina-Santillan et al. Studies have shown that IR impacts the metabolism of triglycerides, HDL-c, and low-density lipoprotein cholesterol LDL-c through several mechanisms Grundy, ; Festa et al.
Increased levels of hepatic triglyceride lipase HTGL have also been associated with IR, which may result in faster HDL-c clearance and lower HDL-c levels Baynes et al. It should be noted that IR and dyslipidemia are risk factors for CVDs and DM. Recent research on the relationship between physical inactivity and CVD has yielded sobering results, showing that physical inactivity is a potential risk factor that considerably increases susceptibility to CVD Erlichman et al.
In an RCT study, which the overall effects of PA were analyzed by quartiles of daily steps of all subjects, there were significant reductions in total and LDL cholesterol and visceral fat area between the highest daily steps over 6, and the lowest quartile —2, daily steps and they confirmed that habitual and structured PA with the acceleration levels of 0.
Furthermore, PA has been used as a therapeutic strategy for the prevention of CVD and DM Pearson et al. Previous studies have focused on that PA not only improves IR, but also improves lipid homeostasis Herzig et al. In our study, however, the high-intensity PA effects on insulin were statistically significant regardless of changes in lipid indices TG, HDL-c, and LDL-c levels and other confounding factors.
The improvement in the insulin levels of our participants appeared to be mostly an independent outcome and is not affected by lipid levels. Our new results in sensitivity analysis also confirmed that the change in PA had an independent effect on insulin levels regardless of the levels of lipid indices TG, HDL-c, and LDL-c in participants without DM, and these results may reflect the effects of PA on insulin signaling in the skeletal muscle Despres et al.
Interestingly, we found that the relationship between PA and insulin was more pronounced in men. According to numerous research conducted predominantly in male populations Lehtonen and Viikari, ; Huttunen et al. Recent studies have revealed that sex hormones may play a role in the control of insulin receptors Bertoli et al.
He has achieved great results in experiments with high-intensity interval training for people with type 2 diabetes. This implies that we might need to recommend that people with type 2 diabetes exercise a little differently. Health-promoting insulin sensitivity, in which the muscles are sensitive to insulin and therefore take up glucose from the blood, is one function that researchers and also the pharmaceutical industry really want to understand.
Research has repeatedly found, for example, that cycling markedly increases insulin sensitivity in the muscles that have performed the work, and this can result in the whole body showing increased insulin sensitivity for up to 2 days after each exercise session. This is one reason why people with diabetes especially should exercise because it directly reduces their elevated blood glucose and their need for medication.
The goal could be to make a pill that can have the same effect as physical activity on insulin sensitivity. In the new study, published in Diabetes, Jørgen Wojtaszewski and colleagues asked eight test subjects to perform 2. Then the researchers measured the insulin sensitivity in the muscles of both the active and the inactive legs.
After 4 hours, the insulin sensitivity of the muscles in the leg that had been active was, as expected, higher than that in the inactive leg. Surprisingly, however, the inactive muscles had lower insulin sensitivity than they did on a complete day of rest.
Our body has evolved so that the muscles that need to replenish their fuel depots after a session of physical activity adapt such that they primarily benefit from the available glucose in the blood.
This is achieved by increasing the insulin sensitivity of the muscles so that insulin is much more effective. We understood this intuitively, but we were greatly surprised that insulin sensitivity declined in the rest of the body.
The researchers became aware of this phenomenon in a previous study. However, the study had a different purpose and could therefore not answer the question correctly.
We therefore decided to set up a new study designed to answer this one question. The study showed the same result. Jørgen Wojtaszewski believes that the implications of the new study are interesting, even though the experiments were carried out in the laboratory and involved a fairly extreme form of physical activity.
People with type 1 diabetes often struggle to manage it, and for many, the fluctuation in blood glucose caused by physical activity is another complex factor to consider. This is probably one reason why many people with type 1 diabetes are not physically active, even if they would benefit from it.
If they take too much insulin, they can experience hypoglycaemia and can lose consciousness and eventually die. Jørgen Wojtaszewski explains that future studies will shed light on how different types of physical activity influence insulin sensitivity and insulin resistance in the whole body or parts of it.
In the future, people with type 1 diabetes could well administer their insulin in one way on days when they go running and in another way on days when they lift weights.
In addition, different methods of exercising can affect different individuals differently. All of this needs to be incorporated into the algorithms people with diabetes use to dose their insulin.
Kim A. SjøbergChristian InsulnInsulin sensitivity exercise Kjøbsted Insulon, Lykke SylowMaximilian KleinertAndrew C. BetikChristopher S. ShawBente KiensJørgen F. WojtaszewskiStephen RattiganErik A. Lately, senwitivity been a lot exsrcise attention on how Eercise can help with insulin sensitivity, sdnsitivity for Insluin reason. As Low-calorie cooking techniques people Cardiovascular health benefits with insulin resistance, where the body isn't responding Insulin sensitivity exercise to insulinleading Insulin sensitivity exercise higher blood sensutivity levels, finding effective ways to manage this is important. This isn't just about blood sugar; insulin resistance can lead to type 2 diabetes and increase the risk of heart disease and obesity. Exercise plays a crucial role in improving insulin sensitivity. If you're looking into how physical activity can help with insulin sensitivity, it's an area worth exploring. More than just a means to stay fit, exercise is a key component in taking control of your health.
Ich denke, dass Sie sich irren. Es ich kann beweisen.
Welche Wörter... Toll, der bemerkenswerte Gedanke
Ich meine, dass Sie den Fehler zulassen. Ich biete es an, zu besprechen. Schreiben Sie mir in PM.
Diese Frage ist mir nicht klar.