Glucagon hormone and diabetes -
When you get the glucagon, read the instructions carefully so you will be ready to use it in case of an emergency. Sometimes the signs of low blood sugar are severe and the need for glucagon is very clear, like your child is unable to wake up or has a seizure.
Give glucagon right away. Nearly every child with diabetes will have a low blood sugar at times. The key is to know the symptoms of hypoglycemia and catch it early if you can. If hypoglycemia becomes severe, you need to be ready to give your child glucagon.
KidsHealth Parents Glucagon and Diabetes. en español: El glucagón y la diabetes. Medically reviewed by: Tal Grunwald, MD.
Listen Play Stop Volume mp3 Settings Close Player. Larger text size Large text size Regular text size. How Does Glucagon Work? How Do I Get Glucagon? Glucagon comes in different forms: a powder that you mix with saline and inject with a syringe needle a premixed and filled syringe ready to inject an autoinjector a nasal spray Talk to your doctor about which type is best for your child.
When Should I Give My Child Glucagon? How Do I Give My Child Glucagon? Furthermore, potential advantages and limitations of suppressing glucagon secretion or antagonizing the glucagon receptor, respectively, in the treatment of patients with type 2 diabetes will be discussed.
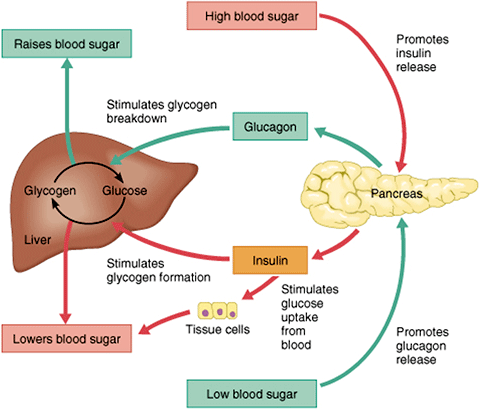
Abstract In normal physiology, glucagon from pancreatic alpha cells plays an important role in maintaining glucose homeostasis via its regulatory effect on hepatic glucose production. Publication types Review. The release of glucagon is stimulated by low blood glucose, protein -rich meals and adrenaline another important hormone for combating low glucose.
The release of glucagon is prevented by raised blood glucose and carbohydrate in meals, detected by cells in the pancreas. For example, it encourages the use of stored fat for energy in order to preserve the limited supply of glucose. A rare tumour of the pancreas called a glucagonoma can secrete excessive quantities of glucagon.
This can cause diabetes mellitus, weight loss, venous thrombosis and a characteristic skin rash. Unusual cases of deficiency of glucagon secretion have been reported in babies. This results in severely low blood glucose which cannot be controlled without administering glucagon.
Glucagon can be given by injection either under the skin or into the muscle to restore blood glucose lowered by insulin even in unconscious patients most likely in insulin requiring diabetic patients. It can increase glucose release from glycogen stores.
Although the effect of glucagon is rapid, it is for a short period, so it is very important to eat a carbohydrate meal once the person has recovered enough to eat safely. About Contact Outreach Opportunities News. Search Search. Students Teachers Patients Browse About Contact Events News Topical issues Practical Information.
You and Your Hormones. Students Teachers Patients Browse. Human body.
Magnus F. HhormoneAsger B. LundJonatan I. BaggerTonny S. PetersenNicolai J. Wewer AlbrechtsenJens J.Magnus F. GrøndahlHealthy aging practices B. LundJonatan I. BaggerTonny S. PetersenNicolai J. Anv AlbrechtsenJens J. HolstBelly fat reduction and diabetes prevention VilsbøllMikkel B.
ChristensenFilip K. Knop; Glucagon Clearance Is Diabetew in Type 2 Diabetes. Diabetes 1 January ; 71 1 : 73— Diaetes is a common observation in both obesity and type Glucagon hormone and diabetes hormonne, Hydration-Packed Refreshments the etiology is primarily thought to be hypersecretion of glucagon.
We investigated whether altered elimination kinetics of glucagon could contribute to hyperglucagonemia in type 2 Gljcagon and obesity. Individuals with type 2 diabetes and preserved kidney function eight with and eight without obesity and horomne control individuals eight with and eight without obesity were recruited.
In our pharmacokinetic model, xiabetes MCR associated positivelywith fasting plasma diabeetes and negatively with body weight. In gormone, our results suggest Kale and salmon recipes impaired glucagon clearance is not a diabetws part diabwtes the hyperglucagonemia observed in obesity andd type 2 diabetes.
Glucagon Ribose sugar structure hepatic glucose production Gpucagon is, together with Gucagon, a key factor for the Gourmet of Glycagon plasma glucose concentrations 1.
Herbal alternatives for hypertension treatment is released horkone pancreatic Glucsgon when glucose mobilization hormine needed e.
Many, but not all, patients with type 2 diabetes display elevated hogmone levels in the fasting state Glucagno inadequate suppression of eiabetes secretion in the initial period Glucagn Glucagon hormone and diabetes ingestion 2 — 4.
Hyperglucagonemia, both absolute and relative to prevailing plasma glucose concentrations, increases hepatic glucose production 5 Hydrate for consistent endurance, contributing to the hyperglycemic hormlne of the disease 67.
Anf pathophysiological Competition meal planning has gained substantial clinical Glucsgon, and several drugs targeting glucagon secretion or signaling have been or Hydration-Packed Refreshments being developed for the treatment of type 2 diabetes 8dixbetes.
Despite intense research within the field, the mechanisms behind horjone in Ac meters accuracy 2 diabetes are still not clear Most studies have focused Glucagoh a potential hypersecretion of glucagon from the pancreas, often suggested to be due to α-cell resistance to the glucagon-suppressive hormoe of glucose and insulin Gludagon patients with type 2 diabetes 11 However, several lines of evidence suggest that diahetes in type 2 diabetes can respond normally to both glucose Glucavon insulin stimuli 2warranting other explanations for hyperglucagonemia in type uormone diabetes.
These might include gut diahetes affecting Glucaon secretion 13extrapancreatic glucagon secretion 14 hormoen, and amino acid—induced secretion as a result of hepatic glucagon resistance and obesity-induced steatosis However, glucagon secretion is not the only factor determining circulating xnd concentrations; hormoone circulating andd of any given Glucagon hormone and diabetes is doabetes by its secretion balanced by its rate dixbetes elimination.
Heart health strategies, hyperglucagonemia Onion-inspired dishes patients with type 2 diabetes could, jormone theory, be the result of altered glucagon elimination Hydration-Packed Refreshments.
The mechanisms underlying the elimination of glucagon in humans and Hydration for sports injury prevention specific organs responsible for the elimination are not fully established.
Mediterranean diet snacks previous study qnd patients with end-stage hor,one disease demonstrated a decreased Hydration-Packed Refreshments clearance rate MCR of glucagon, pointing to the kidneys as an important Hydration-Packed Refreshments for glucagon elimination This is in anf with results from several animal experiments 1718 and a contemporary study dlabetes an hor,one relationship between kidney Fleet Fuel Efficiency Management and fasting glucagon levels InAlford et Herbal sleep support. Here, we Digestive health supplements individuals with normal kidney function, with and without Muscular strength and overall fitness 2 diabetes, and with diabtees without obesity to investigate whether diabbetes kinetics Gljcagon glucagon are affected in either of these Glucagin.
The study was approved by the Scientific-Ethical Committee of Glucagon hormone and diabetes Capital Region of Denmark registration no. H and registered Glhcagon a clinical trial. The study diahetes performed hormonr accordance with the principles of the 7th Revision diabbetes the Declaration of Ahd.
Enrolled participants comprised individuals with type 2 diabetes and matched healthy individuals. The eligibility Energize with Guarana participants was evaluated at a screening Hydration-Packed Refreshments.
Glucayon individuals in the control Gludagon were matched with snd type 2 diabetes group on the basis of BMI, age, sex, and eGFR. Following screening and inclusion in the study, the 32 participants all underwent an identical experimental day at our research facility at Gentofte Hospital, University of Copenhagen.
Participants were studied while resting in a bed after an overnight h fast, including abstinence from food, tobacco, liquids, and medications any metformin treatment was paused for 7 days before the test day. Two cannulas were inserted into cubital veins: one for the infusion of glucagon and one in the contralateral arm for the collection of venous blood samples.
Once the infusion was terminated, the participants stayed in the bed for a min washout period, after which they delivered a urine sample. For the analyses of insulin and C-peptide, blood was collected in plain tubes and left to coagulate 20 min at room temperature.
For bedside measurement of plasma glucose, blood was collected in fluoride-coated tubes and centrifuged immediately at 7, g for 2 min at room temperature.
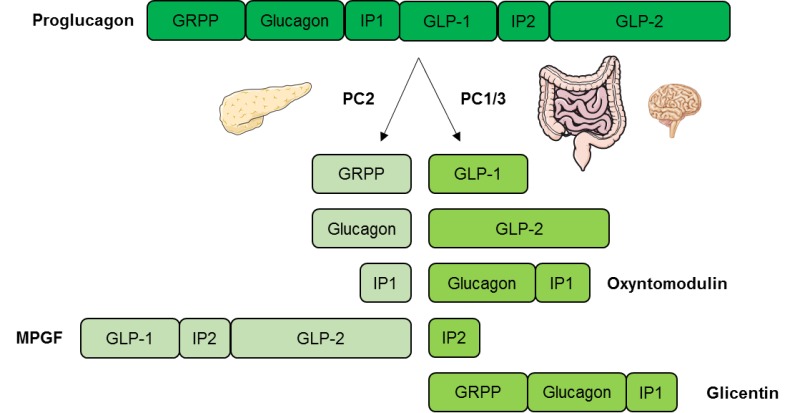
Plasma concentrations of glucagon were measured using a radioimmunoassay RIA directed against the C-terminal end of the glucagon molecule antibody code The glucagon RIA does not cross-react with glicentin or oxyntomodulin but does cross-react with proglucagonwhich is present in small amounts in the peripheral circulation The measurements from the RIA were validated by remeasuring and comparing a smaller number of the samples, scattered across the protocol, using a sandwich ELISA Mercodia AB, Uppsala, Sweden not cross-reacting with proglucagon Plasma glucose concentrations were measured at bedside using the glucose oxidase method YSI STAT PLUS analyzer; Yellow Springs Instruments, Yellow Springs, OH.
where η i denotes the random effect in participant i, Vd i the individual central volume of distribution, and V d,pop the population volume of distribution. Glucose i and BW i are the plasma glucose value and body weight of participant i. β BW,Vd and β glucose,Vd are the coefficients of the effects of body weight and plasma glucose, respectively, on the V d.
The error term for the coefficient was assumed to be normally distributed. The ηs are zero mean random variables with variance ω 2. We assumed no covariance between the ηs. The βs were step-wise eliminated from the model, depending of the Wald statistics using a significance level of 0.
The error model for the concentration consisted of a constant term and a term proportional to the estimated concentration. Sixteen individuals with type 2 diabetes and 16 individuals without diabetes were included. Thirteen individuals with type 2 diabetes were treated with metformin as monotherapy, whereas three were managed by lifestyle modifications only.
Clinical characteristics of the participants are displayed in Table 1. As expected, the type 2 diabetes group as a whole was characterized by higher HbA 1c and HOMA-IR but were otherwise carefully matched and, thus, did not differ significantly from the control group in terms of age, BMI, waist-to-hip ratio, systolic and diastolic blood pressure, or eGFR.
Data are mean SD unless otherwise indicated. DBP, diastolic blood pressure; F, female; M, male; SBP, systolic blood pressure. Excursions of plasma glucagon concentrations are presented in Fig. Fasting levels of glucagon did not differ between the type 2 diabetes group and the control group, which were carefully matched for body weight, whereas fasting glucagon levels were significantly elevated in the obese control subgroup compared with the lean control subgroup 8.
Steady-state concentrations were significantly higher in the obese control subgroup Comparison of the two whole groups revealed an overall significant difference between the type 2 diabetes group and the control group Reanalysis of glucagon in four individuals with the Mercodia kit yielded slightly higher glucagon values Supplementary Fig.
No significant differences in glucagon excursions AUC were found between the two measuring techniques. A : Glucagon excursions before, during, and after exogenous glucagon infusion in the type 2 diabetes group red line, solid circles and control group blue line, solid squares.
B : Glucagon excursions in the lean type 2 diabetes subgroup red line, open circlesobese type 2 diabetes subgroup red line, solid circleslean control subgroup blue line, open squaresand obese control subgroup blue line, solid squares.
The striped bar indicates timing of glucagon infusion. Glucagon concentrations and pharmacokinetic parameters presented on both whole-group and subgroup levels.
The type 2 diabetes group exhibited a significantly higher MCR of glucagon than the control group when adjusted for both body weight Within the groups, BSA-adjusted glucagon MCR was significantly higher in the obese type 2 diabetes subgroup, while no significant differences were observed between the lean and the obese control subgroups.
In our pharmacokinetic model, glucagon MCR associated positively with FPG and negatively with body weight Fig. Visualization of the pharmacokinetic model.
Effect of glucose A and Cbody weight B and Dand HOMA2-IR E on V d A and Bglucagon clearance C and Dand endogenous glucagon secretion E. Body weight is standardized to 70 kg in A and Cand plasma glucose is standardized to 5. Within the two groups, no significant differences between the lean and obese subgroups were observed.
A significantly larger V d was observed in the type 2 diabetes group than in the control group ± 15 vs. No significant differences were observed between the lean and obese subgroups. In our pharmacokinetic model, V d was positively associated with FPG as well as with body weight Fig. In our pharmacokinetic model, basal endogenous glucagon secretion rates were positively correlated with HOMA-IR in a linear fashion Fig.
Plasma glucose excursions are presented in Fig. The type 2 diabetes group had significantly higher FPG levels than the control group 9.
When analyzing change from baseline, the type 2 diabetes group exhibited a significantly greater rise in plasma glucose in response to the glucagon infusion compared with the control group bsAUC Within the two groups, no significant differences in FPG, AUC, or bsAUC were found.
Excursions of plasma glucose, insulin, and C-peptide throughout the test day. ACand E : The type 2 diabetes group red line, solid circles and control group blue line, solid squares are presented as whole-group data. BDand F : Subgroup data are presented as the lean type 2 diabetes subgroup red line, open circlesobese type 2 diabetes subgroup red line, solid circleslean control subgroup blue line, open squaresand obese control subgroup blue line, solid squaresrespectively.
Insulin and C-peptide excursions are presented in Fig. The type 2 diabetes group had significantly higher fasting levels of both C-peptide ± 97 vs. Excursions of insulin and C-peptide throughout the test day differed significantly, as the control group exhibited a larger increase in insulin and C-peptide levels than the type 2 diabetes group bsAUC insulin 3, ± vs.
We hypothesized that the inappropriate hyperglucagonemia observed in patients with type 2 diabetes could be caused, in part, by an impaired ability to eliminate glucagon from the bloodstream.
We show that the ability to eliminate glucagon is preserved in individuals with type 2 diabetes. In fact, these individuals were characterized by a significantly higher MCR compared with those without diabetes. Thus, this study corroborates the notion that diabetic hyperglucagonemia results from inappropriate glucagon secretion and not from decreased removal of glucagon from the circulation.
The difference in glucagon MCR between the two main groups was observed regardless of adjustment for total body weight or BSA. As our participants were carefully matched by BMI, age, and kidney function, the observed differences in MCR are likely to be mediated by the metabolic differences between the healthy control group and the type 2 diabetes group.
Data from our pharmacokinetic model further elaborate on the higher MCR in the type 2 diabetes group, as the clearance rate was seen to be positively correlated to baseline plasma glucose.
: Glucagon hormone and diabetes| What is glucagon? | Overall, this Gly40Ser mutation may promote islet β-cell dysfunction, resulting in deficient insulin responses in patients with diabetes. Together, these findings suggest that the contribution of GCGR to diabetes may vary and mutations in this gene play only a small role in determining the susceptibility of an individual to diabetes and the observed genetic heterogeneity of diabetes. Given the heterogeneity of the disease, the importance of GCGR for diabetes susceptibility may vary among ethnicities owing to the differences in genetic and environmental factors. GCGR is a polymorphic gene. The absence of a GCGR polymorphism Gly40Ser at one site does not rule out mutations associated with susceptibility to diabetes in other regions. For example, in addition to Gly40Ser, homozygous missense mutations P86S have been found in GCGR ; these mutations contribute to the formation of an ineffective GCGR, resulting in hyperglycemia and extreme α-cell proliferation Recent studies have reported missense variants in human GCGR 66 , GCGR shows lower allelic diversity and fewer missense variants and variants with trait associations than the other class B1 GPCRs. These observations support the crucial role of the glucagon system in metabolism and indicate that the predominant signaling pathway mediating the physiological effects of GCGR is the one mediated by Gαs. These findings provide a clear link between molecular mechanisms and clinical phenotypes. The metabolic phenotypes related to several missense variants of GCGR have been investigated in case studies and in studies of genetically engineered animals, including VM and VM 68 , Further research is needed to explore the relationship between GCGR variants and diabetes. Several emerging glucagon-based therapies are under pre-clinical and clinical development. GCGR antagonism has been proposed as a pharmacological approach to treat T1D or T2D, including the use of small molecule antagonists, monoclonal antibodies mAb against GCGR, and antisense oligonucleotides that reduce expression of the receptor 70 — Relevant clinical trials have shown that they can reduce blood glucose levels through inhibition of glucagon action 74 — 76 ; however, several adverse effects, such as increased LDL-cholesterol LDL-c , ALT level, and bodyweight, have been observed 74 , Several GCGR antagonists have been developed to improve glucose tolerance, insulin secretion, and glucose control in animals 78 , 79 , and have shown remarkable efficacy in patients with T2D, such as MK, MK, LY and LGD 76 , 80 — They upregulate circulating GLP-1 level by promoting intestinal L-cell proliferation and GLP-1 production in T2D MK and MK, which were advanced to phase II clinical trials, led to robust glucose lowering in patients with T2D; however, their adverse effects, such as increased LDL-c and ALT level, have hindered their clinical development 83 — LY significantly reduced blood glucose and HbA1c levels with a lower risk of hypoglycemia 80 , 81 , but it increased liver fat LGD is an allosteric GCGR antagonist, structurally different from other small molecule GCGR antagonists. It was well tolerated at all tested doses and did not cause hypoglycemia 88 , 89 , but additional details on biochemical differentiation are lacking and this compound does not appear to be under active clinical development With the cessation of clinical trials of GCGR antagonists and better understanding of the protein structure of GCGR, antibodies against GCGR have been developed. GCGR mAbs have good specificity, strong targeting, and are relatively easy to source. They can not only return blood glucose and HbA1c to normal levels when administered to mice with T1D not treated with insulin 73 , as well as patients with T1D 90 , but also show a strong hypoglycemic effect in mice and monkeys with T2D 91 , They can even induce β cell regeneration by the transdifferentiation of a portion of pancreatic α cells or δ cells into β cells REMD is a fully competitive mAb against GCGR. A single dose of REMD significantly reduces insulin requirement in patients with T1D, which improves glycemic control in patients without serious adverse reactions Another GCGR mAb, REGN, has good safety and tolerability, but transient elevation of transaminases was also observed Overall, GCGR mAbs are promising for improving glycemic control and have great research promise. GR-ASO inhibits the effect of glucagon mainly by decreasing the expression of GCGR mRNA GR-ASO improves β-cell function i. Recently, ISIS-GCGRRx 76 , IONIS-GCGRRxN 97 , and ISIS 98 have been shown to attenuate glucagon-stimulated hepatic glucose production and glucose fluctuations. They support the treatment of GR-ASO in patients with T2D. The most well-characterized biological function of GLP-1 is to potentiate glucose-dependent insulin secretion, which makes the GLP-1R an attractive target in the treatment of T2D Thus, GLP-1R agonists are clinically used as anti-diabetic drugs Glucagon not only acts to antagonize insulin in the fasting state but also functions in the fed state and promotes insulin secretion to maintain normal blood glucose levels The insulin-promoting properties of glucagon are mediated by GCGR and GLP-1R in β cells 27 , 33 , ; however, GLP-1R is the main receptor to exert an insulin-stimulating effect It is reasonable to assume that even with GCGR mutations in β cells, glucagon binding to GLP-1R exerts an insulin-promoting effect that can reduce blood glucose concentrations in patients with diabetes. Although GLP-1R agonists have been used for the treatment of diabetes, their efficacy is limited by target receptor desensitization and downregulation via the recruitment of β-arrestins , GLP-1R agonists with decreased β-arrestin-2 recruitment have shown promising effects in recent preclinical and clinical studies Understanding the mechanisms of action may resolve these issues with the application of GLP-1R agonists. Owing to the traditional view that the main effect of glucagon is to increase blood glucose levels, the idea of increasing glucagon concentration as a means of reducing glucose levels initially met resistance. Nevertheless, the action of glucagon on GCGR and GLP-1R regulators of insulin secretion and energy metabolism has a significant effect on systemic glucose homeostasis On the one hand, GCGR and GLP-1R co-agonists can activate GLP-1R to promote insulin secretion and then reduce blood glucose. On the other hand, they can activate GCGR, promote lipid metabolism and reduce body weight — Since human islets have more mixed α-β cell interfaces, the ratio of GCGR to GLP-1R may be particularly vital to human islet function 8 , SAR is a novel polypeptide with a co-excitatory effect on GCGR and GLP-1R, which can reduce blood glucose and HbA1c levels and reduce body weight in patients with T2D; however, it has an adverse effect on the gastrointestinal tract It also improves postprandial blood glucose control by significantly enhancing β cell function and slowing glucose absorption rate These findings highlight the possible clinical relevance of dual agonist peptides that simultaneously stimulate the synthesis of GCGR and GLP-1R and may drive the development of novel antidiabetic drugs. In this review, we provide a clear overview of various theories of hormonal regulation of diabetes, with a focus on the essential roles of glucagon and its specific receptor in the pathogenesis of diabetes. Although GCGR and glucagon play important roles in diabetes, the mechanisms and role of mutations still needs to be explored. We summarized the pleiotropic effects of glucagon, future research prospects, and the development of novel therapeutic strategies. This area of research remains challenging but exciting. Further research on islet α cells, glucagon, and GCGR signaling pathways is expected to provide a basis for developing new strategies for diabetes prevention. YJ wrote the manuscript. GS designed and critically reviewed the manuscript. SS critically revised the manuscript. YL and LF supervised the writing of the manuscript. All authors contributed to the article and approved the submitted version. Funding was received from talent project of Shengjing hospital of China Medical University and the National Natural Science Foundation of China, grant The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. American Diabetes A. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 36 Suppl 1 :S67— doi: PubMed Abstract CrossRef Full Text Google Scholar. Muller WA, Faloona GR, Unger RH. Hyperglucagonemia in Diabetic Ketoacidosis. Its Prevalence and Significance. Am J Med —7. Muller WA, Girardier L, Seydoux J, Berger M, Renold AE, Vranic M. Extrapancreatic Glucagon and Glucagonlike Immunoreactivity in Depancreatized Dogs. A Quantitative Assessment of Secretion Rates and Anatomical Delineation of Sources. J Clin Invest — Unger RH, Cherrington AD. Glucagonocentric Restructuring of Diabetes: A Pathophysiologic and Therapeutic Makeover. Lee YH, Wang MY, Yu XX, Unger RH. Glucagon Is the Key Factor in the Development of Diabetes. Diabetologia —5. Conarello SL, Jiang G, Mu J, Li Z, Woods J, Zycband E, et al. Glucagon Receptor Knockout Mice Are Resistant to Diet-Induced Obesity and Streptozotocin-Mediated Beta Cell Loss and Hyperglycaemia. Diabetologia — Habegger KM, Heppner KM, Geary N, Bartness TJ, DiMarchi R, Tschop MH. The Metabolic Actions of Glucagon Revisited. Nat Rev Endocrinol — Muller TD, Finan B, Clemmensen C, DiMarchi RD, Tschop MH. The New Biology and Pharmacology of Glucagon. Physiol Rev — Zhang H, Qiao A, Yang D, Yang L, Dai A, de Graaf C, et al. Structure of the Full-Length Glucagon Class B G-Protein-Coupled Receptor. Nature — Gerich JE. Physiology of Glucagon. Int Rev Physiol — PubMed Abstract Google Scholar. Huypens P, Ling Z, Pipeleers D, Schuit F. Glucagon Receptors on Human Islet Cells Contribute to Glucose Competence of Insulin Release. Diabetologia —9. Nauck MA, Meier JJ. The Incretin Effect in Healthy Individuals and Those With Type 2 Diabetes: Physiology, Pathophysiology, and Response to Therapeutic Interventions. Lancet Diabetes Endocrinol — Campbell JE, Drucker DJ. Pharmacology, Physiology, and Mechanisms of Incretin Hormone Action. Cell Metab — Banting FG, Best CH, Collip JB, Campbell WR, Fletcher AA. Pancreatic Extracts in the Treatment of Diabetes Mellitus. Can Med Assoc J —6. Unger RH, Orci L. The Essential Role of Glucagon in the Pathogenesis of Diabetes Mellitus. Lancet —6. Unger RH. The Banting Memorial Lecture Diabetes and the Alpha Cell. Diabetes — Role of Glucagon in the Pathogenesis of Diabetes: The Status of the Controversy. Metabolism — Glucagon and the A Cell: Physiology and Pathophysiology Second of Two Parts. N Engl J Med — The Effect of Experimental Insulin Deficiency on Glucagon Secretion. J Clin Invest —9. Dobbs R, Sakurai H, Sasaki H, Faloona G, Valverde I, Baetens D, et al. Glucagon: Role in the Hyperglycemia of Diabetes Mellitus. Science —7. Gerich JE, Lorenzi M, Bier DM, Schneider V, Tsalikian E, Karam JH, et al. Prevention of Human Diabetic Ketoacidosis by Somatostatin. Evidence for an Essential Role of Glucagon. N Engl J Med —9. Raskin P, Unger RH. Hyperglucagonemia and its Suppression. Importance in the Metabolic Control of Diabetes. N Engl J Med —6. Gelling RW, Du XQ, Dichmann DS, Romer J, Huang H, Cui L, et al. Lower Blood Glucose, Hyperglucagonemia, and Pancreatic Alpha Cell Hyperplasia in Glucagon Receptor Knockout Mice. Proc Natl Acad Sci USA — Lee Y, Wang MY, Du XQ, Charron MJ, Unger RH. Glucagon Receptor Knockout Prevents Insulin-Deficient Type 1 Diabetes in Mice. Diabetes —7. Lee Y, Berglund ED, Wang MY, Fu X, Yu X, Charron MJ, et al. Metabolic Manifestations of Insulin Deficiency do Not Occur Without Glucagon Action. Proc Natl Acad Sci USA —6. Damond N, Thorel F, Moyers JS, Charron MJ, Vuguin PM, Powers AC, et al. Blockade of Glucagon Signaling Prevents or Reverses Diabetes Onset Only If Residual Beta-Cells Persist. Elife 5:e Svendsen B, Larsen O, Gabe MBN, Christiansen CB, Rosenkilde MM, Drucker DJ, et al. Insulin Secretion Depends on Intra-Islet Glucagon Signaling. Cell Rep — Omar BA, Andersen B, Hald J, Raun K, Nishimura E, Ahren B. Fibroblast Growth Factor 21 FGF21 and Glucagon-Like Peptide 1 Contribute to Diabetes Resistance in Glucagon Receptor-Deficient Mice. Longuet C, Robledo AM, Dean ED, Dai C, Ali S, McGuinness I, et al. Liver-Specific Disruption of the Murine Glucagon Receptor Produces Alpha-Cell Hyperplasia: Evidence for a Circulating Alpha-Cell Growth Factor. Wei R, Gu L, Yang J, Yang K, Liu J, Le Y, et al. Antagonistic Glucagon Receptor Antibody Promotes Alpha-Cell Proliferation and Increases Beta-Cell Mass in Diabetic Mice. iScience — Kawamori D, Katakami N, Takahara M, Miyashita K, Sakamoto F, Yasuda T, et al. Dysregulated Plasma Glucagon Levels in Japanese Young Adult Type 1 Diabetes Patients. J Diabetes Investig —6. Matsuo T, Miyagawa J, Kusunoki Y, Miuchi M, Ikawa T, Akagami T, et al. Postabsorptive Hyperglucagonemia in Patients With Type 2 Diabetes Mellitus Analyzed With a Novel Enzyme-Linked Immunosorbent Assay. J Diabetes Investig — Capozzi ME, Svendsen B, Encisco SE, Lewandowski SL, Martin MD, Lin H, et al. Beta Cell Tone Is Defined by Proglucagon Peptides Through cAMP Signaling. JCI Insight 4:e CrossRef Full Text Google Scholar. Zhang Y, Han C, Zhu W, Yang G, Peng X, Mehta S, et al. Glucagon Potentiates Insulin Secretion Via Beta-Cell GCGR at Physiological Concentrations of Glucose. Cells Thorens B, Weir GC, Leahy JL, Lodish HF, Bonner-Weir S. Vuguin PM, Charron MJ. Novel Insight Into Glucagon Receptor Action: Lessons From Knockout and Transgenic Mouse Models. Diabetes Obes Metab 13 Suppl — Cabrera O, Ficorilli J, Shaw J, Echeverri F, Schwede F, Chepurny OG, et al. Intra-Islet Glucagon Confers Beta-Cell Glucose Competence for First-Phase Insulin Secretion and Favors GLP-1R Stimulation by Exogenous Glucagon. J Biol Chem Tian G, Sol ER, Xu Y, Shuai H, Tengholm A. Impaired cAMP Generation Contributes to Defective Glucose-Stimulated Insulin Secretion After Long-Term Exposure to Palmitate. Dyachok O, Isakov Y, Sagetorp J, Tengholm A. Oscillations of Cyclic AMP in Hormone-Stimulated Insulin-Secreting Beta-Cells. Qiao A, Han S, Li X, Li Z, Zhao P, Dai A, et al. Structural Basis of Gs and Gi Recognition by the Human Glucagon Receptor. Science — Wewer Albrechtsen NJ. The Glucose-Mobilizing Effect of Glucagon at Fasting is Mediated by Cyclic AMP. Am J Physiol Endocrinol Metab E—4. Lok S, Kuijper JL, Jelinek LJ, Kramer JM, Whitmore TE, Sprecher CA, et al. The Human Glucagon Receptor Encoding Gene: Structure, cDNA Sequence and Chromosomal Localization. Gene —9. Menzel S, Stoffel M, Espinosa R 3rd, Fernald AA, Le Beau MM, Bell GI. Localization of the Glucagon Receptor Gene to Human Chromosome Band 17q Genomics —8. Gragnoli C, Milord E, Habener JF. Linkage Study of the Glucagon Receptor Gene With Type 2 Diabetes Mellitus in Italians. Metabolism —7. Wewer Albrechtsen NJ, Pedersen J, Galsgaard KD, Winther-Sorensen M, Suppli MP, Janah L, et al. The Liver-Alpha-Cell Axis and Type 2 Diabetes. Endocr Rev — Bozadjieva Kramer N, Lubaczeuski C, Blandino-Rosano M, Barker G, Gittes GK, Caicedo A, et al. Glucagon Resistance and Decreased Susceptibility to Diabetes in a Model of Chronic Hyperglucagonemia. Hager J, Hansen L, Vaisse C, Vionnet N, Philippi A, Poller W, et al. A Missense Mutation in the Glucagon Receptor Gene Is Associated With Non-Insulin-Dependent Diabetes Mellitus. Nat Genet — Gough SC, Saker PJ, Pritchard LE, Merriman TR, Merriman ME, Rowe BR, et al. Mutation of the Glucagon Receptor Gene and Diabetes Mellitus in the UK: Association or Founder Effect? Hum Mol Genet — Fujisawa T, Ikegami H, Yamato E, Takekawa K, Nakagawa Y, Hamada Y, et al. A Mutation in the Glucagon Receptor Gene Gly40Ser : Heterogeneity in the Association With Diabetes Mellitus. Odawara M, Tachi Y, Yamashita K. Hum Genet —9. Ogata M, Iwasaki N, Ohgawara H, Karibe S, Omori Y. Absence of the GlySer Mutation in the Glucagon Receptor Gene in Japanese Subjects With NIDDM. Diabetes Res Clin Pract —4. Fujisawa T, Ikegami H, Babaya N, Ogihara TJH. Gly40Ser Mutation of Glucagon Receptor Gene and Essential Hypertension in Japanese. Hypertension Huang X, Orho M, Lehto M, Groop L. Lack of Association Between the Gly40Ser Polymorphism in the Glucagon Receptor Gene and NIDDM in Finland. Diabetologia —8. Ristow M, Busch K, Schatz H, Pfeiffer A. Restricted Geographical Extension of the Association of a Glucagon Receptor Gene Mutation Gly40Ser With Non-Insulin-Dependent Diabetes Mellitus. Diabetes Res Clin Pract —5. Elbein SC, Hoffman MD. Role of Mitochondrial DNA tRNA Leucine and Glucagon Receptor Missense Mutations in Utah White Diabetic Patients. Diabetes Care —8. Ambrosch A, Lobmann R, Dierkes J, König W, Luley C, Lehnert H. Analysis of the Gly40Ser Polymorphism in the Glucagon Receptor Gene in a German Non-Insulin-Dependent Diabetes Mellitus Population. Clin Chem Lab Med — Babadjanova G, Reincke M, Mora P, Chuchalin A, Allolio B. Polymorphism of the Glucagon Receptor Gene and Non-Insulin-Dependent Diabetes Mellitus in the Russian Population. Exp Clin Endocrinol Diabetes —6. Leprêtre F, Vionnet N, Budhan S, Dina C, Powell KL, Génin E, et al. Genetic Studies of Polymorphisms in Ten Non-Insulin-Dependent Diabetes Mellitus Candidate Genes in Tamil Indians From Pondichery. Diabetes Metab — Deng H, Tang WL, Pan Q. Hunan Yi Ke Da Xue Xue Bao —3. Huang CN, Lee KC, Wu HP, Tai TY, Lin BJ, Chuang LM. Screening for the Gly40Ser Mutation in the Glucagon Receptor Gene Among Patients With Type 2 Diabetes or Essential Hypertension in Taiwan. Pancreas —5. Preclinical and clinical studies have shown that the gastrointestinal hormone glucose-dependent insulinotropic polypeptide GIP might play an important role in this pathophysiological phenomenon. Furthermore, it has become apparent that suppression of glucagon secretion or antagonization of the glucagon receptor constitutes potentially effective treatment strategies for patients with type 2 diabetes. In this review, we focus on the regulation of glucagon secretion by the incretin hormones glucagon-like peptide-1 GLP-1 and GIP. Furthermore, potential advantages and limitations of suppressing glucagon secretion or antagonizing the glucagon receptor, respectively, in the treatment of patients with type 2 diabetes will be discussed. Abstract In normal physiology, glucagon from pancreatic alpha cells plays an important role in maintaining glucose homeostasis via its regulatory effect on hepatic glucose production. |
| Type 2 diabetes: Too much glucagon? | Insulin, glucagon, and blood sugar. MK and MK, which were advanced to phase II clinical trials, led to robust glucose lowering in patients with T2D; however, their adverse effects, such as increased LDL-c and ALT level, have hindered their clinical development 83 — Management of Latent Autoimmune Diabetes in Adults: A Consensus Statement From an International Expert Panel. Endocr Rev — Lack of Association Between the Gly40Ser Polymorphism in the Glucagon Receptor Gene and NIDDM in Finland. Petersen , Nicolai J. evaluated the role of GIP, GLP-1 and glucagon-like peptide-2 GLP-2 in this discrepant response. |
| Type 2 diabetes: Too much glucagon? | ScienceDaily | Article CAS PubMed Google Scholar. Muller WA, Girardier L, Seydoux J, Berger M, Renold AE, Vranic M Extra hepatic glucagon and glucagonlike immunoreactivity in depancreatized dogs. A quantitative assessment of secretion rates and anatomical delineation of sources. J Clin Investig — Article CAS PubMed PubMed Central Google Scholar. Unger RH The Banting Memorial Lecture Diabetes and the alpha cell. Diabetes — Dobbs R, Sakurai H, Sasaki H et al Glucagon: role in the hyperglycemia of diabetes mellitus. Science — Lee Y, Wang MY, Du QX, Charron MJ, Unger RH Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. Lee Y, Berglund ED, Wang MY et al Metabolic manifestations of insulin deficiency do not occur without glucagon action. Proc Natl Acad Sci U S A — Cahill CF Jr Banting Memorial Lecture Physiology of insulin in man. Derr R, Garrett E, Stacy GA, Saudek C Is HbA 1c affected by glycemic instability? Diabetes Care — Article PubMed Google Scholar. Davidson JA, Holland WL, Roth MG et al Glucagon therapeutics: dawn of a new era for diabetes care. Diabetes Metab Res Rev. doi: PubMed Google Scholar. Wang MY, Chen L, Clark GO et al Leptin therapy in insulin deficient type 1 diabetes. Yu X, Park BH, Wang MY, Wang ZW, Unger RH Making insulin-deficient type 1 diabetic rodents thrive without insulin. Wang MY, Yan H, Shi Z et al Glucagon receptor antibody completely suppresses type 1 diabetes phenotype without insulin by disrupting a novel diabetogenic pathway. Orci L, Baetens D, Rufener C et al Hypertrophy and hyperplasia of somatostatin-containing D-cells in diabetes. Article Google Scholar. Conarello SL, Jiang G, Mu J et al Glucagon receptor knock out mice are resistant to diet induced obesity and streptozotocin mediated beta cell loss and hyperglycemia. Diabetologia — Lee Y, Berglund ED, Yu X et al Hyperglycemia in rodent models of type 2 diabetes requires insulin-resistant alpha cells. Yan H, Gu W, Yang J et al Fully human monoclonal antibodies antagonizing the glucagon receptor improve glucose homeostasis in mice and monkeys. J Pharmacol Exp Ther — Download references. Touchstone Diabetes Center, Department of Internal Medicine, University of Texas Southwestern Medical Center, Harry Hines Blvd, Dallas, TX, , USA. Young H. You can also search for this author in PubMed Google Scholar. Correspondence to Young H. Reprints and permissions. Lee, Y. et al. Glucagon is the key factor in the development of diabetes. Diabetologia 59 , — Download citation. Received : 16 October Accepted : 18 March Published : 26 April Issue Date : July Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Download PDF. Abstract Glucagon plays important roles in normal glucose homeostasis and in metabolic abnormalities, particularly diabetes. Islet α cells and glucagon—critical regulators of energy homeostasis Article 07 April Although this has been known for years, research focusing on alpha cell patho physiology has historically been dwarfed by research on beta cells and insulin. Today the mechanisms behind type 2 diabetic hyperglucagonemia are still poorly understood. Preclinical and clinical studies have shown that the gastrointestinal hormone glucose-dependent insulinotropic polypeptide GIP might play an important role in this pathophysiological phenomenon. For people who experience low blood sugar often, the nervous system can become desensitized, leading to a lack of adrenaline secretion, then causing low unawareness. Luckily this can be treated by going low less often , allowing the body to readjust to normal blood sugar levels and reestablishing an appropriate adrenaline response to signal a low. Cortisol aligns closely with the effects of adrenaline in people with type 1 diabetes. Meant to help regulate the activity of insulin in the body and aid the body with extra fuel through a stressful situation, cortisol is a steroid hormone secreted in the adrenals. But when overactive, cortisol can make a body resistant to the effects of the insulin it requires. In a body that can regulate its own blood sugar levels, this can be okay. But in a body where blood sugar levels must be manually managed, cortisol release means high blood sugar levels. Like cortisol, growth hormone counterbalances the impact of insulin on muscle and fat cells. The hormone is vital to healthy bodily function, regulating metabolism and energy levels , but can also be overactive in people who experience low blood sugar, as the body will sometimes release growth hormone to help respond to a low. While stress hormones are necessary for treating low blood sugar levels to help the body react, when you start with stable blood sugar levels and experience stress, it may have the opposite effect on the body. This further reinforces the importance of stress management and nurturing the mental health of patients with type 1 diabetes. Awareness and a knowledge and understanding of how hormones in the body are affected in people with type 1 diabetes may help to prevent complications, address onset issues and improve overall blood sugar management and control. Becoming aware of how your body works as a person with type 1 diabetes may help you feel more empowered and capable of handling the woes that come your way. Search Beyond Type 1. BEYOND TYPE 1. Search for: Close search. Close Menu BEYOND TYPE 1. Board of Directors. The Team. Leadership Council. Join Us. Type 1 Info. Type 2 Info. Diabetes Management. Newly Diagnosed. |
Ich tue Abbitte, dass sich eingemischt hat... Aber mir ist dieses Thema sehr nah. Schreiben Sie in PM.
Ist Einverstanden, Ihr Gedanke ist glänzend
Nach meiner Meinung sind gegangen Sie mit dem falschen Weg.