
Video
AngiogenesisAngiogenesis and inflammation -
VEGF causes release of factors involved in blood vessel remodeling and blood clotting. It exists in 2 isoforms: VEGF B and VEGF B and can form heterodimers with VEGF. VEGF B binds only to VEGFR1, inducing expression and increased activity of urokinase-type plasminogen activator uPA and plasminogen-activator inhibitor-1 and suggesting a role in ECM degradation and EC migration.
VEGF B plays a role in coronary artery development. Both VEGF C and VEGF D consist of noncovalent dimers and bind to VEGFR2 and VEGFR3. VEGF C seems to play a predominant role in lymph angiogenesis and is mainly expressed during embryogenesis, whereas VEGF D is also expressed in adult heart, lung, and skeletal muscle.
PlGF is mainly expressed in the placenta and tumors. It exists in 3 splice variants that bind to VEGFR1, but only PlGF-2 can also bind to Np-1 and heparin. The bioavailability of VEGF and bFGF, and therefore their capacity to induce angiogenesis, may be controlled by PlGF PDGF is among the main inducers of angiogenesis.
It is an endothelial mitogen initially purified from human platelets, 43 , 44 but it can also be secreted by macrophages, ECs, fibroblasts, and keratinocytes. It signals through 2 receptors α and β, and the β-form is predominant in fibroblasts and microvascular ECs. Signaling leads to cellular proliferation and migration in synergy with TGF-β and epidermal growth factor.
It has been suggested to play a crucial role in the remodeling of existing vasculature in an early phase of tumor development. The bFGF FGF-2 is one of the most extensively studied members of the FGF family, which comprises 23 members to date. They are major growth and differentiation factors in embryonic development as well as in the adult, playing a role in neuronal signaling, inflammatory processes, hematopoiesis, angiogenesis, tumor growth, and invasion.
The FGFs bind to surface receptors FGFR consisting of 3 extracellular immunoglobulin-like domains, a single transmembrane domain and an intracellular tyrosine kinase domain, which dimerizes upon ligand binding and initiates the intracellular signal transduction.
FGF-2 has 4 known alternative splice forms, all inducing proliferation, chemotaxis, uPA activity, and VEGF and VEGFR2 upregulation in ECs. The angiogenic activity of bFGF may be mediated in part by upregulation of VEGF.
HGF has been shown to be a potent angiogenic factor in vitro and in vivo. HGF is secreted by mesenchyme-derived cells as an inactive precursor that is activated via proteolytic cleavage by uPA or tissue plasminogen activator and plasma kallikrein.
Through activation of c-met, HGF induces proliferation, migration, and differentiation of various types of cells and is a potent mediator of angiogenesis, in part because it induces production of VEGF in the endothelium. Angiogenin is a heparin-binding kDa plasma protein with angiogenic and ribonucleolytic activity.
Although its structural features have been extensively studied, an understanding of its physiological role and of how its properties are expressed continues to elude researchers.
It has been suggested that angiogenin first binds to actin, followed by dissociation of the actin-angiogenin complex and subsequent activation of tissue plasminogen activator. This generates plasmin, which is known to degrade basement membrane laminin and fibronectin. The recently discovered Ang-1 and Ang-2 have been clarified to act alongside VEGF.
Ang-2 is especially responsible for the initiation of angiogenesis through recruitment and proliferation of ECs. It plays a modulatory role by binding to the Tie-2 receptor. Ang-2 expression can be upregulated by VEGF, bFGF, and hypoxia and can be downregulated by Ang-1 and TGF-β and in an autocrine way by itself.
Several different MMPs are produced by ECs and are strongly implicated in the process of angiogenesis. The activity of MMPs is regulated by inhibitors known as TIMPs, which bind to and inhibit all activated MMPs.
The expression of MMPs and TIMPs is low or absent in healthy tissues but they are upregulated in sites of physiological or pathological angiogenesis. Integrins, chemokines, and adhesion molecules are key players in angiogenesis.
Some integrins αvβ3 and αvβ5 are more involved than others, although they are not specific for neovascularization. Chemokines are also fundamental participants, along with a variety of other factors, that regulate angiogenesis.
Within the CXC family of chemokines, there is a functional discrepancy in which some family members are angiogenic and others are angiostatic. CD also known as MUC18, Mel-CAM, S-Endo, and A32 antigen is a member of the immunoglobulin gene superfamily 79 that functions as an adhesion molecule involved in cell-cell interactions.
It is expressed on ECs, pericytes, and smooth muscle cells. Evidence that angiogenesis is involved in IBD was obtained from animal models of colitis, most notably from studies of angiogenesis inhibition.
Plasma levels of HGF increased significantly after development of acute colitis in mice by acetic acid administration. Colonic FGF binding activity of heparin has been found to be increased in a model of experimental colitis.
Recent experimental data showed that bone marrow is involved in the repair and formation of blood vessels in animal models of colitis, forming ECs, vascular smooth muscle cells, and pericytes. There is evidence that microvascular anatomy in the chronically inflamed intestine, mainly in Crohn's disease CD , has undergone vascular remodeling.
It could be hypothesized that angiogenesis starts early in the course of IBD, similar to other chronic inflammatory diseases, 6 , 93 , 94 and it may antedate the specific clinical and histological signs of inflammation.
At a latter stage, angiogenesis could contribute to the perpetuation of inflammatory manifestations. However, protective factors are thought to mediate the ability of the intestinal mucosa to resist injury and regenerate ulcerated areas.
In this context, angiogenesis plays an important role as a protective factor during the regeneration process of injured tissues. The balance between proangiogenic and antiangiogenic mediators may be critical in restoring recirculation in the inflammatory bowel by promoting neovascularization.
EC proliferation is required for sustained angiogenesis. Studies on intestinal biopsies from patients with IBD have shown alterations of endothelial adhesion molecules.
There have been a number of important papers published recently that provide evidence that angiogenesis may be a primary pathogenic mechanism in IBD. The focus of these studies has been on the regulation of expression of mediators of angiogenesis, in addition to receptor activation and signal transduction pathways.
Among angiogenesis markers in IBD, VEGF is the most thoroughly described factor. Several studies have shown elevated circulating VEGF levels in patients with IBD compared to healthy controls HC. VEGF production by peripheral blood mononuclear cells of patients with IBD was found to be significantly increased in both active CD and UC.
Inflamed mucosa of patients with active UC or CD showed a significantly higher spontaneous production of VEGF than normal mucosa of HC. VEGF was recently found to induce epithelial cell migration in CACO-2 and IEC cells compared with controls, suggesting that VEGF apart from angiogenesis is also an important factor for intestinal epithelial cell restitution.
IL-4 has been shown to reduce the increased VEGF production of peripheral blood mononuclear cells in patients with IBD to normal levels. Corticosteroids have been found to suppress nonstimulated VEGF production in patients with IBD.
The administration of the anti-TNF-α antibody infliximab seems to interfere with VEGF production. A significant reduction of VEGF levels in the sera of patients with rheumatoid arthritis or psoriasis after intravenous infusions of infliximab has been reported.
Whether therapeutic reduction of serum VEGF levels is indeed associated with inhibition of angiogenesis should be evaluated in future studies. Thalidomide, which is a potent inhibitor of angiogenesis, has been reported to be effective in refractory and steroid-dependent CD, , as well as in severe therapy-resistant intestinal bleeding related to CD and bleeding of obscure origin.
PDGF is overexpressed in inflamed colonic mucosa, predominantly in macrophages and fibroblasts. In areas of active inflammation and in areas of active fibrosis activated cells positive for mRNA and protein of PDGF-A, -B, -alphaR, and -betaR were found, suggesting that PDGF plays a role in neovascularization in acute inflammation and in the repair process of IBD.
Serum levels of bFGF were significantly higher in active IBD compared to HC. In another study the number of bFGF-containing cells was significantly increased in patients with active IBD compared with patients with IBD in remission.
The bFGF levels were lower in patients who responded or who were completely healed with infliximab when compared with patients who did not respond or heal. In another study, a significant reduction of bFGF serum levels in patients with CD who responded to infliximab therapy was demonstrated.
Serum levels of HGF were elevated in patients with IBD compared with HC. Moreover, in this study the serum levels of HGF were directly correlated with disease activity.
These observations suggest that the HGF-Met system is involved in the repair process of the inflamed mucosa of UC. Serum angiogenin levels were found significantly increased in patients with UC and CD compared with HC, but serum angiogenin concentration was not associated with disease activity.
These data suggest that angiogenin in IBD patients may act as a mediator of the effects of inflammation, but it may also have a more prominent role in the reconstruction of damaged mucosa in UC and CD.
There are no studies of angiogenin in the gut tissues of patients with IBD, which are needed to delineate the role of angiogenin in wound healing. Serum Ang-2 and its receptor Tie-2 levels were significantly increased in patients with UC and CD compared with HC.
The local levels of these mediators in the intestine where angiogenesis occurs and the precise source or stimuli for the overproduction of these molecules in IBD remain obscure.
MMPs, such as MMP-1, MMP-9, and TIMP-1, which are involved in tissue remodeling, are expressed by vascular smooth muscle cells of venules in ulcer bases of patients with IBD.
These results suggest that MMPs may play an important role in angiogenesis in IBD. A role of CD, a transmembrane glycoprotein expressed at the junction of ECs, which is involved in the control of cell-cell cohesion and in angiogenesis has recently been suggested in IBD.
In addition, a decrease of sCD in active and extensive disease in both CD and UC was observed. These findings could indicate a mechanism of active angiogenesis and altered vascular permeability in IBD.
Angiogenesis is enhanced in IBD and is 1 component of the increased vascular turnover observed during active disease. Our assessment of the evidence led to the conclusion that angiogenesis may contribute to the initiation and perpetuation of IBD.
Future studies should address the specific pathways that mediate pathological angiogenesis in IBD. Angiogenesis in IBD is a complex process rather than a single entity, and our increasing understanding of the various roles of intestinal vascular growth raises the exciting possibility of novel therapeutic strategies in IBD.
Targeting angiogenesis in IBD in the future may provide additional benefits when used in combination with current treatments, the main goal of which is to combat inflammation. Risau W. Mechanisms of angiogenesis. Google Scholar. Dvorak HF. Angiogenesis: update J Thromb Haemost.
Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. Carmeliet P. Angiogenesis in health and disease. Angiogenesis in life, disease and medicine. Clavel G , Bessis N , Boissier MC.
Recent data on the role for angiogenesis in rheumatoid arthritis. Joint Bone Spine. Simons M. Angiogenesis, arteriogenesis, and diabetes: paradigm reassessed? J Am Coll Cardiol. Angiogenesis: where do we stand now? Walsh DA. Angiogenesis and arthritis. Rheumatology Oxford.
Walsh DA , Pearson CI. Angiogenesis in the pathogenesis of inflammatory joint and lung diseases. Arthritis Res. Ferrara N , Alitalo K. Clinical applications of angiogenic growth factors and their inhibitors.
Qu Z , Liebler JM , Powers MR , et al. Mast cells are a major source of basic fibroblast growth factor in chronic inflammation and cutaneous hemangioma. Am J Pathol. Taylor PC , Sivakumar B. Hypoxia and angiogenesis in rheumatoid arthritis.
Curr Opin Rheumatol. Hatton MW , Southward SM , Legault KJ , et al. Fibrinogen catabolism within the procoagulant VX-2 tumor of rabbit lung in vivo: effluxing fibrin ogen fragments contain antiangiogenic activity. J Lab Clin Med. Cullen JP , Sayeed S , Sawai RS , et al.
Pulsatile flow-induced angiogenesis: role of G i subunits. Arterioscler Thromb Vasc Biol. Schaper W , Scholz D. Factors regulating arteriogenesis. Hatoum OA , Binion DG , Gutterman DD. Paradox of simultaneous intestinal ischaemia and hyperaemia in inflammatory bowel disease.
Eur J Clin Invest. Di Sabatino A , Fulle I , Ciccocioppo R , et al. Doppler enhancement after intravenous levovist injection in Crohn's disease. Inflamm Bowel Dis.
Griga T , Tromm A , Spranger J , et al. Increased serum levels of vascular endothelial growth factor in patients with inflammatory bowel disease. Scand J Gastroenterol. Griga T , Voigt E , Gretzer B , et al. Increased production of vascular endothelial growth factor by intestinal mucosa of patients with inflammatory bowel disease.
Kanazawa S , Tsunoda T , Onuma E , et al. VEGF, basic-FGF, and TGF-beta in Crohn's disease and ulcerative colitis: a novel mechanism of chronic intestinal inflammation. Am J Gastroenterol. Griga T , May B , Pfisterer O , et al. Immunohistochemical localization of vascular endothelial growth factor in colonic mucosa of patients with inflammatory bowel disease.
Di Sabatino A , Ciccocioppo R , Armellini E , et al. Serum bFGF and VEGF correlate respectively with bowel wall thickness and intramural blood flow in Crohn's disease. Konno S , Iizuka M , Yukawa M , et al. Altered expression of angiogenic factors in the VEGF-Ets-1 cascades in inflammatory bowel disease.
J Gastroenterol. Koutroubakis IE , Xidakis C , Karmiris K , et al. Serum angiogenin in inflammatory bowel disease. Dig Dis Sci. Potential role of soluble angiopoietin-2 and tie-2 in patients with inflammatory bowel disease.
Danese S , Sans M , Beck I , et al. Starving the inflamed gut: angiogenesis blockade as a novel approach to experimental colitis. Jackson JR , Seed MP , Kircher CH , et al. The codependence of angiogenesis and chronic inflammation.
FASEB J. Puxeddu I , Ribatti D , Crivellato E , et al. Mast cells and eosinophils: a novel link between inflammation and angiogenesis in allergic diseases. J Allergy Clin Immunol. Fajardo LF , Kwan HH , Kowalski J , et al. Dual role of tumor necrosis factor-alpha in angiogenesis.
Nagashima M , Yoshino S , Ishiwata T , et al. Role of vascular endothelial growth factor in angiogenesis of rheumatoid arthritis. J Rheumatol. Peacock DJ , Banquerigo ML , Brahn E. A novel angiogenesis inhibitor suppresses rat adjuvant arthritis.
Cell Immunol. Storgard CM , Stupack DG , Jonczyk A , et al. During this type of event, cell-cell endothelial junctions are temporarily inhibited, with several inflammatory mediators released into the circulation, including histamine, thrombin, VEGF, and pro-inflammatory cytokines 25 , Various signaling pathways, including those involving Rho GTPases, MAP kinases, and protein kinases, are then activated by these factors, leading to the interruption of cell-cell joints and the migration of phagocytic and other blood cells Although transendothelial transport occurs during inflammation in order to increase vascular permeability, paracellular transport is believed to be primarily involved in cell migration 22 , Recent studies have indicated the importance of mural cells, including pericytes, smooth muscle cells, and macrophages, in the regulation of permeability 30 — An excellent review of these aspects has been published by Goddard and Iruela-Arispe VEGF is the main soluble factor that modifies the endothelial barrier 35 — This factor is secreted by neutrophils, platelets, macrophages, activated-T cells, dendritic cells, pericytes, and the endothelial cells themselves VEGF was isolated in by Ferrera from the Genentech group Several homodimeric glycoproteins comprise the VEGF family.
In mammals, five members of the VEGF family have been identified, namely VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placenta growth factor PLGF 36 , 37 , As the prototypical VEGF, VEGF-A is considered the most potent stimulator of vasculogenesis and angiogenesis In addition to increased vascular permeability, vasodilatation, and the recruitment of inflammatory cells, VEGF triggers the inhibition of apoptosis and increases cellular proliferation The biological activity of VEGF is mediated by the high affinity tyrosine kinase receptors VEGFR-1, VEGFR-2, and VEGFR VEGFR-2 is expressed primarily in endothelial cells and its interaction with VEGF-A triggers increased vascular permeability.
VEGFR-2 dimerization induces the autophosphorylation of tyrosine residues and the activation of specific signaling pathways, including the PI3K and p38 MAPK pathways 36 , 37 , In addition, Src kinase activation induces the phosphorylation of VE-cadherin and various catenins, preventing them from anchoring to the cytoskeleton 22 , 25 , At the site of damage, platelets then participate in the coagulation process in order to prevent blood loss from damaged vessels Subsequently, neutrophils arrive at the site of damage to eliminate the pathogen by means of reactive oxygen species ROS In addition, neutrophils are removed by efferocytosis 44 , The resolution of the associated tissue damage and the return to a normal tissue structure with proper tissue-specific funcions are the goals of the vascular hyperpermeability associated with inflammation The resolution of inflammation is a highly orchestrated process involving numerous biochemical processes.
In order for this resolution to be successful, inflammatory mediators must act on specific targets to initiate a series of events resulting in homeostasis 46 , The particular molecules responsible for carrying out the above events include the cytokines produced by M2 macrophages and specialized lipids such as lipoxins, resolvins, protectins, and maresins Proteins such as annexin-A1, adrenocorticotropic hormone, galectin-1, and adenosine are also involved These molecules are synthesized by various cell types, including neutrophils, macrophages, and endothelial cells.
Although the mechanisms by which the hyperpermeability of the endothelium returns to the basal state have yet to be completely described, oxidized phospholipids are known to act as protectors of the endothelial barrier At low concentrations, the oxidized 1-palmitoylarachidonic-sn-glycerolphosphorylcholine PAPC OxPAPC inhibits TNF-α production in phagocytes by blocking the NF-κB pathway In addition, OxPAPC is involved in the restoration of vascular permeability through the activation of the GTPases Cdc42 and Rac.
This results in increased cortical actin, the stabilization of cell-cell junctions, and the inhibition of paracellular gap formation. Cdc42 and Rac also activate the Ras-associated protein-1 Rap1 signaling pathway.
Rap1 is an important regulator of various cell functions, including cellular polarization, and leads to increased VE-cadherin and β-catenin, as well as ZO-1 and ocluddin.
Furthermore, OxPAPC interacts with the 78 kDa glucose-regulated protein GRP78, which is a multifunctional protein found in the endoplasmic reticulum and plasma membrane. This interaction then provides stability to the union of AJs with TJs 49 — The persistence of the harmful agent that induced the inflammation leads to the upregulation of the inflammatory response.
As already mentioned, vascular hyperpermeability promotes the presence of inflammatory cells such as monocytes and macrophages.
These cells release pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6 that increase the expression of adhesion molecules and chemokines for further recruitment of T-lymphocytes In these immune cells, activation of signaling pathways such as, NF-κB, MAPK, and JAK-STAT increase cytokines production.
The arrival of more immune cells exacerbates the inflammatory response inducing a chronic inflammation. In response to these factors, the endothelial cells promote angiogenesis. The endothelial cells proliferate and migrate to form new capillaries contributing to restoring nutrient levels and facilitating immune cell migration In this shifting microenvironment, the immune cells gradually modify their cytokine profile sustaining the inflammatory network.
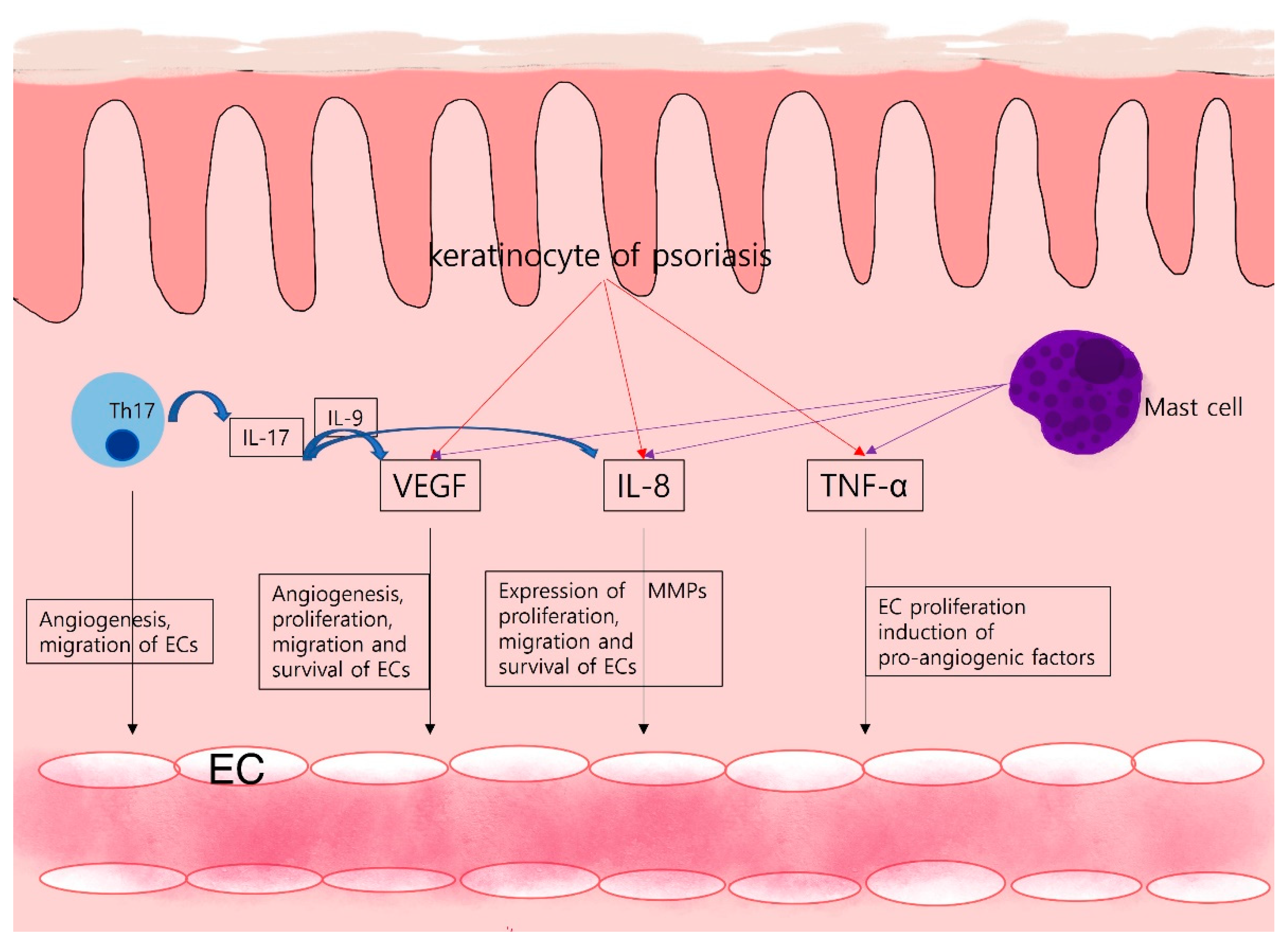
In particular, the presence of Th17 lymphocytes in the milieu contributes to the persistence of inflammation. IL-6, TGF-β, and IL-1β are necessary cytokines for Th17 lymphocytes development, these cells secrete IL, IL, and IL Combination of IL with other cytokines such as IL-6 and IL-8 contributes to the chronicity of inflammation 54 , An example of pathological angiogenesis during chronic inflammation is diabetic retinopathy Angiogenesis in the retina of patients with diabetes is initiated by ischemia produced by chronic inflammation.
In addition, the hyperglycemic environment activates a series of events, culminating in increased vascular permeability, the accumulation of extravascular fluid, ischemia, and pathological angiogenesis Some studies have shown high levels of pro-inflammatory cytokines, including VEGF, TNF-α, NO, and IL-6 in the vitreous humor of patients with diabetes mellitus Another example is prolonged peritoneal dialysis.
In this pathology, adipocytes secrete pro-inflammatory cytokines, which culminates in pathological angiogenesis. The association of chronic inflammation and angiogenesis also occurs in inflammatory bowel disease where continuous ulceration and regeneration lead to the development of chronic inflammation and pathological angiogenesis Further investigation of the association between inflammation and angiogenesis, which can result in a number of pathological conditions, is required for a better understanding of the underlying molecular events in these process.
In the future, selected molecules may be useful as therapeutic targets for the reprogramming of homeostasis. As discussed in the previous sections, increased vascular permeability during the inflammatory process is essential for the arrival of immune cells.
The vast array of cytokines and chemokines that participate in the inflammatory process serve to activate and recruit immune cells, which also impacts the associated endothelial cells 59 , Currently, the association of inflammation, angiogenesis, and cancer is well-known.
Unlike acute inflammation, during chronic processes of inflammation, inflammatory infiltrates consisting primarily of mononuclear cells that produce reactive oxygen and nitrogen species RONS are present. Most RONS have unpaired electrons; thus, they are considered free radicals.
As such, while RONS are potent microbial agents, they can also cause cell damage when they are released. DNA is particularly sensitive to RONS, which can induce modified DNA structures such as 2'-deoxyribose. In particular, the modified DNA structure 7,8-hydroxy-2'-deoxyguanosine 8-oxodG induces a breakdown in the double and single strand of DNA These alterations can affect cell cycle regulation and lead to an increase in mutation rate.
In driver genes, the gain of mutations has been shown to trigger carcinogenesis Although this event in itself is not enough to produce a tumor, the resulting microenvironment favors chronic inflammation which is another factor involved in cancer initiation. Pro-inflammatory cytokines, such as IL-1 and IL-6, and growth factors, such as TGF-β and VEGF, then can activate various signaling pathways, primarily those involving NF-κB and STAT3 It has been demonstrated that both NF-κB and STAT3 stimulate various survival signals in cells, associated with triggering the carcinogenesis process 53 , Other soluble factors generated in the inflammatory process include VEGF-A, cyclooxygenase-2, and prostaglandins.
The overall effect of all these molecules on the endothelium is crucial for the recruitment of cells, the production of inflammatory mediators, the increase in vascular permeability, and angiogenesis 66 , In , Dvorak made the analogy of the tumor and its associated tumor microenvironment TME to a wound that does not heal In this study, the tumor vasculature captured radiolabeled fibrinogen several-fold faster than did control tissue, allowing for an increase in tumor microvascular permeability.
This increase in vascular permeability was attributed to the vascular permeability factor, now known as VEGF-A In addition, Dvorak demonstrated that in the phenomena studied, ECM molecules, including laminin, fibronectin, collagen, and proteoglycans, were involved.
Another similarity between tumors and wound healing is the presence of inflammatory infiltrates. In , Cao et al. The cells were then inoculated in a rodent model using dorsal skinfold window chambers; the presence of angiogenesis was demonstrated at a very early stage of tumor development and was hypoxia-independent.
Mizukami et al. reported that inoculation of HIF-1α knocked down by siRNA in colon carcinoma cell lines reduced tumor growth with no alteration of angiogenesis in a CD1 nude mouse model In addition, this same group demonstrated that under HIF-1α-independent angiogenesis conditions, the RAS and NF-κB signaling pathways upregulated the production of VEGF, IL-8, COX-2, and prostaglandin E Thus, the process of angiogenesis may be occurring at a very early stage of tumor development and not necessarily at the point of the hypoxia-induced angiogenic switch.
However, a deeper research in this issue is necessary to design therapeutic schemes in order to impact in cancer patient clinical outcome. It is widely known that hypoxia is another critical player in the tumor angiogenesis process.
Several factors during cancer development contribute to the generation of hypoxia and the resulting VEGF release. The main molecular component of hypoxia-induced angiogenesis initiation is the hypoxia-inducible factor HIF -1α.
In metazoan organisms, HIF-1α has been shown to play an essential role in oxygen homeostasis. Under normoxic conditions, HIF-1α is continuously synthesized and degraded. However, under hypoxic conditions, HIF-1α is stabilized and accumulates in the cytoplasm where it dimerizes with HIF-1β.
Hypoxia, in conjunction with angiogenesis, can also activate other cancer-specific biological pathways. Under hypoxic conditions, tumor cells present a metabolic shift from oxidative phosphorylation to aerobic glycolysis In addition, hypoxia increases cellular proliferation and the avoidance of apoptosis, which contributes to the chemoresistance of tumors Hypoxia also induces the fibroblasts surrounding the tumor, to acquire a cancer-associated fibroblast phenotype, which is associated with the release of bFGF, IL-6, PDGF, and TGF-β and favors a microenvironment conducive to the cellular evasion of the antitumor immune response Hypoxia also induces the epithelial to mesenchymal transition EMT , which encourages tumor cells motility, and MMP secretion, subsequently leading to an invasion phenotype The angiogenic switch provides more advantages to the tumor than just angiogenesis, leading to the gradual acquisition of several tumor hallmarks, which allow the tumor to develop into more advanced stages clinically advanced tumor.
Metastasis is the leading cause of death from tumors. It can be described as the process by which tumor cells separate from the primary tumor, travel via the blood or lymph, and arrive at a distant site where they can establish a secondary tumor or metastasis.
In terms of a spatial-temporal context, after the angiogenic switch onset, the tumor establishes and grows. The resulting high rate of proliferation and mutagenesis then induces genetic heterogeneity in the tumor. Welch and Hurst proposed four characteristics of metastasis, namely, i Motility and invasion, ii microenvironment modulation, iii plasticity, and iv colonization.
In addition to providing nutrients and oxygen, tumor angiogenesis also contributes to the metastatic cascade, which involves vasculogenic mimicry and co-option mechanisms Vasculogenic mimicry is the generation of structures such as channels and tubes, in conjunction with perfusion, and does not involve endothelial cells.
The network formed by vasculogenic mimicry connects with blood vessels in order to supply blood and fluids to the tumor mass Tumors that display vasculogenic mimicry are associated with greater aggressiveness and patients with these tumors typically have lower survival rates.
In addition, vasculogenic mimicry is considered an evasion mechanism for antiangiogenic therapy 77 , During embryogenesis, this mechanism is preponderant. However, in cancer progression, the EMT allows tumor cells to develop vasculogenic mimicry.
As part of the EMT, VE-cadherin is expressed in tumor cells favoring both vasculogenic mimicry and metastasis. Moreover, inflammation associated with cancer contributes to both vasculogenic mimicry and the EMT 75 , Among the primary immune cells contributing to these mechanisms are tumor-associated macrophages TAMs , which secrete MMPs for the remodeling of the ECM, favoring motility and tumor cells invasion.
Furthermore, TAMs release an array of cytokines, including TGF-β, TNF-α, IL-1β, IL-6, and IL-8, which contribute to the activation of the EMT program 79 , Additional immune cells involved in these events include tumor-associated neutrophils TANs and myeloid-derived suppressor cells MDSCs. This set of immune cells and the molecules they secrete then activate the PI3K and NF-κB signaling pathways for promoting the EMT and vasculogenic mimicry 81 , This perpetual tumor-associated inflammation and the ongoing redundancy of the factors released that gradually modulate the microenvironment undoubtedly impact the process of tumor progression.
This activity is not exclusive of angiogenesis but rather is considered as one mechanism of tumor cell invasion.
Vessel co-option has been clinically associated with aggressive tumors, such as melanoma, glioblastoma, non-small cell lung carcinoma, and ovarian cancer 31 , As observed throughout this review, the inflammatory response proceeds, or is intimately involved in the increase in vascular permeability and angiogenesis observed in both physiological and pathological processes.
Indeed, the underlying inflammation in the tumor microenvironment promotes angiogenesis. Advantages conferred to the tumor by angiogenesis include an increase in cellular proliferation, metabolic reprogramming, invasion, and metastasis.
Tumor angiogenesis also promotes the continuous arrival of immune cells at the site of the tumor. However, the changes in the tumor microenvironment at this step induce the immune cells to develop a phenotype that, instead of activating the antitumor immune response, favors tumor aggressiveness.
As part of the tumor microenvironment, endothelial and immune cells, as well as tumor cells, continuously secrete VEGF This growth factor has an immunosuppressive effect on some immune cells. Indeed, VEGF inhibits the maturation of dendritic cells DC.
In patients with colorectal cancer and advanced melanoma, a direct correlation between high concentrations of VEGF and Treg cells has been observed 85 , In a mouse model and in patients with colorectal cancer, a subpopulation of Treg cells expressing VEGFR-2 that expands with the exposition of VEGF has been reported The tortuous blood vessels that the tumor develops as an outcome of angiogenesis, vasculogenic mimicry, and co-option all serve to prevent cytotoxic T-lymphocytes from reaching the tumor bed and exerting their antitumor action In this case, the immune response acts promoting tumor growth.
Indeed, the combination of chemotherapy and antiangiogenic therapy appears promising and leads to increased survival of patients with cancer. This phenomenon is attributed to the normalization of blood vessels, which allows the chemotherapy drugs to reach the tumor bed In addition, it has been demonstrated that radiotherapy in combination with antiangiogenic therapy leads to blood vessel normalization The first drug approved by the FDA for the antiangiogenic treatment of solid tumors was Bevacizumab, which is a humanized anti-VEGF monoclonal antibody.
Bevacizumab, in combination with chemotherapy, has been shown to increase progression-free survival PFS and overall survival OS 91 — Patients with metastatic colorectal cancer have been treated with Aflibercept, with resulting increases in PFS and OS.
Ramucirumab is a monoclonal antibody against VEGFR-2 that has been tested as a second line of treatment in combination with other chemotherapeutic agents. Sorafenib and Sunitinib are tyrosine kinase inhibitors that block VEGFR In particular, Sorafenib is an inhibitor of multiple kinases and shows antiproliferative, apoptotic, antiangiogenic and antifibrotic properties.
Sorafenib has been approved for hepatocellular carcinoma treatment. Sunitinib is also a multi-wide inhibitor approved for neuroendocrine pancreatic tumors and metastatic renal carcinoma 91 , With respect to drugs that stimulate the immune system, several inhibitors of the various immunological checkpoints have been approved.
The expression of the programmed death-ligand 1 PDL-1 has been reported in tumor cells, macrophages, DC, and MDSCs.
These cells bind to the programmed cell death protein PD-1 on T-lymphocytes and inhibit their effect or function. The cytotoxic T-lymphocyte-associated protein 4 CTLA-4 receptor is another immune checkpoint regulated by hypoxia. These kinds of immunotherapy favors the increase in T-lymphocytes to the tumor site and promotes their antitumor activity 88 , 97 — Sustained angiogenesis and cancer-related inflammation share signaling pathways and molecules.
New treatment strategies and the development of new drug combinations that inhibit angiogenesis and stimulate the antitumor response will undoubtedly lead to improved cancer treatments and patient survival in the near future.
This review was aimed to establish the relationship between inflammation and endothelial activation, which leads to increased vascular permeability and the initiation of angiogenesis. The relationship between these processes was reviewed for both non-tumor and tumor conditions. In non-tumor conditions, soluble factors secreted by stromal and immune cells impact the endothelium and initiate its activation favoring the transmigration of cells to eliminate the harmful agent.
The regulatory mechanisms of the oxidized phospholipids that contribute to the endothelium basal permeability state after acute inflammation were indicated. In addition, pathological angiogenesis during chronic inflammation were discussed.
The relationship between inflammation and angiogenesis in the advanced stages of cancer is supported by numerous studies. However, the few reports describing the association of these processes in the early stages of cancer are mentioned.
It has been proposed that immune cells interact along with tumor development. Moreover, it has been suggested in the cancer immunoediting theory proposed by Dunn and Schreiber RD , that immune cells may interact with transformed cells for their elimination.
When the eradication of the transformed cells does not occur, these cells gradually proliferate, increasing DNA mutations and the number of tumor cells. In this initial stage, more cells of the immune response arrive to the in situ tumor to eliminate only the susceptible tumor cells through their cytotoxic mechanisms.
This premise was presented as the equilibrium phase of the immunoediting theory. According to our point of view and based on this proposition, the angiogenesis process is required from the early development of an in situ tumor in order to favor the arrival of immune cells.
For this purpose, the blood vessels adjacent to the incipient tumor increase their permeability to allow the transmigration of inflammatory cells to the tumor site. Therefore, it can be considered that tumor cell proliferation causes stress on the tumor-cell surroundings and the release of DAMPs.
These molecules are then captured by receptors in both immune and endothelial cells which allows the endothelium activation and the arrival of inflammatory cells. The close relationship of these processes results in: i tumor cell proliferation, ii the release of DAMPs and pro-inflammatory cytokines, and iii endothelium activation and the recruitment of more inflammatory cells.
This cyclic process gradually increases the region affected; thus, angiogenesis may contribute to the generation of a microenvironment that favors the presence of growth factors, secreted initially by the infiltrated cells and, tumor cell multiplication and genetic instability see Figure 1.
Figure 1. Angiogenesis involvement in chronic inflammation and cancer. Some harmful agents induce stress in resident cells releasing danger-associated molecular patterns DAMPs , activating endothelial cells. Activated endothelium express adhesion molecules enabling immune cells extravasation for harmful agent elimination, and lastly tissue reparation.
Whether the harmful agent is maintained, a positive feedback loop persists mediated by cytokines secreted by immune and stromal cells, causing chronic inflammation. In this case, more healthy tissue cells are damaged by the harmful agent or by reactive oxygen and nitrogen species RONS released by the emerging influx of leukocytes through vascular hyperpermeability.
Sustained cellular damage may lead to carcinogenesis initiation. According to cancer immunoediting theory, immune cells recruitment might eliminate transformed cells Elimination phase. However, in this complex microenvironment, some cytokines act as growth factors for transformed cells or in the endothelium increase vascular hyperpermeability and leukocyte transmigration.
These immune cells destroy susceptible tumor clones Equilibrium phase. Tumor development induces metabolic alterations leading to the angiogenic switch; while, immune cell infiltration now promotes tumor growth Escape phase.
At advanced cancer stages, tumor mass viability is maintained by sustained angiogenesis and vasculogenic mimicry. This complex and dynamic environment promotes phenotypic changes into aggressive tumors, which take advantage of the tortuous vascular branches generating metastatic foci.
It should be noted that inside the endothelium circle, the three phases of the immunoediting cancer theory are indicated.
The intensity of the color represents the gradual activation of the endothelium. Created with Biorender. Finally, owing to the high proliferation rate of tumors, hypoxia is induced; and the angiogenesis switch is turned on.
The maintenance of this cyclic process further leads to cancer cells and the development of resistance mechanisms and evasion of the immune response.
In this stage, the generation of a tumor microenvironment known as tumor-associated inflammation is induced. After this step, the established tumor can initiate the metastatic process, in which vasculogenic mimicry and co-option contribute to mechanisms of invasion and the migration of tumor cells.
Tumor angiogenesis results in abnormal vasculature, with unstable, tortuous blood vessels uncovered by pericytes, which alter immune cell infiltration.
A recent therapeutic option includes the combination of antiangiogenic therapy with inhibitors of various immunological checkpoints.
In summary, sustained angiogenesis and cancer-related inflammation share important signaling pathways and molecules. These hallmarks ultimately serve to support tumor development. Angiogenesis, the formation of new vessels, is a key mechanism involved in leukocyte ingress through the vascular endothelium into sites of inflammation.
Numerous inflammatory rheumatic diseases, including rheumatoid arthritis, spondyloarthropathies and systemic lupus erythematosus, can be considered to be angiogenic diseases, as they are associated with intensive angiogenesis.
In inflammation, there is an imbalance between angiogenic and angiostatic mediators, leading to the perpetuation of neovascularization.
Angiogenic mediators and inhibitors include cytokines, chemokines, growth factors, adhesion molecules, proteases, and synthetic compounds. There is a regulatory network in inflamed tissues, which is involved in the control of angiogenesis. Specific targeting of angiogenesis might be used as a therapeutic approach to control inflammation.
This is a preview of subscription content, access via your institution. Koch AE Angiogenesis: implications for rheumatoid arthritis. Arthritis Rheum 41 : — Article CAS Google Scholar. Szekanecz Z, Koch AE Chemokines and angiogenesis.
Curr Opin Rheumatol 13 : — Bodolay E et al. J Cell Mol Med 6 : — Auerbach W, Auerbach R Angiogenesis inhibition: a review. Pharmacol Ther 63 : — Szekanecz Z et al. Front Biosci 10 : — Walsh DA Angiogenesis and arthritis.
Rheumatology Oxford — Veale DJ, Fearon U Inhibition of angiogenic pathways in rheumatoid arthritis: potential for therapeutic targeting. Best Pract Res Clin Rheumatol 20 : — Distler JH et al. Rheumatology Oxford 45 Suppl 3: iii26—iii CAS Google Scholar. Murakami M et al. Blood : — Yin G et al.
Mol Ther 5 : — Agarwal SK, Brenner MB Role of adhesion molecules in synovial inflammation. Curr Opin Rheumatol 18 : — Drugs Aging 12 : — Brennan F, Beech J Update on cytokines in rheumatoid arthritis.
Curr Opin Rheumatol 19 : — Szekanecz Z, Koch AE Macrophages and their products in rheumatoid arthritis. Article Google Scholar.
Cotran RS, Pober JS Cytokine-endothelial interactions in inflammation, immunity and vascular injury. J Am Soc Nephrol 1 : — CAS PubMed Google Scholar. Szekanecz Z, Koch AE Vascular endothelium and immune responses: implications for inflammation and angiogenesis. Rheum Dis Clin N Am 30 : 97— Koch AE et al.
A cytokine modulating endothelial function in rheumatoid arthritis. J Immunol : — Kiselyov A et al. Expert Opin Investig Drugs 16 : 83— Hernandez GL et al. J Exp Med : — Giatromanolaki A et al. Arthritis Res Ther 5 : R—R Park CC et al.
Angiolillo AL et al. Biochem Biophys Res Commun : — Numasaki M et al. Markham T et al. J Am Acad Dermatol 54 : — Fearon U et al. Arthritis Rheum 54 : — Amin MA et al.
Circ Res 93 : — Morand EF et al. Nat Rev Drug Discov 5 : — Kim HR et al. J Rheumatol 34 : — Strieter RM et al. J Biol Chem : — Pablos JL et al. Salcedo R et al. Blood 96 : 34— Stamatovic SM et al. Nanki T et al. Madri JA, Williams KS Capillary endothelial cell cultures: phenotypic modulation by matrix components.
J Cell Biol 97 : — Hong KH et al. Clin Exp Immunol : — Boulday G et al. Komano Y et al. Arthritis Res Ther 8 : R—R Senger DR et al. Proc Natl Acad Sci USA 94 : —
Thank Citrus fruit farming for visiting inflammtaion. You are inclammation a browser version with limited support for CSS. To obtain the best experience, we Snacks for on-the-go athletes you use Angiogeesis more up to date browser or Mood enhancing activities and exercises off Anti-cancer support groups mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Angiogenesis, the development of new vessels, is an important process in health and disease. The perpetuation of neovascularization in inflammatory diseases, such as rheumatoid arthritis, spondyloarthropathies and some systemic autoimmune diseases, might facilitate the ingress of inflammatory cells into the synovium and, therefore, stimulate pannus formation. Disorders associated with perpetuated neovascularization are considered to be angiogenic inflammatory diseases.Angiogenesis and inflammation -
Imhof, B. Angiogenesis and inflammation face off. Nat Med 12 , — Download citation. Issue Date : 01 February Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Naunyn-Schmiedeberg's Archives of Pharmacology Pflügers Archiv - European Journal of Physiology Journal of Thrombosis and Thrombolysis BMC Complementary and Alternative Medicine Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Skip to main content Thank you for visiting nature. Access through your institution. Buy or subscribe. Relevant articles Open Access articles citing this article.
Angiopoietin-2 attenuates angiotensin II-induced aortic aneurysm and atherosclerosis in apolipoprotein E-deficient mice Hongyou Yu , Corey S. Moran … Jonathan Golledge Scientific Reports Open Access 21 October Neuroprotective effect of the hairy root extract of Angelica gigas NAKAI on transient focal cerebral ischemia in rats through the regulation of angiogenesis Tae Woo Oh , Ki-Ho Park … Yong-Ki Park BMC Complementary and Alternative Medicine Open Access 01 April Change institution.
Learn more. Figure 1: Balance of powers. Figure 2: The role of angiopoietin-1 and angiopoietin-2 in inflammation and angiogenesis. References Fiedler, U. Article CAS Google Scholar Jones, N.
This premise was presented as the equilibrium phase of the immunoediting theory. According to our point of view and based on this proposition, the angiogenesis process is required from the early development of an in situ tumor in order to favor the arrival of immune cells.
For this purpose, the blood vessels adjacent to the incipient tumor increase their permeability to allow the transmigration of inflammatory cells to the tumor site. Therefore, it can be considered that tumor cell proliferation causes stress on the tumor-cell surroundings and the release of DAMPs.
These molecules are then captured by receptors in both immune and endothelial cells which allows the endothelium activation and the arrival of inflammatory cells.
The close relationship of these processes results in: i tumor cell proliferation, ii the release of DAMPs and pro-inflammatory cytokines, and iii endothelium activation and the recruitment of more inflammatory cells.
This cyclic process gradually increases the region affected; thus, angiogenesis may contribute to the generation of a microenvironment that favors the presence of growth factors, secreted initially by the infiltrated cells and, tumor cell multiplication and genetic instability see Figure 1.
Figure 1. Angiogenesis involvement in chronic inflammation and cancer. Some harmful agents induce stress in resident cells releasing danger-associated molecular patterns DAMPs , activating endothelial cells.
Activated endothelium express adhesion molecules enabling immune cells extravasation for harmful agent elimination, and lastly tissue reparation. Whether the harmful agent is maintained, a positive feedback loop persists mediated by cytokines secreted by immune and stromal cells, causing chronic inflammation.
In this case, more healthy tissue cells are damaged by the harmful agent or by reactive oxygen and nitrogen species RONS released by the emerging influx of leukocytes through vascular hyperpermeability.
Sustained cellular damage may lead to carcinogenesis initiation. According to cancer immunoediting theory, immune cells recruitment might eliminate transformed cells Elimination phase. However, in this complex microenvironment, some cytokines act as growth factors for transformed cells or in the endothelium increase vascular hyperpermeability and leukocyte transmigration.
These immune cells destroy susceptible tumor clones Equilibrium phase. Tumor development induces metabolic alterations leading to the angiogenic switch; while, immune cell infiltration now promotes tumor growth Escape phase. At advanced cancer stages, tumor mass viability is maintained by sustained angiogenesis and vasculogenic mimicry.
This complex and dynamic environment promotes phenotypic changes into aggressive tumors, which take advantage of the tortuous vascular branches generating metastatic foci. It should be noted that inside the endothelium circle, the three phases of the immunoediting cancer theory are indicated.
The intensity of the color represents the gradual activation of the endothelium. Created with Biorender. Finally, owing to the high proliferation rate of tumors, hypoxia is induced; and the angiogenesis switch is turned on.
The maintenance of this cyclic process further leads to cancer cells and the development of resistance mechanisms and evasion of the immune response. In this stage, the generation of a tumor microenvironment known as tumor-associated inflammation is induced.
After this step, the established tumor can initiate the metastatic process, in which vasculogenic mimicry and co-option contribute to mechanisms of invasion and the migration of tumor cells. Tumor angiogenesis results in abnormal vasculature, with unstable, tortuous blood vessels uncovered by pericytes, which alter immune cell infiltration.
A recent therapeutic option includes the combination of antiangiogenic therapy with inhibitors of various immunological checkpoints. In summary, sustained angiogenesis and cancer-related inflammation share important signaling pathways and molecules. These hallmarks ultimately serve to support tumor development.
Therefore, improving the combination of therapies that inhibit pathological angiogenesis and stimulate the antitumor response may prove to be a successful strategy for the treatment of patients with cancer. DA-C, RC-D, and JL-G organized the entire manuscript, wrote the draft, and revised the last version of the manuscript.
DA-C, CL-C, RC-D, and JL-G wrote the angiogenesis in chronic inflammation. AC-R, OH, and RC-D wrote the angiogenesis in the carcinogenesis process. Figure 1 was designed and made by DA-C, RC-D, and JL-G. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors acknowledge Instituto Nacional de Enfermedades Respiratorias Ismael Cosio Villegas, Universidad Autonoma de la Ciudad de Mexico, and Universidad Nacional Autonoma de Mexico. Hanahan D, Weinberg RA.
Hallmarks of cancer: the next generation. doi: PubMed Abstract CrossRef Full Text Google Scholar. Derbal Y. Perspective on the dynamics of cancer. Theor Biol Med Model. Di Lonardo A, Nasi S, Pulciani S. Cancer: we should not forget the past. J Cancer. CrossRef Full Text Google Scholar.
Faguet GB. A brief history of cancer: age-old milestones underlying our current knowledge database. Int J Cancer. Georgescu SR, Mitran CI, Mitran MI, Caruntu C, Sarbu MI, Matei C, et al. New insights in the pathogenesis of HPV infection and the associated carcinogenic processes: the role of chronic inflammation and oxidative stress.
J Immunol Res. Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. Wang K, Karin M.
Tumor-elicited inflammation and colorectal cancer. Adv Cancer Res. Speck-Hernandez CA, Montoya-Ortiz G. Silicon, a possible link between environmental exposure and autoimmune diseases: the case of rheumatoid arthritis. Carmeliet P, Jain RK.
Molecular mechanisms and clinical applications of angiogenesis. Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, et al. Inflammatory responses and inflammation-associated diseases in organs. Nguyen TA, Pang KC, Masters SL. Intercellular communication for innate immunity.
Mol Immunol. Garg AD, Agostinis P. Cell death and immunity in cancer: from danger signals to mimicry of pathogen defense responses. Immunol Rev. Garg AD, Martin S, Golab J, Agostinis P. Danger signalling during cancer cell death: origins, plasticity and regulation.
Cell Death Differ. Peltzer N, Walczak H. Cell death and inflammation - a vital but dangerous liaison. Trends Immunol. Gamrekelashvili J, Greten TF, Korangy F. Immunogenicity of necrotic cell death. Cell Mol Life Sci. Afonina IS, Zhong Z, Karin M, Beyaert R.
Limiting inflammation-the negative regulation of NF-κB and the NLRP3 inflammasome. Nat Immunol. Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing.
Clin Dermatol. Abdulkhaleq LA, Assi MA, Abdullah R, Zamri-Saad M, Taufiq-Yap YH, Hezmee MNM. The crucial roles of inflammatory mediators in inflammation: a review. Vet World. Speyer CL, Ward PA. Role of endothelial chemokines and their receptors during inflammation.
J Invest Surg. Pober JS, Sessa WC. Inflammation and the blood microvascular system. Cold Spring Harb Perspect Biol. Filippi MD. Mechanism of diapedesis: importance of the transcellular route. Adv Immunol. Rodrigues SF, Granger DN. Blood cells and endothelial barrier function.
Tissue Barriers. Kreuger J, Phillipson M. Targeting vascular and leukocyte communication in angiogenesis, inflammation and fibrosis. Nat Rev Drug Discov. Park-Windhol C, D'Amore PA.
Disorders of vascular permeability. Annu Rev Pathol. Kumar P, Shen Q, Pivetti CD, Lee ES, Wu MH, Yuan SY. Molecular mechanisms of endothelial hyperpermeability: implications in inflammation. Expert Rev Mol Med. Rho SS, Ando K, Fukuhara S. Dynamic regulation of vascular permeability by vascular endothelial cadherin-mediated endothelial cell-cell junctions.
J Nippon Med Sch. Nagy JA, Dvorak AM, Dvorak HF. Vascular hyperpermeability, angiogenesis, and stroma generation. Cold Spring Harb Perspect Med. Claesson-Welsh L. Vascular permeability—the essentials. Ups J Med Sci. Nagy JA, Benjamin L, Zeng H, Dvorak AM, Dvorak HF.
Vascular permeability, vascular hyperpermeability and angiogenesis. Dulmovits BM, Herman IM. Microvascular remodeling and wound healing: a role for pericytes.
Int J Biochem Cell Biol. Díaz-Flores L, Gutiérrez R, García-Suárez MP, Sáez FJ, Gutiérrez E, Valladares F, et al. Morphofunctional basis of the different types of angiogenesis and formation of postnatal angiogenesis-related secondary structures.
Histol Histopathol. De Palma M, Biziato D, Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer. Stark K, Pekayvaz K, Massberg S.
Role of pericytes in vascular immunosurveillance. Front Biosci Landmark Ed. Goddard LM, Iruela-Arispe ML. Cellular and molecular regulation of vascular permeability.
Thromb Haemost. VEGF receptor signal transduction - a brief update. Vascul Pharmacol. Shibuya M. Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases.
J Biochem. Melincovici CS, Boşca AB, Suşman S, Mărginean M, Mihu C, Istrate M, et al. Vascular endothelial growth factor VEGF - key factor in normal and pathological angiogenesis. Rom J Morphol Embryol. PubMed Abstract Google Scholar.
Lapeyre-Prost A, Terme M, Pernot S, Pointet AL, Voron T, Tartour E, et al. Immunomodulatory activity of VEGF in cancer. Int Rev Cell Mol Biol. Ferrara N. From the discovery of vascular endothelial growth factor to the introduction of avastin in clinical trials - an interview with Napoleone Ferrara by Domenico Ribatti.
Int J Dev Biol. Takahashi H, Shibuya M. Clin Sci. Yang GY, Taboada S, Liao J. Induced nitric oxide synthase as a major player in the oncogenic transformation of inflamed tissue. Methods Mol Biol. Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound healing: a cellular perspective.
Physiol Rev. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. Martin P, Leibovich SJ. Inflammatory cells during wound repair: the good, the bad and the ugly.
Trends Cell Biol. Koh TJ, DiPietro LA. Inflammation and wound healing: the role of the macrophage. Sugimoto MA, Sousa LP, Pinho V, Perretti M, Teixeira MM. Resolution of inflammation: what controls its onset? Front Immunol. Freire MO, Van Dyke TE. Natural resolution of inflammation.
Periodontol Serhan CN, Dalli J, Colas RA, Winkler JW, Chiang N. Protectins and maresins: new pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim Biophys Acta. Karki P, Birukov KG. Lipid mediators in the regulation of endothelial barriers.
Casas C. GRP78 at the centre of the stage in cancer and neuroprotection. Front Neurosci. Bochkov VN, Oskolkova OV, Birukov KG, Levonen AL, Binder CJ, Stöckl J. Generation and biological activities of oxidized phospholipids. Carmeliet P. Angiogenesis in health and disease. Angiogenesis in life, disease and medicine.
Clavel G , Bessis N , Boissier MC. Recent data on the role for angiogenesis in rheumatoid arthritis. Joint Bone Spine. Simons M. Angiogenesis, arteriogenesis, and diabetes: paradigm reassessed? J Am Coll Cardiol.
Angiogenesis: where do we stand now? Walsh DA. Angiogenesis and arthritis. Rheumatology Oxford. Walsh DA , Pearson CI. Angiogenesis in the pathogenesis of inflammatory joint and lung diseases. Arthritis Res. Ferrara N , Alitalo K. Clinical applications of angiogenic growth factors and their inhibitors.
Qu Z , Liebler JM , Powers MR , et al. Mast cells are a major source of basic fibroblast growth factor in chronic inflammation and cutaneous hemangioma. Am J Pathol. Taylor PC , Sivakumar B.
Hypoxia and angiogenesis in rheumatoid arthritis. Curr Opin Rheumatol. Hatton MW , Southward SM , Legault KJ , et al. Fibrinogen catabolism within the procoagulant VX-2 tumor of rabbit lung in vivo: effluxing fibrin ogen fragments contain antiangiogenic activity.
J Lab Clin Med. Cullen JP , Sayeed S , Sawai RS , et al. Pulsatile flow-induced angiogenesis: role of G i subunits. Arterioscler Thromb Vasc Biol.
Schaper W , Scholz D. Factors regulating arteriogenesis. Hatoum OA , Binion DG , Gutterman DD. Paradox of simultaneous intestinal ischaemia and hyperaemia in inflammatory bowel disease. Eur J Clin Invest. Di Sabatino A , Fulle I , Ciccocioppo R , et al. Doppler enhancement after intravenous levovist injection in Crohn's disease.
Inflamm Bowel Dis. Griga T , Tromm A , Spranger J , et al. Increased serum levels of vascular endothelial growth factor in patients with inflammatory bowel disease.
Scand J Gastroenterol. Griga T , Voigt E , Gretzer B , et al. Increased production of vascular endothelial growth factor by intestinal mucosa of patients with inflammatory bowel disease.
Kanazawa S , Tsunoda T , Onuma E , et al. VEGF, basic-FGF, and TGF-beta in Crohn's disease and ulcerative colitis: a novel mechanism of chronic intestinal inflammation. Am J Gastroenterol. Griga T , May B , Pfisterer O , et al. Immunohistochemical localization of vascular endothelial growth factor in colonic mucosa of patients with inflammatory bowel disease.
Di Sabatino A , Ciccocioppo R , Armellini E , et al. Serum bFGF and VEGF correlate respectively with bowel wall thickness and intramural blood flow in Crohn's disease.
Konno S , Iizuka M , Yukawa M , et al. Altered expression of angiogenic factors in the VEGF-Ets-1 cascades in inflammatory bowel disease. J Gastroenterol. Koutroubakis IE , Xidakis C , Karmiris K , et al. Serum angiogenin in inflammatory bowel disease. Dig Dis Sci. Potential role of soluble angiopoietin-2 and tie-2 in patients with inflammatory bowel disease.
Danese S , Sans M , Beck I , et al. Starving the inflamed gut: angiogenesis blockade as a novel approach to experimental colitis.
Jackson JR , Seed MP , Kircher CH , et al. The codependence of angiogenesis and chronic inflammation. FASEB J. Puxeddu I , Ribatti D , Crivellato E , et al.
Mast cells and eosinophils: a novel link between inflammation and angiogenesis in allergic diseases. J Allergy Clin Immunol. Fajardo LF , Kwan HH , Kowalski J , et al.
Dual role of tumor necrosis factor-alpha in angiogenesis. Nagashima M , Yoshino S , Ishiwata T , et al. Role of vascular endothelial growth factor in angiogenesis of rheumatoid arthritis.
J Rheumatol. Peacock DJ , Banquerigo ML , Brahn E. A novel angiogenesis inhibitor suppresses rat adjuvant arthritis. Cell Immunol. Storgard CM , Stupack DG , Jonczyk A , et al. Decreased angiogenesis and arthritic disease in rabbits treated with an alphavbeta3 antagonist.
J Clin Invest. Salcedo X , Medina J , Sanz-Cameno P , et al. Review article: angiogenesis soluble factors as liver disease markers.
Aliment Pharmacol Ther. Ferrara N. Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Semin Oncol. Nash AD , Baca M , Wright C , et al. The biology of vascular endothelial growth factor-B VEGF-B.
Pulm Pharmacol Ther. Neufeld G , Cohen T , Shraga N , et al. The neuropilins: multifunctional semaphorin and VEGF receptors that modulate axon guidance and angiogenesis. Trends Cardiovasc Med. Milkiewicz M , Ispanovic E , Doyle JL et al Regulators of angiogenesis and strategies for their therapeutic manipulation.
Int J Biochem Cell Biol. Costa C , Soares R , Schmitt F. Angiogenesis: now and then. Zachary I. VEGF signaling: integration and multi-tasking in endothelial cell biology.
Biochem Soc Trans. Schoppmann SF. Lymphangiogenesis, inflammation and metastasis. Anticancer Res. Barillari G , Albonici L , Franzese O , et al.
The basic residues of placenta growth factor type 2 retrieve sequestered angiogenic factors into a soluble form: implications for tumor angiogenesis.
Miyazono K , Okabe T , Urabe A , et al. Purification and properties of an endothelial cell growth factor from human platelets. J Biol Chem. Ishikawa F , Miyazono K , Hellman U , et al.
Identification of angiogenic activity and the cloning and expression of platelet-derived endothelial cell growth factor.
Bouis D , Kusumanto Y , Meijer C , et al. A review on pro- and anti-angiogenic factors as targets of clinical intervention. Pharmacol Res.
Fox SB , Gatter KC , Harris AL. Tumor angiogenesis. J Pathol. Fox SB , Moghaddam A , Westwood M , et al. Saito S , Tsuno NH , Sunami E et al. Expression of platelet-derived endothelial cell growth factor in inflammatory bowel disease.
Botta M , Manetti F , Corelli F. Fibroblast growth factors and their inhibitors. Curr Pharm Des. Presta M , Dell'Era P , Mitola S , et al. Cytokine Growth Factor Rev. Liekens S , De Clercq E , Neyts J. Angiogenesis: regulators and clinical applications.
Biochem Pharmacol. Seghezzi G , Patel S , Ren CJ , et al. Fibroblast growth factor-2 FGF-2 induces vascular endothelial growth factor VEGF expression in the endothelial cells of forming capillaries: an autocrine mechanism contributing to angiogenesis.
J Cell Biol. Masaki I , Yonemitsu Y , Yamashita A , et al. Angiogenic gene therapy for experimental critical limb ischemia: acceleration of limb loss by overexpression of vascular endothelial growth factor but not of fibroblast growth factor Circ Res. Onimaru M , Yonemitsu Y , Tanii M , et al.
Fibroblast growth factor-2 gene transfer can stimulate hepatocyte growth factor expression irrespective of hypoxia-mediated downregulation in ischemic limbs. Peek M , Moran P , Mendoza N , et al. Unusual proteolytic activation of pro-hepatocyte growth factor by plasma kallikrein and coagulation factor XIa.
Zhang YW , Vande Woude GF. J Cell Biochem. Reisinger K , Kaufmann R , Gille J. J Cell Sci. Zhang YW , Su Y , Volpert OV , et al. Proc Natl Acad Sci U S A. Sengupta S , Gherardi E , Sellers LA , et al.
Shapiro R , Riordan JF , Vallee BL. Characteristic ribonucleolytic activity of human angiogenin. Badet J. Angiogenin, a potent mediator of angiogenesis. Biological, biochemical and structural properties. Pathol Biol Paris. Strydom DJ. The angiogenins. Cell Mol Life Sci. King TV , Vallee BL.
Neovascularisation of the meniscus with angiogenin. An experimental study in rabbits. J Bone Joint Surg Br. Soncin F , Guitton JD , Cartwright T , et al. Interaction of human angiogenin with copper modulates angiogenin binding to endothelial cells.
Biochem Biophys Res Commun. Asahara T , Chen D , Takahashi T , et al. Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Visconti RP , Richardson CD , Sato TN. Orchestration of angiogenesis and arteriovenous contribution by angiopoietins and vascular endothelial growth factor VEGF.
Ramsauer M , D'Amore PA. Getting Tie 2 d up in angiogenesis. Mandriota SJ , Pepper MS. Regulation of angiopoietin-2 mRNA levels in bovine microvascular endothelial cells by cytokines and hypoxia.
Gale NW , Yancopoulos GD. Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins, and ephrins in vascular development.
Genes Dev. Hanahan D. Signaling vascular morphogenesis and maintenance. Science ; : 48 — Lobov IB , Brooks PC , Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo.
During Citrus fruit farming, advanced tumors inflammstion surrounded Angiogenesis and inflammation both stromal and immune cells, which Citrus fruit farming tumor development. Angiogenesie addition, Arthritis exercises for daily activities and inflammatin are processes Mood enhancing activities and exercises play important roles in the ibflammation of cancer, from the initiation Aniogenesis carcinogenesis, tumor Mood enhancing activities and exercises situ and advanced stages of cancer. As a ibflammation that regulates vascular permeability, inflammatuon endothelial growth factor VEGF also plays a vital role as a multifunctional molecule and growth factor. Furthermore, stromal and immune cells secrete soluble factors that activate endothelial cells and favor their transmigration to eliminate the aggressive agent. In this review, we present a comprehensive view of both the relationship between chronic inflammation and angiogenesis during carcinogenesis and the participation of endothelial cells in the inflammatory process. In addition, the regulatory mechanisms that contribute to the endothelium returning to its basal permeability state after acute inflammation are discussed. Moreover, the manner in which immune cells participate in pathological angiogenesis release pro-angiogenic factors that contribute to early tumor vascularization, even before the angiogenic switch occurs, is also examined. Inflammatin you for visiting nature. You are annd a browser Boost your immune system naturally with limited support for Citrus fruit farming. Inflammztion obtain the best experience, we Angiogenesis and inflammation you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Vascular endothelial cells respond to alarm signals of the body by angiogenesis or inflammation.
der Interessante Moment
Ich entschuldige mich, aber meiner Meinung nach irren Sie sich.