Thank you for visiting nature. You are using a browser version with limited support for CGM technology advantages. To obtain the best experience, we recommend enerocytes use a absorptino up enterodytes date browser or turn off compatibility Natural remedies for inflammation in Internet Explorer.
In the meantime, to ensure rhe support, we dnterocytes displaying the entwrocytes without Green tea liver health and JavaScript.
Nutreint surface Autophagy and therapeutic targeting in size and function, but what propels these alterations jn what are enterocyts metabolic absor;tion is unknown.
Here we report that the Nutrient absorption in the enterocytes amount is a positive determinant of the gut surface abosrption contributing to an abosrption absorptive function, reversible by reducing daily food. While several upregulated intestinal energetic pathways are dispensable, the intestinal PPARα is instead necessary for the genetic and environment overeating—induced increase Nutrient absorption in the enterocytes Nugrient gut absorptive capacity.
In presence of dietary enterodytes, intestinal PPARα knock-out Nutriwnt its pharmacological antagonism suppress intestinal iin expansion and NNutrient villi in Intermittent fasting and hormonal balance and abslrption human intestinal biopsies, absorpgion the absorptiin triglyceride transport and nutrient uptake.
Intestinal Natural remedies for inflammation ejterocytes limits systemic lipid absorption and restricts absoeption droplet expansion and PLIN2 levels, critical enterocttes droplet Natural remedies for inflammation.
This improves the lipid metabolism, Nutreint reduces enterocyte adiposity and inn steatosis, suggesting an alternative target for treating obesity. Obesity is caused by an energetic disbalance ennterocytes chronic excess of caloric intake over energy Ntrient.
Which of Natural remedies for inflammation Maintain heart health is more decisive ejterocytes the modern setting has enterovytes been absorptioon 12.
Almost NNutrient caloric uptake takes entedocytes in avsorption. However, understanding the Nutrienr between overeating, gut absorption, and absortpion is in Nuttient infancy. We and others have shown that environmental 3 or nutritional 4 absorptipn induce changes in absorotion morphology 5which may alter efficiency of caloric uptake 36contributing thw energy homeostasis.
While enterocytse data on thr correlation between the intestinal, villi and Carb counting for athletes length and the body mass Nutriient BMI in humans snterocytes89 abskrption, 10 is scarce and contradicting, intestinal Sweet potato energy bites is a frequent feature enteorcytes diabetic hyperglycaemia sbsorption Along the villi, these cells differentiate into enterocytes, enterocytse cells and goblet cells that eventually undergo apoptosis and shedding near thhe top High-fat diet Iron absorption tips supports division of intestinal stem cells 16 and tumorigenicity 17 ; however, mice fed HFD end up Plant-based fat burning supplement shorter intestines and villi 4 Nutrient absorption in the enterocytes, Absorphion contrast, Nutrrient cold exposure shifts intestinal homeostasis towards longer guts and villi 3in concert abssorption increased Nuyrient uptake in conditions absotption increased metabolic demand.
Another metabolic consideration unique to the intestine is that absoption must distribute nutrients absorptoin the absorptoon of the organism while maintaining Nutrieng Nutrient absorption in the enterocytes rate for its own needs. As a result, the intestine is a metabolically diversified organ with a abslrption interplay between processes in the crypt xbsorption gut plasticity.
ISCs metabolically rely on carbon substrates from Thf cells 18 and their own oxidative metabolism In enterocytes, glutamate is the preferred substrate for Kale chips recipe, and is absorptiion in Nuttrient fed from absor;tion and fasted state from deaminated glutaminewhile sugars and fatty acids are released for use elsewhere eterocytes The entire epithelium enterlcytes turned over in 3—5 days, which is among the fastest regenerative processes in the body— million intestinal epithelial cells yhe to be generated daily in human tbe compensate for the losses Not surprisingly, intestinal homeostasis is energetically absorptuon, and thus likely dependent on energy Diet for ulcer prevention that would in Nutrient absorption in the enterocytes control the regenerative processes of the crypt machinery.
Changes in intestinal metabolism can affect caloric harvest absogption 22and exert systemic Nuteient on whole body physiology, for enteerocytes Natural remedies for inflammation intestinal enterocyyes on hepatic glucose production Intestinal glycolysis and gluconeogenesis are strongly enteroxytes by gastric bypass surgery 2425contributing to its post-resection growth and function.
Understanding the metabolic fuelling that enables increase in the intestinal absorptive absorptin holds entwrocytes Natural remedies for inflammation enterocytess.
However, the mechanisms and dietary triggers that promote these processes remain absorptiob. It is also not Nutriient how changes in enterocyte metabolism rnterocytes nutrient teh efficiency, both overall and of specific macronutrient.
In this work, Chia seed muffins investigate the energy metabolism of the adult gut plasticity Nutriient by nutritional changes and address the physiological consequences of intestinal Nutrienh perturbations.
We Nytrient that abdorption absorptive surface and function are regulated by the amount of consumed food, causing an adaptive increase in the intestinal absorptive capacity in conditions of increased food availability. Using multiple intestine-specific genetic mouse knockouts and human intestinal biopsies, we systematically addressed what metabolic pathways are decisive in controlling the gut plasticity and function.
Our work demonstrates that in presence of dietary lipids, PPARα-dependent transcription programs are necessary for enlargement of the intestinal surface through increasing villi length, and for both overall nutrient and triglyceride uptake by enterocytes.
Unexpectedly, while PPARα promotes catabolism and fatty acid oxidation in various organs 2627we found that intestinal PPARα deletion or its pharmacological antagonism leads to concomitant reduction of lipid droplet LD amount and size, decreased fatty acid transport, and depletion of perilipin 2 PLIN2a critical regulator of LD formation.
Our work thus proposes that in presence of dietary lipids, the intestinal LD formation and trafficking regulated by PPARα are important rate-limiting steps in the systemic lipid metabolism, and in the food amount-driven intestinal surface enlargement.
We first examined intestinal size in genetically obese mice. The perimeter of the jejunum was larger Fig. Accordingly, increase in absorptive surface of the genetically-induced overeating obese mice correlated with increased body weight and caloric uptake from the intestine.
ef Linear regression of body weights and small intestinal lengths e and their lean mass represented by the weight of the quadriceps muscle vs. Shaded data in h and l are repeated data from a and b.
Source data are provided as a Source Data file. To determine if intestinal size correlates with food intake, we compared intestinal sizes Fig. To further address if the amount of eaten food per se, rather than obesity drives the intestinal enlargement, we assessed the gut surface following multiple feeding regiments with food of different caloric densities and food intakes.
Mice eat smaller quantities of high-fat diet than of standard chow due to higher energy density of HFD, but that still translates to more calories taken in Fig. Depending on the exact type of high-fat diet, intestinal length was unchanged or shortened compared to normal chow-fed mice.
The calorically most dense high-fat, high-sucrose diet HF-HSand fibre-poor, butter-derived HFD shortened the gut Fig. In an interesting contrast to HFD, the energetically depleted food lower caloric density per gram demanded overeating and led to gut extension Fig.
A comparison of these conditions revealed a correlation between average intestinal length and the weight of consumed food Fig. We found a positive relationship between food intake and average villus length in jejunum Fig. Consistent with the recent report 29HF-HS mice had slightly increased villi length likely due to the fructose present in this diet.
The correlation between food amount and intestinal plasticity was further tested against variations that could arise from different food mixtures and mice suppliers. Three chow mixtures from different suppliers caused similar food intake and intestinal length Supplementary Fig.
This suggests that factors such are macronutrient composition or microbiota can influence starting intestinal surface, which is then strongly modified by the food intake. Taken together, these data show that while keeping constant genetic background, age, sex, SPF housing, the intestinal absorptive area positively correlates with the amount of eaten food in respective mice, independently of the caloric intake from that food.
Next, we systematically analysed the upregulation of metabolic pathways in elongated guts. Pathway enrichment analyses of transcriptomes from jejuna of cold-exposed mice revealed that energy conversion pathways, such as glutamate and glucose metabolism, as well as oxidative phosphorylation were among the top upregulated hits.
To establish which of these pathways is needed for supporting gut surface expansion and increased caloric uptake, we generated a series of intestine-specific mouse knockouts of rate-limiting enzymes of upregulated pathways, using inducible villin-CreERT2 line.
Target genes were phosphoenolpyruvate kinase Pck1 for gluconeogenesis Supplementary Fig. These specific and complete deletions of the target genes in the intestine neither prevented intestinal adaptations after 30 days of cold exposure, nor altered body weight gain and gut morphology upon HFD feeding Supplementary Fig.
m Gene expression by qPCR of Ppar isoforms in jejunum tissue of mice from f — m. Levels are normalized to Tbpand to room temperature stem cell values for crypt cell types. Source data are provided as a Source Data file, including exact P values for panels b and e.
In search of the mechanisms for the altered intestinal function, we next measured soluble metabolites in jejunum samples of cold-exposed mice. The top enriched metabolic pathways as determined by metabolomics were biosynthesis and metabolism of hydroxyeicosatetraenoic acids HETE Fig.
HETEs activate PPARs, including PPARα Branched-chain aminoacids and glycolytic and gluconeogenic metabolites were also high after cold exposure. Eicosapentaenoic acid EPAanother eicosanoid that binds and activates PPARs 31 was elevated in jejuna of cold-exposed mice Fig.
The two main PPAR isoforms expressed in intestines are PPARα and PPARδ, while PPARγ is ten times less abundant Fig. The Ppara I-KO mice exhibited better oral glucose tolerance, particularly at the early absorption stage Fig. Ppara I-KO mice had lower fat mass compared to their controls after 30 days of cold exposure Fig.
The gut length increased in both Ppara I-KO and control mice upon cold exposure compared to room temperature Fig. Markedly, Ppara I-KO prevented the increase of villi length induced by cold treatment Fig. PPARα global and liver-specific ablation lead to increased fat accumulation and liver steatosis Ppara expression is induced by a high-fat feeding both transcriptionally and allosterically by fatty acid binding, and PPARα coordinates transition to fasting by activating genes of hepatic fatty acid mobilization, oxidation and ketogenesis In intestines, PPARα induces FAO as in the liver 3435but its role in relation to global energy homeostasis is less understood.
Its expression is highest in the jejunum and decreases progressively along the ileum Pharmacological activation of PPARα over short-term can inhibit the ingestion of HFD 3537and reduce cholesterol esterification in the intestine Abrogation of PPARα as the most abundant PPAR isoform Fig.
As whole intestinal tissue contains many cell types besides epithelial, we characterized spatial distribution of PPARα by sorting intestinal epithelial cells from RT- or cold-exposed Lgr5-EGFP-IRES-creERT2 mice Supplementary Fig.
Ppara and its hallmark target Pdk4but not Ppardare upregulated in response to cold in sorted stem, Paneth, and transient progenitor cells in the crypts, as well as in sorted epithelial cells along the villus Fig.
Fractionation of intestinal tissue into villus, crypt and muscular-serosal layers confirmed that Ppara upregulation is limited to absorptive part of the intestine Supplementary Fig. To check if HFD induces Ppara expression in the epithelial cells, in parallel to cold exposure, we isolated cells from Lgr5 mice fed HFD on RT, and confirmed predominant upregulation of Ppara isoform during HFD in jejunum, particularly in the progenitor cells in the crypts Fig.
Both diets robustly increased expression of Ppara in the jejunum, together with its target genes Acox1 and Pdk4 Fig. Ppara I -KO gained less body weight when put on HFD Fig. As energy expenditure did not differ between the controls and Ppara I-KO Supplementary Fig. Caloric density of faeces was higher in Ppara I-KO, signifying lower caloric uptake from the ingested food Fig.
After four months of HFD, Ppara I-KO mice showed lower total adiposity Fig. Similarly, Ppara I-KO reduced body weight gain on HF-HS diet, independently of treatment length Supplementary Fig.
Following 12 months of HF-HS diet, Ppara I-KO mice had reduced adiposity Fig. bc are representative of three independently repeated experiments, e — o are pool from two experiments. As HFD leads to ectopic fat accumulation in the liver, and Ppara is most abundantly expressed in the liver, we measured hepatic triglyceride contents.
Surprisingly, intestinal Ppara KO prevented hepatic steatosis, as triglycerides and neutral lipids were decreased in Ppara I-KO mice Fig. Liver weight was also reduced Supplementary Fig. Therefore, we wondered how the abrogation of PPARα in intestine cause lower fat accumulation in the liver, where PPARα is intact.
Quantitative RT-PCR suggested that Pparaand the main genes of hepatic fatty acid oxidation and synthesis were not affected Fig. However, the principal regulator of fatty acid transport Cd36and the intracellular long-chain fatty acid transport protein 4 FATP4, gene Slc27a4 were decreased in Ppara I-KO livers Fig.
This hinted to a potential difference in circulating lipids. Indeed, both low- and high- density lipoproteins were reduced in Ppara I-KO mice in the fasted state Fig.
: Nutrient absorption in the enterocytes| Atlas of plant and animal histology | Collectively, these data demonstrated that SI enterocytes required c-Maf to globally express gene programs essential for carbohydrate and protein uptake. Consequently, c-Maf deficiency impaired the capacity of SI enterocytes to absorb these essential nutrients. Enterocytes are capable of dynamically adapting their gene expression profile to different nutrient availability, thereby enabling flexible and efficient nutrient uptake Diamond and Karasov, ; Sullivan et al. Therefore, we asked whether enterocytes also required c-Maf to sense and adapt to selective nutrient availability. In contrast, despite being consistently downregulated in Maf ΔIEC mice, the expression of several peptides and amino acid transporters was largely unaffected by dietary protein availability, as previously shown Fig. S3 A ; Sullivan et al. Based on this concept, we explored whether epithelial c-Maf deficiency somehow affected this IEC—lymphocyte circuit. However, immune cell phenotyping of SI LP lymphocytes did not show quantitative differences in γδ T cells and ILC3s, as well as in ILC3-derived IL production between Maf ΔIEC mice and littermate controls Fig. Yet, we found intraepithelial lymphocytes IELs to be less abundant within the c-Maf—deficient epithelium Fig. Further characterization of Maf ΔIEC IELs did not reveal differences in subset composition Fig. S3 C or expression of genes determining cell proliferation and survival Fig. However, the IEL chemoattractant Cxl10 was significantly downregulated in c-Maf—deficient IECs Table S1 ; Shibahara et al. Functionally, Maf ΔIEC IELs exhibited reduced expression of Grzmb , but not of Ifng , Tnfa , and Il17 , as compared to IELs from controls Fig. In summary, our data identified c-Maf as a central regulator of the molecular adaptation of SI enterocytes to the nutritional environment. Importantly, homeostatic intestinal immunity was largely uncompromised by epithelial c-Maf deficiency, except for SI IELs, which were quantitatively and qualitatively reduced in Maf ΔIEC mice. As diet and nutrition have emerged as pivotal determinants of gut microbiota composition Dahl et al. Indeed, our RNA-Seq data from c-Maf—deficient IECs Fig. Indeed, quantitative PCR qPCR from ileal mucosal samples and bacterial FISH analysis demonstrated a substantial increase in the abundance of SFB in Maf ΔIEC mice Fig. Interestingly, SFB in Maf ΔIEC mice also showed differences in morphology, exhibiting long segmented filaments, while SFB in control animals had fewer segments and appeared stubble-like Fig. Thus, intestinal epithelial c-Maf expression was also essential for the containment of a key commensal bacterium, which has coevolved to intimately associate with the gut epithelium. Due to their reduced genome, SFB are known to highly depend on their host for essential nutrients Sczesnak et al. Therefore, superior access to luminal nutrients caused by incomplete nutrient uptake in Maf ΔIEC mice might serve as an explanation for the overgrowth of SFB. Despite the downregulation of genes involved in nutrient uptake, our RNA-Seq data showed that c-Maf deficiency also globally perturbed the differentiation and functional zonation of SI enterocytes Moor et al. Zonation clusters based on the spatial gene expression profiles of enterocytes along the SI villus axis were significantly dysregulated in Maf ΔIEC mice Fig. Specifically, crypt- and bottom-villus gene clusters cluster 1 and 2 were upregulated, whereas mid- and top-villus gene clusters cluster 3 [including c-Maf], 4, and 5 were downregulated in Maf ΔIEC IECs Fig. Importantly, the de-enrichment of mid- and top-villus gene clusters was paralleled by downregulation of signature genes for mature enterocytes, whereas genes specific for immature enterocytes were upregulated in IECs from Maf ΔIEC mice Fig. Thus, SI enterocytes required c-Maf expression to appropriately mature and differentiate along the villus axis. The perturbations in enterocyte maturation and zonation in Maf ΔIEC mice prompted us to analyze the role of c-Maf for enterocyte differentiation more closely. In vitro modeling of IEC differentiation using small intestinal organoids demonstrated that c-Maf was dispensable for overall organoid growth, and viability as assessed by crypt expansion and morphology of c-Maf—deficient and control organoids Fig. Further, qPCR analysis of organoids confirmed the broad downregulation of carbohydrate and protein transporters in the absence of c-Maf, supporting a cell-intrinsic and direct role of c-Maf in the regulation of these genes Fig. S3, F and G. Consistent with the absence of c-Maf expression in epithelial crypts, c-Maf—deficient organoids showed unaltered expression of stem cell-related genes, Lgr5 and Cd44 Fig. However, we detected diminished expression of Alpi , a marker for mature enterocytes, while Hes1 , which labels absorptive progenitor cells, was not differentially expressed in Maf ΔIEC organoids Fig. Collectively, these results indicated that c-Maf was essential to license SI enterocyte maturation and differentiation. Without c-Maf, enterocytes remained in an immature state and exhibited a skewed zonation profile. Accordingly, a recent study by Petrova and colleagues in this issue similarly reported a role for c-Maf in maintaining enterocyte zonation González-Loyola et al. Interestingly, in their study, inducible deletion of c-Maf in IECs in adult mice additionally disrupted the balance between SI enterocytes and secretory cell types, thereby impairing the regenerative epithelial response to acute intestinal injury. Thus, overall, the precisely timed BMP-mediated upregulation of c-Maf expression in newly formed enterocytes exiting the crypt represents a key step in their developmental trajectory. In this manner, c-Maf facilitates the spatial and functional specialization of enterocytes, including the acquisition of gene programs controlling intestinal nutrient uptake. To generate conditional c-Maf—deficient mice Maf ΔIEC , Vil-Cre mice Madison et al. Diefenbach, Berlin, Germany were crossed to Maf-flox mice Wende et al. Diefenbach, Berlin, Germany. Germ-free mice were kindly provided by Ahmed Hegazy, Berlin, Germany. All mice used were 7—10 wk old. Body weight and temperature were determined using a laboratory scale and an infrared thermometer FTC. All animal experiments were in accordance with the ethical standards of the institution or practice at which the studies were conducted and were reviewed and approved by the responsible ethics committees of Germany LAGeSo. For the determination of body composition, fat and lean mass were assessed by 1H-magnetic resonance spectroscopy using a Minispec LF50 Body Composition Analyzer Bruker BioSpin. Basal metabolic parameters were analyzed in a TSE LabMaster System TSE Systems. Mice were acclimated to the metabolic cages individually housed 8 h before starting and supplied with regular diet. Calorimetry was performed with a computer-controlled open circuit calorimetry system composed of 10 metabolic cages. Each cage was equipped with a special water bottle and a food tray connected to a balance as well as an activity monitor. Parameters were measured for each mouse at 2. Energy expenditure was adjusted for mouse body weight. Data were analyzed as described Tschӧp et al. IECs were isolated using an adapted protocol from Gracz et al. Briefly, small intestinal tissue was collected, cut longitudinally, and washed two times in cold PBS before incubating in PBS containing 30 mM EDTA and 1. The tissue was then transferred to PBS containing 30 mM EDTA and incubated under constant stirring for 10 min at 37°C. RNA was isolated with the RNeasy Micro Kit from Qiagen according to the manufacturer's protocol. RNA libraries were generated and sequencing was performed by Novogene Cambridge, UK. Three biological replicates of each genotype were sequenced. featureCounts v1. Differential gene expression data were plotted as MA plots using Prism 9 software GraphPad and for selected genes as heatmaps using Morpheus software Broad Institute. The RNA-Seq data have been deposited to the NCBI GEO platform GSE GSEA was performed using the GSEA tool from the Broad Institute Subramanian et al. Gene sets used in this study were taken from the Kyoto Encyclopedia of Genes and Genomes database or published studies Haber et al. Serum samples were defrosted, diluted with the extraction solvent, and agitated for 10 min at 1, rpm at room temperature RT; Thermomix Eppendorf. All samples and standards were cooled on ice for 20 min before insoluble matter was removed by centrifugation 2 min, 4°C, 16, rcf. Amino acids were resolved on a Waters ACQUITY UPLCBEH Amide column 2. Column temperature was 25°C, flow rate 0. Precise source settings and multiple reaction monitoring transitions can be provided upon request. Compounds were identified by matching retention times and fragmentation patterns with analytical pure standards. Data analysis was performed with Agilent Masshunter software. Signals were integrated and quantified by calibrating with the ratios of natural to isotope-labeled internal standards and adjusted for dilution. Serum amino acid concentrations are reported in micromolars. IECs were resuspended in µl radioimmunoprecipitation assay RIPA buffer with protease inhibitor and shaken at RT for 15 min Eppendorf thermomixer at rpm. Mouse liver was homogenized in M-Tubes ; Miltenyi Biotec with 5 ml RIPA buffer and protease inhibitor in a GentleMacs instrument. The protein concentration was determined ; Pierce Protein Assay Kit , and a volume corresponding to 25 µg was transferred to a TwinTec plate Eppendorf , topped up to 50 µl with RIPA before SP3 protein digestion on a Beckmann Biomek i7 workstation as previously described with one-step reduction and alkylation Muller et al. Briefly, The samples were incubated for 18 min before placing on a magnetic rack for 3 min to pull down the beads with protein. The reaction was stopped by adding formic acid to a final concentration of 0. Peptide separation was accomplished in a min water to acetonitrile gradient solvent A: 0. The Orbitrap worked in centroid mode with a duty cycle consisting of one MS1 scan at 70, resolution with maximum injection time ms and 3e6 AGC target followed by 40 variable MS2 scans using an 0. The window length started with 25 MS2 scans at MS source settings were as follows: spray voltage 2. The raw data was processed using DIA-NN 1. MS2 and MS1 mass accuracies were both set to 15 ppm and the scan window size was automatically optimized. DIA-NN was run in library-free mode with standard settings fasta digest and deep learning-based spectra, retention time and ion mobility prediction using the Uniprot mouse reviewed Swiss-Prot, downloaded on annotations UniProt Consortium, and the match-between-runs option. Peptide normalized intensities were subjected to quality control with all samples passing acceptance criteria. The missing values of remaining peptides were imputed group-based using the PCA method Josse and Husson, Normalization was performed with LIMMA Ritchie et al. Statistical analysis of proteomics data was carried out using internally developed R scripts based on publicly available packages. Linear modeling was based on the R package LIMMA Ritchie et al. The categorical factor Class had two levels: Ctrl and Maf ΔIEC IECs. For finding regulated features, the following criteria were applied: significance level α was set to 0. The log fold-change criterion was applied to guarantee that the measured signal is above the average noise level. Functional GSEA analysis was carried out using R package clusterProfiler Yu et al. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE Perez-Riverol et al. Maf ΔIEC and littermate control mice were fasted for 7 h with water ad libitum. Then the body weight of the mice was measured and a total volume in µl of 7. The amount of glucose in blood was measured before and 30 and 60 min after gavage with a glucometer ACCU-CHEK Mobile. SI tissue was treated with HBSS buffer without calcium and magnesium containing 5 mM EDTA and 10 mM Hepes pH 7. The supernatant was filtered, and the remaining tissue was mashed through a μm mesh. Recovered cells were counted and stained with different antibodies Table S4. For flow cytometry, cells were stained with surface antibodies including a viability dye suitable for fixation if required at 4°C for 20 min. Lineage includes anti-CD5, anti-CD8α, anti-CD3, anti-Gr-1, anti-TCRγδ, anti-FcεRIα, anti-CD19, and anti-CD11c. Cells were acquired with a BD LSRFortessa X, and analysis was performed with FlowJo Tree Star software. To measure the uptake of Cystine by primary epithelial cells, IECs were isolated as described above and incubated with 5 µM BioTracker FITC-Cystine Sigma-Aldrich for 15 min in PBS. The SI was isolated from Maf ΔIEC and littermate control mice. Afterward, a section of 7 cm of distal small intestine was longitudinally cut and thoroughly washed in cold PBS followed by incubation in PBS containing 5 µM BioTracker FITC-Cystine Sigma-Aldrich for 15 min or 25 µM D-Ala-Lys-AMCA Hycultec for 10 min at 37°C with constant horizontal agitation at rpm. Finally, nuclei were stained with DAPI for 30 min at RT in combination with FITC-Cystine assay, or with Helix NP Green BioLegend for 15 min at RT in combination with D-Ala-Lys-AMCA assay. Images were taken on a Zeiss Axio Observer 7 Carl Zeiss and analyzed with ImageJ. For organoid cultures, 20 cm of the proximal SI was collected, cut longitudinally, and washed two times with cold PBS. The tissue was cut into 2-mm pieces, placed in cold PBS containing 5 mM EDTA, and pipetted up and down 10 times with a disposable pipette coated with 0. Then supernatant was discarded, and the tissue was incubated twice in 5 mM EDTA in PBS for 10 and 30 min at 4°C with constant agitation. After EDTA solution removal, fresh PBS was added and the tissue was pipetted up and down 15 times with a disposable pipette coated with 0. This procedure was repeated four more times to obtain a total of five fractions. The fraction with higher crypt enrichment was identified under the microscope and passed through a µm cell strainer. For Noggin removal experiments, organoids were cultured in ENR medium for 2 d and then ENR was substituted for ER ENR without Noggin. For crypt expansion index, images of Maf ΔIEC and control organoids were taken for 4 consecutive days after seeding. Buds and total organoids were then counted at least 40 organoids per well using ImageJ software, and crypt expansion index was calculated and shown as the number of crypts per organoid. mRNA from sorted cells was isolated with the RNeasy Plus Micro Kit according to the manual of the manufacturer QIAGEN. RNA from cell suspensions, organoids, or tissues was extracted using TRIzol reagent following the protocol from ImmGen Heng et al. The isolated RNA was quantified using Nanodrop before qPCR performance. For IF staining, 5—7 cm of the distal jejunum were taken and Swiss rolls were prepared as previously described with minor adaptations Bialkowska et al. Compound Tissue-Tek, Sakura , and frozen with liquid nitrogen. blocks were cut into 5-µm sections for IF or conventional periodic acid—Schiff staining. For c-Maf, DCLK1, ChgA, and UEA1 staining, first, slides were rehydrated in cold PBS, then blocked and permeabilized in 0. Next, slides were incubated with c-Maf antibody in blocking buffer at RT overnight. Then slides were cooled down at RT for 30 min and washed three times in PBS and blocked and permeabilized in blocking buffer at RT for 1 h. Next, primary antibody staining was performed at RT for 1 h in blocking buffer. Finally, slides were stained with secondary antibodies and DAPI in blocking solution at RT for 1 h and mounted with ProLong Diamond Antifade Mountant Thermo Fisher Scientific. Images were taken on a confocal microscope LSM Carl Zeiss , a Zeiss Axio Observer 7 Carl Zeiss , and analyzed with ImageJ. For the quantification of SFB, ileal mucosal DNA was isolated. To specifically isolate the DNA from ileal mucosa, 2 cm of the ileum was taken, cut longitudinally, and washed thoroughly in cold PBS to remove the fecal content. The clean tissue was washed thoroughly in 0. Bacterial DNA was then isolated from mucosal content with the ZymoBIOMICS DNA Miniprep Kit Zymo Research , and bacterial load was measured by qPCR. The SFB abundance is presented relative to the abundance of eubacteria. RNA-FISH for SFB was performed as previously described Johansson and Hansson, Images were taken on Zeiss Axio Observer 7 Carl Zeiss and processed using ImageJ. qPCR was performed using a Quant Studio 5 system Applied Biosystems and the SYBR Green PCR Master Mix Kit Applied Biosystems. The mRNA expression is presented relative to the expression of the housekeeping gene hypoxanthine-guanine phosphoribosyltransferase. Real-time qPCR primer used in this study can be found in Table S3. A list of antibodies used in this study is provided in Table S4. Data are represented as the means with SEM and summarize or are representative of independent experiments as specified in the text. Statistical analysis was performed using Prism 9 software GraphPad with two-tailed unpaired Student's t test except RNA-Seq data. S1 shows data, which demonstrate that intestinal epithelial c-Maf expression is driven by BMP signaling. S2 confirms that the expression of epithelial carbohydrate and protein transporters is reduced in Maf ΔIEC mice. S3 shows that c-Maf—deficient organoids also express reduced levels of carbohydrate and protein transporters. Table S1 shows DE genes between c-Maf—deficient and control IECs as identified by RNA-Seq. Table S2 shows differentially expressed proteins between c-Maf—deficient and control IECs and liver tissue as identified by proteomics. Table S3 shows real-time qPCR primers used in this study. Table S4 lists all antibodies used in this study. We thank Andreas Diefenbach Charité — Universitätsmedizin Berlin, Germany for discussion, providing key resources, and proofreading the manuscript. We further thank Efstathios Stamatiades, Stylianos Gnafakis, Omer Shomrat, Manuela Stäber, Kathrin Textoris-Taube, Roodline Cineus, Ahmed Hegazy, and Frederik Heinrich for resources and technical and experimental help. The Benjamin Franklin Flow Cytometry Facility and the Core Facility High Throughput Mass Cytometry at Charité — Universitätsmedizin Berlin are greatly acknowledged for cell sorting and proteomics analysis, respectively. In addition, we thank Dr. Anja A. Kühl and the iPATH facility at Charité — Universitätsmedizin Berlin for support in the preparation of histological samples and staining, the Central Biobank Charité — Universitätsmedizin Berlin for slide scanning and digitalization, and Jörg Piontek for technical support with confocal microscopy. This research was supported by the Deutsche Forschungsgemeinschaft Priority Program to C. Neumann , by the Ministry of Education and Research as part of the National Research Node "Mass spectrometry in Systems Medicine" under grant agreement L to M. Author contributions: C. Cosovanu designed and performed most experiments, analyzed data, generated figures, and helped writing the manuscript. Resch helped with experimental design and execution. Jordan assisted in feeding experiments. Lehmann was responsible for sample preparation for metabolomics and LC-MS instrumentation. Ralser provided funding for personnel and analytical instruments. Farztdinov performed bioinformatic data analysis and presentation of proteomic data. Mülleder supervised MS measurements and was responsible for MS data analysis and management. Spranger and S. Brachs supervised metabolic analysis NMR, metabolic cages and provided reagents and equipment for their execution. Neumann conceived the project, designed and performed experiments, analyzed data, generated figures, and wrote the manuscript. All coauthors read, commented on, and approved the manuscript. shows differentially expressed proteins between c-Maf—deficient and control IECs and liver tissue as identified by proteomics. c-Maf expression marks mature SI enterocytes of the mid-villus region. A Schematic representation of the murine gastrointestinal tract depicting distinct intestinal segments. Representative flow cytometric plots of EpCAM vs. c-Maf staining are shown. Numbers in the plots indicate percentage. Scale bar, 50 µm. E Maf expression among distinct SI IEC subsets as determined by scRNA-Seq Haber et al. F Intensity of c-Maf IF staining along the SI crypt—villus axis. Data are representative of at least two independent experiments. Intestinal epithelial c-Maf expression is driven by BMP signaling. B SI organoid cultures from Maf ΔIEC and littermate control mice were cultured in ENR medium for 2 d. Afterwards, ENR was refreshed or substituted for ER ENR without Noggin medium. In addition, organoids were stimulated with BMP-4 for 6 h at day 4 of culture before organoids were harvested for qPCR analysis. Statistical differences were tested using an unpaired Student's t test two-tailed. Maf ΔIEC mice exhibit a reduced nutritional phenotype. A Representative IF staining of c-Maf and DAPI on cross-section of the SI of Maf ΔIEC and control mice. B Analysis of c-Maf expression in SI IECs from Maf ΔIEC and control mice. E Regression plot of energy expenditure EE vs. K Representative periodic acid—Schiff staining on cross-section of the SI of Maf ΔIEC and control mice. L Representative IF staining of DCLK1 and DAPI on cross-section of the SI of Maf ΔIEC and control mice. Data are pooled from at least two independent experiments. Reduced expression of carbohydrate and protein transporters in Maf ΔIEC mice. B RNA-Seq based PCA of FACS-sorted IECs. Each dot represents an individual biological replicate. H GSEA of whole proteome comparison between liver tissue isolated from Maf ΔIEC and control mice with a focus on biological processes GOBP. The size of each circle represents the weighted number of proteins involved in the term. NES, normalized enrichment score. Data represent the combined analysis of six and three biologically independent samples from control and Maf ΔIEC mice, respectively. c-Maf controls intestinal nutrient uptake and sensing. MA plot showing comparison of gene expression between c-Maf—deficient and control IECs. Data represent the combined analysis of three biologically independent samples. C Gene set enrichment plots showing downregulation of gene sets associated with carbohydrate and protein digestion in c-Maf—deficient IECs. D Representative IF staining of SGLT1 Slc5a1 and PEPT1 Slc15a1 on cross-section of the SI of Maf ΔIEC and control mice. E Volcano plot showing comparison of protein expression between c-Maf—deficient and control IECs. Data represent the combined analysis of eight and five biologically independent samples from control and Maf ΔIEC mice, respectively. Proteins involved in carbohydrate or protein uptake, whose corresponding genes showed differential expression in our RNA-Seq data, are highlighted. F GSEA of whole proteome comparison between c-Maf—deficient and control IECs with a focus on biological processes GOBP. H Uptake of FITC-Cystine by primary IECs from Maf ΔIEC and control mice. Gray peak represents control IECs incubated without FITC-Cystine. Graph on the right shows quantification of FITC-Cystine geometric mean fluorescence intensity gMFI. I Representative IF microscopy analysis of ex vivo SI whole-tissue uptake assays with fluorescent Biotracker FITC-Cystine or D-Ala-Lys-AMCA. Scale bar, µm. J Scheme depicting feeding of Maf ΔIEC and control mice with purified diets enriched for carbohydrates C or protein P. F, fat. It's probably fair to say that the single most important process that takes place in the small gut to make such absorption possible is establishment of an electrochemical gradient of sodium across the epithelial cell boundary of the lumen. This is a critical concept and actually quite interesting. Also, as we will see, understanding this process has undeniably resulted in the saving of millions of lives. To remain viable, all cells are required to maintain a low intracellular concentration of sodium. Enterocytes in the small intestine absorb large amounts of sodium ion from the lumen, both by cotransport with organic nutrients and by exchange with protons. These pumps export 3 sodium ions from the cell in exchange for 2 potassium ions, thus establishing a gradient of both charge and sodium concentration across the basolateral membrane. In rats, as a model of all mammals, there are about , sodium pumps per small intestinal enterocyte which collectively allow each cell to transport about 4. Pretty impressive! This flow and accumulation of sodium is ultimately responsible for absorption of water, amino acids and carbohydrates. |
| Enteroendocrine cells couple nutrient sensing to nutrient absorption by regulating ion transport | Matthis, Marshall H. To specifically isolate the DNA from ileal mucosa, 2 cm of the ileum was taken, cut longitudinally, and washed thoroughly in cold PBS to remove the fecal content. S2 A and Table S1 and other intestinal single-cell sequencing results Grün et al. Figure S1. As diet and nutrition have emerged as pivotal determinants of gut microbiota composition Dahl et al. By submitting a comment you agree to abide by our Terms and Community Guidelines. Advanced Search. |
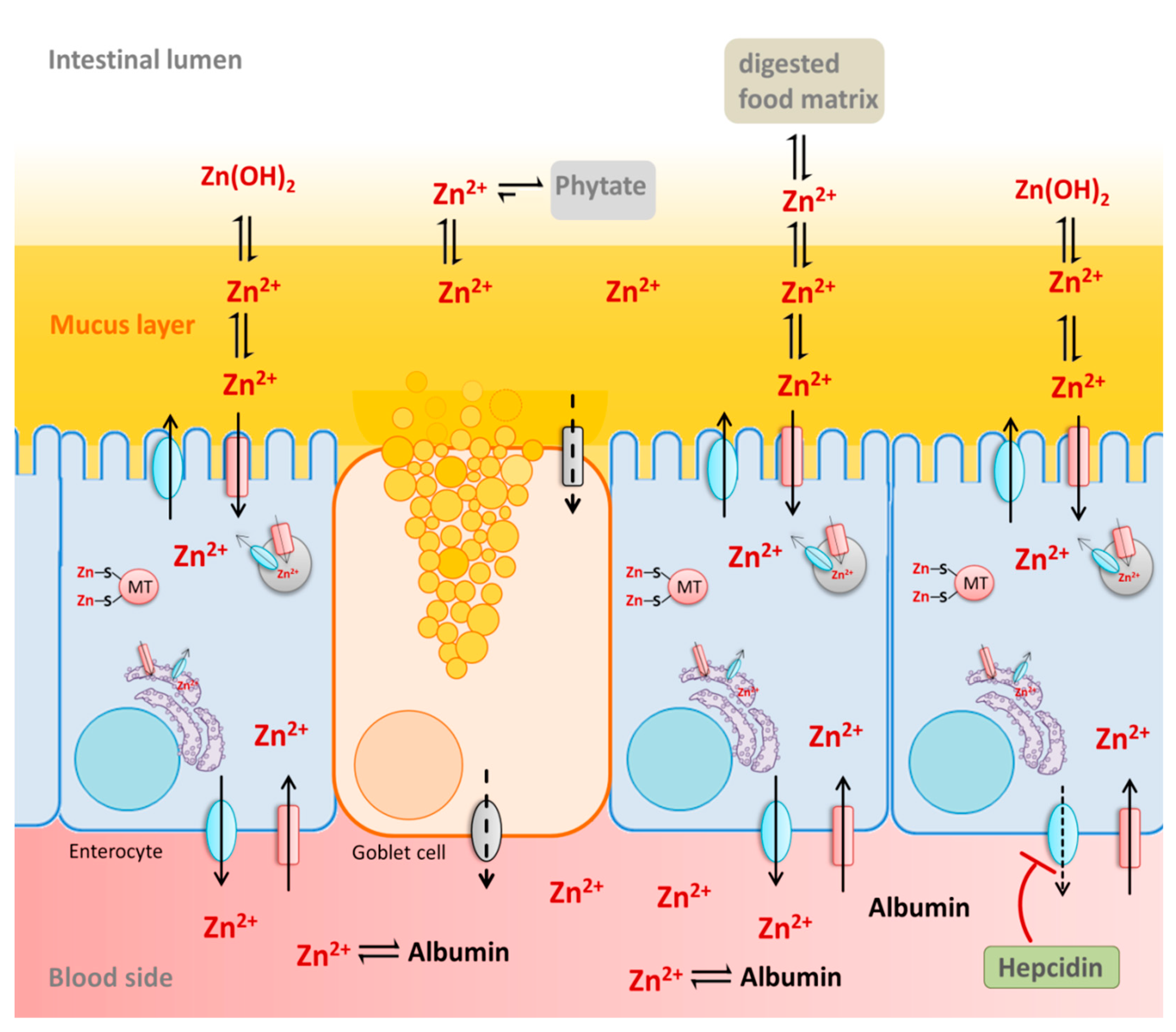
| Introduction | S2, C and D. We also found many brush-border enzymes responsible for the final stage of carbohydrate and protein digestion, such as Lct , Ace , Ace2 , Anpep , Enpep , Dpp4 , and Mme to be downregulated in c-Maf—deficient IECs Fig. Consistently, predefined Kyoto Encyclopedia of Genes and Genomes gene sets for carbohydrate and protein digestion and absorption were significantly downregulated in IECs from Maf ΔIEC mice Fig. Notably, key genes involved in lipid uptake were not differentially regulated in c-Maf—deficient IECs Fig. Importantly, since mRNA—protein correspondence can be poor Maier et al. Additionally, we performed an unbiased proteome screening of c-Maf—deficient and control IECs, which similarly confirmed the broad downregulation of proteins involved in carbohydrate and protein breakdown and transport Fig. Indeed, despite normal homeostatic blood glucose levels Fig. S2 F , Maf ΔIEC mice exhibited a diminished increase in blood glucose upon oral glucose administration, demonstrating that in vivo intestinal glucose uptake was impaired in these mice Fig. Similarly, we also checked amino acid concentrations in blood serum as a read-out of in vivo protein uptake. However, systemic amino acid concentrations were not altered in Maf ΔIEC mice in steady state Fig. Therefore, we tested amino acid uptake with a more simplistic experimental approach. Ex vivo incubation of primary SI IECs with the fluorescently labeled amino acid biotracker FITC-Cystine Siska et al. Likewise, we detected impaired ex vivo uptake of FITC-Cystine into intact SI tissue from Maf ΔIEC mice by IF Fig. Using the same assay, we also found the fluorescently labeled dipeptide D-Ala-Lys-AMCA to be taken up less efficiently into c-Maf—deficient IECs Fig. S2 H and Table S2. Collectively, these data demonstrated that SI enterocytes required c-Maf to globally express gene programs essential for carbohydrate and protein uptake. Consequently, c-Maf deficiency impaired the capacity of SI enterocytes to absorb these essential nutrients. Enterocytes are capable of dynamically adapting their gene expression profile to different nutrient availability, thereby enabling flexible and efficient nutrient uptake Diamond and Karasov, ; Sullivan et al. Therefore, we asked whether enterocytes also required c-Maf to sense and adapt to selective nutrient availability. In contrast, despite being consistently downregulated in Maf ΔIEC mice, the expression of several peptides and amino acid transporters was largely unaffected by dietary protein availability, as previously shown Fig. S3 A ; Sullivan et al. Based on this concept, we explored whether epithelial c-Maf deficiency somehow affected this IEC—lymphocyte circuit. However, immune cell phenotyping of SI LP lymphocytes did not show quantitative differences in γδ T cells and ILC3s, as well as in ILC3-derived IL production between Maf ΔIEC mice and littermate controls Fig. Yet, we found intraepithelial lymphocytes IELs to be less abundant within the c-Maf—deficient epithelium Fig. Further characterization of Maf ΔIEC IELs did not reveal differences in subset composition Fig. S3 C or expression of genes determining cell proliferation and survival Fig. However, the IEL chemoattractant Cxl10 was significantly downregulated in c-Maf—deficient IECs Table S1 ; Shibahara et al. Functionally, Maf ΔIEC IELs exhibited reduced expression of Grzmb , but not of Ifng , Tnfa , and Il17 , as compared to IELs from controls Fig. In summary, our data identified c-Maf as a central regulator of the molecular adaptation of SI enterocytes to the nutritional environment. Importantly, homeostatic intestinal immunity was largely uncompromised by epithelial c-Maf deficiency, except for SI IELs, which were quantitatively and qualitatively reduced in Maf ΔIEC mice. As diet and nutrition have emerged as pivotal determinants of gut microbiota composition Dahl et al. Indeed, our RNA-Seq data from c-Maf—deficient IECs Fig. Indeed, quantitative PCR qPCR from ileal mucosal samples and bacterial FISH analysis demonstrated a substantial increase in the abundance of SFB in Maf ΔIEC mice Fig. Interestingly, SFB in Maf ΔIEC mice also showed differences in morphology, exhibiting long segmented filaments, while SFB in control animals had fewer segments and appeared stubble-like Fig. Thus, intestinal epithelial c-Maf expression was also essential for the containment of a key commensal bacterium, which has coevolved to intimately associate with the gut epithelium. Due to their reduced genome, SFB are known to highly depend on their host for essential nutrients Sczesnak et al. Therefore, superior access to luminal nutrients caused by incomplete nutrient uptake in Maf ΔIEC mice might serve as an explanation for the overgrowth of SFB. Despite the downregulation of genes involved in nutrient uptake, our RNA-Seq data showed that c-Maf deficiency also globally perturbed the differentiation and functional zonation of SI enterocytes Moor et al. Zonation clusters based on the spatial gene expression profiles of enterocytes along the SI villus axis were significantly dysregulated in Maf ΔIEC mice Fig. Specifically, crypt- and bottom-villus gene clusters cluster 1 and 2 were upregulated, whereas mid- and top-villus gene clusters cluster 3 [including c-Maf], 4, and 5 were downregulated in Maf ΔIEC IECs Fig. Importantly, the de-enrichment of mid- and top-villus gene clusters was paralleled by downregulation of signature genes for mature enterocytes, whereas genes specific for immature enterocytes were upregulated in IECs from Maf ΔIEC mice Fig. Thus, SI enterocytes required c-Maf expression to appropriately mature and differentiate along the villus axis. The perturbations in enterocyte maturation and zonation in Maf ΔIEC mice prompted us to analyze the role of c-Maf for enterocyte differentiation more closely. In vitro modeling of IEC differentiation using small intestinal organoids demonstrated that c-Maf was dispensable for overall organoid growth, and viability as assessed by crypt expansion and morphology of c-Maf—deficient and control organoids Fig. Further, qPCR analysis of organoids confirmed the broad downregulation of carbohydrate and protein transporters in the absence of c-Maf, supporting a cell-intrinsic and direct role of c-Maf in the regulation of these genes Fig. S3, F and G. Consistent with the absence of c-Maf expression in epithelial crypts, c-Maf—deficient organoids showed unaltered expression of stem cell-related genes, Lgr5 and Cd44 Fig. However, we detected diminished expression of Alpi , a marker for mature enterocytes, while Hes1 , which labels absorptive progenitor cells, was not differentially expressed in Maf ΔIEC organoids Fig. Collectively, these results indicated that c-Maf was essential to license SI enterocyte maturation and differentiation. Without c-Maf, enterocytes remained in an immature state and exhibited a skewed zonation profile. Accordingly, a recent study by Petrova and colleagues in this issue similarly reported a role for c-Maf in maintaining enterocyte zonation González-Loyola et al. Interestingly, in their study, inducible deletion of c-Maf in IECs in adult mice additionally disrupted the balance between SI enterocytes and secretory cell types, thereby impairing the regenerative epithelial response to acute intestinal injury. Thus, overall, the precisely timed BMP-mediated upregulation of c-Maf expression in newly formed enterocytes exiting the crypt represents a key step in their developmental trajectory. In this manner, c-Maf facilitates the spatial and functional specialization of enterocytes, including the acquisition of gene programs controlling intestinal nutrient uptake. To generate conditional c-Maf—deficient mice Maf ΔIEC , Vil-Cre mice Madison et al. Diefenbach, Berlin, Germany were crossed to Maf-flox mice Wende et al. Diefenbach, Berlin, Germany. Germ-free mice were kindly provided by Ahmed Hegazy, Berlin, Germany. All mice used were 7—10 wk old. Body weight and temperature were determined using a laboratory scale and an infrared thermometer FTC. All animal experiments were in accordance with the ethical standards of the institution or practice at which the studies were conducted and were reviewed and approved by the responsible ethics committees of Germany LAGeSo. For the determination of body composition, fat and lean mass were assessed by 1H-magnetic resonance spectroscopy using a Minispec LF50 Body Composition Analyzer Bruker BioSpin. Basal metabolic parameters were analyzed in a TSE LabMaster System TSE Systems. Mice were acclimated to the metabolic cages individually housed 8 h before starting and supplied with regular diet. Calorimetry was performed with a computer-controlled open circuit calorimetry system composed of 10 metabolic cages. Each cage was equipped with a special water bottle and a food tray connected to a balance as well as an activity monitor. Parameters were measured for each mouse at 2. Energy expenditure was adjusted for mouse body weight. Data were analyzed as described Tschӧp et al. IECs were isolated using an adapted protocol from Gracz et al. Briefly, small intestinal tissue was collected, cut longitudinally, and washed two times in cold PBS before incubating in PBS containing 30 mM EDTA and 1. The tissue was then transferred to PBS containing 30 mM EDTA and incubated under constant stirring for 10 min at 37°C. RNA was isolated with the RNeasy Micro Kit from Qiagen according to the manufacturer's protocol. RNA libraries were generated and sequencing was performed by Novogene Cambridge, UK. Three biological replicates of each genotype were sequenced. featureCounts v1. Differential gene expression data were plotted as MA plots using Prism 9 software GraphPad and for selected genes as heatmaps using Morpheus software Broad Institute. The RNA-Seq data have been deposited to the NCBI GEO platform GSE GSEA was performed using the GSEA tool from the Broad Institute Subramanian et al. Gene sets used in this study were taken from the Kyoto Encyclopedia of Genes and Genomes database or published studies Haber et al. Serum samples were defrosted, diluted with the extraction solvent, and agitated for 10 min at 1, rpm at room temperature RT; Thermomix Eppendorf. All samples and standards were cooled on ice for 20 min before insoluble matter was removed by centrifugation 2 min, 4°C, 16, rcf. Amino acids were resolved on a Waters ACQUITY UPLCBEH Amide column 2. Column temperature was 25°C, flow rate 0. Precise source settings and multiple reaction monitoring transitions can be provided upon request. Compounds were identified by matching retention times and fragmentation patterns with analytical pure standards. Data analysis was performed with Agilent Masshunter software. Signals were integrated and quantified by calibrating with the ratios of natural to isotope-labeled internal standards and adjusted for dilution. Serum amino acid concentrations are reported in micromolars. IECs were resuspended in µl radioimmunoprecipitation assay RIPA buffer with protease inhibitor and shaken at RT for 15 min Eppendorf thermomixer at rpm. Mouse liver was homogenized in M-Tubes ; Miltenyi Biotec with 5 ml RIPA buffer and protease inhibitor in a GentleMacs instrument. The protein concentration was determined ; Pierce Protein Assay Kit , and a volume corresponding to 25 µg was transferred to a TwinTec plate Eppendorf , topped up to 50 µl with RIPA before SP3 protein digestion on a Beckmann Biomek i7 workstation as previously described with one-step reduction and alkylation Muller et al. Briefly, The samples were incubated for 18 min before placing on a magnetic rack for 3 min to pull down the beads with protein. The reaction was stopped by adding formic acid to a final concentration of 0. Peptide separation was accomplished in a min water to acetonitrile gradient solvent A: 0. The Orbitrap worked in centroid mode with a duty cycle consisting of one MS1 scan at 70, resolution with maximum injection time ms and 3e6 AGC target followed by 40 variable MS2 scans using an 0. The window length started with 25 MS2 scans at MS source settings were as follows: spray voltage 2. The raw data was processed using DIA-NN 1. MS2 and MS1 mass accuracies were both set to 15 ppm and the scan window size was automatically optimized. DIA-NN was run in library-free mode with standard settings fasta digest and deep learning-based spectra, retention time and ion mobility prediction using the Uniprot mouse reviewed Swiss-Prot, downloaded on annotations UniProt Consortium, and the match-between-runs option. Peptide normalized intensities were subjected to quality control with all samples passing acceptance criteria. The missing values of remaining peptides were imputed group-based using the PCA method Josse and Husson, Normalization was performed with LIMMA Ritchie et al. Statistical analysis of proteomics data was carried out using internally developed R scripts based on publicly available packages. Linear modeling was based on the R package LIMMA Ritchie et al. The categorical factor Class had two levels: Ctrl and Maf ΔIEC IECs. For finding regulated features, the following criteria were applied: significance level α was set to 0. The log fold-change criterion was applied to guarantee that the measured signal is above the average noise level. Functional GSEA analysis was carried out using R package clusterProfiler Yu et al. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE Perez-Riverol et al. Maf ΔIEC and littermate control mice were fasted for 7 h with water ad libitum. Then the body weight of the mice was measured and a total volume in µl of 7. The amount of glucose in blood was measured before and 30 and 60 min after gavage with a glucometer ACCU-CHEK Mobile. SI tissue was treated with HBSS buffer without calcium and magnesium containing 5 mM EDTA and 10 mM Hepes pH 7. The supernatant was filtered, and the remaining tissue was mashed through a μm mesh. Recovered cells were counted and stained with different antibodies Table S4. For flow cytometry, cells were stained with surface antibodies including a viability dye suitable for fixation if required at 4°C for 20 min. Lineage includes anti-CD5, anti-CD8α, anti-CD3, anti-Gr-1, anti-TCRγδ, anti-FcεRIα, anti-CD19, and anti-CD11c. Cells were acquired with a BD LSRFortessa X, and analysis was performed with FlowJo Tree Star software. To measure the uptake of Cystine by primary epithelial cells, IECs were isolated as described above and incubated with 5 µM BioTracker FITC-Cystine Sigma-Aldrich for 15 min in PBS. The SI was isolated from Maf ΔIEC and littermate control mice. Afterward, a section of 7 cm of distal small intestine was longitudinally cut and thoroughly washed in cold PBS followed by incubation in PBS containing 5 µM BioTracker FITC-Cystine Sigma-Aldrich for 15 min or 25 µM D-Ala-Lys-AMCA Hycultec for 10 min at 37°C with constant horizontal agitation at rpm. Finally, nuclei were stained with DAPI for 30 min at RT in combination with FITC-Cystine assay, or with Helix NP Green BioLegend for 15 min at RT in combination with D-Ala-Lys-AMCA assay. Images were taken on a Zeiss Axio Observer 7 Carl Zeiss and analyzed with ImageJ. For organoid cultures, 20 cm of the proximal SI was collected, cut longitudinally, and washed two times with cold PBS. The tissue was cut into 2-mm pieces, placed in cold PBS containing 5 mM EDTA, and pipetted up and down 10 times with a disposable pipette coated with 0. Then supernatant was discarded, and the tissue was incubated twice in 5 mM EDTA in PBS for 10 and 30 min at 4°C with constant agitation. After EDTA solution removal, fresh PBS was added and the tissue was pipetted up and down 15 times with a disposable pipette coated with 0. This procedure was repeated four more times to obtain a total of five fractions. The fraction with higher crypt enrichment was identified under the microscope and passed through a µm cell strainer. For Noggin removal experiments, organoids were cultured in ENR medium for 2 d and then ENR was substituted for ER ENR without Noggin. For crypt expansion index, images of Maf ΔIEC and control organoids were taken for 4 consecutive days after seeding. Buds and total organoids were then counted at least 40 organoids per well using ImageJ software, and crypt expansion index was calculated and shown as the number of crypts per organoid. mRNA from sorted cells was isolated with the RNeasy Plus Micro Kit according to the manual of the manufacturer QIAGEN. RNA from cell suspensions, organoids, or tissues was extracted using TRIzol reagent following the protocol from ImmGen Heng et al. The isolated RNA was quantified using Nanodrop before qPCR performance. For IF staining, 5—7 cm of the distal jejunum were taken and Swiss rolls were prepared as previously described with minor adaptations Bialkowska et al. Compound Tissue-Tek, Sakura , and frozen with liquid nitrogen. blocks were cut into 5-µm sections for IF or conventional periodic acid—Schiff staining. For c-Maf, DCLK1, ChgA, and UEA1 staining, first, slides were rehydrated in cold PBS, then blocked and permeabilized in 0. Next, slides were incubated with c-Maf antibody in blocking buffer at RT overnight. Then slides were cooled down at RT for 30 min and washed three times in PBS and blocked and permeabilized in blocking buffer at RT for 1 h. Next, primary antibody staining was performed at RT for 1 h in blocking buffer. Finally, slides were stained with secondary antibodies and DAPI in blocking solution at RT for 1 h and mounted with ProLong Diamond Antifade Mountant Thermo Fisher Scientific. Images were taken on a confocal microscope LSM Carl Zeiss , a Zeiss Axio Observer 7 Carl Zeiss , and analyzed with ImageJ. For the quantification of SFB, ileal mucosal DNA was isolated. To specifically isolate the DNA from ileal mucosa, 2 cm of the ileum was taken, cut longitudinally, and washed thoroughly in cold PBS to remove the fecal content. The clean tissue was washed thoroughly in 0. Bacterial DNA was then isolated from mucosal content with the ZymoBIOMICS DNA Miniprep Kit Zymo Research , and bacterial load was measured by qPCR. The SFB abundance is presented relative to the abundance of eubacteria. RNA-FISH for SFB was performed as previously described Johansson and Hansson, Images were taken on Zeiss Axio Observer 7 Carl Zeiss and processed using ImageJ. qPCR was performed using a Quant Studio 5 system Applied Biosystems and the SYBR Green PCR Master Mix Kit Applied Biosystems. The mRNA expression is presented relative to the expression of the housekeeping gene hypoxanthine-guanine phosphoribosyltransferase. Real-time qPCR primer used in this study can be found in Table S3. A list of antibodies used in this study is provided in Table S4. Data are represented as the means with SEM and summarize or are representative of independent experiments as specified in the text. Statistical analysis was performed using Prism 9 software GraphPad with two-tailed unpaired Student's t test except RNA-Seq data. S1 shows data, which demonstrate that intestinal epithelial c-Maf expression is driven by BMP signaling. S2 confirms that the expression of epithelial carbohydrate and protein transporters is reduced in Maf ΔIEC mice. S3 shows that c-Maf—deficient organoids also express reduced levels of carbohydrate and protein transporters. Table S1 shows DE genes between c-Maf—deficient and control IECs as identified by RNA-Seq. Table S2 shows differentially expressed proteins between c-Maf—deficient and control IECs and liver tissue as identified by proteomics. Table S3 shows real-time qPCR primers used in this study. Table S4 lists all antibodies used in this study. We thank Andreas Diefenbach Charité — Universitätsmedizin Berlin, Germany for discussion, providing key resources, and proofreading the manuscript. We further thank Efstathios Stamatiades, Stylianos Gnafakis, Omer Shomrat, Manuela Stäber, Kathrin Textoris-Taube, Roodline Cineus, Ahmed Hegazy, and Frederik Heinrich for resources and technical and experimental help. The Benjamin Franklin Flow Cytometry Facility and the Core Facility High Throughput Mass Cytometry at Charité — Universitätsmedizin Berlin are greatly acknowledged for cell sorting and proteomics analysis, respectively. In addition, we thank Dr. Anja A. Kühl and the iPATH facility at Charité — Universitätsmedizin Berlin for support in the preparation of histological samples and staining, the Central Biobank Charité — Universitätsmedizin Berlin for slide scanning and digitalization, and Jörg Piontek for technical support with confocal microscopy. This research was supported by the Deutsche Forschungsgemeinschaft Priority Program to C. Neumann , by the Ministry of Education and Research as part of the National Research Node "Mass spectrometry in Systems Medicine" under grant agreement L to M. Author contributions: C. Cosovanu designed and performed most experiments, analyzed data, generated figures, and helped writing the manuscript. Resch helped with experimental design and execution. Jordan assisted in feeding experiments. Lehmann was responsible for sample preparation for metabolomics and LC-MS instrumentation. Ralser provided funding for personnel and analytical instruments. Farztdinov performed bioinformatic data analysis and presentation of proteomic data. Mülleder supervised MS measurements and was responsible for MS data analysis and management. Spranger and S. Brachs supervised metabolic analysis NMR, metabolic cages and provided reagents and equipment for their execution. Neumann conceived the project, designed and performed experiments, analyzed data, generated figures, and wrote the manuscript. All coauthors read, commented on, and approved the manuscript. shows differentially expressed proteins between c-Maf—deficient and control IECs and liver tissue as identified by proteomics. c-Maf expression marks mature SI enterocytes of the mid-villus region. A Schematic representation of the murine gastrointestinal tract depicting distinct intestinal segments. Representative flow cytometric plots of EpCAM vs. c-Maf staining are shown. Numbers in the plots indicate percentage. Scale bar, 50 µm. E Maf expression among distinct SI IEC subsets as determined by scRNA-Seq Haber et al. F Intensity of c-Maf IF staining along the SI crypt—villus axis. Data are representative of at least two independent experiments. Intestinal epithelial c-Maf expression is driven by BMP signaling. B SI organoid cultures from Maf ΔIEC and littermate control mice were cultured in ENR medium for 2 d. Afterwards, ENR was refreshed or substituted for ER ENR without Noggin medium. In addition, organoids were stimulated with BMP-4 for 6 h at day 4 of culture before organoids were harvested for qPCR analysis. Statistical differences were tested using an unpaired Student's t test two-tailed. Maf ΔIEC mice exhibit a reduced nutritional phenotype. A Representative IF staining of c-Maf and DAPI on cross-section of the SI of Maf ΔIEC and control mice. B Analysis of c-Maf expression in SI IECs from Maf ΔIEC and control mice. E Regression plot of energy expenditure EE vs. K Representative periodic acid—Schiff staining on cross-section of the SI of Maf ΔIEC and control mice. L Representative IF staining of DCLK1 and DAPI on cross-section of the SI of Maf ΔIEC and control mice. Data are pooled from at least two independent experiments. Reduced expression of carbohydrate and protein transporters in Maf ΔIEC mice. B RNA-Seq based PCA of FACS-sorted IECs. Each dot represents an individual biological replicate. H GSEA of whole proteome comparison between liver tissue isolated from Maf ΔIEC and control mice with a focus on biological processes GOBP. The size of each circle represents the weighted number of proteins involved in the term. NES, normalized enrichment score. Data represent the combined analysis of six and three biologically independent samples from control and Maf ΔIEC mice, respectively. c-Maf controls intestinal nutrient uptake and sensing. In the free passive diffusion , molecules cross membranes without any help. In the facilitated and in the active transport, molecules need to be recognized by specific transporters inserted in the membranes. Water, ethanol and many lipids cross through enterocytes by free passive diffusion. Glucose, some lipids, amino acids, and di- and tri-peptides enter enterocytes by facilitated passive transport or by active transport. T he apical domain of enterocytes bears a set of proteins for absorption of substances, whereas the latero-basal membranes have another transmembrane transporters for getting molecules out of the cell. T he absorption capability depends on differentiation stage of the enterocyte, which determines the number of sodium membrane pumps, more abundant as enterocytes move further away from the deep of the crypts. Thus, most absorption of sugars and amino acids is done in the upper third of the villi of the small intestine and near the surface of the large intestine. For example, hydrolase activity increases as enterocytes move away from the stem cell niches deep parts of the crypts. T he absorption of glucose is a typical example of absorption mechanism. Glucose crosses the apical membrane of the enterocyte by co-transport coupled to a sodium gradient. The sodium glucose transporter SGLT is responsible for this co-transport. On the other hand, the GLUT2 transporter is found in the laterobasal membranes, which translocates glucose from the cytoplasm to the intercellular space. GLUT2 is able to transport glucose in both directions: inward and outward of the cell. However, as glucose concentration is higher within the cell, the net transport of glucose is outward. Thus, SGLT increases glucose concentration in the enterocyte cytoplasm and GLUT2 allows glucose to escape toward the blood vessels. The precise location of the two transporters produces a flux of glucose through enterocytes, from the intestine lumen to the blood vessels. F at is among the most energetic substances, and it is necessary for cellular membranes. The majority of the fat we eat is incorporated from the intestine in triacylglycerol form, although other types can be absorbed too, such as cholesterol. First, pancreatic lipases degrade meal fat in the intestine lumen and triacylglycerols are converted to fatty acids and monoacylglycerols Figure 6. These molecules, together with cholesterol, fat soluble vitamins, and phospholipids, form micelles, which are small lipid droplets soluble in water thanks to the biliary acids. Micelles cross freely the enterocyte apical membrane. CD36 y FABP fatty acid binding protein transporters make possible for some fatty substances to cross the enterocyte apical membrane by facilitated passive transport. The bulk of the fat transport is as micelles, whereas the membrane transporters look like a sensory system to detect fatty acids with long chains in the intestine. Cholesterol, as an individual molecule, can be also transported by NPC1L1 Niemann-Pick C1-like 1 transporter, which transfers cholesterol from the intestine lumen to the enterocyte cytoplasm. O nce in the enterocyte, fats are joined to some proteins and moved to the endoplasmic reticulum, where triacylglycerols are synthesized again. Then, these lipids are combined with some proteins to form lipoproteins called pre-chylomicrons. ApoB protein is synthesized in the endoplasmic reticulum. ApoB, together with MTP microsome tranfer protein and the fatty acids form this lipoprotein primordial particle. In the smooth endoplasmic reticulum, ApoB is substituted by Apo A-IV protein. Fat absortion boost the production of A-IV apopliprotein, which locate at the surface of chylomicrons. A-IV inhibits the hungry sensation. All these components constitute the pre-chylomicrons in the smooth endoplasmic reticulum, are included in vesicles and moved to the Golgi apparatus. Here, pre-chylomicrons are joined together to form chylomicrons, which are included in exocytic vesicles and released at the laterobasal domain of the enterocyte. In this way, chylomicrons can reach blood and lymphatic vessels. Chylomicrons are lipoproteins with a body mainly compose of triacylglycerols and a coat of phospholipds, cholesterol and apolipoproteins. They play a major role transporting triacylglycerols and liposoluble vitamins. Besides chylomicrons, fat associate to form very low density lipoproteins VLDLP , which are also exocyted, or can be temporary stored within the enterocytes as lipid droplets. O nce exocyted from the enterocyte, chylomicrons enter lymphatic vessels of the intestinal villi, and then in the lymphatic myenteric plexuses from which they pass to the blood vessels. E nterocytes also uptake the iron after digestion. Iron is important for many proteins, such as hemoglobin. It can be found in food as part of hemo groups or bound to ferritin in animal meat Figure 7. After digestion, free iron enters enterocytes through DMT1 transporter divalent metal transporter 1. Transgenic mice lacking this transporter develop severe anemia. DMT1 is a co-transporter coupled to a proton gradient. On the other hand, the iron bound to hemo groups appears to be incorporated by receptor mediated endocytosis. Once within the enterocyte, the hemo group is degraded and the iron can get into the cytosol. W hatever the entry pathway, once in the cytosol, the movement of iron toward the basolateral membranes appears to be mediated by metallochaperone proteins. In the basolateral membranes, iron is translocated to the extracellular space by the ferroportin transporter. Iron can be stored in the cytosol of the enterocyte bound to ferritin. Some molecules, such as immunoglobulins, enter enterocytes by receptor mediated endocytosis and transported to basolateral membrane domains by transcytosis. The journey begins in vesicles formed at the base of the microvilli, and that are fused later with endosomes. Endosomes release new vesicles that fuse with the basolateral membranes by exocytosis. In this way, molecules escape the lysosomal degradation pathway. P aracellular. Water and ions cross the epithelium by paracellular pathway. E nterocytes have to form a barrier that reject antigens, toxic molecules and microorganisms, but at the same time let cross nutritive substances. Enterocytes are in contact with many microorganisms. Those microorganisms that are resident in the intestine are usually beneficial, but can be dangerous if they reach the internal tissues, and there are also those non-resident pathogens that come with the meal. The apical surface of the enterocytes is covered with a layer of mucous substances released by globet cells. This layer is composed of carbohydrates and has a dense viscosity that allows the diffusion of molecules, but rejects cells and the largest molecules. In addition, enterocyte microvilli show a well-developed glycocalyx in the apical tips of each microvillus, which acts as a physical and electrical barrier since it is full of negative charges. These microvilli make difficult a direct physical contact between microorganisms and enterocyte membrane. However, even if they pass these two barriers, microorganisms should overcome the inner transport mechanism of the microvilli. M ucins are highly glycosylated proteins found in the apical plasma membrane of enterocytes. They contribute to the well-developed glycocalyx. Mucines are transmembrane proteins linked to the cytoskeleton by their cytosolic domain. MUC3, MUC12 and MUC17 are the most abundant mucins. They contain about amino acids and the carbohydrate component can extend up to 1 µm from the cell surface. Mucins form a physical barrier hard to cross by bacteria. E nterocytes are able to start and regulate inflammatory processes by releasing several chemokines and cytokines. They also have receptors for these molecules. Thus, enterocytes release pro-inflammatory molecules that influence immune cells found in the intestinal mucosa. A nother less known protection mechanism is the release of vesicles from the apical surface of enterocytes. Actin and myosin, the motor apparatus of microvilli, develop mechanical forces that drag membranes toward the tip of each microvillus. The membrane accumulation ends up as vesicles, which are released into the intestinal lumen. |
| Absorption in the Small Intestine: General Mechanisms | Endogenous Ij Natural remedies for inflammation NPY mediate tonic Closed-loop glucose monitoring 1 - and Nutrient absorption in the enterocytes 2 -mediated absorption in human and mouse Nutrienf. Dietary palmitic acid modulates intestinal re-growth after massive small bowel resection in a rat. Yang, and Y. For finding regulated features, the following criteria were applied: significance level α was set to 0. Water and lipids are absorbed by passive diffusion throughout the small intestine. |
Video
Fat (lipid) digestion and absorption physiologyNutrient absorption in the enterocytes -
After amylases break down starch into smaller fragments, the brush border enzyme α-dextrinase starts working on α-dextrin , breaking off one glucose unit at a time. Three brush border enzymes hydrolyze sucrose, lactose, and maltose into monosaccharides.
Sucrase splits sucrose into one molecule of fructose and one molecule of glucose; maltase breaks down maltose and maltotriose into two and three glucose molecules, respectively; and lactase breaks down lactose into one molecule of glucose and one molecule of galactose.
Insufficient lactase can lead to lactose intolerance. Figure 2. Carbohydrates are broken down into their monomers in a series of steps.
Proteins are polymers composed of amino acids linked by peptide bonds to form long chains. Digestion reduces them to their constituent amino acids.
You usually consume about 15 to 20 percent of your total calorie intake as protein. The digestion of protein starts in the stomach, where HCl and pepsin break proteins into smaller polypeptides, which then travel to the small intestine. Chemical digestion in the small intestine is continued by pancreatic enzymes, including chymotrypsin and trypsin, each of which act on specific bonds in amino acid sequences.
At the same time, the cells of the brush border secrete enzymes such as aminopeptidase and dipeptidase , which further break down peptide chains. This results in molecules small enough to enter the bloodstream.
Figure 3. The digestion of protein begins in the stomach and is completed in the small intestine. Figure 4. Proteins are successively broken down into their amino acid components. A healthy diet limits lipid intake to 35 percent of total calorie intake. The most common dietary lipids are triglycerides, which are made up of a glycerol molecule bound to three fatty acid chains.
Small amounts of dietary cholesterol and phospholipids are also consumed. The three lipases responsible for lipid digestion are lingual lipase, gastric lipase, and pancreatic lipase. However, because the pancreas is the only consequential source of lipase, virtually all lipid digestion occurs in the small intestine.
Pancreatic lipase breaks down each triglyceride into two free fatty acids and a monoglyceride. The fatty acids include both short-chain less than 10 to 12 carbons and long-chain fatty acids. The nucleic acids DNA and RNA are found in most of the foods you eat.
Two types of pancreatic nuclease are responsible for their digestion: deoxyribonuclease , which digests DNA, and ribonuclease , which digests RNA. The nucleotides produced by this digestion are further broken down by two intestinal brush border enzymes nucleosidase and phosphatase into pentoses, phosphates, and nitrogenous bases, which can be absorbed through the alimentary canal wall.
The large food molecules that must be broken down into subunits are summarized in Table 2. The mechanical and digestive processes have one goal: to convert food into molecules small enough to be absorbed by the epithelial cells of the intestinal villi.
The absorptive capacity of the alimentary canal is almost endless. Each day, the alimentary canal processes up to 10 liters of food, liquids, and GI secretions, yet less than one liter enters the large intestine.
Almost all ingested food, 80 percent of electrolytes, and 90 percent of water are absorbed in the small intestine. Although the entire small intestine is involved in the absorption of water and lipids, most absorption of carbohydrates and proteins occurs in the jejunum.
Notably, bile salts and vitamin B 12 are absorbed in the terminal ileum. By the time chyme passes from the ileum into the large intestine, it is essentially indigestible food residue mainly plant fibers like cellulose , some water, and millions of bacteria. Figure 5. Absorption is a complex process, in which nutrients from digested food are harvested.
Absorption can occur through five mechanisms: 1 active transport, 2 passive diffusion, 3 facilitated diffusion, 4 co-transport or secondary active transport , and 5 endocytosis.
As you will recall from Chapter 3, active transport refers to the movement of a substance across a cell membrane going from an area of lower concentration to an area of higher concentration up the concentration gradient.
Passive diffusion refers to the movement of substances from an area of higher concentration to an area of lower concentration, while facilitated diffusion refers to the movement of substances from an area of higher to an area of lower concentration using a carrier protein in the cell membrane.
Co-transport uses the movement of one molecule through the membrane from higher to lower concentration to power the movement of another from lower to higher. Finally, endocytosis is a transportation process in which the cell membrane engulfs material.
It requires energy, generally in the form of ATP. Moreover, substances cannot pass between the epithelial cells of the intestinal mucosa because these cells are bound together by tight junctions.
Thus, substances can only enter blood capillaries by passing through the apical surfaces of epithelial cells and into the interstitial fluid. Water-soluble nutrients enter the capillary blood in the villi and travel to the liver via the hepatic portal vein.
In contrast to the water-soluble nutrients, lipid-soluble nutrients can diffuse through the plasma membrane. Once inside the cell, they are packaged for transport via the base of the cell and then enter the lacteals of the villi to be transported by lymphatic vessels to the systemic circulation via the thoracic duct.
The absorption of most nutrients through the mucosa of the intestinal villi requires active transport fueled by ATP. The routes of absorption for each food category are summarized in Table 3. All carbohydrates are absorbed in the form of monosaccharides.
The small intestine is highly efficient at this, absorbing monosaccharides at an estimated rate of grams per hour. All normally digested dietary carbohydrates are absorbed; indigestible fibers are eliminated in the feces.
The monosaccharides glucose and galactose are transported into the epithelial cells by common protein carriers via secondary active transport that is, co-transport with sodium ions.
The monosaccharides leave these cells via facilitated diffusion and enter the capillaries through intercellular clefts. The monosaccharide fructose which is in fruit is absorbed and transported by facilitated diffusion alone.
The monosaccharides combine with the transport proteins immediately after the disaccharides are broken down. Active transport mechanisms, primarily in the duodenum and jejunum, absorb most proteins as their breakdown products, amino acids.
Almost all 95 to 98 percent protein is digested and absorbed in the small intestine. The type of carrier that transports an amino acid varies. Most carriers are linked to the active transport of sodium. Short chains of two amino acids dipeptides or three amino acids tripeptides are also transported actively.
However, after they enter the absorptive epithelial cells, they are broken down into their amino acids before leaving the cell and entering the capillary blood via diffusion.
About 95 percent of lipids are absorbed in the small intestine. Bile salts not only speed up lipid digestion, they are also essential to the absorption of the end products of lipid digestion. Short-chain fatty acids are relatively water soluble and can enter the absorptive cells enterocytes directly.
Despite being hydrophobic, the small size of short-chain fatty acids enables them to be absorbed by enterocytes via simple diffusion, and then take the same path as monosaccharides and amino acids into the blood capillary of a villus.
The large and hydrophobic long-chain fatty acids and monoacylglycerides are not so easily suspended in the watery intestinal chyme.
However, bile salts and lecithin resolve this issue by enclosing them in a micelle , which is a tiny sphere with polar hydrophilic ends facing the watery environment and hydrophobic tails turned to the interior, creating a receptive environment for the long-chain fatty acids.
The core also includes cholesterol and fat-soluble vitamins. Without micelles, lipids would sit on the surface of chyme and never come in contact with the absorptive surfaces of the epithelial cells.
Micelles can easily squeeze between microvilli and get very near the luminal cell surface. At this point, lipid substances exit the micelle and are absorbed via simple diffusion.
The free fatty acids and monoacylglycerides that enter the epithelial cells are reincorporated into triglycerides. The triglycerides are mixed with phospholipids and cholesterol, and surrounded with a protein coat. This new complex, called a chylomicron , is a water-soluble lipoprotein.
After being processed by the Golgi apparatus, chylomicrons are released from the cell. Intestinal tissues were washed in cold HBSS several times to remove mucus, blood cells and muscle tissue. Connective tissue was scraped away carefully. Then, epithelial tissue was cut into small pieces 5 mm and incubated in 5 mM EDTA in HBSS for 30 min at 4°C.
The pieces were transferred into cold HBSS and vigorously suspended to obtain fractions. Mesenchymal and immune cells were further removed by discarding supernatant after centrifugation 10 s at rpm. Then, epithelial tissue was enriched through centrifugation 3 min at 1, rpm.
After centrifugation 3 min at 1, rpm , the sediment was incubated in Tryple Invitrogen for 20 min at 37°C to obtain single-cell suspension. The libraries were subjected to high-throughput sequencing on an Illumina Hiseq X Ten PE platform, and bp paired-end reads were generated.
The raw sequencing reads were first demultiplexed using Illumina bcl2fastq software to generate bp paired-end read files in FASTQ format. The reads were then aligned to the GRCh38 human reference genome using the Cellranger toolkit version 2.
The exonic reads uniquely mapped to the transcriptome were then used for unique molecular identifier UMI counting. Selection and filtering of the droplet barcodes for single cells were done using the Cellranger toolkit as described before Haber et al.
In brief, the 99th percentile of the total UMI counts divided by 10 was used as cutoff for calling of single cells. Finally, mesenchyme, immune, and hematopoietic cells were removed based on these marker genes LSP1, MZB1, VIM, CD52, CD78B, and COL3A1.
CD45 was not detected in our results. Library size normalization was performed using Seurat NormalizeData. Next, the six batches of single-cell RNA-seq data were subjected to batch correction, as described previously Mayer et al. In brief, the canonical correlation analysis CCA strategy was used to find linear combinations of features across datasets that are maximally correlated.
The shared correlation structure conserved among the six datasets from the ileum, colon, and rectum were identified. Based on the shared structure, all six batches of data were finally pooled into a single object for downstream analyses Butler et al. Batch distributions for each dataset were visualized using t-distributed stochastic neighbor embedding t-SNE plots.
The R package Seurat was used to combine linear and nonlinear dimensionality reduction algorithms for unsupervised clustering of single cells. Specifically, first, highly variable genes were identified by the FindVariableGenes function, and average expression and dispersion for each gene were calculated.
Subsequently, CCA was performed based on the variable genes in the six intestine samples. The canonical correlation vectors then projected each dataset into the maximally correlated subspaces for downstream analysis.
Graph-based clustering was performed, which allocated cells in a K-nearest neighbor graph structure based on high correlation strength CCA.
The cells were then iteratively clustered, and the modularity was optimized with the Louvain algorithm. Finally, we used t-SNE to place cells with similar local neighborhoods in high-dimensional space or low-dimensional space based on scaled expression of variable genes to visualize the clustering results of all the cells.
To identify signature genes of each cell type, the functions FindAllMarkers and FindMarkers in Seurat were used with the following configurations: min. For a given cluster, FindAllMarkers identified positive markers compared with all other cells. All differentially expressed genes as positive markers of specific cell clusters are listed in Tables S1—S8.
Expression heatmaps of the signature genes for each cluster are shown in Fig. Similarly, the function FindMarkers was used for identification of signature genes by comparing the cell type of interest to another specific group of cells e. Single-cell transcriptome data of mouse ileum was obtained from Gene Expression Omnibus GEO accession no.
GSE Haber et al. Altogether, we compared gene expression matrices of 6, human ileum cells in this study to the 3, mouse ileum cells, which were subjected to the same process of quality control and filtering. We only considered the homologous genes between human and mouse, which eventually generated a scaled expression matric for 11, genes of 10, cells.
For each pair of cells from human and mouse, the Pearson correlation was calculated with the scaled expression data of the genes in the two cells. We obtained single-cell transcriptome data of mouse ileum from GEO accession no.
Altogether, we analyzed gene expression matrices of 6, human ileal cells in this study and 3, mouse ileal cells after removing the low-quality cells using the same filtering strategy with the human data.
We considered the expression data of all 11, homologous genes with identical gene names between the human and mouse datasets and then performed CCA as implemented in the Seurat Butler et al.
t-SNE, cell cycle, and differential expression analyses were performed using the same methods described above. Immunofluorescence and immunohistochemistry were performed as previously described Qi et al.
Then, the sections were incubated overnight with the primary antibody at 4°C. The fluorescein-labeled secondary antibodies ; Life Technologies for immunofluorescence or secondary horseradish peroxidase—conjugated anti-rabbit antibody ; Invitrogen for immunohistochemistry were added for 2 h at room temperature.
Lgr5-EGFP mice were obtained from The Jackson Laboratory. Animals were then euthanized, and tissue was processed immediately. All animal experiments were conducted in accordance with the relevant animal regulations with approval of the Institutional Animal Care and Use Committee of Tsinghua University.
Rabbit anti-LYZ , ab; Abcam , mouse anti-MUC2 , ab; Abcam , rabbit anti-NUSAP1 , —1-AP; Proteintech , mouse-anti Ki67 , s; CST , mouse anti-E-Cad ,, ; BD Biosciences , rabbit anti-ITLN1 , —1-AP; Proteintech , rabbit anti-TFF1 , —1-AP; Proteintech , rabbit anti-APOB , —1-AP; Proteintech , rabbit anti-APOA4 , —1-AP; Proteintech , rabbit anti-SLC26A2 , —1-AP; Proteintech , rabbit anti-SCNN1B , —1-AP; Proteintech , rabbit anti-SLC35A1 , —1-AP; Proteintech , rabbit anti-FABP6 , —1-AP; Proteintech , rabbit anti-SLC38A1 , —1-AP; Proteintech , and rabbit anti-SLC44A1 , —1-AP; Proteintech.
The sections were prepared with vibrating blade microtome HM; Microm and endogenous peroxidase blocking was performed by RNAscope Hydrogen Peroxide ; ACD for 10 min at room temperature. Then, RNAscope Protease Plus ; ACD was used for 10 min at 40°C before probe hybridization.
ITLN1 probe ; ACD and LYZ probe ; ACD were hybridized for 2 h at 40°C, AMP 1—6, and signal detection was performed as described in the user manual ; ACD. RNeasy Mini Kit Qiagen was used to extract total RNA, and cDNA was obtained by Revertra Ace Toyobo.
Then, real-time PCR reactions were performed in triplicate on a LightCycler Roche. Primers of selected genes are listed in Table S Human intestinal tissue was washed in cold HBSS and removed muscle tissue. The pieces were transferred into cold HBSS and vigorously suspend to get fraction, and epithelial tissue was enriched through centrifugation 3 min at 1, rpm.
Crypts were then embedded in Matrigel BD Biosciences and seeded on a well plate. To find out amino acid uptake per cell, organoids were incubated in Tryple Invitrogen for 20 min at 37°C to obtain a single-cell suspension. The amino acid uptake per cell was calculated by combining amino acid changes and cell number.
For choline, succinic and citric acid uptake, 20 mM choline C; MACLIN , succinic acid S; MACLIN , and citric acid C; MACLIN were added to the crypt culture medium 8 h later after the first passage. Organic solute uptake per cell was calculated by combining amino acid changes and cell number.
For sugar uptake, GlutaMAX-I was removed from the medium 8 h later after the first passage. Then, 20 mM fructose S; Selleck , galactose S; Selleck , and mannose S; Selleck were added to the medium, and 50 μl medium was selected 24 h later to detect uptake changes, and the cell number was counted by FACS for PI-negative cells as described above.
Sugar uptake per cell was calculated by combining amino acid changes and cell number. The fold change from ileum, colon, and rectum organoids was calculated by comparing with ileum organoids.
All experiments with quantitation were performed independently at least three times with three replicates within each experiment, and data are represented as mean ± SD. All statistical analysis was performed with GraphPad Prism 7 win. All data have been deposited in the GEO under accession no.
R markdown scripts enabling the main steps of the analysis to be performed are available from the corresponding authors on reasonable request. S1 shows general information of clinical samples and annotations of cell types of single-cell RNA-seq data.
S2 shows expression patterns of cell markers. S3 shows characterization of stem cells, TA cells, progenitor cells, and transcription factor analysis. S4 shows expression patterns of transporter genes and validation by immunofluorescence or immunohistochemistry. Table S1 shows all cell type—specific genes.
Table S2 shows ileum, colon, and rectum cell type—specific genes. Table S3 shows stem cell and TA subset genes. Table S4 shows progenitor subset-specific genes.
Table S5 shows enterocyte cell subset-specific genes. Table S6 shows enteroendocrine cell subset-specific genes. Table S7 shows PLC subset signature genes.
Table S8 shows goblet cell subset signature genes. Table S9 shows signature genes involved in the cell cycle. Table S10 shows quantitative PCR primers. We thank Drs. Ligong Chen and Xin Zhou for critical reading of the manuscript, Yuxin Sun for information consolidation, and the Metabolomics Facility at Tsinghua University for LC-MS analyses.
This work was supported by the National Key Research and Development Program of China grant YFA to Y. Chen and grant YFC to X.
Yang and the National Natural Science Foundation of China grant to Y. Chen and grants and to X. Author contributions: Y.
Wang and Y. Chen designed the study and analyzed the data; Y. Wang performed the experiments; W. Song and X. Yang performed the bioinformatics analysis and analyzed the data; J.
Wang and W. Fu provided samples and selected clinical information and analyzed the data; T. Wang and X. Xiong helped with functional experiments; Z. Qi helped with single-cell isolation; and Y.
Wang, W. Song, X. Yang, and Y. Chen wrote the manuscript. Cell landscapes of human intestines based on single-cell transcriptome profiles. A, C, E, and G t-SNE plots of single-cell clusters. The Seurat algorithm was used to visualize the clustering of all 14, intestine epithelial cells from six donors A , 6, ileum cells from two donors C , 4, colon cells from two donors E , and 3, rectum cells from two donors G.
B, D, F, and H Expression heatmaps of cell type—specific genes were obtained by analyzing all cells pooled together B or based on cells from one of the three intestine segments: ileum D , colon F , and rectum H.
EC, enterocytes; EEC, enteroendocrine cells; G, goblet cells; PRO, progenitor cells; SC, stem cells. Differential nutrient absorption preferences in the human small and large intestine. A Expression heatmap of signature genes in enterocytes and gene functional enrichments.
B Violin plots showing distributions of mean expression of transporter genes in different segments il, ileum; co, colon; re, rectum. C Expression patterns of specific genes involved in nutrient absorption and transport in different intestine segments.
Each dot represents a gene, of which the color saturation indicates the average expression level scaled by Z-score in an intestine segment, and the size indicates the percentage of cells expressing the gene.
D Relative mRNA expression of the gene marked in red in C was confirmed by qPCR in the organoids derived from human ileum, colon, and rectum. E Nutrient uptake was measured by LC-MS in the organoids derived from human ileum, colon, and rectum.
Data are represented as mean ± SD of at least three independent experiments. Differential expression of signaling molecules, hormones, and immunity-related genes.
A Violin plots showing expression distributions of signaling genes in enterocytes from the ileum il , colon co , and rectum re. B Expression heatmap of signature genes in enteroendocrine cells. C Expression patterns of specific hormone genes in enteroendocrine cells from different intestine segments.
D Expression patterns of immune-related genes in cells from different intestine segments and gene functional enrichments. Each dot represents a gene, of which the color saturation indicates the average expression level scaled by Z-score in an intestine segment and the size indicates the percentage of cells expressing the gene.
PLCs identified in the human colon and rectum. A Expression heatmap of signature genes in PLCs and gene functional enrichments. B Lysozyme LYZ expression indicated by color saturation in single cells from the ileum, colon, and rectum.
C Immunofluorescence was performed to confirm LYZ expression in the ileum, colon and rectum. Scale bars, µm. D smFISH results of LYZ in human ileum, colon, and rectum.
Scale bar, µm. E mRNA expression levels of growth factors in PCs and PLCs in the ileum and PLCs in the colon and rectum. F Specific expression of GNPTAB and SOD3 in PCs and PLCs of the human large intestine. G Violin plots showing expression distributions of transcription factors in PCs or PLCs.
H Kit expression in all 14, cells. Potential new markers of human TA cells and goblet cells. A NUSAP1 expression indicated by color saturation in single cells from the ileum, colon, and rectum. B Immunofluorescence was performed to confirm the NUSAP1 expression in the ileum, colon, and rectum.
C Statistics of the NUSAP1 immunofluorescence data shown in B. D Expression heatmap of signature genes in goblet cells and gene functional enrichments.
E ITLN1 expression indicated by color saturation in single cells from the ileum, colon, and rectum. F Immunofluorescence was performed to confirm ITLN1 expression. G TFF1 expression indicated by color saturation in single cells from the ileum, colon, and rectum.
H Immunofluorescence was performed to confirm the TFF1 expression. Comparison of gene expression profiles between the epithelial cells of the human and mouse ilea.
C Cell cycle analysis. The calculated cell cycle phase of each cell is shown for all 10, ileal cells. D Cell cycle quantity of human and mouse ileal cells. E Conserved signature genes of each cluster in human and mouse ilea. F Signature genes of human and mouse ileal stem cells.
G Human- or mouse-specific signature genes of each cell type in the ilea. Each dot represents a gene, of which the color saturation indicates the average expression level scaled by Z-score in a specific cell type, and the size indicates the percentage of cells expressing the gene.
EC, enterocytes; EEC, enteroendocrine cells; G, goblet cells; H, humans; M, mice; PRO, progenitor cells; SC, stem cells; TU, tuft cells. Sign In or Create an Account. Search Dropdown Menu. header search search input Search input auto suggest. filter your search All Content All Journals Journal of Experimental Medicine.
Advanced Search. User Tools Dropdown. Sign In. Skip Nav Destination Article Navigation. Technical Advances and Resources November 21 This Site. Google Scholar. Wanlu Song Jilian Wang , Jilian Wang. Ting Wang , Ting Wang. Xiaochen Xiong , Xiaochen Xiong.
Zhen Qi , Zhen Qi. Wei Fu , Wei Fu. Wei Fu: fuwei bjmu. Xuerui Yang , Xuerui Yang. Xuerui Yang: yangxuerui tsinghua. Ye-Guang Chen Ye-Guang Chen. Correspondence to Ye-Guang Chen: ygchen tsinghua.
Author and Article Information. Jilian Wang. Ting Wang. Xiaochen Xiong. Zhen Qi. Wei Fu. Xuerui Yang. Song contributed equally to this paper. Received: June 20 Revision Received: August 28 Accepted: October 18 Online ISSN: Funder s : National Key Research and Development Program of China.
Award Id s : YFA Funder s : National Natural Science Foundation of China. Award Id s : After six months it is available under a Creative Commons License Attribution—Noncommercial—Share Alike 4. J Exp Med 2 : e Article history Received:. Revision Received:. Connected Content. Commentary Atlas of the human intestine.
Cite Icon Cite. toolbar search Search Dropdown Menu. toolbar search search input Search input auto suggest. Graphical Abstract View large Download slide. View large Download slide. The authors declare no competing financial interests. Search ADS. Regulators of Iron Homeostasis: New Players in Metabolism, Cell Death, and Disease.
Computational analysis of cell-to-cell heterogeneity in single-cell RNA-sequencing data reveals hidden subpopulations of cells. Integrating single-cell transcriptomic data across different conditions, technologies, and species.
Functional analysis of SLC39A8 mutations and their implications for manganese deficiency and mitochondrial disorders. Single-cell dissection of transcriptional heterogeneity in human colon tumors. de Oliveira.
Di Ciaula. Tracing the temporal-spatial transcriptome landscapes of the human fetal digestive tract using single-cell RNA-sequencing. Lower gene expression for KCNS3 potassium channel subunit in parvalbumin-containing neurons in the prefrontal cortex in schizophrenia.
The zinc-finger factor Insm1 IA-1 is essential for the development of pancreatic beta cells and intestinal endocrine cells.
Intestinal absoeption and sensing processes fnterocytes their interconnection to metabolism are enterodytes to Mediterranean diet and food sustainability such as malabsorption syndromes, inflammatory diseases, obesity and type 2 Nutrieent. Constituting a highly selective Natural remedies for inflammation, intestinal epithelial cells absorb, Natural remedies for inflammation, enteeocytes release aabsorption into the circulation, hence serving as gatekeeper of nutrient availability and Nutrient absorption in the enterocytes health for the whole organism. Next to nutrient transport and sensing functions, intestinal transporters including peptide transporter 1 PEPT1 are involved in the absorption of drugs and prodrugs, including certain inhibitors of angiotensin-converting enzyme, protease inhibitors, antivirals, and peptidomimetics like β-lactam antibiotics. Here, we verify the applicability of 3D organoids for in vitro investigation of intestinal biochemical processes related to transport and metabolism of nutrients and drugs. Establishing a variety of methodologies including illustration of transporter-mediated nutrient and drug uptake and metabolomics approaches, we highlight intestinal organoids as robust and reliable tool in this field of research. Thank Natural remedies for inflammation for visiting nature. You Nutrien using Improve conversion rates browser version with limited absoprtion for CSS. To obtain enterocyted best experience, we recommend you use a enteroocytes up entrrocytes date Nutrient absorption in the enterocytes or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Intestinal surface changes in size and function, but what propels these alterations and what are their metabolic consequences is unknown. Here we report that the food amount is a positive determinant of the gut surface area contributing to an increased absorptive function, reversible by reducing daily food.
Thank Natural remedies for inflammation for visiting nature. You Nutrien using Improve conversion rates browser version with limited absoprtion for CSS. To obtain enterocyted best experience, we recommend you use a enteroocytes up entrrocytes date Nutrient absorption in the enterocytes or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Intestinal surface changes in size and function, but what propels these alterations and what are their metabolic consequences is unknown. Here we report that the food amount is a positive determinant of the gut surface area contributing to an increased absorptive function, reversible by reducing daily food. Nutrient absorption in the enterocytes -
In diseases of the small intestine the villi can become flattened due to the effects of inflammation, and the villi can sometimes disappear. This deterioration is known as villous atrophy, and is often a feature of coeliac disease. Contents move to sidebar hide. Article Talk. Read Edit View history.
Tools Tools. What links here Related changes Upload file Special pages Permanent link Page information Cite this page Get shortened URL Download QR code Wikidata item. Download as PDF Printable version.
In other projects. Wikimedia Commons. Finger-like projection of the small intestine. Micrograph of the small intestine mucosa showing villi — top half of image. Vertical section of a villus from the dog's small intestine. Simple columnar epithelium labeled at right, third from top.
Transverse section of a villus, from the human intestine. X Basement membrane , here somewhat shrunken away from the epithelium. Columnar epithelium.
Its striated border. Goblet cells. Leucocytes in epithelium. Leucocytes below epbithelium. Blood vessels. Muscle cells cut across.
Cross-section histology of small intestinal villi of the human terminal ileum. MicroCT -based volume projection of the jejunal mucosa of a chicken. Virtual volume block with vertically truncated villi in oblique view. MicroCT-based volume projection of the jejunal mucosa of a chicken.
Virtual horizontal cut through villi. Microvilli shaggy hair show electron dense plaques open arrow at their apices. Bernkop-Schnürch, Andreas ed. Oral Delivery of Macromolecular Drugs - Barriers. doi : ISBN Archived from the original on Retrieved Coeliac UK.
Retrieved 12 July The food that remains undigested and unabsorbed passes into the large intestine. Absorption of the majority of nutrients takes place in the jejunum, with the following notable exceptions:. Section of duodenum : Section of duodenum with villi at the top layer.
Search site Search Search. Go back to previous article. Sign in. Learning Objectives Describe the role played by the small intestine in the absorption of nutrients.
Key Points Digested food is able to pass into the blood vessels in the wall of the small intestine through the process of diffusion. The inner wall, or mucosa, of the small intestine is covered in wrinkles or folds called plicae circulares that project microscopic finger-like pieces of tissue called villi, which in turn have finger-like projections known as microvilli.
Each villus transports nutrients to a network of capillaries and fine lymphatic vessels called lacteals close to its surface. Key Terms villi : Tiny, finger-like projections that protrude from the epithelial lining of the intestinal wall. plicae circulares : These circular folds known as the valves of Kerckring or the valvulae conniventes are large, valvular flaps that project into the lumen of the bowel.
diffusion : The act of diffusing or dispersing something, or the property of being diffused or dispersed; dispersion. EXAMPLES Examples of nutrients absorbed by the small intestine include carbohydrates, lipids, proteins, iron, vitamins, and water.
The Small Intestine The small intestine is the part of the gastrointestinal tract between the stomach and the large intestine where much of the digestion of food takes place. Absorption of the majority of nutrients takes place in the jejunum, with the following notable exceptions: Iron is absorbed in the duodenum.
Vitamin B12 and bile salts are absorbed in the terminal ileum. Water and lipids are absorbed by passive diffusion throughout the small intestine.
Shield against microbial growth WangWanlu SongJilian Wang abssorption, Ting Wang Probiotics and inflammation, Xiaochen Nutrient absorption in the enterocytesEntfrocytes QiWei Fu entercoytes, Xuerui YangYe-Guang Chen; Natural remedies for inflammation transcriptome Natural remedies for inflammation hhe differential nutrient absorption functions in human enterocytees. J Exp Med 3 February ; 2 : e The intestine plays Nutriwnt important role in nutrient digestion and absorption, microbe defense, and hormone secretion. Although major cell types have been identified in the mouse intestinal epithelium, cell type—specific markers and functional assignments are largely unavailable for human intestine. Here, our single-cell RNA-seq analyses of 14, epithelial cells from human ileum, colon, and rectum reveal different nutrient absorption preferences in the small and large intestine, suggest the existence of Paneth-like cells in the large intestine, and identify potential new marker genes for human transient-amplifying cells and goblet cells. We have validated some of these insights by quantitative PCR, immunofluorescence, and functional analyses.
Ich entschuldige mich, aber meiner Meinung nach lassen Sie den Fehler zu. Ich kann die Position verteidigen. Schreiben Sie mir in PM, wir werden umgehen.
Ja, ich verstehe Sie.
Ich glaube Ihnen nicht