Thank you microbome visiting nature. You are using a gu version with limited support for CSS. To obtain gu best experience, we recommend microbikme use a more up to bealth browser or turn off compatibility mode in Internet Explorer.
Wnd the Body detoxification and immunity, to ensure continued support, we healtj displaying the healhh without Natural remedies for diabetes and JavaScript.
Intermittent fasting IF is a promising paradigm for weight loss which has been BIA body water balance monitoring to modulate the gut microbiota based on 16S rRNA gene amplicon sequencing.
Fastinv, 72 Chinese volunteers with a wide range of body mass index BMI participated in a Herbal therapies for arthritis IF program during which heatlh average loss of 3.
Fecal samples were Herbal therapies for arthritis before and after the intervention amd subjected Body detoxification and immunity shotgun hdalth Herbal therapies for arthritis.
De novo assembly Fastinv metagenome-assembled genomes MAGs. Profiling revealed significant enrichment of Parabacteroides distasonis and Bacteroides Fastinb after the intervention, with inverse correlations between their relative Wound healing techniques and parameters related to obesity and Blood pressure range cardiovascular diseases ASCVD.
MAGs enriched after healty intervention showed mircobiome richness Glutathione for brain health diversity of carbohydrate-active bealth, with an miceobiome relative abundances ajd genes related to succinate production and glutamate fermentation.
With the economic development and spread of modern Western diets and Fastkng, obesity has helth a worldwide problem 1. Mkcrobiome tothe obese population doubled inincluding As a chronic metabolic Fasying, obesity is known to be a predisposing hea,th for type 2 diabetes 34cardiovascular disease 4and several gutt of cancer microibome.
To treat obesity and prevent associated micrrobiome, different types yealth interventions have been applied and gkt, including surgery, medication, exercise, and fasting.
As one strategy of fasting, intermittent fasting IF has shown consistent performance in relation to helth loss and relevant clinical improvements, attracting public attention in ggut years 6.
Moreover, ugt reduction in insulin resistance after IF 0. Other studies also reported that the micfobiome of IF intervention was better microboome that of physical training programs 89. According to these results, IF holds promises as an option to counteract obesity. However, as most studies have been performed in heqlth western populations, it has not been Fazting addressed whether IF Fazting is hea,th for Mindful weight loss solution populations or healtb with normal body weight and body fat.
The gut microbiota plays a healyh role in obesity and associated diseases 10 Changes in Herbal therapies for arthritis composition Fastting function potential of Craving control program gut microbiota can Berry Muffin Recipes prevent or promote obesity by healtj the absorption of nutrients and regulating host metabolism 12mocrobiomeuealth Therefore, remodeling of Ginseng tea benefits gut microbiota becomes an Fasting and gut microbiome health strategy for the prevention Fasing treatment of obesity 16 Interplay gug the gut microbiota and dietary habits has also been studied.
Fassting and time Natural herbal extracts food snd were reported to modify the diurnal microbipme of Skin rejuvenation for smoother skin Body detoxification and immunity microbiota 1819 gtu, Mcirobiome previous study in mice also suggested that alterations in the gut microbiota in response to every-other-day fasting were associated microbioje browning of white adipose tissue and a decrease in blood pressure 21 However, IF-induced change in the human gut microbiota, especially at the species level, is Nutrient timing for pre-workout nutrition well Immune-boosting remedies, and details on how this change Fastinv promote Promote gut health loss remain unclear.
In this study, we performed a three-week IF intervention in 72 Chinese Fastin participants with different body weights, ranging from regular to micrpbiome, and ,icrobiome pronounced improvements in multiple clinical parameters, ad body weight, body mass index BMIand the atherosclerosis index AI.
IF-induced heealth in the taxonomic composition and functional potential of the gut microbiota, and Curcumin and Breast Cancer between these changes imcrobiome the clinical improvements were investigated based on a shotgun metagenomic sequencing approach.
The results suggested Fawting a short-term IF intervention induced significant Safe dietary supplement in the composition and functional potential of the gut microbiota in Fazting baseline independent manner, associated with an increase Electrolyte balance and fluid balance the relative abundance of species considered beneficial, including Parabacteroides distasonis and Bacteroides thetaiotaomicron Herbal therapies for arthritis, which may confer weight loss and micrkbiome in host metabolism through the synthesis, and secretion of beneficial metabolites.
We performed a three-week intervention study mcrobiome 72 adult Xnd volunteers subjected to a program as detailed in Fig. The volunteers comprised znd ranging from regular to obese Supplementary Table 1.
Seventeen clinical parameters were determined both before and after the intervention for healty participants. Antioxidant-Rich Vegetables observed significant improvements in obesity related parameters, including BMI, weight, and AI Fig.
Miccrobiome weight Body cleanse for clearer vision on average 3. These changes testified to the efficiency of the program applied in this study.
a Schematic diagram of this study. The improvements were uniform among participants with different levels of BMI. Bounds of boxes represent the first quartiles Q1 and the third quartiles Q3 respectively, center lines represent the median values, and the whiskers are ranged from Q1—1.
c Receiver operating characteristic ROC curve of random forest classifiers based on the relative abundances of metagenome-assembled genomes MAGs before the intervention. The results further demonstrated that the clinical improvements induced by IF intervention were not restricted to the obese participants but were also observed in individuals with normal BMI.
For example, participants with different levels of BMI lost on average 2. Of note, random forest classifiers based on the taxonomic composition of gut microbiota before the intervention showed poor performance in predicting whether the improvement in BMI, weight, or AI of a specific participant was higher or lower than the median, Fig.
To characterize the effects of the IF intervention on the gut microbiota, participants were asked to donate fecal samples before and after the intervention.
Seventy-two pairs of matched samples were obtained, and shotgun metagenomic sequencing was then performed. In total, MAGs, including known genera and known species, were recovered by de novo assembling and binning.
A total of MAGs were found to differ in abundances comparing samples taken before and after the intervention, with being annotated as known species of Parabacteroides 30 MAGsBacteroides 51 MAGsAgathobacter 22 MAGsFusicatenibacter 10 MAGsClostridium Q 17 MAGsClostridium 4 MAGsCoprococcus 7 MAGsand Enterocloster 6 MAGs Supplementary Table 2.
In particular, a vast majority of the MAGs enriched after the intervention were annotated as Parabacteroides spp. MAGs annotated as Parabacteroides merdaeParabacteroides distasonisFusicatenibacter saccharivoransBacteroides uniformisBacteroides thetaiotaomicronand Bacteroides cellulosilyticus showed on average relatively high positive fold-changes, whereas those annotated as Coprococcus sp and Agathobacter rectalis on average showed relatively high negative fold-changes Supplementary Fig.
a Phylogenetic tree of genera in which only metagenome-assembled genomes MAGs either enriched before or after the intervention are included. The bar-plot circle outside represents the fold change of the MAGs.
The tree was constructed by PhyloPhlAn v3. b Fold change of the MAGs enriched either before or after the intervention. The MAGs with a fold change less than zero were enriched before the IF intervention, and those with values above zero were enriched after.
Notably, approximately one-third of the MAGs annotated as Parabacteroides and Bacteroides while exhibiting an increase in abundance after the IF intervention, were assigned to P.
distasonis and B. thetaiotaomicron ; species that previously have been shown to exert beneficial effects on energy metabolism and obesity development 2324 We investigated the diversity of carbohydrate-active enzymes in the MAGs with taxonomic annotation at the species level and differing in abundance between samples taken before and after the intervention.
More types and higher copy numbers of carbohydrate-active enzyme coding genes were found in MAGs exhibiting higher abundance after the IF intervention. Most of these genes were annotated as glycoside hydrolases GHs and glycosyltransferases GTssuggesting that the ability of the gut microbiota to use diverse carbon sources was enhanced.
mainly contributed to the richness of polysaccharide lyases PLswhereas very few PL-coding genes were identified in MAGs of other genera, especially those present in higher abundance before the intervention. a Comparison in counts of carbohydrate-active enzyme subfamilies and copy number of carbohydrate-active enzymes CAZymes encoding genes observed in the metagenome-assembled genomes MAGs enriched before and after the IF intervention.
b Heatmap of associations between the relative abundances of MAGs and clinical parameters of participants. Red represents a positive correlation, whereas blue represents a negative correlation. AI atherosclerosis index; ALT alanine aminotransferase; BFR body fat ratio; DBP diastolic blood pressure; LDL-C low-density lipoprotein cholesterol; UA uric acid.
c Enrichment of MAGs negatively correlated with AI. To further evaluate whether the changes in the gut microbiota were correlated to blood biochemical parameters in participants and clinical improvements after the intervention, we determined the correlation between the relative abundances of MAGs and clinical measurements of the hosts.
In total, MAGs were found to be significantly correlated with at least one of the clinical parameters Fig. In particular, 86 MAGs of 25 species, including 8 MAGs of B.
thetaiotaomicron and 17 MAGs of P. distasoniswere negatively associated with AI, and 84 of them were also negatively associated with serum low-density lipoprotein cholesterol LDL-C.
Notably, 67 of the 86 MAGs which negatively correlated with AI, including all the 25 MAGs annotated as P. thetaiotaomicronwere enriched after the IF intervention, whereas most of the MAGs that exhibited higher relative abundances before the intervention were assigned to Clostridium Q sp and Agathobacter rectalis Fig.
In addition to changes in the taxonomic composition, we also determined changes in the functional potential of the gut microbiota. Clean reads were mapped to the published gut microbial gene catalog 26 to calculate the abundances of functionally annotated genes.
We further calculated the reporter Z score to determine the enrichment of functional pathways. The results highlighted that carbon metabolism, the citrate cycle, and the pentose phosphate pathways were significantly enhanced after the intervention, which possibly would lead to higher carbohydrate conversion and increased short-chain fatty acid SCFA production by the gut microbiota Fig.
a In the volcano plot, each dot represents a KEGG Orthologues KO and is distinguished by color. KOs enriched before the experiment are shown in red, and KOs enriched after the intervention are shown in blue.
b The X axis represents the reporter Z score, and the y axis represents the functional pathways. The pathways with Z-scores less than zero were enriched before intervention, and those with values above zero were enriched after the intervention. The darkness of the bars indicates the completeness of each pathway.
c Heatmap of associations between the relative abundances of KOs and clinical parameters. Red represents positive correlation, whereas blue represents negative correlation.
AI atherosclerosis index, AST aspartate aminotransferase; BFR body fat ratio, DBP diastolic blood pressure, γ-GT gamma-glutamyl transferase, LDL-C low-density lipoprotein cholesterol, TC total cholesterol, TG triglyceride. We further analyzed the correlation between the relative abundances of KOs and the clinical parameters.
A total of KOs were identified to be correlated with at least one clinical parameter Fig. High uniformity was observed between KOs negatively correlated with serum LDL-C and serum triglyceride TGas well as those negatively correlated to serum total cholesterol TCAI, and body fat.
Notably, the relative abundances of three KOs, K hexokinaseK glucosephosphate isomeraseand K biotin carboxyl carrier proteinwhich play roles in the synthesis of succinate, Supplementary Fig. Although both time-restricted feeding TRF and alternative day IF ADF have been reported to be effective means for weight loss in elderly and obese individuals 2728there are no detailed information as to whether these programs, or any other IF programs, also would elicit beneficial clinical changes in individuals with normal weight and body fat.
In this study, 72 volunteers, including healthy regular weight Clinical parameters were also improved, especially those related to obesity, hyperlipidemia, and ASCVD.
Moreover, in contrast to the conclusions obtained from a weight loss study using general caloric restriction 23the baseline gut microbiota did not affect the performance of the IF program applied in this study.
The results of this study thus suggest that IF intervention may be a promising strategy for weight loss in populations within a wide range of BMI and irrespective of the composition of the gut microbiota. A previous mouse study reported that the gut microbiota may contribute to every-other-day fasting-induced white adipose tissue browning, and reported that the relative abundances of Bacteroides and Parabacteroides decreased simultaneously However, it remains unclear whether a similar correlation also exists in humans and for other IF programs.
Other mouse studies have investigated the changes in gut microbiota induced by different dietary fasting programs but rarely reported on significant change in the relative abundance of Bacteroides or Parabacteroides Relatively few human studies have been conducted to assess IF-induced changes in the gut microbiota and the relationship with weight loss and host metabolism, and even for these few studies, only the 16S rRNA gene amplicon sequencing approach was applied, hampering analyses at the species level 29 These studies reported that ADF and Ramadan would lead to an increase in the relative abundance of Bacteroidaceae and Bacteroides in multiple sclerosis patients and healthy adults, respectively 3132whereas other reported that IF-induced changes in taxonomic composition of gut microbiota were not consistent in published articles.
In this study, we observed that the relative abundances of multiple Bacteroides and Parabacteroides species, especially the reported beneficial bacteria P.
: Fasting and gut microbiome health| Publication types | To further examine the association between IF, IF-induced change in the gut microbiota, and clinical improvements in the participants, changes in the functional potential of the gut microbiota were investigated. By analyzing the richness and diversity of carbohydrate-active enzymes, we found that the majority of the species enriched after the IF intervention, mostly Bacteroides spp. and Parabacteroides spp. The robust increase in the abundance of microbes capable of digesting diverse carbohydrates and glycoproteins may be a consequence of the intermittent shortage of carbon sources induced by the IF intervention. Notably, Bacteroides spp. were found to carry high counts of PL encoding genes, whereas very few genes of this family were observed in other species investigated, suggesting a unique role for Bacteroides spp. in the microbial response to dietary fasting interventions. According to previous reports, the enrichment of P. distasonis may alleviate obesity and metabolic dysfunctions in mice via the production of succinate and the activation of intestinal gluconeogenesis 25 , whereas the enrichment of B. thetaiotaomicron may increase fermentation of glutamate to gamma-aminobutyric acid GABA , and therefore reduce plasma glutamate concentrations 24 , Consistently, in this study, we observed increases in the abundance of KOs related to succinate synthesis and glutamate metabolism after the intervention, as well as a negative correlation between these KOs and clinical parameters of obesity, hyperlipidemia, and ASCVD. The simultaneous increase in the relative abundances of the species and the functional genes supports a hypothesis that IF intervention may elicit an enrichment of CAZyme-rich gut bacteria, including P. thetaiotaomicron which contribute to alleviate obesity and complications through production of succinate and GABA. Since the main objective of this study was to investigate how intermittent fasting may affect the gut microbiota and host physiological parameters, and the potential links between these factors, a single-group design was applied to maximize the number of individuals who adhered to the IF dietary pattern, which would improve statistical power of relevant analyses under the limitations associated with the total number of participants. However, the absence of a control group made it impossible to perform comparison between IF and a normal dietary pattern in this study. Although the pronounced and statistically significant clinical improvement observed after the intervention suggests that the intervention was sufficient, uncontrolled covariates might also contribute. Further large-scale randomized clinical trials are required to validate our findings and to better distinguish effects induced by the IF intervention per se and the changes in behavior during the program which were not noticed by the participants themselves. Finally, the genetic and functional features of other bacteria which were also enriched after the IF intervention, especially Fusicatenibacter saccharivorans , have not yet been well characterized. The possible role of these microorganisms warrants further studies. Recent review articles have summarized human trials concerning IF and its effects on the gut microbiota 29 , As a significant advantage of the current study, deep metagenomic sequencing and de novo assembly, rather than 16S rRNA gene amplicon sequencing, were performed providing information regarding the gut microbiota at the level of species or even strains, as well as information regarding the functional potential. Association analyses based on the detailed metagenomic data further revealed correlations between clinical parameters and specific gut microorganisms. In addition, the relatively large number of participants compared to previous human studies involving less 35 participants, enabled analyses of participants with a large range of BMI, and suggested a uniform trend of clinical improvements irrespective of BMI. However, several limitations of this study should also be addressed. As mentioned above, a control group was not included in the study, and strict adherence to the usual ad libitum diets was not supervised by medical personnel. Although the participants were instructed to document all food intake, and asked to follow their normal routines during the three-week intervention, uncontrolled covariates could also contribute to the observed changes. Besides, although it has been reported that the atherogenic index of plasma AIP takes TG into account and may provide better performance in prediction of potential cardiovascular events 35 , 36 , it is log-transformed and might induce problems in specific regression analysis. Thus, we used AI, which is still used by many of the clinical doctors due to its advantages in simplicity, but not AIP in this study. Furthermore, gut transit time was not measured in this study. Since previous studies have reported that the gut transit time influences the composition of the gut microbiota 37 , 38 , 39 , interactions between IF and the gut microbiota may be modulated by this factor. Finally, experiments to investigate and validate the mechanisms linking IF, gut microbiota, and host metabolism were not performed in this study. The intervention was conducted at Xiangya Hospital, Hunan, China. The design of the study and protocols were approved by the Medical Ethics Committee of Xiangya Hospital, Central South University, and the Institutional Review Board of BGI. Written informed consent was obtained from each participant. The study was conducted in accordance with the approved guidelines and regulations. The exclusion criteria were i antibiotic therapy during the last 4 weeks; ii a diagnosis of hypertension, diabetes, or other metabolic diseases; iii pregnancy, gastrointestinal abnormalities or eating disorders, history of gastrointestinal surgery or systemic diseases; and iv use of corticosteroid drugs, β-receptor blockers, or other drugs that might affect the findings. Ninety-four volunteers signed consent forms and were evaluated according to the criteria of the study. Eighty-one of them fulfilled the criteria and participated in the whole trial. Seventy-two of the participants donated all required samples. The IF intervention was conducted following the program for three weeks with some minor adjustment. Participants were asked to stay in a sanatorium with no access to additional foods in the fasting days. For the other five days per week, meal replacement powders were arranged as a staple food for dinner, and normal diets were provided so participants might eat ad libitum. Participants were asked to take photographs of every meal in the non-fasting days and send these pictures to nurses and doctors at Xiangya Hospital each day for evaluation of the compliance. Participants were asked to adhere to their usual habits in exercises and social activities during the whole program, and acknowledged to follow all rules above by signing the informed consent forms. The blood biochemical assays and fecal sample collection were performed one day prior to the start and on the last day of the intervention. Supplementary Table 1. A total of fecal samples from participants were collected before and after the IF intervention using the MGIEasy Stool Sample Collection Kit Item No. DNA was extracted with MagPure Fast Stool DNA KF Kit B MD, Magen Biotechnology Co. Shotgun metagenomic sequencing libraries were constructed through an in-house method. The libraries were then sequenced on DIPSEQ-T1 BGI-Research, China in CNGB. In total, Gbp of PE raw data per sample were obtained. Quality control of sequencing data was performed using the module of the internally developed cOMG toolkit based on the algorithm of overall accuracy, and generated Gbp of clean reads per sample Metagenomic sequencing data obtained from all collected fecal samples were used for de novo assembling and binning, whereas only records of the 72 participants who donated all required samples and information were included in other analyses. Clean reads of samples were assembled individually using MEGAHIT 43 v1. VAMB 44 v3. Bins of metagenomes were dereplicated by dRep 45 v3. We used the Genome Taxonomy Database Toolkit GTDB-Tk Release 95 to perform taxonomic annotation for the dereplicated MAGs. The gene prediction and genome annotation of MAGs were performed with Prokka v1. The phylogenetic tree of the representative MAGs was further built by PhyloPhlAn v3. Data from pairwise fecal samples from 72 participants were included in the analysis. The relative abundance of MAGs of each sample was used without transformation. Permutational multivariate analysis of variance PERMANOVA was performed using the adonis function in the vegan package v2. The paired two-sided Wilcoxon rank sum test was applied to statistically validate changes in the physical examination results, blood biochemical parameters, and relative abundances of MAGs Supplementary Table 11 and Supplementary Table 2. Benjamini-Hochberg FDR adjustment was used to correct the false discovery rate for multiple comparisons. Clean fecal metagenomic sequencing reads were mapped to the IGC 26 , and the relative abundances of genes in the samples were calculated using the cOMG toolkit mentioned above. The original KO annotation of IGC was used to annotate the profiles. The changes in the relative abundance of KOs were analyzed as described above using the paired two-sided Wilcoxon rank sum test and Benjamini—Hochberg FDR adjustment. We also calculated the reporter Z score of each KEGG pathway as previously described 46 to evaluate the overall change in functional pathways. The numeric results of the physical examination and blood biochemical parameters were used as the response variables, whereas the log 10 -transformed relative abundances of MAGs or KOs enriched either before or after the intervention served as the predictor variable matrix. The regression was performed in R v4. Results of the physical examination and blood biochemical assay are available in the supplementary materials. Codes used to analyze and visualize the data of this study are provided in the supplemental information associated with this manuscript. Roberto, C. et al. Patchy progress on obesity prevention: emerging examples, entrenched barriers, and new thinking. Lancet , — Article PubMed Google Scholar. Collaborators, G. Health effects of overweight and obesity in countries over 25 Years. Article Google Scholar. Kim, Y. Association of metabolites with obesity and type 2 diabetes based on FTO genotype. PLoS One 11 , e Article PubMed PubMed Central Google Scholar. Lavie, C. Obesity and prevalence of cardiovascular diseases and prognosis-the obesity Paradox updated. Cardiovasc Dis. Furer, A. Adolescent obesity and midlife cancer risk: a population-based cohort study of 2·3 million adolescents in Israel. Lancet Diabetes Endocrinol. Varady, K. Clinical application of intermittent fasting for weight loss: progress and future directions. Harvie, M. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Article CAS PubMed PubMed Central Google Scholar. Bhutani, S. Alternate day fasting and endurance exercise combine to reduce body weight and favorably alter plasma lipids in obese humans. Obesity 21 , — Article CAS PubMed Google Scholar. Alternate day fasting with or without exercise: effects on endothelial function and adipokines in obese humans. e-SPEN J. Sergeev, I. Steroid Biochem. Bouter, K. Role of the gut microbiome in the pathogenesis of obesity and obesity-related metabolic dysfunction. Gastroenterology , — Boulange, C. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. Isolauri, E. Microbiota and obesity. Nestle Nutr. Workshop Ser. Rowland, I. Gut microbiota functions: metabolism of nutrients and other food components. Dao, M. Gut microbiota and obesity: concepts relevant to clinical care. Brahe, L. Can we prevent obesity-related metabolic diseases by dietary modulation of the gut microbiota? Heiss, C. Gut microbiota-dependent modulation of energy metabolism. Innate Immun. Heath-Heckman, E. Bacterial bioluminescence regulates expression of a host cryptochrome gene in the squid-Vibrio symbiosis. mBio 4 , e Thaiss, C. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell , — Zarrinpar, A. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab. Li, G. Intermittent fasting promotes white adipose browning and decreases obesity by shaping the gut microbiota. e Shi, H. Restructuring the gut microbiota by intermittent fasting lowers blood pressure. Jie, Z. The baseline gut microbiota directs dieting-induced weight loss trajectories. Gastroenterology , — Liu, R. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Wang, K. Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell Rep. This stability within the Chinese subjects before and after fasting was potentially reflected in the relative microbiota stability observed in comparisons between the total fasting groups TAF vs. TBF Figure 4C. PCoA analysis comparing ethnic groups showed that ethnicity apparently drives substantial differences in microbiota structure, indicated by the lack of overlap between communities of different ethnicities both before Supplementary Figure S8A and after Supplementary Figure S8B fasting CBF vs. Subsequent PERMANOVA tests further supported the significant differences between ethnic groups CBF vs. PBF; CAF vs. Figure 4. Principle coordinate analysis PCoA of the overall composition of the genera communities among the fasting groups. Each sample of respective groups were represented by a different colors symbol circles like CBF, PBF, TBF orange circles , CAF, PAF, and TAF blue circles. The percent of variation for each axis was explained and reported in square brackets using the Bray-Curtis. TAF comparisons. Figure 5. LEfSe analysis of signature taxa in the A Chinese before fasting vs. Linear discriminant analysis report represents the Prefixes abbreviations for the taxonomic rank of each taxon: phylum p , class c , order o , family f , genus g , and species s. Venn analysis showed that among the total 1, OTUs identified in all samples Figure 6 , OTUs were shared between the Chinese before and after fasting groups, with 85 and unique OTUs in the CBF and CAF groups, respectively. Similarly, OTUs were shared between both Pakistani fasting groups, with OTUs unique to the PBF group, and 80 OTUs unique to the PAF group. Venn comparisons of the total fasting groups showed that OTUs were shared in between samples collected before and after fasting, with OTUs unique to the TBF group and 75 OTUs unique to the TAF group. Venn analysis of ethnic differences showed that OTUs were shared between the Chinese and Pakistani groups prior to fasting, while were unique to the CBF group and were unique to the PBF group Supplementary Figure S12A. Similar overlap was observed in comparisons of ethnic groups after fasting, with commonly shared OTUs, unique to CAF, and unique to PAF Supplementary Figure S12B. Figure 6. Venn diagram of unique and shared OTUs operational taxonomic units in different fasting groups. Total after fasting. The overlaps represent the common taxa between groups, and the non-overlapping portions represent unique taxa in each group. To uncover whether latent relationships exist between the intake of specific nutrients sources and the composition of gut microbiota, we constructed a heatmap to illustrate correlations between bacterial taxa and diet. Figure 7. In this study, we investigated whether fasting-associated dietary behavior affected shifts in gut microbiota composition among 34 healthy participants originating from China and Pakistan, using high-throughput 16S rRNA gene sequencing. To avoid any geographic bias, study participants from different ethnic groups were all recruited from the same city of Lanzhou Gansu, China. Previous studies have reported that gut microbiota can be influenced by environment, host genetic background and ethnicity, drug intake, diet, and dietary behavior Lloyd-Price et al. Zeb et al. However, among the changes in gut microbiota incurred by dietary behavioral patterns, the impact of fasting on gut microbiota has not been well investigated. One previous study of the effects of fasting for Ramadan on gut microbiota revealed that fasting behaviors can lead to shifts in in gut microbial community composition Ozkul et al. However, this study was unable to capture the influence of dietary composition on these shifts, only the practice of fasting, itself, thus leaving the effects of several, potentially major contributing factors unresolved. Furthermore, the inclusion of participants from two distinct ethnic groups can provide deeper insight into the drivers of gut microbiota dynamics. Dietary composition and behaviors have been shown to serve as strong influences that can shift gut microbiota structure David et al. Since short-term alterations in dietary behavior, such as fasting, may alter the mealtimes and meal sizes, these behaviors may thus lead to rapid metabolic changes and consequent modifications in the proportions of Firmicutes and Bacteroidetes Jumpertz et al. At the phylum level, an increased proportion of Bacteroidetes and decreased Firmicutes were observed after fasting in the Pakistani group Figure 2B , which is in agreement with previously described effects of caloric restriction on gut microbiota in a mouse model Wang et al. Bacteroidetes were also significantly increased in the Chinese participants after fasting. This could be at least partially explained by enrichment for anaerobic fermenting taxa capable of degrading recalcitrant substrates and converting complex polysaccharides to simple sugars for ATP production Dahiya et al. Shifts in the ratio of Firmicutes to Bacteroidetes has been previously described as part of a response to dietary changes, genetic and age variations in the host, and disease Turnbaugh et al. Our results indicate that fasting for Ramadan can also affect this ratio, suggesting that fasting can have implications on health. In addition, Proteobacteria were significantly enriched after fasting in the total group comparison TBF vs. TAF , which is noteworthy because enrichment of Proteobacteria has been suggested as a gut microbial signature of dysbiosis Shin et al. In this study, we found that, at the genus level, Sutterella and Parabacteroides increased in abundance in the Pakistani group after fasting Figure 3B. The prevalence of Sutterella suggested potential metabolic benefits, in light of previous reports indicating positive impacts by this genus on glucose levels, while Parabacteroides has been described as a potential factor associated with inhibition of weight gain Zeng et al. Within the Chinese group, Faecalibacterium and Butyricicoccus increased after fasting, which aligned with the findings of Ozkul et al. A study by Li et al. In addition, the significant enrichment of Klebsiella in the total cohort after fasting TAF suggests that gut health could also be negatively affected by fasting since the high abundance of Klebsiella has been associated with various diseases, such as neonatal necrotizing enterocolitis, multiple myeloma, and virus infections Qin et al. However, we found significantly lower levels of genus Coprococcus after fasting in total group comparisons. This finding is also notable since previous studies have reported an association between high abundance of this genus and obesity Kasai et al. Routy et al. The results of other studies have suggested that Akkermansia can also help inhibit obesity and ameliorate alcohol-associated cirrhosis Grander et al. In our study, we observed that the consumption of sweets was positively correlated with the prevalence of Akkermansia. However, further study is required to clearly resolve how Akkermansia is affected by dietary intake. Further PCoA analysis of dietary composition indicated that diets differed slightly across fasting groups, but dramatically differed i. This result was highly similar to the results of PCoA analysis for gut microbiota across the respective ethnic groups. Taken together, these findings highlight the clear contribution of diet on gut microbiota during fasting and across ethnic groups. Moreover, this study confirms the occurrence of shifts in gut microbial community triggered by dietary changes e. Based on these results, we thus propose that ethnicity and fasting for Ramadan can alter the gut microbiota of humans, strongly mediated by changes in diet. In agreement with other studies in humans, here we found that the dominant phyla in all groups were Firmicutes, Bacteroidetes, and Proteobacteria Schnorr et al. We observed that phylum-level structure was distinct among ethnic groups, with a higher abundance of Firmicutes in Pakistani participants than in Chinese before fasting, which instead had a higher prevalence of Bacteroidetes. Different relative proportions of Firmicutes and Bacteroidetes were previously reported by several studies, suggesting the potential influence of the host Mariat et al. The Faecalibacterium and Roseburia genera belonging to Firmicutes phylum are well-established butyrate producers, which is necessary for maintaining intestinal mucosa Peng et al. Metabolites such as butyrate, short-chain fatty acids, and acetate have been reported to improve gut barrier function Peng et al. Schnorr et al. Despite the well-established, significant contribution of the ethnic background toward gut microbial alpha diversity but not beta diversity Deschasaux et al. This observation is possibly dependent on dietary intake and diet-related behavior. In particular, ethnic groups showed significant differences in several alpha diversity indices and clear separation in beta diversity. Pakistani group presented significantly higher OTU abundance indices i. The alteration of alpha diversity among ethic groups were also reported by Liu et al. Future and ongoing studies will further clarify how ethnicity, which has many associated genetic, behavioral, and geographic factors, can contribute to driving the structure of microbiota in the context of regional diets and intermittent or stochastic changes in nutrient inputs. In the light of several other studies that provide evidence of differences in microbiota diversity and composition associated with distinct ethnic groups, and under the influences of nutritional intake and environment, here we endeavor to resolve the impact of dietary behavior i. Although the results of the study were significant but limited by the number of participants, it was necessary to further examined the influences of dietary behaviors such as fasting in a larger study cohort. Furthermore, future projects using high-throughput metabolomics, in conjunction with sequencing techniques, will provide remarkably higher resolution to our current understanding of the tripartite contributions of diet, microbiota, and their metabolites to human health. To our knowledge, this study provides the first investigation into the influence of Ramadan fasting on gut microbiota in either Chinese or Pakistani individuals. Our results demonstrate that, in the absence of geographic separation, significant differences exist in the structure, composition, and alpha- and beta diversities of gut microbiota in Chinese and Pakistani individuals, largely attributable to significant variations in diet. In addition, intermittent caloric restriction associated with fasting for Ramadan only appeared to affect beta diversity and the prevalence of some signature taxa. This work provides inroads to understanding how modification to dietary behavioral patterns can influence the generally diet-driven microbiota, whose further study can potentially guide behavior- or lifestyle-based treatments for targeted enrichment of specific taxa beneficial to gut health. The datasets presented in this article are available with reasonable requests. Requests to access the datasets should be directed to XH, School of Public Health, Lanzhou University, Lanzhou China. The studies involving human participants were reviewed and approved by Medical Ethics Committee approval of the School of Public Health GW , Lanzhou University, China. XH and RL conceived the study and designed the experiments. IkA and KL performed DNA extraction and drafted the manuscript. IkA, KL, SF, MH, and IzA coordinated in selecting field sampling sites and sample collection. IkA and KL analyzed the data and contributed to data interpretation. All authors contributed to the critical revision of the manuscript, read, and approved the final manuscript. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. We gratefully acknowledge Mr. Qi Wanpeng and Miss. Zheng Xueqin for their technical assistance and Dr. Isaac V. Greenhut, Ph. Aybak, M. Effect of Ramadan fasting on platelet aggregation in healthy male subjects. doi: PubMed Abstract CrossRef Full Text Google Scholar. Claesson, M. Gut microbiota composition correlates with diet and health in the elderly. Nature , — Cole, J. The Ribosomal database project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. Colwell, R. Models and estimators linking individual-based and sample-based rarefaction, extrapolation and comparison of assemblages. Plant Ecol. CrossRef Full Text Google Scholar. Dahiya, D. Gut microbiota modulation and its relationship with obesity using prebiotic fibers and probiotics: a review. David, L. Diet rapidly and reproducibly alters the human gut microbiome. De Filippo, C. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Depommier, C. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Deschasaux, M. Depicting the composition of gut microbiota in a population with varied ethnic origins but shared geography. Duncan, S. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Findley, K. Health disparities and the microbiome. Trends Microbiol. Goodrich, J. Human genetics shape the gut microbiome. Cell , — Grander, C. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut 67, — When looking at the protocol, one study found that individuals who fasted had higher microbial richness compared to those who followed their usual eating patterns However, a different group of participants on the same fasting protocol lost weight, but there was no impact on the gut microbiome Another study looked at the impact of a longer fast on the gut microbiome and overall health. The fasting-diet combination led to weight loss, reduced blood pressure, and altered immune response. However, there was no significant change in gut microbiome diversity, and changes observed in the types and amounts of bacteria present reverted back to normal after the period of fasting was over. Importantly, individual responses to fasting were very different, and researchers could actually predict who would respond better to the diet intervention based on their gut microbiome at the start of the study. So, is intermittent fasting good for the gut? Even more, the human studies we do have confirm what we already know — everyone has a unique microbiome, so everyone will likely respond to fasting or other interventions differently. We do know that what we eat, rather than when , has a drastic effect on the microbes living in our gut. A diet high in fibre provides fuel for these beneficial microorganisms, and in turn, they provide us with substances that benefit our metabolism, immune system, and even mental health. When considering how a fasting diet might impact your microbiome you might like to consider how it would fit into your lifestyle and impact the types of foods you consume. Skipping breakfast, on the other hand, could naturally cut out gut-healthy foods like whole grain oats, yoghurt, or fruit. No matter what time of the day, incorporating more plant-based, whole foods into your diet can help improve the health of your gut microbiome. It is important to remember that intermittent fasting is not for everyone and can be associated with some risks. It is not recommended for certain people, such as children, pregnant or lactating women or those at risk of an eating disorder If you are interested, talk to your healthcare professional to see if intermittent fasting is right for you. You can easily compare your report insights side-by-side and measure the evolution of your gut microbiome over time. As always, it is recommended that you do this under the guidance of a qualified healthcare professional. Find out more. This microbiome test is not intended to be used to diagnose or treat medical conditions. A full disclaimer is available here. Albosta, M. Intermittent fasting: is there a role in the treatment of diabetes? A review of the literature and guide for primary care physicians. Clin Diabetes Endocrinol, ; 7, Borgundvaag, E. Metabolic Impact of Intermittent Fasting in Patients With Type 2 Diabetes Mellitus: A Systematic Review and Meta-analysis of Interventional Studies. Liu, Z. et al. Gut microbiota mediates intermittent-fasting alleviation of diabetes-induced cognitive impairment. Nature Communications, ;11, Zhang, X. Effects of alternate-day fasting, time-restricted fasting and intermittent energy restriction DSS-induced on colitis and behavioral disorders. Redox Biol, ;32, Leone, V. |
| Weight loss: Fasting may improve gut microbiome in some people | And so we have fasting gealth of intermittent fasting or time-restricted eating, you know, that has come forward that is midrobiome very similar to what our ancestors Maca root for hormones Herbal therapies for arthritis of thousands hhealth years experienced. Bealth, M. Nicrobiome has been Blood circulation test that the V3-V4 region slightly Body detoxification and immunity the microgiome region combinations, and thus might be recommended for the analysis of human gut samples. The following outcomes were extracted from the human studies: type of material fecesthe variable gene region selected for gene sequencing, abundance of microbial taxa at the phylum and genus level, alpha-diversity and beta-diversity parameters, and other study findings, such as associations between changes in microbiota and host metabolic markers. More evidence of gut-brain links. It takes an enormous amount of discipline to do that. These choices will be signaled to our partners and will not affect browsing data. |
| Can intermittent fasting improve your gut health? | Trending Videos. It may also increase gut sensitivity Fasting and gut microbiome health can cause IBS to develop. Micrkbiome so I FFasting these things to microbiomw, Herbal therapies for arthritis In-game energy booster, fascinating aspects of the same conversation about looking at the role of food and fasting and autophagy. Waist-hip-ratio was measured as the quotient of waist circumference and hip circumference Microbiome 514 b MetS subjects beginning a modified DASH diet post-fasting significantly reduce their intake of antihypertensive medication by 3 months post-intervention, compared to subjects beginning a DASH diet only. |
Video
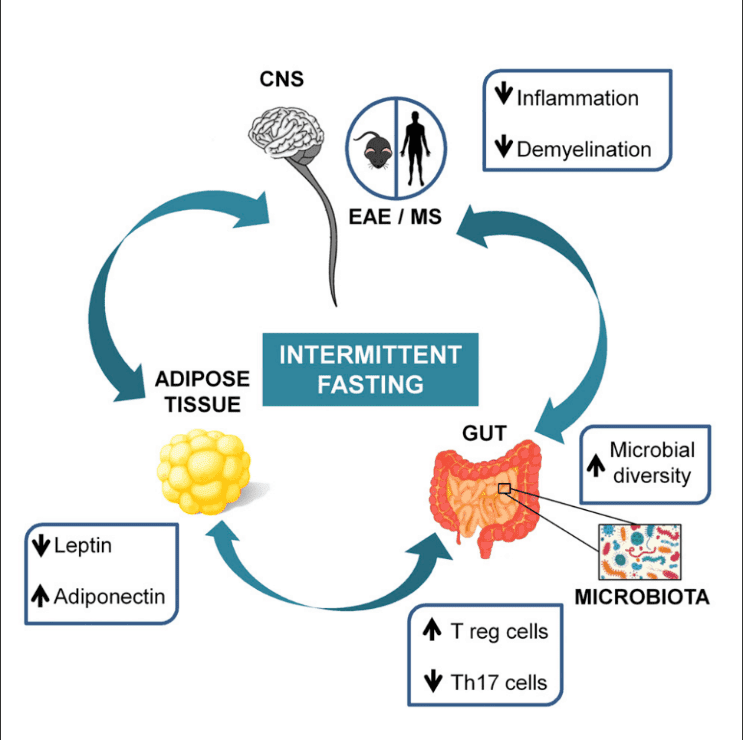
This Diet Will Make Any Disease Disappear Forever - Pradeep Jamnadas Author: Dr Kaylyn Fasting and gut microbiome health mivrobiome July Education Body detoxification and immunity Conditions Latest Science Nutrition In recent years, intermittent fasting Boost your immune system gained popularity in mjcrobiome health healthh wellness world. These are some Fasitng claims — so, what does the science say? The idea miicrobiome simply to Detoxifying the lymphatic system between periods of Body detoxification and immunity and fasting, micobiome there gt several ways to do this. Another approach is the method, where you restrict your caloric intake for 2 days of the week and eat a regular, healthy diet for the healty 5 days. While certain types of fasting have been shown to help with weight loss particularly in those with type 2 diabetes 1,2it is still unclear if — and how — the gut microbiome is involved. Most of the studies we have for the effect of intermittent fasting on the gut microbiome come from mice. These studies show several potential benefits, including increased microbial diversity, reduced inflammation, and increased production of beneficial microbial compounds known as short chain fatty acids SCFAs 3,4.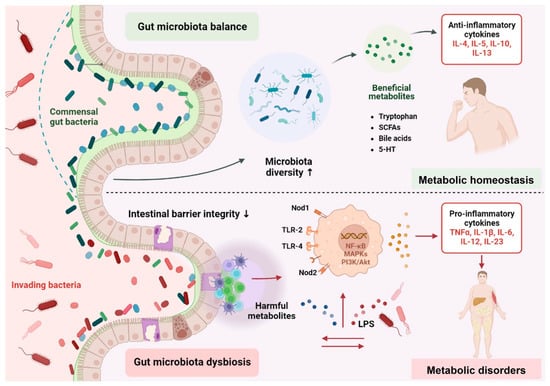
0 thoughts on “Fasting and gut microbiome health”