
Electrolyte balance and fluid balance -
Metabolic pH Imbalance. Acid-base imbalance due to inadequacy of a physiological buffer system is compensated for by the other system. Main Page. Associate Degree Nursing Physiology Review. Fluid Shifts If ECF becomes hypertonic relative to ICF, water moves from ICF to ECF If ECF becomes hypotonic relative to ICF, water moves from ECF into cells.
Regulation of Water Output Obligatory water losses include: Insensible water losses from lungs and skin Water that accompanies undigested food residues in feces Obligatory water loss reflects the fact that: Kidneys excrete mOsm of solutes to maintain blood homeostasis Urine solutes must be flushed out of the body in water Primary Regulatory Hormones 1.
Antidiuretic hormone ADH also called vasopressin Is a hormone made by the hypothalamus, and stored and released in the posterior pituitary gland Primary function of ADH is to decrease the amount of water lost at the kidneys conserve water , which reduces the concentration of electrolytes ADH also causes the constriction of peripheral blood vessels, which helps to increase blood pressure ADH is released in response to such stimuli as a rise in the concentration of electrolytes in the blood or a fall in blood volume or pressure.
These stimuli occur when a person sweats excessively or is dehydrated. Sweating or dehydration increases the blood osmotic pressure.
The increase in osmotic pressure is detected by osmoreceptors within the hypothalamus that constantly monitor the osmolarity "saltiness" of the blood 3. ADH travels through the bloodstream to its target organs : a. Sodium balance. The thyroid gland releases calcitonin CT.
CT binds to receptors on osteoblasts bone-forming cells. This triggers the osteoblasts to deposit calcium salts into bone throughout the skeletal system. This causes the blood calcium levels to fall. CT stops being produced when blood calcium levels return to normal.
When blood calcium levels fall, the parathyroid glands located on posterior surface of the thyroid gland release PTH. PTH binds to receptors on osteoclasts bone-degrading cells within the skeletal system The osteoclasts decompose bone and release calcium into the blood.
The blood calcium level rises PTH stops being produced when blood calcium levels return to normal. Normal pH of body fluids: Arterial blood is 7. Challenges to acid-base balance due to cellular metabolism: produces acids — hydrogen ion donors Acidosis physiological acidosis is a blood pH below 7.
ADH secretion is influenced by several factors note that anything that stimulates ADH secretion also stimulates thirst :. By special receptors in the hypothalamus that are sensitive to increasing plasma osmolarity when the plasma gets too concentrated.
These stimulate ADH secretion. By stretch receptors in the atria of the heart, which are activated by a larger than normal volume of blood returning to the heart from the veins. These inhibit ADH secretion, because the body wants to rid itself of the excess fluid volume.
By stretch receptors in the aorta and carotid arteries, which are stimulated when blood pressure falls. These stimulate ADH secretion, because the body wants to maintain enough volume to generate the blood pressure necessary to deliver blood to the tissues.
In addition to regulating total volume, the osmolarity the amount of solute per unit volume of bodily fluids is also tightly regulated. Extreme variation in osmolarity causes cells to shrink or swell, damaging or destroying cellular structure and disrupting normal cellular function. Regulation of osmolarity is achieved by balancing the intake and excretion of sodium with that of water.
Sodium is by far the major solute in extracellular fluids, so it effectively determines the osmolarity of extracellular fluids. An important concept is that regulation of osmolarity must be integrated with regulation of volume, because changes in water volume alone have diluting or concentrating effects on the bodily fluids.
For example, when you become dehydrated you lose proportionately more water than solute sodium , so the osmolarity of your bodily fluids increases.
In this situation the body must conserve water but not sodium, thus stemming the rise in osmolarity. The extracellular water compartment is subdivided into the spaces between cells also known as interstitial, blood plasma, and other bodily fluids such as the cerebrospinal fluid which surrounds and protects the brain and spinal cord Figure 3.
The composition of solutes differs between the fluid compartments. For instance, more protein is inside cells than outside and more chloride anions exist outside of cells than inside.
One of the essential homeostatic functions of the body is to maintain fluid balance and the differences in solute composition between cells and their surrounding environment. Osmoregulation is the control of fluid balance and composition in the body.
The processes involved keep fluids from becoming too dilute or too concentrated. Fluid compartments are separated by selectively permeable membranes, which allow some things, such as water, to move through while other substances require special transport proteins, channels, and often energy. The movement of water between fluid compartments happens by osmosis, which is simply the movement of water through a selectively permeable membrane from an area where it is highly concentrated to an area where it is not so concentrated.
Water is never transported actively; that is, it never takes energy for water to move between compartments. Although cells do not directly control water movement, they do control movement of electrolytes and other solutes and thus indirectly regulate water movement by controlling where there will be regions of high and low concentrations.
Cells maintain their water volume at a constant level, but the composition of solutes in a cell is in a continuous state of flux. This is because cells are bringing nutrients in, metabolizing them, and disposing of waste products. To maintain water balance a cell controls the movement of electrolytes to keep the total number of dissolved particles, called osmolality the same inside and outside Figure 3.
The total number of dissolved substances is the same inside and outside a cell, but the composition of the fluids differs between compartments. For example, sodium exists in extracellular fluid at fourteen times the concentration as compared to that inside a cell.
Body Galance Compartments Composition of Body Fluids Electrolyte balance and fluid balance Composition of Body Fluids Multivitamin for sleep support and Intracellular Fluids Fluid Movement Balancd Compartments Fluid Shifts Regulation Electrolyge Fluids And Electrolytes Water Balanxe and ECF Osmolality Water Output Regulation galance Water Intake Regulation of Water Output Primary Regulatory Hormones Disorders of Water Balance Electrolyte Balance Sodium in Fluid and Electrolyte Balance Sodium balance Regulation of Sodium Balance: Aldosterone Atrial Natriuretic Hormone ANH Potassium Balance Regulation of Potassium Balance Regulation of Calcium Regulation of Anions Acid-Base Balance Sources of Hydrogen Ions Hydrogen Ion Regulation Chemical Buffer Systems -- 1. Bicarbonate Buffer System - - 2. Phosphate Buffer System -- 3. Composition of Body Fluids. Electrolyte Composition of Body Fluids.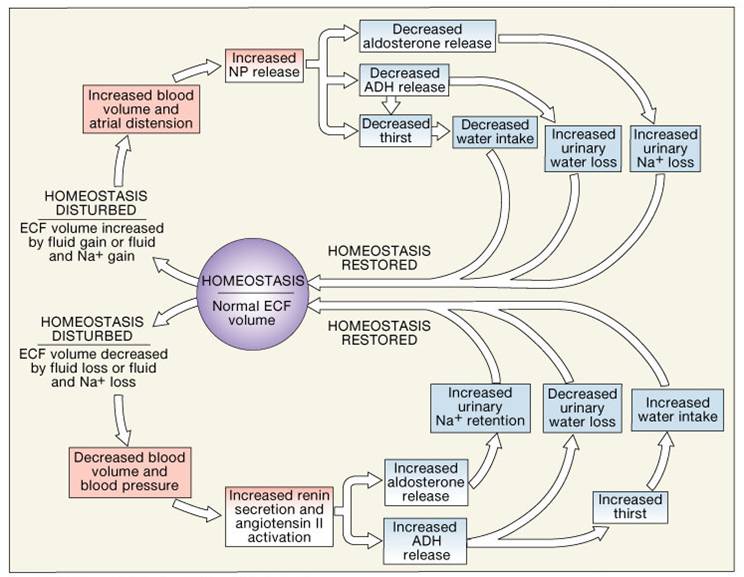
0 thoughts on “Electrolyte balance and fluid balance”