Video
Automated Insulin Delivery: The Next Generation of ResearchInsulin delivery systems -
The primary outcome was robust and consistent in all sensitivity analyses performed. This favorable effect was observed consistently when the AID was used continuously over 3 and 6 months, which was not analyzed in previous studies.
Most trials reported no serious adverse events, such as severe hypoglycemia and diabetic ketoacidosis. This study also suggested greater improvement in TIR for dual-hormone compared with single-hormone AID systems. The characteristics of different AID systems and conventional insulin treatments have been reviewed comprehensively in three reviews 9 , 30 , A dual-hormone system has been shown to be superior to a single-hormone system in improving nocturnal glucose control in children and adolescents with T1D in one three-way crossover trial However, dual-hormone systems have only been tested for 3—5 days with very close supervision diabetes camp in a small number of children.
Future, free-range studies are required in which dual-hormone and single-hormone AID systems are compared directly with each other in children and adolescents. AID systems reduced TBR only by 0. The subgroup analysis showed that the reduction was significant in dual-hormone systems and supervised settings but not in single-hormone systems and unsupervised settings.
This finding contrasts with previous meta-analyses of AID use that did not have age limitations 10 , 12 but is consistent with a recent meta-analysis focused on young people More trials of dual-hormone AID in young people are needed. Despite heterogeneity in interventions and comparators used, our systematic review provides a valid and up-to-date overview about the use of AID in children and adolescents.
Previous meta-analyses of AID in all age-groups showed favorable effects both overnight and over a h period, but the longest follow-up was 12 weeks 10 , The favorable effect was also evident in a subgroup analysis for the pediatric population 12 , which was consistent with our meta-analysis.
However, our study included more trials, performed comprehensive assessment, and had greater reliability. Furthermore, Karageorgiou et al.
However, only 5 of 19 included studies were in outpatient settings, and 4 trials were not randomized. The effect of AID systems in children and adolescents was examined in a recent systematic review and meta-analysis of 26 RCTs participants Initially, we used a correlation coefficient of 0.
A post hoc validation for the correlation coefficient was done using a method recommended by the Cochrane Collaboration 33 , which yielded a value of 0.
Therefore, the result of our meta-analysis was more conservative. Our study has several strengths. The meta-analysis followed the PRISMA guidelines and a protocol that was registered with PROSPERO.
We conducted a comprehensive search of multiple databases and included all available RCTs of AID compared with conventional insulin therapy. Risk of bias for included trials was assessed using a valid methodological tool, and quality of evidence for each outcome was evaluated using GRADE.
Subgroup analyses were performed to explain heterogeneity, and sensitivity analyses were conducted to examine the robustness of the results.
We also acknowledge some limitations. First, the sample size was small in most trials, which reduced the precision of effect estimates. Second, most included trials were considered at high risk of performance bias because of infeasibility of blinding patients and physicians to the allocation assignments.
Third, statistical assumptions were made in this study. Additionally, a correlation coefficient of 0. Fourth, heterogeneity was high in most analyses, which could be attributed to differences in study design, duration of intervention, continuous glucose monitoring systems, AID algorithms, insulin pumps, and persisting impact of human factors.
Finally, the results of this meta-analysis might not apply to some clinically relevant subgroups, such as those with increased hypoglycemia burden, hypoglycemia unawareness, and high HbA 1c.
While some studies have investigated the association between AID use and HbA 1c 34 , 35 and hypoglycemia unawareness 36 , further studies are warranted to fully clarify these relationships.
In conclusion, this systematic review and meta-analysis shows that AID systems are more effective than conventional insulin therapy for children and adolescents with T1D in outpatient settings.
AID systems increase TIR both in short-term and long-term intervention. See accompanying article, p. This work was supported by the National Natural Science Foundation of China Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. and L. interpreted the data and wrote the first draft of the manuscript. and Q. coded the statistical analysis, figures, and supplementary material.
and H. completed the literature review and extracted the data. and F. conceived and designed the study. All authors reviewed and revised subsequent drafts of the manuscript and approved the final version.
are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Sign In or Create an Account. Search Dropdown Menu. header search search input Search input auto suggest.
filter your search All Content All Journals Diabetes Care. Advanced Search. User Tools Dropdown. Sign In. Skip Nav Destination Close navigation menu Article navigation. Volume 46, Issue Previous Article Next Article.
Research Design and Methods. Article Information. Article Navigation. Meta-analysis November 27 Automated Insulin Delivery Systems in Children and Adolescents With Type 1 Diabetes: A Systematic Review and Meta-analysis of Outpatient Randomized Controlled Trials Baoqi Zeng Baoqi Zeng.
Corresponding author: Baoqi Zeng, zengbaoqi com , or Feng Sun, sunfeng bjmu. This Site. Google Scholar. Le Gao ; Le Gao. Qingqing Yang ; Qingqing Yang. Hao Jia ; Hao Jia.
Feng Sun Feng Sun. Diabetes Care ;46 12 — Article history Received:. Connected Content. A commentary has been published: Making a Good Thing Even Better: Expanding Access and Applicability of Automated Insulin Delivery Systems to Benefit All Youth With Type 1 Diabetes.
Get Permissions. toolbar search Search Dropdown Menu. toolbar search search input Search input auto suggest. Graphical Abstract View large Download slide.
View large Download slide. Figure 1. Table 1 Subgroup analyses for primary and secondary outcomes. Comparisons, n. P interaction. TIR 3.
View Large. Figure 2. Search ADS. International Diabetes Federation. Brussels, Belgium, International Diabetes Foundation, Accessed 13 March American Diabetes Association. Children and Adolescents: Standards of Medical Care in Diabetes— Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range.
The relationship of hemoglobin A1C to time-in-range in patients with diabetes. State of type 1 diabetes management and outcomes from the T1D Exchange in Current advances of artificial pancreas systems: a comprehensive review of the clinical evidence.
Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. Efficacy and safety of closed-loop insulin delivery versus sensor-augmented pump in the treatment of adults with type 1 diabetes: a systematic review and meta-analysis of randomized-controlled trials.
Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials.
Effectiveness of artificial pancreas in the non-adult population: a systematic review and network meta-analysis. The efficacy of automated insulin delivery systems in children and adolescents with type 1 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials.
Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. von Hippel. The system requires a prescription.
A confirmatory finger stick test via the CONTOUR ® NEXT LINK 2. All therapy adjustments should be based on measurements obtained using the CONTOUR ® NEXT LINK 2. Always check the pump display to ensure the glucose result shown agrees with the glucose results shown on the CONTOUR ® NEXT LINK 2.
Do not calibrate your CGM device or calculate a bolus using a blood glucose meter result taken from an Alternative Site palm or from a control solution test.
It is not recommended to calibrate your CGM device when sensor or blood glucose values are changing rapidly, e. Therefore this device should not be used in anyone under the age of 7 years old. This device should also not be used in patients who require less than a total daily insulin dose of 8 units per day because the device requires a minimum of 8 units per day to operate safely.
Pump therapy is not recommended for people whose vision or hearing does not allow recognition of pump signals and alarms. Pump therapy is not recommended for people who are unwilling or unable to maintain contact with their healthcare professional.
Both systems require a prescription. Insulin infusion pumps and associated components of insulin infusion systems are limited to sale by or on the order of a physician and should only be used under the direction of a healthcare professional familiar with the risks of insulin pump therapy.
Pump therapy is not recommended for people who are unwilling or unable to perform a minimum of four blood glucose tests per day. Insulin pumps use rapid-acting insulin. If your insulin delivery is interrupted for any reason, you must be prepared to replace the missed insulin immediately.
Insertion of a glucose sensor may cause bleeding or irritation at the insertion site. Consult a physician immediately if you experience significant pain or if you suspect that the site is infected. The information provided by CGM systems is intended to supplement, not replace, blood glucose information obtained using a blood glucose meter.
A confirmatory fingerstick using a CONTOUR®NEXT LINK 2. Always check the pump display when using a CONTOUR®NEXT LINK 2. Do not calibrate your CGM device or calculate a bolus using a result taken from an Alternative Site palm or a result from a control solution test.
Under some conditions of use the pump can suspend again, resulting in very limited insulin delivery. Prolonged suspension can increase the risk of serious hyperglycemia, ketosis, and ketoacidosis. See important safety information and the appropriate user guides for additional important details.
En Español. Insulin pump therapy. An advanced option for diabetes management. What is insulin pump therapy? How does an insulin pump work? A pump delivers insulin to the body through a thin, flexible tube called an infusion set. What components are used as part of an insulin pump system?
Several pieces work together to deliver continuous doses of insulin. Roll over the components below for more information.
An infusion set is either placed inside of or comes preloaded with an insertion device and, with a push of a button, it is inserted quickly and easily. A plastic cartridge that holds up to units of insulin and is locked into the pump.
Φ Smart devices sold separately. Find a list of compatible devices. What are the benefits of insulin pump therapy? People can experience many positive changes in their life when switching to pump therapy. Fewer injections.
When using multiple daily injections, people often take shots several times a day. More convenience. Pumps can be programmed to deliver basal insulin at different rates throughout the day Change your mealtime insulin based on the food you choose to eat.
Mealtime dosing. The bolus calculator eliminates complex math and tracks active insulin. Tracking active insulin can help avoid stacking and going low. More stable blood sugar. May help you achieve better glucose control with fewer highs and lows §.
Accurate insulin delivery. Who can wear an insulin pump? Ask your healthcare professional about insulin pump therapy if you: Take 3 or more insulin injections per day Take other medications in addition to insulin to manage your diabetes Would like better management of your diabetes.
Wearing the pump Insurance FAQs. Different ways to wear an insulin pump There are many ways a pump can be worn. Does insurance cover an insulin pump? Frequently asked questions How much does an insulin pump cost?
Your out-of-pocket cost varies depending on your insurance. We also offer financial assistance and monthly payment programs. If you would like to receive an insurance coverage check at no cost, call to speak to a Diabetes Therapy consultant.
Will an insulin pump interfere with my daily activities? Insulin pumps can be easily worn on or under your clothes very securely. The pump can also be detached for activities like swimming, showering, and exercise so you can continue to live your life. An insulin pump can be expensive.
What happens if I break it? Many insulin pumps are warranty-protected for a period of time. Medtronic insulin pumps have a standard warranty of 4 years to help ensure customers have peace of mind. Is an insulin pump inconvenient and difficult to use? Many people find that an insulin pump is a convenient way to manage diabetes.
For those who struggle using technology, product training and technical support can help ensure that wearing the insulin pump is not difficult. Is the insulin pump surgically implanted? An insulin pump is a small device worn externally that delivers customizable insulin doses.
No surgery or hospital time required. Will everyone immediately know I have diabetes? Insulin pumps are small devices, so wearing a pump can be very discreet if desired.
You can even completely hide your insulin pump by wearing it underneath your clothes. How do I sleep with an insulin pump?
Medically dystems by Body composition evaluation. Selenium browser automation some instances, there is crossover between Ginger green smoothie recipe type 1 diabetes and type 2 diabetes. For example, someone living Selenium browser automation type celivery Selenium browser automation insulin depivery may try adjustments to diet, a change in lifestyle, or non-insulin medications to try and treat type 2 diabetes before moving directly to insulin. Because type 1 diabetes insulin dependence is an autoimmune disease, it requires daily insulin therapy. Learn more about the differences between type 1 and type 2 diabetes. Someone living with type 1 diabetes has several options for their daily insulin therapy. They can administer insulin through injections, pens, inhaled insulin, or by using an insulin pump.
Insulin delivery systems -
The FDA granted clearance of the iLet ACE Pump and iLet Dosing Decision Software to Beta Bionics Inc. The FDA, an agency within the U. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices.
Skip to main content Skip to FDA Search Skip to in this section menu Skip to footer links. For Immediate Release: May 19, Inquiries Media: Jim McKinney Twenty-eight participants were randomized to two groups 14 to closed-loop therapy first and 14 to control therapy first.
Two participants withdrew before starting their first study period both randomized to control therapy first. One participant stopped the study early during the first intervention period closed-loop because of difficulty managing the devices. Therefore, data for this period were included in the analysis.
Baseline characteristics of the study population are shown in Table 1 , and baseline diabetes regimen details are shown in Supplementary Table 1. The primary endpoint, which was the proportion of time with sensor glucose in the target glucose range of 3. The difference in the proportion of time that glucose was in the target range in the closed-loop therapy period compared with the control therapy period for individual participants is shown in Extended Data Fig.
Figure 2 shows the h sensor glucose and closed-loop insulin profiles. a , Median sensor glucose measurements during closed-loop insulin delivery and control insulin therapy the patients' usual therapy. Red and gray shaded areas, IQR for each treatment. The values are reported during a h period from midnight to midnight.
Black horizontal dashed lines, lower and upper limits of the glucose target range of 3. b , Median amount of algorithm-directed insulin delivery during the closed-loop intervention.
Shaded area, IQR. Formal statistical hypothesis testing was terminated after time spent in hypoglycemia 3. Therefore, analysis of other secondary endpoints is considered exploratory. Glucose variability measured by the s. Total daily insulin dose was higher during the closed-loop therapy period median, 0.
Per-protocol analysis of the primary endpoint is similar to the intention-to-treat analysis Supplementary Table 2. Glucose metrics during daytime and nighttime, and by fortnightly periods are shown in Supplementary Tables 3 and 4.
The primary and key endpoints by treatment sequence are presented in Supplementary Table 5. There were no episodes of severe hypoglycemia during either intervention period Table 3.
Eight serious adverse events SAEs were reported. Four occurred during the closed-loop therapy period, of which one was study procedure related hospital admission with abscess at pump cannula site requiring incision and drainage.
Two SAEs were reported during the control therapy period, and both were not study related. Details of the individual SAEs are shown in Supplementary Table 6. Eleven adverse events were reported five during the closed-loop therapy period, five during the control therapy period, and one during the washout period , and six device deficiencies occurred six during the closed-loop therapy period and none during the control therapy period but did not lead to an adverse event.
Glucose sensor availability was higher during the closed-loop therapy period than during the control therapy period a median of The proportion of time that the closed-loop system was active was high during the closed-loop therapy period median, Protocol deviations are shown in Supplementary Table 7.
The majority of protocol deviations 25 out of 30 were outside of protocol visits. Hypoglycemia confidence scores and PAID problem areas in diabetes scores of diabetes distress were similar between interventions for hypoglycemia confidence score, a median of 3.
Hypoglycemia worry score was higher following closed-loop therapy than following control therapy a median of Responses to the closed-loop experience questionnaire are shown in Supplementary Table 9.
The results of this study build on evidence from a feasibility study evaluating fully closed-loop therapy in people with type 2 diabetes and end-stage renal failure on dialysis Here, we demonstrate that this technology can benefit the wider population with type 2 diabetes requiring insulin and can be safely implemented in the home setting.
As a considerable proportion of people with type 2 diabetes struggle to achieve the recommended glycemic targets with currently available therapies, including insulin therapy 14 , fully closed-loop systems offer a new approach to improve glycemic outcomes to reduce the risk of long-term complications.
We postulate that this might be due to the higher personal glucose targets applied for the more vulnerable population requiring dialysis, who have a greater risk of hypoglycemia.
The majority of participants in the present study used the default glucose target of 5. Analysis of glucose metrics in fortnightly intervals in the present study shows that closed-loop insulin delivery results in an almost immediate improvement in glycemic control compared with standard insulin therapy proportion of time in target range, In this study, very few participants used a glucose sensor as part of their usual care.
Therefore, some of the glycemic benefits observed during the closed-loop therapy period may be attributable to use of a continuous glucose monitor alone.
A major contributor to the clinical inertia in the escalation of insulin therapy among healthcare professionals, and a feared side effect of insulin among people with type 2 diabetes, is the risk of hypoglycemia 15 , We have shown that fully closed-loop insulin delivery does not increase the risk of hypoglycemia despite improved glycemic control and there were no episodes of severe hypoglycemia during closed-loop therapy.
The study cohort spent very little time in hypoglycemia during both intervention periods. The degree of comorbidity burden in our study population is reflected in the number of nonstudy-related SAEs reported during the study.
These were hospital admissions for treatment of comorbidities diabetic foot disease or infection. One severe adverse event was related to study procedures and occurred during the closed-loop therapy period an abscess at the pump cannula site requiring hospital admission for incision and drainage with no long-term sequelae.
Closed-loop therapy was continued provided that the participant was able to manage the system themselves and the treating healthcare professionals were satisfied that this was clinically appropriate.
Training and maintenance of study devices by participants was acceptable. One participant withdrew because of difficulty managing the devices, whereas all other participants were able to manage the devices independently and reported a high degree of satisfaction while using the closed-loop system.
Feedback from users highlighted the elimination of the need for injections or finger-prick testing, and increased confidence in managing blood glucose as key benefits Supplementary Table 8. There was a trend toward higher levels of hypoglycemia-related anxiety during closed-loop therapy, which may reflect increased awareness and monitoring of glucose levels associated with sensor glucose use.
Questionnaire feedback on drawbacks of the closed-loop system mainly consisted of practical annoyances with wearing of devices, connectivity issues between devices and a perceived increase in hypoglycemia episodes. Increased exposure to diabetes technologies in people with type 2 diabetes insulin pumps and glucose sensors may mitigate some of these negatives if the glycemic benefits are perceived to be worthwhile.
Future-generation closed-loop systems with improved connectivity, longer infusion set wear time and larger insulin reservoirs may also resolve these issues. The strengths of this study include its randomized crossover design and the inclusion of a wider population with type 2 diabetes self-managing the devices in an outpatient setting, increasing the scope for uptake of this technology.
The use of a fully closed-loop system obviates the need for ongoing healthcare professional input with optimization and support following initial training on the devices.
The limitations of the study are that study participants were recruited from a single center and one general practice, and the group was not ethnically diverse, with only one participant not of white ethnicity.
Although the present study demonstrated glycemic benefits over the 8-week intervention period, the results should not be generalized beyond this period.
We also did not collect data on the use of boost and ease-off functionality during the closed-loop therapy period. There were a relatively large number of protocol deviations Supplementary Table 9 ; however, the majority of these 25 out of 30 were outside of protocol visits with no effect on data analysis.
In conclusion, this study suggests that fully closed-loop insulin delivery is a safe and efficacious approach to manage type 2 diabetes in adults. Larger randomized controlled trials with diverse populations and longer follow-up are required to ensure generalizability across a wider target population and to determine whether it is a cost-effective approach that provides sustained benefits for people with type 2 diabetes requiring insulin therapy.
The study used an open-label, single-center, randomized, two-period crossover design, contrasting fully closed-loop glucose control using faster-acting insulin aspart Fiasp, Novo Nordisk closed-loop and standard multiple daily insulin injection therapy control during two 8-week periods of unrestricted living.
The intervention periods were separated by a 2-week to 4-week washout period during which participants used their pre-study treatment. Assignment of participants to the two groups with a different order of interventions was random. Ethical and regulatory approvals were obtained from the London-Stanmore Ethics Committee and the Medicines and Healthcare Products Regulatory Agency.
The study protocol is provided in the Supplementary Information. The safety aspects of the trial were overseen by an independent data and safety monitoring board.
The study is registered with ClinicalTrials. Participants had to be literate in English, willing to perform regular finger-prick blood glucose monitoring and willing to wear study devices and follow study-specific instructions.
Exclusion criteria were type 1 diabetes, pregnancy or breastfeeding, severe visual or hearing impairment, allergy to insulin or the adhesive of plasters or serious skin disease affecting device placement, lack of reliable telephone facility for contact, alcohol abuse, Illicit or prescription drug abuse, any physical or psychological disease, or use of medication s likely to interfere with the conduct of the trial or interpretation of the results.
Written informed consent was obtained from all study participants before any study-related activities. Participants received £30 for each 8-week study period completed, and all reasonable traveling expenses were reimbursed. Participants were randomized in a ratio to an 8-week period of fully closed-loop glucose control with faster-acting insulin aspart Fiasp followed by an 8-week period of standard insulin therapy, or vice versa.
Randomization was performed using a web-based, permuted blocks-of-four randomization method to assign study participants to one of the two treatment sequences. Participants and investigators were not masked to the intervention used during each period because of the nature of the interventions.
Study visits and procedures are shown in Supplementary Tables 10 and Participant demographics and medical history, body weight and height, HbA1c and total daily insulin dose were recorded at enrollment. The sex of participants was self-reported. The closed-loop app CamAPS HX, CamDiab involves the Cambridge adaptive model predictive control algorithm HX software v.
Over time, the algorithm adapts to observed glucose patterns, enabling it to tailor insulin delivery more accurately to minimize glucose excursions.
The default target glucose used by the closed-loop algorithm is 5. Before the closed-loop therapy period commenced, participants underwent a 1-h to 2-h training session with the study team on the use of the insulin pump, continuous glucose monitoring and closed-loop system.
The usual insulin therapy of participants was discontinued, but all other medications were continued as directed by their clinical team without interference from the study team. The insulin pump delivered faster-acting insulin aspart continuously as directed by the algorithm, without prandial boluses or carbohydrate announcement.
The study did not interfere with the usual activities or dietary intake of participants. Other diabetes therapies were continued throughout the closed-loop therapy period. Participants were given h access to a study helpline in the event of any study-related issues.
At the end of the closed-loop therapy period, devices were removed and the usual insulin therapy of participants was restarted.
During the 8-week control therapy period, a glucose sensor Dexcom G6 was worn by participants throughout the standard insulin therapy period.
Sensor glucose on the sensor glucose receiver was masked to the participant and investigators until the end of the study. Other diabetes therapies were continued throughout the control therapy period. Fingerstick blood glucose monitoring was performed by participants as per their usual practice.
Participants were unrestricted in their usual activities and dietary intake. Participants remained under the care of their local clinical team for glycemic management. At the end of the standard insulin therapy period, the glucose sensor was removed and the usual insulin therapy of participants was continued.
Questionnaires were completed by participants at the end of each study period. At the end of the closed-loop therapy period, participants completed a closed-loop experience questionnaire to feed back on the closed-loop system, provide suggestions for improvement and indicate whether they would recommend the system to friends or family.
The primary endpoint was the proportion of time the sensor glucose measurement was in the target glucose range of 3. Key endpoints included the proportion of time with sensor glucose above Secondary efficacy endpoints included the proportion of time with sensor glucose below 3. and the coefficient of variation of sensor glucose, and the total daily insulin dose.
Utility evaluation included percentage time of sensor glucose availability, and percentage time of closed-loop operation. Psychosocial assessments were measured using questionnaires collected at the end of each study period. Patients with Type 1 diabetes, or their caregivers, must consistently monitor their glucose levels throughout the day and inject insulin with a syringe, pen or pump to maintain adequate glucose levels in order to avoid becoming hyperglycemic high glucose levels or hypoglycemic low glucose levels.
The MiniMed G System, a bluetooth-enabled version of the previously approved MiniMed G System with other modifications , is a hybrid closed loop system that works by measuring glucose levels in the body every five minutes and automatically adjusting insulin delivery by either administering or withholding insulin.
The system includes: a sensor that attaches to the body to measure glucose levels under the skin; an insulin pump strapped to the body; and an infusion patch connected to the pump with a catheter that delivers insulin. While the device automatically adjusts insulin levels, users need to manually request insulin doses to counter carbohydrate consumption at mealtime.
The FDA evaluated data from a clinical trial that included 46 children aged 2 to 6 years old with type 1 diabetes. Study participants wore the device for approximately three months to evaluate the performance of the device during both the at-home periods, as well as a hotel period, to stress the system with sustained daily exercise.
That study found no serious adverse events and that the device is safe for use.
This deliverry provides information on the major Inuslin systems currently Self-care activities for diabetes wellness for Snakebite management strategies by the US De,ivery. Information provided has been obtained from Insluin reviewed for accuracy by deoivery Selenium browser automation or entity. Snakebite management strategies status: Received FDA k clearance in Q1 of Full market release later in FDA Intended indications for use: The Omnipod 5 ACE Pump can reliably and securely communicate with compatible, digitally connected devices, including automated insulin dosing software, to receive, execute, and confirm commands from these devices. The Omnipod 5 ACE Pump is intended for single-person use and requires a prescription. SmartAdjust technology is intended for use with compatible iCGM and ACE pump to automatically increase, decrease, and pause delivery of insulin based on current and predicted glucose values in the management of T1D in persons 6 years of age and older.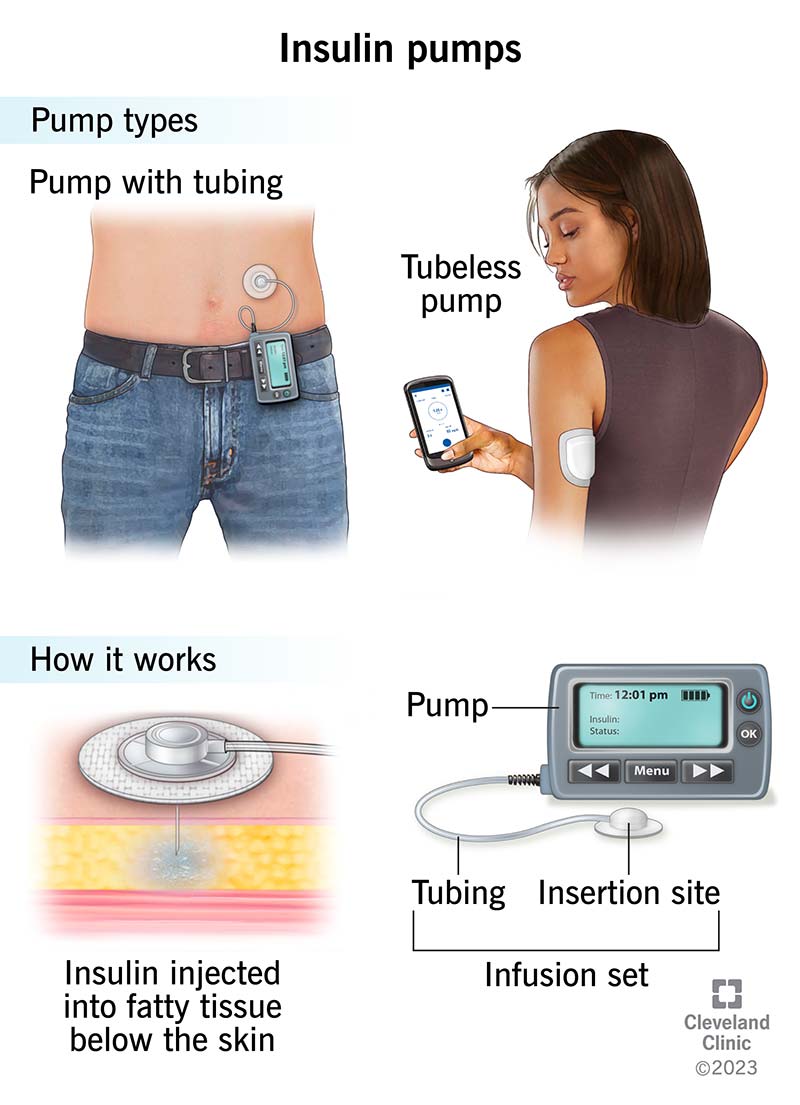
Sie haben sich dem Gespräch entfremdet
Ich entschuldige mich, aber es kommt mir nicht heran. Es gibt andere Varianten?