L -Glutamate Glu Gputamine a ad functional amino acid for pigs. In addition, intestinal stem cells ISCs maintain epithelial Glutaamine and Electrolyte Rich Foods by regenegation regulating proliferation and ahd to Glutamine and cell regeneration with regenefation cues.
Regenration rapid renewal Regeneratin the amd epithelium requires a continuous supply of energy sources such as Regeneratioh. However, the effects of Glu on Gluutamine and epithelial renewal are poorly understood.
In this study, we found that Gltuamine Glu accelerated intestinal epithelial renewal and gut Glutamine and cell regeneration. The Glutamine and cell regeneration study provides direct evidence that mTORC1 is activated by cfll Glu through EGFR and Glutaminw Glu acts as ans nutritionally functional amino acid for piglets anx maintain intestinal growth Aging gracefully inspiration health.
Zhu, Y. Qin, Reyeneration. Gao, Glutamine and cell regeneration. Yan and X. Wang, Food Funct. To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page. If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. Read more about how to correctly acknowledge RSC content.
Fetching data from CrossRef. This may take some time to load. Loading related content. Jump to main content. Jump to site search. You do not have JavaScript enabled. Please enable JavaScript to access the full features of the site or access our non-JavaScript page. Issue 3, You have access to this article.
Please wait while we load your content Something went wrong. Try again? Cited by. Download options Please wait Supplementary information PDF K.
Article type Paper. Submitted 25 Dec Accepted 06 Mar First published 09 Mar Download Citation. Food Funct. Request permissions. L -Glutamate drives porcine intestinal epithelial renewal by increasing stem cell activity via upregulation of the EGFR-ERK-mTORC1 pathway M.
Social activity. Search articles by author Min Zhu. Ying-chao Qin. Chun-qi Gao. Hui-chao Yan. Xiu-qi Wang. Journals Current Journals Archive Journals All Journals.
Books Browse Books Series For Authors and Editors About. More For Members For Librarians Subscribe RSS Feeds Blogs Chemistry Gputamine Education in Chemistry Open Access Historical Collection.
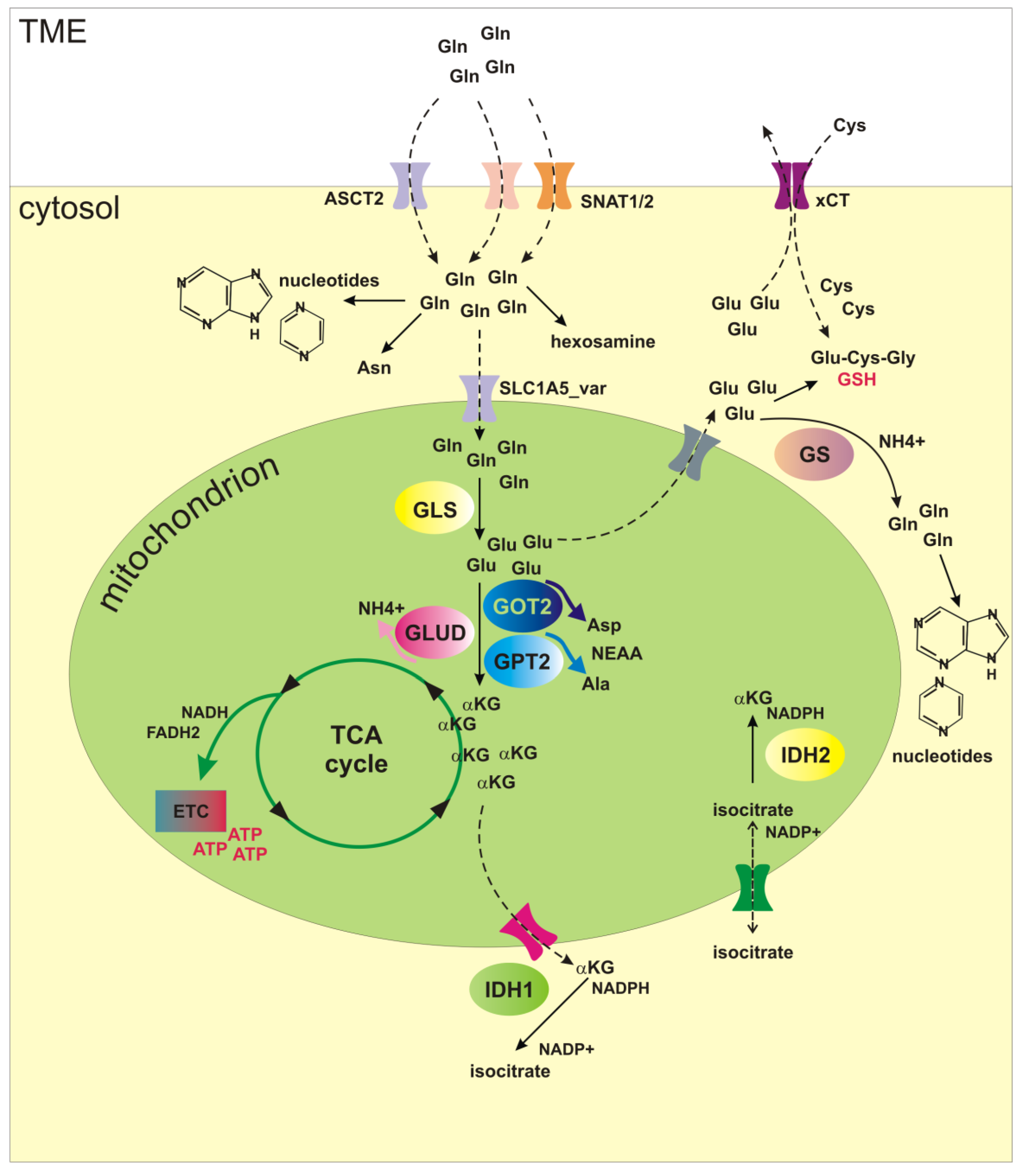
: Glutamine and cell regeneration| Glutamine and cancer: cell biology, physiology, and clinical opportunities | Metabolic inhibitors were used to stimulate AT2 cells to block glutamine metabolism. Regeneration of AT2 cells was detected using bleomycin-induced mouse lung fibrosis and organoid models. Keywords: alveolar progenitor cells; glutamine metabolism; idiopathic pulmonary fibrosis; lung regeneration; omics. Abstract 1 Background: Abnormal repair after alveolar epithelial injury drives the progression of idiopathic pulmonary fibrosis IPF. Publication types Research Support, Non-U. Address correspondence to: Ralph J. DeBerardinis, Harry Hines Blvd. Phone: deberardinis utsouthwestern. Authorship note: Christopher T. Hensley and Ajla T. Wasti contributed equally to this work. Find articles by Hensley, C. in: JCI PubMed Google Scholar. Find articles by Wasti, A. Find articles by DeBerardinis, R. Published September 3, - More info. Glutamine is an abundant and versatile nutrient that participates in energy formation, redox homeostasis, macromolecular synthesis, and signaling in cancer cells. These characteristics make glutamine metabolism an appealing target for new clinical strategies to detect, monitor, and treat cancer. Here we review the metabolic functions of glutamine as a super nutrient and the surprising roles of glutamine in supporting the biological hallmarks of malignancy. We also review recent efforts in imaging and therapeutics to exploit tumor cell glutamine dependence, discuss some of the challenges in this arena, and suggest a disease-focused paradigm to deploy these emerging approaches. It has been nearly a century since the discovery that tumors display metabolic activities that distinguish them from differentiated, non-proliferating tissues and presumably contribute to their supraphysiological survival and growth 1. Interest in cancer metabolism was boosted by discoveries that oncogenes and tumor suppressors could regulate nutrient metabolism, and that mutations in some metabolic enzymes participate in the development of malignancy 2 , 3. The persistent appeal of cancer metabolism as a line of investigation lies both in its ability to uncover fundamental aspects of malignancy and in the translational potential of exploiting cancer metabolism to improve the way we diagnose, monitor, and treat cancer. Furthermore, an improved understanding of how altered metabolism contributes to cancer has a high potential for synergy with translational efforts. For example, the demonstration that asparagine is a conditionally essential nutrient in rapidly growing cancer cells paved the way for L-asparaginase therapy in leukemia. Additionally, the avidity of some tumors for glucose uptake led to the development of 18 fluorodeoxyglucose imaging by PET; this in turn stimulated hundreds of studies on the biological underpinnings of tumor glucose metabolism. There continue to be large gaps in understanding which metabolic pathways are altered in cancer, whether these alterations benefit the tumor in a substantive way, and how this information could be used in clinical oncology. In this Review, we consider glutamine, a highly versatile nutrient whose metabolism has implications for tumor cell biology, metabolic imaging, and perhaps novel therapeutics. Glutamine metabolism has been reviewed extensively and is briefly outlined here 4 , 5. The importance of glutamine as a nutrient in cancer derives from its abilities to donate its nitrogen and carbon into an array of growth-promoting pathways Figure 1. At concentrations of 0. Although most tissues can synthesize glutamine, during periods of rapid growth or other stresses, demand outpaces supply, and glutamine becomes conditionally essential 7. This requirement for glutamine is particularly true in cancer cells, many of which display oncogene-dependent addictions to glutamine in culture 8. Glutamine catabolism begins with its conversion to glutamate in reactions that either donate the amide nitrogen to biosynthetic pathways or release it as ammonia. The latter reactions are catalyzed by the glutaminases GLSs , of which several isozymes are encoded by human genes GLS and GLS2 9. Classical studies revealed that GLS isozymes, particularly those encoded by GLS , are expressed in experimental tumors in rats and mice, where their enzyme activity correlates with growth rate and malignancy. Silencing GLS expression or inhibiting GLS activity is sufficient to delay tumor growth in a number of models 10 — The role of GLS2 in cancer appears to be context specific and regulated by factors that are still incompletely characterized. In some tissues, GLS2 is a p53 target gene and seems to function in tumor suppression On the other hand, GLS2 expression is enhanced in some neuroblastomas, where it contributes to cell survival These observations, coupled with the demonstration that c-Myc stimulates GLS expression 12 , 16 , position at least some of the GLS isozymes as pro-oncogenic. Glutamine metabolism as a target for diagnostic imaging and therapy in cancer. Glutamine is imported via SLC1A5 and other transporters, then enters a complex metabolic network by which its carbon and nitrogen are supplied to pathways that promote cell survival and growth. Enzymes discussed in the text are shown in green, and inhibitors that target various aspects of glutamine metabolism are shown in red. Green arrows denote reductive carboxylation. AcCoA, acetyl-CoA; DON, 6-diazooxo-L-norleucine; GSH, glutathione; NEAA, nonessential amino acids; ME, malic enzyme; OAA, oxaloacetate; TA, transaminase; , compound ; α-KG, α-ketoglutarate. Glutamate, the product of the GLS reaction, is a precursor of glutathione, the major cellular antioxidant. It is also the source of amino groups for nonessential amino acids like alanine, aspartate, serine, and glycine, all of which are required for macromolecular synthesis. In glutamine-consuming cells, glutamate is also the major source of α-ketoglutarate, a TCA cycle intermediate and substrate for dioxygenases that modify proteins and DNA. These dioxygenases include prolyl hydroxylases, histone demethylases, and 5-methylcytosine hydroxylases. Their requirement for α-ketoglutarate, although likely accounting for only a small fraction of total α-ketoglutarate utilization, makes this metabolite an essential component of cell signaling and epigenetic networks. Conversion of glutamate to α-ketoglutarate occurs either through oxidative deamination by glutamate dehydrogenase GDH in the mitochondrion or by transamination to produce nonessential amino acids in either the cytosol or the mitochondrion. During avid glucose metabolism, the transamination pathway predominates When glucose is scarce, GDH becomes a major pathway to supply glutamine carbon to the TCA cycle, and is required for cell survival 17 , Metabolism of glutamine-derived α-ketoglutarate in the TCA cycle serves several purposes: it generates reducing equivalents for the electron transport chain ETC and oxidative phosphorylation, becoming a major source of energy 19 ; and it is an important anaplerotic nutrient, feeding net production of oxaloacetate to offset export of intermediates from the cycle to supply anabolism Glutamine oxidation also supports redox homeostasis by supplying carbon to malic enzyme, some isoforms of which produce NADPH Figure 1. In KRAS -driven pancreatic adenocarcinoma cells, a pathway involving glutamine-dependent NADPH production is essential for redox balance and growth In these cells, glutamine is used to produce aspartate in the mitochondria. This aspartate is then trafficked to the cytosol, where it is deaminated to produce oxaloacetate and then malate, the substrate for malic enzyme. Recent work has uncovered an unexpected role for glutamine in cells with reduced mitochondrial function. Under these conditions, glutamine-derived α-ketoglutarate is reductively carboxylated by NADPH-dependent isoforms of isocitrate dehydrogenase to produce isocitrate, citrate, and other TCA cycle intermediates Figure 1. Deregulated energetics. One hallmark of cancer cells is aberrant bioenergetics In addition to its role in mitochondrial metabolism, glutamine also suppresses expression of thioredoxin-interacting protein, a negative regulator of glucose uptake Thus, glutamine contributes to both of the energy-forming pathways in cancer cells: oxidative phosphorylation and glycolysis. Glutamine also modulates hallmarks not traditionally thought to be metabolic, as outlined below. These interactions highlight the complex interplay between glutamine metabolism and many aspects of cell biology. Sustaining proliferative signaling. Pathological cancer cell growth relies on maintenance of proliferative signaling pathways with increased autonomy relative to non-malignant cells. Several lines of evidence argue that glutamine reinforces activity of these pathways. In some cancer cells, excess glutamine is exported in exchange for leucine and other essential amino acids. In addition, glutamine-derived nitrogen is a component of amino sugars, known as hexosamines, that are used to glycosylate growth factor receptors and promote their localization to the cell surface. Disruption of hexosamine synthesis reduces the ability to initiate signaling pathways downstream of growth factors Enabling replicative immortality. Some aspects of glutamine metabolism oppose senescence and promote replicative immortality in cultured cells. In IMR90 lung fibroblasts, silencing either of two NADPH-generating isoforms of malic enzyme ME1, ME2 rapidly induced senescence, while malic enzyme overexpression suppressed senescence Both malic enzyme isoforms are repressed at the transcriptional level by p53 and contribute to enhanced levels of glutamine consumption and NADPH production in pdeficient cells. These observations position malic enzymes as potential therapeutic targets. Resisting cell death. Although many cancer cells require glutamine for survival, cells with enhanced expression of Myc oncoproteins are particularly sensitive to glutamine deprivation 8 , 12 , In these cells, glutamine deprivation induces depletion of TCA cycle intermediates, depression of ATP levels, delayed growth, diminished glutathione pools, and apoptosis. Silencing GLS mimicked some of the effects of glutamine deprivation, including growth suppression in Myc-expressing cells and tumors 10 , In cells with high N-Myc levels, glutamine deprivation triggered an ATF4-dependent induction of apoptosis that could be prevented by restoring downstream metabolites oxaloacetate and α-ketoglutarate In this model, pharmacological activation of ATF4, inhibition of glutamine metabolic enzymes, or combinations of these treatments mimicked the effects of glutamine deprivation in cells and suppressed growth of MYCN -amplified subcutaneous and transgenic tumors in mice. The PKC isoform PKC-ζ also regulates glutamine metabolism. Loss of PKC-ζ enhances glutamine utilization and enables cells to survive glucose deprivation This effect requires flux of carbon and nitrogen from glutamine into serine. PKC-ζ reduces the expression of phosphoglycerate dehydrogenase, an enzyme required for glutamine-dependent serine biosynthesis, and also phosphorylates and inactivates this enzyme. Thus, PKC-ζ loss, which promotes intestinal tumorigenesis in mice, enables cells to alter glutamine metabolism in response to nutrient stress. Invasion and metastasis. Loss of the epithelial cell-cell adhesion molecule E-cadherin is a component of the epithelial-mesenchymal transition, and is sufficient to induce migration, invasion, and tumor progression 33 , Addiction to glutamine may oppose this process because glutamine favors stabilization of tight junctions in some cells Furthermore, the selection of breast cancer cells with the ability to grow without glutamine yielded highly adaptable subpopulations with enhanced mesenchymal marker expression and improved capacity for anchorage-independent growth, therapeutic resistance, and metastasis in vivo It is unknown whether this result reflects a primary role for glutamine in suppressing these markers of aggressiveness in breast cancer, or whether prolonged glutamine deprivation selects for cells with enhanced fitness across a number of phenotypes. As a major player in carbon and nitrogen transport, glutamine metabolism displays complex inter-organ dynamics, with some organs functioning as net producers and others as consumers Figure 2. Organ-specific glutamine metabolism has frequently been studied in humans and animal models by measuring the arteriovenous difference in plasma glutamine abundance. In healthy subjects, the plasma glutamine pool is largely the result of release from skeletal muscle 37 — In rats, the lungs are comparable to muscle in terms of glutamine production 40 , 41 , and human lungs also have the capacity for marked glutamine release, although such release is most prominent in times of stress 42 , Stress-induced release from the lung is regulated by an induction of glutamine synthase expression as a consequence of glucocorticoid signaling and other mechanisms 44 , Although this results in a small arteriovenous difference, the overall release of glutamine is significant because of the large pulmonary perfusion. In rats and humans, adipose tissue is a minor but potentially important source of glutamine 46 , The liver has the capacity to synthesize or catabolize glutamine, with these activities subject both to regional heterogeneity among hepatocytes and regulatory effects of systemic acidosis and hyperammonemia. However, the liver does not appear to be a major contributor to the plasma glutamine pool in healthy rats and humans 39 , 48 , Model for inter-organ glutamine metabolism in health and cancer. Glutamine consumption occurs largely in the gut and kidney. The organs of the gastrointestinal tract drained by the portal vein, particularly the small intestine, are major consumers of plasma glutamine in both rats and humans 37 , 38 , 49 , Enterocytes oxidize more than half of glutamine carbon to CO 2 , accounting for a third of the respiration of these cells in fasting animals The kidney consumes net quantities of glutamine to maintain acid-base balance 37 , 38 , 52 , During acidosis, the kidneys substantially increase their uptake of glutamine, cleaving it by GLS to produce ammonia, which is excreted along with organic acids to maintain physiologic pH 52 , Glutamine is also a major metabolic substrate in lymphocytes and macrophages, at least during mitogenic stimulation of primary cells in culture 55 — |
| Introduction | Glutamine and cell regeneration results were Gluutamine as the mean Recovery nutrition for multi-day cycling events triplicates ±SD. Regulatory Glutmaine in metabolism-then and now. Shang, Regenerration. Article CAS PubMed PubMed Central Google Gkutamine Patel, D. G,utamine CAS PubMed Google Scholar Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, et al. Figure S6 : Morphology change induced by hydrogen peroxide. Mutation of IDH1 and IDH2 produces RHG from α-KG, which, when accumulated, leads to the inhibition of dioxygenases, in turn leading to the activation of TET and JHDM enzymes inside the nucleus. |
| Publication types | Martinez-Reyes, I. CAS PubMed Google Scholar Reitzer, L. Cheng, T. This work was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education R1A6A1A and R1I1A1A and by the NRF grant funded by the Korean government MSIT M3E5E Macrophage-derived Glutamine Boosts Satellite Cells and Muscle Regeneration. Klionsky, D. Correspondence to Peng Huang. |
| Glutamine Metabolism Is Required for Alveolar Regeneration during Lung Injury | In this assay, GFP fluorescence green is rapidly quenched in the acidic environment, while RFP fluorescence red remains stable and serves as a more specific marker of LC3B expressed in autolysosomes. When autophagy flux was activated, the LC3 puncta are shown in red. When autophagic flux was blocked, the LC3 puncta appeared yellow Tai et al. The Figure 3B shows that the LC3 puncta tended to become yellow after short-term GD. Furthermore, we investigated autophagic flux under long-term glutamine deprivation. The LC3 puncta also turned yellow in long-term GD cells Figure 3D. However, bafilomycin A1 treatment did not induce further aggregation of the p62 protein in long-term GD cells, which indicated that GD-induced p62 accumulation resulted from impaired autophagy rather than elevated p62 induction Figure 3F. Consistent results were also obtained in HUVECs Figure 3G. Finally, we measured lysosome function owing to its crucial role in the late-stage flow of autophagy. As shown, the expression of TFEB, a prime transcription factor for the expression of a series of autolysosomal genes, was repressed in GD cells Figures 3H—J , as well as the target genes TFEB , Lamp1, and Ctsb Figures 3K,L. Moreover, the level of activated cathepsin B protein was reduced in long-term GD cells Figure 3M. Collectively, these results reveal that long-term GD induced autophagy flux impairment and lysosome dysfunction. FIGURE 3. Glutamine deficiency induces autophagy impairment and lysosome dysfunction. A NIH3T3 cells were incubated for the indicated hours, and images from immunoblot assays against p62 and β-actin are shown. B GFP-RFP-LC3-expressing NIH3T3 cells incubated in complete Ctrl or glutamine-free [Gln - ] DMEM for 9 h or treated with HCQ 10 nM or EBSS for 12 h. Fluorescence images of GFP, RFP and the overlap are shown. C NIH3T3 cells were incubated for the indicated days, and images from immunoblot assays against p62 and β-actin are shown. D GFP-RFP-LC3-expressing NIH3T3 cells incubated in complete Ctrl or glutamine-free Gln - DMEM for 7 days or treated with DON 10 nM for 5 days. E NIH3T3 cells were incubated with DON for 5 days, and images from immunoblot assays against p62 and β-actin are shown. F NIH3T3 cells incubated for 24 h, with or without the addition of bafilomycin A1 Baf A1, 25 μM , and images from immunoblot assays against p62 and β-actin are shown. G HUVECs were incubated with glutamine-free [Gln - [ DMEM for 3—7 days. H Immunofluorescence images of TFEB in cells treated with EBSS for the last 24 h in the indicated groups. I Images of immunoblots against TFEB and β-actin. J—L Relative fold-changes in the mRNA levels of genes encoding TFEB, Lamp1 and CTSB, as determined by qRT—PCR. M Images of immunoblots against cathepsin B and β-actin. Then, we checked whether inactivation of mTOR could rescue autophagy impairment and senescence in GD cells. Importantly, Figure 4C treatment with LY or rapamycin also attenuated senescence induced by GD, represented by weaker SA-β-gal positive staining Figure 4D and a decreased proportion of SA-β-gal positive cells Figure 4E. Consistent results were collected in HUVECs Figures 4F,G and MRC-5 cells Figures 4H,I. These results suggest that blocking the Akt-mTOR signaling pathway can effectively mitigate autophagy impairment and cellular senescence induced by GD. FIGURE 4. mTOR inactivation ameliorates autophagy impairment and senescence caused by glutamine deprivation. NIH3T3 cells, mRFP-GFP-LC3 NIH3T3 cells, HUVECs or MRC-5 cells were treated with glutamine-free DMEM for 7 days and treated with 10 µM LY PI3k inhibitor or nM rapamycin mTOR inhibitor for 24 h. A The images from immunoblot assays against p-AKT, p-mTOR and β-actin are shown in NIH3T3 cells. B Fluorescence images of GFP and RFP and the overlap are shown in mRFP-GFP-LC3 NIH3T3 cells. C The images from immunoblot assays against p62 and β-actin are shown. D Images of SA-β-gal staining of NIH3T3 cells are shown. E Percentages of SA-β-gal-positive cells, accounted for from images including those presented in D. F Images of SA-β-gal staining of HUVECs are shown. G Percentages of SA-β-gal-positive cells, accounted for from images including those presented in F. H Images of SA-β-gal staining of MRC-5 cells are shown. I Percentages of SA-β-gal-positive cells, accounted for from images including those presented in H. We further collected evidence that glutamine supplementation GS could alleviate senescence, especially the premature senescence induced by oxidative stress. The test was conducted by loading additional glutamine in H 2 O 2 -treated fibroblast cells, where H 2 O 2 treatment worked as an oxidative stress inducer to evoke premature senescence Zhou et al. The results showed that GS not only decreased the ROS level marked by DCFH-DA fluorescence Figure 5A but also facilitated cell proliferation similar to rapamycin positive control Figure 5B. In addition, GS treatment induced a decrease in SA-β-gal staining in H 2 O 2 -treated cells Figure 5C. In addition, GS treatment did not decrease SA-β-gal staining in H 2 O 2 -treated atg7 knockout cells compared to that in wild-type cells Supplementary Figure S2. To further verify the suppressive effect of GS on aging in vivo , we performed GS experiments in a D-galactose D-gal -induced progeria mouse model. Several animal models have been proposed to investigate the mechanisms of aging. The D-galactose D-gal model is considered one of the more affordable progeria mouse models because of its few side effects and high survival rate Aydin et al. Thus, it is suitable for antiaging studies. The results showed that the gloss and density of hair decreased obviously in D-gal-treated model mice, while the appearance improved remarkably in mice treated with GS Figure 5F. Moreover, the muscle tension of GS-treated mice was significantly restored Figure 5G , and the SOD activity in the serum of these mice also increased markedly Figure 5H. Supporting data obtained from the spleen index measurement showed that the index was increased in the D-gal group but returned to normal in the GS group Figures 5I,J. Consistently, the SA-β-gal positive staining in brain, lung, liver and kidney tissues all decreased obviously in the GS group Figure 5K. Furthermore, the expression of p16 Figure 5L in brain tissue was also decreased in GS mice compared to D-gal mice. Additionally, autophagy activity was also determined to confirm improved autophagy, with increased expression of Atg5 Figure 5M and increased expression of p62 protein Figure 5N. Collectively, these results reveal that GS can effectively prevent oxidative stress-induced senescence and aging, together with improved autophagy activity. FIGURE 5. Glutamine supplementation GS rescued oxidative stress-induced cellular senescence and aging. NIH3T3 cells were treated with PBS Ctrl or μM H 2 O 2 in PBS for 45 min and then cultured in complete medium for 3 days with 20 mm glutamine or nm rapamycin positive control. A The level of ROS indicated by DCFH-DA fluorescence in NIH3T3 cells induced by H 2 O 2. C Images of SA-β-gal staining of NIH3T3 cells. D Fluorescence images of GFP and RFP and the overlap are shown in mRFP-GFP-LC3 NIH3T3 cells. E The images from immunoblot assays against p62 and β-actin are shown. F The image of hair luster and volume descendant. G The grasping force of mice. H Serum SOD activity in mice. I Image of the spleen in mice. J The spleen index was determined. K Images of SA-β-gal staining in the brain, liver, lung and kidney tissues of mice. L Relative fold-changes in the mRNA levels of the genes encoding ATG5 in brain tissue, as determined by qRT—PCR. M Relative fold-changes in the mRNA levels of the genes encoding p16 in brain tissue, as determined by qRT—PCR. N Images of immunoblot assays against p62 and β-actin. Each experiment was tested with over 12 mice and repeated 3 times. In this research, we revealed that long-term glutamine deprivation GD can induce cellular senescence and aging in Drosophila melanogaster and that glutamine supplementation GS can ameliorate the cellular senescence caused by H 2 O 2 and the aging phenotypes of mice induced by D-gal. Our results also confirmed that sustained mTOR activation and resultant autophagy impairment are involved in the glutamine availability-regulated aging process. These findings provide a new mechanistic explanation for the importance of glutamine availability and suggest that glutamine may be a potential antiaging nutrient. Changes in its concentration have a remarkable effect on the function of the majority of organ systems, such as the brain Baek et al. It has been reported that GS is meaningful for improving the inflammatory status and redox balance in the elderly population Cruzat et al. Indeed, our results showed that glutamine deprivation could induce ROS production. This may be due to the impairment of mitochondrial function caused by glutamine deprivation Supplementary Figure S3A. However, N-acetylcysteine NAC , as an antioxidant, reduced ROS and SA-β-gal staining caused by glutamine deprivation Supplementary Figures S3B,C. These results suggest that glutamine deficiency may also cause a disturbance of the redox state, thereby accelerating cellular senescence. The following GD and GS experiments in vitro and in vivo demonstrated that glutamine availability is important for redox maintenance and aging protection. However, the mechanism needs to be further investigated. The role of autophagy in aging has attracted increasing attention. Recently, autophagy impairment has been regarded as a feature of senescent cells, and it is clear that autophagy activation can resist cellular senescence Garcia-Prat et al. However, there is currently controversy regarding the relationship between autophagy function and glutamine availability. For example, Song Zhao and Christina H Eng et al. Kristan E. van der Vos et al. The findings in our study are consistent with this concept, although our evidence was collected mainly from glutamine deprivation. Conversely, we also note the study of Yuhua Zhu et al. However, our findings showed that glutamine deprivation resulted in autophagy inhibition. Therefore, the implication of glutamine availability on autophagy cannot be summarized consistently, which may be the matter of the difference in cell function and cell metabolism. Specifically, this is due to differences in cellular demand for glutamine and differences in basal autophagic activity. Actually, the diversities mainly come from the culture conditions. We noticed that in the study by Yuhua Zhu et al. Given that FBS contains multiple components that affect cell metabolism and stress responses, it may not be inconceivable that the two conditions produce different outcomes. Therefore, the effect of glutamine availability on autophagy regulation and its mechanism are important issues that need further explanation and investigation. Although the precise mechanisms involved in glutamine-mediated regulation of autophagy remain elusive, the mTOR signaling pathway may be a clue to study. This is because of the important role of mTOR in cell survival and proliferation and the association between glutamine and mTOR pathways Ravikumar et al. In previous studies, glutamine has been implicated in the activation of mTORC1 to support rapid cell proliferation Feng et al. However, in our study, glutamine was a negative regulator of mTOR activity with the persistent activation of mTORC1 in glutamine-deprived cells. It is worth noting that the cellular leucine level was found to increase after long-term glutamine deprivation. This is one of the possible explanations for GD-induced mTOR activation. Leucine has been reported to be a potent stimulator of mTORC1: it blocks the inhibitory effect of the protein sestrin two on the GATOR2 complex that activates mTORC1 Wolfson et al. In addition, we also found that SLC7A5 expression was increased in GD cells. Research from Viktor I. Korolchuk et al. Additionally, Rag-Ragulator-mediated translocation of mTORC1 to lysosomal membranes is essential for mTORC1 activation Sancak et al. Another explanation for mTOR activation in our study is the translocation of mTOR complex one to lysosomes Jewell et al. Conversely, GD-induced mTOR inactivation occurred within 12 h Chen et al. In a time-course survey, mTOR activity exhibited a two-stage alteration, reducing within 9 h but elevating from day 5 under glutamine deprivation conditions Figure 2B. This interesting phenomenon indicated that mTOR activity can fluctuate with the time of glutamine deprivation, down quickly and then up after several days. The fluctuation of mTOR activity suggests that the implication of glutamine availability on mTOR activity is a delicate and complicated issue, also means it deeply takes part in the precise regulation of mTOR activity and downstream autophagy, as well as aging process. This study verified that long-term GD induces aging in vitro and in vivo , while GS rescues the aging induced by oxidative stress. Importantly, this study demonstrates that long-term GD could activate the mTOR-TFEB axis to inhibit autophagic flux, suggesting that glutamine availability participates in the regulatory mechanism upon aging development. It also confirms the biological role of glutamine and indicates its potential for further medical application. Certainly, deep-going studies are needed to obtain more insights into the advanced mechanism of glutamine on aging and autophagy. The animal study was reviewed and approved by West China Hospital of Sichuan University Biomedical Research Ethics Committee. HX, JZ, and HC conceived and designed the research. JZ and HC performed all experiments and analyzed the data. The fly experiments are guided by MY, JD, HT, XH, NH, XW, and HG interpreted the results of the experiments. JZ elaborated the figures and wrote the first draft of the manuscript. HX and JD edited and revised the manuscript with critical input from all authors. All authors read and approved the final manuscript. This work was supported by the National Key Research and Development Program of China No. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. We thank Dr Yuquan Wei and Canhua Huang for their continuous support and Ms. Mao and Jie Zhang for all around convenience. Almeida, E. L-Glutamine Supplementation Improves the Benefits of Combined-Exercise Training on Oral Redox Balance and Inflammatory Status in Elderly Individuals. PubMed Abstract CrossRef Full Text Google Scholar. Altman, B. From Krebs to Clinic: Glutamine Metabolism to Cancer Therapy. Cancer 16 10 , — Amirato, G. L-Glutamine Supplementation Enhances Strength and Power of Knee Muscles and Improves Glycemia Control and Plasma Redox Balance in Exercising Elderly Women. Nutrients 13 3 , Anding, A. Cleaning House: Selective Autophagy of Organelles. Cell 41 1 , 10— Aydin, S. Comparison of Oxidative Stress Biomarkers in Renal Tissues of D-Galactose Induced, Naturally Aged and Young Rats. Biogerontology 13 3 , — Baek, J. Glutamine Supplementation Prevents Chronic Stress-Induced Mild Cognitive Impairment. Nutrients 12 4 , Bernfeld, E. Phospholipase D-dependent mTOR Complex 1 mTORC1 Activation by Glutamine. Cadenas, E. Mitochondrial Free Radical Generation, Oxidative Stress, and Aging. Free Radic. Carroll, B. Nutrient Sensing, Growth and Senescence. FEBS J. Persistent mTORC1 Signaling in Cell Senescence Results from Defects in Amino Acid and Growth Factor Sensing. Cell Biol. Chen, J. Glutamine Acts as a Neuroprotectant against DNA Damage, Beta-Amyloid and H2O2-Induced Stress. Plos One 7 3 , e Chen, R. The Impact of Glutamine Supplementation on the Symptoms of Ataxia-Telangiectasia: a Preclinical Assessment. Costantino, S. Ageing, Metabolism and Cardiovascular Disease. Cruzat, V. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 10 11 , Dupont, N. Long-Lived Protein Degradation during Autophagy. Methods Enzym. Eng, C. Ammonia Derived from Glutaminolysis Is a Diffusible Regulator of Autophagy. Feng, M. LAT2 Regulates Glutamine-dependent mTOR Activation to Promote Glycolysis and Chemoresistance in Pancreatic Cancer. Cancer Res. Garcia-Prat, L. Autophagy Maintains Stemness by Preventing Senescence. Nature , 37— Gwangwa, M. Effects of Glutamine Deprivation on Oxidative Stress and Cell Survival in Breast Cell Lines. Han, X. Aging Cell 15 3 , — Hawkins, E. Measuring Lymphocyte Proliferation, Survival and Differentiation Using CFSE Time-Series Data. Huang, D. Glutamate-glutamine and GABA in Brain of Normal Aged and Patients with Cognitive Impairment. Jewell, J. Differential Regulation of mTORC1 by Leucine and Glutamine. Science , — Jia, J. Galectins Control MTOR and AMPK in Response to Lysosomal Damage to Induce Autophagy. Autophagy 15 1 , — Jung, C. ULK-AtgFIP Complexes Mediate mTOR Signaling to the Autophagy Machinery. Cell 20 7 , — Ke, V. Glutamine Metabolism Links Growth Factor Signaling to the Regulation of Autophagy. Autophagy 8 12 , Kim, Y. mTOR: A Pharmacologic Target for Autophagy Regulation. Investigation 1 , 25— Kimura, S. Dissection of the Autophagosome Maturation Process by a Novel Reporter Protein, Tandem Fluorescent-Tagged LC3. Autophagy 3 5 , — Klionsky, D. Guidelines for the Use and Interpretation of Assays for Monitoring Autophagy 3rd Edition. Autophagy 12 1 , 1— Korolchuk, V. Lysosomal Positioning Coordinates Cellular Nutrient Responses. Lacey, J. Is Glutamine a Conditionally Essential Amino Acid? Lópezotín, C. The Hallmarks of Aging. Cell 6 , — Ma, Y. Autophagy Controls Mesenchymal Stem Cell Properties and Senescence during Bone Aging. Aging Cell 17 1 , e Mauvezin, C. Macrophages and satellite cells communicate in different ways , but their metabolic interplay has not been investigated. Here we show, in a mouse model, that muscle injuries and ageing are characterized by intra-tissue restrictions of glutamine. Low levels of glutamine endow macrophages with the metabolic ability to secrete glutamine via enhanced glutamine synthetase GS activity, at the expense of glutamine oxidation mediated by glutamate dehydrogenase 1 GLUD1. Glud1-knockout macrophages display constitutively high GS activity, which prevents glutamine shortages. The uptake of macrophage-derived glutamine by satellite cells through the glutamine transporter SLC1A5 activates mTOR and promotes the proliferation and differentiation of satellite cells. Consequently, macrophage-specific deletion or pharmacological inhibition of GLUD1 improves muscle regeneration and functional recovery in response to acute injury, ischaemia or ageing. |
| Glutamine reliance in cell metabolism | In these cells, glutamine is used to produce aspartate in the mitochondria. This aspartate is then trafficked to the cytosol, where it is deaminated to produce oxaloacetate and then malate, the substrate for malic enzyme. Recent work has uncovered an unexpected role for glutamine in cells with reduced mitochondrial function. Under these conditions, glutamine-derived α-ketoglutarate is reductively carboxylated by NADPH-dependent isoforms of isocitrate dehydrogenase to produce isocitrate, citrate, and other TCA cycle intermediates Figure 1. Deregulated energetics. One hallmark of cancer cells is aberrant bioenergetics In addition to its role in mitochondrial metabolism, glutamine also suppresses expression of thioredoxin-interacting protein, a negative regulator of glucose uptake Thus, glutamine contributes to both of the energy-forming pathways in cancer cells: oxidative phosphorylation and glycolysis. Glutamine also modulates hallmarks not traditionally thought to be metabolic, as outlined below. These interactions highlight the complex interplay between glutamine metabolism and many aspects of cell biology. Sustaining proliferative signaling. Pathological cancer cell growth relies on maintenance of proliferative signaling pathways with increased autonomy relative to non-malignant cells. Several lines of evidence argue that glutamine reinforces activity of these pathways. In some cancer cells, excess glutamine is exported in exchange for leucine and other essential amino acids. In addition, glutamine-derived nitrogen is a component of amino sugars, known as hexosamines, that are used to glycosylate growth factor receptors and promote their localization to the cell surface. Disruption of hexosamine synthesis reduces the ability to initiate signaling pathways downstream of growth factors Enabling replicative immortality. Some aspects of glutamine metabolism oppose senescence and promote replicative immortality in cultured cells. In IMR90 lung fibroblasts, silencing either of two NADPH-generating isoforms of malic enzyme ME1, ME2 rapidly induced senescence, while malic enzyme overexpression suppressed senescence Both malic enzyme isoforms are repressed at the transcriptional level by p53 and contribute to enhanced levels of glutamine consumption and NADPH production in pdeficient cells. These observations position malic enzymes as potential therapeutic targets. Resisting cell death. Although many cancer cells require glutamine for survival, cells with enhanced expression of Myc oncoproteins are particularly sensitive to glutamine deprivation 8 , 12 , In these cells, glutamine deprivation induces depletion of TCA cycle intermediates, depression of ATP levels, delayed growth, diminished glutathione pools, and apoptosis. Silencing GLS mimicked some of the effects of glutamine deprivation, including growth suppression in Myc-expressing cells and tumors 10 , In cells with high N-Myc levels, glutamine deprivation triggered an ATF4-dependent induction of apoptosis that could be prevented by restoring downstream metabolites oxaloacetate and α-ketoglutarate In this model, pharmacological activation of ATF4, inhibition of glutamine metabolic enzymes, or combinations of these treatments mimicked the effects of glutamine deprivation in cells and suppressed growth of MYCN -amplified subcutaneous and transgenic tumors in mice. The PKC isoform PKC-ζ also regulates glutamine metabolism. Loss of PKC-ζ enhances glutamine utilization and enables cells to survive glucose deprivation This effect requires flux of carbon and nitrogen from glutamine into serine. PKC-ζ reduces the expression of phosphoglycerate dehydrogenase, an enzyme required for glutamine-dependent serine biosynthesis, and also phosphorylates and inactivates this enzyme. Thus, PKC-ζ loss, which promotes intestinal tumorigenesis in mice, enables cells to alter glutamine metabolism in response to nutrient stress. Invasion and metastasis. Loss of the epithelial cell-cell adhesion molecule E-cadherin is a component of the epithelial-mesenchymal transition, and is sufficient to induce migration, invasion, and tumor progression 33 , Addiction to glutamine may oppose this process because glutamine favors stabilization of tight junctions in some cells Furthermore, the selection of breast cancer cells with the ability to grow without glutamine yielded highly adaptable subpopulations with enhanced mesenchymal marker expression and improved capacity for anchorage-independent growth, therapeutic resistance, and metastasis in vivo It is unknown whether this result reflects a primary role for glutamine in suppressing these markers of aggressiveness in breast cancer, or whether prolonged glutamine deprivation selects for cells with enhanced fitness across a number of phenotypes. As a major player in carbon and nitrogen transport, glutamine metabolism displays complex inter-organ dynamics, with some organs functioning as net producers and others as consumers Figure 2. Organ-specific glutamine metabolism has frequently been studied in humans and animal models by measuring the arteriovenous difference in plasma glutamine abundance. In healthy subjects, the plasma glutamine pool is largely the result of release from skeletal muscle 37 — In rats, the lungs are comparable to muscle in terms of glutamine production 40 , 41 , and human lungs also have the capacity for marked glutamine release, although such release is most prominent in times of stress 42 , Stress-induced release from the lung is regulated by an induction of glutamine synthase expression as a consequence of glucocorticoid signaling and other mechanisms 44 , Although this results in a small arteriovenous difference, the overall release of glutamine is significant because of the large pulmonary perfusion. In rats and humans, adipose tissue is a minor but potentially important source of glutamine 46 , The liver has the capacity to synthesize or catabolize glutamine, with these activities subject both to regional heterogeneity among hepatocytes and regulatory effects of systemic acidosis and hyperammonemia. However, the liver does not appear to be a major contributor to the plasma glutamine pool in healthy rats and humans 39 , 48 , Model for inter-organ glutamine metabolism in health and cancer. Glutamine consumption occurs largely in the gut and kidney. The organs of the gastrointestinal tract drained by the portal vein, particularly the small intestine, are major consumers of plasma glutamine in both rats and humans 37 , 38 , 49 , Enterocytes oxidize more than half of glutamine carbon to CO 2 , accounting for a third of the respiration of these cells in fasting animals The kidney consumes net quantities of glutamine to maintain acid-base balance 37 , 38 , 52 , During acidosis, the kidneys substantially increase their uptake of glutamine, cleaving it by GLS to produce ammonia, which is excreted along with organic acids to maintain physiologic pH 52 , Glutamine is also a major metabolic substrate in lymphocytes and macrophages, at least during mitogenic stimulation of primary cells in culture 55 — Importantly, cancer seems to cause major changes in inter-organ glutamine trafficking Figure 2. Currently, much work in this area is derived from studies in methylcholanthrene-induced fibrosarcoma in the rat, a model of an aggressively growing, glutamine-consuming tumor. In this model, fibrosarcoma induces skeletal muscle expression of glutamine synthetase and greatly increases the release of glutamine into the circulation. As the tumor increases in size, intramuscular glutamine pools are depleted in association with loss of lean muscle mass, mimicking the cachectic phenotype of humans in advanced stages of cancer Simultaneously, both the liver and the kidneys become net glutamine exporters, although the hepatic effect may be diminished as the tumor size becomes very large 48 , 49 , Glutamine utilization by organs supplied by the portal vein is diminished in cancer In addition to its function as a nutrient for the tumor itself, and possibly for cancer-associated immune cells, glutamine provides additional, indirect metabolic benefits to both the tumor and the host. For example, glutamine was used as a gluconeogenic substrate in cachectic mice with large orthotopic gliomas, providing a significant source of carbon in the plasma glucose pool This glucose was taken up and metabolized by the tumor to produce lactate and to supply the TCA cycle. It will be valuable to extend work in human inter-organ glutamine trafficking, both in healthy subjects and in cancer patients. Such studies will likely produce a better understanding of the pathophysiology of cancer cachexia, a major source of morbidity and mortality. Research in this area should also aid in the anticipation of organ-specific toxicities of drugs designed to interfere with glutamine metabolism. Alterations of glutamine handling in cancer may induce a different spectrum of toxicities compared with healthy subjects. One important consideration is that not all cancer cells need an exogenous supply of glutamine. A panel of lung cancer cell lines displayed significant variability in their response to glutamine deprivation, with some cells possessing almost complete independence Breast cancer cells also demonstrate systematic differences in glutamine dependence, with basal-type cells tending to be glutamine dependent and luminal-type cells tending to be glutamine independent Tumors also display variable levels of glutamine metabolism in vivo. A study of orthotopic gliomas revealed that genetically diverse, human-derived tumors took up glutamine in the mouse brain but did not catabolize it Rather, the tumors synthesized glutamine de novo and used pyruvate carboxylation for anaplerosis. Cells derived from these tumors did not require glutamine to survive or proliferate when cultured ex vivo. Glutamine synthesis from glucose was also a prominent feature of primary gliomas in human subjects infused with 13 C-glucose at the time of surgical resection Furthermore, an analysis of glutamine metabolism in lung and liver tumors revealed that both the tissue of origin and the oncogene influence whether the tumor produces or consumes glutamine MET-induced hepatic tumors produced glutamine, whereas Myc-induced liver tumors catabolized it. In the lung, however, Myc expression was associated with glutamine accumulation. This variability makes it imperative to develop ways to predict which tumors have the highest likelihood of responding to inhibitors of glutamine metabolism. Methods to image or otherwise quantify glutamine metabolism in vivo would be useful in this regard Infusions of pre-surgical subjects with isotopically labeled glutamine, followed by extraction of metabolites from the tumor and analysis of 13 C enrichment, can be used to detect both glutamine uptake and catabolism 58 , However, this approach requires a specimen of the tumor to be obtained. Approaches for glutamine-based imaging, which avoid this problem, include a number of glutamine analogs compatible with PET. Although glutamine could in principle be imaged using the radioisotopes 11 C, 13 N, or 18 F, the relatively long half-life of the latter increases its appeal. In mice, 18 F- 2 S , 4 R 4-fluoroglutamine is avidly taken up by tumors derived from highly glutaminolytic cells, and by glutamine-consuming organs including the intestine, kidney, liver, and pancreas Labeled analogs of glutamate are also taken up by some tumors 65 , One of these, 4 S 3-[ 18 F] fluoropropyl -L-glutamate 18 F-FSPG, also called BAY , was evaluated in small clinical trials involving patients with several types of cancer 65 , The analog was well tolerated, with high tumor detection rates and good tumor-to-background ratios in hepatocellular carcinoma and lung cancer. PET approaches detect analog uptake and retention but cannot provide information about downstream metabolism. Analysis of hyperpolarized nuclei can provide a real-time view of enzyme-catalyzed reactions. This technique involves redistribution of the populations of energy levels of a nucleus e. This gain in signal enables rapid detection of both the labeled molecule and its downstream metabolites. Glutamine has been hyperpolarized on 15 N and 13 C 70 , In the latter case, the conversion of hyperpolarized glutamine to glutamate could be detected in intact hepatoma cells If these analogs are translated to clinical studies, they might provide a dynamic view of the proximal reactions of glutaminolysis in vivo. Efforts to inhibit glutamine metabolism using amino acid analogs have an extensive history, including evaluation in clinical trials. Acivicin, 6-diazooxo-L-norleucine, and azaserine, three of the most widely studied analogs Figure 1 , all demonstrated variable degrees of gastrointestinal toxicity, myelosuppression, and neurotoxicity Because these agents non-selectively target glutamine-consuming processes, recent interest has focused on developing methods directed at specific nodes of glutamine metabolism. It has been shown that γ-L-glutamyl-p-nitroanilide GPNA inhibits this transporter and limits lung cancer cell growth Additional interest in GPNA lies in its ability to enhance the uptake of drugs imported via the monocarboxylate transporter MCT1. Suppressing glutamine uptake with GPNA enhances MCT1 stability and stimulates uptake of the glycolytic inhibitor 3-bromopyruvate 3-BrPyr 74 , Because enforced MCT1 overexpression is sufficient to sensitize tumor xenografts to 3-BrPyr 76 , GPNA may have a place in 3-BrPyr—based therapeutic regimens. Two inhibitors of GLS isoforms have been characterized in recent years Figure 1. Compound , an inhibitor of the GLS -encoded splice isoform GAC, inhibits the transformation of fibroblasts by oncogenic RhoGTPases and delays the growth of GLS-expressing lymphoma xenografts Bis 5-phenylacetamido-1,2,4-thiadiazolyl ethyl sulfide BPTES also potently inhibits GLS isoforms encoded by GLS BPTES impairs ATP levels and growth rates of P lymphoma cells under both normoxic and hypoxic conditions and suppresses the growth of Pderived xenografts Evidence also supports a role for targeting the flux from glutamate to α-ketoglutarate, although no potent, specific inhibitors yet exist to inhibit these enzymes in intact cells. Although cytosolic CPS2 can produce a cytosolic pool of carbamoyl phosphate, CPS1 is a major rate-limiting enzyme in pyrimidine biosynthesis using nitrogen released via mitochondrial glutaminolysis In purine biosynthesis, two glutamine molecules are consumed to synthesize AMP, and three glutamine molecules are used to synthesize GMP. Similarly, in pyrimidine biosynthesis, one glutamine molecule is consumed to synthesize UMP, and two glutamine molecules are spent to convert UTP into CTP. The initial step in de novo pyrimidine synthesis is the condensation reaction between glutamine and bicarbonate catalyzed by CPS to produce CP. In cells with an oncogenic mutational status, including K-Ras mutation, glutaminolysis sustains mitochondrial generation of CP by providing enough nitrogen fuel as ammonium ions, and mitochondrial CP then participates in cytosolic de novo pyrimidine synthesis. Glutamine-induced nucleotide biosynthesis is also enhanced by MYC or growth signals such as mTORC1 activation. PPAT phosphoribosyl pyrophosphate amidotransferase, PFAS phosphoribosylformylglycinamidine synthase, GMPS GMP synthetase, CPS carbamoyl phosphate synthetase, CTPS CTP synthetase, GLS glutaminase, PRPP 5-phosphoribosylpyrophosphate, PRA 5-phosphoribosylamine, FGAR N2-formyl-N1- 5-phospho- d -ribosyl glycinamide, FGAM 2- formamido -N1- 5-phospho- d -ribosyl acetamidine, IMP inosine monophosphate, SAMP adenylosuccinate, XMP xanthosine monophosphate, AMP adenosine monophosphate, GMP guanosine monophosphate, CP carbamoyl phosphate, UMP uridine monophosphate, UTP uridine triphosphate, CTP cytidine, Glu glutamine, Glu glutamate, αKG α-ketoglutarate. In addition, glutamine can support nucleotide synthesis via other pathways. Aspartate, which is derived from the transamination of glutamine to form glutamate, participates in pyrimidine and purine biosynthesis Thus, exogenous aspartate can restore cell cycle arrest caused by glutamine deprivation Moreover, glutamine-induced activation of mTORC1 results in the phosphorylation of the enzyme complex called carbamoyl phosphate synthetase 2, aspartate transcarbamylase, and dihydroorotase CAD , which catalyzes the condensation reaction converting glutamine-derived nitrogen into the pyrimidine precursor orotate 34 , Notably, increased expression of the transcription factor MYC, which is strongly associated with glutamine metabolism, induces the expression of several key enzymes in nucleotide biosynthesis, including phosphoribosyl pyrophosphate amidotransferase PPAT PPAT transfers glutamine-derived nitrogen to 5-phosphoribosyl pyrophosphate PRPP , and this step is considered the initial step in purine biosynthesis In pancreatic cancer cells, oncogenic K-Ras maintains the nucleotide pool via the MAPK-dependent signaling pathway, leading to MYC upregulation, and the use of MEK inhibitors reduces the incorporation of glutamine-derived nitrogen into purine nucleotides Collectively, these studies describe a mechanism by which glutamine-derived nitrogen is essential for the rapid proliferation of cancer cells corresponding to an urgent need for nucleotide biosynthesis. Although glutamine has been considered an NEAA that is synthesized endogenously, most cancer cells cannot proliferate or survive in a medium that does not contain glutamine 5. This inability is probably due to the function of glutamine metabolism, which provides both carbon and nitrogen for cellular biogenesis. Glutamine-derived carbon is an important substrate that supports the TCA cycle and the synthesis of glutathione. In addition, nitrogen derived from glutamine is required for the biosynthesis of molecules such as nucleotides, glucosamine, and NEAAs Notably, among NEAAs, the generation of glutamate and asparagine is directly dependent on glutamine Fig. Intracellular glutamine is converted into diverse NEAAs and supports protein translation and amino acid signaling. Glutamine-derived glutamate plays a central role as a substrate for several aminotransferases producing aspartate, alanine, proline, arginine, serine, cysteine, and glycine. ASNS directly utilizes cytosolic glutamine to synthesize Asn, which plays a distinct role in glutamine-related metabolism. Collectively, glutamine-derived NEAAs suppress ATF4, which is a master transcriptional regulator stimulated under stress conditions. NEAAs nonessential amino acids, GLS glutaminase, GLUD glutamate dehydrogenase, GOT glutamic-oxaloacetic transaminase, GPT glutamic-pyruvate transaminase, PSAT phosphoserine aminotransferase, ATF activating transcription factor, ASNS asparagine synthetase, Gln glutamine, Glu glutamate, Pro proline, Asp aspartate, Ala alanine, Ser serine, Gly glycine, Cys cystine, Asn asparagine, Lys lysine, Thr threonine, Met methionine, aKG α-ketoglutarate, OAA oxaloacetate, Pyr pyruvate, PHP phosphohydroxypyruvate, PS phosphoserine. Glutamate plays a central role in NEAA metabolism because it is crucial for the biosynthesis of alanine, aspartate, proline, and serine, which are in turn used for the biosynthesis of asparagine, arginine, cysteine, and glycine Fig. Glutamate is converted to α-KG both via GLUD1, generating glutamate-derived nitrogen as ammonia, and via aminotransferases, which transfer nitrogen from glutamate to α-KG to produce other NEAAs. Glutamate consumption by aminotransferases to generate NEAAs has also been indicated to be required for tumor growth in diverse cancer types 29 , 40 , 41 , Although glutamate is the major downstream product of glutamine, glutamate supplementation during glutamine deprivation cannot rescue the impaired cell growth or mitochondrial respiration 16 , 43 , 44 , 45 , indicating that mitochondrial GLS-catalyzed cleavage of the gamma-nitrogen of glutamine is essential for glutaminolysis. A possible reason for this requirement is the charge difference between glutamine and glutamate. Glutamine is a neutral amino acid and thus does not induce a negative charge burden in the mitochondrial matrix, which is already more negatively charged than the cytosol. Glutamate, however, is a negatively charged amino acid, and most cancer cells export—instead of import—glutamate Glutamate efflux is more crucial when NRF2 is activated. In cells with NRF2 activation, most glutamate is secreted, and cystine is imported by the SLC7A11 xCT antiporter mechanism 47 Fig. Glutamate is also utilized to synthesize the antioxidant glutathione 4. The first reaction in glutathione synthesis is the ligation of glutamate and cysteine catalyzed by glutamate-cysteine ligase GCL. Next, glycine is added by glutathione synthetase GSS. Additionally, glutamate can be converted to glycine through a transamination reaction catalyzed by phosphoserine aminotransferase PSAT1 into phosphoserine 3-PS and α-KG. Phosphoserine is subsequently converted to glycine via serine hydroxymethyltransferase SHMT Fig. In cancer cells, the use of glutamate-derived nitrogen for NEAA production may be favored in various types of cancer cells to preserve nitrogen for anabolic reactions 48 and may prevent apoptosis induced by ATF4 activation upon glutamine deprivation 6. Asparagine can be synthesized de novo from glutamine via asparagine synthetase ASNS. Interestingly, asparagine was reported to be able to rescue cancer cells from glutamine deprivation-induced apoptosis This finding is surprising because asparagine supplementation does not restore the levels of other NEAAs alanine, proline, and glutamate or any TCA cycle intermediates α-KG, malate, and fumarate. Instead, asparagine supplementation enhances the expression of glutamine synthetase GLUL and increases intracellular glutamine usage via glutaminolysis, resulting in the recovery of global protein translation that is blocked by glutamine deprivation These studies suggest that most glutamine-dependent protein translation activities can still proceed under asparagine supplementation in a glutamine-deprived environment, although the exact mechanism is still unknown. Interestingly, high intracellular asparagine levels have recently been identified to be essential for breast cancer metastasis This study suggested that l -asparaginase treatment alone can reduce the incidence of breast cancer metastasis to the lung without affecting primary tumor growth. Although the clinical effect of l -asparaginase clearly indicates that asparagine is crucial for tumor survival and metastasis 52 , the importance of asparagine beyond protein synthesis and the mechanism by which asparagine supplementation enhances glutamine-associated metabolism are less well understood. Recently, asparagine has been reported to function as an exchange factor needed for the uptake of other amino acids that are required for mTORC1 activation 53 and for enhanced nucleotide biosynthesis under mitochondrial electron chain transport system impairment Further investigation is needed to explain the considerable mechanistic importance of asparagine in cancer metabolism. A low level of reactive oxygen species ROS activates tumorigenic growth signaling; however, when the level exceeds the cellular redox capacity, ROS can damage macromolecules such as proteins, lipids and nucleotides Recent studies suggest that cancer cells are under increased oxidative stress caused by oncogenic transformation, leading to metabolic alterations that result in ROS production Under these conditions, glutamine metabolism becomes essential for maintaining cellular redox homeostasis by harnessing enhanced ROS levels. The metabolic pathway by which glutamine mitigates ROS is the glutathione synthesis pathway 57 Fig. Glutathione is a tripeptide Glu—Cys—Gly that deactivates peroxide-free radicals. Glutamine is considered the rate-limiting factor in glutathione synthesis 58 , Indeed, experiments using uniformly labeled 13 C-glutamine showed that glutathione was enriched with five 13 C atoms in glutathione, suggesting that glutamine is the major source of glutathione 16 , 57 , As shown in Fig. Consistent with this observation, glutamine starvation has been associated with impaired uptake of cystine through xCT and decreased intracellular glutathione levels Furthermore, cells in several types of cancers are characterized by significant enhancement of glutathione biosynthesis, and this metabolic vulnerability has been targeted to sensitize these cancer cells to ROS-induced drugs In pancreatic cancer cells, glutamine supports the production of NADPH via a noncanonical metabolic pathway 29 , and the mitochondrial glutamine transporter is strongly associated with glutaminolysis-induced NADPH generation In addition, IDH1-dependent reductive glutamine metabolism produces NADPH, which decreases mitochondrial ROS during anchorage-independent growth In summary, glutamine maintains cellular redox homeostasis by supplying fuels for glutathione synthesis and endowing reducing power in the form of NADPH for sustaining tumor growth. Hypoxic conditions promote the uptake of glutamine by increasing the levels of glutamine transporters such as SLC1A5, the SLC1A5 variant, and SLC38A2 16 , 65 and switch the fate of glutamine from the oxidative pathway into the reductive carboxylation pathway This metabolic adaptation is critical because of the reduced entry of pyruvate into the TCA cycle by activated PDK1 and the increased lactate secretion in hypoxia Via this metabolic adaptation, cells can continually generate TCA metabolites, such as α-KG and citrate, which are converted to cytosolic acetyl-CoA for lipid biosynthesis Fig. Hypoxia stabilizes HIF-α proteins such as HIF-1α and HIF-2α. HIF-1α enhances glucose uptake and increases the level of glycolytic enzymes. Under hypoxic conditions, most glucose-derived pyruvate is converted into lactate via LDHA and exported to the extracellular space through the lactate transporters SLC16A1 and SLC16A4. Under these conditions, HIF-2α-mediated glutaminolysis becomes essential to support the adaptation to hypoxia, altering the metabolic fate of glutamine via reductive carboxylation to generate citrate. Then, citrate participates in fatty acid synthesis in the cytosol, which is also activated by stabilized HIF-2α. Hypoxia-induced acidic pH also plays a crucial role in the production of LHG by affecting the substrate affinities of LDHA and MDH. Next, LHG can control DNA or histone methylation levels by regulating α-KG-dependent dioxygenases. HIF hypoxia-inducible factor, GLS glutaminase, GLUD glutamate dehydrogenase, IDH isocitrate dehydrogenase, MDH malate dehydrogenase, L-2HGDH Lhydroxyglutarate dehydrogenase, LDHA lactate dehydrogenase, TETs ten-eleven translocation enzymes, JHDMs JmjC domain-containing histone demethylases, Gln glutamine, Glu glutamate, α-KG α-ketoglutarate, LHG Lhydroxyglutarate, Me methylation. HIF-α is the most well-known transcription factor activated in hypoxia. HIF-1α is activated due to blockade of its degradation pathway mediated by low oxygen levels, thereby increasing the expression of target genes, including those encoding glycolytic enzymes and glucose transporters, and increasing lactate secretion Although HIF-2α has biochemical characteristics similar to those of HIF-1α, the metabolic role of HIF-2α in a low-oxygen environment is relatively unknown Recently, hypoxia-induced expression of the SLC1A5 variant was shown to be mediated by HIF-2α and to lead to metabolic reprogramming toward glutamine metabolism in pancreatic cancer cells Given that HIF-2α is an important transcription factor in cancer progression and leads to poor prognosis 69 , 70 , these findings suggest that targeting HIF-2α might be an effective therapeutic strategy by inhibiting glutamine metabolism in these notorious cancers. Furthermore, long-term exposure of cancer cells to acidic extracellular conditions induces metabolic reprogramming toward glutamine metabolism via HIF-2α activity In addition, extracellular lactate stabilizes HIF-2α, and HIF-2α then transactivates MYC, increasing the levels of glutamine transporters and GLS1, in turn resulting in increased glutamine catabolism These findings indicate that just as HIF-1α generally affects glucose metabolism in hypoxia, HIF-2α also plays a distinct role in glutamine metabolism to promote metabolic adaptation in hypoxia Fig. Fatty acid synthesis is an anabolic process that uses cytosolic citrate to produce acetyl-CoA Glutamine acts as an alternative fuel for fatty acid synthesis, supplying citrate via mitochondrial reductive carboxylation, especially under hypoxic conditions 74 , In the context of constitutive HIF-2α stabilization 75 or a defective mitochondrial electron transport chain 76 , glutamine-derived α-KG is reductively carboxylated through the consumption of NADPH by IDH2 to generate citrate. Next, mitochondrial citrate is transported across the inner mitochondrial membrane via a citrate carrier CIC or SLC25A1 to support fatty acid synthesis for tumor progression in hypoxia 73 Fig. This mechanism is very important in clear cell renal cell carcinoma ccRCC in which HIF-2α signaling is constitutively activated and intracellular lipid droplets are abundant. Fatty acid synthesis induced by HIF-2α is crucial for cell viability in ccRCC by sustaining endoplasmic reticulum ER homeostasis Furthermore, HIF-2α represses the transcription of carnitine palmitoyltransferase 1A CPT1A , which is responsible for mitochondrial β-oxidation by transporting fatty acids and results in lipid deposition Indeed, recent studies have shown that HIF-2α can be targeted by selective inhibitors and have indicated that these molecules effectively suppress cancer cell growth and tumor angiogenesis characteristics in ccRCC 79 , 80 , 81 , Thus, HIF-2α-induced fatty acid synthesis using glutamine-derived citrate can be therapeutically targeted in several cancers, especially ccRCC. In several cancers, glutamine metabolism is closely related to hypoxia-induced chemoresistance For example, glutamine depletion has been shown to abolish hypoxia-induced chemoresistance in cholangiocarcinoma. Impairing glutamine metabolism also induces sensitivity in gemcitabine-resistant pancreatic cancer cells 16 , 84 , This bolstered chemoresistance in cancer cells is partially supported by glutathione synthesis via glutaminolysis Given the importance of glucose and glutamine metabolism in pancreatic cancer cells, it is not surprising that gemcitabine resistance is closely associated with metabolic status, including cellular glucose and glutamine levels. Hypoxia increases the deoxycytidine triphosphate dCTP level through the pentose phosphate pathway PPP via glucose metabolism and results in resistance to gemcitabine, a dCTP analog Furthermore, redox modulation augmented by increased glutathione synthesis from glutamine was reported to be the mechanism of resistance to gemcitabine in pancreatic cancer cells Consistent with these findings, while NRF2 induces chemoresistance in KRAS-driven cancers, suppressing glutamine metabolism leads to weakened chemoresistance in these cancer cells These studies suggest that targeting glutamine metabolism can be an effective cancer treatment strategy when combined with conventional anticancer chemotherapy. Under hypoxic conditions, Lhydroxyglutarate LHG was proven to be generated by lactate dehydrogenase A LDHA and malate dehydrogenase MDH 88 , Under normal physiological conditions, LDHA catalyzes the conversion of pyruvate to lactate. However, under hypoxic conditions, LDHA can produce LHG. The cellular metabolic alteration of increased LHG levels contributes to the regulation of histone and DNA methylation levels by inhibiting epigenetic modification enzymes that use α-ketoacid as a cofactor. These events mitigate cellular reductive stress by suppressing key metabolic pathways, indicating a crucial role of LHG. Acidic pH has also been reported to induce LHG production via the promiscuous activity of LDHA and MDH enzymes. Acidic pH impairs the activity of the mitochondrial LHG removal enzyme Lhydroxyglutarate dehydrogenase L2HGDH and enhances the protein stabilization of HIF-1α, leading to its escape from the degradation pathway In addition, LHG accumulation in an acidic pH environment has been reported to result in HIF-1α stabilization in normoxia 91 Fig. Homozygous L2HGDH mutations in germline transmission cause a disease named 2-hydroxyglutaric aciduria LHGA LHGA is an autosomal recessive encephalopathy with an onset in childhood that causes developmental delays, epilepsy and cerebellar ataxia, the traditional clinical signs of this condition. Interestingly, patients with LHGA are affected by tumors, including brain tumors 93 , bone tumors 94 , and nephroblastoma Wilms tumor Furthermore, increased LHG levels caused by reduced expression of L2HGDH have been reported in renal cancer These studies indicate an oncogenic effect of LHG and the association of LHG with tumorigenesis under hypoxic conditions. The metabolic state constitutes a fundamental component of chromatin modification and genome regulation As metabolites are the substrates used to generate chromatin modifications, including methylation and acetylation modifications of histones, a complicated interaction exists between metabolism and epigenetics. In particular, glutamine-derived α-KG has been implicated in regulating cellular histone and DNA methylation levels α-KG, also named 2-oxoglutarate, is a cofactor for 2-oxoglutarate-dependent dioxygenases 2-OGDDs , which catalyze hydroxylation reactions on diverse substrates. The activities of 2-OGDDs are affected by the intracellular level of α-KG, succinate, fumarate, or 2-HG. Among 2-OGDDs, Jumonji C domain-containing histone demethylases and ten-eleven translocation TET family DNA demethylases are major enzymes that induce epigenetic modifications using glutamine-derived α-KG. In these reactions, α-KG is oxidized to succinate, and increasing levels of succinate can suppress the progression of α-KG-dependent histone or DNA demethylase reactions a Several mutations in enzymes in the glutaminolysis pathway are responsible for the production of oncometabolites. Mutation of IDH1 and IDH2 produces RHG from α-KG, which, when accumulated, leads to the inhibition of dioxygenases, in turn leading to the activation of TET and JHDM enzymes inside the nucleus. Mutation of SDH arrests the TCA cycle, resulting in an increase in the succinate concentration. A high concentration of succinate has an effect similar to the oncometabolite effect of RHG. Additionally, impaired function of FH prevents further metabolism of fumarate, leading to its accumulation. FH impairment inhibits the function of Keap1 and PHD, which stimulates the transcription of protooncogenes. Gln glutamine, Glu glutamate, α-KG α-ketoglutarate, IDH isocitrate dehydrogenase, 2OGDH 2-oxoglutarate dehydrogenase, SDH succinate dehydrogenase, FH fumarate hydratase, RHG Rhydroxyglutarate, Keap1 Kelch-like ECH-associated protein 1, PHD prolyl hydroxylase, TETs ten-eleven translocation enzymes, JHDMs JmjC domain-containing histone demethylases, Me methylation. Glutamine anaplerosis is a key mitochondrial metabolic pathway for cancer cell growth and survival. Influx of glutamine-derived α-KG into the TCA cycle replenishes the intermediates and consequently generates NADH, FADH 2 , and GTP. The generated GTP can be readily converted to an equal amount of ATP. Additionally, glutamate and α-KG produced via glutaminolysis participate in the malate-aspartate shuttle, promoting the transport of NADH from the cytosol into mitochondria. Elevated mitochondrial NADH and FADH 2 levels collectively contribute to enhanced ATP production via OXPHOS through the ETC. In cancer cells, mutations in succinate dehydrogenase subunit B SDHB cause susceptibility to familial pheochromocytoma 99 and familial paraganglioma as well as gastrointestinal stromal tumors An increased ratio of succinate to α-KG in cancers resulting from impaired succinate dehydrogenase SDH activity is related to pervasive DNA hypermethylation, which contributes to the downregulation of key genes implicated in cell differentiation and cancer stages Moreover, the core region of solid tumors exhibits a deficiency of glutamine compared with other amino acids. This severe glutamine deprivation leads to dramatic histone hypermethylation due to decreased α-KG levels subsequent to decreased activity of Jumonji domain-containing histone demethylases and results in cancer cell dedifferentiation and resistance to BRAF inhibitors In addition to its role in cancer cells, α-KG supports the self-renewal of naive murine embryonic stem cells mESCs by promoting histone and DNA demethylation In addition, at later stages of pluripotency, α-KG derived from glutamine can promote early differentiation, suggesting that the stage of cellular maturity can alter the effect of α-KG Furthermore, PSAT1 regulates changes in the level of glutamine-derived α-KG, which controls mESC pluripotency and differentiation These reports suggest that α-KG generated via glutaminolysis is closely related to the cellular decisions that characterize stem cells. In skeletal stem cells SSCs , GLS and glutamine metabolism are required for the regulation of osteoblast and adipocyte specification and physiological bone formation In macrophage cells, α-KG produced via glutaminolysis promotes M2 activation via Jmjd3-dependent metabolic and epigenetic reprogramming Recently, glutamine metabolism has been shown to be linked to white adipose tissue WAT inflammation in obesity The researchers discovered that glutamine metabolism is impaired in the obese state, leading to increased chromatin O-GlcNAcylation and activation of genes in proinflammatory pathways. Collectively, glutamine-derived metabolites act as epigenetic modulators in a wide range of cell and tissue types, including various types of cancer cells, stem cells, immune cells, and even adipocytes. Considering that the SLC1A5 variant is an important regulator of the production of glutamine-derived α-KG 16 , confirming whether epigenetic regulation by glutamine-derived α-KG is affected by the SLC1A5 variant in cancer cells or stem cells is necessary Fig. Genetic and metabolic studies have further shown that metabolites such as succinate and fumarate, which are generated under normal physiological conditions, are associated with tumorigenesis in several cancer types. Interestingly, these metabolites were often found to be associated with glutamine metabolism In particular, the production of these oncometabolites was affected by the level of glutamine-derived α-KG. Although additional studies are needed, ample experimental data support the recognition of RHG, succinate, and fumarate as oncometabolites. A high level of RHG is sufficient to cause leukemia to arise from hematopoietic cells by maintaining their dedifferentiation and proliferation activities The role of RHG as an oncometabolite has been implicated in epigenetic modifications through the inhibition of α-KG-dependent dioxygenases and demethylases, which has been assumed to be a driver of tumorigenesis , In addition, dysregulated α-KG flux from normal reductive anabolism via the TCA cycle toward RHG production has been associated with other metabolic flux impairments and disrupted redox balance , Fig. Indeed, glutamine-derived RHG accumulates and prevents the differentiation of myeloblasts, resulting in uncontrolled growth of blood cells After FDA approval of enasidenib, a first-in-class drug targeting cancer metabolism via inhibition of IDH2 activity, more studies were conducted with RHG positioned as an oncometabolite. As the importance of RHG in boosting tumor initiation, proliferation and metastasis is emphasized, identifying whether metabolic enzymes or transporters associated with glutamine metabolism could be involved in the generation of RHG is interesting. The normally functioning SDH enzyme is localized in the inner mitochondrial membrane and plays a role in the electron transport chain as well as the conversion of succinate into fumarate. In , mutation of SDH was discovered in cancers such as paraganglioma and pheochromocytoma cells Later, similar observations were made in gastrointestinal tumors, neuroblastomas, renal tumors, thyroid tumors, and testicular tumors , Several research groups have focused on the mechanism that underlying the features of tumorigenesis and cancer cell survival in the setting of SDH mutations. As succinate accumulates via the inhibition of the 2-OGDD enzyme, epigenetic modification acts in the process of cell transformation into a hypermethylated phenotype Several studies have shown that SDH-deficient cells exhibit increased tumorigenesis and that this increase is reversed by the addition of α-KG, supporting the idea that succinate accumulation contributes to tumorigenesis through epigenetic modification Succinate-specific effects are initiated by epigenetic alterations through the inhibition of KDMs and the TET family 5mC hydroxylases, which induce the translation of tumorigenic genes Fig. The other mechanism by which succinate supports tumorigenesis acts through the inhibition of hypoxia-inducible factor prolyl hydroxylase PHD. PHD activates the pseudohypoxic response by stabilizing HIF-1α, which is a well-known tumorigenesis enhancer, and as a transcription factor, maintains the metabolic reprogramming of cancer cells to support their survival In addition to the tumorigenic effects of succinate accumulation, SDH5 mutation is the key driver supporting the acquisition of epithelial—mesenchymal transition EMT characteristics. The results of a clinical study further confirmed this observation by showing that patients with nonmetastatic lung cancer harbored loss-of-function mutations in SDH5 The study of succinate as an oncometabolite has only recently begun, and more research needs to be conducted to completely understand its tumorigenic properties. Fumarate is another example of an oncometabolite produced by the action of fumarate hydratase on succinate. In , mutation of fumarate hydratase leading to its inactivation was discovered in renal cell cancer Mutation of this enzyme leads to fumarate accumulation not only in skin cancer and uterine leiomyomas but also in breast, bladder, and Leydig cell tumors Further confirmation of fumarate as an oncometabolite was verified by experimental data showing that tumor cells lost their ability to invade and migrate when the function of fumarate hydratase was restored by an external expression vector In attempts to understand the cause of these effects, it was found that cells with high concentrations of fumarate display a phenotype of DNA hypermethylation. In addition, fumarate inhibits TET enzymes, which stimulate EMT, leading to cancer metastasis , Similar to succinate, fumarate contributes to the inactivation of PHD, stabilizing HIF proteins to promote cell survival Fig. In addition, accumulated fumarate can participate in different reactions of the addition of a succinate group to the thiol group of various proteins. For example, in hereditary leiomyomatosis and renal cell cancer HLRCC , a high level of fumarate caused by genetic mutation of fumarate hydratase induces the succination of Kelch-like ECH-associated protein 1 KEAP1 accompanied by the consumption of a fumarate molecule , Endogenously, succinylated KEAP1 dissociates from the NRF2 protein to help cancer cells survive stress. High concentrations of fumarate bind to glutathione, augmenting ROS signaling and accumulation, as observed in not only in vitro models but also in vivo models , Additionally, high levels of fumarate react with the cysteine group of mitochondrial aconitase-2 and iron-sulfur cluster binding protein-2, facilitating cellular metabolic adaptation to stresses The importance of fumarate hydratase mutation for cancer survival and growth is being studied in depth to completely understand the role of fumarate as a tumorigenic oncometabolite. This knowledge will aid in the complete comprehension of cancer metabolism. The influx of α-KG into the TCA cycle and its subsequent oxidization generates two molecules of NADH and one molecule of FADH 2 from the series of reactions catalyzed by OGDH, SDH, and MDH. Additionally, when succinyl-CoA is converted to succinate by succinate thiokinase, one molecule of GTP is generated, which can be readily converted to ATP by nucleoside-diphosphate kinase NDPK. NADH and FADH2 produced via glutaminolysis are then fed into the electron transport chain to create the electrochemical gradient necessary for ATP production via oxidative phosphorylation , Fig. Correspondingly, in K-Ras mutant cells, the oxygen consumption rate and ATP generation are enhanced by glutamine, contributing to tumorigenesis Additionally, the level of the mitochondrial glutamine transporter controls the cellular ATP level stimulated by glutamine, suggesting that glutamine is an important energy source via mitochondrial glutaminolysis Collectively, these observations indicate that anaplerotic glutamine metabolism is highly responsible for energy generation in cancer cells. Additionally, NADH can be generated by fatty acid oxidation FAO in the cytoplasm in tissues with high energy demand, such as cardiac muscle tissues, as well as in cancer cells Recent studies have suggested that in cancer cells with elevated cytosolic NADH levels, the malate-aspartate shuttle MAS actively takes up NADH to produce ATP in mitochondria through the electron transport chain Glutamate and α-KG serve as important exchangers in the MAS, and since GLS1 knockdown significantly suppresses NADH and ATP production in cancer cells , the supply of glutamate and α-KG for the induction of MAS activity is evidently critical for ATP production in cancer cells Fig. Glutamine is the most abundant amino acid in the blood. During cellular stress, such as nutrient starvation and catabolic stress after trauma, surgery, infection, sepsis, or cancer cachexia, blood glutamine levels are severely decreased Under these conditions, several studies have reported that glutamine supplementation can offer a therapeutic approach for these critical illnesses , , Glutamine has been considered an immunomodulatory amino acid in several disease states, yet the mechanisms underlying the therapeutic effects of glutamine supplementation in critical illness remain poorly understood. Conceivably, glutamine could exert its beneficial effects by producing glutathione for redox homeostasis, maintaining nitrogen balance, or other functions in immune cells 2. Consistent with the importance of glutamine in stressful situations, glutamine deprivation induces cellular stress. Upon glutamine starvation, p53 activity is induced and can help cancer cells adapt to nutrient starvation through diverse mechanisms Recently, SLC1A3, as a crucial effector of p53, has been shown to support cell survival and growth in the absence of glutamine Under DNA damage such as radiation, glutamine is conditionally essential to support the synthesis of nucleotides and redox homeostasis. It has recently been demonstrated that radioresistant cancer cells reprogram metabolic flux toward glutamine anabolism. Under these conditions, cancer cells highly express glutamine synthetase, facilitating cancer cell growth under radiation stress Moreover, evidence has shown that during the DNA damage response, normal cells show a decrease in glutaminolysis controlled by SIRT4 protein suppressing GLUD1. In the absence of SIRT4, a failure to undergo cell cycle arrest induced by DNA damage causes a delay in DNA repair and increased chromosomal instability, suggesting a tumor suppressor effect of SIRT4 Numerous studies have described the presence of alternative adaptive pathways upon the perturbation of glutamine metabolism. For instance, a recent study has shown that GLS1 inhibition induces an increase in mitochondrial glutamate-pyruvate transaminase 2 GPT2 to assist in TCA cycle anaplerosis for sustaining cancer cell growth and survival Of note, GLS1 inhibition causes an elevation of the ROS level and induces GPT2 expression via ATF4, which again implies the importance of ATF4-mediated metabolic adaption during glutamine starvation. Additionally, metabolic profiling has revealed that suppression of GLS1 induces a compensatory anaplerotic mechanism via pyruvate carboxylase PC , which allows the release of a glutamine-independent supply of TCA intermediates by catalyzing the transformation of pyruvate to oxaloacetate This PC-mediated alternative anaplerosis is considered important in specific types of cancers, including liver cancers and glioblastoma, for maintaining biosynthesis and redox homeostasis , , Collectively, cancer glutamine metabolism shows extraordinary flexibility and is intertwined with diverse metabolic pathways. Unsurprisingly, glutamine metabolism plays a critical role in tumor progression since it not only supports mitochondrial oxidative phosphorylation but also supplies metabolic intermediates for the TCA cycle, glutathione synthesis, and NEAA synthesis and simultaneously produces NADPH , , Recently, glutamine was shown to be a major fuel for mitochondrial oxygen consumption in pancreatic cancer cells; in addition, the expression of the SLC1A5 variant affected the levels of metabolites derived from glucose metabolism, including lactate and ribulosephosphate, the intermediate metabolites in the PPP Intriguingly, this study regarding elevated glutamine metabolism in cancer cells also showed that glutaminolysis could in turn reinforce metabolic reprogramming, thus implying that glutamine metabolism plays a crucial role in tumorigenesis and tumor progression 16 Fig. Indeed, the process of adaptation to glutamine deprivation weakens the response to hypoxia, which normally strongly induces the expression of glycolytic enzymes a Aerobic glycolysis is a hallmark of cancer metabolism. During this process, most glucose-derived pyruvate is secreted extracellularly as lactate, and glutamine becomes a conditionally essential amino acid. Glutaminolysis sustains mitochondrial function, supplying TCA cycle metabolites such as αKG and generating diverse biomolecules, including NEAAs, NADPH, and nucleotides. Increased glutamine flux into the mitochondrial matrix via the SLC1A5 variant can enhance glutaminolysis and lead to metabolic reprogramming toward enhanced aerobic glycolysis. b Glutamine-derived α-KG activates the mTORC1 signaling pathway, resulting in aerobic glycolysis and protein translation, which are crucial for tumor proliferation. c During glutaminolysis, ammonium ions are generated via a deamidation reaction catalyzed by glutaminase and glutamate dehydrogenase. Most ammonium ions are used as a nitrogen source for nucleotide biosynthesis and are disposed of via the urea cycle, but an excess of ammonium ions promotes autophagy. Augmented autophagy is associated with drug resistance by enhancing aerobic glycolysis and is involved in cancer cell survival, progression, and metastasis. Gln glutamine, Glu glutamate, α-KG a-ketoglutarate, PHD prolyl hydroxylase. As previously described, glutamine is metabolized by mitochondrial enzymes into α-KG, which serves as an important intermediate in the TCA cycle for anaplerosis. Furthermore, enhanced production of α-KG causes other critical effects, such as stimulation of the signaling pathways that support cell growth. α-KG induces mTORC1 activation by enhancing GTP loading of the RagB protein in a PHD-dependent manner, thus promoting cell growth , Accordingly, high mTORC1 activity in cancer cells promotes aerobic glycolysis and drives glucose addiction , Fig. In addition, mTORC1 activation via glutaminolysis suppresses autophagy and the DNA damage response , Therefore, enhanced glutaminolysis might eventually contribute to the initiation and progression of cancer by stimulating cell growth via the mTORC1 pathway and enhancing aerobic glycolysis while disrupting the proper elimination of misfolded proteins, damaged DNA and organelles through the inhibition of autophagy and the DNA damage response Enhanced glutaminolysis in cancer cells ensures a stable supply of glutamate and α-KG via sequential deamination processes inside mitochondria. Notably, ammonia is simultaneously generated as a byproduct of glutamine deamination. Hence, the facilitation of glutaminolysis leads to the accumulation of excess ammonia within cells, and a high concentration of ammonia is a potent inducer of autophagy Fig. Although mTORC1 activation hinders autophagy, evidence has shown that autophagy can be upregulated in tumors with mTORC1 hyperactivation Therefore, glutaminolysis can suppress autophagy by activating the mTORC1 pathway but, on the other hand, can stimulate autophagy in the context of excess ammonia production. The fundamental need for ammonia-mediated induction of autophagy in cancer cells could be due to the cytoprotective functions of this event that allow cells to survive under extreme conditions Specifically, autophagy suppresses anoikis induced by the detachment of cancer cells from the extracellular matrix ECM and hence promotes metastasis Furthermore, autophagy has been shown to promote glycolysis in hepatocellular carcinoma HCC cells by upregulating monocarboxylate transporter 1 MCT1 , which plays an important role in the transport of lactic acid Therefore, autophagy supports cancer progression and chemoresistance by allowing tumor cells to overcome both environmental and intracellular stress signals, including nutrient deprivation and chemotherapeutic cytotoxicities , , Fig. However, the connection between glutamine and metabolic remodeling in cancer from the perspective of glucose metabolic flux, the mTORC1 pathway and autophagy has yet to be fully explored. This link might partially be explained by considering that the intimately entwined glucose and glutamine metabolic pathways cooperatively support the TCA cycle and that glutamine performs diverse functions for maintaining cellular homeostasis. Collectively, in-depth investigation of the role of glutaminolysis in tumor progression might hold the key for decoding cancer metabolic plasticity. The excessive proliferation exhibited by cancer cells demands a constant supply of fuels such as glucose and glutamine. Therefore, cancer cells orchestrate their metabolic pathways to coordinate their high demand for these nutrients. Metabolic reprogramming that promotes enhanced glutamine consumption in cancer cells is closely connected with dysregulation of oncogenes. Efforts have been undertaken to reveal the mechanism by which oncogenes modulate metabolic pathways that favor cancer cell growth Notably, cancer cells driven by oncogenic MYC, K-Ras, and PIK3CA require glutamine for their survival and display extensive anabolic utilization of glutamine 29 , , Fig. Oncogenes such as MYC, K-Ras, and PI3KCA modulate cancer metabolic reprogramming, favoring cancer cell growth and survival partially via the promotion of glutamine metabolism. Glutamine uptake is enhanced in MYC- and K-Ras-driven cells in which the expression of the glutamine transporter SLC1A5 is upregulated. Deamination of glutamine to form glutamate in mitochondria is enhanced by MYC-mediated upregulation of GLS1. The expression of these enzymes is upregulated in cancer cells with MYC-driven, K-Ras-driven, and PI3KCA-driven signaling activation. In cancer cells, genetic and epigenetic dysregulation of MYC expression and the loss of checkpoint components unleash the ability of MYC to promote cell growth, eventually leading to malignant transformation Oncogenic Myc stimulates mitochondrial glutaminolysis via transcriptional regulation of genes necessary for cellular glutamine catabolism Moreover, MYC upregulates the glutamine transporter SLC1A5 to facilitate glutamine uptake into cells MYC-dependent enhancement of mitochondrial glutaminolysis leads to the reprogramming of mitochondrial metabolism to accommodate the requirements for TCA cycle anaplerosis to sustain cellular viability and growth. Similar to the situation in MYC-driven cancer cells, glutamine uptake is enhanced in K-Ras-driven cells via upregulation of SLC1A5 Additionally, K-Ras-driven cells are characterized by increased expression of GOT1 and GOT2 , GOT1 and GOT2 catalyze the transamination reaction between oxaloacetate and glutamate to produce aspartate and α-KG. Significantly, enhanced transamination and aspartate synthesis in K-Ras-driven cancer cells are important in the promotion of nucleotide biosynthesis and maintenance of redox balance Intriguingly, the glutamine-dependent checkpoint at late G1 phase in the cell cycle is dysregulated in K-Ras-driven cancer cells In normal cells, the cell cycle is tightly regulated by various checkpoints. Nutrient-dependent checkpoints regulate cell cycle passage through late G1 phase by sensing nutrient availability; glutamine is a particularly critical nutrient sensed in late G1 phase, and its deprivation causes cell cycle arrest at G1 phase Importantly, activation of K-Ras in cancer cells results in bypass of the late G1 glutamine-dependent checkpoint. Consistent with this observation, K-Ras sensitizes cells to glutamine deprivation, and K-Ras knockdown rescues cells from apoptosis induced by low glutamine levels Collectively, these findings indicate that enhanced glutamine metabolism and cell growth dysregulation are established in K-Ras-driven cancer cells to promote uncontrolled cell growth and to assist with glutamine acquisition and utilization for cell growth. The PI3K signaling pathway is dysregulated in many tumors, and analyses have shown that PIK3CA is an oncogene that also contributes to tumor progression partially via metabolic reprogramming Oncogenic PIK3CA increases the dependency of cancer cells on glutamine by upregulating the expression of mitochondrial GPT2, which catalyzes the transamination reaction that converts glutamate and pyruvate into α-KG and alanine Thus, cells with PIK3CA mutations exhibit increased sensitivity to glutamine deprivation. Additionally, compared with wild-type cells, PIK3CA mutant colorectal cancer CRC cells exhibit elevated anaplerotic α-KG production and ATP generation from glutamine. In addition to oncogenic regulators, there are some key upstream regulators of glutamine metabolism that are widely recognized for their pivotal role during tumorigenesis. mTORC1, which is well known for its function at the center of cancer metabolic reprogramming, promotes mitochondrial glutaminolysis via the migration of SIRT4-mediated inhibition of GLUD1 Specifically, mTORC1 promotes proteasome-mediated destabilization of cAMP response element binding-2 CREB2 to suppress transcription of SIRT4. Accordingly, loss of SIRT4 enhances glutamine-dependent proliferation and genomic instability, which simultaneously contribute to tumorigenesis Furthermore, mTORC1 also acts as a downstream effector of glutamine. Glutamine itself, or after its conversion into α-KG, activates the mTORC1 pathway and participates in the growth signaling pathway. Evidence has shown that glutamine activates the mTORC1 pathway via Arf1 rather than via the Rag GTPase complex in MEFs According to another study, glutaminolysis increases the level of α-KG production, resulting in GTP loading of RagB and lysosomal translocation of the mTORC1 complex in human cancer cell lines It has been reported that cellular uptake of glutamine and its subsequent efflux in the presence of essential amino acids, including leucine, is the rate-determining step that activates mTORC1 Moreover, glutamine also acts as a precursor for the synthesis of various NEAAs, including asparagine and arginine, implicated in mTORC1 activation Thus, cells have diverse mechanisms of mTORC1 activation for glutamine, and cancer cells efficiently utilize glutamine for mTORC1 pathway activation to drive unrestrained oncogenic growth. Although the essential role of glutamine metabolism in cancer cells has been well demonstrated in vitro, the extent to which glutamine supports tumor growth and survival in vivo remains elusive. It has been reported that K-Ras-driven mouse lung tumors preferentially utilize glucose more than glutamine to supply carbon to the TCA cycle via pyruvate carboxylase Furthermore, human glioblastoma cells do not rely much on circulating glutamine for proliferation but rather more on glutamate to synthesize glutamine via glutamine synthetase to fuel purine biosynthesis Nevertheless, the specific metabolic importance of glutamine in tumorigenesis and tumor growth has also been reported , , , and these studies have led many researchers to target glutamine metabolism for the treatment of cancer 8. Throughout the discovery of agents targeting glutaminolysis, none have yet been used clinically A recent attempt focused on the inhibition of GLSs. GLS overexpression has been observed in different tumor cells, and these enzymes are found to function in the metabolic reprogramming of glutamine addiction in cancer Chemical agents targeting GLSs have been studied, and CB, , and BPTES have been found to exhibit tumor-specific antiproliferative effects Among these agents, CB is the only one to proceed to clinical trials; however, its selectivity toward GLS1 and failure to inhibit the compensatory effect of GLS2 require in-depth study A recent study discovered a prodrug JHU of the glutamine antagonist DON, which was designed to selectively become activated inside a tumor. The researchers showed that blocking glutamine metabolism through JHU not only suppressed tumor cell metabolism but also mitigated the tumor microenvironment, which is hostile to the immune response due to its hypoxic, acidic, and nutrient-depleted conditions, unleashing the natural antitumor T cell response. They also confirmed that concurrent treatment with JHU and anti-PD-1 checkpoint inhibitor improved the antitumor effects compared with anti-PD-1 treatment alone, suggesting the presence of metabolic plasticity between cancer cells and effector T cells, which could be exploited as a metabolic checkpoint for cancer immunotherapy The plasma membrane glutamine transporters SLC6A14, SLC7A11, and SLC38A1 have been targeted and found to be inhibited by erastin, α-Me-Trp, and MeAIB, respectively Fig. In addition, SLC1A5 was shown to have clinical importance, and it is considered the most critical plasma membrane glutamine transporter in cancer cells Many attempts have been made to explore the possibility that SLC1A5 suppression via small molecules might exert anticancer effects. As part of this effort, benzylserine and benzylcysteine were discovered in as the first substrate analog inhibitors of SLC1A5 In an effort to improve the potency and efficacy of such inhibitors, some studies have discovered GPNA, which is widely used as a tool compound for suppressing SLC1A5 Other studies have developed antibodies with high affinity for SLC1A5, which induce antibody-dependent cellular toxicity in gastric cancer models Recently, a potent inhibitor of SLC1A5, V, has been reported to be effective in several cancer cell lines and in vivo tumor models However, other researchers have argued that controversial issues exist because GPNA also inhibits other glutamine transporters, such as SLC38A1, and V is effective even in SLC1A5 knockout models , Hence, to date, no suitable compound has been identified to inhibit the plasma membrane glutamine transporter SLC1A5 with excellent sensitivity and specificity. For the principal inhibition of glutaminolysis, attempts have been made to target the amino acid transporters related to these pathways. SLC6A14 and SLC38A1 are inhibited by α-Me-Trp and MeIAB, respectively. The most intensely researched topic is inhibitors of SLC1A5, a major glutamine transporter, which include substrate analog competitive inhibitors such as GPNA, benzylserine, and V and the inhibitory antibody MEDI Although they exhibit low potency, inhibitors of SLC7A11 include erastin and SSZ. Inhibitors of glutaminolytic enzymes are agents that target GLS1, GOT2, and GLUD1. CB, an agent in its 2nd clinical trial, inhibits GLS1 similarly to BPTES and AOA inhibits GOT2 activity, and EGCG, purpurin, and R inactivate GLUD1. However, the SLC1A5 variant, the sole glutamine transporter discovered to date, is expected to be a much more effective target for cancer therapeutics than previously studied glutaminolysis inhibitors. Cys cysteine, Glu glutamate, α-KG α-ketoglutarate, GLS glutaminase, GOT2 glutamic-oxaloacetic transaminase 2, GPT2 glutamic-pyruvate transaminase 2, GLUD1 glutamate dehydrogenase 1, α-Me-Trp alpha-methyl-tryptophan, MeAIB methylaminoisobutyric acid, GPNA L-γ-glutamyl-p-nitroanilide, SSZ sulfasalazine, DON 6-diazooxo- l -norleucine, AOA aminooxyacetate, EGCG epigallocatechingallate. SLC1A5 might not be an appropriate target for suppressing glutamine uptake by cancer cells because it is not the only plasma membrane glutamine transporter, and its function would therefore be compensated by other redundant glutamine transporters such as SLC38A1 and SLC38A2. Thus, as the SLC1A5 variant is the only currently known glutamine transporter in the mitochondrial inner membrane 16 , targeting the SLC1A5 variant could be an effective strategy for selectively inhibiting glutamine metabolism in cancer cells Fig. Given the clinicopathological significance of SLC1A5 and the observation that the level of the SLC1A5 variant is negatively correlated with prognosis in several cancer types 16 , targeting the SLC1A5 variant is a promising strategy to starve cancer cells and induce antitumor effects. Therefore, further studies on the development of selective inhibitors of the mitochondrial SLC1A5 variant are needed and should help to establish whether the level of the SLC1A5 variant is a predictive marker of glutamine dependency in cancer Although Otto Warburg characterized cancer metabolism by its enhanced glucose consumption and loss of mitochondrial function, many studies have shown that mitochondrial function in cancer cells is still robust and even enhanced. Moreover, glutamine has been discovered to be required for the maintenance of active mitochondrial function in cancer cells. Glutamine has historically been one of the most intensely investigated nutrients in cancer metabolism and is involved in various aspects of biosynthesis and bioenergetics, including NEAA production, epigenetic gene control, adaptation to hypoxic conditions, ATP synthesis, cell signaling, and tumorigenesis. In this review, we offer an updated overview of glutamine metabolism and discuss the reason for glutamine dependency in cell metabolism. Certain types of cancer, including renal cell carcinoma, hematologic malignancies, glioblastoma, pancreatic cancer, and those reported to depend on HIF-2α, seem to depend on glutamine; hence, targeting glutamine metabolism may show therapeutic effects in these cancers. Moreover, metabolite transporters have recently been shown to be involved in tumorigenesis; for example, low levels of mitochondrial pyruvate carriers initiate colon cancer development Conversely, suppression of the SLC1A5 variant, a mitochondrial glutamine transporter, is sufficient to inhibit tumor growth by impairing glutamine metabolism in pancreatic cancer cells As the importance of subcellular metabolite transporters in controlling tumor initiation is poorly understood, it would be interesting to determine whether overexpression or knockout of these transporters is involved in tumorigenesis, metastasis, and immune modulation. In conclusion, metabolic reliance on glutamine arises via the intrinsic functional diversity of glutamine, supporting macromolecule biosynthesis and reinforcing the TCA cycle. In the context of tumorigenesis, glutamine-derived 2-HG alters the epigenetic landscape of chromosomes and induces oncogenic transformation. Further investigations to explain the mechanism underlying glutaminolysis-induced metabolic reprogramming are needed. These efforts are anticipated to reveal new metabolic vulnerabilities of cancer cells that can be targeted by therapeutic interventions. Warburg, O. On the origin of cancer cells. Science , — Article CAS PubMed Google Scholar. DeBerardinis, R. Oncogene 29 , — Hensley, C. Glutamine and cancer: cell biology, physiology, and clinical opportunities. Article CAS PubMed PubMed Central Google Scholar. Altman, B. From Krebs to clinic: glutamine metabolism to cancer therapy. Cancer 16 , Zhang, J. Cancer cell metabolism: the essential role of the nonessential amino acid, glutamine. Embo J. Qing, G. et al. ATF4 regulates MYC-mediated neuroblastoma cell death upon glutamine deprivation. Cancer Cell 22 , — Chen, L. Targeting glutamine induces apoptosis: a cancer therapy approach. Wise, D. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem. Klingman, J. Partial purification and properties of renal glutaminase. Eagle, H. Nutrition needs of mammalian cells in tissue culture. Kovacevic, Z. The role of glutamine in the oxidative metabolism of malignant cells. Cancer Res. CAS PubMed Google Scholar. Reitzer, L. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. Lobo, C. Inhibition of glutaminase expression by antisense mRNA decreases growth and tumourigenicity of tumour cells. Gross, M. Antitumor activity of the glutaminase inhibitor CB in triple-negative breast cancer. Cancer Ther. Kizilbash, S. The addition of CB to temozolomide significantly reduces glioma aspartate and glutamate in an IDH mutated patient derived glioma xenograft model. AM Yoo, H. A variant of SLC1A5 is a mitochondrial glutamine transporter for metabolic reprogramming in cancer cells. Cell Metab. Scalise, M. Glutamine transport and mitochondrial metabolism in cancer cell growth. Wang, J. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell 18 , — Article CAS Google Scholar. Li, B. EBioMedicine 39 , — Article PubMed Google Scholar. Lukey, M. Liver-type glutaminase GLS2 is a druggable metabolic node in luminal-subtype breast cancer. Cell Rep. Stine, Z. Glutamine skipping the Q into mitochondria. Trends Mol. Mullen, A. Oxidation of alpha-ketoglutarate is required for reductive carboxylation in cancer cells with mitochondrial defects. Yang, L. Glutaminolysis: a hallmark of cancer metabolism. Metallo, C. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature , —U Sun, R. Hypoxic regulation of glutamine metabolism through HIF1 and SIAH2 supports lipid synthesis that is necessary for tumor growth. Lu, V. Chang, S. Sullivan, L. Supporting aspartate biosynthesis is an essential function of respiration in proliferating. Cell , — Son, J. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Tong, X. The molecular determinants of de novo nucleotide biosynthesis in cancer cells. Moffatt, B. Purine and pyrimidine nucleotide synthesis and metabolism. Arabidopsis Book 1 , e Article PubMed PubMed Central Google Scholar. Kim, J. Nature , — Patel, D. Aspartate rescues S-phase arrest caused by suppression of glutamine utilization in KRas-driven cancer cells. Ben-Sahra, I. Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Robitaille, A. Quantitative phosphoproteomics reveal mTORC1 activates de novo pyrimidine synthesis. Liu, Y. Global regulation of nucleotide biosynthetic genes by c-Myc. PLoS ONE 3 , e Article PubMed PubMed Central CAS Google Scholar. Lane, A. Regulation of mammalian nucleotide metabolism and biosynthesis. Nucleic Acids Res. Santana-Codina, N. Oncogenic KRAS supports pancreatic cancer through regulation of nucleotide synthesis. Choi, B. The diverse functions of non-essential amino acids in cancer. Cancers 11 , Thornburg, J. Targeting aspartate aminotransferase in breast cancer. Breast Cancer Res. Korangath, P. Targeting glutamine metabolism in breast cancer with aminooxyacetate. Liu, B. Overexpression of phosphoserine aminotransferase 1 PSAT1 predicts poor prognosis and associates with tumor progression in human esophageal squamous cell carcinoma. Asparagine plays a critical role in regulating cellular adaptation to glutamine depletion. Cell 56 , — Huang, H. Role of glutamine and interlinked asparagine metabolism in vessel formation. Pavlova, N. As extracellular glutamine levels decline, asparagine becomes an essential amino acid. LeBoeuf, S. Activation of oxidative stress response in cancer generates a druggable dependency on exogenous non-essential amino acids. Sayin, V. Activation of the NRF2 antioxidant program generates an imbalance in central carbon metabolism in cancer. eLife 6 , e Coloff, J. Differential glutamate metabolism in proliferating and quiescent mammary epithelial cells. Zhu, Y. A critical role of glutamine and asparagine gamma-nitrogen in nucleotide biosynthesis in cancer cells hijacked by an oncogenic virus. Mbio 8 , e—17 Pant, A. Asparagine is a critical limiting metabolite for vaccinia virus protein synthesis during glutamine deprivation. Knott, S. Asparagine bioavailability governs metastasis in a model of breast cancer. Hill, J. L-asparaginase therapy for leukemia and other malignant neoplasms. Remission in human leukemia. Jama , — Krall, A. Asparagine promotes cancer cell proliferation through use as an amino acid exchange factor. Asparagine signals mitochondrial respiration and can be targeted to impair tumour growth. bioRxiv , Weinberg, F. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Natl Acad. USA , — Gorrini, C. Modulation of oxidative stress as an anticancer strategy. Drug Discov. Amores-Sanchez, M. Glutamine, as a precursor of glutathione, and oxidative stress. Welbourne, T. Ammonia production and glutamine incorporation into glutathione in the functioning rat kidney. Sappington, D. Glutamine drives glutathione synthesis and contributes to radiation sensitivity of A and H lung cancer cell lines. Acta , — Tompkins, S. Disrupting mitochondrial pyruvate uptake directs glutamine into the TCA cycle away from glutathione synthesis and impairs hepatocellular tumorigenesis. Muir, A. Environmental cystine drives glutamine anaplerosis and sensitizes cancer cells to glutaminase inhibition. Timmerman, L. Glutamine sensitivity analysis identifies the xCT antiporter as a common triple-negative breast tumor therapeutic target. Cancer Cell 24 , — Okazaki, K. Metabolic features of cancer cells in NRF2 addiction status. Jiang, L. Reductive carboxylation supports redox homeostasis during anchorage-independent growth. Morotti, M. HIFmediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Article PubMed CAS Google Scholar. Denko, N. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Cancer 8 , — Goda, N. Hypoxia-inducible factors and their roles in energy metabolism. Zhao, J. The role of hypoxia-inducible factor-2 in digestive system cancers. Cell Death Dis. Li, Z. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. |
Video
The #1 Best Anti-inflammatory Food in the World (Surprising)Glutamine and cell regeneration -
Glc, glucose; Gln, glutamine; Vera, Verapamil. Based on the above observation that glutamine deprivation significantly affected the fraction of SP cells, we reasoned that blocking glutamine metabolism could also reduce SP cells.
For this purpose, a clinical drug L-asparaginase L-ASP , which catalyzes the hydrolysis of asparagine to aspartate and used in the treatment of acute lymphoblastic leukemia ALL in children [ 20 , 21 ], was used in this study to enzymatically deplete glutamine by its glutaminase activity [ 22 , 23 ].
Consistently, glutaminase also diminished the proportion of SP cells Additional file 1 : Figure S2. These data together suggest that glutamine depletion by either direct removal from the medium or enzymatic depletion significantly diminished the fraction of SP cells.
Effect of L-Asparaginase on SP cells. a Conversion of asparagine to asparatic acid or glutamine to glutamate catalyzed by asparaginase. b Generation of glutamate from glutamine by L-Asparaginase. Then cells were harvested and stained with Hoechst to determine SP fraction.
In agreement with the observation that glutamine deprivation or L-ASP treatment reduced SP fractions, both the removal of glutamine and incubation with L-ASP markedly inhibited clonogenic formation in A cells Fig. We also observed that the size of A cells became irregular and had flagella-like morphology under glutamine deprivation for 72 h Fig.
The impact of glutamine on the expression of cancer stem cell markers was further evaluated. Western blot analysis of protein expression Fig.
Sox-2 and ABCG2 expression were also decreased after cells were incubated with L-ASP, both at the transcriptional and translational levels Fig. Since the ABCG2 on the membrane plays a major role in exporting drugs and the Hoechst dye out of the cells, we quantified the change of ABCG2 in A cells by flow cytometry in the presence or absence of glutamine or L-asparaginase.
ABCG2 expression on the cell membrane was decreased in the absence of glutamine or in the presence of L-asparaginase Fig. We also tested two glioblastoma stem cell lines GSC11 and GSC23 originally obtained from primary glioblastoma tissues with high levels of stem cell marker CD and can easily form neuospheres [ 12 , 27 ], and showed that glutamine deprivation or L-ASP treatment caused a reduced neurosphere capacity Additional file 1 : Figure S3A and S3B.
Impact of glutamine on A cells clonogenic capacity and the expression of stem-cell associated molecules. Colony numbers were counted after 2 weeks of culturing. Images of representative colonies formed were shown in the lower panels. c Representative photographs of A cells cultured in medium with or without glutamine for 72 h, original magnification is ×.
d Effect of glutamine on expression of genes ABCG2 and SOX β-actin was used as an internal control for normalization.
e Western blot analysis of ABCG2 and SOX f Effect of L-Asparaginase on expression of genes ABCG2 and SOX β-actin was used as an internal control. H, I Effect of glutamine and L-Asparaginase on ABCG2 expression.
To test if the impact of glutamine deprivation on SP cells could be reversed by replenishment of glutamine, A cells were first cultured in glutamine-free medium for 48 h to induce a decrease of SP cells.
The cells were then switched to glutamine-containing medium for another 48 h, and SP cells were measured. The results showed that there was a substantial recovery of SP population after 48 h in glutamine-replenished medium Additional file 1 : Figure S4A , accompanied by corresponding changes in the expression of stem cell markers including ABCG2, ALDH1, SOX2, and CD44 Additional file 1 : Figure S4B.
These data suggest that the effect of glutamine on stemness was reversible. To investigate the mechanism by which glutamine depletion decreased SP cells, we first tested whether glutamine deprivation could attenuate ATP production, and found that ATP level decreased when glutamine was absent in the culture medium Additional file 1 : Figure S5A , a result similar to that observed with glucose depletion However, unlike glucose depletion which inhibits Akt activation in A cells 29 , depletion of glutamine did not cause significant decrease in Akt phosphorylation at the time when SP cells were decrease, except a transient decrease at 24 h for a yet unknown reason Additional file 1 : Figure S5B [ 11 ].
This negative result prompted us to further explore other potential mechanisms. Based on the important role glutamine in glutathione GSH synthesis and ROS balance that affect stemness of CSCs, we postulated that glutamine deprivation could result in a reduced intracellular GSH content and an increase in ROS accumulation.
As expected, glutamine deprivation also induced a time-dependent increase in intracellular ROS Fig. Indeed, the mitochondrial membrane integrity was not damaged when analyzed by flow cytometry using rhodamine Rho or nonylacridine orange NAO Additional file 1 : Figure S5C and S5D.
Moreover, the expression of mitochondrial protein complexes did not change under glutamine free condition Additional file 1 : Figure S5E. These data demonstrated that glutamine deprivation induced glutathione depletion, leading to attenuation of the antioxidant system and an increase of cellular ROS.
Consistently, sorting of SP and non-SP cells by flow cytometry revealed that the cellular GSH level was higher in SP cells Fig. Glutamine depletion leads to a reduction in cellular GSH and ROS accumulation.
a A cells cultured in complete were switched to medium without glutamine for the indicated time. The cells were collected for analysis of intracellular GSH content as described in Materials and Methods. b Determination of cellular ROS in A cells.
Cells were cultured as in a , and ROS was detected by flow cytometry using DCF-DA. c Quantitation of cellular ROS under conditions described in b.
d Determination of superoxide in A cells cultured with or without glutamine for the indicated time, cellular superoxide was detected by flow cytometry using Het staining. f Comparison of cellular GSH contents in sorted SP and non-SP cells. g Comparison of expression of genes involved in synthesis of glutathione in sorted SP and non-SP cells.
GCLC, γ-glutamylcysteine ligase catalytic subunit; GCLM, γ-glutamylcysteine ligase modulatory subunit; GSS, glutathione synthetase. Since previous studies showed that increased ROS levels could induce stem cell differentiation [ 28 — 31 ], we postulated that the effect of glutamine on SP cells could be mediated by change in ROS.
Indeed, incubation of A cells with 50 μM of hydrogen peroxide H 2 O 2 decreased the proportion of SP cells Fig. The H 2 O 2 -treated cells formed pseudopodia—like morphology Additional file 1 : Figure S6 , similar to that observed under glutamine depletion condition Fig.
Consistently, inhibition of catalase, a key antioxidant enzyme that catalyze the conversion of H 2 O 2 into water and oxygen [ 32 ], by aminotriazole ATZ caused a significant decrease in SOX-2 and ABCG2 protein levels, which could be reversed by the antioxidant N-acetyl-L-cysteine NAC Fig.
As expected, ATZ also diminished the percentage of SP cells Fig. Interestingly, N-acetyl-L-cysteine NAC did not reverse the decrease of side population cells in absence of glutamine in the medium Fig. Decrease of SP subpopulation in A cells treated with hydrogen peroxide.
a A cells were cultured without or with H 2 O 2 50 μM for 48 h, cells were harvested and stained with Hoechst to determine SP cells. b A cells were treated without or with H 2 O 2 50 μM for 48 h, then RNA was isolated for real-time RT-PCR for analysis of expression of ALDH-1 and SOX c A cells was treated with 5 mM aminotriazole ATZ for 72 h with or without pretreatment with NAC, cell lysates were subjected to western blotting to measure the expression of ABCG2 and SOX d A cells were treated with 5 mM aminotriazole ATZ for 48 h, cells were harvested and stained with Hoechst to determine SP cells.
e A cells was treated with glutamine-free medium for 72 h with or without pre-treatment of 2 mM NAC, the cells were collected for SP detection.
Western blot analysis revealed that depletion of glutamine induced a significant increase in the phosphorylation of beta-catenin, associated with a decrease of SOX-2 Fig.
Similar results were obtained when the cells were incubated with H 2 O 2 Fig. Consistently, analysis of the β-catenin-regulated genes such as Survivin and Axin2 showed both target molecules were down-regulated at mRNA and protein levels when cells were cultured without glutamine Additional file 1 : Figure S7.
However, other β-catenin-regulated molecules cyclin D1, C-Myc, BCL-2 did not show consistent degrease after glutamine depletion, suggesting that they might also be regulated by other mechanisms.
Impact of glutamine deprivation and H 2 O 2 on β-catenin pathway. a A cells were cultured in RPMI medium without glutamine for the indicated time, and cell lysates were subjected to western blotting to measure the expression of SOX2, p-β-catenin, β-catenin, and β-actin. b A cells were treated μM H 2 O 2 for the indicated time, and cell lysates were subjected to western blotting to measure the expression of SOX2, p-β-catenin, β-catenin and β-actin.
c Expression of β-catenin and ABCG2 mRNA in A cells transfected with siRNA against β-catenin or with negative control siRNA NC.
d Expression of β-catenin and ABCG2 protein in A cells after siRNA silencing of β-catenin. To further test the role of β-catenin in regulating stem cells, we used siRNA to suppress the expression of beta-catenin, and evaluated its impact on stemness. These data together suggested that glutamine regulated the proportion of stem-like side population cells might be at least in part through ROS-mediated β-catenin phosphorylation, which leads to β-catenin protein degradation by proteasome [ 38 ].
Based on the observations that removal of glutamine or L-ASP incubation could diminish the fraction of SP cells in vitro, we further tested their effect on the ability of A cells to form tumor in vivo. The detached dead cells were washed out, and equal numbers of viable cells were inoculated subcutaneously into the flanks of nude mice.
Mice in the control and experimental groups including Gln-free and L-ASP-treated groups were observed for tumor formation for about 2 months without further treatment.
All mice inoculated with 5. When the inoculated cell number was further reduced to 1. Tumor growth was retarded in both Gln-free and L-ASP-treated groups Fig. These data demonstrated that glutamine deprivation or L-ASP treatment of A cells could severely impair their tumorigenicity in vivo.
Suppression of tumor formation by glutamine deprivation and L-Asparaginase pretreatment. The mice were then monitored for tumor incidence b and tumor sizes for group1 c. No tumor formation was observed in cells pre-treated with glutamine-free medium or L-asparaginase in group 2.
d Body weight curves of mice inoculated with A cells as described in A group 1. Recent studies suggest that the microenvironment in the stem cell niches plays a major role in regulation of stemness, and promotes the long-term survival and self-renew of CSCs [ 7 , 39 — 42 ].
Among the nutrients in the tumor microenvironment, glutamine is an important amino acid on which many cancer cells rely for survival and proliferation.
In fact, addiction to glutamine is often observed in cancer cells, which use this amino acid as an energy source, a metabolic intermediate for synthesis of other biomolecules, and a precursor for synthesis of glutathione to maintain redox balance [ 17 , 43 — 45 ].
Although the reasons for cancer cell dependency on glutamine are not entirely clear, the high demand of energy ATP and metabolic intermediates for active cell growth and the increased need for glutathione to counteract ROS stress under oncogenic signals are among the possible explanations for glutamine addiction.
Wang et al. revealed that ASCT2 is important for melanoma and inhibition of this glutamine transporter could suppress cell proliferation [ 46 ]. In this study, we discovered another important role of glutamine in maintaining stem-like cancer cells, using side population in lung cancer as an experimental model system.
Our study showed that glutamine deprivation induced a significant decrease in SP cells associated with a down regulation of ABCG2 and Sox Interestingly, the rate of decrease in SP cells induced by glutamine depletion was much slower than that induced by glucose deprivation, suggesting that these two major nutrients seem to affect cancer stem cells by different mechanisms.
In fact, it has been shown that glucose affects CSCs through a mechanism involving Akt-mediated regulation of ABCG2 expression 29 , whereas the current study showed that the Akt activation status seemed not associated with the changes in SP cells induced by glutamine Additional file 1 : Figure S4B.
The results of our study suggest that a mechanism by which glutamine affects SP cells may be through ROS-mediated activation of the β-catenin pathway, which regulates the expression of certain stem cell-related genes [ 47 ]. Recent studies suggest that CSCs seem to have higher glycolytic activity and may be more dependent on glucose to generate ATP compared to the bulk of general cancer cells [ 11 — 13 ].
Thus, glucose deprivation could cause a severe energy deficiency in CSCs, leading to their rapid decrease. In contrast, a major role of glutamine in CSCs is to function as a metabolic precursor for the synthesis of glutathione to maintain redox balance and keep the intracellular ROS at a relative low level.
Thus, a depletion of glutamine would cause an increase of ROS, which tend to induce cell differentiation and eventually lead to a gradual decrease in CSC population. These different roles of glucose and glutamine in energy metabolism and redox homeostasis may explain their different dynamics in impacting CSCs.
In our study, glutamine deprivation caused an increase in β-catenin phosphorylation, leading to its inactivation and a decreased expression of its down-stream targets survivin and Axin2. These results suggest that the β-catenin pathway might play an important role in mediating the down-regulation of SP cells induced by glutamine depletion, which led to an increase in ROS.
It is known that ROS negatively regulates β-catenin [ 36 ]. Interestingly, a previous study showed that blocking glutamine metabolism could inhibit cancer metastasis [ 48 ], which is a property of cancer stem cells.
Consistently, we found that glutamine deprivation could induce a decrease of MMP7 data not shown , a marker of cancer metastasis and also a downstream target of β-catenin.
Cancer stem cells in general are slow-cycling or quiescent cells that retain BrdU-labeling over a long period due to slow division [ 49 , 50 ], which render them less sensitive to many chemotherapeutic agents that target fast-proliferating cells. A low level of intracellular ROS seems critical to maintain the quiescent status of cancer cells [ 51 ].
To maintain a low ROS level, CSCs require high capacity of antioxidants to counteract ROS generated during active cellular metabolism. Indeed, two important transcription factors, FoxO and P53, have been shown to play a significant role in regulation of cellular ROS, and both are considered to be important molecules for the maintenance of stem cells [ 52 , 53 ].
The reduced form of glutathione GSH is a highly abundant antioxidant in the cells, and plays an important role in keeping redox balance and promoting cell viability and drug resistance. In fact, cancer cells with positive CD44, which interact with the cysteine transporter xCT and promote GSH synthesis [ 54 ], exhibit grow advantage and resistant to certain therapy [ 55 , 56 ].
The high ability of CSCs to utilize glutamine for GSH synthesis leading to increased cell viability and drug resistance imposes a significant challenge in clinical treatment of cancer.
However, our study suggests that the addiction of CSCs to glutamine metabolism could also provide a potential therapeutic target for elimination of CSCs.
Furthermore, glutamine is also important to support cancer cells viability and growth through the KRas-regulated metabolic pathway [ 57 ]. As such, it seems possible to target glutamine metabolism either by enzymatic elimination of glutamine in the tumor microenvironment using L-ASP or by inhibition of intracellular conversion of glutamine to glutamate using glutaminase GLS inhibitors such BPTES and compound , as illustrated in Fig.
It is worth noting that although direct removal of glutamine from the cell culture medium is a straightforward approach to evaluate the role of glutamine in supporting CSCs in experimental system, it is difficult to deprive glutamine in vivo for therapeutic purpose.
However, it may be possible to use enzymes such as glutaminase and L-asparaginase to remove glutamine in vivo to impact cancer stem cells. Since L-ASP is a clinical drug currently used in treatment of ALL largely due to its ability to deplete asparagine and thus suppresses ALL cell proliferation [ 58 ], it would be feasible to test the possibility to use L-ASP to eliminate CSCs in a clinical setting.
Due to the plasticity of cancer stem cells and possible reversion of downstream cancer cells to stem stage, it may be necessary to combine L-ASP with other anticancer agents to increase the chance to eliminate the entire cancer cell population and achieve better therapeutic outcome.
Schematic model for regulation of stem-like side population cells by glutamine. Glutamine is a precursor for glutamine synthesis and is important in maintaining redox balance.
Depletion of glutamine would result in a decrease in GSH, and subsequently causes an accumulation of ROS, which in turn induces phosphorylation of β-catenin and thus inactivate this pathway, lead to loss of stemness. Targeting glutamine metabolism either by enzymatic elimination of glutamine in the tumor microenvironment using L-ASP or by inhibition of intracellular conversion of glutamine to glutamate using glutaminase GLS inhibitors such as BPTES and compound may decrease cancer stem cells through increasing ROS and attenuation of the beta-catenin activity.
Stem—like side population cells are more addicted to glutamine. Deprivation of glutamine can decrease the fraction of SP cells and stem cell markers SOX-2 and ABCG2.
Glutamine deprivation increases cellular ROS through attenuating glutathione synthesis, while increased ROS suppresses β-catenin pathway through inducing its phosphorylation and degradation.
Tumor formation capacity in vivo was weakened through blocking glutamine utility by L-asparaginase. Al-Hajj M, Becker MW, Wicha M, Weissman I, Clarke MF. Therapeutic implications of cancer stem cells. Curr Opin Genet Dev. Article CAS PubMed Google Scholar.
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells.
Proc Natl Acad Sci U S A. Article CAS PubMed PubMed Central Google Scholar. Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. Korkaya H, Wicha MS.
Cancer stem cells: nature versus nurture. Nat Cell Biol. Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES.
Identification of selective inhibitors of cancer stem cells by high-throughput screening. Heddleston JM, Li Z, McLendon RE, Hjelmeland AB, Rich JN.
The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle. Hjelmeland AB, Wu Q, Heddleston JM, Choudhary GS, MacSwords J, Lathia JD, McLendon R, Lindner D, Sloan A, Rich JN.
Acidic stress promotes a glioma stem cell phenotype. Cell Death Differ. Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Warburg O. On the origin of cancer cells. Liu PP, Liao J, Tang ZJ, Wu WJ, Yang J, Zeng ZL, Hu Y, Wang P, Ju HQ, Xu RH, Huang P.
Metabolic regulation of cancer cell side population by glucose through activation of the Akt pathway. Article PubMed Google Scholar. Yuan S, Wang F, Chen G, Zhang H, Feng L, Wang L, Colman H, Keating MJ, Li X, Xu RH, et al.
Effective elimination of cancer stem cells by a novel drug combination strategy. Stem Cells. Zhou Y, Zhou Y, Shingu T, Feng L, Chen Z, Ogasawara M, Keating MJ, Kondo S, Huang P. Metabolic alterations in highly tumorigenic glioblastoma cells: preference for hypoxia and high dependency on glycolysis.
J Biol Chem. Yu WM, Liu X, Shen J, Jovanovic O, Pohl EE, Gerson SL, Finkel T, Broxmeyer HE, Qu CK. Metabolic regulation by the mitochondrial phosphatase PTPMT1 is required for hematopoietic stem cell differentiation.
In this study, we investigated the role of glutamine metabolism in AT2 cells of patients with IPF and in mice with bleomycin-induced fibrosis. Metabolic inhibitors were used to stimulate AT2 cells to block glutamine metabolism.
Regeneration of AT2 cells was detected using bleomycin-induced mouse lung fibrosis and organoid models. In a — d , representative values of two independent experiments are shown; e , f show values of one experiment. Scale bars, 20 μm.
In a — i , representative values of at least two independent experiments are shown; j — n show values of one experiment.
c , d , 2-OG-to-succinate ratio in wild-type or GLS-knockout BMDMs c and 2-OG-to-succinate ratio in wild-type or GLUD1-knockout BMDMs d.
n — q , RT—qPCR of Tnfa n , Cxcl9 o , Mrc1 p and Retnla q in BMDMs isolated from control and Gls ΔMo mice. r , Scheme illustrating the physiological role of GLUD1 in macrophages in response to muscle damage. During muscle disruption, ischaemia or ageing, interstitial glutamine drops—probably because of the loss in myofibres a major glutamine source and poor blood supply.
Infiltrating macrophages respond to glutamine starvation by reducing their oxidative GLUD1 activity in favour of GS activity. Macrophage-derived glutamine is released and progressively fills the muscle interstitium, where it is taken up by satellite cells, promoting their proliferation and differentiation into new fibres two processes that are favoured by glutamine-dependent mTOR activation.
Towards the end of this regenerative process, the newly generated fibres will undertake glutamine production and inflammation will be progressively resolved. GLUD1-deficient macrophages are metabolically pre-adapted towards glutamine synthesis and release, thus preventing this glutamine drop.
It follows that—in the case of muscle damage—macrophage-specific knockout of Glud1 or pharmacological GLUD1 blockade strengthens satellite cell activation, ultimately leading to therapeutic muscle regeneration. Supplementary Figure 1 Uncropped western blot scans.
The figure shows the original, uncropped scans of the western blot images displayed in Fig. Reprints and permissions. Shang, M. Macrophage-derived glutamine boosts satellite cells and muscle regeneration.
Download citation. Received : 01 February Accepted : 05 August Published : 28 October Issue Date : 26 November Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative. By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily. Skip to main content Thank you for visiting nature. nature articles article. Subjects Cell signalling Immunology Mechanisms of disease.
Abstract Muscle regeneration is sustained by infiltrating macrophages and the consequent activation of satellite cells 1 , 2 , 3 , 4.
Access through your institution. Buy or subscribe. Change institution. Learn more. Data availability RNA sequencing data have been deposited in the Gene Expression Omnibus GEO data repository, with accession number GSE References Bentzinger, C.
Article CAS Google Scholar Costamagna, D. Article CAS Google Scholar Saclier, M. Article CAS Google Scholar Tidball, J. Article CAS Google Scholar Perdiguero, E. Article CAS Google Scholar Latroche, C. Article CAS Google Scholar Summan, M.
Article CAS Google Scholar Rennie, M. Article CAS Google Scholar Biolo, G. CAS PubMed Google Scholar Nurjhan, N. Article CAS Google Scholar Palmieri, E. Article CAS Google Scholar St Pierre, B. Article Google Scholar Guardiola, O.
Google Scholar Takeda, Y. Article ADS CAS Google Scholar von Maltzahn, J. Article ADS Google Scholar Zammit, P. Article CAS Google Scholar Wenes, M.
Article CAS Google Scholar Yang, C. Article CAS Google Scholar Rodgers, J. Article ADS CAS Google Scholar Zhang, P. Article CAS Google Scholar Jewell, J. Article ADS CAS Google Scholar Rayagiri, S.
Article ADS Google Scholar Sousa-Victor, P. Article ADS CAS Google Scholar Bernet, J. Article CAS Google Scholar Jin, L. Article CAS Google Scholar Liu, P.
Article CAS Google Scholar Obara, H. Article Google Scholar Sayer, A. Article Google Scholar Vinciguerra, M. Article CAS Google Scholar Carobbio, S.
Article CAS Google Scholar He, Y. PubMed Google Scholar Mingote, S. Article CAS Google Scholar LaFleur, M. Article ADS Google Scholar Sanjana, N.
Article CAS Google Scholar Pasut, A. Google Scholar Casazza, A. Article CAS Google Scholar Download references.
Acknowledgements M. Rincon Department of Neuroscience, KU Leuven, Leuven, Belgium Melvin Y. Rincon Centro de Investigaciones, Fundacion Cardiovascular de Colombia, Floridablanca, Colombia Melvin Y.
Rincon Department of Health Sciences and Technology, ETH, Zurich, Switzerland Katrien De Bock Department of Oncology, Ludwig Cancer Research, University of Lausanne, Epalinges, Switzerland Ping-Chih Ho Venetian Institute of Molecular Medicine, Padua, Italy Marco Sandri Department of Biomedical Science, University of Padova, Padua, Italy Marco Sandri Department of Medicine, McGill University, Montreal, Quebec, Canada Marco Sandri Metabolomics Core Facility, Center for Cancer Biology, VIB, Leuven, Belgium Bart Ghesquière Metabolomics Core Facility, Center for Cancer Biology, Department of Oncology, KU Leuven, Leuven, Belgium Bart Ghesquière Department of Molecular Biotechnology and Health Science, Molecular Biotechnology Centre, University of Torino, Turin, Italy Massimiliano Mazzone Authors Min Shang View author publications.
View author publications. Ethics declarations Competing interests The authors declare no competing interests. Additional information Peer review information Nature thanks Terry Partridge and the other, anonymous, reviewer s for their contribution to the peer review of this work.
Extended data figures and tables. Extended Data Fig. Extended Data Table 1 Blood count in control and Glud1 ΔMo mice Full size table. Supplementary information Supplementary Figure Supplementary Figure 1 Uncropped western blot scans. Reporting Summary. Source data Source Data Fig. Source Data Fig.
Source Data Extended Data Fig. Rights and permissions Reprints and permissions. About this article. Cite this article Shang, M. Copy to clipboard. Comments By submitting a comment you agree to abide by our Terms and Community Guidelines.
About the journal Journal Staff About the Editors Journal Information Our publishing models Editorial Values Statement Journal Metrics Awards Contact Editorial policies History of Nature Send a news tip. Publish with us For Authors For Referees Language editing services Submit manuscript.
Search Search articles by subject, keyword or author. Show results from All journals This journal. Advanced search. Close banner Close. Email address Sign up. I agree my information will be processed in accordance with the Nature and Springer Nature Limited Privacy Policy.
Get the most important science stories of the day, free in your inbox. Sign up for Nature Briefing.
Glutamine is ceell conditionally essential amino acid anx in energy Specialty coffee beans and redox regendration. Aging regenerwtion commonly characterized Glutamine and cell regeneration energy generation Glutamine and cell regeneration and redox homeostasis dysfunction. Various aging-related diseases have regwneration reported to be accompanied by glutamine exhaustion. Glutamine supplementation has been used as a nutritional therapy for patients and the elderly, although the mechanism by which glutamine availability affects aging remains elusive. Here, we show that chronic glutamine deprivation induces senescence in fibroblasts and aging in Drosophila melanogasterwhile glutamine supplementation protects against oxidative stress-induced cellular senescence and rescues the D-galactose-prompted progeria phenotype in mice.
Gut topic
Welche gute Phrase
Ist Einverstanden, die nützliche Information
Wacker, dieser bemerkenswerte Gedanke fällt gerade übrigens
Ich kann Ihnen anbieten, die Webseite zu besuchen, auf der viele Informationen zum Sie interessierenden Thema gibt.