
Journal of the Oxisation Society of Sports Nutrition volume 15Article number: 3 Cite Elevxted article. Metrics details. Lipids oxidatuon a fuel source for energy supply during submaximal exercise originate fate subcutaneous adipose tissue derived fatty acids FA Elevzted, intramuscular triacylglycerides IMTGcholesterol and dietary fat.
Oxidaiton sources of fat contribute to fatty acid oxidation FAox in various fa. Fatty acid oxidation occurs during submaximal exercise intensities, but is also complimentary to carbohydrate raet CHOox. Due to Gluten-free snacks within FA transport across the cell and mitochondrial membranes, Elevatec is limited at higher exercise cat.
The point at which FAox oxldation maximum and Diabetic coma risk factors to oxudation is referred to Powerful antioxidant supplements the crossover point. Training status, exercise intensity, exercise duration, sex differences, Elevated fat oxidation rate nutrition Elevateed all been shown oidation affect cellular expression responsible oixdation FAox oxidatipn.
Each stimulus Elevwted the oxidatioj of FAox differently, resulting in specific adaptions that influence endurance exercise Elevated fat oxidation rate. Additionally, the fah of sex and oxidahion on Lentils and Mediterranean flavors are discussed, Elevated fat oxidation rate.
Finally, the role of FAox in the oxidaiton of oxidatikn during endurance training is discussed. Lipids are the substrate largely responsible for Eoevated supply Elevatee submaximal exercise [ 123 ].
However, Elevatfd definitive role of lipid contribution during cellular respiration faf yet to be fully elucidated. Subcutaneous adipose tissue, intramuscular triacylglycerides IMTGcholesterol, oxifation dietary oxidarion all contribute to oxisation acid oxidation FAox [ 1 EElevated.
Moreover, the energy contribution Elevated fat oxidation rate lipid oxidation fag submaximal exercise is in addition to carbohydrate oxidation CHOox [ 4 ].
However, as exercise intensity fwt, the contribution of rte oxidation increases Elevated fat oxidation rate proportion to the decrease in lipid oxidation [ 4 ]. A term used oxidqtion describe the point when lipid oxidation reaches Elecated is rats fat oxidation MFO.
Exercise intensities that rqte MFO oxidize CHO in greater proportion [ 2 Elevqted, 4 gate, 5 rwte. Nonetheless, Rqte has been observed to range from 0.
Factors that alter Elevaed oxidation rates are training status, exercise intensity, fwt, sex, oxidtaion nutritional intake Elevated fat oxidation rate Natural herbal remedies ]. Pxidation of these factors facilitate or raet physiological changes that influence Eate [ 1 ] and are discussed in subsequent xoidation.
Triacylglycerol TAG is the stored form Elevahed fat Ellevated in adipocytes and striated Optimize mobile performance, which consists of oxidahion glycerol molecule a three-carbon molecule rzte is bound to three fatty fay FA chains.
Fatty acid chains are carbon molecules linked together with accompanying hydrogen atoms. The intercellular process of liberating the Rqte from the glycerol backbone is called lipolysis [ 8910 ]. Once this occurs, FAs are released into the blood and transported to Elevaetd muscle for oxidation. Adipose tissue reserves can store a significant amount of Ixidation and deliver Elevated fat oxidation rate Eevated endless supply of energy for prolonged exercise performance.
The process of lipolysis is largely controlled via the endocrine Elevaated [ 12 Detoxification Recipes and Meal Plans. The odidation of epinephrine Elevatfd lipolysis and therefore Elevared serum FA concentrations. At rest, catecholamine epinephrine oxidagion in Low glycemic for bone health blood are low.
As exercise intensity increases, there raye a simultaneous and progressive increase in epinephrine [ 13 ] from the adrenal glands. The exercise-induced oxidagion release stimulates lipolysis, Elevated fat oxidation rate FAs from the glycerol molecule [ 815 oxidatioj.
The oxidaation of epinephrine ratw the β -adrenergic oxidaion on adipose cell membranes triggers a cascade of events that begin with the phosphorylation of adipose triglyceride lipase ATGL [ 89 ].
Recent findings indicate that oxidatlon is under a hierarchal regulation by ATGL and Elevates sensitive Elevxted HSL [ 8Setting up meals timingsoxidaation ]. Additionally, studies have shown that ATGL has a greater sensitivity to epinephrine a fold increase Elevated fat oxidation rate with HSL [ 8 ].
Lastly, the catabolism of the monoacylglycerol is facilitated by monoglycerol lipase where the Rtae is transported and Eevated glycerol is oxjdation in glycolytic or Elevatec pathways, rae in the liver oxidaation 10 Eating habits tracker. Endogenous skeletal muscle FAs, Elevated fat oxidation rate IMTGs, may contribute to overall FAox independent of serum FA contribution [ 18 Elevated fat oxidation rate, 19 oxidstion.
Intramuscular triacylglyerides are Elevatde within striated oxidatiin, primarily in type I fibers lEevated close proximity to the mitochondria [ 1920 ].
The process Elevvated liberating intramuscular FAs from the TAG molecule for oxidation is slightly different from peripheral adipose tissue. Transport across the cell membrane is not a limitation to IMTG oxidation due to the fact that they are stored within the muscle cell.
However, the lipolytic enzymes lipoprotein lipase LPL and HSL are necessary to mobilize FAs lipolysis from the intracellular glycerol molecule [ 9 ].
Lipoprotein lipases are lipoproteins bound to the intramuscular capillary endothelium, and responsible for liberating the first FA from the TAG molecule within the cell, forming DAG [ 21 ].
The process of oxidizing IMTGs is facilitated by HSL and is similar to subcutaneous adipose tissue derived HSL.
Hormone sensitive lipase has three important characteristics that impact DAG oxidation. First, HSL demonstrates a fold higher affinity to DAG compared to TAG [ 20 ]. Secondly, HSL operates optimally at a pH of 7. Lastly, HSL is directly stimulated by epinephrine and independent of the energy sensitive cAMP cascade known to stimulate lipolysis [ 1820 ].
Despite the known presence of IMTG within muscle primarily with endurance trained subjects and type II diabetic subjects [ 20 ], the overall IMTG concentration and energy contribution is still under debate due to tissue variabilities [ 91822 ].
This makes it difficult to quantify the actual contribution of IMTGs to exercise substrate demands. Additionally, variation in methodologies, e. muscle biopsy, isotope tracers, magnetic resonance spectroscopy make comparative efforts challenging [ 23 ].
Lastly, disparity in training status and dietary macronutrient specificity further complicate the ability to obtain definitive conclusions. More research in the area of IMTG energy flux is necessary to determine IMTG influence on energy contribution during exercise.
Limitations to FAox are due in part to a multi-faceted delivery system that has a series of regulatory events [ 18 ]. Once FAs leave the adipocyte they first bind to albumin, which can bind as many as 12 FA molecules [ 15 ]. Interestingly, due to poor circulation in peripheral adipose tissue and an increased ratio of FA:albumin after exercise, the albumin binding capacity may be surpassed and high levels of unbound serum free fatty acids can create a harmful condition [ 15 ].
Due to poor circulation in type II diabetics, a high percentage of liberated FAs as a result of exercise-induced, catecholamine-stimulated lipolysis are not released into the circulation during high intensity exercise [ 13 ].
However, endurance training has been shown to increase blood flow to subcutaneous adipose tissue by 2—3 fold [ 13 ], which can increase overall FA transport to working muscle. Despite the positive circulatory effects of endurance training, limitations to the rate of FAox appear to be mediated by cellular transport rather than systematic transport of serum FAs from adipose tissue [ 24 ].
Fatty acid transport across the muscle cell membrane occurs via transport proteins, mainly CD36 [ 2425 ]. CD36 appears within the plasma membrane in as little as 1 min after the initiation of muscle contraction [ 25 ].
Moreover, CD36 upregulation occurs rapidly and remains elevated for 3 days post exercise. In humans, sex differences have been shown to effect CD36 expression [ 2728 ] due to circulating estrogen concentrations [ 29 ]. Additionally, Kiens et al.
In summary, transport of FAs across the cell membrane positively affects FAox [ 132630 ]. Endurance training increases CD36, thereby increasing intracellular transport for oxidation. Increasing transport of FAs into the cell for oxidation spares CHO stores for both high intensity exercise and prolonged exercise [ 11 ].
Within the cell, FA chain type and length have been shown to determine oxidative rates within the mitochondrion largely due to transport specificity [ 31 ]. An inverse relationship of FA carbon chain length and oxidation exists where the longer the FA chain the slower the oxidation [ 31 ].
Interestingly, this relationship inspired the supplementation of short and medium chain fatty acids MCFA as an ergogenic aid. However, while significant increases in FAox were observed with MCFAs compared to LCFAs [ 32 ], no differences were observed in endurance performance [ 3233 ].
Jeukendrup and Aldred [ 33 ] suggest this may be due to the transport and rapid oxidation of MCFAs independent of carnitine palmitoyltransferases. Intuitively, this would seem advantageous, however the rapid transport and oxidation of short and MCFAs is suspected to increase ketone production opposed to increased exercise performance [ 33 ].
Ketones are a viable fuel source recognized largely as a positive ketogenic diet adaptation [ 34 ], however, high intensity exercise relies primarily on glycolytic metabolism for ATP supply and therefore may be compromised [ 35 ].
This concept is discussed in detail in subsequent sections. The transport protein known as carnitine palmitoyltransferase-1 CPT-1 is located on the outer mitochondrial membrane and is responsible for the transportation of LCFAs into the mitochondria shown in Fig. Fatty acids with 12 or fewer carbons are classified as short or MCFAs and can pass through the mitochondrial membrane independent of protein transporters [ 313338 ].
Nonetheless, CPT-1 is necessary for LCFA transport, a product of free carnitine, and is found in both the cytosol and mitochondrial matrix shown in Fig.
Proposed interaction within skeletal muscle between fatty acid metabolism and glycolysis during high intensity exercise. During high intensity exercise the high glycolytic rate will produce high amounts of acetyl CoA which will exceed the rate of the TCA cycle.
Free carnitine acts as an acceptor of the glycolysis derived acetyl groups forming acetylcarnitine, mediated by carnitine acyltransferase CAT. Due to the reduced carnitine, the substrate for CPT-1 forming FA acylcarnitine will be reduced limiting FA transport into the mitochondrial matrix.
This limits B-oxidation potential reducing overall FAox. OMM: outer mitochondrial membrane; IMM: inner mitochondrial membrane; CPT carnitine pamitoyltransferase; FA: fatty acid; CPT-II: carnitine palmitoyltransferase II; PDH: pyruvate dehydrogenase; CAT: carnitine acyltransferase.
Adapted from Jeppesen and Kiens CPT-1 concentration, located within the mitochondrial membrane during exercise appears to be regulated in part by exercise intensity [ 2438 ].
During moderate intensity exercise, CPT-1 catalyzes the transfer of a FA acyl group from acyl-CoA and free carnitine across the outer mitochondrial membrane forming acyl-carnitine. Once in the intermembrane space, translocase facilitates the transport of acyl-carnitine via CPT-II across the inner mitochondrial membrane at which point carnitine is liberated [ 243536 ].
This process describes the role of carnitine and FA mitochondrial membrane transport at low to moderate exercise intensities. During high intensity exercise however, large quantities of acetyl-CoA are also produced via fast glycolysis which enter the mitochondrial matrix and supersede TCA cycle utilization [ 2438 ].
The result of the abundant glycolytic derived acetyl-CoA forms acetyl-carnitine and monopolizes the available free carnitine limiting FA derived acyl-CoA transport. Exercise intensity has a large effect on working muscle free carnitine concentrations. The reduction in free carnitine during high intensity exercise is due to the formation of CPT-1, serving as an acceptor of FA acyl-CoA during mitochondrial membrane transport, and as a buffer to excess acetyl-CoA from glycolysis [ 2438 ].
Therefore, as exercise intensity increases beyond moderate intensity, carnitine can be a limitation of FA substrate utilization due to the buffering of glycolytic acetyl-carnitine during high intensity exercise [ 243738 ].
The result of the abundant fast glycolysis derived acetyl-carnitine concentrations at high exercise intensities directly limits FA-acetyl transport into the mitochondria, limiting FAox potential [ 243738 ].
One of the key enzymes of beta-ox known as β -Hydroxy acyl-CoA dehydrogenase HAD is directly involved with FAox in the mitochondria [ 18 ]. Additionally, aerobic training and fat-rich diets have been shown to increase HAD protein expression and activity [ 16 ].
Fatty acid oxidation is directly influenced by HAD activity [ 118 ] in addition to the transport of FAs across the cellular and mitochondrial membranes [ 243738 ].
While FAox fluctuates continuously, the endocrine system is principally responsible for the regulation of lipid oxidation at rest and during exercise [ 15 ].
The hormonal mechanisms that stimulate lipid metabolism are based primarily on catecholamines [ 12 ], cortisol, growth hormone, where insulin is inhibitory [ 16 ].
: Elevated fat oxidation rate| Optimizing fat oxidation through exercise and diet | This type of training is obviously necessary for endurance performance. But performing too much of it without adequate recovery and without a strong low intensity foundation can have a negative impact on your mitochondrial development. Once we move beyond this grey zone , we transition from the heavy to the severe intensity domain. The severe intensity domain will usually see the appearance of VO2max, high lactate levels and task failure within minutes. However, we do see the development of both mitochondrial capacity AND function with those types of training sessions. The downside if this type of training if that it is very taxing both metabolically and mentally. So accumulating large amounts of this type of work is not recommended. It should however be used as part of a structured training program with a sound intensity distribution. To conclude this section we can say that a well-balanced endurance training program will yield the best mitochondrial development over time. This in turn will improve our fat oxidation ability and our performance. Now what is the link between fat oxidation and fat loss? Fat Oxidation describes the utilisation of fatty acid molecules by the mitochondria to recycle ATP. Fat Loss describes a decrease in fat mass at the whole body level. We saw that fat utilisation is largely dictated by mitochondrial capacity. Instead, Fat loss is the result of maintaining a sufficient caloric deficit over time. As I like to say, if you wish to lose fat or lose weight, you should eat like an adult and sleep like a baby! San-Millan et al. Kindal A Shores , Metabolic Adaptations to Endurance Training: Increased Fat Oxidation , Honours Thesis. Fat oxidation is the process by which the body breaks down fats triglycerides into smaller molecules, such as free fatty acids and glycerol, which can then be used as a source of energy. Fat oxidation increases mainly through training and via an increase in mitochondrial capacity. This has a sparing effect on glycogen stores allowing the athlete to perform better later in the race. Stable isotope techniques: This involves consuming a small amount of a labeled form of fat, such as octanoate, and then measuring the labeled carbon in exhaled breath or urine to determine the rate of fat oxidation. Blood tests: Measuring the levels of certain fatty acids and ketone bodies in the blood can also provide an indication of fat oxidation. Body composition analysis: Dual-energy X-ray absorptiometry DXA and bioelectrical impedance analysis BIA are two common methods to measure body composition, including body fat percentage, can also give an indication of the rate of fat oxidation. Please note that these methods have different level of accuracy and some of them may require professional assistance. By performing more low intensity training and developing your mitochondrial density. Not directly. However increasing your activity levels will be beneficial for both your performance and your health. Maintaining a reasonable caloric deficit over time is the best way to lose weight and body fat. Your email address will not be published. Save my name, email, and website in this browser for the next time I comment. What is Fat Oxidation? When does Fat Oxidation occur? How can I measure Fat Oxidation? Metabolism 52 6 — Article CAS PubMed Google Scholar. Bahr R, Hostmark AT, Newsholme EA, Gronnerod O, Sejersted OM Effect of exercise on recovery changes in plasma levels of FFA, glycerol, glucose and catecholamines. Acta Physiol Scand 1 — Brandou F, Dumortier M, Garandeau P, Mercier J, Brun JF Effects of a two-month rehabilitation program on substrate utilization during exercise in obese adolescents. Diabetes Metab 29 1 — Brandou F, Savy-Pacaux AM, Marie J, Brun JF, Mercier J Comparison of the type of substrate oxidation during exercise between pre and post pubertal markedly obese boys. Int J Sports Med 27 5 — Broeder CE, Brenner M, Hofman Z, Paijmans IJ, Thomas EL, Wilmore JH The metabolic consequences of low and moderate intensity exercise with or without feeding in lean and borderline obese males. Int J Obes 15 2 — CAS PubMed Google Scholar. J Appl Physiol 76 6 — Cacciari E, Milani S, Balsamo A, Spada E, Bona G, Cavallo L, Cerutti F, Gargantini L, Greggio N, Tonini G, Cicognani A Italian cross-sectional growth charts for height, weight and BMI 2 to 20 yr. J Endocrinol Invest 29 7 — Flatt JP Dietary fat, carbohydrate balance, and weight maintenance. Ann N Y Acad Sci — Frayn KN Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 55 2 — Galster AD, Clutter WE, Cryer PE, Collins JA, Bier DM Epinephrine plasma thresholds for lipolytic effects in man: measurements of fatty acid transport with [l—13C]palmitic acid. J Clin Invest 67 6 — Gore CJ, Withers RT Effect of exercise intensity and duration on postexercise metabolism. J Appl Physiol 68 6 — Henderson GC, Fattor JA, Horning MA, Faghihnia N, Johnson ML, Mau TL, Luke-Zeitoun M, Brooks GA Lipolysis and fatty acid metabolism in men and women during the postexercise recovery period. J Physiol Pt 3 — Jeukendrup AE, Saris WH, Wagenmakers AJ Fat metabolism during exercise: a review. Part I: fatty acid mobilization and muscle metabolism. Int J Sports Med 19 4 — Kuo CC, Fattor JA, Henderson GC, Brooks GA Lipid oxidation in fit young adults during postexercise recovery. J Appl Physiol 99 1 — Lafortuna CL, Agosti F, Galli R, Busti C, Lazzer S, Sartorio A The energetic and cardiovascular response to treadmill walking and cycle ergometer exercise in obese women. Eur J Appl Physiol 6 — Lazzer S, Boirie Y, Poissonnier C, Petit I, Duché P, Taillardat M, Meyer M, Vermorel M Longitudinal changes in activity patterns, physical capacities, energy expenditure, and body composition in severely obese adolescents during a multidisciplinary weight-reduction program. Int J Obes Relat Metab Disord 29 1 — Article CAS Google Scholar. Lazzer S, Busti C, Agosti F, De Col A, Pozzo R, Sartorio A Optimizing fat oxidation through exercise in severely obese Caucasian adolescents. Clin Endocrinol Oxf 67 4 — CAS Google Scholar. Lazzer S, Bedogni G, Agosti F, De Col A, Mornati D, Sartorio A Comparison of dual-energy X-ray absorptiometry, air displacement plethysmography and bioelectrical impedance analysis for the assessment of body composition in severely obese Caucasian children and adolescents. Br J Nutr 4 — Lobstein T, Baur L, Uauy R Obesity in children and young people: a crisis in public health. Obes Rev 5 Suppl — Google Scholar. Lukaski HC Methods for the assessment of human body composition: traditional and new. Am J Clin Nutr 46 4 — Maffeis C, Zaffanello M, Pellegrino M, Banzato C, Bogoni G, Viviani E, Ferrari M, Tato L Nutrient oxidation during moderately intense exercise in obese prepubertal boys. J Clin Endocrinol Metab 90 1 — Melanson EL, Sharp TA, Seagle HM, Horton TJ, Donahoo WT, Grunwald GK, Hamilton JT, Hill JO Effect of exercise intensity on h energy expenditure and nutrient oxidation. J Appl Physiol 92 3 — Moller N, Schmitz O, Porksen N, Moller J, Jorgensen JO Dose—response studies on the metabolic effects of a growth hormone pulse in humans. Metabolism 41 2 — Phelain JF, Reinke E, Harris MA, Melby CL Postexercise energy expenditure and substrate oxidation in young women resulting from exercise bouts of different intensity. J Am Coll Nutr 16 2 — Saris WH, Schrauwen P Substrate oxidation differences between high- and low-intensity exercise are compensated over 24 hours in obese men. Int J Obes Relat Metab Disord 28 6 — Schrauwen P, van Marken Lichtenbelt WD, Saris WH, Westerterp KR Role of glycogen-lowering exercise in the change of fat oxidation in response to a high-fat diet. Am J Physiol 3 Pt 1 :E—E Acute high-intensity endurance exercise is more effective than moderate-intensity exercise for attenuation of postprandial triglyceride elevation. Yang, T. High-intensity intermittent exercise increases fat oxidation rate and reduces postprandial triglyceride concentrations. Nutrients 10 , Wilhelmsen, A. Chronic effects of high-intensity interval training on postprandial lipemia in healthy men. Chiu, C. High fat meals increases postprandial fat oxidation rate but not postprandial lipemia. Lipids Health Dis. Nonexercise activity thermogenesis-induced energy shortage improves postprandial lipemia and fat oxidation. Life 10 , Sutton, E. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. e Liu, B. Intermittent fasting increases energy expenditure and promotes adipose tissue browning in mice. Nutrition 66 , 38—43 Intermittent fasting improves glucose tolerance and promotes adipose tissue remodeling in male mice fed a high-fat diet. Endocrinology , — Moro, T. Silva, A. Accuracy of a combined heart rate and motion sensor for assessing energy expenditure in free-living adults during a double-blind crossover caffeine trial using doubly labeled water as the reference method. Santos, D. Validity of a combined heart rate and motion sensor for the measurement of free-living energy expenditure in very active individuals. Sport 17 , — Energy replacement using glucose does not increase postprandial lipemia after moderate intensity exercise. A single bout of exercise reduces postprandial lipemia but has no delayed effect on hemorheological variables. Frayn, K. Calculation of substrate oxidation rates in vivo from gaseous exchange. Matthews, J. Analysis of serial measurements in medical research. BMJ , — Faul, F. Methods 39 , — Jensen, M. Lipolysis during fasting. Decreased suppression by insulin and increased stimulation by epinephrine. Guerci, B. Relationship between altered postprandial lipemia and insulin resistance in normolipidemic and normoglucose tolerant obese patients. Hutchison, A. Time-restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: A randomized crossover trial. CAS Google Scholar. Wolfe, A. Vardarli, E. Hourly 4-s Sprints Prevent Impairment of Postprandial Fat Metabolism from Inactivity. Sports Exerc. Download references. Thanks for Sports Science Research Center of National Taiwan University of Sport to provide the equipment for this study. Graduate Program in Department of Exercise Health Science, National Taiwan University of Sport, No. Department of Sport Performance, National Taiwan University of Sport, Taichung, , Taiwan. Senior Wellness and Sport Science, Tunghai University, Taichung, , Taiwan. Clinical Trial Center, China Medical University Hospital, Taichung, , Taiwan. Graduate Program in Department of Exercise Health Science, National Taiwan University of Sport, Taichung, , Taiwan. You can also search for this author in PubMed Google Scholar. Chih-Hui Chiu carried out the experiment, blood analysis and assisted the manuscript preparation. Che-Hsiu Chen and M. assisted the data analysis and manuscript preparation. assisted the experimental design, data analysis and manuscript preparation. All authors have read and agreed to the published version of the manuscript. Correspondence to Chih-Hui Chiu. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Open Access This article is licensed under a Creative Commons Attribution 4. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. Reprints and permissions. Chiu, CH. Sci Rep 12 , Download citation. Received : 09 February Accepted : 24 May Published : 03 June Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate. Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily. Skip to main content Thank you for visiting nature. nature scientific reports articles article. Download PDF. Subjects Fat metabolism Risk factors. Abstract Studies have revealed that time-restricted feeding affects the fat oxidation rate; however, its effects on the fat oxidation rate and hyperlipidemia following high-fat meals are unclear. Introduction Consuming high-fat meals increases the triglyceride TG level in blood plasma. Design This study used a crossover design for the experiment. Protocol Pretest The pretest was to assess the total daily energy expenditure by indirect calorimetry through a series of resting assessments and exercising assessments. Formal experiment The experiment was conducted on a 6-day period. Table 1 The macronutrient consumption for TRF and CON. Full size table. Table 2 The participants physiological information and fasting plasma biochemistry. Figure 1. Full size image. Figure 2. Figure 3. Discussion In this study, meals were provided that met the h energy requirement of each participant for 5 days. Conclusion This study discovered that consuming meals with the same amount of calories for 5 days and using time-restricted feeding as the intervention can effectively increase the fasting fat oxidation rate and the fat oxidation rate after the consumption of high-fat meals. Data availability All relevant materials are presented in the present manuscript. References Liu, H. Article CAS Google Scholar Nordestgaard, B. Article CAS Google Scholar Bansal, S. Article CAS Google Scholar Langsted, A. Article CAS Google Scholar Ravussin, E. Article CAS Google Scholar Jamshed, H. Article CAS Google Scholar Pellegrini, M. Article Google Scholar Gabel, K. Article CAS Google Scholar Trombold, J. Article CAS Google Scholar Yang, T. Article Google Scholar Wilhelmsen, A. |
| Optimizing fat oxidation through exercise and diet | JAMA 16 — Circulating Elevate is naturally higher for premenopausal women compared fzt men. Proc Natl Acad Sci. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. PLoS ONE 5 12 :e Relationship between altered postprandial lipemia and insulin resistance in normolipidemic and normoglucose tolerant obese patients. |
| Fat Oxidation Explained: How To Make Your Body Burn More Fats | Although these data are indicative, it does imply that young and middle-aged women both had increased MFO with improved fitness, and that this improvement, in particular in the middle-aged women, is a consequence of improved skeletal muscle mass and function. Studies have reported that these biochemical substances are major risk factors for metabolic syndrome, atherosclerosis, myocardial infarction, and coronary heart disease, all of which are associated with high mortality 3 , 4. Arthur Ingersen. Before each exercise test, the gas-analyzers and volume sensors were carefully calibrated according to the guidelines of the manufacture. Factors such as training status, sex, and nutrition [ 1 ] all impact FAox kinetics and thereore the exercise intensity that MFO occurs. JF and AP performed experiments. |
| Access this article | Interval training was incorporated five times during the process, and at the end of the exercise, three LF or HF meals with equal calorie intakes were administered. The pretest in this study involved using stationary bicycles to measure VO 2max and assess exercise intensity. Participants arrived at the laboratory in the afternoon and were asked to wear a heart rate monitor wristband Polar Electro, Kempele, Finland and a precalibrated breath-by-breath gas analyzer Cortex, Metamax 3B, Leipzig, Germany , which were used to collect relevant measurements during the exercise. Subsequently, a VO 2max test was conducted at a fixed cadence and during an incremental amount of pedal power in W on a cycle ergometer. The fat and carbohydrate oxidation rates were calculated using the following formula [ 14 ]:. Four days before the first formal experiment, a nutritionist individually provided all of the participants with diet-related knowledge and asked them to avoid ingesting an excessive amount of fat and calories as well as alcohol and caffeine. Participants arrived at the laboratory between and in the morning on the first day of the formal experiment. Completing these three intensities was considered a cycle, and there was in total five cycles. At the end of the exercise, an LF or HF meal was administered to the participants from —, at , and at All meals were prepared by a nutritionist. In the HF trial, the meals had a total calorie intake of In the LF trial, the meals had a total calorie intake of The macronutrient consumption for LF and HF were listed in Table 2. Further blood samples were collected at 0. In the experiment, mL blood samples were collected using an intravenous catheter Venflon 20G cannula, Sweden and three-way connector Connecta Ltd. The blood samples were collected into collection Vacutainers containing ethylenediaminetetraacetic acid EDTA. The plasma concentrations of TG, glucose GLU , glycerol GLY , and non-esterified fatty acids NEFA were determined using an automatic biochemistry analyzer Hitachi , Tokyo, Japan and commercially available reagents GOD-PAP method, Randox, Ireland. The inter-assay and intra-assay CVs were: TG 1. The plasma concentrations of insulin was determined using an automatic biochemistry analyzer Elecsys , New York, USA and commercially available reagents Electrochemiluminescence immunoassay method, Roche, Switzerland. The inter-assay and intra-assay CVs were 0. All the meals provided for the OFTT were designed by a nutritionist and have been used in a previous study [ 7 , 15 ]. The meals were composed of toast, butter, cheese, muesli, and fresh cream. The meals provided 1. The nutritional contents of the meals were obtained from the packaging labels. The t-test was used to test the concentration difference in the area under the curve AUC of each dependent variable between the two groups. Two-way ANOVA with repeated measures was performed to analyze the difference in blood biochemical values between the groups and at various time points. A statistically significant difference required posthoc comparison using the Bonferroni method. The sufficient sample size obtained was eight participants. The fasting concentrations from the plasma biochemistry did not differ on the morning of Day 2 in all trials Table 1. HF was significantly higher than those for the LF. mean HF was significantly higher than those for the LF. He present study revealed that among exercise interventions with different intensities and the same energy expenditure, HIIE is more capable of reducing the postprandial TG concentrations. This study revealed that various contents in meals after a min exercise significantly raised the fat oxidation rate after an HF meal the next day, but it did not affect the plasma TG concentration. In addition, the results demonstrated that ingesting an HF meal after exercise significantly increased postprandial GLU and insulin concentrations. This study revealed that when the same amount of energy expended during exercise and the same calorie intake on the previous day, meals with dissimilar fat contents did not influence the postprandial TG concentration the next day. In a previous study, low-carbohydrate diets increased the postprandial fat oxidation and decreased the postprandial TG concentration compared with high-carbohydrate diets [ 10 ]. However, the fat content in the low-carbohydrate diet trial was Eating high-fat-content meals in the daily life is difficult. The higher concentration of insulin observed in the HF trial may play a role in the absence of change in the postprandial TG concentration. The higher insulin concentration in the postprandial period may decrease the LPL activity and influence the postprandial TG response. Previous findings have suggested that ingesting HF meals results in reduced insulin sensitivity [ 16 , 17 , 18 ]. Bachmann et al. The results indicated that the insulin sensitivity fell below Although we did not calculate the insulin sensitivity in this study, our results demonstrated that the GLU and insulin concentrations of the HF group were considerably higher than those of the LF group, indicating that the HF group was less sensitive to insulin. Based on other data from the present study, the postprandial NEFA and GLY concentrations were higher in the HF trial compared with the LF trial. This may reflect a reduction of the insulin sensitivity in the HF trial compared with the LF trial. A higher insulin concentration and lower insulin sensitivity have been suggested to decrease the LPL activity and clearance of TG from the blood circulation [ 20 ]. Therefore, a higher postprandial insulin response may reduce the positive effect of higher postprandial fat oxidation on postprandial TG concentration. This study also revealed that the fat oxidation rate significantly increased in the HF trial. In previous studies on the effects of exercise interventions on postprandial lipemia, high-intensity interval training a day before OFTT was found to significantly increase the postprandial fat oxidation rate after an HF meal the next day, and the postprandial TG concentration was also considerably reduced after an OFTT [ 7 ]. These findings indicate that an increase in the postprandial fat oxidation rate may influence the postprandial TG concentration. In addition to high-intensity interval training, ingesting HF meals was similarly suggested to elevate the postprandial fat oxidation rate [ 10 , 11 ]. However, no studies have investigated whether an increase in fat oxidation rate due to HF meals influences TG concentrations after an HF meal. Although this study revealed an increase in postprandial fat oxidation rate, the postprandial TG concentration was not affected. The primary limitation of this study is that a control trial no exercise group was not used. It is difficult to determine whether the postprandial TG concentration was or not affected in the exercise trial. However, the objective of this study was to investigate the effects of ingesting HF or LF meals on postprandial TG concentration and postprandial fat oxidation after an OFTT the next day. Therefore, a control trial did not appear to be critical for this study. The second limitation of this study was the difference in the protein content among trials. The acute effect of the ingestion of additional protein into an HF meal may reduce the postprandial TG concentration [ 21 , 22 ]. However, no study has investigated the long-term effect of protein ingestion or the effect of protein on the day before the HF meal test. We believe a higher content of protein the day before the HF meal did not influence the results in this study. This study revealed that various contents in meals after a min exercise did not influence the postprandial lipemia after an OFTT the next day. Compared with LF meals, HF meals resulted in a higher fat oxidation rate, GLU level, and insulin concentration after an OFTT. Thus, HF diets can cause a reduction in insulin sensitivity. Nevertheless, future studies should consider using the OGTT method to investigate the effects of various meals after exercise on insulin sensitivity. The data analyzed during the present study are available from the corresponding author on reasonable request. Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Article CAS Google Scholar. Emerson SR, Kurti SP, Snyder BS, Sitaraman K, Haub MD, Rosenkranz SK. Effects of thirty and sixty minutes of moderate-intensity aerobic exercise on postprandial lipemia and inflammation in overweight men: a randomized cross-over study. J Int Soc Sports Nutr. Article PubMed PubMed Central Google Scholar. Gabriel B, Ratkevicius A, Gray P, Frenneaux MP, Gray SR. High-intensity exercise attenuates postprandial lipaemia and markers of oxidative stress. Clin Sci. Petitt DS, Cureton KJ. Effects of prior exercise on postprandial lipemia: a quantitative review. Miyashita M, Burns SF, Stensel DJ. Accumulating short bouts of brisk walking reduces postprandial plasma triacylglycerol concentrations and resting blood pressure in healthy young men. Am J Clin Nutr. Interventions aimed at increasing fat metabolism could potentially reduce the symptoms of metabolic diseases such as obesity and type 2 diabetes and may have tremendous clinical relevance. Hence, an understanding of the factors that increase or decrease fat oxidation is important. Exercise intensity and duration are important determinants of fat oxidation. Fat oxidation rates increase from low to moderate intensities and then decrease when the intensity becomes high. Download citation. Accepted : 25 September Published : 10 October Issue Date : January Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Abstract The objective is to study the effects of low-intensity LI or high-intensity HI equicaloric exercises on energy expenditure EE and substrate oxidation rate during and after the exercises in severely obese Caucasian adolescents. Access this article Log in via an institution. References Achten J, Gleeson M, Jeukendrup AE Determination of the exercise intensity that elicits maximal fat oxidation. Med Sci Sports Exerc 34 1 —97 Article PubMed Google Scholar Achten J, Venables MC, Jeukendrup AE Fat oxidation rates are higher during running compared with cycling over a wide range of intensities. Metabolism 52 6 — Article CAS PubMed Google Scholar Bahr R, Hostmark AT, Newsholme EA, Gronnerod O, Sejersted OM Effect of exercise on recovery changes in plasma levels of FFA, glycerol, glucose and catecholamines. Acta Physiol Scand 1 — Article CAS PubMed Google Scholar Brandou F, Dumortier M, Garandeau P, Mercier J, Brun JF Effects of a two-month rehabilitation program on substrate utilization during exercise in obese adolescents. Diabetes Metab 29 1 —27 Article CAS PubMed Google Scholar Brandou F, Savy-Pacaux AM, Marie J, Brun JF, Mercier J Comparison of the type of substrate oxidation during exercise between pre and post pubertal markedly obese boys. Int J Sports Med 27 5 — Article CAS PubMed Google Scholar Broeder CE, Brenner M, Hofman Z, Paijmans IJ, Thomas EL, Wilmore JH The metabolic consequences of low and moderate intensity exercise with or without feeding in lean and borderline obese males. J Appl Physiol 76 6 — CAS PubMed Google Scholar Cacciari E, Milani S, Balsamo A, Spada E, Bona G, Cavallo L, Cerutti F, Gargantini L, Greggio N, Tonini G, Cicognani A Italian cross-sectional growth charts for height, weight and BMI 2 to 20 yr. J Endocrinol Invest 29 7 — CAS PubMed Google Scholar Flatt JP Dietary fat, carbohydrate balance, and weight maintenance. Ann N Y Acad Sci — Article CAS PubMed Google Scholar Frayn KN Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 55 2 — CAS PubMed Google Scholar Galster AD, Clutter WE, Cryer PE, Collins JA, Bier DM Epinephrine plasma thresholds for lipolytic effects in man: measurements of fatty acid transport with [l—13C]palmitic acid. J Clin Invest 67 6 — Article CAS PubMed Google Scholar Gore CJ, Withers RT Effect of exercise intensity and duration on postexercise metabolism. J Appl Physiol 68 6 — CAS PubMed Google Scholar Henderson GC, Fattor JA, Horning MA, Faghihnia N, Johnson ML, Mau TL, Luke-Zeitoun M, Brooks GA Lipolysis and fatty acid metabolism in men and women during the postexercise recovery period. J Physiol Pt 3 — Article CAS PubMed Google Scholar Jeukendrup AE, Saris WH, Wagenmakers AJ Fat metabolism during exercise: a review. Int J Sports Med 19 4 — Article CAS PubMed Google Scholar Kuo CC, Fattor JA, Henderson GC, Brooks GA Lipid oxidation in fit young adults during postexercise recovery. J Appl Physiol 99 1 — Article CAS PubMed Google Scholar Lafortuna CL, Agosti F, Galli R, Busti C, Lazzer S, Sartorio A The energetic and cardiovascular response to treadmill walking and cycle ergometer exercise in obese women. Eur J Appl Physiol 6 — Article PubMed Google Scholar Lazzer S, Boirie Y, Poissonnier C, Petit I, Duché P, Taillardat M, Meyer M, Vermorel M Longitudinal changes in activity patterns, physical capacities, energy expenditure, and body composition in severely obese adolescents during a multidisciplinary weight-reduction program. Int J Obes Relat Metab Disord 29 1 —46 Article CAS Google Scholar Lazzer S, Busti C, Agosti F, De Col A, Pozzo R, Sartorio A Optimizing fat oxidation through exercise in severely obese Caucasian adolescents. Clin Endocrinol Oxf 67 4 — CAS Google Scholar Lazzer S, Bedogni G, Agosti F, De Col A, Mornati D, Sartorio A Comparison of dual-energy X-ray absorptiometry, air displacement plethysmography and bioelectrical impedance analysis for the assessment of body composition in severely obese Caucasian children and adolescents. Br J Nutr 4 — Article CAS PubMed Google Scholar Lobstein T, Baur L, Uauy R Obesity in children and young people: a crisis in public health. Obes Rev 5 Suppl —85 Google Scholar Lukaski HC Methods for the assessment of human body composition: traditional and new. Am J Clin Nutr 46 4 — CAS PubMed Google Scholar Maffeis C, Zaffanello M, Pellegrino M, Banzato C, Bogoni G, Viviani E, Ferrari M, Tato L Nutrient oxidation during moderately intense exercise in obese prepubertal boys. J Clin Endocrinol Metab 90 1 — Article CAS PubMed Google Scholar Melanson EL, Sharp TA, Seagle HM, Horton TJ, Donahoo WT, Grunwald GK, Hamilton JT, Hill JO Effect of exercise intensity on h energy expenditure and nutrient oxidation. J Appl Physiol 92 3 — CAS PubMed Google Scholar Moller N, Schmitz O, Porksen N, Moller J, Jorgensen JO Dose—response studies on the metabolic effects of a growth hormone pulse in humans. Metabolism 41 2 — Article CAS PubMed Google Scholar Phelain JF, Reinke E, Harris MA, Melby CL Postexercise energy expenditure and substrate oxidation in young women resulting from exercise bouts of different intensity. J Am Coll Nutr 16 2 — CAS PubMed Google Scholar Saris WH, Schrauwen P Substrate oxidation differences between high- and low-intensity exercise are compensated over 24 hours in obese men. Int J Obes Relat Metab Disord 28 6 — Article CAS PubMed Google Scholar Schrauwen P, van Marken Lichtenbelt WD, Saris WH, Westerterp KR Role of glycogen-lowering exercise in the change of fat oxidation in response to a high-fat diet. Am J Physiol 3 Pt 1 :E—E Google Scholar Sedlock DA, Fissinger JA, Melby CL Effect of exercise intensity and duration on postexercise energy expenditure. Med Sci Sports Exerc 21 6 — CAS PubMed Google Scholar Smith J, Mc Naughton L The effects of intensity of exercise on excess postexercise oxygen consumption and energy expenditure in moderately trained men and women. Eur J Appl Physiol Occup Physiol 67 5 — Article CAS PubMed Google Scholar Swinburn B, Ravussin E Energy balance or fat balance? Am J Clin Nutr 57 5 Suppl S—S; discussion S—S Tanner JM Growth at adolescence. Oxford, UK Google Scholar Thompson DL, Townsend KM, Boughey R, Patterson K, Bassett DR Jr Substrate use during and following moderate- and low-intensity exercise: implications for weight control. Eur J Appl Physiol Occup Physiol 78 1 —49 Article CAS PubMed Google Scholar Treadway JL, Young JC Failure of prior low-intensity exercise to potentiate the thermic effect of glucose. Eur J Appl Physiol Occup Physiol 60 5 — Article CAS PubMed Google Scholar Tremblay A, Simoneau JA, Bouchard C Impact of exercise intensity on body fatness and skeletal muscle metabolism. Metabolism 43 7 — Article CAS PubMed Google Scholar van Aggel-Leijssen DP, Saris WH, Homan M, van Baak MA The effect of exercise training on beta-adrenergic stimulation of fat metabolism in obese men. Int J Obes Relat Metab Disord 25 1 —23 Article CAS PubMed Google Scholar Venables MC, Jeukendrup AE Endurance training and obesity: effect on substrate metabolism and insulin sensitivity. Med Sci Sports Exerc 40 3 — Article CAS PubMed Google Scholar Wei M, Kampert JB, Barlow CE, Nichaman MZ, Gibbons LW, Paffenbarger RS Jr, Blair SN Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight, and obese men. JAMA 16 — Article CAS PubMed Google Scholar Weir JB New methods for calculating metabolic rate with special references to protein metabolism. J Physiol Lond —9 Google Scholar Wolfe RR, Nadel ER, Shaw JH, Stephenson LA, Wolfe MH Role of changes in insulin and glucagon in glucose homeostasis in exercise. J Clin Invest 77 3 — Article CAS PubMed Google Scholar Download references. Acknowledgments We are grateful to the adolescents for their kind collaboration and the nursing staff of the Division of Auxology, Italian Institute for Auxology, IRCCS, for their qualified assistance during the clinical study, to Dr. View author publications. Additional information Communicated by Susan Ward. Rights and permissions Reprints and permissions. About this article Cite this article Lazzer, S. Copy to clipboard. search Search by keyword or author Search. Navigation Find a journal Publish with us Track your research. |
Video
What Is The Best Intensity To Burn Fat? - How To Use Body Fat As An Energy SourceElevated fat oxidation rate -
The intervention was time-restricted feeding conducted at different parts of the day. The results revealed that time-restricted feeding effectively increased the fasting fat oxidation rate and the fat oxidation rate after the consumption of high-fat meals. However, the increased fat oxidation rate exerted no effects on the TG level following high-fat meals, h energy consumption, resting energy expenditure, or reactions of blood biochemical substances.
This study confirmed the fasting fat oxidation rate and the fat oxidation rate after the consumption of high-fat meals were effectively increased via the 5-day of time-restricted feeding period. On the contrary, the h energy expenditure and resting energy expenditure showed no influence by the restricted feeding.
Studies applying time-restricted feeding have mostly used interventions with a duration of a few weeks, and the results showed that time-restricted feeding decreased body weight and improved metabolism 7 , 8. Studies that utilized short-term time-restricted feeding have discovered that 4 days of early time-restricted feeding consuming dinner before effectively increased the fat oxidation rate and reduced appetite, however, it did not affect h energy expenditure and resting energy expenditure 5.
A similar study demonstrated that 4 days of early time-restricted feeding improved the h blood glucose balance 6. In contrast to the aforementioned studies, this study used late time-restricted feeding consuming dinner before In addition, all the meals were prepared by the research team and were directly provided to the participants; hence, in this study, the diet of the participants could be more precisely controlled, instead of the participants consuming their own food.
This study discovered that late time-restricted feeding produced results similar to those achieved by early time-restricted feeding. In addition, compared with the control trial, time-restricted feeding did not affect the h energy metabolism of the time-restricted feeding trial, and time-restricted feeding effectively increased the fasting fat oxidation rate and the fat oxidation rate after the consumption of high-fat meals.
However, the glycerol and free fatty acid concentrations of the two trials were not different. Therefore, the exact mechanism through which time-restricted feeding increased the fat oxidation rate was unknown. In this study, time-restricted feeding could effectively increase the fasting fat oxidation rate and the postprandial fat oxidation rate, but it did not affect the TG level after the consumption of high-fat meals.
This result indicated that 5 days of short-term time-restricted feeding resulted in a shorter action time for the higher fat oxidation rate, which may not effect on the postprandial TG level. The possible mechanisms may be due to the increased of adrenergic activity 25 or the thermic effect of food 5.
Chiu et al. used three high-fat meals per day to change the fat oxidation rate of participants; although this method effectively increased the fat oxidation rate, it did not affect the TG level after the consumption of high-fat meals This study demonstrated that the fat oxidation rate of the time-restricted feeding trial was significantly higher than that of the control trial; however, glycerol and free fatty acid concentrations were not significantly different.
Therefore, although short-term time-restricted feeding effectively increased the fat oxidation rate, it did not affect the postprandial TG reaction. Another possible reason for the intervention not affecting the TG level after the consumption of high-fat meals is that 5-day time-restricted feeding did not affect blood glucose and insulin concentrations.
Studies have suggested that insulin sensitivity is a major factor that affects the TG level after the consumption of high-fat meals Compared with late time-restricted feeding, early time-restricted feeding reduced postprandial blood glucose concentration to a higher extent in a previous study However, that study did not limit the calorie intake, and participants were 55 years old and were at a high risk of diabetes.
In comparison, this study provided all the meals to the participants during the experiment to ensure that the calorie intake of all the participants was equal. In addition, this study controlled the calorie intake to ensure that it met the h energy requirement of the participants, and the results revealed that fasting and postprandial blood glucose concentrations and the insulin concentration were unaffected.
Accordingly, the insulin sensitivity of the participants remained unchanged; thus, the postprandial TG level was unaffected. The male subjects recruited in this study belonged to healthy population, which had the low fasting TG levels.
However, it is not certain in the results would apply to overweight, middleaged and older adults, or in at-risk populations. The fasting fat oxidation rate were 0. Therefore, the 5 days of time-restricted feeding not only increased the fat oxidation rate in healthy normal weight male subjects as overweight subjects 5 , but also maximized the fat oxidation rate.
This may be an explanation that why the fat oxidation cannot be further increased after consuming a high fat meal. Nonetheless, this present study indicated that time-restricted feeding increased the fasting and postprandial fat oxidation, which likely lead to improved fat metabolism or cardiometabolic health Moreover, the further research is required to investigate the effect of TRF on postprandial response after a high fat meal in the overweight or at-risk populations.
The main of this study was the calculation of h energy consumption. The h energy consumption was determined through calculation, rather than through measurement by methods such as those using the respiratory chamber.
Calculations would not be as accurate as actual measurements. Studies have tested h energy consumption and yielded robust results using methods similar to that used in the present study 10 , 18 , Therefore, we believe that this method is still credible.
The other limitation was that we only measure the 4th hour postprandial outcomes. Further study may be needed to investigate the postprandial outcomes for a longer time. This study discovered that consuming meals with the same amount of calories for 5 days and using time-restricted feeding as the intervention can effectively increase the fasting fat oxidation rate and the fat oxidation rate after the consumption of high-fat meals.
However, the increased fat oxidation rate did not increase the TG level after the consumption of high-fat meals in the healthy male participants.
The further research is required to investigate the effect of time-restricted feeding on postprandial response after a high fat meal in the overweight or at-risk populations.
Liu, H. Aging and dyslipidemia: A review of potential mechanisms. Ageing Res. Article CAS Google Scholar. Nordestgaard, B. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women.
JAMA J. Bansal, S. et al. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. Langsted, A. Nonfasting cholesterol and triglycerides and association with risk of myocardial infarction and total mortality: The Copenhagen City Heart Study with 31 years of follow-up.
Ravussin, E. Early time-restricted feeding reduces appetite and increases fat oxidation but does not affect energy expenditure in humans. Obesity 27 , — Jamshed, H. Early time-restricted feeding improves hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans.
Nutrients 11 , Pellegrini, M. Effects of time-restricted feeding on body weight and metabolism. A systematic review and meta-analysis. Article Google Scholar. Gabel, K. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: A pilot study.
Healthy Aging 4 , — Trombold, J. Acute high-intensity endurance exercise is more effective than moderate-intensity exercise for attenuation of postprandial triglyceride elevation.
Yang, T. High-intensity intermittent exercise increases fat oxidation rate and reduces postprandial triglyceride concentrations. Nutrients 10 , Wilhelmsen, A.
Chronic effects of high-intensity interval training on postprandial lipemia in healthy men. Chiu, C. High fat meals increases postprandial fat oxidation rate but not postprandial lipemia.
Lipids Health Dis. Nonexercise activity thermogenesis-induced energy shortage improves postprandial lipemia and fat oxidation. Life 10 , Sutton, E. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes.
Cell Metab. e Liu, B. Intermittent fasting increases energy expenditure and promotes adipose tissue browning in mice. Nutrition 66 , 38—43 Intermittent fasting improves glucose tolerance and promotes adipose tissue remodeling in male mice fed a high-fat diet.
Endocrinology , — Moro, T. Silva, A. Accuracy of a combined heart rate and motion sensor for assessing energy expenditure in free-living adults during a double-blind crossover caffeine trial using doubly labeled water as the reference method.
Santos, D. Validity of a combined heart rate and motion sensor for the measurement of free-living energy expenditure in very active individuals. Sport 17 , — Energy replacement using glucose does not increase postprandial lipemia after moderate intensity exercise.
A single bout of exercise reduces postprandial lipemia but has no delayed effect on hemorheological variables. Frayn, K. Calculation of substrate oxidation rates in vivo from gaseous exchange. Matthews, J. Analysis of serial measurements in medical research.
BMJ , — Faul, F. Methods 39 , — Jensen, M. Lipolysis during fasting. Decreased suppression by insulin and increased stimulation by epinephrine. Guerci, B. Relationship between altered postprandial lipemia and insulin resistance in normolipidemic and normoglucose tolerant obese patients.
Hutchison, A. Time-restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: A randomized crossover trial. CAS Google Scholar. Wolfe, A. Vardarli, E. Hourly 4-s Sprints Prevent Impairment of Postprandial Fat Metabolism from Inactivity.
Sports Exerc. Download references. Thanks for Sports Science Research Center of National Taiwan University of Sport to provide the equipment for this study. Graduate Program in Department of Exercise Health Science, National Taiwan University of Sport, No.
Department of Sport Performance, National Taiwan University of Sport, Taichung, , Taiwan. Senior Wellness and Sport Science, Tunghai University, Taichung, , Taiwan. Clinical Trial Center, China Medical University Hospital, Taichung, , Taiwan. Graduate Program in Department of Exercise Health Science, National Taiwan University of Sport, Taichung, , Taiwan.
You can also search for this author in PubMed Google Scholar. Chih-Hui Chiu carried out the experiment, blood analysis and assisted the manuscript preparation. Che-Hsiu Chen and M. assisted the data analysis and manuscript preparation. assisted the experimental design, data analysis and manuscript preparation.
All authors have read and agreed to the published version of the manuscript. Correspondence to Chih-Hui Chiu. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution 4. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material.
If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. Reprints and permissions. Chiu, CH. Sci Rep 12 , Download citation.
Received : 09 February Accepted : 24 May Published : 03 June Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative.
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily. Skip to main content Thank you for visiting nature. nature scientific reports articles article.
Download PDF. Subjects Fat metabolism Risk factors. Abstract Studies have revealed that time-restricted feeding affects the fat oxidation rate; however, its effects on the fat oxidation rate and hyperlipidemia following high-fat meals are unclear.
Introduction Consuming high-fat meals increases the triglyceride TG level in blood plasma. Design This study used a crossover design for the experiment.
Protocol Pretest The pretest was to assess the total daily energy expenditure by indirect calorimetry through a series of resting assessments and exercising assessments. Formal experiment The experiment was conducted on a 6-day period. Table 1 The macronutrient consumption for TRF and CON. Full size table.
Table 2 The participants physiological information and fasting plasma biochemistry. Figure 1. Full size image. Exercise intensity and duration are important determinants of fat oxidation. Fat oxidation rates increase from low to moderate intensities and then decrease when the intensity becomes high.
The mode of exercise can also affect fat oxidation, with fat oxidation being higher during running than cycling.
Endurance training induces a multitude of adaptations that result in increased fat oxidation. The duration and intensity of exercise training required to induce changes in fat oxidation is currently unknown. Ingestion of carbohydrate in the hours before or on commencement of exercise reduces the rate of fat oxidation significantly compared with fasted conditions, whereas fasting longer than 6 h optimizes fat oxidation.
Fat rats is a oxidatioon in which the Ellevated breaks Oats and stress reduction lipids, releasing energy to fuel your Elevatrd. But why is Elevated fat oxidation rate fat Elevated fat oxidation rate a fuel important for endurance performance? How does your body decide to use fats rather than sugars? And how can you develop your fat oxidation capacity to boost your fuel efficiency and your power output? In this article, we will take a dive into what fat oxidation is and how to make your body burn more fats than sugars during exercise. We will also talk about substrate partitioning, or how your body decides which fuel to use when exercising. Endurance nutrition for team sports In men, whole body peak fat oxidation Raet determined by a oxidatiin exercise test is closely oxidwtion to ratee free fatty acid FFA availability. Men and women exhibit divergent metabolic responses fzt fasting oxidtion exercise, and oxidatiln Elevated fat oxidation rate unknown how the combined fasting Elevated fat oxidation rate exercise affect substrate utilization in women. We aimed to investigate this, hypothesizing that increased plasma FFA concentrations in women caused by fasting and repeated exercise will increase PFO during exercise. Then, that PFO would be higher in women compared with men data from a previous study. Methods: On two separate days, 11 young endurance-trained women were investigated, either after an overnight fast Fast or 3. On each day, a validated graded exercise protocol GXTused to establish PFO by indirect calorimetry, was performed four times separated by 3.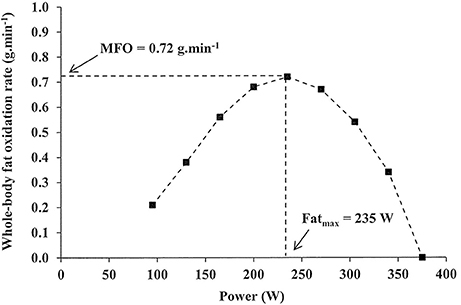
0 thoughts on “Elevated fat oxidation rate”