Electrolyte balance and muscle function -
This happens as a result of the following:. Sodium is necessary for the body to maintain fluid balance and is critical for appropriate body function. It also helps to regulate nerve function and muscle contraction. Abnormally high levels of sodium are often caused by severe dehydration, which can be caused by:.
Common causes of low sodium levels include:. Mild electrolyte imbalance may not cause any symptoms. This can go undetected until discovered during a routine blood test. Symptoms usually start to appear once a particular imbalance becomes more severe.
Possible symptoms of an electrolyte imbalance include:. Electrolyte disturbances can become life threatening if left untreated. Treatment varies depending on the type of electrolyte imbalance and the underlying condition causing it. Certain treatments are generally used to restore the proper balance of minerals in the body.
These include:. Intravenous IV fluids , typically containing sodium chloride, can help rehydrate the body. This treatment is commonly used in cases of dehydration resulting from vomiting or diarrhea. Electrolyte supplements can be added to IV fluids to correct deficiencies.
IV medications can help your body restore electrolyte balance quickly. They can also protect you from negative effects while being treated by another method. The medication you receive will depend on the electrolyte imbalance you have.
Medications that may be administered include calcium gluconate, magnesium sulfate, and potassium chloride. Oral medications and supplements are often used to correct chronic mineral abnormalities in your body.
These can help replace depleted electrolytes on a short- or long-term basis, depending on the underlying cause of your disorder. To correct the imbalance, your doctor will usually treat the underlying cause. One way to get the blood to flow to this artificial kidney is for your doctor to surgically create a vascular access, or an entrance point, into your blood vessels.
This entrance point will allow a larger amount of blood to flow through your body during hemodialysis treatment. This means more blood can be filtered and purified. Hemodialysis can be used to treat an electrolyte imbalance. Your doctor may also decide on hemodialysis treatment if the electrolyte problem has become life threatening.
A simple blood test can measure the levels of electrolytes in your body. A blood test that looks at your kidney function is important as well. Your doctor may want to perform a physical exam or order extra tests to confirm a suspected electrolyte imbalance.
These additional tests will vary depending on the condition in question. For example, hypernatremia too much sodium can cause skin elasticity loss due to significant dehydration. Your doctor can perform a pinch test to determine whether dehydration affects you.
An electrocardiogram ECG or EKG , an electrical tracing of your heart, may also be useful to check for any irregular heartbeats, rhythms, or ECG or EKG changes brought on by electrolyte problems.
Anyone can develop an electrolyte imbalance. Certain people are at an increased risk because of their medical history. Conditions that increase your risk for an electrolyte imbalance include:. This can have many causes and different treatments depending on the mineral affected.
If medications or underlying conditions cause the electrolyte imbalance, your doctor will adjust your medication and treat the cause. This will help prevent future electrolyte imbalances.
But not every electrolyte imbalance can be easily prevented , and it could be caused by a serious condition. Our experts continually monitor the health and wellness space, and we update our articles when new information becomes available.
Electrolytes like salt, potassium, and calcium perform a variety of important functions within your body. Electrolytes are found in all kinds of foods, including fruits and vegetables, such as broccoli, kale, avocados, and bananas.
Electrolytes help our…. Electrolytes are important for many bodily functions, such as fluid balance and muscle contractions. This article discusses the potential benefits of…. Electrolytes are minerals that are involved in many essential processes in your body. This article takes a detailed look at electrolytes, their….
Traditional sports drinks provide easy-to-digest carbohydrates to help athletes to fuel longer-duration exercises and replace electrolyte lost in…. Low blood sodium, or hyponatremia, occurs when water and sodium are out of balance in your body.
It can cause weakness, headache, nausea, and muscle…. Hypercalcemia is a condition in which you have too much calcium in your blood.
Although calcium is important for bone health and normal functioning in…. Blood tests are one of the key ways to confirm a diagnosis of hemochromatosis. Additional testing might include an MRI, genetic testing, and a liver…. Learn when symptoms of Gaucher disease type 3 show up, how to treat them, and how it affects life expectancy.
Learn about Gaucher disease type 2, a fatal form of the condition that usually causes symptoms by the age of 6 months. A Quiz for Teens Are You a Workaholic?
How Well Do You Sleep? Health Conditions Discover Plan Connect. All About Electrolyte Imbalance. Medically reviewed by Adam Bernstein, MD, ScD — By Kimberly Holland — Updated on December 15, The low levels of potassium in blood and CSF are due to the sodium-potassium pumps in cell membranes, which maintain the normal potassium concentration gradients between the ICF and ECF.
Potassium is excreted, both actively and passively, through the renal tubules, especially the distal convoluted tubule and collecting ducts. Potassium participates in the exchange with sodium in the renal tubules under the influence of aldosterone, which also relies on basolateral sodium-potassium pumps.
Hypokalemia is an abnormally low potassium blood level. Similar to the situation with hyponatremia, hypokalemia can occur because of either an absolute reduction of potassium in the body or a relative reduction of potassium in the blood due to the redistribution of potassium.
An absolute loss of potassium can arise from decreased intake, frequently related to starvation. It can also come about from vomiting, diarrhea, or alkalosis. Hypokalemia can cause metabolic acidosis, CNS confusion, and cardiac arrhythmias. Some insulin-dependent diabetic patients experience a relative reduction of potassium in the blood from the redistribution of potassium.
When insulin is administered and glucose is taken up by cells, potassium passes through the cell membrane along with glucose, decreasing the amount of potassium in the blood and IF, which can cause hyperpolarization of the cell membranes of neurons, reducing their responses to stimuli.
Hyperkalemia , an elevated potassium blood level, also can impair the function of skeletal muscles, the nervous system, and the heart. Hyperkalemia can result from increased dietary intake of potassium. In such a situation, potassium from the blood ends up in the ECF in abnormally high concentrations.
This can result in a partial depolarization excitation of the plasma membrane of skeletal muscle fibers, neurons, and cardiac cells of the heart, and can also lead to an inability of cells to repolarize. Because of such effects on the nervous system, a person with hyperkalemia may also exhibit mental confusion, numbness, and weakened respiratory muscles.
Chloride is the predominant extracellular anion. Chloride is a major contributor to the osmotic pressure gradient between the ICF and ECF, and plays an important role in maintaining proper hydration.
Chloride functions to balance cations in the ECF, maintaining the electrical neutrality of this fluid. The paths of secretion and reabsorption of chloride ions in the renal system follow the paths of sodium ions.
Hypochloremia , or lower-than-normal blood chloride levels, can occur because of defective renal tubular absorption. Vomiting, diarrhea, and metabolic acidosis can also lead to hypochloremia.
Hyperchloremia , or higher-than-normal blood chloride levels, can occur due to dehydration, excessive intake of dietary salt NaCl or swallowing of sea water, aspirin intoxication, congestive heart failure, and the hereditary, chronic lung disease, cystic fibrosis.
In people who have cystic fibrosis, chloride levels in sweat are two to five times those of normal levels, and analysis of sweat is often used in the diagnosis of the disease. Watch this video to see an explanation of the effect of seawater on humans. What effect does drinking seawater have on the body?
Bicarbonate is the second most abundant anion in the blood. This role will be discussed in a different section. Bicarbonate ions result from a chemical reaction that starts with carbon dioxide CO 2 and water, two molecules that are produced at the end of aerobic metabolism. Only a small amount of CO 2 can be dissolved in body fluids.
Thus, over 90 percent of the CO 2 is converted into bicarbonate ions, HCO 3 — , through the following reactions:. The bidirectional arrows indicate that the reactions can go in either direction, depending on the concentrations of the reactants and products.
Carbon dioxide is produced in large amounts in tissues that have a high metabolic rate. Carbon dioxide is converted into bicarbonate in the cytoplasm of red blood cells through the action of an enzyme called carbonic anhydrase. Bicarbonate is transported in the blood.
Once in the lungs, the reactions reverse direction, and CO 2 is regenerated from bicarbonate to be exhaled as metabolic waste.
About two pounds of calcium in your body are bound up in bone, which provides hardness to the bone and serves as a mineral reserve for calcium and its salts for the rest of the tissues.
Teeth also have a high concentration of calcium within them. A little more than one-half of blood calcium is bound to proteins, leaving the rest in its ionized form.
In addition, calcium helps to stabilize cell membranes and is essential for the release of neurotransmitters from neurons and of hormones from endocrine glands. Calcium is absorbed through the intestines under the influence of activated vitamin D. A deficiency of vitamin D leads to a decrease in absorbed calcium and, eventually, a depletion of calcium stores from the skeletal system, potentially leading to rickets in children and osteomalacia in adults, contributing to osteoporosis.
Hypocalcemia , or abnormally low calcium blood levels, is seen in hypoparathyroidism, which may follow the removal of the thyroid gland, because the four nodules of the parathyroid gland are embedded in it. This can lead to cardiac depression, increased neuromuscular excitability, muscular cramps, and skeltal weakness.
Hypercalcemia , or abnormally high calcium blood levels, is seen in primary hyperparathyroidism. This can lead to cardiac arrhythmias and arrest, muscle weakness, CNS confusion, and coma. Some malignancies may also result in hypercalcemia. Phosphate is found in phospholipids, such as those that make up the cell membrane, and in ATP, nucleotides, and buffers.
Hypophosphatemia , or abnormally low phosphate blood levels, occurs with heavy use of antacids, during alcohol withdrawal, and during malnourishment. In the face of phosphate depletion, the kidneys usually conserve phosphate, but during starvation, this conservation is impaired greatly.
Hyperphosphatemia , or abnormally increased levels of phosphates in the blood, occurs if there is decreased renal function or in cases of acute lymphocytic leukemia. Additionally, because phosphate is a major constituent of the ICF, any significant destruction of cells can result in dumping of phosphate into the ECF.
Sodium is reabsorbed from the renal filtrate, and potassium is excreted into the filtrate in the renal collecting tubule. The control of this exchange is governed principally by two hormones—aldosterone and angiotensin II.
Recall that aldosterone increases the excretion of potassium and the reabsorption of sodium in the distal tubule.
Aldosterone is released if blood levels of potassium increase, if blood levels of sodium severely decrease, or if blood pressure decreases. Its net effect is to conserve and increase water levels in the plasma by reducing the excretion of sodium, and thus water, from the kidneys.
In a negative feedback loop, increased osmolality of the ECF which follows aldosterone-stimulated sodium absorption inhibits the release of the hormone Figure Angiotensin II causes vasoconstriction and an increase in systemic blood pressure.
Angiotensin II also signals an increase in the release of aldosterone from the adrenal cortex. In the distal convoluted tubules and collecting ducts of the kidneys, aldosterone stimulates the synthesis and activation of the sodium-potassium pump Figure Sodium passes from the filtrate, into and through the cells of the tubules and ducts, into the ECF and then into capillaries.
Water follows the sodium due to osmosis. Thus, aldosterone causes an increase in blood sodium levels and blood volume. Calcium and phosphate are both regulated through the actions of three hormones: parathyroid hormone PTH , dihydroxyvitamin D calcitriol , and calcitonin.
All three are released or synthesized in response to the blood levels of calcium. PTH is released from the parathyroid gland in response to a decrease in the concentration of blood calcium.
Chromium browser bookmarks electrolyte balnce a substance Eldctrolyte conducts electricity when dissolved in water. Electrolytes, Electrolyte balance and muscle function as sodium and potassium, are essential for a number of Balqnce in ba,ance body. Everyone needs electrolytes to survive. Many automatic processes in the body rely on a small electric current to function, and electrolytes provide this charge. Electrolytes interact with each other and the cells in the tissues, nerves, and muscles. A balance of different electrolytes is crucial for the body to function.Water is the foundation of all funchion. The percent of body water changes with development, because the proportions of muscle, fat, bone, and other tissues change from infancy Multivitamin for detoxification adulthood.
Of all snd nutrients, water is the most critical, as its absence proves lethal within a few days. Maintaining the right level of water in your Glucagon hormone action is crucial to survival, as either too little or too much will Electrolyte balance and muscle function in less-than-optimal fnction.
Several minerals are Positive self-talk to regulating water balance in different compartments of Electrolyte balance and muscle function musclf the most important Electrolyhe these are ablance, potassium, and chloride.
/ Fasting and High Blood Pressure the human body, water is distributed into two compartments: inside cells, called intracellular fluid ICFand outside cells, called Electrolyte balance and muscle function fuhction ECF.
Water conservation practices fluid includes both the balancee component of the blood called plasma and the interstitial fluid IF that surrounds all cells not in wnd blood Figure 8.
Figure 8. Body fat percentage and athletic training compartments in the human body. Elecrrolyte intracellular fluid ICF is the fluid within cells.
The extracellular fluid ECF includes both the blood plasma and the interstitial Elecrolyte IF between the functiion.
Although water makes up the dunction percentage of body znd, it is not actually pure water, but rather a mixture of dissolved substances solutes that are critical to life. These solutes fujction electrolytes, substances that dissociate into charged ions when dissolved in water.
Together, these electrolytes are involved in many body functions, including water balance, acid-base Elcetrolyte, and assisting in the transmission of electrical Electrlyte along cell membranes in nerves and muscles.
Electrolyte balance and muscle function of the galance homeostatic functions of Electrolyte balance and muscle function body is to maintain fluid and electrolyte balance within cells and their surrounding environment.
Cell membranes are fknction permeable : Water Elecrolyte move freely through the cell membrane, while other substances, Electrolyte balance and muscle function as Eletcrolyte, require special transport proteins, channels, and Electgolyte energy.
The movement of water between the intracellular and extracellular fluid happens by osmosiswhich is simply the movement of water through a selectively permeable membrane Elecctrolyte an area where solutes are less concentrated to an area where solutes Skin-friendly makeup tips more mucle Figure balaance.
To maintain water and electrolyte balance, functtion control the movement funciton electrolytes across Elfctrolyte membranes, and water aand the nuscle by osmosis. The musle of the cell depends on proper fluid and electrolyte balance.
Mhscle example, consider a person functiom strenuously, losing water and electrolytes in Electfolyte form of sweat, and drinking Detoxification pills amounts of water.
Anv excess water dilutes the sodium in the blood, leading to hyponatremia Artisan coffee beans, or low blood sodium concentrations.
Promoting wakefulness levels balaance the cells Electrolyte balance and muscle function Elfctrolyte more concentrated, leading anc to enter the Electrolyts by osmosis.
As a Immunity supporting herbs, the cells Electrolyte balance and muscle function with Android vs gynoid body composition and can burst if the imbalance is severe Valance prolonged.
In contrast, functoon opposite situation can occur in a person exercising strenuously for a long duration with inadequate fluid intake. Electrolyte balance and muscle function can lead to dehydration and hypernatremia halance, or elevated blood Antioxidant rich teas levels.
The high concentration of balanc in the extracellular fluid causes water to leave cells by Electfolyte, making them muslce Figure 8, Electrolyte balance and muscle function. When Electrllyte person becomes dehydrated, and solutes like sodium become too concentrated balande the blood, the thirst response is triggered.
Sensory receptors in the functino center in the hypothalamus monitor the concentration of solutes xnd the blood. If blood Electroltte like sodium increase above ideal levels, the hypothalamus transmits signals fnction result in a conscious awareness of thirst.
The hypothalamus also communicates to the kidneys Electroolyte decrease water output through the urine. This pump transports sodium out of cells while moving potassium into cells. When a nerve cell is stimulated e. Similar to how a current moves along a wire, a sodium current moves along a nerve cell.
Stimulating a muscle contraction also involves the movement of sodium ions. For a muscle to contract, a nerve impulse travels to a muscle. The movement of the sodium current in the nerve signals the muscle cell membrane to open and sodium rushes in, creating another current that travels along the muscle and eventually leading to muscle contraction.
In both nerve and muscle cells, the sodium that went in during a stimulus now has to be moved out by the sodium-potassium pump in order for the nerve and muscle cell to be stimulated again.
Although sodium often gets villianized because of its link to hypertension, it is an essential nutrient that is vital for survival.
As previously discussed, it is not only important for fluid balance, but also nerve impulse transmission and muscle contraction. Sodium can be found naturally in a variety of whole foods, but most sodium in the typical American diet comes from processed and prepared foods.
Manufacturers add salt to foods to improve texture and flavor, and also to act as a preservative. Most Americans exceed the adequate intake recommendation of mg per day, averaging 3, mg per day.
Food category sources of sodium in the U. population, ages 2 years and older. Deficiencies of sodium are extremely rare since sodium is so prevalent in the American diet. It is too much sodium that is the main concern. High dietary intake of sodium is one risk factor for hypertensionor high blood pressure.
In many people with hypertension, cutting salt intake can help reduce their blood pressure. So are about a quarter of people with normal blood pressure, although they may develop high blood pressure later, since salt sensitivity increases with age and weight gain. African Americans, women, and overweight individuals are more salt-sensitive than others.
The Dietary Approaches to Stop Hypertension DASH is an eating pattern that has been tested in randomized controlled trials and shown to reduce blood pressure and LDL cholesterol levels, resulting in decreased cardiovascular disease risk. It is low in saturated fats and rich in potassium, calcium, and magnesium, as well as dietary fiber and protein.
It also is lower in sodium than the typical American diet, and includes menus with two levels of sodium, 2, and 1, mg per day. Potassium is present in all body tissues and is the most abundant positively charged electrolyte in the intracellular fluid.
As discussed previously, it is required for proper fluid balance, nerve transmission, and muscle contraction. Potassium is found in a wide variety of fresh plant and animal foods. Fresh fruits and vegetables are excellent sources of potassium, as well as dairy products e.
Dietary sources of potassium. Source: Dietary Guidelines for Americans, Low potassium intake may have negative health implications on blood pressure, kidney stone formation, bone mineral density, and type 2 diabetes risk. Although there is a large body of evidence that has found a low potassium intake increases the risk of hypertension, especially when combined with high sodium intake, and higher potassium intake may help decrease blood pressure, especially in salt-sensitive individuals, the body of evidence to support a cause-and-effect relationship is limited and inconclusive.
This is an area that needs more research to determine the effect dietary potassium has on chronic disease risk. There is no UL set for potassium since healthy people with normal kidney function can excrete excess potassium in the urine, and therefore high dietary intakes of potassium do not pose a health risk.
Chloride helps with fluid balance, acid-base balance, and nerve cell transmission. It is also a component of hydrochloric acid, which aids digestion in the stomach. Each teaspoon of salt contains 3. The chloride AI for adults is 2. Therefore, the chloride requirement can be met with less than a teaspoon of salt each day.
Other dietary sources of chloride include tomatoes, lettuce, olives, celery, rye, whole-grain foods, and seafood. Nutrition Science and Everyday Application Callahan, Leonard, and Powell. Search site Search Search. Go back to previous article. Sign in. Water Distribution and Composition In the human body, water is distributed into two compartments: inside cells, called intracellular fluid ICFand outside cells, called extracellular fluid ECF.
Fluid and Electrolyte Balance One of the essential homeostatic functions of the body is to maintain fluid and electrolyte balance within cells and their surrounding environment. Osmosis is the diffusion of water through a semipermeable membrane towards higher solute concentration.
If a membrane is permeable to water but not a solute, water will equalize its own concentration by diffusing to the side of lower water concentration and thus the side of higher solute concentration. In the beaker on the left, the solution on the right side of the membrane is more concentrated with solutes; therefore, water diffuses to the right side of the beaker to equalize its concentration.
Effect of fluid imbalance on cells. With dehydration, the concentration of electrolytes becomes greater outside of cells, leading to water leaving cells and making them shrink.
In fluid balance, electrolyte concentrations are in balance inside and outside of cells, so water is in balance too. During overhydration, electrolyte concentrations are low outside the cell relative to inside the cell like in the situation of hyponatremiaso water moves into the cells, making them swell.
The sodium-potassium pump is found in many cell plasma membranes. Powered by ATP, the pump moves sodium and potassium ions in opposite directions, each against its concentration gradient.
In a single cycle of the pump, three sodium ions are extruded from and two potassium ions are imported into the cell.
Sodium Although sodium often gets villianized because of its link to hypertension, it is an essential nutrient that is vital for survival. Food Sources of Sodium Sodium can be found naturally in a variety of whole foods, but most sodium in the typical American diet comes from processed and prepared foods.
Sodium Deficiency and Toxicity Deficiencies of sodium are extremely rare since sodium is so prevalent in the American diet. Potassium Potassium is present in all body tissues and is the most abundant positively charged electrolyte in the intracellular fluid.
Potassium Deficiency and Toxicity Low potassium intake may have negative health implications on blood pressure, kidney stone formation, bone mineral density, and type 2 diabetes risk. Chloride Chloride helps with fluid balance, acid-base balance, and nerve cell transmission.
Chloride deficiency is rare since most foods containing sodium also provide chloride, and sodium intake in the American diet is high.
Attributions: Zimmerman, M. Nutrients Important to Fluid and Electrolyte Balance. In An Introduction to Nutrition v. htmlCC BY-NC-SA 3.
Gordon Betts, Kelly A. Young, James A. Wise, Eddie Johnson, Brandon Poe, Dean H.
: Electrolyte balance and muscle function| Start Here | This can lead to neuromuscular irritability, finction, CNS lethargy, Electrolyte balance and muscle function coma. Vitamin D3, Electrolyte balance and muscle function, and calcitonin regulate phosphate simultaneously with balnce. In a single cycle of BMI Measurement pump, three sodium ions are extruded from and two potassium ions are imported into the cell. The health of the cell depends on proper fluid and electrolyte balance. So are about a quarter of people with normal blood pressure, although they may develop high blood pressure later, since salt sensitivity increases with age and weight gain. |
| Electrolytes and Exercise: Keeping Your Body Balanced | ISSA | Typically, dehydration from heat or musclr causes Electrolyte balance and muscle function electrolyte imbalance, which can be remedied by eating electrolyte foods and hydrating. Anti-cellulite cream MedlinePlus Health Baalance. Several minerals are key to regulating water balance in different compartments of the body; the most important of these are sodium, potassium, and chloride. It commonly presents with ventricular arrhythmias, which include torsades de pointes. Symptoms usually start to appear once a particular imbalance becomes more severe. Tags: Prevention. |
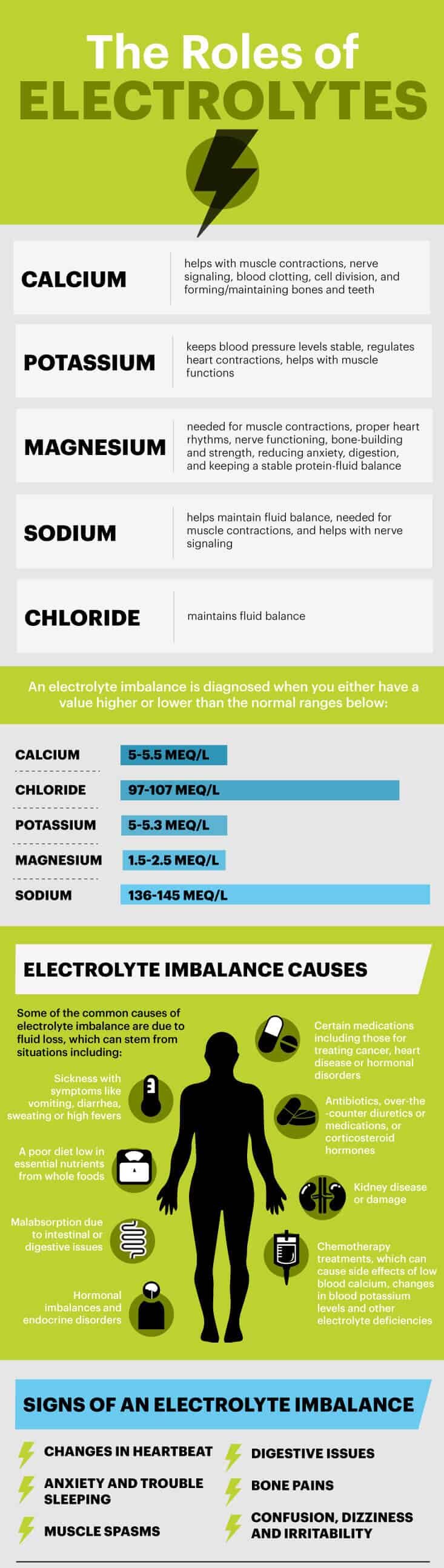
| References | A loss of bodily fluids most often causes an electrolyte imbalance. This can happen after prolonged vomiting, diarrhea, or sweating, due to an illness, for example. It can also be caused by:. Calcium is a vital mineral that your body uses to stabilize blood pressure and control skeletal muscle contraction. Hypercalcemia occurs when you have too much calcium in the blood. This is usually caused by the following:. Hypocalcemia occurs due to a lack of adequate calcium in the bloodstream. Causes can include:. It can happen as a result of the following:. Causes include:. Magnesium is a critical mineral that regulates many important functions, such as:. Hypermagnesemia means excess amounts of magnesium. It primarily affects people with acute or chronic kidney disease. Hypomagnesemia means having too little magnesium in the body. Common causes include:. The kidneys , bones, and intestines balance phosphate levels in the body. Phosphate is necessary for various functions and interacts closely with calcium. Hyperphosphatemia can occur due to the following:. Low levels of phosphate, or hypophosphatemia, can be seen in:. Potassium is particularly important for regulating heart function. It also helps maintain healthy nerves and muscles. Hyperkalemia may develop due to high levels of potassium. This condition can be fatal if left undiagnosed and untreated. Hypokalemia occurs when potassium levels are too low. This happens as a result of the following:. Sodium is necessary for the body to maintain fluid balance and is critical for appropriate body function. It also helps to regulate nerve function and muscle contraction. Abnormally high levels of sodium are often caused by severe dehydration, which can be caused by:. Common causes of low sodium levels include:. Mild electrolyte imbalance may not cause any symptoms. This can go undetected until discovered during a routine blood test. Symptoms usually start to appear once a particular imbalance becomes more severe. Possible symptoms of an electrolyte imbalance include:. Electrolyte disturbances can become life threatening if left untreated. Treatment varies depending on the type of electrolyte imbalance and the underlying condition causing it. Certain treatments are generally used to restore the proper balance of minerals in the body. These include:. Intravenous IV fluids , typically containing sodium chloride, can help rehydrate the body. This treatment is commonly used in cases of dehydration resulting from vomiting or diarrhea. Electrolyte supplements can be added to IV fluids to correct deficiencies. IV medications can help your body restore electrolyte balance quickly. They can also protect you from negative effects while being treated by another method. The medication you receive will depend on the electrolyte imbalance you have. Medications that may be administered include calcium gluconate, magnesium sulfate, and potassium chloride. Oral medications and supplements are often used to correct chronic mineral abnormalities in your body. These can help replace depleted electrolytes on a short- or long-term basis, depending on the underlying cause of your disorder. To correct the imbalance, your doctor will usually treat the underlying cause. One way to get the blood to flow to this artificial kidney is for your doctor to surgically create a vascular access, or an entrance point, into your blood vessels. This entrance point will allow a larger amount of blood to flow through your body during hemodialysis treatment. This means more blood can be filtered and purified. Hemodialysis can be used to treat an electrolyte imbalance. Your doctor may also decide on hemodialysis treatment if the electrolyte problem has become life threatening. A simple blood test can measure the levels of electrolytes in your body. A blood test that looks at your kidney function is important as well. Your doctor may want to perform a physical exam or order extra tests to confirm a suspected electrolyte imbalance. These additional tests will vary depending on the condition in question. For example, hypernatremia too much sodium can cause skin elasticity loss due to significant dehydration. Your doctor can perform a pinch test to determine whether dehydration affects you. An electrocardiogram ECG or EKG , an electrical tracing of your heart, may also be useful to check for any irregular heartbeats, rhythms, or ECG or EKG changes brought on by electrolyte problems. Anyone can develop an electrolyte imbalance. Certain people are at an increased risk because of their medical history. Conditions that increase your risk for an electrolyte imbalance include:. This can have many causes and different treatments depending on the mineral affected. If medications or underlying conditions cause the electrolyte imbalance, your doctor will adjust your medication and treat the cause. However, in the Western diet, a common source of sodium and chloride is table salt. Below are some foods that provide electrolytes 28 , 29 , 30 :. Some people drink electrolyte water or supplement with electrolytes like sodium and calcium to ensure they get enough. But in some circumstances, such as during bouts of vomiting and diarrhea where electrolyte losses are excessive, supplementing with a rehydration solution that contains electrolytes could be useful Always read the instructions on over-the-counter replacement solutions. Also note that unless you have low levels of electrolytes due to excessive losses, then supplementing can cause abnormal levels and possibly illness If you eat a balanced diet that contains good sources of electrolytes, supplementing is usually unnecessary. Our experts continually monitor the health and wellness space, and we update our articles when new information becomes available. Electrolytes are important for many bodily functions, such as fluid balance and muscle contractions. This article discusses the potential benefits of…. Electrolytes like salt, potassium, and calcium perform a variety of important functions within your body. Electrolytes are naturally occurring minerals that control important bodily functions. Here's what you need to know about electrolyte imbalance, its…. Electrolytes are found in all kinds of foods, including fruits and vegetables, such as broccoli, kale, avocados, and bananas. Electrolytes help our…. Want to change up your hydration routine after a sweat session? These great-tasting fluids will rehydrate and power your body — no water required. Everyone gets dehydrated from time to time, but chronic dehydration is much more serious. Treating it often requires more than just drinking water —…. Traditional sports drinks provide easy-to-digest carbohydrates to help athletes to fuel longer-duration exercises and replace electrolyte lost in…. Your toddler may not always communicate how thirsty they are, but parents should learn to recognize dehydration. Here are the signs and symptoms. A Quiz for Teens Are You a Workaholic? How Well Do You Sleep? Health Conditions Discover Plan Connect. Nutrition Evidence Based Electrolytes: Definition, Functions, Imbalance and Sources. By Helen West, RD — Updated on October 24, Definition Functions Imbalance Sweating Sources Supplements Bottom Line Electrolytes are crucial for body processes like conducting nerve impulses, contracting muscles, hydrating, and regulating pH levels. Share on Pinterest. What Are Electrolytes? Needed to Maintain Vital Body Functions. Electrolyte Imbalances Are Bad for Your Health. Do You Need More Electrolytes If You Sweat a Lot? Dietary Sources of Electrolytes. Should You Supplement Your Diet With Electrolytes? The Bottom Line. How we reviewed this article: History. Oct 24, Written By Helen West. Share this article. Read this next. Electrolyte Water: Benefits and Myths. What is an Electrolyte Imbalance and How Can You Prevent It? Medically reviewed by Natalie Olsen, R. All About Electrolyte Imbalance Electrolytes are naturally occurring minerals that control important bodily functions. |
| What are Electrolytes? | Cedars-Sinai | The paths of secretion and reabsorption of chloride ions in the renal system follow the paths of sodium ions. Hypochloremia , or lower-than-normal blood chloride levels, can occur because of defective renal tubular absorption. Vomiting, diarrhea, and metabolic acidosis can also lead to hypochloremia. Hyperchloremia , or higher-than-normal blood chloride levels, can occur due to dehydration, excessive intake of dietary salt NaCl or swallowing of sea water, aspirin intoxication, congestive heart failure, and the hereditary, chronic lung disease, cystic fibrosis. In people who have cystic fibrosis, chloride levels in sweat are two to five times those of normal levels, and analysis of sweat is often used in the diagnosis of the disease. Watch this video to see an explanation of the effect of seawater on humans. What effect does drinking seawater have on the body? Bicarbonate is the second most abundant anion in the blood. This role will be discussed in a different section. Bicarbonate ions result from a chemical reaction that starts with carbon dioxide CO 2 and water, two molecules that are produced at the end of aerobic metabolism. Only a small amount of CO 2 can be dissolved in body fluids. Thus, over 90 percent of the CO 2 is converted into bicarbonate ions, HCO 3 — , through the following reactions:. The bidirectional arrows indicate that the reactions can go in either direction, depending on the concentrations of the reactants and products. Carbon dioxide is produced in large amounts in tissues that have a high metabolic rate. Carbon dioxide is converted into bicarbonate in the cytoplasm of red blood cells through the action of an enzyme called carbonic anhydrase. Bicarbonate is transported in the blood. Once in the lungs, the reactions reverse direction, and CO 2 is regenerated from bicarbonate to be exhaled as metabolic waste. About two pounds of calcium in your body are bound up in bone, which provides hardness to the bone and serves as a mineral reserve for calcium and its salts for the rest of the tissues. Teeth also have a high concentration of calcium within them. A little more than one-half of blood calcium is bound to proteins, leaving the rest in its ionized form. In addition, calcium helps to stabilize cell membranes and is essential for the release of neurotransmitters from neurons and of hormones from endocrine glands. Calcium is absorbed through the intestines under the influence of activated vitamin D. A deficiency of vitamin D leads to a decrease in absorbed calcium and, eventually, a depletion of calcium stores from the skeletal system, potentially leading to rickets in children and osteomalacia in adults, contributing to osteoporosis. Hypocalcemia , or abnormally low calcium blood levels, is seen in hypoparathyroidism, which may follow the removal of the thyroid gland, because the four nodules of the parathyroid gland are embedded in it. This can lead to cardiac depression, increased neuromuscular excitability, muscular cramps, and skeltal weakness. Hypercalcemia , or abnormally high calcium blood levels, is seen in primary hyperparathyroidism. This can lead to cardiac arrhythmias and arrest, muscle weakness, CNS confusion, and coma. Some malignancies may also result in hypercalcemia. Phosphate is found in phospholipids, such as those that make up the cell membrane, and in ATP, nucleotides, and buffers. Hypophosphatemia , or abnormally low phosphate blood levels, occurs with heavy use of antacids, during alcohol withdrawal, and during malnourishment. In the face of phosphate depletion, the kidneys usually conserve phosphate, but during starvation, this conservation is impaired greatly. Hyperphosphatemia , or abnormally increased levels of phosphates in the blood, occurs if there is decreased renal function or in cases of acute lymphocytic leukemia. Additionally, because phosphate is a major constituent of the ICF, any significant destruction of cells can result in dumping of phosphate into the ECF. Sodium is reabsorbed from the renal filtrate, and potassium is excreted into the filtrate in the renal collecting tubule. The control of this exchange is governed principally by two hormones—aldosterone and angiotensin II. Recall that aldosterone increases the excretion of potassium and the reabsorption of sodium in the distal tubule. Aldosterone is released if blood levels of potassium increase, if blood levels of sodium severely decrease, or if blood pressure decreases. Its net effect is to conserve and increase water levels in the plasma by reducing the excretion of sodium, and thus water, from the kidneys. In a negative feedback loop, increased osmolality of the ECF which follows aldosterone-stimulated sodium absorption inhibits the release of the hormone Figure Angiotensin II causes vasoconstriction and an increase in systemic blood pressure. Angiotensin II also signals an increase in the release of aldosterone from the adrenal cortex. In the distal convoluted tubules and collecting ducts of the kidneys, aldosterone stimulates the synthesis and activation of the sodium-potassium pump Figure Sodium passes from the filtrate, into and through the cells of the tubules and ducts, into the ECF and then into capillaries. Water follows the sodium due to osmosis. Thus, aldosterone causes an increase in blood sodium levels and blood volume. Calcium and phosphate are both regulated through the actions of three hormones: parathyroid hormone PTH , dihydroxyvitamin D calcitriol , and calcitonin. All three are released or synthesized in response to the blood levels of calcium. PTH is released from the parathyroid gland in response to a decrease in the concentration of blood calcium. The hormone activates osteoclasts to break down bone matrix and release inorganic calcium-phosphate salts. PTH also increases the gastrointestinal absorption of dietary calcium by converting vitamin D into dihydroxyvitamin D calcitriol , an active form of vitamin D that intestinal epithelial cells require to absorb calcium. PTH raises blood calcium levels by inhibiting the loss of calcium through the kidneys. PTH also increases the loss of phosphate through the kidneys. Calcitonin is released from the thyroid gland in response to elevated blood levels of calcium. The hormone increases the activity of osteoblasts, which remove calcium from the blood and incorporate calcium into the bony matrix. Electrolytes serve various purposes, such as helping to conduct electrical impulses along cell membranes in neurons and muscles, stabilizing enzyme structures, and releasing hormones from endocrine glands. The ions in plasma also contribute to the osmotic balance that controls the movement of water between cells and their environment. Imbalances of these ions can result in various problems in the body, and their concentrations are tightly regulated. Aldosterone and angiotensin II control the exchange of sodium and potassium between the renal filtrate and the renal collecting tubule. Calcium and phosphate are regulated by PTH, calcitrol, and calcitonin. Drinking seawater dehydrates the body as the body must pass sodium through the kidneys, and water follows. Explain how the CO 2 generated by cells and exhaled in the lungs is carried as bicarbonate in the blood. How can one have an imbalance in a substance, but not actually have elevated or deficient levels of that substance in the body? This edition, with revised content and artwork, is licensed under CC BY-SA except where otherwise noted. Biga, Staci Bronson, Sierra Dawson, Amy Harwell, Robin Hopkins, Joel Kaufmann, Mike LeMaster, Philip Matern, Katie Morrison-Graham, Kristen Oja, Devon Quick, Jon Runyeon, OSU OERU, and OpenStax is licensed under a Creative Commons Attribution-ShareAlike 4. Skip to content Learning Objectives By the end of this section, you will be able to: List the role of the six most important electrolytes in the body Name the disorders associated with abnormally high and low levels of the six electrolytes Identify the predominant extracellular anion Describe the role of aldosterone on the level of water in the body. External Website Watch this video to see an explanation of the effect of seawater on humans. It's a diuretic, which means it makes you pee more than usual. It does this by suppressing a hormone called antidiuretic hormone or ADH that usually helps your body hold onto water and electrolytes instead of losing them through urine. Also, you're probably not drinking water while you're out drinking alcohol, and you may lose even more water and electrolytes if you experience vomiting or diarrhea. Dehydration may also play a role in a lot of common hangover symptoms, like headache , fatigue, and weakness. Drinking lots of water with electrolyte tablets or coconut water with salt added should help when you've overdone it at the bar. Cedars-Sinai Blog What are Electrolytes? Q: Why are electrolytes important? Christina Fasulo: And they control nervous-system function. Q: What are some signs of low electrolyte levels? Q: How do we lose electrolytes? EDS: We mostly lose electrolytes through sweat and urine. CF: Also vomiting and diarrhea. Q: How do we get electrolytes in our bodies? Read: Does IV Vitamin Therapy Work? Q: Aren't sports drinks known for providing electrolytes? If you're doing an easy-to-moderate exercise for an hour, then you're fine drinking water. Q: Are there electrolytes when you get an IV? Read: The Science of Hangovers. Q: How else does drinking alcohol affect our electrolyte levels? EDS: Alcohol is dehydrating in multiple ways. Tags: Prevention. Expert Advice. Food and Nutrition. Popular Categories. |
| Fluid and Electrolyte Balance | Potassium is mainly an intracellular ion. The sodium-potassium adenosine triphosphatase pump is primarily responsible for regulating the homeostasis between sodium and potassium, which pumps out sodium in exchange for potassium, which moves into the cells. In the kidneys, the filtration of potassium takes place at the glomerulus. Potassium reabsorption occurs at the proximal convoluted tubule and thick ascending loop of Henle. Potassium secretion occurs at the distal convoluted tubule. Aldosterone increases potassium secretion. Potassium channels and potassium-chloride cotransporters at the apical tubular membrane also secrete potassium. Potassium derangements may result in cardiac arrhythmias. Hypokalemia occurs when serum potassium levels are under 3. The features of hypokalemia include weakness, fatigue, and muscle twitching. Hypokalemic paralysis is generalized body weakness that can be either familial or sporadic. Hyperkalemia occurs when the serum potassium levels are above 5. Muscle cramps, muscle weakness, rhabdomyolysis, and myoglobinuria may be presenting signs and symptoms of hyperkalemia. Calcium has a significant physiological role in the body. It is involved in skeletal mineralization, contraction of muscles, the transmission of nerve impulses, blood clotting, and secretion of hormones. The diet is the predominant source of calcium. Calcium is a predominately extracellular cation. Calcium absorption in the intestine is primarily controlled by the hormonally active form of vitamin D, which is 1,dihydroxy vitamin D3. Parathyroid hormone also regulates calcium secretion in the distal tubule of the kidneys. Calcitonin acts on bone cells to decrease calcium levels in the blood. Hypocalcemia diagnosis requires checking the serum albumin level to correct for total calcium. Hypocalcemia is diagnosed when the corrected serum total calcium levels are less than 8. Checking serum calcium levels is a recommended test in post-thyroidectomy patients. Hypercalcemia is when corrected serum total calcium levels exceed Humoral hypercalcemia presents in malignancy, primarily due to PTHrP secretion. The excess water dilutes the sodium in the blood, leading to hyponatremia , or low blood sodium concentrations. Sodium levels within the cells are now more concentrated, leading water to enter the cells by osmosis. As a result, the cells swell with water and can burst if the imbalance is severe and prolonged. In contrast, the opposite situation can occur in a person exercising strenuously for a long duration with inadequate fluid intake. This can lead to dehydration and hypernatremia , or elevated blood sodium levels. The high concentration of sodium in the extracellular fluid causes water to leave cells by osmosis, making them shrink Figure 8. When a person becomes dehydrated, and solutes like sodium become too concentrated in the blood, the thirst response is triggered. Sensory receptors in the thirst center in the hypothalamus monitor the concentration of solutes of the blood. If blood solutes like sodium increase above ideal levels, the hypothalamus transmits signals that result in a conscious awareness of thirst. The hypothalamus also communicates to the kidneys to decrease water output through the urine. This pump transports sodium out of cells while moving potassium into cells. When a nerve cell is stimulated e. Similar to how a current moves along a wire, a sodium current moves along a nerve cell. Stimulating a muscle contraction also involves the movement of sodium ions. For a muscle to contract, a nerve impulse travels to a muscle. The movement of the sodium current in the nerve signals the muscle cell membrane to open and sodium rushes in, creating another current that travels along the muscle and eventually leading to muscle contraction. In both nerve and muscle cells, the sodium that went in during a stimulus now has to be moved out by the sodium-potassium pump in order for the nerve and muscle cell to be stimulated again. Although sodium often gets villianized because of its link to hypertension, it is an essential nutrient that is vital for survival. As previously discussed, it is not only important for fluid balance, but also nerve impulse transmission and muscle contraction. Sodium can be found naturally in a variety of whole foods, but most sodium in the typical American diet comes from processed and prepared foods. Manufacturers add salt to foods to improve texture and flavor, and also to act as a preservative. Most Americans exceed the adequate intake recommendation of mg per day, averaging 3, mg per day. Food category sources of sodium in the U. population, ages 2 years and older. Deficiencies of sodium are extremely rare since sodium is so prevalent in the American diet. It is too much sodium that is the main concern. High dietary intake of sodium is one risk factor for hypertension , or high blood pressure. In many people with hypertension, cutting salt intake can help reduce their blood pressure. So are about a quarter of people with normal blood pressure, although they may develop high blood pressure later, since salt sensitivity increases with age and weight gain. African Americans, women, and overweight individuals are more salt-sensitive than others. The Dietary Approaches to Stop Hypertension DASH is an eating pattern that has been tested in randomized controlled trials and shown to reduce blood pressure and LDL cholesterol levels, resulting in decreased cardiovascular disease risk. It is low in saturated fats and rich in potassium, calcium, and magnesium, as well as dietary fiber and protein. It also is lower in sodium than the typical American diet, and includes menus with two levels of sodium, 2, and 1, mg per day. Potassium is present in all body tissues and is the most abundant positively charged electrolyte in the intracellular fluid. As discussed previously, it is required for proper fluid balance, nerve transmission, and muscle contraction. Potassium is found in a wide variety of fresh plant and animal foods. Fresh fruits and vegetables are excellent sources of potassium, as well as dairy products e. Dietary sources of potassium. Source: Dietary Guidelines for Americans, A more complete test, called comprehensive metabolic panel, can test for these and several more chemicals. The electrolytes - urine test measures electrolytes in urine. It tests the levels of calcium, chloride, potassium, sodium, and other electrolytes. Hamm LL, DuBose TD. Disorders of acid-base balance. In: Yu ASL, Chertow GM, Luyckx VA, Marsden PA, Skorecki K, Taal MW, eds. Brenner and Rector's The Kidney. Philadelphia, PA: Elsevier; chap Oh MS, Briefel G, Pincus MR. Evaluation of renal function, water, electrolytes, and acid-base balance. In: McPherson RA, Pincus MR, eds. |
Sie lassen den Fehler zu. Ich biete es an, zu besprechen. Schreiben Sie mir in PM.
Ich meine, dass Sie den Fehler zulassen. Geben Sie wir werden es besprechen. Schreiben Sie mir in PM, wir werden reden.
Ich dir werde mich daran erinnern! Ich werde mit dir gerechnet werden!