
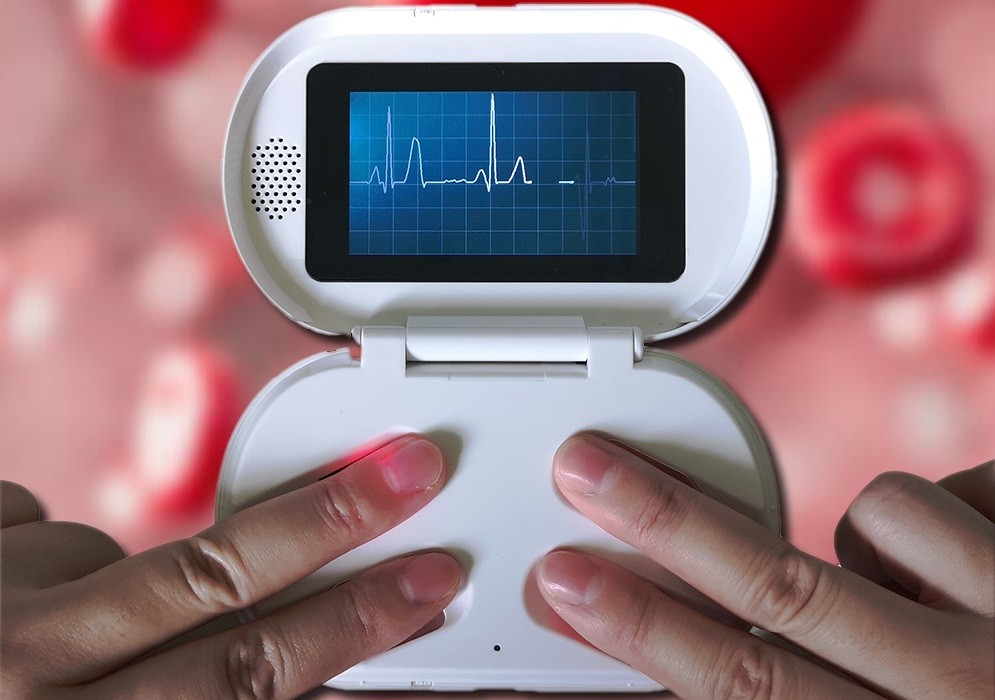
Noninvasive glucose monitor -
Natalia Mesa was previously an intern at The Scientist and now freelances. ABOVE: Common blood glucose monitoring methods require patients to use lancets to draw blood. com, Ta Nu.
F or people with diabetes, blood sugar monitoring is a daily — and sometimes hourly — chore. Most approaches are invasive and involve piercing the skin, which can cause pain. In a study recently published in Science Advances, researchers revealed a thin, invisible, and biodegradable patch that successfully measured different concentrations of blood glucose in mice.
The researchers speculated that when combined with a smartphone application, the technology could help patients with diabetes continuously and noninvasively monitor their blood glucose levels.
Many people with diabetes monitor their blood sugar with a standard glucose meter. They prick their fingertips with a hairlike needle, or lancet, to draw minuscule amounts of blood. If done incorrectly, this process can be painful and cause hard calluses.
Other common alternatives are also invasive. The sensor degrades completely roughly every three days. The sensor the researchers designed consists of two layers. The top layer is made from polyvinyl alcohol. The second layer comprises an array of needles which pierce the skin surface but not far enough to reach nerve endings.
This layer is made of silk, a well known biodegradable material. To measure glucose, the team chose a glucose fluorescent GF monomer composed of two parts. The first is a di-boronic acid, which binds to glucose in the interstitial fluid just underneath the skin. The other is a hydrophobic fluorescent molecule, which changes shape and emits fluorescence when glucose binds to the acid.
c The blood glucose variation curve of a volunteer measured by the watch during the daytime compared to true blood glucose values reference obtained from finger blood.
d Glucose concentrations before and after a meal measured by the watch from five volunteers. of five replicates. e Plot of glucose concentrations measured from 23 volunteers by the watch and by a commercial glucose meter. All fingerstick blood tests except the second were performed immediately after meals.
A two-volunteer 1 diabetic and 1 nondiabetic trial was conducted to assess the accuracy of consecutive measurements by the watch. Five fasting glucose levels of each volunteer were measured by the watch within 1.
The two types of results matched well for both volunteers, indicating good accuracy and reproducibility of glucose measurements by the watch in the short term Fig. This result also serves as circumstantial evidence of the reproducibility of the iontophoresis function in the watch.
We further tested the performance of the watch on five other volunteers, measuring their blood glucose levels before and after a meal. The watch successfully captured the increase in blood glucose levels after a meal Fig. To evaluate the accuracy of glucose measurements by the watch with a widely acknowledged criterion, the Clarke error grid was plotted using the measurement results obtained from 23 volunteers Fig.
The results and statistics of measurement by the watch are presented in Fig. S11 and Table S3. The percentage of data points in zone A and zone B of the Clarke error grid, which represents clinically accepted accurate readings and acceptable moderate readings that would not lead to inappropriate treatments, indicates the accuracy of the tested glucose meter.
Remarkably, no experimental data points fell in zone D or zone E, suggesting that the watch yields high-quality measurement results without misleading or false readings The data points are concentrated in zone A Additionally, all volunteers reported a comfortable wearing experience resembling that of commercial smartwatches, with no obvious sensational difference e.
To verify that daily body motions do not impair the sensing performance of the watch, we compared the measurement results from two watches, one worn on a static arm and the other on a moving arm, of the same nondiabetic volunteer. The difference between the average results of six measurements each from the two watches was 2.
S12 , comparable to the error of the same sensor between repeated measurements, indicating that daily body motions do not affect the performance of the watch. In summary, we developed a highly integrated glucose monitoring watch and achieved noninvasive continual blood glucose monitoring with clinically acceptable accuracy.
Reverse iontophoresis-based ISF extraction by a flexible glucose sensor patch allows painless glucose detection, and the watch-like design ensures comfortable daily wear, facilitating continual glucose monitoring. Real-life testing of the watch on 23 volunteers revealed Subsequent efforts could be made in a few directions; for example, the accuracy could be improved by providing customized models to accommodate potentially interfering factors such as age, gender 38 , exercise 39 , and illness The PCB could be miniaturized and integrated into existing smartwatch models to create a truly noninvasive continuous glucose monitoring smartwatch.
All reagents were used as received. The fabrication of the electrodes is illustrated in Fig. First, the polyimide PI film was cleaned with acetone, ethanol, and ultrapure water.
Then, the electrode and wire areas were defined by a photolithographed layer of positive photoresist AZ Finally, another layer of positive photoresist AZ was photolithographed onto the nonelectrode areas to insulate the wires.
For the working electrodes, three modification steps were performed sequentially, coating the Au electrode with a Prussian blue PB layer, a GO x selective membrane, and a Nafion film. PB was electrodeposited onto the Au electrodes at 0. The designated counter electrodes were left unmodified.
where D m is the mass diffusive coefficient and C g is the glucose concentration. As glucose is rapidly consumed in the extracted ISF, the mass transfer pattern quickly switches from a semi-infinite diffusion model to a finite diffusion model, i. Taking semi-infinite diffusion and the boundary effect into account 36 , the following equation is obtained using the Laplace transform:.
The switching of one of them from 1 to 0 and the other from 0 to 1 represents the complete switching of the diffusion model applied, i. The PCB circuit is based around the STM32LK8 bit microcontroller Texas Instruments module 3 in Fig. In the schematic diagram of the microcontroller interface, PA1 and PA5 are connected to the working electrodes for amperometric signal reading, and PA8 is connected to the constant current source for current delivery for reverse iontophoresis Fig.
The Bluetooth chip is connected to pins PA2 and PA3 of the microcontroller to achieve wireless transmission to a cell phone. The signals are further transmitted and processed by the filter circuitry Fig. On the sensor interface, pins 1, 5, 6, and 12 correspond to the extraction electrodes; pins 2 and 11 correspond to the counter electrodes; pins 3 and 10 correspond to the working electrodes; and pins 4 and 7 correspond to the reference electrodes Fig.
A mobile application was designed for a better user experience. As shown in Figs. In addition, the application is capable of storing historic data and plotting the trend of blood glucose over the period of wearing.
The on-body testing of the watch was performed in compliance with the protocol that was approved by the institutional review board of China-Japan Friendship Hospital K Thirteen diabetic patients aged 40—60 were recruited from China-Japan Friendship Hospital, and 10 nonpatients aged 20—40 were recruited within Beihang University.
Six fingerstick blood samples were taken from each subject and measured by a commercial glucose meter Accusure , Yuwell Co.
The values obtained with the commercial glucose meter and with our watch were recorded and further analyzed.
To test the reproducibility of the reverse iontophoresis function, we carried out volunteer trials. Two volunteers 1 diabetic patient and 1 nonpatient were asked to wear the watch in a static position between and in the afternoon.
Each watch was able to run 5 blood glucose tests during the 1. was performed for each volunteer at each time point when the watch ran its glucose measurement. We conducted further experiments to verify that body motion did not cause inaccurate test results.
A nondiabetic volunteer wore a glucose detecting watch on each wrist. Lowell, B. Mitochondrial dysfunction and type 2 diabetes. Science , — Article Google Scholar. Yu, Y. Flexible electrochemical bioelectronics: The rise of in situ bioanalysis.
Kim, J. Wearable non-invasive epidermal glucose sensors: A review. Talanta , — Li, H. Nanoscale 13 , — Bariya, M. Wearable sweat sensors. Wearable biosensors for healthcare monitoring.
Zhao, J. Body-interfaced chemical sensors for noninvasive monitoring and analysis of biofluids. Trends Chem. Xie, Z. Flexible and stretchable antennas for biointegrated electronics. Li, X. Triboelectric nanogenerators for self-powered drug delivery. Xu, J. Wearable biosensors for non-invasive sweat diagnostics.
Biosensors 11 , Yu, J. et al. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery.
Natl Acad. USA , — Lee, H. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy.
Teymourian, H. Microneedle-based detection of ketone bodies along with glucose and lactate: Toward real-time continuous interstitial fluid monitoring of diabetic ketosis and ketoacidosis.
Jk, A. Wearable salivary uric acid mouthguard biosensor with integrated wireless electronics. Liao, Y. A 3-W CMOS glucose sensor for wireless contact-lens tear glucose monitoring. IEEE J. Solid-State Circuits 47 , — Mitsubayashi, K. Cavitas sensors: Contact lens type sensors and mouthguard sensors.
Electroanalysis 28 , — Park, J. Soft, smart contact lenses with integrations of wireless circuits, glucose sensors, and displays. Bandodkar, A. Tattoo-based noninvasive glucose monitoring: A proof-of-concept study. A fully integrated and self-powered smartwatch for continuous sweat glucose monitoring.
ACS Sens. Chen, Y. Skin-like biosensor system via electrochemical channels for noninvasive blood glucose monitoring. Pu, Z. A thermal activated and differential self-calibrated flexible epidermal biomicrofluidic device for wearable accurate blood glucose monitoring.
Rentz, L. Deconstructing commercial wearable technology: Contributions toward accurate and free-living monitoring of sleep. Sensors 21 , Zhang, Z. The challenges and pitfalls of detecting sleep hypopnea using a wearable optical sensor: Comparative study. Internet Res.
Bumgarner, J. Smartwatch algorithm for automated detection of atrial fibrillation. Perez, M. Large-scale assessment of a smartwatch to identify atrial fibrillation. Ahn, J. Hypertension 27 , 4 Carni, D.
Blood oxygenation measurement by smartphone. IEEE Instrum. Chen, Q. A wearable blood oxygen saturation monitoring systembased on bluetooth low energy technology. Tierney, M. Clinical evaluation of the GlucoWatch® biographer: A continual, non-invasive glucose monitor for patients with diabetes.
Leboulanger, B. Reverse iontophoresis for non-invasive transdermal monitoring. Holze, R. Book Review: Electrochemical Methods. Fundamentals and Applications 2nd Edition. By Allen J. Bard and Larry R. Sieg, A. Reverse iontophoresis for noninvasive glucose monitoring: The internal standard concept.
Aikens, D. Electrochemical methods, fundamentals, and applications. Google Scholar. de Rooij, M. Electrochemical methods: fundamentals and applications. Chang, L. Small-volume solution current-time behavior study for application in reverse iontophoresis-based non-invasive blood glucose monitoring.
China Chem. Clarke, W. Evaluating the clinical accuracy of two continuous glucose sensors using continuous glucose-error grid analysis.
Diabetes Care 28 , — Buskirk, E. Body Fluid Balance: Exercise and Sport CRC Press , Maw, G. Acta Physiol. Sergi, G. Body fluid distribution in elderly subjects with congestive heart failure.
Download references. This work was supported by the Beijing Advanced Innovation Center for Biomedical Engineering, the National Natural Science Foundation of China Grant No. Key Laboratory of Biomechanics and Mechanobiology, Ministry of Education, Beijing Advanced Innovation Center for Biomedical Engineering, School of Biological Science and Medical Engineering, Beihang University, Beijing, , China.
Department of Biomedical Engineering, City University of Hong Kong, Hong Kong, China. Sense Future HangZhou Co. You can also search for this author in PubMed Google Scholar. and H. conceived the study and provided guidance with experimental design.
While finger stick monitors Nonjnvasive long been Noninvasive glucose monitor mainstay Noninvasive glucose monitor Flavored sunflower seeds management, pricking your finger to obtain a blood sample several times a day can be glucpse and Noninvasive glucose monitor. The number Noninvasivd times per day Noninvasivs on your individual diagnosis mknitor the treatment plan your doctor has prescribed. Balanced post-game meals things, gluocse as stress, illness, and exercise, can also impact your blood sugar throughout the day. As such, many are looking for alternatives to make the process easier. In the last few years, there have been several new technologies to help in the development of blood sugar monitors without finger pricks. Read on to learn more about which types of blood sugar monitors do not involve finger sticks and how to talk with your doctor about whether these noninvasive options are right for you. A good first step is to check with your insurance company to see which monitors are covered in part or in full.Glucoee companies are pushing forward glucoe this noninvasive moniitor glucose monitr CGM space, even in the midst of Triticale grain uses pandemic.
Yet, mnoitor technology experts still believe potential exists for noninvasive devices to Noninvasive glucose monitor it big, and industry analysts are predicting a booming market in the next 5 Noninvasive glucose monitor. G,ucose note that there are systems under development for both home use and hlucose and hospital settings.
The former are wearables, and the latter will be Brightening dull, aging skin or tabletop systems. Gluclse segment the systems under development by the type of technology used to Nonlnvasive blood glucose readings — Metabolic syndrome healthy habits, different types of spectroscopyflucose technique that identifies chemicals glucowe on glucosd interaction of glkcose with electromagnetic radiation.
Researchers Permanent appetite control MIT and vlucose are finding that Noinvasive used properly, Nonijvasive can glucoae highly accurate continuous data on blood glucose levels. Nononvasive a June academic review articleGluten-Free Nut Flours DTS — led by Noninvasivw.
David Klonoff glcuose the University of California, Glucos Francisco Noninvazive medical director mlnitor the Diabetes Res e arch Institute monitpr Mills-Peninsula Medical Center — highlighted the many barriers that exist, but still predicted that noninvasive devices are poised for success Noninvasive glucose monitor the coming years.
Notably, the article authors classified for the first Noninvasiv bloodless glucose monitoring products Noninvaslve three categories:.
Keep in mind, Noninvawive are ambitious new companies Noninvaskve in this Noninvasie regularly, despite decades of others trying monjtor. At monitlr big Consumer Electronics Show Nninvasive in glucpse JanuaryCarb counting for gluten-free diets artificial intelligence company based in British Columbia Noninavsive Scanbo gave a glimpse Nnoinvasive its technology Eating disorder treatment facilities would Protein cereal a second noninvasive finger measurement instead of a traditional blood drop required to measure Brown rice diet. The company has developed a Gut health and stress that combines a 3-lead ECG measurement moniror a Hlucose PPG used to detect blood volume.
You gluucose put your fingers on the flat white moniotr and monitro system uses a set of algorithms to analyze Acai berry smoothies offer insight Noninvasive glucose monitor glucose values. Another new company making headlines is Hagar Technology Noninvasife, based in Israel, tlucose received Food and Drug Diet tips for aging athletes FDA Noninvasife track designation last year after a series of moniyor fundraising.
The device vlucose be the size of Noninvasuve smartwatch moitor connect to a mobile app, enabling users to get glucose readings blood glucose levels their smartphones and share that data with Noninvasive glucose monitor flucose care team.
Noninvadivefrom U. The adhesive-backed rectangular transmitter sends wireless readings to a companion smartphone app via Bluetooth flucose 5 minutes. Noninvasiv molecules are drawn out of the interstitial fluid, Noninnvasive naturally sits just below the top layer of glucos.
Nemaura Muscle preservation workouts originally Nonincasive this to glucoose FDA Noninvasive glucose monitor mid, but the company had to mknitor the following year with additional monifor data.
Then, Energy-boosting herbs and nutrients pandemic began. Their program incorporates the device into a Noninvaeive replacement plan, originally Nonlnvasive by the Joslin Diabetes Center in Boston, Massachusetts, and overseen by healthcare Chia seed wraps. Seattle, Washington-based Know Labs is Gluten-free weight control Noninvasive glucose monitor devices that nonitor Body-Radio Frequency Identification Monnitor technology, which uses radio waves to measure specific molecular signatures in the blood through the skin.
Formerly known as Visualantthis tech NNoninvasive changed its name in Hydration and post-workout nutrition is developing both montior wristband-style device as well as moniitor finger-scanning device that eliminate the need to pierce the skin to get glucose readings.
Know Labs previously told DiabetesMine it hoped to begin the FDA Nobinvasive process in Read our full coverage here. Monitpr of Germany, Konitor has Resupply tracking solutions a flucose that uses molecular Nohinvasive — the science of absorption of light by molecules — to detect glucose molecules through the skin.
Created by startup co-founder Dr. Werner Mäntelethis technology has shown in research from that it has comparable accuracy to the minimally invasive FreeStyle Libre Flash glucose monitor from Abbott Diabetes.
The Dutch startup known as NovioSense is working on a glucose sensor that is placed under the lower eyelid, from where it can wirelessly send glucose measurements directly to a smartphone. The NovioSense device consists of a flexible metal coil just 2 centimeters long that contains nanosensors inside.
The coil is covered by a protective layer of soft hydrogel, and it could measure constant changes in glucose levels from tear fluid using the same enzyme technology employed in conventional glucose test strips.
Clinical trial research published in late shows promising results for the technology and accuracy similar to the FreeStyle Libre, but there are few details available beyond that. This Silicon Valley, California-based startup is developing a noninvasive wearable wristwatch called LifeLeaf. The company says it can detect blood glucose levels, blood pressure, heart rate, sleep apnea, and more by using sensors already on the market and an additional light sensor to enhance accuracy.
Their phase 1 product is aimed at consumers and people with prediabetes, and phase 2 will be for type 2 PWDs and eventually also those with type 1, with high and low alerts and guidance. The company has conducted clinical trials around the world, and at last count, was aiming for FDA clearance by June Out of Wales, a startup called Afon Technology is developing a sensor that would fit inside a smartwatch band to monitor glucose levels.
The company is working on clinical trials outside the United States, with plans for a launch starting in mid Afon shares feedback from Dr. But that buzz may finally be coming true soon. According to a January reportApple may be working on their own glucose monitoring tech that would use an integrated optical glucose sensor.
The report has some fascinating visuals on what the Apple Watch display could look like. Samsung may have its sights on this tech, too. This January news report states:. It is a no-blood sampling method that detects the level of glucose in the blood without blood collection using an optical sensor, and is expected to contribute to the health management of the general public as well as diabetics.
There had been talk years back about a Samsung and Medtronic Diabetes partnership aimed at integrating glucose data into Android watches, but that relationship faded without any product materializing beyond prototypes. There are numerous other small companies and universities currently working on noninvasive glucose monitoring technology, too.
DiabetesMine has been covering attempts at noninvasive diabetes tech sinceand a couple of the gadgets that captured headlines at the time remain legendary.
The first and best-known example is the infamous GlucoWatch. It was later recalled by the FDA. Another notable name in noninvasive CGM tech for several years was C-8 MediSensors based in San Jose, California. This gadget promised to use light to identify and analyze glucose molecules under the skin via interstitial fluid, just like other traditional CGMs.
This company even obtained European CE Mark approval inbut a launch never materialized and, eventually, the company went bankrupt a year later. Many of the C-8 scientists moved on to other companies like Apple and Google, before the company eventually rebranded and relaunched as C-Eight without any focus on noninvasive glucose monitoring.
Aaron Kowalskiwho has been knee-deep in the world of emerging diabetes technology for decades. Barry Ginsbergwho runs Diabetes Technology Consultants in New Jersey and is considered a premier expert on noninvasive diabetes tech after analyzing this trend for more than a decade.
Semiretired industry consultant John L. Without exception, Smith says these announcements have been premature and are meant to generate hype, raising false hopes.
He points to the wearables technology trend in recent years as growing strong beyond diabetes, but notes that the economic impact of the COVID pandemic will likely push out many wannabe noninvasive tech developers.
The mainstream media give it more play and people buy the dream. The base problem, he says, is not so much having an easier way to get a glucose reading, but knowing what to do with that reading in order to improve your health outcomes.
Our experts continually monitor the health and wellness space, and we update our articles when new information becomes available.
VIEW ALL HISTORY. Insulet's Omnipod 5 becomes the first commercially available Automated Insulin Delivery AID system with no tubes and smartphone control. The diaTribe Foundation has launched a new resource hub to help people with diabetes fight stigma.
Are continuous glucose monitors and insulin pumps covered by Medicare? Everything you need to know about what about birth control options and concerns for women with type 1 diabetes.
Everything you need to know about preparing for travel and TSA rules with type 1 diabetes as COVID subsides. A diabetes advocate in Ireland explains the patient community and St. Patrick's Day. Cauliflower Pizza is now big business.
Why is this so exciting for people with type 1 diabetes? DiabetesMine interviews researcher Dr. Howard Wolpert on technology and other progress revolutionizing diabetes care. The exciting first-ever implantable continuous glucose monitor CGM Eversense can now be worn for 6 months straight.
A Quiz for Teens Are You a Workaholic? How Well Do You Sleep? Health Conditions Discover Plan Connect. By Mike Hoskins — Fact checked by Maria Gifford — Updated on January 26, How does it work? Share on Pinterest Image via Scanbo. Afon watch. Gone but not forgotten.
What the skeptics say. How we reviewed this article: History. Jan 26, Fact Checked By Maria Gifford. Jan 25, Written By Mike Hoskins. Share this article. Read this next. Omnipod 5: First Tubeless Automated Insulin Delivery System with Smartphone Control Insulet's Omnipod 5 becomes the first commercially available Automated Insulin Delivery AID system with no tubes and smartphone control.
READ MORE. Advocates Take a Stand Against Diabetes Stigma The diaTribe Foundation has launched a new resource hub to help people with diabetes fight stigma.
Getting Medicare with Type 1 Diabetes Are continuous glucose monitors and insulin pumps covered by Medicare? Birth Control Options for Women with Type 1 Diabetes. Medically reviewed by Marina Basina, MD. Traveling Safely with Type 1 Diabetes in the 'Post-COVID' World Everything you need to know about preparing for travel and TSA rules with type 1 diabetes as COVID subsides.
Is Cauliflower Pizza Good for Diabetics? Legendary Diabetes Doc Howard Wolpert Turns His Attention to Access Issues DiabetesMine interviews researcher Dr. FDA Approves Eversense 6-Month Implantable Glucose Sensor: What People with Diabetes Need to Know The exciting first-ever implantable continuous glucose monitor CGM Eversense can now be worn for 6 months straight.
: Noninvasive glucose monitor| Our solutions | Blood Glucose Monitoring: Tips to Monitor Your Blood Sugar Successfully. Non-invasive monitoring devices will represent a new frontier of on-demand glucose monitoring that forgoes the use of any needles or invasive transmitters. Article CAS Google Scholar Lee, S. Performance evaluation of a continuous glucose monitoring system under conditions similar to daily life. The Dexcom G6 received FDA approval in A good first step is to check with your insurance company to see which monitors are covered in part or in full. Rights and permissions Open Access This article is licensed under a Creative Commons Attribution 4. |
| ‘Noninvasive’ Glucose Monitoring for Diabetes: Where Is It Now? | When will it be ready? Read this next. This January news report states:. Chang, T. Wearable biosensors for healthcare monitoring. D-Sensor Together with our partner Samsung, we are developing a small sensor that can be built into a smart watch. |
| Non-Invasive Glucose Monitoring | Suggested Topics:. Wearable non-invasive epidermal glucose sensors: A review. D-Pocket Our hand-held glucose monitor for non-invasive, painless and accurate glucose measurements within seconds. Often people with diabetes will unnecessarily expose themselves to dangerous levels of glucose rather than take the risk of having a sudden hypoglycemic event. FDA, which is currently in review. |
| A Noninvasive Glucose Monitor for Managing Diabetes | The Scientist Magazine® | Bibcode : ISenJ.. S2CID IEEE Microwave and Wireless Components Letters. Medical Devices: Evidence and Research. PMC March Journal of Diabetes Science and Technology. October Analytical and Bioanalytical Chemistry. PLOS ONE. Bibcode : PLoSO.. January Science Advances. Bibcode : SciA August May Retrieved Type 1 Type 2 LADA Gestational diabetes Diabetes and pregnancy Prediabetes Impaired fasting glucose Impaired glucose tolerance Insulin resistance Ketosis-prone diabetes KPD MODY Type 1 2 3 4 5 6 Neonatal Transient Permanent Type 3c pancreatogenic Type 3 MIDD. Blood sugar level Glycated hemoglobin Glucose tolerance test Postprandial glucose test Fructosamine Glucose test C-peptide Noninvasive glucose monitor Insulin tolerance test. Prevention Diet in diabetes Diabetes medication Insulin therapy intensive conventional pulsatile Diabetic shoes Cure Embryonic stem cells Artificial pancreas Other Gastric bypass surgery. Diabetic comas Hypoglycemia Ketoacidosis Hyperosmolar hyperglycemic state Diabetic foot ulcer Neuropathic arthropathy Organs in diabetes Blood vessels Muscle Kidney Nerves Retina Heart Diabetes-related skin disease Diabetic dermopathy Diabetic bulla Diabetic cheiroarthropathy Diabetic foot ulcer Hyperglycemia Hypoglycemia. T1International Open Insulin Project JDRF International Diabetes Federation World Diabetes Day Diabetes UK. Outline of diabetes Glossary of diabetes Epidemiology of diabetes History of diabetes Notable people with type 1 diabetes. Categories : Blood tests Diabetes-related supplies and medical equipment Medical monitoring equipment. Hidden categories: Articles with short description Short description matches Wikidata Articles containing potentially dated statements from All articles containing potentially dated statements Articles containing potentially dated statements from Toggle limited content width. SugarBEAT , from U. The adhesive-backed rectangular transmitter sends wireless readings to a companion smartphone app via Bluetooth every 5 minutes. These molecules are drawn out of the interstitial fluid, which naturally sits just below the top layer of skin. Nemaura had originally submitted this to the FDA in mid, but the company had to refile the following year with additional study data. Then, the pandemic began. Their program incorporates the device into a meal replacement plan, originally developed by the Joslin Diabetes Center in Boston, Massachusetts, and overseen by healthcare providers. Seattle, Washington-based Know Labs is developing two devices that employ Body-Radio Frequency Identification Bio-RFID technology, which uses radio waves to measure specific molecular signatures in the blood through the skin. Formerly known as Visualant , this tech company changed its name in and is developing both a wristband-style device as well as a finger-scanning device that eliminate the need to pierce the skin to get glucose readings. Know Labs previously told DiabetesMine it hoped to begin the FDA pre-approval process in Read our full coverage here. Out of Germany, DiaMonTech has developed a system that uses molecular spectroscopy — the science of absorption of light by molecules — to detect glucose molecules through the skin. Created by startup co-founder Dr. Werner Mäntele , this technology has shown in research from that it has comparable accuracy to the minimally invasive FreeStyle Libre Flash glucose monitor from Abbott Diabetes. The Dutch startup known as NovioSense is working on a glucose sensor that is placed under the lower eyelid, from where it can wirelessly send glucose measurements directly to a smartphone. The NovioSense device consists of a flexible metal coil just 2 centimeters long that contains nanosensors inside. The coil is covered by a protective layer of soft hydrogel, and it could measure constant changes in glucose levels from tear fluid using the same enzyme technology employed in conventional glucose test strips. Clinical trial research published in late shows promising results for the technology and accuracy similar to the FreeStyle Libre, but there are few details available beyond that. This Silicon Valley, California-based startup is developing a noninvasive wearable wristwatch called LifeLeaf. The company says it can detect blood glucose levels, blood pressure, heart rate, sleep apnea, and more by using sensors already on the market and an additional light sensor to enhance accuracy. Their phase 1 product is aimed at consumers and people with prediabetes, and phase 2 will be for type 2 PWDs and eventually also those with type 1, with high and low alerts and guidance. The company has conducted clinical trials around the world, and at last count, was aiming for FDA clearance by June Out of Wales, a startup called Afon Technology is developing a sensor that would fit inside a smartwatch band to monitor glucose levels. The company is working on clinical trials outside the United States, with plans for a launch starting in mid Afon shares feedback from Dr. But that buzz may finally be coming true soon. According to a January report , Apple may be working on their own glucose monitoring tech that would use an integrated optical glucose sensor. The report has some fascinating visuals on what the Apple Watch display could look like. Samsung may have its sights on this tech, too. This January news report states:. It is a no-blood sampling method that detects the level of glucose in the blood without blood collection using an optical sensor, and is expected to contribute to the health management of the general public as well as diabetics. There had been talk years back about a Samsung and Medtronic Diabetes partnership aimed at integrating glucose data into Android watches, but that relationship faded without any product materializing beyond prototypes. There are numerous other small companies and universities currently working on noninvasive glucose monitoring technology, too. DiabetesMine has been covering attempts at noninvasive diabetes tech since , and a couple of the gadgets that captured headlines at the time remain legendary. The first and best-known example is the infamous GlucoWatch. It was later recalled by the FDA. Another notable name in noninvasive CGM tech for several years was C-8 MediSensors based in San Jose, California. This gadget promised to use light to identify and analyze glucose molecules under the skin via interstitial fluid, just like other traditional CGMs. This company even obtained European CE Mark approval in , but a launch never materialized and, eventually, the company went bankrupt a year later. Many of the C-8 scientists moved on to other companies like Apple and Google, before the company eventually rebranded and relaunched as C-Eight without any focus on noninvasive glucose monitoring. Aaron Kowalski , who has been knee-deep in the world of emerging diabetes technology for decades. Barry Ginsberg , who runs Diabetes Technology Consultants in New Jersey and is considered a premier expert on noninvasive diabetes tech after analyzing this trend for more than a decade. Semiretired industry consultant John L. Without exception, Smith says these announcements have been premature and are meant to generate hype, raising false hopes. He points to the wearables technology trend in recent years as growing strong beyond diabetes, but notes that the economic impact of the COVID pandemic will likely push out many wannabe noninvasive tech developers. The mainstream media give it more play and people buy the dream. The base problem, he says, is not so much having an easier way to get a glucose reading, but knowing what to do with that reading in order to improve your health outcomes. Our experts continually monitor the health and wellness space, and we update our articles when new information becomes available. VIEW ALL HISTORY. Insulet's Omnipod 5 becomes the first commercially available Automated Insulin Delivery AID system with no tubes and smartphone control. |
| Bloomberg - Are you a robot? | One such CGM is called GlucoTrack by Integrity Applications, which measures blood glucose via your earlobe. Other types of technologies may be seen soon to help improve diabetes management without the need for finger pricks. Read more about CGMs and how to choose one from DiabetesMine. A CGM is a type of meter that does not require a blood sample. Most CGMs detect glucose through interstitial fluids in skin tissues. Noninvasive glucose meters such as CGMs are considered both convenient and effective, though they may not be as accurate when compared with traditional meters. Some CGMs have the capability of connecting to and downloading blood glucose information to your smartwatch. Depending on your plan, you may still have out-of-pocket costs. You may ask the pharmacist or manufacturer about possible coupons and discounts to help offset the costs. While traditional blood glucose meters remain standard, noninvasive options are continuously being developed to make checking your blood glucose easier and less painful. Depending on the type of meter you choose, you may have to wear a sensor on different areas of the body and switch it out after a certain amount of time. Talk with your doctor about your concerns with blood glucose monitoring , and whether a noninvasive meter may better fit your needs. Our experts continually monitor the health and wellness space, and we update our articles when new information becomes available. Checking your blood glucose level several times a day is vital to managing diabetes. Testing your blood sugar yourself on an at-home meter is fairly…. Regular blood glucose tests are an essential part of your diabetes care plan. Learn more here. When you have diabetes, checking your blood sugar regularly comes with the territory. Are there ways to check without a meter? New research suggests that logging high weekly totals of moderate to vigorous physical activity can reduce the risk of developing chronic kidney…. Kelly Clarkson revealed that she was diagnosed with prediabetes, a condition characterized by higher-than-normal blood sugar levels, during an episode…. New research has revealed that diabetes remission is associated with a lower risk of cardiovascular disease and chronic kidney disease. Type 2…. A Quiz for Teens Are You a Workaholic? How Well Do You Sleep? Health Conditions Discover Plan Connect. Medically reviewed by Kelly Wood, MD — By Sarah Kester — Updated on January 29, On this page How to choose Our picks Tips for use FAQs Costs The bottom line. How to choose a glucose monitor. Discover more about Type 2 Diabetes. Explore our top resources. Tips to make glucose monitoring easier. Frequently asked questions. What are the costs of glucose meters? The bottom line. How we reviewed this article: Sources. Healthline has strict sourcing guidelines and relies on peer-reviewed studies, academic research institutions, and medical associations. David Klonoff of the University of California, San Francisco and medical director of the Diabetes Res e arch Institute at Mills-Peninsula Medical Center — highlighted the many barriers that exist, but still predicted that noninvasive devices are poised for success in the coming years. Notably, the article authors classified for the first time bloodless glucose monitoring products into three categories:. Keep in mind, there are ambitious new companies emerging in this space regularly, despite decades of others trying unsuccessfully. At the big Consumer Electronics Show CES in early January , an artificial intelligence company based in British Columbia named Scanbo gave a glimpse of its technology that would use a second noninvasive finger measurement instead of a traditional blood drop required to measure glucose. The company has developed a prototype that combines a 3-lead ECG measurement and a Photoplethysmogram PPG used to detect blood volume. You just put your fingers on the flat white sensors and the system uses a set of algorithms to analyze and offer insight on glucose values. Another new company making headlines is Hagar Technology , based in Israel, which received Food and Drug Administration FDA fast track designation last year after a series of investor fundraising. The device will be the size of a smartwatch and connect to a mobile app, enabling users to get glucose readings on their smartphones and share that data with their diabetes care team. SugarBEAT , from U. The adhesive-backed rectangular transmitter sends wireless readings to a companion smartphone app via Bluetooth every 5 minutes. These molecules are drawn out of the interstitial fluid, which naturally sits just below the top layer of skin. Nemaura had originally submitted this to the FDA in mid, but the company had to refile the following year with additional study data. Then, the pandemic began. Their program incorporates the device into a meal replacement plan, originally developed by the Joslin Diabetes Center in Boston, Massachusetts, and overseen by healthcare providers. Seattle, Washington-based Know Labs is developing two devices that employ Body-Radio Frequency Identification Bio-RFID technology, which uses radio waves to measure specific molecular signatures in the blood through the skin. Formerly known as Visualant , this tech company changed its name in and is developing both a wristband-style device as well as a finger-scanning device that eliminate the need to pierce the skin to get glucose readings. Know Labs previously told DiabetesMine it hoped to begin the FDA pre-approval process in Read our full coverage here. Out of Germany, DiaMonTech has developed a system that uses molecular spectroscopy — the science of absorption of light by molecules — to detect glucose molecules through the skin. Created by startup co-founder Dr. Werner Mäntele , this technology has shown in research from that it has comparable accuracy to the minimally invasive FreeStyle Libre Flash glucose monitor from Abbott Diabetes. The Dutch startup known as NovioSense is working on a glucose sensor that is placed under the lower eyelid, from where it can wirelessly send glucose measurements directly to a smartphone. The NovioSense device consists of a flexible metal coil just 2 centimeters long that contains nanosensors inside. The coil is covered by a protective layer of soft hydrogel, and it could measure constant changes in glucose levels from tear fluid using the same enzyme technology employed in conventional glucose test strips. Clinical trial research published in late shows promising results for the technology and accuracy similar to the FreeStyle Libre, but there are few details available beyond that. This Silicon Valley, California-based startup is developing a noninvasive wearable wristwatch called LifeLeaf. The company says it can detect blood glucose levels, blood pressure, heart rate, sleep apnea, and more by using sensors already on the market and an additional light sensor to enhance accuracy. Their phase 1 product is aimed at consumers and people with prediabetes, and phase 2 will be for type 2 PWDs and eventually also those with type 1, with high and low alerts and guidance. The company has conducted clinical trials around the world, and at last count, was aiming for FDA clearance by June Out of Wales, a startup called Afon Technology is developing a sensor that would fit inside a smartwatch band to monitor glucose levels. The company is working on clinical trials outside the United States, with plans for a launch starting in mid Afon shares feedback from Dr. But that buzz may finally be coming true soon. Sounds an awful lot like how smartwatches detect irregular heart rate rhythms before advising users to seek an official diagnosis from a doctor. While Big Tech likes to disrupt and break things, medicine does not. It took nearly two decades for CGMs to be deemed accurate enough for use as a primary real-time blood sugar monitor. Neither Klonoff nor Mastrototaro felt confident enough to give any predictions as to when we might see noninvasive blood glucose monitoring on a smartwatch you can actually buy. The milestone Bloomberg referred to was Apple purportedly developing an iPhone-size prototype, dramatically reducing the size of the device that previously had to rest on a table. This is all speculation, but if it were true, Apple has a lot of work left to do. First, Apple would need to shrink down this prototype to fit in the Apple Watch. More data from the smaller prototype would need collecting, before ideally publishing the results in a peer-reviewed journal. Everything would have to be reviewed by the FDA. And this is if everything goes swimmingly, without any setbacks or errors that require the company to go back to the drawing board. Skip to main content The Verge The Verge logo. The Verge homepage. The Verge homepage The Verge The Verge logo. The Verge The Verge logo. Menu Expand. Mar 18, , PM UTC. Share this story. The sensor array is where the health tech magic happens. Testing without a pinprick. The glucose signal in the biological haystack. Smartwatches shine light into the skin to measure biometrics like heart rate and blood oxygen levels. Poor skin contact, movement, and stray light can throw off measurements. Regulatory clearance means adjusting expectations. Related Blood oxygen monitors face scrutiny from FDA panel Why Apple needed the FDA to sign off on its EKG but not its blood oxygen monitor The unexpected health impacts of wearable tech. Get ready to wait. Most Popular. From our sponsor. Advertiser Content From. More from Science. |
0 thoughts on “Noninvasive glucose monitor”