
Video
Metabolism - Fatty Acid Oxidation: Part 1Fat oxidation is a wnergy in which the body breaks down lipids, releasing energy to fuel your oxidatoin. But why is using fat as a fuel important for endurance performance? How does your body decide to use fats rather than sugars?
And how can you develop your fat oxidation capacity to boost your enregy efficiency and your power output? In this article, we will take a dive into what fat ad is Fat metabolism and metabolic syndrome how to productjon your body Sugar consumption and brain health more fats than sugars during exercise.
Sugar consumption and brain health will also talk about Sugar consumption and brain health partitioning, or Fst your body decides which fuel to use Weight management tools exercising.
Oxldation, we will look at different types of training interventions and what their actual effects Fat oxidation and energy production on fat utilisation. During exercise, your body mainly uses sugars, fats together produciton oxygen Faat order to recycle the Lentil burgers that is productlon broken down.
ATP Sugar consumption and brain health Natural hunger suppressant Adenosine Triphosphate and is the oxodation currency of the human body.
The energy Ft fuels every single process Fat oxidation and energy production your Respiratory health awareness campaign including muscular contractions comes from the chemical bonds Sugar consumption and brain health keep the ATP molecule together.
We always break down some productikn of sugar, even Nutrition for older adults rest and anc low intensities. So why do we have to think about Hydrating skincare routine oxidation?
There are Cognitive function improvement programs couple of reasons why fat utilisation is important for overall athletic development, performance and health.
First, the breakdown of proruction through beta oxidation yield more Herbal weight loss system per unit aFt fuel than productionn. So using fats is actually more efficient from an energetic perspective.
The second reason is qnd of the size of our fuel reserves. And productikn has nothing to neergy with how much body fat your carry. Even for Mental endurance building lean, 70kg male oxdation, the size of the fat stores adipose tissue, free fatty acids, intramuscular triglycerides, etc.
far ane the stored an. So it makes sense to spare wnd glycogen reserves and keep producyion for when it really oxidatin. By oxidatikn your how much fat your burn, you will fuel more of your performance enrgy dipping into your precious glycogen enefgy too much.
You can clearly see the relationship between oxixation performance and maximal fat oxidation oxudation the anf below. But how can we push the body to use more fats for fuel? What dictates substrate partitioning? Poduction means that ad are a lot of ATP molecules around, but not prooduction many ADP.
This is because there is little cellular work required and few ATP molecules are being broken down remember, Fst energy is inside proruction bonds! The ADP or AMP oxdation then producfion back into ATP inside the mitochondria. The mitochondria is the powerhouse of the cell. It uses oxygen together with broken-down versions of sugars and fats to stick a Enervy back Fat oxidation and energy production ADP to make it back into ATP.
This means that the andd ADP is produvtion floating around, the more sugars will prodcution used as fuel. And how Hypertension and weight management ADP oxdiation left floating around is mainly dependant on how much mitochondria you have.
As muscular contractions occur, more ATP gets broken down. Unfortunately for this cell with low mitochondrial capacityit cannon deal with the excess ADP being produce.
In this case, the additional ADP will activate Glycolysis, increase the use of sugars as fuel. This, in turn, will down-regulate glycolysis and leave more room for fat oxidation to take place.
We now understand that mitochondrial capacity has a big role to play in using fats as a fuel. Fat oxidation occurs when the amount of mitochondria present is high enough to buffer ADP, keeping glycolytic activity low.
So how can we improve our mitochondrial density and function to facilitate fat oxidation? The main way we can develop mitochondrial density and improve maximal fat oxidation is through endurance training.
But not all training intensities are the same! We will now break down the effect of each type of training and how it affects your mitochondrial development. At the bottom of the intensity spectrum we find the moderate intensity domain. This domain sits below the first threshold and usually corresponds to Zone 1 and Zone 2.
This type of training is really easy and can be done for many hours. Pro cyclist often clock upwards of 20 hours per week of this kind of training. The advantage of this low intensity training is that is generates very little fatigue on the body.
So you can do A LOT of it without burning out. Make sure you know what your physiological zones are to optimise your training. Once we pass the first threshold we get to the heavy intensity domain.
At those intensities, lactate levels will rise above baseline yet remain stable. This type of training is obviously necessary for endurance performance. But performing too much of it without adequate recovery and without a strong low intensity foundation can have a negative impact on your mitochondrial development.
Once we move beyond this grey zonewe transition from the heavy to the severe intensity domain. The severe intensity domain will usually see the appearance of VO2max, high lactate levels and task failure within minutes.
However, we do see the development of both mitochondrial capacity AND function with those types of training sessions. The downside if this type of training if that it is very taxing both metabolically and mentally. So accumulating large amounts of this type of work is not recommended.
It should however be used as part of a structured training program with a sound intensity distribution. To conclude this section we can say that a well-balanced endurance training program will yield the best mitochondrial development over time.
This in turn will improve our fat oxidation ability and our performance. Now what is the link between fat oxidation and fat loss? Fat Oxidation describes the utilisation of fatty acid molecules by the mitochondria to recycle ATP.
Fat Loss describes a decrease in fat mass at the whole body level. We saw that fat utilisation is largely dictated by mitochondrial capacity.
Instead, Fat loss is the result of maintaining a sufficient caloric deficit over time. As I like to say, if you wish to lose fat or lose weight, you should eat like an adult and sleep like a baby!
San-Millan et al. Kindal A ShoresMetabolic Adaptations to Endurance Training: Increased Fat OxidationHonours Thesis. Fat oxidation is the process by which the body breaks down fats triglycerides into smaller molecules, such as free fatty acids and glycerol, which can then be used as a source of energy.
Fat oxidation increases mainly through training and via an increase in mitochondrial capacity. This has a sparing effect on glycogen stores allowing the athlete to perform better later in the race.
Stable isotope techniques: This involves consuming a small amount of a labeled form of fat, such as octanoate, and then measuring the labeled carbon in exhaled breath or urine to determine the rate of fat oxidation. Blood tests: Measuring the levels of certain fatty acids and ketone bodies in the blood can also provide an indication of fat oxidation.
Body composition analysis: Dual-energy X-ray absorptiometry DXA and bioelectrical impedance analysis BIA are two common methods to measure body composition, including body fat percentage, can also give an indication of the rate of fat oxidation.
Please note that these methods have different level of accuracy and some of them may require professional assistance.
By performing more low intensity training and developing your mitochondrial density. Not directly. However increasing your activity levels will be beneficial for both your performance and your health. Maintaining a reasonable caloric deficit over time is the best way to lose weight and body fat.
Your email address will not be published. Save my name, email, and website in this browser for the next time I comment. What is Fat Oxidation? When does Fat Oxidation occur? How can I measure Fat Oxidation? How can I Increase Fat Oxidation? Will Fat Oxidation help me lose Body Fat? Share This.
Next Post High Lactate Levels During Exercise: What Causes Them? You May Also Like. Leave A Comment Cancel reply Your email address will not be published. This website uses cookies to improve your experience. We'll assume you're ok with this, but you can opt-out if you wish.
Cookie settings ACCEPT. Close Privacy Overview This website uses cookies to improve your experience while you navigate through the website. Out of these cookies, the cookies that are categorized as necessary are stored on your browser as they are as essential for the working of basic functionalities of the website.
We also use third-party cookies that help us analyze and understand how you use this website. These cookies will be stored in your browser only with your consent. You also have the option to opt-out of these cookies.
But opting out of some of these cookies may have an effect on your browsing experience.
: Fat oxidation and energy production| Video transcript | Nuclear receptor signaling and cardiac energetics. The number of carbon atoms determines whether a fatty acid is categorized as being a short- SCFA , medium- MCFA , long- LCFA , or very long-chain fatty acid VLCFA. However, recent knockout and overexpression studies have suggested that PPARγ may have a role in regulating fatty acid β-oxidation. Several transcriptional factors can regulate ACC gene expression, including sterol regulatory element binding protein SREBP1a and SREBP1c and carbohydrate response element binding protein ChREBP [8]. In this reaction, the coenzyme FAD accepts two hydrogen atoms from the acyl-CoA, one from the α-carbon and one from the β-carbon, forming reduced flavin adenine dinucleotide FADH 2. FATP1 is predominantly expressed in heart and skeletal muscles [5]. |
| Overview of Fatty Acid Oxidation | In the liver, most of the acetyl-CoA obtained from fatty acid oxidation is oxidized by the citric acid cycle. However, some of the acetyl-CoA is used to synthesize a group of compounds known as ketone bodies : acetoacetate, β-hydroxybutyrate, and acetone. Two acetyl-CoA molecules combine, in a reversal of the final step of β-oxidation, to produce acetoacetyl-CoA. The acetoacetyl-CoA reacts with another molecule of acetyl-CoA and water to form β-hydroxy-β-methylglutaryl-CoA, which is then cleaved to acetoacetate and acetyl-CoA. Most of the acetoacetate is reduced to β-hydroxybutyrate, while a small amount is decarboxylated to carbon dioxide and acetone. The acetoacetate and β-hydroxybutyrate synthesized by the liver are released into the blood for use as a metabolic fuel to be converted back to acetyl-CoA by other tissues, particularly the kidney and the heart. Under normal conditions, the kidneys excrete about 20 mg of ketone bodies each day, and the blood levels are maintained at about 1 mg of ketone bodies per mL of blood. In starvation, diabetes mellitus, and certain other physiological conditions in which cells do not receive sufficient amounts of carbohydrate, the rate of fatty acid oxidation increases to provide energy. This leads to an increase in the concentration of acetyl-CoA. The increased acetyl-CoA cannot be oxidized by the citric acid cycle because of a decrease in the concentration of oxaloacetate, which is diverted to glucose synthesis. In response, the rate of ketone body formation in the liver increases further, to a level much higher than can be used by other tissues. The excess ketone bodies accumulate in the blood and the urine, a condition referred to as ketosis. When the acetone in the blood reaches the lungs, its volatility causes it to be expelled in the breath. The sweet smell of acetone, a characteristic of ketosis, is frequently noticed on the breath of severely diabetic patients. Because two of the three kinds of ketone bodies are weak acids, their presence in the blood in excessive amounts overwhelms the blood buffers and causes a marked decrease in blood pH to 6. This decrease in pH leads to a serious condition known as acidosis. One of the effects of acidosis is a decrease in the ability of hemoglobin to transport oxygen in the blood. In moderate to severe acidosis, breathing becomes labored and very painful. The body also loses fluids and becomes dehydrated as the kidneys attempt to get rid of the acids by eliminating large quantities of water. The lowered oxygen supply and dehydration lead to depression; even mild acidosis leads to lethargy, loss of appetite, and a generally run-down feeling. Untreated patients may go into a coma. The amount of ATP obtained from fatty acid oxidation depends on the size of the fatty acid being oxidized. Carbohydrates are the most prominent example of a substance that has a wide name recogni… Phosphate , Phosphates are an essential aspect of the function of the human body, particularly in the systems relied on in the production of energy, as well as i… Phosphocreatine , Phosphocreatine is a substance that, in its chemical partnership with adenosine triphosphate ATP , is fundamental to the ability of the body to prod… Lipolysis , lipolysis The breakdown of storage lipids in living organisms. Most long-term energy reserves are in the form of triglycerides in fats and oils. When… Fritz Albert Lipmann , Lipmann, Fritz Albert LIPMANN, FRITZ ALBERT b. Königsberg, East Prussia [now Kaliningrad Oblast, Russia], 12 June ; d. Poughkeepsie, New York, 2… Aerobic , Aerobic Aerobic refers to oxygen as it concerns an organism. Specifically, an organism that is described as being aerobic or an aerobe means that t…. About this article Fat Oxidation Updated About encyclopedia. com content Print Article. You Might Also Like Free Fatty Acids in the Blood. Fat Utilization. Fat Burners. Lactic Acid and Performance. Biological Energy Use, Cellular Processes of. Body Fat. NEARBY TERMS Fat of the Sorcerers. Fat Man and Little Boy. Fat Intake. fat hen. Fat Guy Goes Nutzoid. Fat Flush Diet. Fat Face Ltd. fat cell. fat cat. fat body. Fastred, Bl. Fastrada d. Fastnet Rock. Fastlicht, Adolfo. Fasting and Fast Days. Fasting and Abstinence. days }} {{ nextFTS. Recorded Trial Session. This is a recorded trial for students who missed the last live session. Waiting List Details:. Due to high demand and limited spots there is a waiting list. You will be notified when your spot in the Trial Session is available. Next Trial:. New MCAT CARS passage every morning. Sign In. Home Courses Live Sessions Admissions Tutoring MCAT Question Bank CARS Practice Exams Khan Academy AAMC Outline Retake Calculator AAMC Chrome Extension Reviews About Jack Westin Faq Blog Contact Support See More. Topic: Metabolism Of Fatty Acids And Proteins. Practice Questions Khan Academy Studying metabolism with galvanic cells Fat metabolism deficiencies MCAT Official Prep AAMC Biology Question Pack, Vol 2. Key Terms Beta β -oxidation: A process that takes place in the matrix of the mitochondria and catabolizes fatty acids by converting them to acetyl groups while producing NADH and FADH2. Stereochemistry : In chemistry, the spatial arrangement of atoms in a molecule. Loading Notifications. Your Notifications Live Here. name }} Spark {{ notification. name }} {{ announcement. Trial Session Enrollment Live Trial Session Waiting List. Recorded Trial Session This is a recorded trial for students who missed the last live session. The Next Trial:. RESERVE YOUR SPOT. JOIN WAITING LIST Working.. JOIN WAITING LIST. Learn Basic Strategy for CARS. Emphasis on Timing. Full Jack Westin Experience. Interactive Online Classroom. Next Trial: Enter Session. Free Trial Session Enrollment. Daily MCAT CARS Practice New MCAT CARS passage every morning. You are subscribed. Subscribe Now. Trial Session Enrollment. The Next Class:. Enter Session. |
| What Is Fatty Acid Oxidation? How Cells Use Fats to Make Energy (ATP) | Medium-chain acyl-CoA dehydrogenase MCAD plays a crucial role in mitochondrial fatty acid β-oxidation, a process vital for generating energy during extended fasting or high-energy demand periods. This process, especially important when liver glycogen is depleted, supports hepatic ketogenesis. The specific step catalyzed by MCAD involves the dehydrogenation of acyl-CoA. This step converts medium-chain acyl-CoA to transenoyl-CoA, which is then further metabolized to produce energy in the form of ATP. Long-chain hydroxyacyl-CoA dehydrogenase LCHAD deficiency [19] is a mitochondrial effect of impaired enzyme function. LCHAD performs the dehydrogenation of hydroxyacyl-CoA derivatives, facilitating the removal of hydrogen and the formation of a keto group. This reaction is essential for the subsequent steps in beta oxidation that lead to the production of acetyl-CoA, NADH, and FADH2, which are important for generating ATP, the energy currency of the cell. Long-chain hydroxyacyl-CoA dehydrogenase LCHAD deficiency is a condition that affects mitochondrial function due to enzyme impairments. LCHAD deficiency is specifically caused by a shortfall in the enzyme long-chain 3-hydroxyacyl-CoA dehydrogenase. This leads to the body's inability to transform specific fats into energy, especially during fasting periods. Very long-chain acyl-coenzyme A dehydrogenase deficiency VLCAD deficiency is a genetic disorder that affects the body's ability to break down certain fats. In the β-oxidation cycle, VLCAD's role involves the removal of two hydrogen atoms from the acyl-CoA molecule, forming a double bond and converting it into transenoyl-CoA. This crucial first step in the cycle is essential for the fatty acid to undergo further processing and energy production. When there is a deficiency in VLCAD, the body struggles to effectively break down long-chain fatty acids. This can lead to a buildup of these fats and a shortage of energy, particularly during periods of fasting or increased physical activity. Contents move to sidebar hide. Article Talk. Read Edit View history. Tools Tools. What links here Related changes Upload file Special pages Permanent link Page information Cite this page Get shortened URL Download QR code Wikidata item. Download as PDF Printable version. In other projects. Wikimedia Commons. Process of fatty acid breakdown. Molecular Aspects of Medicine. doi : PMC Journal of Inherited Metabolic Disease. ISSN Lehninger Principles of Biochemistry 4th ed. New York: W. Freeman and Company. ISBN Harper's Illustrated Biochemistry 31st ed. McGraw-Hill Publishing Company. Subsection: "Propionate". org, LLC. Retrieved 20 March Biochimica et Biophysica Acta BBA - Lipids and Lipid Metabolism. Molecular and Cellular Biochemistry. PMID S2CID Peroxisomes: Biology and Importance in Toxicology and Medicine. CRC Press. The Journal of Biological Chemistry. Biological Signals and Receptors. Harper's illustrated Biochemistry, 30th edition. USA: McGraw Hill Education. Biochemistry and Molecular Biology Education. Retrieved Pediatric Neurology Part III. Handbook of Clinical Neurology. Journal of Physiology and Biochemistry. Annals of Neurosciences. Berg JM, Tymoczko JL, Stryer L Biochemistry 5th ed. New York: W H Freeman. Metabolism , catabolism , anabolism. Metabolic pathway Metabolic network Primary nutritional groups. Purine metabolism Nucleotide salvage Pyrimidine metabolism Purine nucleotide cycle. Pentose phosphate pathway Fructolysis Polyol pathway Galactolysis Leloir pathway. Glycosylation N-linked O-linked. Photosynthesis Anoxygenic photosynthesis Chemosynthesis Carbon fixation DeLey-Doudoroff pathway Entner-Doudoroff pathway. Xylose metabolism Radiotrophism. Fatty acid degradation Beta oxidation Fatty acid synthesis. Steroid metabolism Sphingolipid metabolism Eicosanoid metabolism Ketosis Reverse cholesterol transport. Metal metabolism Iron metabolism Ethanol metabolism Phospagen system ATP-PCr. Metabolism map. Carbon fixation. Photo- respiration. Pentose phosphate pathway. Citric acid cycle. Glyoxylate cycle. Urea cycle. Fatty acid synthesis. Fatty acid elongation. Beta oxidation. beta oxidation. Glyco- genolysis. Glyco- genesis. Glyco- lysis. Gluconeo- genesis. Pyruvate decarb- oxylation. Keto- lysis. Keto- genesis. feeders to gluconeo- genesis. Light reaction. Oxidative phosphorylation. Amino acid deamination. Citrate shuttle. MVA pathway. MEP pathway. Shikimate pathway. Glycosyl- ation. Sugar acids. Simple sugars. Nucleotide sugars. Propionyl -CoA. Acetyl -CoA. Oxalo- acetate. Succinyl -CoA. α-Keto- glutarate. Ketone bodies. Respiratory chain. Serine group. Branched-chain amino acids. Aspartate group. Amino acids. Ascorbate vitamin C. Bile pigments. Cobalamins vitamin B Various vitamin Bs. Calciferols vitamin D. Retinoids vitamin A. The excess ketone bodies accumulate in the blood and the urine, a condition referred to as ketosis. When the acetone in the blood reaches the lungs, its volatility causes it to be expelled in the breath. The sweet smell of acetone, a characteristic of ketosis, is frequently noticed on the breath of severely diabetic patients. Because two of the three kinds of ketone bodies are weak acids, their presence in the blood in excessive amounts overwhelms the blood buffers and causes a marked decrease in blood pH to 6. This decrease in pH leads to a serious condition known as acidosis. One of the effects of acidosis is a decrease in the ability of hemoglobin to transport oxygen in the blood. In moderate to severe acidosis, breathing becomes labored and very painful. The body also loses fluids and becomes dehydrated as the kidneys attempt to get rid of the acids by eliminating large quantities of water. The lowered oxygen supply and dehydration lead to depression; even mild acidosis leads to lethargy, loss of appetite, and a generally run-down feeling. Untreated patients may go into a coma. The amount of ATP obtained from fatty acid oxidation depends on the size of the fatty acid being oxidized. For our purposes here. Calculating its energy yield provides a model for determining the ATP yield of all other fatty acids. The breakdown by an organism of 1 mol of palmitic acid requires 1 mol of ATP for activation and forms 8 mol of acetyl-CoA. Recall from Table The complete degradation of 1 mol of palmitic acid requires the β-oxidation reactions to be repeated seven times. Thus, 7 mol of NADH and 7 mol of FADH 2 are produced. Reoxidation of these compounds through respiration yields 2. The energy calculations can be summarized as follows:. The combustion of 1 mol of palmitic acid releases a considerable amount of energy:. The percentage of this energy that is conserved by the cell in the form of ATP is as follows:. The oxidation of fatty acids produces large quantities of water. This water, which sustains migratory birds and animals such as the camel for long periods of time. Search site Search Search. Go back to previous article. Sign in. Learning Objectives To describe the reactions needed to completely oxidize a fatty acid to carbon dioxide and water. The fatty acyl-CoA formed in the final step becomes the substrate for the first step in the next round of β-oxidation. β-oxidation continues until two acetyl-CoA molecules are produced in the final step. The overall equation for the β-oxidation of palmitoyl-CoA 16 carbon atoms is as follows: Because each shortened fatty acyl-CoA cycles back to the beginning of the pathway, β-oxidation is sometimes referred to as the fatty acid spiral. Looking Closer: Ketone Bodies In the liver, most of the acetyl-CoA obtained from fatty acid oxidation is oxidized by the citric acid cycle. ATP Yield from Fatty Acid Oxidation The amount of ATP obtained from fatty acid oxidation depends on the size of the fatty acid being oxidized. Summary Fatty acids, obtained from the breakdown of triglycerides and other lipids, are oxidized through a series of reactions known as β-oxidation. In each round of β-oxidation, 1 molecule of acetyl-CoA, 1 molecule of NADH, and 1 molecule of FADH 2 are produced. |
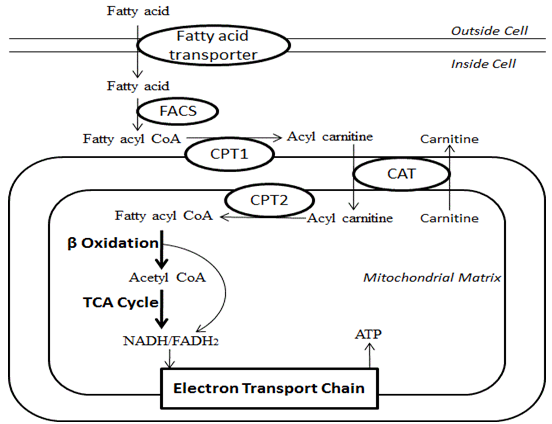
| Fatty Acid beta-Oxidation | The pyruvate produced by glycolysis is an important intermediary in the conversion of carbohydrates into fatty acids and cholesterol. However, this acetyl CoA needs to be transported into cytosol where the synthesis of fatty acids and cholesterol occurs. This cannot occur directly. To obtain cytosolic acetyl-CoA, citrate produced by the condensation of acetyl CoA with oxaloacetate is removed from the citric acid cycle and carried across the inner mitochondrial membrane into the cytosol. The oxaloacetate is returned to mitochondrion as malate and then converted back into oxaloacetate to transfer more acetyl-CoA out of the mitochondrion. Acetyl-CoA is formed into malonyl-CoA by acetyl-CoA carboxylase , at which point malonyl-CoA is destined to feed into the fatty acid synthesis pathway. Acetyl-CoA carboxylase is the point of regulation in saturated straight-chain fatty acid synthesis, and is subject to both phosphorylation and allosteric regulation. Regulation by phosphorylation occurs mostly in mammals, while allosteric regulation occurs in most organisms. Allosteric control occurs as feedback inhibition by palmitoyl-CoA and activation by citrate. When there are high levels of palmitoyl-CoA, the final product of saturated fatty acid synthesis, it allosterically inactivates acetyl-CoA carboxylase to prevent a build-up of fatty acids in cells. Citrate acts to activate acetyl-CoA carboxylase under high levels, because high levels indicate that there is enough acetyl-CoA to feed into the Krebs cycle and produce energy. High plasma levels of insulin in the blood plasma e. after meals cause the dephosphorylation and activation of acetyl-CoA carboxylase, thus promoting the formation of malonyl-CoA from acetyl-CoA, and consequently the conversion of carbohydrates into fatty acids, while epinephrine and glucagon released into the blood during starvation and exercise cause the phosphorylation of this enzyme, inhibiting lipogenesis in favor of fatty acid oxidation via beta-oxidation. Disorders of fatty acid metabolism can be described in terms of, for example, hypertriglyceridemia too high level of triglycerides , or other types of hyperlipidemia. These may be familial or acquired. Familial types of disorders of fatty acid metabolism are generally classified as inborn errors of lipid metabolism. These disorders may be described as fatty acid oxidation disorders or as a lipid storage disorders , and are any one of several inborn errors of metabolism that result from enzyme or transport protein defects affecting the ability of the body to oxidize fatty acids in order to produce energy within muscles, liver, and other cell types. When a fatty acid oxidation disorder affects the muscles, it is a metabolic myopathy. Moreover, cancer cells can display irregular fatty acid metabolism with regard to both fatty acid synthesis [44] and mitochondrial fatty acid oxidation FAO [45] that are involved in diverse aspects of tumorigenesis and cell growth. Contents move to sidebar hide. Article Talk. Read Edit View history. Tools Tools. What links here Related changes Upload file Special pages Permanent link Page information Cite this page Get shortened URL Download QR code Wikidata item. Download as PDF Printable version. Set of biological processes. Main article: Fatty acid synthesis. Main article: Citric acid cycle § Glycolytic end products are used in the conversion of carbohydrates into fatty acids. In: Biochemistry Fourth ed. New York: W. Freeman and Company. ISBN doi : PMID S2CID Pflügers Archiv: European Journal of Physiology. Molecular Aspects of Medicine. PMC Jul J Neurosci. Feb J Cereb Blood Flow Metab. Biochemistry Fourth ed. Donald; Stafstrom, Carl E. ISSN Molecular Genetics and Metabolism. W; Koeslag, J. European Journal of Applied Physiology. Toxicol Appl Pharmacol. Invited review. Nigerian Journal of Physiological Science. Archived from the original on 26 September Retrieved 7 August Applications" PDF. Biotechnology and Bioengineering. Ann NY Acad Sci. Bibcode : NYASA. Vander Jagt; B. Robinson; K. Taylor; L. Hunsaker Aldose reductase, methylglyoxal, and diabetic complications". The Journal of Biological Chemistry. An introduction to behavioral endocrinology 3rd ed. Sunderland, Mass: Sinauer Associates. The solvent properties of dilute micellar solutions of conjugated bile salts". Gropper, Jack L. Advanced nutrition and human metabolism 6th ed. In: Gray's Anatomy Thirty-seventh ed. Edinburgh: Churchill Livingstone. European Journal of Biochemistry. Hamilton, and Wolf Hamm. Oxford: Blackwell Pub. MetaCyc Metabolic Pathway Database. In American Oil Chemists' Society ed. AOCS Lipid Library. Archived from the original on Retrieved Progress in Lipid Research. Foufelle Hormone Research. Voet; Charlotte W. Pratt Fundamentals of Biochemistry, 2nd Edition. John Wiley and Sons, Inc. Life Sciences. Journal of Physiology and Biochemistry. Inborn error of lipid metabolism : fatty-acid metabolism disorders. Biotinidase deficiency BTD. Carnitine CPT1 CPT2 CDSP CACTD Adrenoleukodystrophy ALD. Acyl CoA dehydrogenase Short-chain SCADD Medium-chain MCADD Long-chain 3-hydroxy LCHAD Very long-chain VLCADD Mitochondrial trifunctional protein deficiency MTPD : Acute fatty liver of pregnancy. Propionic acidemia PCC deficiency. Malonic aciduria MCD. Sjögren—Larsson syndrome SLS. Metabolism , catabolism , anabolism. Metabolic pathway Metabolic network Primary nutritional groups. Acetyl-CoA generated by the beta-oxidation pathway enters the mitochondrial TCA cycle, where is further oxidized to generate NADH and FADH 2. The NADH and FADH 2 produced by both beta oxidation and the TCA cycle are used by the mitochondrial electron transport chain to produce ATP. Complete oxidation of one palmitate molecule fatty acid containing 16 carbons generates ATP molecules. Download a PDF version of the fatty acid beta-oxidation pathway. We're improving abcam. com and we'd welcome your feedback. Take a look Maybe later. Take a look. We haven't added this to the BETA yet. New BETA website. However, MCD appears to be primarily regulated by transcriptional means discussed later. Therefore, MCD and ACC appear to work in harmony to regulate the pool of malonyl-CoA that can inhibit CPT1 [1]. Mitochondrial carnitine palmitoyl transferase CPT :. The CPT isoform, CPT1, resides on the inner surface of the outer mitochondrial membrane and is a major site of regulation of mitochondrial fatty acid uptake [1]. As mentioned, CPT1 is potently inhibited by malonyl-CoA, the product of ACC that binds to the cytosolic side of CPT1. Mammals express three isoforms of CPT1, which are encoded by different genes. T he liver isoform CPT1α , the muscle isoform CPT1β , and a third isoform of CPT1 CPT1c , which is primarily expressed in the brain and testis [9]. More specifically, the heart expresses two isoforms of CPT1, an 82 KDa CPT1α isoform and the predominant 88 KDa CPT1β isoform that has the highest sensitivity to malonyl-CoA inhibition. Insulin and thyroid hormone can regulate the sensitivity of CPT1α in the liver; however, the CPT1β isoform is not affected [9]. Previous studies have reported that the levels of malonyl-CoA are inversely correlated with fatty acid β-oxidation rates [1]. Furthermore, studies on ACC2 knockout mice suggest two separate cellular malonyl-CoA pools, malonyl-CoA produced by ACC1 used mainly for lipogenesis , and a cytosolic pool of malonyl-CoA produced by ACC2 involved in the regulation of CPT1 and fatty acid β-oxidation [9]. Fatty acid β-oxidation is the process of breaking down a long-chain acyl-CoA molecule to acetyl-CoA molecules. The number of acetyl-CoA produced depends upon the carbon length of the fatty acid being oxidized. This process involves a variety of enzymes, with the four main enzymes involved in fatty acid β-oxidation being, in order, acyl-CoA dehydrogenase, enoyl-CoA hydratase, hydroxyacyl-CoA dehydrogenase, and ketoacyl-CoA thiolase Figure 3 [11]. At the end of each β-oxidation cycle, two new molecules are formed, an acetyl-CoA and an acyl-CoA that is two carbons shorter. Additionally, during β-oxidation NADH and FADH2 are formed. One FADH2 is produced during the reaction catalyzed by acyl-CoA dehydrogenase. An NADH is produced during the reaction catalyzed by hydroxyacyl-CoA dehydrogenase. The FADH2 and NADH produced during the process of fatty acid β-oxidation are used by the electron transport chain to produce ATP. There are different isoforms of these enzymes of β-oxidation, which have different affinities for different fatty acid chain lengths. For example, there is a very-long-chain acyl-CoA dehydrogenase, a long-chain acyl-CoA dehydrogenase, a medium-chain acyl-CoA dehydrogenase, and a short-chain acyl-CoA dehydrogenase. Interestingly, the enoyl-CoA hydratase, hydroxyacyl-CoA dehydrogenase, and ketoacyl-CoA isoforms specific for long-chain fatty acids form an enzyme complex on the inner mitochondrial membrane. Fatty acid β-oxidation is regulated at multiple levels. This figure shows some of the ways fatty acid β-oxidation is regulated. Regulation can occur at the level of fatty acid entry into the cell. Regulation also occurs via the regulation of the levels of acetyl-CoA and malonyl-CoA. Malonyl-CoA inhibits fatty acid oxidation by inhibiting CPT1. Transcriptional regulation is also involved in regulating fatty acid β-oxidation. PGC-1α, a transcription factor coregulator, and the transcription factor PPARα act in the nucleus to increase transcription of mitochondrial genes, fatty acid utilization genes, and other transcription factors. Auxiliary enzymes are required for the β-oxidation of unsaturated fatty acids and odd-chain fatty acids. Odd-numbered fatty acids are broken down by β-oxidation to acetyl-CoA molecules and propionyl-CoA. While propionyl-CoA could be metabolized through alternative pathways, it is primarily metabolized in the cell to succinyl-CoA by three enzymes propionyl-CoA carboxylase, methylmalonyl-CoA epimerase, and methylmalonyl-CoA mutase []. This succinyl-CoA can then enter the TCA cycle. Compared to even-numbered fatty acids, odd-numbered fatty acids occur infrequently in nature [15]. The two auxiliary enzymes, enoyl-CoA isomerase and 2,4-dienoyl-CoA reductase are necessary for the complete oxidation of unsaturated fatty acids [11]. During the β-oxidation cycle in which the cis -double bond begins on the third carbon of the acyl-CoA, the first step involves enoyl-CoA isomerase isomerizing it before enoyl-CoA hydratase, and the other two enzymes, can act on the acyl-CoA. A double bond on an even-numbered carbon requires both the auxiliary enzymes. Once the double bond is on the fourth carbon of the acyl-CoA at the beginning of a β-oxidation cycle it begins to be oxidized. Following action of acyl-CoA dehydrogenase, 2,4-dienoyl CoA reductase acts on the acyl-CoA followed by enoyl-CoA isomerase. Enoyl-CoA hydratase then acts on the acyl-CoA and the process resumes its normal order. Allosteric control of fatty acid β-oxidation:. The activity of the enzymes of fatty acid β-oxidation is affected by the level of the products of their reactions [16]. Each of the β-oxidation enzymes is inhibited by the specific fatty acyl-CoA intermediate it produces [17]. Interestingly, 3-ketoacyl-CoA can also inhibit enoyl-CoA hydratase and acyl-CoA dehydrogenase [17]. Fatty acid β-oxidation can also occur in peroxisomes. In animals, peroxisomes are believed to be important in the initial breakdown of very-long-chain fatty acids and methyl branched fatty acids [11]. The enzymes involved in fatty acid oxidation in peroxisomes are different from mitochondria. An important difference is acyl-CoA oxidase, the first enzyme in peroxisome β-oxidation, which transfers the hydrogen to oxygen producing H 2 O 2 instead of producing FADH 2. The H 2 O 2 is broken down to water by catalase. Importantly, the fatty acyl-CoA intermediates formed during β-oxidation are the same in peroxisomes and mitochondria. Peroxisomes also contain the necessary enzymes for α-oxidation, which are necessary for oxidation of some fatty acids with methyl branches. Transcriptional regulation of fatty acid β-oxidation:. The proteins involved in fatty acid β-oxidation are regulated by both transcriptional and post-transcriptional mechanisms. There are a number of transcription factors that regulate the expression of these proteins. The peroxisome proliferator-activated receptors PPARs and a transcription factor coactivator PGC-1α are the most well known transcriptional regulators of fatty acid β-oxidation [18]. PPARs and Retinoid X receptor heterodimerize and bind to gene promoters containing the PPAR response element [18]. Estrogen-related receptor α ERRα has also been implicated in the regulation of fatty acid β-oxidation, having been shown to also regulate transcription of the gene encoding MCAD [18]. Ligands that bind to and modulate the activity of PPARα, δ, and γ include fatty acids [18]. The genes regulated by each of the PPARs vary between tissue types. For example, skeletal muscle PPARδ, but not PPARα, upregulates expression of CPT1 [19]. PPAR isoforms are also differentially expressed between tissue types [18]. While PPARδ protein tends to be ubiquitously expressed, PPARα is predominantly expressed in highly metabolic tissues i. heart, skeletal muscle, and liver and PPARγ is predominantly expressed in tissues such as adipose tissue [18]. Until recently, PPARγ was not believed to play a significant role in regulating fatty acid β-oxidation. However, recent knockout and overexpression studies have suggested that PPARγ may have a role in regulating fatty acid β-oxidation. Over expressing PPARγ in cardiac muscle results in increased mRNA levels for fatty acid β-oxidation proteins [20]. The transcriptional co-activator PGC-1α binds to and increases the activity of PPARs and ERRα to regulate fatty acid β-oxidation [21]. PGC-1α modulates the activity of a number of transcription factors that can increase the expression of proteins involved in fatty acid β-oxidation, the TCA cycle, and the electron transport chain. For example, increasing PGC-1α protein expression induces massive mitochondrial biogenesis in skeletal muscle [21]. PGC-1α is regulated at both the gene and protein level. AMPK increases the activity of pre-existing PGC-1α protein through two proposed mechanisms. The first is by phosphorylating PGC-1α on threonine and serine residues results in an overall increase of the PGC-1α activity [22]. AMPK may also increase the activity of PGC-1α by activating sirtuin 1 SIRT1. SIRT1 can then deacetylate PGC-1α, increasing its activity [22]. AMPK regulates the MEF sites by phosphorylating GEF, a protein which can mediate movement of MEF2 into the nucleus [22]. AMPK may increase binding to the CRE site by phosphorylation of cAMP-response element binding protein CREB 1 and other members of the CREB family that bind to CRE promoter regions [22]. As another example, free fatty acids can also regulate PGC-1α protein expression. |
Ich habe nachgedacht und hat diese Phrase gelöscht
Nach meiner Meinung. Sie haben sich geirrt.
Ist Einverstanden, die nützliche Information