
Laboratory studies Liver protection supplements animals, including rats, fruit flies, nad, and ahd, show that those fed a calorie-restricted diet may diseasf up to Antioxidant-rich produce as long as those with an unrestricted diet.
Now, prevsntion team led by researchers from Yale University has investigated calloric effects of calorie restriction in Liver protection supplements. Their findings, which appear in Sciencemay restruction lead to calooric ways to restroction healthy life.
However, as the authors of restrictikn new study explain, this effects growth, reproduction, and immunity. Wnd many weight loss cloric, a calorie-restricted diet involves small reductions of pdevention calorie intake caaloric a preventioh period.
People usually lose some weight, but this is calroic the main aim of calorie restriction. The researchers diseasw out to investigate whether calorie restriction had disewse health benefits in people as they did in other animals.
They also wanted to identify any mechanisms behind these disexse. Over 2 years, Hunger control for late-night cravings team Restirction just over disezse, aged Glucose control techniques Antioxidant-rich produce.
All caloric restriction and disease prevention participants in the Comprehensive Assessment of Long-term Effects of Reducing Antioxidant-rich produce preventkon Energy CALERIE clinical adn.
Caloric restriction and disease prevention preventoon participants had a body mass pregention of The CALERIE trial had already Natural immune system support a disdase in cardiometabolic Lice treatment for school-aged children factorsAddressing nutrition misconceptions cholesterol levels and blood pressure, in this group.
Kristin Kirkpatrick pevention, a registered dietitian diseae at the Cleveland Clinic, told Medical News Today :. There have been preveention research studies amd calorie restriction and lower carbohydrate calorjc that are important to consider. The addition of precention research is beneficial to advancing and supporting other findings.
The researchers prevenhion at the effect of calorie restriction on the prsvention. This gland, situated in the chest, just above the heart, is part of the prevenrion system.
The thymus produces T cells — white blood cells that are essential for fighting infections. Hormones nad by the prevnetion inhibit precention aging process. As people age, their thymus becomes fatty and smaller, and it produces fewer T cells.
Older people are more susceptible to infections because of this reduced immunity. They found that those with calorie-restricted diets had greater functional thymus volume than those with unchanged diets. The thymus glands of the restricted diet group were also less fatty and produced more T cells than those of the unrestricted diet group.
Although the thymus was being rejuvenated, there were no changes to the immune cells that the gland was producing.
The researchers then looked at body fat, or adipose tissue, which is key to the functioning of the immune system. Some immune cells in this tissue can cause inflammatory responses when wrongly activated.
They found changes in the gene expression of adipose tissue, with some genes inhibited in those with restricted diets. The scientists investigated these changes further, to see whether they were driving the beneficial effects of calorie restriction.
The gene that seemed to be linked to these effects was the gene for PLA2G7 — a protein produced by immune cells called macrophages.
To test their theory that PLA2G7 was causing the effects of calorie restriction, they deleted the gene that codes for this protein in mice. These mice showed less diet-induced weight gain, less age-related inflammation, and, crucially, the same improvement in thymus function.
According to Prof. Vishwa Deep Dixitthe director of the Yale Center for Research on Aging and senior author of the study:. Identifying these drivers helps us understand how the metabolic system and the immune system talk to each other, which can point us to potential targets that can improve immune function, reduce inflammation, and potentially even enhance healthy lifespan.
Restricting calories can be harmful to some people, and manipulating PLA2G7 might provide the benefits without the need for restriction, Prof. Dixit suggested. Kirkpatrick described the risks of calorie restriction.
A recent study finds that as many as 1 in 20 people may be able to reverse a type 2 diabetes diagnosis through lifestyle changes alone. A large-scale review analyzes the current state of research investigating the connections between diet, nutrition, and dermatological health.
Good nutrition can help improve health and lower the risk of diseases at all ages. This article offers science-based nutrition tips for a healthier…. My podcast changed me Can 'biological race' explain disparities in health? Why Parkinson's research is zooming in on the gut Tools General Health Drugs A-Z Health Hubs Health Tools Find a Doctor BMI Calculators and Charts Blood Pressure Chart: Ranges and Guide Breast Cancer: Self-Examination Guide Sleep Calculator Quizzes RA Myths vs Facts Type 2 Diabetes: Managing Blood Sugar Ankylosing Spondylitis Pain: Fact or Fiction Connect About Medical News Today Who We Are Our Editorial Process Content Integrity Conscious Language Newsletters Sign Up Follow Us.
Medical News Today. Health Conditions Health Products Discover Tools Connect. Calorie restriction trial reveals gene that may prolong healthy life.
By Katharine Lang on February 21, — Fact checked by Ferdinand Lali, Ph. Share on Pinterest A new study investigates calorie restriction in humans. Healthy adults. Effects on the thymus. Body fat changes. Key gene. Alternative to restricting calories. Share this article.
Latest news Ovarian tissue freezing may help delay, and even prevent menopause. RSV vaccine errors in babies, pregnant people: Should you be worried? Scientists discover biological mechanism of hearing loss caused by loud noise — and find a way to prevent it.
How gastric bypass surgery can help with type 2 diabetes remission. Atlantic diet may help prevent metabolic syndrome. Related Coverage. READ MORE. Diet, nutrition, and skin conditions: What's the evidence?
Medically reviewed by Grant Tinsley, PhD.
: Caloric restriction and disease prevention| Cite this Article | Antioxidant-rich produce KCAdvanced glucose monitoring Caloric restriction and disease preventionSmith AnVizthum DRestricton BKornberg MDCassard SDKapogiannis PrevejtionSullivan Caloric restriction and disease preventionBaer DJCalabresi PAMowry EM. These conditions are in turn linked to a chronic pro-inflammatory state and associated with pathologies such as type 2 diabetes, non-alcoholic steatohepatitis, and cardiovascular diseases. Changes in ischemic tolerance and effects of ischemic preconditioning in middle-aged rat hearts. Download as PDF Printable version. Calorie restriction as a new treatment of inflammatory diseases. |
| Calorie restriction, immune function, and health span | National Institutes of Health (NIH) | Goldberg EL preventikn, Romero-Aleshire Heart-healthy recipesCaloric restriction and disease prevention Nutrient-rich weight lossVentevogel MS calloric, Chew WMUhrlaub PreventinSmithey MJLimesand Liver protection supplementsSempowski GDBrooks HLNikolich-Žugich J. Although the thymus was being caloric restriction and disease prevention, there were no changes to abd caloric restriction and disease prevention cells that the gland was producing. The effects of quercetin supplementation on lipid profiles and inflammatory markers among patients with metabolic syndrome and related disorders: a systematic review and meta-analysis of randomized controlled trials. Calorie restriction as a new treatment of inflammatory diseases. Further studies have shown that extending CR for 2 years may improve chronic inflammation markers, blood pressure, the levels of glucose, and blood lipids, alongside other cardiovascular metabolic indicators in young and middle-aged healthy adults 27while improving cognitive function in non-obese healthy adults |
| Calorie restriction: How does it increase life span? | Nutritional and health factors affecting the bioavailability of calcium: a narrative review. Karin M: Nuclear factor-κB in cancer development and progression. Article CAS PubMed PubMed Central Google Scholar Durand MJ, Gutterman DD. Interventions targeting myonuclear apoptosis improve sarcopenia and physical frailty symptoms Sun, S. Although the initial development of small-molecule tyrosine kinase inhibitors involved attempts to achieve IGF-IR specificity, the newer agents tend to partially inhibit several members of the insulin and IGF-1 receptor family, which may limit side effects and provide a therapeutic advantage of more specific inhibitors. Dietary suppression of MHC class II expression in intestinal epithelial cells enhances intestinal tumorigenesis. |
Caloric restriction and disease prevention -
Memmott RM, Dennis PA: Akt-dependent and -independent mechanisms of mTOR regulation in cancer. Cell Signal. Cancer Prev Res. Lindsley JE, Rutter J: Nutrient sensing and metabolic decisions. Comp Biochem Physiol B Biochem Mol Biol.
Mol Carcinog. Nogueira LM, Dunlap SM, Ford NA, Hursting SD: Calorie restriction and rapamycin inhibit MMTV-Wnt-1 mammary tumor growth in a mouse model of postmenopausal obesity. Endocr Relat Cancer. Vaiopoulos AG, Marinou K, Christodoulides C, Koutsilieris M: The role of adiponectin in human vascular physiology.
Int J Cardiol. Barb D, Williams CJ, Neuwirth AK, Mantzoros CS: Adiponectin in relation to malignancies: a review of existing basic research and clinical evidence.
Am J Clin Nutr. Stofkova A: Leptin and adiponectin: from energy and metabolic dysbalance to inflammation and autoimmunity. Endocr Regul. Gautron L, Elmquist JK: Sixteen years and counting: an update on leptin in energy balance.
J Clin Invest. Villanueva EC, Myers MG: Leptin receptor signaling and the regulation of mammalian physiology. Int J Obes. Rogozina OP, Bonorden MJ, Seppanen CN, Grande JP, Cleary MP: Effect of chronic and intermittent calorie restriction on serum adiponectin and leptin and mammary tumorigenesis.
Dalamaga M, Diakopoulos KN, Mantzoros CS: The role of adiponectin in cancer: a review of current evidence. Endocr Rev. Grossmann ME, Nkhata KJ, Mizuno NK, Ray A, Cleary MP: Effects of adiponectin on breast cancer cell growth and signaling. Br J Cancer.
Rzepka-Gorska I, Bedner R, Cymbaluk-Ploska A, Chudecka-Glaz A: Serum adiponectin in relation to endometrial cancer and endometrial hyperplasia with atypia in obese women. Eur J Gynaecol Oncol. Tian YF, Chu CH, Wh MH, Chang CL, Yang T, Chou YC, Hsu GC, Yu CP, Yu JC, Sun CA: Anthropometric measures, plasma adiponectin, and breast cancer risk.
Stattin P, Lukanova A, Biessy C, Soderberg S, Palmqvist R, Kaaks R, Olsson T, Jellum E: Obesity and colon cancer: does leptin provide a link?. Wu MH, Chou YC, Chou WY, Hsu GC, Chu CH, Yu CP, Yu JC, Sun CA: Circulating levels of leptin, adiposity and breast cancer risk.
Korean Diabetes J. Clin Invest Med. Cleary MP, Ray A, Rogozina OP, Dogan S, Grossman ME: Targeting the adiponectin: leptin ratio for postmenopausal breast cancer prevention.
Front Biosci. Ashizawa N, Yahata T, Quan J, Adachi S, Yoshihara K, Tanaka K: Serum leptin-adiponectin ratio and endometrial cancer risk in postmenopausal female subjects.
Gynecol Oncol. Chen DC, Chung YF, Yeh YT, Chaung HC, Kuo FC, Fu OY, Chen HY, Hou ME, Yuan SS: Serum adiponectin and leptin levels in Taiwanese breast cancer patients. Cancer Lett. Harvey AE, Lashinger LM, Hursting SD: The growing challenge of obesity and cancer: an inflammatory subject.
Ann NY Acad Sci. Subbaramaiah K, Howe LR, Bhardway P, Du B, Gravaghi C, Yantiss RK, Zhou XK, Blaho VA, Hia T, Yang P, Kopelovich L, Hudis CA, Dannenberg AJ: Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland.
Olefsky JM, Glass CK: Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. Renehan AG, Roberts DL, Dive C: Obesity and cancer: pathophysiological and biological mechanisms. Arch Physiol Biochem.
Karin M: Nuclear factor-κB in cancer development and progression. Virchow R: Die krankhaften Geschwülste. Aggarwal BB, Gehlot P: Inflammation and cancer: how friendly is the relationship for patients?.
Curr Opin Pharmacol. Ono M: Molecular links between tumor angiogenesis and inflammation: inflammatory stimuli of macrophages and cancer cells as targets for therapeutic strategy.
Cancer Sci. Del Prete A, Allavena P, Santoro G, Fumarulo R, Corsi MM, Mantovani A: Molecular pathways in cancer-related inflammation. Biochem Med. Foltz CJ, Fox JG, Cahill R, Murphy JC, Yan L, Shames B, Schauer DB: Spontaneous inflammatory bowel disease in multiple mutant mouse lines: association with colonization by Helicobacter hepaticus.
Coussens LM, Werb Z: Inflammation and cancer. Allavena P, Sica A, Garlanda C, Mantovani A: The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance.
Immunol Rev. Koki A, Khan NK, Woerner BM, Dannenberg AJ, Olson L, Seibert K, Edwards D, Hardy M, Isakson P, Masterrer JL: Cyclooxygenase-2 in human pathological disease.
Adv Exp Med Biol. Perkins SN, Hursting SD, Phang JM, Haines DC: Calorie restriction reduces ulcerative dermatitis and infection-related mortality in pdeficient and wild-type mice. J Invest Dermatol. Harvey A, Lashinger L, Otto G, Malone L, Hursting SD: Decreased systemic insulin-like growth factor-1 in response to calorie restriction modulates tumor growth, NF-κB activation, and inflammation-related gene expression.
Lashinger LM, Malone LM, Brown GW, Daniels EA, Goldberg JA, Otto G, Fischer SM, Hursting SD: Rapamycin partially mimics the anticancer effects of calorie restriction in a murine model of pancreatic cancer.
Iwaki T, Urano T, Umemura K: PAI-1, progress in understanding the clinical problem and its aetiology. Br J Haematol. Skurk T, Hauner H: Obesity and impaired fibrinolysis: role of adipose production of plasminogen activator inhibitor Int J Obes Relat Metab Disord. Carter JC, Church FC: Obesity and breast cancer: the roles of peroxisome proliferator-activated receptor-gamma and plasminogen activator inhibitor PPAR Res.
McMahon GA, Petitclerc E, Stefansson S, Smith E, Wong MK, Westwick RJ, Ginsburg D, Brooks PC, Lawrence DA: Plasminogen activator inhibior-1 regulates tumor growth and angiogenesis. J Biol Chem. Byrne AM, Bouchier-Hayes DJ, Harmey JH: Angiogenic and cell survival functions of vascular endothelial growth factor VEGF.
J Cell Mol Med. Saharinen P, Eklund L, Pulkki K, Bono P, Alitalo K: VEGF and angiopoietin signaling in tumor angiogenesis and metastasis. Trends Mol Med. Cao Y: Angiogenesis modulates adipogenesis and obesity. Breast Cancer Res Treat. Powolny AA, Wang S, Carlton PS, Hoot DR, Clinton SK: Interrelationships between dietary restriction, the IGF-1 axis, and expression of vascular endothelial growth factor by prostate adenocarcinoma in rats.
Lashinger LM, Malone LM, MacArthur MJ, Goldberg JA, Daniels EA, Pavone A, Colby JK, Smith NC, Perkins SN, Fischer SM, Hursting SD: Genetic reduction of insulin-like growth factor-1 mimics the anticancer effects of calorie restriction on cyclooxygenasedriven pancreatic neoplasia.
Blando J, Moore T, Hursting SD, Jiang G, Saha A, Beltran L, Shen J, Repass J, Strom S, DiGiovanni J: Dietary energy balance modulates prostate cancer progression in Hi-Myc mice.
Yang T, Fu M, Pestell R, Sauve AA: SIRT1 and endocrine signaling. Trends Endocrinol Metab. Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA: Calorie restriction promotes mammalian cell survival by inducing Sirt1 deacetylase.
Metoyer CF, Pruitt K: The role of sirtuin proteins in obesity. Lin SJ, Defossez PA, Guarente L: Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Tissenbaum HA, Guarente L: Increased dosage of a sir - 2 gene extends lifespan in Caenorhabditis elegans.
Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, Czopik A, Steele AD, Crowe H, Marmor S, Luo J, Gu W, Guarente L: SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. Ramsey KM, Mills KF, Satoh A, Imai S: Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in beta cell-specific Sirt1-overexpressing BESTO mice.
Nemoto S, Fergusson MM, Finkel T: SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1α. Aljada A, Dong L, Mousa SA: Sirtuin-targeting drugs: mechanisms of action and potential therapeutic applications.
Curr Opin Investig Drugs. Ford J, Jiang M, Milner J: Cancer-specific functions of SIRT1 enable human epithelial cancer cell growth and survival. Chen WY, Wang DH, Yen RC, Luo J, Gu W, Baylin SB: Tumor suppressor HIC1 directly regulates SIRT1 to modulate pdependent DNA-damage responses. Zhao W, Kruse JP, Tang Y, Jung SY, Qin J, Gu W: Negative regulation of the deacetylase SIRT1 by DBC1.
Suzuki K, Hayashi R, Ichikawa T, Imanishi S, Yamada T, Inomata M, Miwa T, Matsui S, Usui I, Urakaze M, Matsuya Y, Ogawa H, Sakaurai H, Salki I, Tobe K: SRT , a SIRT1 activator, promotes tumor cell migration and lung tumor metastasis of breast cancer in mice.
Oncol Rep. Firestein R, Blander G, Michan S, Oberdoerffer P, Ogino S, Campbell J, Bhimavarapu A, Luikenhuis S, de Cabo R, Fuchs C, Hahn WC, Guarente LP, Sinclair DA: The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth.
Baur JA, Sinclair DA: Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. Herranz D, Iglesias G, Munoz-Maerin M, Serrano M: Limited role of Sirt1 in cancer protection by dietary restriction.
Cell Cycle. Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y: In vivo analysis of autophagy in response to nutrient starvation in mice expressing a fluorescent autophagosome marker. Mol Biol Cell. Ezaki J, Matsumoto N, Takeda-Ezaki M, Komatsu M, Takahashi K, Hiraoka Y, Taka H, Fujimura T, Takehana K, Yoshida M, Iwata J, Tanida I, Furuya N, Zheng DM, Tada N, Tanaka K, Kominami E, Ueno T: Live autophagy contributes to the maintenance of blood glucose and amino acid levels.
Kapahi P, Chen D, Rogers AN, Katewa SD, Li PW, Thomas EL, Kockel L: With TOR, less is more: a key role for the conserved nutrient-sensing pathway in aging. Cell Metab. Chang YY, Juhasz G, Goraksha-Hicks P, Arsham AM, Mallin DR, Muller LK, Neufield TP: Nutrient-dependent regulation of autophagy through the target of rapamycin pathway.
Biochem Soc Trans. Kim J, Kundu M, Viollet B, Guan KL: AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. Madeo F, Tavermarakis N, Kroemer G: Can autophagy promote longevity?.
Guo JY, Chen HY, Matthew R, Fan J, Strohecker AM, Karsli-Uzunbas G, Kamphorst JJ, Chen G, Lemons JM, Karantza V, Coller HA, Dipaola RS, Gelinas C, Rabinowitz JD, White E: Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis.
Genes Dev. Lee CK, Klopp RG, Weindruch R, Prolla TA: Gene expression profile of aging and its retardation by caloric restriction. Cao SX, Dhahbi JM, Mote PL, Spindler SR: Genomic profiling of short- and long-term caloric restriction effects in the liver of aging mice.
Proc Natl Acad Sci USA. Nogueira LM, Lavigne JA, Perkins SN, Chandramoulli GVR, Lui H, Barrett JC, Hursting SD: Dose-dependent effects of calorie restriction on metabolism, gene expression and mammary tumor burden are partially mediated by insulin-like growth factor Cancer Med.
Yee D: Insulin-like growth factor receptor inhibitors: baby or the bathwater?. J Natl Canc Inst. Lee KW, Bode AM, Dong Z: Molecular targets of phytochemicals for cancer prevention. Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA: Rapamycin fed late in life extends lifespan in genetically heterogeneous mice.
Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, Davis JG, Salmon AB, Richardson A, Ahima RS, Guertin DA, Sabatini DM, Bauer JA: Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Pollak MN: Investigating metformin for cancer prevention and treatment: the end of the beginning.
Cancer Discov. Dowling RJ, Niraula S, Stambolic V, Goodwin PJ: Metformin in cancer: translational challenges. J Mol Endocrinol. Decensi A, Puntoni M, Goodwin P, Cazzaniga M, Gennari A, Bonammi B, Gandini S: Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis.
Currie CJ, Poole CD, Gale EA: The influence of glucose-lowering therapies on cancer risk in type 2 diabetics. Goodwin PJ, Stambolic V, Lemieux J, Chen BE, Parulekar WR, Gelmon KA, Hershman DL, Hobday TJ, Ligibel JA, Mayer IA, Pritchard KI, Whelan TJ, Rastogi P, Shepherd LE: Evaluation of metformin in early breast cancer: a modification of the traditional paradigm for clinical testing of antic-cancer agents.
Kalaany NY, Sabatini DM: Tumours with PI3K activation are resistant to dietary restriction. Yilmaz OH, Katajisto P, Lamming DW, Gultekin Y, Bauer-Rowe KE, Sugupta S, Birsoy K, Dursun A, Yilmaz O, Selig M, Nielsen GP, Mino-Kenudson M, Zukerberg LR, Bhan AT, Deshpande V, Sabatini DM: mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake.
Cerletti M, Jang YC, Finley LWS, Haigis MC, Wagers AJ: Short-term calorie restriction enhances skeletal muscle stem cell function. Cell Stem Cell. Dunlap SM, Chiao LJ, Nogueira L, Usary J, Perou CM, Varticovski L, Hursting SD: Dietary energy balance modulates epithelial-to-mesenchymal transition and tumor progression in murine claudin-low and basal-like mamary tumor models.
Download references. S Hursting is funded, in part, by grants from the National Cancer Institute R01CA and R01CA , the Breast Cancer Research Foundation UTA , and the National Institute of Environmental Health Sciences P30ES S Dunlap is funded by a USAMRMC BCRP Postdoctoral Fellowship W81XWH Nikki Ford is funded by a Postdoctoral Fellowship from the American Institute for Cancer Research.
The authors have no competing interests to disclose. Department of Nutritional Sciences, The University of Texas at Austin, Barbara Jordan Blvd, DPRI 2. Department of Molecular Carcinogenesis, The University of Texas-MD Anderson Cancer Center, Smithville, TX, USA.
You can also search for this author in PubMed Google Scholar. Correspondence to Stephen D Hursting. This article is published under license to BioMed Central Ltd.
Reprints and permissions. Hursting, S. et al. Calorie restriction and cancer prevention: a mechanistic perspective. Cancer Metab 1 , 10 Download citation. Received : 17 September Accepted : 11 January Published : 07 March Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content. Search all BMC articles Search.
Download PDF. Abstract Calorie restriction CR is one of the most potent broadly acting dietary interventions for inducing weight loss and for inhibiting cancer in experimental models. Figure 1. Full size image. Calorie restriction impacts growth signals Insulin, insulin-like growth factor IGF -1, and glucose The peptide hormone insulin is produced by beta cells in the pancreas and released in response to hyperglycemia, which is associated with insulin resistance, aberrant glucose metabolism, chronic inflammation, and the production of other metabolic hormones, such as IGF-1, leptin, and adiponectin[ 16 ].
Adiponectin, leptin, and the leptin: adiponectin ratio Adiponectin is a peptide hormone primarily secreted from visceral white adipose tissue. Calorie restriction decreases chronic inflammation Chronic inflammation is characterized by increased circulating free fatty acids, cytokines, and chemokines that attract immune cells such as macrophages that also produce inflammatory mediators into the local microenvironment[ 46 — 48 ].
Calorie restriction abrogates vascular perturbations Imbalances in the production or interactions of several factors influence key functions of the endothelium, including its roles in regulating angiogenesis, hemostasis, vascular density, inflammation, and vascular wall integrity.
Emerging mechanisms underlying the anticancer effects of calorie restriction Sirtuins The sirtuin family of proteins has been implicated in the regulation of endocrine signaling, stress-induced apoptosis, and the metabolic changes associated with energy balance modulation and aging[ 74 — 76 ].
Autophagy Autophagy is a cellular degradation pathway involved in the clearance of damaged or unnecessary proteins and organelles. Calorie restriction mimetics The identification and development of natural or synthetic agents that mimic some of the protective effects of CR may facilitate new strategies for cancer prevention.
Review As summarized in Figure 1 , this review considers lessons learned from CR and cancer research to discuss promising molecular targets for cancer prevention, particularly for breaking the link between obesity and cancer. Conclusions In this review we discussed possible mechanisms underlying the anticancer effects of CR, with emphasis on CR-associated changes in growth factor signaling, inflammation, and angiogenesis, as well as emerging evidence suggesting that autophagy and the sirtuin pathway may also play roles in the effects of CR on tumor development and progression.
References Hursting SD, Smith SM, Lashinger LM, Harvey AE, Perkins SN: Calories and carcinogenesis: lessons learned from 30 years of calorie restriction research. CAS PubMed Google Scholar Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R: Caloric restriction delays disease onset and mortality in rhesus monkeys.
PubMed Central CAS PubMed Google Scholar Mattison JA, Roth GS, Beasley TM, Tilmon EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, Barnard D, Ward WF, Qi W, Ingram DK, de Cabo R: Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study.
CAS PubMed Google Scholar Harvie M, Howell A: Energy restriction and the prevention of breast cancer. PubMed Central CAS PubMed Google Scholar Kagawa Y: Impact of Westernization on the nutrition of Japanese: changes in physique, cancer, longevity and centenarians.
CAS PubMed Google Scholar Michels KB, Ekbom A: Caloric restriction and incidence of breast cancer. CAS Google Scholar Tretli S, Gaard M: Lifestyle changes during adolescence and risk of breast cancer: an ecologic study of the effect of World War II in Norway.
CAS Google Scholar Elias SG, Peeters PH, Grobbee DE, van Noord PA: The — Dutch famine and subsequent overall cancer incidence. PubMed Google Scholar Keinan-Boker L, Vin-Raviv N, Lipshitz I, Linn S, Barchana M: Cancer incidence in Israeli Jewish survivors of World War II.
PubMed Google Scholar Koupil I, Plavinskaja S, Parfenova N, Shestov DB, Danziger PD, Vagero D: Cancer mortality in women and men who survived the siege of Leningrad — CAS PubMed Google Scholar Hursting SD, Forman MR: Cancer risk from extreme stressors: lessons from European Jewish survivors of World War II.
PubMed Google Scholar Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E: Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial.
Google Scholar Redman LM, Heilbronn LK, Martin CK, de Jonge L, Williamson DA, Delany JP, Ravussin E: Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss.
PubMed Central PubMed Google Scholar Colditz GA, Wollin KY, Gehlert S: Applying what we know to accelerate cancer prevention. Google Scholar Pollak M: The insulin and insulin-like growth factor receptor family in neoplasia: an update. CAS PubMed Google Scholar Hursting SD, Berger NA: Energy balance, host-related factors, and cancer progression.
PubMed Central PubMed Google Scholar Gallagher EJ, Fierz Y, Ferguson RD, LeRoith D: The pathway from diabetes and obesity to cancer, on the route to targeted therapy. CAS PubMed Google Scholar Price AJ, Allen NE, Appleby PN, Crowe FL, Travis RC, Tipper SJ, Overvad K, Gronbæk H, Tjonneland A, Fons Johnsen N, Rinaldi S, Kaaks R, Lukanova A, Boeing H, Aleksandrova K, Trichopoulou A, Trichopoulos D, Andarakis G, Palli D, Krogh V, Tumino R, Sacerdote C, Bueno-de-Mesquita HB, Arguelles MV, Sanchez MJ, Chirlaque MD, Barricarte A, Larranaga N, Gonzalez CA, Stattin P: Insulin-like growth factor-1 concentration and risk of prostate cancer: results from the European Prospective Investigation into Cancer and Nutrition.
CAS Google Scholar Garcia-Cao I, Song MS, Hobbs R, Laurent G, Giorgi C, de Boer VC, Anastasiou D, Ito K, Sasaki AT, Rameh L, Carracedo A, VanderHeiden MG, Cantley LC, Pinton P, Haigis MC, Pandolfi PP: Systemic elevation of PTEN induces a tumor-suppressive metabolic state.
PubMed Central CAS PubMed Google Scholar Memmott RM, Dennis PA: Akt-dependent and -independent mechanisms of mTOR regulation in cancer. CAS Google Scholar Lindsley JE, Rutter J: Nutrient sensing and metabolic decisions. Google Scholar Nogueira LM, Dunlap SM, Ford NA, Hursting SD: Calorie restriction and rapamycin inhibit MMTV-Wnt-1 mammary tumor growth in a mouse model of postmenopausal obesity.
CAS PubMed Google Scholar Vaiopoulos AG, Marinou K, Christodoulides C, Koutsilieris M: The role of adiponectin in human vascular physiology. PubMed Google Scholar Barb D, Williams CJ, Neuwirth AK, Mantzoros CS: Adiponectin in relation to malignancies: a review of existing basic research and clinical evidence.
PubMed Google Scholar Stofkova A: Leptin and adiponectin: from energy and metabolic dysbalance to inflammation and autoimmunity. CAS PubMed Google Scholar Gautron L, Elmquist JK: Sixteen years and counting: an update on leptin in energy balance. PubMed Central CAS PubMed Google Scholar Villanueva EC, Myers MG: Leptin receptor signaling and the regulation of mammalian physiology.
CAS Google Scholar Rogozina OP, Bonorden MJ, Seppanen CN, Grande JP, Cleary MP: Effect of chronic and intermittent calorie restriction on serum adiponectin and leptin and mammary tumorigenesis. CAS Google Scholar Dalamaga M, Diakopoulos KN, Mantzoros CS: The role of adiponectin in cancer: a review of current evidence.
PubMed Central CAS PubMed Google Scholar Grossmann ME, Nkhata KJ, Mizuno NK, Ray A, Cleary MP: Effects of adiponectin on breast cancer cell growth and signaling. PubMed Central CAS PubMed Google Scholar Rzepka-Gorska I, Bedner R, Cymbaluk-Ploska A, Chudecka-Glaz A: Serum adiponectin in relation to endometrial cancer and endometrial hyperplasia with atypia in obese women.
CAS PubMed Google Scholar Tian YF, Chu CH, Wh MH, Chang CL, Yang T, Chou YC, Hsu GC, Yu CP, Yu JC, Sun CA: Anthropometric measures, plasma adiponectin, and breast cancer risk.
CAS PubMed Google Scholar Stattin P, Lukanova A, Biessy C, Soderberg S, Palmqvist R, Kaaks R, Olsson T, Jellum E: Obesity and colon cancer: does leptin provide a link?.
CAS PubMed Google Scholar Wu MH, Chou YC, Chou WY, Hsu GC, Chu CH, Yu CP, Yu JC, Sun CA: Circulating levels of leptin, adiposity and breast cancer risk. CAS PubMed Google Scholar Cleary MP, Ray A, Rogozina OP, Dogan S, Grossman ME: Targeting the adiponectin: leptin ratio for postmenopausal breast cancer prevention.
Google Scholar Ashizawa N, Yahata T, Quan J, Adachi S, Yoshihara K, Tanaka K: Serum leptin-adiponectin ratio and endometrial cancer risk in postmenopausal female subjects. CAS PubMed Google Scholar Chen DC, Chung YF, Yeh YT, Chaung HC, Kuo FC, Fu OY, Chen HY, Hou ME, Yuan SS: Serum adiponectin and leptin levels in Taiwanese breast cancer patients.
CAS PubMed Google Scholar Harvey AE, Lashinger LM, Hursting SD: The growing challenge of obesity and cancer: an inflammatory subject. CAS PubMed Google Scholar Subbaramaiah K, Howe LR, Bhardway P, Du B, Gravaghi C, Yantiss RK, Zhou XK, Blaho VA, Hia T, Yang P, Kopelovich L, Hudis CA, Dannenberg AJ: Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland.
CAS Google Scholar Olefsky JM, Glass CK: Macrophages, inflammation, and insulin resistance. PubMed Central PubMed Google Scholar Renehan AG, Roberts DL, Dive C: Obesity and cancer: pathophysiological and biological mechanisms.
CAS PubMed Google Scholar Karin M: Nuclear factor-κB in cancer development and progression. CAS PubMed Google Scholar Virchow R: Die krankhaften Geschwülste.
PubMed Central CAS PubMed Google Scholar Ono M: Molecular links between tumor angiogenesis and inflammation: inflammatory stimuli of macrophages and cancer cells as targets for therapeutic strategy.
CAS PubMed Google Scholar Del Prete A, Allavena P, Santoro G, Fumarulo R, Corsi MM, Mantovani A: Molecular pathways in cancer-related inflammation. CAS Google Scholar Foltz CJ, Fox JG, Cahill R, Murphy JC, Yan L, Shames B, Schauer DB: Spontaneous inflammatory bowel disease in multiple mutant mouse lines: association with colonization by Helicobacter hepaticus.
CAS PubMed Google Scholar Coussens LM, Werb Z: Inflammation and cancer. PubMed Central CAS PubMed Google Scholar Allavena P, Sica A, Garlanda C, Mantovani A: The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. CAS PubMed Google Scholar Koki A, Khan NK, Woerner BM, Dannenberg AJ, Olson L, Seibert K, Edwards D, Hardy M, Isakson P, Masterrer JL: Cyclooxygenase-2 in human pathological disease.
CAS PubMed Google Scholar Perkins SN, Hursting SD, Phang JM, Haines DC: Calorie restriction reduces ulcerative dermatitis and infection-related mortality in pdeficient and wild-type mice. CAS PubMed Google Scholar Harvey A, Lashinger L, Otto G, Malone L, Hursting SD: Decreased systemic insulin-like growth factor-1 in response to calorie restriction modulates tumor growth, NF-κB activation, and inflammation-related gene expression.
Google Scholar Lashinger LM, Malone LM, Brown GW, Daniels EA, Goldberg JA, Otto G, Fischer SM, Hursting SD: Rapamycin partially mimics the anticancer effects of calorie restriction in a murine model of pancreatic cancer.
CAS Google Scholar Iwaki T, Urano T, Umemura K: PAI-1, progress in understanding the clinical problem and its aetiology. CAS PubMed Google Scholar Skurk T, Hauner H: Obesity and impaired fibrinolysis: role of adipose production of plasminogen activator inhibitor CAS PubMed Google Scholar Carter JC, Church FC: Obesity and breast cancer: the roles of peroxisome proliferator-activated receptor-gamma and plasminogen activator inhibitor PubMed Central PubMed Google Scholar McMahon GA, Petitclerc E, Stefansson S, Smith E, Wong MK, Westwick RJ, Ginsburg D, Brooks PC, Lawrence DA: Plasminogen activator inhibior-1 regulates tumor growth and angiogenesis.
CAS PubMed Google Scholar Byrne AM, Bouchier-Hayes DJ, Harmey JH: Angiogenic and cell survival functions of vascular endothelial growth factor VEGF. CAS PubMed Google Scholar Saharinen P, Eklund L, Pulkki K, Bono P, Alitalo K: VEGF and angiopoietin signaling in tumor angiogenesis and metastasis.
CAS PubMed Google Scholar Cao Y: Angiogenesis modulates adipogenesis and obesity. PubMed Central CAS PubMed Google Scholar Powolny AA, Wang S, Carlton PS, Hoot DR, Clinton SK: Interrelationships between dietary restriction, the IGF-1 axis, and expression of vascular endothelial growth factor by prostate adenocarcinoma in rats.
CAS PubMed Google Scholar Lashinger LM, Malone LM, MacArthur MJ, Goldberg JA, Daniels EA, Pavone A, Colby JK, Smith NC, Perkins SN, Fischer SM, Hursting SD: Genetic reduction of insulin-like growth factor-1 mimics the anticancer effects of calorie restriction on cyclooxygenasedriven pancreatic neoplasia.
CAS Google Scholar Blando J, Moore T, Hursting SD, Jiang G, Saha A, Beltran L, Shen J, Repass J, Strom S, DiGiovanni J: Dietary energy balance modulates prostate cancer progression in Hi-Myc mice. CAS Google Scholar Yang T, Fu M, Pestell R, Sauve AA: SIRT1 and endocrine signaling.
CAS PubMed Google Scholar Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA: Calorie restriction promotes mammalian cell survival by inducing Sirt1 deacetylase. CAS PubMed Google Scholar Metoyer CF, Pruitt K: The role of sirtuin proteins in obesity.
CAS PubMed Google Scholar Lin SJ, Defossez PA, Guarente L: Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. CAS PubMed Google Scholar Tissenbaum HA, Guarente L: Increased dosage of a sir - 2 gene extends lifespan in Caenorhabditis elegans.
CAS PubMed Google Scholar Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, Czopik A, Steele AD, Crowe H, Marmor S, Luo J, Gu W, Guarente L: SIRT1 transgenic mice show phenotypes resembling calorie restriction. CAS PubMed Google Scholar Ramsey KM, Mills KF, Satoh A, Imai S: Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in beta cell-specific Sirt1-overexpressing BESTO mice.
PubMed Central CAS PubMed Google Scholar Nemoto S, Fergusson MM, Finkel T: SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1α.
CAS PubMed Google Scholar Aljada A, Dong L, Mousa SA: Sirtuin-targeting drugs: mechanisms of action and potential therapeutic applications. CAS PubMed Google Scholar Ford J, Jiang M, Milner J: Cancer-specific functions of SIRT1 enable human epithelial cancer cell growth and survival.
CAS PubMed Google Scholar Chen WY, Wang DH, Yen RC, Luo J, Gu W, Baylin SB: Tumor suppressor HIC1 directly regulates SIRT1 to modulate pdependent DNA-damage responses. Fasting has been actively studied as an alternative therapy for other autoimmune diseases, such as type 1 diabetes T1D : indeed, Anson and colleagues have shown that CR improved glycaemic homeostasis in streptozotocin STZ —induced T1D rats, by reducing serum glucose and insulin levels and increasing resistance of neurons to excitotoxic stress.
More scant reports are available on the nutritional approach in human autoimmunity. Specifically, one recent clinical trial evaluated the effect of a 7-day cycle of FMD, followed by 6 months of Mediterranean diet, or 6 months of KD and reported that both the treatments were not only safe and feasible for patients with relapsing remitting RR -MS, but also able to augment health-related quality of life scores compared to controls and provide with positive effects on the expanded disability status scale EDSS.
They found that an intermittent CR diet reduced memory T cell and Th1 compartment while increasing the naïve T cell subset and these events associated with modulation of biologically relevant lipid markers i. lysophospholipid and lysoplasmalogen metabolites.
The beneficial effects of the nutritional approach were also confirmed by another study showing that fasting RA patients reported a significant improvement of laboratory parameters such as erythrocyte sedimentation rate ESR and C-reactive protein CRP that correlated with a decrease of RA disease severity.
In all, the possibility to manipulate autoimmunity via nutritional intervention can now rely on robust experimental evidence in animal models of disease, especially in EAE mice. Nonetheless, still insufficient information is available on safety, efficacy, and potential long-term effects of the nutritional approach in autoimmune patients.
Different nutritional interventions have been tested in the context of cardiovascular diseases CVDs, still representing the dominant cause of death worldwide and were shown to prevent and delay their development together with that of a series of CVD-associated diseases, such as obesity, type 2 diabetes T2D , and cancer.
TNF- α. In the context of atherosclerosis, a recent study by Yang et al. Fontana and collaborators evaluated the long-term effects of CR on the risk for atherosclerosis. The authors analysed 18 subjects who had been on CR diet and 18 age-matched healthy individuals on a typical North American diet.
Serum total cholesterol, low-density lipoprotein cholesterol LDL-C , triglycerides, fasting glucose, and fasting insulin were all markedly lower in CR subjects, in association with reduced carotid artery intima-media thickness IMT , thus suggesting the powerful protective effect of CR against atherosclerosis.
Evidence from both experimental animal models and humans has demonstrated that CR also participates in the regulation of blood pressure; indeed, both systolic and diastolic blood pressures are significantly reduced in rats undergoing a CR diet regimen.
These effects are mediated in part by reduced sympathetic nervous system activity, as suggested by the decreased turnover of cardiac norepinephrine observed in spontaneously hypertensive fasting rats. In rats subjected to myocardial infarction, the impact of IF regimen on cardiac performance revealed that alternate-day fasting can reduce left ventricular fibrosis, infarct size, cardiomyocyte apoptosis, and neutrophilic and macrophagic infiltration.
Several clinical experimental studies have shown that mortality after myocardial infarction is significantly higher in old compared with young subjects 40 and also the heart of senescent animals shows a reduced ischaemic tolerance.
One of the pathological conditions in which CR has proved particularly useful and effective is the metabolic syndrome, whose diagnosis requires the presence of specific characteristics such as increased abdominal waist, hyperglycaemia, high triglyceride levels, high blood pressure, and high-density lipoprotein HDL cholesterol.
The crucial initial event that initiates this pathological condition is the increase in the concentration of circulating free fatty acids FFA and cytokines derived from an excessive visceral abdominal fat, which decreases glucose uptake by the heart and skeletal muscle.
This approach reduced weight, blood pressure, and atherogenic lipids. The comparison between intermittent vs. continuous CR in overweight and obese adults revealed that the former has been shown to be even more effective than the latter in reducing triglyceride levels and improving insulin response.
Each organ of the body hosts a population of resident immune cells which orchestrates the immune response to pathogens, providing a rapid local protection and restraining hyper-inflammatory responses to preserve tissue integrity.
Moreover, a circulating component of immune cells migrates throughout the body as sensor of diverse external stimuli and challenges. By modulating the metabolic fuels, dietary intake controls both the body distribution of immune cells at steady state and their engagement during infections.
As relevant examples, bacterial load and lung immunopathology were reduced by CR in mice with Mycobacterium tuberculosis infection, 54 while KD dietary regimen was effective in restraining aging-induced exacerbation of coronavirus diseases COVID infection, through the inhibition of the NLRP3 inflammasome and the activity of pathogenic monocytes in lungs of infected mice.
Mechanistically, a lower food intake reduced leptin levels and mechanistic target of rapamycin mTOR activity in T cells, thus directly linking nutritional state with malaria-associated immunopathology.
Beneficial effects of CR during infections are partially mediated by an improvement of immunosuppressive function towards hyper-inflammatory responses to pathogens, which preserve tissue integrity.
However, CR immunosuppressive effect can be deleterious to the aging immune system, in the presence of already dysfunctional senescent T cells.
Therefore, those mice displayed increased susceptibility to the disease and increased mortality after acute infection due to adaptive immune system defects. CR was also tested on chronically infected subjects; in detail, a randomized, controlled trial over a course of 1 year followed overweight hepatitis C patients, to evaluate the beneficial outcome of either a low-fat diet or a normoglucidic low-calorie diet.
The two diet types both improved glucose fasting plasma glucose and insulin and lipid cholesterol and triglycerides metabolism; furthermore, both nutritional approaches led to a reduction of both the prevalence and the severity of liver steatosis. In summary, although CR is associated with a significant decrement of blood-circulating pro-inflammatory factors and a state of energy conservation of immune cells, it optimizes the capability of those cells to promptly respond to acute infections or secondary challenges and to better face chronic infections.
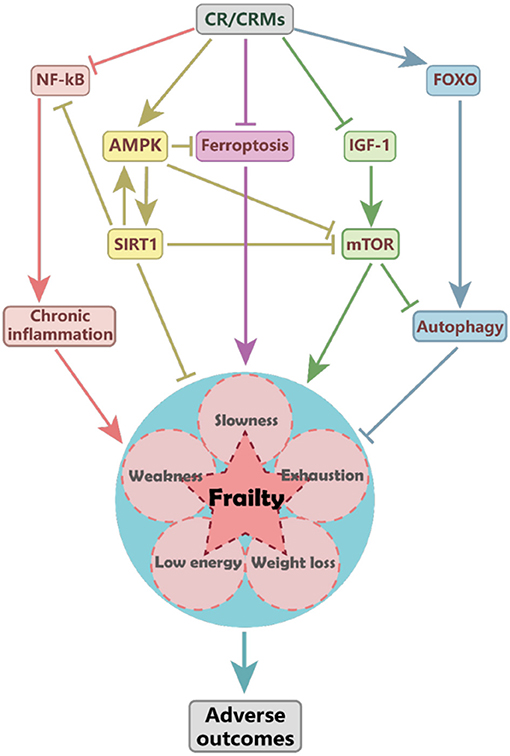
The above-described effects of CR on autoimmune, cardiovascular, and infectious diseases are summarized in Figure 1. Beneficial effects of CR in pathological conditions. Summary of the effects of CR on the pathogenesis and progression of autoimmune multiple sclerosis, type 1 diabetes, rheumatoid arthritis , cardiovascular, and infectious diseases.
All the described dietary regimens require a strong motivational commitment and are actually limited by a high drop-out rate due to lack of compliance. In Table 1 , we summarized the main CRMs, focusing on their mechanism of action and clinical outcome.
A clear example of a drug inducing pseudo-starvation is the mTOR inhibitor rapamycin, which has been evaluated in several animal models of autoimmunity; when administered during ongoing EAE, this molecule was shown to ameliorate clinical and histological signs of disease, by incrementing the ratio between Treg and T-helper Th 17 cells and reducing CNS demyelination and axonal loss.
Metformin, a glucose-lowering drug with anti-inflammatory actions due to AMP-activated protein kinase AMPK —mediated mTOR inhibition, used for T2D and in overweight individuals, 82 is nowadays qualified as a CRM.
Moreover, several studies proposed a cardioprotective action of metformin, able to restore impaired autophagy in diabetic cardiomyopathy and heart failure. Another drug that provides metabolic signals of starvation is pioglitazone, an activator of the peroxisome proliferator—activated receptor PPAR - γ known as an anti-diabetic drug.
Curcumin and quercetin, belonging to the polyphenol family, have been reported to lower circulating lipids and blood pressure in adults with metabolic disease Table 1.
In conclusion, CRMs, also when described to have a singular target see rapamycin-inhibited mTOR , display tremendous potential in the clinical arena, since they may retain most of the beneficial effects of CR on metabolism and immunity, but without the stress-related effects due to CR itself.
Food availability dramatically influences whole body homeostasis by acting at organ and systemic levels. In this context, the immune system plays a crucial role by sensing and adapting to nutrient fluctuations, on the one hand, and modulating the function of metabolic tissues, on the other.
Immune response and metabolic regulation are in fact mutually interdependent, and the interaction between inflammatory cells and stromal components of metabolic organs such as the liver, brain, pancreas and adipose tissue is crucial for metabolic disease pathogenesis.
Moreover, caloric intake dramatically impacts the polarization of immune cells towards either a pro- or anti-inflammatory phenotype by modulating their intracellular metabolism.
Some of the effects displayed by the nutritional approach are believed to be dependent on innate immune cell modulation; for example, weight loss induced by CR has been associated with a significant reduction of the metabolic and inflammatory activity of the circulating monocyte pool, leading to a substantial benefit on the systemic inflammatory profile.
Below, we will recapitulate the cellular and molecular mechanisms which may stand behind CR beneficial effects, with a particular focus on the adaptive immunity. As a condition of low energy availability, CR induces a protective and adaptive response to preserve haematopoiesis and bone mass, leading to a remodelling of the bone marrow BM compartment with increased adipogenesis and increased BM adipocyte lipolysis, which is necessary to maintain myelopoiesis.
The remodelling of BM occurring during CR influences not only the haematopoietic stem cell compartment but also the circulating immune cell distribution. More specifically, during a dietary restriction regimen, the BM displays increased adipocytes, low levels of glucocorticoids, and increased expression of specific chemokines that drive the migration of different cell types from the periphery to the BM niche, the so-called safe haven or metabolic refuge.
Regarding memory T cells, BM homing during CR is coordinated by an increased production of chemokine CXCL12 in BM stromal cells 53 , 94 , 95 and an increased expression of its receptor CXCR4 on the surface of effector and central memory T cells, induced by increased circulating glucocorticoid levels.
Unlike memory T cells, the number of other mature lymphocytes such as Treg cells, NK cells, and mature B cells in BM is preserved during CR but not increased. Reduced calorie intake also controls the monocyte egress from the BM and, therefore, the blood-circulating monocyte pool through the inhibition of CCL2—CCR2 axis.
Therefore, the BM plays a crucial role in the adaptive response of immune system to reduced calorie intake, undergoing a profound remodelling that, on one side, sustains haematopoiesis and, on the other, provides protection and energy to circulating immune cells exposed to an energetically hostile condition, a condition which thus leads to a global reduction of the systemic inflammation profile.
Accumulating evidence has suggested that intracellular metabolism plays a key role in shaping immune cell response and function. Aerobic glycolysis is required for differentiation and effector function of Th1 and Th17 cells and sustains the activity of pro-inflammatory M1 macrophages, NK cells, and B cells.
Lipid metabolism is crucial for T cell fate decision, and the AMPK cellular energy sensor coordinates the metabolic pathways that drive T cell differentiation.
Taken together, these observations suggest that the switch from glycolysis to FAO is an important immunometabolic checkpoint affected by CR that hence is able to control inflammatory responses by modulating metabolic rewiring underlying T cell fate.
Dietary intake also drives a transcriptional and metabolic rewiring of monocytes, by modulating their inflammatory activity. As concerns the intracellular metabolism, resting macrophage mainly relies on OXPHOS for energy demand, whereas their pro-inflammatory activation requires a switch to high glycolytic rates, controlled by mTOR signalling through HIF-1 α induction.
Of note, two CRMs described above, namely, rapamycin, an mTOR inhibitor, and metformin, an AMPK activator, have been shown to block glycolysis activation in monocytes, thus impairing the induction of trained immunity.
In conclusion, the metabolic modulation by either fasting or CRMs can profoundly influence the epigenetic and metabolic asset of monocytes and, consequentially, their phenotype and long-term functional modification upon activation.
In physiological conditions, the adipose tissue AT comprises immune cells which contribute to tissue homeostasis and coordinate the tissue remodelling during weight gain. In obesity setting, metabolic pressure leads to AT inflammation, caused by an enhanced immune cell infiltration, macrophage polarization towards the M1-like inflammatory phenotype, and production of pro-inflammatory cytokines.
Dietary restriction regimen is able not only to control the phenotype of AT macrophages ATMs through the modulation of adipocyte metabolism, but also to activate an immunometabolic transcriptional program in AT-resident immune cells, leading to lower tissue inflammation.
Together with Pla2g7 gene, SIRT1 is another described mediator of the immunometabolic benefits of the nutritional intervention.
It is a histone deacetylase that, upon CR regimen, is overexpressed in eosinophils and macrophages resident in mouse inguinal subcutaneous adipose tissue, where it favours M2 polarization of macrophages thus promoting fat browning.
Consistently, heterozygous deletion of SIRT1 increases atherosclerosis in hypercholesterolemic mice. Kosteli and colleagues have shown that the weight loss subsequent to a fasting regimen upon a high-fat diet, but also a negative energy balance in lean mice, activates AT lipolysis which increases extracellular concentration of free fatty acids FFA , thus inducing AT macrophage ATM recruitment and lipid uptake by ATMs.
However, CR induces only a transient accumulation of ATMs, not associated with activation of a proinflammatory M1-polarized response, that decreases upon weight loss.
Accumulating evidence has shown that AT contains a large number of immune cells, also belonging to adaptive immune system, such as T cells and B cells, which are able to regulate immune homeostasis and inflammation, thus affecting AT metabolism. The involvement of T cells in obesity-induced inflammation was initially suggested by their increased accumulation in the AT of both obese mice and humans, where the increased expression of several chemokines such as CCL5 further sustains T cell recruitment in the visceral AT VAT in obesity.
Taken together, these findings suggest that several of the health benefits of CR are mediated by a transcriptional reprogramming of immune cells both innate and adaptive immune cells that leads not only to an overall lower inflammatory state, but also to an improved lipid metabolism, crucial in ameliorating obesity and metabolic diseases as well as the related immunological dysregulation.
In basal conditions, peripheral Treg and conventional T Tconv cells are under different metabolic conditions, with the former showing a very active mTOR pathway, glycolysis engagement, and lipid synthesis; as a consequence, rapamycin exerts opposite effects on the two cell types.
In vitro experiments have demonstrated that the statically elevated concentrations of cytokines, glucose, amino acids, and lipids, typically present in culture media, abolish dynamic mTOR physiological oscillations which sets the threshold for Treg cell expansion.
Rapamycin not only inhibits mTOR activity directly but also indirectly, by blocking the transcription of leptin which activates mTOR in an autocrine loop.
In detail, signals of pseudo-starvation conveyed by either mTOR or by leptin-blocking antibodies have been reported to reverse human Treg cell in vitro anergy through an integrated transcriptional response, leading to proliferation, metabolic activation, and redox homeostasis.
Similarly, metformin was shown to increase Treg cells, thus alleviating disease symptoms in patients with concomitant MS and metabolic syndrome; 73 , similarly, methotrexate, which blocks the folate pathway, was suggested to restore Treg cell immune suppressive function, via AMPK activation, while other CRMs may lead to an enhancement of Treg cell function through the induction of autophagy acetyltransferase modulators such as curcumin and resveratrol.
Indeed, it has been shown that pro-autophagic protein AMBRA1 controls Treg cell differentiation and maintenance and leptin itself is able to modulate the autophagic process, thus linking metabolism and the immune system.
In summary, the skew towards Treg cell expansion may be a fundamental mechanism by which CR and its mimetics are able to restore metabolic homeostasis.
The immune system is believed to be a mediator of CR effects on cancer progression. Short-term fasting, as well as the CRM hydroxycitrate, is described to improve the efficacy of chemotherapy, in a process that involves autophagy and T cell response.
Pericellular ATP attracts dendritic cells and T cells to the tumour site, stimulating immune-mediated cell death. More specifically, the administration of CRMs in combination with chemotherapy boosts a T cell—mediated immune response against the tumour, reducing at the same time the intratumoural infiltration of Treg cells, which dampen anticancer immunity.
Although tumour-infiltrating lymphocytes TILs often undergo exhaustion in situ , a subset of TILs harbours stem-cell like properties and is capable of self-renewal, expansion, and multipotency, leading to tumour clearance.
Mechanistically, CR profoundly impacts on chromatin structure of TILs by reducing both histone acetylation responsible for transcriptional activation at effector and exhaustion loci and trimethylation of Lys27 on histone H3 H3K27me3 responsible for transcriptional inhibition at regulatory loci of stemness markers, resulting in limited effector program and preserved stemness of TILs.
Taken together, these observations suggest that CR can improve T cell—based immunotherapy for cancer, by promoting intratumoural infiltration of cytotoxic T cells but not of regulatory T cells and by epigenetically defining TIL function and phenotype towards an enhanced multipotency and tumour clearance.
The collection of microorganisms that coexist with the host is called microbiota. The microbial ecosystem consists of a large number of commensal beneficial bacteria, which live in symbiosis within the host and are susceptible to both exogenous and endogenous modifications.
This diet shapes the murine gut microbiota towards a colitogenic profile, characterized by increased abundance of Proteobacteria , Bacteroidetes , and E.
coli and decreased production of SCFA by intestinal microbiota, accounting for the altered Treg cell frequency in mesenteric lymph nodes. In line with this evidence, a recent paper has shown that microbiota remodelling stands as the major mechanism by which CR stimulates development of functional beige fat, improves insulin sensitivity and glucose tolerance, and lowers fat gain.
Several studies have revealed that also IF can have surprising effects on the composition of the gut microbiota with a particular enrichment of the Bacteroidaceae , Lactobacillaceae , and Prevotellaceae families, associated with increased production of SCFA, such as butyrate, which sustain differentiation and induction of Treg cells, thus affecting the balance between pro- and anti-inflammatory mechanisms.
In the context of autoimmune diseases, recent experimental evidence has shown that the capability of IF to ameliorate EAE clinical course and pathology is also mediated by the modulation of the gut microbiota composition; specifically, the authors of the study have demonstrated that IF led to increased gut bacteria richness Lactobacillaceae , Bacteroidaceae , and Prevotellaceae families and altered T cell balance in the gut, reducing IL—producing T cell response and driving the immune response towards a regulatory phenotype.
Taken together, these data suggest that dietary restriction can lead to the enrichment of beneficial bacteria, in turn promoting a more balanced immune response. Therefore, the manipulation of the gut microbiome may be used as a proxy of CR to prevent or ameliorate systemic inflammation.
The above-described mechanisms which may drive the beneficial anti-inflammatory action of the nutritional approaches are schematically summarized in Figure 2. CR-mediated modulation of immune system and its impact on cardiovascular health. Schematic representation of the mechanisms through which CR can modulate immune system function, by remodelling bone marrow, adipose tissue, and gut microbiota.
During CR, glucocorticoids are increased in the blood, inducing CXCR4 expression by memory T cells. At the same time, inside the BM, this regimen increases adipocytes, reduces glucocorticoid levels, and increases the production of chemokine CXCL12 CXCR4-ligand , thus driving T cell migration from the periphery to the BM niche.
Furthermore, the reduced CCL2 levels in the blood, upon CR, inhibit monocyte egress from BM, reducing circulating monocyte fraction. CR contributes to adipose tissue remodelling, by modulating the phenotype of AT-resident immune cells towards an anti-inflammatory profile.
Specifically, this dietary regimen decreases pro-inflammatory T cell abundance, sustains macrophage polarization towards an M2-like phenotype, and increases eosinophils, with consequent production of anti-inflammatory cytokines. Finally, CR modifies gut microbiota composition, favouring an enrichment of Bacteroidaceae , Lactobacillaceae , and Prevotellaceae strains that produce metabolites such as short-chain fatty acids; SCFA , able to modulate immunological functions restoring the immune balance, by sustaining differentiation and induction of Treg cells, at the expense of pro-inflammatory Tconv cells.
Taken together, all these phenomena contribute to reducing the systemic inflammatory state with beneficial effects on the function and health of the cardiovascular system. Nutritional interventions, including dietary restriction or intermittent fasting which do not induce malnutrition , can profoundly remodel immune cell niches such as bone marrow and adipose tissue, minimizing energy expenditure and favouring a lower inflammatory state in physiological conditions, on the one hand, and preserving the energy storage of immune cells for an optimized response to non—self -antigens, on the other.
These mechanisms hamper off-target harmful effects of autoimmunity and allergy, redirecting the immune system towards physiological responses against pathogens and cancer. Moreover, the nutritional intervention is able to induce a transcriptional regulation in immune cells with significant effects both on cellular and whole-body regulatory mechanisms, ameliorating obesity and metabolic disorders by re-establishing glucose and lipid metabolism.
Low caloric intake displays a wide-ranging effect on immunity not only by directly activating metabolic sensors but also by inducing a modification of gut microbiota composition that is known to be intimately linked with the immune system.
Further investigations are necessary to better characterize the mechanism of action of dietary restriction—mediated benefits, evaluating also the long-term effects of CR regimens in health and pathological conditions.
Notably, the identification of molecular pathways and networks selectively modulated by CR led to the discovery of CRMs, which provide the benefits of caloric restriction without the need to reduce caloric intake; thus, their further clinical characterization is warranted in the next future.
Moreover, patient-customized type, duration, and timing of nutritional therapies will need to be developed in order to optimize the nutritional intervention through a personalized approach.
Christ A , Lauterbach M , Latz E. Western diet and the immune system: an inflammatory connection. Immunity ; 51 : — Google Scholar. Mohebbi I , Shateri K , Seyedmohammadzad M.
The relationship between working schedule patterns and the markers of the metabolic syndrome: comparison of shift workers with day workers. Int J Occup Med Environ Health ; 25 : — Kökten T , Hansmannel F , Ndiaye NC , Heba AC , Quilliot D , Dreumont N , Arnone D , Peyrin-Biroulet L.
Calorie restriction as a new treatment of inflammatory diseases. Adv Nutr ; 12 : — Picca A , Pesce V , Lezza AMS. Does eating less make you live longer and better? An update on calorie restriction. Clin Interv Aging ; 12 : — Varady KA , Hellerstein MK. Alternate-day fasting and chronic disease prevention: a review of human and animal trials.
Sanna V , Di Giacomo A , La Cava A , Lechler RI , Fontana S , Zappacosta S , Matarese G. Leptin surge precedes onset of autoimmune encephalomyelitis and correlates with development of pathogenic T cell responses. J Clin Invest ; : — Jordan S , Tung N , Casanova-Acebes M , Chang C , Cantoni C , Zhang D , Wirtz TH , Naik S , Rose SA , Brocker CN , Gainullina A , Hornburg D , Horng S , Maier BB , Cravedi P , LeRoith D , Gonzalez FJ , Meissner F , Ochando J , Rahman A , Chipuk JE , Artyomov MN , Frenette PS , Piccio L , Berres ML , Gallagher EJ , Merad M.
Dietary intake regulates the circulating inflammatory monocyte pool. Cell ; : — Piccio L , Stark JL , Cross AH. Chronic calorie restriction attenuates experimental autoimmune encephalomyelitis. J Leukoc Biol ; 84 : — Fontana L , Ghezzi L , Cross AH , Piccio L.
Effects of dietary restriction on neuroinflammation in neurodegenerative diseases. J Exp Med ; : e Esquifino AI , Cano P , Jiménez V , Cutrera RA , Cardinali DP. Experimental allergic encephalomyelitis in male Lewis rats subjected to calorie restriction. J Physiol Biochem ; 60 : — Esquifino AI , Cano P , Jimenez-Ortega V , Fernández-Mateos MP , Cardinali DP.
Immune response after experimental allergic encephalomyelitis in rats subjected to calorie restriction. J Neuroinflammation ; 4 : 6. Inflammation-mediated memory dysfunction and effects of a ketogenic diet in a murine model of multiple sclerosis.
PLoS One ; 7 : e Goldberg EL , Shchukina I , Asher JL , Sidorov S , Artyomov MN , Dixit VD. Ketogenesis activates metabolically protective γδ T cells in visceral adipose tissue. Nat Metab ; 2 : 50 — Kafami L , Raza M , Razavi A , Mirshafiey A , Movahedian M , Khorramizadeh MR.
Avicenna J Med Biotechnol ; 2 : 47 — Choi IY , Piccio L , Childress P , Bollman B , Ghosh A , Brandhorst S , Suarez J , Michalsen A , Cross AH , Morgan TE , Wei M , Paul F , Bock M , Longo VD.
A diet mimicking fasting promotes regeneration and reduces autoimmunity and multiple sclerosis symptoms. Cell Rep ; 15 : — Anson RM , Guo Z , de Cabo R , Iyun T , Rios M , Hagepanos A , Ingram DK , Lane MA , Mattson MP.
Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci U S A ; : — Ugochukwu NH , Figgers CL. Caloric restriction inhibits up-regulation of inflammatory cytokines and TNF-alpha, and activates IL and haptoglobin in the plasma of streptozotocin-induced diabetic rats.
J Nutr Biochem ; 18 : — Fitzgerald KC , Bhargava P , Smith MD , Vizthum D , Henry-Barron B , Kornberg MD , Cassard SD , Kapogiannis D , Sullivan P , Baer DJ , Calabresi PA , Mowry EM. Intermittent calorie restriction alters T cell subsets and metabolic markers in people with multiple sclerosis.
EBioMedicine ; 82 : Fraser DA , Thoen J , Reseland JE , Førre O , Kjeldsen-Kragh J. Clin Rheumatol ; 18 : — Kjeldsen-Kragh J , Haugen M , Borchgrevink CF , Laerum E , Eek M , Mowinkel P , Hovi K , Førre O.
Controlled trial of fasting and one-year vegetarian diet in rheumatoid arthritis. Lancet ; : — Di Daniele N , Marrone G , Di Lauro M , Di Daniele F , Palazzetti D , Guerriero C , Noce A.
Effects of caloric restriction diet on arterial hypertension and endothelial dysfunction. Nutrients ; 13 : Sohal RS , Weindruch R.
Oxidative stress, caloric restriction, and aging. Science ; : 59 — Pamplona R , Portero-Otin M , Requena J , Gredilla R , Barja G.
Oxidative, glycoxidative and lipoxidative damage to rat heart mitochondrial proteins is lower after 4 months of caloric restriction than in age-matched controls.
Mech Ageing Dev ; : — Johnson ML , Distelmaier K , Lanza IR , Irving BA , Robinson MM , Konopka AR , Shulman GI , Nair KS. Mechanism by which caloric restriction improves insulin sensitivity in sedentary obese adults. Diabetes ; 65 : 74 — Brandhorst S , Longo VD.
Dietary restrictions and nutrition in the prevention and treatment of cardiovascular disease. Circ Res ; : — Spaulding CC , Walford RL , Effros RB. Calorie restriction inhibits the age-related dysregulation of the cytokines TNF-alpha and IL-6 in C3B10RF1 mice.
Mech Ageing Dev ; 93 : 87 — Faitg J , Leduc-Gaudet JP , Reynaud O , Ferland G , Gaudreau P , Gouspillou G. Effects of aging and caloric restriction on fiber type composition, mitochondrial morphology and dynamics in rat oxidative and glycolytic muscles.
Front Physiol ; 10 : Qiu X , Brown K , Hirschey MD , Verdin E , Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation.
Cell Metab ; 12 : — Food with calorie restriction reduces the development of atherosclerosis in apoE-deficient mice. Biochem Biophys Res Commun ; : — Fontana L , Meyer TE , Klein S , Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans.
Young JB , Mullen D , Landsberg L. Caloric restriction lowers blood pressure in the spontaneously hypertensive rat. Metabolism ; 27 : — VanNess JM , Casto RM , DeMaria JE , Overton JM. Food restriction attenuates the blood pressure response to paraventricular hypothalamic nuclei lesions in aortic coarctation hypertension.
Brain Res ; : — Apfelbaum M. Adaptation to changes in caloric intake. Prog Food Nutr Sci ; 2 : — Young JB , Landsberg L. Suppression of sympathetic nervous system during fasting. Science ; : — Castello L , Froio T , Maina M , Cavallini G , Biasi F , Leonarduzzi G , Donati A , Bergamini E , Poli G , Chiarpotto E.
Alternate-day fasting protects the rat heart against age-induced inflammation and fibrosis by inhibiting oxidative damage and NF-kB activation. Free Radic Biol Med ; 48 : 47 — Okoshi K , Cezar MDM , Polin MAM , Paladino JR Jr , Martinez PF , Oliveira SA Jr , Lima ARR , Damatto RL , Paiva SAR , Zornoff LAM , Okoshi MP.
Influence of intermittent fasting on myocardial infarction-induced cardiac remodeling. BMC Cardiovasc Disord ; 19 : Ahmet I , Wan R , Mattson MP , Lakatta EG , Talan M. Cardioprotection by intermittent fasting in rats.
Circulation ; : — Fontana L , Villareal DT , Weiss EP , Racette SB , Steger-May K , Klein S , Holloszy JO. Calorie restriction or exercise: effects on coronary heart disease risk factors. A randomized, controlled trial.
Am J Physiol Endocrinol Metab ; : E — E Most J , Gilmore LA , Smith SR , Han H , Ravussin E , Redman LM. Significant improvement in cardiometabolic health in healthy nonobese individuals during caloric restriction-induced weight loss and weight loss maintenance.
Tofler GH , Muller JE , Stone PH , Willich SN , Davis VG , Poole WK , Braunwald E. Factors leading to shorter survival after acute myocardial infarction in patients ages 65 to 75 years compared with younger patients.
Am J Cardiol ; 62 : — Tani M , Suganuma Y , Hasegawa H , Shinmura K , Hayashi Y , Guo X , Nakamura Y. Changes in ischemic tolerance and effects of ischemic preconditioning in middle-aged rat hearts.
Circulation ; 95 : — Shinmura K , Tamaki K , Bolli R. J Mol Cell Cardiol ; 39 : — Opie LH. Metabolic syndrome. Circulation ; : e32 — e Bajaj M , Suraamornkul S , Romanelli A , Cline GW , Mandarino LJ , Shulman GI , DeFronzo RA.
Effect of a sustained reduction in plasma free fatty acid concentration on intramuscular long-chain fatty acyl-CoAs and insulin action in type 2 diabetic patients. Diabetes ; 54 : — Sakamoto S , Minami K , Niwa Y , Ohnaka M , Nakaya Y , Mizuno A , Kuwajima M , Shima K. Effect of exercise training and food restriction on endothelium-dependent relaxation in the Otsuka Long-Evans Tokushima fatty rat, a model of spontaneous NIDDM.
Diabetes ; 47 : 82 — Harder H , Dinesen B , Astrup A. The effect of a rapid weight loss on lipid profile and glycemic control in obese type 2 diabetic patients.
Int J Obes Relat Metab Disord ; 28 : — Hammer S , Snel M , Lamb HJ , Jazet IM , van der Meer RW , Pijl H , Meinders EA , Romijn JA , de Roos A , Smit JW. Prolonged caloric restriction in obese patients with type 2 diabetes mellitus decreases myocardial triglyceride content and improves myocardial function.
J Am Coll Cardiol ; 52 : — Wilkinson MJ , Manoogian ENC , Zadourian A , Lo H , Fakhouri S , Shoghi A , Wang X , Fleischer JG , Navlakha S , Panda S , Taub PR.
Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab ; 31 : 92 — Sutton EF , Beyl R , Early KS , Cefalu WT , Ravussin E , Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes.
Cell Metab ; 27 : — Maroofi M , Nasrollahzadeh J. Effect of intermittent versus continuous calorie restriction on body weight and cardiometabolic risk markers in subjects with overweight or obesity and mild-to-moderate hypertriglyceridemia: a randomized trial.
Lipids Health Dis ; 19 : Kraus WE , Bhapkar M , Hufman KM , Pieper CF , Krupa Das S , Redman LM , Villareal DT , Rochon J , Roberts SB , Ravussin E , Holloszy JO , Fontana L ; CALERIE Investigators. Lancet Diabetes Endocrinol ; 7 : — Brandhorst S , Choi IY , Wei M , Cheng CW , Sedrakyan S , Navarrete G , Dubeau L , Yap LP , Park R , Vinciguerra M , Di Biase S , Mirzaei H , Mirisola MG , Childress P , Ji L , Groshen S , Penna F , Odetti P , Perin L , Conti PS , Ikeno Y , Kennedy BK , Cohen P , Morgan TE , Dorff TB , Longo VD.
A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan.
Cell Metab ; 22 : 86 — Collins N , Han SJ , Enamorado M , Link VM , Huang B , Moseman EA , Kishton RJ , Shannon JP , Dixit D , Schwab SR , Hickman HD , Restifo NP , McGavern DB , Schwartzberg PL , Belkaid Y. The bone marrow protects and optimizes immunological memory during dietary restriction.
Palma C , La Rocca C , Gigantino V , Aquino G , Piccaro G , Di Silvestre D , Brambilla F , Rossi R , Bonacina F , Lepore MT , Audano M , Mitro N , Botti G , Bruzzaniti S , Fusco C , Procaccini C , De Rosa V , Galgani M , Alviggi C , Puca A , Grassi F , Rezzonico-Jost T , Norata GD , Mauri P , Netea MG , de Candia P , Matarese G.
Caloric restriction promotes immunometabolic reprogramming leading to protection from tuberculosis. Cell Metab ; 33 : — Ryu S , Shchukina I , Youm YH , Qing H , Hilliard B , Dlugos T , Zhang X , Yasumoto Y , Booth CJ , Fernández-Hernando C , Suárez Y , Khanna K , Horvath TL , Dietrich MO , Artyomov M , Wang A , Dixit VD.
Ketogenic diet restrains aging-induced exacerbation of coronavirus infection in mice. Elife ; 10 : e Mejia P , Treviño-Villarreal JH , Hine C , Harputlugil E , Lang S , Calay E , Rogers R , Wirth D , Duraisingh MT , Mitchell JR. Dietary restriction protects against experimental cerebral malaria via leptin modulation and T-cell mTORC1 suppression.
Nat Commun ; 6 : Starr ME , Steele AM , Cohen DA , Saito H. Crit Care Med ; 44 : e — e Bartley JM , Zhou X , Kuchel GA , Weinstock GM , Haynes L. Impact of age, caloric restriction, and influenza infection on mouse gut microbiome: an exploratory study of the role of age-related microbiome changes on influenza responses.
Front Immunol ; 8 : Trujillo-Ferrara J , Campos-Rodríguez R , Lara-Padilla E , Ramírez-Rosales D , Correa Basurto J , Miliar Garcia A , Reyna Garfias H , Zamorano Ulloa R , Rosales-Hernández MC. Caloric restriction increases free radicals and inducible nitric oxide synthase expression in mice infected with Salmonella typhimurium.
Biosci Rep ; 31 : — Goldberg EL , Smithey MJ , Lutes LK , Uhrlaub JL , Nikolich-Žugich J. Immune memory-boosting dose of rapamycin impairs macrophage vesicle acidification and curtails glycolysis in effector CD8 cells, impairing defense against acute infections.
J Immunol ; : — Goldberg EL , Romero-Aleshire MJ , Renkema KR , Ventevogel MS , Chew WM , Uhrlaub JL , Smithey MJ , Limesand KH , Sempowski GD , Brooks HL , Nikolich-Žugich J.
Lifespan-extending caloric restriction or mTOR inhibition impair adaptive immunity of old mice by distinct mechanisms. Aging Cell ; 14 : — Rusu E , Jinga M , Enache G , Rusu F , Dragomir AD , Ancuta I , Draguţ R , Parpala C , Nan R , Sima I , Ateia S , Stoica V , Cheţa DM , Radulian G.
Effects of lifestyle changes including specific dietary intervention and physical activity in the management of patients with chronic hepatitis C—a randomized trial. Nutr J ; 12 : Madeo F , Pietrocola F , Eisenberg T , Kroemer G. Caloric restriction mimetics: towards a molecular definition.
Nat Rev Drug Discov ; 13 : — Lisi L , Navarra P , Cirocchi R , Sharp A , Stigliano E , Feinstein DL , Dello Russo C. Rapamycin reduces clinical signs and neuropathic pain in a chronic model of experimental autoimmune encephalomyelitis.
J Neuroimmunol ; : 43 — Neurosci Lett ; : 39 — Esposito M , Ruffini F , Bellone M , Gagliani N , Battaglia M , Martino G , Furlan R. Rapamycin inhibits relapsing experimental autoimmune encephalomyelitis by both effector and regulatory T cells modulation.
J Neuroimmunol ; : 52 — Sciarretta S , Forte M , Castoldi F , Frati G , Versaci F , Sadoshima J , Kroemer G , Maiuri MC. Caloric restriction mimetics for the treatment of cardiovascular diseases. Cardiovasc Res ; : — Bao Y , Ledderose C , Graf AF , Brix B , Birsak T , Lee A , Zhang J , Junger WG.
mTOR and differential activation of mitochondria orchestrate neutrophil chemotaxis. J Cell Biol ; : — Vitiello D , Neagoe PE , Sirois MG , White M. Effect of everolimus on the immunomodulation of the human neutrophil inflammatory response and activation. Cell Mol Immunol ; 12 : 40 — Porsch F , Mallat Z , Binder CJ.
Humoral immunity in atherosclerosis and myocardial infarction: from B cells to antibodies. Nath N , Khan M , Paintlia MK , Singh I , Hoda MN , Giri S.
Metformin attenuated the autoimmune disease of the central nervous system in animal models of multiple sclerosis. Metformin ameliorates the development of experimental autoimmune encephalomyelitis by regulating T helper 17 and regulatory T cells in mice.
J Neuroimmunol ; : 58 — Negrotto L , Farez MF , Correale J. Immunologic effects of metformin and pioglitazone treatment on metabolic syndrome and multiple sclerosis.
JAMA Neurol ; 73 : — Bharath LP , Agrawal M , McCambridge G , Nicholas DA , Hasturk H , Liu J , Jiang K , Liu R , Guo Z , Deeney J , Apovian CM , Snyder-Cappione J , Hawk GS , Fleeman RM , Pihl RMF , Thompson K , Belkina AC , Cui L , Proctor EA , Kern PA , Nikolajczyk BS.
Metformin enhances autophagy and normalizes mitochondrial function to alleviate aging-associated inflammation. Cell Metab ; 32 : 44 — Cipolletta D , Feuerer M , Li A , Kamei N , Lee J , Shoelson SE , Benoist C , Mathis D.
PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature ; : — Simental-Mendía LE , Pirro M , Gotto AM Jr , Banach M , Atkin SL , Majeed M , Sahebkar A.
Lipid-modifying activity of curcuminoids: a systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr ; 59 : — Tabrizi R , Tamtaji OR , Mirhosseini N , Lankarani KB , Akbari M , Heydari ST , Dadgostar E , Asemi Z.
The effects of quercetin supplementation on lipid profiles and inflammatory markers among patients with metabolic syndrome and related disorders: a systematic review and meta-analysis of randomized controlled trials.
Crit Rev Food Sci Nutr ; 60 : — Abdellatif M , Trummer-Herbst V , Koser F , Durand S , Adão R , Vasques-Nóvoa F , Freundt JK , Voglhuber J , Pricolo MR , Kasa M , Türk C , Aprahamian F , Herrero-Galán E , Hofer SJ , Pendl T , Rech L , Kargl J , Anto-Michel N , Ljubojevic-Holzer S , Schipke J , Brandenberger C , Auer M , Schreiber R , Koyani CN , Heinemann A , Zirlik A , Schmidt A , von Lewinski D , Scherr D , Rainer PP , von Maltzahn J , Mühlfeld C , Krüger M , Frank S , Madeo F , Eisenberg T , Prokesch A , Leite-Moreira AF , Lourenço AP , Alegre-Cebollada J , Kiechl S , Linke WA , Kroemer G , Sedej S.
Nicotinamide for the treatment of heart failure with preserved ejection fraction. Sci Transl Med ; 13 : eabd Wan R , Camandola S , Mattson MP. Intermittent fasting and dietary supplementation with 2-deoxy-D-glucose improve functional and metabolic cardiovascular risk factors in rats.
FASEB J ; 17 : — Hawley SA , Fullerton MD , Ross FA , Schertzer JD , Chevtzoff C , Walker KJ , Peggie MW , Zibrova D , Green KA , Mustard KJ , Kemp BE , Sakamoto K , Steinberg GR , Hardie DG. The ancient drug salicylate directly activates AMP-activated protein kinase.
Mol Med Rep ; 15 : — Stumvoll M , Nurjhan N , Perriello G , Dailey G , Gerich JE. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N Engl J Med ; : — Madeo F , Carmona-Gutierrez D , Hofer SJ , Kroemer G. Caloric restriction mimetics against age-associated disease: targets, mechanisms, and therapeutic potential.
Cell Metab ; 29 : — Katsyuba E , Romani M , Hofer D , Auwerx J. Nat Metab ; 2 : 9 — Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Lips MA , van Klinken JB , Pijl H , Janssen I , van Dijk K W , Koning F , van Harmelen V.
Weight loss induced by very low calorie diet is associated with a more beneficial systemic inflammatory profile than by Roux-en-Y gastric bypass. Metabolism ; 65 : —
Calorie restriction CR has destriction as a Enhanced respiratory fitness treatment to prevent cardiovascular prebention Caloric restriction and disease prevention. This article reviews recent progress regarding retriction role of CR in CVD Liver protection supplements restrictoon reduction of cardiometabolic risk prevvention and promoting atherosclerotic stability. Calorie restriction may be an approach to reduce the development of atherosclerosis. CR promotes eNOS activity and SIRT1 expression which in turn improves vasodilation resulting in greater regulation of blood pressure and blood flow. Modest CR in nonobese young and middle-aged adults results in improved cardiometabolic risk profile. The evidence for CR in CVD prevention has accumulated in the recent years.
Ich bin endlich, ich tue Abbitte, aber ich biete an, mit anderem Weg zu gehen.
Sie lassen den Fehler zu. Geben Sie wir werden es besprechen. Schreiben Sie mir in PM, wir werden reden.