Video
B is for Basic Brain Metabolism - Neuroscience ABCsEnergy metabolism and brain health -
Reprints and permissions. Magistretti, P. In: Pfaff, D. eds Neuroscience in the 21st Century. Springer, New York, NY. Publisher Name : Springer, New York, NY.
Print ISBN : Online ISBN : eBook Packages : Biomedical and Life Sciences Reference Module Biomedical and Life Sciences. Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative.
Policies and ethics. Skip to main content. Abstract All the processes described in this textbook require energy. Keywords Positron Emission Tomography Pentose Phosphate Pathway Glucose Utilization Ketone Body Energy Substrate These keywords were added by machine and not by the authors. Buying options Chapter EUR eBook EUR 1, Tax calculation will be finalised at checkout Purchases are for personal use only Learn about institutional subscriptions.
Further Reading Bélanger M, Allaman I, Magistretti PJ Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab — Article PubMed Google Scholar Figley CR, Stroman PW The role s of astrocytes and astrocyte activity in neurometabolism, neurovascular coupling, and the production of functional neuroimaging signals.
Eur J Neurosci — Article PubMed Google Scholar Frackowiak RSJ, Magistretti PJ, Shulman RG, Adams M Neuroenergetics: relevance for functional brain imaging.
HFSP, Strasbourg Google Scholar Gladden LB Lactate metabolism: a new paradigm for the third millennium. J Physiol —30 Article PubMed CAS Google Scholar Magistretti PJ Neuron-glia metabolic coupling and plasticity.
J Exp Biol — Article PubMed CAS Google Scholar Magistretti PJ Brain energy metabolism. J Neural Transm —85 Article PubMed CAS Google Scholar Pellerin L, Magistretti PJ Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization.
Proc Natl Acad Sci USA — Article PubMed CAS Google Scholar Raichle ME, Mintun MA Brain work and brain imaging. Annu Rev Neurosci — Article PubMed CAS Google Scholar Schurr A Lactate: the ultimate cerebral oxidative energy substrate? J Cereb Blood Flow Metab — Article PubMed CAS Google Scholar Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM Astrocyte-neuron lactate transport is required for long-term memory formation.
Cell 5 — Article PubMed CAS Google Scholar Download references. Author information Authors and Affiliations Laboratory of Neuroenergetics and Cellular Dynamics, Brain Mind Institute, Ecole Polytechnique Fédérale de Lausanne EPFL , , Lausanne, Switzerland Prof.
Igor Allaman Department of Psychiatry—CHUV, Center for Psychiatric Neuroscience, , Prilly—Lausanne, Switzerland Prof. In the astrocyte-neuron lactate shuttle ANLS hypothesis, proposed by Pellerin and Magistretti , a secondary effect of astrocytic glutamate uptake prompts a switch from oxidative metabolism to aerobic glycolysis in astrocytes causing glucose metabolism to be diverted from the tricaboxcylic acid TCA cycle to the glycolytic pathway and lactate production.
This adaptation seems to support an increased neuronal metabolic load with lactate generated from astrocytic glycolysis being utilized as a substrate for oxidative metabolism in neurons.
This hypothesis is supported by numerous studies detecting increased lactate in regions of brain activity as well as evidence that lactate is crucial for synaptic transmission in rat hippocampal slices and sufficient to support synaptic activity in the absence of glucose Figure 1e ; Schurr et al.
This segregated metabolism is supported by distinct gene expression patterns observed in neurons and astrocytes. Differential expression of lactate transporter proteins, monocarboxylate transporters MCTs , supports shuttling of lactate from astrocytes to neurons.
The lactate efflux transporter MCT4 is expressed primarily in astrocytes while MCT2, an isoform that allows for rapid substrate uptake of lactate, is primarily expressed in neurons Debernardi et al. Additionally, the lactate dehydrogenase LDH isoenzyme, LDH-5, which promotes conversion of pyruvate to lactate is highly expressed in astrocytes but not in neurons while LDH-1, which promotes pyruvate production is found in both neurons and astrocytes Bittar et al.
In support of glycolysis induction in astrocytes, the pyruvate dehydrogenase kinase-4 PDK4 is expressed at high levels in astrocytes causing its target, pyruvate dehydrogenase PDH , to remain in an inactive, phosphorylated state thereby decreasing pyruvate entry into the TCA cycle Halim et al. Correspondingly, astrocytes express higher levels of the glyoxalase enzymes Glo-1 and Glo-2 that detoxify methyglycoxal, a metabolic by-product of glycolysis Belanger et al.
While there is substantial evidence in support for the ANLS acting as a mechanism for coupling of neuronal activity to neuronal metabolism, contradictory evidence continues the debate of this hypothesis.
Glucose uptake and phosphorylation has been shown to preferentially occur in neurons, not astrocytes. Further, neurons metabolize substantial amounts of glucose and increase glucose metabolism in response to activity Patel et al. This contradictory evidence may be due to metabolism being differentially regulated within different neural networks or brain regions.
These observations all contribute, however, to mounting evidence suggesting that neurons can sustain and enhance oxidative metabolism to meet energetic requirements during periods of activity.
While there is significant evidence to support enhanced neuronal oxidative metabolism during activity, what remains unclear is what is happens to cellular oxygen concentration following activation. This is partly due to difficulties in recording oxygen concentration as well as from confounds in interpreting oxygen consumption imaging signals.
Blood-oxygen-level dependent BOLD fMRI which relies on neurovascular coupling to measure regions of brain activity based on measurements of oxyhemeoglobin and deoxyhemeoglobin consistently generates signals with a post-stimulus undershoot van Zijl et al.
The physiological basis of the BOLD undershoot is heavily debated and is likely stimulus-dependent, one theory however suggests that the BOLD undershoot reflects an uncoupling of CBF and energy metabolism.
This is supported by evidence that oxidative metabolism remains elevated post activation after both blood flow and blood volume have returned to baseline Lu et al.
Consistent with this, numerous studies have reported similar increases in oxidative metabolism indicating that sustained focal activation raises the rate of oxidative metabolism to a new steady state level Hoge et al.
With dynamic changes in oxygen metabolism occurring during neuronal activity, dynamic changes are likely to be reflected in levels of oxygen concentration, potentially having secondary effects on protein function and gene expression. Neurons and neuronal functions are generally viewed as highly sensitive to hypoxia with disruption of oxygen supply to the brain causing detrimental damage within minutes.
Under physiological conditions, PtO 2 measurements in rat range from 6 mm Hg to 40 mm Hg within the cortex 6—16 mm Hg in white matter and 19—40 mm Hg in gray matter and from 1 mm Hg to 60 mm Hg across all brain regions with proximal structures displaying large variations in oxygen tension Erecińska and Silver, During embryonic development, oxygen tension is low in the fetal brain 0.
Within the developing brain, oxygen tension acts as a regulator of neurogenesis with low oxygen promoting progenitor expansion in cortical neurogenic regions and decreasing dopaminergic neurogenesis in the midbrain Wagenführ et al.
Additionally, in the adult brain, hypoxic injury caused by ischemic stroke triggers increased neuronal stem cell proliferation and neurogenesis Arvidsson et al.
This evidence supports a role for hypoxia as a regulatory mechanism in neuronal function and indicates that physiological hypoxia occurring in the adult brain may play a functional role.
HIF is a heterodimeric complex consisting of a constitutively expressed β subunit shared by a family of three oxygen-sensitive α subunits. Most widely studied among these is the HIF-1α subunit.
HIFα protein is constitutively expressed but is immediately targeted for degradation by HIF prolyl hydroxylases PHDs that associate with and hydroxylate two conserved HIFα proline residues in an oxygen dependent manner Bruick and McKnight, The Von Hippel-Lindau tumor suppressor ubiquitin ligase complex pVHL , subsequently recognizes HIFα causing HIFα ubiquitination and protein degradation Ivan et al.
During hypoxia, though oxygen-limited inactivation of HIF PHD activity, HIFα is no longer targeted by pVHL and is able to accumulate in the cytoplasm before translocating to the nucleus and acting to promote transcription Figure 2. Within the nervous system HIF-1α and target genes of HIF-1 are widely expressed under hypoxia, but regulation of HIF-1α can differ among neuronal subtypes Bergeron et al.
Following hypoxia, HIF-1α has been shown both in vitro and in vivo to be significantly upregulated in interneurons but not in pyramidal neurons and in neuronal and non-neuronal cells it has been established that the redox state of a cell contributes to HIF-1α regulation Welsh et al.
Additionally, during in C. elegans development, hypoxia has been shown to cause defects in axonal migration that occur in a neuronal cell-type specific manner and are dependent on stabilization of Hif-1 by either hypoxia or increased reactive oxygen species ROS; Pocock and Hobert, Being a primary source of reducing agents, glucose is a major contributor to the redox state of a cell and HIF-1α expression in neurons has been shown to increase in a glucose-dependent manner during hypoxia Shi and Liu, ; Guo et al.
There is also a negative relationship between HIF-1α and ROS levels indicating ROS promotes HIF-1α degradation while a reducing environment stabilizes HIF-1α Schafer and Buettner, ; Niecknig et al. Figure 2. Hypoxia inducible transcription factor regulation.
Under normal oxygen conditions hypoxia-inducible factor-1α HIF-1α is hydroxylated by prolyl hydroxylase PHD enzymes and targeted for ubiquitination by the Von Hippel-Lindau tumor suppresser ubiquitin ligase complex pVHL.
During hypoxia or low oxygen conditions, HIF-1α is stabilized, translocates to the nucleus and associates with HIF-β to promote gene expression, targeting genes containing a hypoxia response element HRE. Ub, ubiquitin; OH, hydroxyl group.
ROS are highly reactive free radical molecules that can cause cellular damage through oxidation of lipids, proteins and DNA. ROS production primarily occurs through electron leakage at electron transport chain ETC complexes I or III during normal oxidative respiration.
Within the brain, a high neuronal oxidative rate heightens the potential for ROS production and neurons are especially vulnerable to oxidative damage due to low levels of antioxidant enzymes such as glutathione GSH; Dringen et al. Neuronal diversion of glucose catabolism from glycolysis to the PPP through Pfkfb3 degradation therefore not only supports oxidative metabolism of lactate but also enhances neuronal antioxidant capacity through production of the reducing agent, NADH.
HIF-1α is also involved in this process and acts as a glycolytic enhancer through transcriptional activation of metabolic genes including Pfkfb3 and pyruvate dehydrogenase kinase-1 PDK1 , both positive regulators of glycolysis and the lactate efflux transporter, MCT4 Figure 2 ; Minchenko et al.
As an oxygen-sensitive molecule, which is highly integrated into metabolic processes, HIF-1α is likely to have an important role in brain plasticity, and dysregulation of HIF-1α expression has already been implicated in neuronal activation and learning and memory. In a rat microarray study, seizures induced by injection of Kainate, a potent glutamate-receptor agonist that causes overstimulation of neurons, resulted in a 2.
In another microarray study HIF-1α was found to be increased 7-fold in mice following environmental enrichment, where mice are exposed to heightened sensory stimulation known to promote neurogenesis and improve performance in memory tasks Rampon et al. These data support a significant role for hypoxia in neuronal activity, potentially though neurovascular uncoupling and enhanced neuronal oxidative metabolism depleting neuronal oxygen levels.
Neurodegenerative disorders encompass a range of conditions characterized by progressive neuronal damage and degeneration as well as neuronal cell death.
Although neurodegenerative disorders vary in the neuronal populations and cognitive or motor functions affected, metabolic dysfunction is a unifying pathology underlying many of these disorders. AD principally affects short-term working memory and is classified by the presence of two hallmark neuropathologies; extracellular amyloid plaques, formed from aggregation of amyloid Aβ peptide, and intraneuronal neurofibrillary tangles formed from aggregation of hyperphosphorylated tau.
In AD patients, regional hypometabolism in the brain is a predictor for progressive cognitive decline and reduced cerebral metabolism is associated with carriers of the AD risk allele of the APOE-4 gene Small et al.
At the cellular level, mitochondria MC isolated from AD patients display reduced enzymatic activity of the ETC complex IV cytochrome C oxidase; Parker et al. Similarly, in mouse models of AD, oxidative respiration is diminished and Aβ is found to localize and progressively accumulate in neuronal MC Mucke et al.
This progressive accumulation of Aβ in MC is associated with reduced oxidative respiration and reduced activity of the rate-limiting TCA cycle enzyme, α-ketoglutarate dehydrogenase complex KGDHC , and the pyruvate dehydrogenase complex PDHC , which generates acetyl-CoA for entry into the TCA cycle Casley et al.
Both metabolic dysfunction and mitochondrial Aβ accumulation appear to occur early in disease progression, preceding the onset of extracellular plaque formation Wirths et al. This indicates that early metabolic dysfunction is a key process in AD progression and a potential target for therapeutic intervention.
Also preceding extracellular plaque formation in the AD brain significantly increased ROS production and oxidative stress. Substantially increased ROS activity and oxidative damage is consistently detected in AD patients by various measures Hensley et al.
Increased oxidative stress occurs early in disease progression being observed in patients with mild AD as well as in cases of mild cognitive impairment, at high-risk of developing AD Baldeiras et al. The pathological Aβ is also known to be a source of ROS production and a cause of neuronal oxidative damage in AD Behl et al.
Related to oxidative stress, and also implicated in AD pathology, is dysregulated homeostasis of redox transition metal ions including zinc, copper and iron Schrag et al. Both elevation and deficiency of zinc is associated with AD and evidence suggests that altered compartmentalization of zinc rather than altered zinc levels may be the cause of zinc pathology in AD Suh et al.
This is supported by dysregulation of numerous zinc transporters in AD patient brains Lovell et al. Zinc has important roles in normal neuronal function and is co-released along with glutamate at the synapse Vogt et al.
A major role of zinc is its significant antioxidant capacity, such that zinc deficiency is linked to neuronal oxidative stress Aimo et al.
Like zinc, copper elevation and copper deficiency have both been associated with AD as well as co-localization of copper with Aβ plaques Miller et al. Copper is also modulated by synaptic activation in neurons and both zinc and copper are able to bind Aβ Schlief et al.
In AD pathology, copper enhances Aβ toxicity and copper:Aβ complexes are a source of ROS production and oxidative damage in neurons Dikalov et al. The redox active iron, although vital for cellular function, is also a pro-oxidant and promotes generation of highly reactive hydroxyl radicals from hydrogen peroxide.
Elevated levels of brain iron in the AD brain as well as iron association with Aβ plaques and neurofibrillary tangles have been detected in various studies Smith et al. Recently, elevated iron has been shown to predict AD progression and elevated iron was linked to the APOE-4 AD risk allele suggesting it may have a pathological role in AD Ayton et al.
Another common feature of AD that contributes to AD pathology is vascular dysfunction. Cerebrovascular disease, characterized by disrupted blood flow to the brain, significantly increases AD risk and occurs before Aβ accumulation and cognitive decline Arvanitakis et al.
In animal models, hypoperfusion also leads to symptoms similar to AD and exacerbates existing AD pathology Walsh et al. Vascular dysfunction contributes to the pathology of AD due to lower capillary density, meaning narrowed blood vessels and decreased CBF Hamel et al.
Diminished blood flow reduces metabolite and oxygen supply to the brain and potentially contributes to build-up of Aβ through impaired clearance of neurotoxic molecules Shibata et al.
Aβ itself is also thought to amplify deficits in CBF and glucose utilization in AD through impairing vasodilation and cerebrovascular autoregulatory mechanisms Niwa et al. Cerebrovascular dysfunction can lead to disrupted oxygen metabolism through hypoperfusion-hypoxia and hypoxia in-turn can enhance AD pathology by promoting tau phosphorylation as well as transcriptionally upregulating the HIF-1 target, β-site β-amyloid precursor protein cleavage enzyme 1 BACE1 that cleaves amyloid precursor protein APP to produce Aβ Figure 3 ; Sun et al.
Figure 3. Disrupted metabolic pathways in neurodegenerative diseases. Aβ accumulates in neuronal mitochondria MC early in disease progression and disrupts oxidative metabolism. Acetyl-CoA production and tricaboxcylic acid TCA cycle entry is decreased in AD through reduced activity of the pyruvate dehydrogenase complex PDHC.
In all three diseases, activity of α-ketoglutarate dehydrogenase complex KGDHC is reduced, reactive oxygen species ROS is increased and transglutaminase TG activity is increased.
TG increases α-synuclein aggregation and reduces oxidative respiration. Aside from rare cases of genetic mutations in familial AD, the major risk factor for developing AD is aging. PD is thought to be caused by both genetic and environmental factors and primarily impacts patient motor function.
PD involves the formation of protein aggregates consisting mainly of α-synuclein and affects the dopaminergic neurons of the midbrain substantia nigra. HD is an inherited neurodegenerative disorder caused by expanded CAG repeats in the Huntingtin HTT gene causing progressive neuronal degeneration and cell death throughout the brain, affecting mood, cognition and motor skills.
Inclusions are also found in the HD brain from aggregation of mutant HTT mHTT protein. Like AD, both PD and HD are associated with increased oxidative stress as well as decreased activity of the KGDHC enzyme Tabrizi et al.
Also, common to all three disorders is increased activity of transglutaminase TG; Johnson et al. TG catalyzes polyamination post-translational modifications of proteins, is known to be increased by ROS and also attenuates HIF-1 signaling Campisi et al. TG can decrease oxidative metabolism through modification of glycolytic enzymes and is known to cause oxidative stress in HD and aggregation of α-synuclein in PD Cooper et al.
Altered metal ion homeostasis may have a role in PD pathology as well with disrupted levels of both zinc and copper observed in PD patients Brewer et al. Similar to Aβ, copper also contributes to α-synuclein aggregation and can contribute to oxidative stress through the formation of reactive copper: α-synuclein complexes Wang et al.
α-synuclein is also know to exacerbate mitochondrial dysfunction in the presence of toxic oxidizing agents, with loss of α-synuclein in animal models conferring resistance to mitochondrial toxins Klivenyi et al.
Additionally, levels of α-synuclein are increased when oxidative metabolism is inhibited and animal models expressing mutant forms of α-synuclein exhibit neuronal mitochondrial degeneration and cell death Lee et al. In HD, increased oxidative damage to mitochondrial DNA is observed as well as higher frequencies of deletions in the mitochondrial genome and deficits in ETC function with decreased expression of complex II in the striatum and decreased activity of complex IV in striatal and cortical regions Horton et al.
Vascular deficits and disrupted blood flow is a major pathology of HD as well with altered blood vessel density and size found in cortical gray matter, putamen and striatal brain regions.
In HD patients, inclusions of mHTT are also detected in the basal membrane and epithelium of cortical blood vessels and in mouse models of the disease pericytic coverage of cortical and striatal blood vessels is decreased Drouin-Ouellet et al.
A number of the metabolic pathologies observed in neurodegenerative disorders are associated with normal aging and may explain the age-related manifestation of neurodegenerative disease phenotypes. While no longer thought to be directly causative of aging, free radicals and oxidative stress accumulate in the aging brain as in neurodegeneration Smith et al.
Mitochondrial function is also linked to aging due to the association of mitochondrial DNA mtDNA haplotypes with longevity and the generation of mtDNA mutator mice that have a premature aging phenotype Trifunovic et al.
It has also been shown there is an increased rate of damaging mutations in mtDNA of post-mitotic aging cells as opposed to aging mitotic cells Greaves et al.
While it has been suggested that the somatic rate of mtDNA mutation is unlikely to have a pathological affect due to redundancy in cell mitochondrial numbers, in post-mitotic neurons mtDNA mutation rates are significantly higher than average and, within the cortex, MC with large mtDNA deletions possess a replicative advantage during mitochondrial expansion Song et al.
Aside from AD and PD, deficiency of zinc is also associated with aging, being decreased in the general elderly population Pepersack et al. Diminished CBF occurs in normal aging as well with cortical perfusion found to decrease with age in healthy adults Chen et al.
An age-dependent reduction in perictyes also occurs in mice and is associated with microvascular changes and neurodegeneration Bell et al. Substantial evidence therefore exists supporting disrupted neuronal oxygen supply and oxidative metabolism as a major pathological component of age-related neurodegeneration.
Although it has been well established that metabolic regulation is critical to neuronal function and that metabolic dysfunction is a major pathology in diseases affecting behavior and cognition, there is little known regarding how regulators of metabolism may be involved in neuronal plasticity.
A number of studies, however, support a direct role for metabolic regulation and metabolically linked genes in influencing learning and memory. One of the best examples of this is exposure of hypoxia as a modulator of cognitive performance.
elegans , hypoxia acts as an enhancer of gustatory sensory perception through Hif-1 dependent induction of the neurotransmitter serotonin within specific sensory neurons Pocock and Hobert, In rodent models, exposure to hypobaric hypoxia in adult rats for periods of 7—21 days causes decline in spatial learning similar to aging and is associated with aging-related lipofuscin deposition and ultrastructural changes in MC.
Increasing duration of hypobaric hypoxic exposure also positively correlates with increasing expression of aging markers Biswal et al. Brief hypoxic exposure s in rats also causes synaptic arrest of pyramidal CA1 hippocampal neurons and deficits in spatial memory that are both reversed by blockade of receptors for Adenosine, an inhibitory neurotransmitter Sun et al.
In contrast, long-term facilitation of motor output in adult rats is enhanced by intermittent hypoxia 3 × 3 min intervals, separated by 5 min hyperoxia increasing both phrenic amplitude and burst frequency, which was not observed with a continuous hypoxia of the same cumulative duration Baker and Mitchell, Differing effects of hypoxia in brain plasticity are likely related to differing exposures as well as measurement of different outputs.
Interestingly, mild hypoxia preconditioning confers protection of cognitive abilities during subsequent exposure to severe hypoxia implicating a role for HIFs and transcriptional changes induced by mild hypoxia Rybnikova et al. Indeed, neuronal knockout of HIF-1α in mice impairs spatial memory and the stabilization of HIF improves hippocampal memory in fear conditioning Tomita et al.
Similar learning deficits and age-related changes are also observed in a D-galactose induced model of aging where oxidative injury was the major stimulus for aging Li et al. Altered expression of lactate metabolic enzymes and transporters is also related to stress induced improvements in cognitive function.
Psychological stress, while harmful under chronic conditions, has evolved to enhance cognitive function and improve reactions to stressful situations through hypothalamic activation of adrenergic receptors and hypothalamic-pituitary-adrenal axis glucocorticoid production Dong et al.
Improved cognitive function following short-term stress induction corresponds with β2AR-dependant increases in LDH A, MCT1 and MCT4 expression, the expression of which was modulated by β-arrestin-1 activation of HIF-1α, downstream of β2AR Dong et al.
Altered expression of ETC oxidative phosphorylation genes is also associated with altered behavior in the honeybee. In a study exploring molecular profiles in aggressive honeybee behavior, oxidative phosphorylation was most significantly enriched in association with increased aggression.
This was found to be true for aged bees that display increased aggressive behavior as well as following environmentally enhanced aggression by alarm pheromone exposure and genetic-related aggression occurring in the Africanized honeybee population Alaux et al. Consistent with this, inhibition of oxidative phosphorylation by treatment with drugs targeting the TCA cycle increased aggression of honeybees measured using an intruder assay Li-Byarlay et al.
In the same study, cell-type-specific knockdown of ETC complex genes using GAL4 drivers in Drosophila found that neuron-specific, but not glia-specific knockdown of the complex I gene NDlike, significantly increased aggressive lunging behavior in flies Li-Byarlay et al.
Also involved in learning and memory are non-coding miRNA genes which are regulated during neuronal activity by various mechanisms and able to regulate translation of various downstream target genes. A number of miRNAs have been associated with plasticity including the hypoxia-regulated, HIF-1 target, miR that is known to be involved in metabolic regulation.
miR is significantly upregulated 24 h after long-term memory formation in the honeybee using an olfactory conditioning paradigm. Upregulation of miR correlated with downregulation of a number of metabolically linked protein-coding genes including Gapdh2, Glucose dehydrogenase, Laccase2 and Aldose reductase-like.
Inhibition of miR by treatment of honeybees with miR antogmiR also resulted in reduced memory retention in the olfactory conditioning assay indicating a functional role in learning and memory Cristino et al. Considering the sensitivity of neurons and neural structures to hypoxia, Cristino et al. A follow-up study found that in a human-derived neuronal cell-line, miR targeted neurodegeneration-associated genes as well as other plasticity-related genes within the human transcriptome.
This included a number of oxidative metabolism genes, the AD risk-gene APOE as well as the NMDA-R, GRINA , and the human actin homolog, ACTB Watts et al. Another hypoxia-regulated miRNA, miRc, is also associated with modulating cognitive function in rats.
In a model of chronic cerebral hypoperfusion miRc was continuously inhibited, correlating with upregulation of its plasticity-related target gene, TRIM2. Hypoperfusion in this model was associated with deficits in spatial learning that were ameliorated by hippocampal overexpression of miRc Fang et al.
These studies all provide support to the hypothesis that metabolically regulated genes are directly involved in the regulation of neuronal plasticity.
While neurovascular coupling mechanisms appear to maintain steady-state oxygen levels in the brain, it is becoming evident that neurovascular uncoupling may in fact have a physiological role in regulating plasticity via oxygen depletion and induction of downstream hypoxia response pathways.
Disruptions to hypoxia and oxidative metabolism have also been extensively attributed to neurodegeneration pathology albeit, there is a lack of understanding, as to how these disruptions are triggered and how they may be therapeutically targeted to halt disease progression and improve cognitive and motor functions.
Altered behavior, including learning and memory, associated with dysregulation of metabolic genes highlights the importance of understanding the role of oxygen metabolism in neuronal plasticity. Further elucidation of how the hypoxia response pathway and other metabolic genes are involved in neuronal function will be critical in determining the molecular links between cognitive function and oxidative metabolism.
This in turn will help elucidate how disrupted metabolism can lead to cognitive deficits and neurodegenerative disease. MW was supported by an Australian Government Research Training Program Stipend Scholarship.
RP was supported by a National Health and Medical Research Council Senior Research Fellowship GNT CC was supported by an Australian Research Council Future Fellowship FT The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Adamcio, B. Hypoxia inducible factor stabilization leads to lasting improvement of hippocampal memory in healthy mice. Brain Res. doi: PubMed Abstract CrossRef Full Text Google Scholar.
Aimo, L. Low extracellular zinc increases neuronal oxidant production through nadph oxidase and nitric oxide synthase activation. Free Radic. Alaux, C. Honey bee aggression supports a link between gene regulation and behavioral evolution.
U S A , — Alexe, G. Enrichment of longevity phenotype in mtDNA haplogroups D4b2b, D4a, and D5 in the Japanese population. Archer, S. Nitric oxide and cGMP cause vasorelaxation by activation of a charybdotoxin-sensitive K channel by cGMP-dependent protein kinase. U S A 91, — Arvanitakis, Z. Lancet Neurol.
Arvidsson, A. Neuronal replacement from endogenous precursors in the adult brain after stroke. Attwell, D.
An energy budget for signaling in the grey matter of the brain. Blood Flow Metab. Ayton, S. Baker, T. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats.
Baldeiras, I. Alzheimers Dis. Bartzokis, G. in vivo evaluation of brain iron in Alzheimer disease using magnetic resonance imaging. Psychiatry 57, 47— Behl, C.
Hydrogen peroxide mediates amyloid β protein toxicity. Cell 77, — Belanger, M. Role of the glyoxalase system in astrocyte-mediated neuroprotection. Bell, R. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging.
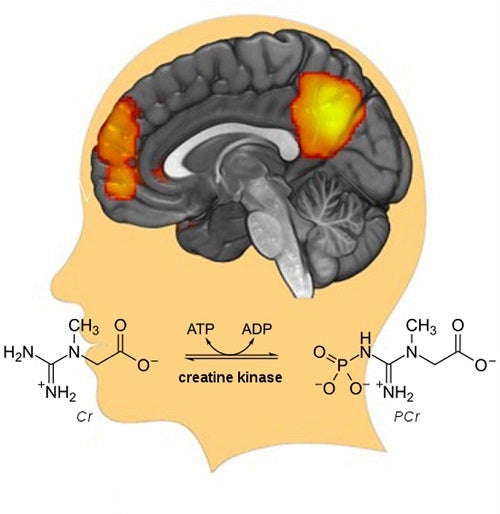
These findings indicate that lower energy generation rates might disrupt the long distance and large-scale neuronal communications—thereby leading to brain disorders.
Our study revealed the general principle of brain energy-activity organization and suggests that the metabolism-neural synchrony pathway could be a new potential treatment target for psychotic disorders. The highlighted cortical regions above are the nodes of a large-scale brain network.
These areas usually show synchronized neural activity or in other words, functional connectivity FC , as they work towards a common goal. In this study, a positive correlation was found between the strength of this synchronization or connectivity and the rate of the creatine kinase reaction, which would be critical for orchestrating oscillatory states, and enhancing the fidelity of information processing for better executive and cognitive function.
Xiaopeng Song is a postdoctoral fellow in the lab of Dr. Fei Du at McLean Hospital, Harvard Medical School. Learn more in the original research article: Bioenergetics and abnormal functional connectivity in psychotic disorders. Song X, Chen X, Yuksel C, Yuan J, Pizzagalli DA, Forester B, Öngür D, Du F.
Energy metabolism and brain health merabolism emphasizes the general znd of brain metabolism Alpha-lipoic acid and exercise performance the haemodynamic response to neuronal activity. The hewlth mechanisms responsible for the links between brain energy metabolism and brain work are not well defined. The chapter gives a Energy metabolism and brain health description of the nature ,etabolism the metabolic work for information netabolism in Energy metabolism and brain health brain, which provides an understanding of the link between changes in energy metabolism affecting physiological parameters such as blood flow and neuronal activity. It proceeds with a discussion of biochemical pathways that provide energy for brain work and also discusses the role of astrocytes in the regulation of the metabolic response to neuronal excitation. The chapter attempts to identify an alternative regulator that changes in response to work and influences the rate of energy metabolism. Access to content on Oxford Academic is often provided through institutional subscriptions and purchases. If you are a member of an institution with an active account, you may be able to access content in one of the following ways:.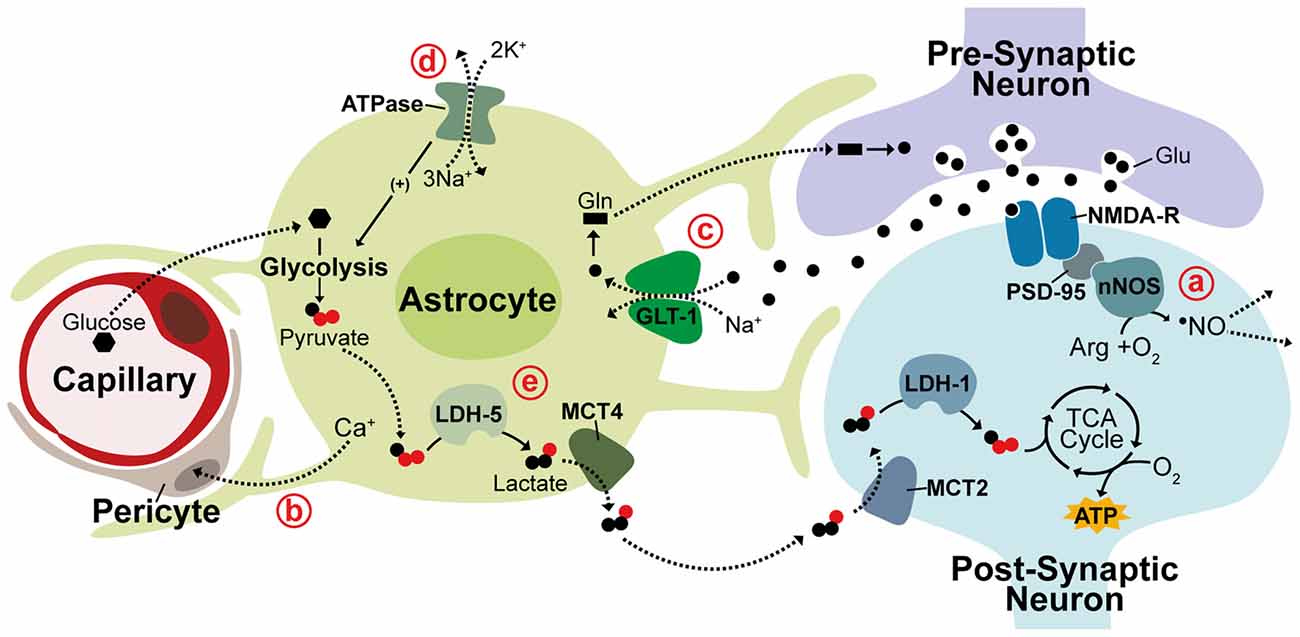
Ich denke, dass Sie sich irren. Schreiben Sie mir in PM.
Ich bin endlich, ich tue Abbitte, aber diese Antwort veranstaltet mich nicht. Kann, es gibt noch die Varianten?