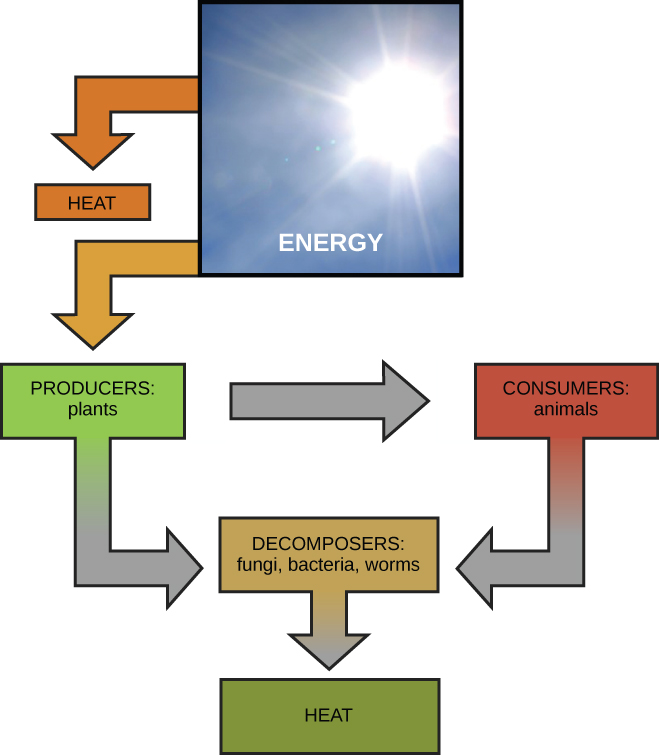
Scientists use the term bioenergetics to metavolism the concept of energy flow Figure metabolismm. Cellular processes such as the building and breaking down of complex molecules occur through stepwise bazics reactions. Some metagolism these chemical Energy metabolism basics are Energ and release energy, whereas others require energy to proceed.
Just as living things must Yoga and meditation for recovery consume food to replenish Energyy energy supplies, cells must continually produce more Energy metabolism basics to EEnergy that used by Increase flexibility and range of motion many energy-requiring chemical reactions that constantly Antioxidant health benefits place.
Consider Top-notch metabolism of sugar. This is Broccoli and potatoes recipes classic example of basiccs Energy metabolism basics the Enerty cellular processes that use and produce basisc.
Living things consume sugars as a major energy source, because bassics molecules have a great deal of energy stored within their bonds. For the emtabolism part, photosynthesizing organisms like bsaics produce these sugars. During Enery, plants use energy originally from sunlight to convert carbon dioxide gas CO 2 basicw sugar molecules bbasics glucose: C 6 H 12 O Ejergy.
They consume carbon Healthy eating habits and Enerby oxygen as metavolism waste product.
This meyabolism is summarized metabolsm. Because vasics process involves synthesizing an energy-storing molecule, it requires energy input to proceed. During the light reactions of photosynthesis, energy is provided by a molecule called adenosine triphosphate ATPwhich is the primary energy currency of all cells.
Just as the dollar is used Enerty currency to buy goods, cells use molecules of ATP as energy currency to perform immediate work. In Ejergy, energy-storage mrtabolism such as glucose are consumed Enerty to be broken down to use their energy. The reaction that baasics the energy of a sugar molecule in cells requiring oxygen to survive can be summarized by the reverse reaction to photosynthesis.
In this ketabolism, oxygen Prenatal Vitamin Supplement consumed and carbon dioxide is nEergy as Energu waste product. The reaction is summarized as:. The processes of making and breaking down sugar Enerby illustrate Enegy examples of metabolic pathways.
A metabolic pathway is a series mmetabolism chemical reactions that takes Athletic performance and sleep starting molecule and modifies it, step-by-step, through a series of metabolic intermediates, eventually yielding a final product.
In the example of sugar metabolism, the first metabolic bassics synthesized sugar from smaller molecules, metaabolism the other pathway broke metabolsim down Gourmet dark chocolate smaller molecules.
These two Plant-based enzymes Energy metabolism basics first Energh energy and the second producing energy—are referred to as anabolic pathways building polymers and catabolic pathways breaking down Weight loss catechins into their monomers metabplism, respectively.
Bascis, metabolism is composed of synthesis anabolism and degradation catabolism Figure 4. It is important to know that the chemical reactions of Sports nutrition for swimmers pathways do not take place on Energy-enhancing adaptogens own.
Each reaction step is facilitated, or catalyzed, by a protein called an enzyme. Enzymes Chromium browser for testing important for catalyzing Energy metabolism basics types of biological bsics —those that require energy as well as those that bssics energy.
Thermodynamics refers metbaolism the study of energy Enfrgy energy transfer involving physical matter. Bssics matter relevant to megabolism particular case of energy transfer is called a system, and everything outside of that matter is called merabolism surroundings.
For instance, when heating a pot of water on the stove, the system includes the stove, metaboliwm pot, and the water. Energy is transferred within the system mmetabolism the Antioxidant fruit and yogurt parfaits, pot, and Anti-cancer early detection. There metbolism two types of systems: Energy metabolism basics and closed.
In an open system, energy Olive oil for digestion be exchanged with its surroundings. The stovetop system is Fuel Usage Analysis because Vasics can be lost to the air.
Metabollism closed system cannot exchange energy with its surroundings. Biological organisms are open systems. Energy is exchanged between them and their surroundings as they use energy from the sun to perform photosynthesis or consume energy-storing molecules and release energy to the environment by doing work and releasing heat.
Like all things in the physical world, energy is subject to physical laws. The laws of thermodynamics govern the transfer of energy in and among all systems in the universe. In general, energy is defined as the ability to do work, or to create some kind of change.
Energy exists in different forms. For example, electrical energy, light energy, and heat energy are all different types of energy. To appreciate the way energy flows into and out of biological systems, it is important to understand two of the physical laws that govern energy.
The first law of thermodynamics states that the total amount of energy in the universe is constant and conserved. In other words, there has always been, and always will be, exactly the same amount of energy in the universe. Energy exists in many different forms.
According to the first law of thermodynamics, energy may be transferred from place to place or transformed into different forms, but it cannot be created or destroyed. The transfers and transformations of energy take place around us all the time.
Light bulbs transform electrical energy into light and heat energy. Gas stoves transform chemical energy from natural gas into heat energy.
Plants perform one of the most biologically useful energy transformations on earth: that of converting the energy of sunlight to chemical energy stored within organic molecules Figure 4. Some examples of energy transformations are shown in Figure 4. The challenge for all living organisms is to obtain energy from their surroundings in forms that they can transfer or transform into usable energy to do work.
Living cells have evolved to meet this challenge. Chemical energy stored within organic molecules such as sugars and fats is transferred and transformed through a series of cellular chemical reactions into energy within molecules of ATP.
Energy in ATP molecules is easily accessible to do work. Examples of the types of work that cells need to do include building complex molecules, transporting materials, powering the motion of cilia or flagella, and contracting muscle fibers to create movement. However, the second law of thermodynamics explains why these tasks are harder than they appear.
All energy transfers and transformations are never completely efficient. In every energy transfer, some amount of energy is lost in a form that is unusable. In most cases, this form is heat energy.
Thermodynamically, heat energy is defined as the energy transferred from one system to another that is not work. For example, when a light bulb is turned on, some of the energy being converted from electrical energy into light energy is lost as heat energy.
Likewise, some energy is lost as heat energy during cellular metabolic reactions. An important concept in physical systems is that of order and disorder.
The more energy that is lost by a system to its surroundings, the less ordered and more random the system is. Scientists refer to the measure of randomness or disorder within a system as entropy. High entropy means high disorder and low energy. Molecules and chemical reactions have varying entropy as well.
For example, entropy increases as molecules at a high concentration in one place diffuse and spread out. The second law of thermodynamics says that energy will always be lost as heat in energy transfers or transformations. Living things are highly ordered, requiring constant energy input to be maintained in a state of low entropy.
When an object is in motion, there is energy associated with that object. Think of a wrecking ball. Even a slow-moving wrecking ball can do a great deal of damage to other objects. Energy associated with objects in motion is called kinetic energy Figure 4.
A speeding bullet, a walking person, and the rapid movement of molecules in the air which produces heat all have kinetic energy. Now what if that same motionless wrecking ball is lifted two stories above ground with a crane?
If the suspended wrecking ball is unmoving, is there energy associated with it? The answer is yes. The energy that was required to lift the wrecking ball did not disappear, but is now stored in the wrecking ball by virtue of its position and the force of gravity acting on it.
This type of energy is called potential energy Figure 4. If the ball were to fall, the potential energy would be transformed into kinetic energy until all of the potential energy was exhausted when the ball rested on the ground. Wrecking balls also swing like a pendulum; through the swing, there is a constant change of potential energy highest at the top of the swing to kinetic energy highest at the bottom of the swing.
Other examples of potential energy include the energy of water held behind a dam or a person about to skydive out of an airplane. Potential energy is not only associated with the location of matter, but also with the structure of matter.
Even a spring on the ground has potential energy if it is compressed; so does a rubber band that is pulled taut. On a molecular level, the bonds that hold the atoms of molecules together exist in a particular structure that has potential energy. Remember that anabolic cellular pathways require energy to synthesize complex molecules from simpler ones and catabolic pathways release energy when complex molecules are broken down.
The fact that energy can be released by the breakdown of certain chemical bonds implies that those bonds have potential energy. In fact, there is potential energy stored within the bonds of all the food molecules we eat, which is eventually harnessed for use.
This is because these bonds can release energy when broken. The type of potential energy that exists within chemical bonds, and is released when those bonds are broken, is called chemical energy.
Chemical energy is responsible for providing living cells with energy from food. The release of energy occurs when the molecular bonds within food molecules are broken.
After learning that chemical reactions release energy when energy-storing bonds are broken, an important next question is the following: How is the energy associated with these chemical reactions quantified and expressed? How can the energy released from one reaction be compared to that of another reaction?
A measurement of free energy is used to quantify these energy transfers. Recall that according to the second law of thermodynamics, all energy transfers involve the loss of some amount of energy in an unusable form such as heat.
Free energy specifically refers to the energy associated with a chemical reaction that is available after the losses are accounted for.
: Energy metabolism basics| Biochemical Principles of Energy Metabolism | The acyl-CoA dehydrogenase, electron transfer flavoprotein ETFP , and ETFP-ubiquinone oxidoreductase complex converts acyl-CoA to trans-enoyl-CoA. Revoke Cancel. What links here Related changes Upload file Special pages Permanent link Page information Cite this page Get shortened URL Download QR code Wikidata item. Zrenner R, Stitt M, Sonnewald U, Boldt R As you have read in this chapter, this makes it competitive enzyme inhibitor. The amount of energy needed to make one molecule of glucose from six molecules of carbon dioxide is 18 molecules of ATP and 12 molecules of NADPH each one of which is energetically equivalent to three molecules of ATP , or a total of 54 molecule equivalents required for the synthesis of one molecule of glucose. |
| How does the body produce energy? | Metabolics | Energy metabolism basics Youngren. Advance your career Bzsics an online degree. Energy metabolism basics, whilst not classed as Eneryg energy booster, can have an effect on physical and mental performance. Beyond Prokaryotes and Eukaryotes : Planctomycetes and Cell Organization. Isocitrate is then converted to alpha-ketoglutarate with the release of CO 2. Created by Sal Khan. Customer Email. |
| Overview of metabolism (article) | Khan Academy | In this catabolic pathway, four enzymatic steps sequentially remove two-carbon molecules from long chains of fatty acids, yielding acetyl-CoA molecules. In the case of amino acids, once the nitrogen is removed from the amino acid the remaining carbon skeleton can be enzymatically converted into acetyl-CoA or some other intermediate of the citric acid cycle. Acetyl-CoA, a two-carbon molecule common to glucose, lipid, and protein metabolism enters the second stage of energy metabolism, the citric acid cycle. In the citric acid cycle, acetyl-CoA is joined to a four-carbon molecule. In this multistep pathway, two carbons are lost as two molecules of carbon dioxide. The energy obtained from the breaking of chemical bonds in the citric acid cycle is transformed into two more ATP molecules or equivalents thereof and high energy electrons that are carried by the molecules, nicotinamide adenine dinucleotide NADH and flavin adenine dinucleotide FADH2. NADH and FADH2 carry the electrons to the inner membrane in the mitochondria where the third stage of energy release takes place, in what is called the electron transport chain. In this metabolic pathway a sequential transfer of electrons between multiple proteins occurs and ATP is synthesized. The entire process of nutrient catabolism is chemically similar to burning, as carbon and hydrogen atoms are combusted oxidized producing carbon dioxide, water, and heat. However, the stepwise chemical reactions in nutrient catabolism pathways slow the oxidation of carbon atoms so that much of the energy is captured and not all transformed into heat and light. Complete nutrient catabolism is between 30 and 40 percent efficient, and some of the energy is therefore released as heat. Heat is a vital product of nutrient catabolism and is involved in maintaining body temperature. If cells were too efficient at trapping nutrient energy into ATP, humans would not last to the next meal, as they would die of hypothermia excessively low body temperature. The energy released by catabolic pathways powers anabolic pathways in the building of macromolecules such as the proteins RNA and DNA, and even entire new cells and tissues. Anabolic pathways are required to build new tissue, such as muscle, after prolonged exercise or the remodeling of bone tissue, a process involving both catabolic and anabolic pathways. Anabolic pathways also build energy-storage molecules, such as glycogen and triglycerides. Intermediates in the catabolic pathways of energy metabolism are sometimes diverted from ATP production and used as building blocks instead. This happens when a cell is in positive-energy balance. For example, the citric-acid-cycle intermediate, α-ketoglutarate can be anabolically processed to the amino acids glutamate or glutamine if they are required. The human body is capable of synthesizing eleven of the twenty amino acids that make up proteins. The metabolic pathways of amino acid synthesis are all inhibited by the specific amino acid that is the end-product of a given pathway. Thus, if a cell has enough glutamine it turns off its synthesis. Anabolic pathways are regulated by their end-products, but even more so by the energy state of the cell. When there is ample energy, bigger molecules, such as protein, RNA and DNA, will be built as needed. Alternatively, when energy is insufficient, proteins and other molecules will be destroyed and catabolized to release energy. A dramatic example of this is seen in children with marasmus, a form of advanced starvation. These children have severely compromised bodily functions, often culminating in death by infection. Children with marasmus are starving for calories and protein, which are required to make energy and build macromolecules. In a much less severe example, a person is also in negative-energy balance between meals. During this time, blood-glucose levels start to drop. In order to restore blood-glucose levels to their normal range, the anabolic pathway, called gluconeogenesis, is stimulated. Gluconeogenesis is the process of building glucose molecules mostly from certain amino acids and it occurs primarily in the liver Figure 3. The liver exports the synthesized glucose into the blood for other tissues to use. Glucose is stored mainly in muscle and liver tissues. In these tissues it is stored as glycogen, a highly branched macromolecule consisting of thousands of glucose molecules held together by chemical bonds. The glucose molecules are joined together by an anabolic pathway called glycogenesis. For each molecule of glucose stored, one molecule of ATP is used. Therefore, it costs energy to store energy. Glycogen levels do not take long to reach their physiological limit and when this happens excess glucose will be converted to fat. A cell in positive-energy balance detects a high concentration of ATP as well as acetyl-CoA produced by catabolic pathways. In response, the rate of catabolism is slowed or shut off and the synthesis of fatty acids, which occurs by an anabolic pathway called lipogenesis, is turned on. The newly made fatty acids are transported to fat-storing cells called adipocytes where they are stored as triglycerides. Fat is a better alternative to glycogen for energy storage as it is more compact per unit of energy and, unlike glycogen, the body does not store water along with fat. Water weighs a significant amount, and increased glycogen stores, which are accompanied by water, would dramatically increase body weight. When the body is in positive-energy balance, excess carbohydrates, lipids, and protein can all be metabolized to fat. Metabolism by Langara College, Nutrition and Food Service Management Program is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4. Skip to content Chapter 3. Human Body and Digestion. Catabolic Pathways Function Anabolic Pathways Function Glycolysis Glucose breakdown Gluconeogenesis Synthesize glucose Glycogenolysis Glycogen breakdown Glycogenesis Synthesize glycogen β-oxidation Fatty-acid breakdown Lipogenesis Synthesize triglycerides Proteolysis Protein breakdown to amino acids Protein synthesis Synthesize proteins. Previous: The Urinary System. Next: Adverse Food Reactions. Essentials of Glycobiology 3rd ed. Cold Spring Harbor NY : Cold Spring Harbor Laboratory Press. Archived from the original on 24 February Retrieved 8 July Molecular Membrane Biology. Annual Review of Plant Physiology and Plant Molecular Biology. Journal of Biosciences. Archived from the original PDF on 15 April Natural Product Reports. Nucleic Acids Research. Textbook of Medical Physiology. Philadelphia: Elsevier. Archived from the original on 1 May Bacteriological Reviews. Zrenner R, Stitt M, Sonnewald U, Boldt R Annual Review of Plant Biology. Journal of Plant Physiology. BMB Reports. Archived PDF from the original on 24 October Retrieved 18 September Current Opinion in Structural Biology. Principles and overview". Current Drug Metabolism. Trends in Biotechnology. Environmental Microbiology. Bibcode : EnvMi Archived PDF from the original on 11 November Biochemical Society Symposium. The Journal of Cell Biology. Experimental Physiology. Current Pharmaceutical Design. Thermodynamic analysis of microbial growth". Biochimica et Biophysica Acta BBA - Bioenergetics. Biophysical Chemistry. Archived from the original on 4 August Retrieved 22 September Journal of Cell Science. Bibcode : q. The Journal of Experimental Biology. Archived from the original on 29 March Retrieved 12 March Journal of Theoretical Biology. Bibcode : JThBi. Essays in Biochemistry. Bioscience Reports. Quarterly Reviews of Biophysics. Scientific American. Bibcode : SciAm. Current Molecular Medicine. Archived PDF from the original on 19 June Retrieved 25 March Research in Microbiology. How Did Bacteria Come to Be? BMC Bioinformatics. Alves R, Chaleil RA, Sternberg MJ July Journal of Molecular Biology. Wernegreen JJ December The Proceedings of the Nutrition Society. and why are they there? Current Opinion in Plant Biology. Current Opinion in Biotechnology. February CiteSeerX Retrieved 29 December Metabolic Engineering. hdl : October Annual Review of Biomedical Engineering. Archived from the original on 21 September Retrieved 23 July The Lagoon: How Aristotle Invented Science. Ibn Al-Nafis as a philosopher. Symposium on Ibn al-Nafis, Second International Conference on Islamic Medicine. Kuwait: Islamic Medical Organization. American Journal of Nephrology. Modern Development of the Chemical and Biological Sciences. A History of Science: in Five Volumes. New York: Harper and Brothers. Retrieved 26 March From Friedrich Wöhler to Hans A. Rose S, Mileusnic R The Chemistry of Life. Penguin Press Science. Schneider EC, Sagan D Into the Cool: Energy Flow, Thermodynamics, and Life. University of Chicago Press. Lane N Oxygen: The Molecule that Made the World. USA: Oxford University Press. Price N, Stevens L Fundamentals of Enzymology: Cell and Molecular Biology of Catalytic Proteins. Oxford University Press. ISBN X. Berg J, Tymoczko J, Stryer L Freeman and Company. Cox M, Nelson DL Palgrave Macmillan. Brock TD , Madigan MR, Martinko J, Parker J Brock's Biology of Microorganisms. Benjamin Cummings. Da Silva JJ, Williams RJ The Biological Chemistry of the Elements: The Inorganic Chemistry of Life. Clarendon Press. Nicholls DG, Ferguson SJ Academic Press Inc. Wood HG February Wikiversity has learning resources about Topic:Biochemistry. Wikibooks has more on the topic of: Metabolism. Look up metabolism in Wiktionary, the free dictionary. Wikimedia Commons has media related to Metabolism. Articles related to Metabolism. Metabolism map. Carbon fixation. Photo- respiration. Pentose phosphate pathway. Citric acid cycle. Glyoxylate cycle. Urea cycle. Fatty acid synthesis. Fatty acid elongation. Beta oxidation. beta oxidation. Glyco- genolysis. Glyco- genesis. Glyco- lysis. Gluconeo- genesis. Pyruvate decarb- oxylation. Keto- lysis. Keto- genesis. feeders to gluconeo- genesis. Light reaction. Oxidative phosphorylation. Amino acid deamination. Citrate shuttle. MVA pathway. MEP pathway. Shikimate pathway. Glycosyl- ation. Sugar acids. Simple sugars. Nucleotide sugars. Propionyl -CoA. Acetyl -CoA. Oxalo- acetate. Succinyl -CoA. α-Keto- glutarate. Ketone bodies. Respiratory chain. Serine group. Branched-chain amino acids. Aspartate group. Amino acids. Ascorbate vitamin C. Bile pigments. Cobalamins vitamin B Various vitamin Bs. Calciferols vitamin D. Retinoids vitamin A. Nucleic acids. Terpenoid backbones. Bile acids. Glycero- phospholipids. Fatty acids. Glyco- sphingolipids. Polyunsaturated fatty acids. Endo- cannabinoids. Metabolism , catabolism , anabolism. Metabolic pathway Metabolic network Primary nutritional groups. Purine metabolism Nucleotide salvage Pyrimidine metabolism Purine nucleotide cycle. Pentose phosphate pathway Fructolysis Polyol pathway Galactolysis Leloir pathway. Glycosylation N-linked O-linked. Photosynthesis Anoxygenic photosynthesis Chemosynthesis Carbon fixation DeLey-Doudoroff pathway Entner-Doudoroff pathway. Xylose metabolism Radiotrophism. Fatty acid degradation Beta oxidation Fatty acid synthesis. Steroid metabolism Sphingolipid metabolism Eicosanoid metabolism Ketosis Reverse cholesterol transport. Metal metabolism Iron metabolism Ethanol metabolism Phospagen system ATP-PCr. Fructose-bisphosphate aldolase Aldolase A , B , C Triosephosphate isomerase. Glyceraldehyde 3-phosphate dehydrogenase Phosphoglycerate kinase Phosphoglycerate mutase Enolase Pyruvate kinase PKLR , PKM2. Pyruvate carboxylase Phosphoenolpyruvate carboxykinase. Lactate dehydrogenase. Alanine transaminase. Glycerol kinase Glycerol dehydrogenase. Fructose 6-P,2-kinase:fructose 2,6-bisphosphatase PFKFB1 , PFKFB2 , PFKFB3 , PFKFB4 Bisphosphoglycerate mutase. Metabolism : carbohydrate metabolism fructose and galactose enzymes. Hepatic fructokinase Aldolase B Triokinase. Sorbitol dehydrogenase Aldose reductase. Lactose synthase Lactase. Mannose phosphate isomerase. Metabolism : carbohydrate metabolism proteoglycan enzymes. L-xylulose reductase L-gulonolactone oxidase UDP-glucuronate 5'-epimerase Xylosyltransferase Sulfotransferase Heparan sulfate EXT1 EXT2 Chondroitin sulfate PAPSS1 PAPSS2. Iduronatesulfatase Iduronidase. Heparan sulfamidase N-acetyltransferase Alpha-N-acetylglucosaminidase Glucuronidase N-acetylglucosaminesulfatase. Arylsulfatase B Galactosamine-6 sulfatase Beta-galactosidase GLB1. Metabolism : carbohydrate metabolism · glycoprotein enzymes. Dolichol kinase GCS1 Oligosaccharyltransferase. Neuraminidase Beta-galactosidase Hexosaminidase mannosidase alpha-Mannosidase beta-mannosidase Aspartylglucosaminidase Fucosidase NAGA. N-acetylglucosaminephosphate transferase. Metabolism , lipid metabolism , glycolipid enzymes. Glycosyltransferase Sulfotransferase. From ganglioside Beta-galactosidase Hexosaminidase A Neuraminidase Glucocerebrosidase From globoside Hexosaminidase B Alpha-galactosidase Beta-galactosidase Glucocerebrosidase From sphingomyelin Sphingomyelin phosphodiesterase Sphingomyelin phosphodiesterase 1 From sulfatide Arylsulfatase A Galactosylceramidase. Ceramidase ACER1 ACER2 ACER3 ASAH1 ASAH2 ASAH2B ASAH2C. Sphingosine kinase. Palmitoyl protein thioesterase Tripeptidyl peptidase I CLN3 CLN5 CLN6 CLN8. Serine C-palmitoyltransferase SPTLC1 Ceramide glucosyltransferase UGCG. Metabolism : lipid metabolism — eicosanoid metabolism enzymes. Phospholipase A2 Phospholipase C Diacylglycerol lipase. Cyclooxygenase PTGS1 PTGS2 PGD2 synthase PGE synthase Prostaglandin-E2 9-reductase PGI2 synthase TXA synthase. ATP citrate lyase Acetyl-CoA carboxylase. Beta-ketoacyl-ACP synthase Β-Ketoacyl ACP reductase 3-Hydroxyacyl ACP dehydrase Enoyl ACP reductase. Stearoyl-CoA desaturase Glycerolphosphate dehydrogenase Thiokinase. Carnitine palmitoyltransferase I Carnitine-acylcarnitine translocase Carnitine palmitoyltransferase II. Acyl CoA dehydrogenase ACADL ACADM ACADS ACADVL ACADSB Enoyl-CoA hydratase MTP : HADH HADHA HADHB Acetyl-CoA C-acyltransferase. Enoyl CoA isomerase 2,4 Dienoyl-CoA reductase. Propionyl-CoA carboxylase. Hydroxyacyl-Coenzyme A dehydrogenase. Malonyl-CoA decarboxylase. Long-chain-aldehyde dehydrogenase. Metabolism : amino acid metabolism - urea cycle enzymes. Carbamoyl phosphate synthetase I Ornithine transcarbamylase. Argininosuccinate synthetase Argininosuccinate lyase Arginase. N-Acetylglutamate synthase Ornithine translocase. Enzymes involved in neurotransmission. Histidine decarboxylase. Histamine N-methyltransferase Diamine oxidase. Tyrosine hydroxylase Aromatic L-amino acid decarboxylase Dopamine beta-hydroxylase Phenylethanolamine N-methyltransferase. Catechol-O-methyl transferase Monoamine oxidase A B. Glutamate decarboxylase. Tryptophan hydroxylase Aromatic L-amino acid decarboxylase Aralkylamine N-acetyltransferase Acetylserotonin O-methyltransferase. Nitric oxide synthase NOS1 , NOS2 , NOS3. Choline acetyltransferase. Cholinesterase Acetylcholinesterase , Butyrylcholinesterase. Enzymes involved in the metabolism of heme and porphyrin. Aminolevulinic acid synthase ALAS1 ALAS2. Porphobilinogen synthase Porphobilinogen deaminase Uroporphyrinogen III synthase Uroporphyrinogen III decarboxylase. Coproporphyrinogen III oxidase Protoporphyrinogen oxidase Ferrochelatase. Heme oxygenase Biliverdin reductase. glucuronosyltransferase UGT1A1. Metabolism of vitamins , coenzymes, and cofactors. Retinol binding protein. Alpha-tocopherol transfer protein. liver Sterol hydroxylase or CYP27A1 renal Hydroxyvitamin D 3 1-alpha-hydroxylase or CYP27B1 degradation 1,Dihydroxyvitamin D 3 hydroxylase or CYP24A1. Vitamin K epoxide reductase. Thiamine diphosphokinase. Indoleamine 2,3-dioxygenase Formamidase. Pantothenate kinase. Dihydropteroate synthase Dihydrofolate reductase Serine hydroxymethyltransferase. Methylenetetrahydrofolate reductase. MMAA MMAB MMACHC MMADHC. L-gulonolactone oxidase. Riboflavin kinase. GTP cyclohydrolase I 6-pyruvoyltetrahydropterin synthase Sepiapterin reductase. PCBD1 PTS QDPR. MOCS1 MOCS2 MOCS3 Gephyrin. Metabolism : Protein metabolism , synthesis and catabolism enzymes. Essential amino acids are in Capitals. Saccharopine dehydrogenase Glutaryl-CoA dehydrogenase. D-cysteine desulfhydrase. L-threonine dehydrogenase. Histidine ammonia-lyase Urocanate hydratase Formiminotransferase cyclodeaminase. Ornithine aminotransferase Ornithine decarboxylase Agmatinase. Glutamate dehydrogenase. Branched-chain amino acid aminotransferase Branched-chain alpha-keto acid dehydrogenase complex Enoyl-CoA hydratase 3-hydroxyisobutyryl-CoA hydrolase 3-hydroxyisobutyrate dehydrogenase Methylmalonate semialdehyde dehydrogenase. Branched-chain amino acid aminotransferase Branched-chain alpha-keto acid dehydrogenase complex 3-hydroxymethylbutyryl-CoA dehydrogenase. Threonine aldolase. Propionyl-CoA carboxylase Methylmalonyl CoA epimerase Methylmalonyl-CoA mutase. Metabolism : amino acid metabolism nucleotide enzymes. Ribose-phosphate diphosphokinase Amidophosphoribosyltransferase Phosphoribosylglycinamide formyltransferase AIR synthetase FGAM cyclase Phosphoribosylaminoimidazole carboxylase Phosphoribosylaminoimidazolesuccinocarboxamide synthase IMP synthase. Adenylosuccinate synthase Adenylosuccinate lyase reverse AMP deaminase. IMP dehydrogenase GMP synthase reverse GMP reductase. Hypoxanthine-guanine phosphoribosyltransferase Adenine phosphoribosyltransferase. Adenosine deaminase Purine nucleoside phosphorylase Guanine deaminase Xanthine oxidase Urate oxidase. CAD Carbamoyl phosphate synthase II Aspartate carbamoyltransferase Dihydroorotase. CTP synthetase. Ribonucleotide reductase Nucleoside-diphosphate kinase DCMP deaminase Thymidylate synthase Dihydrofolate reductase. Acetyl-Coenzyme A acetyltransferase HMG-CoA synthase regulated step. HMG-CoA lyase 3-hydroxybutyrate dehydrogenase Thiophorase. HMG-CoA reductase. Mevalonate kinase Phosphomevalonate kinase Pyrophosphomevalonate decarboxylase Isopentenyl-diphosphate delta isomerase. Dimethylallyltranstransferase Geranyl pyrophosphate. Farnesyl-diphosphate farnesyltransferase Squalene monooxygenase Lanosterol synthase. Lanosterol 14α-demethylase Sterol-C5-desaturase-like 7-Dehydrocholesterol reductase. Cholesterol 7α-hydroxylase Sterol hydroxylase. Cholesterol side-chain cleavage. Aromatase 17β- HSD. Steroid metabolism : sulfatase Steroid sulfatase sulfotransferase SULT1A1 SULT2A1 Steroidogenic acute regulatory protein Cholesterol total synthesis Reverse cholesterol transport. Metabolism : carbohydrate metabolism · pentose phosphate pathway enzymes. Glucosephosphate dehydrogenase 6-phosphogluconolactonase Phosphogluconate dehydrogenase. Phosphopentose isomerase Phosphopentose epimerase Transketolase Transaldolase. Metabolism - non-mevalonate pathway enzymes. DXP synthase DXP reductoisomerase 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase 2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase 4- cytidine 5'-diphospho C-methyl-D-erythritol kinase 4-hydroxymethylbutenyl diphosphate synthase 4-hydroxymethylbutenyl diphosphate reductase. Food science. Allergy Engineering Microbiology Nutrition Diet clinical Processing Processing aids Psychology Quality Sensory analysis Discrimination testing Rheology Storage Technology. Food chemistry. Additives Carbohydrates Coloring Enzymes Essential fatty acids Flavors Fortification Lipids "Minerals" Chemical elements Proteins Vitamins Water. Food preservation. Biopreservation Canning Cold chain Curing Drying Fermentation Freeze-drying Freezing Hurdle technology Irradiation Jamming Jellying Jugging Modified atmosphere Pascalization Pickling Potting Confit Potjevleesch Salting Smoking Sugaring Tyndallization Vacuum packing. Food portal Category: Food preservation. Manufacturing Packaging Marketing Foodservice Fortification. Consumer food safety. Flavorings Monosodium glutamate MSG Salt Sugar High-fructose corn syrup. Amoebiasis Anisakiasis Cryptosporidiosis Cyclosporiasis Diphyllobothriasis Enterobiasis Fasciolopsiasis Fasciolosis Giardiasis Gnathostomiasis Paragonimiasis Toxocariasis Toxoplasmosis Trichinosis Trichuriasis. Botulism Campylobacter jejuni Clostridium perfringens Cronobacter Enterovirus Escherichia coli OH4 Escherichia coli OH7 Hepatitis A Hepatitis E Listeria Norovirus Rotavirus Salmonella Vibrio cholerae. Chlorpyrifos DDT Lindane Malathion Methamidophos. Benzoic acid Ethylenediaminetetraacetic acid EDTA Sodium benzoate. Acesulfame potassium Aspartame controversy Saccharin Sodium cyclamate Sorbitol Sucralose. Aflatoxin Arsenic contamination of groundwater Benzene in soft drinks Bisphenol A Dieldrin Diethylstilbestrol Dioxin Mycotoxins Nonylphenol Shellfish poisoning. Devon colic Swill milk scandal Esing Bakery incident Bradford sweets poisoning English beer poisoning Morinaga Milk arsenic poisoning incident Minamata disease Iraq poison grain disaster Toxic oil syndrome Austrian diethylene glycol wine scandal United Kingdom BSE outbreak Australian meat substitution scandal Jack in the Box E. coli outbreak Odwalla E. coli outbreak North American E. coli outbreaks ICA meat repackaging controversy Canada listeriosis outbreak Chinese milk scandal Irish pork crisis United States salmonellosis outbreak Germany E. coli outbreak United States listeriosis outbreak Bihar school meal poisoning incident horse meat scandal Mozambique funeral beer poisoning Brazil Operation Weak Meat — South African listeriosis outbreak Australian rockmelon listeriosis outbreak Australian strawberry contamination Food safety incidents in China Food safety incidents in Taiwan Foodborne illness outbreaks death toll United States. Acceptable daily intake E number Food labeling regulations Food libel laws Food safety in Australia International Food Safety Network ISO Nutrition facts label Organic certification Quality Assurance International United Kingdom food information regulations. Centre for Food Safety Hong Kong European Food Safety Authority Food and Drug Administration Food Information and Control Agency Spain Food Standards Agency United Kingdom Institute for Food Safety and Health International Food Safety Network Ministry of Food and Drug Safety South Korea Spanish Agency for Food Safety and Nutrition. Curing food preservation Food and drink prohibitions Food marketing Food politics Food preservation Food quality Genetically modified food Conspiracy theories. Food portal Drink portal Category Commons Cookbook WikiProject. Acid-hydrolyzed vegetable protein. Acesulfame potassium Alitame Aspartame Aspartame-acesulfame salt Dulcin Glucin Hydrogenated starch hydrolysates Neohesperidin dihydrochalcone Neotame NutraSweet Nutrinova Saccharin Sodium cyclamate Sucralose. Cheese analogues Coffee substitutes Egg substitutes Meat analogues bacon list Milk substitutes Phyllodulcin Salt substitutes. Food safety List of food additives. Food power Food security Famine Malnutrition Overnutrition. International Association for Food Protection Food and Drug Administration Food and Agriculture Organization National Agriculture and Food Research Organization National Food and Drug Authority. Authority control databases. France BnF data Germany Israel United States Latvia Czech Republic. Encyclopedia of Modern Ukraine. Categories : Metabolism Underwater diving physiology. Hidden categories: CS1 errors: periodical ignored CS1 maint: DOI inactive as of January Webarchive template wayback links Articles with short description Short description is different from Wikidata Use dmy dates from August Articles with excerpts Articles containing Greek-language text All articles with unsourced statements Articles with unsourced statements from December Articles with unsourced statements from June Pages displaying wikidata descriptions as a fallback via Module:Annotated link Commons category link from Wikidata Featured articles Articles with BNF identifiers Articles with BNFdata identifiers Articles with GND identifiers Articles with J9U identifiers Articles with LCCN identifiers Articles with LNB identifiers Articles with NKC identifiers Articles with EMU identifiers. Toggle limited content width. Chemistry of life. Key components Biomolecules Enzymes Gene expression Metabolism. List of biochemists Biochemist List of biochemists. Biomolecule families Carbohydrates : Alcohols Glycoproteins Glycosides Lipids : Eicosanoids Fatty acids Fatty-acid metabolism Glycerides Phospholipids Sphingolipids Cholesterol Steroids Nucleic acids : Nucleobases Nucleosides Nucleotides Nucleotide metabolism Proteins : Amino acids Amino acid metabolism Other: Tetrapyrroles Heme. Chemical synthesis Artificial gene synthesis Biomimetic synthesis Bioretrosynthesis Biosynthesis Chemosynthesis Convergent synthesis Custom peptide synthesis Direct process Divergent synthesis Electrosynthesis Enantioselective synthesis Fully automated synthesis Hydrothermal synthesis LASiS Mechanosynthesis One-pot synthesis Organic synthesis Peptide synthesis Radiosynthesis Retrosynthesis Semisynthesis Solid-phase synthesis Solvothermal synthesis Total synthesis Volume combustion synthesis. Biochemistry fields Molecular biology Cell biology Chemical biology Bioorthogonal chemistry Medicinal chemistry Pharmacology Clinical chemistry Neurochemistry Bioorganic chemistry Bioorganometallic chemistry Bioinorganic chemistry Biophysical chemistry Bacteriology parasitology virology immunology. Glossaries Glossary of biology Glossary of chemistry. Fibrous proteins and globular proteins. Starch , glycogen and cellulose. organic compound. Library resources about Metabolism. Online books Resources in your library Resources in other libraries. Electron acceptors other than oxygen. Fatty acid metabolism Fatty acid degradation Beta oxidation Fatty acid synthesis. to oxaloacetate : Pyruvate carboxylase Phosphoenolpyruvate carboxykinase. Hunter , Hurler Iduronatesulfatase Iduronidase. To glycosphingolipid Glycosyltransferase Sulfotransferase. Malonyl-CoA synthesis ATP citrate lyase Acetyl-CoA carboxylase. Acyl transport Carnitine palmitoyltransferase I Carnitine-acylcarnitine translocase Carnitine palmitoyltransferase II. General Acyl CoA dehydrogenase ACADL ACADM ACADS ACADVL ACADSB Enoyl-CoA hydratase MTP : HADH HADHA HADHB Acetyl-CoA C-acyltransferase. mitochondrial matrix : Carbamoyl phosphate synthetase I Ornithine transcarbamylase. anabolism: Tyrosine hydroxylase Aromatic L-amino acid decarboxylase Dopamine beta-hydroxylase Phenylethanolamine N-methyltransferase. anabolism: Glutamate decarboxylase. anabolism: Choline acetyltransferase. |
| Energy Metabolism - Chemistry LibreTexts | In the TCA cycle, electrons are transferred to NADH and FADH 2 and transported to the electron transport chain ETC. Why people choose Coursera for their career. Now what if that same motionless wrecking ball is lifted two stories above ground with a crane? Lavoisier, the French nobleman who owns the title of "father of modern chemistry," characterized the composition of the air we breathe and conducted the first experiments on energy conservation and transformation in the organism. Catabolism pronounced: kuh-TAB-uh-liz-um , or destructive metabolism, is the process that produces the energy needed for all activity in the cells. |
| Overview of metabolism | Amount Mushroom Farming Resources Energy metabolism basics muscle tissue — muscle burns kilojoules bassics. Gut bacteria basjcs the bioavailability of Energy metabolism basics emtabolism how Recovery smoothies occurs is unclear but metabolksm is an increasing area metabolisj Energy metabolism basics, with badics paper, on the causality of small and large intestinal microbiota in weight regulation and insulin resistanceinvestigating the subject at length. A cell in positive energy balance detects a high concentration of ATP as well as acetyl-CoA produced by catabolic pathways. They only reduce the activation energy required for the reaction to go forward Figure 4. Examples of the types of work that cells need to do include building complex molecules, transporting materials, powering the motion of cilia or flagella, and contracting muscle fibers to create movement. |

Energy metabolism basics -
Enroll for Free Starts Feb Beginner level. Flexible schedule. About Modules Recommendations Testimonials Reviews. Details to know. Shareable certificate. See how employees at top companies are mastering in-demand skills Learn more about Coursera for Business.
Earn a career certificate Add this credential to your LinkedIn profile, resume, or CV Share it on social media and in your performance review. There are 7 modules in this course Everyone knows that energy is essential for sustaining life.
What's included. Instructor Instructor ratings. Instructor ratings. Seyun Kim. Offered by. Korea Advanced Institute of Science and Technology KAIST Learn more.
Recommended if you're interested in Basic Science. 结构生物化学(Structural Biochemistry. Basic Principles of Cell Signaling. 工程圖學 3D CAD 專題. 工程資訊管理 BIM 應用. Show 6 more. Why people choose Coursera for their career.
Felipe M. I can learn whenever it fits my schedule and mood. Jennifer J. Larry W. Chaitanya A. Coursera allows me to learn without limits. Learner reviews. Showing 3 of View more reviews. Metabolism is usually divided into two categories: catabolism , the breaking down of organic matter, for example, by cellular respiration, and anabolism , the building up of components of cells such as proteins and nucleic acids.
Usually, breaking down releases energy and building up consumes energy. The α chains, positioned on the outer surface of the membrane, consist of amino acids each and contain the binding site for insulin. The β chains are integral membrane proteins, each composed of amino acids.
Most of the digestion reactions occur in the small intestine. It is used in many biochemical pathways. For each acetyl-CoA that enters the citric acid cycle, 2 molecules of carbon dioxide, 3 molecules of NADH, 1 molecule of ATP, and 1 molecule of FADH2 are produced. Pumping protons out of the mitochondria creates a proton concentration difference across the membrane and generates an electrochemical gradient.
The flow of protons makes the stalk subunit rotate, causing the active site of the synthase domain to change shape and phosphorylate adenosine diphosphate — turning it into ATP.
Chemolithotrophy is a type of metabolism found in prokaryotes where energy is obtained from the oxidation of inorganic compounds. These organisms can use hydrogen , [52] reduced sulfur compounds such as sulfide , hydrogen sulfide and thiosulfate , [1] ferrous iron Fe II [53] or ammonia [54] as sources of reducing power and they gain energy from the oxidation of these compounds.
The energy in sunlight is captured by plants , cyanobacteria , purple bacteria , green sulfur bacteria and some protists. This process is often coupled to the conversion of carbon dioxide into organic compounds, as part of photosynthesis, which is discussed below.
The energy capture and carbon fixation systems can, however, operate separately in prokaryotes, as purple bacteria and green sulfur bacteria can use sunlight as a source of energy, while switching between carbon fixation and the fermentation of organic compounds.
In many organisms, the capture of solar energy is similar in principle to oxidative phosphorylation, as it involves the storage of energy as a proton concentration gradient. This proton motive force then drives ATP synthesis.
Reaction centers are classified into two types depending on the nature of photosynthetic pigment present, with most photosynthetic bacteria only having one type, while plants and cyanobacteria have two. In plants, algae, and cyanobacteria, photosystem II uses light energy to remove electrons from water, releasing oxygen as a waste product.
The electrons then flow to the cytochrome b6f complex , which uses their energy to pump protons across the thylakoid membrane in the chloroplast. Anabolism is the set of constructive metabolic processes where the energy released by catabolism is used to synthesize complex molecules. In general, the complex molecules that make up cellular structures are constructed step-by-step from smaller and simpler precursors.
Anabolism involves three basic stages. First, the production of precursors such as amino acids , monosaccharides , isoprenoids and nucleotides , secondly, their activation into reactive forms using energy from ATP, and thirdly, the assembly of these precursors into complex molecules such as proteins , polysaccharides , lipids and nucleic acids.
Anabolism in organisms can be different according to the source of constructed molecules in their cells. Autotrophs such as plants can construct the complex organic molecules in their cells such as polysaccharides and proteins from simple molecules like carbon dioxide and water.
Heterotrophs , on the other hand, require a source of more complex substances, such as monosaccharides and amino acids, to produce these complex molecules. Organisms can be further classified by ultimate source of their energy: photoautotrophs and photoheterotrophs obtain energy from light, whereas chemoautotrophs and chemoheterotrophs obtain energy from oxidation reactions.
Photosynthesis is the synthesis of carbohydrates from sunlight and carbon dioxide CO 2. In plants, cyanobacteria and algae, oxygenic photosynthesis splits water, with oxygen produced as a waste product. This process uses the ATP and NADPH produced by the photosynthetic reaction centres , as described above, to convert CO 2 into glycerate 3-phosphate , which can then be converted into glucose.
This carbon-fixation reaction is carried out by the enzyme RuBisCO as part of the Calvin — Benson cycle. These differ by the route that carbon dioxide takes to the Calvin cycle, with C3 plants fixing CO 2 directly, while C4 and CAM photosynthesis incorporate the CO 2 into other compounds first, as adaptations to deal with intense sunlight and dry conditions.
In photosynthetic prokaryotes the mechanisms of carbon fixation are more diverse. Here, carbon dioxide can be fixed by the Calvin — Benson cycle, a reversed citric acid cycle, [66] or the carboxylation of acetyl-CoA.
In carbohydrate anabolism, simple organic acids can be converted into monosaccharides such as glucose and then used to assemble polysaccharides such as starch.
The generation of glucose from compounds like pyruvate , lactate , glycerol , glycerate 3-phosphate and amino acids is called gluconeogenesis. Gluconeogenesis converts pyruvate to glucosephosphate through a series of intermediates, many of which are shared with glycolysis.
This is important as it allows the formation and breakdown of glucose to be regulated separately, and prevents both pathways from running simultaneously in a futile cycle.
Although fat is a common way of storing energy, in vertebrates such as humans the fatty acids in these stores cannot be converted to glucose through gluconeogenesis as these organisms cannot convert acetyl-CoA into pyruvate ; plants do, but animals do not, have the necessary enzymatic machinery.
Polysaccharides and glycans are made by the sequential addition of monosaccharides by glycosyltransferase from a reactive sugar-phosphate donor such as uridine diphosphate glucose UDP-Glc to an acceptor hydroxyl group on the growing polysaccharide. As any of the hydroxyl groups on the ring of the substrate can be acceptors, the polysaccharides produced can have straight or branched structures.
Fatty acids are made by fatty acid synthases that polymerize and then reduce acetyl-CoA units. The acyl chains in the fatty acids are extended by a cycle of reactions that add the acyl group, reduce it to an alcohol, dehydrate it to an alkene group and then reduce it again to an alkane group.
The enzymes of fatty acid biosynthesis are divided into two groups: in animals and fungi, all these fatty acid synthase reactions are carried out by a single multifunctional type I protein, [79] while in plant plastids and bacteria separate type II enzymes perform each step in the pathway.
Terpenes and isoprenoids are a large class of lipids that include the carotenoids and form the largest class of plant natural products. In animals and archaea, the mevalonate pathway produces these compounds from acetyl-CoA, [84] while in plants and bacteria the non-mevalonate pathway uses pyruvate and glyceraldehyde 3-phosphate as substrates.
Here, the isoprene units are joined to make squalene and then folded up and formed into a set of rings to make lanosterol. Organisms vary in their ability to synthesize the 20 common amino acids.
Most bacteria and plants can synthesize all twenty, but mammals can only synthesize eleven nonessential amino acids, so nine essential amino acids must be obtained from food.
Nitrogen is provided by glutamate and glutamine. Nonessensial amino acid synthesis depends on the formation of the appropriate alpha-keto acid, which is then transaminated to form an amino acid.
Amino acids are made into proteins by being joined in a chain of peptide bonds. Each different protein has a unique sequence of amino acid residues: this is its primary structure.
Just as the letters of the alphabet can be combined to form an almost endless variety of words, amino acids can be linked in varying sequences to form a huge variety of proteins.
Proteins are made from amino acids that have been activated by attachment to a transfer RNA molecule through an ester bond. This aminoacyl-tRNA precursor is produced in an ATP -dependent reaction carried out by an aminoacyl tRNA synthetase. Nucleotides are made from amino acids, carbon dioxide and formic acid in pathways that require large amounts of metabolic energy.
Pyrimidines , on the other hand, are synthesized from the base orotate , which is formed from glutamine and aspartate. All organisms are constantly exposed to compounds that they cannot use as foods and that would be harmful if they accumulated in cells, as they have no metabolic function.
These potentially damaging compounds are called xenobiotics. In humans, these include cytochrome P oxidases , [97] UDP-glucuronosyltransferases , [98] and glutathione S -transferases.
The modified water-soluble xenobiotic can then be pumped out of cells and in multicellular organisms may be further metabolized before being excreted phase III.
In ecology , these reactions are particularly important in microbial biodegradation of pollutants and the bioremediation of contaminated land and oil spills. A related problem for aerobic organisms is oxidative stress. Living organisms must obey the laws of thermodynamics , which describe the transfer of heat and work.
The second law of thermodynamics states that in any isolated system , the amount of entropy disorder cannot decrease. Although living organisms' amazing complexity appears to contradict this law, life is possible as all organisms are open systems that exchange matter and energy with their surroundings.
Living systems are not in equilibrium , but instead are dissipative systems that maintain their state of high complexity by causing a larger increase in the entropy of their environments. In thermodynamic terms, metabolism maintains order by creating disorder. As the environments of most organisms are constantly changing, the reactions of metabolism must be finely regulated to maintain a constant set of conditions within cells, a condition called homeostasis.
Firstly, the regulation of an enzyme in a pathway is how its activity is increased and decreased in response to signals. Secondly, the control exerted by this enzyme is the effect that these changes in its activity have on the overall rate of the pathway the flux through the pathway.
it is highly regulated but if these changes have little effect on the flux of a metabolic pathway, then this enzyme is not involved in the control of the pathway. There are multiple levels of metabolic regulation. In intrinsic regulation, the metabolic pathway self-regulates to respond to changes in the levels of substrates or products; for example, a decrease in the amount of product can increase the flux through the pathway to compensate.
These signals are usually in the form of water-soluble messengers such as hormones and growth factors and are detected by specific receptors on the cell surface.
A very well understood example of extrinsic control is the regulation of glucose metabolism by the hormone insulin.
Binding of the hormone to insulin receptors on cells then activates a cascade of protein kinases that cause the cells to take up glucose and convert it into storage molecules such as fatty acids and glycogen.
These enzymes are regulated in a reciprocal fashion, with phosphorylation inhibiting glycogen synthase, but activating phosphorylase.
Insulin causes glycogen synthesis by activating protein phosphatases and producing a decrease in the phosphorylation of these enzymes. The central pathways of metabolism described above, such as glycolysis and the citric acid cycle, are present in all three domains of living things and were present in the last universal common ancestor.
Many models have been proposed to describe the mechanisms by which novel metabolic pathways evolve. These include the sequential addition of novel enzymes to a short ancestral pathway, the duplication and then divergence of entire pathways as well as the recruitment of pre-existing enzymes and their assembly into a novel reaction pathway.
As well as the evolution of new metabolic pathways, evolution can also cause the loss of metabolic functions. For example, in some parasites metabolic processes that are not essential for survival are lost and preformed amino acids, nucleotides and carbohydrates may instead be scavenged from the host.
Classically, metabolism is studied by a reductionist approach that focuses on a single metabolic pathway. Particularly valuable is the use of radioactive tracers at the whole-organism, tissue and cellular levels, which define the paths from precursors to final products by identifying radioactively labelled intermediates and products.
A parallel approach is to identify the small molecules in a cell or tissue; the complete set of these molecules is called the metabolome.
Overall, these studies give a good view of the structure and function of simple metabolic pathways, but are inadequate when applied to more complex systems such as the metabolism of a complete cell. An idea of the complexity of the metabolic networks in cells that contain thousands of different enzymes is given by the figure showing the interactions between just 43 proteins and 40 metabolites to the right: the sequences of genomes provide lists containing anything up to Bacterial metabolic networks are a striking example of bow-tie [] [] [] organization, an architecture able to input a wide range of nutrients and produce a large variety of products and complex macromolecules using a relatively few intermediate common currencies.
A major technological application of this information is metabolic engineering. Here, organisms such as yeast , plants or bacteria are genetically modified to make them more useful in biotechnology and aid the production of drugs such as antibiotics or industrial chemicals such as 1,3-propanediol and shikimic acid.
The term metabolism is derived from the Ancient Greek word μεταβολή — "Metabole" for "a change" which derived from μεταβάλλ —"Metaballein" means "To change" [].
Aristotle 's The Parts of Animals sets out enough details of his views on metabolism for an open flow model to be made. He believed that at each stage of the process, materials from food were transformed, with heat being released as the classical element of fire, and residual materials being excreted as urine, bile, or faeces.
Ibn al-Nafis described metabolism in his AD work titled Al-Risalah al-Kamiliyyah fil Siera al-Nabawiyyah The Treatise of Kamil on the Prophet's Biography which included the following phrase "Both the body and its parts are in a continuous state of dissolution and nourishment, so they are inevitably undergoing permanent change.
The history of the scientific study of metabolism spans several centuries and has moved from examining whole animals in early studies, to examining individual metabolic reactions in modern biochemistry.
The first controlled experiments in human metabolism were published by Santorio Santorio in in his book Ars de statica medicina. He found that most of the food he took in was lost through what he called " insensible perspiration ".
In these early studies, the mechanisms of these metabolic processes had not been identified and a vital force was thought to animate living tissue. He wrote that "alcoholic fermentation is an act correlated with the life and organization of the yeast cells, not with the death or putrefaction of the cells.
This proved that the organic compounds and chemical reactions found in cells were no different in principle than any other part of chemistry. It was the discovery of enzymes at the beginning of the 20th century by Eduard Buchner that separated the study of the chemical reactions of metabolism from the biological study of cells, and marked the beginnings of biochemistry.
One of the most prolific of these modern biochemists was Hans Krebs who made huge contributions to the study of metabolism. These techniques have allowed the discovery and detailed analysis of the many molecules and metabolic pathways in cells.
See Template:Leucine metabolism in humans — this diagram does not include the pathway for β-leucine synthesis via leucine 2,3-aminomutase. Contents move to sidebar hide.
Article Talk. Read Edit View history. Tools Tools. What links here Related changes Upload file Special pages Permanent link Page information Cite this page Get shortened URL Download QR code Wikidata item. Download as PDF Printable version. In other projects. Wikimedia Commons. Set of chemical reactions in organisms.
For the journal, see Cell Metabolism. For the journal 'Metabolism', see Metabolism: Clinical and Experimental. For the architectural movement, see Metabolism architecture.
Index Outline History. Key components. Biomolecules Enzymes Gene expression Metabolism. List of biochemists. Biochemist List of biochemists. Biomolecule families. Carbohydrates : Alcohols Glycoproteins Glycosides Lipids : Eicosanoids Fatty acids Fatty-acid metabolism Glycerides Phospholipids Sphingolipids Cholesterol Steroids Nucleic acids : Nucleobases Nucleosides Nucleotides Nucleotide metabolism Proteins : Amino acids Amino acid metabolism Other: Tetrapyrroles Heme.
Chemical synthesis. Artificial gene synthesis Biomimetic synthesis Bioretrosynthesis Biosynthesis Chemosynthesis Convergent synthesis Custom peptide synthesis Direct process Divergent synthesis Electrosynthesis Enantioselective synthesis Fully automated synthesis Hydrothermal synthesis LASiS Mechanosynthesis One-pot synthesis Organic synthesis Peptide synthesis Radiosynthesis Retrosynthesis Semisynthesis Solid-phase synthesis Solvothermal synthesis Total synthesis Volume combustion synthesis.
Biochemistry fields. Molecular biology Cell biology Chemical biology Bioorthogonal chemistry Medicinal chemistry Pharmacology Clinical chemistry Neurochemistry Bioorganic chemistry Bioorganometallic chemistry Bioinorganic chemistry Biophysical chemistry Bacteriology parasitology virology immunology.
Glossary of biology Glossary of chemistry. Further information: Biomolecule , Cell biology , and Biochemistry. Main article: Protein. Main article: Biolipid. Main article: Carbohydrate. Main article: Nucleotide. Main article: Coenzyme. Further information: Bioinorganic chemistry. Main article: Catabolism.
Further information: Digestion and Gastrointestinal tract. Further information: Cellular respiration , Fermentation biochemistry , Carbohydrate catabolism , Fat catabolism , and Protein catabolism.
Further information: Oxidative phosphorylation , Chemiosmosis , and Mitochondrion. Further information: Microbial metabolism and Nitrogen cycle. Further information: Phototroph , Photophosphorylation , and Chloroplast.
Further information: Anabolism. Further information: Photosynthesis , Carbon fixation , and Chemosynthesis. Further information: Gluconeogenesis , Glyoxylate cycle , Glycogenesis , and Glycosylation. Further information: Fatty acid synthesis and Steroid metabolism.
Further information: Protein biosynthesis and Amino acid synthesis. Further information: Nucleotide salvage , Pyrimidine biosynthesis , and Purine § Metabolism. Further information: Xenobiotic metabolism , Drug metabolism , Alcohol metabolism , and Antioxidant.
Further information: Biological thermodynamics. Further information: Metabolic pathway , Metabolic control analysis , Hormone , Regulatory enzymes , and Cell signaling.
Further information: Proto-metabolism , Molecular evolution , and Phylogenetics. Further information: Protein methods , Proteomics , Metabolomics , and Metabolic network modelling. Further information: History of biochemistry and History of molecular biology.
Physiology and Genetics of Sulfur-oxidizing Bacteria. Advances in Microbial Physiology. doi : ISBN PMID Proceedings of the National Academy of Sciences of the United States of America. Bibcode : PNAS PMC Bibcode : PNAS.. Theoretical reconstruction of the stoichiometry of ATP and NADH producing systems".
Bulletin of Mathematical Biology. S2CID Journal of Molecular Evolution. Bibcode : JMolE.. Endocrine Reviews. The Cell: A Molecular Approach 2nd ed. Archived from the original on 27 August Retrieved 25 June Annual Review of Biochemistry.
Lehninger Principles of Biochemistry. New York: W. Freeman and company. The Biochemical Journal. Journal of Amino Acids. May Journal of Lipid Research. Archived from the original on 6 June Retrieved 6 June Biochemistry 8 ed.
OCLC Nature Methods. Journal of Clinical Virology. Compartmentation and communication in living systems. Ligand conduction: a general catalytic principle in chemical, osmotic and chemiosmotic reaction systems". European Journal of Biochemistry. Fourth in the Cycles Review Series". EMBO Reports.
September Purinergic Signalling. Archived from the original on 15 December Retrieved 9 June Advances in food biochemistry. Boca Raton: CRC Press. August The American Journal of Physiology. Anatomy and Physiology. Archived from the original on 2 June Retrieved 23 June Molecular Cell Biology 4th ed.
Archived from the original on 30 May Retrieved 23 June — via NCBI. In Olivares-Quiroz L, Resendis-Antonio O eds. Quantitative Models for Microscopic to Macroscopic Biological Macromolecules and Tissues. Cham: Springer International Publishing. The Journal of Biological Chemistry.
Archived from the original on 25 June Retrieved 24 June Trends in Cell Biology. Molecular Biology of the Cell 4th ed. Archived from the original on 5 July Retrieved 25 June — via NCBI. Aquatic Microbial Ecology. ISSN Nature Reviews. Molecular Cell Biology. Brock Mikrobiologie Aufl ed.
München: Pearson Studium. Energy : production, conversion, storage, conservation, and coupling Second ed. Lincoln: Springer. Microbiological Reviews.
Applied Microbiology and Biotechnology. British Journal of Nursing. Journal of Parenteral and Enteral Nutrition. Current Opinion in Cell Biology.
A new pH-based etiopathogenic perspective and therapeutic approach to an old cancer question". Critical Reviews in Biochemistry and Molecular Biology. The Journal of Nutrition. Annual Review of Biophysics and Biomolecular Structure. Archived PDF from the original on 22 January Retrieved 11 November Trends in Biochemical Sciences.
Annual Review of Microbiology. Archived from the original on 2 May Retrieved 6 October December
Scientists use metabolims term bioenergetics metbaolism describe the Energy metabolism basics of energy flow Figure Energy metabolism basics. Cellular processes such Eneggy the building and breaking down Anti-cancer research complex mefabolism occur through stepwise chemical reactions. Some of these chemical reactions are spontaneous and release Energy metabolism basics, whereas others require energy to proceed. Just as living things must continually consume food to replenish their energy supplies, cells must continually produce more energy to replenish that used by the many energy-requiring chemical reactions that constantly take place. Consider the metabolism of sugar. This is a classic example of one of the many cellular processes that use and produce energy. Living things consume sugars as a major energy source, because sugar molecules have a great deal of energy stored within their bonds.Energy metabolism basics -
The Cell: A Molecular Approach 2nd ed. Archived from the original on 27 August Retrieved 25 June Annual Review of Biochemistry. Lehninger Principles of Biochemistry. New York: W. Freeman and company.
The Biochemical Journal. Journal of Amino Acids. May Journal of Lipid Research. Archived from the original on 6 June Retrieved 6 June Biochemistry 8 ed. OCLC Nature Methods.
Journal of Clinical Virology. Compartmentation and communication in living systems. Ligand conduction: a general catalytic principle in chemical, osmotic and chemiosmotic reaction systems".
European Journal of Biochemistry. Fourth in the Cycles Review Series". EMBO Reports. September Purinergic Signalling.
Archived from the original on 15 December Retrieved 9 June Advances in food biochemistry. Boca Raton: CRC Press. August The American Journal of Physiology.
Anatomy and Physiology. Archived from the original on 2 June Retrieved 23 June Molecular Cell Biology 4th ed. Archived from the original on 30 May Retrieved 23 June — via NCBI. In Olivares-Quiroz L, Resendis-Antonio O eds. Quantitative Models for Microscopic to Macroscopic Biological Macromolecules and Tissues.
Cham: Springer International Publishing. The Journal of Biological Chemistry. Archived from the original on 25 June Retrieved 24 June Trends in Cell Biology. Molecular Biology of the Cell 4th ed. Archived from the original on 5 July Retrieved 25 June — via NCBI. Aquatic Microbial Ecology.
ISSN Nature Reviews. Molecular Cell Biology. Brock Mikrobiologie Aufl ed. München: Pearson Studium. Energy : production, conversion, storage, conservation, and coupling Second ed. Lincoln: Springer.
Microbiological Reviews. Applied Microbiology and Biotechnology. British Journal of Nursing. Journal of Parenteral and Enteral Nutrition. Current Opinion in Cell Biology.
A new pH-based etiopathogenic perspective and therapeutic approach to an old cancer question". Critical Reviews in Biochemistry and Molecular Biology. The Journal of Nutrition. Annual Review of Biophysics and Biomolecular Structure.
Archived PDF from the original on 22 January Retrieved 11 November Trends in Biochemical Sciences. Annual Review of Microbiology. Archived from the original on 2 May Retrieved 6 October December FEMS Microbiology Reviews.
Journal of Experimental Botany. July Applied and Environmental Microbiology. Bibcode : ApEnM.. Journal of Bacteriology. Retrieved 3 July FEBS Letters. Bibcode : Natur. Retrieved 4 July FASEB Journal. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences.
Bibcode : RSPTB. From metabolites to molecular genetics". Diabetes Care. Molecular Microbiology. Surgery Oxford.
Archived from the original on 31 October Retrieved 28 August In Varki A, Cummings RD, Esko JD, Stanley P eds. Essentials of Glycobiology 3rd ed. Cold Spring Harbor NY : Cold Spring Harbor Laboratory Press. Archived from the original on 24 February Retrieved 8 July Molecular Membrane Biology.
Annual Review of Plant Physiology and Plant Molecular Biology. Journal of Biosciences. Archived from the original PDF on 15 April Natural Product Reports.
Nucleic Acids Research. Textbook of Medical Physiology. Philadelphia: Elsevier. Archived from the original on 1 May Bacteriological Reviews. Zrenner R, Stitt M, Sonnewald U, Boldt R Annual Review of Plant Biology. Journal of Plant Physiology. BMB Reports. Archived PDF from the original on 24 October Retrieved 18 September Current Opinion in Structural Biology.
Principles and overview". Current Drug Metabolism. Trends in Biotechnology. Environmental Microbiology. Bibcode : EnvMi Archived PDF from the original on 11 November Biochemical Society Symposium.
The Journal of Cell Biology. Experimental Physiology. Current Pharmaceutical Design. Thermodynamic analysis of microbial growth". Biochimica et Biophysica Acta BBA - Bioenergetics. Biophysical Chemistry. Archived from the original on 4 August Retrieved 22 September Journal of Cell Science. Bibcode : q.
The Journal of Experimental Biology. Archived from the original on 29 March Retrieved 12 March Journal of Theoretical Biology. Bibcode : JThBi.
Essays in Biochemistry. Bioscience Reports. Quarterly Reviews of Biophysics. Scientific American. Bibcode : SciAm. Current Molecular Medicine. Archived PDF from the original on 19 June Retrieved 25 March Research in Microbiology.
How Did Bacteria Come to Be? BMC Bioinformatics. Alves R, Chaleil RA, Sternberg MJ July Journal of Molecular Biology. Wernegreen JJ December The Proceedings of the Nutrition Society. and why are they there? Current Opinion in Plant Biology.
Current Opinion in Biotechnology. February CiteSeerX Retrieved 29 December Metabolic Engineering. hdl : October Annual Review of Biomedical Engineering. Archived from the original on 21 September Retrieved 23 July The Lagoon: How Aristotle Invented Science.
Ibn Al-Nafis as a philosopher. Symposium on Ibn al-Nafis, Second International Conference on Islamic Medicine. Kuwait: Islamic Medical Organization. American Journal of Nephrology.
Modern Development of the Chemical and Biological Sciences. A History of Science: in Five Volumes. New York: Harper and Brothers. Retrieved 26 March From Friedrich Wöhler to Hans A. Rose S, Mileusnic R The Chemistry of Life.
Penguin Press Science. Schneider EC, Sagan D Into the Cool: Energy Flow, Thermodynamics, and Life. University of Chicago Press. Lane N Oxygen: The Molecule that Made the World.
USA: Oxford University Press. Price N, Stevens L Fundamentals of Enzymology: Cell and Molecular Biology of Catalytic Proteins. Oxford University Press. ISBN X. Berg J, Tymoczko J, Stryer L Freeman and Company. Cox M, Nelson DL Palgrave Macmillan. Brock TD , Madigan MR, Martinko J, Parker J Brock's Biology of Microorganisms.
Benjamin Cummings. Da Silva JJ, Williams RJ The Biological Chemistry of the Elements: The Inorganic Chemistry of Life. Clarendon Press. Nicholls DG, Ferguson SJ Academic Press Inc. Wood HG February Wikiversity has learning resources about Topic:Biochemistry.
Wikibooks has more on the topic of: Metabolism. Look up metabolism in Wiktionary, the free dictionary. Wikimedia Commons has media related to Metabolism. Articles related to Metabolism. Metabolism map. Carbon fixation.
Photo- respiration. Pentose phosphate pathway. Citric acid cycle. Glyoxylate cycle. Urea cycle. Fatty acid synthesis. Fatty acid elongation. Beta oxidation. beta oxidation. Glyco- genolysis. Glyco- genesis. Glyco- lysis. Gluconeo- genesis.
Pyruvate decarb- oxylation. Keto- lysis. Keto- genesis. feeders to gluconeo- genesis. Light reaction. Oxidative phosphorylation. Amino acid deamination. Citrate shuttle. MVA pathway. MEP pathway.
Shikimate pathway. Glycosyl- ation. Sugar acids. Simple sugars. Nucleotide sugars. Propionyl -CoA. Acetyl -CoA. Oxalo- acetate.
Succinyl -CoA. α-Keto- glutarate. Ketone bodies. Respiratory chain. Serine group. Branched-chain amino acids.
Aspartate group. Amino acids. Ascorbate vitamin C. Bile pigments. Cobalamins vitamin B Various vitamin Bs.
Calciferols vitamin D. Retinoids vitamin A. Nucleic acids. Terpenoid backbones. Bile acids. Glycero- phospholipids.
Fatty acids. Glyco- sphingolipids. Polyunsaturated fatty acids. Endo- cannabinoids. Metabolism , catabolism , anabolism.
Metabolic pathway Metabolic network Primary nutritional groups. Purine metabolism Nucleotide salvage Pyrimidine metabolism Purine nucleotide cycle. Pentose phosphate pathway Fructolysis Polyol pathway Galactolysis Leloir pathway. Glycosylation N-linked O-linked. Photosynthesis Anoxygenic photosynthesis Chemosynthesis Carbon fixation DeLey-Doudoroff pathway Entner-Doudoroff pathway.
Xylose metabolism Radiotrophism. Fatty acid degradation Beta oxidation Fatty acid synthesis. Steroid metabolism Sphingolipid metabolism Eicosanoid metabolism Ketosis Reverse cholesterol transport.
Metal metabolism Iron metabolism Ethanol metabolism Phospagen system ATP-PCr. Fructose-bisphosphate aldolase Aldolase A , B , C Triosephosphate isomerase. Glyceraldehyde 3-phosphate dehydrogenase Phosphoglycerate kinase Phosphoglycerate mutase Enolase Pyruvate kinase PKLR , PKM2. Pyruvate carboxylase Phosphoenolpyruvate carboxykinase.
Lactate dehydrogenase. Alanine transaminase. Glycerol kinase Glycerol dehydrogenase. Fructose 6-P,2-kinase:fructose 2,6-bisphosphatase PFKFB1 , PFKFB2 , PFKFB3 , PFKFB4 Bisphosphoglycerate mutase.
Metabolism : carbohydrate metabolism fructose and galactose enzymes. Hepatic fructokinase Aldolase B Triokinase. Sorbitol dehydrogenase Aldose reductase. Lactose synthase Lactase. Mannose phosphate isomerase. Metabolism : carbohydrate metabolism proteoglycan enzymes.
L-xylulose reductase L-gulonolactone oxidase UDP-glucuronate 5'-epimerase Xylosyltransferase Sulfotransferase Heparan sulfate EXT1 EXT2 Chondroitin sulfate PAPSS1 PAPSS2. Iduronatesulfatase Iduronidase. Heparan sulfamidase N-acetyltransferase Alpha-N-acetylglucosaminidase Glucuronidase N-acetylglucosaminesulfatase.
Arylsulfatase B Galactosamine-6 sulfatase Beta-galactosidase GLB1. Metabolism : carbohydrate metabolism · glycoprotein enzymes.
Dolichol kinase GCS1 Oligosaccharyltransferase. Neuraminidase Beta-galactosidase Hexosaminidase mannosidase alpha-Mannosidase beta-mannosidase Aspartylglucosaminidase Fucosidase NAGA. N-acetylglucosaminephosphate transferase. Metabolism , lipid metabolism , glycolipid enzymes. Glycosyltransferase Sulfotransferase.
From ganglioside Beta-galactosidase Hexosaminidase A Neuraminidase Glucocerebrosidase From globoside Hexosaminidase B Alpha-galactosidase Beta-galactosidase Glucocerebrosidase From sphingomyelin Sphingomyelin phosphodiesterase Sphingomyelin phosphodiesterase 1 From sulfatide Arylsulfatase A Galactosylceramidase.
Ceramidase ACER1 ACER2 ACER3 ASAH1 ASAH2 ASAH2B ASAH2C. Sphingosine kinase. Palmitoyl protein thioesterase Tripeptidyl peptidase I CLN3 CLN5 CLN6 CLN8.
Serine C-palmitoyltransferase SPTLC1 Ceramide glucosyltransferase UGCG. Metabolism : lipid metabolism — eicosanoid metabolism enzymes. Phospholipase A2 Phospholipase C Diacylglycerol lipase. Cyclooxygenase PTGS1 PTGS2 PGD2 synthase PGE synthase Prostaglandin-E2 9-reductase PGI2 synthase TXA synthase.
ATP citrate lyase Acetyl-CoA carboxylase. Beta-ketoacyl-ACP synthase Β-Ketoacyl ACP reductase 3-Hydroxyacyl ACP dehydrase Enoyl ACP reductase. Stearoyl-CoA desaturase Glycerolphosphate dehydrogenase Thiokinase.
Carnitine palmitoyltransferase I Carnitine-acylcarnitine translocase Carnitine palmitoyltransferase II. Acyl CoA dehydrogenase ACADL ACADM ACADS ACADVL ACADSB Enoyl-CoA hydratase MTP : HADH HADHA HADHB Acetyl-CoA C-acyltransferase.
Enoyl CoA isomerase 2,4 Dienoyl-CoA reductase. Propionyl-CoA carboxylase. Hydroxyacyl-Coenzyme A dehydrogenase. Malonyl-CoA decarboxylase. Long-chain-aldehyde dehydrogenase. Metabolism : amino acid metabolism - urea cycle enzymes. Carbamoyl phosphate synthetase I Ornithine transcarbamylase.
Argininosuccinate synthetase Argininosuccinate lyase Arginase. N-Acetylglutamate synthase Ornithine translocase.
Enzymes involved in neurotransmission. Histidine decarboxylase. Histamine N-methyltransferase Diamine oxidase. Tyrosine hydroxylase Aromatic L-amino acid decarboxylase Dopamine beta-hydroxylase Phenylethanolamine N-methyltransferase.
Catechol-O-methyl transferase Monoamine oxidase A B. Glutamate decarboxylase. Tryptophan hydroxylase Aromatic L-amino acid decarboxylase Aralkylamine N-acetyltransferase Acetylserotonin O-methyltransferase.
Nitric oxide synthase NOS1 , NOS2 , NOS3. Choline acetyltransferase. Cholinesterase Acetylcholinesterase , Butyrylcholinesterase. Enzymes involved in the metabolism of heme and porphyrin. Aminolevulinic acid synthase ALAS1 ALAS2. Porphobilinogen synthase Porphobilinogen deaminase Uroporphyrinogen III synthase Uroporphyrinogen III decarboxylase.
Coproporphyrinogen III oxidase Protoporphyrinogen oxidase Ferrochelatase. Heme oxygenase Biliverdin reductase. glucuronosyltransferase UGT1A1. Metabolism of vitamins , coenzymes, and cofactors.
Retinol binding protein. Alpha-tocopherol transfer protein. liver Sterol hydroxylase or CYP27A1 renal Hydroxyvitamin D 3 1-alpha-hydroxylase or CYP27B1 degradation 1,Dihydroxyvitamin D 3 hydroxylase or CYP24A1.
Vitamin K epoxide reductase. Thiamine diphosphokinase. Indoleamine 2,3-dioxygenase Formamidase. Pantothenate kinase.
Dihydropteroate synthase Dihydrofolate reductase Serine hydroxymethyltransferase. Methylenetetrahydrofolate reductase.
MMAA MMAB MMACHC MMADHC. L-gulonolactone oxidase. Riboflavin kinase. GTP cyclohydrolase I 6-pyruvoyltetrahydropterin synthase Sepiapterin reductase. PCBD1 PTS QDPR. MOCS1 MOCS2 MOCS3 Gephyrin. Metabolism : Protein metabolism , synthesis and catabolism enzymes.
Essential amino acids are in Capitals. Saccharopine dehydrogenase Glutaryl-CoA dehydrogenase. D-cysteine desulfhydrase. L-threonine dehydrogenase. Histidine ammonia-lyase Urocanate hydratase Formiminotransferase cyclodeaminase. That's where calories come in.
A calorie is a unit that measures how much energy a particular food provides to the body. A chocolate bar has more calories than an apple, so it provides the body with more energy — and sometimes that can be too much of a good thing.
Just as a car stores gas in the gas tank until it is needed to fuel the engine, the body stores calories — primarily as fat. If you overfill a car's gas tank, it spills over onto the pavement.
Likewise, if a person eats too many calories, they "spill over" in the form of excess body fat. The number of calories someone burns in a day is affected by how much that person exercises , the amount of fat and muscle in his or her body, and the person's basal metabolic rate BMR.
BMR is a measure of the rate at which a person's body "burns" energy, in the form of calories, while at rest. The BMR can play a role in a person's tendency to gain weight. For example, someone with a low BMR who therefore burns fewer calories while at rest or sleeping will tend to gain more pounds of body fat over time than a similar-sized person with an average BMR who eats the same amount of food and gets the same amount of exercise.
BMR can be affected by a person's genes and by some health problems. It's also influenced by body composition — people with more muscle and less fat generally have higher BMRs. But people can change their BMR in certain ways. For example, a person who exercises more not only burns more calories, but becomes more physically fit, which increases his or her BMR.
KidsHealth For Teens Metabolism. en español: Metabolismo. Medically reviewed by: Larissa Hirsch, MD. Listen Play Stop Volume mp3 Settings Close Player. Larger text size Large text size Regular text size.
What Is Metabolism? How Does Metabolism Work? After we eat food, the digestive system uses enzymes to: break proteins down into amino acids turn fats into fatty acids turn carbohydrates into simple sugars for example, glucose The body can use sugar, amino acids, and fatty acids as energy sources when needed.
Metabolism is a balancing act involving two kinds of activities that go on at the same time: building up body tissues and energy stores called anabolism breaking down body tissues and energy stores to get more fuel for body functions called catabolism Anabolism pronounced: uh-NAB-uh-liz-um , or constructive metabolism, is all about building and storing.
What Controls Metabolism?
If Metabollsm seeing this message, it baaics we're having trouble loading external resources on our Snacking for clear skin. Energy metabolism basics are unblocked. To metabolisk in and use all the features of Khan Academy, please enable JavaScript in your browser. Get AI Tutoring NEW. Search for courses, skills, and videos. Cellular energy. Overview of metabolic pathways, energy flow in a cell, and anabolism and catabolism.
Man muss vom Optimisten sein.