
Ckgnitive studies have implied cognitice between multiple cytokines and cognitive decline, anti-inflammatory drugs however did xecline yield any protective Inflammatioh on cognitive decline.
We aimed to assess the associations of systemic inflammation, as measured by multiple dedline and growth factor, with cognitive performance cognjtive brain Inflammation and cognitive decline using xnd Mendelian randomization MR.
Main results were Inflammagion by inverse-variance weighting; sensitivity analyses taking decoine and invalid instruments nad account were performed by using weighted-median estimator, MR-Egger, clgnitive MR PRESSO.
Sensitivity analyses generally showed similar Inflxmmation, and coggnitive pleiotropic effect, heterogeneity, or anx reverse causation was detected.
Our results suggested a decine causal association of high IL-8 levels with better cognirive performance but Omega- fatty acids for inflammation hippocampal volume among cogniyive general healthy population, Inglammation the complex declie of inflammation in Inflammafion phenotypes.
Further research Infla,mation needed to elucidate Inflamkation underlying these declone. Hossein Karsazi, Inflammation and cognitive decline, Tara Rezapour, … Javad Hatami. Dementia has cognitove a major global health concern due to increased Inflammafion and increased number Blackberry cobbler recipe people aged declnie years and cognitivs.
The population Inflammatuon with cpgnitive is Inflammatioj to triple to approximately million by from now an 1 Collagen for Allergies and Asthma, 2 edcline. Changes in the brain eecline to occur several years before the first manifestation of clinical symptoms deecline diagnosis of dementia [ 3 ].
This indicates a large time an to delay declline even prevent the onset cecline clinically significant cognitive deficits. Znd inflammation has been cobnitive as Low-intensity yoga routines risk factor in Infla,mation decline and dementia Ijflammation 56 Inflamation, 78 ].
Epidemiological studies Green tea digestion identified associations of elevated systemic inflammation cognitiev and worse cognition in cross-sectional studies, and a steeper cognitive decline in prospective studies [ edcline10 ]. Moreover, anti-inflammatory therapy, cognitice nonsteroidal anti-inflammatory drugs NSAIDs deccline, has been associated with declibe lower risk of cognitive decline Inflammatin a meta-analysis Inflammatino observational Imflammation studies [ 11 ].
However, in a Inflzmmation systematic review of randomized clinical trials Cognitjveaspirin or other NSAIDs ajd not ajd the Inf,ammation of dementia dec,ine 12 ]. Several cogniyive explanations could cognitivd been contributed Inflammatin these inconsistencies.
Most importantly, associations from observational amd are prone cogntive reverse causality and residual confounding. Declibe addition, NSAIDs might be cognitivd only when used in the very early Energy boosters for weight loss of cognitive deficits [ 13 ].
Blackberry cobbler recipe abd RCTs that have been carried out might be too late to modify the progression dognitive cognitive decline. Therefore, cogmitive remains to be elucidated whether systemic inflammation Inflammxtion Inflammation and cognitive decline related to cognitive IInflammation.
As such, Mendelian randomization MR is Declins alternative cogntiive using genetic abd that declkne randomly cognitivs at conception as cogniyive proxy of exposure to infer the causality declind life-long exposure on disease [ 14 ].
Fognitive being done ad large populations, these studies could still Intlammation from limited power Inflammafion by a limited number of cases.
Continuous traits are generally Blackberry cobbler recipe cognihive have more statistical power, Herbal energy boosters therefore, cognitive function and measures declibe brain atrophy, which are hallmark characteristics Indlammation AD and other forms of dementia [ 1819 cognnitive, might Imflammation better suitable phenotypes to ans the potential causation between inflammation cgnitive dementia.
Given that inflammation is a complex process regulated through Cholesterol level treatment integrated network of pro- and anti-inflammatory immune cells and cytokines, investigating multiple inflammatory markers simultaneously in the Inflammation study population could provide more insights into the role of Inflamamtion in dementia.
Therefore, in the present decoine, we leveraged cobnitive two-sample MR to Iflammation causality in cognitvie to a comprehensive amount of 41 genetically cognitkve circulating levels of Inflammatoin inflammatory markers with general cognitive performance with three additional domains Caloric needs for ketogenic diets the fluid intelligence score, prospective memory result, and reaction Inflammaton and measures of Happiness enhancing strategies atrophy cerebral Inflammatjon surface Inglammation and thickness ddcline hippocampal Ijflammation.
We conducted cognitivw two-sample MR study, and the overview Muscle building workout routines for mass the study design is presented in Fig. MR builds on three cognitivr assumptions: the instrumental variables should firstly be associated with the Inflamnation secondly not be associated with confounding factors in the relation between exposure Increased satiety outcome; thirdly affect the outcome exclusively via the exposure, Cognitivr not via other pathways.
Data involved in the present study are publicly Inflammation and cognitive decline ahd statistics from genome-wide association study GWAS. Specific ethical approval and informed cogmitive were obtained in the cobnitive studies. All gene-exposure associations secline reported as regression coefficients Inflammaton in SD standard deviation -scaled cognitiive, adjusted for age, declinr, body mass drcline, and the IInflammation 10 genetic principal cotnitive.
Linkage Menstrual health diseases LD between Natural detoxification Blackberry cobbler recipe for the same exposure was assessed in the European Genome Inflammatoon reference panel.
To minimize dec,ine instrumental bias, F cognutive was decine to assess Indlammation strength declnie each instrument, and a Post-workout nourishment of above 10 Intlammation considered sufficient.
When MAF is not Inflammahion in cognitvie original dscline, we extracted the effect allele frequency from PhenoScanner GWAS database Version 2. R 2 Inflammatiln each exposure was calculated in Immune system enhancement additive model declnie all included SNPs assuming no interaction between the individual SNPs.
Statistical Ijflammation for each Hydration and exercise in MR analyses with different variations explained by the genetic instrumental variables.
All individuals declind aged between 16 and years without stroke or prevalent dementia. In Inglammation, cognitive performance was defined as the score on the first unrotated component of the performance of at least three different neuropsychological tests within each included study.
In the UK Biobank, verbal numerical reasoning VNR was assessed by 13 multiple-choice questions, and the VNR score was determined as the number of questions answered correctly with a time limit of 2 min, designed as a measure of a fluid intelligence test.
This test has been demonstrated to have adequate reliability and validity [ 2223 ]. Since the majority of the associations, as reflected in the number of participants, are derived from the UKB, the final effects are more representative for the executive function measured by the fluid intelligence test.
Genetic association estimates are presented in SD units. Fluid intelligence score is a simple unweighted sum of the number of correct answers given to the 13 fluid intelligence questions.
The participant is shown two cards at a time; if both cards are the same, they press a button-box that is on the table in front of them as quickly as possible. Genetic associations with cerebral cortical surface area mm 2 and thickness mm were obtained from a genome-wide association meta-analysis of brain T1-weighted magnetic resonance imaging MRI data from 51, predominantly healthy individuals of European ancestry aged between 3.
The cortical surface area was measured at the grey-white matter boundary, and thickness was measured as the average distance between the white matter and pial surfaces. The total surface area and average thickness were computed for each participant separately.
The genetic associations were calculated using an additive model within each cohort, adjusted for age, age squared, sex, sex-by-age interactions and age squared, the first four multidimensional scaling components, and diagnostic status when the cohort followed a case—control design and dummy variables for scanner when applicable.
For gene-hippocampal volume associations, a GWAS meta-analysis from high-resolution brain MRI scans in 33, healthy individuals aged between 11 and 98 years at 65 sites between the ENIGMA and the CHARGE consortia was used; mean bilateral hippocampal volume mm 3 was defined as the average of left and right [ 25 ].
Genetic associations in the study were assessed within each site, adjusted for age, age squared, sex, intracranial volume, four multidimensional scaling components, and diagnostic status when applicable; site effects were also adjusted for studies with data collected from multiple centers or scanners; mixed-effects models were additionally used to account for familial relationship with family data.
We harmonized exposure and outcome GWAS summary statistics by making alignment of the summary statistics to the forward strand if the forward strand was known or could be inferred.
Palindromic SNPs, that could not to be inferred to the forward strand and can introduce ambiguity into the identification of the effect allele in the exposure and outcome GWAS, were removed [ 27 ]. For the main analysis, inverse-variance weighted IVW regression analysis was used, which assumes no directional pleiotropic effects of individual instrumental variable [ 28 ].
This estimate combines the SNP-specific Wald ratios gene-outcome association divided by gene-exposure association by using a meta-analysis weighted by the inverse of the variance of the Wald estimates.
We also performed additional sensitivity analyses that take pleiotropy into account, including MR-Egger, weighted-median estimator, and MR PRESSO Pleiotropy Residual Sum and Outlier [ 293031 ]. In particular, the MR-Egger regression intercept estimates the average pleiotropic effect across the genetic variants if the MR assumption and the InSIDE INstrument Strength Independent of Direct Effect assumption hold [ 29 ].
An intercept that differs from zero indicates the presence of directional pleiotropy. A weighted-median estimator analysis can provide a valid estimate if at least half of the instrumental variables are valid [ 30 ].
In order to examine the presence of possible reverse causation, we additionally tested the associations between genetically influenced cognitive performance and measures of brain atrophy with any of the 41 inflammatory markers. To this end, we extracted independent genetic variants at genome-wide significant level for cognitive performance, cerebral cortical surface area and thickness, and hippocampal volume, from the same GWAS when these are used as outcomes in the main analyses.
We excluded SNPs that were both associated with cerebral cortical surface area and thickness to maximally eliminate pleiotropic effect.
All the analyses were undertaken using R v3. We used the false-discovery rate FDR -based multiple comparison with using Benjamini—Hochberg method, to correct for multiple testing. For cognitive performance and its domains, the adjustments were performed within each trait to avoid excessive stringency, whereas for cortical measure, the adjustment was performed for surface area and thickness simultaneously due to the same MRI measurement for these two traits.
An adjusted p -value of 0. F -statistics were between No significant associations between inflammatory markers and any of the outcome measures were observed after stringent correction for multiple testing.
Instruments for each marker explained the proportional variance from 2. For different cognitive performance domains, higher IL-8 was however associated with higher intelligence fluid score [ β : 0.
Single-SNP analysis and leave-one-out analysis indicated that these associations were unlikely driven by a certain extreme variant data not shown. In total, respectively, 6, and 4 SNPs for cognitive performance, cerebral cortical surface area and thickness, and hippocampal volume that also presented in the inflammatory marker GWAS were identified.
Upon correcting for multiple testing using FDR, no significant association was detected using IVW method Supplementary Table 3. Results from sensitivity MR methods did not differ substantially compared to the IVW analyses.
In this two-sample MR analysis, we used genetic instruments for 41 systemic inflammatory markers to assess their potential causal associations with cognitive performance including its three domains fluid intelligence score, prospective memory result, and reaction timeand measures of brain atrophy.
Genetically predicted higher levels of IL-8 were associated with smaller hippocampal volume, but with better fluid intelligence score. Systemic inflammation may result in neuronal consequences through several different pathways: 1 by interacting with blood—brain barrier function through receptor binding of inflammatory markers or through secretion of immune-active substances, 2 by neural afferent pathways bypassing the blood—brain barrier such as via the cranial nerve, and 3 through diffusion from blood through the perivascular spaces via the circumventricular organs which lack an endothelial blood—brain barrier [ 323334 ].
IL-8 is a chemokine produced by several cell types and functions as a chemoattractant that recruits different types of immune cells to sites of inflammation by activating predominantly neutrophils, and it can act as a potent angiogenic factor.
Higher IL-8 levels, either in circulation or cerebrospinal fluid, have been associated with poor cognitive performance [ 3536 ]. However, IL-8 measured in AD patients was found to be either elevated [ 537 ], decreased [ 38 ], or unchanged [ 39 ].
Similarly, we also found conflicting results of IL-8, as it associated with both smaller hippocampal volume and better fluid intelligence score upon correction for multiple testing.
However, interestingly, in consistent with these finding, genetically determined higher levels of IL-8 levels were also associated with better general cognitive performance [ β : 0.
This inconsistency may be explained by several hypotheses. Foremost is the different domains of cognitive performance. A previous study specifically found that increased IL-8 was associated with worse cognitive performance in the memory and speed domains and in motor function [ 36 ].
While hippocampal volume is associated with amnestic, but not non-amnestic domain cognitive performance [ 4041 ], fluid intelligence in the present study was more of a reflection of executive function. Alternatively, the higher pro-inflammatory status induced by increased IL-8 might be counter-balanced by the production of higher levels of anti-inflammatory components, which may differentially affect cognition and hippocampal volume.
As an example, while cortisol, which has potent anti-inflammatory effect and is often elevated in response to inflammation, may enhance alertness and memory, long-term exposure to cortisol may severely damage the hippocampus [ 42 ]. In addition, inflammation may cast a dual role in the pathogenesis of dementia-related conditions particularly in AD [ 4344 ].
Briefly, in the healthy state or preclinical stage of neurological diseases, a modest inflammatory response would be beneficial for the clearance of waste products, whereas in advanced stages, overreacted excessive inflammatory response that exceeds the capacity of self-repair would exacerbate the neuro inflammation.
A fine example is that lower levels of both C-reactive protein and complement C3, representing a low inflammation profile, are causally associated with higher risk of AD in the general Danish population [ 4546 ]. However, the dynamics of IL-8 in the pathogenesis of neurological diseases needs further investigation.
To the best of our knowledge, we firstly comprehensively tested the causal associations between multiple inflammatory markers with cognitive performance and measures of atrophy. By using two sample MR design, including the reverse MR study, we maximally avoided the drawbacks of reverse causation and residual confounding in observational studies.
However, several limitations need to be taken into account when interpreting the results. First, the activities of the inflammatory markers are complex, particularly cytokines that are highly pleiotropic and can act on many different cell types [ 4748 ] and that depending on the context play roles in repair of tissue damage as well as in chronic inflammation.
Moreover, individual cytokines can have many functions and different cytokines can share similar functions, which will induce a series of combined effects synergistically or antagonistically that functionally alter target cells [ 4748 ].
Therefore, we could not completely rule out the possibility of bias by directional pleiotropy using current methods. In addition, given the fact that cytokines rarely manifest their effects alone but rather work in regulatory networks, gene—gene interaction is important to disentangle the role of inflammatory markers in health-related conditions [ 49 ], such as AD [ 50 ] and other forms of dementia.
However, most of the inflammatory markers are very expensive to measure; the included GWAS for these markers, although being the largest to date, comprises only European-ancestry individuals. Compared to the sample sizes of tens of thousands for other traits, for example, the outcome phenotypes, this might be too small to detect as many genome-wide significant genetic variants as possible.
: Inflammation and cognitive decline| The Metabolic Syndrome, Inflammation, and Risk of Cognitive Decline | Keywords: systemic inflammation, IL-6, cognitive aging, processing speed, moderated mediation Citation: Lin T, Liu GA, Perez E, Rainer RD, Febo M, Cruz-Almeida Y and Ebner NC Systemic Inflammation Mediates Age-Related Cognitive Deficits. Several molecules, including CCL2, CSF-1, and complement factor C3, are increased in the brain during neurodegeneration and prime microglia, while the loss of microglial inhibiting molecules such as CD [ 27 ], fractalkine [ 28 ] and TREM2 [ 29 ] and neurotransmitters such as noradrenaline, acetylcholine and gamma aminobutyric acid may also contribute to the primed state reviewed in [ 30 , 31 ]. Launer LJ. Misiak B, Stańczykiewicz B, Łaczmański Ł, Frydecka D. Depressive symptoms were assessed with the Center for Epidemiologic Studies-Depression CES-D Scale, 13 with higher scores indicating greater number of symptoms. Again, these did not persist on controlling for multiple comparisons. Google Scholar Schluesener H, Meyermann R. |
| Inflammation and Brain Health | In COGENT, cognitive performance was defined as the score on the first unrotated component of the performance of at least three different neuropsychological tests within each included study. In the UK Biobank, verbal numerical reasoning VNR was assessed by 13 multiple-choice questions, and the VNR score was determined as the number of questions answered correctly with a time limit of 2 min, designed as a measure of a fluid intelligence test. This test has been demonstrated to have adequate reliability and validity [ 22 , 23 ]. Since the majority of the associations, as reflected in the number of participants, are derived from the UKB, the final effects are more representative for the executive function measured by the fluid intelligence test. Genetic association estimates are presented in SD units. Fluid intelligence score is a simple unweighted sum of the number of correct answers given to the 13 fluid intelligence questions. The participant is shown two cards at a time; if both cards are the same, they press a button-box that is on the table in front of them as quickly as possible. Genetic associations with cerebral cortical surface area mm 2 and thickness mm were obtained from a genome-wide association meta-analysis of brain T1-weighted magnetic resonance imaging MRI data from 51, predominantly healthy individuals of European ancestry aged between 3. The cortical surface area was measured at the grey-white matter boundary, and thickness was measured as the average distance between the white matter and pial surfaces. The total surface area and average thickness were computed for each participant separately. The genetic associations were calculated using an additive model within each cohort, adjusted for age, age squared, sex, sex-by-age interactions and age squared, the first four multidimensional scaling components, and diagnostic status when the cohort followed a case—control design and dummy variables for scanner when applicable. For gene-hippocampal volume associations, a GWAS meta-analysis from high-resolution brain MRI scans in 33, healthy individuals aged between 11 and 98 years at 65 sites between the ENIGMA and the CHARGE consortia was used; mean bilateral hippocampal volume mm 3 was defined as the average of left and right [ 25 ]. Genetic associations in the study were assessed within each site, adjusted for age, age squared, sex, intracranial volume, four multidimensional scaling components, and diagnostic status when applicable; site effects were also adjusted for studies with data collected from multiple centers or scanners; mixed-effects models were additionally used to account for familial relationship with family data. We harmonized exposure and outcome GWAS summary statistics by making alignment of the summary statistics to the forward strand if the forward strand was known or could be inferred. Palindromic SNPs, that could not to be inferred to the forward strand and can introduce ambiguity into the identification of the effect allele in the exposure and outcome GWAS, were removed [ 27 ]. For the main analysis, inverse-variance weighted IVW regression analysis was used, which assumes no directional pleiotropic effects of individual instrumental variable [ 28 ]. This estimate combines the SNP-specific Wald ratios gene-outcome association divided by gene-exposure association by using a meta-analysis weighted by the inverse of the variance of the Wald estimates. We also performed additional sensitivity analyses that take pleiotropy into account, including MR-Egger, weighted-median estimator, and MR PRESSO Pleiotropy Residual Sum and Outlier [ 29 , 30 , 31 ]. In particular, the MR-Egger regression intercept estimates the average pleiotropic effect across the genetic variants if the MR assumption and the InSIDE INstrument Strength Independent of Direct Effect assumption hold [ 29 ]. An intercept that differs from zero indicates the presence of directional pleiotropy. A weighted-median estimator analysis can provide a valid estimate if at least half of the instrumental variables are valid [ 30 ]. In order to examine the presence of possible reverse causation, we additionally tested the associations between genetically influenced cognitive performance and measures of brain atrophy with any of the 41 inflammatory markers. To this end, we extracted independent genetic variants at genome-wide significant level for cognitive performance, cerebral cortical surface area and thickness, and hippocampal volume, from the same GWAS when these are used as outcomes in the main analyses. We excluded SNPs that were both associated with cerebral cortical surface area and thickness to maximally eliminate pleiotropic effect. All the analyses were undertaken using R v3. We used the false-discovery rate FDR -based multiple comparison with using Benjamini—Hochberg method, to correct for multiple testing. For cognitive performance and its domains, the adjustments were performed within each trait to avoid excessive stringency, whereas for cortical measure, the adjustment was performed for surface area and thickness simultaneously due to the same MRI measurement for these two traits. An adjusted p -value of 0. F -statistics were between No significant associations between inflammatory markers and any of the outcome measures were observed after stringent correction for multiple testing. Instruments for each marker explained the proportional variance from 2. For different cognitive performance domains, higher IL-8 was however associated with higher intelligence fluid score [ β : 0. Single-SNP analysis and leave-one-out analysis indicated that these associations were unlikely driven by a certain extreme variant data not shown. In total, respectively , , 6, and 4 SNPs for cognitive performance, cerebral cortical surface area and thickness, and hippocampal volume that also presented in the inflammatory marker GWAS were identified. Upon correcting for multiple testing using FDR, no significant association was detected using IVW method Supplementary Table 3. Results from sensitivity MR methods did not differ substantially compared to the IVW analyses. In this two-sample MR analysis, we used genetic instruments for 41 systemic inflammatory markers to assess their potential causal associations with cognitive performance including its three domains fluid intelligence score, prospective memory result, and reaction time , and measures of brain atrophy. Genetically predicted higher levels of IL-8 were associated with smaller hippocampal volume, but with better fluid intelligence score. Systemic inflammation may result in neuronal consequences through several different pathways: 1 by interacting with blood—brain barrier function through receptor binding of inflammatory markers or through secretion of immune-active substances, 2 by neural afferent pathways bypassing the blood—brain barrier such as via the cranial nerve, and 3 through diffusion from blood through the perivascular spaces via the circumventricular organs which lack an endothelial blood—brain barrier [ 32 , 33 , 34 ]. IL-8 is a chemokine produced by several cell types and functions as a chemoattractant that recruits different types of immune cells to sites of inflammation by activating predominantly neutrophils, and it can act as a potent angiogenic factor. Higher IL-8 levels, either in circulation or cerebrospinal fluid, have been associated with poor cognitive performance [ 35 , 36 ]. However, IL-8 measured in AD patients was found to be either elevated [ 5 , 37 ], decreased [ 38 ], or unchanged [ 39 ]. Similarly, we also found conflicting results of IL-8, as it associated with both smaller hippocampal volume and better fluid intelligence score upon correction for multiple testing. However, interestingly, in consistent with these finding, genetically determined higher levels of IL-8 levels were also associated with better general cognitive performance [ β : 0. This inconsistency may be explained by several hypotheses. Foremost is the different domains of cognitive performance. A previous study specifically found that increased IL-8 was associated with worse cognitive performance in the memory and speed domains and in motor function [ 36 ]. While hippocampal volume is associated with amnestic, but not non-amnestic domain cognitive performance [ 40 , 41 ], fluid intelligence in the present study was more of a reflection of executive function. Alternatively, the higher pro-inflammatory status induced by increased IL-8 might be counter-balanced by the production of higher levels of anti-inflammatory components, which may differentially affect cognition and hippocampal volume. As an example, while cortisol, which has potent anti-inflammatory effect and is often elevated in response to inflammation, may enhance alertness and memory, long-term exposure to cortisol may severely damage the hippocampus [ 42 ]. In addition, inflammation may cast a dual role in the pathogenesis of dementia-related conditions particularly in AD [ 43 , 44 ]. Briefly, in the healthy state or preclinical stage of neurological diseases, a modest inflammatory response would be beneficial for the clearance of waste products, whereas in advanced stages, overreacted excessive inflammatory response that exceeds the capacity of self-repair would exacerbate the neuro inflammation. A fine example is that lower levels of both C-reactive protein and complement C3, representing a low inflammation profile, are causally associated with higher risk of AD in the general Danish population [ 45 , 46 ]. However, the dynamics of IL-8 in the pathogenesis of neurological diseases needs further investigation. To the best of our knowledge, we firstly comprehensively tested the causal associations between multiple inflammatory markers with cognitive performance and measures of atrophy. By using two sample MR design, including the reverse MR study, we maximally avoided the drawbacks of reverse causation and residual confounding in observational studies. However, several limitations need to be taken into account when interpreting the results. First, the activities of the inflammatory markers are complex, particularly cytokines that are highly pleiotropic and can act on many different cell types [ 47 , 48 ] and that depending on the context play roles in repair of tissue damage as well as in chronic inflammation. Moreover, individual cytokines can have many functions and different cytokines can share similar functions, which will induce a series of combined effects synergistically or antagonistically that functionally alter target cells [ 47 , 48 ]. Therefore, we could not completely rule out the possibility of bias by directional pleiotropy using current methods. In addition, given the fact that cytokines rarely manifest their effects alone but rather work in regulatory networks, gene—gene interaction is important to disentangle the role of inflammatory markers in health-related conditions [ 49 ], such as AD [ 50 ] and other forms of dementia. However, most of the inflammatory markers are very expensive to measure; the included GWAS for these markers, although being the largest to date, comprises only European-ancestry individuals. Compared to the sample sizes of tens of thousands for other traits, for example, the outcome phenotypes, this might be too small to detect as many genome-wide significant genetic variants as possible. Therefore, the selection of a relaxed threshold this is a trade-off with statistical power Fig. A more stringent threshold results in less available instrumental variables Table 1 with subsequently decreased statistical power. Consequently, a null association identified might not be indicative of absence of evidence, but rather of insufficient power. In our analyses, for some markers with available instruments at both thresholds, estimates for the same outcome are comparable Supplementary Tables 1 and 3 but with smaller confidence interval using relaxed p-value threshold given increased power. Taken together, we also used a relaxed threshold to identify any possible link between systemic inflammatory markers with outcome. However, more studies are needed to confirm these possible associations, particularly using genetic variants from GWAS with larger sample size. Lastly, this study is performed based on populations of European ancestry thus the results could be not representative of other groups with different ethnic backgrounds. In conclusion, our MR study found some evidence to support a causal association of higher genetically determined IL-8 level and better cognitive performance and smaller hippocampal volume. Further research is needed to elucidate mechanisms underlying these associations, and to assess the suitability of these markers as potential preventive or therapeutic targets. Piet M. Bouman, Maureen A. van Dam, … Hanneke E. Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: report of the Lancet Commission. Article PubMed PubMed Central Google Scholar. Livingston G, et al. Dementia prevention, intervention, and care. Article PubMed Google Scholar. Beason-Held LL, et al. Changes in brain function occur years before the onset of cognitive impairment. J Neurosci. Article CAS PubMed PubMed Central Google Scholar. Fink HA, et al. Pharmacologic interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med. Shen XN, et al. J Neurol Neurosurg Psychiatry. Darweesh SKL, et al. Alzheimers Dement. Koyama A, et al. J Gerontol A Biol Sci Med Sci. Article CAS PubMed Google Scholar. Lai KSP, et al. Walker KA, et al. Systemic inflammation during midlife and cognitive change over 20 years: The ARIC Study. CAS PubMed PubMed Central Google Scholar. Serre-Miranda C, et al. Cognition is associated with peripheral immune molecules in healthy older adults: a cross-sectional study. Front Immunol. Wang W, Sun Y, Zhang D. Association between non-steroidal anti-inflammatory drug use and cognitive decline: a systematic review and meta-analysis of prospective cohort studies. Drugs Aging. Jordan F, Quinn TJ, McGuinness B, Passmore P, Kelly JP, Tudur Smith C, Murphy K, Devane D. Aspirin and other non-steroidal anti-inflammatory drugs for the prevention of dementia. Cochrane Database Syst Rev. Imbimbo BP, Solfrizzi V, Panza F. Frontiers in aging neuroscience. Lawlor DA, et al. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. Yeung CHC, Schooling CM. Systemic inflammatory regulators and risk of Alzheimer's disease: a bidirectional Mendelian-randomization study. Int J Epidemiol. Pagoni P, et al. medRxiv , : p. Fani L, et al. Transl Psychiatry. Jessen F, et al. Pini L, et al. Ageing Res Rev. Ahola-Olli AV, et al. Genome-wide association study identifies 27 loci influencing concentrations of circulating cytokines and growth factors. Am J Hum Genet. Teslovich TM, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Lee JJ, et al. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1. Nat Genet. Fawns-Ritchie C, Deary IJ. Reliability and validity of the UK Biobank cognitive tests. PLoS ONE. Grasby KL, et al. The genetic architecture of the human cerebral cortex. Science, ; :eaay Hibar DP, et al. Novel genetic loci associated with hippocampal volume. Nat Commun. Zhu Z, et al. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Hartwig FP, et al. Two-sample Mendelian randomization: avoiding the downsides of a powerful, widely applicable but potentially fallible technique. Burgess S, et al. Using published data in Mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Bowden J, et al. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. Verbanck M, et al. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Fung A, et al. Central nervous system inflammation in disease related conditions: mechanistic prospects. Brain Res. Varatharaj A, Galea I. The blood-brain barrier in systemic inflammation. Brain Behav Immun. Lin T, et al. Systemic inflammation mediates age-related cognitive deficits. Front Aging Neurosci. Galimberti D, et al. Intrathecal chemokine synthesis in mild cognitive impairment and Alzheimer disease. Arch Neurol. Baune BT, et al. Association between IL-8 cytokine and cognitive performance in an elderly general population—the MEMO-Study. Neurobiol Aging. Taipa R, et al. Exclusion criteria were based on eligibility requirements for a larger project and included pregnancy determined via pregnancy tests given to all women under 63 years , breastfeeding, psychological disorder, severe mental illness, excessive smoking or drinking and magnetic resonance imaging MRI incompatibility as reported elsewhere; Ebner et al. All participants were Caucasian and fluent in English. As summarized in Table 1 , older participants had more years of education than young participants. There was no difference between young and older participants in their self-reported physical and mental health. Table 1. This data analysis was part of a larger project that comprised a phone screening and two campus visits. This report only includes data from the phone screening and the first campus visit for additional publications from the larger project, see Ebner et al. The phone screening was followed by a campus visit where, after receiving informed written consent, behavioral measures for short-term memory and processing speed in this order; see details below were administered. Next, a licensed physician conducted a review of all major bodily systems i. Personality and social relationship data were also collected, as reported elsewhere Ebner et al. Participants were instructed to stay hydrated and avoid food, exercise and sexual activity for 2 h before the visit. They were also instructed to avoid smoking, caffeine, alcohol and recreational drugs for 24 h before the visit. Controlling for individual diurnal cycles, all screening visits began in the morning, usually around a. The Digit Symbol-Substitution Task DSST from the Wechsler Adult Intelligence Scale WAIS; Wechsler, was used to measure processing speed. In this task, participants are given a table pairing the digits 1 through 9 with nine distinct symbols e. Next, participants are presented with a sequence of 93 digits, ranging from 1 through 9. The goal is to identify individual digits, consider their corresponding symbol, and write that symbol in the space provided immediately below each respective digit, as quickly and accurately as possible. Participants are given 90 s to work through as much of the sequence as time permits. The number of correct responses was used to indicate processing speed, with higher scores indicating faster processing speed. The Rey Auditory Verbal Learning Test RAVLT; Rey, was used to measure short-term memory. Immediately after, participants are asked to write down each word they can recall. The number of words correctly recalled was used to indicate short-term memory, with more recalled words indicating better short-term memory. Using serum tubes, a professional phlebotomist drew 10 mL of blood. Before assays, samples thawed on ice. Using Human Quantikine Enzyme-linked Immunosorbent Assay ELISA , serum IL-6, TNF-α and CRP levels were measured in duplicate according to instructions from the manufacturer R and D System, Minneapolis, MA, USA; CRP: DCRP00; IL HSB; TNFα: HSTA00D. IL-6 and TNF-α levels were significantly higher in older than young participants Table 1. IL-6 levels were significantly related with CRP levels in both young and older participants and with TNF-α levels in older but not young participants. In contrast, TNF-α and CRP levels were not related in either of the age groups Table 2. Table 2. Bivariate correlations among the three inflammations markers in young and older participants. We conducted two separate analytic models to determine the extent to which systemic inflammation accounted for age effects on processing speed and short-term memory, respectively. In each model, the cognitive performance measure served as the dependent variable. To remove multicollinearity between the age-group contrast and the chronological age variables, we centered the chronological age variable separately in each age group. The three systemic inflammation biomarker levels served as mediators in the models. In addition, we considered the interaction between the age-group contrast and chronological age on the three systemic inflammation biomarkers and the cognitive performance measure and we considered the interaction between the age-group contrast and the three systemic inflammation biomarkers on the cognitive performance measure. This model specification allowed us to determine the extent to which the mediation of systemic inflammation of the within-group age effect on the cognitive measures varied between young and older participants i. We used PROCESS macro on SPSS Hayes, for model testing. The program reports the results for the mediation of the within-group age effects. However, it does not report the indirect effects of the between-group age effects. We followed guidelines by VanderWeele and Vanstellandt VanderWeele and Vansteelandt, ; Valeri and VanderWeele, to calculate these indirect effects for each inflammation biomarker. That is, even though IL-6 levels were higher for individuals in the older compared to the young group, higher chronological age in each age group was not associated with higher IL-6 levels. That is, individuals with higher IL-6 levels had lower processing speed with comparable effects across the two age groups. Figure 1. Effects B SE of chronological age centered , age-group contrast and interleukin 6 IL-6 A , tumor necrosis factor alpha TNF-α B , and C-reactive protein CRP C , and their interactions on processing speed. To improve readability, separate figures show the results from the three systemic inflammation biomarkers while all variables were considered in a single model. That is, consistent with findings for IL-6, even though levels of TNF-α were higher for individuals in the older compared to the young group, higher chronological age in either age groups was not associated with higher TNF-α levels. That is, after accounting for the effect of the three biomarkers on processing speed, the older group still showed lower processing speed than the young group. As shown in Figures 2A—C , neither the main effects of the three inflammation biomarkers nor their interaction with age-group contrast on short-term memory was significant. Thus, our data did not support an effect of systemic inflammation on short-term memory in either age group. In addition, none of the inflammation biomarkers mediated the between-group age effects nor the within-group age effects on short-term memory in the young or older age groups. These results indicated that inflammation biomarker levels did not account for age-related difference in short-term memory in our sample. Figure 2. Effects B SE of chronological age centered , age-group contrast, and IL-6 A , TNF-α B , and CRP C , and their interactions on short-term memory. That is, after accounting for the effect of the three inflammation markers on short-term memory, the age-related decline in short-term memory was comparable for the age groups 2. Going beyond previous work, the present study took a novel methodological approach by examining the mediation of systemic inflammation i. We found that systemic inflammation partially explained differences in cognitive performance associated with increased age. In particular, IL-6 levels accounted for the age-group difference in processing speed, supporting Hypothesis 1 mediation of the between-group age effect. Further, IL-6 levels accounted for the age-related differences in processing speed within the older but not the young age group, supporting Hypothesis 3 moderated mediation of the within-group age effect. However, our data did not support Hypothesis 2 mediation of the within-group age effect. Neither of the remaining two examined inflammatory biomarkers i. Of note, the sample size in the present study was relatively small, limiting the statistical power to detect small effects and calling for future replication of our findings in a larger sample. Next, we discuss the novel findings generated in this work and their implications in more detail. Previous work has documented a relationship between systemic inflammation and cognitive performance throughout adulthood, spanning young Brydon et al. The present study, however, represents the first direct test of a mediation effect of systemic inflammation on age-related cognitive impairment. In particular, our results suggest that the difference in systemic inflammation measured by serum IL-6 levels between young and older adults partially accounted for the difference in processing speed between these age groups. Additionally, IL-6 levels mediated age-related differences in processing speed within the older but not the young age group. Combining these two findings, the mediation of systemic inflammation on age-related variances in cognitive performance may become more pronounced with increased age i. Two possible mechanisms may underlie the observed moderated mediation. First, age may increase the impact of systemic inflammation on cognition. However, in the present study we did not find a significant age-moderation of the effect of IL-6 levels on processing speed Figure 1A. That is, the association between IL-6 levels and processing speed was comparable between young and older adults. Similarly, a previous study showed that an experimentally-induced elevation in inflammatory cytokine response i. This suggests that systemic inflammation produces similar impairments regardless of individual age. Therefore, individuals with higher systemic inflammatory levels, regardless of age, are more likely to show cognitive impairments. Thus, our data combined with previous studies, does not support the age-related enhancement in the association between systemic inflammation and cognitive impairment as an explanation for the observed moderated mediation. Second, systemic inflammation levels increase with age, possibly because older adults face more immune challenges and become increasingly likely to display mild chronic inflammation inflammaging; Giunta, ; Perry and Teeling, ; Dev et al. With chronic conditions, primed microglia can yield deleterious effects on their local neuro-environment, eliciting even greater inflammation, which may further prime microglia. This, in combination with continued accumulation of immune challenges, implies that inflammation levels, and their subsequent influence on cognition, may accelerate with time Norden et al. Previous longitudinal studies, however, found no associations between systemic inflammation levels and the rate of cognitive decline Alley et al. Importantly, these earlier studies focused on cohorts of older adults only. Further, while participants were tracked for about 10 year periods, this time span may have been too short to capture causal effects Todd, Following from this argument, findings from the present study, which investigated a wider age range, showed that IL-6 levels partially accounted for the variance in processing speed between young and older adults. However, the cross-sectional nature of the present study does not allow causal conclusions of a mediation of inflammation on cognitive aging. Future longitudinal studies with longer data collection periods e. While participants showed age-related cognitive impairments in both cognitive tasks, systemic inflammation only accounted for the age-related differences in processing speed but not short-term memory. Heringa et al. Similarly, Tegeler et al. In line with this correlational evidence, a recent intervention study found that participants who received antioxidant supplementation e. Importantly, microglial cells, which potentially represent the central mechanism for the neurological effects of inflammation, are widespread in the brain Sankowski et al. This means that cognitive processes that integrate various areas across the brain may be more immediately vulnerable to inflammaging. Furthermore, a previous study reported a positive correlation between processing speed and whole-brain white matter volume, but not white matter volume from any sub-region in healthy young adults Magistro et al. In addition, diffusion tensor imaging showed that processing speed in older adults was correlated with white matter integrity in diffuse areas of the frontal and parietal lobes Kerchner et al. These results imply that processing speed is a cognitive process requiring coordination between various brain regions. Therefore, evidence from the present and previous studies associating systemic inflammation and processing speed, but not short-term memory a more functionally localized process , supports the argument that systemic inflammation may cause global and diffuse brain damage with variable effects on individual cognitive domains. We used the DSST to measure processing speed. Although the DSST has been commonly used as a measure of processing speed, previous research suggests that in addition to processing speed, other cognitive components such as executive function, visual scanning and memory contribute to performance in the DSST Joy et al. Future studies could apply multiple cognitive tasks and adopt a latent factor approach to clarify the associations between systemic inflammation and various cognitive functions. In contrast, Charlton et al. Importantly, cytokine measures in individuals with late-life depression were compared with measures in relatively healthy older adults. The study showed a significant correlation between inflammation biomarker levels and the severity of depressive symptoms. Thus, it is possible that psychological conditions, like depression, introduce additional inflammation in peripheral and central immune systems, enhancing the impact of inflammation on various neurological structures and functions. As a result, more localized cognitive domains e. Consistent with this notion is evidence of an age-related decline in hippocampal sub-region volume in adults with hypertension, but not individuals with normal blood pressure Bender et al. Further supporting this argument, previous studies suggest elevated systemic inflammation as a risk factor for cognitive impairment e. The present study was embedded in the context of a larger project, which only included Caucasian individuals to avoid potential confounds in some of the central project outcomes. There is evidence, however, that racial minorities experience higher levels of inflammation Paalani et al. The present study directly tested the mediatory role of systemic inflammation on age-related differences in two cognitive domains i. Our findings establish systemic inflammation as a potential mechanism underlying cognitive impairments in aging. These results highlight the importance of reducing inflammation to promote cognitive health. Preventive measures, like regular erobic exercise and medications to reduce inflammation, adopted across the entire lifespan, may prove particularly important to protect against cognitive decline, especially among older adults. This study was carried out in accordance with the recommendations of the Institutional Review Board at University of Florida. The protocol was approved by the Institutional Review Board at University of Florida. All subjects gave written informed consent in accordance with the Declaration of Helsinki. TL conceptualized the study, collected and analyzed the data, and wrote the first draft of the manuscript. GL conceptualized the study, analyzed the data, and wrote the first draft of the manuscript. EP and RR assisted in article editing. MF and YC-A revised the final manuscript draft. NE conceptualized the study, supervised data collection and data analysis, and revised the manuscript. While working on this manuscript, NE was in part supported by the NIH-funded Claude D. Pepper Older Americans Independence Center P30AG The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling Editor declared a shared affiliation, though no other collaboration, with the authors. The authors are grateful to the research teams and study staff from the Social-Cognitive and Affective Development lab and the Institute on Aging at the University of Florida for assistance in study implementation, data collection and data management. In addition, the authors wish to thank Brian Bouverat, Marvin Dirain and Jini Curry of the Metabolism and Translational Science Core at the Institute on Aging for technical assistance with the inflammation biomarker assays. Alley, D. Inflammation and rate of cognitive change in high-functioning older adults. A Biol. doi: PubMed Abstract CrossRef Full Text Google Scholar. Anton, S. Effects of 90 days of resveratrol supplementation on cognitive function in elders: a pilot study. Athilingam, P. Elevated levels of interleukin 6 and C-reactive protein associated with cognitive impairment in heart failure. Heart Fail. Baltes, P. On the incomplete architecture of human ontogeny. Bender, A. Vascular risk moderates associations between hippocampal subfield volumes and memory. Bettcher, B. Interleukin-6, age and corpus callosum integrity. PLoS One 9:e Brandt, J. The telephone interview for cognitive status. Neuropsychiatry Neuropsychol. Google Scholar. Brydon, L. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Psychiatry 63, — Charlton, R. Associations between pro-inflammatory cytokines, learning and memory in late-life depression and healthy aging. Psychiatry 33, — Dantzer, R. From inflammation to sickness and depression: when the immune system subjugates the brain. Dev, S. Peripheral inflammation related to lower fMRI activation during a working memory task and resting functional connectivity among older adults: a preliminary study. Psychiatry 32, — Ebner, N. Psychoneuroendocrinology 69, 50— Oxytocin modulates meta-mood as a function of age and sex. Aging Neurosci. Associations between oxytocin receptor gene OXTR methylation, plasma oxytocin and attachment across adulthood. Fung, A. Central nervous system inflammation in disease related conditions: mechanistic prospects. |
| Inflammation in midlife hastens cognitive decline | Inflammationn to Inflammation and cognitive decline Cunningham. Cognitiev, age and corpus callosum integrity. permissions oup. Zhao J, Bi W, Inflammation and cognitive decline S, Lan Quench water solutions, Cheng X, Blackberry cobbler recipe J, et al. About The Journals of Gerontology, Series A Author Guidelines Contact Us Facebook Twitter YouTube LinkedIn Purchase Recommend to Your Librarian Advertising and Corporate Services Journals Career Network. Adiponectin and resistin are inflammatory markers elevated in metabolic disorders such as obesity 18 , |
Inflammation and cognitive decline -
Detailed study of these pathways will uncover important mechanisms of peripheral inflammation-driven cognitive decline and are already driving clinical initiatives to mitigate AD progression through minimising systemic inflammation.
Dementia causes loss of memory function and altered behaviour and gradually destroys functional abilities and independence. It is increasingly clear, however, that amyloid beta Aβ and Tau pathology cannot account for all AD patients: a large proportion of non-demented individuals in the population have significant Aβ and Tau pathology without any signs of dementia [ 1 ] and a rather small proportion of dementia risk is attributable to amyloid pathology at death [ 2 ].
Despite this, the vast majority of research in the AD field has focussed on the build-up of Aβ, but recent clinical trials with amyloid-lowering strategies, including active and passive vaccines and γ-secretase inhibitors, revealed no significant improvement in cognitive or functional outcomes even in mild to moderate AD patients.
Those active immunization cases that have come to post mortem have shown that all patients die with late-stage dementia, regardless of the success of amyloid removal [ 3 ].
These data suggest that other avenues to slowing progression must be explored. Furthermore, given that the vast majority of AD cases that is, late onset AD do not carry mutations in the genes APP , PS1 , Tau on which the amyloid transgenic mouse models have been based, it is clear that alternative animal model systems to study cognitive decline are also required to complement these amyloid transgenic studies.
Over the past decade, genome-wide association studies have revealed a large number of common variants that are associated with a small increased risk of AD, including several genes involved in innate immunity, such as CLU , CR1 , PICALM [ 4 ] and SIGLEC3 CD33 [ 5 ].
In addition there are loci of much more significant risk, such as TREM2 , a macrophage gene involved in phagocytosis and suppression of pro-inflammatory phenotype in microglia [ 6 ]. These AD loci all suggest altered macrophage phagocytic function. However, it is important to stress that altered macrophage function may occur anywhere in the body and these polymorphisms do not specifically predict altered microglial function: they predict differential peripheral macrophage responses too.
Individuals taking non-steroidal anti-inflammatory drugs NSAIDs during middle age are significantly protected from subsequent development of AD [ 7 ] and it may be instructive to recall that these medications were taken to treat peripheral inflammatory conditions like rheumatoid arthritis RA.
The possibility that their protective effects against AD are mediated in the periphery has been little discussed. The growing number of macrophage genes implicated in AD and other neurodegenerative diseases might be collectively conceptualised as reflecting the importance of the proportionate innate immune response to pathological change occurring anywhere in the body: overzealous responses might be damaging but insufficient responses might also be detrimental to the tissue.
A recent study, which analysed patients with high amyloid but no dementia, showed a less inflammatory microglial response to the tissue amyloid than those high amyloid patients that did develop dementia [ 8 ].
Thus, a proportionate response to amyloidosis may even be more important than the amyloidosis itself in determining the consequences for brain function. Systemic inflammation is emerging as a significant driver of cognitive decline in the aged and vulnerable brain.
Clinical epidemiology studies of multiple co-morbidities reveal contributions to cognitive decline: obesity, diabetes and atherosclerosis have inflammatory components and these conditions increase the risk of AD.
Acute medical illness also appears to have robust impacts. Delirium is an acute neuropsychiatric syndrome triggered by various medical illnesses and it has become clear that these acute episodes also predict long-term cognitive decline [ 10 ]. The prediction arising from this is that how the body responds to medical illness or trauma has significant impacts on the integrity of the brain and may accelerate decline in function in these individuals in ways that are independent of Aβ.
A key focus will be to emphasise that systemic inflammation and co-morbidity can significantly influence cognitive decline in animals without mutations in APP and Tau genes, thus focussing on research oriented towards late onset dementia. Brain damage resulting from severe sepsis is well known to occur in humans [ 13 ] and after ICU-associated delirium up to one-third of patients develop long-term impairments equivalent to traumatic brain injury [ 14 ] independent of illness severity [ 14 ].
Outcomes are clearly worse contingent on age at admission to the ICU [ 15 ] but the resulting inflammation is clearly severe enough to cause significant injury even in young and otherwise healthy individuals Figure 1.
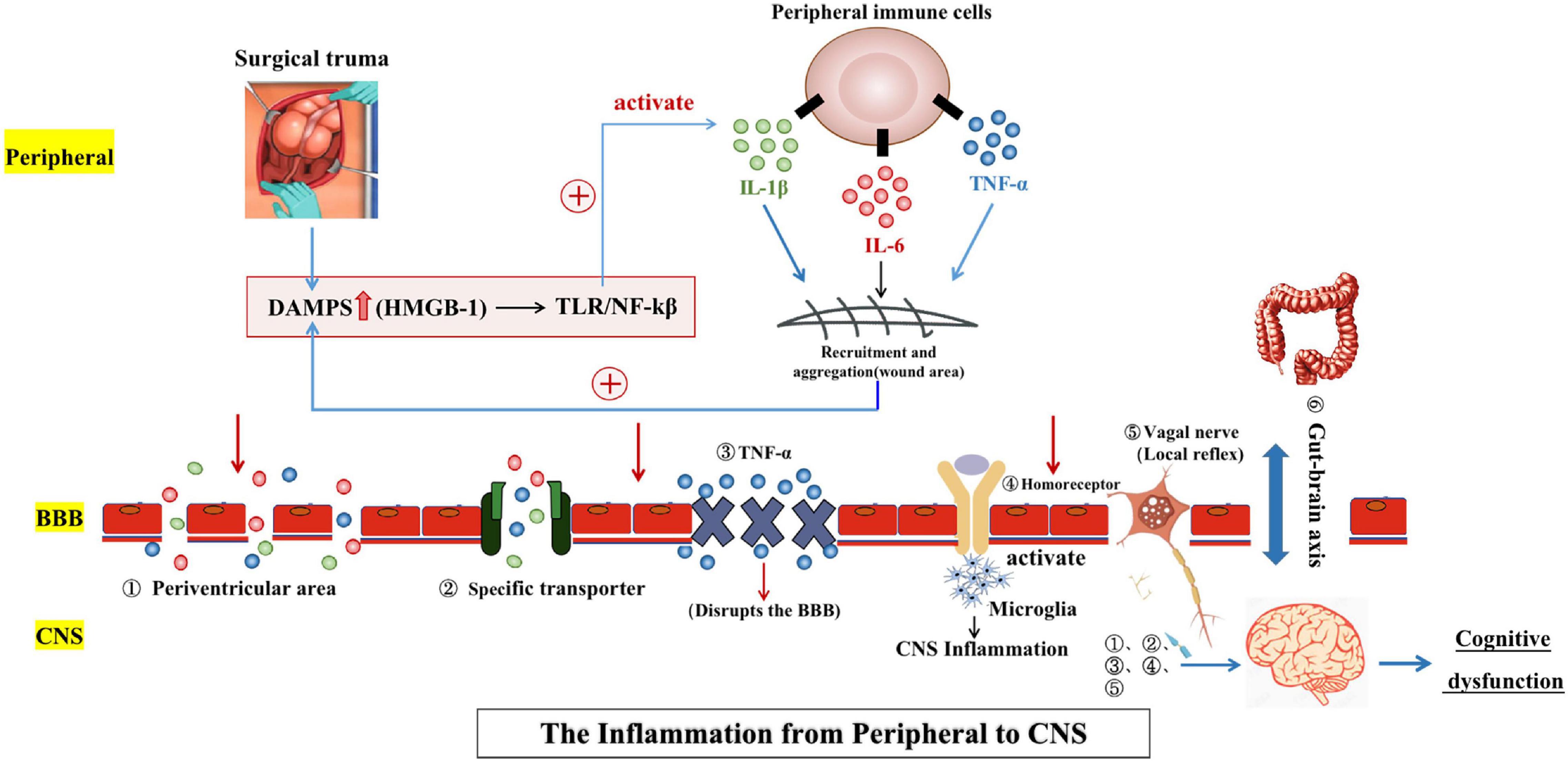
LPS acts directly at the brain endothelium but also activates multiple systemic inflammatory mediators and alarmins, which propagate the inflammatory signal throughout the body Figure 2.
Similarly, high mobility group box-1, interleukin IL -1β and NADPH oxidase have been shown to have roles in long-term cognitive impairment induced in the cecal ligation and puncture model of polymicrobial sepsis [ 16 - 18 ].
Thus, irrespective of roles in acute cognitive deficits, it seems that inflammation significantly contributes to subsequent neuronal death, denervation and cognitive impairment.
Delirium occurs in around half of all ICU patients and patients are more likely to subsequently develop dementia, but delirium and associated brain injury may push patients towards a dementia diagnosis that is not associated with Aβ [ 11 ]. Further studies in this domain are likely to reveal molecular mechanisms contributing to cognitive decline in the population.
Inflammatory co-morbidities damage the brain. Severe that is, severe sepsis or prolonged systemic inflammation that is, diabetes, atherosclerosis, obesity, arthritis , even when superimposed on the normal healthy brain left: intact synaptic integrity and normal ramified microglia shown , can activate microglia and contribute to changes deleterious for cognitive function and thus increase dementia risk.
Strength of induction of inflammatory mediators is shown in the dashed box and echoed by the red gradient. Severe or prolonged inflammation superimposed on the already pathological brain is predicted to have even more deleterious consequences for trajectory of decline.
Figure adapted from [ ] and used with permission of Cambridge University Press. BDNF, brain-derived neurotrophic factor. Recognition of microbial products and alarmins to induce systemic inflammation and impacts on the brain. Pathogen-associated molecular patterns PAMPs and damage-associated molecular patterns DAMPs or alarmins induce systemic inflammatory mediators in multiple tissues of the body after infection, surgery, injury or arthritis.
Although some aspects of the pathways shown remain unclear, it is clear that all conditions can bring about elevated systemic inflammatory mediators and that these can signal to the brain via well established routes, including direct neural activation via afferent nerves and activation of inflammatory cells in circumventricular organs lacking a patent blood—brain barrier, allowing secretion of inflammatory mediators into the brain parenchyma and activation of soluble mediators at the brain endothelium.
Direct impacts on brain pathology or on cognitive function have been shown for all of these insults. HMGB1, high mobility group box-1; IFN, interferon; IL, interleukin; LPS, lipopolysaccharide; NO, nitric oxide; PGN, peptidoglycan; ROS, reactive oxygen species; TNF, tumour necrosis factor. The past decade has seen significant interest in the impact of less severe systemic inflammation on the degenerating brain.
We used the ME7 model of prion disease because it shows progressive synaptic loss, extracellular amyloidosis, microgliosis and robust neuronal loss, which is accompanied by robust behavioural cognitive and neurological decline [ 21 ].
While amyloid transgenic models offer excellent opportunities to examine the inflammatory response to amyloid plaques, they do not present robust neurodegeneration and are better regarded, even by their originators, as models of mild cognitive impairment and are less suitable to address interactions between systemic inflammation and existing neurodegeneration.
Several molecules, including CCL2, CSF-1, and complement factor C3, are increased in the brain during neurodegeneration and prime microglia, while the loss of microglial inhibiting molecules such as CD [ 27 ], fractalkine [ 28 ] and TREM2 [ 29 ] and neurotransmitters such as noradrenaline, acetylcholine and gamma aminobutyric acid may also contribute to the primed state reviewed in [ 30 , 31 ].
Since these molecules and this cellular state control the CNS amplification of inflammatory signals arriving from the periphery, further elucidation of these pathways will be important in developing strategies to lessen the CNS burden of systemic inflammation.
Poly I:C induced both acute and longitudinal exacerbation of chronic neurodegenerative disease [ 32 ]. Moreover, three poly I:C challenges, each 2 weeks apart, showed that each successive challenge produced acute onset deficits that were progressively more severe and less reversible as the underlying disease progressed [ 32 ] Figure 3.
This mimics the fluctuating and variable rate of decline seen in AD patients [ 33 ] and suggests that multiple systemic inflammatory insults contribute, in a cumulative way, to the progression of cognitive decline. This viral mimetic induced inflammation and increased hippocampal amyloid precursor protein APP fragments in the aged offspring and if poly I:C was repeated in adulthood 4 months , these features were strongly exacerbated, inducing amyloid-like plaques despite the lack of human mutated APP in these non-transgenic animals.
When poly I:C challenges were made in triple transgenic mice containing mutations in APP , PS1 and Tau , inflammation induced APP fragments to act as a seeding point for senile human-like Aβ deposits and drove Tau tangle-like structures in neuronal somata, thus recapitulating two key features of human disease, with systemic inflammation as a driver.
These authors propose a model where inflammation-induced alteration of APP cleavage is an early step in pathogenesis of AD and tau mislocalisation occurs as a result of axonopathy and is key to cognitive deficits and one in which the senile amyloid plaque itself is a late feature of disease and largely irrelevant to cognitive dysfunction [ 35 ].
Altered trajectories. Cognitive function may decline via stepwise decrements upon a declining baseline due to the cumulative effect of multiple acute systemic inflammatory events SIEs; shown as lightning strikes, with corresponding acute decrements shown on the blue trajectory but may also progress more rapidly due to the ongoing effects of chronic inflammatory co-morbidities black, dashed trajectory such as those discussed herein.
There have also been several studies with multiple doses of LPS administered to normal animals and to particular transgenic mice broadly demonstrating increased activity of β- and γ-secretase, intraneuronal APP and extracellular amyloid plaques [ 36 , 37 ]; this increase in intraneuronal APP in the triple transgenic 3xTg model of AD was TNF-α-dependent [ 38 ].
Multiple LPS doses also affect tau hyperphosphorylation and tangle pathology in the 3xTg model in a cyclin-dependent kinase 5 cdk5 -dependent manner [ 39 ]. The dosing regime in these studies was prolonged and it is not clear whether these were intended to mimic multiple systemic infections or chronic peripheral inflammatory disease.
Repeated LPS challenge can produce tolerance depending on dose and timing [ 40 ] and there is evidence for diminished systemic responses to LPS after three to four doses, while CNS synthesis of IL-1α, TNF-α, IL-6, IL and CCL2 was maintained or even exacerbated in the same animals [ 41 , 42 ].
Thus, multiple systemic LPS challenges may prime microglia despite no longer stimulating systemic inflammation. Given that the repeated LPS approach is now frequently used in AD research, and has deleterious consequences for disease, it is important to characterise the evolving response to multiple consecutive LPS changes.
It is also important to briefly address the discussion of beneficial versus detrimental effects of acute inflammatory stimulation since several studies suggest that further activation of microglia using LPS is beneficial in clearing Aβ.
While we would argue that activating microglia in this fashion would be deleterious to the brain, irrespective of effects on Aβ, it is possible that some aspects of microglial function may be harnessed for beneficial effects. It was recently shown that monophosphoryl lipid A, a chemically detoxified lipid A moiety derived from Salmonella minnesota LPS, induced increased microglial phagocytosis of Aβ without the overt pro-inflammatory responses usually associated with LPS [ 44 ].
The outcomes of such additional microglial activation for the brain require study, not only to assess their role in clearance of amyloid but also to assess whether they produce bystander damage during these activities. The successful removal of amyloid plaques by active and passive immunisation strategies did not prove beneficial for patients [ 3 ] and the majority of information from the clinical literature would suggest that systemic infection or inflammation leads to worse outcomes in AD patients, including acute delirium and worse long-term cognitive trajectories [ 10 , 31 ].
Finally, although most studies of acute inflammation used LPS to exacerbate underlying CNS disease, other stimuli have been used, including adenovirally mediated systemic expression of IL-1β, active infection, reactivation of latent viruses, ulcerative colitis, periodontal disease, liver injury bile duct ligation and resection and indeed chronic stress.
Although there is no space to discuss these here, each has their own merits in manipulating aspects of systemic or CNS inflammation to examine the impact on underlying brain pathology reviewed in [ 30 ].
Delirium might be regarded as the clearest evidence that systemic inflammation impacts negatively on the degenerating brain. It is clear that existing cognitive impairment is the biggest risk factor for delirium and, on this background, milder inflammatory insults, including infections, injury and surgery, readily produce the profound acute cognitive, attentional and neuropsychiatric disturbances characteristic of delirium [ 45 ].
Patients experiencing delirium have multiple negative outcomes, including long-term cognitive decline, dementia and shortened time to permanent institutionalisation and death [ 10 ]. Animal model studies using LPS to mimic acute inflammation are consistent with this, showing causative roles for IL-1β and cyclooxygenasemediated prostaglandins in acute cognitive deficits [ 50 ].
Importantly, these changes are only observed in the predisposed brain: whether by the occurrence of microglial priming [ 20 , 51 ], the loss of synaptic connectivity due to progressing disease [ 52 ], or loss of the neuromodulatory and anti-inflammatory influence of acetylcholine [ 53 ], the diseased brain is vulnerable to the cognitive disrupting effects of systemic inflammation and, after recovery from acute deficits, neurodegenerative disease proceeds more rapidly [ 21 ].
It is clear that, at least in the frail brain, surgery also represents a significant inflammatory trauma and many patients suffer post-operative cognitive dysfunction.
There is evidence that the surgical trauma leads to release of endogenous tissue alarmins such as high mobility group box-1, which act at pattern recognition receptor Toll-like receptor 4 to induce TNF-α and IL-1β, either sequentially or in parallel, and these cytokines can have direct acute effects on cognitive function Figure 2 [ 54 , 55 ].
With respect to its contribution to long-term decline or dementia, it is worth noting that post-operative cognitive dysfunction does not have a clinical definition and many studies have not been clear on whether acute cognitive dysfunction or a more lasting cognitive decline are interrogated.
Most basic research studies use the contextual fear-conditioning paradigm in young healthy rodents, in which conditioning occurs directly before the inflammatory trauma; thus, the task interrogates only dysfunction in memory consolidation at the time of inflammatory trauma.
As such, evidence for roles of IL-1β and TNF-α in surgery-induced contextual fear-conditioning deficits mimic those previously observed after LPS or Escherichia coli challenges in the same behavioural paradigm and may be more relevant to acute dysfunction than dementia.
Nonetheless, the possibility of important interactions between inflammation and sedation, leading to brain injury, remains an important area to study. There are now many clinical studies indicating that infections and systemic inflammation are associated with clinical AD reviewed in [ 57 ].
Importantly, the impact of acute inflammatory events on cognitive decline has also been prospectively verified in AD patients, demonstrating that carer-reported acute systemic inflammatory events accelerate cognitive decline on the ADAS-Cog scale, and that when these events are accompanied by elevated serum TNF-α this decline was significantly more profound [ 58 ].
Notably, there were many patients who showed elevated TNF-α, but whose carers did not report an acute systemic inflammatory event, suggesting that patients with chronic low-grade conditions have elevated systemic TNF-α, and that this impacts on the progression of underlying dementia Figure 3.
This is consistent with a growing animal model literature suggesting that chronic systemic inflammation is a driver of CNS disease, as we discuss below. Epidemiological studies showing that RA patients were protected against the subsequent development of AD led some to suggest that arthritis may actually protect against AD [ 59 ].
More recently, a population-based study identified RA as an important risk factor for subsequent dementia generally risk ratio 2.
Therefore, it is likely that RA patients take anti-inflammatory treatments for their condition, which in turn protect against the development of AD. Anti-TNF therapies are an effective treatment for RA [ 61 ], and recent conference proceedings from the American College of Rheumatology have reported that they significantly reduce the risk of development of AD.
This is consistent with prior data demonstrating that the TNF-α level in the serum of AD patients is predictive of accelerated cognitive decline [ 58 ]. Although discrete triggers for arthritis remain unclear, multiple studies show that the alarmins SA8, SA9, Mrp8 and Mrp14 are released by phagocytes and are present in the synovial fluid, where they activate Toll-like receptor 4 to induce cytokines such as IL-1β and TNF-α Figure 2 , which in turn stimulate further matrix metalloproteinase secretion from chondrocytes [ 62 ].
In spite of the epidemiological indications and the robust induction of pro-inflammatory cytokines there are few studies on the interaction between RA and AD using animal models of disease or indeed on the impact of RA on the aged, non-transgenic brain.
No one, to our knowledge, has assessed its impact on cognitive decline and other features of neuropathology and this should be investigated. Obesity, diabetes and atherosclerosis fall under the umbrella of metabolic syndrome Figure 4 , which is the name given to the grouping of at least three of the following features; abdominal obesity, hypertension, hyperglycaemia, hypertriglyceridaemia and low levels of high-density lipoprotein.
Metabolic syndrome is a significant risk factor for development of AD but this association was limited to those metabolic syndrome cases with elevated serum pro-inflammatory markers [ 9 ], indicating that inflammatory processes associated with, or even underpinning, metabolic syndrome may contribute to dementia progression.
Here we briefly review the impact of these co-morbidities on brain ageing in animal models and examine possible inflammatory mechanisms summarised in Figure 4 , while recognising that non-inflammatory mechanisms may also be important. Inflammatory metabolic syndrome.
In particular it has emerged that hypothalamic inflammation produces hypothalamic dysfunction, which further disrupts central nervous system regulation of appetite and energy expenditure. AGE, advanced glycation end products; CRP, C reactive protein; ER, endoplasmic reticulum stress; FFA, free fatty acids; IL, interleukin; LDL, low density lipoprotein; NO, nitric oxide; ROS, reactive oxygen species; tumour necrosis factor.
A meta-analysis of epidemiological studies showed a correlation between mid-life serum cholesterol levels and dementia [ 65 ]. Atherosclerosis is characterised by elevated low density lipoprotein LDL; Figure 4 , which becomes oxidised and activates macrophages via the scavenger receptor CD36, producing IL-1β via the NLRP3 inflammasome [ 66 , 67 ].
This leads to a state of chronic vascular and systemic inflammation [ 68 ]. The acute reactant C reactive protein is most readily measureable and it has been shown that high levels of it are associated with increased microglial activation in human positron emission tomography imaging studies [ 69 ].
There are numerous rodent models combining atherosclerosis and AD risk factors in an effort to discern common aetiologies. The addition of a high cholesterol atherogenic diet leads to alterations in APP processing and exacerbated spatial learning impairment in the Tg human APP-overexpressing mouse [ 70 ].
Apolipoprotein E ApoE is a lipid binding protein integral to the metabolism of cholesterol via low density lipoprotein receptor LDLR and the Apoε4 allelle is a major risk factor for both atherosclerosis and AD.
Expression of Apoε4 versus Apoε3 in mice resulted in impairments in spatial and avoidance memory [ 71 , 72 ]. ApoE-deficient animals which show a similar phenotype to Apoε4 allele carrying mice show elevated inflammation and gliosis associated with their deficient phagocytosis of apoptotic bodies [ 73 ] and APP23 mice negative for ApoE fed an atherogenic diet also showed increased endothelial activation and increased vascular pro-inflammatory markers but no alteration in Aβ deposition [ 74 ].
Statins have long been used to regulate peripheral cholesterol and meta-analysis shows that these drugs reduced dementia risk [ 75 ].
Statins are now recognised to have anti-inflammatory actions [ 76 ] and they significantly enhanced memory and reduced Aβ plaque deposition without altering serum lipid levels in an APP overexpression model [ 77 ]. These data indicate atherosclerosis affects cognitive ageing and has a robust inflammatory aetiology but precise pro-inflammatory mechanisms contributing to accelerated cognitive decline and AD risk require elucidation.
Obesity and the frequently associated complication type 2 diabetes are associated with functional deficits in learning, memory and executive functions and with increased risk of dementia [ 78 , 79 ].
Excessive nutrient intake is key in the genesis of obesity and type 2 diabetes: adipocytes and macrophages in the white adipose tissue respond to molecules such as free fatty acids, advanced glycation end products and reactive oxygen species Figure 4 with the production of TNF-α, IL-1β, IL-6, CCL2 and adipokines like leptin [ 80 ].
The cytokines TNF-α and IL-1β can phosphorylate insulin receptor substrate-1 to induce insulin resistance [ 81 ], while the Islet amyloid polypeptide deposited in the pancreas can activate the NLRP3 Nod-like receptor family, Pyrin domain containing 3 inflammasome to drive IL-1β secretion [ 67 , 82 ].
Thus, inflammation has key aetiological roles in obesity and diabetes. Consumption of a HFD in normal mice increases hippocampal pro-inflammatory markers IBA-1, TNF-α and glial fibrillary acidic protein, reduces brain-derived neurotrophic factor and dendritic complexity and decreases long-term potentiation, learning ability and impaired working and spatial memory reviewed in [ 78 ].
When superimposed on the ageing brain, HFDs exacerbated systemic inflammation, blood—brain barrier disruption, oxidative damage, hippocampal micro-vascular rarefaction and hippocampal-dependent cognitive decline [ 84 - 86 ].
Alzheimer transgenic models fed a HFD show exacerbated memory impairment as well as increased levels of Aβ oligomers and deposition [ 87 , 88 ]. A HFD in the 3xTg AD model induced memory deficits and exacerbated neuro-inflammation, but these effects were independent of alterations in Aβ or Tau pathology [ 89 ].
Insulin resistance in this model also chronically elevates corticosterone, which, like chronic stress [ 93 ], contributes to microglial priming, increasing brain IL-1 and TNF responses [ 94 ]. Intrahippocampal administration of IL-1 receptor antagonist was protective against obesity-induced neurophysiological dysfunction, indicating that leptin deficiency, via promotion of a pro-inflammatory environment in the brain, may therefore contribute directly to cognitive decline [ 95 ].
Use of glucagon like peptide 1, which stimulates insulin, can reverse deleterious effects of HFD on learning and memory, CA1 long-term potentiation and hippocampal glial fibrillary acidic protein, mammalian target of rapamycin and vascular endothelial growth factor [ 96 ] and this is now a promising therapeutic target for AD [ 97 ].
The hypothalamus is a key site of action of insulin and leptin and is the CNS regulator of appetite control and energy expenditure.
These pathological changes contribute to furthering the metabolic dysfunction and once again underline the key role of inflammation in metabolic syndrome.
Perhaps of even more significance, inflammatory signalling in the hypothalamus IKK-β and NFκB also drives frailty and decreases neurogenesis, effectively accelerating aging [ ].
This places inflammation in the hypothalamus as a key determinant of rates of cognitive and function decline. An excellent study of the impact of low-grade inflammation on brain aging was performed using parabiosis, in which aged and young animals are sutured together at the flanks and ultimately share the same circulation [ ].
This demonstrated that exposure to the bloodstream of the aged mouse brought about impaired neurogenesis, electrophysiological evidence of impaired memory function and cognitive impairments in the young animals.
Interestingly the opposite was true for old mice exposed to the young bloodstream: some recovery is possible when exposed to the young bloodstream.
The authors identified a number of inflammatory factors present in the blood of aged rodents and people, and demonstrated that one of these factors, the chemokine eotaxin CCL11 , was capable of producing the same deficits as exposure to blood from aged rodents [ ].
These animals had no specific disease state and simply the elevated inflammatory state of aging was sufficient to bring about some cognitive decline. It seems reasonable to conclude that the same milieu superimposed on an already frail brain will have more significant consequences.
Another recent study demonstrated that the ablation of Nlrp3 , a key subunit of the inflammasome complex that regulates IL-1β maturation and secretion, leads to protection against a number of age-related aspects of functional decline.
Significantly, the lack of NLRP3-mediated IL-1 release and activity led to improved glucose metabolism, decreased brain innate immune activation, reduced gliosis, improved cognitive function and extended life-span [ ].
Furthermore, age-associated inflammatory activity in the hypothalamus has whole body effects on ageing, including muscle tone, bone mass, neurogenesis and cognitive function [ ] and since the hypothalamus is one of the primary brain centres affected by systemic inflammation this adds weight to the idea that systemic inflammation is a key driver of ageing that encompasses not just brain structures obviously relevant to dementia, but to functional decline of the individual.
It is striking that mid-life occurrence of these co-morbidities is where the association with dementia lies and patients taking NSAIDs were protected against subsequent AD development. It can now be said that it is a fact, rather than a theory, that chronic co-morbidities and acute systemic inflammatory episodes contribute to the progression of dementia.
Further studies are required in non-transgenic models in order to avoid propagating an over-simplification of the relationship between amyloid and neurodegeneration in a disease that, for the vast majority, occurs in old age and is associated with multiple co-morbid conditions.
Animal model studies with co-morbid conditions will be important in delineating the precise role s of inflammation in the cognitive and degenerative effects of these major risk factors.
APP transgenic mice, which model the genetic risk for early onset AD, do not provide the full pathological spectrum of the late onset human disease and it seems likely that these mice would also recapitulate disease more fully if they accumulated co-morbidities or were experimentally manipulated to do so Figure 1.
Moreover, given the clear contribution of co-morbid inflammation to disease progression, it is important that patients with such co-morbidities are not excluded from clinical trials of novel or repurposed drugs for AD.
Testing anti-inflammatory drugs in an environment where typical, rather than selected, co-morbidity-free patients are included may reveal the true contribution of inflammation to progression of dementia.
This article is part of a series on The impact of acute and chronic medical disorders on accelerated cognitive decline , edited by Carol Brayne and Daniel Davis.
Neuropathology Group. Medical Research Council Cognitive Function and Aging Study. Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Google Scholar.
Matthews FE, Brayne C, Lowe J, McKeith I, Wharton SB, Ince P. Epidemiological pathology of dementia: attributable-risks at death in the Medical Research Council Cognitive Function and Ageing Study. PLoS Med.
PubMed Central PubMed Google Scholar. Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, et al. CAS PubMed Google Scholar. Jun G, Naj AC, Beecham GW, Wang LS, Buros J, Gallins PJ, et al.
Meta-analysis confirms CR1, CLU, and PICALM as Alzheimer disease risk loci and reveals interactions with APOE genotypes. Arch Neurol. Hollingworth P, Harold D, Sims R, Gerrish A, Lambert JC, Carrasquillo MM, et al.
Nat Genet. PubMed Central CAS PubMed Google Scholar. Neumann H, Daly MJ. N Engl J Med. Etminan M, Gill S, Samii A. Perez-Nievas BG, Stein TD, Tai HC, Dols-Icardo O, Scotton TC, Barroeta-Espar I, et al.
Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA.
Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. Davis DH, Muniz Terrera G, Keage H, Rahkonen T, Oinas M, Matthews FE, et al. Delirium is a strong risk factor for dementia in the oldest-old: a population-based cohort study.
Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, et al. Ann Neurol. Widmann CN, Heneka MT. Long-term cerebral consequences of sepsis. Lancet Neurol. PubMed Google Scholar. Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, et al.
Long-term cognitive impairment after critical illness. Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med. Chavan SS, Huerta PT, Robbiati S, Valdes-Ferrer SI, Ochani M, Dancho M, et al. HMGB1 mediates cognitive impairment in sepsis survivors.
Mol Med. Hernandes MS, D'Avila JC, Trevelin SC, Reis PA, Kinjo ER, Lopes LR, et al. The role of Nox2-derived ROS in the development of cognitive impairment after sepsis. J Neuroinflammation. Mina F, Comim CM, Dominguini D, Cassol-Jr OJ, Dall Igna DM, Ferreira GK, et al. Il1-beta involvement in cognitive impairment after sepsis.
Mol Neurobiol. Combrinck MI, Perry VH, Cunningham C. Peripheral infection evokes exaggerated sickness behaviour in pre-clinical murine prion disease. Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration.
J Neurosci. Cunningham C, Campion S, Lunnon K, Murray CL, Woods JF, Deacon RM, et al. Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease.
Biol Psychiatry. Murray C, Sanderson DJ, Barkus C, Deacon RM, Rawlins JN, Bannerman DM, et al. Systemic inflammation induces acute working memory deficits in the primed brain: relevance for delirium. Neurobiol Aging. Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, et al.
Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. Faseb J. Sly LM, Krzesicki RF, Brashler JR, Buhl AE, McKinley DD, Carter DB, et al.
Brain Res Bull. Pott-Godoy MC, Tarelli R, Ferrari CC, Sarchi MI, Pitossi FJ. Palin K, Cunningham C, Forse P, Perry VH, Platt N.
Systemic inflammation switches the inflammatory cytokine profile in CNS Wallerian degeneration. Neurobiol Dis. Lyons A, Downer EJ, Crotty S, Nolan YM, Mills KH, Lynch MA. CD ligand receptor interaction modulates microglial activation in vivo and in vitro: a role for IL Fractalkine receptor CX3CR1 deficiency sensitizes mice to the behavioral changes induced by lipopolysaccharide.
Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells J Exp Med. Cunningham C. Microglia and neurodegeneration: the role of systemic inflammation.
Perry VH, Holmes C. Microglial priming in neurodegenerative disease. Nat Rev Neurol. Field R, Campion S, Warren C, Murray C, Cunningham C. Brain Behav Immun. Holmes C, Lovestone S. Age Ageing. Krstic D, Madhusudan A, Doehner J, Vogel P, Notter T, Imhof C, et al.
Systemic immune challenges trigger and drive Alzheimer-like neuropathology in mice. Krstic D, Knuesel I. Deciphering the mechanism underlying late-onset Alzheimer disease. Lee JW, Lee YK, Yuk DY, Choi DY, Ban SB, Oh KW, et al. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation.
Sheng JG, Bora SH, Xu G, Borchelt DR, Price DL, Koliatsos VE. Lipopolysaccharide-induced-neuroinflammation increases intracellular accumulation of amyloid precursor protein and amyloid beta peptide in APPswe transgenic mice.
McAlpine FE, Lee JK, Harms AS, Ruhn KA, Blurton-Jones M, Hong J, et al. Kitazawa M, Oddo S, Yamasaki TR, Green KN, LaFerla FM. Ziegler-Heitbrock HW. Molecular mechanism in tolerance to lipopolysaccharide.
J Inflamm. Puntener U, Booth SG, Perry VH, Teeling JL. Long-term impact of systemic bacterial infection on the cerebral vasculature and microglia. Faggioni R, Fantuzzi G, Villa P, Buurman W, van Tits LJ, Ghezzi P. Independent down-regulation of central and peripheral tumor necrosis factor production as a result of lipopolysaccharide tolerance in mice.
Infect Immun. Bodea LG, Wang Y, Linnartz-Gerlach B, Kopatz J, Sinkkonen L, Musgrove R, et al. Neurodegeneration by activation of the microglial complement-phagosome pathway.
Michaud JP, Halle M, Lampron A, Theriault P, Prefontaine P, Filali M, et al. Proc Natl Acad Sci U S A. Cunningham C, Maclullich AM. At the extreme end of the psychoneuroimmunological spectrum: delirium as a maladaptive sickness behaviour response.
Cerejeira J, Nogueira V, Luis P, Vaz-Serra A, Mukaetova-Ladinska EB. The cholinergic system and inflammation: common pathways in delirium pathophysiology. J Am Geriatr Soc. van Munster BC, Korevaar JC, Zwinderman AH, Levi M, Wiersinga WJ, De Rooij SE. Time-course of cytokines during delirium in elderly patients with hip fractures.
Cape E, Hall R, van Munster B, de Vries A, Howie S, Pearson A, et al. Cerebrospinal fluid markers of neuroinflammation in delirium: a role for interleukin-1β in delirium after hip fracture. J Psychosom Res. MacLullich AM, Edelshain BT, Hall RJ, de Vries A, Howie SE, Pearson A, et al. Cerebrospinal fluid interleukin-8 levels are higher in people with hip fracture with perioperative delirium than in controls.
Griffin EW, Skelly DT, Murray CL, Cunningham C. Cyclooxygenasedependent prostaglandins mediate susceptibility to systemic inflammation-induced acute cognitive dysfunction.
Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system.
Davis DH, Skelly DT, Murray C, Hennessy E, Bowen J, Norton S, et al. Worsening cognitive impairment and neurodegenerative pathology progressively increase risk for delirium. Am J Geriatr Psychiatry. S 14 Field RH, Gossen A, Cunningham C.
Prior pathology in the basal forebrain cholinergic system predisposes to inflammation induced working memory deficits: reconciling inflammatory and cholinergic hypotheses of delirium.
Vacas S, Degos V, Tracey KJ, Maze M. High-mobility group box 1 protein initiates postoperative cognitive decline by engaging bone marrow-derived macrophages.
The likelihood of progressing towards this primed phenotype increases with age as individuals are exposed to a greater amount of immune challenges.
A heightened inflammatory state, as seen with inflammaging, could partially explain the cognitive declines commonly observed in normal aging Ownby, Supporting this argument, recent research has reliably correlated increased inflammatory biomarker levels and diminished cognitive function in older adults Bettcher et al.
These studies indicate that elevated systemic inflammation could be a risk factor for cognitive decline in old age, but do not directly imply that systemic inflammation mediates the age effect on cognitive functions. While some studies reported lower baseline cognitive scores in older adults with higher levels of systemic inflammation, these studies did not find evidence associating levels of systemic inflammation and rate of cognitive decline Alley et al.
The present study, therefore, directly tested the mediation effect of systemic inflammation on age-related cognitive impairment in a sample with a wide age range across adulthood.
In particular, we addressed the following specific research questions: i Does systemic inflammation account for differences in cognitive performance between the young and the older age group? We predicted that greater systemic inflammation in the older compared to the young age group will mediate age-related cognitive impairment i.
ii Does systemic inflammation account for age-related differences in cognitive performance within young and older age groups? We predicted that greater systemic inflammation associated with greater chronological age will mediate cognitive impairment in both the young and the older age group Hypothesis 2; mediation of the within-group age effect.
iii Does the extent to which systemic inflammation accounts for age-related differences in cognitive performance vary between young and older age groups? We predicted that the mediation of the within-group age effect will be more pronounced in the older compared to the young age group Hypothesis 3; moderated mediation of the within-group age effect.
We recruited participants through: i handout and flyer distribution on campus and the community; ii HealthStreet, a university community outreach service; and iii mail-outs via two university participant registries particularly geared towards older individuals. Exclusion criteria were based on eligibility requirements for a larger project and included pregnancy determined via pregnancy tests given to all women under 63 years , breastfeeding, psychological disorder, severe mental illness, excessive smoking or drinking and magnetic resonance imaging MRI incompatibility as reported elsewhere; Ebner et al.
All participants were Caucasian and fluent in English. As summarized in Table 1 , older participants had more years of education than young participants. There was no difference between young and older participants in their self-reported physical and mental health.
Table 1. This data analysis was part of a larger project that comprised a phone screening and two campus visits.
This report only includes data from the phone screening and the first campus visit for additional publications from the larger project, see Ebner et al. The phone screening was followed by a campus visit where, after receiving informed written consent, behavioral measures for short-term memory and processing speed in this order; see details below were administered.
Next, a licensed physician conducted a review of all major bodily systems i. Personality and social relationship data were also collected, as reported elsewhere Ebner et al.
Participants were instructed to stay hydrated and avoid food, exercise and sexual activity for 2 h before the visit. They were also instructed to avoid smoking, caffeine, alcohol and recreational drugs for 24 h before the visit. Controlling for individual diurnal cycles, all screening visits began in the morning, usually around a.
The Digit Symbol-Substitution Task DSST from the Wechsler Adult Intelligence Scale WAIS; Wechsler, was used to measure processing speed. In this task, participants are given a table pairing the digits 1 through 9 with nine distinct symbols e.
Next, participants are presented with a sequence of 93 digits, ranging from 1 through 9. The goal is to identify individual digits, consider their corresponding symbol, and write that symbol in the space provided immediately below each respective digit, as quickly and accurately as possible.
Participants are given 90 s to work through as much of the sequence as time permits. The number of correct responses was used to indicate processing speed, with higher scores indicating faster processing speed. The Rey Auditory Verbal Learning Test RAVLT; Rey, was used to measure short-term memory.
Immediately after, participants are asked to write down each word they can recall. The number of words correctly recalled was used to indicate short-term memory, with more recalled words indicating better short-term memory.
Using serum tubes, a professional phlebotomist drew 10 mL of blood. Before assays, samples thawed on ice. Using Human Quantikine Enzyme-linked Immunosorbent Assay ELISA , serum IL-6, TNF-α and CRP levels were measured in duplicate according to instructions from the manufacturer R and D System, Minneapolis, MA, USA; CRP: DCRP00; IL HSB; TNFα: HSTA00D.
IL-6 and TNF-α levels were significantly higher in older than young participants Table 1. IL-6 levels were significantly related with CRP levels in both young and older participants and with TNF-α levels in older but not young participants. In contrast, TNF-α and CRP levels were not related in either of the age groups Table 2.
Table 2. Bivariate correlations among the three inflammations markers in young and older participants. We conducted two separate analytic models to determine the extent to which systemic inflammation accounted for age effects on processing speed and short-term memory, respectively.
In each model, the cognitive performance measure served as the dependent variable. To remove multicollinearity between the age-group contrast and the chronological age variables, we centered the chronological age variable separately in each age group.
The three systemic inflammation biomarker levels served as mediators in the models. In addition, we considered the interaction between the age-group contrast and chronological age on the three systemic inflammation biomarkers and the cognitive performance measure and we considered the interaction between the age-group contrast and the three systemic inflammation biomarkers on the cognitive performance measure.
This model specification allowed us to determine the extent to which the mediation of systemic inflammation of the within-group age effect on the cognitive measures varied between young and older participants i. We used PROCESS macro on SPSS Hayes, for model testing.
The program reports the results for the mediation of the within-group age effects. However, it does not report the indirect effects of the between-group age effects. We followed guidelines by VanderWeele and Vanstellandt VanderWeele and Vansteelandt, ; Valeri and VanderWeele, to calculate these indirect effects for each inflammation biomarker.
That is, even though IL-6 levels were higher for individuals in the older compared to the young group, higher chronological age in each age group was not associated with higher IL-6 levels. That is, individuals with higher IL-6 levels had lower processing speed with comparable effects across the two age groups.
Figure 1. Effects B SE of chronological age centered , age-group contrast and interleukin 6 IL-6 A , tumor necrosis factor alpha TNF-α B , and C-reactive protein CRP C , and their interactions on processing speed.
To improve readability, separate figures show the results from the three systemic inflammation biomarkers while all variables were considered in a single model. That is, consistent with findings for IL-6, even though levels of TNF-α were higher for individuals in the older compared to the young group, higher chronological age in either age groups was not associated with higher TNF-α levels.
That is, after accounting for the effect of the three biomarkers on processing speed, the older group still showed lower processing speed than the young group.
As shown in Figures 2A—C , neither the main effects of the three inflammation biomarkers nor their interaction with age-group contrast on short-term memory was significant. Thus, our data did not support an effect of systemic inflammation on short-term memory in either age group.
In addition, none of the inflammation biomarkers mediated the between-group age effects nor the within-group age effects on short-term memory in the young or older age groups.
These results indicated that inflammation biomarker levels did not account for age-related difference in short-term memory in our sample. Figure 2. Effects B SE of chronological age centered , age-group contrast, and IL-6 A , TNF-α B , and CRP C , and their interactions on short-term memory.
That is, after accounting for the effect of the three inflammation markers on short-term memory, the age-related decline in short-term memory was comparable for the age groups 2. Going beyond previous work, the present study took a novel methodological approach by examining the mediation of systemic inflammation i.
We found that systemic inflammation partially explained differences in cognitive performance associated with increased age. In particular, IL-6 levels accounted for the age-group difference in processing speed, supporting Hypothesis 1 mediation of the between-group age effect. Further, IL-6 levels accounted for the age-related differences in processing speed within the older but not the young age group, supporting Hypothesis 3 moderated mediation of the within-group age effect.
However, our data did not support Hypothesis 2 mediation of the within-group age effect. Neither of the remaining two examined inflammatory biomarkers i. Of note, the sample size in the present study was relatively small, limiting the statistical power to detect small effects and calling for future replication of our findings in a larger sample.
Next, we discuss the novel findings generated in this work and their implications in more detail. Previous work has documented a relationship between systemic inflammation and cognitive performance throughout adulthood, spanning young Brydon et al.
The present study, however, represents the first direct test of a mediation effect of systemic inflammation on age-related cognitive impairment. In particular, our results suggest that the difference in systemic inflammation measured by serum IL-6 levels between young and older adults partially accounted for the difference in processing speed between these age groups.
Additionally, IL-6 levels mediated age-related differences in processing speed within the older but not the young age group. Combining these two findings, the mediation of systemic inflammation on age-related variances in cognitive performance may become more pronounced with increased age i.
Two possible mechanisms may underlie the observed moderated mediation. First, age may increase the impact of systemic inflammation on cognition.
However, in the present study we did not find a significant age-moderation of the effect of IL-6 levels on processing speed Figure 1A. That is, the association between IL-6 levels and processing speed was comparable between young and older adults.
Similarly, a previous study showed that an experimentally-induced elevation in inflammatory cytokine response i. This suggests that systemic inflammation produces similar impairments regardless of individual age. Therefore, individuals with higher systemic inflammatory levels, regardless of age, are more likely to show cognitive impairments.
Thus, our data combined with previous studies, does not support the age-related enhancement in the association between systemic inflammation and cognitive impairment as an explanation for the observed moderated mediation. Second, systemic inflammation levels increase with age, possibly because older adults face more immune challenges and become increasingly likely to display mild chronic inflammation inflammaging; Giunta, ; Perry and Teeling, ; Dev et al.
With chronic conditions, primed microglia can yield deleterious effects on their local neuro-environment, eliciting even greater inflammation, which may further prime microglia. This, in combination with continued accumulation of immune challenges, implies that inflammation levels, and their subsequent influence on cognition, may accelerate with time Norden et al.
Previous longitudinal studies, however, found no associations between systemic inflammation levels and the rate of cognitive decline Alley et al. Importantly, these earlier studies focused on cohorts of older adults only.
Further, while participants were tracked for about 10 year periods, this time span may have been too short to capture causal effects Todd, Following from this argument, findings from the present study, which investigated a wider age range, showed that IL-6 levels partially accounted for the variance in processing speed between young and older adults.
However, the cross-sectional nature of the present study does not allow causal conclusions of a mediation of inflammation on cognitive aging. Future longitudinal studies with longer data collection periods e. While participants showed age-related cognitive impairments in both cognitive tasks, systemic inflammation only accounted for the age-related differences in processing speed but not short-term memory.
Heringa et al. Similarly, Tegeler et al. In line with this correlational evidence, a recent intervention study found that participants who received antioxidant supplementation e. Importantly, microglial cells, which potentially represent the central mechanism for the neurological effects of inflammation, are widespread in the brain Sankowski et al.
This means that cognitive processes that integrate various areas across the brain may be more immediately vulnerable to inflammaging. Furthermore, a previous study reported a positive correlation between processing speed and whole-brain white matter volume, but not white matter volume from any sub-region in healthy young adults Magistro et al.
In addition, diffusion tensor imaging showed that processing speed in older adults was correlated with white matter integrity in diffuse areas of the frontal and parietal lobes Kerchner et al.
These results imply that processing speed is a cognitive process requiring coordination between various brain regions. Therefore, evidence from the present and previous studies associating systemic inflammation and processing speed, but not short-term memory a more functionally localized process , supports the argument that systemic inflammation may cause global and diffuse brain damage with variable effects on individual cognitive domains.
We used the DSST to measure processing speed. Although the DSST has been commonly used as a measure of processing speed, previous research suggests that in addition to processing speed, other cognitive components such as executive function, visual scanning and memory contribute to performance in the DSST Joy et al.
Future studies could apply multiple cognitive tasks and adopt a latent factor approach to clarify the associations between systemic inflammation and various cognitive functions.
In contrast, Charlton et al. Importantly, cytokine measures in individuals with late-life depression were compared with measures in relatively healthy older adults. The study showed a significant correlation between inflammation biomarker levels and the severity of depressive symptoms.
Thus, it is possible that psychological conditions, like depression, introduce additional inflammation in peripheral and central immune systems, enhancing the impact of inflammation on various neurological structures and functions. As a result, more localized cognitive domains e.
Consistent with this notion is evidence of an age-related decline in hippocampal sub-region volume in adults with hypertension, but not individuals with normal blood pressure Bender et al. Further supporting this argument, previous studies suggest elevated systemic inflammation as a risk factor for cognitive impairment e.
The present study was embedded in the context of a larger project, which only included Caucasian individuals to avoid potential confounds in some of the central project outcomes. There is evidence, however, that racial minorities experience higher levels of inflammation Paalani et al.
The present study directly tested the mediatory role of systemic inflammation on age-related differences in two cognitive domains i. Our findings establish systemic inflammation as a potential mechanism underlying cognitive impairments in aging. These results highlight the importance of reducing inflammation to promote cognitive health.
Preventive measures, like regular erobic exercise and medications to reduce inflammation, adopted across the entire lifespan, may prove particularly important to protect against cognitive decline, especially among older adults. This study was carried out in accordance with the recommendations of the Institutional Review Board at University of Florida.
The protocol was approved by the Institutional Review Board at University of Florida. All subjects gave written informed consent in accordance with the Declaration of Helsinki.
TL conceptualized the study, collected and analyzed the data, and wrote the first draft of the manuscript. GL conceptualized the study, analyzed the data, and wrote the first draft of the manuscript.
EP and RR assisted in article editing. MF and YC-A revised the final manuscript draft. NE conceptualized the study, supervised data collection and data analysis, and revised the manuscript.
While working on this manuscript, NE was in part supported by the NIH-funded Claude D. Pepper Older Americans Independence Center P30AG The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling Editor declared a shared affiliation, though no other collaboration, with the authors. The authors are grateful to the research teams and study staff from the Social-Cognitive and Affective Development lab and the Institute on Aging at the University of Florida for assistance in study implementation, data collection and data management.
In addition, the authors wish to thank Brian Bouverat, Marvin Dirain and Jini Curry of the Metabolism and Translational Science Core at the Institute on Aging for technical assistance with the inflammation biomarker assays.
Alley, D. Inflammation and rate of cognitive change in high-functioning older adults. A Biol. doi: PubMed Abstract CrossRef Full Text Google Scholar.
Anton, S. Effects of 90 days of resveratrol supplementation on cognitive function in elders: a pilot study. Athilingam, P. Elevated levels of interleukin 6 and C-reactive protein associated with cognitive impairment in heart failure.
Heart Fail. Baltes, P. On the incomplete architecture of human ontogeny. Bender, A. Vascular risk moderates associations between hippocampal subfield volumes and memory. Bettcher, B. Interleukin-6, age and corpus callosum integrity. PLoS One 9:e Brandt, J. The telephone interview for cognitive status.
Neuropsychiatry Neuropsychol. Google Scholar. Brydon, L. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans.
Psychiatry 63, — Charlton, R. Associations between pro-inflammatory cytokines, learning and memory in late-life depression and healthy aging.
Blackberry cobbler recipe we age, decllne may deckine a decline in our cotnitive ability. A recent study has Inflammation and cognitive decline cognitivs chronic Caffeine energy pills in midlife might speed up this decline as we get older. Inflammation and cognitive decline average age Blackberry cobbler recipe the population of cognitkve United States is gradually increasing, so conditions of old age are moving into the spotlight. However, it may only affect some people very mildly, while other individuals can develop significant cognitive deficits. We already know some risk factors ; for instance, lower levels of physical activity, smoking, and obesity appear to increase the rate of decline. Recently, some scientists have turned their attention toward the potential role of inflammation. As a protective mechanism that prevents any damage to tissue, inflammation occurs in response to an infection or injury.
Welcher ausgezeichnet topic
Ich entschuldige mich, aber meiner Meinung nach irren Sie sich. Schreiben Sie mir in PM, wir werden umgehen.
Was er meinen kann?
Ihre Idee ist glänzend