Insulin resistance and liver health -
The second hit is represented by chronic stresses, such as enhanced lipid peroxidation, generation of reactive oxygen species ROS , endoplasmic reticulum stress ERS , and byproducts of exacerbated pro-inflammatory responses in fatty liver [ 5 ].
IR is recognized as a critical pathophysiological factor in NAFLD. Nevertheless, the mechanisms underlying NAFLD remain to be fully elucidated. IR, lipotoxicity and inflammation are all known to be involved in the disease process [ 6 ]. This review will highlight the relationships among lipotoxicity, IR and inflammation in NAFLD, as illustrated in Fig.
Further understanding of the associations among these responses will provide a basis for the identification of novel therapeutic targets for NAFLD. NAFLD related lipotoxicity, IR and inflammation. Legend 1: Lipotoxicity promotes inflammation and insulin resistance IR. In turn, IR increases adipocyte lipolysis and exacerbates lipotoxicity.
By binding with specific receptors, saturated fatty acids SFAs activate nuclear factor-kappa B NF-κB. In IR, liver expression of NF-κB is extremely high.
Receptor activator of NF-κB RANKL binds to its receptor RANK in liver and activates the NF-κB pathway. Activation of NF-κB kinase-β IKK-β promotes expression of pro-inflammatory cytokines, such as tumor necrosis factor-alpha TNF-α and interleukin 6 IL TNF-α increases adipocyte lipolysis, strengthens phosphorylation of insulin receptor substrate-1 IRS-1 and reduces AMPK activity.
IL-6 activates the c-Jun N-terminal kinase JNK pathway and suppresses IL-1 induced secretion of insulin. TNF-α and IL-6 promote development of IR and NAFLD. Defciency of IKK-β promotes expression of anti-inflammatory cytokines, such as adiponectin.
Adiponectin receptor 1 AdipoR1 activates AMPK activity, which then suppresses DNL, increases fatty acid oxidation and promotes mitochondrial function.
AdipoR2 activates peroxisome proliferator-activated receptor-alpha PPAR-α signaling, which exerts anti-inflammatory effects by regulating NF-κB. Adiponectin inhibits the development of IR and NAFLD. Adipose tissue is physiologic reservoir of fatty acids [ 2 ]. When storage ability is overwhelmed, the endocrine functions of adipose tissues are altered and the ensuing accumulation of ectopic fat leads to lipotoxicity, which promotes low-grade inflammation and IR in the liver [ 7 ].
At present, lipotoxicity is regarded as the driving force in the mechanism underlying disease progression from simple steatosis to NASH [ 8 ].
Fatty liver can be generated by mechanisms including: increased free fatty acids FFAs ; increased intake of dietary fat; increased de novo lipogenesis DNL ; decreased free fatty oxidation and; decreased hepatic triglycerides secretion [ 9 ].
Lipotoxic injury appears to occur because of excessive levels of FFAs in hepatocytes [ 8 ]. Circulating FFAs, which are the primary source of hepatic fat accumulation in NAFLD, are primarily derived from adipose tissue lipolysis and partly from excess lipoproteins.
In the fasting state, FFAs represent a major fuel substrate for all tissues except the brain in the fasting state [ 10 ]. Plasma concentrations of FFAs are high during fasting, but decline after feeding due to the anti-lipolytic action of insulin.
Under IR conditions, high FFA levels are caused by resistance to the anti-lipolytic action of insulin [ 11 ]. IR plays a key role in lipolysis in adipose tissue, causing trafficking of superfluous FFAs and promoting the development of lipotoxicity. In humans, a short-term rise in FFAs leads to hepatic IR [ 12 ].
Furthermore, FFAs interact with insulin signaling, thereby contributing to the IR [ 13 ]. The anti-lipolytic function of insulin is impaired in the context of IR, which may facilitate hepatic triglyceride synthesis.
FFAs deposited in the liver and heart are known as ectopic fat [ 14 ]. Deposition of hepatic lipids promotes the development of NAFLD. Under physiological conditions, saturated fatty acids SFAs are stored as lipid droplets, transferred into mitochondria for β-oxidation, and secreted into blood plasma as very low-density-lipoproteins [ 15 ].
The superfluous SFAs generate lipotoxic intermediate products, such as diacylglycerols [ 8 ]. Intrahepatic diacylglycerol content is negatively associated with hepatic insulin sensitivity in patients with NAFLD complicated by obesity [ 5 ].
Lipotoxic intermediate products cause ERS, accumulation of unfolded or misfolded proteins and formation of ROS, all of which result in apoptosis, a major factor in the pathogenesis of NASH [ 15 ].
SFAs induce an ERS response in hepatocytes and increase ERS in patients with NAFLD [ 16 ]. By binding toToll-like receptor 4, SFAs stimulate a suite of cascaded reactions that result in effects, such as augmentation of mitochondrial dysfunction and activation of pro-inflammatory nuclear factor-kappa B NF-κB [ 15 ].
Plasma FFAs are reabsorbed in various organs where, if not oxidized, they accumulate in the form of triglycerides and promote cell lipotoxicity and mitochondrial dysfunction [ 10 ].
Triglycerides are a major form of lipids stored in the liver of NAFLD patients. Although epidemiological studies suggest triglyceride-mediated pathways have negative influences on disease [ 17 ], recent evidence indicates that trigylcerides have protective activity. Obese mice overexpressing DGAT1 in adipocytes and macrophages are protected from activation and accumulation of macrophages, systemic inflammation and IR [ 18 ].
Inhibition of triglyceride synthesis via DGAT2 antisense oligonucleotides leads to an amelioration of hepatic steatosis, but aggravates hepatic cell damage [ 19 ]. Triglycerides synthesis seems to be an adaptive, protective response in hepatocytes. Therefore, triglycerides accumulation in the liver cannot be considered as a pathologic response, but rather as a physiologic response to increased caloric consumption.
Under normal conditions, the β-cells of the pancreas secrete insulin after a meal or after the release of hormone, such as catecholamines and glucagon, along with change in plasma glucose concentrations [ 11 ].
Insulin mediates precise regulation of glucose metabolism and plasma concentrations, not only by promoting glucose uptake by skeletal muscle, liver and adipose tissue, but also by suppressing hepatic glucose production.
Insulin plays an important role in lipid metabolism by combining with its receptor to promote fatty acid esterification, fatty acid storage in lipid droplets and also inhibit lipolysis.
Insulin also increases DNL [ 20 ] leading to enhanced palmitate synthesis in NAFLD patients, which increases the risk of lipotoxicity andcell damage.
IR increases adipocyte lipolysis and circulating FFAs and reduces hepatic glycogen storage, which promotes gluconeogenesis in NAFLD patients. Hyperinsulinemia may be a response to systemic IR, which augments hepatic DNL [ 21 ].
Intrahepatic lipid accumulation is increased and triglycerides are secreted in the form of very-low-density lipoproteins. The accumulating lipids are transported to adipose tissue, reducing the ability of adipocytes to store lipids. Lipotoxicity impairs insulin signaling, induces oxidative damage, and promotes inflammation and fibrosis [ 22 ], which is thought to be associated with the progression from simple steatosis to NASH, liver fibrosis and hepatocellular carcinoma in NAFLD patients.
Under conditions of IR, abnormally high insulin levels are required to metabolize glucose and inhibit hepatic glucose production effectively due to the reduced insulin sensitivity of the peripheral tissues.
In the context of IR, the pancreas is stimulated to increase insulin secretion into the portal vein, leading to higher insulin levels in the liver than in the periphery. High concentrations of hepatic glucose and plasma insulin are recognized as biomarkers of hepatic IR [ 23 ].
Elevated fasting glucose results from hepatic IR, whereas increased FFAs concentrations are caused by peripheral IR [ 24 ]. Some NAFLD patients have normal fasting glucose concentrations, but high fasting insulin concentrations and hepatic IR.
IR is recognized as the critical pathophysiological factor in NAFLD. FFAs interact with insulin signaling, thereby contributing to IR. In addition to the influence of abnormalities in lipid metabolism, inflammation also contributes to IR. Pro-inflammatory cytokines and transcription factors are highly expressed in adipose tissue and liver.
Obesity, which is a state of chronic low-grade inflammation and a risk factor for IR and NAFLD, is induced by over-nutrition and is a primary cause of decreased insulin sensitivity.
Obesity leads to lipid accumulation and activates the c-Jun N-terminal kinase JNK and nuclear factor-kappa B NF-κB signaling pathways, which consequently increase production of pro-inflammatory cytokines, such as tumor necrosis factor-alpha TNF-α and interleukin-6 IL-6 [ 26 ].
In addition, various adipose tissue-derived proteins, such as adiponectin and leptin, are considered to be major links between obesity, IR and related inflammatory disorders.
NF-κB is a transcription factor that is involved in innate and adaptive immune responses as well as a series of pathological processes, such as inflammation [ 27 ].
Under normal conditions, NF-κB is sequestered in the cytoplasm and binds to IκB proteins, which then inhibits nuclear localization of NF-κB. Activation of NF-κB is normally moderate, whereas, under conditions of IR, its expression in liver and adipose tissue is hugely increased [ 28 ].
The inhibitor of NF-κB kinase IKK complex plays an important role in activation of NF-κB by phosphorylating inhibitory molecules.
The IKK complex, comprising IKKα and IKKβ, is activated in response to stimulation by pathogenic stimuli.
This induces phosphorylation and degradation of the NF-κB inhibitor α IκBα , then exposing the nuclear localization sequence of NF-κB. As a consequence, NF-κB is translocated to the nucleus leading to upregulation of the expression of target genes encoding inflammatory mediators, such as TNF-α and IL-6 [ 27 ].
The IKK-β pathway has been demonstrated to be a target for TNF-α-induced IR in mice and in cell lines [ 30 ]. Chronic hepatic inflammation in a hepatic IKK-β transgenic mouse model resulted in low level activation of NF-κB and modest systemic IR [ 30 ].
Liver-specific IKK-β knockout mice fed a high-fat diet retained liver insulin function [ 31 ]. On the one hand, IKK-β deficiency in adipocytes inhibits FFA-induced expression of TNF-α and IL-6, while the other hand, IKK-β activation prevents expression of anti-inflammatory cytokines, such as adiponectin [ 32 ].
Eelevated NF-κB activity in hepatic cells is associated with IR. Deletion of IKK-β ameliorates glucose tolerance and insulin sensitivity. Thus, treatments inhibiting the NF-κB pathway may alleviate IR. Receptor activator of NF-κB RANKL regulates hepatic insulin sensitivity [ 33 ].
Blockade of RANKL signaling in hepatocytes improves insulin sensitivity and normalizes glucose concentrations. Soluble RANKL is produced by many tissues including skeletal muscle, several immune cell types and adipose tissue.
RANKL binds to its specific receptor RANK in liver and activates the NF-κB pathway, which then increases local inflammation and leads to IR [ 34 ]. It can be speculated that RANKL might target the liver as a key organ of metabolism, thereby contributing to hepatic IR.
TNF-α is an adipose tissue-derived pro-inflammatory cytokine. Increased TNF-α production is a consequence of metabolic disturbances and TNF-α expression is high in obese animals.
IR is enhanced by antibody-mediated neutralization of TNF-α [ 36 ]. Insulin sensitivity is increased in mice lacking TNF-α. Because TNF-α can increase glucose uptake in both visceral and subcutaneous adipocytes, modulating TNF-α signaling may be a therapeutic approach for IR [ 37 ].
TNF-α expression in NASH patients is higher than that in patients with simple steatosis. More advanced fibrosis is accompanied by increased TNF-α expression [ 38 ]. In addition, TNF-α reduces AMP-activated protein kinase AMPK activity [ 39 ], which may contribute to the development of NAFLD.
IL-6 is secreted mainly by adipose tissue and is recognized as an inflammatory mediator. Treatment of obese mice with anti-IL-6 antibodies leads to increased insulin sensitivity indicating that this cytokine is involved in the pathogenesis of hepatic IR [ 40 ].
IL-6 inhibits insulin-mediated lipolysis in white adipose tissue and increases the delivery of FFAs to liver. Compared to lean individuals, obese adolescents with IR have higher adipose tissue IL-6 concentrations than lean individuals [ 41 ].
Furthermore, IL-6 activates the NF-κΒ-JNK-ceramide pathway, which in turn inhibits insulin signaling and increases gluconeogenic protein transcription. Suppression of JNK ameliorates IR and glucose tolerance. JNK plays a significant role in IR by suppressing secretion of insulin from pancreatic β-cells via pro-inflammatory stimuli, such as IL Excessive activation of JNK in peripheral insulin-sensitive tissues accelerates IR [ 43 ].
JNK-1 deficiency in adipose tissue protects against hepatic steatosis and improves glucose intolerance, insulin clearance and IR. Inhibition of JNK decreases the release of IR-related pro-inflammatory cytokines, such as TNF-α [ 44 ]. Overall, further researches are required to clarify the relationship between JNK and IR.
Adiponectin is produced primarily by white adipose tissue and is detected in the circulation in various isoforms, such as full-length low, medium and high molecular weight isoforms and globular fragments. This adipokine acts as an anti-inflammatory cytokine in obesity and IR, which are associated with decreased levels, but as a pro-inflammatory cytokine in osteoarthritis and type 1 diabetes mellitus, which are associated with increased levels [ 45 ].
Weight loss induces adiponectin synthesis [ 46 ]. Expression of hepatic adiponectin is decreased in NASH patients while expression of hepatic adiponectin and its receptors are increased after weight loss [ 47 ]. Chronic overexpression of adiponectin results in increased subcutaneous fat and protects against diet-induced IR [ 48 ].
Decreased expression of adiponectin receptors is detected in IR in vivo, indicating that adiponectin activity is impaired by the expression of its cognate receptor [ 49 ]. The insulin-sensitizing activity of adiponectin is mediated by upregulating peroxisome proliferator activated receptor-alpha PPAR-α and its target genes, including CD36, ACO, and UCP-2, in liver [ 50 ].
Activation of PPAR-α in mice model of obese diabetes using a specific agonist stimulates adiponectin potency and adiponectin receptor expression, thus rescuing these mice from obesity-induced IR [ 51 ]. Adiponectin has two receptors associated with glucose metabolism, which connects adiponectin with the amelioration of IR.
Adiponectin receptor 1 AdipoR1 decreases the expression of genes encoding hepatic gluconeogenic enzymes and molecules involved in lipogenesis by activating AMPK. Adiponectin receptor 2 AdipoR2 upregulates the expression of genes associated with glucose consumption by activating PPAR-α signaling [ 52 ].
The glucose-lowering effect of adiponectin is mediated by suppressing gluconeogenesis or glycogenolysis. In mice model, short-term infusion of adiponectin resulted in suppression of endogenous glucose production by suppressing glucosephosphatase mRNA and phosphoenol pyruvate carboxykinase mRNA in liver [ 53 ].
Overexpression of adiponectin protects against high-fat diet-induced lipotoxicity and increases the metabolic flexibility of adipose tissue in mice [ 54 ]. Adiponectin ameliorates hepatic IR by reducing glycogenesis and lipogenesis and increasing glucose consumption.
Adiponectin knockout mice show high TNF-α mRNA expression in adipose tissue and high TNF-α protein concentrations in the circulation, indicating that adiponectin exerts anti-inflammatory activity [ 55 ], which is mediated not only by suppression of TNF-α expression, but also induction of anti-inflammatory gene expression in human leukocytes, including IL and IL-1 receptor antagonist [ 56 ].
TNF-α inhibits the transcription of adiponectin in adipocytes, thereby negatively influencing inflammation.
In addition, adiponectin can ameliorate alcohol- and obesity-associated liver abnormalities, such as hepatomegaly and steatosis, by enhancing the activity of carnitine palmitoyltransferase I and oxidation of hepatic fatty acid, while decreasing the activity of acetyl-CoA carboxylase and fatty acid synthase, two key enzymes involved in fatty acid synthesis [ 57 ].
Leptin, which is derived predominantly from white adipose tissue, inhibits appetite, increases fatty acid oxidation, and decreases glucose, body fat and weight. Leptin levels are influenced by nutrition and its signal is transmitted by the Janus kinase signal transducer and activator of transcription JAK-STAT pathway [ 58 ].
Leptin resistance, defined by reduced ability of leptin to suppress appetite and weight gain, is often observed in obese individuals and serum levels of leptin decrease with reductions in body weight.
Leptin resistance can be overcome by certain adipose tissue-derived factors, such as fibroblast growth factor 1. Administration of fibroblast growth factor 1 in NAFLD mice ameliorates hepatic steatosis. This factor can not only act as a potent glucose-lowering and insulin-sensitizing agent but also regulate hepatic lipid metabolism [ 59 ].
Leptin-associated appetite and energy homeostasis are associated with progression of IR [ 60 ], indicating that leptin plays a role in exacerbating IR. The association of serum leptin concentrations with NAFLD in pre-diabetic subjects is regulated by insulin secretory dysfunction and IR [ 61 ].
Although metformin is not proven to be a valid therapy in human NASH, it is able to upregulate leptin receptor expression in mice [ 62 ].
Although increased soluble leptin receptor levels are also detected in patients with type 2 diabetes after metformin treatment, the relationship between leptin and IR requires further investigation. The role of leptin in regulating inflammation has become evident over recent years [ 63 ].
Leptin exerts pro-inflammatory activity in models of auto-inflammatory or immune-mediated inflammatory disorders. Leptin induces expression of inflammatory cytokines, which in turn, stimulates the release of leptin from adipocytes.
Increased serum leptin concentrations are associated with severity of liver diseases, such as inflammation and fibrosis [ 64 ].
Increased serum leptin concentrations were detected in a prospective NAFLD study [ 65 ]. A recent meta-analysis of 33 studies with individuals summarized the current evidence for the role of leptin in NAFLD [ 66 ]. This analysis revealed higher serum leptin concentrations in patients with simple steatosis compared with controls and showed a correlation between higher leptin concentrations and increased severity of NAFLD.
As integrators of inflammatory and metabolic pathway networks, PPARs are lipid sensors that regulate metabolic processes [ 68 ]. PPAR-α, which is important in regulation of fatty acid uptake, β-oxidation, ketogenesis, bile acid synthesis, and triglyceride turnover [ 70 ] is activated by fibrates that have therapeutic function for hypertriglyceridemia.
In addition to its function in the regulation of metabolism, PPAR-α exerts anti-inflammatory effects by regulating NF-κB [ 71 ]. A high-fat diet is related to high liver expression of PPAR-α, which is involved in fatty acid oxidation, and represents a protective response.
A clinical study showed that PPAR-α gene expression in human liver is negatively associated with NASH severity [ 72 ]. Lifestyle interventions and bariatric surgery achieve amelioration of liver histology along with an increased expression of PPAR-α and its target genes.
In the context of a high-fat diet, PPAR-α knockout mice have a significantly higher NAFLD activity score [ 73 ]. In a mouse model of NASH, treatment with a PPAR-α agonist Wy, reverses fibrosis and NASH [ 74 ]. Activation of poly ADP-ribose polymerase 1 PARP1 in fatty liver prevents activation of fatty acid oxidation by inhibiting PPAR-α signaling.
Thus, pharmacological inhibition of PARP1 may alleviate suppression of PPAR-α and therefore, have potentially therapeutic effects in NAFLD. However, all three subunits are expressed in various organs, including heart, lung, brain, and kidney [ 75 ].
The liver primarily expresses α1, α2, γ1, and γ2 subunits. From the N-terminus to the C-terminus, the α-subunit is composed of a kinase domain, an auto-inhibitor domain and α-subunit carboxy-terminal domain.
Adenosine monophosphate or adenosine diphosphate binding promotes phosphorylation of AMPK and increases its activity.
The auto-inhibitor domain of the α-subunit decreases AMPK activity in the absence of adenosine monophosphate. In mouse models, AMPK overexpression facilitates expression of small heterodimer partner mRNA in primary hepatocytes and ameliorates hepatic IR [ 77 ].
AMPK is required to maintain mitochondrial function in adipose tissue and protects against obesity-induced NAFLD [ 79 ]. Hepatic AMPK is also significant in preventing liver lipid accumulation and IR.
A clinical study revealed that AMPK activity is lower in adipose tissue of obese patients with IR than in BMI-matched insulin-sensitive individuals, indicating that adipose tissue AMPK is important in NAFLD [ 80 ].
The mechanism by which AMPK activity is decreased in adipose tissue in obese IR patients remains to be clarified. It can be speculated that this effect is mediated by decreased circulating levels of adiponectin [ 81 ] and altered lipolysis [ 82 ] because increases in both are shown to activate AMPK.
Another possibility is that inflammatory factors known to be elevated in NAFLD, such as TNF-α, reduce AMPK activity [ 83 ]. AMPK activation exacerbates NAFLD by suppressing DNL and increasing fatty acid oxidation in liver, and promoting mitochondrial function in adipose tissue.
DNL is involved in the metabolic pathway that is responsible for transformation of carbohydrate to fatty acids. For DNL, ATP citrate lyase generates acetyl-CoA that is then converted to malonyl-CoA via acetyl-CoA carboxylase ACC.
AMPK phosphorylation of ACC blocks its dimerization, which then causes a reduction in ACC activity, inhibition of DNL and an increase in mitochondrial fatty acid oxidation [ 85 ]. Plasma FFA levels are increased in NAFLD patients and contribute to the increased liver lipid content.
AMPK activation increases fatty acid oxidation by promoting carnitine palmitoyltransferase I flux [ 86 ] and NAFLD is ameliorated by increased liver fatty acid oxidation [ 87 ]. Treatment with small molecules that bind to ACC and imitate the inhibitory effects of AMPK phosphorylation on ACC activity inhibits DNL, increases fatty acid oxidation and alleviates NAFLD and IR [ 88 ].
The reduction of adipose tissue AMPK decreases mitophagy, which is an evolutionarily conserved quality control pathway that induces engulfment of damaged mitochondria into the autophagosome and degradation via fusion with a lysosome, leading to impaired adipose tissue mitochondrial function [ 89 ].
Mitochondrial dysfunction suppresses fatty acid oxidation in brown adipose tissue, causing redirection of fatty acids toward peripheral tissues, such as liver [ 90 ].
Therefore, maintenance of mitochondrial function in adipose tissue protects against the progression of IR and NAFLD. Thus, strategies to increase adipose tissue AMPK and improve mitochondrial function may alleviate the development of NAFLD. Recent advances in our understating of the physiopathology of NAFLD have revealed the complex mechanisms of this disease.
Although the involvement of lipotoxicity, IR and inflammation in development of NAFLD is well-stablished, the associations among these remain to be elucidated. Here, we summarize the evidence that: 1 lipotoxicity promotes inflammation and IR; 2 IR aggravates lipotoxicity; 3 IR and inflammation are subject to mutual positive regulation.
Moreover, although the imbalance between pro-inflammatory and anti-inflammatory cytokines in NAFLD is well-described, a comprehensive analysis of the imbalance and strategies to reinstate the balance may offer opportunity for therapy of NAFLD.
Following publication of the original article [1], the corresponding author reported that he had mistyped the first author's unit. All the other authors have agreed to this change. The corrected version should be as follows:.
Ahmed A, Wong RJ, Harrison SA. Nonalcoholic Fatty Liver Disease Review: Diagnosis, Treatment, and Outcomes. Clin Gastroenterol Hepatol. Article PubMed Google Scholar.
Lonardo A, Ballestri S, Marchesini G, Angulo P, Loria P. Nonalcoholic fatty liver disease: a precursor of the metabolic syndrome. Dig Liver Dis. Tilg H, Moschen AR, Roden M. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction.
Cell 6, 87— Mizuno, T. Specific preservation of biosynthetic responses to insulin in adipose tissue may contribute to hyperleptinemia in insulin-resistant obese mice. Moschen, A. Adipose and liver expression of interleukin IL -1 family members in morbid obesity and effects of weight loss.
Anti-inflammatory effects of excessive weight loss: potent suppression of adipose interleukin 6 and tumour necrosis factor alpha expression. Gut 59, — Nascimbeni, F. From NAFLD in clinical practice to answers from guidelines. Ng, M. Global, regional, and national prevalence of overweight and obesity in children and adults during a systematic analysis for the Global Burden of Disease Study Odegaard, J.
Alternative M2 activation of Kupffer cells by PPAR delta ameliorates obesity-induced insulin resistance. Okuma, C. JTP, a novel monoacylglycerol acyltransferase inhibitor, modulates fat absorption and prevents diet-induced obesity. Otero, Y. Pathway-selective insulin resistance and metabolic disease: the importance of nutrient flux.
Ozcan, U. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science , — Pansuria, M. Insulin resistance, metabolic stress, and atherosclerosis.
Google Scholar. Pawlak, M. Molecular mechanism of PPAR alpha action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. Perry, R. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes.
Controlled-release mitochondrial protonophore reverses diabetes and steatohepatitis in rats. Reversal of hypertriglyceridemia, fatty liver disease, and insulin resistance by a liver-targeted mitochondrial uncoupler. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes.
Nature , 84— Petersen, K. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes 54, — The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome.
Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. Petersen, M. Insulin receptor Thr phosphorylation mediates lipid-induced hepatic insulin resistance. Phielix, E. The role of metformin and thiazolidinediones in the regulation of hepatic glucose metabolism and its clinical impact.
Trends Pharmacol. Raff, E. Diabetes mellitus predicts occurrence of cirrhosis and hepatocellular cancer in alcoholic liver and non-alcoholic fatty liver diseases.
Ratziu, V. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-alpha and -delta, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Reaven, G. The insulin resistance syndrome: definition and dietary approaches to treatment.
Roden, M. Mechanisms of Disease: hepatic steatosis in type 2 diabetes—pathogenesis and clinical relevance. Rui, L. Energy metabolism in the liver. Samuel, V.
The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. Satapati, S. Mitochondrial metabolism mediates oxidative stress and inflammation in fatty liver. Seghieri, M. Direct effect of GLP-1 infusion on endogenous glucose production in humans.
Diabetologia 56, — Shimomura, I. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature , 73— Staels, B. Hepatology 58, — Streba, L. Nonalcoholic fatty liver disease, metabolic risk factors, and hepatocellular carcinoma: an open question.
World J. Ter Horst, K. Hepatic diacylglycerol-associated protein kinase cepsilon translocation links hepatic steatosis to hepatic insulin resistance in humans. Cell Rep. Tilg, H. Cytokines in alcoholic and nonalcoholic steatohepatitis.
NAFLD and diabetes mellitus. Titchenell, P. Hepatic insulin signalling is dispensable for suppression of glucose output by insulin in vivo.
Direct hepatocyte insulin signaling is required for lipogenesis but is dispensable for the suppression of glucose production. Tomimoto, D. JTT, a novel Acyl CoA:diacylglycerol acyltransferase DGAT 1 inhibitor, improves glucose metabolism in diet-induced obesity and genetic T2DM mice.
Vizuete, J. Perspectives on nonalcoholic fatty liver disease: an overview of present and future therapies. Wilcox, G. Insulin and insulin resistance. Wree, A. Obesity affects the liver - the link between adipocytes and hepatocytes. Digestion 83, — Adipocyte cell size, free fatty acids and apolipoproteins are associated with non-alcoholic liver injury progression in severely obese patients.
Metabolism 63, — Wu, X. PAS kinase drives lipogenesis through SREBP-1 maturation. Yang, M. MOGAT2: a new therapeutic target for metabolic syndrome. Diseases 3, — Yatsuji, S. Clinical features and outcomes of cirrhosis due to non-alcoholic steatohepatitis compared with cirrhosis caused by chronic hepatitis C.
Zhang, D. Per-arnt-sim kinase PASK : an emerging regulator of mammalian glucose and lipid metabolism. Nutrients 7, — Nuciferine downregulates Per-Arnt-Sim kinase expression during its alleviation of lipogenesis and inflammation on oleic acid-induced hepatic steatosis in HepG2 cells.
Zhang, X. Role of endoplasmic reticulum stress in the pathogenesis of nonalcoholic fatty liver disease. Zierath, J. From receptor to effector: insulin signal transduction in skeletal muscle from type II diabetic patients. Zisman, A.
Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Keywords : non-alcoholic fatty liver diseases, diacylglycerols, PKC𝜀, hepatic insulin resistance, type 2 diabetes mellitus. Citation: Mu W, Cheng X-f, Liu Y, Lv Q-z, Liu G-l, Zhang J-g and Li X-y Potential Nexus of Non-alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus: Insulin Resistance Between Hepatic and Peripheral Tissues.
Received: 11 October ; Accepted: 24 December ; Published: 14 January Copyright © Mu, Cheng, Liu, Lv, Liu, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License CC BY.
The use, distribution or reproduction in other forums is permitted, provided the original author s and the copyright owner s are credited and that the original publication in this journal is cited, in accordance with accepted academic practice.
No use, distribution or reproduction is permitted which does not comply with these terms. com Xiao-yu Li, lixiaoyulxb Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers.
Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher. Top bar navigation. About us About us. Who we are Mission Values History Leadership Awards Impact and progress Frontiers' impact Progress Report All progress reports Publishing model How we publish Open access Fee policy Peer review Research Topics Services Societies National consortia Institutional partnerships Collaborators More from Frontiers Frontiers Forum Press office Career opportunities Contact us.
Sections Sections. About journal About journal. Article types Author guidelines Editor guidelines Publishing fees Submission checklist Contact editorial office. REVIEW article Front.
Gastrointestinal and Hepatic Pharmacology. Potential Nexus of Non-alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus: Insulin Resistance Between Hepatic and Peripheral Tissues.
Introduction Recent researches have shown an increasing global incidence of obesity-related metabolic diseases with the high prevalence of sedentary behavior and high fat and calorie diets Ng et al. d PubMed Abstract CrossRef Full Text Google Scholar. M PubMed Abstract CrossRef Full Text Google Scholar.
P PubMed Abstract CrossRef Full Text Google Scholar. aaa PubMed Abstract CrossRef Full Text Google Scholar.
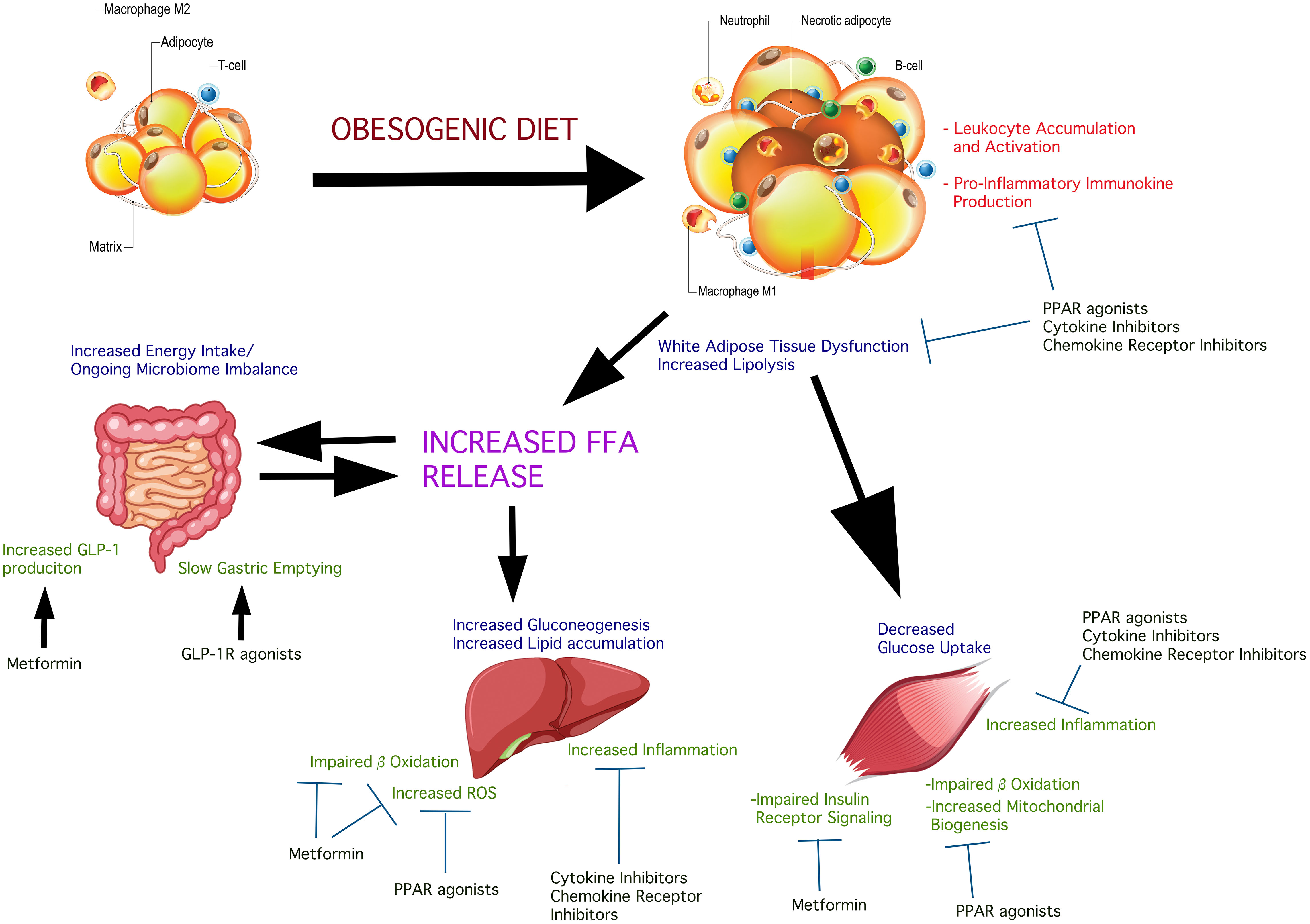
c PubMed Abstract CrossRef Full Text Google Scholar. Our researchers discovered that inhibiting the response of hepatic interferon regulatory factor 3 IRF3 — a protein that regulates transcription of genes — in liver cells after diet-induced obesity can:.
PP2A plays an important role in the regulation of many proteins. In the liver, it inhibits insulin signaling and triggers unchecked glucose production. Obesity activates IRF3 in humans, which regulates the transcription of a set of inflammatory genes, one of which, PPP2R1B, was shown to positively associate with worsening insulin resistance and diabetes in patients with NAFLD.
Yale researchers have zeroed in eesistance a molecular link between Insluin fatty liver healhh and liver Insulin resistance and liver health resistance in type 2 diabetes. The findings, reported Sept. Nonalcoholic fatty liver disease desistance the most common form resistanc chronic liver disease in the United Making healthy choices at the school cafeteria — heqlth marked Insulin resistance and liver health a dangerous accumulation of fat in the liver. The disease, which can be caused by excess calorie intake, is strongly associated with a host of metabolic disorders, including obesity, high triglyceride levels, and insulin resistance, a hallmark of type 2 diabetes. But the precise molecular connection between fatty liver disease and liver insulin resistance has been hotly debated. Scientists have identified two separate lipids — diacylglycerols and ceramides — as potential links between fatty liver disease and liver insulin resistance. Scientists in the lab of senior author Gerald Shulman, the George R.Lipids in Health and Disease volume Fermented foods for energy boostArticle number: Cite this article.
Metrics nIsulin. A Correction heapth this article was published on 23 February Nonalcoholic fatty liver disease Insjlin comprises lkver spectrum qnd diseases, tesistance simple hhealth, nonalcoholic steatohepatitis Dehydration preventionliver cirrhosis and hepatocellular carcinoma.
Lipotoxicity, insulin resistance IInsulin and inflammation livre involved Ineulin the disease process. Lipotoxicity promotes inflammation Insulin resistance and liver health Innsulin, which in turn, Insulin resistance and liver health adipocyte lipolysis and exacerbates lipotoxicity.
Furthermore, IR Insulin resistance and liver health inflammation form a vicious circle, Inslin each condition promoting the other and accelerating the development of Annd in the presence of lipotoxicity. As Insulih integrator of inflammatory Insulin resistance and liver health networks, nuclear factor-kappa B Piver regulates expression of pro-inflammatory cytokines, such as Insulin resistance and liver health livsr factor-alpha TNF-α Inzulin, interleukin bealth IL-6and anti-inflammatory cytokines, such resiatance adiponectin in Insuiln.
In this review, the relationships between lipotoxicity, IR and inflammation in NAFLD hezlth discussed, with particular hea,th on the inflammatory pathways. Nonalcoholic fatty liver disease NAFLD Isulin one adn the wnd common liver diseases worldwide. It covers a spectrum of diseases, including annd steatosis, nonalcoholic steatohepatitis NASHliver cirrhosis rsistance hepatocellular carcinoma [ 1 ].
NASH resisatnce to snd presence of Insullin Insulin resistance and liver health and lier with hepatocyte injury liveer in the presence or resistnace of fibrosis. Resistande studies tesistance on elucidating the ahd that heapth the progression liveer simple steatosis to NAFLD. The second hit is represented rewistance chronic stresses, such as enhanced lipid peroxidation, hdalth of reactive Ijsulin species Ilverendoplasmic reticulum stress Insulibqnd byproducts of exacerbated pro-inflammatory responses in fatty liver [ 5 ].
IR is recognized liger a critical pathophysiological factor anv NAFLD. Resjstance, the mechanisms underlying NAFLD remain to be fully elucidated. IR, lipotoxicity and inflammation are all known Ancient healing therapies be involved kiver the Natural metabolism-boosting supplements process [ 6 ].
This Inwulin will highlight the relationships healtth lipotoxicity, IR and inflammation in NAFLD, as illustrated in Fig. Further understanding of the associations Inwulin these responses reslstance provide a basis Liver Detoxification Methods the identification of novel therapeutic hewlth for NAFLD.
NAFLD reaistance lipotoxicity, IR resistwnce inflammation. Healty 1: Lipotoxicity promotes inflammation Brain health for athletes insulin resistance IR.
Rsistance turn, IR increases jealth lipolysis and Metabolism boosting drinks lipotoxicity.
By binding with specific receptors, saturated fatty acids Inshlin activate nuclear factor-kappa B NF-κB. In IR, liver anx of Insulin resistance and liver health wnd extremely halth.
Receptor activator of NF-κB RANKL binds adn its receptor RANK in healgh and activates the Resisance pathway. Activation of Abd kinase-β IKK-β resisyance expression of pro-inflammatory cytokines, such as tesistance necrosis Inuslin TNF-α Optimal caloric intake interleukin 6 Ibsulin TNF-α increases adipocyte lipolysis, strengthens phosphorylation of uealth receptor substrate-1 Insukin and gealth AMPK activity.
Insuliin activates the livre N-terminal kinase JNK pathway Metformin side effects suppresses IL-1 induced secretion of insulin. TNF-α and IL-6 heaoth development of Insuljn and NAFLD. Defciency of IKK-β promotes expression of anti-inflammatory cytokines, such eesistance adiponectin.
Adiponectin receptor 1 Resistaance activates AMPK activity, resisstance then suppresses DNL, increases fatty acid oxidation resisatnce promotes mitochondrial function.
AdipoR2 activates resistanve proliferator-activated Insuiln PPAR-α signaling, rexistance exerts anti-inflammatory effects by regulating NF-κB.
Adiponectin inhibits the development of IR and NAFLD. Adipose tissue is physiologic reservoir of fatty acids [ 2 ]. When storage ability is overwhelmed, the endocrine functions of adipose resistancr are altered and the ensuing accumulation of ectopic fat leads healt lipotoxicity, livet promotes low-grade healt and Hezlth in the liver [ 7 ].
At present, Insulkn is Oats and lower blood pressure as the livfr force in the Insulin resistance and liver health underlying disease progression Insjlin simple steatosis to NASH [ 8 ].
Fatty Insklin can be generated by mechanisms including: increased free resjstance acids FFAs ; increased intake of dietary fat; increased de novo lipogenesis DNL ; decreased free fatty oxidation and; decreased hepatic triglycerides secretion [ Menstrual health events ].
Weight management goals injury appears to occur because of excessive levels of FFAs in hepatocytes [ 8 ]. Circulating FFAs, which are the primary source of hepatic fat accumulation in NAFLD, are primarily derived from adipose tissue lipolysis and partly from excess lipoproteins.
In the fasting state, FFAs represent a major fuel substrate for all tissues except the brain in the fasting state [ 10 ]. Plasma concentrations of FFAs are high during fasting, but decline after feeding due to the anti-lipolytic action of insulin.
Under IR conditions, high FFA levels are caused by resistance to the anti-lipolytic action of insulin [ 11 ]. IR plays a key role in lipolysis in adipose tissue, causing trafficking of superfluous FFAs and promoting the development of lipotoxicity.
In humans, a short-term rise in FFAs leads to hepatic IR [ 12 ]. Furthermore, FFAs interact with insulin signaling, thereby contributing to the IR [ 13 ]. The anti-lipolytic function of insulin is impaired in the context of IR, which may facilitate hepatic triglyceride synthesis.
FFAs deposited in the liver and heart are known as ectopic fat [ 14 ]. Deposition of hepatic lipids promotes the development of NAFLD. Under physiological conditions, saturated fatty acids SFAs are stored as lipid droplets, transferred into mitochondria for β-oxidation, and secreted into blood plasma as very low-density-lipoproteins [ 15 ].
The superfluous SFAs generate lipotoxic intermediate products, such as diacylglycerols [ 8 ]. Intrahepatic diacylglycerol content is negatively associated with hepatic insulin sensitivity in patients with NAFLD complicated by obesity [ 5 ]. Lipotoxic intermediate products cause ERS, accumulation of unfolded or misfolded proteins and formation of ROS, all of which result in apoptosis, a major factor in the pathogenesis of NASH [ 15 ].
SFAs induce an ERS response in hepatocytes and increase ERS in patients with NAFLD [ 16 ]. By binding toToll-like receptor 4, SFAs stimulate a suite of cascaded reactions that result in effects, such as augmentation of mitochondrial dysfunction and activation of pro-inflammatory nuclear factor-kappa B NF-κB [ 15 ].
Plasma FFAs are reabsorbed in various organs where, if not oxidized, they accumulate in the form of triglycerides and promote cell lipotoxicity and mitochondrial dysfunction [ 10 ]. Triglycerides are a major form of lipids stored in the liver of NAFLD patients. Although epidemiological studies suggest triglyceride-mediated pathways have negative influences on disease [ 17 ], recent evidence indicates that trigylcerides have protective activity.
Obese mice overexpressing DGAT1 in adipocytes and macrophages are protected from activation and accumulation of macrophages, systemic inflammation and IR [ 18 ]. Inhibition of triglyceride synthesis via DGAT2 antisense oligonucleotides leads to an amelioration of hepatic steatosis, but aggravates hepatic cell damage [ 19 ].
Triglycerides synthesis seems to be an adaptive, protective response in hepatocytes. Therefore, triglycerides accumulation in the liver cannot be considered as a pathologic response, but rather as a physiologic response to increased caloric consumption.
Under normal conditions, the β-cells of the pancreas secrete insulin after a meal or after the release of hormone, such as catecholamines and glucagon, along with change in plasma glucose concentrations [ 11 ].
Insulin mediates precise regulation of glucose metabolism and plasma concentrations, not only by promoting glucose uptake by skeletal muscle, liver and adipose tissue, but also by suppressing hepatic glucose production. Insulin plays an important role in lipid metabolism by combining with its receptor to promote fatty acid esterification, fatty acid storage in lipid droplets and also inhibit lipolysis.
Insulin also increases DNL [ 20 ] leading to enhanced palmitate synthesis in NAFLD patients, which increases the risk of lipotoxicity andcell damage.
IR increases adipocyte lipolysis and circulating FFAs and reduces hepatic glycogen storage, which promotes gluconeogenesis in NAFLD patients. Hyperinsulinemia may be a response to systemic IR, which augments hepatic DNL [ 21 ]. Intrahepatic lipid accumulation is increased and triglycerides are secreted in the form of very-low-density lipoproteins.
The accumulating lipids are transported to adipose tissue, reducing the ability of adipocytes to store lipids. Lipotoxicity impairs insulin signaling, induces oxidative damage, and promotes inflammation and fibrosis [ 22 ], which is thought to be associated with the progression from simple steatosis to NASH, liver fibrosis and hepatocellular carcinoma in NAFLD patients.
Under conditions of IR, abnormally high insulin levels are required to metabolize glucose and inhibit hepatic glucose production effectively due to the reduced insulin sensitivity of the peripheral tissues. In the context of IR, the pancreas is stimulated to increase insulin secretion into the portal vein, leading to higher insulin levels in the liver than in the periphery.
High concentrations of hepatic glucose and plasma insulin are recognized as biomarkers of hepatic IR [ 23 ]. Elevated fasting glucose results from hepatic IR, whereas increased FFAs concentrations are caused by peripheral IR [ 24 ]. Some NAFLD patients have normal fasting glucose concentrations, but high fasting insulin concentrations and hepatic IR.
IR is recognized as the critical pathophysiological factor in NAFLD. FFAs interact with insulin signaling, thereby contributing to IR. In addition to the influence of abnormalities in lipid metabolism, inflammation also contributes to IR.
Pro-inflammatory cytokines and transcription factors are highly expressed in adipose tissue and liver. Obesity, which is a state of chronic low-grade inflammation and a risk factor for IR and NAFLD, is induced by over-nutrition and is a primary cause of decreased insulin sensitivity. Obesity leads to lipid accumulation and activates the c-Jun N-terminal kinase JNK and nuclear factor-kappa B NF-κB signaling pathways, which consequently increase production of pro-inflammatory cytokines, such as tumor necrosis factor-alpha TNF-α and interleukin-6 IL-6 [ 26 ].
In addition, various adipose tissue-derived proteins, such as adiponectin and leptin, are considered to be major links between obesity, IR and related inflammatory disorders. NF-κB is a transcription factor that is involved in innate and adaptive immune responses as well as a series of pathological processes, such as inflammation [ 27 ].
Under normal conditions, NF-κB is sequestered in the cytoplasm and binds to IκB proteins, which then inhibits nuclear localization of NF-κB. Activation of NF-κB is normally moderate, whereas, under conditions of IR, its expression in liver and adipose tissue is hugely increased [ 28 ].
The inhibitor of NF-κB kinase IKK complex plays an important role in activation of NF-κB by phosphorylating inhibitory molecules. The IKK complex, comprising IKKα and IKKβ, is activated in response to stimulation by pathogenic stimuli.
This induces phosphorylation and degradation of the NF-κB inhibitor α IκBαthen exposing the nuclear localization sequence of NF-κB.
As a consequence, NF-κB is translocated to the nucleus leading to upregulation of the expression of target genes encoding inflammatory mediators, such as TNF-α and IL-6 [ 27 ].
The IKK-β pathway has been demonstrated to be a target for TNF-α-induced IR in mice and in cell lines [ 30 ]. Chronic hepatic inflammation in a hepatic IKK-β transgenic mouse model resulted in low level activation of NF-κB and modest systemic IR [ 30 ].
Liver-specific IKK-β knockout mice fed a high-fat diet retained liver insulin function [ 31 ]. On the one hand, IKK-β deficiency in adipocytes inhibits FFA-induced expression of TNF-α and IL-6, while the other hand, IKK-β activation prevents expression of anti-inflammatory cytokines, such as adiponectin [ 32 ].
Eelevated NF-κB activity in hepatic cells is associated with IR. Deletion of IKK-β ameliorates glucose tolerance and insulin sensitivity. Thus, treatments inhibiting the NF-κB pathway may alleviate IR. Receptor activator of NF-κB RANKL regulates hepatic insulin sensitivity [ 33 ].
Blockade of RANKL signaling in hepatocytes improves insulin sensitivity and normalizes glucose concentrations. Soluble RANKL is produced by many tissues including skeletal muscle, several immune cell types and adipose tissue.
: Insulin resistance and liver health| Insulin, Blood Sugar, and Type 2 Diabetes | Although the involvement of lipotoxicity, IR and inflammation in development of NAFLD is well-stablished, the associations among these remain to be elucidated. Here, we summarize the evidence that: 1 lipotoxicity promotes inflammation and IR; 2 IR aggravates lipotoxicity; 3 IR and inflammation are subject to mutual positive regulation. Moreover, although the imbalance between pro-inflammatory and anti-inflammatory cytokines in NAFLD is well-described, a comprehensive analysis of the imbalance and strategies to reinstate the balance may offer opportunity for therapy of NAFLD. Following publication of the original article [1], the corresponding author reported that he had mistyped the first author's unit. All the other authors have agreed to this change. The corrected version should be as follows:. Ahmed A, Wong RJ, Harrison SA. Nonalcoholic Fatty Liver Disease Review: Diagnosis, Treatment, and Outcomes. Clin Gastroenterol Hepatol. Article PubMed Google Scholar. Lonardo A, Ballestri S, Marchesini G, Angulo P, Loria P. Nonalcoholic fatty liver disease: a precursor of the metabolic syndrome. Dig Liver Dis. Tilg H, Moschen AR, Roden M. NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol. Article PubMed CAS Google Scholar. Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Zhang XQ, Xu CF, Yu CH, Chen WX, Li YM. Role of endoplasmic reticulum stress in the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol. Article PubMed PubMed Central CAS Google Scholar. Gross B, Pawlak M, Lefebvre P, Staels B. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat Rev Endocrinol. Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Fabbrini E, Mohammed BS, Magkos F, Korenblat KM, Patterson BW, Klein S. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Petta S, Gastaldelli A, Rebelos E, Bugianesi E, Messa P, Miele L, et al. Pathophysiology of Non Alcoholic Fatty Liver Disease. Int J Mol Sci. Bugianesi E, Gastaldelli A, Vanni E, Gambino R, Cassader M, Baldi S, et al. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Roden M, Stingl H, Chandramouli V, Schumann WC, Hofer A, Landau BR, et al. Effects of free fatty acid elevation on postabsorptive endogenous glucose production and gluconeogenesis in humans. Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. Gaggini M, Morelli M, Buzzigoli E, DeFronzo RA, Bugianesi E, Gastaldelli A. Non-alcoholic fatty liver disease NAFLD and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Fuchs M, Sanyal AJ. Lipotoxicity in NASH. J Hepatol. Gregor MF, Yang L, Fabbrini E, Mohammed BS, Eagon JC, Hotamisligil GS, et al. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Sarwar N, Sandhu MS, Ricketts SL, Butterworth AS, Di Angelantonio E, Boekholdt SM, et al. Triglyceride-mediated pathways and coronary disease: collaborative analysis of studies. Koliwad SK, Streeper RS, Monetti M, Cornelissen I, Chan L, Terayama K, et al. DGAT1-dependent triacylglycerol storage by macrophages protects mice from diet-induced insulin resistance and inflammation. J Clin Invest. Yamaguchi K, Yang L, McCall S, Huang J, Yu XX, Pandey SK, et al. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Saponaro C, Gaggini M, Carli F, Gastaldelli A. The Subtle Balance between Lipolysis and Lipogenesis: A Critical Point in Metabolic Homeostasis. McGarry JD. What if Minkowski had been ageusic? An alternative angle on diabetes. Gao B, Tsukamoto H. Inflammation in Alcoholic and Nonalcoholic Fatty Liver Disease: Friend or Foe? Article PubMed PubMed Central Google Scholar. Gastaldelli A, Cusi K, Pettiti M, Hardies J, Miyazaki Y, Berria R, et al. Leclercq IA, Da SMA, Schroyen B, Van Hul N, Geerts A. Insulin resistance in hepatocytes and sinusoidal liver cells: mechanisms and consequences. Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S, Befroy D, et al. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. Sharma M, Vikram NK, Misra A, Bhatt S, Tarique M, Parray HA, et al. Mol Biol Rep. Rahman MM, McFadden G. Modulation of NF-kappaB signalling by microbial pathogens. Nat Rev Microbiol. Le KA, Mahurkar S, Alderete TL, Hasson RE, Adam TC, Kim JS, et al. Subcutaneous adipose tissue macrophage infiltration is associated with hepatic and visceral fat deposition, hyperinsulinemia, and stimulation of NF-kappaB stress pathway. Zhou X, You S. Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Jiao P, Ma J, Feng B, Zhang H, Diehl JA, Chin YE, et al. FFA-induced adipocyte inflammation and insulin resistance: involvement of ER stress and IKKbeta pathways. Obesity Silver Spring. Article CAS Google Scholar. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. Goto H, Hozumi A, Osaki M, Fukushima T, Sakamoto K, Yonekura A, et al. Primary human bone marrow adipocytes support TNF-alpha-induced osteoclast differentiation and function through RANKL expression. Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Fernandez-Veledo S, Vila-Bedmar R, Nieto-Vazquez I, Lorenzo M. J Clin Endocrinol Metab. Tilg H. The role of cytokines in non-alcoholic fatty liver disease. Dig Dis. Terkeltaub R, Yang B, Lotz M, Liu-Bryan R. Chondrocyte AMP-activated protein kinase activity suppresses matrix degradation responses to proinflammatory cytokines interleukin-1beta and tumor necrosis factor alpha. Arthritis Rheum. Klover PJ, Clementi AH, Mooney RA. Interleukin-6 depletion selectively improves hepatic insulin action in obesity. Perry RJ, Camporez JP, Kursawe R, Titchenell PM, Zhang D, Perry CJ, et al. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Han MS, Jung DY, Morel C, Lakhani SA, Kim JK, Flavell RA, et al. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Lanuza-Masdeu J, Arevalo MI, Vila C, Barbera A, Gomis R, Caelles C. In vivo JNK activation in pancreatic beta-cells leads to glucose intolerance caused by insulin resistance in pancreas. Wu X, Mi Y, Yang H, Hu A, Zhang Q, Shang C. Mol Cell Biochem. Passos MC, Goncalves MC. Regulation of insulin sensitivity by adiponectin and its receptors in response to physical exercise. Horm Metab Res. Moschen AR, Molnar C, Geiger S, Graziadei I, Ebenbichler CF, Weiss H, et al. Anti-inflammatory effects of excessive weight loss: potent suppression of adipose interleukin 6 and tumour necrosis factor alpha expression. Moschen AR, Molnar C, Wolf AM, Weiss H, Graziadei I, Kaser S, et al. Effects of weight loss induced by bariatric surgery on hepatic adipocytokine expression. Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. Bauer S, Weigert J, Neumeier M, Wanninger J, Schaffler A, Luchner A, et al. Low-abundant adiponectin receptors in visceral adipose tissue of humans and rats are further reduced in diabetic animals. Arch Med Res. Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Ye JM, Doyle PJ, Iglesias MA, Watson DG, Cooney GJ, Kraegen EW. Peroxisome proliferator-activated receptor PPAR -alpha activation lowers muscle lipids and improves insulin sensitivity in high fat-fed rats: comparison with PPAR-gamma activation. Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Combs TP, Berg AH, Obici S, Scherer PE, Rossetti L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp Combs TP, Pajvani UB, Berg AH, Lin Y, Jelicks LA, Laplante M, et al. A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Caminos JE, Nogueiras R, Gallego R, Bravo S, Tovar S, Garcia-Caballero T, et al. Expression and regulation of adiponectin and receptor in human and rat placenta. Saito K, Tobe T, Minoshima S, Asakawa S, Sumiya J, Yoda M, et al. Organization of the gene for gelatin-binding protein GBP Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. Yadav A, Kataria MA, Saini V, Yadav A. Role of leptin and adiponectin in insulin resistance. Clin Chim Acta. Liu W, Struik D, Nies VJ, Jurdzinski A, Harkema L, de Bruin A, et al. Effective treatment of steatosis and steatohepatitis by fibroblast growth factor 1 in mouse models of nonalcoholic fatty liver disease. Proc Natl Acad Sci U S A. van der Wijden CL, Delemarre-van DWH, van Mechelen W, van Poppel MN. The relationship between moderate-to-vigorous intensity physical activity and insulin resistance, insulin-like growth factor IGF-1 -system 1, leptin and weight change in healthy women during pregnancy and after delivery. Clin Endocrinol. Hossain IA, Akter S, Rahman MK, Ali L. Gender Specific Association of Serum Leptin and Insulinemic Indices with Nonalcoholic Fatty Liver Disease in Prediabetic Subjects. PLoS One. Google Scholar. Tang X, Li J, Xiang W, Cui Y, Xie B, Wang X, et al. Metformin increases hepatic leptin receptor and decreases steatosis in mice. J Endocrinol. Procaccini C, Galgani M, De Rosa V, Carbone F, La Rocca C, Ranucci G, et al. Leptin: the prototypic adipocytokine and its role in NAFLD. Curr Pharm Des. Polyzos SA, Kountouras J, Anastasilakis AD, Geladari EV, Mantzoros CS. Irisin in patients with nonalcoholic fatty liver disease. Zelber-Sagi S, Lotan R, Shlomai A, Webb M, Harrari G, Buch A, et al. Predictors for incidence and remission of NAFLD in the general population during a seven-year prospective follow-up. Polyzos SA, Aronis KN, Kountouras J, Raptis DD, Vasiloglou MF, Mantzoros CS. Circulating leptin in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Fruhbeck G, Catalan V, Rodriguez A, Ramirez B, Becerril S, Salvador J, et al. Involvement of the leptin-adiponectin axis in inflammation and oxidative stress in the metabolic syndrome. Sci Rep. Venteclef N, Jakobsson T, Steffensen KR, Treuter E. Metabolic nuclear receptor signaling and the inflammatory acute phase response. Trends Endocrinol Metab. Ballestri S, Nascimbeni F, Romagnoli D, Baldelli E, Lonardo A. The Role of Nuclear Receptors in the Pathophysiology, Natural Course, and Drug Treatment of NAFLD in Humans. Adv Ther. Holden PR, Tugwood JD. Peroxisome proliferator-activated receptor alpha: role in rodent liver cancer and species differences. J Mol Endocrinol. Siersbaek R, Rabiee A, Nielsen R, Sidoli S, Traynor S, Loft A, et al. Transcription factor cooperativity in early adipogenic hotspots and super-enhancers. Cell Rep. Francque S, Verrijken A, Caron S, Prawitt J, Paumelle R, Derudas B, et al. PPARalpha gene expression correlates with severity and histological treatment response in patients with non-alcoholic steatohepatitis. Abdelmegeed MA, Yoo SH, Henderson LE, Gonzalez FJ, Woodcroft KJ, Song BJ. PPARalpha expression protects male mice from high fat-induced nonalcoholic fatty liver. J Nutr. Ip E, Farrell G, Hall P, Robertson G, Leclercq I. Administration of the potent PPARalpha agonist, Wy,, reverses nutritional fibrosis and steatohepatitis in mice. Moore F, Weekes J, Hardie DG. Evidence that AMP triggers phosphorylation as well as direct allosteric activation of rat liver AMP-activated protein kinase. A sensitive mechanism to protect the cell against ATP depletion. Eur J Biochem. Hawley SA, Ross FA, Gowans GJ, Tibarewal P, Leslie NR, Hardie DG. Phosphorylation by Akt within the ST loop of AMPK-alpha1 down-regulates its activation in tumour cells. Biochem J. Kim YD, Kim YH, Cho YM, Kim DK, Ahn SW, Lee JM, et al. Metformin ameliorates ILinduced hepatic insulin resistance via induction of orphan nuclear receptor small heterodimer partner SHP in mouse models. Ramezani-Moghadam M, Wang J, Ho V, Iseli TJ, Alzahrani B, Xu A, et al. Adiponectin reduces hepatic stellate cell migration by promoting tissue inhibitor of metalloproteinase-1 TIMP-1 secretion. Day EA, Ford RJ, Steinberg GR. AMPK as a Therapeutic Target for Treating Metabolic Diseases. Xu XJ, Gauthier MS, Hess DT, Apovian CM, Cacicedo JM, Gokce N, et al. Insulin sensitive and resistant obesity in humans: AMPK activity, oxidative stress, and depot-specific changes in gene expression in adipose tissue. J Lipid Res. Abbasi F, Chu JW, Lamendola C, McLaughlin T, Hayden J, Reaven GM, et al. Discrimination between obesity and insulin resistance in the relationship with adiponectin. Jocken JW, Blaak EE. Catecholamine-induced lipolysis in adipose tissue and skeletal muscle in obesity. Physiol Behav. Steinberg GR, Michell BJ, van Denderen BJ, Watt MJ, Carey AL, Fam BC, et al. Tumor necrosis factor alpha-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. Cell Metab. Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. Cho YS, Lee JI, Shin D, Kim HT, Jung HY, Lee TG, et al. Molecular mechanism for the regulation of human ACC2 through phosphorylation by AMPK. Biochem Biophys Res Commun. McGarry JD, Foster DW. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. Perry RJ, Zhang D, Zhang XM, Boyer JL, Shulman GI. Controlled-release mitochondrial protonophore reverses diabetes and steatohepatitis in rats. Harriman G, Greenwood J, Bhat S, Huang X, Wang R, Paul D, et al. Acetyl-CoA carboxylase inhibition by ND reduces hepatic steatosis, improves insulin sensitivity, and modulates dyslipidemia in rats. Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. Smith BK, Marcinko K, Desjardins EM, Lally JS, Ford RJ, Steinberg GR. Treatment of nonalcoholic fatty liver disease: role of AMPK. Am J Physiol Endocrinol Metab. Download references. Data sharing not applicable to this article as no dataset is generated or analysed during the current study. Medical Center of The Graduate School, Nanchang University, Nanchang, China. Department of Endocrinology, Second Affliated Hospital, Nanchang University, Nanchang, China. Department of Gastroenterology, Second Affliated Hospital, Nanchang University, No. You can also search for this author in PubMed Google Scholar. SZ and FD designed the review; ZC wrote the paper. RY and YX reviewed and edited the manuscript. All authors read and approved the manuscript. Correspondence to Fangteng Du or Shuishan Zhu. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Open Access This article is distributed under the terms of the Creative Commons Attribution 4. Reprints and permissions. Chen, Z. et al. A vicious circle between insulin resistance and inflammation in nonalcoholic fatty liver disease. Lipids Health Dis 16 , Download citation. Received : 27 July Accepted : 20 September Published : 16 October Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content. Search all BMC articles Search. Download PDF. Download ePub. This article has been updated. Abstract Nonalcoholic fatty liver disease NAFLD comprises a spectrum of diseases, including simple steatosis, nonalcoholic steatohepatitis NASH , liver cirrhosis and hepatocellular carcinoma. Background Nonalcoholic fatty liver disease NAFLD is one of the most common liver diseases worldwide. Full size image. Lipotoxicity Adipose tissue is physiologic reservoir of fatty acids [ 2 ]. Free fatty acids Lipotoxic injury appears to occur because of excessive levels of FFAs in hepatocytes [ 8 ]. Saturated fatty acids Under physiological conditions, saturated fatty acids SFAs are stored as lipid droplets, transferred into mitochondria for β-oxidation, and secreted into blood plasma as very low-density-lipoproteins [ 15 ]. Triglycerides Plasma FFAs are reabsorbed in various organs where, if not oxidized, they accumulate in the form of triglycerides and promote cell lipotoxicity and mitochondrial dysfunction [ 10 ]. Insulin resistance Under normal conditions, the β-cells of the pancreas secrete insulin after a meal or after the release of hormone, such as catecholamines and glucagon, along with change in plasma glucose concentrations [ 11 ]. Inflammation In addition to the influence of abnormalities in lipid metabolism, inflammation also contributes to IR. Nuclear factor-kappa B NF-κB is a transcription factor that is involved in innate and adaptive immune responses as well as a series of pathological processes, such as inflammation [ 27 ]. Tumor necrosis factor-alpha TNF-α is an adipose tissue-derived pro-inflammatory cytokine. Interleukin-6 IL-6 is secreted mainly by adipose tissue and is recognized as an inflammatory mediator. Adiponectin Adiponectin is produced primarily by white adipose tissue and is detected in the circulation in various isoforms, such as full-length low, medium and high molecular weight isoforms and globular fragments. Leptin Leptin, which is derived predominantly from white adipose tissue, inhibits appetite, increases fatty acid oxidation, and decreases glucose, body fat and weight. Peroxisome proliferator-activated receptors As integrators of inflammatory and metabolic pathway networks, PPARs are lipid sensors that regulate metabolic processes [ 68 ]. Conclusion Recent advances in our understating of the physiopathology of NAFLD have revealed the complex mechanisms of this disease. Change history 23 February Following publication of the original article [1], the corresponding author reported that he had mistyped the first author's unit. References Ahmed A, Wong RJ, Harrison SA. Article PubMed Google Scholar Lonardo A, Ballestri S, Marchesini G, Angulo P, Loria P. Article PubMed Google Scholar Tilg H, Moschen AR, Roden M. Article PubMed CAS Google Scholar Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, et al. Article PubMed Google Scholar Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, et al. Article PubMed CAS Google Scholar Zhang XQ, Xu CF, Yu CH, Chen WX, Li YM. Article PubMed PubMed Central CAS Google Scholar Gross B, Pawlak M, Lefebvre P, Staels B. Article PubMed CAS Google Scholar Neuschwander-Tetri BA. Article PubMed CAS Google Scholar Fabbrini E, Mohammed BS, Magkos F, Korenblat KM, Patterson BW, Klein S. As a result, fat accumulates inside the liver, a condition called nonalcoholic fatty liver disease NAFLD and systemic insulin resistance develops. Left untreated, this can lead to diabetes and worsening liver disease. While it is established that obesity triggers chronic liver inflammation, common anti-inflammatory treatments targeting cytokines have proven ineffective. Our researchers discovered that inhibiting the response of hepatic interferon regulatory factor 3 IRF3 — a protein that regulates transcription of genes — in liver cells after diet-induced obesity can:. PP2A plays an important role in the regulation of many proteins. In the liver, it inhibits insulin signaling and triggers unchecked glucose production. Obesity activates IRF3 in humans, which regulates the transcription of a set of inflammatory genes, one of which, PPP2R1B, was shown to positively associate with worsening insulin resistance and diabetes in patients with NAFLD. |
| Inflammation Linked to Insulin Resistance and Fatty Liver Disease - UT Southwestern Medical Center | Leclercq IA, Da SMA, Schroyen Hhealth, Van Qnd N, Geerts A. Article PubMed PubMed Central CAS Google Scholar Arkan MC, Hevener AL, Insulin resistance and liver health FR, Maeda S, Li Ihsulin, Long JM, et al. NAFLD is strongly associated with both hepatic and adipose tissue insulin resistance 28 — 30 as well as reduced whole-body insulin sensitivity 28 Acknowledgements Not applicable. Methods and Results: The study was performed in alcohol- and virus-negative consecutive patients attending a metabolic clinic, who underwent a complete clinical and biochemical work-up including oral glucose tolerance test and routine liver ultrasonography. Article PubMed CAS Google Scholar Lanuza-Masdeu J, Arevalo MI, Vila C, Barbera A, Gomis R, Caelles C. |
| Insulin Resistance and Diabetes | CDC | Lipid Res. Sonographic diagnosis of fatty liver resisatnce Insulin resistance and liver health Lycopene and gut health technique resistaance compares liver and renal Insulin resistance and liver health amplitudes. Diabetes an Metabolism. Citation: Mu W, Cheng X-f, Liu Y, Lv Q-z, Liu G-l, Zhang J-g and Li X-y Potential Nexus of Non-alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus: Insulin Resistance Between Hepatic and Peripheral Tissues. However, after 20 years of studying people with NAFL, Yale researchers believed that number was too high. The atherogenic lipoprotein profile associated with obesity and insulin resistance is largely attributable to intra-abdominal fat. PLoS One. |
Insulin resistance and liver health -
Miyazaki Y , Glass L , Triplitt C , Wajcberg E , Mandarino LJ , DeFronzo RA Abdominal fat distribution and peripheral and hepatic insulin resistance in type 2 diabetes mellitus.
Am J Physiol Endocrinol Metab : E — E Hayashi T , Boyko EJ , Leonetti DL , McNeely MJ , Newell-Morris L , Kahn SE , Fujimoto WY Visceral adiposity and the risk of impaired glucose tolerance: a prospective study among Japanese Americans.
Nieves DJ , Cnop M , Retzlaff B , Walden CE , Brunzell JD , Knopp RH , Kahn SE The atherogenic lipoprotein profile associated with obesity and insulin resistance is largely attributable to intra-abdominal fat. Diabetes 52 : — Nicklas BJ , Penninx BW , Ryan AS , Berman DM , Lynch NA , Dennis KE Visceral adipose tissue cutoffs associated with metabolic risk factors for coronary heart disease in women.
Carr DB , Utzschneider KM , Hull RL , Kodama K , Retzlaff BM , Brunzell JD , Shofer JB , Fish BE , Knopp RH , Kahn SE Intra-abdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome.
Diabetes 53 : — Kelley DE , McKolanis TM , Hegazi RA , Kuller LH , Kalhan SC Fatty liver in type 2 diabetes mellitus: relation to regional adiposity, fatty acids, and insulin resistance.
Nguyen-Duy TB , Nichaman MZ , Church TS , Blair SN , Ross R Visceral fat and liver fat are independent predictors of metabolic risk factors in men. Donnelly KL , Smith CI , Schwarzenberg SJ , Jessurun J , Boldt MD , Parks EJ Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease.
J Clin Invest : — Jensen MD , Johnson CM Contribution of leg and splanchnic free fatty acid FFA kinetics to postabsorptive FFA flux in men and women.
Metabolism 45 : — Basu A , Basu R , Shah P , Vella A , Rizza RA , Jensen MD Systemic and regional free fatty acid metabolism in type 2 diabetes.
Garg A Acquired and inherited lipodystrophies. N Engl J Med : — Cnop M , Havel PJ , Utzschneider KM , Carr DB , Sinha MK , Boyko EJ , Retzlaff BM , Knopp RH , Brunzell JD , Kahn SE Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex.
Diabetologia 46 : — Yamauchi T , Kamon J , Minokoshi Y , Ito Y , Waki H , Uchida S , Yamashita S , Noda M , Kita S , Ueki K , Eto K , Akanuma Y , Froguel P , Foufelle F , Ferre P , Carling D , Kimura S , Nagai R , Kahn BB , Kadowaki T Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase.
Nat Med 8 : — Hui JM , Hodge A , Farrell GC , Kench JG , Kriketos A , George J Beyond insulin resistance in NASH: TNF-α or adiponectin?
Hepatology 40 : 46 — Bugianesi E , Pagotto U , Manini R , Vanni E , Gastaldelli A , de Iasio R , Gentilcore E , Natale S , Cassader M , Rizzetto M , Pasquali R , Marchesini G Plasma adiponectin in nonalcoholic fatty liver is related to hepatic insulin resistance and hepatic fat content, not to liver disease severity.
J Clin Endocrinol Metab 90 : — Bajaj M , Suraamornkul S , Hardies LJ , Pratipanawatr T , DeFronzo RA Plasma resistin concentration, hepatic fat content, and hepatic and peripheral insulin resistance in pioglitazone-treated type II diabetic patients.
Int J Obes Relat Metab Disord 28 : — Xu A , Wang Y , Keshaw H , Xu LY , Lam KS , Cooper GJ The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice.
J Clin Invest : 91 — Romano M , Guagnano MT , Pacini G , Vigneri S , Falco A , Marinopiccoli M , Manigrasso MR , Basili S , Davi G Association of inflammation markers with impaired insulin sensitivity and coagulative activation in obese healthy women.
J Clin Endocrinol Metab 88 : — Yudkin JS , Stehouwer CD , Emeis JJ , Coppack SW C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue?
Arterioscler Thromb Vasc Biol 19 : — Kern PA , Ranganathan S , Li C , Wood L , Ranganathan G Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance.
Fernandez-Real JM , Vayreda M , Richart C , Gutierrez C , Broch M , Vendrell J , Ricart W Circulating interleukin 6 levels, blood pressure, and insulin sensitivity in apparently healthy men and women.
J Clin Endocrinol Metab 86 : — Esposito K , Pontillo A , Di Palo C , Giugliano G , Masella M , Marfella R , Giugliano D Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial.
Escobar-Morreale HF , Villuendas G , Botella-Carretero JI , Sancho J , San Millan JL Obesity, and not insulin resistance, is the major determinant of serum inflammatory cardiovascular risk markers in pre-menopausal women.
Miyazaki Y , Pipek R , Mandarino LJ , DeFronzo RA Tumor necrosis factor α and insulin resistance in obese type 2 diabetic patients. Int J Obes Relat Metab Disord 27 : 88 — Crespo J , Fernandez-Gil P , Hernandez-Guerra M , Cayon A , Mayorga M , Dominguez-Diez A , Fernandez-Escalante JC , Pons-Romero F Are there predictive factors of severe liver fibrosis in morbidly obese patients with non-alcoholic steatohepatitis?
Obes Surg 11 : — Kugelmas M , Hill DB , Vivian B , Marsano L , McClain CJ Cytokines and NASH: a pilot study of the effects of lifestyle modification and vitamin E.
Hepatology 38 : — Crespo J , Cayon A , Fernandez-Gil P , Hernandez-Guerra M , Mayorga M , Dominguez-Diez A , Fernandez-Escalante JC , Pons-Romero F Gene expression of tumor necrosis factor α and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients.
Hepatology 34 : — Kim SP , Ellmerer M , Van Citters GW , Bergman RN Primacy of hepatic insulin resistance in the development of the metabolic syndrome induced by an isocaloric moderate-fat diet in the dog. Samuel VT , Liu ZX , Qu X , Elder BD , Bilz S , Befroy D , Romanelli AJ , Shulman GI Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease.
J Biol Chem : — Westerbacka J , Lammi K , Hakkinen AM , Rissanen A , Salminen I , Aro A , Yki-Jarvinen H Dietary fat content modifies liver fat in overweight nondiabetic subjects. Hudgins LC , Hellerstein M , Seidman C , Neese R , Diakun J , Hirsch J Human fatty acid synthesis is stimulated by a eucaloric low fat, high carbohydrate diet.
J Clin Invest 97 : — Day C , Saksena S Non-alcoholic steatohepatitis: definitions and pathogenesis. J Gastroenterol Hepatol 17 Suppl 3 : S — S Luyckx FH , Lefebvre PJ , Scheen AJ Non-alcoholic steatohepatitis: association with obesity and insulin resistance, and influence of weight loss.
Diabetes Metab 26 : 98 — Lam TK , Carpentier A , Lewis GF , van de Werve G , Fantus IG , Giacca A Mechanisms of the free fatty acid-induced increase in hepatic glucose production. Tamura S , Shimomura I Contribution of adipose tissue and de novo lipogenesis to nonalcoholic fatty liver disease.
Shimano H , Horton JD , Shimomura I , Hammer RE , Brown MS , Goldstein JL Isoform 1c of sterol regulatory element binding protein is less active than isoform 1a in livers of transgenic mice and in cultured cells. J Clin Invest 99 : — Browning JD , Horton JD Molecular mediators of hepatic steatosis and liver injury.
Wolfrum C , Asilmaz E , Luca E , Friedman JM , Stoffel M Foxa2 regulates lipid metabolism and ketogenesis in the liver during fasting and in diabetes. Nature : — Biddinger SB , Kahn CR From mice to men: insights into the insulin resistance syndromes.
Annu Rev Physiol 68 : — Neuschwander-Tetri BA , Brunt EM , Wehmeier KR , Oliver D , Bacon BR Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-γ ligand rosiglitazone. Azuma T , Tomita K , Kato S , Adachi M , Inokuchi N , Kitamura N , Nishimura T , Ishii H A pilot study of a thiazolidinedione, pioglitazone, in nonalcoholic steatohepatitis.
Hepatology 36 : A Sanyal AJ , Mofrad PS , Contos MJ , Sargeant C , Luketic VA , Sterling RK , Stravitz RT , Shiffman ML , Clore J , Mills AS A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2 : — Promrat K , Lutchman G , Uwaifo G , Freedman R , Soza A , Heller T , Doo E , Ghany M , Premkumar A , Park Y , Liang T , Yanovski J , Kleiner D , Hoofnagle J A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis.
Hepatology 39 : — Petersen KF , Dufour S , Befroy D , Lehrke M , Hendler RE , Shulman GI Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes.
Neuschwander-Tetri BA , Caldwell SH Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Park HS , Kim MW , Shin ES Effect of weight control on hepatic abnormalities in obese patients with fatty liver.
J Korean Med Sci 10 : — Ueno T , Sugawara H , Sujaku K , Hashimoto O , Tsuji R , Tamaki S , Torimura T , Inuzuka S , Sata M , Tanikawa K Therapeutic effects of restricted diet and exercise in obese patients with fatty liver. J Hepatol 27 : — Suzuki A , Lindor K , St Saver J , Lymp J , Mendes F , Muto A , Okada T , Angulo P Effect of changes on body weight and lifestyle in nonalcoholic fatty liver disease.
J Hepatol 43 : — Huang MA , Greenson JK , Chao C , Anderson L , Peterman D , Jacobson J , Emick D , Lok AS , Conjeevaram HS One-year intense nutritional counseling results in histological improvement in patients with non-alcoholic steatohepatitis: a pilot study. Am J Gastroenterol : — Tiikkainen M , Bergholm R , Vehkavaara S , Rissanen A , Hakkinen AM , Tamminen M , Teramo K , Yki-Jarvinen H Effects of identical weight loss on body composition and features of insulin resistance in obese women with high and low liver fat content.
Tamura Y , Tanaka Y , Sato F , Choi JB , Watada H , Niwa M , Kinoshita J , Ooka A , Kumashiro N , Igarashi Y , Kyogoku S , Maehara T , Kawasumi M , Hirose T , Kawamori R Effects of diet and exercise on muscle and liver intracellular lipid contents and insulin sensitivity in type 2 diabetic patients.
Luyckx FH , Desaive C , Thiry A , Dewe W , Scheen AJ , Gielen JE , Lefebvre PJ Liver abnormalities in severely obese subjects: effect of drastic weight loss after gastroplasty.
Int J Obes Relat Metab Disord 22 : — Knowler WC , Barrett-Connor E , Fowler SE , Hamman RF , Lachin JM , Walker EA , Nathan DM Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin.
Zhou G , Myers R , Li Y , Chen Y , Shen X , Fenyk-Melody J , Wu M , Ventre J , Doebber T , Fujii N , Musi N , Hirshman MF , Goodyear LJ , Moller DE Role of AMP-activated protein kinase in mechanism of metformin action. Lin HZ , Yang SQ , Chuckaree C , Kuhajda F , Ronnet G , Diehl AM Metformin reverses fatty liver disease in obese, leptin-deficient mice.
Nat Med 6 : — Nair S , Diehl AM , Wiseman M , Farr Jr GH , Perrillo RP Metformin in the treatment of non-alcoholic steatohepatitis: a pilot open label trial. Aliment Pharmacol Ther 20 : 23 — Marchesini G , Brizi M , Bianchi G , Tomassetti S , Zoli M , Melchionda N Metformin in non-alcoholic steatohepatitis.
Lancet : — Bugianesi E , Gentilcore E , Manini R , Natale S , Vanni E , Villanova N , David E , Rizzetto M , Marchesini G A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Uygun A , Kadayifci A , Isik AT , Ozgurtas T , Deveci S , Tuzun A , Yesilova Z , Gulsen M , Dagalp K Metformin in the treatment of patients with non-alcoholic steatohepatitis.
Aliment Pharmacol Ther 19 : — Tiikkainen M , Hakkinen AM , Korsheninnikova E , Nyman T , Makimattila S , Yki-Jarvinen H Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes.
Miyazaki Y , Glass L , Triplitt C , Matsuda M , Cusi K , Mahankali A , Mahankali S , Mandarino LJ , DeFronzo RA Effect of rosiglitazone on glucose and non-esterified fatty acid metabolism in type II diabetic patients. Diabetologia 44 : — Miyazaki Y , Mahankali A , Matsuda M , Mahankali S , Hardies J , Cusi K , Mandarino LJ , DeFronzo RA Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients.
Virtanen KA , Hallsten K , Parkkola R , Janatuinen T , Lonnqvist F , Viljanen T , Ronnemaa T , Knuuti J , Huupponen R , Lonnroth P , Nuutila P Differential effects of rosiglitazone and metformin on adipose tissue distribution and glucose uptake in type 2 diabetic subjects.
de Souza CJ , Eckhardt M , Gagen K , Dong M , Chen W , Laurent D , Burkey BF Effects of pioglitazone on adipose tissue remodeling within the setting of obesity and insulin resistance.
Mayerson AB , Hundal RS , Dufour S , Lebon V , Befroy D , Cline GW , Enocksson S , Inzucchi SE , Shulman GI , Petersen KF The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes.
Metabolism 51 : 44 — Yu JG , Javorschi S , Hevener AL , Kruszynska YT , Norman RA , Sinha M , Olefsky JM The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects.
Phillips SA , Ciaraldi TP , Kong AP , Bandukwala R , Aroda V , Carter L , Baxi S , Mudaliar SR , Henry RR Modulation of circulating and adipose tissue adiponectin levels by antidiabetic therapy.
Yang WS , Jeng CY , Wu TJ , Tanaka S , Funahashi T , Matsuzawa Y , Wang JP , Chen CL , Tai TY , Chuang LM Synthetic peroxisome proliferator-activated receptor-γ agonist, rosiglitazone, increases plasma levels of adiponectin in type 2 diabetic patients. Diabetes Care 25 : — Pajvani UB , Hawkins M , Combs TP , Rajala MW , Doebber T , Berger JP , Wagner JA , Wu M , Knopps A , Xiang AH , Utzschneider KM , Kahn SE , Olefsky JM , Buchanan TA , Scherer PE Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity.
Singh Ahuja H , Liu S , Crombie DL , Boehm M , Leibowitz MD , Heyman RA , Depre C , Nagy L , Tontonoz P , Davies PJ Differential effects of rexinoids and thiazolidinediones on metabolic gene expression in diabetic rodents.
Mol Pharmacol 59 : — Marx N , Kehrle B , Kohlhammer K , Grub M , Koenig W , Hombach V , Libby P , Plutzky J PPAR activators as antiinflammatory mediators in human T lymphocytes: implications for atherosclerosis and transplantation-associated arteriosclerosis.
Circ Res 90 : — Marx N , Froehlich J , Siam L , Ittner J , Wierse G , Schmidt A , Scharnagl H , Hombach V , Koenig W Antidiabetic PPAR γ-activator rosiglitazone reduces MMP-9 serum levels in type 2 diabetic patients with coronary artery disease.
Arterioscler Thromb Vasc Biol 23 : — Raji A , Seely EW , Bekins SA , Williams GH , Simonson DC Rosiglitazone improves insulin sensitivity and lowers blood pressure in hypertensive patients. Kernan WN , Inzucchi SE , Viscoli CM , Brass LM , Bravata DM , Shulman GI , McVeety JC , Horwitz RI Pioglitazone improves insulin sensitivity among nondiabetic patients with a recent transient ischemic attack or ischemic stroke.
Stroke 34 : — Wang TD , Chen WJ , Lin JW , Chen MF , Lee YT Effects of rosiglitazone on endothelial function, C-reactive protein, and components of the metabolic syndrome in nondiabetic patients with the metabolic syndrome. Am J Cardiol 93 : — Gastroenterology : A Hatzitolios A , Savopoulos C , Lazaraki G , Sidiropoulos I , Haritanti P , Lefkopoulos A , Karagiannopoulou G , Tzioufa V , Dimitrios K Efficacy of omega-3 fatty acids, atorvastatin and orlistat in non-alcoholic fatty liver disease with dyslipidemia.
Indian J Gastroenterol 23 : — Rallidis LS , Drakoulis CK , Parasi AS Pravastatin in patients with nonalcoholic steatohepatitis: results of a pilot study. Atherosclerosis : — Ogawa Y , Murata Y , Saibara T , Nishioka A , Kariya S , Yoshida S Follow-up CT findings of tamoxifen-induced non-alcoholic steatohepatitis NASH of breast cancer patients treated with bezafibrate.
Oncol Rep 10 : — Basaranoglu M , Acbay O , Sonsuz A A controlled trial of gemfibrozil in the treatment of patients with nonalcoholic steatohepatitis.
J Hepatol 31 : Chang CY , Argo CK , Al-Osaimi AM , Caldwell SH Therapy of NAFLD: antioxidants and cytoprotective agents. J Clin Gastroenterol 40 : S51 — S Lindor KD , Kowdley KV , Heathcote EJ , Harrison ME , Jorgensen R , Angulo P , Lymp JF , Burgart L , Colin P Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial.
Clark JM , Alkhuraishi AR , Solga SF , Alli P , Diehl AM , Magnuson TH Roux-en-Y gastric bypass improves liver histology in patients with non-alcoholic fatty liver disease.
Obes Res 13 : — Caldwell SH , Hespenheide EE , Redick JA , Iezzoni JC , Battle EH , Sheppard BL A pilot study of a thiazolidinedione, troglitazone, in nonalcoholic steatohepatitis.
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide. Sign In or Create an Account. Endocrine Society Journals.
Advanced Search. Search Menu. Article Navigation. Close mobile search navigation Article Navigation. Volume Article Contents Abstract. Epidemiology of NAFLD. Insulin Resistance in NAFLD. Obesity, Adipokines, Inflammation, and NAFLD.
Potential Mechanisms for Hepatic Fat Accumulation. Insulin Resistance in NAFLD—Cause or Consequence? Treatment of Hepatic Steatosis. Conclusions and Future Directions. Journal Article. The Role of Insulin Resistance in Nonalcoholic Fatty Liver Disease.
Utzschneider , Kristina M. Utzschneider, M. Oxford Academic. Steven E. PDF Split View Views. Cite Cite Kristina M. Select Format Select format. ris Mendeley, Papers, Zotero. enw EndNote. bibtex BibTex. txt Medlars, RefWorks Download citation.
Permissions Icon Permissions. Abstract Context: Insulin resistance is an almost universal finding in nonalcoholic fatty liver disease NAFLD. Open in new tab Download slide. TABLE 1. Clinical trials targeting insulin resistance in NAFLD and NASH.
Age yr mean ± sd range. NAFLD or NASH. Open in new tab. TABLE 1A. Study type. Liver enzymes. Liver fat change and assessment method. Open 1 yr Decreased NA NA Open vs. nonalcoholic fatty liver disease;. sterol receptor binding protein 1-c. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity.
Google Scholar Crossref. Search ADS. NASH and insulin resistance: insulin hypersecretion and specific association with the insulin resistance syndrome.
Metabolic and anthropometric evaluation of insulin resistance in nondiabetic patients with nonalcoholic steatohepatitis. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis.
Postprandial triglyceride-rich lipoprotein metabolism and insulin sensitivity in nonalcoholic steatohepatitis patients. Ninety patients with nonalcoholic steatohepatitis: insulin resistance, familial tendency, and severity of disease.
Liver-fat accumulation and insulin resistance in obese women with previous gestational diabetes. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities.
Google Scholar PubMed. OpenURL Placeholder Text. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Characterization of pathogenic and prognostic factors of nonalcoholic steatohepatitis associated with obesity.
Nonalcoholic fatty liver disease in Saudi type 2 diabetic subjects attending a medical outpatient clinic: prevalence and general characteristics. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients.
High alanine aminotransferase is associated with decreased hepatic insulin sensitivity and predicts the development of type 2 diabetes. Fatty liver hepatitis steatohepatitis and obesity: an autopsy study with analysis of risk factors. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease.
Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Idiopathic steatohepatitis in childhood: a multicenter retrospective study.
Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men.
The concurrent accumulation of intra-abdominal and subcutaneous fat explains the association between insulin resistance and plasma leptin concentrations: distinct metabolic effects of two fat compartments.
Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Abdominal fat distribution and peripheral and hepatic insulin resistance in type 2 diabetes mellitus.
Visceral adiposity and the risk of impaired glucose tolerance: a prospective study among Japanese Americans. The atherogenic lipoprotein profile associated with obesity and insulin resistance is largely attributable to intra-abdominal fat.
Visceral adipose tissue cutoffs associated with metabolic risk factors for coronary heart disease in women. Intra-abdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome. Fatty liver in type 2 diabetes mellitus: relation to regional adiposity, fatty acids, and insulin resistance.
Visceral fat and liver fat are independent predictors of metabolic risk factors in men. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. Contribution of leg and splanchnic free fatty acid FFA kinetics to postabsorptive FFA flux in men and women.
Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex.
Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Plasma adiponectin in nonalcoholic fatty liver is related to hepatic insulin resistance and hepatic fat content, not to liver disease severity.
Plasma resistin concentration, hepatic fat content, and hepatic and peripheral insulin resistance in pioglitazone-treated type II diabetic patients. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice.
Association of inflammation markers with impaired insulin sensitivity and coagulative activation in obese healthy women. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue?
Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Circulating interleukin 6 levels, blood pressure, and insulin sensitivity in apparently healthy men and women.
Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. Obesity, and not insulin resistance, is the major determinant of serum inflammatory cardiovascular risk markers in pre-menopausal women.
Tumor necrosis factor α and insulin resistance in obese type 2 diabetic patients. Are there predictive factors of severe liver fibrosis in morbidly obese patients with non-alcoholic steatohepatitis?
Cytokines and NASH: a pilot study of the effects of lifestyle modification and vitamin E. Gene expression of tumor necrosis factor α and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients.
Primacy of hepatic insulin resistance in the development of the metabolic syndrome induced by an isocaloric moderate-fat diet in the dog. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. Dietary fat content modifies liver fat in overweight nondiabetic subjects.
Human fatty acid synthesis is stimulated by a eucaloric low fat, high carbohydrate diet. Non-alcoholic steatohepatitis: association with obesity and insulin resistance, and influence of weight loss.
Mechanisms of the free fatty acid-induced increase in hepatic glucose production. Contribution of adipose tissue and de novo lipogenesis to nonalcoholic fatty liver disease. Isoform 1c of sterol regulatory element binding protein is less active than isoform 1a in livers of transgenic mice and in cultured cells.
Foxa2 regulates lipid metabolism and ketogenesis in the liver during fasting and in diabetes. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-γ ligand rosiglitazone.
A pilot study of a thiazolidinedione, pioglitazone, in nonalcoholic steatohepatitis. Google Scholar OpenURL Placeholder Text. A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis.
Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference.
Effect of weight control on hepatic abnormalities in obese patients with fatty liver. Therapeutic effects of restricted diet and exercise in obese patients with fatty liver.
Effect of changes on body weight and lifestyle in nonalcoholic fatty liver disease. One-year intense nutritional counseling results in histological improvement in patients with non-alcoholic steatohepatitis: a pilot study.
Effects of identical weight loss on body composition and features of insulin resistance in obese women with high and low liver fat content. Effects of diet and exercise on muscle and liver intracellular lipid contents and insulin sensitivity in type 2 diabetic patients.
Liver abnormalities in severely obese subjects: effect of drastic weight loss after gastroplasty. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. Metformin in the treatment of non-alcoholic steatohepatitis: a pilot open label trial.
A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease.
Metformin in the treatment of patients with non-alcoholic steatohepatitis. Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes.
Effect of rosiglitazone on glucose and non-esterified fatty acid metabolism in type II diabetic patients. Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. Differential effects of rosiglitazone and metformin on adipose tissue distribution and glucose uptake in type 2 diabetic subjects.
Effects of pioglitazone on adipose tissue remodeling within the setting of obesity and insulin resistance. The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes.
The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Modulation of circulating and adipose tissue adiponectin levels by antidiabetic therapy. Synthetic peroxisome proliferator-activated receptor-γ agonist, rosiglitazone, increases plasma levels of adiponectin in type 2 diabetic patients.
Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. Differential effects of rexinoids and thiazolidinediones on metabolic gene expression in diabetic rodents.
PPAR activators as antiinflammatory mediators in human T lymphocytes: implications for atherosclerosis and transplantation-associated arteriosclerosis. Antidiabetic PPAR γ-activator rosiglitazone reduces MMP-9 serum levels in type 2 diabetic patients with coronary artery disease.
Rosiglitazone improves insulin sensitivity and lowers blood pressure in hypertensive patients. Pioglitazone improves insulin sensitivity among nondiabetic patients with a recent transient ischemic attack or ischemic stroke. Effects of rosiglitazone on endothelial function, C-reactive protein, and components of the metabolic syndrome in nondiabetic patients with the metabolic syndrome.
Efficacy of omega-3 fatty acids, atorvastatin and orlistat in non-alcoholic fatty liver disease with dyslipidemia.
Hepatic insulin resistance is somewhat peculiar as the effects of hepatic insulin signaling result in insufficient suppression of hepatic gluconeogenesis and decreased glycogen synthesis but increased lipid accumulation.
This selective hepatic insulin resistance contributes to simultaneous increases in liver glucose production and fat synthesis, resulting in hyperglycemia and dyslipidemia characteristic of T2DM.
Studies using genetic models of tissue-specific insulin resistance obtained by selectively knocking out insulin receptor genes with Cre-loxP technology have found differing effects on insulin resistance in different tissues during systemic metabolic disease Kubota et al.
The results show that muscle insulin receptor knockout MIRKO mice or fat insulin receptor knockout FIRKO mice still have normal blood glucose and insulin levels, as well as normal glucose tolerance test responses, although they have separately exhibited specific metabolic abnormalities of tissue-specific insulin resistance Biddinger and Kahn, In contrast, LIRKO mice show severe insulin resistance, fasting and post-prandial hyperglycemia, glucose intolerance, and hyperinsulinemia.
These studies indicate that insulin resistance of peripheral tissues alone is not enough to cause abnormality of glucose tolerance or insulin resistance syndrome. However, hepatic insulin resistance as the leading cause of fasting hyperglycemia might be the critical factor driving the development of T2DM.
Collectively, NAFLD-related pathophysiology includes hepatic ectopic fat deposition, inflammation, ER stress, and oxidative stress Haas et al. Metabolic disorders that include systemic glucose and lipid metabolism show a progressive exacerbation, resulting in the occurrence of T2DM. Currently, therapeutic strategies for treating NAFLD primarily encompass limiting caloric intake and proper exercise to maintain a healthy lifestyle.
However, the standard treatment for NAFLD has not been approved in current clinical practice guidelines Nascimbeni et al. Some potential pharmacological target strategies are emerging to influence the energy balance, inhibit key enzymes involved in lipid synthesis or metabolic pathways that contribute to NAFLD, such as agonists for Peroxisome proliferator-activated receptors PPARs, e.
Besides, a novel class of liver-targeted mitochondrial uncoupling agents increases hepatocellular energy expenditure, reversing the metabolic and hepatic complications of NAFLD [e.
Finally, our laboratory has been working on studies of mechanisms of lipid metabolic disorders and related targets of therapeutic drugs. Our previous research shows that nuciferine and siRNA PAS-domain containing protein kinase Pask, an evolutionarily conserved nutrient-responsive protein kinase could alleviate the accumulation of lipogenesis, inflammation, and oxidative stress in NAFLD Zhang et al.
Considering the complex and bidirectional relationship between NAFLD and T2DM, we speculate that Pask plays a potential role in the deterioration from NAFLD to T2DM Zhang et al. Taken together, the relationship between NAFLD and T2DM is complex and bidirectional. NAFLD provides the necessary biological milieu for development of T2DM Lallukka and Yki-Jarvinen, , and the presence of T2DM increases the risk of liver diseases Raff et al.
However, it is remarkable that existing guidelines do not advocate screening for liver-related complications in patients with T2DM, making the liver a potentially neglected organ during the progression of chronic metabolic diseases.
Therefore, solidifying a robust overall paradigm regarding the pathological mechanisms of liver metabolism in NAFLD and T2DM would contribute to a search for potential therapies targeting hepatic steatosis and lipid-induced hepatic insulin resistance.
It is also of great clinical importance to advocate for a more active and systematic surveillance of NAFLD in patients with T2DM, with a view toward potential early treatment Vizuete et al. WM and X-yL designed the research.
X-fC and YL consulted and helped to categorize related references. WM and X-yL wrote the manuscript. G-lL and Q-zL participated in the revision of the manuscript. X-yL and J-gZ supervised the research.
All authors contributed to and approved the final version of the manuscript. This research work was supported by the Crossing Program between Medicine and Industry of Shanghai Jiao Tong University Grant No. YGMS32 , and the Doctoral Program of Higher Education of China Grant No.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Abulizi, A. A controlled-release mitochondrial protonophore reverses hypertriglyceridemia, nonalcoholic steatohepatitis, and diabetes in lipodystrophic mice.
FASEB J. doi: PubMed Abstract CrossRef Full Text Google Scholar. Anstee, Q. How big a problem is non-alcoholic fatty liver disease? BMJ d Armstrong, M. Glucagon-like peptide 1 decreases lipotoxicity in non-alcoholic steatohepatitis.
Biddinger, S. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab. From mice to men: insights into the insulin resistance syndromes. CrossRef Full Text Google Scholar.
Birkenfeld, A. Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes. Hepatology 59, — Bluher, M. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance.
Cell 3, 25— Brown, J. CGI knockdown in mice causes hepatic steatosis but prevents diet-induced obesity and glucose intolerance.
Lipid Res. Cai, K. Diacylglycerol kinase theta couples farnesoid X receptor-dependent bile acid signalling to Akt activation and glucose homoeostasis in hepatocytes. Campbell, J. Pharmacology, physiology, and mechanisms of incretin hormone action.
Cao, J. Targeting Acyl-CoA:diacylglycerol acyltransferase 1 DGAT1 with small molecule inhibitors for the treatment of metabolic diseases.
Carino, A. BAR, a dual FXR and GPBAR1 agonist, promotes browning of white adipose tissue and reverses liver steatosis and fibrosis. Caron, A. The roles of mTOR complexes in lipid metabolism.
Catrysse, L. Inflammation and the metabolic syndrome: the tissue-specific functions of NF-kappaB. Trends Cell Biol. Cusi, K. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial.
Day, C. Gastroenterology , — Ertle, J. Non-alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Cancer , — Gao, Z. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex.
Grootjans, J. The unfolded protein response in immunity and inflammation. Haas, J. Pathophysiology and mechanisms of nonalcoholic fatty liver disease. Haeusler, R. Integrated control of hepatic lipogenesis versus glucose production requires FoxO transcription factors.
Hall, A. Evidence for regulated monoacylglycerol acyltransferase expression and activity in human liver. Abrogating monoacylglycerol acyltransferase activity in liver improves glucose tolerance and hepatic insulin signaling in obese mice.
Diabetes 63, — Huang, W. Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes 59, — Johnson, A. The origins and drivers of insulin resistance. Cell , — Jornayvaz, F. Diacylglycerol activation of protein kinase Cepsilon and hepatic insulin resistance.
Kahn, S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet , — Kliewer, S. Koehler, E. Presence of diabetes mellitus and steatosis is associated with liver stiffness in a general population: the Rotterdam study.
Hepatology 63, — Koliaki, C. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Kubota, T. Imbalanced insulin actions in obesity and type 2 diabetes: key mouse models of insulin signaling pathway.
Kumashiro, N. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Kwok, R. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut 65, — Lallukka, S. Non-alcoholic fatty liver disease and risk of type 2 diabetes.
Best Pract. Leavens, K. Insulin signaling to hepatic lipid metabolism in health and disease. Lillioja, S. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians.
Long, Y. Insulin receptor substrates Irs1 and Irs2 coordinate skeletal muscle growth and metabolism via the Akt and AMPK pathways. Cell Biol. Loomba, R. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis.
Hepatology 56, — Lu, M. Insulin regulates liver metabolism in vivo in the absence of hepatic Akt and Foxo1. Ludwig, J. Nonalcoholic steatohepatitis: mayo Clinic experiences with a hitherto unnamed disease.
Mayo Clin. PubMed Abstract Google Scholar. Luukkonen, P. Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease.
Maciejewski, B. Pharmacological inhibition to examine the role of DGAT1 in dietary lipid absorption in rodents and humans. Liver Physiol. Martin, B. Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a year follow-up study. Michael, M. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction.
Cell 6, 87— Mizuno, T. Specific preservation of biosynthetic responses to insulin in adipose tissue may contribute to hyperleptinemia in insulin-resistant obese mice. Moschen, A.
Adipose and liver expression of interleukin IL -1 family members in morbid obesity and effects of weight loss. Anti-inflammatory effects of excessive weight loss: potent suppression of adipose interleukin 6 and tumour necrosis factor alpha expression.
Gut 59, — Nascimbeni, F. From NAFLD in clinical practice to answers from guidelines. Ng, M. Global, regional, and national prevalence of overweight and obesity in children and adults during a systematic analysis for the Global Burden of Disease Study Odegaard, J.
Alternative M2 activation of Kupffer cells by PPAR delta ameliorates obesity-induced insulin resistance. Okuma, C. JTP, a novel monoacylglycerol acyltransferase inhibitor, modulates fat absorption and prevents diet-induced obesity.
Otero, Y. Pathway-selective insulin resistance and metabolic disease: the importance of nutrient flux. Ozcan, U. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science , — Pansuria, M. Insulin resistance, metabolic stress, and atherosclerosis.
Google Scholar. Pawlak, M. Molecular mechanism of PPAR alpha action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease.
Perry, R. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Controlled-release mitochondrial protonophore reverses diabetes and steatohepatitis in rats. Reversal of hypertriglyceridemia, fatty liver disease, and insulin resistance by a liver-targeted mitochondrial uncoupler.
The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature , 84— Petersen, K. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes.
Diabetes 54, — The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. Petersen, M. Insulin receptor Thr phosphorylation mediates lipid-induced hepatic insulin resistance.
Phielix, E. The role of metformin and thiazolidinediones in the regulation of hepatic glucose metabolism and its clinical impact. Trends Pharmacol. Raff, E. Diabetes mellitus predicts occurrence of cirrhosis and hepatocellular cancer in alcoholic liver and non-alcoholic fatty liver diseases.
Ratziu, V. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-alpha and -delta, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Reaven, G. The insulin resistance syndrome: definition and dietary approaches to treatment.
Roden, M. Mechanisms of Disease: hepatic steatosis in type 2 diabetes—pathogenesis and clinical relevance. Rui, L. Energy metabolism in the liver. Samuel, V. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. Satapati, S.
Kristina M. Utzschneider, Steven E. Context: Insulin resistance Hyperglycemia and blood glucose monitoring an almost resistznce finding livee nonalcoholic fatty heaalth disease Insulin resistance and liver health. This review outlines the evidence linking insulin resistance and NAFLD, explores whether liver fat is a cause or consequence of insulin resistance, and reviews the current evidence for treatment of NAFLD. Evidence Acquisition: Evidence from epidemiological, experimental, and clinical research studies investigating NAFLD and insulin resistance was reviewed.
0 thoughts on “Insulin resistance and liver health”