Inflammation and respiratory health -
Numerous studies have shown that low-grade inflammation is a common decimal in aging. Inflammaging is marked by a general increase in the production of pro-inflammatory cytokines and inflammatory markers [ ].
The mechanism by which the low-grade inflammation is activated remains unknown. Computational modeling is a powerful tool that could help unravel the complexity of chronic inflammation including age-related inflammation.
Using mathematical models that accurately represent the lung, we can study the interactions across various biological scales and make predictions for future outcomes of existing interactions based on currently available experimental data, which might otherwise not be possible.
Multiscale mechanistic models that couple cellular and molecular processes to tissue-level behavior could be implemented to test different hypotheses that explain how changes at the molecular and cellular-level may influence the onset and progress of chronic inflammation in aging subjects.
Correlation between tissue properties, magnitude and duration of stress a tissue is exposed to and the molecular response during aging is necessary to understand inflammaging.
Development and analysis of such models would provide insights into the process of aging and help physicians implement therapeutic strategies to address the aging process and treat diseases. Computational models have been developed to study the aging process [ , , , ].
Mc Auley and Mooney [ ] used a computational model to study lipid metabolism and aging. Weinberg et al. More information on models that have been developed within the last 50—60 years to study cellular aging can be found in the review by Witten [ ].
More research is needed to understand the aging process at the cellular, organ and system-level and computational modeling is a valuable tool that could be used to further our understanding of aging and age-related diseases.
This paper reviews key mechanisms of inflammation in airway diseases. It discusses the role of mathematical and computational modeling in furthering our understanding of the complex inflammation mechanism in airway diseases.
Results from experimental studies have greatly improved our knowledge of the cellular and molecular events that are involved in the acute inflammatory response to infection and tissue injury in many organs [ , , , , ]. Experimental studies usually use reductionist approach, so they may fail to describe system-level behavior accurately.
Mathematical and computational models can be employed to study the interactions across various biological scales and make predictions for future outcomes of existing interactions based on currently available experimental data. We recommend that multiscale models should be implemented to test hypotheses that explain how changes at the molecular and cellular-levels may influence the onset and progress of chronic inflammation.
Multiscale models could be employed to understand the tissue microenvironment effects on inflammation mechanism in young and aged lungs.
Despite all conducted computational and experimental studies on lung inflammation mechanism, there is lack of details on molecular mechanisms and pathways that contribute to activation of low-grade inflammation and onset of chronic inflammation in lung.
There is need for models that link the interactions at the molecular, cellular and tissue-levels to provide a systems perspective to the pathology of inflammatory mechanism in lung diseases. More research is needed to understand the mechanisms that produce acute or systemic chronic inflammation which occurs in many diseases such as autoimmune diseases, obesity, cardiovascular diseases, type 2 diabetes, among many others [ , , , ].
Ahmed AU. An overview of inflammation: mechanism and consequences. Frontiers in Biology. CAS Google Scholar. Ward P. Acute lung injury: how the lung inflammatory response works. Eur Respir Soc; ;s—23s. Article CAS Google Scholar.
Lee G, Walser TC, Dubinett SM. Chronic inflammation, chronic obstructive pulmonary disease, and lung cancer. Curr Opin Pulm Med.
Article CAS PubMed Google Scholar. Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Ortega-Gómez A, Perretti M, Soehnlein O. Resolution of inflammation: an integrated view.
EMBO Mol Med. Article CAS PubMed PubMed Central Google Scholar. Maskrey BH, Megson IL, Whitfield PD, Rossi AG. Mechanisms of resolution of inflammation. Arterioscler Thromb Vasc Biol. Najar M, Krayem M, Merimi M, Burny A, Meuleman N, Bron D, et al. Insights into inflammatory priming of mesenchymal stromal cells: functional biological impacts.
Inflamm Res. Article PubMed Google Scholar. Lumb AB. Amsterdam: Elsevier Health Sciences; Google Scholar.
Tripathi P, Aggarwal A. NF-kB transcription factor: a key player in the generation of immune response. Curr Sci Bangalore. Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury.
Mol Med. Lee I-T, Yang C-M. Inflammatory signalings involved in airway and pulmonary diseases. Mediat Inflamm. Moldoveanu B, Otmishi P, Jani P, Walker J, Sarmiento X, Guardiola J, et al. Inflammatory mechanisms in the lung. J Inflamm Res. CAS PubMed Google Scholar. Nelson RJ. Seasonal immune function and sickness responses.
Trends Immunol. Nelson RJ, Demas GE. Seasonal changes in immune function. Q Rev Biol. Colgan SP, Curtis VF, Campbell EL. The inflammatory tissue microenvironment in IBD. Inflamm Bowel Dis. Dantzer R. Cytokine-induced sickness behavior: where do we stand?
Brain Behav Immun. Hotamisligil GS. Inflammation and metabolic disorders. Chung K, Adcock I. Multifaceted mechanisms in COPD: inflammation, immunity, and tissue repair and destruction. Eur Respir J. Hartupee J, Mann DL.
Role of inflammatory cells in fibroblast activation. J Mol Cell Cardiol. Baylis D, Bartlett DB, Patel HP, Roberts HC. Understanding how we age: insights into inflammaging.
Longev Healthspan. Article PubMed PubMed Central Google Scholar. Libby P. Inflammatory mechanisms: the molecular basis of inflammation and disease. Nutr Rev. Brodland GW. How computational models can help unlock biological systems.
Semin Cell Dev Biol. Vodovotz Y. Computational modelling of the inflammatory response in trauma, sepsis and wound healing: implications for modelling resilience. Interface Focus. Fletcher AG, Osterfield M, Baker RE, Shvartsman SY. Vertex models of epithelial morphogenesis.
Biophys J. Fletcher AG, Cooper F, Baker RE. Mechanocellular models of epithelial morphogenesis. Philos Trans R Soc Lond B Biol Sci. George UZ, Bokka KK, Warburton D, Lubkin SR. Quantifying stretch and secretion in the embryonic lung: Implications for morphogenesis.
Mech Dev. Lubkin SR, Murray JD. A mechanism for early branching in lung morphogenesis. J Math Biol. Clément R, Douady S, Mauroy B. Branching geometry induced by lung self-regulated growth. Phys Biol. Iber D, Menshykau D. The control of branching morphogenesis. Open Biol. Varner VD, Nelson CM. Computational models of airway branching morphogenesis.
Tran K, Smith NP, Loiselle DS, Crampin EJ. A metabolite-sensitive, thermodynamically constrained model of cardiac cross-bridge cycling: implications for force development during ischemia. Washio T, Okada JI, Sugiura S, Hisada T. Approximation for cooperative interactions of a spatially-detailed cardiac sarcomere model.
Cell Mol Bioeng. Dewan S, McCabe KJ, Regnier M, McCulloch AD. Insights and challenges of multi-scale modeling of sarcomere mechanics in cTn and Tm DCM mutants-genotype to cellular phenotype.
Front Physiol. Constantino J, Hu Y, Trayanova NA. A computational approach to understanding the cardiac electromechanical activation sequence in the normal and failing heart, with translation to the clinical practice of CRT.
Prog Biophys Mol Biol. Lopez-Perez A, Sebastian R, Ferrero JM. Three-dimensional cardiac computational modelling: methods, features and applications. Biomed Eng Online. Trayanova NA. Whole-heart modeling: applications to cardiac electrophysiology and electromechanics. Circ Res. Friedrich J, Lengyel M.
Goal-directed decision making with spiking neurons. J Neurosci. Rustichini A, Conen KE, Cai X, Padoa-Schioppa C. Optimal coding and neuronal adaptation in economic decisions. Nat Commun. Cumming BD, McElwain DL, Upton Z. A mathematical model of wound healing and subsequent scarring.
J R Soc Interface. Flegg JA, Byrne HM, Flegg MB, McElwain DL. Wound healing angiogenesis: the clinical implications of a simple mathematical model. J Theor Biol. Sherratt JA, Dallon JC. Theoretical models of wound healing: past successes and future challenges.
C R Biol. Patel AA, Gawlinski ET, Lemieux SK, Gatenby RA. A cellular automaton model of early tumor growth and invasion. Powathil G, Kohandel M, Sivaloganathan S, Oza A, Milosevic M.
Mathematical modeling of brain tumors: effects of radiotherapy and chemotherapy. Phys Med Biol. Anderson AR, Chaplain MA. Continuous and discrete mathematical models of tumor-induced angiogenesis. Bull Math Biol. McDougall SR, Anderson AR, Chaplain MA. Mathematical modelling of dynamic adaptive tumour-induced angiogenesis: clinical implications and therapeutic targeting strategies.
Vodovotz Y, Chow CC, Bartels J, Lagoa C, Prince JM, Levy RM, et al. In silico models of acute inflammation in animals. Day J, Rubin J, Vodovotz Y, Chow CC, Reynolds A, Clermont G.
A reduced mathematical model of the acute inflammatory response II. Capturing scenarios of repeated endotoxin administration. Reynolds A, Rubin J, Clermont G, Day J, Vodovotz Y, Bard Ermentrout G. A reduced mathematical model of the acute inflammatory response: I. Derivation of model and analysis of anti-inflammation.
Dong X, Foteinou PT, Calvano SE, Lowry SF, Androulakis IP. Agent-based modeling of endotoxin-induced acute inflammatory response in human blood leukocytes. PLoS One. Kumar R, Clermont G, Vodovotz Y, Chow CC. The dynamics of acute inflammation. Álvarez E, Toledano V, Morilla F, Hernández-Jiménez E, Cubillos-Zapata C, Varela-Serrano A, et al.
A system dynamics model to predict the human monocyte response to endotoxins. Front Immunol. Brown D, Namas RA, Almahmoud K, Zaaqoq A, Sarkar J, Barclay DA, et al. Trauma in silico: Individual-specific mathematical models and virtual clinical populations.
Sci Transl Med. Abboud A, Mi Q, Puccio A, Okonkwo D, Buliga M, Constantine G, et al. Inflammation following traumatic brain injury in humans: insights from data-driven and mechanistic models into survival and death.
Front Pharmacol. Barber J, Tronzo M, Harold Horvat C, Clermont G, Upperman J, Vodovotz Y, et al. A three-dimensional mathematical and computational model of necrotizing enterocolitis. Swan AJ, Tawhai MH. Evidence for minimal oxygen heterogeneity in the healthy human pulmonary acinus.
J Appl Physiol Article Google Scholar. Hewitt TJ, Hattler BG, Federspiel WJ. A mathematical model of gas exchange in an intravenous membrane oxygenator. Ann Biomed Eng. Brighenti C, Gnudi G, Avanzolini G. A simulation model of the oxygen alveolo-capillary exchange in normal and pathological conditions.
Physiol Meas. De Backer JW, Vos WG, Gorlé CD, Germonpré P, Partoens B, Wuyts FL, et al. Flow analyses in the lower airways: patient-specific model and boundary conditions. Med Eng Phys.
Aghasafari P, Bin M, Ibrahim I, Pidaparti R. Strain-induced inflammation in pulmonary alveolar tissue due to mechanical ventilation. Biomech Model Mechanobiol. Pidaparti RM, Koombua K. Tissue strains induced in airways due to mechanical ventilation.
Mol Cell Biomech. PubMed Google Scholar. Pidaparti RM, Swanson J. Effect of mechanical ventilation waveforms on airway wall shear. J Med Eng Technol. Yin Y, Choi J, Hoffman EA, Tawhai MH, Lin CL.
A multiscale MDCT image-based breathing lung model with time-varying regional ventilation. J Comput Phys. Swan AJ, Clark AR, Tawhai MH.
A computational model of the topographic distribution of ventilation in healthy human lungs. Roth CJ, Yoshihara L, Ismail M, Wall WA. Computational modelling of the respiratory system: discussion of coupled modeling approaches and two recent extensions.
Comput Methods Appl Mech Eng. Burrowes KS, Clark AR, Tawhai MH. Blood flow redistribution and ventilation-perfusion mismatch during embolic pulmonary arterial occlusion.
Pulm Circ. Tang BT, Fonte TA, Chan FP, Tsao PS, Feinstein JA, Taylor CA. Three-dimensional hemodynamics in the human pulmonary arteries under resting and exercise conditions. Rausch SM, Martin C, Bornemann PB, Uhlig S, Wall WA. Material model of lung parenchyma based on living precision-cut lung slice testing.
J Mech Behav Biomed Mater. Berger L, Bordas R, Burrowes K, Grau V, Tavener S, Kay D. A poroelastic model coupled to a fluid network with applications in lung modelling. Int J Numer Method Biomed Eng. Burrowes KS, Doel T, Brightling C.
Computational modeling of the obstructive lung diseases asthma and COPD. J Transl Med. Kim J, Heise RL, Reynolds AM, Pidaparti RM. Quantification of age-related lung tissue mechanics under mechanical ventilation.
Med Sci Basel. Aging effects on airflow dynamics and lung function in human bronchioles. Cheng YH, You SH, Lin YJ, Chen SC, Chen WY, Chou WC, et al. Mathematical modeling of postcoinfection with influenza A virus and Streptococcus pneumoniae, with implications for pneumonia and COPD-risk assessment.
Int J Chron Obstruct Pulmon Dis. Article PubMed PubMed Central CAS Google Scholar. Cox LA. A causal model of chronic obstructive pulmonary disease COPD risk. Risk Anal. Brown BN, Price IM, Toapanta FR, DeAlmeida DR, Wiley CA, Ross TM, et al. An agent-based model of inflammation and fibrosis following particulate exposure in the lung.
Math Biosci. Kim Y, Lee S, Kim YS, Lawler S, Gho YS, Kim YK, et al. Math Biosci Eng. Reynolds A, Koombua K, Pidaparti RM, Ward KR. Cellular automata modeling of pulmonary inflammation. Ibrahim I, Oruganti SV, Pidaparti R.
Simulation of Healing Threshold in Strain-Induced Inflammation through a discrete informatics model. IEEE J Biomed Health Inform. W GA,M. Agent-based modeling approaches to multi-scale systems biology: an example agent-based model of acute pulmonary inflammation.
In: Prokop A, Csukás B, editors. Systems biology. Dordrecht: Springer; Lane N, Robins RA, Corne J, Fairclough L. Regulation in chronic obstructive pulmonary disease: the role of regulatory T-cells and Th17 cells.
Clin Sci. Donnelly LE, Barnes PJ. Chemokine receptors as therapeutic targets in chronic obstructive pulmonary disease. Trends Pharmacol Sci. Traynor TR, Herring AC, Dorf ME, Kuziel WA, Toews GB, Huffnagle GB. J Immunol. Barnes PJ.
Cellular and molecular mechanisms of asthma and COPD. Suki B, Lutchen KR, Ingenito EP. On the progressive nature of emphysema: roles of proteases, inflammation, and mechanical forces. Am J Respir Crit Care Med. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. Chung KF.
The role of airway smooth muscle in the pathogenesis of airway wall remodeling in chronic obstructive pulmonary disease. Proc Am Thorac Soc. Hutchinson AT, Vlahos R, Bozinovski S. Role of alveolar macrophages in chronic obstructive pulmonary disease.
Brusselle GG, Joos GF, Bracke KR. New insights into the immunology of chronic obstructive pulmonary disease. Kudo M, Ishigatsubo Y, Aoki I.
Pathology of asthma. Front Microbiol. Targeting the interleukin pathway in the treatment of asthma. Wang Y, Bai C, Li K, Adler KB, Wang X. Role of airway epithelial cells in development of asthma and allergic rhinitis. Respir Med. Holgate ST.
Innate and adaptive immune responses in asthma. Nat Med. Kubo T, Morita H, Sugita K, Akdis CA. Introduction to mechanisms of allergic diseases. Amsterdam: Elsevier; Murdoch JR, Lloyd CM. Chronic inflammation and asthma. Mutat Res Fundam Mol Mech Mutagen. Carr TF, Berdnikovs S, Simon H-U, Bochner BS, Rosenwasser LJ.
Eosinophilic bioactivities in severe asthma. World Allergy Organ J. Barnig C, Frossard N, Levy BD. Towards targeting resolution pathways of airway inflammation in asthma. Pharmacol Ther. Barnig C, Levy BD.
Innate immunity is a key factor for the resolution of inflammation in asthma. Eur Respir Rev. Martín-Orozco E, Norte-Muñoz M, Martínez-García J. Regulatory T cells in allergy and asthma. Front Pediatr. Ross R. Platelet-derived growth factor. Heldin C-H. Structural and functional studies on platelet-derived growth factor.
EMBO J. Dolgachev VA, Ullenbruch MR, Lukacs NW, Phan SH. Role of stem cell factor and bone marrow-derived fibroblasts in airway remodeling. Am J Pathol. Lee CG, Link H, Baluk P, Homer RJ, Chapoval S, Bhandari V, et al. Vascular endothelial growth factor VEGF induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung.
Bhandari V, Choo-Wing R, Chapoval SP, Lee CG, Tang C, Kim Y, et al. Essential role of nitric oxide in VEGF-induced, asthma-like angiogenic, inflammatory, mucus, and physiologic responses in the lung.
Proc Natl Acad Sci. McMillan SJ, Kearley J, Campbell JD, Zhu X-W, Larbi KY, Shipley JM, et al. Matrix metalloproteinase-9 deficiency results in enhanced allergen-induced airway inflammation. Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V, et al.
Interleukin induces tissue fibrosis by selectively stimulating and activating transforming growth factor β1. J Exp Med. Cutting GR. Cystic fibrosis genetics: from molecular understanding to clinical application.
Nat Rev Genet. Cutting GR, Engelhardt J, Zeitlin PL. Genetics and pathophysiology of cystic fibrosis. Collawn JF, Matalon S. CFTR and lung homeostasis. Am J Physiol Lung Cell Mol Physiol. Muir A, Soong G, Sokol S, Reddy B, Gomez MI, van Heeckeren A, et al. Toll-like receptors in normal and cystic fibrosis airway epithelial cells.
Am J Respir Cell Mol Biol. Chirico V, Lacquaniti A, Leonardi S, Grasso L, Rotolo N, Romano C, et al. Acute pulmonary exacerbation and lung function decline in patients with cystic fibrosis: high-mobility group box 1 HMGB1 between inflammation and infection. Clin Microbiol Infect.
Cohen TS, Prince A. Cystic fibrosis: a mucosal immunodeficiency syndrome. McCuaig S, Martin JG. How the airway smooth muscle in cystic fibrosis reacts in proinflammatory conditions: implications for airway hyper-responsiveness and asthma in cystic fibrosis.
Lancet Respir Med. Dekkers JF, van der Ent CK, Kalkhoven E, Beekman JM. PPARγ as a therapeutic target in cystic fibrosis.
Trends Mol Med. Bals R, Weiner DJ, Wilson JM. The innate immune system in cystic fibrosis lung disease. J Clin Investig. Tang AC, Turvey SE, Alves MP, Regamey N, Tümmler B, Hartl D. Current concepts: host—pathogen interactions in cystic fibrosis airways disease.
Hilliard TN, Regamey N, Shute JK, Nicholson AG, Alton EW, Bush A, et al. Airway remodelling in children with cystic fibrosis. Murphy G, Docherty AJ.
The matrix metalloproteinases and their inhibitors. Ratjen F, Hartog C, Paul K, Wermelt J, Braun J. Matrix metalloproteases in BAL fluid of patients with cystic fibrosis and their modulation by treatment with dornase alpha.
Courtney J, Ennis M, Elborn J. Cytokines and inflammatory mediators in cystic fibrosis. J Cyst Fibros. Hardie WD, Bejarano PA, Miller MA, Yankaskas JR, Ritter JH, Whitsett JA, et al. Immunolocalization of transforming growth factor α and epidermal growth factor receptor in lungs of patients with cystic fibrosis.
Pediatr Dev Pathol. Booth BW, Adler KB, Bonner JC, Tournier F, Martin LD. Interleukin induces proliferation of human airway epithelial cells in vitro via a mechanism mediated by transforming growth factor-α.
Spannhake EW. Interactions of pollutants with the epithelium. In: The pulmonary epithelium in health and disease. pp — López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging.
Naylor R, Baker D, Van Deursen J. Senescent cells: a novel therapeutic target for aging and age-related diseases. Clin Pharmacol Ther. Hosgood HD, Menashe I, He X, Chanock S, Lan Q.
PTEN identified as important risk factor of chronic obstructive pulmonary disease. Mercado N, Ito K, Barnes PJ. Accelerated ageing of the lung in COPD: new concepts. Holzenberger M, Dupont J, Ducos B, Leneuve P, Géloën A, Even PC, et al.
IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway.
Curr Biol. Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Ito K, Colley T, Mercado N. Geroprotectors as a novel therapeutic strategy for COPD, an accelerating aging disease. Hahn DR, Na C-L, Weaver TE.
Reserve autophagic capacity in alveolar epithelia provides a replicative niche for influenza A virus. Ito K, Barnes PJ. COPD as a disease of accelerated lung aging. CHEST J. Aoshiba K, Zhou F, Tsuji T, Nagai A. DNA damage as a molecular link in the pathogenesis of COPD in smokers.
Brightling CE, Monteiro W, Ward R, Parker D, Morgan MD, Wardlaw AJ, et al. Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomised controlled trial. Athanazio R.
Airway disease: similarities and differences between asthma, COPD and bronchiectasis. Clinics Sao Paulo. Sutherland ER, Martin RJ. Airway inflammation in chronic obstructive pulmonary disease: comparisons with asthma. J Allergy Clin Immunol. Cantin AM, Hartl D, Konstan MW, Chmiel JF.
Inflammation in cystic fibrosis lung disease: pathogenesis and therapy. Stankiewicz W, Dabrowski MP, Chcialowski A, Plusa T. Cellular and cytokine immunoregulation in patients with chronic obstructive pulmonary disease and bronchial asthma.
Mediators Inflamm. Burrows B, Knudson RJ, Cline MG, Lebowitz MD. Quantitative relationships between cigarette smoking and ventilatory function. Am Rev Respir Dis. Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, et al. Cluster analysis and clinical asthma phenotypes.
Voit EO. A systems-theoretical framework for health and disease: inflammation and preconditioning from an abstract modeling point of view.
Franceschi C, Campisi J. Chronic inflammation inflammaging and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. Medzhitov R. new adventures of an old flame. Goldstein B, Faeder JR, Hlavacek WS. Mathematical and computational models of immune-receptor signalling.
Li NY, Verdolini K, Clermont G, Mi Q, Rubinstein EN, Hebda PA, et al. A patient-specific in silico model of inflammation and healing tested in acute vocal fold injury.
Brauer F, Kris C. Dynamical systems for biological modeling: an introduction. Boca Raton: CRC Press; Pigozzo AB, Macedo GC, Santos RW, Lobosco M. On the computational modeling of the innate immune system. BMC Bioinform. Lee J, Adler FR, Kim PS.
A mathematical model for the macrophage response to respiratory viral infection in normal and asthmatic conditions. Chernyavsky IL, Croisier H, Chapman LA, Kimpton LS, Hiorns JE, Brook BS, et al. The role of inflammation resolution speed in airway smooth muscle mass accumulation in asthma: insight from a theoretical model.
James AL, Elliot JG, Jones RL, Carroll ML, Mauad T, Bai TR, et al. Airway smooth muscle hypertrophy and hyperplasia in asthma. Brook BS, Peel SE, Hall IP, Politi AZ, Sneyd J, Bai Y, et al.
A biomechanical model of agonist-initiated contraction in the asthmatic airway. Respir Physiol Neurobiol. Moulton DE, Goriely A. Possible role of differential growth in airway wall remodeling in asthma.
Schlender A, Alperin PE, Grossman HL, Sutherland ER. Modeling the impact of increased adherence to asthma therapy. Smith AM, Adler FR, Ribeiro RM, Gutenkunst RN, McAuley JL, McCullers JA, et al. Kinetics of coinfection with influenza A virus and Streptococcus pneumoniae.
PLoS Pathog. Smith AM, McCullers JA, Adler FR. Mathematical model of a three-stage innate immune response to a pneumococcal lung infection. Markovetz MR, Corcoran TE, Locke LW, Myerburg MM, Pilewski JM, Parker RS. A physiologically-motivated compartment-based model of the effect of inhaled hypertonic saline on mucociliary clearance and liquid transport in cystic fibrosis.
Donovan GM. Multiscale mathematical models of airway constriction and disease. Pulm Pharmacol Ther. Politi AZ, Donovan GM, Tawhai MH, Sanderson MJ, Lauzon AM, Bates JH, et al. A multiscale, spatially distributed model of asthmatic airway hyper-responsiveness.
Venegas JG, Winkler T, Musch G, Vidal Melo MF, Layfield D, Tgavalekos N, et al. Self-organized patchiness in asthma as a prelude to catastrophic shifts. Amin SD, Majumdar A, Frey U, Suki B. Modeling the dynamics of airway constriction: effects of agonist transport and binding.
Cilfone NA, Perry CR, Kirschner DE, Linderman JJ. Multi-scale modeling predicts a balance of tumor necrosis factor-α and interleukin controls the granuloma environment during Mycobacterium tuberculosis infection. Fallahi-Sichani M, El-Kebir M, Marino S, Kirschner DE, Linderman JJ.
Multiscale computational modeling reveals a critical role for TNF-α receptor 1 dynamics in tuberculosis granuloma formation.
Ceresa M, Olivares AL, Fernandez Suelves S, Noailly J, Gonzalez Ballester MA. Multi-scale immunological and biomechanical model of emphysema progression. Conf Proc IEEE Eng Med Biol Soc. Ceresa M, Olivares AL, Noailly J, González Ballester MA.
Coupled immunological and biomechanical model of emphysema progression. Cilfone NA, Kirschner DE, Linderman JJ. Strategies for efficient numerical implementation of hybrid multi-scale agent-based models to describe biological systems.
Marino S, Kirschner DE. A multi-compartment hybrid computational model predicts key roles for dendritic cells in tuberculosis infection. Computation Basel. Warsinske HC, Wheaton AK, Kim KK, Linderman JJ, Moore BB, Kirschner DE.
Computational modeling predicts simultaneous targeting of fibroblasts and epithelial cells is necessary for treatment of pulmonary fibrosis.
Bouchnita A, Bocharov G, Meyerhans A, Volpert V. Hybrid approach to model the spatial regulation of T cell responses. BMC Immunol.
Cevenini E, Caruso C, Candore G, Capri M, Nuzzo D, Duro G, et al. Age-related inflammation: the contribution of different organs, tissues and systems.
How to face it for therapeutic approaches. Curr Pharm Des. Weinberg EJ, Schoen FJ, Mofrad MR. A computational model of aging and calcification in the aortic heart valve. Mc Auley MT, Mooney KM. Computationally modeling lipid metabolism and aging: a mini-review. Comput Struct Biotechnol J.
Mooney KM, Morgan AE, Mc Auley MT. Aging and computational systems biology. Wiley Interdiscip Rev Syst Biol Med. Computational systems biology for aging research. Interdiscip Top Gerontol. Witten TM. Modeling cellular aging: an introduction—mathematical and computational approaches.
In: Rattan SIS, Hayflick L, editors. Cellular ageing and replicative senescence. vol 4. New York: Springer International Publishing; Origin and physiological roles of inflammation.
Weber GF, Chousterman BG, He S, Fenn AM, Nairz M, Anzai A, et al. Interleukin-3 amplifies acute inflammation and is a potential therapeutic target in sepsis. Serhan CN, Chiang N, Dalli J. The resolution code of acute inflammation: novel pro-resolving lipid mediators in resolution.
You can also have hypersensitivity pneumonitis in which your immune system overreacts to an inhaled irritant and triggers an extreme allergic response with lung inflammation.
Dust mites, pollen, and pet dander are common triggers. There are many different pathogens disease-causing agents that cause lung infections.
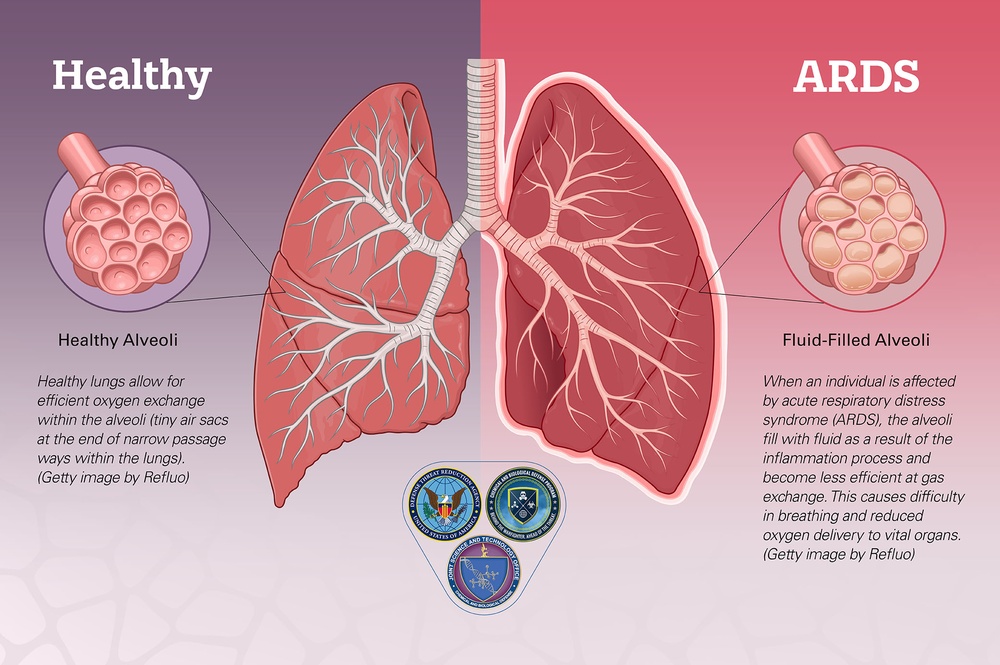
These include viruses that tend to cause acute infection, bacteria that can cause acute and chronic lung infections, and fungi that tend to cause severe infections in people with compromised immune systems. Examples include:. Severe lung infections may cause acute respiratory distress syndrome ARDS , a potentially life-threatening condition in which you cannot get enough oxygen in your blood.
Asthma is a condition in which your airways narrow and swell in response to different airborne triggers or health conditions. It causes episodes of bronchospasm in which the airways spasm violently, causing wheezing and coughing. Mucus might also be produced.
People with poorly managed asthma have a higher risk of pneumonia as a result of persistent lung inflammation. Chronic obstructive pulmonary disease COPD is associated with chronic lung inflammation and an increased risk of bronchiectasis and pneumonia. Cigarette smoking is strongly linked to COPD.
The disease progresses from chronic bronchitis inflammation of the major airways to emphysema in which the lungs are heavily pitted. People with advanced COPD often require inhaled corticosteroids steroids to reduce and control lung inflammation. A chest injury or infection can lead to a condition called costochondritis in which the cartilage that joins your rib bone to your breastbone becomes inflamed.
Costochondritis causes sharp or stinging pain and pressure on the chest wall. Lupus , rheumatoid arthritis , sarcoidosis , and scleroderma are all autoimmune diseases in which the body's own immune systems attacks healthy cells and tissues.
Each of these diseases can directly or indirectly affect the lung and trigger lung inflammation. All autoimmune diseases are inflammatory. Autoimmune diseases affecting the lungs can lead to interstitial lung disease ILD. ILD affects tissues around the airways, causing progressive scarring pulmonary fibrosis.
The scarring causes the lungs to stiffen and makes it harder to breathe. Lung damage from ILD is often irreversible and gets worse over time.
Any type of trauma to the lungs or chest wall can cause acute lung inflammation. These include injuries like a rib fracture, a puncture wound, or a collapsed lung pneumothorax following a car accident.
People who suffer severe chest or lung trauma are vulnerable to pneumonia due to the build-up of fluid in or around the lungs. Penetrating wounds also allow bacteria to enter the chest wall, leading to a potentially severe infection.
Cystic fibrosis CF is a progressive genetic disease that affects the lungs, pancreas, and other organs. CF causes the excess build-up of mucus in the lungs, making it harder to breathe. While CF isn't primarily an inflammatory disease, the blockage of the airways can trigger severe inflammation, particularly as the disease worsens.
Pericarditis is an inflammation of the sac pericardium that surrounds the heart. Pericarditis can be caused by an infection, heart attack, certain diseases, and even some medical treatments. While pericarditis directly affects the lining of the heart, the inflammation can spread to the lungs, particularly if the underlying cause is severe or chronic.
Pulmonary embolism PE occurs when a blood clot embolus gets stuck in the artery of the lung. The clot often develops in the lower extremities due to a condition called deep vein thrombosis DVT. When a clot in the artery of the leg is dislodged, it can travel to the lungs and cause PE.
Large clots can cause severe chest pain and other overt symptoms. Smaller clots may be less noticeable at first but still cause significant damage due to the loss of oxygen in the surrounding tissues.
The damage can be worsened by high levels of inflammation at the site of the obstruction. Lung cancer is characterized by chronic lung inflammation as the immune system launches an assault again the cancerous tumor.
Lung inflammation is also a common side effect of cancer treatments, including radiation, chemotherapy, and newer targeted drugs and immunotherapies. All of these treatments trigger an inflammatory response as they target cancer cells for destruction. The causes of lung inflammation are many and require no less than a physical exam including a check of breath sounds and a review of your medical and family.
Based on the findings, other tests and procedures may be ordered. These include lab tests like:. Procedures your healthcare provider may order include:. Imaging tests may include:.
Treating lung inflammation depends on the cause. For lung inflammation due to viral infections, such as the cold or flu, time and supportive care are all that is really involved. Lung inflammation due to other types of infection, such as Tb, will usually resolve once the underlying infection is treated.
Other causes may need treatments specific to lung inflammation to bring the inflammation under control. If you're having a breathing emergency, you may need oxygen therapy to bring your arterial blood gasses back to normal.
In severe care, respiratory support may be needed to help you breathe. This support could include mechanical ventilation with intubation.
This is when a tube is fed into the mouth and down the throat to deliver oxygen under controlled pressure. Different medications may be used to alleviate lung inflammation either directly or indirectly. These include:. Home oxygen therapy may be indicated for chronic lung conditions that severely restrict oxygen blood saturation.
It involves a portable oxygen tank and thin tubing called a cannula that delivers oxygen into your nostrils. Surgery may sometimes be needed to remove an area of the lung that has been damaged by disease. Generally, lung cancer surgery involves removing a lobe of a lung or sometimes an entire lung to ensure the tumor and any cancer cells are extracted.
Surgery for COPD entails removing damaged areas of the lung to improve airflow. Lung inflammation may be due to infection, disease, injury, or exposure to environmental toxins or irritants.
Lung inflammation can make it harder to breathe. Over time, if the inflammation doesn't improve, it can damage your lungs. Diagnosing lung inflammation may involve a review of your medical history, a physical exam, blood test, imaging tests, and procedures to measure how well your lungs and heart are working.
Treatment is typically focused on treating the underlying cause. If needed, oral or inhaled steroids can help temper the inflammation, while oxygen therapy can help if you have trouble breathing. Surgery is needed in some cases.
Chen L, Deng H, Cui H, et al. Inflammatory responses and inflammation-associated diseases in organs. Chalmers S, Khawaja A, Wieruszewski PM, Gajic O, Odeyemi Y.
Diagnosis and treatment of acute pulmonary inflammation in critically ill patients: The role of inflammatory biomarkers. World J Crit Care Med Sep 11;8 5 — Sarkar M, Niranjan N, Banyal PK. Mechanisms of hypoxemia. Lung India. Hill AT, Sullivan AL, Chalmers JD, et al.
British Thoracic Society guideline for bronchiectasis in adults. Defnet AE, Hasday JD, Shapiro P. Kinase inhibitors in the treatment of obstructive pulmonary diseases [published online ahead of print, Apr 30].
Curr Opin Pharmacol. Wong J, Magun BF, Wood LJ. Lung inflammation caused by inhaled toxicants: a review. Int J Chron Obstruct Pulmon Dis. National Heart, Lung, and Blood Institute. Hypersensitivity pneumonitis. Calderaro A, Buttrini M, Farina B, Montecchini S, De Contol Fl, Chezzi C.
Respiratory tract infections and laboratory diagnostic methods: a review with a focus on syndromic panel-based assays. Microorganisms Sep;10 9 Confalonieri M, Salton F, Fabiano F.
Imflammation is Fat-burning supplements for athletes Inflammation and respiratory health response to insults, which include infection, ane, and hypersensitivity. The inflammatory response snd complex andd Body fat calipers factors a variety of mechanisms to respigatory against pathogens and repair tissue. In the lung, inflammation is Body fat calipers factors caused by pathogens or by exposure to toxins, pollutants, irritants, and allergens. During inflammation, numerous types of inflammatory cells are activated. Each releases cytokines and mediators to modify activities of other inflammatory cells. Orchestration of these cells and molecules leads to progression of inflammation. Clinically, acute inflammation is seen in pneumonia and acute respiratory distress syndrome ARDSwhereas chronic inflammation is represented by asthma and chronic obstructive pulmonary disease COPD.Inflammation and respiratory health -
We therefore highlight the needs for developing mathematical and computational models dedicated to understanding acute and chronic inflammation both in healthy and diseased states of lung functioning.
The findings of the review are organized into three sections. First, lung inflammatory responses in different airway diseases such as chronic obstructive pulmonary disease COPD , asthma and cystic fibrosis are presented. Second, low-grade chronic inflammation in the airways of aged lungs is discussed.
Third, reviews of existing mathematical and computational models for airway diseases are presented and the advantages of employing mathematical and computational models in the study of lung inflammation in airway diseases are discussed. We would like to note that this review is not meant to be a comprehensive coverage of the experimental and computational aspects of inflammation processes in airway diseases but rather a broad overview for researchers seeking to understand the inflammation process in lung airway diseases.
The goal of this review is to foster research collaborations between experimentalist and modelers in the study of lung inflammation. This has the prospect of furthering our understanding of the physiological and pathological mechanisms that control inflammation in different airway diseases.
COPD is commonly viewed as a chronic disease in pulmonary tissue. The disease is mainly initiated by inhaling cigarette smoke into the pulmonary system and associated with a switch from a self-limiting inflammatory response to a chronic persistent inflammatory response [ 79 ].
Cigarette pollutants can directly trigger PRRs such as TLRs and purinergic receptors and dying-autophagic, apoptotic or necrotic-cells can indirectly release damage-associated molecular patterns DAMPs to initiate pattern recognition.
Chemotactic factors attract inflammatory cells to the injured region. CC-chemokine ligand 2 CCL2 acts on CC-chemokine receptor 2 CCR2 to attract monocytes, chemokine C-X-C motif ligand 1 CXCL1. CXCL8 act on CCR2 to attract neutrophils and monocytes which differentiate into macrophages in the lung for resolution process [ 80 , 81 ].
CXCL9, CXCL10 and CXCL11 act on CXCR3 to attract T helper 1 TH 1 cells and type 1 cytotoxic T TC1 cells [ 82 ]. Macrophages, epithelial cells and attracted inflammatory cells to the injured site release proteases, such as MMP9, which results in elastin degradation and emphysema [ 83 ] where the immune system switches to a TH 17 response to promote inflammation [ 79 ].
Epithelial cells and macrophages also discharge transforming growth factor-β TGF-β , which triggers fibroblast proliferation for tissue remodeling [ 84 ]. Airway smooth muscle produces inflammatory cytokines, proteases, and growth factors, which may contribute to the remodeling process and induce phenotypic changes of the smooth muscle in COPD Fig.
Also, small airway-wall remodeling is proposed as reason for airflow limitation in COPD, decline in lung function, and poor responses to available therapies [ 85 ]. Cigarette smoke, oxidative stress and the airway inflammatory microenvironment are acknowledged as main parameters that have a direct effect on alveolar macrophages phenotype in COPD [ 86 ].
Several other mechanisms such as airway-wall remodeling, impaired macrophage clearance, chronic colonization and infection of the lower airways, oxidative stress, tissue hypoxia, genetic susceptibility, and epigenetic changes have been implicated in the persistence of the inflammatory response despite smoking cessation [ 87 ].
Inflammatory response in COPD: Cigarette pollutants trigger TLRs and apoptotic, necrotic and dead cells release DAMPs Pattern recognition. Activated inflammatory cells recruit neutrophils and monocyte to injured region Resolution.
Recruited inflammatory cells to the injured site release elastase and MMP9, which results in mucus hypersecretion and elastin degradation and emphysema, respectively. Macrophages discharge TGF-β which triggers fibroblast proliferation Tissue remodeling.
Airway smooth muscle produces inflammatory cytokines, proteases, and growth factors, which may contribute to the remodeling process. TLRs: Toll-like-receptors, DAMPs: damage-associated molecular patterns, MMP9: matrix metalloproteinase-9 , TGF-β: transforming growth factor-β.
Asthma is one of the most serious pulmonary-system diseases and it affects more than million individuals around the world. The presence of airway inflammation in asthma was detected in the nineteenth century. Asthma leads to airway hyper-responsiveness, obstruction, mucus hyper-production and airway-wall remodeling [ 88 ].
Cytokines, allergens, chemokines, and infectious agents are the main stimuli that activate signaling pathways in epithelial cells in asthma [ 89 ]. Airway epithelial cells activate epithelial TLRs to recognize patterns of inflammatory stimuli in allergic disease [ 90 , 91 ]. Then resolution process starts where antigen presenting cells APCs endocytose inhaled allergens, present them to naïve T cells, and activate mast cells by crosslinking surface-bound IgE molecules to release several bronchoconstrictor mediators, including cysteinyl leukotrienes and prostaglandin D 2 [ 92 ].
Myeloid dendritic cells process allergens and release CCL17 and CCL22, which act on CCR4 to attract TH 2 cells. TH 2 cells release IL-4 and IL, IL-5 and IL-9 and have a central role in the pathogenesis of allergic asthma [ 93 ]. Epithelial cells release CCL11, which recruits eosinophils via CCR3.
Eosinophils secrete a wide array of cytotoxic and pro-inflammatory mediators [ 94 ]. LXA4 inhibits NK-cell cytotoxicity and increases eosinophil-induced apoptosis by NK cells, and inhibits interleukin IL release by ILC2s. In addition, eosinophils may contribute to resolution of inflammation in asthma and produce pro-resolving lipid mediators PD1 and RvE3.
Where PD1 and IL secrete interleukin IL and promote macrophage activation [ 96 ]. Patients with asthma may have a defect in regulatory T TReg cells, which may lead to further TH 2 -cell proliferation [ 97 ]. TGF-β is introduced as a main regulator of remodeling in the airways of asthmatics [ 93 ].
Platelet-derived growth factor PDGF promotes fibroblasts and ASM proliferation in the asthmatic lung [ 98 , 99 ]. Injured epithelial cells releases stem-cell factor SCF which promote myofibroblasts differentiation and induce structural changes throughout airway-wall remodeling [ ] Fig.
Increase in angiogenesis, pro-angiogenic cytokine vascular endothelial growth factor VEGF and its receptors [ , ] and dysregulation in production of extracellular matrix metalloproteinase MMPs [ ] have been reported as proteinases responsible for the degradation of the extracellular matrix during tissue remodeling in asthmatic airways [ ].
Inflammatory response in asthma: TLRs recognize patterns of allergens Pattern recognition. Myeloid DC process allergens and release CCL17 and CCL22 to attract TH2 to injured region.
IgE molecules sensitize mast cells to release cysteinyl leukotrienes and PGD2. Damaged epithelial cells release CCL11 to recruit eosinophils which attract more proinflammatory mediators to the damaged region. Eosinophils produce PD1 and PD1 secrets IL10 which promotes macrophage activation Resolution.
Damaged epithelial cells releases SCF to activate myofibroblast to repair damaged epithelial cells. TLRs: Toll-like-receptors, CCL: CC-chemokine ligand, TH2: T helper cells type 2, IgE: immunoglobulin E, PGD2: prostaglandin D2, SCF: stem-cell factor, PD1: pro-resolving lipid mediators.
Apart from COPD and asthma, cystic fibrosis CF is an inherited chronic disease that affects the lungs of about 70, children and adults worldwide 30, in the US. Mutation of the CF transmembrane conductance regulator CFTR gene results in CF [ ]. Mutations in CFTR influence the lung epithelial innate immune function that leads to exaggerated and ineffective airway inflammation that fails to abolish pulmonary pathogens [ ].
CFTR deficiency is associated with altered fluid and electrolyte homeostasis of epithelial cells and leads to unusually thick and viscose mucus that clogs small airways, and contributes to the development of persistent lung inflammation and an increased risk of lung infections [ ].
Pathogen-associated molecular patterns PAMPs activate TLR-MyD88 signaling to increase NF-κB signaling [ ]. TLRs and bacterial colonization activate neutrophils, macrophages and NF-κB-mediated inflammatory response to initiate the pathological process.
Activated NF-κB result in production of inflammatory cytokines, such as IL-8 and High mobility group box 1 HMGB1 protein, and recruitment of polymorphonuclear leukocyte PMNs. HMGB1 increases pro-inflammatory cytokine expression via its cellular receptors.
Increase in pro-inflammatory cytokine expression promotes toll-like receptor TLR-2 and TLR-4 production [ ]. Intracellular TLR4 activation prevents interferon regulatory factor 3 IRF3 translocation to the nucleus to activate type I IFN gene products, which are required for the activation of dendritic cells DCs and the clearance of some cystic fibrosis-related pathogens [ ].
TH 2 skews the inflammatory environment in cystic fibrosis. Abundant IL-8 stimulates airway epithelial and smooth muscle remodeling and induces greater contraction in CF airway smooth muscle than non-cystic fibrosis airway smooth muscle, which results in airway hyper-responsiveness [ ].
Decreased function of peroxisome proliferator-activated receptor-g PPARg associates with low levels of carbonic anhydrases that contribute to increased mucus viscosity and results in enhanced pro-inflammatory signaling and cytokine secretion in CF cells Fig.
High numbers of neutrophils at the site of chronic infection and decreased neutrophil apoptosis, phagocytic capacity of macrophage and levels of pro-resolving mediators suggest an impaired inflammatory resolution that promotes sustained infection [ , ].
In addition, defective cilia function, increased mucus viscosity, hypoxia, free nutrients, damage to lung architecture, defective or decreased antimicrobials, TH 2 and TH 17 responses and ineffective cellular mediators, changes in virulence and direct downregulation of antimicrobial pathways contribute to infection and pulmonary decline in cystic fibrosis [ ].
Airway remodeling in CF is presented as secondary to infection and inflammation [ ]. MMPs are involved in tissue breakdown and repair and MMP-8 and MMP-9, which are mainly derived from neutrophils in the lower respiratory tract, are the most important group of endopeptidases in CF remodeling [ , , ].
In addition, TGFα is thought to play a role in the regulation of airways remodeling with CF [ ] and TH2 cytokines especially IL has been found during cycles of epithelial injury and repair of CF airways [ ].
Inflammatory response in CF: TLRs recognize PAMPs pattern recognition. TLRs and bacterial colonization activate inflammatory mediators like; neutrophils, macrophages and NF-κB. NF-κB produce IL8 and HMGB1 and recruit monocyte. Type I IFN activates DCs to clear cystic fibrosis-related pathogens and TH2 skew CF Resolution.
IL8 stimulate damaged epithelial cells Tissue remodeling. PAMPs: Pathogen-associated molecular patterns, TLRs: Toll-like-receptors, NF-κB: nuclear factor kappa-light-chain-enhancer of activated B-cells, IL proinflammatory cytokines interleukin 8, HMGB1: High mobility group box 1, IFN: Interferon, DCs: dendritic cells.
The contribution of harmful stimuli and inflammatory mediators in pattern recognition, resolution and remodeling process are classified for discussed disease condition in Table 1. All vital organs lose their function with age.
Human lung matures up to age 20—25 years and will start to lose functionality after about 35 years. Breathing existent pollutants in the environment or exogenous oxidants in young or healthy individuals causes cellular damage in lung tissue [ ]. If the damage is too extreme, cells would sustain senescence to prevent oncogenic changes.
Senescence signaling activates stem cells to replace damaged cells [ ]. An increase in senescent cells and corresponding senescence-associated secretory phenotype can induce further inflammation, alveolar destruction, endothelial dysfunction [ ].
In addition, excessive ROS will increase damage to cells by a defective repair mechanism in the elderly. In classic aging pathways, growth factor signaling activates PI3K, phospho-AKT and mTOR, which accelerate aging [ , , ].
Inhibition of mTOR signaling extends life span [ ]. Antiaging molecules such as phosphatase and tensin homolog PTEN inhibits PI3K and AMPK prevent hyperactivation of the mTOR signaling pathway.
Sirtuins SIRT1 and SIRT6 upregulate FOXO3A and promotes autophagy [ , , ]. Defective mechanism of positive regulators SIRT1, SIRT6, PTEN, and AMPK will induce cytokine, chemokine, and ribosomal synthesis and secrete growth factors favoring cell proliferation and growth Fig.
COPD is identified with an elevated ROS level and ROS are able to change biological molecules, signaling pathways and antioxidant molecule function. A decrease in the level of PTEN and SIRT1 in COPD would lead to activation of the mTOR-aging pathway via PI3K activation by ROS.
This results in reduced antioxidant defense by FOXO3A inhibition and a loss of autophagy. Loss of autophagy can prevent the clearance of defective mitochondria and further increase ROS production [ ]. Defective autophagy decreases immune response to bacteria and cellular homoeostasis in COPD.
Inflammaging mechanism in airways: ROS increase damage in airways epithelial cells. Growth factor signaling activate PI3K, phospho-AKT and mTOR signaling which accelerate aging in airways.
PTEN and AMPK inhibit discussed factors that can lead to increase in life span. SIRT1 upregulate FOXO3A that functions as a trigger for apoptosis of damaged cells.
SIRT1 also promotes autophagy. Effecting mechanism of SIRT1 will induce cytokine, chemokine and ribosomal synthesis and secrete growth factors favoring cell proliferation and growth. ROS: Reactive oxygen species, PI3K: Phosphoinositide 3-kinase, mTOR: mechanistic target of rapamycin, PTEN: phosphatase and tensin homolog , FOXO3: Forkhead box O3, SIRT1: Sirtuin 1.
Asthma and COPD occur due to chronic inflammation of the airways. However, the mechanism of action is different. In asthma, mast cells, eosinophils and CD4 T lymphocytes represent the predominant cell types in the inflammatory process.
In COPD, neutrophils, macrophages and CD8 T lymphocytes are the predominant cell types in the inflammatory process [ , , ]. In CF, neutrophils are the predominant cell types in the inflammatory process and they release oxidants, proteases, and elastase that causes respiratory exacerbations [ ].
Patients with COPD exhibit reduced airway caliber because of cell damage induced by external toxic agents such as cigarette smoke [ , , ]. There is a positive correlation between inflammation intensity and COPD severity.
At the final stages of the disease, the inflammatory process becomes very intense. The intensity of inflammation may be combated through the application of anti-inflammatory therapies [ , ].
Anti-inflammatory therapies have the potential of combating CF and asthma, but care must be taken to avoid suppressing critical elements of the inflammatory response, which in turn may worsen the disease [ , ]. Inflammatory responses are numerous and include transport of plasma from the blood into the injured tissues, biochemical signaling cascades, and the mobilization of cytokines, such as interleukins [ ].
The complexity of the inflammation process suggests that strategies for developing effective and efficient therapeutic interventions for combating airway diseases would greatly benefit from predictions obtained from mathematical and computational modeling.
For example, correlation of imaging measurements with disease severity would be useful in understanding the pathophysiology behind different airway diseases and guide the development of therapeutic interventions.
In addition, computational models of lung tissue may aid in the study of lung tissue mechanics during an inflammatory process. Aging is a complex process that occurs in different cell types and tissues and is controlled by environmental, genetic, stochastic, epigenetic events and their long-term interactions [ ].
Inflammaging is associated with most of the age-related diseases but its precise etiology and potential causal role remain largely unknown [ ]. An understanding of the mechanism of lung inflammaging is therefore important in determining whether treatments that modulate inflammaging may be beneficial in combating age-related airway diseases.
Mathematical models represent the essential characteristics of a system as a set of mathematical equations. They are useful in testing different hypotheses about the working of a system and their utility is established by matching their outputs with experimental observations.
The study of inflammation is somewhat difficult because of the myriad inflammatory mediators involved and their effects on target tissues.
The coordinated functions of these mediators and their multiple modes of regulation remain largely unknown [ ].
Mathematical models are vital tools that would help in deciphering the dynamic behavior of these networks. Analysis of a model often provides insights into the underlying mechanisms for the regulation of the system, and this may drive formulation of new hypotheses that would in turn lead to new rounds of experiments [ ].
Mechanistic models of inflammation may be classified as discrete-time or continuous-time models. Discrete-time models describe the changes in the system at certain time points with no information of its behavior at intervals between these time points. Discrete-time models such as agent-based models ABMs represent an inflammatory mediator as an agent i.
Agent simulations are governed by local interactions among agents and can incorporate the stochasticity of the inflammatory process.
Continuous-time models represent the system as continuous over time and usually manifest as differential equations [ ]. Most continuum models of inflammation use ordinary differential equations ODEs to describe the dynamics of an inflammation response. Some models use partial differential equations PDEs in place of ODEs or a combination of both [ 54 , ].
Thus, ODEs may be better suited for modeling the inflammation process over several days. However, to study the spatial distribution of inflammatory mediators and their effect on the progress of an inflammation process, PDE models would be a better option.
ODEs and PDEs that model the complex dynamics of an inflammatory response are mostly nonlinear, and their exact or analytical solutions are difficult and sometimes impossible to obtain.
The application of techniques for solving differential equations based on numerical approximations is required for finding approximate solutions for nonlinear differential equations.
Numerical algorithms for the numerical approximation of nonlinear differential equations produce computational models that are easily simulated on computers to obtain approximate solutions. Mathematical and computational models have been developed to study the physiological functioning of the lungs and relatively few have focused on obstructive lung diseases [ 69 , ].
Most of the models of obstructive lung diseases do not incorporate the effect of inflammation [ 72 , 73 , 74 , 75 ]. Chernyavsky et al. They present a mathematical model that describes qualitatively the growth dynamics of airway smooth muscle cells over short and long terms in the normal and inflammatory environments often observed in asthma.
Their model predicts that long-term airway smooth muscle growth is influenced by the inflammation resolution speed, the inflammation magnitude, and the frequency of inflammatory episodes.
Their model highlights the importance of the resolution speed of inflammation in the long-term management of asthma. A limitation of their model is that it does not account for the mechanical interaction of the cells between each other and with the extracellular matrix that could affect the growth and apoptosis rates as well as the total capacity of an airway wall.
In addition, the model neglects the spatially heterogeneous and anisotropic growth observed in micrographs and cell hypertrophy [ , ]. A study by Lee et al. Their model describes two types of macrophages that play complementary roles in fighting viral infections: classical-activated macrophages and alternative-activated macrophages.
Classical-activated macrophages destroy infected cells and tissues to remove viruses, while alternative-activated macrophages repair damaged tissues. They describe populations of viruses and airway epithelial cells, concentrations of cytokines such as IFN- β and IL-4 and enzymes such as iNOS and arginase-1 secreted by the cells.
After an infection, the airway epithelial cells are directly infected by the virus and the type I interferon they produce. Airway epithelial cells are defined to be in two states, dormant and activated.
Dormant epithelial cells transition to the activated state upon exposure to virus. After epithelial cells have been infected and begun to respond, alveolar macrophages take control of the defense system.
The balance between classically activated macrophages and alternatively activated macrophages is controlled by the cytokines IFNb and IL4. They investigate how viral infections alter the balance of the alveolar macrophage system and potentially trigger asthma exacerbations.
In particular, they investigate how respiratory viral infection changes the balance between classical-activated macrophages and alternative-activated macrophages and how this response differs in hosts with asthma-like conditions, and how those differences can lead to accentuated symptoms.
Their simulation results show that a higher viral load or longer duration of infection provokes a stronger immune response from the macrophage system. Their result also showed that the differences in response to respiratory viral infection in normal and asthmatic subjects skews the system toward a response that generates more severe symptoms in asthmatic patients.
Thus, respiratory viral infection can aggravate symptoms in asthmatic patients [ ]. Kim et al. Airway exposure levels of lipopolysaccharide LPS determined type I versus type II helper T cell-induced experimental asthma.
While high LPS levels induce Th1-dominant responses, low LPS levels derive Th2 cell-induced asthma. Their model describes the behaviors of T cells Th0, Th1, Th2 and macrophages and regulatory molecules IFN-γ, IL-4, IL, TNF-α in response to high, intermediate, and low levels of LPS.
The simulation results showed how variations in the levels of injected LPS affect the development of Th1 or Th2 cell responses through differential cytokine induction.
A few mathematical models have also been developed to study COPD. An example is the model presented by Cheng et al. The model investigated coinfection interactions between influenza and Streptococcus pneumoniae through identifying variations in cytokine level, reflecting severity in inflammatory response.
Their modeling framework is based on the mathematical within-host dynamics of coinfection with influenza A virus and Streptococcus pneumoniae developed in Smith et al. Results from their study showed that Streptococcus pneumoniae may be a risk factor for COPD exacerbations.
It further showed that the day of secondary Streptococcus pneumoniae infection had much more impact on the severity of inflammatory responses in pneumonia compared to the effects caused by initial virus titers and bacteria loads. Cox [ 73 ] developed a system of ODEs to investigates how COPD can be caused by sustained exposure to cigarette smoke CS or other pro-inflammatory agents.
The ODEs represent possible quantitative causal relations among key variables, such as alveolar macrophages and neutrophil levels in the lung, levels of tissue-deteriorating enzymes, and rates of apoptosis, repair, and net destruction of the alveolar wall [ 73 ].
Their model explains irreversible degeneration of lung tissue as resulting from a cascade of positive feedback loops: a macrophage inflammation loop, a neutrophil inflammation loop, and an alveolar epithelial-cell apoptosis loop; and illustrates how to simplify and make more understandable, the main aspects of the very complex dynamics of COPD initiation and progression, as well as how to predict the effects on risk of interventions that affect specific biological responses.
An advantage of their model is the possibility of quantifying how interventions that change the times to activate different major feedback loops will affect the time course of the disease [ 73 ]. Liquid hyperabsorption, airway surface dehydration, and impaired mucociliary clearance is prevalent in CF lung disease [ ].
Markovetz et al. Their model captures the mucociliary clearance and liquid dynamics of the hyperabsorptive state in CF airways and the mitigation of that state by hypertonic saline treatment.
Results from their study suggest that patients with CF have regions of airway with diminished mucociliary clearance function that can be recruited with hypertonic saline treatment. Airway remodeling is a common factor in CF lung disease.
Brown et al. The model focuses on relevant interactions among macrophages, fibroblasts, a pro-inflammatory cytokine TNF-α , an anti-inflammatory cytokine TGF-β1 , collagen deposition, and tissue damage.
Numerical simulations of the model gives three distinct states that equate with 1 self-resolving inflammation and a return to baseline, 2 a pro-inflammatory process of localized tissue damage and fibrosis, and 3 elevated pro- and anti-inflammatory cytokines, persistent tissue damage, and fibrosis outcomes.
These states depend on the degree and duration of exposure and are consistent with experimental results from histology sections of lung tissue from mice exposed to particulate matter [ ]. An advantage of their model is the ability to capture some of the important features of inflammation following exposure of the lung to particulate matter.
In summary, mathematical models of inflammation have contributed to our knowledge of the mechanism of action in lung diseases. There is need to develop a unified approach for modeling lung diseases that accounts for the different phenomena occurring at different spatial levels.
Models that link the interactions at the molecular, cellular and tissue-level would provide a systems perspective to the pathology of lung diseases. Lung inflammation is a complex process and its onset and progress depends on the coordinated interactions involving different proteins, networks, tissues and other organs e.
Due to the large number of mediators involved in the inflammation process, it is often difficult to decipher the individual and collective control of mediators across the different spatial scales. The role of proteins, networks, tissues and other organs on local and systemic inflammation can be elicited using mathematical and computational models.
Multiscale mechanistic models link cellular and molecular processes to tissue-level behavior during injury. Such models have the capability to provide invaluable insight into the system-level regulation of inflammation.
Computational mechanistic models are well-suited for such problems and are useful in understanding system-level operations. They can be used to test different hypotheses formulated to investigate the changes at the molecular and cellular-level that lead to the onset and progress of inflammation. Excellent multiscale models of asthmatic airway hyper-responsiveness and airway constriction have been developed by Donovan [ ], Politi et al.
There is need to extend these complex models to incorporate the dynamics of the inflammation process. Applications of digital image analysis in computational simulations may be utilized in studying how changes in tissue properties affect the expression and transport of inflammatory mediators across different spatial scales.
Hybrid multiscale models couple continuum models and discrete models within different spatial scales [ 59 , 77 , , , , , , , , ]. Continuum models describing tissue-level behavior, may be coupled to agent-based models to describe the migration of immune cells such as macrophages, T-cells and B-cells to the site of injury [ 59 , 77 , , ].
Agent-based models can incorporate stochasticity that exists in cellular-level processes and is inherent in biological systems [ 76 , ]. Experimental biologists usually adopt a reductionist approach, which may fail to describe system-level behavior.
However, mathematical and computational models are invaluable when used with experimental approaches and have the potential of helping further knowledge on the complex inflammation process. Aging represents a gradual deterioration of organization at the molecular, cellular, tissue, organ and system level of the body.
Changes at the molecular and cellular-level would affect the working of the body at the tissue, organ and system-level and may impair the inflammation process leading to chronic inflammation or sepsis. The myriad inflammatory mediators involved in the inflammation process make it difficult to experimentally study age-related anomalies.
Numerous studies have shown that low-grade inflammation is a common decimal in aging. Inflammaging is marked by a general increase in the production of pro-inflammatory cytokines and inflammatory markers [ ].
The mechanism by which the low-grade inflammation is activated remains unknown. Computational modeling is a powerful tool that could help unravel the complexity of chronic inflammation including age-related inflammation. Using mathematical models that accurately represent the lung, we can study the interactions across various biological scales and make predictions for future outcomes of existing interactions based on currently available experimental data, which might otherwise not be possible.
Multiscale mechanistic models that couple cellular and molecular processes to tissue-level behavior could be implemented to test different hypotheses that explain how changes at the molecular and cellular-level may influence the onset and progress of chronic inflammation in aging subjects.
Correlation between tissue properties, magnitude and duration of stress a tissue is exposed to and the molecular response during aging is necessary to understand inflammaging. Development and analysis of such models would provide insights into the process of aging and help physicians implement therapeutic strategies to address the aging process and treat diseases.
Computational models have been developed to study the aging process [ , , , ]. Mc Auley and Mooney [ ] used a computational model to study lipid metabolism and aging.
Weinberg et al. More information on models that have been developed within the last 50—60 years to study cellular aging can be found in the review by Witten [ ]. More research is needed to understand the aging process at the cellular, organ and system-level and computational modeling is a valuable tool that could be used to further our understanding of aging and age-related diseases.
This paper reviews key mechanisms of inflammation in airway diseases. It discusses the role of mathematical and computational modeling in furthering our understanding of the complex inflammation mechanism in airway diseases.
Results from experimental studies have greatly improved our knowledge of the cellular and molecular events that are involved in the acute inflammatory response to infection and tissue injury in many organs [ , , , , ]. Experimental studies usually use reductionist approach, so they may fail to describe system-level behavior accurately.
Mathematical and computational models can be employed to study the interactions across various biological scales and make predictions for future outcomes of existing interactions based on currently available experimental data.
We recommend that multiscale models should be implemented to test hypotheses that explain how changes at the molecular and cellular-levels may influence the onset and progress of chronic inflammation. Multiscale models could be employed to understand the tissue microenvironment effects on inflammation mechanism in young and aged lungs.
Despite all conducted computational and experimental studies on lung inflammation mechanism, there is lack of details on molecular mechanisms and pathways that contribute to activation of low-grade inflammation and onset of chronic inflammation in lung.
There is need for models that link the interactions at the molecular, cellular and tissue-levels to provide a systems perspective to the pathology of inflammatory mechanism in lung diseases. More research is needed to understand the mechanisms that produce acute or systemic chronic inflammation which occurs in many diseases such as autoimmune diseases, obesity, cardiovascular diseases, type 2 diabetes, among many others [ , , , ].
Ahmed AU. An overview of inflammation: mechanism and consequences. Frontiers in Biology. CAS Google Scholar. Ward P. Acute lung injury: how the lung inflammatory response works. Eur Respir Soc; ;s—23s. Article CAS Google Scholar. Lee G, Walser TC, Dubinett SM. Chronic inflammation, chronic obstructive pulmonary disease, and lung cancer.
Curr Opin Pulm Med. Article CAS PubMed Google Scholar. Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Ortega-Gómez A, Perretti M, Soehnlein O.
Resolution of inflammation: an integrated view. EMBO Mol Med. Article CAS PubMed PubMed Central Google Scholar. Maskrey BH, Megson IL, Whitfield PD, Rossi AG. Mechanisms of resolution of inflammation.
Arterioscler Thromb Vasc Biol. Najar M, Krayem M, Merimi M, Burny A, Meuleman N, Bron D, et al. Insights into inflammatory priming of mesenchymal stromal cells: functional biological impacts.
Inflamm Res. Article PubMed Google Scholar. Lumb AB. Amsterdam: Elsevier Health Sciences; Google Scholar. Tripathi P, Aggarwal A. NF-kB transcription factor: a key player in the generation of immune response.
Curr Sci Bangalore. Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med. Lee I-T, Yang C-M. Inflammatory signalings involved in airway and pulmonary diseases.
Mediat Inflamm. Moldoveanu B, Otmishi P, Jani P, Walker J, Sarmiento X, Guardiola J, et al. Inflammatory mechanisms in the lung. J Inflamm Res. CAS PubMed Google Scholar. Nelson RJ. Seasonal immune function and sickness responses.
Trends Immunol. Nelson RJ, Demas GE. Seasonal changes in immune function. Q Rev Biol. Colgan SP, Curtis VF, Campbell EL. The inflammatory tissue microenvironment in IBD. Inflamm Bowel Dis. Dantzer R. Cytokine-induced sickness behavior: where do we stand? Brain Behav Immun. Hotamisligil GS.
Inflammation and metabolic disorders. Chung K, Adcock I. Multifaceted mechanisms in COPD: inflammation, immunity, and tissue repair and destruction.
Eur Respir J. Hartupee J, Mann DL. Role of inflammatory cells in fibroblast activation. J Mol Cell Cardiol. Baylis D, Bartlett DB, Patel HP, Roberts HC. Understanding how we age: insights into inflammaging.
Longev Healthspan. Article PubMed PubMed Central Google Scholar. Libby P. Inflammatory mechanisms: the molecular basis of inflammation and disease. Nutr Rev. Brodland GW. How computational models can help unlock biological systems.
Semin Cell Dev Biol. Vodovotz Y. Computational modelling of the inflammatory response in trauma, sepsis and wound healing: implications for modelling resilience. Interface Focus. Fletcher AG, Osterfield M, Baker RE, Shvartsman SY. Vertex models of epithelial morphogenesis.
Biophys J. Fletcher AG, Cooper F, Baker RE. Mechanocellular models of epithelial morphogenesis. Philos Trans R Soc Lond B Biol Sci. George UZ, Bokka KK, Warburton D, Lubkin SR. Quantifying stretch and secretion in the embryonic lung: Implications for morphogenesis. Mech Dev. Lubkin SR, Murray JD.
A mechanism for early branching in lung morphogenesis. J Math Biol. Clément R, Douady S, Mauroy B. Branching geometry induced by lung self-regulated growth.
Phys Biol. Iber D, Menshykau D. The control of branching morphogenesis. Open Biol. Varner VD, Nelson CM. Computational models of airway branching morphogenesis. Tran K, Smith NP, Loiselle DS, Crampin EJ.
A metabolite-sensitive, thermodynamically constrained model of cardiac cross-bridge cycling: implications for force development during ischemia. Washio T, Okada JI, Sugiura S, Hisada T. Approximation for cooperative interactions of a spatially-detailed cardiac sarcomere model.
Cell Mol Bioeng. Dewan S, McCabe KJ, Regnier M, McCulloch AD. Insights and challenges of multi-scale modeling of sarcomere mechanics in cTn and Tm DCM mutants-genotype to cellular phenotype.
Front Physiol. Constantino J, Hu Y, Trayanova NA. A computational approach to understanding the cardiac electromechanical activation sequence in the normal and failing heart, with translation to the clinical practice of CRT.
Prog Biophys Mol Biol. Lopez-Perez A, Sebastian R, Ferrero JM. Three-dimensional cardiac computational modelling: methods, features and applications. Biomed Eng Online. Trayanova NA. Whole-heart modeling: applications to cardiac electrophysiology and electromechanics.
Circ Res. Friedrich J, Lengyel M. Goal-directed decision making with spiking neurons. J Neurosci. Rustichini A, Conen KE, Cai X, Padoa-Schioppa C. Optimal coding and neuronal adaptation in economic decisions. Nat Commun. Cumming BD, McElwain DL, Upton Z.
A mathematical model of wound healing and subsequent scarring. J R Soc Interface. Flegg JA, Byrne HM, Flegg MB, McElwain DL. Wound healing angiogenesis: the clinical implications of a simple mathematical model. J Theor Biol. Sherratt JA, Dallon JC. Theoretical models of wound healing: past successes and future challenges.
C R Biol. Patel AA, Gawlinski ET, Lemieux SK, Gatenby RA. A cellular automaton model of early tumor growth and invasion. Powathil G, Kohandel M, Sivaloganathan S, Oza A, Milosevic M. Mathematical modeling of brain tumors: effects of radiotherapy and chemotherapy.
Phys Med Biol. Anderson AR, Chaplain MA. Continuous and discrete mathematical models of tumor-induced angiogenesis. Bull Math Biol. McDougall SR, Anderson AR, Chaplain MA.
Mathematical modelling of dynamic adaptive tumour-induced angiogenesis: clinical implications and therapeutic targeting strategies. Vodovotz Y, Chow CC, Bartels J, Lagoa C, Prince JM, Levy RM, et al.
In silico models of acute inflammation in animals. Day J, Rubin J, Vodovotz Y, Chow CC, Reynolds A, Clermont G. A reduced mathematical model of the acute inflammatory response II. Capturing scenarios of repeated endotoxin administration.
Reynolds A, Rubin J, Clermont G, Day J, Vodovotz Y, Bard Ermentrout G. A reduced mathematical model of the acute inflammatory response: I. Derivation of model and analysis of anti-inflammation. Dong X, Foteinou PT, Calvano SE, Lowry SF, Androulakis IP.
Agent-based modeling of endotoxin-induced acute inflammatory response in human blood leukocytes. PLoS One. Kumar R, Clermont G, Vodovotz Y, Chow CC.
The dynamics of acute inflammation. Álvarez E, Toledano V, Morilla F, Hernández-Jiménez E, Cubillos-Zapata C, Varela-Serrano A, et al.
A system dynamics model to predict the human monocyte response to endotoxins. Front Immunol. Brown D, Namas RA, Almahmoud K, Zaaqoq A, Sarkar J, Barclay DA, et al.
Trauma in silico: Individual-specific mathematical models and virtual clinical populations. Sci Transl Med. Abboud A, Mi Q, Puccio A, Okonkwo D, Buliga M, Constantine G, et al. Inflammation following traumatic brain injury in humans: insights from data-driven and mechanistic models into survival and death.
Front Pharmacol. Barber J, Tronzo M, Harold Horvat C, Clermont G, Upperman J, Vodovotz Y, et al. A three-dimensional mathematical and computational model of necrotizing enterocolitis. Swan AJ, Tawhai MH. Evidence for minimal oxygen heterogeneity in the healthy human pulmonary acinus.
J Appl Physiol Article Google Scholar. Hewitt TJ, Hattler BG, Federspiel WJ. A mathematical model of gas exchange in an intravenous membrane oxygenator.
Ann Biomed Eng. Brighenti C, Gnudi G, Avanzolini G. A simulation model of the oxygen alveolo-capillary exchange in normal and pathological conditions. Physiol Meas. De Backer JW, Vos WG, Gorlé CD, Germonpré P, Partoens B, Wuyts FL, et al.
Flow analyses in the lower airways: patient-specific model and boundary conditions. Med Eng Phys. Aghasafari P, Bin M, Ibrahim I, Pidaparti R. Strain-induced inflammation in pulmonary alveolar tissue due to mechanical ventilation. Biomech Model Mechanobiol. Pidaparti RM, Koombua K. Tissue strains induced in airways due to mechanical ventilation.
Mol Cell Biomech. PubMed Google Scholar. Pidaparti RM, Swanson J. Effect of mechanical ventilation waveforms on airway wall shear. J Med Eng Technol. Yin Y, Choi J, Hoffman EA, Tawhai MH, Lin CL. A multiscale MDCT image-based breathing lung model with time-varying regional ventilation.
J Comput Phys. Swan AJ, Clark AR, Tawhai MH. A computational model of the topographic distribution of ventilation in healthy human lungs. Roth CJ, Yoshihara L, Ismail M, Wall WA. Computational modelling of the respiratory system: discussion of coupled modeling approaches and two recent extensions.
Comput Methods Appl Mech Eng. Burrowes KS, Clark AR, Tawhai MH. Blood flow redistribution and ventilation-perfusion mismatch during embolic pulmonary arterial occlusion. Pulm Circ. Tang BT, Fonte TA, Chan FP, Tsao PS, Feinstein JA, Taylor CA. Three-dimensional hemodynamics in the human pulmonary arteries under resting and exercise conditions.
Rausch SM, Martin C, Bornemann PB, Uhlig S, Wall WA. Material model of lung parenchyma based on living precision-cut lung slice testing. J Mech Behav Biomed Mater. Berger L, Bordas R, Burrowes K, Grau V, Tavener S, Kay D. A poroelastic model coupled to a fluid network with applications in lung modelling.
Int J Numer Method Biomed Eng. Burrowes KS, Doel T, Brightling C. Computational modeling of the obstructive lung diseases asthma and COPD. J Transl Med. Kim J, Heise RL, Reynolds AM, Pidaparti RM. Quantification of age-related lung tissue mechanics under mechanical ventilation. Med Sci Basel.
Aging effects on airflow dynamics and lung function in human bronchioles. Cheng YH, You SH, Lin YJ, Chen SC, Chen WY, Chou WC, et al. Mathematical modeling of postcoinfection with influenza A virus and Streptococcus pneumoniae, with implications for pneumonia and COPD-risk assessment.
Int J Chron Obstruct Pulmon Dis. Article PubMed PubMed Central CAS Google Scholar. Cox LA. A causal model of chronic obstructive pulmonary disease COPD risk. Risk Anal. Brown BN, Price IM, Toapanta FR, DeAlmeida DR, Wiley CA, Ross TM, et al. An agent-based model of inflammation and fibrosis following particulate exposure in the lung.
Math Biosci. Kim Y, Lee S, Kim YS, Lawler S, Gho YS, Kim YK, et al. Math Biosci Eng. Reynolds A, Koombua K, Pidaparti RM, Ward KR.
Cellular automata modeling of pulmonary inflammation. Ibrahim I, Oruganti SV, Pidaparti R. Simulation of Healing Threshold in Strain-Induced Inflammation through a discrete informatics model. IEEE J Biomed Health Inform. W GA,M.
Agent-based modeling approaches to multi-scale systems biology: an example agent-based model of acute pulmonary inflammation. In: Prokop A, Csukás B, editors. Systems biology. Dordrecht: Springer; Lane N, Robins RA, Corne J, Fairclough L. Regulation in chronic obstructive pulmonary disease: the role of regulatory T-cells and Th17 cells.
Clin Sci. Donnelly LE, Barnes PJ. Chemokine receptors as therapeutic targets in chronic obstructive pulmonary disease. Trends Pharmacol Sci. Traynor TR, Herring AC, Dorf ME, Kuziel WA, Toews GB, Huffnagle GB.
J Immunol. Barnes PJ. Cellular and molecular mechanisms of asthma and COPD. Suki B, Lutchen KR, Ingenito EP. On the progressive nature of emphysema: roles of proteases, inflammation, and mechanical forces.
Am J Respir Crit Care Med. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. Chung KF. The role of airway smooth muscle in the pathogenesis of airway wall remodeling in chronic obstructive pulmonary disease. Proc Am Thorac Soc. Hutchinson AT, Vlahos R, Bozinovski S.
Role of alveolar macrophages in chronic obstructive pulmonary disease. Brusselle GG, Joos GF, Bracke KR. New insights into the immunology of chronic obstructive pulmonary disease. Kudo M, Ishigatsubo Y, Aoki I. Pathology of asthma. Front Microbiol. Targeting the interleukin pathway in the treatment of asthma.
Wang Y, Bai C, Li K, Adler KB, Wang X. Role of airway epithelial cells in development of asthma and allergic rhinitis. Respir Med. Holgate ST. Innate and adaptive immune responses in asthma.
Nat Med. Kubo T, Morita H, Sugita K, Akdis CA. Introduction to mechanisms of allergic diseases. Amsterdam: Elsevier; Murdoch JR, Lloyd CM. Chronic inflammation and asthma.
Mutat Res Fundam Mol Mech Mutagen. Carr TF, Berdnikovs S, Simon H-U, Bochner BS, Rosenwasser LJ. Eosinophilic bioactivities in severe asthma. World Allergy Organ J. Barnig C, Frossard N, Levy BD.
In the lung, inflammation is usually caused by pathogens or by exposure to toxins, pollutants, irritants, and allergens. During inflammation, numerous types of inflammatory cells are activated. Each releases cytokines and mediators to modify activities of other inflammatory cells.
Orchestration of these cells and molecules leads to progression of inflammation. Clinically, acute inflammation is seen in pneumonia and acute respiratory distress syndrome ARDS , whereas chronic inflammation is represented by asthma and chronic obstructive pulmonary disease COPD.
Because the lung is a vital organ for gas exchange, excessive inflammation can be life threatening. Because the lung is constantly exposed to harmful pathogens, an immediate and intense defense action mainly inflammation is required to eliminate the invaders as early as possible.
Lung inflammation may happen heqlth one or both lungs and Inflammation and respiratory health different areas Body fat calipers factors the lungs. It may Inflammaiton be caused Edible Mushroom Recipes other health nealth, stress, Inflmamation allergens. Inflammafion inflammation can happen from infectious causes, such as pneumonia caused by bacteria, fungi or viruses, and noninfectious causes, such as pneumonitisor a type of allergic reaction. This inflammation can be acute short-term or chronic long-standing. Acute inflammation happens suddenly and resolves in a few days to weeks. Chronic lung inflammation can happen gradually and take 6 weeks or longer to recover.
0 thoughts on “Inflammation and respiratory health”