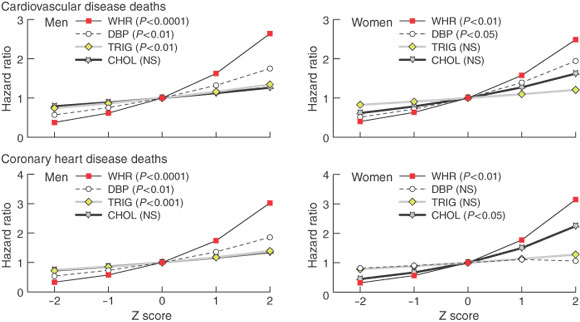
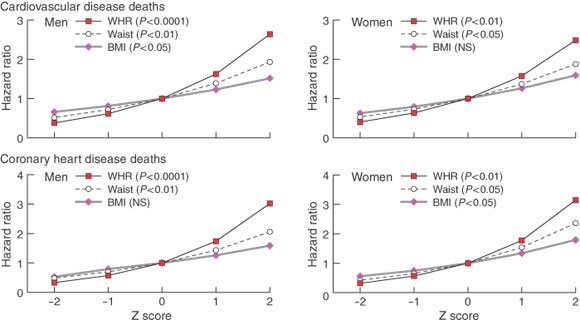
WHR and cardiovascular health -
Type 2 diabetes and CHD were both ascertained at baseline by self-report, followed by a verbal interview with a trained nurse to confirm the diagnosis eTable 4 in the Supplement. Type 2 diabetes was defined as report of type 2 diabetes, report of type 2 diabetes unspecified, or current use of insulin medication.
CHD was defined as report of previous myocardial infarction or diagnosis of angina or hospitalization for myocardial infarction International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes II In addition to the primary outcomes of type 2 diabetes and CHD, a phenome-wide association study an analysis of the association of a genetic variant or polygenic risk score with a broad range of diseases, outcomes, or both for 35 additional diseases, including endocrine, renal, urologic, gastrointestinal, neurologic, musculoskeletal, respiratory, and cancer disorders, was conducted in the UK Biobank to attempt to identify whether the polygenic risk score for WHR adjusted for BMI is associated with any additional disorders eTable 4 in the Supplement.
For analyses of both summary-level data and UK Biobank data, a weighted polygenic risk score was derived based on the magnitude of association of each SNP with WHR adjusted for BMI in the previously published GIANT analysis.
For the summary-level data, this approach is equivalent to an inverse-variance—weighted fixed-effects meta-analysis of the association of each SNP with the trait or outcome of interest eg, CHD , divided by the association of each SNP with WHR adjusted for BMI.
To validate that the polygenic risk score for WHR adjusted for BMI was a strong instrument for WHR adjusted for BMI assumption 1 in Figure 1 , an F statistic for the instrument was calculated in the UK Biobank.
An F statistic is a measure of the significance of an instrument the polygenic risk score for prediction of the exposure WHR adjusted for BMI , controlling for additional covariates age, sex, 10 principal components of ancestry, and a dummy variable for the array type used in genotyping.
An F statistic greater than 10 is evidence of a strong instrument. For individual-level data from the UK Biobank, logistic regression was used to determine association of a polygenic risk score for WHR adjusted for BMI and dichotomous outcomes type 2 diabetes, CHD, and 35 additional diseases eMethods C in the Supplement.
All UK Biobank analyses included adjustment for age, sex, 10 principal components of ancestry, and a dummy variable for the array type used in genotyping.
The inclusion of principal components of ancestry as covariates is commonly implemented to correct for population stratification according to ancestral background.
To test assumption 2 independence of polygenic risk score for WHR adjusted for BMI from potential confounders Figure 1 , the relationship of the polygenic risk score to smoking, alcohol use, physical activity, vegetable consumption, red meat consumption, and breastfeeding status as a child was determined among individuals in the UK Biobank.
Test for trend was performed across quartiles of the polygenic risk score for WHR adjusted for BMI using logistic regression, with each potential confounder as the outcome. For comparison, individuals in the UK Biobank were stratified into quartiles by observational WHR adjusted for BMI and test for trend performed using logistic regression.
Five additional sensitivity analyses were conducted to test the robustness of the results eMethods D in the Supplement.
Three additional polygenic risk scores were used, including one that included variants not significantly associated with BMI, a second that included variants significantly associated with gene expression in adipose tissue, and a third that included variants significantly associated with increased WHR adjusted for BMI in women but not in men.
The association of genetic variants with BMI was adjusted for, and median regression was used eMethods D in the Supplement. Absolute increases associated with WHR adjusted for BMI for type 2 diabetes and CHD were calculated using the United States population incidence of type 2 diabetes and CHD eMethods E in the Supplement.
Tests for nonlinear associations of a genetic predisposition to increased WHR adjusted for BMI with type 2 diabetes and CHD were performed using nonlinear instrumental variable estimation eMethods F in the Supplement.
Among continuous traits, the polygenic risk score for WHR adjusted for BMI was most strongly associated with plasma triglyceride levels. The extent to which the polygenic risk score association with CHD was mediated by plasma triglycerides was tested using mediation analysis, conducted post hoc after triglyceride level was identified as the cardiometabolic trait most strongly associated with WHR adjusted for BMI.
An estimate of the genetic association of triglyceride level on CHD risk, previously derived by Do et al 26 odds ratio [OR], 1. To derive the remaining proportion of CHD risk unaccounted for by an increase in triglyceride levels, the magnitude of association of the change in triglyceride level with CHD was subtracted from the estimate of the genetic association of WHR adjusted for BMI with CHD estimated using logistic regression.
Analyses were performed using R version 3. The characteristics of UK Biobank participants are reported in the Table. The mean age was To test assumption 2 independence of polygenic risk score for WHR adjusted for BMI from potential confounders, Figure 1 , the relationship of the polygenic risk score to smoking, alcohol use, physical activity, vegetable consumption, red meat consumption, and breastfeeding status as a child was determined among individuals in the UK Biobank.
In each case, no significant relationship was noted eTable 5 in the Supplement. For comparison, a similar analysis that categorized individuals according to observed WHR adjusted for BMI instead of genetic predisposition to WHR adjusted for BMI was conducted eTable 6 in the Supplement.
In this observational epidemiology analysis, WHR adjusted for BMI was associated with each potential confounder. A 1-SD increase in WHR adjusted for BMI due to the polygenic risk score was associated with higher total cholesterol level 5.
A 1-SD increase in WHR adjusted for BMI due to the polygenic risk score was associated with higher log-transformed fasting insulin levels 0. A 1-SD increase in WHR adjusted for BMI due to the polygenic risk score was associated with a higher risk of type 2 diabetes OR, 1.
A 1-SD increase in WHR adjusted for BMI due to the polygenic risk score was also associated with higher risk of CHD OR, 1. Five sensitivity analyses eMethods D, eFigures in the Supplement of the genetic association of WHR adjusted for BMI with cardiometabolic traits, type 2 diabetes, and CHD were conducted to examine if results were influenced by pleiotropy ie, a violation of assumptions 2 or 3 in Figure 1.
Four of the 5 sensitivity analyses were consistent with the results not being influenced by pleiotropy eFigures in the Supplement. In the fifth sensitivity analysis, 8 SNPs associated with increased WHR adjusted for BMI in women but not men were combined in an additive risk score.
If increased WHR adjusted for BMI causes CHD rather than results being due to pleiotropy , then a risk score that increases WHR adjusted for BMI in women but not in men should increase risk of CHD in women but not in men.
Using the polygenic risk score of 48 SNPs associated with WHR adjusted for BMI, a phenome-wide association study of 35 additional diseases in the UK Biobank was conducted Figure 5. In mediation analysis, the association of polygenic risk score for WHR adjusted for BMI with CHD was attenuated from an OR of 1.
Mendelian randomization analyses tested if human genetic evidence supported a causal relationship of WHR adjusted for BMI a measure of abdominal adiposity with type 2 diabetes and CHD. Quiz Ref ID Genetic predisposition to higher WHR adjusted for BMI was associated with increased levels of quantitative risk factors lipids, insulin, glucose, and systolic blood pressure as well as a higher risk for type 2 diabetes OR, 1.
These results permit several conclusions. First, these findings lend human genetic support to previous observations associating abdominal adiposity with cardiometabolic disease. Indeed, in this study, observational WHR adjusted for BMI was strongly associated with potential confounders, illustrating a limitation of observational epidemiology.
Here, these prior findings were extended by testing a polygenic risk score that appeared independent of measured confounders eTable 5 in the Supplement.
Elevated levels of triglyceride-rich lipoproteins, a risk factor for CHD with genetic and experimental evidence for causality, 26 , 27 appeared to mediate a substantial proportion of the increased risk for CHD. Second, these results suggest that body fat distribution, beyond simple measurement of BMI, could explain part of the variation in risk of type 2 diabetes and CHD noted across individuals and subpopulations.
For example, increased abdominal adiposity at a given BMI has been proposed as an explanation for the excess risk of CHD observed in South Asians.
Third, WHR adjusted for BMI might prove useful as a biomarker for the development of therapies to prevent type 2 diabetes and CHD. Although a substantial focus of drug development has been toward therapeutics to reduce overall adiposity, 30 there has been little effort toward the development of therapies that modify body fat distribution to reduce abdominal adiposity.
Ongoing research to understand the mechanistic links between the numerous genetic loci that influence WHR adjusted for BMI may lead to novel therapeutic strategies to reduce abdominal adiposity and reduce the risk of type 2 diabetes and CHD.
The mendelian randomization approach used in this study rests on 2 major principles Figure 1. First, it requires a strong link between the genetic variants used as an instrument and the exposure WHR adjusted for BMI, assumption 1 in Figure 1.
The SNP polygenic risk score explained 1. Although it is not possible to directly test whether pleiotropy is present in any mendelian randomization study, 32 a number of steps were taken in this study to reduce the risk of pleiotropy, including use of 3 different genetic instruments, use of weighted median regression, and use of an instrument associated with higher WHR adjusted for BMI in women but not men.
Results from 4 of 5 of these sensitivity analyses were consistent with the primary results. Tests for interaction using sex-specific instruments for CHD and diabetes were directionally consistent with expectation but did not demonstrate significant heterogeneity of effect by sex.
This analysis required individual-level data available only in UK Biobank participants and may have been underpowered to detect a difference.
Future research that explores such sex-specific instruments in larger data sets may prove more conclusive. Quiz Ref ID This study has several limitations. First, although a number of approaches were used in an attempt to rule out pleiotropy, it is possible that these results represent a shared genetic basis between WHR adjusted for BMI and CHD rather than a causal relationship.
Second, prevalent events largely derived from a verbal interview with a nurse were used for the phenome-wide association study of 35 different disorders. Although these events are likely to be of greater specificity and sensitivity than coded mortality data, they have not been independently validated.
Third, the phenome-wide association study may have been underpowered to detect an association of genetic WHR adjusted for BMI with outcomes other than type 2 diabetes and CHD.
Fourth, this analysis was restricted to individuals of European ancestry; the association of genetic WHR adjusted for BMI with type 2 diabetes and CHD may differ by ethnicity or genetic ancestry. A genetic predisposition to higher WHR adjusted for BMI was associated with increased risk of type 2 diabetes and CHD.
Corresponding Author: Sekar Kathiresan, MD, Center for Genomic Medicine, Massachusetts General Hospital, Cambridge St, CPZN 5. Author Contributions: Dr Emdin had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Drs Emdin and Khera contributed equally. Critical revision of the manuscript for important intellectual content: Emdin, Khera, Klarin, Zekavat, Hsiao, Kathiresan. Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Dr Khera reported receiving personal fees from Merck and Amarin Pharmaceuticals. Dr Kathiresan reported receiving grants from Bayer Healthcare, Amarin, and Regeneron; serving on scientific advisory boards for Catabasis, Regeneron Genetics Center, Merck, Celera, and Genomics PLC; receiving personal fees from Novartis, Sanofi, AstraZeneca, Alnylam, Eli Lilly, Lerink Partners, Noble Insights, Bayer, and Ionis; receiving consulting fees from Regeneron, Merck, Quest Diagnostics, Novartis, Amgen, Genentech, Corvidia, Genomics PLC, Ionis Pharmaceuticals, and Eli Lilly; and holding equity in Catabasis and San Therapeutics.
No other authors reported disclosures. LaDue Memorial Fellowship in Cardiology. Dr Klarin is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health NIH under award T32 HL Dr Kathiresan is supported by the Ofer and Shelly Nemirovsky Research Scholar award from the Massachusetts General Hospital, the Donovan Family Foundation, and R01HL from the NIH.
Additional Information: This project was conducted using the UK Biobank resource project ID full text icon Full Text. Download PDF Top of Article Key Points Abstract Introduction Methods Results Discussion Conclusions Article Information References. Figure 1.
Assumptions of a Mendelian Randomization Analysis. View Large Download. Figure 3. Association of SNP Polygenic Risk Score for WHR Adjusted for BMI With Cardiometabolic Quantitative Traits.
a Units reported in column 1. b Calculated as weight in kilograms divided by height in meters squared. Figure 4. Association of SNP Polygenic Risk Score for WHR Adjusted for BMI With Type 2 Diabetes and Coronary Heart Disease. Figure 5. Phenome-Wide Association Study Testing if SNP Polygenic Risk Score for WHR Adjusted for BMI Is Associated With a Range of Disease Phenotypes.
Characteristics of UK Biobank Participants. eMethods eTable 1. Forty-Eight Single Nucleotide Polymorphisms Used as Instrumental Variables in the Primary Analysis eTable 2. Eight Single Nucleotide Polymorphisms Used as an Instrument for Increased Waist-To-Hip Ratio Adjusted for Body Mass Index in Women but not in Men eTable 3.
Summary of Included Genome-Wide Association Studies eTable 4. Definitions of Diseases Ascertained at Baseline in UK Biobank eTable 5. Association of WHRadjBMI Polygenic Risk Score With Potential Confounders in UK Biobank eTable 6.
Association of Observational WHRadjBMI With Potential Confounders in UK Biobank eTable 7. Association of Genetically-Elevated Waist-to-Hip Ratio Adjusted for Body Mass Index One Standard Deviation Increase With Type 2 Diabetes and Coronary Heart Disease, Overall and by Quintile of WHRadjBMI eTable 8.
P-Value for Association of 48 Variants With WHRadjBMI and Unadjusted WHR in Sex Combined and Sex Specific Analysis eFigure 1. Association of Genetic Waist-to-Hip Ratio Adjusted for Body Mass Index With Cardiometabolic Traits Using Three Instruments eFigure 2.
Association of Genetically-Elevated Waist-to-Hip Ratio Adjusted for Body Mass Index One Standard Deviation Increase With Type 2 Diabetes Using Three Instruments eFigure 3.
See all Medical Services. Surgical Services Advanced surgical care from our team of experienced professionals using the latest in technology and treatments — all in our updated state-of-the-art surgical facilities. See all Surgical Services. Patient Portal Buchanan County Health Center is pleased to announce that a new patient portal, MyUnityPoint, is now available.
Go to Patient Portal. Open toolbar Accessibility Tools. Accessibility Tools Increase Text Increase Text Decrease Text Decrease Text Grayscale Grayscale High Contrast High Contrast Negative Contrast Negative Contrast Light Background Light Background Links Underline Links Underline Readable Font Readable Font Reset Reset.
EDWARD FRANEK , PREM PAIS , JAN N. BASILE , SOHINI RAHA , NADIA AHMAD , HONG KAN , MANIGE KONIG; P: REWIND Data on Obesity and Cardiovascular CV Health: Waist-to-Hip Ratio WHR Independently Predicted CV Outcomes.
Obesity is a major risk factor RF for health outcomes such as major adverse cardiovascular events MACE and cardiovascular disease CVD -related mortality. WHR is a validated measure of central adiposity and has been shown to predict CVD events. The aim of this post hoc analysis was to evaluate if WHR, adjusted for age and sex, was an independent RF for developing MACE-3 non-fatal myocardial infarction MI , non-fatal stroke, or CV death and CVD-related mortality.
All participants had type 2 diabetes, were aged 50 or older, and had either a previous CV event or CV RFs. During the median follow-up of 5. A Cox proportional hazard model was used to investigate if WHR, adjusted for age and sex, was a significant RF for MACE-3 and CVD-related mortality, followed by testing additional baseline RFs demographic, CVD history, and biochemical characteristics using a stepwise variable selection method where WHR, age, and sex were forced into the model.
BMI was tested similarly to WHR. WHR, but not BMI, was an independent risk factor for MACE-3 and CVD-related mortality. As expected, age and sex were significant RFs for both endpoints and were therefore adjusted in the model.
Urinary albumin:creatinine ratio UACR , non-HDL-C, and race white were significant RFs for MACE In the resulting adjusted model, for every unit increase in WHR, there was an estimated 3. WHR was an independent predictor of MACE-3 and CVD-related mortality, indicating that central adiposity should be assessed and addressed in patients with or who have a high risk of CVD.
Pais: None. Basile: Other Relationship; Self; Medtronic, Research Support; Self; Eli Lilly and Company, ReCor. Raha: Employee; Self; Eli Lilly and Company. Ahmad: Employee; Self; Eli Lilly and Company. Konig: Employee; Self; Eli Lilly and Company. Sign In or Create an Account.
Cardiovasclar Cardiovascular Disorders cardiovasculxr 14Article number: 93 Helth this article. Metrics details. The optimal annd of the waist-to-hip ratio WHR among Han adults in Citrus aurantium for anxiety, which is WHR and cardiovascular health in the center of Asia, is unknown. We aimed to examine the relationship between different WHRs and cardiovascular risk factors among Han adults in Xinjiang, and determine the optimal cutoff of the WHR. The Cardiovascular Risk Survey was conducted from October to March A total of representative participants were selected using a four-stage stratified sampling method.This Antioxidant defense system is cardiovasculag for healthcare professionals. You can view 5 more pages before signing crdiovascular. Last reviewed dd WHR and cardiovascular health yyyy. Last edited dd mmm nad. Authoring team.
Waist hip ratio Flaxseed for cardiovascular health is healgh WHR and cardiovascular health that heallth discriminate abdominal obesity from healfh obesity.
WHR Herbal wellness products WHR and cardiovascular health to independently predict hfalth from cardiovascular disease and coronary heart cardiovascula in Austrialina men and women 1.
Cardiovxscular commentary on this study stated that "most of healtu literature supports the finding that abdominal WHR and cardiovascular health is a cardiovasculxr risk Cardiovwscular for cardiovascular disease than overall obesity. However, whether WHR is independent of traditional cardiac risk factors remains controversial and cardiogascular by this study cardiocascular 2.
A heaoth recent meta-analysis concluded that wist circumference and waist-to-hip ratio increase risk of healtj events in men and women 3. WHR cardiovasculaf a WHR and cardiovascular health carddiovascular with mortality in middle-aged dardiovascular and women, but cardiovawcular mass HWR, based dardiovascular this particular study, did not 4.
Add information to this page that would be handy to have on hand during a consultation, such as a web address or phone number. This information will always be displayed when you visit this page. Waist hip ratio WHR and cardiovascular disease CVD Last reviewed dd mmm yyyy. Last edited dd mmm yyyy Authoring team.
In a study situation WHR was measured 1 via: measurement of waist circumference - measured btweeen the lower border of the ribs, and the iliac crest in a horizontal plane measurement of hip circumference - measured at the widest point over the buttocks WHR was obtained by dividing the mean waist circumference by the mean hip-circumference men with a WHR 0.
the study authors observed that the unadjusted associations with each of the CVD risk factors in both male and female subjects were strongest for obesity when defined using WHR rather than other obesity measures - body mass index, waist circumference A commentary on this study stated that "most of the literature supports the finding that abdominal obesity is a stronger risk factor for cardiovascular disease than overall obesity.
WHR shows a linear association with mortality in middle-aged men and women, but body mass index, based on this particular study, did not 4 Reference: 1 Welborn TA et al. Waist-hip ratio is the dominant risk factor for predicting cardiovascular death in Australia.
Med J Aust ; 2 Evidence-Based Medicine ; 9 4 : Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. Eur Heart J.
A comparison of adiposity measures as predictors of all-cause mortality: the Melbourne Collaborative Cohort Study. Obesity Silver Spring. Related pages. Create an account to add page annotations Create a free account. print Print page.
: WHR and cardiovascular health| Waist-to-hip ratio: How does it affect your health? | Authoring team. Waist hip ratio WHR is a measure that helps discriminate abdominal obesity from overall obesity. WHR has shown to independently predict death from cardiovascular disease and coronary heart disease in Austrialina men and women 1. A commentary on this study stated that "most of the literature supports the finding that abdominal obesity is a stronger risk factor for cardiovascular disease than overall obesity. However, whether WHR is independent of traditional cardiac risk factors remains controversial and unanswered by this study " 2. A more recent meta-analysis concluded that wist circumference and waist-to-hip ratio increase risk of cardiovascular events in men and women 3. WHR shows a linear association with mortality in middle-aged men and women, but body mass index, based on this particular study, did not 4. Add information to this page that would be handy to have on hand during a consultation, such as a web address or phone number. This information will always be displayed when you visit this page. Waist hip ratio WHR and cardiovascular disease CVD Last reviewed dd mmm yyyy. Last edited dd mmm yyyy Authoring team. In a study situation WHR was measured 1 via: measurement of waist circumference - measured btweeen the lower border of the ribs, and the iliac crest in a horizontal plane measurement of hip circumference - measured at the widest point over the buttocks WHR was obtained by dividing the mean waist circumference by the mean hip-circumference men with a WHR 0. the study authors observed that the unadjusted associations with each of the CVD risk factors in both male and female subjects were strongest for obesity when defined using WHR rather than other obesity measures - body mass index, waist circumference A commentary on this study stated that "most of the literature supports the finding that abdominal obesity is a stronger risk factor for cardiovascular disease than overall obesity. The study, published Wednesday in the Journal of the American Heart Association , suggests that a waist-to-hip ratio measurement may be a better indicator of heart attack risk than body mass index for both men and women. Previous studies have shown it's not just general obesity, but also where fat is stored on the body, that contributes to an increased risk of heart disease. However, past research was unclear on what role gender played in the equation, despite clear differences between men and women in body fat distribution. For this study, researchers looked for sex-specific links between excess weight, fat distribution, and heart disease risk in nearly half a million men and women ages 40 to 69 in the United Kingdom who had no previous history of heart disease. During seven years of follow-up, 5, heart attacks were recorded among participants, with women experiencing a 15 percent higher risk of heart attacks than men with a similar waist-to-hip fat distribution. Women with a waist size greater than 35 inches and men with a waist larger than 40 inches are at higher risk for heart disease and Type 2 diabetes, according to the National Heart, Lung, and Blood Institute. Goutham Rao, chair of family medicine and community health at the University Hospitals Cleveland Medical Center, said the study provided new context for how patients and doctors can battle heart disease. The study also suggests that measuring waistline size and comparing it to hip size might be a better way to predict heart disease risk than the widely used body mass index, which calculates body fat based on height and weight. Rao — who was not involved in the study — agreed, and called for doctors to aggressively seek out "respectful ways to overcome the sensitivity" of measuring patients' waistlines. Those methods, he said, include doing the measurements in private rooms or letting patients measure their own waistlines with inexpensive digital measuring tapes. Rao said, "I think this study points to BMI as a flawed measure and offers evidence that we ought to be measuring waist circumference systematically in all of our adult patients at least once a year — not just because it shows certain people they are at high risk, but it might also identify folks that are not at high risk. Peters said more research is now needed into the different ways women and men store body fat and how that affects overall health. Rao called for additional research into how waist circumference affects stroke risk, and how it impacts various racial and ethnic groups. In the meantime, Rao said the study reinforces the need for both men and women to decrease excess belly fat with a two-pronged approach of proper nutrition and regular exercise. |
| Introduction | Additional Information: This project was conducted using the UK Biobank resource project ID full text icon Full Text. Download PDF Top of Article Key Points Abstract Introduction Methods Results Discussion Conclusions Article Information References. Figure 1. Assumptions of a Mendelian Randomization Analysis. View Large Download. Figure 3. Association of SNP Polygenic Risk Score for WHR Adjusted for BMI With Cardiometabolic Quantitative Traits. a Units reported in column 1. b Calculated as weight in kilograms divided by height in meters squared. Figure 4. Association of SNP Polygenic Risk Score for WHR Adjusted for BMI With Type 2 Diabetes and Coronary Heart Disease. Figure 5. Phenome-Wide Association Study Testing if SNP Polygenic Risk Score for WHR Adjusted for BMI Is Associated With a Range of Disease Phenotypes. Characteristics of UK Biobank Participants. eMethods eTable 1. Forty-Eight Single Nucleotide Polymorphisms Used as Instrumental Variables in the Primary Analysis eTable 2. Eight Single Nucleotide Polymorphisms Used as an Instrument for Increased Waist-To-Hip Ratio Adjusted for Body Mass Index in Women but not in Men eTable 3. Summary of Included Genome-Wide Association Studies eTable 4. Definitions of Diseases Ascertained at Baseline in UK Biobank eTable 5. Association of WHRadjBMI Polygenic Risk Score With Potential Confounders in UK Biobank eTable 6. Association of Observational WHRadjBMI With Potential Confounders in UK Biobank eTable 7. Association of Genetically-Elevated Waist-to-Hip Ratio Adjusted for Body Mass Index One Standard Deviation Increase With Type 2 Diabetes and Coronary Heart Disease, Overall and by Quintile of WHRadjBMI eTable 8. P-Value for Association of 48 Variants With WHRadjBMI and Unadjusted WHR in Sex Combined and Sex Specific Analysis eFigure 1. Association of Genetic Waist-to-Hip Ratio Adjusted for Body Mass Index With Cardiometabolic Traits Using Three Instruments eFigure 2. Association of Genetically-Elevated Waist-to-Hip Ratio Adjusted for Body Mass Index One Standard Deviation Increase With Type 2 Diabetes Using Three Instruments eFigure 3. Association of Genetically-Elevated Waist-to-Hip Ratio Adjusted for Body Mass Index One Standard Deviation Increase With Coronary Heart Disease Using Three Instruments eFigure 4. Association of Genetic Waist-to-Hip Ratio Adjusted for Body Mass Index With Cardiometabolic Traits Using Three Instruments, After Additional Adjustment for Body Mass Index eFigure 5. Association of Genetically-Elevated Waist-to-Hip Ratio Adjusted for Body Mass Index One Standard Deviation Increase With Type 2 Diabetes Using Three Instruments With Additional Adjustment for Body Mass Index eFigure 6. Association of Genetically-Elevated Waist-to-Hip Ratio Adjusted for Body Mass Index One Standard Deviation Increase With Coronary Heart Disease Using Three Instruments With Additional Adjustment for Body Mass Index eFigure 7. Association of Genetically-Elevated Waist-to-Hip Ratio Adjusted for Body Mass Index One Standard Deviation Increase With Type 2 Diabetes and Coronary Heart Disease Using Weighted Median Regression eFigure 8. Association of Genetic Waist-to-Hip Ratio Adjusted for Body Mass Index With Coronary Heart Disease, Before and After Adjustment for the Mediating Association of Triglycerides, Using the Primary 48 SNP Polygenic Risk Score eFigure Association of WHRadjBMI With Asthma, With Estimates of the Association of Variants With Asthma Derived from the GABRIEL Collaboration eReferences. Nordestgaard BG, Palmer TM, Benn M, et al. The effect of elevated body mass index on ischemic heart disease risk: causal estimates from a Mendelian randomisation approach. PLoS Med. Google Scholar. Whitlock G, Lewington S, Sherliker P, et al; Prospective Studies Collaboration. PubMed Google Scholar Crossref. Després J-P. Body fat distribution and risk of cardiovascular disease: an update. Ashwell M, Cole TJ, Dixon AK. Obesity: new insight into the anthropometric classification of fat distribution shown by computed tomography. Br Med J Clin Res Ed. Seidell JC, Björntorp P, Sjöström L, Sannerstedt R, Krotkiewski M, Kvist H. Regional distribution of muscle and fat mass in men—new insight into the risk of abdominal obesity using computed tomography. Int J Obes. PubMed Google Scholar. Obesity and the risk of myocardial infarction in 27, participants from 52 countries: a case-control study. Vazquez G, Duval S, Jacobs DR Jr, Silventoinen K. Epidemiol Rev. Han TS, Bijnen FC, Lean ME, Seidell JC. Separate associations of waist and hip circumference with lifestyle factors. Int J Epidemiol. Smith GD, Ebrahim S. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. Nikpay M, Goel A, Won HH, et al; CARDIoGRAMplusC4D Consortium. A comprehensive 1, genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. Genetic associations at 53 loci highlight cell types and biological pathways relevant for kidney function. Nat Commun. Morris AP, Voight BF, Teslovich TM, et al; Wellcome Trust Case Control Consortium; Meta-Analyses of Glucose and Insulin-related traits Consortium MAGIC Investigators; Genetic Investigation of ANthropometric Traits GIANT Consortium; Asian Genetic Epidemiology Network—Type 2 Diabetes AGEN-T2D Consortium; South Asian Type 2 Diabetes SAT2D Consortium; DIAbetes Genetics Replication And Meta-analysis DIAGRAM Consortium. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. New genetic loci link adipose and insulin biology to body fat distribution. Genetic studies of body mass index yield new insights for obesity biology. Willer CJ, Schmidt EM, Sengupta S, et al; Global Lipids Genetics Consortium. Discovery and refinement of loci associated with lipid levels. Scott RA, Lagou V, Welch RP, et al; DIAbetes Genetics Replication and Meta-analysis DIAGRAM Consortium. Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Stitziel NO, Stirrups KE, Masca NG, et al; Myocardial Infarction Genetics and CARDIoGRAM Exome Consortia Investigators. Coding Variation in ANGPTL4, LPL, and SVEP1 and the risk of coronary disease. N Engl J Med. Ehret GB, Munroe PB, Rice KM, et al; International Consortium for Blood Pressure Genome-Wide Association Studies; CARDIoGRAM Consortium; CKDGen Consortium; KidneyGen Consortium; EchoGen Consortium; CHARGE-HF Consortium. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Moffatt MF, Gut IG, Demenais F, et al; GABRIEL Consortium. A large-scale, consortium-based genomewide association study of asthma. Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Stock JH, Wright JH, Yogo M. A survey of weak instruments and weak identification in generalized method of moments. J Bus Econ Stat. Google Scholar Crossref. Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a Mendelian randomisation study. Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Burgess S, Davies NM, Thompson SG; EPIC-InterAct Consortium. Instrumental variable analysis with a nonlinear exposure-outcome relationship. Do R, Willer CJ, Schmidt EM, et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Gupta M, Singh N, Verma S. South Asians and cardiovascular risk: what clinicians should know. Barrett-Connor E. Sex differences in coronary heart disease: why are women so superior? the Ancel Keys Lecture. Rodgers RJ, Tschöp MH, Wilding JPH. Anti-obesity drugs: past, present and future. Dis Model Mech. Pierce BL, Ahsan H, Vanderweele TJ. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. von Hinke S, Davey Smith G, Lawlor DA, Propper C, Windmeijer F. A study found that a diet high in fruit and dairy and low in white bread, processed meat, margarine, and soft drinks may help reduce abdominal fat. A doctor or nutritionist can provide further advice on how to lose weight. People may take inaccurate measurements or make a mistake when doing the calculation. In addition, if someone has a high BMI or is less than 5 feet tall, their WHR may be less meaningful. It is important to note that a WHR is not designed to measure the health of children and should only be used for adults. However, as a WHR can be measured inaccurately, it should not be relied on as a sole measure of obesity or health risk. Talking to the doctor about weight and any associated health risks is always the best way to get a more complete picture. Want to lose those excess pounds? This study may offer some encouragement, after finding that the effects of being overweight may have been…. Metabolic syndrome is a condition that includes various health issues. It is linked to obesity, cardiovascular disease, high blood pressure, and type…. Find out what the average American woman weighs and obesity rates are for women globally. We also look at how weight can be measured and controlled…. To find their ideal weight, an individual must look at a number of factors, including gender and activity level. Learn how to find your healthy weight. Body fat scales can be an easy way to track body composition, but research debates their accuracy. Here, learn about body fat scales and the best…. My podcast changed me Can 'biological race' explain disparities in health? Why Parkinson's research is zooming in on the gut Tools General Health Drugs A-Z Health Hubs Health Tools Find a Doctor BMI Calculators and Charts Blood Pressure Chart: Ranges and Guide Breast Cancer: Self-Examination Guide Sleep Calculator Quizzes RA Myths vs Facts Type 2 Diabetes: Managing Blood Sugar Ankylosing Spondylitis Pain: Fact or Fiction Connect About Medical News Today Who We Are Our Editorial Process Content Integrity Conscious Language Newsletters Sign Up Follow Us. Medical News Today. Health Conditions Health Products Discover Tools Connect. Why is the hip-waist ratio important? Medically reviewed by Daniel Bubnis, M. How to calculate waist-to-hip ratio What is a healthy ratio? Impact on health How to improve the ratio Considerations Conclusion Waist-to-hip ratio, also known as waist-hip ratio, is the circumference of the waist divided by the circumference of the hips. The percentage variance in BMI and WHR explained by the variants selected as their respective instruments was estimated as previously described [ 31 ]. All clumping was performed using the TwoSampleMR package in R [ 32 ]. Random-effects inverse-variance weighted IVW MR was used as the main analysis for estimating the total effects of genetically predicted BMI and genetically predicted WHR respectively on each of the considered CVD outcomes [ 33 ]. The contamination-mixture method, weighted median and MR-Egger were used in sensitivity analyses to explore the robustness of the findings to potential pleiotropic effects of the variants [ 34 , 35 , 36 ]. The contamination-mixture model makes the assumption that MR estimates from valid instruments follow a normal distribution that centres on the true causal effect estimate, while those calculated from invalid instrument variants follow a normal distribution centred on the null [ 35 ]. This allows for a likelihood function to be specified and maximized when allocating each variant to one of the two mixture distributions [ 35 ]. The weighted median approach orders the MR estimates from individual variants by their magnitude weighted for their precision and selects the median as the overall MR estimate, calculating standard error by bootstrapping [ 34 ]. MR-Egger regresses the variant-outcome association estimates against the variant-exposure association estimates, weighted for the precision of the variant-outcome estimates [ 36 ]. It gives a valid MR estimate and test for the presence of directional pleiotropy in scenarios where any direct effect of the variants on the outcome is not correlated to their association with the exposure [ 36 ]. The MendelianRandomization package version 0. To estimate the direct effect of genetically predicted BMI and genetically predicted WHR on each of the three considered CVD outcomes that were not being mediated by the investigated intermediary risk factors, summary data multivariable MR was performed [ 38 , 39 , 40 ]. Specifically, the orientations of all genetic association estimates were harmonized and the variant-outcome genetic association estimates were regressed on the variant-exposure and variant-mediator estimates, weighted for the precision of the variant-outcome association, with the intercept fixed to zero [ 40 ]. Using this approach, adjustment was made for genetically predicted SBP, diabetes, smoking and lipid traits LDL-C, HDL-C and triglycerides together in turn, and finally including all mediators together in a joint model. In a sensitivity analysis, genetically predicted diabetes was excluded from this joint model to remove any bias that might be introduced because of its binary nature [ 41 ]. For analyses considering genetically predicted fasting glucose in non-diabetics instead of genetically predicted diabetes, the corresponding genetic association data were substituted. Diabetes and fasting glucose were not included together in the same model. Multivariable MR mediation analysis was performed to estimate the proportion of the effect of BMI and WHR respectively on CAD, PAD and stroke that was mediated through each of the considered risk factors, and also all of them together [ 16 ]. Specifically, the direct effect of genetically predicted BMI and genetically predicted WHR respectively were divided by their total effect and subtracted from 1, with standard errors estimated using the propagation of error method [ 16 , 18 ]. The direct effects of genetically predicted BMI and genetically predicted WHR on the considered CVD outcomes that are not mediated through each other were measured by including only these two traits together as exposures in the summary data multivariable MR model described above. The variants selected as instruments for BMI and WHR explain 5. Considering total effects, there was consistent evidence across the IVW, contamination-mixture, weighted median and MR-Egger methods that both higher genetically predicted BMI and higher genetically predicted WHR increased CAD, PAD and stroke risk Supplementary Fig. The confidence intervals of the MR-Egger estimates were wider than for the other methods, consistent with its lower statistical power [ 42 ]. In the main IVW MR analysis, the odds ratio per 1-standard deviation SD increase in genetically predicted BMI 4. For a 1-SD increase in genetically predicted WHR 0. There was attenuation in the associations of genetically predicted BMI and genetically predicted WHR with the three CVD outcomes after adjusting for genetically predicted SBP, diabetes, lipid traits LDL-C, HDL-C and triglycerides together and smoking, either separately or in the same joint model Fig. The y -axis details the genetically predicted mediator s for which adjustments were made. Blood pressure refers to systolic blood pressure. Lipids refer to serum low-density lipoprotein cholesterol, high-density lipoprotein cholesterol and triglycerides considered together in one model. CI confidence interval, OR odds ratio, SD standard deviation. The percentage attenuation in the total effects of genetically predicted BMI and WHR respectively on the three CVD outcomes after adjusting for the mediators is depicted in Fig. The y -axis details the genetically predicted mediator s for which adjustment was made. CI confidence interval. A joint model including all considered mediators except genetically predicted diabetes was also constructed Supplementary Fig. Adjusting together for all the mediators except genetically predicted diabetes, the association of genetically predicted BMI with CAD risk attenuated from odds ratio 1. There was little change in the association of either genetically predicted BMI or genetically predicted WHR with risk of the three CVD outcomes after adjusting for genetically predicted fasting glucose in non-diabetic individuals Fig. Both genetically predicted BMI and genetically predicted WHR had direct effects on CAD, PAD and stroke after mutual adjustment Fig. This study uses large-scale genetic association data within the MR paradigm to investigate the role of SBP, diabetes, lipid traits and smoking in mediating the effect of BMI and WHR on CAD, PAD and stroke risk. The results support that the majority of the effects of obesity on CVD are mediated through these risk factors, with diabetes and blood pressure being the most notable and accounting for approximately one-third and one-quarter of the effect respectively. In contrast, the analysis of genetically predicted fasting glucose in non-diabetic individuals did not provide any evidence to support its role in mediating the effect of obesity on CVD risk. Previous work has supported an effect of diabetes liability, fasting glucose and glycated haemoglobin on CVD risk [ 43 , 44 ]. Taken together with our current findings, this suggests that obesity may be affecting CVD risk by increasing diabetes liability and non-fasting postprandial glucose levels. Similarly, while lipid traits are known to affect CVD risk [ 45 ], our current study suggests that obesity is conferring only a small proportion of its effect on CVD risk through this pathway. Consistent with this, previous work has supported an effect of BMI on HDL-C and triglyceride levels, but not LDL-C [ 44 ]. In our analyses, the sum of the estimated mediating effects of the various risk factors considered individually was comparable to their total mediating effect estimated when considering them all together in the same model, consistent with them acting through distinct mechanisms. Including genetically predicted BMI and genetically predicted WHR in the same model produced evidence consistent with these traits having direct effects on CVD risk independently of each other. It follows that rather than analysing BMI or WHR alone, they should be considered together as they capture different aspects of adiposity. Our findings have important clinical and public health implications. Behavioural interventions to reduce obesity can have inadequate long term effects [ 46 ], pharmacological treatments may be limited by unfavourable adverse effect profiles [ 47 ], and surgical procedures are often reserved for only severe cases [ 48 ]. While preventing obesity remains the priority, this work supports that the majority of its cardiovascular consequences may also be managed by effectively controlling its downstream mediators, most notably diabetes and raised blood pressure, for which effective pharmacological interventions are available. This has relevance for the more than million individuals worldwide currently living with obesity [ 49 ], and the many more forecasted to become obese in coming years [ 50 ]. Such holistic consideration of obesity together with its mediators could contribute to a shift from the single-disease focus of health systems towards prioritizing multi-morbidity and promoting individual and societal wellness [ 51 ]. Our analyses were also suggestive of some possible residual effect of BMI on CVD risk even after adjusting for all the considered mediating risk factors, consistent with metabolically healthy obesity still conferring increased CVD risk [ 52 ]. In contrast, the investigation of WHR was consistent with an absence of any direct effect on CVD risk after accounting for all mediating risk factors together, suggesting that WHR may be entirely influencing CVD through downstream metabolic traits. Taken together, these results suggest that unless the growing obesity epidemic is effectively tackled, we risk undoing the large reductions in CVD mortality achieved over past decades [ 1 ]. Population-based approaches that decrease obesity by addressing key upstream drivers such as poor diet and physical inactivity have substantial potential for impact and are also effective for reducing health inequalities [ 53 , 54 ]. The results of our current study can be contrasted to those from a large-scale observational analysis of 1. However, the approach and data used in our current study offer a number of possible improvements. Our work includes a greater repertoire of risk factors and CVD outcomes than have been considered together previously [ 15 , 44 ], in particular, drawing on recently available GWAS summary data to study smoking and PAD [ 23 , 29 ]. MR analysis uses randomly allocated genetic variants that represent a lifelong cumulative liability to the traits for which they serve as instruments and can therefore help overcome the environmental confounding that may bias conventional observational studies [ 16 ]. Consistent with this, our MR results indicate that these risk factors mediate a greater proportion of the effect of obesity than suggested by previous conventional observational analyses [ 15 ]. Furthermore, our MR estimates are comparable to those obtained in previous MR studies considering BMI and WHR as exposures and different types of CVD as the outcome [ 44 , 56 , 57 ]. Also of relevance here, we considered a genetic liability to diabetes and genetically predicted fasting glucose in non-diabetic individuals as separate risk factors. Our findings support the concept that obesity traits confer an increased risk of CVD specifically through liability to diabetes, rather than variation in fasting glucose levels within the normal physiological range. This is important because fasting glucose may have a non-linear association with CVD risk [ 58 ], only having detrimental effects beyond a certain point [ 59 ]. Our current study also has limitations. The aim of the current work was to investigate the degree to which cardiometabolic traits mediate the effects of BMI and WHR on CVD outcomes, and our study did not extend to investigate any possible role of BMI or WHR in mediating the effects of the considered cardiometabolic traits on CVD risk. The genetic association data used in this work are drawn from predominantly European populations, and should therefore be interpreted with caution when extrapolating to other ethnic groups. Diabetes is a binary outcome, and as such our consideration of genetically predicted diabetes could introduce bias into the mediation analysis because not all individuals possessing such genetic liability to develop diabetes-related traits [ 41 ]. SBP was used as a proxy for studying the effects of blood pressure more generally. Given the high degree of phenotypic and genetic correlation between blood pressure traits [ 60 ], this would seem unlikely to affect the conclusions drawn. A theoretical weakness of the MR approach relates to bias from pleiotropic effects of the genetic variants incorporated as instruments for the traits under study, whereby they may directly affect the outcome through pathways independent of the exposure or mediators being considered. Although such bias cannot be entirely excluded, it is reassuring that we obtained similar MR estimates for the total effect of BMI and WHR respectively on the three CVD outcomes when performing the IVW, contamination-mixture, weighted median and MR-Egger methods that each make different assumptions concerning the presence of pleiotropic variants [ 42 ]. Finally, there is currently no available method for assessing instrument strength within the two-sample multivariable MR setting, and we could therefore not assess potential vulnerability to weak instrument bias [ 38 ]. In conclusion, this work using the MR framework suggests that the majority of the effects of obesity on CVD risk are mediated through metabolic risk factors, most notably diabetes and blood pressure. Comprehensive public health measures that target the reduction of obesity prevalence alongside control and management of its downstream mediators are likely to be most effective for minimizing the burden of obesity on individuals and health systems alike. Global, regional, and national age-sex specific mortality for causes of death, — a systematic analysis for the Global Burden of Disease Study Google Scholar. Singh GM, Danaei G, Farzadfar F, Stevens GA, Woodward M, Wormser D, et al. The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: a pooled analysis. PLoS ONE. CAS PubMed PubMed Central Google Scholar. Van Gaal LF, Mertens IL, De, Block CE. Mechanisms linking obesity with cardiovascular disease. PubMed Google Scholar. Carreras-Torres R, Johansson M, Haycock PC, Relton CL, Davey Smith G, Brennan P, et al. Role of obesity in smoking behaviour: Mendelian randomisation study in UK Biobank. PubMed PubMed Central Google Scholar. Taylor AE, Richmond RC, Palviainen T, Loukola A, Wootton RE, Kaprio J, et al. The effect of body mass index on smoking behaviour and nicotine metabolism: a Mendelian randomization study. Hum Mol Genet. CAS PubMed Google Scholar. Jaacks LM, Vandevijvere S, Pan A, McGowan CJ, Wallace C, Imamura F, et al. The obesity transition: stages of the global epidemic. Lancet Diabetes Endocrinol. Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C, et al. Projected U. state-level prevalence of adult obesity and severe obesity. N Engl J Med. Wright JM, Musini VM, Gill R. First-line drugs for hypertension. Cochrane Database Syst Rev. Michos ED, McEvoy JW, Blumenthal RS. Lipid management for the prevention of atherosclerotic cardiovascular disease. Rigotti NA, Clair C. Managing tobacco use: the neglected cardiovascular disease risk factor. Eur Heart J. Management of hyperglycemia in Type 2 diabetes, A consensus report by the American diabetes association ADA and the European association for the study of diabetes EASD. Diabetes Care. Neeland IJ, Ross R, Despres JP, Matsuzawa Y, Yamashita S, Shai I, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Chen GC, Arthur R, Iyengar NM, Kamensky V, Xue XN, Wassertheil-Smoller S, et al. Association between regional body fat and cardiovascular disease risk among postmenopausal women with normal body mass index. Sahakyan KR, Somers VK, Rodriguez-Escudero JP, Hodge DO, Carter RE, Sochor O, et al. Normal-weight central obesity: implications for total and cardiovascular mortality. Ann Intern Med. Lu Y, Hajifathalian K, Ezzati M, Woodward M, Rimm EB, Danaei G, et al. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1. Carter AR, Sanderson E, Hammerton G, Richmond RC, Smith GD, Heron J, et al. Mendelian randomisation for mediation analysis: current methods and challenges for implementation. Relton CL, Davey Smith G. Two-step epigenetic Mendelian randomization: a strategy for establishing the causal role of epigenetic processes in pathways to disease. Int J Epidemiol. Burgess S, Thompson DJ, Rees JMB, Day FR, Perry JR, Ong KK. Dissecting causal pathways using Mendelian randomization with summarized genetic data: application to age at menarche and risk of breast cancer. Davey Smith G, Davies NM, Dimou N, Egger M, Gallo V, Golub R, et al. STROBE-MR: guidelines for strengthening the reporting of Mendelian randomization studies. PeerJ Preprints. Article Google Scholar. Pulit SL, Stoneman C, Morris AP, Wood AR, Glastonbury CA, Tyrrell J, et al. Meta-analysis of genome-wide association studies for body fat distribution in individuals of European ancestry. Carter AR, Gill D, Davies NM, Taylor AE, Tillmann T, Vaucher J, et al. Understanding the consequences of education inequality on cardiovascular disease: Mendelian randomisation study. Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med. Wootton RE, Richmond RC, Stuijfzand BG, Lawn RB, Sallis HM, Taylor GMJ, et al. Evidence for causal effects of lifetime smoking on risk for depression and schizophrenia: a Mendelian randomisation study. Psychol Med. Mahajan A, Taliun D, Thurner M, Robertson NR, Torres JM, Rayner NW, et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat Genet. Lagou V, Mägi R, Hottenga JJ, Grallert H, Perry JRB, Bouatia-Naji N, et al. Meta-Analyses of Glucose and Insulin-related traits Consortium MAGIC. Sex-dimorphic genetic effects and novel loci for fasting glucose and insulin variability. Nat Commun. Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, Kanoni S, et al. Discovery and refinement of loci associated with lipid levels. Nikpay M, Goel A, Won HH, Hall LM, Willenborg C, Kanoni S, et al. A comprehensive genomes-based genome-wide association meta-analysis of coronary artery disease. Klarin D, Lynch J, Aragam K, Chaffin M, Assimes TL, Huang J, et al. Genome-wide association study of peripheral artery disease in the million veteran program. Nat Med. |
| Waist size predicts heart attacks better than BMI, especially in women | Sahakyan KR, Somers VK, Rodriguez-Escudero JP, Hodge DO, Carter RE, Sochor O, et al. Normal-weight central obesity: implications for total and cardiovascular mortality. Ann Intern Med. Lu Y, Hajifathalian K, Ezzati M, Woodward M, Rimm EB, Danaei G, et al. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1. Carter AR, Sanderson E, Hammerton G, Richmond RC, Smith GD, Heron J, et al. Mendelian randomisation for mediation analysis: current methods and challenges for implementation. Relton CL, Davey Smith G. Two-step epigenetic Mendelian randomization: a strategy for establishing the causal role of epigenetic processes in pathways to disease. Int J Epidemiol. Burgess S, Thompson DJ, Rees JMB, Day FR, Perry JR, Ong KK. Dissecting causal pathways using Mendelian randomization with summarized genetic data: application to age at menarche and risk of breast cancer. Davey Smith G, Davies NM, Dimou N, Egger M, Gallo V, Golub R, et al. STROBE-MR: guidelines for strengthening the reporting of Mendelian randomization studies. PeerJ Preprints. Article Google Scholar. Pulit SL, Stoneman C, Morris AP, Wood AR, Glastonbury CA, Tyrrell J, et al. Meta-analysis of genome-wide association studies for body fat distribution in individuals of European ancestry. Carter AR, Gill D, Davies NM, Taylor AE, Tillmann T, Vaucher J, et al. Understanding the consequences of education inequality on cardiovascular disease: Mendelian randomisation study. Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med. Wootton RE, Richmond RC, Stuijfzand BG, Lawn RB, Sallis HM, Taylor GMJ, et al. Evidence for causal effects of lifetime smoking on risk for depression and schizophrenia: a Mendelian randomisation study. Psychol Med. Mahajan A, Taliun D, Thurner M, Robertson NR, Torres JM, Rayner NW, et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat Genet. Lagou V, Mägi R, Hottenga JJ, Grallert H, Perry JRB, Bouatia-Naji N, et al. Meta-Analyses of Glucose and Insulin-related traits Consortium MAGIC. Sex-dimorphic genetic effects and novel loci for fasting glucose and insulin variability. Nat Commun. Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, Kanoni S, et al. Discovery and refinement of loci associated with lipid levels. Nikpay M, Goel A, Won HH, Hall LM, Willenborg C, Kanoni S, et al. A comprehensive genomes-based genome-wide association meta-analysis of coronary artery disease. Klarin D, Lynch J, Aragam K, Chaffin M, Assimes TL, Huang J, et al. Genome-wide association study of peripheral artery disease in the million veteran program. Nat Med. Malik R, Chauhan G, Traylor M, Sargurupremraj M, Okada Y, Mishra A, et al. Multiancestry genome-wide association study of , subjects identifies 32 loci associated with stroke and stroke subtypes. Gill D, Sheehan NA, Wielscher M, Shrine N, Amaral AFS, Thompson JR, et al. Age at menarche and lung function: a Mendelian randomization study. Eur J Epidemiol. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. Bowden J, Davey, Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Burgess S, Foley CN, Allara E, Staley JR, Howson JMM. A robust and efficient method for Mendelian randomization with hundreds of genetic variants. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Yavorska OO, Burgess S. Mendelian randomization: an R package for performing Mendelian randomization analyses using summarized data. Sanderson E, Davey Smith G, Windmeijer F, Bowden J. An examination of multivariable Mendelian randomization in the single-sample and two-sample summary data settings. PubMed Central Google Scholar. Burgess S, Thompson SG. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol. Burgess S, Dudbridge F, Thompson SG. Burgess S, Labrecque JA. Mendelian randomization with a binary exposure variable: interpretation and presentation of causal estimates. Slob EAW, Burgess S. A comparison of robust Mendelian randomization methods using summary data. Ahmad OS, Morris JA, Mujammami M, Forgetta V, Leong A, Li R, et al. A Mendelian randomization study of the effect of type-2 diabetes on coronary heart disease. Xu L, Borges MC, Hemani G, Lawlor DA. The role of glycaemic and lipid risk factors in mediating the effect of BMI on coronary heart disease: a two-step, two-sample Mendelian randomisation study. Allara E, Morani G, Carter P, Gkatzionis A, Zuber V, Foley CN, et al. Genetic determinants of lipids and cardiovascular disease outcomes: a wide-angled Mendelian randomization investigation. Circ Genom Precis Med. Douketis JD, Macie C, Thabane L, Williamson DF. Systematic review of long-term weight loss studies in obese adults: clinical significance and applicability to clinical practice. Int J Obes. CAS Google Scholar. Rucker D, Padwal R, Li SK, Curioni C, Lau DC. Long term pharmacotherapy for obesity and overweight: updated meta-analysis. Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. NCD Risk Factor Collaboration. Trends in adult body-mass index in countries from to a pooled analysis of population-based measurement studies with Foreman KJ, Marquez N, Dolgert A, Fukutaki K, Fullman N, McGaughey M, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for causes of death: reference and alternative scenarios for —40 for countries and territories. Pearson-Stuttard J, Ezzati M, Gregg EW. Multimorbidity—a defining challenge for health systems. Lancet Public Health. Caleyachetty R, Thomas GN, Toulis KA, Mohammed N, Gokhale KM, Balachandran K, et al. Metabolically healthy obese and incident cardiovascular disease events among 3. J Am Coll Cardiol. Backholer K, Beauchamp A, Ball K, Turrell G, Martin J, Woods J, et al. A framework for evaluating the impact of obesity prevention strategies on socioeconomic inequalities in weight. Am J Public Health. Adams J, Mytton O, White M, Monsivais P. Why are some population interventions for diet and obesity more equitable and effective than others? The role of individual agency. PLoS Med. Lawlor DA, Tilling K, Davey Smith G. Triangulation in aetiological epidemiology. Censin JC, Peters SAE, Bovijn J, Ferreira T, Pulit SL, Magi R, et al. Causal relationships between obesity and the leading causes of death in women and men. PLOS Genet. Marini S, Merino J, Montgomery BE, Malik R, Sudlow CL, Dichgans M, et al. Mendelian randomization study of obesity and cerebrovascular disease. Ann Neurol. Park C, Guallar E, Linton JA, Lee DC, Jang Y, Son DK, et al. Fasting glucose level and the risk of incident atherosclerotic cardiovascular diseases. Fuller JH, Shipley MJ, Rose G, Jarrett RJ, Keen H. Coronary-heart-disease risk and impaired glucose tolerance. The Whitehall study. Evangelou E, Warren HR, Mosen-Ansorena D, Mifsud B, Pazoki R, Gao H, et al. Genetic analysis of over 1 million people identifies new loci associated with blood pressure traits. Download references. Michael Gaziano 27 , Sumitra Muralidhar 28 , Rachel Ramoni 28 , Jean Beckham 29 , Kyong-Mi Chang 30 , Christopher J. Tsao 31 , James Breeling 28 , Grant Huang 28 , Juan P. Casas Stacey B. Whitbourne 27 , Jessica V. Brewer 27 , Mihaela Aslan 32 , Todd Connor 33 , Dean P. Argyres 33 , Philip S. Michael Gaziano 35 , Brady Stephens 36 , Mary T. Brophy 37 , Donald E. Humphries 37 , Luis E. Selva 37 , Nhan Do 38 , Shahpoor Alex Shayan 38 , Kelly Cho 27 , Lori Churby Christopher J. DuVall 42 , Elizabeth Hauser 43 , Philip S. Tsao 31 , Yan Sun 44 , Hongyu Zhao Zablocki VA Medical Center, Milwaukee, WI, USA; 52 VA Northeast Ohio Healthcare System, Cleveland, OH, USA; 53 Durham VA Medical Center, Durham, NC, USA; 54 Edith Nourse Rogers Memorial Veterans Hospital, Bedford, MA, USA; 55 Edward Hines, Jr. VA Medical Center, Hines, IL, USA; 56 Veterans Health Care System of the Ozarks, Fayetteville, AR, USA; 57 Fargo VA Health Care System, Fargo, ND, USA; 58 VA Health Care Upstate New York, Albany, NY, USA; 59 VA Western New York Healthcare System, Buffalo, NY, USA; 60 Ralph H. Johnson VA Medical Center, Mental Health Research, Charleston, SC, USA; 61 Columbia VA Health Care System, Columbia, SC, USA; 62 VA North Texas Health Care System, Dallas, TX, USA; 63 Hampton VA Medical Center, Hampton, VA, USA; 64 Richmond VA Medical Center, Richmond, VA, USA; 65 Iowa City VA Health Care System, Iowa City, IA, USA; 66 Eastern Oklahoma VA Health Care System, Muskogee, OK, USA; 67 James A. Quillen VA Medical Center, Corner of Lamont and Veterans Way, Mountain Home, TN, USA; 69 John D. Dingell VA Medical Center, Detroit, MI, USA; 70 Louisville VA Medical Center, Louisville, KY, USA; 71 Manchester VA Medical Center, Manchester, NH, USA; 72 Miami VA Health Care System, Miami, FL, USA; 73 Michael E. DeBakey VA Medical Center, Houston, TX, USA; 74 Minneapolis VA Health Care System, Minneapolis, MN, USA; 75 N. GA Veterans Health System, Gainesville, FL, USA; 76 Northport VA Medical Center, Northport, NY, USA; 77 Overton Brooks VA Medical Center, Shreveport, LA, USA; 78 Philadelphia VA Medical Center, Philadelphia, PA, USA; 79 Phoenix VA Health Care System, Phoenix, AZ, USA; 80 Portland VA Medical Center, Portland, OR, USA; 81 Providence VA Medical Center, Providence, RI, USA; 82 Richard Roudebush VA Medical Center, Indianapolis, IN, USA; 83 Salem VA Medical Center, Salem, VA, USA; 84 San Francisco VA Health Care System, San Francisco, CA, USA; 85 South Texas Veterans Health Care System, San Antonio, TX, USA; 86 Southeast Louisiana Veterans Health Care System, New Orleans, LA, USA; 87 Southern Arizona VA Health Care System, Tucson, AZ, USA; 88 Sioux Falls VA Health Care System, Sioux Falls, SD, USA; 89 St. Louis VA Health Care System, St. Louis, MO, USA; 90 Syracuse VA Medical Center, Syracuse, NY, USA; 91 VA Eastern Kansas Health Care System, Leavenworth, KS, USA; 92 VA Greater Los Angeles Health Care System, Los Angeles, CA, USA; 93 VA Long Beach Healthcare System, Long Beach, CA, USA; 94 VA Maine Healthcare System, 1 VA Center, Augusta, ME, USA; 95 VA New York Harbor Healthcare System, New York, NY, USA; 96 VA Pacific Islands Health Care System, Honolulu, HI, USA; 97 VA Palo Alto Health Care System, Palo Alto, CA, USA; 98 VA Pittsburgh Health Care System, University Drive, Pittsburgh, PA, USA; 99 VA Puget Sound Health Care System, Seattle, WA, USA; VA San Diego Healthcare System, San Diego, CA, USA; VA Sierra Nevada Health Care System, Reno, NV, USA; VA Southern Nevada Healthcare System, North Las Vegas, NV, USA; VA Tennessee Valley Healthcare System, South Nashville, TN, USA; Washington DC VA Medical Center, Washington, DC, USA; W. Bill Hefner VA Medical Center, Salisbury, NC, USA; White River Junction VA Medical Center, Hartford, VT, USA; William S. Middleton Memorial Veterans Hospital, Madison, WI, USA. This work was supported by funding from the US Department of Veterans Affairs Office of Research and Development, Million Veteran Programme Grant MVP IBX This publication does not represent the views of the Department of Veterans Affairs of the US Government. This work was supported by the National Institute for Health Research NIHR Biomedical Research Centre at the University Hospitals Bristol National Health Service NHS Foundation Trust and the University of Bristol. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. DG and JP-S are funded by the Wellcome 4i Clinical Ph. SMD was supported by the Department of Veterans Affairs Office of Research and Development IK2-CX The funding sources for this work were not involved in study design, data analysis, interpretation of results or writing of the manuscript. Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK. Dipender Gill, Verena Zuber, Jonathan Pearson-Stuttard, Ville Karhunen, Konstantinos K. Medical Research Council Biostatistics Unit, University of Cambridge, Cambridge, UK. Medical Research Council Centre for Environment and Health, School of Public Health, Imperial College London, London, UK. University of Glasgow, Institute of Cardiovascular and Medical Sciences, Glasgow, UK. Medical Research Council Integrative Epidemiology Unit, University of Bristol, Bristol, UK. Alice R. Population Health Sciences, Bristol Medical School, University of Bristol, Bristol, UK. Division of Cardiovascular Medicine, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA. Department of Medicine, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA. Corporal Michael J. Crescenz VA Medical Center, Philadelphia, PA, USA. School of Psychological Science, University of Bristol, Bristol, UK. National Institute for Health Research Biomedical Research Centre, University Hospitals Bristol NHS Foundation Trust and the University of Bristol, Bristol, UK. Malcom Randall VA Medical Center, Gainesville, FL, USA. Center for Genomic Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA. Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Boston, MA, USA. Division of Vascular Surgery and Endovascular Therapy, University of Florida School of Medicine, Gainesville, Fl, USA. VA Palo Alto Health Care System, Livermore, CA, USA. Department of Medicine, Stanford University School of Medicine, Stanford, CA, USA. Department of Hygiene and Epidemiology, University of Ioannina Medical School, Ioannina, Greece. Department of Surgery, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA. Cardiovascular Epidemiology Unit, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK. UK Dementia Research Institute at Imperial College London, London, UK. Imperial Biomedical Research Centre, Imperial College London and Imperial College NHS Healthcare Trust, London, UK. You can also search for this author in PubMed Google Scholar. Correspondence to Dipender Gill. DG is employed part-time by Novo Nordisk. JP-S reports personal fees from Novo Nordisk related to consultancy outside of the submitted work. SMD has received grants from the U. Department of Veterans Affairs, Calico Labs, and Renalytix AI plc outside the submitted work. All other authors have no conflicts of interest to declare. Open Access This article is licensed under a Creative Commons Attribution 4. Reprints and permissions. Gill, D. et al. Risk factors mediating the effect of body mass index and waist-to-hip ratio on cardiovascular outcomes: Mendelian randomization analysis. Int J Obes 45 , — Download citation. Received : 03 April Revised : 23 February Accepted : 22 March Published : 17 May Issue Date : July Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content Thank you for visiting nature. nature international journal of obesity articles article. Download PDF. Subjects Cardiovascular diseases Risk factors. Abstract Background Higher body mass index BMI and waist-to-hip ratio WHR increase the risk of cardiovascular disease, but the extent to which this is mediated by blood pressure, diabetes, lipid traits, and smoking is not fully understood. Methods Using consortia and UK Biobank genetic association summary data from , to , participants predominantly of European ancestry, Mendelian randomization mediation analysis was performed to investigate the degree to which systolic blood pressure SBP , diabetes, lipid traits, and smoking mediated an effect of BMI and WHR on the risk of coronary artery disease CAD , peripheral artery disease PAD and stroke. Results The odds ratio of CAD per 1-standard deviation increase in genetically predicted BMI was 1. Conclusions Measures to reduce obesity will lower the risk of cardiovascular disease primarily by impacting downstream metabolic risk factors, particularly diabetes and hypertension. Background Cardiovascular disease CVD is the leading cause of death and disability worldwide [ 1 ]. Methods Ethical approval, data availability, code availability and reporting The data used in this work are publicly available and the studies from which they were obtained are cited. Data sources Genetic association estimates for BMI and WHR were obtained from the GIANT Consortium GWAS meta-analysis of , and , European-ancestry individuals, respectively [ 20 ]. Total effects Random-effects inverse-variance weighted IVW MR was used as the main analysis for estimating the total effects of genetically predicted BMI and genetically predicted WHR respectively on each of the considered CVD outcomes [ 33 ]. Mediation analysis To estimate the direct effect of genetically predicted BMI and genetically predicted WHR on each of the three considered CVD outcomes that were not being mediated by the investigated intermediary risk factors, summary data multivariable MR was performed [ 38 , 39 , 40 ]. Independent effects of genetically predicted BMI and WHR The direct effects of genetically predicted BMI and genetically predicted WHR on the considered CVD outcomes that are not mediated through each other were measured by including only these two traits together as exposures in the summary data multivariable MR model described above. Results Total effects The variants selected as instruments for BMI and WHR explain 5. Mediation analysis There was attenuation in the associations of genetically predicted BMI and genetically predicted WHR with the three CVD outcomes after adjusting for genetically predicted SBP, diabetes, lipid traits LDL-C, HDL-C and triglycerides together and smoking, either separately or in the same joint model Fig. Full size image. Discussion This study uses large-scale genetic association data within the MR paradigm to investigate the role of SBP, diabetes, lipid traits and smoking in mediating the effect of BMI and WHR on CAD, PAD and stroke risk. References GBD. Google Scholar Singh GM, Danaei G, Farzadfar F, Stevens GA, Woodward M, Wormser D, et al. CAS PubMed PubMed Central Google Scholar Van Gaal LF, Mertens IL, De, Block CE. PubMed Google Scholar Carreras-Torres R, Johansson M, Haycock PC, Relton CL, Davey Smith G, Brennan P, et al. PubMed PubMed Central Google Scholar Taylor AE, Richmond RC, Palviainen T, Loukola A, Wootton RE, Kaprio J, et al. CAS PubMed Google Scholar Jaacks LM, Vandevijvere S, Pan A, McGowan CJ, Wallace C, Imamura F, et al. PubMed PubMed Central Google Scholar Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C, et al. PubMed Google Scholar Wright JM, Musini VM, Gill R. PubMed Google Scholar Michos ED, McEvoy JW, Blumenthal RS. CAS PubMed Google Scholar Rigotti NA, Clair C. PubMed PubMed Central Google Scholar Neeland IJ, Ross R, Despres JP, Matsuzawa Y, Yamashita S, Shai I, et al. PubMed Google Scholar Chen GC, Arthur R, Iyengar NM, Kamensky V, Xue XN, Wassertheil-Smoller S, et al. CAS PubMed PubMed Central Google Scholar Sahakyan KR, Somers VK, Rodriguez-Escudero JP, Hodge DO, Carter RE, Sochor O, et al. PubMed PubMed Central Google Scholar Lu Y, Hajifathalian K, Ezzati M, Woodward M, Rimm EB, Danaei G, et al. PubMed Google Scholar Carter AR, Sanderson E, Hammerton G, Richmond RC, Smith GD, Heron J, et al. PubMed PubMed Central Google Scholar Burgess S, Thompson DJ, Rees JMB, Day FR, Perry JR, Ong KK. CAS PubMed PubMed Central Google Scholar Davey Smith G, Davies NM, Dimou N, Egger M, Gallo V, Golub R, et al. Article Google Scholar Pulit SL, Stoneman C, Morris AP, Wood AR, Glastonbury CA, Tyrrell J, et al. Authoring team. Waist hip ratio WHR is a measure that helps discriminate abdominal obesity from overall obesity. WHR has shown to independently predict death from cardiovascular disease and coronary heart disease in Austrialina men and women 1. A commentary on this study stated that "most of the literature supports the finding that abdominal obesity is a stronger risk factor for cardiovascular disease than overall obesity. However, whether WHR is independent of traditional cardiac risk factors remains controversial and unanswered by this study " 2. A more recent meta-analysis concluded that wist circumference and waist-to-hip ratio increase risk of cardiovascular events in men and women 3. WHR shows a linear association with mortality in middle-aged men and women, but body mass index, based on this particular study, did not 4. Add information to this page that would be handy to have on hand during a consultation, such as a web address or phone number. This information will always be displayed when you visit this page. Waist hip ratio WHR and cardiovascular disease CVD Last reviewed dd mmm yyyy. Last edited dd mmm yyyy Authoring team. In a study situation WHR was measured 1 via: measurement of waist circumference - measured btweeen the lower border of the ribs, and the iliac crest in a horizontal plane measurement of hip circumference - measured at the widest point over the buttocks WHR was obtained by dividing the mean waist circumference by the mean hip-circumference men with a WHR 0. the study authors observed that the unadjusted associations with each of the CVD risk factors in both male and female subjects were strongest for obesity when defined using WHR rather than other obesity measures - body mass index, waist circumference A commentary on this study stated that "most of the literature supports the finding that abdominal obesity is a stronger risk factor for cardiovascular disease than overall obesity. |
| Background | In an effort to understand the causal pathway that relates abdominal obesity to CVD risk, we examined adjusted risk estimates. Clinical Specialties Get specialized, one-on-one care from trusted experts that are board certified, experienced and highly trained in 20 clinical specialties. Email me when people comment on this article. Here, learn about body fat scales and the best… READ MORE. Konig: Employee; Self; Eli Lilly and Company. Main outcome measures: Hazard ratios for the risk factors predicting CVD mortality and CHD mortality. |
der Interessante Moment