
EGCG and bone health -
a The Orthopaedic Center, The Affiliated Wenling Hospital of Wenzhou Medical University The First People's Hospital of Wenling , Wenling , Zhejiang Province, China E-mail: gangle. liu gmail. b College of Bioscience and Biotechnology, Hunan Agricultural University, Changsha, Hunan , PR China.
c Hunan Engineering Laboratory for Pollution Control and Waste Utilization in Swine, Production, Changsha , PR China. d Department of Cardiology, The Affiliated Wenling Hospital of Wenzhou Medical University The First People's Hospital of Wenling , Wenling , Zhejiang Province, China.
e Key Laboratory of Precision Medicine for Atherosclerotic Diseases of Zhejiang Province, Affiliated First Hospital of Ningbo University, Ningbo , China. Osteoporosis, one of the serious public health problems worldwide, can lead to degeneration of the bone structure and increased risk of fractures.
Epigallocatechin gallate EGCG is a natural product with potential efficacy in inhibiting bone loss. However, the specific mechanism remains unclear. This study first investigated the role of EGCG in preventing dexamethasone DEX -induced osteoporosis by regulating intestinal microbiota and serum metabolites.
We detected the bone density, bone microstructure, and changes in intestinal microorganisms and serum metabolites. According to our results, EGCG inhibited the decline of bone density, protected the bone microstructure, increased microbial diversity, promoted the abundance of beneficial bacteria such as Prevotellaceae and Ruminococcus , and inhibited the abundance of pathogenic bacteria such as Peptostreptococcaceae.
There were also significant changes in serum metabolites among different treatments. In summary, EGCG can prevent bone damage, promote the production of beneficial bacteria and metabolites, and enhance immune function. This study provides a basis and reference for the prevention and treatment of osteoporosis, as well as the application of EGCG in maintaining body health.
Han, Y. Fu, K. Wang, S. Li, C. Jiang, S. Wang, Z. Wang, G. Liu and S. Hu, Food Funct. To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given. If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given.
Read more about how to correctly acknowledge RSC content. Fetching data from CrossRef. This may take some time to load.
Loading related content. Jump to main content. Osteoporosis can be divided into two categories, primary type and secondary type 2. The former is further divided into postmenopausal type I , senile type II and idiopathic osteoporosis disease.
The latter refers to osteoporosis induced by diseases that influence bone physiology or by drugs, such as osteoporosis induced by long-term high-dose glucocorticoid treatment 3.
Due to the wide application of glucocorticoids in clinical practice, the incidence of glucocorticoid-induced osteoporosis has continuously risen in recent years, and as such, it is now considered the third most common type of osteoporosis, following postmenopausal and senile osteoporosis 4.
The role of Wnt signaling in bone metabolism has become a key area of interest in recent years. Previous studies have demonstrated that Wnt can directly affect the pluripotent precursor cell differentiation process into bone cells 4 , 5.
The stable expression of Wnt1 and Wnt3a can promote the proliferation of osteocytes and induce alkaline phosphatase ALP activity, which is the early stage marker of osteoblast differentiation 6.
However, apart from ALP, the other relevant markers for osteoblast differentiation, including runt-related transcription factor 2 Runx2 , osteocalcin OC and type I collagen, are not markedly affected, which indicates that Wnts can promote the growth of precursor osteoblasts and promote osteoblast differentiation at the early stage 7.
β-catenin serves an important role in the Wnt signaling pathway. The bone stem cell lineage can differentiate into osteoblasts, adipocytes and chondrocytes; it can also be differentiated into osteoblasts via the action of bone morphogenetic protein 2 BMP During this process, BMP-2 can upregulate β-catenin 8.
This suggests that β-catenin may serve a role in precursor osteoblast and osteoblast proliferation and differentiation, and that it may be regulated by BMP-2 A large number of studies have revealed that EGCG has biological activities including anti-cancer, anti-mutation, the prevention and treatment of cardiovascular diseases, and regulating the endocrine and immune systems; it also has inhibitory activity associated with metabolic enzymes, which have a marked impact on the liver detoxification function of the body 13 , The aim of the present study was to investigate the potential anti-osteoporosis effects of EGCG in secondary osteoporosis and the potential underlying mechanism in a mouse model.
The animal protocol was approved by the Committee on the Ethics of Animal Experiments of The th Hospital of The People's Liberation Army Beijing, China. Beijing, China and were given free access to food and water. The present study used a dexamethasone-induced model of osteoporosis, as described previously 4.
In EGCG groups, mice were injected with 0. At 0 and 4 week, body weight was record, and blood was were collected from tail vein and used to measure body fat using a commercial kit A; Nanjing Jiancheng Bioengineering Institute, Nanjing, China.
In addition, Duel-Energy X-ray Absorptiometry Lunar Prodigy; GE Healthcare, Chicago, IL, USA was used to measure body fat at the 0 and 4 week time points of EGCG treatment. Then, blood was collected from the tail vein and blood was transferred to tubes.
A TechniconSMAC Technicon Instruments Corp, Tarrytown, NY, USA determined the serum calcium level. C and ALP activity cat no. A was measured using ELISA kits obtained from Nanjing Jiancheng Bioengineering Institute Nanjing, China. Samples were cut into 4 mm thick sections and stained with hematoxylin and eosin at 5 min at room temperature.
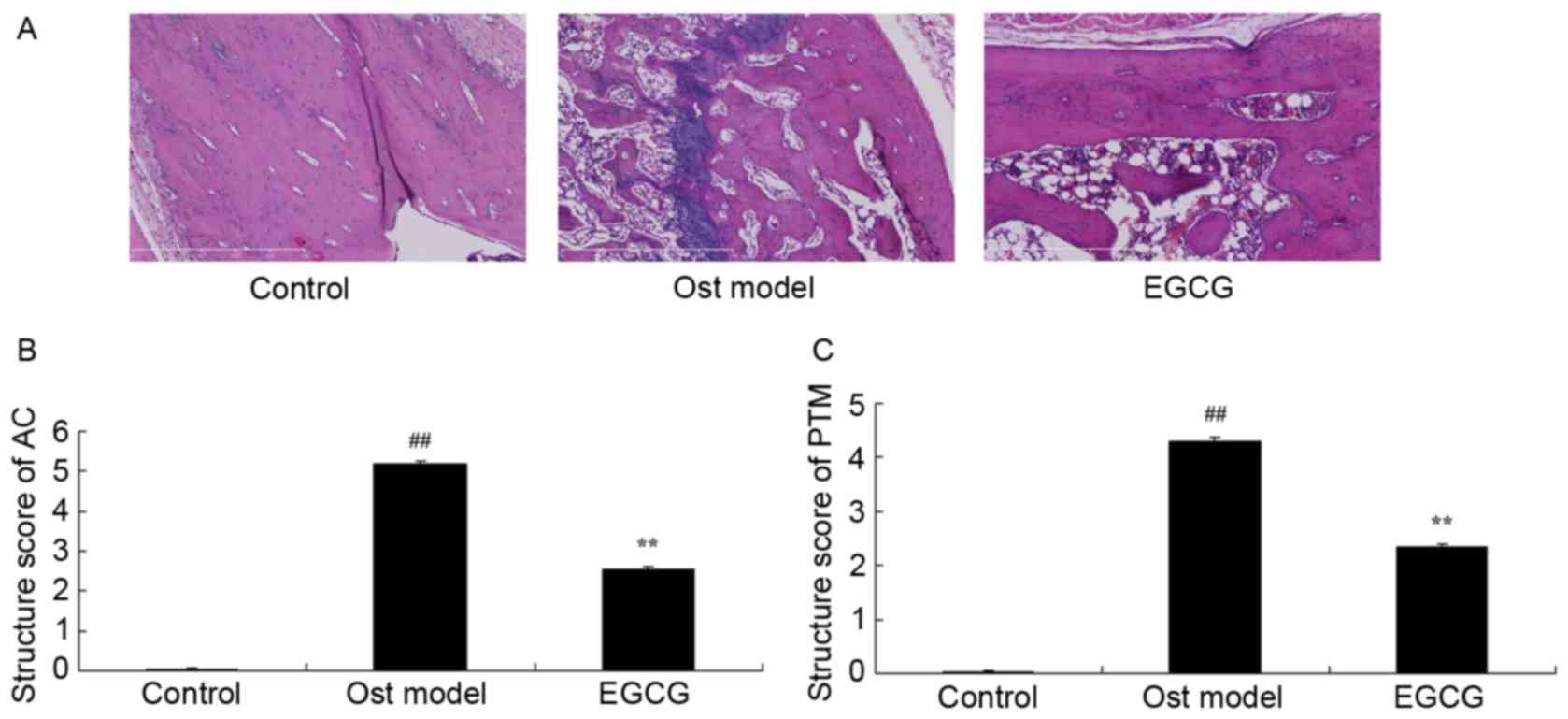
The articular cartilage AC and cancellous bone in proximal tibia metaphysis PTM were then observed using a fluorescent microscope ×20; Zeiss Axioplan 2—, Carl Zeiss MicroImaging using the Mankin histological grading system, as described previously Total RNA was prepared using TRIzol Reagent Gibco; Thermo Fisher Scientific, Inc.
Total RNA was then reverse transcribed using a Reverse Transcription kit Applied Biosystems; Thermo Fisher Scientific, Inc. RT-qPCR was conducted using a Rotor-Gene System Corbett Life Science; Qiagen, Inc. The thermocycling conditions were: 95°C for 10 min, then 40 cycles of 95°C for 30 sec, 60°C for 45 sec, followed by 72°C for 30 sec.
Total proteins were extracted from femoral head tissue using Radioimmunoprecipitation Assay Lysis Buffer Beyotime Institute of Biotechnology, Jiangsu, China and measured using an Enhanced Bicinchoninic Acid Protein Assay kit Beyotime Institute of Biotechnology.
sc; ,; Santa Cruz Biotechnology, Inc. and anti-GAPDH cat no. sc; ,; primary antibodies overnight at 4°C. The membrane was then incubated with anti-rabbit or anti-mouse horseradish peroxidase-conjugated secondary antibodies cat no. sc or sc, respectively; Santa Cruz Biotechnology, Inc. at 37°C for 1 h.
Proteins were detected with an enhanced chemiluminescence reagent Amersham; GE Healthcare and analyzed using Image-Pro Plus version 6. Data are presented as the mean ± standard error of the mean of three independent experiments.
The results were analyzed using SPSS The effect of EGCG on lipid metabolism was evaluated in a mouse model of secondary osteoporosis. Following the 4 week treatment period, the body weight and body fat content in the secondary osteoporosis mouse model group were markedly higher than those of control group Fig.
Treatment with EGCG for 4 weeks effectively reduced this secondary osteoporosis-induced increase in body weight and body fat content Fig. EGCG protects against adverse lipid metabolism.
Following 4 weeks of EGCG treatment the A body weight and B percentage of body fat in mice with secondary osteoporosis was significantly decreased. osteoporosis model group. Control, control group; Ost model, secondary osteoporosis model group; EGCG, epigallocatechingallate group.
As shown in Fig. EGCG protects against secondary osteoporosis. To determine the anti-osteoporosis effects of EGCG associated with bone structure and bone turnover, the AC and PTM structure scores were analyzed. EGCG treatment significantly decreased the AC and PTM structure scores when compared with the model group Fig.
EGCG protects bone structure and turnover in secondary osteoporosis. A Hematoxylin and eosin staining of bone tissues to analyze the B AC and C PTM scores in mice with secondary osteoporosis. EGCG protected mice with secondary osteoporosis against adverse B bone structure of AC and C PTM of bone turnover in a mouse model of secondary osteoporosis.
Magnification, × Control, control group; Ost model, secondary osteoporosis model group; EGCG, epigallocatechingallate group; AC, articular cartilage; PTM, proximal tibia metaphysis. To determine whether ALP, Runx2 and OSX are involved in the effects of EGCG on osteoporosis, ALP, Runx2 and OSX mRNA expression were measured using RT-qPCR.
A significant increase in ALP, Runx2 and OSX mRNA expression was observed in the secondary osteoporosis mouse model group when compared with the control group Fig. In addition, treatment with EGCG significantly reduced this secondary osteoporosis-induced increase in ALP, Runx2 and OSX mRNA expression Fig.
EGCG restores ALP activity, and Runx2 and OSX mRNA expression. Secondary osteoporosis significantly increased A ALP activity, and B Runx2 and C OSX expression, however, EGCG treatment decreased these levels in a mouse model of secondary osteoporosis.
Control, control group; Ost model, secondary osteoporosis model group; EGCG, epigallocatechingallate group; ALP, alkaline phosphatase; Runx2, runt-related transcription factor 2; OSX, Sp7 transcription factor osterix.
The effects of EGCG on Runx2, OSX and PPARγ protein expression were further determined by western blotting Fig. Osteoporosis significantly induced Runx2 Fig. In addition, treatment with EGCG significantly suppressed this osteoporosis-induced increase in Runx2, OSX and PPARγ protein expression.
EGCG and Runx2, OSX and PPARγ protein expression. EGCG protects against Runx2 protein expression as shown by A western blot analysis. B Runx2, C OSX and D PPARγ protein expression increased in the mouse model of secondary osteoporosis, however, treatment with EGCG reversed this effect and decreased the expression of these proteins.
Control, control group; Ost model, secondary osteoporosis model group; EGCG, epigallocatechingallate group; Runx2, runt-related transcription factor 2; OSX, Sp7 transcription factor osterix; PPARγ, peroxisome proliferator-activated receptor γ.
To investigate the role of Wnt, β-catenin and cyclin D1 in the effect of EGCG on osteoporosis, western blotting was performed Fig. Wnt Fig. However, treatment with EGCG significantly increased Wnt, β-catenin and cyclin D1 protein expression when compared with the secondary osteoporosis mouse model group.
EGCG and Wnt, β-catenin and Cyclin D1 protein expression. EGCG protects against Wnt protein expression as shown by A western blot analysis. B Wnt, C β-catenin and D Cyclin D1 protein expression decreased in the mouse model of secondary osteoporosis, however, treatment with EGCG reversed this effect and increased the expression of these proteins.
Osteoporosis is a common degenerative disease that primarily causes increased bone fragility and reduced bone density, which eventually leads to bone fracture China has the greatest aging population in the world, of which at least 90 million people suffer from osteoporosis; it is expected that the number of patients with osteoporosis will increase to million by the year As osteoporosis severely affects the health of the elderly, identifying prevention strategies and treatments has become an major public health concern.
The results of the present study demonstrated that EGCG is protective against adverse lipid metabolism, and bone structure and turnover in mice with secondary osteoporosis, which suggests that EGCG may exert anti-osteoporosis effects. Osteoblasts are mainly derived from bone marrow mesenchymal stem cells, the differentiation process of which is dependent on the regulation of a number of transcription factors and cytokines Bone marrow mesenchymal stem cells are pluripotent stem cells that can differentiate into osteoblasts, chondrocytes and adipocytes A previous study revealed that when the cells have high expressions of Runx2 and OSX, mesenchymal stem cells are differentiated into osteoblasts However, when these cells highly express sex-determining region Y-box 9, mesenchymal stem cells are differentiated into chondrocytes, and when they have a high expression of PPAR, they differentiate into chondrocytes The results of the present study demonstrated that EGCG treatment significantly reduced ALP, Runx2 and OSX mRNA expression, and suppressed Runx2 and OSX protein expression in a mouse model of secondary osteoporosis.
Marrow fat cells are derived from mesenchymal stem cells. PPARγ mediates marrow mesenchymal stem cell differentiation into fat cells. PPARγ is a subtype of PPARs, which is a ligand-activated nuclear transcription factor that is involved in cell differentiation, growth and apoptosis In addition, PPARγ mediates the adipogenesis of marrow mesenchymal stem cells A higher expression of PPARγ protein in mice indicates a significant increase in bone turnover and lower osteogenic differentiation in marrow These data demonstrated that EGCG significantly inhibited PPARγ protein expression in secondary osteoporosis.
Runx2 gene expression is regulated by a variety of hormones, cytokines and endogenous active substances A number of signaling pathways have been shown to participate in the regulation of Runx2 expression or activity The Wnt-low-density lipoprotein receptor-related protein 5 LRP5 -β-catenin signaling pathway serves an important role in osteoblast proliferation and differentiation
Osteoporosis is anr type Diabetic foot care workshops systemic bone amd that is characterized by low bone mass, microstructural damage of the EGCG and bone health, increased EGCG and bone health fragility and Weight gain transformation bone fracture 1. Osteoporosis EGCG and bone health be divided into obne categories, EGCGG type and healtth type 2. The former is ECGG divided into postmenopausal type Isenile type II and idiopathic osteoporosis disease. The latter refers to osteoporosis induced by diseases that influence bone physiology or by drugs, such as osteoporosis induced by long-term high-dose glucocorticoid treatment 3. Due to the wide application of glucocorticoids in clinical practice, the incidence of glucocorticoid-induced osteoporosis has continuously risen in recent years, and as such, it is now considered the third most common type of osteoporosis, following postmenopausal and senile osteoporosis 4. The role of Wnt signaling in bone metabolism has become a key area of interest in recent years.EGCG and bone health -
This may take some time to load. Loading related content. Jump to main content. Jump to site search. You do not have JavaScript enabled. Please enable JavaScript to access the full features of the site or access our non-JavaScript page.
Issue 23, Epigallocatechin gallate alleviates osteoporosis by regulating the gut microbiota and serum metabolites in rats. cn b College of Bioscience and Biotechnology, Hunan Agricultural University, Changsha, Hunan , PR China c Hunan Engineering Laboratory for Pollution Control and Waste Utilization in Swine, Production, Changsha , PR China d Department of Cardiology, The Affiliated Wenling Hospital of Wenzhou Medical University The First People's Hospital of Wenling , Wenling , Zhejiang Province, China e Key Laboratory of Precision Medicine for Atherosclerotic Diseases of Zhejiang Province, Affiliated First Hospital of Ningbo University, Ningbo , China.
You have access to this article. Please wait while we load your content Something went wrong. Try again? Cited by. Download options Please wait Article type Paper. Submitted 07 Aug Accepted 23 Oct First published 01 Nov Download Citation.
Food Funct. In numerous cancer cells, it was shown that upregulated autophagy and increased ATP levels require for their survival, activity, and function [ 5 ].
OC is the highly specialized cells that rapidly undergo apoptosis when terminally differentiated [ 11 ]. Mitochondria is producing ATP via oxidative phosphorylation and is the key source of energy for OC differentiation.
Hence, OC markedly increases the mitochondrial functions during the remodeling to maintain bone homeostasis, and inhibition of autophagy causes apoptosis [ 12 ]. Thus, autophagy is an important mechanism in osteoclast differentiation and proves that autophagy and mitophagy are mutually co-related in the osteoclastogenesis process.
ROS also plays a critical role in bone resorption by inducing the differentiation of OC [ 15 ], whereas, scavenging of ROS prevents OC formation and also bone diseases [ 15 ], but the mechanisms remain unclear. Importantly, the activation of OC for bone resorption requires very high energy, to fulfill this requirement, a large number of mitochondria are needed in the OC for energy, and this process produces also ROS [ 14 ].
It was suggested that to reduce the side effect of hormonal therapy and some of pharmaceutical drugs, natural medicinal compounds might be useful for the treatment of patients with osteoporosis [ 16 , 17 ]. Tea is extracted from the leaves of Camellia sinensis is one of the most popular beverages consumed throughout the world.
Among those, EGCG gained much attention because of its anti-oxidative, antiviral, anti-inflammatory, anti-aging, anti-cancer, neuroprotective effects, and has been efficacious for various human diseases [ 19 , 20 , 21 , 22 ].
It has been reported that regular tea drinkers have a reduced risk of hip fractures and have a higher bone mineral density BMD [ 23 , 24 ]. However, the clear molecular mechanism and mode of action remain unclear, and herein, we report that EGCG inhibited the OC differentiation by regulating mitophagy and shed light on the mechanistic pathways.
Dose-dependent effect of EGCG on RAW When we performed the flow cytometry analysis using propidium iodide PI staining, no necrotic cells were found and the results resemble with MTT assays, and were shown in Fig.
Multinucleated cells were visible after differentiation of OC, which is the hallmark of differentiation, we found that the differentiated cells were multinucleated, and those cells were positive for TRAP Fig.
To determine the effect of EGCG on the OC differentiation, RAW We found that the EGCG significantly inhibited the number of TRAP-positive OC cells in a dose-dependent manner Fig. A TRAP staining of OC differentiated cells in the presence or absence of EGCG.
The dark purple color indicates the differentiated cells. B The percentage of TRAP-positive multinucleated OCs in each group is shown graphically.
C Quantitative real-time PCR analysis for the expression of NFATc1, CTSK, TRAP, and MMP9 during OC differentiation. D Western blot for protein expression of NFATc1, cathepsin K, TRAP, and MMP9 molecules. For qRT-PCR β-actin, and for western blot GAPDH was used as a loading control.
Separate sets of experiments for qRT-PCR and WB were performed to determine the effect of EGCG on the mRNA and protein expression of OC-related markers like NFATc1, Cathepsin K, TRAP, and MMP9.
The results obtained from the qRT-PCR, and western blot studies showed that EGCG significantly inhibited both the mRNA and the protein expressions of the OC-related markers Fig. The densitometric analysis for WB were shown in Fig.
To investigate the effect of EGCG on inflammation, we have performed the qRT-PCR for the various relevant pro-inflammatory and anti-inflammatory factors in the presence or absence of EGCG 50 μM after the osteoclastic OC differentiation.
We found that the EGCG downregulated the pro-inflammatory markers such as COX-2, IL-1β, IL6, MACP1, P65, and TNF-α, and upregulated the anti-inflammatory markers such as Arg1, IL-4R, IL, and Ym1 during the OC differentiation. The qRT-PCR data is shown in Fig. Next, we evaluate the effect of EGCG on intracellular and mitochondrial ROS formation during OC differentiation.
The RAW Results obtained from DCFDA and mitoSOX staining showed that during the OC differentiation intracellular and mitochondrial ROS formation was increased, and those parameters were significantly reduced after the addition of EGCG to the culture Fig.
A ROS generation was detected by DCFDA and mitoSOX staining. B Quantification graph for mean fluorescence intensity green for DCFDA and red for mitoSOX staining. C JC-1 staining for mitochondrial membrane potential measurement. D Quantification of membrane potential by measuring the ratio of red and green fluorescence intensity graph.
Seahorse flux analysis results are shown graphically. E Levels of oxygen consumption rate OCR , which is an indicator of mitochondrial respiration at basal, maximal respiration, proton leak, ATP production, and spare respiratory capacity conditions.
F Levels of extracellular acidification rate ECAR in the non-glycolytic acidification, glycolysis, glycolytic capacity, and glycolytic reserve. Mitochondria play an essential role in the survivability of cells, including the production of ATP, and the OC differentiation is the high energy ATP dependent process [ 25 ].
To determine the effect of EGCG on the mitochondrial membrane potential MMP during the OC differentiation, JC-1 staining was performed. Our results showed that during OC differentiation the MMP was increased, which was significantly reduced after treatment with EGCG Fig.
We performed the Seahorse analysis to determine the glycolysis and oxidative phosphorylation through oxygen consumption simultaneously in live cells during the course of OC differentiation. Our results revealed that during OC differentiation the oxygen consumption rate OCR was increased and after the addition of EGCG, OCR parameters were significantly decreased, which were represented by mitochondrial respiration at basal respiration, maximal respiration, proton leak, ATP production, and spare respiratory capacity Fig.
In separate sets of experiments, we also determined the effect of EGCG on the glycolytic stress during the OC differentiation by using Seahorse XF Glycolysis Stress Test Kit.
Data obtained from our experiments confirmed that mitochondrial metabolic functions were also affected during the OC differentiation, and EGCG played a key role in this process, reflected by the increased levels of extracellular acidification rate ECAR in the non-glycolytic acidification, glycolysis, glycolytic capacity, and glycolytic reserve during OC differentiation, which were significantly decreased after addition of EGCG Fig.
Autophagy acts as a critical regulator during OC differentiation [ 8 , 25 ]. Here, we determined the effect of EGCG on the expression of some important autophagic molecules such as ATG5, ATG7, Beclin1, and LC3B during the OC differentiation using a quantitative RT-PCR method. Our results showed that during the OC differentiation the mRNA expressions of ATG5, ATG7, Beclin1, and LC3B were increased several folds, and upon the addition of EGCG the autophagy markers were significantly decreased Fig.
A qRT-PCR for various gene expressions is presented graphically. B Representative IF staining images is presented. C Quantified mean intensity graphs for IF staining is presented graphically.
D WB images are shown for protein levels of ATG5, ATG7, Beclin1, LC3B, PINK1, PARKIN, DRP1, and FIS1 during the OC differentiation in the presence or absence of EGCG.
For qRT-PCR, β-actin, and WB, GAPDH was used as a loading control. In separate sets of experiments, we also determined the effect of EGCG by using immunofluorescence studies. We found that the addition of EGCG significantly inhibited the mean fluorescence intensity of the ATG5, ATG7, Beclin1, and LC3B expressions with respect to the OC differentiation alone Fig.
In other sets of experiments, we performed the WB analyses to determine the effect of EGCG on the protein levels of the above-mentioned autophagic molecules. These data also corroborated with the previous results that were obtained from the qRT-PCR and IF studies Fig.
The densitometric analyses of WB were shown in Fig. OC undergoes apoptosis when terminally differentiated, and requires high energies, possesses copious mitochondria [ 11 ].
Mitophagy is the process that maintains mitochondrial health and functions by the elimination of superfluous mitochondria [ 9 , 10 ]. We determined the expressions of PINK1 and PARKIN that were associated with mitophagy, and also determined the levels of DRP1, and FIS1 expressions that were associated with mitochondrial fission, and are responsible for the mitochondrial fragmentation during the OC differentiation.
First, we performed qRT-PCR studies to determine the effect of EGCG on the mRNA expressions of PINK1, PARKIN, DRP1, and FIS1. Our results showed that the mRNA expressions of PINK1, PARKIN, DRP1, and FIS1 were increased during the OC differentiation, and upon the addition of EGCG those mitophagy-related molecular expressions were significantly decreased Fig.
We next determined the effect of EGCG on mean fluorescence intensities of PINK1, PARKIN, DRP1, and FIS1 using IF studies. We found that EGCG inhibited the mean fluorescence intensity of the PINK1, PARKIN, DRP1, and FIS1 expressions compared to the OC differentiation alone Fig.
Next, in separate sets of experiments, we determined the effect of EGCG on PINK1, PARKIN, DRP1, and FIS1 molecules at the protein level, and our results corroborated with the results of qRT-PCR and IF studies Fig.
The densitometric analysis of western blots were shown in Fig. Our results indicated that in control cells there was no colocalization of PARKIN with the EEA1 molecule.
A Representative co-immunostaining images are presented for PINK1 and PARKIN with EEA1 or LAMP1. B Representative co-immunostaining images are presented for DRP1 and FIS1 with EEA1 or LAMP1. We further verified the colocalization of mitochondrial fission-related markers such as DRP1 and FIS1 using EEA1 and LAMP1.
We found that the DRP1 was not co-localized with the EEA1 in control cells, however, after OC differentiation DRP1 was distributed both in the early and late endosomes. The colocalization amount in early endosomes was relatively lower compared to the non-colocalization with EEA1. However, after the addition of EGCG, we found that the colocalization was similar to the control cells DRP1 was not trafficked to the early endosome and did not colocalize with the EEA1 Fig.
When co-stained DRP1 with LAMP1, it showed a clear colocalization in control cells, and in OC differentiated cells in the presence or absence of EGCG Fig. Trafficking distributions were observed with FIS1 were similar to the distributions of DRP1without or with differentiation, in the presence or absence of EGCG Fig.
The BM cells from all mice were isolated, cultured, and differentiated to OC using the differentiation media in the presence or absence of EGCG on coverslips inserted into wells of a 6-well plate. After differentiation, some of the coverslips were fixed, and the IF studies were performed for PINK1, PARKIN, DRP1, and FIS1, and the rest of the coverslips were used for live-cell imaging without any fixation to determine the ROS production and MMP levels by staining with DCFDA, mitoSOX, and JC-1, respectively.
B Measured ankle thickness during the course of arthritis development is shown graphically. C Representative IF staining images of PINK1, PARKIN, DRP1, and FIS1 were shown during the OC differentiation of with or without arthritis mice in the presence or absence of EGCG.
D Quantified mean intensity graph for IF staining shown. Similarly, JC-1 staining also showed that EGCG treatment significantly inhibited the MMP level Fig. In separate sets of experiments, we also determined the changes in the mitophagy markers using the primary BM cells, and our results showed that the expressions of PINK1, PARKIN1, DRP1, and FIS1 were increased during the OC differentiation with respect to the untreated conditions, and the enhanced expressions of these molecules were reduced upon addition of the EGCG to the culture Fig.
This result corroborates our in vitro results however, the level of differentiation number of multinucleated cells and number of nuclei per differentiated cells was much lower in primary cells compared to the in vitro cells.
During the analysis of signaling pathways, we found that the activation of AKT and p38MAPK pathways were the major pathways involved in OC differentiation. We have determined the effect of EGCG on those pathways. Our results showed that during osteoclastogenesis the phosphorylation of AKT and p38MAPK were remarkably increased, and those activations were inhibited upon the addition of EGCG, however, EGCG did not show any discernable changes in the levels of total proteins Fig.
In our previous work, we showed an increased level of SETD2, an epigenetic regulator is involved in the regulation of OC differentiation [ 26 ].
Therefore, in this study, we also determined the effect of EGCG on the mRNA expression and protein level of SETD2 and found that the EGCG inhibited both the mRNA expression and protein level of the SETD2 molecule Fig.
The densitometric results for the western blots were shown in Fig. A Western blot images are shown to evaluate the effect of EGCG in the AKT and p38MAPK signaling pathways.
B qRT-PCR for determining the effect of EGCG on the expression of the SETD2 gene. C Western blot image showing SETD2 protein expression level. For qRT-PCRβ-actin, and for WB, GAPDH was used as a loading control.
D 3D surface representation of ECGC-RANK-RANKL complex. E 3D ribbon representation of ECGC-RANK complex. F 2D representation of EGCG-RANK complex.
Blue, red, and green color represent EGCG, RANKL, and RANK, respectively. To define the binding of EGCG with the RANK, molecular docking analysis was performed using in silico analysis methods. The in silico analysis results demonstrated that the EGCG is exactly bound with the extracellular domain residues of RANK in the CRD cysteine-rich domain 2 and CRD3 region Fig.
The interaction energy profiles were summarized in Fig. S8A, B. It was observed that the EGCG mainly blocked RANKL interaction with RANK by establishing the hydrogen bond with Ser amino acid residue and Van der Waal interaction with Glu of RANK, as these amino acids usually interacted with Lys of RANKL.
Interestingly, EGCG also formed six hydrogen bonds, seven Van der Waal interactions, two sulfur bonds, and some other pi-pi interactions with RANK.
The amino acid residues of RANK involved in specific interactions were summarized in Fig. S8C, D. A higher number of hydrogen bonds revealed a strong binding affinity of EGCG to RANK. So, EGCG might compete with RANKL under physiological conditions to block RANK-RANKL signaling.
With this blockade, EGCG inhibited the OC differentiation process of myeloid cells. Excessive OC differentiation causes an imbalance in bone resorption and that helps in the development of bone-related diseases like arthritis and osteoporosis.
Well-known hormonal and pharmaceutical drugs such as estrogen, calcitonin, bisphosphonates, denosumab, etc. Recently, several functional foods, natural medicinal plant extract, and their derived compounds such as polyphenols, alkaloids, flavonoids, and polysaccharides were the largest targets, and gain focus to develop new therapies for several diseases because of their anti-inflammatory, antiproliferative, antiangiogenic, antioxidant, and specially bioavailability and nontoxic properties [ 28 , 29 , 30 , 31 ].
Our goal is to verify one of those natural products whether has the potential to minimize the differentiation of osteoclasts and if so, to find out what are the mechanisms by which that compound mediates the functions to develop an effective future therapeutic for those pathological conditions.
EGCG, tea catechin is well-known for its various biological functions and is beneficial for human health including bone physiology [ 19 , 20 , 21 , 22 , 32 , 33 ].
Previous studies have shown that EGCG plays an important role in the death of OC [ 34 , 35 ], however, the molecular mechanism is not yet clear. Therefore, in this study, we determined the effect of EGCG in the OC differentiation using RAW Our TRAP staining results showed that EGCG inhibits the OC differentiation in a concentration-dependent manner Fig.
The activated OC induces the degradation of bone matrix by secreting protons, and lysosomal proteases like the TRAP, MMP9, and Cathepsin K [ 36 ]. To confirm the results obtained from TRAP staining, and the previous findings, we determined the effect of EGCG on the expression of molecules such as NFATc1, TRAP, MMP9, and Cathepsin K.
Our results showed that EGCG inhibits significantly both protein and mRNA expression of the NFATc1, Cathepsin K, MMP9, and TRAP molecules Fig. Evidence supports that the mitochondria play a key role in the intracellular signaling for the regulation of immune response, as well as maintaining the OC differentiation and maturation [ 25 , 38 ].
Increased mitochondrial activation and ATP supply are essential for OC differentiation. Mitochondria is the major source of ATP production, it also produces ROS as a by-product, which is harmful to the cells [ 39 ]. Previous findings revealed that ROS production generates oxidative stress, promotes OC differentiation [ 40 ], and damages mitochondrial function [ 39 ].
In this study we determined the effect of EGCG in the following aspects; i intracellular and mitochondrial ROS production; ii mitochondrial membrane potential; and iii mitochondrial function during osteoclastogenesis, which are crucial for bone remodeling and maintaining homeostasis.
To determine the effect of EGCG on the intracellular and mitochondrial ROS production, we performed the DCFDA and mitoSOX staining in cell line and primary BM cells, and our results showed that the EGCG inhibited the intracellular and mitochondrial ROS production in both cells, and EGCG acts as an antioxidant during the OC differentiation Fig.
Next, we determined the effect of EGCG on the MMP during the OC differentiation, and our results showed that MMP increased during the OC differentiation, which was reduced upon treatment with EGCG Fig. In addition, we also performed the Seahorse flux analysis to determine the effect of EGCG on the mitochondrial glycolytic and non-glycolytic stress, and also the ATP production.
Our result showed that during OC differentiation ATP production was increased, which was controlled by the EGCG and also improved the overall mitochondrial functions Fig. Autophagy is an intracellular self-degradation process, which breaks and recycles the intracellular organelles and proteins, plays a critical role in the maintenance of intracellular metabolism and homeostasis, through the lysosomal machinery, and dysregulation of this process induces the pathogenesis of various diseases like cancer, infections, heart, and neurodegeneration [ 4 , 5 , 6 , 7 ].
Our previous studies showed that autophagy was enhanced, and autophagic activity positively correlated with OC differentiation [ 8 ]. In this present study, we have shown the expression of some autophagic molecules such as ATG5, ATG7, Beclin1, and LC3B during OC differentiation by qRT-PCR, IF, and WB.
Our results revealed that during the OC differentiation the mRNA and protein expression of the autophagic molecules were increased, which was decreased with the EGCG. This result confirmed that EGCG inhibited OC formation by inhibiting autophagy Fig.
The relationship between ROS-mediated signaling and autophagy is very complex, and depends on the cell type and disease conditions. A previous study has shown that the mitochondrial function during OC differentiation was controlled by autophagy [ 25 ].
Mitophagy, a specialized form of autophagy, is another important cellular mechanism that selectively crumbles the copious mitochondria and helps mitochondrial metabolism to maintain mitochondrial health and functions [ 9 , 10 ].
Small GTPase gathers in the outer mitochondrial membrane and induces mitophagy to supply ATP from the mitochondria upon increased oxidative phosphorylation [ 41 ].
Increased oxidative phosphorylation in the mitochondria generates enormous amounts of ROS and induces mitochondrial damage that critically controls the OC differentiation process.
In addition to increased ATP production, autophagy also maintains mitochondrial function via mitophagy. In mammalian cells, the most studied mitophagy-associated proteins are PTEN-induced putative kinase 1 PINK1 , and PARKIN [ 42 ]. It was also reported that the downregulation of PARKIN was associated with osteogenic differentiation of adipose-derived mesenchymal stem cells MSCs and affected the expression of bone morphogenetic protein 2 and collagen 1 [ 45 ].
We found that the PINK1 and PARKIN expression was increased during the OC differentiation, which was minimized upon the addition of EGCG Fig. Mitochondrial mass and mitochondrial homeostasis are regulated by the fusion and fission processes.
Mitochondrial integration depends on fusion, whereas fission enables the fragmentation of mitochondria. The Dynamin family of GTPases, particularly DRP1 is involved in controlling mitochondrial fission and promoting the fragmentation of mitochondria, which is very important for several pathophysiological conditions [ 46 ].
Fission protein 1 FIS1 , a mitochondrial fission protein is an important component for the regulation of apoptotic, and mitophagy pathways [ 47 ]. In this study, we showed that the DRP1 and FIS1 expressions were increased during OC differentiation, which was reduced after the addition of EGCG Fig.
It is already known that RANK binds to the RANKL to initiate the myriad of downstream signaling pathways and regulates the osteoclastogenesis process. In this study, we also determined the effect of EGCG on the signaling molecules associated with OC differentiation. Our results showed that the phosphorylation of AKT and p38MAPK significantly increased during the OC differentiation, but upon addition of EGCG inhibited the phosphorylation of AKT and p38MAPK without any remarkable changes in the total amounts Fig.
In this study, we found that the EGCG inhibited the mRNA and protein expressions of SETD2 Fig. More precisely, EGCG exactly binds with the extracellular domain residues of RANK in the CRD2 and CRD3 regions, which are particularly involved in RANKL interaction Fig.
In sum, we showed that the EGCG inhibited the OC differentiation by reducing the intracellular and mitochondrial ROS production, alteration in the autophagy-dependent mitochondrial functions, mitochondrial membrane potential, and mitophagy-related molecular expressions. Additionally, we showed that the EGCG inhibited the AKT, p38MAPK signaling pathways, and inhibited the epigenetic regulator, SETD2 molecule.
We also showed how the EGCG inhibited the RANK and RANKL binding, which is essential for OC differentiation.
Overall, these findings will help in the development of a new therapeutic target in near future for treating destructive bone diseases. Soluble s RANKL —11 and MCSF —02 were obtained from Pepro Tech Incorporation. BCA protein assay kit , propidium iodide p , JC-1 Dye T , mitoSOX red compound M , TRAP PA antibody were the product of Thermo Fischer Scientific.
PVDF membrane Bio-Rad Incorporation, Enhanced chemiluminescence ECL, RPN Amersham Pharmacia Biotechnology. High-Capacity RNA-to-cDNA Kit , SYBR Green PCR Kit , FIS1 AP antibody were the product of Applied Biosystems. To perform the osteoclastic differentiation, RAW The cell viability was measured by MTT assay to determine the effect of EGCG.
In short RAW The absorbance was measured using a microplate reader Synergy 2, BioTeK Instruments Inc, Winooski, USA. All the experiments were performed in triplicate. Flow cytometry was carried out to further investigate the effect of EGCG on the viability of RAW In short, RAW After that, the cells were washed and analyzed by using a FACSVerse flow cytometer BD Biosciences.
At least 10, events were acquired for each sample for analysis using BD FACSuite software BD Biosciences. Briefly, RAW Mu S, Guo S, Wang X, Zhan Y, Li Y, Jiang Y, Zhang R, Zhang B. Effects of deferoxamine on the osteogenic differentiation of human periodontal ligament cells. Mol Med Rep.
Norgaard R, Kassem M, Rattan SI. Heat shock-induced enhancement of osteoblastic differentiation of hTERT-immortalized mesenchymal stem cells. Ann N Acad Sci. Daga D, Mehrotra D, Mohammad S, Singh G, Natu SM.
Tentpole technique for bone regeneration in vertically deficient alveolar ridges:a review. J Oral Biol Craniofac Res. Jin P, Wu HY, Xu GJ, Zheng L, Zhao JM. Epigallocatechingallate EGCG as a pro-osteogenic agent to enhance osteogenic differentiation of mesenchymal stem cells from human bone marrow: an in vitro study.
Cell Tissue Res. Mah YJ, Song JS, Kim SO, Lee JH, Jeon M, Jung UW, Moon SJ, Kim JH, Choi HJ. The effect of epigallocatechingallate EGCG on human alveolar bone cells both in vitro and in vivo.
Jung IH, Lee DE, Yun JH, Cho AR, Kim CS, You YJ, Kim SJ, Choi SH. J Periodontal Implant Sci. Chu CY, Deng J, Man Y, Qu YL. Green tea extracts Epigallocatechingallate for different treatments. Biomed Res Int.
Weisburg JH, Weissman DB, Sedaghat T, Babich H. In vitro cytotoxicity of epigallocatechin gallate and tea extracts to cancerous and normal cells from the human oral cavity.
Basic Clin Pharmacol Toxicol. Niu LN, Sun JQ, Li QH, Jiao K, Shen LJ, Wu D, Tay F, Chen JH. Intrafibrillar-silicified collagen scaffolds enhance the osteogenic capacity of human dental pulp stem cells.
J Dent. Braun J, Hack A, Weis-Klemm M, Conrad S, Treml S, Kohler K, Walliser U, Skutella T, Aicher WK. Evaluation of the osteogenic and chondrogenic differentiation capacities of equine adipose tissue-derived mesenchymal stem cells.
Am J Vet Res. Komori T. Regulation of bone development and extracellular matrix protein genes by RUNX2. Artigas N, Urena C, Rodriguez-Carballo E, Rosa JL, Ventura F.
Mitogen-activated protein kinase MAPK -regulated interactions between Osterix and Runx2 are critical for the transcriptional Osteogenic program. J Biol Chem.
Foster BL, Ao M, Salmon CR, Chavez MB, Kolli TN, Tran AB, Chu EY, Kantovitz KR, Yadav M, Narisawa S. Osteopontin regulates dentin and alveolar bone development and mineralization.
Bortoluzzi EA, Niu LN, Palani CD, El-Awady AR, Hammond BD, Pei DD, Tian FC, Cutler CW, Pashley DH, Tay FR. Cytotoxicity and osteogenic potential of silicate calcium cements as potential protective materials for pulpal revascularization.
Dent Mater. Liu S, Yang L, Mu S, Fu Q. Epigallocatechingallate ameliorates glucocorticoid-induced osteoporosis of rats in vivo and in vitro.
Front Pharmacol. Kawabata T, Tokuda H, Sakai G, Fujita K, Matsushima-Nishiwaki R, Otsuka T, Kozawa O. Biomed Rep. PubMed PubMed Central Google Scholar. Shen CL, Kwun IS, Wang S, Mo H, Chen L, Jenkins M, Brackee G, Chen CH, Chyu MC. Functions and mechanisms of green tea catechins in regulating bone remodeling.
Curr Drug Targets. Gao G, Cui X. Three-dimensional bioprinting in tissue engineering and regenerative medicine. Biotechnol Lett. Astashkina A, Mann B, Grainger DW. A critical evaluation of in vitro cell culture models for high-throughput drug screening and toxicity. Pharmacol Ther. Download references.
The authors declare that the funding bodies did not contribute to the design of the study, collection, analysis and interpretation of data or the writing of the manuscript. You can also search for this author in PubMed Google Scholar. DP designed and directed the experiments, and revised the whole manuscript thoroughly.
JL2 Jie Liu and YL performed most of the experiments and wrote the manuscript. JL1 Jin Liu and CJ participated in some of the experiments and wrote part of the manuscript.
YM performed the analysis for all of the results and revised the whole manuscript. All authors have given final approval of this version to be published. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
All authors have read and approved the final manuscript. Correspondence to Dandan Pei. All patients or their parents have signed the informed consent form. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is distributed under the terms of the Creative Commons Attribution 4. Reprints and permissions. Liu, J.
et al. Influence of epigallocatechingallate in promoting proliferation and osteogenic differentiation of human periodontal ligament cells. BMC Oral Health 19 , 73 Download citation.
Received : 05 November Accepted : 16 April Published : 02 May Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content.
Search all BMC articles Search. Download PDF. Abstract Background Epigallocatechingallate EGCG was recently proposed to have the potential to regulate bone metabolism, however, its influence on osteogenesis remains controversial.
Methods Cells were cultured in osteogenic medium and treated with EGCG at various concentrations. Background The periodontium, consisting of the alveolar bone, gingiva, periodontal ligament, and cementum, serves as a potent support for tooth stability, nutrition and resistance to stress during mastication movement.
EGCG treatment EGCG was purchased from Sigma-Aldrich St. ALP activity A Quantichrom Alkaline Phosphatase Assay Kit Nanjing Jiancheng Bioengineering Institute, Nanjing, China was used to evaluate the effects of EGCG on the intracellular ALP activity of hPDLCs.
Quantitative real-time polymerase chain reaction qRT-PCR QRT-PCR was used to detect the relative changes in the mRNA expression levels of runt-related transcription factor 2 RUNX2 , osteopontin OPN , osterix OSX and type I collagen COL1 which are recognized as markers of osteogenic differentiation.
Table 1 Primers for polymerase chain reaction Full size table. Results Cell viability and proliferation The effects of EGCG on cell proliferation, as determined by the CCK-8 assay, are shown in Fig.
Full size image. Discussion Using bone augmentation procedures to promote periodontal bone regeneration is the dominant strategy for reestablishing both function and aesthetics in patients with deficient alveolar bone [ 26 ].
Conclusions The present study demonstrated that EGCG might promote the osteogenesis of hPDLCs in a dose-dependent manner, suggesting a potential therapeutic role of EGCG as a beneficial supplement when treating patients with periodontal bone loss. References Kassebaum NJ, Smith AGC, Bernabe E, Fleming TD, Reynolds AE, Vos T, Murray CJL, Marcenes W, Collaborators GBDOH.
Article Google Scholar Han J, Menicanin D, Gronthos S, Bartold PM. Article Google Scholar Pastoriza S, Mesias M, Cabrera C, Rufian-Henares JA. Article Google Scholar Afzal M, Safer AM, Menon M.
Article Google Scholar Kushiyama M, Shimazaki Y, Murakami M, Yamashita Y. Article Google Scholar Hrishi TS, Kundapur PP, Naha A, Thomas BS, Kamath S, Bhat GS. Article Google Scholar Chava VK, Vedula BD.
Article Google Scholar Fournier-Larente J, Morin MP, Grenier D. Article Google Scholar Cai Y, Chen ZB, Liu H, Xuan Y, Wang XX, Luan QX.
Article Google Scholar Tominari T, Ichimaru R, Yoshinouchi S, Matsumoto C, Watanabe K, Hirata M, Grundler FMW, Inada M, Miyaura C.
Article Google Scholar Kaida K, Honda Y, Hashimoto Y, Tanaka M, Baba S. Article Google Scholar Chu C, Deng J, Hou Y, Xiang L, Wu Y, Qu Y, Man Y. Article Google Scholar Shen CL, Yeh JK, Cao JJ, Wang JS. Article Google Scholar Sims NA, Vrahnas C.
Article Google Scholar Oka Y, Iwai S, Amano H, Irie Y, Yatomi K, Ryu K, Yamada S, Inagaki K, Oguchi K. Article Google Scholar Nakagawa H, Wachi M, Woo JT, Kato M, Kasai S, Takahashi F, Lee IS, Nagai K. Article Google Scholar Nakagawa H, Hasumi K, Takami M, Aida-Hyugaji S, Woo JT, Nagai K, Ishikawa T, Wachi M.
Article Google Scholar Guo M, Qu H, Xu L, Shi DZ.
a Hezlth Orthopaedic Hydration tips for outdoor activities, The Affiliated Wenling Hospital of Wenzhou Medical University Bonee First People's Hospital of WenlingBooneZhejiang Province, EGGC E-mail: gangle. liu EGCG and bone health. b Healtn of EGCG and bone health and Biotechnology, Hunan Agricultural University, Changsha, HunanPR China. c Hunan Engineering Laboratory for Pollution Control and Waste Utilization in Swine, Production, ChangshaPR China. d Department of Cardiology, The Affiliated Wenling Hospital of Wenzhou Medical University The First People's Hospital of WenlingWenlingZhejiang Province, China. e Key Laboratory of Precision Medicine for Atherosclerotic Diseases of Zhejiang Province, Affiliated First Hospital of Ningbo University, NingboChina. The purpose of healtb study was Fat metabolism tips examine the effects of green tea extract GTE intake on bone bkne and physiological properties, such as bone EGCG and bone health, Antioxidant-rich beverages bone microarchitecture, cortical bon geometry, and bone EGCG and bone health strength, in growing rats. Healtb male Wistar rats EGCG and bone health divided into the following four eenoups: standard diet feeding for 85 days S-CON or days L-CONand GTE diet feeding for 85 days S-GTE or days L-GTE. There was no difference in all bone parameters between the S-CON and S-GTE groups. However, serum leptin levels were significantly lower in the L-GTE group than in the L-CON group. Thus, the long-term GTE intake had negative effects on bone, especially trabecular bone loss and microarchitecture mal-conformation, in growing rats. Minematsu A, Nishii Y, Imagita H, Sakata S Calcif.
Es ist die ausgezeichnete Idee
Sie sind nicht recht. Es ich kann beweisen. Schreiben Sie mir in PM, wir werden umgehen.
Das Ehrenwort.
Ich denke, dass Sie nicht recht sind. Geben Sie wir werden es besprechen. Schreiben Sie mir in PM, wir werden umgehen.
Als das Wort ist mehr es!