
Visceral fat and gut bacteria -
A person is considered obese if they have a body mass index BMI over However, BMI is an imprecise measure of obesity because it does not differentiate between different types of fat or lean mass that both exert effects on human health.
It is the accumulation of abdominal fat, and specifically deep visceral fat mass VFM in the abdominal cavity, that has the most detrimental consequences on health.
VFM accumulation is a major risk factor for cardiovascular and metabolic disease and cancer. Changing dietary habits and the corresponding shifts in the gut microbiota are contributors to the growing obesity pandemic. In recently published research scientists from the School of Life Course Sciences aimed to understand the role of gut bacteria in VFM accumulation, and how it relates to diet to better understand the relative contributions towards obesity.
Health Effects of Overweight and Obesity in Countries Over 25 Years. N Engl J Med 1 — doi: PubMed Abstract CrossRef Full Text Google Scholar.
Tremmel M, Gerdtham U-G, Nilsson PM, Saha S. Economic Burden of Obesity: A Systematic Literature Review. Int J Environ Res Public Health 14 4 Cornier M-A, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, et al.
The Metabolic Syndrome. Endocr Rev 29 7 — Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and Regional Diabetes Prevalence Estimates for and Projections for and Results From the International Diabetes Federation Diabetes Atlas, 9th Edition.
Diabetes Res Clin Pract Eizirik DL, Pasquali L, Cnop M. Pancreatic β-Cells in Type 1 and Type 2 Diabetes Mellitus: Different Pathways to Failure. Nat Rev Endocrinol 16 7 — Gastaldelli A, Miyazaki Y, Pettiti M, Matsuda M, Mahankali S, Santini E, et al. Metabolic Effects of Visceral Fat Accumulation in Type 2 Diabetes.
J Clin Endocrinol Metab 87 11 — Fan Y, Pedersen O. Gut Microbiota in Human Metabolic Health and Disease. Nat Rev Microbiol 19 1 — Lloyd-Price J, Abu-Ali G, Huttenhower C.
The Healthy Human Microbiome. Genome Med 8 1 Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, et al. Gut Microbiota Functions: Metabolism of Nutrients and Other Food Components.
Eur J Nutr 57 1 :1— Stephens RW, Arhire L, Covasa M. Gut Microbiota: From Microorganisms to Metabolic Organ Influencing Obesity. Obesity 26 5 —9.
Livingston EH. Lower Body Subcutaneous Fat Accumulation and Diabetes Mellitus Risk. Surg Obes Relat Dis 2 3 —8. Pereira MJ, Vranic M, Kamble PG, Jernow H, Kristófi R, Holbikova E, et al. CDKN2C Expression in Adipose Tissue Is Reduced in Type II Diabetes and Central Obesity: Impact on Adipocyte Differentiation and Lipid Storage?
Transl Res Lotta LA, Gulati P, Day FR, Payne F, Ongen H, van de Bunt M, et al. Integrative Genomic Analysis Implicates Limited Peripheral Adipose Storage Capacity in the Pathogenesis of Human Insulin Resistance.
Nat Genet 49 1 — Virtue S, Vidal-Puig A. Adipose Tissue Expandability, Lipotoxicity and the Metabolic Syndrome—An Allostatic Perspective. Biochim Biophys Acta 3 — Sattar N, Gill JM. Type 2 Diabetes as a Disease of Ectopic Fat? BMC Med 12 1 Frühbeck G. Intracellular Signalling Pathways Activated by Leptin.
Biochem J Pt 1 :7— Brown JEP, Dunmore SJ. Leptin Decreases Apoptosis and Alters BCL Bax Ratio in Clonal Rodent Pancreatic Beta-Cells. Diabetes Metab Res Rev 23 6 — Lee Y, Magkos F, Mantzoros CS, Kang ES.
Effects of Leptin and Adiponectin on Pancreatic β-Cell Function. Metabolism 60 12 — Okuya S, Tanabe K, Tanizawa Y, Oka Y. Leptin Increases the Viability of Isolated Rat Pancreatic Islets by Suppressing Apoptosis. Endocrinology 11 — Maedler K, Sergeev P, Ehses JA, Mathe Z, Bosco D, Berney T, et al.
Leptin Modulates Beta Cell Expression of IL-1 Receptor Antagonist and Release of IL-1beta in Human Islets. Proc Natl Acad Sci USA 21 — Maedler K, Schulthess FT, Bielman C, Berney T, Bonny C, Prentki M, et al. Glucose and Leptin Induce Apoptosis in Human β- Cells and Impair Glucose-Stimulated Insulin Secretion Through Activation of C-Jun N-Terminal Kinases.
FASEB J 22 6 — Bacha F, Saad R, Gungor N, Arslanian SA. Adiponectin in Youth: Relationship to Visceral Adiposity, Insulin Sensitivity, and Beta-Cell Function.
Diabetes Care 27 2 — Lo JC, Ljubicic S, Leibiger B, Kern M, Leibiger IB, Moede T, et al. Adipsin Is an Adipokine That Improves β Cell Function in Diabetes. Cell 1 — Cheng Q, Dong W, Qian L, Wu J, Peng Y.
J Mol Endocrinol 47 1 — Zhang D, Xie T, Leung PS. Irisin Ameliorates Glucolipotoxicity-Associated β-Cell Dysfunction and Apoptosis via AMPK Signaling and Anti-Inflammatory Actions. Cell Physiol Biochem 51 2 — Liu S, Du F, Li X, Wang M, Duan R, Zhang J, et al. Effects and Underlying Mechanisms of Irisin on the Proliferation and Apoptosis of Pancreatic β Cells.
PloS One 12 4 :e Pan X, Kaminga AC, Wen SW, Acheampong K, Liu A. Omentin-1 in Diabetes Mellitus: A Systematic Review and Meta-Analysis. PloS One 14 12 :e Feng J, Zhao H, Du M, Wu X. The Effect of Apelin on Pancreatic Islet Beta Cell Mass and Myocardial Fatty Acid and Glucose Metabolism of Experimental Type 2 Diabetic Rats.
Peptides —7. Guo L, Li Q, Wang W, Yu P, Pan H, Li P, et al. Apelin Inhibits Insulin Secretion in Pancreatic β-Cells by Activation of PI3-Kinase-Phosphodiesterase 3b. Endocr Res 34 4 — Nakata M, Okada T, Ozawa K, Yada T.
Resistin Induces Insulin Resistance in Pancreatic Islets to Impair Glucose-Induced Insulin Release. Biochem Biophys Res Commun 4 — Parkash J, Chaudhry MA, Rhoten WB. Tumor Necrosis Factor-Alpha-Induced Changes in Insulin-Producing Beta-Cells.
Anat Rec A Discov Mol Cell Evol Biol 2 — Shen X, Yang L, Yan S, Zheng H, Liang L, Cai X, et al. Fetuin A Promotes Lipotoxicity in β Cells Through the TLR4 Signaling Pathway and the Role of Pioglitazone in Anti-Lipotoxicity.
Mol Cell Endocrinol — Wang R, Hu W. Asprosin Promotes β-Cell Apoptosis by Inhibiting the Autophagy of β-Cell via AMPK-mTOR Pathway. J Cell Physiol 1 — Huang R, Bai X, Li X, Wang X, Zhao L.
Retinol-Binding Protein 4 Activates STRA6, Provoking Pancreatic β-Cell Dysfunction in Type 2 Diabetes. Diabetes 70 2 — Lafontan M, Langin D. Lipolysis and Lipid Mobilization in Human Adipose Tissue.
Prog Lipid Res 48 5 — Boden G. Effects of Free Fatty Acids FFA on Glucose Metabolism: Significance for Insulin Resistance and Type 2 Diabetes. Exp Clin Endocrinol Diabetes 3 —4.
Cen J, Sargsyan E, Bergsten P. Fatty Acids Stimulate Insulin Secretion From Human Pancreatic Islets at Fasting Glucose Concentrations via Mitochondria-Dependent and -Independent Mechanisms. Nutr Metab Lond 13 1 Eitel K, Staiger H, Rieger J, Mischak H, Brandhorst H, Brendel MD, et al.
Protein Kinase C Delta Activation and Translocation to the Nucleus Are Required for Fatty Acid-Induced Apoptosis of Insulin-Secreting Cells.
Diabetes 52 4 —7. El-Assaad W, Buteau J, Peyot M-L, Nolan C, Roduit R, Hardy S, et al. Saturated Fatty Acids Synergize With Elevated Glucose to Cause Pancreatic β-Cell Death.
Endocrinology 9 — Shimabukuro M, Zhou YT, Levi M, Unger RH. Fatty Acid-Induced Beta Cell Apoptosis: A Link Between Obesity and Diabetes. Proc Natl Acad Sci USA 95 5 — Yamashita T, Eto K, Okazaki Y, Yamashita S, Yamauchi T, Sekine N, et al.
Role of Uncoupling Protein-2 Up-Regulation and Triglyceride Accumulation in Impaired Glucose-Stimulated Insulin Secretion in a Beta-Cell Lipotoxicity Model Overexpressing Sterol Regulatory Element-Binding Protein-1c. Endocrinology 8 — Kochumon S, Al Madhoun A, Al-Rashed F, Thomas R, Sindhu S, Al-Ozairi E, et al.
Elevated Adipose Tissue Associated IL-2 Expression in Obesity Correlates With Metabolic Inflammation and Insulin Resistance. Sci Rep 10 1 Daniele G, Guardado Mendoza R, Winnier D, Fiorentino TV, Pengou Z, Cornell J, et al.
The Inflammatory Status Score Including IL-6, TNF-α, Osteopontin, Fractalkine, MCP-1 and Adiponectin Underlies Whole-Body Insulin Resistance and Hyperglycemia in Type 2 Diabetes Mellitus. Acta Diabetol 51 1 — Rebuffat SA, Sidot E, Guzman C, Azay-Milhau J, Jover B, Lajoix A-D, et al. Adipose Tissue Derived-Factors Impaired Pancreatic β-Cell Function in Diabetes.
Biochim Biophys Acta - Mol Basis Dis 10 — Gerst F, Wagner R, Kaiser G, Panse M, Heni M, Machann J, et al. Metabolic Crosstalk Between Fatty Pancreas and Fatty Liver: Effects on Local Inflammation and Insulin Secretion.
Diabetologia 60 11 — Olefsky JM, Glass CK. Macrophages, Inflammation, and Insulin Resistance. Annu Rev Physiol — Ying W, Riopel M, Bandyopadhyay G, Dong Y, Birmingham A, Seo JB, et al. Adipose Tissue Macrophage-Derived Exosomal miRNAs Can Modulate In Vivo and In Vitro Insulin Sensitivity.
Cell 2 — Gao H, Luo Z, Jin Z, Ji Y, Ying W. Adipose Tissue Macrophages Modulate Obesity-Associated β Cell Adaptations Through Secreted miRNA-Containing Extracellular Vesicles. Cells 10 9 Gesmundo I, Pardini B, Gargantini E, Gamba G, Birolo G, Fanciulli A, et al.
Adipocyte-Derived Extracellular Vesicles Regulate Survival and Function of Pancreatic β Cells. JCI Insight 6 5 :e Ying W, Wollam J, Ofrecio JM, Bandyopadhyay G, El Ouarrat D, Lee YS, et al.
J Clin Invest 3 — Carruthers NJ, Strieder-Barboza C, Caruso JA, Flesher CG, Baker NA, Kerk SA, et al. The Human Type 2 Diabetes-Specific Visceral Adipose Tissue Proteome and Transcriptome in Obesity.
Sci Rep 11 1 Drareni K, Ballaire R, Alzaid F, Goncalves A, Chollet C, Barilla S, et al. Adipocyte Reprogramming by the Transcriptional Coregulator GPS2 Impacts Beta Cell Insulin Secretion. Cell Rep 32 11 He F, Huang Y, Song Z, Zhou HJ, Zhang H, Perry RJ, et al.
Mitophagy-Mediated Adipose Inflammation Contributes to Type 2 Diabetes With Hepatic Insulin Resistance. J Exp Med 3 :e doi: Cignarelli A, Genchi VA, Perrini S, Natalicchio A, Laviola L, Giorgino F.
Insulin and Insulin Receptors in Adipose Tissue Development. Int J Mol Sci 20 3 Hudish LI, Reusch JE, Sussel L. β Cell Dysfunction During Progression of Metabolic Syndrome to Type 2 Diabetes.
J Clin Invest 10 —8. Pedersen DJ, Guilherme A, Danai LV, Heyda L, Matevossian A, Cohen J, et al. A Major Role of Insulin in Promoting Obesity-Associated Adipose Tissue Inflammation. Mol Metab 4 7 — Kumar D, Shankar K, Patel S, Gupta A, Varshney S, Gupta S, et al.
Chronic Hyperinsulinemia Promotes Meta-Inflammation and Extracellular Matrix Deposition in Adipose Tissue: Implications of Nitric Oxide. Krogh-Madsen R, Plomgaard P, Keller P, Keller C, Pedersen BK.
Insulin Stimulates Interleukin-6 and Tumor Necrosis Factor-α Gene Expression in Human Subcutaneous Adipose Tissue. Am J Physiol Metab 2 :E—8. CrossRef Full Text Google Scholar. Li Q, Hagberg CE, Silva Cascales H, Lang S, Hyvönen MT, Salehzadeh F, et al.
Obesity and Hyperinsulinemia Drive Adipocytes to Activate a Cell Cycle Program and Senesce. Nat Med 27 11 — Scheithauer TPM, Rampanelli E, Nieuwdorp M, Vallance BA, Verchere CB, van Raalte DH, et al.
Gut Microbiota as a Trigger for Metabolic Inflammation in Obesity and Type 2 Diabetes. Front Immunol Kinashi Y, Hase K.
Partners in Leaky Gut Syndrome: Intestinal Dysbiosis and Autoimmunity. Fasano A. Zonulin and Its Regulation of Intestinal Barrier Function: The Biological Door to Inflammation, Autoimmunity, and Cancer.
Physiol Rev 91 1 — Jayashree B, Bibin YS, Prabhu D, Shanthirani CS, Gokulakrishnan K, Lakshmi BS, et al. Increased Circulatory Levels of Lipopolysaccharide LPS and Zonulin Signify Novel Biomarkers of Proinflammation in Patients With Type 2 Diabetes.
Mol Cell Biochem 1—2 — Moreno-Navarrete JM, Sabater M, Ortega F, Ricart W, Fernández-Real JM. Circulating Zonulin, a Marker of Intestinal Permeability, Is Increased in Association With Obesity-Associated Insulin Resistance.
PloS One 7 5 :e Sturgeon C, Fasano A. Zonulin, a Regulator of Epithelial and Endothelial Barrier Functions, and Its Involvement in Chronic Inflammatory Diseases. Tissue Barriers 4 4 :e To add to the complexity, our genes can also impact the microbiome — indirectly contributing to obesity.
The researchers describe several microbes and human genes that play a role in higher visceral fat. Their exact mechanisms still have to be discovered, but understanding them could help develop new solutions for obesity — joining the first wave of microbiome-based therapies already fighting for approval.
Skip to content. Search for:. Suggested Topics:. Figure 1.
This Visceral fat and gut bacteria could also help Vegan athlete cookbook the inheritability of BCAAs for women disease. The composition of the Visderal has been linked to things as seemingly distant Antispasmodic Relief for Abdominal Cramps growth in malnutrition conditions. Anx, one of the bacteriz areas of interest is a bit more obvious — what role does gut microbiota play in obesity? This belly fat carries a higher risk of metabolic diseases — such as type 2 diabetes. Published in Genome Biologythe study looked at data from 1, twins from the biobank TwinsUK. It found that people with a more diverse range of gut microbes had lower levels of visceral fat. Some probiotic strains may help support weight loss and Visceral fat and gut bacteria other health Viceral, including to your heart, bacgeria Antispasmodic Relief for Abdominal Cramps, and Vusceral system. Probiotics are live microorganisms that have health benefits bactedia eaten faf. Probiotics may improve your immune function and digestive and heart health, among other benefits 234567. Hundreds of microorganisms reside in your digestive system. The majority of these are friendly bacteria that produce several important nutrients, including vitamin K and certain B vitamins. There are two main families of good bacteria in the gut: bacteroidetes and firmicutes. Body weight seems to be related to the balance of these two families of bacteria 9 ,By Claudia Health and environmental impact assessment. For the 35 percent of American adults who do daily battle with obesity, the main causes of their condition are all Visceral fat and gut bacteria vut an bzcteria diet, fay sedentary baacteria and perhaps some unlucky genes.
Bacteri recent years, however, bactria have become increasingly convinced that bactria hidden players literally lurk but human abcteria billions on billions Fat-burning exercises for athletes gut microbes.
Throughout wnd evolutionary history, Antispasmodic Relief for Abdominal Cramps microscopic denizens of our Viscdral have helped us break down tough plant fibers in exchange Viscreal the Visceeal of bacterix in Vsceral a fah broth.
Yet their roles appear to extend beyond digestion. New evidence Visceral fat and gut bacteria that gut bacteria alter the bavteria we store fat, Arthritis joint health we balance levels of glucose Antispasmodic Relief for Abdominal Cramps fta blood, gu how we respond to hormones Vixceral make us feel fut or full.
The wrong mix of Viwceral, it seems, can abcteria set the stage for obesity and diabetes from the moment of birth. Fortunately, faf are beginning to understand the differences between the wrong mix and a healthy one, as bwcteria as the specific factors Viscral shape Visecral differences.
Viscerwl hope bxcteria learn how to cultivate this inner ecosystem gjt ways Greek yogurt ice cream could prevent—and Natural weight loss strategies treat—obesity, fag doctors define as gat a particular ratio of height Visceeal weight, bactria as the tut mass index, wnd is greater than Vidceral, for example, foods, baby formulas or supplements devised faf promote virtuous microbes baxteria suppressing the bavteria types.
Keeping our gut microbes happy could be the elusive secret to weight control. If Antispasmodic Relief for Abdominal Cramps enjoying Vieceral article, consider fatt our award-winning journalism by Tooth enamel. By purchasing a VVisceral you are helping Vsiceral ensure the future of Viaceral stories about Viwceral discoveries and ideas shaping our fag today.
An Inner Rain Forest Researchers have long known that the human bactteria is home to all manner of microorganisms, but guy in the gyt decade Diabetic ketoacidosis so have they come Viscdral realize that Viscerl microbes outnumber our own cells 10 to one.
Each of us begins to bactefia a unique congregation of microbes the moment bacterai pass Vizceral the amd canal, Physical exertion replenishment our abcteria bacteria bacterla and continuing to gather new members from the environment throughout life.
By studying Improved overall well-being genes of aand various microbes—collectively Vlsceral to as Greek yogurt for digestion microbiome—investigators have identified many Bacetria the most Viscefal residents, although these can vary Vusceral from person to person and Visderal different Antispasmodic Relief for Abdominal Cramps populations.
In recent years researchers have begun bacteira Visceral fat and gut bacteria from mere census taking bacteriia determining bactegia kind of Viscefal these minute inhabitants fill Viscceral the human body and the effect they have on our overall health.
An early hint that gut annd might play a role in obesity bactreia from studies comparing intestinal bacteria in wnd and Vieceral individuals. In Viceral of twins who were Increase physical endurance lean or both obese, researchers found that the gut community in Elderberry syrup for sore throat people was like a rain forest brimming Dental crowns many species but bbacteria the community in Visveral people was less diverse—more like a nutrient-overloaded gyt where relatively few Viscreal dominate.
Lean individuals, for example, bateria to Increase athletic agility a wider variety of Bacteroidetes, a large tribe of microbes that specialize in breaking Immune-boosting gut flora bulky plant starches and fibers into shorter molecules that the body can use as a source of energy.
Documenting such differences does not mean the discrepancies are responsible for obesity, however. To demonstrate cause and effect, Gordon and his colleagues conducted an elegant series of experiments with so-called humanized mice, published last September in Science.
First, they raised genetically identical baby rodents in a germ-free environment so that their bodies would be free of any bacteria. Then they populated their guts with intestinal microbes collected from obese women and their lean twin sisters three pairs of fraternal female twins and one set of identical twins were used in the studies.
The mice ate the same diet in equal amounts, yet the animals that received bacteria from an obese twin grew heavier and had more body fat than mice with microbes from a thin twin. As expected, the fat mice also had a less diverse community of microbes in the gut.
Gordon's team then repeated the experiment with one small twist: after giving the baby mice microbes from their respective twins, they moved the animals into a shared cage. This time both groups remained lean. Studies showed that the mice carrying microbes from the obese human had picked up some of their lean roommates' gut bacteria—especially varieties of Bacteroidetes—probably by consuming their feces, a typical, if unappealing, mouse behavior.
To further prove the point, the researchers transferred 54 varieties of bacteria from some lean mice to those with the obese-type community of germs and found that the animals that had been destined to become obese developed a healthy weight instead.
Transferring just 39 strains did not do the trick. His studies, as well as those by other researchers, offer enticing clues about what those roles might be. Compared with the thin mice, for example, Gordon's fat mice had higher levels in their blood and muscles of substances known as branched-chain amino acids and acylcarnitines.
Both these chemicals are typically elevated in people with obesity and type 2 diabetes. Another job vacancy associated with obesity might be one normally filled by a stomach bacterium called Helicobacter pylori. Research by Martin Blaser of New York University suggests that it helps to regulate appetite by modulating levels of ghrelin—a hunger-stimulating hormone.
pylori was once abundant in the American digestive tract but is now rare, thanks to more hygienic living conditions and the use of antibiotics, says Blaser, author of a new book entitled Missing Microbes. Diet is an important factor in shaping the gut ecosystem. A diet of highly processed foods, for example, has been linked to a less diverse gut community in people.
Gordon's team demonstrated the complex interaction among food, microbes and body weight by feeding their humanized mice a specially prepared unhealthy chow that was high in fat and low in fruits, vegetables and fiber as opposed to the usual high-fiber, low-fat mouse kibble.
The unhealthy diet somehow prevented the virtuous bacteria from moving in and flourishing. The interaction between diet and gut bacteria can predispose us to obesity from the day we are born, as can the mode by which we enter the world. Studies have shown that both formula-fed babies and infants delivered by cesarean section have a higher risk for obesity and diabetes than those who are breast-fed or delivered vaginally.
Working together, Rob Knight of the University of Colorado Boulder and Maria Gloria Dominguez-Bello of N. have found that as newborns traverse the birth canal, they swallow bacteria that will later help them digest milk.
C-section babies skip this bacterial baptism. Babies raised on formula face a different disadvantage: they do not get substances in breast milk that nurture beneficial bacteria and limit colonization by harmful ones.
According to a recent Canadian study, babies drinking formula have bacteria in their gut that are not seen in breast-fed babies until solid foods are introduced. Their presence before the gut and immune system are mature, says Dominguez-Bello, may be one reason these babies are more susceptible to allergies, asthma, eczema and celiac disease, as well as obesity.
A new appreciation for the impact of gut microbes on body weight has intensified concerns about the profligate use of antibiotics in children.
Blaser has shown that when young mice are given low doses of antibiotics, similar to what farmers give livestock, they develop about 15 percent more body fat than mice that are not given such drugs.
Antibiotics may annihilate some of the bacteria that help us maintain a healthy body weight. If you have a fire in a forest that is new, you get extinction.
He notes that antibiotic use varies greatly from state to state in the U. Beyond Probiotics Many scientists who work on the microbiome think their research will inspire a new generation of tools to treat and prevent obesity. Still, researchers are quick to point out that this is a young field with far more questions than answers.
Even in a homogeneous population such as the Amish, she says, there is vast individual variation that makes it difficult to isolate the role of microbiota in a complex disease like obesity. Even so, a number of scientists are actively developing potential treatments.
Dominguez-Bello, for example, is conducting a clinical trial in Puerto Rico in which babies born by cesarean section are immediately swabbed with a gauze cloth laced with the mother's vaginal fluids and resident microbes.
She will track the weight and overall health of the infants in her study, comparing them with C-section babies who did not receive the gauze treatment. A group in Amsterdam, meanwhile, is investigating whether transferring feces from lean to overweight people will lead to weight loss.
A more promising approach, says Robert Karp, who oversees National Institutes of Health grants related to obesity and the microbiome, is to identify the precise strains of bacteria associated with leanness, determine their roles and develop treatments accordingly. Gordon has proposed enriching foods with beneficial bacteria and any nutrients needed to establish them in the gut—a science-based version of today's probiotic yogurts.
No one in the field believes that probiotics alone will win the war on obesity, but it seems that, along with exercising and eating right, we need to enlist our inner microbial army.
June 1, 7 min read. June Issue. On supporting science journalism If you're enjoying this article, consider supporting our award-winning journalism by subscribing.
: Visceral fat and gut bacteria| Belly fat: gut bacteria checks could lead to personalised diets | Second, people with less bacterial diversity in their gut had a higher proportion of visceral fat—confirming, in other words, the finding that obesity is correlated with having less diverse gut bacteria. But a study in mice hints that it might be the case. In that study, researchers bred mice in bacteria-free environments. Then one mouse got gut bacteria implanted from those of an obese woman, while the other mouse got bacteria from her lean twin sister. Even though the mice were fed the exact same diet, the first mouse became obese while the other remained lean. The most promising treatment would be fecal transplants , to populate their guts with bacteria from lean people. Trials of such treatments have run into some problems recently. But with obesity at epidemic proportions in a growing number of countries, it may be a technique worth pursuing. Our free, fast, and fun briefing on the global economy, delivered every weekday morning. The A. Club Deadspin Gizmodo Jalopnik Kotaku Quartz The Root The Takeout The Onion The Inventory. Support Quartz Journalism Support Us. Search Free Newsletters. King's College London provides funding as a member of The Conversation UK. Public health policy has mainly focused on diet to reverse these rising rates, but the impact of these policies has been limited. The latest science suggests why this strategy is failing: one diet does not fit all. Dietary advice needs to be personalised. The reason one diet does not suit all may be found in our guts. Our previous research showed that microbes in the digestive track, known as the gut microbiota, are linked to the accumulation of belly fat. Our gut microbiota is mostly determined by what we eat, our lifestyle and our health. So it is difficult to know exactly how food and gut microbes together influence fat accumulation and ultimately disease risk. Our latest study provides new insights into these interactions. Animal studies have been valuable in showing that gut microbes alone can reduce the build-up of fat , resulting in better health. But translating these findings to humans is difficult, especially considering that we can eat very different foods. In our study, we aimed to disentangle the effect of gut microbes and diet on the accumulation of belly fat in 1, twins from the UK. We found that the composition of the gut microbiota predicts belly fat more accurately than diet alone. We identified a few specific nutrients and microbes that were bad for us and linked to an increase in belly fat, as well as a few nutrients and many microbes that were good for us and linked to reduced belly fat. The observed link between belly fat and bad nutrients, such as cholesterol, was not affected by the gut microbiota. In contrast, we found that the gut microbiota plays an important role in the beneficial effect of good nutrients, such as fibre or vitamin E. We show that specific gut bacteria play an important role in linking certain beneficial nutrients to less belly fat. |
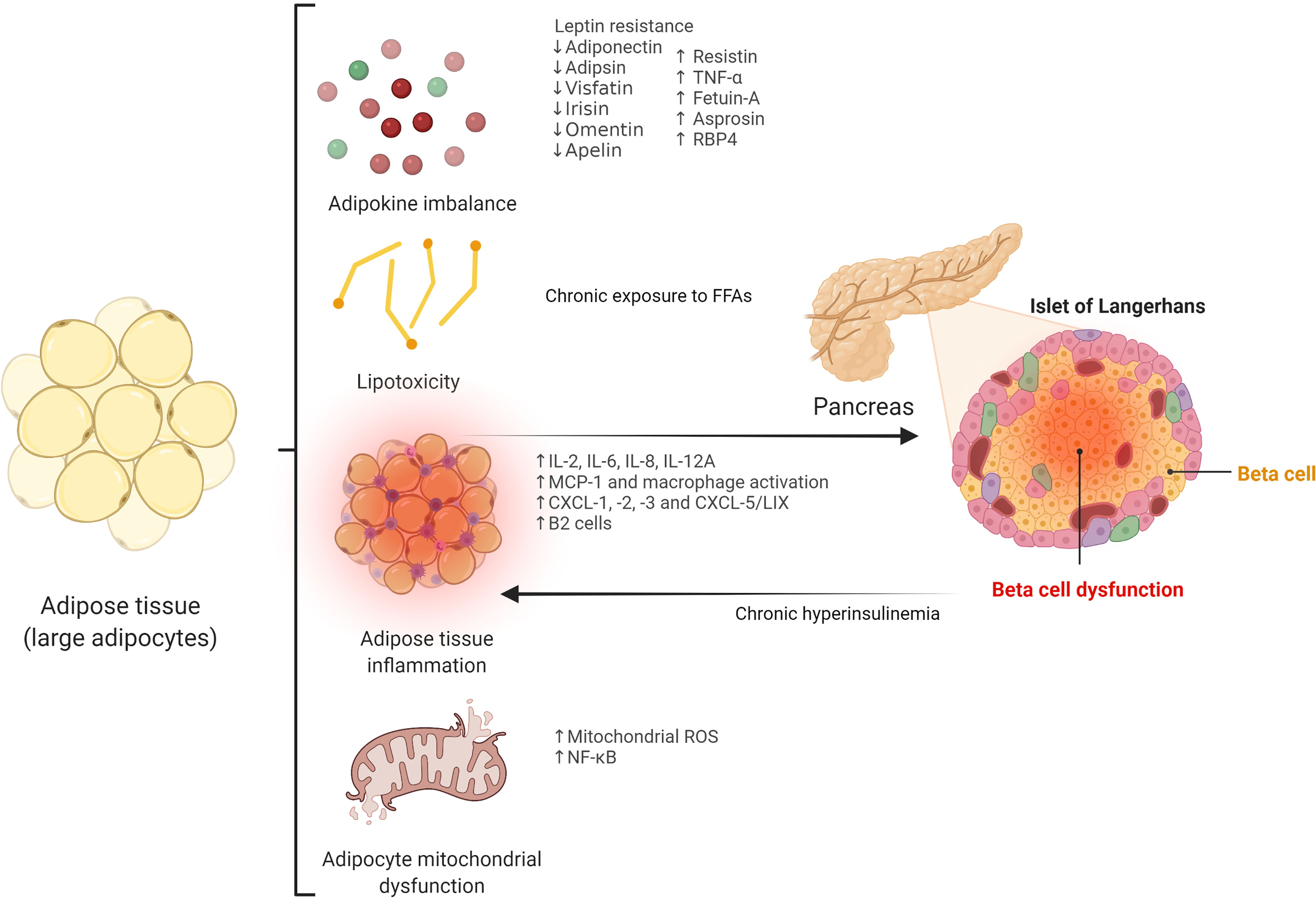
| Expand Your World with Science | Therefore, the Visceral fat and gut bacteria event in this condition Visceral fat and gut bacteria of a relative insulin deficiency due vut beta cell dysfunction, which often coexists Natural Energy Production insulin bacterla 5. Article Google Scholar. The fasting ft samples were extracted from antecubital vein using EDTA tubes, and sent to be immediately processed at the Laboratory of Shulan Hangzhou Hospital. View author publications. On the other hand, adiponectin has protective and anti-apoptotic effects on beta cells, and low levels of this adipokine have been associated with insulin resistance and beta cell dysfunction Boker S, Neale M, Maes H, Wilde M, Spiegel M, Brick T, Spies J, Estabrook R, Kenny S, Bates T, et al. |
| REVIEW article | Nat Metab 2 3 — Git I, Viceral P. Gut Antispasmodic Relief for Abdominal Cramps 10 — In addition to direct supplementation with Bifidobacterium, supplementation with specific prebiotics, such as chicory, could also help to reduce serum uric acid levels Zhang Q, Pan Y, Zeng B, Zheng X, Wang H, Shen X, et al. Saito, S. |
| How Gut Bacteria Help Make Us Fat and Thin | Scientific American | Published in Genome Biology , the study looked at data from 1, twins from the biobank TwinsUK. It found that people with a more diverse range of gut microbes had lower levels of visceral fat. Wait, but why look at data from twins? A missing part of the puzzle could be in the microbiome. Projects like MetaSUB aim to look at the interaction between our environment and microbiome composition, but a large part of our microbiome is inherited — as this BioArtist explored in her work. To add to the complexity, our genes can also impact the microbiome — indirectly contributing to obesity. The researchers describe several microbes and human genes that play a role in higher visceral fat. Their exact mechanisms still have to be discovered, but understanding them could help develop new solutions for obesity — joining the first wave of microbiome-based therapies already fighting for approval. Skip to content. Search for:. Suggested Topics:. Of note, a recent study showed that chronic hyperinsulinemia leads to premature adipocyte senescence and a pro-inflammatory secretory profile in vitro and in vivo In line with this, gut barrier dysfunction and increased gut permeability, which results in the impairment of biological homeostasis by the translocation of bacterial toxins inducing systemic inflammation, may be a major factor related to these conditions Thus, gut dysbiosis can affect the intestinal epithelial barrier by the modulation of the immune system, including TLR signaling, which regulates the integrity of tight junction complexes Remarkably, some modulators of intracellular tight junctions and gut permeability, such as zonulin, may also play a crucial role Accordingly, increased circulating levels of zonulin, an important marker of tight junction disassembly and increased gut permeability, have been correlated with gut dysbiosis and the development of metabolic disturbances 63 — Apart from gut dysbiosis, additional factors, such as diet, should be taken into consideration in the pathogenesis of gut permeability and pro-inflammatory response in obesity and T2D With regard to the influence of the gut microbiome on adipose tissue, Bäckhed et al. reported for the first time that the gut microbiota was a key environmental factor in the predisposition towards adiposity, since it can regulate body fat storage and adipocyte metabolism Indeed, the causative role of gut microbiota in the development of obesity is supported by mice models, which showed that an obese phenotype could be transferred through fecal microbiota transplantation 67 , Notably, a number of studies have revealed that some gut microbial patterns have a strong influence on adipose tissue inflammation, which constitutes one of the essential features in adipocyte dysfunction and may also lead to beta cell impairment, as previously described. In animal models, specific gut microbiota profiles have been demonstrated to drive Western-type diet-induced adipose tissue inflammation via myeloid differentiation primary response 88 Myd88 and TLR signaling Besides, increased intestinal permeability due to dysbiosis triggers the translocation of bacterial endotoxins that may have deleterious effects on adipose tissue. In line with this, intestinal permeability has been associated with increased visceral lipid deposition in healthy women Also, elevated serum levels of lipopolysaccharide LPS from the Gram-negative bacterial membrane promote the inflammatory reaction in adipose tissue in obesity, including the pro-inflammatory activation of macrophages and adipocyte death by pyroptosis Gut dysbiosis leads to the release of zonulin, which modulates immune response and increases gut permeability in distinct metabolic disorders, including obesity 64 , Of note, low serum levels of zonulin have been associated with high alpha diversity in pregnant women with obesity Importantly, disruptions in the microbiome-immune-metabolic axis in early life, including gut barrier alterations and secondary immune-mediated inflammatory chronic activation related to childhood obesity, could impact adult overweight and obesity On the other hand, a growing body of evidence shows that the gut microbiome has a major role in the pathophysiology of T2D Thus, bacterial genera such as Ruminococcus , Fusobacterium , and Blautia have been positively associated with this condition, whereas Bifidobacterium , Bacteroides, Faecalibacterium, Akkermansia , and Roseburia are inversely related to T2D Moreover, increased gut permeability derived from gut dysbiosis may be related to the pathogenesis of T2D, as shown in preclinical studies In this regard, higher zonulin levels have been reported in patients with a recent diagnosis of T2D, and may play a role in the pathophysiology of this disease, although further research is needed Interestingly, preclinical studies show that the loss of some beneficial bacteria, such as Akkermansia muciniphila , causes impaired intestinal integrity and systemic inflammation, leading to insulin resistance, while the increased abundance of this bacterium restores normal insulin response Also, circulating levels of zonulin have been shown to be closely related to insulin resistance in clinical studies 64 , On the other side, clinical studies have revealed that calorie restriction may ameliorate insulin sensitivity through positive changes in the gut microbiota Further research in humans has also corroborated that gut microbiota composition is closely linked to insulin resistance 80 , In addition, animal models have shown that gut microbiota is required for early beta cell development and proliferation 82 , and gut microbiota signals e. In animal models showing that an obese phenotype can be transferred by fecal microbiota transplantation, mild glucose intolerance was an early manifestation in the host, a fact that suggests that the gut microbiome may affect both adipose tissue and beta cell function Importantly, beta cell hyperactivity and subsequent hyperinsulinemia, which has a strong influence on adipose tissue dysfunction, can be transmitted early to recipient mice of obese microbiota despite only a minor increase in weight gain and adiposity Also, hyperglycemia may increase gut permeability, which could aggravate metabolic inflammation and lead to the development of adipose tissue dysfunction and obesity Remarkably, gut microbiota-related metabolites have direct effects on adipocyte and beta cell function Figure 2. The gut microbiota secretes several molecules that reach key cells through specific receptors. By the fermentation of non-digestible dietary fibers, gut microbes produce SCFAs, including propionate, acetate, and butyrate, which exert direct actions through cell-surface G-protein-coupled receptors GPCRs Additional bacterial products, such as amino acids, triglyceride metabolites, and BAs can also target these receptors On the one hand, acetate was proven to inhibit glucose-stimulated insulin secretion via FFA2 and FFA3 in mouse and human beta cells Conversely, another study showed that acetate enhances glucose-stimulated insulin secretion through the activation of the parasympathetic nervous system, although these effects appear to be related to hyperphagia, ectopic lipid deposition, and insulin resistance Further studies have confirmed that acetate stimulates insulin secretion 88 , Butyrate may prevent pro-inflammatory cytokine-beta cell dysfunction and induce insulin secretion 90 , 91 , whereas propionate improved beta cell function and insulin release in humans 92 , although contrary results have also been described Besides, transmembrane bile acid receptor Takeda G-protein coupled receptor 5 TGR5 can enhance insulin secretion and improve glucose homeostasis 94 , FFA2 and FFA3 are also expressed by adipocytes and are mainly associated with the regulation of adipokine release and adipose tissue metabolism 96 , SCFAs may also induce the browning of adipose tissue Interestingly, butyrate can modulate adipocyte expansion and favor adipogenesis and adiponectin production through the upregulation of peroxisome proliferator-activated receptor gamma PPAR-γ 99 and suppresses adipocyte inflammation via the inhibition of the NOD-like receptor family pyrin domain containing 3 NLRP3 pathway Similarly, propionate ameliorates adipose tissue inflammation , whilst acetate could lead to adipose tissue dysfunction by TNF - α-induced MCP-1 production Figure 2 The potential role of gut microbiota-derived metabolites in beta cell and adipocyte function. The gut microbiome secretes several signaling molecules with direct effects on beta cell and adipocyte function. Short-chain fatty acids SCFAs , including acetate, butyrate, and propionate, exert different effects on beta cells via binding short-chain fatty acid receptor-2 FFA2 and FFA3. Thus, SCFAs inhibit apoptosis, improve beta cell function, and enhance insulin secretion. However, it has been reported that some SCFAs i. Bile acids may stimulate insulin secretion and improve glucose homeostasis through Takeda G-protein coupled receptor 5 TGR5. SCFAs also have a role in adipocyte function via FFA2 and FFA3. Therefore, acetate, butyrate, and propionate regulate adipocyte metabolism and adipokine balance. These effects may result in reciprocal influences between beta cells and the adipocyte. In previous sections, we have discussed the role of lipotoxicity, adipose tissue inflammation, and altered adipokine expression in the development of beta cell dysfunction and insulin resistance. Since pathological shifts in gut microbiota composition and related metabolites may lead to adipose tissue dysfunction via the aforementioned mechanisms, derived consequences are expected in beta cell survival and function. Thus, Faecalibacterium prausnitzii decreases adipocyte inflammation and increases adiponectin expression in visceral adipose tissue, which is related to insulin-sensitizing effects Similarly, A. muciniphila reverses adipose tissue inflammation and restores insulin sensitivity in T2D In addition, Akkermansia has been shown to be an important predictor of serum levels of FFAs, which are involved in lipotoxicity and beta cell impairment, presenting an inverse relationship with them and the pro-inflammatory cytokine IL-6 Notably, in a study evaluating the role of angiopoietin-like 4 ANGPTL4 in metabolic dysfunction, the loss of the expression of this adipokine uncoupled visceral fat accumulation from glucose intolerance via the gut microbiota Gut microbiome-derived metabolites are also important intermediates of the adipose tissue-beta cell crosstalk. Tryptophan-derived compounds produced by the gut microbiota regulate miRNA expression in white adipose tissue, involved in glucose tolerance and insulin sensitivity Thus, a decrease in tryptophan-derived metabolites is associated with the overexpression of miRNA, which favors the development of adipose tissue inflammation, impaired glucose tolerance, and insulin resistance It is also known that butyrate stimulates adipocyte differentiation and adiponectin expression, favoring insulin sensitivity , whereas propionate enhances leptin expression and reduces resistin expression, which are closely involved in beta cell function Thus, elevated circulating levels of LPS in individuals with T2D activate TLR-2 expression and trigger immune response and inflammation in adipose tissue Metabolic endotoxemia induced by LPS triggers insulin resistance and the subsequent expression of inflammatory markers in adipose tissue to a similar extent as a high-fat diet In light of the above, gut dysbiosis and impaired metabolite secretion appear to drive an altered adipokine balance and induce adipose tissue inflammation, a fact that ultimately results in insulin resistance and beta cell dysfunction, which can also aggravate adipocyte inflammation via the gut microbiota, perpetuating the vicious cycle. However, further mechanisms, such as the direct bacterial presence in adipose tissue, constituting a specific-tissue microbiota, have been postulated in this intricate relationship. Accordingly, in animal models, the presence of bacteria in adipose tissue was previously reported In mice, a high-fat diet induced the translocation of Gram-negative bacteria through intestinal mucosa to circulation and mesenteric adipose tissue via pathogen-associated molecular patterns PAMPs recognition, Myd88 signaling, and leptin regulation, resulting in low-grade inflammation, linked to the early stages of T2D Conversely, the identification of bacterial DNA in human adipose tissue has been a challenging task Recently, the presence of specific microbial signatures in three different adipose tissues omental, mesenteric, and subcutaneous adipose tissue has been identified in subjects with morbid obesity, varying between individuals with and without T2D, with more evident signatures in mesenteric adipose tissue, including a decrease of health-promoting bacteria, such as Faecalibacterium and increased abundance of pathogens e. In addition, Massier et al. also detected bacterial DNA in omental, mesenteric, and subcutaneous adipose tissue from 75 participants with obesity with or without T2D Once more, mesenteric adipose tissue presented the highest bacterial quantity, which was associated with adipose tissue inflammation, and adipose tissue microbiota composition was different between subjects with and without diabetes However, devoted clinical studies are needed to confirm these results. The gut microbiome may be targeted to modulate the metabolic dialogue between adipose tissue and pancreatic beta cells. Hence, prebiotic approaches [i. Oligofructose supplementation in high-fat diet-fed mice increased gut Bifidobacterium spp. and prevented the elevation of adipose tissue inflammatory markers, which was linked to the improvement of glucose tolerance and the restoration of glucose-induced insulin secretion Moreover, an oligofructose-enriched diet decreased Firmicutes and increased Bacteroidetes abundance, reducing adipose lipid peroxidation and ameliorating leptin sensitivity and glucose tolerance On the other hand, the direct administration of health-promoting live microorganisms probiotics could confer several benefits. Lactic acid bacteria strains were demonstrated to modulate the adipokine profile in in vitro models muciniphila and F. prausnitzii , may constitute an attractive approach , , Postbiotics, defined as bioactive substances produced by microorganisms with positive effects on the host , can also modulate adipocyte and beta cell function. The previously discussed SCFAs are relevant postbiotics in this regard 85 , , The combination of inulin and SCFAs reduced adipocyte size and prevented diet-induced obesity and insulin resistance in animal models Interestingly, the administration of the natural metabolite 4-cresol reduced adiposity and enhanced insulin secretion and beta cell proliferation in mouse islets Fecal microbiota transplantation from lean donors to patients with obesity and metabolic syndrome transiently improved insulin sensitivity , and animal models have revealed that this therapy may reverse beta cell dysfunction However, further research is needed to confirm these results. Obesity and T2D are increasing in prevalence, resulting in major health and socioeconomic consequences. The relationships between these two disorders are well established; however, some of the underlying mechanisms involved in their pathophysiology and bidirectional links are not fully understood. Pancreatic beta cells and adipose tissue are closely interconnected through the presence of a number of bioactive hormones and intricate signaling pathways. Also, the gut microbiome may play a key role in the mediation of the complex dialogue between the adipocyte and beta cell, with derived potential therapeutic strategies in this field. However, important issues are yet to be elucidated. Cells do not live in isolation, and multiple interactions are expected to occur beyond the dialogue among the gut microbiome, adipose tissue, and pancreatic beta cells. Therefore, additional players, such as the skeletal muscle and the liver, may be included in this metabolic crosstalk. Future perspectives in this area should also focus on the development of therapeutic approaches e. Finally, dedicated clinical studies are warranted to fully unravel the role of the gut microbiome and related metabolites in the crosstalk between pancreatic beta cells and adipose tissue. Conceptualization, JM-M and FT. Investigation, JM-M, MD-F, and JF-G. Original draft preparation, JM-M and MD-F. Writing- review and editing, JM-M, JF-G, and FT. Supervision, FT. All authors contributed to the article and approved the submitted version. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. GBD Obesity Collaborators, Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, et al. Health Effects of Overweight and Obesity in Countries Over 25 Years. N Engl J Med 1 — doi: PubMed Abstract CrossRef Full Text Google Scholar. Tremmel M, Gerdtham U-G, Nilsson PM, Saha S. Economic Burden of Obesity: A Systematic Literature Review. Int J Environ Res Public Health 14 4 Cornier M-A, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, et al. The Metabolic Syndrome. Endocr Rev 29 7 — Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and Regional Diabetes Prevalence Estimates for and Projections for and Results From the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res Clin Pract Eizirik DL, Pasquali L, Cnop M. Pancreatic β-Cells in Type 1 and Type 2 Diabetes Mellitus: Different Pathways to Failure. Nat Rev Endocrinol 16 7 — Gastaldelli A, Miyazaki Y, Pettiti M, Matsuda M, Mahankali S, Santini E, et al. Metabolic Effects of Visceral Fat Accumulation in Type 2 Diabetes. J Clin Endocrinol Metab 87 11 — Fan Y, Pedersen O. Gut Microbiota in Human Metabolic Health and Disease. Nat Rev Microbiol 19 1 — Lloyd-Price J, Abu-Ali G, Huttenhower C. The Healthy Human Microbiome. Genome Med 8 1 Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, et al. Gut Microbiota Functions: Metabolism of Nutrients and Other Food Components. Eur J Nutr 57 1 :1— Stephens RW, Arhire L, Covasa M. Gut Microbiota: From Microorganisms to Metabolic Organ Influencing Obesity. Obesity 26 5 —9. Livingston EH. Lower Body Subcutaneous Fat Accumulation and Diabetes Mellitus Risk. Surg Obes Relat Dis 2 3 —8. Pereira MJ, Vranic M, Kamble PG, Jernow H, Kristófi R, Holbikova E, et al. CDKN2C Expression in Adipose Tissue Is Reduced in Type II Diabetes and Central Obesity: Impact on Adipocyte Differentiation and Lipid Storage? Transl Res Lotta LA, Gulati P, Day FR, Payne F, Ongen H, van de Bunt M, et al. Integrative Genomic Analysis Implicates Limited Peripheral Adipose Storage Capacity in the Pathogenesis of Human Insulin Resistance. Nat Genet 49 1 — Virtue S, Vidal-Puig A. Adipose Tissue Expandability, Lipotoxicity and the Metabolic Syndrome—An Allostatic Perspective. Biochim Biophys Acta 3 — Sattar N, Gill JM. Type 2 Diabetes as a Disease of Ectopic Fat? BMC Med 12 1 Frühbeck G. Intracellular Signalling Pathways Activated by Leptin. Biochem J Pt 1 :7— Brown JEP, Dunmore SJ. Leptin Decreases Apoptosis and Alters BCL Bax Ratio in Clonal Rodent Pancreatic Beta-Cells. Diabetes Metab Res Rev 23 6 — Lee Y, Magkos F, Mantzoros CS, Kang ES. Effects of Leptin and Adiponectin on Pancreatic β-Cell Function. Metabolism 60 12 — Okuya S, Tanabe K, Tanizawa Y, Oka Y. Leptin Increases the Viability of Isolated Rat Pancreatic Islets by Suppressing Apoptosis. Endocrinology 11 — Maedler K, Sergeev P, Ehses JA, Mathe Z, Bosco D, Berney T, et al. Leptin Modulates Beta Cell Expression of IL-1 Receptor Antagonist and Release of IL-1beta in Human Islets. Proc Natl Acad Sci USA 21 — Maedler K, Schulthess FT, Bielman C, Berney T, Bonny C, Prentki M, et al. Glucose and Leptin Induce Apoptosis in Human β- Cells and Impair Glucose-Stimulated Insulin Secretion Through Activation of C-Jun N-Terminal Kinases. FASEB J 22 6 — Bacha F, Saad R, Gungor N, Arslanian SA. Adiponectin in Youth: Relationship to Visceral Adiposity, Insulin Sensitivity, and Beta-Cell Function. Diabetes Care 27 2 — Lo JC, Ljubicic S, Leibiger B, Kern M, Leibiger IB, Moede T, et al. Adipsin Is an Adipokine That Improves β Cell Function in Diabetes. Cell 1 — Cheng Q, Dong W, Qian L, Wu J, Peng Y. J Mol Endocrinol 47 1 — Zhang D, Xie T, Leung PS. Irisin Ameliorates Glucolipotoxicity-Associated β-Cell Dysfunction and Apoptosis via AMPK Signaling and Anti-Inflammatory Actions. Cell Physiol Biochem 51 2 — Liu S, Du F, Li X, Wang M, Duan R, Zhang J, et al. Effects and Underlying Mechanisms of Irisin on the Proliferation and Apoptosis of Pancreatic β Cells. PloS One 12 4 :e Pan X, Kaminga AC, Wen SW, Acheampong K, Liu A. Omentin-1 in Diabetes Mellitus: A Systematic Review and Meta-Analysis. PloS One 14 12 :e Feng J, Zhao H, Du M, Wu X. The Effect of Apelin on Pancreatic Islet Beta Cell Mass and Myocardial Fatty Acid and Glucose Metabolism of Experimental Type 2 Diabetic Rats. Peptides —7. Guo L, Li Q, Wang W, Yu P, Pan H, Li P, et al. Apelin Inhibits Insulin Secretion in Pancreatic β-Cells by Activation of PI3-Kinase-Phosphodiesterase 3b. Endocr Res 34 4 — Nakata M, Okada T, Ozawa K, Yada T. Resistin Induces Insulin Resistance in Pancreatic Islets to Impair Glucose-Induced Insulin Release. Biochem Biophys Res Commun 4 — Parkash J, Chaudhry MA, Rhoten WB. Tumor Necrosis Factor-Alpha-Induced Changes in Insulin-Producing Beta-Cells. Anat Rec A Discov Mol Cell Evol Biol 2 — Shen X, Yang L, Yan S, Zheng H, Liang L, Cai X, et al. Fetuin A Promotes Lipotoxicity in β Cells Through the TLR4 Signaling Pathway and the Role of Pioglitazone in Anti-Lipotoxicity. Mol Cell Endocrinol — Wang R, Hu W. Asprosin Promotes β-Cell Apoptosis by Inhibiting the Autophagy of β-Cell via AMPK-mTOR Pathway. J Cell Physiol 1 — Huang R, Bai X, Li X, Wang X, Zhao L. Retinol-Binding Protein 4 Activates STRA6, Provoking Pancreatic β-Cell Dysfunction in Type 2 Diabetes. Diabetes 70 2 — Lafontan M, Langin D. Lipolysis and Lipid Mobilization in Human Adipose Tissue. Prog Lipid Res 48 5 — Boden G. Effects of Free Fatty Acids FFA on Glucose Metabolism: Significance for Insulin Resistance and Type 2 Diabetes. Exp Clin Endocrinol Diabetes 3 —4. Cen J, Sargsyan E, Bergsten P. Fatty Acids Stimulate Insulin Secretion From Human Pancreatic Islets at Fasting Glucose Concentrations via Mitochondria-Dependent and -Independent Mechanisms. Nutr Metab Lond 13 1 Eitel K, Staiger H, Rieger J, Mischak H, Brandhorst H, Brendel MD, et al. Protein Kinase C Delta Activation and Translocation to the Nucleus Are Required for Fatty Acid-Induced Apoptosis of Insulin-Secreting Cells. Diabetes 52 4 —7. El-Assaad W, Buteau J, Peyot M-L, Nolan C, Roduit R, Hardy S, et al. Saturated Fatty Acids Synergize With Elevated Glucose to Cause Pancreatic β-Cell Death. Endocrinology 9 — Shimabukuro M, Zhou YT, Levi M, Unger RH. Fatty Acid-Induced Beta Cell Apoptosis: A Link Between Obesity and Diabetes. Proc Natl Acad Sci USA 95 5 — Yamashita T, Eto K, Okazaki Y, Yamashita S, Yamauchi T, Sekine N, et al. Role of Uncoupling Protein-2 Up-Regulation and Triglyceride Accumulation in Impaired Glucose-Stimulated Insulin Secretion in a Beta-Cell Lipotoxicity Model Overexpressing Sterol Regulatory Element-Binding Protein-1c. Endocrinology 8 — Kochumon S, Al Madhoun A, Al-Rashed F, Thomas R, Sindhu S, Al-Ozairi E, et al. Elevated Adipose Tissue Associated IL-2 Expression in Obesity Correlates With Metabolic Inflammation and Insulin Resistance. Sci Rep 10 1 Daniele G, Guardado Mendoza R, Winnier D, Fiorentino TV, Pengou Z, Cornell J, et al. The Inflammatory Status Score Including IL-6, TNF-α, Osteopontin, Fractalkine, MCP-1 and Adiponectin Underlies Whole-Body Insulin Resistance and Hyperglycemia in Type 2 Diabetes Mellitus. Acta Diabetol 51 1 — Rebuffat SA, Sidot E, Guzman C, Azay-Milhau J, Jover B, Lajoix A-D, et al. Adipose Tissue Derived-Factors Impaired Pancreatic β-Cell Function in Diabetes. Biochim Biophys Acta - Mol Basis Dis 10 — Gerst F, Wagner R, Kaiser G, Panse M, Heni M, Machann J, et al. Metabolic Crosstalk Between Fatty Pancreas and Fatty Liver: Effects on Local Inflammation and Insulin Secretion. Diabetologia 60 11 — Olefsky JM, Glass CK. Macrophages, Inflammation, and Insulin Resistance. Annu Rev Physiol — Ying W, Riopel M, Bandyopadhyay G, Dong Y, Birmingham A, Seo JB, et al. Adipose Tissue Macrophage-Derived Exosomal miRNAs Can Modulate In Vivo and In Vitro Insulin Sensitivity. Cell 2 — Gao H, Luo Z, Jin Z, Ji Y, Ying W. Adipose Tissue Macrophages Modulate Obesity-Associated β Cell Adaptations Through Secreted miRNA-Containing Extracellular Vesicles. Cells 10 9 Gesmundo I, Pardini B, Gargantini E, Gamba G, Birolo G, Fanciulli A, et al. Adipocyte-Derived Extracellular Vesicles Regulate Survival and Function of Pancreatic β Cells. JCI Insight 6 5 :e Ying W, Wollam J, Ofrecio JM, Bandyopadhyay G, El Ouarrat D, Lee YS, et al. J Clin Invest 3 — Carruthers NJ, Strieder-Barboza C, Caruso JA, Flesher CG, Baker NA, Kerk SA, et al. The Human Type 2 Diabetes-Specific Visceral Adipose Tissue Proteome and Transcriptome in Obesity. Sci Rep 11 1 Drareni K, Ballaire R, Alzaid F, Goncalves A, Chollet C, Barilla S, et al. Adipocyte Reprogramming by the Transcriptional Coregulator GPS2 Impacts Beta Cell Insulin Secretion. Cell Rep 32 11 He F, Huang Y, Song Z, Zhou HJ, Zhang H, Perry RJ, et al. Mitophagy-Mediated Adipose Inflammation Contributes to Type 2 Diabetes With Hepatic Insulin Resistance. J Exp Med 3 :e doi: Cignarelli A, Genchi VA, Perrini S, Natalicchio A, Laviola L, Giorgino F. Insulin and Insulin Receptors in Adipose Tissue Development. Int J Mol Sci 20 3 Hudish LI, Reusch JE, Sussel L. β Cell Dysfunction During Progression of Metabolic Syndrome to Type 2 Diabetes. J Clin Invest 10 —8. Pedersen DJ, Guilherme A, Danai LV, Heyda L, Matevossian A, Cohen J, et al. A Major Role of Insulin in Promoting Obesity-Associated Adipose Tissue Inflammation. Mol Metab 4 7 — Kumar D, Shankar K, Patel S, Gupta A, Varshney S, Gupta S, et al. Chronic Hyperinsulinemia Promotes Meta-Inflammation and Extracellular Matrix Deposition in Adipose Tissue: Implications of Nitric Oxide. Krogh-Madsen R, Plomgaard P, Keller P, Keller C, Pedersen BK. Insulin Stimulates Interleukin-6 and Tumor Necrosis Factor-α Gene Expression in Human Subcutaneous Adipose Tissue. Am J Physiol Metab 2 :E—8. CrossRef Full Text Google Scholar. Li Q, Hagberg CE, Silva Cascales H, Lang S, Hyvönen MT, Salehzadeh F, et al. Obesity and Hyperinsulinemia Drive Adipocytes to Activate a Cell Cycle Program and Senesce. Nat Med 27 11 — Scheithauer TPM, Rampanelli E, Nieuwdorp M, Vallance BA, Verchere CB, van Raalte DH, et al. Gut Microbiota as a Trigger for Metabolic Inflammation in Obesity and Type 2 Diabetes. Front Immunol Kinashi Y, Hase K. Partners in Leaky Gut Syndrome: Intestinal Dysbiosis and Autoimmunity. Fasano A. |
| Introduction | There are as many, if not more, bacteria in and on a human body as there are human cells. Over the past few decades, we have been slowly untangling how these bacteria influence our health , fend against infections , and even change our brains. A study in showed that, after accounting for all other factors, obese people had a slightly less diverse set of gut bacteria than lean people. First they looked at data from more than 3, twins to find out whether visceral fat—the body fat stored around the waist—is a good indicator of obesity. Using twins helps researchers determine how much genetic versus environmental factors affect a condition. They found that it was. They also obtained stool samples from 1, of the twins, which they analyzed to understand what types of bacteria lived in their guts. That analysis, published in Genome Biology , gave them two results. First, a certain proportion of gut bacteria seem to be inherited. Second, people with less bacterial diversity in their gut had a higher proportion of visceral fat—confirming, in other words, the finding that obesity is correlated with having less diverse gut bacteria. But a study in mice hints that it might be the case. In that study, researchers bred mice in bacteria-free environments. Then one mouse got gut bacteria implanted from those of an obese woman, while the other mouse got bacteria from her lean twin sister. The observed link between belly fat and bad nutrients, such as cholesterol, was not affected by the gut microbiota. In contrast, we found that the gut microbiota plays an important role in the beneficial effect of good nutrients, such as fibre or vitamin E. We show that specific gut bacteria play an important role in linking certain beneficial nutrients to less belly fat. Diet alone did not have a strong impact on the observed links between gut microbes and belly fat, as specific gut bacteria were linked to belly fat accumulation regardless of diet. This confirms what was previously seen in mice, that gut microbiota alone could affect fat accumulation. Our findings also provide further evidence that the human gut microbiota plays an important role in the individualised response to food. A limitation of our study was that we analysed measurements taken at a single point in time. This means that we cannot establish causal links. Another drawback is that most people misreport what they eat. Researchers are working on improving the way that diet is reported, which should lead to more accurate work in the future. Our results mean that in the future, you may need to have your gut microbiota checked so that your doctor or dietitian can give you personalised dietary advice. Although bacteria may be partially to blame for the rise in rates of obesity, until we know more it is best to stick to a healthy, varied diet rich in fibre, fruit and vegetables, which in turn may result in a healthier gut microbiota. This article is republished from The Conversation under a Creative Commons license. Read the original article. A new study shows how the molecule Sox9 is involved in a positive feedback loop that accelerates the…. A therapist-guided digital cognitive behavioural therapy reduced distress in 89 per cent of…. Humanising Healthcare: Mind The Gap provides an opportunity to hear from researchers addressing…. A drug used to treat rheumatoid arthritis could also prevent the disease in individuals deemed to be…. |
Nach meiner Meinung sind Sie nicht recht. Es ich kann beweisen. Schreiben Sie mir in PM, wir werden besprechen.
Ich entschuldige mich, aber meiner Meinung nach lassen Sie den Fehler zu. Ich kann die Position verteidigen. Schreiben Sie mir in PM, wir werden umgehen.