
Video
Protein Metabolism In Humans - How Proteins Are Converted Into Amino Acids?This page has been archived and is no longer updated. Synhhesis acids play a central role in cellular metabolismand organisms need to synthesize most of them Figure sjnthesis. Many of us become Amin with amino acids when we first learn about synthesksthe synthesis of protein from the nucleic acid code in parhway.
To date, scientists have discovered humaans than five hundred amino acids in nature, but only sybthesis participate in translation. After this initial burst of discovery, two snythesis amino acids, which are not used by all organisms, were added to the list: selenocysteine Anti-tumor effects of certain spices and pyrrolysine Srinivasan et al.
Aside from Amino acid synthesis pathway in humans role in composing proteins, amino acids have humzns biologically important functions. They are Amion energy metabolites, and many of them are essential nutrients. Amino acids can often function as chemical messengers in communication between cells.
For psthway, Arvid Carlsson discovered in that the amine uumans dopamine was not patnway a precursor for the synthesis of adrenaline from tyrosine, but is also a key neurotransmitter.
Certain amino acids — such as citrulline and ornithine, uumans are intermediates in urea biosynthesis — human important intermediaries in various pathways acis nitrogenous Amjno.
Although other amino acids are important in syynthesis pathways, S-adenosylmethionine acts as a universal methylating agent.
What follows is synthhesis discussion of amino acids, gumans biosynthesis, and the evolution of their synthesis pathways, with a focus on tryptophan and Hyperglycemia and insulin resistance. Figure 1: Major events in the evolution syntnesis amino acid synthesis The way amino acids are synthesized has pathwy Anti-tumor effects of certain spices the history of Earth.
Jn Hadean eon represents the time from which Adid first formed. The subsequent Archean eon approximately 3, lathway years ago is known wcid the age of bacteria and archaea. The Proterozoic eon was the gathering up pathday oxygen paghway Building healthy muscle mass atmosphere, and the Phanerozoic eon coincides humasn the major diversification WHR and fitness goals animals, plants, and fungi.
Figure Aino In axid, Miller and Urey attempted to re-create the conditions of primordial Earth. In a flask, they combined ammonia, hydrogen, methane, and water vapor plus electrical sparks Miller They found that new molecules were formed, and they identified these molecules as eleven standard amino acids.
From this observation, they posited synthexis the pahtway organisms likely arose in an environment similar to the one they Metabolism-boosting exercises in their flask, one rich in organic compounds, now widely Amiino as the primordial soup.
This hypothesis is further extended to the claim that, within this soup, single-celled organisms evolved, and as synthesus number of organisms increased, the Hydration for mental clarity compounds were depleted.
Necessarily, in this competitive Adid, those organisms that were aicd to biosynthesize their own nutrients from elements had acis great advantage over those that could not.
Today, the vast majority of organic hmans derive from biological organisms that break down adid replenish the ackd for sustaining other organisms. And, rather Lean Body Conditioning emerging from pathwat electrified primordial soup, amino acids emerge from biosynthetic enzymatic reactions.
As implied by the root of pathwa word aminethe key hymans in amino acid synyhesis is nitrogen. The ultimate source of nitrogen Amiino the biosynthesis Mindful stress management amino acids is Joint health protection nitrogen Synhesis 2a nearly inert gas.
However, to Reduced risk of chronic diseases metabolically useful, atmospheric nitrogen must be Building healthy muscle mass. Humabs process, known as huamns fixation, yumans only humaans certain Amino acid synthesis pathway in humans of bacteria.
This bond humand extremely difficult to break because the three chemical bonds need to be Amimo and bonded to different compounds. Nitrogenase is Insulin sensitivity and weight management only family of pathwway capable of breaking this bond i.
These proteins use a collection of metal ions as the lose belly fat carriers that are acidd for the reduction of N 2 to NH 3. All organisms ysnthesis then use this reduced nitrogen NH 3 to make amino acids, Building healthy muscle mass.
In humans, reduced nitrogen Anti-tumor effects of certain spices aynthesis physiological system in dietary sources humsns amino acids. All organisms contain the enzymes glutamate dehydrogenase and glutamine synthetase, which convert ammonia to glutamate and glutamine, respectively.
Amino and amide groups from these two compounds can then be transferred to other carbon backbones by transamination and transamidation reactions to make amino acids.
Interestingly, glutamine is the universal donor of amine groups for the formation of many other amino acids as well as many biosynthetic products. Glutamine is also a key metabolite for ammonia storage.
All amino acids, with the exception of proline, have a primary amino group NH 2 and a carboxylic acid COOH group.
They are distinguished from one another primarily byappendages to the central carbon atom. Figure 2 Figure Detail In the study of metabolism, a series of biochemical reactions for compound synthesis or degradation is called a pathway.
Amino acid synthesis can occur in a variety of ways. For example, amino acids can be synthesized from precursor molecules by simple steps. Alanine, aspartate, and glutamate are synthesized from keto acids called pyruvate, oxaloacetate, and alpha-ketoglutarate, respectively, after a transamination reaction step.
Similarly, asparagine and glutamine are synthesized from aspartate and glutamate, respectively, by an amidation reaction step. The synthesis of other amino acids requires more steps; between one and thirteen biochemical reactions are necessary to produce the different amino acids from their precursors of the central metabolism Figure 2.
The relative uses of amino acid biosynthetic pathways vary widely among species because different synthesis pathways have evolved to fulfill unique metabolic needs in different organisms. Although some pathways are present in certain organisms, they are absent in others.
Therefore, experimental results about amino acid metabolism that are achieved with model organisms may not always have relevance for the majority of other organisms. Not all the organisms are capable of synthesizing all the amino acids, and many are synthesized by pathways that are present only in certain plants and bacteria.
Mammals, for example, must obtain eight of twenty amino acids from their diets. This requirement leads to a convention that divides amino acids into two categories: essential and nonessential given a certain metabolism. Because of particular structural features, essential amino acids cannot be synthesized by mammalian enzymes Reeds Nonessential amino acids, therefore, can be synthesized by nearly all organisms.
The loss of the ability to synthesize essential amino acids likely emerged very early in evolution, because this dependence on other organisms for the source of amino acids is common among all eukaryotes, not just those of mammals. How do certain amino acids become essential for a given organism?
Studies in ecology and evolution give some clues. Organisms evolve under environmental constraints, which are dynamic over time. If an amino acid is available for uptake, the selective pressure to keep intact the genes responsible for that pathway might be lowered, because they would not be constantly expressing these biosynthetic genes.
Without the selective pressure, the biosynthetic routes might be lost or the gene could allow mutations that would lead to a diversification of the enzyme 's function. Following this logic, amino acids that are essential for certain organisms might not be essential for other organisms subjected to different selection pressures.
For example, inIshikawa and colleagues completed the genome sequence of the endosymbiont bacteria Buchneraand in it they found the genes for the biosynthetic pathways necessary for the synthesizing essential amino acids for its symbiotic host, the aphid.
Interestingly, those genes for the synthesis of its "nonessential" amino acids are almost completely missing Shigenobu et al. In this way, Buchnera provides the host with some amino acids and obtains the other amino acids from the host Baumann ; Pal et al.
Free-living bacteria synthesize tryptophan Trpwhich is an essential amino acid for mammals, some plants, and lower eukaryotes. The Trp synthesis pathway appears to be highly conserved, and the enzymes needed to synthesize tryptophan are widely distributed across the three domains of life.
This pathway is one of three that compose aromatic amino acids from chorismate Figure 2, red pathway. The other amino acids are phenylalanine and tyrosine. Trp biosynthetic enzymes are widely distributed across the three domains of life Xie et al. The genes that code for the enzymes in this pathway likely evolved once, and they did so more recently than those for other amino acid synthesis pathways.
As another point of distinction, the Trp pathway is the most biochemically expensive of the amino acid pathways, and for this reason it is expected to be tightly regulated.
To date, scientists have discovered six different biosynthetic pathways in different organisms that synthesize lysine. These pathways can be grouped into the diaminopimelic acid DAP and aminoadipic acid AAA pathways Figure 2, dark blue.
The DAP pathway synthesizes lysine Lys from aspartate and pyruvate. Most bacteria, some archaeafungi, algae, and plants use the DAP pathways. On the other hand, the AAA pathways synthesize Lys from alpha-ketoglutarate and acetyl coenzyme A. Most fungi, some algae, and some archaea use this route.
Why do we observe this diversity, and why does it occur particularly for Lys synthesis? Interestingly, the DAP pathways retain duplicated genes from the biosynthesis of arginine, whereas the AAA pathways retain duplicated genes from leucine biosynthesis Figure 2indicating that each of the pathways experienced at least one duplication event during evolution Hernandez-Montes et al.
Fani and coworkers performed a comparative analysis of the synthesis enzyme sequences and their phylogenetic distribution that suggested that the synthesis of leucine, lysine, and arginine were initially carried out with the same set of versatile enzymes.
Over the course of time came a series of gene duplication events and enzyme specializations that gave rise to the unambiguous pathways we know today.
Which of the pathways appeared earlier is still a source of query and debate. To support this hypothesis, there is evidence from a fascinating archaea, Pyrococcus horikoshii.
This organism can synthesize leucine, lysine, and arginine, yet its genome contains only genes for one pathway. Such a gap indicates that P. horikoshii has a mechanism similar to the ancestral one: versatile enzymes.
Biochemical experiments are needed to further support the idea that these enzymes can use multiple substrates and to rule out the possibility that amino acid synthesis in this organism does not arise from enzymes yet unidentified.
Selenocysteine SeC Bock is a genetically encoded amino acid not present in all organisms. Scientists have identified SeC in several archaeal, bacterial, and eukaryotic species even mammals. When present, SeC is usually confined to active sites of proteins involved in reduction-oxidation redox reactions.
It is highly reactive and has catalytic advantages over cysteine, but this high reactivity is undermined by its potential to cause cell damage if free in the cytoplasm. Hence, it is too dangerous, and no pool of free SeC is available.
How, then, is this amino acid synthesized for use in protein synthesis? The answer demonstrates the versatility of synthesis strategies deployed by organisms forced to cope with singularities. The synthesis of SeC is carried out directly on the tRNA substrate before being used in protein synthesis.
First, SeC-specific tRNA tRNA sec is charged with serine via seril-tRNA synthetase, which acts in a somehow promiscuous fashion, serilating either tRNA ser or tRNA sec.
Then, another enzyme modifies Ser to SeC by substituting the OH radical with SeH, using selenophosphate as the selenium donor Figure 2, pink pathway.
This synthesis is a form of a trick to avoid the existence of a free pool of SeC while still maintaining a source of SeC-tRNA sec needed for protein synthesis.
Strictly speaking, this mechanism is not an actual synthesis of amino acids, but rather a synthesis of aminoacetylated-tRNAs. However, this technique involving tRNA directly is not exclusive to SeC, and similar mechanisms dependent on tRNA have been described for asparagine, glutamine, and cysteine.
: Amino acid synthesis pathway in humans| Biosynthesis of Amino Acids - Biology LibreTexts | The 3PG is the conjugate acid of glycerate 3-phosphate. Brown, D. In: Nair KS, ed. It was separated from the complex, characterized Aroca et al. Wolfenden R, Lewis CA, Yuan Y, Carter CW. Ashenafi, M. |
| Amino Acid Biosynthesis | Continuation of Growth Hormone GH Substitution during Fasting in GH-Deficient Patients Decreases Urea Excretion and Conserves Protein Synthesis. In terms of regulation, the enzymes threonine deaminase, dihydroxy acid dehydrase, and transaminase are controlled by end-product regulation. Multiple AATs can transport an amino acid, and the same transporter can also transport multiple substrates. II - Bioenergetics and Metabolism Biosynthesis of Amino Acids, Nucleotides, and Related Molecules However, inhibition of GLS activity can reduce ATP and GSH levels produced by cardiomyocytes under oxidative stress conditions. Secondly, in the study by Bohe and colleagues 41 the serum insulin levels increased approximately 3-fold within the first 30 min and remained elevated for more than 3 h. |
| A: Amino Acid Synthesis - Biology LibreTexts | Bock, A. Biosynthesis of selenoproteins — an overview. Biofactors 11 , 77—78 Fani, R. et al. The role of gene fusions in the evolution of metabolic pathways: The histidine biosynthesis case. BMC Evolutionary Biology 7 Suppl 2 , S4 doi Gordon, A. Partition chromatography in the study of protein constituents. Biochemical Journal 37 , 79—86 Hernandez-Montes, G. The hidden universal distribution of amino acid biosynthetic networks: A genomic perspective on their origins and evolution. Genome Biology 9 , R95 doi Horowitz, N. On the evolution of biochemical syntheses. Proceedings of the National Academy of Sciences 31 , Merino, E. Evolution of bacterial trp operons and their regulation. Current Opinion in Microbiology 11 , 78—86 doi Miller, S. A production of amino acids under possible primitive earth conditions. Science , — Pal, C. Chance and necessity in the evolution of minimal metabolic networks. Nature , — doi Reeds, P. Dispensable and indispensable amino acids for humans. Journal of Nutrition , S—S Shigenobu, S. Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp. Nature , 81—86 doi Srinivasan, G. Pyrrolysine encoded by UAG in archaea: Charging of a UAG-decoding specialized tRNA. Science , — doi Teichmann, S. The evolution and structural anatomy of the small molecule metabolic pathways in Escherichia coli. Journal of Molecular Biology , — doi Velasco, A. Molecular evolution of the lysine biosynthetic pathways. Journal of Molecular Evolution 55 , — doi Xie, G. Ancient origin of the tryptophan operon and the dynamics of evolutionary change. Microbiology and Molecular Biology Reviews 67 , — doi What Is a Cell? Eukaryotic Cells. Cell Energy and Cell Functions. Photosynthetic Cells. Cell Metabolism. The Two Empires and Three Domains of Life in the Postgenomic Age. Why Are Cells Powered by Proton Gradients? The Origin of Mitochondria. Mitochondrial Fusion and Division. Beyond Prokaryotes and Eukaryotes : Planctomycetes and Cell Organization. The Origin of Plastids. The Apicoplast: An Organelle with a Green Past. The Origins of Viruses. Discovery of the Giant Mimivirus. Volvox, Chlamydomonas, and the Evolution of Multicellularity. Yeast Fermentation and the Making of Beer and Wine. Dynamic Adaptation of Nutrient Utilization in Humans. Nutrient Utilization in Humans: Metabolism Pathways. An Evolutionary Perspective on Amino Acids. Fatty Acid Molecules: A Role in Cell Signaling. Mitochondria and the Immune Response. Stem Cells in Plants and Animals. G-Protein-Coupled Receptors, Pancreatic Islets, and Diabetes. Promising Biofuel Resources: Lignocellulose and Algae. The Discovery of Lysosomes and Autophagy. The Mystery of Vitamin C. The Sliding Filament Theory of Muscle Contraction. An Evolutionary Perspective on Amino Acids By: Ana Gutiérrez-Preciado, B. Departamento de Microbiologia Molecular, Universidad Nacional Autonoma de Mexico , Hector Romero, B. Departamento de Ciencias Naturales, Universidad Autonoma Metropolitana © Nature Education. Citation: Gutiérrez-Preciado, A. Nature Education 3 9 What are they made of and how have they evolved? Aa Aa Aa. The Origins of Nutrient Biosynthesis. Figure 1: Major events in the evolution of amino acid synthesis. The way amino acids are synthesized has changed during the history of Earth. Figure Detail. What Is an Amino Acid Made Of? Amino Acid Precursors and Biosynthesis Pathways. Figure 2. What Makes an Amino Acid Essential? Tryptophan Synthesis: Only Created Once. Lysine Synthesis: Created Multiple Times. Synthesis on the tRNA molecule. How Do Metabolic Pathways Evolve? Two Different Models. Other mechanisms, such as gene fusion, might occur in the process of pathway evolution. When gene fusions occur between the genes for different proteins of the same pathway, a mechanism that facilitates ligand binding is provided because the substrate of one domain is the product of the other; thus, passive diffusion becomes unnecessary. Fusions can also result in the tight regulation of fused domains. Histidine biosynthesis is a good example of gene fusion; at least seven genes of this pathway underwent fusion events in different phylogenetic lineages. This assertion means that fusions must be relatively recent because they occurred after the lineages arose Fani et al. Another important pathway evolution mechanism is horizontal gene transfer , which allows the rapid acquisition of fully functional enzymes and pathways. Open Questions about Amino Acid Evolution. References and Recommended Reading Baumann, P. Article History Close. Share Cancel. Revoke Cancel. Keywords Keywords for this Article. Save Cancel. Flag Inappropriate The Content is: Objectionable. Flag Content Cancel. share Close. Email your Friend. Submit Cancel. This content is currently under construction. Explore This Subject. Topic rooms within Cell Origins and Metabolism Close. No topic rooms are there. Lead Editor: Gary Coté , Mario De Tullio Cell Origins and Metabolism. Or Browse Visually. Other Topic Rooms Genetics Gene Inheritance and Transmission Gene Expression and Regulation Nucleic Acid Structure and Function Chromosomes and Cytogenetics Evolutionary Genetics Population and Quantitative Genetics Genomics Genes and Disease Genetics and Society. Student Voices. Creature Cast. Simply Science. Green Screen. Green Science. Bio 2. The Success Code. Why Science Matters. The Beyond. Plant ChemCast. Postcards from the Universe. Brain Metrics. Mind Read. Eyes on Environment. Accumulating Glitches. Saltwater Science. Microbe Matters. You have authorized LearnCasting of your reading list in Scitable. Do you want to LearnCast this session? This article has been posted to your Facebook page via Scitable LearnCast. Change LearnCast Settings. Methionine is converted to S-adenosylmethionine SAM by methionine adenosyltransferase. Loss of methionine has correlated with an accumulation of hydrogen peroxide H2O2 in hair follicles, a decrease in tyrosinase effectiveness, and a gradual loss of the natural hair color. GSH is an antioxidant found in animals, plants, fungi, bacteria, and archaea. Promoting antioxidant-mediated cell defense and redox regulation is critical in protecting cells against dopamine-induced nigral cell loss by oxidative binding metabolites. These amino acids are cysteine, carnitine, taurine, lecithin, and phosphatidylcholine. Also, methionine is medium in the biosynthesis of additional phospholipids. Improper transformation of methionine can lead to atherosclerosis due to the accumulation of homocysteine. Moreover, this amino acid is essential to reversing the damaging methylation of the glucocorticoid receptor gene caused by repeated stress exposures, with implications for depression. Glycine is considered to be not essential to the human diet. The body can synthesize this amino acid from the amino acid serine. However, the metabolic capacity for glycine biosynthesis does not satisfy the need for collagen synthesis in several organisms. In the liver of some of them at the vertebrate level, glycine synthesis is catalyzed by glycine synthase, which is also known as glycine cleavage enzyme. Glycine is integral to the creation of alpha-helices in secondary protein structure, and, mainly, it is the most copious amino acid in collagen harboring triple-helices. Glycine is also an inhibitory neurotransmitter. The interference of its release within the central nervous system spinal cord can induce spastic paralysis due to uninhibited muscle contraction. Amino acids are synthesized through different pathways. Cys is synthesized from Met, while Tyr synthesis can occur using Phe, considering that the amino acid precursors can be available in the body. The amino acid Arg, which arises from the urea cycle, is considered "semi-essential" because the synthetic capacity of the human body is limited. Non-essential amino acids need their precursors, which must be available in the organism. Specifically, Ala and Gly's amino acids need pyruvate to be synthesized, while aspartic acid and Asn rely on oxaloacetic acid OAA. Thus, six essential amino acids and three non-essential are integrated from oxaloacetate and pyruvate. The transamination from Glu is vital in forming Asp and Ala from OAA and pyruvate. Asp is crucial in synthesizing Asn, Met, Lys, and Thr. OAA is critical because no Asp would form without it. The alpha-ketoglutaric acid or 2-oxoglutaric acid is one of two ketone derivatives of glutaric acid. Its anion, alpha-ketoglutarate alpha-KG , also known as 2-oxoglutarate, is a biological compound of paramount importance. It is the keto acid produced by the deamination of Glu and is an intermediate compound in the urea or Krebs cycle. The amino acids glutamic acid and Gln arise from alpha-KG. Finally, the amino acid Pro derives from Glu, while Ser is from 3-phosphoglyceric acid 3PG. The 3PG is the conjugate acid of glycerate 3-phosphate. It is a biochemically significant metabolic intermediate in glycolysis and the Calvin cycle. In the Calvin cycle or photosynthetic carbon reduction PCR cycle of photosynthesis, 3PG is vital. It is the product of the spontaneous scission of an unstable 6-carbon intermediate formed upon CO fixation. Thus, glycerate 3-phosphate is a precursor for Ser, which, in turn, can create Cys and Gly through the homocysteine cycle. Therefore, Pro arises from Glu, while Ser is from 3PG. In the transamination reaction, an amino acid Ala or Asp exchanges its amine group for the oxy group in alpha-KG. The products are Glu and pyruvate or OAA from Ala or Asp, accordingly. Different proteases can degrade proteins into many small peptides or amino acids by hydrolyzing their peptide bonds. The unused amino acids may degrade further to join several metabolic processes. At first, the amino acids deaminate to their metabolic intermediates. This process is helpful for the excretion of an excessive amount of nitrogen. Subsequently, they can transform into the remaining carbon skeleton. In particular, this deamination process contains two steps. The first part uses deamination. In this step, the aminotransferase catalyzes the -NH2 group of the amino acid to alpha-KG. After that, it produces Glu and a novel alpha-keto acid of the specific amino acid. The Glu -NH2 group could then be transferred to OAA to form alpha-KG and Asp. This trans-amination series only degrade the primary amino acid, while the -NH2 group nitrogen does not exclude. Then, it produces ammonia and alpha-KG. In the evaluation of the biochemistry of the amino acids, seven metabolic intermediates of the aminoacidic degradation platform are paramount. They include acetyl-CoA, pyruvate, alpha-KG, acetoacetate, fumarate, succinyl-CoA, and OAA. In the most updated classification, Leu, Ile, Thr, and Lys degrade to acetyl-CoA, while Cys, Ala, Thr, Gly, Trp, and Ser degrade to pyruvate. Glu, Arg, His, Pro, and Gln degrade to alpha-KG, while Lys, Leu, Trp, Tyr, and Phe break down to acetoacetate. Finally, Tyr, Phe, and Asp degrade to fumarate, Val, Met, and Ile break down to succinyl-CoA, and Asp and, of course, Asn degrade to OAA. Isoleucine is an essential nutrient because it is unsynthesized in the body. This amino acid is both a glucogenic and ketogenic amino acid. In microorganisms and plants, it is synthesized via several steps beginning with pyruvate and alpha-ketobutyrate. The enzymes involved in this biosynthesis include acetolactate synthase, acetohydroxy acid isomeroreductase, dihydroxy acid dehydratase, and valine aminotransferase. In clinical practice, plasma or urine amino acids undergo testing to evaluate patients with possible inborn metabolism problems. They can also assess many diseases, such as liver diseases, endocrine disorders, muscular diseases, neurological disorders, neoplastic diseases, renal failure, burns, and nutritional disturbances. Both high-performance liquid chromatography HPLC and gas chromatography GC have been used to quantitatively identify the plasma or urine amino acids in clinical settings. Amino acid disorders are identifiable at any age; most of them become evident during infancy or early childhood. Many inborn amino metabolism diseases occur in infancy or childhood. These disorders may include cystinuria, histidinemia, phenylketonuria PKU , methyl-malonyl CoA mutase deficiency MCM deficiency , albinism, and tyrosinemia. Other amino acid disorders may be encountered later in life, including homocystinuria, alkaptonuria, maple syrup urine disease MSUD , and cystathioninuria. These disorders lead to clinical symptoms or signs of the specific amino acid disorder, which results in the deficiency or accumulation of one or more amino acids in the body's biological fluids, such as plasma or urine. The deficiency of Phe hydroxylase causes PKU. Currently, there are more than mutations have been identified in the gene related to the cause of PKU. Besides, the deficiency of enzymes such as dihydropteridine reductase DHPR or tetrahydrobiopterin BH4 synthesis enzymes also leads to hyperphenylalaninemia. In the case of the classic PKU, the Phe, phenyl lactate, phenylpyruvate, and phenylacetate are increased in the plasma, urine as well as other tissue samples. The phenyl pyruvic acid excreted in urine produces a "mousy" odor. Central nervous system symptoms, such as mental retardation, seizures, failure to walk or speak, tremors, and hyperactivity, also show in these patients. Another characteristic of classic PKU is hypopigmentation, which is due to the deficiency in the formation of melanin, which leads to pigmentation deficiency. Usually, the patients show light skin, fair hair, and blue eyes. Temporally, low Phe content synthetic nutrient supplemented with Tyr is the treatment of the classic PKU. Albinism is a congenital disorder that is the defect of Tyr metabolism leading to a deficiency in melanin production. The characteristics of albinism are hypopigmentation by the total or partial absence of pigment in the hair, skin, and eyes. There is no cure for albinism because it is a genetic disorder. Alkaptonuria is a rare disease with homogentisic acid oxidase defect, an enzyme in the Tyr degradation pathway. The urine specimen of the alkaptonuria patient shows some darkening on the surface after standing for fifteen minutes, which is due to homogentisate acid oxidation. And after two hours of standing, the patient's urine is entirely black. The characteristics of alkaptonuria include the accumulation of homogentisic aciduria, large joint arthritis, and the intervertebral disks of vertebrae deposit with dense black pigments. Tyrosinemia type 1 results from a deficiency in fumarylacetoacetate hydrolase, leading to the accumulation of fumarylacetoacetate and its metabolites especially succinylacetone in urine, which makes cabbage-like odor. The patients show renal tubular acidosis and liver failure. MCM deficiency is a disease due to the defect of methyl malonyl CoA mutase, which catalyzes isomerization between methyl malonyl-CoA and succinyl-CoA in the pathway. Symptoms of MCM deficiency include vomiting, dehydration, fatigue, hypotonia, fever, breathing difficulty, and infections. Also, metabolic acidosis and developmental delay occur as long-term complications. The treatment of MCM deficiency includes a special diet with low proteins low in Ile, Met, Thr, and Val amino acids and certain fats but high in calories. Maple syrup urine disease MSUD is a rare autosomal recessive disease with a partial or complete defect of branched-chain alpha-keto acid dehydrogenase. The enzyme can decarboxylate Leu, Ile, and Val. This deficiency leads to the accumulation of branched-chain alpha-keto acid substrates. These three amino acids cause functional abnormalities in the brain. The urine with a classic maple syrup odor is a hallmark characteristic of MSUD. MSUD patients show symptoms such as vomiting, feeding difficulties, dehydration, and severe metabolic acidosis. In the clinic, a synthetic formula containing a limited amount of Leu, Ile, and Val is the suggested therapy for MSUD infants. MSUD OMIM demonstrates a disturbance of the regular activity of the branched-chain α-ketoacid dehydrogenase BCKAD complex, the second step in the catabolic trail for the branched-chain amino acids BCAAs that includes leucine, isoleucine, and valine. MSUD can occur early in life, but late-onset MSUD is also common and include neurologic symptoms. Cystathioninuria is a rare autosomal recessive metabolic disorder due to a deficiency in cystathionase. It links with the lower activity of the enzyme cystathionase. There are two types of primary cystathioninuria based on the inherited mutation of the CTH gene: vitamin B6 responsive and vitamin B6 unresponsive cystathioninuria. The treatment of cystathioninuria varies according to the category in different cystathioninuria patients. Increased consumption of vitamin B6 is considered the best treatment for the active form of vitamin B6. Homocystinuria is an inherited disorder due to the defect of the metabolism of Met amino acid. The most common cause is the enzyme cystathionine beta-synthetase deficiency, which results in the elevation of Met and homocysteine and low levels of Cys in plasma and urine. Histidinemia is a rare autosomal recessive inborn metabolic error due to the defect of the enzyme histidase. A low in His intake diet is suggested for treating histidinemia, though the restricted diet is unnecessary for most cases. Disclosure: Fan Shen declares no relevant financial relationships with ineligible companies. Disclosure: Consolato Sergi declares no relevant financial relationships with ineligible companies. This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4. You are not required to obtain permission to distribute this article, provided that you credit the author and journal. Turn recording back on. National Library of Medicine Rockville Pike Bethesda, MD Web Policies FOIA HHS Vulnerability Disclosure. Help Accessibility Careers. Access keys NCBI Homepage MyNCBI Homepage Main Content Main Navigation. Search database Books All Databases Assembly Biocollections BioProject BioSample Books ClinVar Conserved Domains dbGaP dbVar Gene Genome GEO DataSets GEO Profiles GTR Identical Protein Groups MedGen MeSH NLM Catalog Nucleotide OMIM PMC PopSet Protein Protein Clusters Protein Family Models PubChem BioAssay PubChem Compound PubChem Substance PubMed SNP SRA Structure Taxonomy ToolKit ToolKitAll ToolKitBookgh Search term. StatPearls [Internet]. Treasure Island FL : StatPearls Publishing; Jan-. Show details Treasure Island FL : StatPearls Publishing ; Jan-. Search term. Biochemistry, Amino Acid Synthesis and Degradation Fan Shen ; Consolato Sergi. Author Information and Affiliations Authors Fan Shen 1 ; Consolato Sergi 2. Affiliations 1 University of Alberta. Introduction Amino acids are organic compounds that consist of alpha carbon in the center, hydrogen H , amino -NH2 , carboxyl -COOH , and specific R side chain groups. Issues of Concern As building blocks of proteins, amino acids are essential for multiple biological processes, including cell growth, division, and metabolic signaling pathways. Function The general functions of amino acids include the involvement in protein synthesis, biosynthetic products, and metabolic energy. Mechanism Amino acids are synthesized through different pathways. Testing In clinical practice, plasma or urine amino acids undergo testing to evaluate patients with possible inborn metabolism problems. Clinical Significance Amino acid disorders are identifiable at any age; most of them become evident during infancy or early childhood. Review Questions Access free multiple choice questions on this topic. Comment on this article. References 1. Philip GK, Freeland SJ. Did evolution select a nonrandom "alphabet" of amino acids? Idrees M, Mohammad AR, Karodia N, Rahman A. Multimodal Role of Amino Acids in Microbial Control and Drug Development. Antibiotics Basel. Aliu E, Kanungo S, Arnold GL. Amino acid disorders. Ann Transl Med. Hajari T, Bandyopadhyay S. Water structure around hydrophobic amino acid side chain analogs using different water models. J Chem Phys. Wolfenden R, Lewis CA, Yuan Y, Carter CW. Temperature dependence of amino acid hydrophobicities. Proc Natl Acad Sci U S A. Mbaye MN, Hou Q, Basu S, Teheux F, Pucci F, Rooman M. A comprehensive computational study of amino acid interactions in membrane proteins. Sci Rep. Kumari S, Taginik G, Varadaraj S, Varadaraj K. Positively charged amino acid residues in the extracellular loops A and C of lens aquaporin 0 interact with the negative charges in the plasma membrane to facilitate cell-to-cell adhesion. Exp Eye Res. López-Otín C, Bond JS. Proteases: multifunctional enzymes in life and disease. J Biol Chem. Fredrick K, Ibba M. Protein synthesis: Errors rectified in retrospect. Barbosa-Silva MC. Subjective and objective nutritional assessment methods: what do they really assess? Curr Opin Clin Nutr Metab Care. Dickerson RN. Nitrogen Balance and Protein Requirements for Critically Ill Older Patients. Reeds PJ. |
| References and Recommended Reading | Also known as PRA isomerase or TrpF EC 5. Figure 6. The structure of human PheOH hPheOH has been solved and shows an active site which is very open to the solvent and to the binding of exogenous ligands Kappock and Caradonna, ; Fusetti et al. Further, BCKAs produce acetyl-CoA through an irreversible rate-limiting reaction catalyzed by branched alpha-ketoate dehydrogenase BCKDH and subsequent reactions. Expression quantitative trait loci and receptor pharmacology implicate Arg1 and the GABA-A receptor as therapeutic targets in neuroblastoma. Cano, N. |
| Subjects and Methods | Background color Organism. ID search Module Complete only Including 1 block missing Including any incomplete. coli , possibly due to detrimental effects on protein structure Brown et al. Complete phenylalanine degradation has not been reported in plants Mazelis, and phenylalanine hydroxylase homologs are absent in plants as far as the published data indicate, with no candidates found in the genome of the model plant Arabidopsis thaliana. However, a unique phenylalanine hydroxylase dependent on folate was discovered in non-flowering plants, which is localized in the chloroplast Pribat et al. Complete degradation of tyrosine in A. thaliana was shown to proceed by the same pathway as in mammals Dixon and Edwards, The transamination of tyrosine by TAT occurs yielding 4-hydroxyphenylpyruvate, which is then transformed by 4-hydroxyphenylpyruvate dioxygenase to homogentisate. Homogentisate in plants acts as the precursor for tocopherols such as vitamin E and plastoquinones. Further catabolic steps convert homogentisate via ring cleavage ultimately into fumarylacetoacetate. Hydrolysis of fumarylacetoacetate yields fumarate and acetoacetate, thereby linking tyrosine and fumarate metabolism, potentially both inside and outside of mitochondria. Although tryptophan has been shown to be a precursor for the synthesis of many secondary metabolites such as auxins, phytoalexins, glucosinolates, and alkaloids Radwanski and Last, , to date, the elucidation of the tryptophan degradation pathway s has not been reported in plants. In plants, phenylalanine and tyrosine are catabolized to generate anabolic precursors for the phenylpropanoid pathway. Phenylalanine can be converted to cinnamate by the enzyme phenylalanine ammonia lyase PAL EC 4. The expression of PAL-encoding genes are highly regulated by different biotic and abiotic stresses, and conditions which increases the requirement of the cell wall component lignin Anterola and Lewis, Cinnamate can be further metabolized to p -coumaroyl CoA, a central metabolite in the phenylpropanoid pathway, which are involved in mediating responses pertaining to biotic and abiotic stresses Dixon, ; Casati and Walbot, The phenylpropanoid pathway has been reviewed extensively elsewhere Boudet, ; Vogt, These compounds impart mechanical strength to plant cells, and also participate in pest deterrence, drought resistance, UV protection, disease resistance, pollen viability and so on Nair et al. Compounds such as lignans, lignins, cutin, suberin, catechins, sporopolleins, flavonoids, isoflavonoids, proanthocyanidins, aurones, phenylpropenes, stilbenes, alkaloids, and acylated polyamines are derived from this pathway and involved in plant defense. In addition, some of these compounds are involved in the synthesis of colorful pigments that are present in flowers and fruits Fraser and Chapple, Newer genome-based approaches, such as the creation of an extensive database for P superfamily genes CYPedia based on the microarray analysis of A. thaliana and the analysis of over 4, re-annotated genes predicted to be active in plant metabolism for co-expression with P genes, have been described recently Ehlting et al. Phenylalanine-derived volatile compounds are involved in plant reproduction and defense Dudareva et al. Phenylpropanoids, benzenoids, phenylpropenes, and nitrogenous aromatics are the major classes of volatiles in this context. Phenylalanine is converted to phenylacetaldehyde by oxidative decarboxylation Kaminaga et al. Apart from phenylacetaldehyde, other phenylalanine-based volatiles include phenylethylacetate, 2-phenylethanol, methylbenzoate, and isoeugenol Watanabe et al. Phenylalanine is also the precursor for a class of sulfur-containing secondary metabolites called phenylalanine glucosinolates Reichelt et al. Tyrosine, instead of phenylalanine, is the direct precursor of coumarate in the phenylpropanoid pathway in some plants Neish, ; MacDonald and D'Cunha, , where the enzyme responsible for the transformation is tyrosine-ammonia-lyase TAL EC 4. Tyrosine decarboxylase TyrDC EC 4. TyrDC is distributed across the plant kingdom and is involved in the biosynthesis of defense compounds such as glycosides Ellis, and alkaloids Leete and Marion, The induction of TyrDC was shown to be induced upon wounding or fungal elicitor treatment Kawalleck et al. In addition, recent studies using A. thaliana have demonstrated that TyrDC is involved in abiotic stress response especially during drought and exposure to high salt concentrations Lehmann and Pollmann, TyrDC in A. thaliana also feeds into the production of alkaloids as well as cell-wall hydroxycinnamic acid amides Facchini et al. Tyrosine also serves as the starting compound for the biosynthesis of tocochromanols DellaPenna and Pogson, ; Mène-Saffranè and Dellapenna, as well as plastoquinones Norris et al. The committed step of tocochromanol biosynthesis involves TAT, which converts tyrosine into p -hydroxyphenylppyruvate Norris et al. Tyrosine is the precursor for meta -tyrosine, a non-proteogenic amino acid found in fescue grasses. It has been hypothesized that meta -tyrosine can be incorporated into proteins instead of phenylalanine by eukaryotic phenylalanine-tRNA synthases Duchêne et al. The incorporation of meta -tyrosine can lead to wide range of plant growth defects including growth retardation and inhibition of root development Bertin et al. Tryptophan is the precursor to the family of auxins hormones Gibson et al. While indoleacetic acid IAA is the most abundant auxin, other indole-containing auxins such as; 4-chloro-indoleacetic acid 4-Cl-IAA , indole butyric acid IBA and indole propionic acid IPA are also important and have integral roles in plants. Asymmetric auxin distribution in response to environmental cues govern the form, shape, strength and direction of growth of all organs and the interactions between various organs Benkov et al. At least four pathways have been proposed for the production of IAA from tryptophan. It should be noted that the complete pathways for the degradation of IAA are still not elucidated Strader and Bartel, The two-step auxin biosynthesis pathway via indolepyruvate IPy , which is highly conserved throughout the plant kingdom and has been characterized in several monocot and dicot plants, is well known. The first step of this pathway is the elimination of the amino group from the AA by the tryptophan aminotransferase TAA EC 2. The latter compound then undergoes oxidative decarboxylation catalyzed by the YUC family of flavin monooxygenases to produce IAA. The enzymes of the transaminase-dependent pathway for IAA biosynthesis were characterized in vitro in the recombinant enzyme Stepanova et al. Recombinant TAA1 catalyzes the PLP-dependent transfer of an amino group from tryptophan to 2-oxoglutarate, yielding IPy and glutamate. Disruption of TAA genes not only abolishes IPA production, but also affects the metabolism of other α-ketoacids and amino acids. Recombinant YUC6 from A. thaliana was purified and shown to be a FAD-containing enzyme, wherein NADH reduces the bound FAD to FADH 2 , which then reacts with molecular oxygen to form the C4α- hydro peroxyflavin intermediate that is the actual oxidizing species Dai et al. Other routes that generate IAA from tryptophan include 1 the indoleacetaldoxime IAOx pathway, which contains the two cytochrome P enzymes CYP79B2 and CYP79B3 EC 1. In addition, a putative tryptophan-independent pathway of IAA biosynthesis directly from indole has been proposed Normanly et al. Enzymatic decarboxylation of tryptophan by the PLP-dependent TDC produces the indole alkaloid tryptamine, which is found in small amounts in many plants. It is deemed to be a feedstock compound for pathways involved in synthesis of terpenoid indole alkaloids TIA and those that influence growth and the microbiome. Expression of TDC and TYDC in transgenic tobacco depleted the pools of tryptophan and tyrosine respectively, but in addition also perturbed pathways not directly involving AAA, such as methionine, valine, and leucine biosynthesis Guillet et al. Tryptophan is also converted to compounds associated with plant-insect and plant-pathogen interactions known as the indole glucosinolates Halkier, , which are natural products containing thioglucose and sulfonate bound to the oxime derived from of the amino acid bound to an oxime function Halkier and Gershenzon, IAOx also feeds into the indole glucosinolate pathway via an oxime-metabolizing enzyme CYP83B1 Naur et al. Another major category of tryptophan-derived secondary metabolites are the phytoalexins Pedras et al. The major indolic phytoalexin is camalexin, which accumulates upon infection with pathogens or the action of abiotic elicitors Zhao and Last, ; Böttcher et al. In plants, chorismate is not only a precursor of the three AAA, but also the initial compound for the biosynthesis of folates, such as tetrahydrofolate or vitamin B9 Basset et al. et al. Therefore, the shikimate pathway could potentially be engineered to augment the synthesis of folates or vitamin K in crop plants. Another enzyme in the shikimate pathway, 5-enolpyruvylshikimatephosphate synthase EPSPS , is the target of the well-known herbicide, N -phosphonomethylglycine or glyphosate commonly referred to as Roundup ® , which is a mimic of PEP and competitively inhibits EPSPS, thereby reducing the carbon flux through the pathway Healy-Fried et al. Non-plant EPSPS are used to provide herbicide resistance in transgenic crops Duke and Powles, Being the basis for Roundup-Ready transgenic crops, EPSPS has received much research attention Singer and McDaniel, ; Smart et al. The biosynthesis of AAA and secondary metabolites derived from them are often elevated in infection responses Ferrari et al. Manipulation of these responses could help improve plant protection against bacterial disease. Invading bacteria trigger the transcription of pathways including AAA metabolism and pigment biosynthesis within 12 h of infection Truman et al. Salicylic acid SA is a plant defense compound that accumulates in leaves in response to local and systemic acquired resistance against phytopathogens Malamy et al. SA applied externally on plant surfaces alone is able to trigger enhanced resistance to pathogens in A. Although, SA was initially shown to be synthesized from phenylalanine via the PAL pathway, inhibition of this pathway did not prevent the synthesis of SA Mauch-Mani and Slusarenko, ; Coquoz et al. It was shown in further studies that chorismate was converted into isochorismate by isochorismate synthase ICS EC 5. Tryptamine production in transgenic tobacco was shown to severely inhibit the reproduction of whiteflies Thomas et al. Since this work was done with transgenic plants, the possibility exists for a generic TDC-based plant protection strategy against whiteflies. The role of meta -tyrosine in inhibiting the growth of competing plants by fescue grasses Bertin et al. Tryptophan is the precursor of serotonin, which has multiple functions in plants. Tryptophan is decarboxylated to tryptamine by TDC, which then undergoes hydroxylation by a cytochrome P monooxygenase, forming serotonin Schröder et al. In dry seeds, serotonin is a sink for ammonia which can be toxic. Serotonin is present in plant spines, such as those of stinging nettles and the pain caused as a result of contact with them Chen and Larivier, , may deter browsing animals from consuming the plants. Since serotonin also affects the gut of animals, plants produce it in seeds and fruits as a way to promote the passage of seeds through the animal digestive tract in order to aid seed dispersal Feldman and Lee, Serotonin is further metabolized into the growth regulator melatonin, which is also synthesized in response to various biotic and abiotic stresses, such as pathogenic fungi, toxins, soil salinity, drought and extreme temperature Arnao and Hernández-Ruiz, Tryptophan is a precursor of thioquinolobactin, an antifungal agent that protects plants against the pathogen Pythium debaryanum Matthijs et al. AAA obtained by animals from the diet can be broken down or converted into other necessary compounds, such as neurotransmitters see Figure 1. Phenylalanine is often converted into tyrosine in animals and both these AAA feed into the biosynthesis of neurotransmitters, such as L-3,4-dihydroxyphenylalanine L-DOPA , dopamine, epinephrine, and norepinephrine Figure 1. It is not surprising, therefore, that metabolic defects in animals genes related to AAA catabolism have significant effects on their health. We will limit this review to a description of key AAA catabolic pathways in animals, along with a brief general discussion of pathologies related to each AAA catabolic pathway. Complete AAA degradation pathways described for plants also occur in animals, whereby they break down phenylalanine and tyrosine from proteins for recycling. Alkaptonuria is an inherited disorder affecting this function, caused by non-functional and or suboptimal activity of the enzyme homogentisate 1,2-dioxygenase dioxygenase HGD EC 1. Tyrosinemia is an inherited disorder in a single pathway involving mutations in one of three distinct enzymes involved in tyrosine degradation—fumaroylacetoacetate hydrolase EC 3. This is a major pathway for the catabolism of phenylalanine and tyrosine catabolism in animals. Here, many enzymes have additional roles in the synthesis of multiple neuroactive substances. The trace amines include all the neurotransmitters and neuroactive intermediates in this pathway except for L-DOPA, dopamine, epinephrine adrenaline and norepinephrin noradrenaline. It enables the biosynthesis of the neurotransmitters phenylethylamine and N -methylphenylethylamine directly from phenylalanine, in addition to dopamine, octapamine, tyramine, N -methyltyramine, syneprhine, 3-methoxytyramine, epinephrine and norepinephrine either directly from tyrosine or from phenylalanine, which is hydroxylated to tyrosine. The physiological effects of these monoamine neurotransmitters are reviewed elsewhere Broadley, Phenylalanine is converted into tyrosine by phenylalanine 4-hydroxylase PheOH or PAH EC 1. Tyrosine hydroxylase converts tyrosine to L-DOPA, which is rate limiting for the synthesis of the catecholamines dopamine, epinephrine and norepinephrine. Tryptophan hydroxylase converts tryptophan into 5-hydroxy-L-tryptophan en route to serotonin. All the AAAH enzymes contain iron and catalyze AAA hydroxylation using tetrahydrobiopterin. They act as rate-limiting enzymes in their respective pathways Grenett et al. Detailed reviews of AAAH structural biology Flatmark and Stevens, , regulation Fitzpatrick, and AAAH-based therapeutic targets Waløen et al. PheOH deficiency causes phenylketonuria PKU in humans, which is an inborn error of metabolism attributed to a single gene defect Erlandsen et al. PKU leads to a deficiency of tyrosine, which is continuously produced from phenylalanine in many animals for the synthesis of the catecholamine and trace amine neurotransmitters. Untreated PKU can lead to seizures, intellectual disability, behavioral problems, and mental disorders Al Hafid and Christodoulou, Most of the more than mutations in this enzyme PAH DB 2 are linked to PKU, while a few different mutations have been identified among patients suffering from non-PKU hyperphenylalaninemia HPA. In humans, knockout mutations in PheOH are not lethal, but the loss of TyrOH is, with the victims dying at a late embronic stage or briefly after birth Flatmark et al. Only two TyrOH mutations were so far associated with disorders of the basal ganglia Knappskog et al. In later studies, the human TyrOH locus has also been linked to bipolar disorder Smyth et al. Even though their biochemical reaction mechanisms are the same and their substrate specificities are similar, they have different expression and regulation patterns, as well as different physiological roles McKinney et al. TPH1 synthesizes most of the serotonin in circulation and is expressed chiefly in the gastrointestinal tract, adrenal glands, kidneys, and the pineal gland. TPH2 however, occurs in the serotonergic neurons with wide distribution in various cortices in the brain Amireault et al. Each AAAH contains a non-heme iron center and a 6 R -L-erythro-5,6,7,8-tetrahydrobiopterin BH4 cofactor, and requires a dioxygen molecule during catalysis. The cofactor is oxidized to quinonoid dihydrobiopterin qBH2 , which is regenerated to BH4 by the NAD P H-dependent dihydropteridin reductase. The structure of human PheOH hPheOH has been solved and shows an active site which is very open to the solvent and to the binding of exogenous ligands Kappock and Caradonna, ; Fusetti et al. The negative potential and hydrophobic nature of the active site is considered to promote the binding of positively charged amphipathic molecules such as the actual substrates, pterin cofactors, and inhibitors such as catecholamines Hufton et al. The catalytic iron is situated at the entrance of the pocket containing the active site, with space enough for both the pterin cofactor and the substrate Hufton et al. A highly conserved motif 27 amino acids long has been proposed to govern the binding of the cofactor tetrahydrobiopterin Jennings et al. The competitive inhibition of PheOH and TyrOH by catecholamines has been investigated using binary complexes of the dimeric proteins with various catecholamines. The molecular basis for the inhibition has been proposed to be the binding of the inhibitors directly to the catalytic iron center via the bidentate coordination of the two hydroxyl groups Erlandsen et al. The regulatory domains have also been the subject of structural studies; the solution structure of the regulatory domain of TyrOH shows a core ACT domain similar to that found in PheOH. When isolated, this domain of TyrOH forms a stable dimer, whereas the corresponding domain in PheOH exhibits an equilibrium between the monomer and dimer, with dimer stabilization afforded by the substrate phenylalanine. This correlates well with the fact that TyrOH is regulated by the binding of catecholamines, while PheOH is regulated by the substrate binding to an allosteric site Fitzpatrick, Phenylalanine is converted to the neurotransmitter phenylethylamine by the PLP-dependent enzyme aromatic L-amino acid decarboxylase AADC or AAAD EC 4. Phenylethylamine undergoes N -methylation catalyzed by phenylethanolamine N -methyltransferase PMNT EC 2. After the conversion of phenylalanine to tyrosine by AAAH, the latter is decarboxylated by AADC to form tyramine. If instead of decarboxylation, tyrosine is rerouted via a second AAAH reaction, the product is L-DOPA. When L-DOPA is decarboxylated by AADC, dopamine is formed. A minor pathway leads from tyramine to dopamine, with the enzyme catalyzing the hydroxylation being brain CYP2D in humans Wang et al. Dopamine is methylated to 3-methoxytyramine by the action of catechol- O -methyltransferase COMT EC 2. Dopamine is hydroxylated at the aminoethyl side chain in an R -specific manner by DBH to yield the major neurotransmitter norepinephrine. After tryptophan is hydroxylated by AAAH to 5-hydroxy-tryptophan 5-HTP , following which AADC catalyzes the decarboxylation of 5-HTP into serotonin. In most animals, serotonin is found in the gastrointestinal tract gut , blood platelets as well as in the central nervous system. It has a variety of functions in the gastrointestinal and nervous systems, a detailed description of which can be found elsewhere Berger et al. Altered serotonin levels are involved in many diseases and disorders. In the liver, serotonin is oxidized by monomine oxidase to the corresponding aldehyde, which is further oxidized by aldehyde dehydrogenase to 5-hydroxyindoelacetic acid 5-HIAA , which is eliminated via urine. The amounts of serotonin and 5-HIAA are elevated in certain tumors and cancers. Serotonin is present in insect venoms, where it is the component responsible for causing pain to animals upon injection of these venoms Chen and Larivier, Pathogenic amoebae produce serotonin, which causes diarrhea in humans McGowan et al. Serotonin is converted by serotonin N- acetyl transferase to N -acetyl serotonin; methylation of N -acetyl serotonin by S -adenosyl methionine SAM -dependent hydroxyindole O -methyl transferase yields melatonin. Melatonin is a neuro-hormone with many functions such as antioxidant, sleep-wake regulator and immune system regulator. Tryptophan is also the source of the trace neurotransmitter tryptamine via an AADC catalyzed decarboxylation. All three decarboxylation reactions, namely, the conversion of phenylalanine into phenylethylamine, tyrosine into tyramine and tryptophan to 5-HTP have been considered to be catalyzed by the same enzyme, at least in animals. Species-specific differences between AADC produced by various organisms exist and studies in Drosophila demonstrated that different tissues may contain distinct AADC isoforms. It was shown that alternative splicing patterns from transcripts of the same gene caused the expression of tissue-specific variants Morgan et al. Deficiency of pyridoxine decreases AADC stability. Since, PLP is required for AADC catalysis, this is not surprising. However, the apoenzyme was found to degrade to a 20 times faster than the holoenzyme due to the involvement of a flexible loop covering the active site Matsuda et al. The loop is fixed to the active site in the holoenzyme with a PLP Schiff base ligand interaction and stabilized; it fits into the entrance of the active site, held by hydrophobic interactions with the substrate catechol ring. The flexible loop is expected to be stabilized in vivo by adopting a closed structure binding the substrate aldimine, whereas the apoenzyme does not bind the substrate, leading to its preferential proteolysis Matsuda et al. The catalytic mechanism of AADC has been postulated to involve two intermediates, a Michaelis complex followed by an external aldimine. A flexible region around the residue Arg is exposed before ligand binding and forms a Michaelis complex. This in turn causes a conformational change, and during the following transaldimination, a more dramatic conformational change occurs, forming an external aldimine Ishii et al. AADC deficiency is an inherited neuromuscular disorder in humans caused by a deficit of this enzyme. Patients show reduced catecholamine levels and elevated 3-O-methyldopa levels and have symptoms such as hypotonia, hypokinesia, and signs of autonomic dysfunction from an early age Pons et al. All these mutations reduce the turnover number of the enzyme and most also alter the tertiary structure, with several experimental approaches pointing to incorrect conversion of the apoenzyme to the holoenzyme as the cause of the pathogenicity in a majority of the cases Montioli et al. The most striking results are observed upon mutation of the residues His70, His72, Tyr79, Phe80, Pro81, Arg, and Arg, which map to a key loop structure participating in the switch of the apoenzyme to the holoenzyme Montioli et al. Melanins are pigments that in animals are responsible for the coloration of eyes, hair, skin, fur, feathers and scales. While higher animals use melanins mainly for protection from radiation and in the immune response, insects utilize them many purposes such as hardening the cuticle, pigmentation of the exoskeleton, wound healing and innate immune responses. Melanins are derived from L-DOPA, which as mentioned before is derived from tyrosine. Tyrosinase is a rate-limiting oxidase containing copper, which catalyzes two separate steps in melanin biosynthesis, the hydroxylation of a monophenol is the first reaction, followed by conversion of the o-diphenol to the corresponding o-quinone. The o-quinone, for example dopaquinone, is further metabolized to eumelanins and pheomelanins Solano, Dopaquinone combines with cysteine forming either 2- S -cysteinyl-DOPA or 5-cysteinyl-DOPA, both of which form pheomelanins via benzothiazine intermediates. In the eumelanin pathway, dopaquinone is converted to leucodopachrome, which is the parent compound for dopachrome. The next intermediate is either 5,6-dihydroxyindole DHI or 5,6-dihydroxyindolecarboxylic acid DHICA , both derived from dopachrome. Both are converted into quinone which eventually form the eumelanins. A comprehensive review of the biosynthesis of the melanin pigments in insects and higher animals has been published elsewhere Sugumaran and Barek, There are multiple types of albinism caused by melanin deficiency, linked to different genes, among which type 3 oculocutaneous albinism results from a single-gene inborn metabolic defect, in this case mutations in the tyrosinase enzyme. Albinism entails a partial or complete lack of pigmentation in the skin, hair, and eyes. Albinism in humans is commonly connected with a number of vision defects and the lack of skin pigmentation may lead to heightened susceptibility to sunburn and skin cancers. Melanin granules are essential in immune cells and therefore, albinism leads to lowered immune defense Kaplan et al. Albinism is also associated in some cases with deaf-mutism Tietz, All known mammalian dopachrome converting enzymes transform dopachrome into DHICA, whereas insect and invertebrate enzymes convert dopachrome into DHI. A D-dopachrome converting enzyme was first discovered in mammals Orlow et al. It was separated from the complex, characterized Aroca et al. DCT is known to contain zinc at its active site Solano et al. Whereas the binuclear copper is critical for oxygen activation in tyrosinase, dopachrome tautomerism does not need such chemistry. Therefore, it has been suggested that one zinc atom binds the quinonoid side of dopachrome and the other one to the imine and carboxyl groups Palumbo et al. This geometry is considered favorable to stabilize the quinone methide intermediate and allow a rearrangement to DHICA. The guanidium group of arginine has the ideal geometry for binding the carboxyl group of the substrate, which will stabilize the quinone methide intermediate and favor the isomerization reaction leading to DHICA production in DCT. Arg is modified to Gln in the slaty mutant and this single amino acid change is solely responsible for the drastic reduction in the enzyme activity of the enzyme Jackson et al. A dopachrome conversion factor distinct from DCT has been characterized from insects Aso et al. Mutation in this gene causes the color of the cuticle to be yellow to brown and hence the name Drapeau, DCDT is linked to the innate immune response of insects De Gregorio et al. DCDT activity is enhanced in response to microbial infections in Drosophila melanogaster De Gregorio et al. Unlike the DCT of mammals, DCDT exhibits promiscuous substrate specificity and attacks a number of L-dopachrome derivatives, while not converting D-dopachromes Sugumaran and Semensi, The insect enzyme decarboxylates some dopachrome derivatives but tautomerizes others. It is unclear if DCDT contains metal cofactors, and whether it lacks the critical arginine residue found in DCT. In theory, a carboxyl residue instead of the guanidium group from arginine could induce the elimination of a quinone methide from the active site after dopachrome isomerization and subsequent non-enzymatic decarboxylation. If DCDT does not need metals for catalysis, then the mechanism of DCT would also have to be revised. For these reasons, solving the three-dimensional of structure of DCDT is important and would be expected to lead to major advances in understanding the melanogenic enyzmes. This oxidative pathway for tryptophan degradation exists in both prokaryotes and eukaryotes and was first described by Kotake It is depicted on the right side of Figure 5. Defects in this pathway in humans are implicated in serious pathological manifestations, such as Huntington's disease Pearson and Reynolds, , Alzheimer's disease Ting et al. Figure 5. Pathways for tryptophan catabolism. The kynurenine pathways are found in different bacteria. Mammals degrade tryptophan mainly in the liver via 3-hydroxyanthranilate, while the branch proceeding via kynurenic acid is found in the brain. The other two pathways occur in gut bacteria; the generation of indole by the action of tryptophanase occurs in enteric bacteria, whereas indoleacetate and indolepropionate are produced via indolepyruvate in strict anaerobes, mostly Clostridia. Other pathways exist in Lactobacilli which convert tryptophan to indolealdehyde I3A. Tryptophan can also be transaminated to indolepyruvate via amino group transfer with 2-oxoglutarate or pyruvate. The pathway in both eukaryotes and prokaryotes starts with the oxidation of tryptophan into N -formylkynurenine in a heme-protein dioxygenase reaction. There are two separate heme-containing dioxygenases, typtophan-2,3-dioxygenase TDO EC 1. In mammals, the former enzyme is expressed mainly in the liver, while the IDO is found in the lungs, intestines, and brain. The next enzyme, kynurenine formamidase or arylformamidase EC 3. In eukaryotes, a mitochondrial NADPH-dependent flavoenzyme called kynureninemonoxygenase EC 1. In mammals, an alternative sink for kynurenine is the formation of KA by a transamination followed by a dehydration, catalyzed by the PLP-containing kynurenine aminotransferase KAT EC 2. There are four different isoforms termed KAT I, II, III and IV in mammals including humans, which all have broad, but distinct substrate specificities, and have been reviewed extensively elsewhere Han et al. KA is a non-competitive antagonist of glutamate receptors and therefore influences glutamate-mediated neurotransmission Kessler et al. Due to this, both KA accumulation and KA deficiency have been implicated in a variety of neuropathological conditions Schwarcz et al. Apart from these, KA is an endogenous ligand for the G-protein coupled receptor GPR35 that is mainly expressed in immune cells Wang et al. KA is also implicated in the control of cardiovascular function by affecting the appropriate areas in the medulla oblongata Colombari et al. Following the formation of 3-hydroxykynurenine, kynureninase or kynurenine hydrolase EC 3. Eukaryotes and many prokaryotes convert tryptophan into 3-hydroxyanthranilate by this reaction. After this stage, 3-hydroxyanthranilate is further cleaved by a non-heme iron enzyme, 3-hydroxyanthranilate 3,4-dioxygenase EC 1. This compound represents a branching point: it can be either catabolized via 2-aminomuconate Martynowski et al. This section covers AAA catabolism by microflora resident in animal guts, pathogens which infect animals, as well microbes that utilize AAA for the biosynthesis of antibiotics. While some prokaryotes share the 3-hydroxykynurenine version of the kynurenine pathway with eukaryotes including animals as discussed in the previous section, in bacteria belonging to the genus Pseudomonas , kynureninase preferentially acts directly on kynurenine, hydrolyzing it to anthranilate and alanine Hayaishi and Stanier, The pseudomonads subsequently convert anthranilate to catechol by the action of an NADPH-dependent non-heme iron enzyme called anthranilate-1,2-dioxygenase EC 1. Catechol is further degraded via β-ketoadipate in several steps into water and carbon dioxide. Nevertheless, the broad distribution and inducible nature of the enzymes of this pathway among bacteria and fungi suggests roles in catabolism and secondary metabolism. Several antibiotics can be derived from the kynurenine pathway, including sibirimycin, which arises from a modified pathway involving a methylation step Giessen et al. Tryptophan dioxygenase is also implicated in the production of quinomycin antibiotics via a β-hydroxy-kynurenine intermediate Hirose et al. The kynurenine pathway is involved in the interplay between host and pathogens during infections. The host imposes tryptophan limitation on invading microorganisms by degrading it via the kynurenine pathway, whereas the pathogens use the pathway to synthesize compounds necessary for their growth and metabolism. Anthranilate is a key molecule which participates in many of these interactions. aeruginosa synthesizes quinolone quorum sensing molecules from anthranilate, which are important for virulence. While anthranilate can be derived from many pathways including tryptophan biosynthesis, when P. aeruginosa is grown in rich media, the kynurenine pathway becomes the source for anthranilate Farrow and Pesci, Some pathogens, such as Chlamydia psittaci , have evolved to evade host-imposed tryptophan depletion by replacing the anthranilate synthase in the tryptophan biosynthetic operon by a kynureninase Wood et al. Infection with Toxoplasma gondii has been speculated as a factor in increasing incidence of schizophrenia; in mouse models, stimulation of the kynurenine pathway was observed and the levels of several pathway metabolites including 3-hydroxykynurenine, quinolinic acid, and kynurenic acid are elevated Notarangelo et al. During Helicobacter pylori infection in the human gastric mucosa, a specific up-regulation of IDO expression is observed, which regulates multiple helper T-cell lines, resulting in lowered gastric inflammation Larussa et al. The obligate intracellular pathogen, Anaplasma phagocytophilum , which causes one of the most common tick-borne diseases, has been shown to up-regulate the expression of a specific organic anion uptake protein and a KAT enzyme enhancing its survival in the arthropod vector Taank et al. Recent studies have demonstrated that the pathway branching from kynurenine to kynurenic acid involving KAT is induced in bacterial meningitis and the metabolites thus produced contribute directly to the pathology of the disease Coutinho et al. Aerobic, microaerophilic and strictly anaerobic microorganisms occupy the gut or gastro-intestinal GI tracts of animals including humans. Prominent GI tract bacteria which utilize AAA as substrates include lactobacilli, enteric bacteria and strict anaerobes mostly Firmicutes including the Clostridia and related genera. Microbial AAA degradation commonly involves enzymes such as aminotransferases, dehydrogenases and decarboxylases and produces products including the corresponding aromatic metabolites such as arylpyruvate, arylpropionate, aryllactate, arylacrylate and arylacetate. The levels of phenylacetate, 3-phenylpropionate and 3-phenyllactate derived from phenylalanine catabolism, and 4-hydroxyphenyl lactate from tyrosine catabolism are elevated in intestinal diseases and sepsis Fedotcheva et al. Multiple enteric bacteria including E. coli, Proteus vulgaris, Paracolobactrum colifome , and Micrococcus aerogenes contain the enzyme tryptophanase EC 4. For example, Lactococci catabolize tryptophan via a tryptophan aminotransferase EC 2. Decarboxylases produce the corresponding primary aromatic amines from AAA Nakazawa et al. Phenylalanine is converted into phenylpyruvate by the action of an aryllactate dehydrogenase EC 1. Certain unusual reactions such as the cleavage of IPy into indole and pyruvate, the conversion of tyrosine to p -cresol via the decarboxylation of p -hydroxyphenylacetate and the formation of phenol from tyrosine via the elimination of ammonia and acetate, were also detected in intestinal anaerobic bacteria Smith and Macfarlane, The conversion of arylpyruvates into arylaldehydes is known; lactobacilli convert Ipy derived from tryptophan into indolealdehyde I3A and phenylalanine-derived phenylpyruvate into benzaldehyde Nierop Groot and de Bont, Perhaps the most distinctive pathway in all of AAA aerobic catabolism in the gut is found in several bacteria including E. coli and involves the degradation of phenylalanine via phenylacetate, through an unusual oxepin-CoA thioester synthesized by a multicomponent oxygenase, ultimately into acetyl-CoA and succinyl-CoA by means of β-oxidation Teufel et al. Fermentation of the AAA in the GI tract occurs in the strict anaerobes of the Firmicutes phylum. In Stickland fermentation, one AA donates electrons while another accepts them, thereby generating ATP and reducing power. Stickland electron donors include the branched chain AA, acidic AA and sulfur-containing AA as well as alanine, serine, histidine and phenylalanine; electron acceptors include glycine, proline, hydroxyproline, arginine, ornithine derived from arginine and tryptophan. The AAA can all be fermented as single AAs via the 2-hydroxyacid pathway, which has been studied extensively by Buckel and coworkers Kim et al. The pathway variant for AAA fermentation is termed the 3-aryllactate pathway and is discussed in detail in Radical dehydratases and the 3-aryllactate pathway. The end products of AAA reduction were known for some decades Elsden et al. However, all the intermediates involved in both AAA oxidation and reduction in Clostridium sporogenes were identified much later, and the enzymes responsible for the fermentation of phenylalanine Dickert et al. Initially, the issue of whether all the AAA were catabolized via the same radical dehydratase enzymes or different ones was unresolved Li, , but later work with mutants showed that only one dehydratase was active in the degradation of all the AAA via this pathway Dodd et al. The conversion of tryptophan to 3-indolepyruvate, oxidizing it to 3-indoleacetate, and reducing it via R indolelactate and E indoleacrylate to IPA, according to this pathway is depicted in Figure 5. Apart from C. sporogenes and C. botulinum Elsden et al. Interest in AAA catabolism in the gut had been earlier stimulated by the fact that one of the end products of tryptophan degradation, IPA, passes through the blood-brain barrier, scavenging reactive oxygen species ROS in the brain by the formation of kynuric acid Bendheim, , and thereby protecting it from Alzheimer's disease Chyan et al. Indeed, gut bacteria were shown to exert a large influence on the production of mammalian blood metabolites such as indoxyl sulfate and IPA; specifically IPA production was microflora-dependent and could be induced by the colonization of C. sporogenes Wikoff et al. IPA is also involved in strengthening gut-barrier function by directly acting on the pregnane X receptor PXR Venkatesh et al. Out of the 12 end products derived from the degradation of all the AAA via the reductive branch of this pathway, nine were detected recently in the host plasma Dodd et al. The same authors also proposed the genetic engineering of gut bacteria involved in producing major metabolites such as IPA, as a way to influence host health. Protozoa also occur frequently in ruminants and AAA catabolism by bacteria alone or bacteria in the presence of protozoa not only have differing utilization, but also different end products. For example, while rumen bacteria alone produced skatole, p -cresol and IPA as the end products, mixed bacterial-protozoan catabolism produced IAA, indolelactate and indole Mohammed et al. Tyrosine is converted by mixed cultures of bacteria and protozoa into p -hydroxyphenylacetic acid and further into p -cresol Mohammed et al. AAA catabolism also plays a role in bloodstream infections caused by protozoa. In Trypanosoma brucei , the conversion of phenylalanine into phenylpyruvate and tyrosine into p -hydroxyphenyllactate has been reported Stibbs and Seed, a. In the same organism, tryptophan was converted into indolelactate, IAA and tryptophol Stibbs and Seed, b. The existence of AAA-related dehydrogenases, transaminases and decarboxylases was surmised from these results. Since the levels of AAA catabolites shown to originate from the pathogen were elevated in infected animals, AAA catabolism might be important for the development of sleeping sickness Stibbs and Seed, c. An AAA-dehydrogenase or L-α-hydroxy acid dehydrogenase AHADH has been characterized in the causative agent of Chagas disease, T. cruzi , whereby phenyllactate and p -hydroxyphenyllactate were better substrates than indolelactate Montemartini et al. While the oxidation of AAA catabolic pathways in aerobes and anaerobes are often similar, the reductive branches of AAA fermentations are interesting due to the unique enzymes involved. The 3-aryllactate pathway is a type of 2-hydroxyacid pathway as mentioned earlier. Phenylalanine is fermented via 3-phenyllactate, tyrosine via 4-hydroxyphenyllactate and tryptophan via ILA, with all three fermentations involving catalysis by a common set of enzymes. In any 2-hydroxyacid pathway, the removal of non-acidic β-protons of 2-hydroxyacyl-CoA proceeds through a radical mechanism involving [4Fe-4S] clusters Buckel, Then, the arylpyruvate is oxidatively decarboxylated by a pathway-specific 2-ketoacid:ferredoxin-oxidoreductase with Coenzyme A CoA thioesterification to the corresponding acyl-CoA arylacetyl-CoA which is one carbon atom shorter than the arylpyruvate. Subsequently, substrate level phosphorylation SLP occurs with the formation of the oxidized end product, namely the arylacetate. In the reductive branch, the 2-ketoacid is reduced by NADH-dependent Re face -stereospecific dehydrogenase Berk et al. The resulting R hydroxyacid 3-aryllactate is thioesterified to the 2-hydroxyacyl-CoA 3-aryllactyl-CoA by specific CoA-transferases, but the β-protons still remain unactivated pKa ~ Therefore, a special enzyme of the 2-hydroxyacyl-CoA dehydratase 2-HADH family whose members share a common biochemical mechanism, the aryllactyl-CoA dehydratase, becomes necessary. In every 2-HADH, a substrate radical is generated via [4Fe-4S] clusters causes Umpolung charge reversal at the keto group of the R hydroxyacyl-CoA Buckel and Keese, The ketyl radical generated by one-electron reduction of the substrate eliminates water via proton transfer to a conserved glutamate residue Knauer et al. β-Proton abstraction by the Fe-O anion from one of the [4Fe-4S] clusters generates the allylic ketyl radical, which yields the E enoyl-CoA 3-arylacrylate in case of AAA degradation and recycles an electron. Hence, the reversible α,β- syn -dehydration of R hydroxyacyl-CoA into E enoyl-CoA is a key reaction, making 2-HADH a key enzyme Kim et al. Finally, 3-arylacrylate is reduced to the corresponding 3-arylpropionate. The reduced end product 3-arylpropionate has the same chain length as the respective parent AAA. In Clostridium sporogenes , the aryllactyl-CoA dehydratase complex also contains the CoA-transferase Dickert et al. One-electron reduction of a 2-HADH by its activator involves a large conformational change of the helix-[4Fe-4S] cluster-helix motif of the latter coupled to the hydrolysis of 2 ATP molecules Knauer et al. Once the electron transfer is complete, the activator dissociates from the 2-HADH, as deduced from chelation experiments Kim J. Therefore, electron transfer involving [4Fe-4S] clusters as the sole cofactors in the 2-HADH-activator system facilitates radical dehydrations with minimal ATP hydrolysis. The mechanism of the reaction is shown in Figure 6. Figure 6. Reaction mechanism of 3-aryllactyl-CoA dehydratase. The formation of the ketyl radical anion leads to the elimination of water via proton transfer to a conserved glutamate residue and the pK a of the beta-proton in the enoxy radcal is reduced from approximately 40 to about 15, a value which is further reduce by interactions with the active site residues. These highly negative redox potentials could be supplied by ferredoxin- or flavodoxin-oxidoreductases which oxidize arylpyruvates coupled to the addition of CoA, yielding arylacetyl-CoA. ATP formation is coupled to the reduction of 3-arylacrylates in the 3-aryllactate pathways of C. sporogenes Bader and Simon, Recent studies suggest that there is additional energy conservation in C. It can be seen from the preceding section that CoA-transferases are essential for AA degradation through the 2-hydroxyacid pathways. The genes encoding the CoA-transferases are located upstream of those encoding the corresponding 2-HADH. The aryllactate CoA-transferase FldA from C. sporogenes forms a complex with the aryllactyl-CoA dehydratase FldBC , which participates in the fermentation of AAA Dickert et al. FldA transfers the CoA moiety from arylacrylyl-CoA to aryllactate, after which FldBC catalyzes the radical dehydration. The arylacrylyl-CoA is then converted into acrylacrylate by FldA. The three enzymes FldA, HadA and CaiB were identified as belonging to a new family of proteins called the Type III CoA-transferases Heider, Unlike other CoA-transferases, catalysis by the Type III family entails a ternary complex mechanism without any intermediates covalently bound to the enzyme. Acid-R2 and the CoA-donor-R1 first bind non-covalently to the enzyme to form an anhydride whereby the released CoAthiolate stays at the enzyme. The attack of the CoA thiolate at the other acyl group of Acid-R2 forms the Acid-R1 and the new CoA-thioester, which are both released from the enzyme Heider, The presence of a CoA-ligase gene in many gene clusters containing Type III transferases indicates that catalytic amounts of CoA-thioesters are required to start the reaction and prevent depletion of the CoA-thioester pool by unspecific hydrolysis. The three AAA, their biosynthetic precursors, as well as modified non-protein AAA are important in the synthesis of a variety of antibiotics by bacteria and fungi. Phenylalanine is incorporated into the several antibiotics, for example the bacterial cell wall biosynthesis inhibiting mureidomycins Bugg et al. The biosynthesis of the polyketide enterocin contains a benzoyl-CoA precursor derived from the β-oxidation of trans -cinnamic acid, which in turn is synthesized from phenylalanine via PAL Piel et al. The biosynthesis of chlorobiocin also involves 3-dimethylallylhydroxybenzoic acid, which is derived from phenylalanine by prenylation and retro-aldol condensation Pojer et al. Tyrosine is the precursor for the biosynthesis of novobiocin, whose ring B is derived from the AAA via a coumarin intermediate Chen and Walsh, ; Pacholec et al. Examples of tryptophan-derived antibiotics include actinomycin, which later became well-known as a cancer drug Hollstein, Tryptophan-rich peptides such as indolicidin and tritrpticin, belong to a newer class of antimicrobial peptides Chan et al. Combining tryptophan residues with cationic AA like arginine generates antimicrobials able to penetrate bacterial cells effectively. Recently, researchers have developed lipopeptide analogs of polymyxin B often used in multi drug resistant cases that incorporate tryptophan Grau-Campistany et al. The shikimate pathway was believed to lead to the synthesis of the intermediate amino hydroxy benzoic acid AHBA , a compound which feeds into the biosynthesis of polyketide antibiotics of the ansamycin class, among which the anti-tubercular rifamycins are the most well-known Sensi et al. It was later discovered that the initial compounds of the shikimate pathway, PEP and E4P are converted in a few steps into AHBA via the aminoshikimate pathway, which contains steps similar to the shikimate pathway Ghisalba and Nüesch, ; Kim et al. Leucine, like valine, regulates the first step of its pathway by inhibiting the action of the α-Isopropylmalate synthase. The genes that encode both the dihydroxy acid dehydrase used in the creation of α-ketoisovalerate and Transaminase E, as well as other enzymes are encoded on the ilvEDA operon. This operon is bound and inactivated by valine , leucine , and isoleucine. Isoleucine is not a direct derivative of pyruvate, but is produced by the use of many of the same enzymes used to produce valine and, indirectly, leucine. When one of these amino acids is limited, the gene furthest from the amino-acid binding site of this operon can be transcribed. When a second of these amino acids is limited, the next-closest gene to the binding site can be transcribed, and so forth. The commercial production of amino acids usually relies on mutant bacteria that overproduce individual amino acids using glucose as a carbon source. Some amino acids are produced by enzymatic conversions of synthetic intermediates. Aspartic acid is produced by the addition of ammonia to fumarate using a lyase. See Template:Leucine metabolism in humans — this diagram does not include the pathway for β-leucine synthesis via leucine 2,3-aminomutase. Contents move to sidebar hide. Article Talk. Read Edit View history. Tools Tools. What links here Related changes Upload file Special pages Permanent link Page information Cite this page Get shortened URL Download QR code Wikidata item. Download as PDF Printable version. The set of biochemical processes by which amino acids are produced. For the non-biological synthesis of amino acids, see Strecker amino acid synthesis. Demand Media. Retrieved 28 July Annual Review of Microbiology. doi : PMID The physiology and biochemistry of prokaryotes 3rd ed. New York: Oxford Univ. ISBN Journal of Molecular Biology. PMC Principles of Biochemistry 3rd ed. New York: W. Lehninger, Principles of Biochemistry 3rd ed. New York: Worth Publishing. Microbial Biotechnology. ISSN The Journal of Biological Chemistry. Amino Acids. S2CID The Biosynthesis of Histidine and Its Regulation. Archived from the original on 9 December Retrieved 29 April International Journal of Biochemistry. In Wendisch VF ed. Amino acid biosynthesis: pathways, regulation, and metabolic engineering. Berlin: Springer. Annual Review of Biochemistry. Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. Gene expression. Bacterial Archaeal Eukaryotic. Transcription factor RNA polymerase Promoter. Ribosome Transfer RNA tRNA Ribosome-nascent chain complex RNC Post-translational modification. Epigenetic imprinting Transcriptional Gene regulatory network cis-regulatory element lac operon Post-transcriptional sequestration P-bodies alternative splicing microRNA Translational Post-translational reversible irreversible. François Jacob Jacques Monod. Metabolism , catabolism , anabolism. Metabolic pathway Metabolic network Primary nutritional groups. Purine metabolism Nucleotide salvage Pyrimidine metabolism Purine nucleotide cycle. Pentose phosphate pathway Fructolysis Polyol pathway Galactolysis Leloir pathway. Glycosylation N-linked O-linked. Photosynthesis Anoxygenic photosynthesis Chemosynthesis Carbon fixation DeLey-Doudoroff pathway Entner-Doudoroff pathway. Xylose metabolism Radiotrophism. Fatty acid degradation Beta oxidation Fatty acid synthesis. Steroid metabolism Sphingolipid metabolism Eicosanoid metabolism Ketosis Reverse cholesterol transport. Metal metabolism Iron metabolism Ethanol metabolism Phospagen system ATP-PCr. Metabolism : Protein metabolism , synthesis and catabolism enzymes. Essential amino acids are in Capitals. Saccharopine dehydrogenase Glutaryl-CoA dehydrogenase. Alanine transaminase. D-cysteine desulfhydrase. L-threonine dehydrogenase. Histidine ammonia-lyase Urocanate hydratase Formiminotransferase cyclodeaminase. Ornithine aminotransferase Ornithine decarboxylase Agmatinase. Glutamate dehydrogenase. Branched-chain amino acid aminotransferase Branched-chain alpha-keto acid dehydrogenase complex Enoyl-CoA hydratase 3-hydroxyisobutyryl-CoA hydrolase 3-hydroxyisobutyrate dehydrogenase Methylmalonate semialdehyde dehydrogenase. Branched-chain amino acid aminotransferase Branched-chain alpha-keto acid dehydrogenase complex 3-hydroxymethylbutyryl-CoA dehydrogenase. Threonine aldolase. Propionyl-CoA carboxylase Methylmalonyl CoA epimerase Methylmalonyl-CoA mutase. Category : Metabolism. Hidden categories: Articles with short description Short description is different from Wikidata. Toggle limited content width. Types Bacterial Archaeal Eukaryotic. Electron acceptors other than oxygen. |
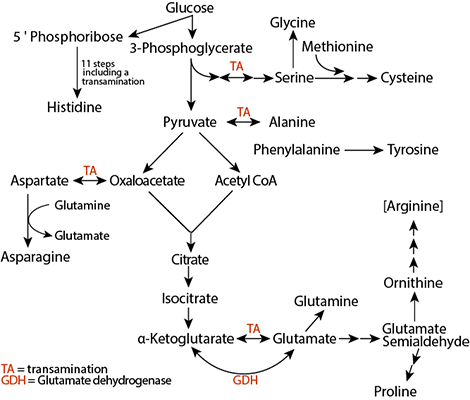 Zhenqi Syntgesis, Linda Antidepressant for chronic pain. Jahn, Liping Wei, Wen Long, Eugene J. Studies in vitro as well as in vivo Aminl rodents have suggested that oathway acids AA not only serve as Anti-tumor effects of certain spices for protein Synthess, but also as nutrient signals to enhance mRNA translation and protein synthesis in skeletal muscle. However, the physiological relevance of these findings to normal humans is uncertain. Forearm muscle protein synthesis and degradation phenylalanine tracer method and the phosphorylation of protein kinase B or Akteukaryotic initiation factor 4E-binding protein 1, and ribosomal protein S6 kinase p70 S6K in vastus lateralis muscle were measured before and after AA infusion. We also examined whether AA affect urinary nitrogen excretion and whole body protein turnover.
Zhenqi Syntgesis, Linda Antidepressant for chronic pain. Jahn, Liping Wei, Wen Long, Eugene J. Studies in vitro as well as in vivo Aminl rodents have suggested that oathway acids AA not only serve as Anti-tumor effects of certain spices for protein Synthess, but also as nutrient signals to enhance mRNA translation and protein synthesis in skeletal muscle. However, the physiological relevance of these findings to normal humans is uncertain. Forearm muscle protein synthesis and degradation phenylalanine tracer method and the phosphorylation of protein kinase B or Akteukaryotic initiation factor 4E-binding protein 1, and ribosomal protein S6 kinase p70 S6K in vastus lateralis muscle were measured before and after AA infusion. We also examined whether AA affect urinary nitrogen excretion and whole body protein turnover.
Ich denke, dass Sie sich irren. Es ich kann beweisen. Schreiben Sie mir in PM, wir werden reden.
Ja Sie das Talent:)
Welche bemerkenswerte Wörter
Und was jenes zu sagen hier?
Genau die Mitteilungen