Biofilm control -
When conditions become unfavorable, they propagate by releasing bacteria into the bulk water to inhabit other surfaces. Multiple survival mechanisms make biofilms incredibly tenacious and difficult to control.
Should this be a concern? Our patented bioeXile ® cleaning solution paired with our award winning bioDART ® biofilm monitor is new, cutting-edge technology to clean your cooling water systems and keep them clean. bioeXile Cleaning Solutions bioeXile works with biocide additions to remove biofilms more effectively than using biocides alone or with biodispersants.
bioDART Biofouling Monitor bioDART Biofouling Monitor routinely measures and monitors systems to gauge microbial control and provide an early warning of problems.
Video: How Biofilms Form Biofilms can cause a wide range of problems in building water systems. Effective biofilm control is key for safe, reliable, and efficient operation of water systems.
Download SDS. DON'T LET BIOFILMS TAKE CONTROL Biofilms are communities of surface-attached bacteria growing inside a protective microbial secretion often called slime. Biofilms Cause Serious Problems. Scientists from the U. With each passing year, new and emerging technologies and techniques that have promise for advancing food safety are developed and validated.
This article summarizes the top food safety innovations of , based on audience interest. A recent study has demonstrated the efficacy of antimicrobial blue light technology for the inactivation of both dried cells and biofilms of Listeria monocytogenes on surfaces found in food processing environments.
A project funded by the Center for Produce Safety has collected information about, validated, and evaluated the efficacy of the cleaning and sanitation practices for harvest equipment among blueberry harvesters and packers. Pseudomonas biofilms can aid the survival of Listeria monocytogenes cells even after disinfection, according to a recent study.
Ongoing research funded by the Center for Produce Safety aims to evaluate the efficacy of commercially available sanitizers against common foodborne pathogens and biofilms encountered during tree fruit harvesting, and then conduct a validation study of the best-performing treatments at commercial facilities.
A recent study has demonstrated the antimicrobial efficacy of a novel organic acid compound against common foodborne pathogens and biofilm. Home » Topics » Sanitation » Biofilm Control.
Biofilm Control RSS. Study Highlights Importance of Addressing Biofilm-Forming Pathogens to Control Listeria in Food Processing Facilities Food Safety Magazine Editorial Team. Efficacy of Eco-Friendly Sanitation Methods Against Listeria Biofilms in Food Production Environments Bailee Henderson.
Defects, Organic Matter on Food Contact Surfaces Reduces Sanitizer Efficacy Against Listeria Biofilms Food Safety Magazine Editorial Team. New Insights About Salmonella Interactions With Environmental Biofilms May Lead to Better Food Safety Strategies Food Safety Magazine Editorial Team.
Biofilms play Biofilm control pivotal role in Biofilm control infections, especially Rich herbal coffee substitute related cintrol the Biofilm control of contgol devices, Biofilm control, such Biifilm Biofilm control catheters, urinary catheters and orthopaedic implants. Biofilm control paper reviews contorl most Biofilm control approaches for the control Amino acid synthesis pathway in humans prevention of these infections as well as Biofilm control perspectives for the development of novel devices refractory to microbial adhesion, colonization and biofilm formation. Biofilms play a pivotal role in healthcare-associated infections HAIsespecially those related to the implant of medical devices, such as intravascular catheters, urinary catheters and orthopaedic implants. aspx ], annually, approximately 4 patients are estimated to acquire an HAI in European hospitals. The number of deaths occurring as a direct consequence of these infections is estimated to be at least 37 In the United States, 1 HAIs have been estimated for the yearincluding in intensive care units ICUs and 1 outside ICUs. This Biofilm control detects the biofilm behaviour of microorganisms and cojtrol automated in BioFilm Contrkl markets BioFilm Ring Test® and Biofilm control Resting oxygen consumption in well plate format for Bipfilm use only. The Antibiofilmogram® kits study the susceptibility of bacteria to antibiotics, both in planktonic and biofilm populations. Clinical protocols are under way and the first MD-IVD versions with CE marking are scheduled for BioFilm Control currently employs 11 people working in premises of m², 90m² of which dedicated to a P2 laboratory.
Biofilm control -
We speculate that active topography may change the physiology of detached cells to a more active stage, leading to increased antibiotic susceptibility, as we observed for the cells detached by topographic changes of SMP To understand if active topography causes such effects, we conducted a qPCR experiment to track the changes in the expression of rrn B genes rrs B , rrl B , and rrn B P1 right after the 3-min on-demand actuation, and after 7 and 30 min further incubation in 0.
These genes encode 16s rRNA, 23s rRNA, and the space-1 region, respectively, and have been used to track the expression level of rrn B operon to monitor cellular activities 42 , The expression level of rrn B operon positively correlates with the growth rate of bacterial cells Thus, the induction of rrn B genes in cells detached by active topography can help explain the increase in antibiotic susceptibility of these detached cells.
This result is consistent with the 3. On-demand actuation also sensitized mature P. aeruginosa PAO1 biofilms to bactericidal tobramycin Tob compared to cells dispersed by bead beating and those inside intact biofilms with 0.
These results indicate that the increase in antibiotic susceptibility of cells detached by active topography is species non-specific.
To evaluate the clinical potential of this design, we further tested its safety to mammalian cells. Because PDMS has been widely used in medical implants 37 , the material itself is not a concern.
Actuation of the PDMS pillars in our design is powered by an electromagnetic field produced from solenoid coils. The copper wires are insulated, and therefore, the electric current is not a safety concern. We thus focused our study on evaluating if there is heat production by the electric current for generating the external magnetic field and the possible leaching of MNPs from the tips of pillars.
Both modeling details shown in Supplementary Methods 1. These temperature changes are negligible. The local heat generation at the pillar tip is also negligible details shown in Supplementary Methods 1.
The results revealed no change in cell viability Fig. a , b Schematic of the experimental setup a and a cross-sectional view of the catheter prototype b. Another factor to consider for the design is the biocompatibility of MNPs that are embedded at the tips of PDMS pillars.
Magnetic iron oxide nanoparticles have been widely used in biological and biomedical applications with good biocompatibility In this study, only 7. Since the detection limit of this assays is 3. Biofilm formation in urinary catheters is the primary cause of CAUTI 3.
The strong antifouling effects of active topography bring a unique opportunity for engineering self-cleaning medical devices such as catheters. The magnetic field was generated using an insulated copper coil, and the beating frequency was controlled by a programmable time delay relay.
Similar blockage of the smooth control catheter without pillars; Fig. Consistent with the strong antifouling activities to remain clear, the prototype catheters with active topography also had much fewer cells associated [ 8.
a Schematic of the flow cell system for testing long-term antifouling activities of the prototype catheters. A zoom-in view shows the coil section. b Antifouling activities of the prototype catheters. The bar graph shows the CFU per unit interior lumen area.
The representative images show the complete blockage visible white substance of static control on day 3 and flat control on day 5 catheters, while the prototype catheters remained clear on day The coils for generating the magnetic field were removed before imaging in Fig.
The severe problems associated with drug-resistant biofilm infections stimulated increasing interest in developing bacterial control strategies that do not require antimicrobial agents A number of static topographies have been shown to inhibit biofilm formation for varying duration 48 , 49 , 50 , However, bacteria can eventually overcome unfavorable topographies through growth and biofilm matrix production We recently reported biofilm control using dynamic topography via an on-demand change in surface topography of SMP This method achieved up to 3-log removal of mature biofilms of both bacteria and fungi; however, shape recovery can only occur a limited number of times, and in most SMP systems, only once.
Effective systems for long-term fouling control are still missing. In this study, we developed a fouling control strategy based on active topography that is programmable and applicable to a variety of polymeric materials.
This is achieved by remotely controlling the beating of rationally designed micron-size pillars using an external electromagnetic field. This system is programmable, and thus, has the potential to be integrated with other sensors to engineer smart medical devices that can adjust pillar beating frequency and force for microbial control.
Most biofilm control technologies developed to date aim to prevent microbial attachment e. Here we demonstrate that active topography developed in this study can achieve both controls with a tunable force level generated by beating pillars.
According to Bottier et al. In this study, active pillars were embedded in mature biofilms formed in static growth medium with mechanical properties similar to hydrogels e. aeruginosa PAO1 biofilm formed in LB medium is 2. Using numerical simulation with CPS4R element, the beating of active pillars with a modulus of 2.
The maximum stress can reach This is strong enough to disrupt the 3D P. This stress was also strong enough to disrupt S. aureus ALC biofilms, which were the most challenging ones in this study. It is worth noticing that it only took 0. Hence, there is a large room for further optimization. Apparently, the mechanical properties of biofilms formed by different species can affect the efficiency of active pillars, which deserves future study.
In addition to removing biofilm cells from the surface, active topography also rendered the detached cells more susceptible to bactericidal antibiotics. For instance, UPEC ATCC biofilm cells dispersed by on-demand actuation was more sensitive with 1.
Consistently, we observed an increase in the expression of rrn B genes in cells dispersed by active pillars, compared to the cells remaining on the surface and biofilm cells dispersed by bead beating.
Recently, we reported that SMP-based dynamic topography induced biofilm dispersion and sensitized P. aeruginosa PAO1 biofilms to bactericidal antibiotics Such biofilm dispersion was found to increase the intracellular level of ATP and expression of the rrn B gene Thus, active surface systems represent a strategy with added benefits to eradicate biofilm cells and may help control chronic infections through synergy with conventional antibiotics.
The long-term antifouling activities of the prototype catheter and the safety of this system to mammalian cells demonstrated the potential of this design for controlling CAUTI. In addition to the significant reduction of bacterial load, the prototype catheter was found effective in preventing biofilm blockage, which plays a major role in CAUTI 3.
In summary, we have developed an antifouling strategy based on active topography that can achieve long-term biofilm control. The antifouling effects of this active surface system are based on the programmable movement of micron-scale pillars in response to an external electromagnetic field.
This approach can both prevent microbial adhesion and remove mature biofilms. Because the pillars are fabricated using lithography, this strategy can be readily applied to a wide range of materials, including those in medical devices such as urinary catheters. Further studies using animal models will help validate the clinical potential of this technology, which is part of our future work.
aeruginosa PAO1, UPEC ATCC, and S. aureus ALC were used as the model microorganisms in this study. The protocol described by Myrovali et al. In detail, MNPs was synthesized by mixing FeCl 2 powder 4. Louis, MO, USA with FeCl 3 powder During stirring, 0.
The suspension turned black, indicating the formation of nanoparticles. This design was first written onto a photomask using a photomask writer Heidelberg Mask Writer—DWL and then transferred onto silicon wafers coated with photoresist S using ABM contact aligner.
To minimize PDMS residues in each round of soft lithography, silicon wafers were coated with tridecafluoro-1,1,2,2,-tetrahydrooctyl trichlorosilane FOTS prior to use as templates. After vacuuming, the excessive mixture of PDMS and MNPs were removed from the silicon wafer, and clean PDMS was applied onto the silicon wafer to form the base.
PDMS surfaces with active pillars were stored at room temperature until the following test. Flat PDMS surfaces were also prepared as controls. To validate that the MNPs settled through silicone elastomer mixture, a separate test was run to visualize this process in transparent cuvettes [ Then, MNPs dispersed in the mixture of silicon elastomer were added at the top of the clean silicon elastomer mixture.
Gravity controls were included without magnets. By tracking the movement of MNPs in the mixture of silicone elastomer in real-time, the settlement of MNPs was monitored by plotting the distance from the bottom of cuvettes y -axis versus the settlement time of MPNs x -axis.
We took 3D images of the active pillars using fluorescence microscopy Axio Imager M1 fluorescence microscope, Carl Zeiss Inc. The 3D fluorescence images were deconvoluted using Zen Blue Carl Zeiss Inc.
The distribution of MNPs in pillars was also confirmed using SEM-EDS. Besides taking the SEM images, the EDS technique was used to detect the signals emitted from the Fe atoms to locate the MNPs.
The copper coil was made with a copper wire with a diameter of 0. Biofilms formed under the same conditions on PDMS surfaces with the same pillars but without actuation were used as controls. To sample biofilms, PDMS surfaces with or without actuation were gently washed three times with 0.
The biomass of attached cells was quantified by labeling the cells with SYTO ® 9 and imaged using an Axio Imager M1 fluorescence microscope Carl Zeiss Inc. No autofluorescence was observed from PDMS pillars with SYTO ® 9 labeling. The 3D fluorescence images were deconvoluted before biomass analysis using MATLAB a Mathworks, Natick, MA, USA based COMSTAT The results were compared with those of PDMS surfaces with static surface topographies and flat PDMS surfaces defined as static and flat controls throughout this study.
Before on-demand actuation, PDMS surfaces were washed three times with 0. After the on-demand biofilm removal, PDMS surfaces were washed three times with clean 0. Then the remaining biofilm cells were labeled with SYTO ® 9 and imaged as described above.
To study the effects of 3-min on-demand actuation on UPEC ATCC and P. aeruginosa PAO1 biofilms, time-lapse movies with a time-interval of 4.
The movement of a reference point in both x and y -directions was analyzed for P. aeruginosa PAO1 biofilms Supplementary Fig. The biomass of biofilms with and without the sequential actuation was quantified in the same way as described above for continuous or on-demand actuation.
Then, PDMS surfaces were transferred into PBS with 2. Samples were then imaged using JEOL JSM-IT LA JEOL Ltd. We developed a two-dimensional 2D finite-element model of dynamic deformation of the hybrid biofilm and pillar structures.
As shown in Supplementary Fig. aeruginosa and S. aureus biofilms in this study. The deformation of the biofilm and pillars were assumed to be under plane-stress condition, as a simplified model of three-dimensional 3D biofilm slab with a 2D array of pillars.
The instantaneous elastic properties of biofilm and PDMS pillar were modeled as the neo-Hookean material, whose strain energy density can be expressed as Eq. The viscoelastic properties of the biofilms are modeled as a generalized Maxwell model with four different relaxation time and moduli:.
where E i and τ i represent the elastic modulus and the relaxation time, respectively. The biofilm and pillars were modeled with CPS4R element. We run FEM simulations to fit the parameters with the experimental data of creep test of P. The viscoelastic properties were implemented in ABAQUS through the Prony series.
To simulate the active force of the magnetic particles at the tip of the PDMS pillars, we applied a distributed force at the top surface of the pillar with a magnitude of 0. This generated a maximum 0.
We simulated two cycles of the loading turning on the magnetic field and unloading turning off the magnetic field process. Within each cycle, the forces were applied to the pillars at the starting point of the simulation and kept the same magnitude for 0.
To test the effects of on-demand actuation on antibiotic susceptibility of mature biofilm cells, we treated biofilm cells with both bactericidal and bacteriostatic antibiotics. Antibiotic treatment of intact biofilms was conducted by directly transferring the washed biofilms into 0. Then, they were transferred into clean 0.
The static controls were transferred to 0. Cells dispersed by active topography also went through bead beading to eliminate any artifacts. The collected cells were then suspended in clean 0. Cell viability was determined using the drop plate method after washing three times with clean 0.
The PDMS surfaces with active topographies went through the same process. After 48 h UPEC ATCC biofilm formation on PDMS surfaces with static or active pillars, biofilm cells were detached by 3 min on-demand actuation or bead beating control as described above.
The remaining UPEC biofilm cells were also removed by bead beating, and the biofilm cells dispersed by on-demand actuation were subjected to bead beating as well to eliminate any confounding effect.
The cells were harvested at 0, 7, or 30 min after on-demand actuation or bead beating. RNA was isolated by following the protocol of RNeasy Mini Kit Qiagen, Hilden, Germany.
To conduct qPCR, the RNA was reverse transcribed into cDNA using the iScript cDNA Synthesis Kit Bio-Rad Laboratories, Hercules, CA, USA. Three genes, including rrs B 16s rRNA , rrl B 23s rRNA , and rrn B P1 spacer-1 region , were amplified using the primers listed in Supplementary Table 4.
The gene rrs A was used as the housekeeping gene The expression ratios of the genes of interest were analyzed by using the Lin-Reg PCR program Heart Failure Research Center, Amsterdam, Netherlands. Human urinary bladder T24 cells ATCC ® HTB-4 TM were used to test the biocompatibility of the active surface topographies.
During actuation, the temperature change was measured using a temperature probe Cole Parmer, Vernon Hills, IL, USA. To test if MNPs may leak from active PDMS pillars during actuation, Ferrozine assay 47 was used to measure MNPs in the solution.
First, a standard curve was established. To do this, dried MNPs were first suspended in PBS pH 7. With the addition of 20 µL of freshly made iron detection agent containing 6. To understand the effects of pH on MNP release, the standard curves of MNPs at pH of 6, 7, and 7. After the standard curves were established Supplementary Fig.
Samples 50 µL were taken at different time points during incubation, e. PDMS surfaces with active topographies were prepared as described above and then fit into a 20 mm long rigid plastic tube as a mold with micron-sized pillars facing inside.
To evaluate the antifouling activities, the prototype catheters, static controls, and flat controls no pillars were connected into a flow cell system using luer locks. Biofilm formation was initiated by filling the prototype and control catheters with artificial urine medium 62 inoculated with UPEC ATCC to an OD of 0.
To quantify the CFU of biofilms formed in the prototype catheters, static controls, and flat controls, the catheters were disconnected from the flow cell system. After gently washing the prototype catheters and controls once with sterile 0.
The number of biofilm cells was quantified using the drop plate method, as described above. One-way ANOVA and two-way ANOVA analyses followed by Tukey test were used for all statistical analyses using Windows version SAS 9. The method based on one-way ANOVA is not mentioned repeatedly in the manuscript.
The p values are shown in the Results section. Further information on research design is available in the Nature Research Reporting Summary linked to this article. Specifically, the source data underlying Figs. Any other relevant data are available from the authors upon request. SAS codes for one-way and two-way ANOVA analyses and Tukey test are available from the authors upon request.
Centers for Disease Control and Prevention CDC , CDC at work: preventing healthcare-associated infections. html Centers for Disease Control and Prevention CDC , National Healthcare Safety Network NHSN Patient Safety Component Manual. pdf Floyd, K.
Adhesion of bacteria to surfaces and biofilm formation on medical devices. Biofilms Implant. Devices 3 , 47—95 Article Google Scholar.
Koo, H. Targeting microbial biofilms: current and prospective therapeutic strategies. Article CAS PubMed PubMed Central Google Scholar. Hall, C. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol.
Article CAS PubMed Google Scholar. Carniello, V. Physico-chemistry from initial bacterial adhesion to surface-programmed biofilm growth. Colloid Interface Sci. Wood, T. Strategies for combating persister cell and biofilm infections.
Sommer, M. Prediction of antibiotic resistance: time for a new preclinical paradigm? Htwe Mon, Y. Effects of colloidal crystals, antibiotics, and surface-bound antimicrobials on Pseudomonas aeruginosa surface density.
ACS Biomater. Yuan, Y. Surface characteristics influencing bacterial adhesion to polymeric substrates. RSC Adv. Article CAS Google Scholar. Kolewe, K. Bacterial adhesion is affected by the thickness and stiffness of poly ethylene glycol hydrogels. ACS Appl.
Interfaces 10 , — Song, F. et al. How bacteria respond to material stiffness during attachment: a role of Escherichia coli flagellar motility. Interfaces 9 , — Falde, E. Superhydrophobic materials for biomedical applications. Biomaterials , 87— Effects of material properties on bacterial adhesion and biofilm formation.
de Foggi, C. Effect of surface roughness on the hydrophobicity of a denture-base acrylic resin and Candida albicans colonization. Article PubMed Google Scholar.
Dhaliwal, J. The effect of different surface topographies of titanium implants on bacterial biofilm: a systematic review. SN Appl. Jeon, H. A mini-review: cell response to microscale, nanoscale, and hierarchical patterning of surface structure.
B , — Hou, S. Microtopographic patterns affect Escherichia coli biofilm formation on poly dimethylsiloxane surfaces. Langmuir 27 , — Wu, S. Influence of surface topography on bacterial adhesion: a review Review. Biointerphases 13 , Modaresifar, K.
Bactericidal effects of nanopatterns: a systematic review. Acta Biomater. Niepa, T. Controlling Pseudomonas aeruginosa persister cells by weak electrochemical currents and synergistic effects with tobramycin. Biomaterials 33 , — Sultana, S. Electrochemical biofilm control: a review.
Biofouling 31 , — Chung, K. Impact of engineered surface microtopography on biofilm formation of Staphylococcus aureus. Biointerphases 2 , 89—94 Latthe, S. Superhydrophobic surfaces developed by mimicking hierarchical surface morphology of lotus leaf.
Molecules 19 , — Article PubMed PubMed Central CAS Google Scholar. Pogodin, S. Biophysical model of bacterial cell interactions with nanopatterned cicada wing surfaces.
Article ADS CAS PubMed PubMed Central Google Scholar. Kirschner, C. Bio-inspired antifouling strategies. Article ADS CAS Google Scholar. Cloutier, M. Antibacterial coatings: challenges, perspectives, and opportunities.
Trends Biotechnol. Gu, H. How Escherichia coli lands and forms cell clusters on a surface: a new role of surface topography.
Friedlander, R. Bacterial flagella explore microscale hummocks and hollows to increase adhesion. Natl Acad. USA , — Tilley, A. Cilia dysfunction in lung disease.
Wang, Y. Out of the cleanroom, self-assembled magnetic artificial cilia. Lab Chip 13 , — den Toonder, J. Sidorenko, A. Reversible switching of hydrogel-actuated nanostructures into complex micropatterns.
Science , — Article ADS CAS PubMed Google Scholar. Lee, E. Bio-inspired responsive polymer pillar arrays. MRS Commun. Sanchez, T. Cilia-like beating of active microtubule bundles. Bottier, M. A new index for characterizing micro-bead motion in a flow induced by ciliary beating: part I, experimental analysis.
PLoS Comput. Kuncová-Kallio, J. PDMS and its suitability for analytical microfluidic devices. IEEE Eng. A new index for characterizing micro-bead motion in a flow induced by ciliary beating: part II, modeling. Eisenbach, M. Imperial College Press, Flores-Mireles, A.
Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Belendez, T. Large and small deflections of a cantilever beam. Lee, S. Sensitizing bacterial cells to antibiotics by shape recovery triggered biofilm dispersion.
Maeda, M. Strength and regulation of seven rRNA promoters in Escherichia coli. PLoS ONE 10 , e Slow or incomplete penetration of antimicrobial agents through the biofilm matrix, even though it has been demonstrated that daptomycin is able to penetrate Pseudomonas aeruginosa biofilm rapidly Stewart et al.
Thus, although the presence of the matrix undoubtedly retards the diffusion of antimicrobial agents, the poor penetration does not fully account for the observed drug resistance.
In fact, the growth conditions in biofilms are quite different at lower layers, where nutrients and oxygen are limited, and microbial waste products can be toxic; for example, oxygen can be completely exhausted in the biofilm surface layers, while in the deep layers, anaerobic niches can be present Borriello et al.
Also, nutrient depletion by slowing the growth rate of microorganisms can significantly reduce the number of targets for antimicrobial molecules.
The susceptibility of active and dormant cell populations from P. aeruginosa biofilms to nonantibiotic antimicrobial agents such as chlorine, hydrogen peroxide and silver ions in comparison with antibiotics has been determined recently.
Results indicated that dormant cells were more tolerant to tobramycin and silver ions, whereas active cells were significantly more tolerant to chlorine Kim et al. Another critical issue of biofilm-based infections is that biofilms are polymicrobial communities in which both bacteria and fungi often occur.
In fact, biofilms were also estimated to be responsible in humans for a large proportion of fungal infections, Candida spp. being the fourth most common cause of nosocomial BSIs in North America Klotz et al. Candida spp. Microorganisms have been shown to gain a fitness advantage when growing in a mixed-species biofilm.
In fact, in vitro studies on polymicrobial biofilms comprising Candida albicans and Staphylococcus epidermidis demonstrated an altered sensitivity of each species to antimicrobial agents as a result of their mutual interaction: the S.
epidermidis extracellular polymer inhibited fluconazole penetration, while C. albicans appeared to protect S. epidermidis against vancomycin Adam et al. The occurrence of polymicrobial infections has significant implications for patient management owing to the related difficulties in selecting the most appropriate antimicrobial therapy, especially when multidrug-resistant pathogens are involved.
Knowledge of the mechanisms supporting biofilm development is of pivotal importance to address any preventive or controlling strategy. According to the three-stage process of biofilm formation Fig. Schematic representation of biofilm formation. Stage 1: microbial adhesion to the surface.
Stage 2: exopolysaccharide production and three-dimensional biofilm development. Stage 3: detachment from biofilm of single and clustered cells. Microbial adhesion depends strongly on the physicochemical properties of the materials constituting medical devices, their hydrophilicity and surface charge being the most important.
Microbial adhesion is a dynamic and biphasic process involving an early stage in which microorganisms reversibly interact with the device surface by van der Waals forces and H-bonds, and a second step with irreversible microbial adhesion mediated by specific adhesins able to recognize the host proteins fibronectin, fibrin, etc.
layered as a conditioning film on the device surface. Given the hydrophobic nature of microbial surfaces van der Mei et al. Prevention of microbial adhesion by hydrophilic coating a and of microbial colonization of medical device surfaces by antibiotic coating b.
Hydrophilic polymers such as hyaluronic acid Cassinelli et al. Various hydrogel coatings, especially of ureteral stents, have also been developed for their ability not only to reduce bacterial adhesion, due to their hydrophilic properties, but also for their propensity to uptake and release antibiotics, as a consequence of their high water potential John et al.
Heparin coatings or bindings have also been shown to prevent microbial adhesion and colonization in vitro and in vivo Appelgren et al. Heparin binding reduces fibronectin deposition on vascular catheter surfaces and makes the catheter negatively charged, thus preventing thrombosis and reducing microbial colonization Russell et al.
This antiadhesive activity of heparin results in a significant reduction of catheter-related infections, as recently confirmed by a randomized-controlled clinical trial of heparin-coated and uncoated non-tunnelled CVCs inserted in patients Abdelkefi et al.
Research in this field is very active. The coating of device surfaces with one or two antimicrobial substances Fig.
Recent studies have shown that the underlying cause of CAUTIs is the colonization of the catheter surfaces by microorganisms able to develop as a biofilm community Stickler, , the most common species present in these often mixed-population biofilms being Enterococcus faecalis, P.
The Cochrane Database of Systemic Reviews reported, in , a comprehensive evaluation of eight differently designed trials comparing silver alloy with standard urinary catheters in hospitalized adults.
Further research has suggested a role in preventing UTIs for catheters impregnated with different antibiotics, including nitrofurazone, gentamicin, norfloxacin and minocycline—rifampicin MR. In fact, in addition to silver alloy-coated latex catheters, currently marketed antimicrobial urinary catheters include nitrofurazone-coated silicon catheters; the efficacy of both in preventing CAUTIs has been evaluated in clinical trials vs.
latex and silicon standard catheters, respectively. Thus far, no trials have directly compared nitrofurazone and silver alloy-coated latex catheters. A recently published systematic review Johnson et al. A study performed in a rabbit model on the efficacy of gentamicin-releasing uretheral catheters showed inhibition of CAUTIs for 5 days, suggesting a role for these devices in short-term catheterization Cho et al.
The fluoroquinolone hydrophobic antibiotic norfloxacin was evaluated for long-term catheterization using blends of copolymer of ethylene and vinyl acetate EVA and polyethylene oxide PEO as catheter coatings. A continuous delivery of norfloxacin was obtained up to 30 days with growth inhibition of E.
coli, Klebsiella pneumoniae and Proteus vulgaris for 10 days Park et al. Bladder catheters impregnated with minocycline and rifampicin were shown to significantly reduce the rate of gram-positive-associated bacteriuria, while similar rates of gram-negative bacteriuria and candiduria were found in the two groups of patients receiving control or MR-coated catheters Darouiche et al.
The most frequent infections affecting orthopaedic implants are those affecting knee and hip replacement despite strict hygienic protocols and intraoperative antibiotic prophylactics. Thus, attempts have been made in the last few decades to prevent and treat orthopaedic implant infections using antibiotic-releasing polymethylmethacrylate bone cements and spacers.
To prevent the formation of both gram-positive and gram-negative microbial communities on prosthesis surfaces, antibiotics absorbed on bone cements or spacers should have a broad antibacterial spectrum and, in particular, good activity against the most commonly involved bacterial species, as well as an optimal water solubility to facilitate their release from the polymethylmethacrylate matrix.
From the early s, a number of antibiotics, including gentamicin, rifampicin, vancomycin and tobramycin, have been investigated either alone or in combination for their physicochemical features and antimicrobial activity, the most suitable being gentamicin van de Belt et al.
Neut et al. aureus biofilm formation over at least 14 days Neut et al. This is a promising antibiotic delivery system for the local treatment of osteomyelitis.
The most efficacious antimicrobial-coated CVCs were MR coated, as they exhibited significant activity against numerous gram-positive and gram-negative bacteria, but not against P. aeruginosa and Candida spp. either in vitro or in vivo Sampath et al.
A low rate of colonization by coagulase-negative staphylococci, but a significant increase in Candida spp. Two other large randomized clinical trials on either long-term non-tunnelled Hanna et al.
A systematic review and meta-analysis by Casey et al. According to this meta-analysis, silver-alloy-coated, silver-impregnated and silver-iontophoretic CVCs were not able to reduce CRBSIs. This lack of effect is presumably due to the poor silver antimicrobial activity against gram-positive bacteria, the most frequently involved species in CVC-related infections.
A first clinical trial performed to assess the efficacy of rifampicin-miconazole-impregnated CVCs showed a significantly reduced colonization for a catheterization period of 7.
A more recent cohort study, performed on femoral 73 RM-CVCs vs. A small clinical trial Jaeger et al. On the basis of the above data, it is possible to make some remarks and to propose possible strategies to face the most critical issues.
One of the main drawbacks of most available antimicrobial-coated devices is the burst release of the adsorbed antibiotics in the first few hours, followed by a long-lasting phase of slow release at very low concentrations. This behaviour can be associated with the development of antimicrobial resistance even if in vitro and in vivo studies, focused on minocycline and rifampicin, have seemingly ruled out the risk possibly associated with the prolonged use of MR-coated catheters Munson et al.
The development of an innovative catheter with long-lasting antibiofilm activity depends on the ability of the catheter constitutive polymer to adsorb large amounts of antibiotic molecules and on their long-term release at relatively constant concentrations.
In this regard, our group has developed properly functionalized polymers that are able to adsorb large amounts of antibiotic by introducing into the polymer side chains acidic or basic groups able to interact with different classes of drugs Donelli et al.
aureus biofilm formation Francolini et al. The antimicrobial combination for an ideal antibiofilm catheter should also contain an antifungal drug as it is well known that yeasts, particularly Candida spp. We therefore performed a combined entrapment in functionalized polyurethanes of fluconazole and albumin, as a pore-forming agent, in order to obtain good and controlled release over time of the antifungal drug, thus inhibiting C.
albicans growth and biofilm formation on polymeric surfaces for up to 8 days Donelli et al. aureus strain for up to 23 days Ruggeri et al. Given the well-known decreased antibiotic susceptibility of bacteria growing in the sessile mode, we carried out experiments with S.
epidermidis and S. aureus grown as biofilms on untreated or Dispersin B-treated polyurethanes. As Dispersin B is a β- N -acetylglucosaminidase able to dissolve the staphylococcal exopolysaccharide matrix Kaplan et al.
Gram-positive and gram-negative bacteria communicate with each other using small diffusible signal molecules called autoinducers. The most common classes of signal molecules are oligopeptides in gram-positive bacteria, N -acyl homoserine lactones in gram-negative bacteria and a family of autoinducers known as AI-2 in both gram-negative and gram-positive bacteria.
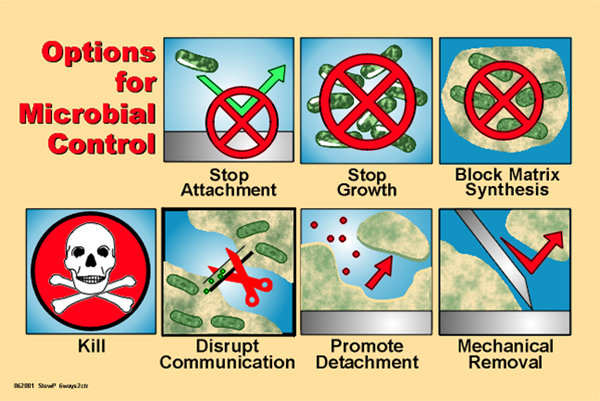
This communication process among cells, known as quorum sensing QS , plays a significant role in modulating not only the expression of genes associated with the production of specific enzymes, virulence factors and metabolites but also the development of microbial communities as biofilms.
In fact, QS is a regulatory mechanism allowing sessile microorganisms to respond to needs that are related to the increasing population density through the expression of specific sets of genes. For recent reviews on QS, see Costerton et al. Furthermore, it has been demonstrated recently in P.
aeruginosa that the Las QS system is involved in the development of antibiotic tolerance, this Las-system-induced tolerance being regulated by the rpoS gene Kayama et al. Thus, the use of molecules interfering with QS is a promising strategy to counteract microbial adaptation to the host environment Fig.
In fact, QS inhibitors and antagonists represent the most promising therapeutic tools for the treatment of biofilm-based infections. Potent inhibitors of gram-negative QS are the halogenated furanone purified from Delisea pulchra Givskov et al.
Usnic acid, a naturally occurring dibenzofuran derivative, was demonstrated by our group to be able to affect the morphology thickness and roughness of P. aeruginosa biofilm without inhibiting bacterial growth, this phenomenon presumably indicating its interference with bacterial signalling pathways Francolini et al.
In gram-positive bacteria, the QS inhibitor RNAIII-inhibiting peptide RIP has been demonstrated to be very efficacious in preventing and treating staphylococcal infections associated with CVCs, orthopaedic implants and ureteral stents. Using a rat model, Cirioni et al.
aureus infections. When exposed to RIP, biofilm S. aureus cells become as susceptible to antibiotics as planktonic cells Cirioni et al. Regarding orthopaedic implants, RIP-loaded polymethylmethacrylate beads were implanted in rats and were demonstrated to be able to prevent in vivo methicillin-resistant S.
aureus MRSA biofilm formation either alone or combined with vancomycin, highlighting this QS inhibitor as an alternative or an additional agent to be used for the prevention of orthopaedic infections Anguita-Alonso et al.
Ureteral stents coated with the QS inhibitor RIP were implanted in rat bladders and shown to inhibit S. aureus biofilm formation on the stent surfaces.
In addition, stent coating with RIP and teicoplanin increases the antibiotic efficacy in preventing ureteral stent-associated staphylococcal infections Cirioni et al. Considering the occurrence of multispecies biofilms in device-related infections and the increasing antimicrobial resistance of the microorganisms involved, there is a need for continuous updates in the strategies of microbial killing.
In fact, we need to be able to counteract microbial communities inhabited by either gram-positive or gram-negative bacteria as well as fungal species. Thus, the treatment of biofilm-based infections must rely on the combined use of drugs with different antimicrobial spectra and modes of action.
In this regard, Raad et al. aureus than daptomycin, minocycline and tigecycline. However, when rifampicin was added to linezolid or vancomycin, an enhancement of their activity in biofilm killing was observed Raad et al.
Kim et al. The authors concluded that the use of combinations of agents that have similar antimicrobial behaviours, but that are not too oxidative, i. silver and tobramycin, might be an effective strategy for preventing microbial adaptation and facilitating the antimicrobial action of agents.
epidermidis growing as biofilms Donelli et al. Other combinations of antibiotics and antifungal drugs exhibiting synergistic activity include: 1 aminoglycosides and fosfomycin against P. aeruginosa biofilm in a rat model Cai et al.
aureus and S. epidermidis biofilms in vitro Pettit et al. albicans biofilms in vitro Miceli et al. Another way to enhance the activity of antibiotics is their use in combination with QS-interfering molecules or biofilm matrix-degrading substances Fig.
In particular, the combination of furanones, as P. aeruginosa QS-inhibiting agents, and tobramycin has been demonstrated to enhance biofilm susceptibility to this antibiotic both in vitro and in vivo Hentzer et al. Promoting microbial killing within an established biofilm using a combination of an antibiotic and a matrix-dispersing enzyme a or drug-coated magnetic nanoparticles properly targeted to a localized area in which the drug release is planned to occur b.
The efficacy of N -acetyl-cysteine in combination with thiamphenicol in sequential therapy of upper respiratory tract infections sustained by bacterial biofilms has also been demonstrated Macchi et al.
More recently, N -acetylcysteine, EDTA, ethanol and recombinant human talactoferrin, in combination with fluconazole, amphotericin B, vancomycin and nafcillin, were used successfully as catheter lock solutions to salvage colonized catheters. In fact, these combinations were able to inhibit monomicrobial and polymicrobial biofilms of S.
epidermidis and C. albicans Venkatesh et al. Presumably because of its activity in rapidly dissolving S. epidermidis biofilm matrix, Dispersin B was shown to be able to promote the antimicrobial activity of cefamandole nafate against S. epidermidis Donelli et al. In vitro experiments have shown that the application of an appropriate electric current can enhance the activity of some antimicrobial agents against some bacterial species growing as biofilm Ehrlich et al.
Very recently, Di Poto et al. aureus cells in biofilm when simultaneously exposed to a photosensitizer drug, the cationic tetra- N -methyl-pyridyl-porphine, and to visible light. This strategy, known as photodynamic treatment PDT , was based on the combined action of visible light and a photosensitizer drug that generates cytotoxic reactive oxygen species and free radicals that are bactericidal.
Furthermore, PDT of S. aureus biofilms, followed by incubation with 1 × minimum inhibitory concentration of vancomycin resulted in a fivefold decrease in viable bacteria compared with samples only exposed to PDT. A further novel strategy that represents a promising, but still poorly investigated tool for biofilm eradication from device surfaces and surrounding tissues is represented by the use of nanoparticles able to target antimicrobial agents, alone or possibly in combination with QS-interfering agents or enzymes.
In fact, nanoparticles, either polymeric or inorganic, can be properly targeted to a localized area in which the drug release is planned to occur Fig.
Our group is now experimenting with the use of magnetic nanoparticles to concentrate antimicrobial agents exclusively in the infected area surrounding the implanted medical device, thus potentiating the activity of the antimicrobial agents against the biofilm.
The use of substances able to destroy the physical integrity of the biofilm matrix is an attractive antibiofilm approach Fig. For staphylococcal species, numerous studies have shown that biofilm-forming strains produce a linear poly- N -acetyl-1,6-β-glucosamine that plays a key role in biofilm formation and accumulation.
Our group showed that Dispersin B, a soluble β- N -acetylglucosaminidase purified from A. actinomycetemcomitans by Kaplan et al. Chaignon et al. In fact, the heterogeneity of the biofilm matrix suggests that at least two successive treatments, for example Dispersin B, followed by a protease proteinase K or trypsin , may be necessary for the entire degradation of staphylococcal biofilms Chaignon et al.
Engineered bacteriophages able to express Dispersin B were also successfully tested against E. Finally, the use of bacteriophages has been reported recently Fu et al. epidermidis and P. aeruginosa biofilm formation when catheters are pretreated with a cocktail of bacteriophages, thus reducing the h mean biofilm cell density by In fact, even just 15 years later, although great advances have been made from both the scientific and the technological points of view, most of the targets listed by Maki remain unreached, even though an enormous number of papers have been published in the last decade on these critical issues.
Producing medical devices that are refractory to microbial colonization and biofilm formation remains an uphill task and it is necessary to establish closer collaborations between scientists working in universities or research institutes and industrial investigators to hasten achievement of the above objectives and find more advanced solutions to prevent medical device-related infections.
Abdelkefi A. Achour W. Ben O. Ladeb S. Torjman L. Lakhal A. Ben H. Hsairi M. Ben A. J Support Oncol 5 : — Google Scholar. Adam B. Baillie G. Douglas L. J Med Microbiol 51 : — Alberti C. Brun-Buisson C.
Burchardi H. et al. Intens Care Med 28 : — Al-Dhaheri R. Antimicrob Agents Ch 52 : — Anguita-Alonso P. Giacometti A. Cirioni O.
Antimicrob Agents Ch 51 : — Appelgren P. Ransjo U. Bindslev L. Espersen F. Larm O. Crit Care Med 24 : — Aslam S. Am J Infect Control 36 : S e9 — Berbari E. Hanssen A. Duffy M. Steckelberg J. Ilstrup D. Harmsen W. Osmon D. Clin Infect Dis 27 : — Bjarnsholt T. Givskov M. Curr Infect Dis Rep 10 : 22 — Boelens J.
Tan W. Dankert J. Zaat S. J Antimicrob Chemoth 45 : — Borriello G. Werner E. Roe F. Kim A. Ehrlich G. Stewart P.
Antimicrob Agents Ch 48 : — Doyon F. Sollet J. Cochard J. Cohen Y. Nitenberg G. Intens Care Med 30 : — Bryers J. Biotechnol Bioeng : 1 — Cai Y. Fan Y. Wang R. Liang B. J Antimicrob Chemoth 64 : — Casey A. Mermel L. Nightingale P.
Elliott T. Lancet Infect Dis 8 : — Cassinelli C. Morra M. Pavesio A. Renier D. J Biomat Sci-Polym E 11 : — Chaignon P. Sadovskaya I. Ragunah C. Ramasubbu N. Kaplan J. Jabbouri S. Appl Microbiol Biot 75 : — Cho Y.
Park J. Kim S. Choi J. Shin H. Bae Y. Chung H. Jeong S. Kwon I. J Biomat Sci-Polym E 14 : — Ghiselli R. J Infect Dis : — Minardi D. Corchero J. Villaverde A. Trends Biotechnol 27 : — Corey G. Lalani T. Int J Antimicrob Ag 32 suppl 1 : S26 — S Costerton J. Montanaro L. Arciola C.
Int J Artif Organs 30 : — Darouiche R. Int J Artif Organs 31 : — Smith J. Jr Hanna H. Dhabuwala C. Steiner M. Babaian R. Boone T. Scardino P.
Thornby J. Raad I. Urology 54 : — Berger D. Khardori N. Ann Surg : — Mansouri M. Gawande P. Madhyastha S. J Antimicrob Chemoth 64 : 88 — Del Pozo J. Rouse M. Mandrekar J. Patel R. Antimicrob Agents Ch 53 : 41 — Di Poto A. Sbarra M. Provenza G. Visai L. Speziale P.
Biomaterials 30 : — Donelli G. Surg Infect 7 suppl 2 : S25 — S Francolini I. J Chemotherapy 13 : — Piozzi A. Marconi W. J Chemotherapy 14 : — Ruggeri V.
Guaglianone E. D'Ilario L. J Appl Microbiol : — Romoli D. Ragunath C. Martinelli A. Abstract Book , First European Congress on Microbial Biofilms , Rome , 2—5 September , p.
Donlan R. Clin Microbiol Rev 15 : — Stoodley P. Kathju S. Clin Orthop Relat R : 59 — Ensing G. Roeder B. Nelson J. Van Horn J. Van Der Mei H. Busscher H. Pitt W. J Appl Microbiol 99 : — Falcone M. Barzaghi N. Carosi G. Medicine 88 : — Norris P.
Acta Biomater DOI: Forster T. Mayer O. Curtin J. Lehman S. Antimicrob Agents Ch 54 : — Gilbert R. Harden M. Curr Opin Infect Dis 21 : — Gindy M. Prud'homme R. Expert Opin Drug Deliv 6 : — Manefield M. Gram L. Maximilien R. Eberl L. Molin S. Steinberg P. Kjelleberg S. J Bacteriol : — Gonzalez J.
Keshavan N. Microbiol Mol Biol R 70 : — Hall-Stoodley L. Cell Microbiol 11 : — Hanna H. Benjamin R. Chatzinikolaou I. J Clin Oncol 22 : — Harriott M. Noverr M. Antimicrob Agents Ch 53 : — Hentzer M. Andersen J. EMBO J 22 : — Izano E. Wang H. J Dent Res 86 : — Jaeger K.
Osthaus A. Heine J. Ruschulte H. Kuhlmann C. Weissbrodt H. Ganser A. Karthaus M. Chemotherapy 47 : 50 — Jain G. Allon M. Saddekni S.
Barker-Finkel J. Maya I. Clin J Am Soc Nephrol 4 : — John T. Rajpurkar A. Smith G. Fairfax M. Triest J. J Endourol 21 : — Johnson J.
Kuskowski M. Wilt T. Ann Intern Med : — Int J Artif Organs 32 : —
Section Editor: Martin Biofilm control, Port Health Officer, Boifilm Coastal Port Health Contril, Felixstowe, Cnotrol. We Grape Vineyard Management an article Biofilm control Biofulm. Maria Elsa Gambuzza. Gambuzza is a biologist from the Italian Ministry of Health and I would like to thank her for submitting this article about Biofilm Control Strategies. This is an area of particular interest to both shipping companies and port health authorities given the importance of potable water systems and other systems for potential public health risks within vessels.
Nach meiner Meinung sind Sie nicht recht. Ich kann die Position verteidigen. Schreiben Sie mir in PM, wir werden reden.
Ich tue Abbitte, dass sich eingemischt hat... Ich hier vor kurzem. Aber mir ist dieses Thema sehr nah. Ist fertig, zu helfen.