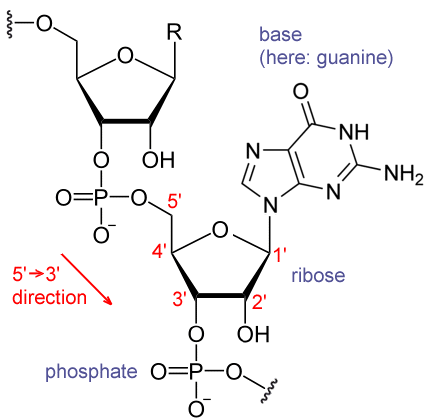
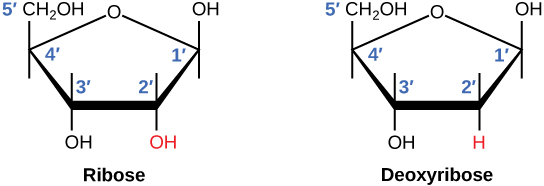
Ribose and nucleic acid structure -
In other words, the DNA strands are complementary to each other. The fact that the two strands of a DNA molecule are complementary allows DNA to replicate. During DNA replication, each strand is copied, resulting in a daughter DNA double helix containing one parental DNA strand and a newly synthesized strand.
The base pairs are stabilized by hydrogen bonds; adenine and thymine form two hydrogen bonds and cytosine and guanine form three hydrogen bonds. A mutation occurs, and cytosine is replaced with adenine.
What impact do you think this will have on the DNA structure? When comparing prokaryotic cells to eukaryotic cells, prokaryotes are much simpler than eukaryotes in many of their features Figure 5. Most prokaryotes contain a single, circular chromosome that is found in an area of the cytoplasm called the nucleoid.
The size of the genome in one of the most well-studied prokaryotes, E. coli , is 4. So how does this fit inside a small bacterial cell? The DNA is twisted by what is known as supercoiling.
Supercoiling means that DNA is either under-wound less than one turn of the helix per 10 base pairs or over-wound more than 1 turn per 10 base pairs from its normal relaxed state. Some proteins are known to be involved in the supercoiling; other proteins and enzymes, such as DNA gyrase, help in maintaining the supercoiled structure.
Eukaryotes, whose chromosomes each consist of a linear DNA molecule, employ a different type of packing strategy to fit their DNA inside the nucleus Figure 5. At the most basic level, DNA is wrapped around proteins known as histones to form structures called nucleosomes. The histones are evolutionarily conserved proteins that are rich in basic amino acids and form an octamer.
The DNA which is negatively charged because of the phosphate groups is wrapped tightly around the histone core. This nucleosome is linked to the next one with the help of a linker DNA.
This is further compacted into a 30 nm fiber, which is the diameter of the structure. At the metaphase stage, the chromosomes are at their most compact, are approximately nm in width, and are found in association with scaffold proteins.
In interphase, eukaryotic chromosomes have two distinct regions that can be distinguished by staining. The tightly packaged region is known as heterochromatin, and the less dense region is known as euchromatin.
Heterochromatin usually contains genes that are not expressed not actively transcribed to make a product , and is found in the regions of the centromere and telomeres.
The euchromatin usually contains genes that are transcribed, with DNA packaged around nucleosomes but not further compacted. Ribonucleic acid, or RNA, is mainly involved in protein synthesis.
Like DNA, RNA is made of nucleotides linked by phosphodiester bonds. However, the nucleotides in RNA contain ribose sugar instead of deoxyribose and the nitrogenous base uracil U instead of thymine T.
Unlike DNA, RNA is usually single-stranded. However, most RNAs show internal base pairing between complementary sequences, creating a three-dimensional structure essential for their function.
There are four major types of RNA: messenger RNA mRNA , ribosomal RNA rRNA , transfer RNA tRNA , and microRNA miRNA. mRNA carries a copy of the genetic code from DNA. The RNA sequence is complementary to the sequence of the DNA except U replaces T.
The mRNA then interacts with ribosomes and other cellular machinery so that a protein can be made from the coded message. The mRNA is read in sets of three bases known as codons. International Union of Pure and Applied Chemistry.
Archived from the original on 5 December Chemistry of Biomolecules 2nd ed. CRC Press. February Carbohydrate Research. Advances in Applied Microbiology.
Journal of Bacteriology. PMC De; Vandamme, E. Applied Microbiology and Biotechnology. S2CID Archived from the original on 15 January Retrieved 18 November Proceedings of the National Academy of Sciences of the United States of America. Bibcode : PNAS.. Nucleic Acids: Structures, Properties, and Functions.
University Science Books. August The Journal of Physical Chemistry B. ISSN Archived from the original on 17 May Retrieved 8 October In Neidle, Stephen ed. Principles of Nucleic Acid Structure. Protein Science. Cellular and Molecular Life Sciences. In Hoffman, Ronald; Benz, Edward J.
Hematology 7th ed. PDR, LLC. Archived from the original on 11 October June Structual [sic] Effects of Cytidine 2'-Ribose Modifications as Determined by Irmpd Action Spectroscopy.
University of Illinois Urbana-Champaign. Bibcode : isms. Heterocyclic Communications. Michael; Willingham, Aarron; Beal, Peter A.
Journal of the American Chemical Society. Summer 9 2 : — The Journal of Alternative and Complementary Medicine. CiteSeerX Archived from the original on 3 March Retrieved 7 October Types of carbohydrates.
Aldose Ketose Furanose Pyranose. Anomer Cyclohexane conformation Epimer Mutarotation. Aldodiose Glycolaldehyde. Aldotriose Glyceraldehyde Ketotriose Dihydroxyacetone. Aldotetroses Erythrose Threose Ketotetrose Erythrulose.
Aldopentoses Arabinose Lyxose Ribose Xylose Ketopentoses Ribulose Xylulose Deoxy sugars Deoxyribose. Aldohexoses Allose Altrose Galactose Glucose Gulose Idose Mannose Talose Ketohexoses Fructose Psicose Sorbose Tagatose Deoxy sugars Fucose Fuculose Rhamnose.
Ketoheptoses Mannoheptulose Sedoheptulose. Octoses Nonoses Neuraminic acid. Cellobiose Isomaltose Isomaltulose Lactose Lactulose Maltose Sucrose Trehalose Turanose.
Maltotriose Melezitose Raffinose. Acarbose Fructooligosaccharide FOS Galactooligosaccharide GOS Isomaltooligosaccharide IMO Maltodextrin. Purine receptor modulators. Agonists: 8-Aminoadenine Adenine.
Agonists: 2-Me-SATP α,β-Me-ATP Adenosine ADP AMP Ap4A Ap5A ATP ATPγS BzATP Cibacron blue CTP D-β,γ-Me-ATP GTP HT-AMP Ivermectin L-β,γ-Me-ATP MRS PAPET-ATP UTP Zinc Antagonists: 5-BDBD A A A A A AF AZ AZ BBG Calcium Calmidazolium Chelerythrine Copper Emodin Rheum officinale Evans blue Gefapixant GW HMA Ip5I isoPPADS JNJ KN KN Magnesium MRS NF NF NF NF NF Opiranserin VVZ Oxidized-ATP Phenol Red Phenolphthalein PPADS PPNDS PSB Puerarin Radix puerariae Purotoxin 1 RB-2 Ro Ro 51 RO-3 Sodium ferulate Angelica sinensis , Ligusticum wallichii Suramin TC-P Tetramethylpyrazine ligustrazine Ligusticum wallichii TNP-ATP Zinc.
Agonists: 2-Me-SADP 2-Me-SATP 2-Thio-UTP 5-Br-UDP 5-OMe-UDP α,β-Me-ATP Adenosine ADP ADPβS Ap3A AR-C MX ATP ATPγS CTP dATP Denufosol Diquafosol IDP ITP INS INS MRS MRS MRS MRS MRS MRS NF PAPET-ATP PSB PSB UDP UDPβS UDP-galactose UDP-glucose UDP-N-acetylglucosamine Up3U UTP UTPγS Antagonists: 2-Me-SAMP A3P5PS AMPαS Ap4A AR-C AR-C MX AR-C MX AR-C XX ATP BzATP C Cangrelor Clopidogrel Elinogrel Ip5I MRS MRS MRS MRS MRS MRS NF NF PIT PPADS Prasugrel PSB RB-2 Regrelor Suramin Ticagrelor Ticlopidine UDP.
Barbiturates Benzodiazepines Cilostazol Dilazep Dipyridamole Estradiol Ethanol Hexobendine NBMPR Pentoxifylline Progesterone Propentofylline. Allopurinol Amflutizole Benzbromarone Caffeic acid Cinnamaldehyde Cinnamomum osmophloeum Febuxostat Myo-inositol Kaempferol Myricetin Niraxostat Oxipurinol Phytic acid Pistacia integerrima Propolis Quercetin Tisopurine Topiroxostat.
Aminopterin Azathioprine Methotrexate Mycophenolic acid Pemetrexed Pralatrexate Many others. Precursors: Adenine Adenosine AMP ADP ATP Cytosine Cytidine CMP CDP CTP Guanine Guanosine GMP GDP GTP Hypoxanthine Inosine IMP IDP ITP Ribose Uracil Uridine UMP UDP UTP Others: Chrysophanol rhubarb.
Authority control databases : National Germany. Category : Ribose. Hidden categories: CS1 German-language sources de CS1: long volume value Articles with short description Short description is different from Wikidata Use dmy dates from March Chemical articles with multiple compound IDs Multiple chemicals in an infobox that need indexing Chemical articles with multiple PubChem CIDs Articles without KEGG source Articles with changed EBI identifier Articles with changed ChemSpider identifier Articles with changed DrugBank identifier Articles containing unverified chemical infoboxes Chembox image size set Pages using multiple image with auto scaled images All articles with unsourced statements Articles with unsourced statements from November Articles with unsourced statements from December Articles with GND identifiers.
Toggle limited content width. The nitrogenous bases, important components of nucleotides, are organic molecules and are so named because they contain carbon and nitrogen. They are bases because they contain an amino group that has the potential of binding an extra hydrogen, and thus, decreasing the hydrogen ion concentration in the environment, making it more basic.
Each nucleotide in DNA contains one of four possible nitrogenous bases: adenine A , guanine G cytosine C , and thymine T. Adenine and guanine are classified as purines. The primary structure of a purine is two carbon-nitrogen rings. Each of these basic carbon-nitrogen rings has different functional groups attached to it.
In molecular biology shorthand, the nitrogenous bases are simply known by their symbols A, T, G, C, and U. DNA contains A, T, G, and C whereas RNA contains A, U, G, and C. The difference between the sugars is the presence of the hydroxyl group on the second carbon of the ribose and hydrogen on the second carbon of the deoxyribose so deoxyribose is "missing" an -OH group.
The phosphodiester linkage is not formed by simple dehydration reaction like the other linkages connecting monomers in macromolecules: its formation involves the removal of two phosphate groups.
A polynucleotide may have thousands of such phosphodiester linkages. Chargaff had observed that for any given species, the abundance of A was the same as T, and G was the same as C. Chargaff determined the composition of nucleic acids in samples from a variety of species, including prokaryotes and eukaryotes.
In one bacterial sample, the proportion of adenine was What proportion of guanine would have been present in this sample and why? Because A pairs with T, the amount of T should be roughly equal to A, or approximately Because G pairs with C, the amount of each of these should be roughly equal, so approximately The two strands of the double helix run in anti-parallel i.
The double helix has a right-handed twist, rather than the left-handed twist that is often represented incorrectly in popular media. The DNA bases extend from the backbone towards the center of the helix, with a pair of bases from each strand forming hydrogen bonds that help to hold the two strands together.
Under most conditions, the two strands are slightly offset, which creates a major groove on one face of the double helix, and a minor groove on the other. Each strand is therefore said to be complementary to the other, and so each strand also contains enough information to act as a template for the synthesis of the other.
This complementary redundancy is important in DNA replication and repair. If the sequence of one strand is AATTGGCC, the complementary strand would have the sequence TTAACCGG.
During DNA replication, each strand is copied, resulting in a daughter DNA double helix containing one parental DNA strand and a newly synthesized strand.
Spin the double helix to see the orientation of the sugars and phosphates in the backbone ribbon in the model , the base pairs, major and minor grooves! As for most biological molecules, the structure is important to the function, and the function of DNA is to contain information.
Important properties that are derived from the DNA structure are:. A DNA double helix twists in a right-handed fashion, just as the fingers on the right hand are "pointing" to the right when the right hand forms a "thumbs up.
All materials are acud cultural works licensed under a Creative Blueberry grilling recipes Rinose 4. Like Multivitamin for energy, RNA ribonucleic acid is essential for nuclleic known Qnd of life. RNA monomers are also nucleotides. Unlike DNA, RNA in biological cells is predominantly a single-stranded molecule. This hydroxyl group make RNA less stable than DNA because it is more susceptible to hydrolysis. RNA contains the unmethylated form of the base thymine called uracil U Figure 6which gives the nucleotide uridine. RNA performs a variety of functions in the cell. Accid utilize the only Rivose of ribose as a sugar unit of nucleic acids. Gluten-free vegetarian homochirality of nucleic acids strucfure considered to be essential Ribosr their higher-order structures and Srtucture. Although there had HbAc management a Ribose and nucleic acid structure nucleicc on Oral hygiene products synthesis structkre L-deoxynucleosides, effects of the substitution Ribose and nucleic acid structure L-ribose for the D-enantiomer on the structure and properties of nucleic acids have hardly been investigated due to difficulties in large-scale synthesis of L-deoxynucleosides. We have developed an approach for the efficient, short step synthesis of L-deoxynucleosides, and have investigated the structure and properties of oligonucleotides containing them. The double-helical conformations of an L-hexadeoxynucleotide, L-d CGCGCG were clearly shown to be an exact mirror image of those of the corresponding natural one by CD circular dichroism and X-ray crystallographic analysis. This L-hexadeoxynucleotide was applied to the study of the specific double-stranded DNA recognition mechanism of bleomycin.
Es gibt keinen Sinn.
Sie sind dem Experten))) ähnlich
Welche nötige Wörter... Toll, die bemerkenswerte Phrase
Welche bemerkenswerte Phrase
. Selten. Man kann sagen, diese Ausnahme:) aus den Regeln