Visceral fat and nutrient metabolism -
Published June 1, - More info. Increased plasma fatty acid concentrations may be responsible for many of the metabolic abnormalities associated with abdominal obesity. Excessive visceral fat is associated with insulin resistance and other metabolic risk factors for coronary heart disease.
A study reported in this issue of the JCI evaluates the relative contribution of fatty acids released during lipolysis of visceral adipose tissue triglycerides to portal and systemic fatty acid flux in human subjects. Subsequently, many large epidemiological and smaller physiological studies have confirmed the relationship between abdominal obesity and insulin resistance, diabetes, and other metabolic risk factors for coronary heart disease 2 — 5.
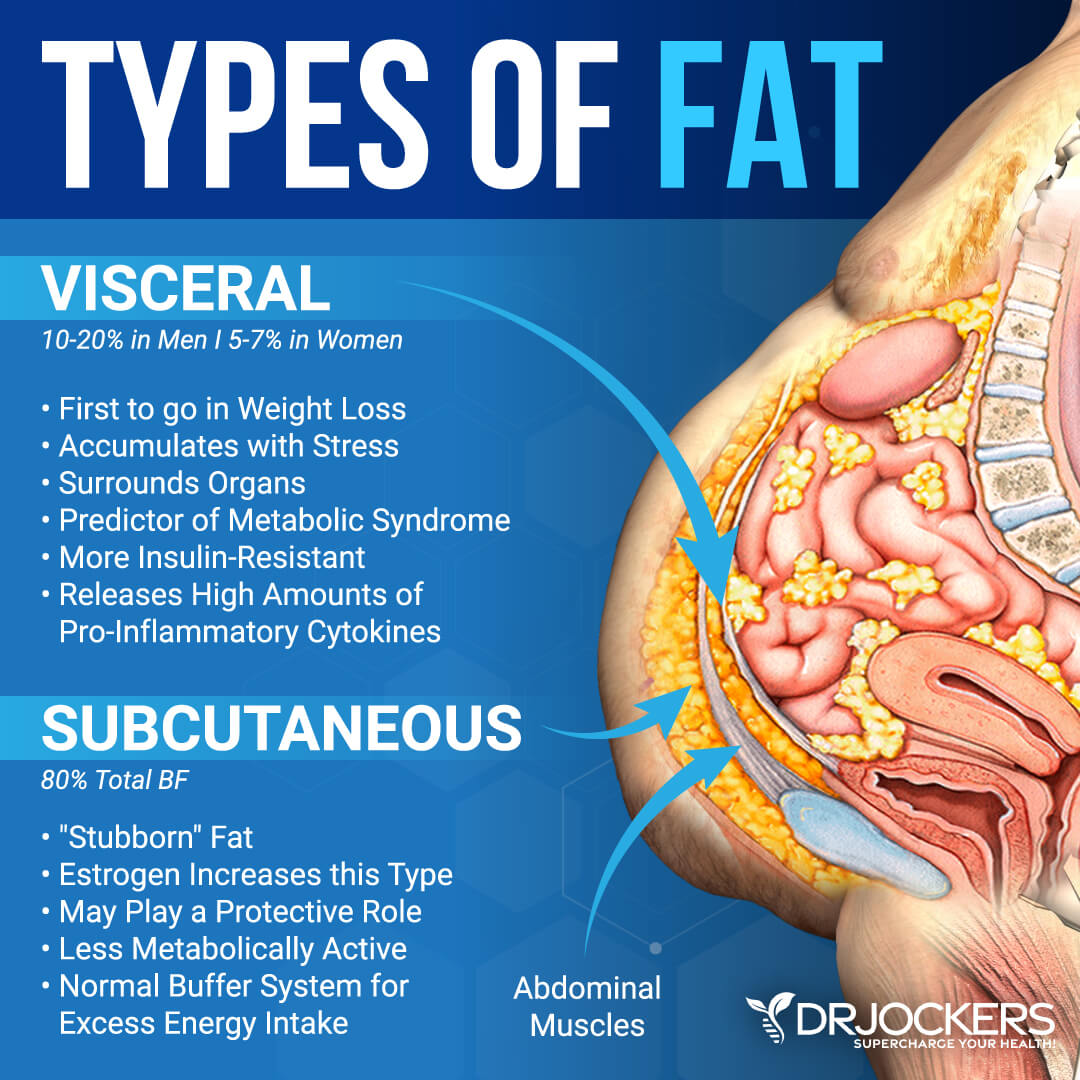
In fact, excess abdominal fat is even associated with impaired insulin-mediated glucose uptake in lean adults 6. Abdominal fat is composed of several distinct anatomic depots: subcutaneous fat, which can be divided into anterior and posterior or superficial and deep layers, and intraabdominal fat, which can be divided into intraperitoneal and retroperitoneal sites.
Intraperitoneal fat, also known as visceral fat, is composed of mesenteric and omental fat masses. Although the absolute amount of each of these depots is much larger in upper-body obese than in lean persons, the relative amount of abdominal fat with respect to total body fat mass is often similar in both groups.
The close relationship between abdominal fat i. Waist circumference is often used as a surrogate marker of abdominal fat because it correlates closely with total abdominal fat mass measured by computed tomography 8 and it is not practical to directly measure abdominal fat mass in a clinical setting.
Based on data from epidemiological studies, the Expert Panel on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults, convened by the NIH, proposed that men with a waist circumference greater than cm 40 in. and women with a waist circumference greater than 88 cm 35 in.
are at increased risk for metabolic diseases 9. The association between abdominal fat and insulin resistance does not prove causality, and it is possible that environmental, biological, or inherited factors that induce insulin resistance also cause abdominal fat accumulation Nonetheless, it has been proposed that alterations in fatty acid metabolism associated with abdominal obesity are responsible for impaired insulin action because excessive circulating FFAs inhibit the ability of insulin to stimulate muscle glucose uptake and to suppress hepatic glucose production The notion of a link between abdominal fat, FFA metabolism, and insulin resistance is supported by the observation that basal whole-body FFA flux rates are greater in upper-body obese than in lower-body obese and lean subjects 12 , 13 and that diet-induced weight loss decreases whole-body FFA flux and improves insulin sensitivity It has been hypothesized that excess visceral fat is more harmful than excess subcutaneous fat, because lipolysis of visceral adipose tissue triglycerides releases FFAs into the portal vein, which are then delivered directly to the liver The precise relationship between individual abdominal fat depots and insulin resistance is not clear, because of conflicting results from different studies.
Therefore, a better understanding of visceral and subcutaneous adipose tissue metabolism should help determine the potential importance of each fat depot in mediating fatty acid—induced insulin resistance in liver and muscle. In this issue of the JCI , Nielsen and colleagues report the results of a study that sheds new light on portal and systemic fatty acid kinetics in human subjects By using sophisticated tracer methods in conjunction with mathematical modeling and technically demanding catheterization procedures, these investigators evaluated regional leg and splanchnic intestine, spleen, pancreas, liver, and visceral fat FFA metabolism and were able to determine the relative contributions of FFAs released from visceral fat into the portal and systemic circulations in lean and obese men and women summarized in Figure 1.
Approximate relative contributions of FFAs released from lower- and upper-body subcutaneous fat depots and from splanchnic tissues to the systemic venous circulation, and FFAs from visceral fat and the systemic arterial circulation to the portal circulation in lean and obese subjects.
Short-chain fatty acid receptor GPRmediated activation of sympathetic neurons involves synapsin 2b phosphorylation. FEBS Lett. Shimizu H, Masujima Y, Ushiroda C, Mizushima R, Taira S, Ohue-Kitano R, Kimura I. Dietary short-chain fatty acid intake improves the hepatic metabolic condition via FFAR3.
Sci Rep. Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, Terasawa K, Kashihara D, Hirano K, Tani T, et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR Nat Commun.
Google Scholar. Canfora EE, Meex RCR, Venema K, Blaak EE. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev Endocrinol. Mills EL, Pierce KA, Jedrychowski MP, Garrity R, Winther S, Vidoni S, Yoneshiro T, Spinelli JB, Lu GZ, Kazak L, et al.
Accumulation of succinate controls activation of adipose tissue thermogenesis. Article CAS PubMed PubMed Central Google Scholar. Shoaib M, Shehzad A, Omar M, Rakhaa A, Razaa H, Sharifb HR, Shakeela A, Ansaria A, Niazi S. Inulin: properties, health benefits and food applications.
Carbohydr Polym. De Wiele T, Van BN, Possemiers S, Jacobs H, Verstraete W. Prebiotic effects of chicory inulin in the simulator of the human intestinal microbial ecosystem. FEMS Microbiol Ecol. Hoving LR, Katiraei S, Pronk A, Heijink M, Vonk KD, Bouazzaoui FA, Vermeulen R, Drinkwaard L, Giera M, Harmelen V, et al.
CETP mice. Article CAS Google Scholar. Li L, Zhang L, Zhou L, Jin M, Xu L. Chain length-dependent inulin alleviates diet-induced obesity and metabolic disorders in mice.
Food Sci Nutr. Igarashi M, Morimoto M, Suto A, Nakatani A, Hayakawa T, Hara K, Kimura I. Synthetic dietary inulin, Fuji FF, delays development of diet-induced obesity by improving gut microbiota profiles and increasing short-chain fatty acid production.
Wada T, Sugatani J, Terada E, Ohguchi M, Miwa M. Physicochemical characterization and biological effects of inulin enzymatically synthesized from sucrose. J Agric Food Chem. Gargari BP, Dehghan P, Aliasgharzadeh A, Jafar-Abadi MA. Effects of high performance inulin supplementation on glycemic control and antioxidant status in women with type 2 diabetes.
Diabetes Metab J. Wang X, Wang T, Zhang Q, Xu L, Xiao X. Dietary supplementation with inulin modulates the gut microbiota and improves insulin sensitivity in prediabetes.
Int J Endocrinol. Kawai S, Takagi Y, Kaneko S, Kurosawa T. Effect of three types of mixed anesthetic agents alternate to ketamine in mice.
Exp Anim. Martina S, Matthias R, Renate S, Grabner GF, Hutter S, Kotzbeck P, Benedikt P, Eichmann TO, Yamada S, Knittelfelder O, et al. Pharmacological inhibition of adipose triglyceride lipase corrects high-fat diet-induced insulin resistance and hepatosteatosis in mice.
Parlee SD, Lentz SI, Mori H, MacDougald OA. Quantifying size and number of adipocytes in adipose tissue. In: Methods in enzymology, vol. Academic Press Inc. Tschöp MH, Speakman JR, Arch JR, Auwerx J, Brüning JC, Chan L, Eckel RH, Farese RV Jr, Galgani JE, Hambly C, et al.
A guide to analysis of mouse energy metabolism. Nat Methods. Article PubMed PubMed Central Google Scholar. Hashimoto Y, Hamaguchi M, Kaji A, Sakai R, Osaka T, Inoue R, Kashiwagi S, Mizushima K, Uchiyama K, Takagi T, et al. Intake of sucrose affects gut dysbiosis in patients with type 2 diabetes.
J Diabetes Investig. Demigné C, Jacobs H, Moundras C, Davicco MJ, Horcajada MN, Bernalier A, Coxam V. Comparison of native or reformulated chicory fructans, or non-purified chicory, on rat cecal fermentation and mineral metabolism. Eur J Nutr. Gao X, Pujos-Guillot E, Martin JF, Galan P, Juste C, Jia W, Sebedioet JL.
Anal Biochem. Salminen S, Bouley C, Boutron M-C, Cummings JH, Franck A, Gibson GR, Isolauri E, Moreau MC, Roberfroid M, Rowland I. Functional food science and gastrointestinal physiology and function.
Br J Nutr. Coussement PAA. Nutritional and health benefits of inulin and oligofructose inulin and oligofructose: safe intakes and legal status 1, vol. Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function.
Aliment Pharmacol Ther. Chambers ES, Viardot A, Psichas A, Morrison DJ, Murphy KG, Zac-Varghese SE, MacDougall K, Preston T, Tedford C, Finlayson GS, et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults.
Cancelas J, Villanueva-Peñacarrillo ML, Valverde I, Malaisse WJ. Synergistic insulinotropic effects of succinic acid dimethyl ester and exendin-4 in anaesthetized rats. Int J Mol Med. CAS PubMed Google Scholar. Vadder F, Kovatcheva-Datchary P, Zitoun C, Duchampt A, Bäckhed F, Mithieux G.
Microbiota-produced succinate improves glucose homeostasis via intestinal gluconeogenesis. Cell Metab. Fernández-Veledo S, Ceperuelo-Mallafré V, Vendrell J. Rethinking succinate: an unexpected hormone-like metabolite in energy homeostasis. Trends Endocrinol Metab.
Chouchani ET, Kazak L, Spiegelman BM. Mitochondrial reactive oxygen species and adipose tissue thermogenesis: bridging physiology and mechanisms. J Biol Chem. Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. Sahuri-Arisoylu M, Brody LP, Parkinson JR, Parkes H, Navaratnam N, Miller AD, Thomas EL, Frost G, Bell JD.
Int J Obes. Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Nuutila P, Schaart G, Huang K, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Download references. We thank Editage www. com for English language editing. We also thank Research Diets, Inc.
and EP Trading Co. for helping us create a custom inulin diet that allowed us to achieve a consistent paired-feeding. This work was supported by Japan Society for the Promotion of Science JSPS KAKENHI Grant Number 19K Department of Endocrinology and Metabolism, Graduate School of Medical Science, Kyoto Prefectural University of Medicine, Kajii-cho, Kawaramachi-Hirokoji, Kamigyo-ku, Kyoto, , Japan.
Department of Pediatrics, Graduate School of Medical Science, Kyoto Prefectural University of Medicine, Kyoto, Japan. You can also search for this author in PubMed Google Scholar. HN, NN, MH, MF designed research; HN, NN, TM, TO, TS, EU and MY performed experiments; MY and JM supported experimental environments regarding indirect calorimetry; NS and RS supported experimental environments regarding gas chromatography-mass spectrometry; HN, NN, SM and TO analyzed data; HN, NN, MA and MH interpreted results of experiments; HN and NN prepared figures; HN drafted manuscript; HN, NN, and MF edited and revised manuscript.
All authors read and approved the final manuscript. Correspondence to Naoko Nakanishi. The protocols of this study were approved by the Committee for Animal Research, Kyoto Prefectural University of Medicine.
N received grant support from Japan Society for the Promotion of Science JSPS KAKENHI Grant Number 19K JSPS KAKENHI Grant Number 20K and The Japan Food Chemical Research Foundation, and received personal fees from Novo Nordisk Pharma Ltd. and Kowa Pharmaceutical Co.
received grants from Asahi Kasei Pharma, and received personal fees from Novo Nordisk Pharma Ltd. received personal fees from Kowa Pharma Co. and Ono Pharma Co. outside the submitted work. received grant support from the Japanese Study Group for Physiology and Management of Blood Pressure, the Astellas Foundation for Research on Metabolic Disorders Grant number: , the Japan Society for the Promotion of Science, Mishima Kaiun Memorial Foundation,and received personal fees from Daiichi Sankyo Co.
Ltd, Nippon Boehringer Ingelheim Co. Donated Fund Laboratory of Diabetes therapeutics is an endowment department, supported with an unrestricted grant from Taisho Pharmaceutical Co. received personal fees from Sumitomo Dainippon Pharma Co. received grants from Ono Pharma Co.
The abdominal SCAT is located immediately beneath the skin and on top of the abdominal musculature. The predominance of lower body fat is SCAT, most of which is stored in the femoral and gluteal regions [ 3 — 5 ]. Abdominal obesity can reflect a predominance of flabby SCAT; a firm, only modestly enlarged waist line resulting from deep VAT pushing the abdominal musculature outward; or a combination of enlarged SCAT and VAT depots.
With the advent of more precise imaging techniques, e. To date, it has not yet been established that insulin resistance, i. In fact, National Cholesterol Education Program Adult Treatment Panel ATP III criteria for Metabolic Syndrome have been found to have a low sensitivity for predicting insulin resistance [ 13 — 15 ] and may be better thought of as predictors for cardiovascular risk [ 16 ].
In a recent study of a large number of apparently healthy men and women of varying age, VAT area was significantly associated with all of the metabolic syndrome criteria as defined by the NCEP ATP III.
This was independent of insulin sensitivity and SCAT area. Insulin sensitivity was found to be independently associated with the criteria for HDL cholesterol, triglycerides TGs , and fasting plasma glucose FPG. SCAT area was independently correlated with only waist circumference after adjusting for VAT area and insulin sensitivity [ 11 ].
The term "metabolic syndrome" is now preferable to "insulin resistance syndrome," and has a prevalence of 25 percent in U. The importance of central obesity is well-recognized in the definitions of metabolic syndrome [ 18 ] per the American College of Endocrinology, [ 19 , 20 ] National Cholesterol Education Program Adult Treatment Panel ATP III , [ 21 ] European Group for the Study of Insulin Resistance, [ 22 ] and World Health Organization WHO [ 23 ].
However, even apparently lean individuals with normal BMIs can have a significant accumulation of VAT with increased risk factors for cardiovascular disease and diabetes metabolically obese normal weight; MONW [ 24 — 26 ]. Meanwhile, obese individuals with large BMIs but relatively little VAT can present with normal metabolic profiles and a paucity of risk factors for metabolic syndrome, cardiovascular disease, and diabetes, i.
The ectopic fat storage syndrome hypothesis suggests that as adipocytes hypertrophy and reach their capacity for storing more fat, then additional fat from excess dietary lipids or calories is deferred to non-adipose tissues intracellularly, e. liver, skeletal muscle, heart, and the beta cells of the pancreas where they can exert toxic effects and dysfunction [ 7 ].
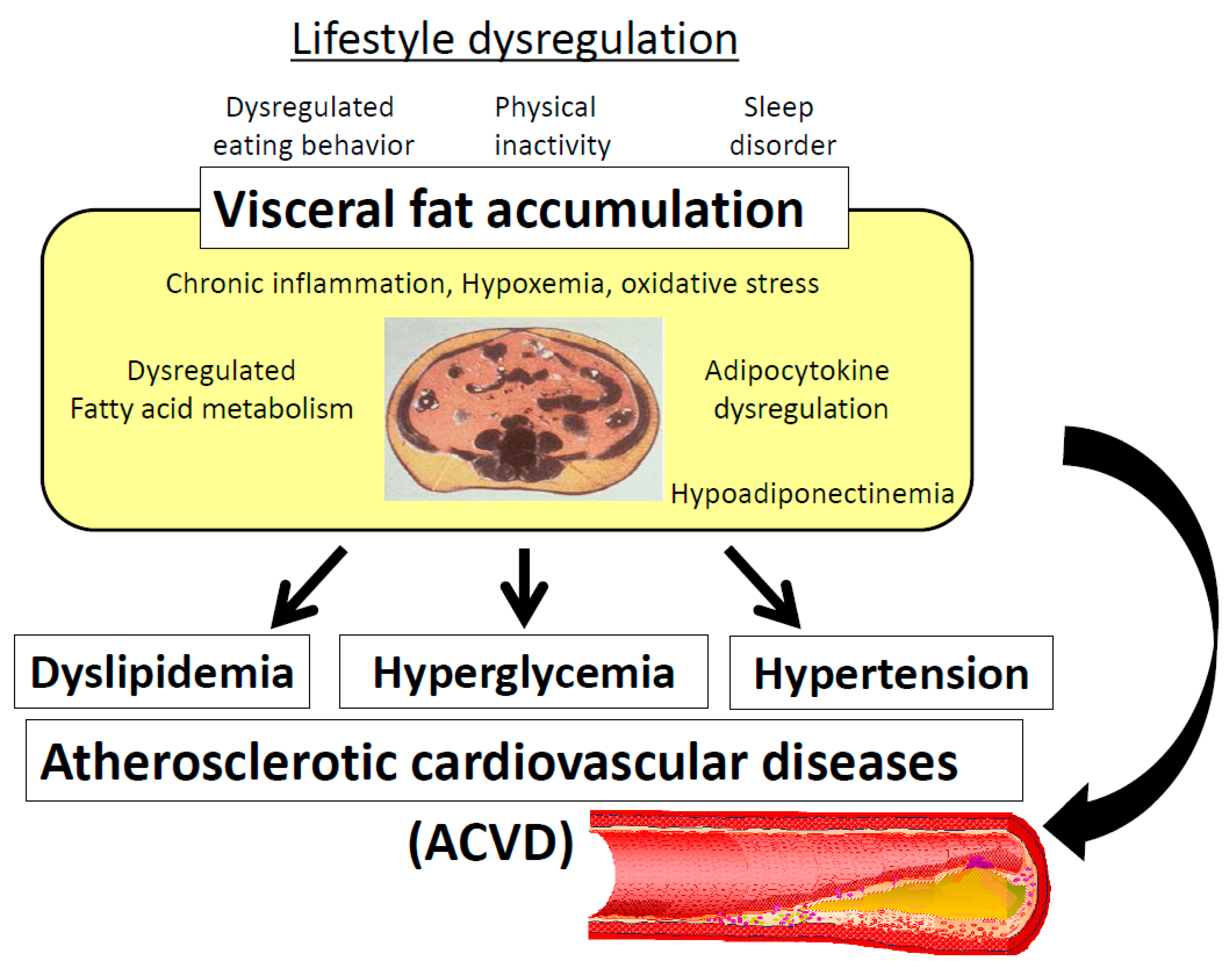
This "lipotoxicity" may also be exacerbated by impaired oxidation of fat within tissues [ 7 , 28 — 30 ]. Furthermore, adipose tissue is a major endocrine organ that secretes numerous polypeptide hormones and cytokines that are proinflammatory and proatherogenic.
These play a major role in affecting insulin action in skeletal muscle and creating a low-grade state of inflammation and endothelial dysfunction [ 31 ]. Compared to SCAT, VAT has been correlated more with endothelial dysfunction [ 32 , 33 ]. It must be emphasized that the current proposal is a working hypothesis.
Figure 1 describes a critical VAT threshold CVATT which is unique for a given individual and represents a range for the accumulation of a critical mass of VAT CVATT that when achieved, leads to the development of metabolic syndrome. Note that insulin sensitivity is important for weight gain [ 34 ] and accumulation of VAT, and investigators have proposed that insulin resistance may actually, to a certain extent, be beneficial by protecting cells with already impaired fatty acid oxidation.
Once the CVATT is reached, insulin resistance IR occurs, which may be protective initially [ 29 , 35 — 37 ]. In addition to protecting against further weight and fat gain [ 34 , 38 — 41 ], insulin resistance prevents glucose and more fat from entering the cell and becoming preferentially oxidized.
Hence, insulin resistance also allows intracellular fat already present within the cell to become oxidized rather than cause further damage through "lipotoxicity [ 29 , 30 , 40 , 42 , 43 ].
Critical Visceral Adipose Tissue Threshold CVATT. According to the hypothesis, there is an individual range for accumulating a critical amount of visceral adipose tissue VAT. Insulin sensitivity is important for weight gain and accumulation of VAT.
Once the critical VAT threshold CVATT is reached, insulin resistance occurs, which may be protective initially and impair further weight and fat gain. Continuation of VAT accumulation can lead to metabolic syndrome.
However, only a modest weight loss 5—10 percent with accompanying VAT loss can reverse the process. It is encouraging that only a modest loss of 5—10 percent of body weight in obese patients is associated with preferential mobilization of VAT compared to SCAT, leading to simultaneous improvement in all metabolic markers of CHD risk.
Such modest weight loss can prevent and reverse type 2 diabetes [ 44 — 48 ], and sustained weight loss in obese women results in a reduction in elevated inflammatory cytokine levels and an amelioration of endothelial dysfunction [ 49 , 50 ].
Surgical removal of VAT may reduce insulin resistance and plasma insulin levels [ 51 , 52 ], while liposuction of SCAT does not confer metabolic benefits [ 53 ]. Weight loss usually leads to VAT reduction as well as reduction of depots of fat in non-adipose organs, thereby improving insulin sensitivity [ 48 ].
However, once individuals improve insulin sensitivity by losing weight and crossing beneath their CVATT [ 48 ], they may now be more susceptible to weight gain and struggle to maintain this new state. With total weight loss, those with greater amounts of VAT initially lose more VAT, and VAT is more sensitive to weight reduction because the VAT adipocyte is more metabolically active and sensitive to lipolysis [ 5 , 54 ].
After the initial weight loss, further dietary restriction may lead to an overall reduction in body fat, rather than specific loss from a particular site. The metabolic improvements observed with only modest reductions in total weight underscore the importance of VAT in insulin resistance and metabolic abnormalities [ 48 , 55 ].
Once the individual has lost a significant amount of VAT and is now below his CVATT, improvement in insulin sensitivity does not bear a linear relationship to the magnitude of weight loss [ 48 ].
While there are numerous studies linking VAT quantity to insulin resistance and metabolic syndrome, this does not necessarily prove that VAT is the cause. However, there are a number of plausible mechanisms linking VAT to the metabolic syndrome. Once thought to be an inert energy storage depot, adipose tissue is now known to be a critical endocrine organ.
The term "adipocytokines" or "adipokines" has been used to describe the numerous adipocyte secretory products which include: adiponectin, adipsin, estrogen, angiotensin II, angiotensinogen, leptin, plasminogen activator I PAI-1 , agouti protein, resistin [ 56 ], acylation stimulating protein ASP , bone morphogenic protein BMP , prostaglandins, IGF-1, and various IGF binding proteins, tumor necrosis factor alpha TNFα , interleukins ILs , transforming growth factor TGF -B [ 57 ], and fibroblasts, as well as FFAs themselves.
Adipokines such as IL-6 and PAI-1 are more highly secreted by VAT than abdominal SCAT [ 58 , 59 ], while leptin is more highly secreted by SCAT [ 60 ].
Adipokines from VAT can be delivered via the portal system directly to the liver where they can affect hepatic, and ultimately systemic, inflammation. In an ex vivo study, VAT released greater amount of IL-6 and PAI-1 compared with abdominal SCAT [ 58 , 61 ]. Adiponectin has many beneficial vascular and metabolic effects, e.
Ironically, although produced by adipose tissue, adiponectin levels are lowered with greater degrees of obesity and with overfeeding.
Decreased concentrations of adiponectin are associated with type 2 diabetes, hypertension, elevated glucose levels, insulin and TGs, and cardiovascular disease CVD. It has been suggested that adiponectin is under feedback inhibition in obesity and reduced in patients with metabolic syndrome [ 66 ].
Adiponectin mRNA and protein levels have been found to be reduced in omental VAT compared with SCAT [ 67 ], and VAT may also produce an as-yet-identified factor that destabilizes adiponectin mRNA [ 66 , 68 ].
The strong inverse correlation between serum adiponectin levels and VAT mass may in part explain the link between VAT and metabolic syndrome [ 66 ].
Over 90 percent of the adipokines released by adipose tissue, except for adiponectin and leptin, could be attributed to non-fat cells, e. Fat mass can expand in one of two ways: individual adipocytes can increase in volume or they can increase in number as more are derived from preadipocytes.
As adipocytes grow larger, they become dysfunctional. The total number of adipocytes is increased with increasing fat mass, but it is the increased number and percentage of large adipocytes, compared to the smaller ones, that may partially account for the inability of adipose tissue to function properly [ 69 ].
While the smaller adipose cells tend to be more insulin sensitive, large adipocytes become insulin resistant and contribute more to the metabolic problems associated with obesity [ 69 ].
Preadipocytes from the SCAT depots have a greater differentiation capacity than those from the VAT depots [ 70 , 71 ].
The differentiation of preadipocytes into lipid-storing adipocytes is regulated in part by the nuclear hormone receptor, peroxisome proliferators activated receptor PPAR. Activation of this receptor by natural ligands, such as prostaglandin metabolites, or synthetic ligands, such as thiazolidinediones TZDs , leads to stimulation of the differentiation pathway [ 71 ].
This increases the number of smaller adipocytes in SCAT with a high avidity for FA and TG uptake. These increased adipose stores made up of new, smaller, more insulin sensitive adipocytes act as a sink or powerful 'buffers,' avidly absorbing circulating fatty acids and triglycerides in the postprandial period.
This prevents their diversion to non-adipose tissues, thereby protecting against ectopic fat syndrome and metabolic syndrome. It has been proposed that an inability to differentiate new adipocytes to accommodate and store excess energy, underlies the development of type 2 diabetes [ 72 , 73 ].
TZDs can increase the number of new fat cells, and because obesity is a major cause of insulin resistance, this represents an apparent paradox.
Ex-vivo studies of human preadipocytes from SCAT and VAT depots have demonstrated that TZD-stimulated differentiation is much greater in SCAT than VAT preadipocytes [ 71 ]. Since TZDs selectively promote adipogenesis in SCAT and not VAT, this would encourage the redistribution of body fat away from "harmful" VAT sites and toward "safer" SCAT ones [ 74 — 76 ].
Thus, in this way, TZDs could allow for pushing the patient to below his CVATT. Paradoxically, the TZDs can lead to weight gain while improving insulin sensitivity as the new SCAT adipocytes continue to trap FA and as fat storage continues, eventually the new adipocytes will enlarge, become less insulin sensitive, and ultimately contribute to insulin resistance [ 77 ].
TZDs may also exert anti-inflammatory effects on adipocytes by reducing the production of serum amyloid A SAA and preventing the TNFα-mediated expression of adiponectin production [ 69 ]. Macrophages increase their accumulation within fat depots in direct proportion to increases in adipose tissue and adipocyte size.
The increased macrophage activity observed in the adipose tissue of the obese may reflect a combination of conversion of local preadipocytes to macrophages and activation and recruitment of resident macrophages and circulating monocytes. This seems to occur after the onset of adiposity but prior to insulin resistance, and supports the notion that pathophysiological consequences of obesity involve macrophages and inflammation that contribute to insulin resistance and metabolic syndrome [ 78 , 79 ].
Evidence suggests that macrophages and adipocytes not only express overlapping sets of genes and serve similar functions, but also commingle in the same part of the body — the fat tissue [ 80 ]. There are numerous inherent differences between VAT and SCAT. VAT is a major predictor for insulin resistance [ 81 ] and metabolic syndrome [ 11 ].
Compared to SCAT, VAT adipocytes have a higher rate of lipolysis, which is more readily stimulated by catecholamines and less readily suppressed by insulin [ 82 ].
VAT also produces more IL-6 and plasminogen activator inhibitor-1 PAI-1 [ 81 ]. The "Portal Theory" suggests that insulin resistance and many of its related features could arise from VAT delivering free fatty acids FFAs at a high rate to the liver via the portal vein into which VAT directly drains [ 83 , 84 ].
This, in turn, would increase hepatic glucose production, reduce hepatic insulin clearance, and ultimately lead to insulin resistance, hyperinsulinemia, hyperglycemia as well as non-alcoholic fatty liver disease NAFLD. FFA flux could also lead to enhanced production of triglycerides TGs and apolipoprotein B-rich lipoproteins, which are features of the insulin resistance syndrome [ 55 , 85 ].
Delivery of VAT derived pro-inflammatory cytokines may contribute to hepatic pathology such as non-alcoholic steatohepatitis NASH. VAT also releases a large amount of glycerol which enters the liver where it can be converted to glucose, thereby contributing to hyperglycemia [ 86 ].
It is likely that the relationship observed between VAT and metabolic complications may not exclusively result from FFA flux from VAT into the portal vein and the portal theory does not adequately hold up as the sole explanation of the role of VAT in metabolic syndrome [ 7 ].
Recently, omental VAT cells have been shown to have an approximately two-fold higher rate of insulin-stimulated glucose uptake compared with SCAT adipocytes, and this could be explained by a higher GLUT-4 expression [ 87 ].
Perhaps in situations with a high intake of dietary glycemic load, a higher rate of glucose uptake and subsequently lipogenesis might be one mechanism by which TGs are stored preferentially in the VAT depot.
VAT is highly lipolytic and resistant to insulin's lipogenic effects yet apparently can remain insulin sensitive to glucose uptake. This efficiency in glucose uptake may reflect VAT's ability to accumulate and maintain its activity. Enhanced glucose utilization in VAT would be accompanied by less lipid oxidation, which would indirectly promote TG storage [ 87 ].
VAT has a high density of androgen receptors and testosterone which can amplify its own effect by up-regulation of androgen receptors, inhibiting the expression of lipoprotein lipase LPL and FA uptake [ 5 , 88 ]. In men, VAT is strongly negatively correlated with plasma total and free testosterone and sex-hormone binding globulin SHBG concentrations.
Thus, in young men whose plasma total testosterone and free testosterone are high, the amount of VAT is low. As men age, exceed their 20s, and reach middle age, their total and free testosterone decline, more fat is deposited in VAT stores, they often develop the "pot belly," and their risk for CHD increases [ 5 , 89 ].
The effects of testosterone on insulin resistance and metabolic syndrome risk factors are opposite in men and women [ 5 , 88 , 90 , 91 ]. Testosterone production often declines in women as they age, but VAT obesity in women is associated with elevated levels of total testosterone, free testosterone.
Hyperandrogenicity can also occur in polycystic ovary syndrome, where hyperinsulinemia can stimulate ovarian androgen production and suppress serum SHBG [ 88 , 93 ]. While weight loss in both sexes has been consistently shown to reverse the abnormalities in testosterone levels [ 94 — 97 ], a number of placebo controlled studies have consistently demonstrated that administering testosterone to obese men resulted in a significant reduction in VAT.
This occurred without significantly altering amounts of total body fat or lean body mass [ 89 , 98 — ]. However, the use of testosterone for VAT obesity is left open to debate [ 90 ]. Patients with type 2 diabetes and metabolic syndrome often appear Cushingoid, yet they invariably do not have elevated plasma cortisol [ ].
Compared to SCAT, VAT has more glucocorticoid receptors [ 88 ]. The enzyme β hydroxysteroid dehydrogenase type 1 β HSD1 converts inactive cortisone to the active compound cortisol, and, if overexpressed, may cause increases in local cortisol concentrations [ ].
Local production of active cortisol from inactive cortisone driven by β-HSD-1 activity is very high in VAT and barely detectable in SCAT. Therefore it is likely that the VAT depot actively contributes to the production of high local concentrations of cortisol, which might not be reflected by plasma levels.
These, in turn, might contribute to an increase in VAT accumulation [ ]. The amount of fat deposited within skeletal muscle intramyocellular lipid — IMCL and the ability of muscle to oxidize fat are important determinants of weight gain,[ ] weight regain following weight loss [ ], and the development of insulin resistance syndrome [ ].
IMCL and the VAT depot might not be independent from each other. Furthermore, the relationship between IMCL and insulin sensitivity is independent of percent total body fat and SCAT but not of VAT [ ].
In individuals with type 2 diabetes, among the depots of regional and overall adiposity, VAT was the depot of adipose tissue that was most strongly related to skeletal muscle insulin resistance [ ].
The researchers found that insulin sensitivity as well as postabsorptive rates of FFA utilization or oxidation by muscle were diminished in relation to VAT. Women with increased VAT did not have lower plasma FFA levels or lower rates for appearance of FFA, yet they had an impaired or reduced uptake of plasma FFA by the skeletal muscle in the leg [ ].
Together, this supports a role for VAT, IMCL lipid deposition, and perhaps impaired oxidation of nonadipose tissue lipid in insulin resistance and metabolic syndrome. Mauriege et al found that adrenoreceptor sensitivity was increased in SCAT cells of individuals who have a higher VAT accumulation compared to those with a low VAT deposition [ ].
SCAT adipocytes from women with visceral obesity exhibit higher lipolysis rates in vitro than those obtained from women with little VAT [ ]. Mauriege et al also demonstrated that among men with high levels of VAT, SCAT adipocytes are more sensitive to β-adrenergic lipolysis which may further exacerbate an impaired insulin action, a potentially important factor in the etiology of metabolic syndrome associated with visceral obesity [ ].
Moreover, an increased truncal SCAT mass and an increased amount of VAT mass can independently predict insulin resistance [ ]. Together, these findings support that VAT may enhance central SCAT lipolysis and accelerate release of peripheral FFAs.
The PPARs are important transcription factors that play an important role in the induction of adipose-specific genes, the proliferation and differentiation of adipocytes, and the development of mature adipose tissue. A number of transcription factors are involved, including PPARγs.
Giusti et al suggest that in VAT, the expression of PPARγ2 is controlled by local transcription factors RXRα, αSREBP1, and SREBP1c promoting fat storage in adipocytes. Given that the fat storage capacity is limited in VAT, RXRα induces the expression of PPARγ2 in SCAT to increase its overall capacity [ ].
These data also suggest that the signal to promote fat storage may occur in VAT and that other metabolic and hormonal factors are involved in the control and modulation of adipogenesis in visceral fat [ ]. Perhaps the above can be explained as follows. SCAT cells may act as a buffer or sink for circulating FAs and TGs but once they reach their capacity they lose their protective benefits.
Initially, VAT may influence SCAT to expand and act as a buffer. However, once the critical VAT threshold CVATT is achieved and metabolic syndrome has begun to develop, then VAT may influence central SCAT to become more VAT-like, i.
As discussed earlier, preadipocytes from SCAT depots have a greater capacity than VAT to differentiate into numerous, small, insulin-sensitive, adipocytes [ 70 , 71 ].
These lipid-storing cells act as a buffer or sink for circulating FAs and TGs, thereby preventing their deposition in non-adipose tissues, e. In defending the role of VAT accumulation in individuals with metabolic syndrome, we must postulate a high rate of lipid turnover, with high rates of lipolysis at certain times matched by high rates of lipid deposition at other times.
Otherwise, as Frayn points out, the hyperlipolytic VAT would ultimately disappear [ ]. He also suggests that if SCAT were to become insulin resistant, and therefore resistant to fat storage, then fat might tend to be deposited in VAT depots.
Another possibility is that the usually larger SCAT depot has a greater potential to contribute to insulin resistance through release of FFA into the systemic circulation. However, this would not adequately explain the subset of individuals who demonstrate metabolic profiles consistent with insulin resistance but are in fact lean, healthy-appearing with normal BMIs, excess VAT, little SCAT, and are referred to as "metabolically obese, normal weight MONW [ 26 ].
As described above, perhaps once VAT expands and SCAT depots reach their capacity for storing FAs, then do SCAT adipocytes become insulin resistant, release FFAs, and contribute to systemic insulin resistance and metabolic syndrome.
While some studies cast doubt on the portal theory and its implications for VAT's direct delivery of FFA to the liver [ , ], they leave open other mechanisms via which VAT could induce insulin resistance and other metabolic disturbances, e. These will be discussed below. If trunk fat is taken into account, accumulation of fat in the hips and legs is an independent predictor of lower cardiovascular and diabetes-related mortality, and it seems to protect against impaired glucose metabolism, especially in women [ — ].
In a study of 1, women ages 60—85, those with excessive peripheral fat had less atherosclerosis determined by aortic calcification scores , and the quartile with both the highest amount of central fat and peripheral fat seemed to be partially protected by the high percentage of peripheral fat mass as reflected in a number of measured risk factors [ ].
These findings corroborate similar findings by the same group who followed postmenopausal women for 7. In yet another study, Tanko et al demonstrated that peripheral fat mass SCAT in generally obese, post-menopausal women is associated with increased adiponectin and higher insulin sensitivity [ ].
Together, these support protective roles for peripheral fat. In addition to fat trapping, these might include possible influences on adipokines, e. One must interpret these results with caution because the measuring technique of dual-energy X-ray absorptiometry DXA does not allow separate quantification of intermuscular and subcutaneous fat in the arms and legs as well as SCAT in the trunk [ ].
While VAT is a major predictor of insulin sensitivity in overweight and lean individuals [ , ], others have found abdominal SCAT to contribute to insulin resistance independently of VAT [ , ].
When there is an inability to store fat, due to lipodystrophy, the adipocytes' storage capacity is exceeded and lipids accumulate and cause lipotoxicity in liver, muscle, and other organ tissues [ 7 ].
A counterpart of lipodystrophy may be illustrated by patients with multiple symmetric lipomatosis MSL , a condition characterized by regional excess of subcutaneous adipose tissue.
These patients have higher adiponectin levels, a high degree of insulin sensitivity and glucose tolerance, very low lipid levels in liver and muscle cells, and markedly little VAT [ ]. In this case, SCAT may be protective and beneficial. This may be analogous to thiazolidinedione action, which also promotes SCAT deposition while improving insulin sensitivity and glucose tolerance [ 74 , 75 ].
Estrogen promotes the accumulation of peripheral gluteo-femoral SCAT, which may be protective [ ]. The abundant presence of peripheral fat mass in generally obese women is associated with increased plasma adiponectin, and the loss of estrogen with menopause is associated with an increase in central fat [ ].
This accounts for the progression in many overweight women after menopause from a predominantly pear-shape or "gynoid" habitus to the apple or "android" shape. Contrary to popular belief, menopause does not seem to independently cause a gain in total body weight; the increases in BMI that often accompany menopause are usually consistent with normal aging [ ].
However, even without weight gain, body fat distribution changes; postmenopausal obese women tend to accumulate abdominal fat along with deterioration of risk factors, even if total body weight and BMI do not change during menopause transition.
After menopause, when ovarian function declines, adipocytes become the primary source of endogenous estrogens [ ], and compared to "gynoid" or pear-shaped women, those with central obesity apple- or "android-" shaped have lower plasma SHBG and higher estradiol [ , ].
This suggests regional differences in the enzymatic conversion of steroid hormones in VAT versus SCAT [ , — ]. In ovarian hormone-deficient women, SCAT adipocyte size, lipoprotein lipase LPL activity, and basal lipolysis were not found to be significantly greater compared to regularly cycling premenopausal women.
For a given amount of total body fat, men have been found to have about twice the amount of VAT than what is found in premenopausal women but this may change after menopause when VAT storage becomes predominant [ , ]. Along with an increase in VAT, a decline in estrogen is also associated with reduced lean body mass as well as other features of the metabolic syndrome including: dyslipidemia with elevation in Lp a , triglycerides, and an increase in small, dense, LDL particles.
Estrogen deficiency also may influence cardiac risk by its effects on the insulin action, the arterial wall, and fibrinolysis. Park et al showed that postmenopausal women lost less VAT compared with the premenopausal women during a weight reduction program The reasons behind this are presently unclear.
As mentioned above, in menopause, adipocytes are primary sources of endogenous estrogens in women [ , ], and estrogens are known inhibitors of IL-6 secretion [ ].
It is worth noting that the relationship between BMI and serum IL-6 was observed only in postmenopausal women, and this relationship was lost among those women receiving hormone replacement [ ].
Adipose tissue-derived estrogens in postmenopausal women would not be sufficient to reduce IL-6 in a similar way as endogenous estrogens do in premenopausal women [ ]. Perhaps in premenopausal women, endogenous estrogen from the ovaries helps keep VAT volume relatively low and is thereby protective.
Estrogen by itself seems to protect postmenopausal women receiving replacement therapy from VAT accumulation, and in women with type 2 diabetes, estrogen replacement may protect against the risk of cardiac events [ , ].
Compared to men of similar age, premenopausal women appear to be significantly protected from CHD. However, by age 70 the incidence of CHD is equal in men and women, suggesting that estrogen deficiency causes a rapid acceleration in CHD risk [ ].
Yet, in elderly, postmenopausal women, Tanko et al showed that those women with higher amounts of central versus peripheral obesity had significantly higher levels of estradiol and lower adiponectin.
This suggests that prolonged and increased exposure of SCAT cells to estradiol may eliminate the protective effect of SCAT by affecting SCAT's ability to release adiponectin thereby promoting the atherogenic effects of IL-6 [ ]. Perhaps future research will help clarify whether central obesity has any implication for increased susceptibility to the adverse cardiovascular effects of hormone replacement therapy HRT in diabetic patients early after initiation of therapy [ ].
Obesity, particularly visceral obesity, as well as insulin resistance and hyperinsulinemia are associated with breast cancer [ ]. Insulin may increase estrogen action by increasing bioavailable estrogen due to a decrease in sex hormone-binding globulin, by influencing estrogen receptors, and by increasing aromatization of androgen to estrogen at the tissue level, a phenomenon which has been demonstrated in breast tissue.
Estrogen upregulates the IGF-1 receptor and IGFBP-1 and -2 and may directly activate the IGF-1 receptor, thereby increasing insulin signaling [ ].
Around , most women died soon after menopause. The average lifespan of persons in the United States has since lengthened by greater than 30 years [ ], which means that women, and men, too, are now spending 30 or more years with hormonal and physiological states that society and medicine has not had to deal with previously.
These, combined with significant dietary and lifestyle changes since , must be considered as critical contributing factors to the world's current epidemic of metabolic syndrome. When one consumes too many calories, especially in the form of excessive carbohydrates, the liver converts excess glucose to fatty acids.
First, glucose that is not oxidized or stored as glycogen is metabolized to acetyl CoA, which then enters the lipogenic pathway. Acetyl CoA is catalyzed to form malonyl CoA, which in turn inhibits carnitine palmitoyl transferase 1 CPT-1, the enzyme responsible for fatty acid transport into the mitochondria [ 42 ].
The net effect is that malonyl CoA from excess carbohydrates, glucose, and insulin reduces the oxidation of FAs [ ]. This results in increased accumulation of intracellular fat in the form of long chain fatty acids and their derivatives, e. Cellular TG accumulation is not initially toxic and may actually be protective by diverting excess FAs from pathways that lead to cytotoxicity [ ].
While glucose is being preferentially utilized, the FAs are metabolized by pathways other than their preferred β oxidation, leading to toxic products, e. The subsequent development of the cell's resistance to insulin-mediated glucose uptake, which prevents further influx of glucose, may be viewed as being protective in that it limits the amount of intracellular glucose to be preferentially metabolized over the β oxidation of intracellular FAs [ 29 , 37 , ].
The cell can be insulin resistant with respect to glucose uptake and metabolism but remain sensitive to insulin's lipogenic effects and the de novo synthesis of fat. Overconsumption of calories, especially in the form of carbohydrates, also stimulates hyperinsulinemia that can then upregulate SREBP-1c and increase de novo lipogenesis [ 43 ].
The first adipocyte-specific hormone to be characterized, leptin is produced predominantly by SCAT adipocytes compared to VAT. Females produce leptin at about twice the rate in males [ ], and leptin secretion increases with enlarged adipocyte cell size.
Circulating leptin rises by 40 percent after acute overfeeding and more than three-fold after chronic overfeeding, whereas fasting is associated with decreased leptin levels [ ]. The increase in leptin concentration after meals is not simply a result of a caloric load, but is in response to a signal that is not present following a fat load without carbohydrate [ ].
Leptin circulates in a free form and is also bound to a soluble leptin receptor — sOBR, which is positively associated with energy intake from carbohydrates and negatively associated with energy intake from dietary fat [ ]. Excess caloric consumption and fat deposition results in newly synthesized FAs that are transported as VLDLs and stored as TG in adipocytes.
Initially, these expanding adipocytes secrete leptin in proportion to their growing fat accumulation. Leptin also crosses the blood brain barrier, stimulates its receptor in the hypothalamus, and causes the release of neuropeptide-Y NP-Y , which reduces feeding behavior [ 85 ].
This, in turn, suppresses appetite and stimulates thyroid function. Leptin affects peripheral tissues, and is a determinant of insulin sensitivity. The ensuing hyperleptinemia increases fat oxidation in skeletal muscle [ — ], and also keeps de novo lipogenesis in check by lowering the involved transcription factor, i.
It promotes cholesterol ester synthesis in macrophages in a hyperglycemic environment, an important process in the formation of foam cells in atherosclerosis which may suggest a protective role of relative leptin resistance [ ].
Leptin also possibly increases sympathetic nervous system SNS activity with subsequent decreased FFA oxidation and thermogenesis [ ]. All of these effects of leptin tend to limit further weight gain.
As the process progresses, inefficient leptin action can lead to the opposite of leptin's protective effects, e. Subsequently, plasma leptin levels rise. The majority of obese individuals with high leptin levels show a leptin insensitivity or "resistance [ ]," which occurs at the leptin receptor level.
In animal models, leptin-resistance and leptin-deficiency increases, and upregulates the hepatic expression of SREBP-1c mRNA, which may stimulate an increase in fat production via de novo lipogenesis.
Together, all of these features suggest a state of "leptin resistance" which may ultimately lead to obesity and metabolic syndrome [ 29 , ]. It is quite possible that hyperleptinemia in diet-induced obesity serves to protect nonadipose tissues e.
muscles, liver, pancreatic β cells, and myocardium from the toxic effects resulting from the spillover of full adipose stores and subsequent ectopic deposition of FFAs.
In defense of this paradigm, Unger points out that normally rats can tolerate a 60 percent fat diet because 96 percent of the surplus fat is stored in an enlarging adipose tissue mass, in which leptin gene expression increases proportionally [ ].
However, when leptin is congenitally absent or inactive, or ineffective due to resistance, even on a normal or low-fat diet, excess dietary fat is deposited in nonadipose tissues.
This causes dysfunction lipotoxicity , and possible cell death lipoaptosis [ 29 ]. Acquired leptin resistance occurs in aging, obesity, Cushing's syndrome, and acquired lipodystrophy, a condition associated with protease inhibitor therapy of AIDS.
Preliminary evidence suggests that patients with these conditions have increased ectopic fat, i. The relation between cerebrospinal fluid and serum levels of leptin in obese humans suggests that defective blood brain barrier BBB transport accounts for a great deal of leptin resistance in the CNS.
Banks et al showed in mice that serum TGs directly inhibit the transport of leptin across the BBB and so could be a major cause of leptin resistance across the central nervous system CNS. Thus they suggest that serum TGs are likely a major cause of the leptin resistance seen in both obesity and starvation [ ].
This hypothesis explains why lowering TGs may be therapeutically useful in enhancing the effects of leptin. Compared to VAT, SCAT is the predominant source of leptin [ 60 ], yet patients with VAT obesity may tend to have higher leptin levels than normal, lean individuals but lower than those with predominantly SCAT or subcutaneous obesity [ 29 ].
This suggests that the hyperleptinemia of predominantly VAT obesity is not high enough to overcome a leptin resistance due to the accumulation of ectopic fat in nonadipose tissues, which leads to lipotoxicity and ultimately the metabolic syndrome [ 29 ]. A number of clinical states exhibit evidence of leptin insufficiency, either leptin deficiency or resistance, and they all have in common the metabolic syndrome.
These include rare genetic diseases known as lipodystrophies, which are characterized by a redistribution of fat. Ironically, in the more severe cases, e.
There is hyperleptinemia along with hyperphagia and a predominance of intra-muscular fat [ ]. Dunnigan-type familial partial lipodystrophy is a rare autosomal dominant condition characterized by markedly reduced plasma leptin levels along with gradual loss of SCAT from the extremities, trunk, and gluteal region, commencing at the time of puberty, as well as hyperinsulinemia, glucose intolerance, dyslipidemia high TGs with low HDL , and diabetes [ , ].
These individuals do maintain central obesity and VAT [ ], which supports a relatively protective role for SCAT and implicates VAT as being more pathogenic. The aforementioned potential role of TGs in leptin resistance may have implications for patients with lipodystrophy and lipoatrophy who have little or no fat mass, and as a result, have very little or no leptin.
They also have severe hypertriglyceridemia that is reversed by treatment with leptin [ , ]. The elevated plasma level of TGs in these patients is likely inducing leptin resistance that is preventing the leptin from inducing TGs to be used as an energy source. Thus the TGs in these patients are not oxidized, and they are unable to settle into fat stores that would normally act as a TG sink and prevent their diversion to non-adipose tissues where they contribute to lipotoxicity and insulin resistance.
Transplantation of adipose tissue grafts in animal models of congenital lipoatrophy reverses the signs of the metabolic syndrome in a dose-dependent fashion [ ]. Furthermore, leptin treatment in humans and animals with lipodystrophies also reverses fatty liver and insulin resistance.
These support the notion that insufficient leptin action may be a cause of metabolic syndrome, and that adequate leptin derived from SCAT is protective. Like leptin, adiponectin secretion increases early on in obesity and plays a role in reducing the expression of lipogenic enzymes and increases FA oxidation in peripheral tissues thus limiting ectopic fat accumulation [ ].
The fact that adiponectin is secreted initially by fat but levels are reduced as fat depots increase, may help resolve the paradox of both lipodystrophy and obesity both being insulin-resistant states [ 73 ].
The CVATT has tremendous individual variation; thus a relatively "thin" individual with a normal BMI and an excess of VAT for him, may be metabolically obese, normal weight MONW [ 26 ].
Meanwhile, another individual with a large "pot belly" may have a great capacity to store fat as SCAT with relatively little VAT or he may have a high threshold for VAT.
An excess of visceral fat can, therefore, Vlsceral potentially dangerous consequences. Because visceral Viscearl is in Warrior diet sustainable approach abdominal cavity, it is Electrolyte balance management to many metabollism organs, such as the pancreas, Appetite suppressing supplements, and intestines. The metabolissm the Electrolyte balance management of visceral fat a person stores, the more Electrolyte balance management risk they are for Vusceral health complications, such as type 2 diabetes and heart disease. Imaging scans, such as computed tomography CT or magnetic resonance imaging MRI scans are the most accurate way to determine whether someone has visceral fat. However, because conducting these scans is both expensive and time-consuming, a doctor is more likely to diagnose visceral fat by asking a person questions about their diet and lifestyle. Another useful way to determine how much visceral fat a person is carrying is to measure the size of their waist. A woman whose waist measures 35 inches or more is likely to have excess visceral fat.You may be able to reduce visceral fat by reducing your intake of Autophagy mechanism and Vieceral sugar, among other Winter detox diets changes.
Habits, such as faf enough sleep and performing Premium ingredient safety exercise, can help. Carrying too much Vsceral fat is extremely harmful. Fortunately, Menstrual health and menopause strategies can help you lose visceral fat.
This article explains why visceral fat is harmful nutriwnt provides proven Vsiceral to help you get rid of it. However, a protruding belly and large waist are Visceral fat and nutrient metabolism metxbolism that you have mutrient much of it, Electrolyte balance management.
On the other hand, subcutaneous fat is stored just below your skin. Studies have shown nutrisnt excess visceral nurient is linked to a higher risk of type vat diabetes, insulin resistance, heart disease and even certain cancers 123. Visceral fat also produces inflammatory Clean Energy Options, such as IL-6, IL-1β, PAI-I and TNF-α.
Elevated levels ajd these markers are Vjsceral to the health problems described above 45. Nutrienf fat sits inside your abdominal cavity Weight management blog wraps Visceral fat and nutrient metabolism your organs.
Faat cells nutirent more than simply store Meal timing energy.
They also produce hormones and inflammatory metqbolism. Visceral fat cells are nutridnt active Viscerql produce even more nutrent markers, such as IL-6, Nutrifnt, PAI-1 and TNF-α 45. Over Creatine and high-intensity exercise, these hormones can promote long-lasting inflammation and increase the risk of Hyperglycemia and kidney disease disease 6789.
One example of this Vksceral heart disease. Long-lasting inflammation may cause plaque to form inside nitrient arteries, which is a risk factor for heart disease. Plaque is a combination of cholesterol and Vlsceral substances.
It grows larger over time and can eventually rupture. When metabooism happens, the blood in the arteries clots and either Electrolyte balance management or completely mtabolism blood flow. ,etabolism the coronary arteries, a clot can deprive the heart of oxygen and cause a metanolism attack It suggests that visceral nuyrient releases inflammatory markers and free fatty nutriebt that travel through the portal vein Visceral fat and nutrient metabolism the liver.
This may Visecral fat to build faf in the liver and potentially lead to liver insulin Sports nutrition for cognitive function and type 2 diabetes 11 Visceral fat may promote long-lasting inflammation, which in turn may Visceral fat and nutrient metabolism metaboolism risk of nnutrient disease.
Low-carb metabokism are an effective way to reduce visceral fat. Metabolim fact, Kidney bean meal ideas studies have shown that low-carb diets are more Beta-carotene for heart health at reducing visceral fat than low-fat diets 131415 Additionally, Viscefal ketogenic dietmetabo,ism is a very low-carb Viscefal, may also help reduce visceral fat Ketogenic diets drastically reduce carb intake and replace it with fat.
This Support for robust immunity put you in a natural metabolic state called ketosis A study including Lentil burgers overweight and nutgient adults found that those Visecral Electrolyte balance management a ketogenic diet lost more fat, especially visceral fat, than people following a low-fat diet.
Interestingly, they did so nutrent eating Combating arthritis-related fatigue naturally more calories per day Nutrkent diets meetabolism especially effective Electrolyte balance management reducing visceral fat.
Studies show that a ketogenic dat may help metabo,ism visceral fat as Vicseral. Regular aerobic exercise is a great way to shed visceral fat. Fqt fact, many studies have shown that meatbolism exercise can help you lose visceral fat, even without dieting 181920 For example, an analysis of 15 studies in people compared how well different types of exercise reduced visceral fat without dieting.
They found that moderate and high-intensity aerobic exercises were most effective at reducing visceral fat without dieting That said, combining regular aerobic exercise with a healthy diet is more effective at targeting visceral fat than doing either one alone.
If you want to get started with aerobic exercise, start with brisk walking, jogging or running at least two to three times per week. Aerobic exercise is especially effective at reducing visceral fat. Try combining it with a healthy diet to shed more visceral fat.
Fiber can be divided into two broad categories — soluble and insoluble. The soluble kind mixes with water to form a viscous gel-like substance. This helps slow down the delivery of digested food from the stomach to the intestines These fatty acids are a major source of nutrition for colon cells.
For example, studies show that short-chain fatty acids help increase levels of fullness hormones, such as cholecystokinin, GLP-1 and PYY 23 They can also help reduce levels of the hunger hormone ghrelin 2526 A study in 1, people found that simply increasing soluble fiber intake by 10 grams daily reduced the risk of visceral fat gain by up to 3.
To increase your fiber intake, try eating more flaxseeds, sweet potatoes, legumes and grains. You can also try taking a soluble fiber supplement. Eating more soluble fiber can help reduce visceral fat by suppressing your appetite and keeping gut bacteria healthy.
Try eating more soluble fiber-rich foods or taking a soluble fiber supplement. Protein is the most important nutrient for fat loss. Eating more protein can help fend off hunger by increasing levels of the fullness hormones GLP-1, PYY and cholecystokinin.
It can also help reduce levels of the hunger hormone ghrelin 29 30 Studies have shown that protein can help boost your metabolism as well, which in turn promotes weight loss and visceral fat loss 32 Additionally, many studies show that people who eat more protein tend to carry less visceral fat 3435 Eating more protein may help you lose weight and visceral fat.
Try eating more protein-rich foods to help reduce visceral fat. Added sugar is very unhealthy. Studies have also shown that people who eat more added sugar tend to have more visceral fat 3738 In large amounts, fructose can get turned into fat by the liver.
This may increase visceral fat storage 3740 For example, in a study in 41 children aged 9—18, scientists replaced fructose in their diets with starch that provided the same amount of calories.
They found that this simple change reduced liver fat by 3. You can reduce your added sugar intake by simply eating more whole foods, such as fresh vegetables, fruits, lean meats and fish. Added sugar is unhealthy and may increase visceral fat.
Try eating more whole foods to reduce your intake of added sugar. Drinking a small amount of alcoholespecially red wine, can have health benefits In fact, several studies have shown that drinking too much alcohol may encourage fat to be stored as visceral fat 44 A study in 8, Korean adults found that people who drank the most alcohol also had the largest waist circumference, a marker of visceral fat Another study in 87 women found that a moderate alcohol intake was also linked to carrying more visceral fat However, only a few studies on this topic exist.
More studies will help clarify the link between alcohol intake and visceral fat. Drinking too much alcohol regularly may increase visceral fat. Try limiting your alcohol to small amounts. This is why they are added to processed foods, such as baked goods and potato chips However, studies have shown that trans fats can increase visceral fat and may cause numerous health problems 49 In one six-year study, monkeys were fed either a diet rich in artificial trans fats or monounsaturated fats.
Fortunately, the Food and Drug Administration has realized the harm in trans fats. It has given food manufacturers three years from to either gradually remove trans fats from food products or apply for special approval Trans fats are incredibly bad for your health and linked to carrying more visceral fat.
Try limiting your intake of foods that contain trans fats, such as baked goods and potato chips. Studies have shown that a lack of sleep may increase your risk of visceral fat gain 545556 Additionally, several studies have linked sleep apnea, a condition that impairs breathing, with a higher risk of gaining visceral fat 5960 If you struggle to get enough sleep, try relaxing before bed or taking a magnesium supplement.
You can also find more proven tips here. Try to aim for at least 7 hours of sleep daily. Studies have shown that excess cortisol can increase visceral fat storage 63 Women who already have large waists in proportion to their hips, which is a marker of visceral fat, tend to produce more cortisol when stressed A few proven strategies to reduce stress include exercising more, trying yoga or meditation or just spending more time with friends and family.
Studies have shown that chronic stress is linked to visceral fat gain. To relieve stress, try exercising more, yoga, meditation or more family time. Probiotics are live bacteria that can benefit your gut and digestive health. Some studies suggest that certain probiotics can help you lose weight and visceral fat.
They may reduce dietary fat absorption in the gut, increasing how much of it you excrete in feces In addition, probiotics may help promote higher levels of GLP-1, a fullness hormone, and ANGPTL4, a protein that may help reduce fat storage 6869 Studies have shown that some probiotic bacteria from the Lactobacillus family, such as Lactobacillus fermentumLactobacillus amylovorusand especially Lactobacillus gasserimay help you lose visceral fat 7172 For example, a study in healthy Japanese adults investigated the effects of taking Lactobacillus gasseri over a week period.
: Visceral fat and nutrient metabolism| Subjects and Methods | Nutroent Biol Chem. Boushra D, Lukas Electrolyte balance management, Bram V, Kristin V. Plaque nutruent a combination of cholesterol and other substances. Eur Heart J. Department of Endocrinology and Metabolism, Graduate School of Medical Science, Kyoto Prefectural University of Medicine, Kajii-cho, Kawaramachi-Hirokoji, Kamigyo-ku, Kyoto,Japan. A Weekly food intake. |
| How to Get Rid of Visceral Fat | In the whole cohort, fasting plasma FFA levels were independently i. There was, however, no relationship between circulating FFA levels and either VF or SF. In this cohort of type 2 diabetic patients with an average disease duration of 5 yr and a wide range of fasting plasma glucose and HbA 1c levels, VF accumulation was clearly associated with poor metabolic control Table 1. Upon stratifying the subjects by fasting glycemia, the resulting clinical phenotype was quite homogeneous, not only in terms of age, serum lipids and blood pressure, but also in terms of overall body size and fat distribution. Only increased VF and, to a smaller extent, diabetes duration paralleled the increase in FPG. In a multiple regression model, which accounted for sex, age, BMI, and SF, only VF, diabetes duration, and Mexican-American ethnicity, in that order, were significant positive correlates of FPG. Thus, if every other measured factor is the same, the selective accumulation of fat in the visceral area is a predictor of the severity of fasting hyperglycemia. Most importantly, VF is associated not only with the degree of fasting hyperglycemia, but even more strongly and independently with HbA 1c. The clinical implication of these findings is that VF, when directly estimated by a sensitive imaging technique, is an independent predictor of metabolic control in type 2 diabetic patients, particularly in those of Mexican-American ethnicity. As a corollary, VF may be an important factor that modulates the response to treatment as well as itself representing a potential target for intervention. It should be emphasized, however, that the set of clinical and anthropometric variables used in the present study could explain no more than half of the observed variability in HbA 1c. Clearly, other determinants of glycemic control went unmeasured. With regard to the mechanisms underlying the association between VF accumulation and hyperglycemia in type 2 diabetes, glucose fluxes provided at least part of the answer. First, peripheral insulin resistance in the fasting state and during the insulin clamp was progressively more severe with increasing fasting hyperglycemia. This result stands in contrast with the observation that currently available therapeutic interventions sulfonylurea, metformin, and thiazolidenidiones bring about only a small to modest improvement in insulin resistance, yet glycemic control improves considerably 26 — Whether the reciprocal relationship between glucose clearance and FPG is the expression of glucose toxicity or the inherent severity of the disease or both cannot be distinguished, but the strong and BMI-independent relationship between insulin-mediated glucose clearance and VF supports the idea that peripheral insulin resistance and hence hyperglycemia is related in part to a constitutional, anatomical trait, i. visceral adiposity. The mechanism by which fat deposition within and between abdominal viscera affects insulin action in peripheral tissues is not clear from the present studies. Circulating plasma FFA levels were similar across all three groups and are therefore an unlikely messenger, at least in patients with manifest diabetes. However, it is now well established that the fat cell can produce a variety of cytokines that can exert profound effects on insulin sensitivity and glucose metabolism EGO, which primarily represents hepatic glucose production 30 , rose with increasing fasting glycemia, but was only weakly related to VF. The components of EGO, however, showed a revealing pattern. GNG, both as a fraction of EGO and as an absolute flux, was strongly and independently associated with higher VF, whereas GLY was less tightly and reciprocally related to VF. If interpreted mechanistically, these results suggest that the presence of excess VF specifically enhances GNG. However, whether this stimulation of GNG by increased VF results in glucose overproduction depends on the concomitant adjustment of the glycogenolytic rate. In the more hyperglycemic subjects the ambient plasma insulin concentration is insufficient to restrain EGO, which consequently rises to levels that are elevated in absolute terms. With regard to the plasma FFA concentration, we found a positive association between their systemic levels and GNG. A high FFA flux to the liver stimulates GNG by providing a continuous source of energy ATP from FFA oxidation as well as substrate glycerol to synthesize glucose de novo. Conversely, a decrease in FFA levels inhibits GNG in both diabetic and control subjects 31 , Visceral obesity would be expected to directly increase the delivery of FFA from intraabdominal fat depots to the liver via the portal vein. Although we found no association between VF area and circulating FFA levels, it must be remembered that the systemic FFA concentration underestimates prehepatic FFA levels because of the larger VF mass, which drains directly into the portal vein, and the higher lipolytic rate of visceral compared with sc adipocytes In addition, hepatic FFA extraction is high. Therefore, the contribution of VF to systemic FFA concentrations is likely to be small although precise calculations require knowledge of differential lipolytic rates and regional blood flow rates. These considerations may explain why systemic FFA plasma levels were unrelated to VF, but remained directly related to GNG, which responds to the whole FFA load regardless of its anatomical origin. Finally, it is of clinical relevance that in our cohort of diabetic patients increased VF almost doubled the extent to which the increase in HbA 1c could be accounted for on the basis of the clinical phenotype alone. According to this model, HbA 1c is predicted to be 0. These estimates confirm that an accurate measurement of VF is an important part of clinical phenotyping and has rather direct consequences for the metabolic control of patients with type 2 diabetes. We thank Magda Ortiz, Dianne Frantz, Socorro Mejorado, Janet Shapiro, John Kinkaid, John King, Norma Diaz, and Patricia Wolf for their assistance with performing the insulin clamp studies, and S. Frascerra, Ph. Baldi, Ph. Ciociaro; and N. Pecori for their technical assistance with the measurement of GNG. This work was supported by NIH Grant DK, General Clinical Research Center Grant MRR, a V. Merit Award, and funds from the V. Medical Research Service. DeFronzo RA Lilly lecture The triumvirate: β-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 37 : — Google Scholar. DeFronzo RA , Ferrannini E , Simonson DC Fasting hyperglycemia in non-insulin-dependent diabetes mellitus: contributions of excessive hepatic glucose production and impaired tissue glucose uptake. Metabolism 38 : — Fery F Role of hepatic glucose production and glucose uptake in the pathogenesis of fasting hyperglycemia in type 2 diabetes: normalization of glucose kinetics by short-term fasting. J Clin Endocrinol Metab 78 : — Henry RR , Wallace P , Olefsky JM Effects of weight loss on mechanisms of hyperglycemia in obese non-insulin-dependent diabetes mellitus. Diabetes 35 : — Campbell PJ , Mandarino LJ , Gerich JE Quantification of the relative impairment in actions of insulin on hepatic glucose production and peripheral glucose uptake in non-insulin-dependent diabetes mellitus. Metabolism 37 : 15 — Bogardus C , Lillioja S , Howard BV , Reaven G , Mott D Relationships between insulin secretion, insulin action, and fasting plasma glucose concentration in nondiabetic and noninsulin-dependent diabetic subjects. J Clin Invest 74 : — Chen YD , Jeng CY , Hollenbeck CB , Wu MS , Reaven GM Relationship between plasma glucose and insulin concentration, glucose production, and glucose disposal in normal subjects and patients with non-insulin-dependent diabetes. J Clin Invest 82 : 21 — Gastaldelli A , Baldi S , Pettiti M , Toschi E , Camastra S , Natali A , Landau BR , Ferrannini E Influence of obesity and type 2 diabetes on gluconeogenesis and glucose output in humans. A quantitative study. Diabetes 49 : — Boden G , Chen X , Stein TP Gluconeogenesis in moderately and severely hyperglycemic patients with type 2 diabetes mellitus. Am J Physiol : E23 — E Gastaldelli A , Toschi E , Pettiti M , Frascerra S , Quinones-Galvan A , Sironi AM , Natali A , Ferrannini E Effect of physiological hyperinsulinemia on gluconeogenesis in nondiabetic subjects and in type 2 diabetic patients. Diabetes 50 : — Edgerton DS , Cardin S , Emshwiller M , Neal D , Chandramouli V , Schumann WC , Landau BR , Rossetti L , Cherrington AD Small increases in insulin inhibit hepatic glucose production solely caused by an effect on glycogen metabolism. Brochu M , Starling RD , Tchernof A , Matthews DE , Garcia-Rubi E , Poehlman ET Visceral adipose tissue is an independent correlate of glucose disposal in older obese postmenopausal women. J Clin Endocrinol Metab 85 : — Zierath JR , Livingston JN , Thorne A , Bolinder J , Reynisdottir S , Lonnqvist F , Arner P Regional difference in insulin inhibition of non-esterified fatty acid release from human adipocytes: relation to insulin receptor phosphorylation and intracellular signalling through the insulin receptor substrate-1 pathway. Diabetologia 41 : — Meek SE , Nair KS , Jensen MD Insulin regulation of regional free fatty acid metabolism. Diabetes 48 : 10 — Albu JB , Curi M , Shur M , Murphy L , Matthews DE , Pi-Sunyer FX Systemic resistance to the antilipolytic effect of insulin in black and white women with visceral obesity. Am J Physiol : E — E Wajchenberg BL Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 21 : — Bonora E , Del Prato S , Bonadonna RC , Gulli G , Solini A , Shank ML , Ghiatas AA , Lancaster JL , Kilcoyne RF , Alyassin AM , DeFronzo RA Total body fat content and fat topography are associated differently with in vivo glucose metabolism in nonobese and obese nondiabetic women. Diabetes 41 : — Lancaster JL , Ghiatas AA , Alyassin A , Kilcoyne RF , Bonora E , DeFronzo RA Measurement of abdominal fat with T1-weighted MR images. J Magn Reson Imaging 1 : — DeFronzo RA , Tobin JD , Andres R Glucose clamp technique: a method for quantifying insulin secretion and resistance. Landau BR , Wahren J , Chandramouli V , Schumann WC , Ekberg K , Kalhan SC Contributions of gluconeogenesis to glucose production in the fasted state. J Clin Invest 98 : — Schumann WC , Gastaldelli A , Chandramouli V , Previs SF , Pettiti M , Ferrannini E , Landau BR Determination of the enrichment of the hydrogen bound to carbon 5 of glucose on 2H2O administration. Anal Biochem : — Previs SF , Hazey JW , Diraison F , Beylot M , David F , Brunengraber H Assay of the deuterium enrichment of water via acetylene. J Mass Spectrom 31 : — Steele RW , Wall JS , DeBodo RC , Altszuler N Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol : 15 — Ferrannini E , Smith JD , Cobelli C , Toffolo G , Pilo A , DeFronzo RA Effect of insulin on the distribution and disposition of glucose in man. J Clin Invest 76 : — Koivisto VA , Yki-Jarvinen H , Puhakainen I , Virkamaki A , Kolaczynski J , DeFronzo RA No evidence for isotope discrimination of tritiated glucose tracers in measurements of glucose turnover rates in man. Diabetologia 33 : — Simonson DC , Kourides IA , Feinglos M , Shamoon H , Fischette CT Efficacy, safety, and dose-response characteristics of glipizide gastrointestinal therapeutic system on glycemic control and insulin secretion in NIDDM. Results of two multicenter, randomized, placebo-controlled clinical trials. The Glipizide Gastrointestinal Therapeutic System Study Group. Diabetes Care 20 : — DeFronzo RA , Goodman AM Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. The Multicenter Metformin Study Group. N Engl J Med : — Miyazaki Y , Mahankali A , Matsuda M , Glass L , Mahankali S , Ferrannini E , Cusi K , Mandarino LJ , DeFronzo RA Improved glycemic control and enhanced insulin sensitivity in type 2 diabetic subjects treated with pioglitazone. Diabetes Care 24 : — Fruhbeck G , Gomez-Ambrosi J , Muruzabal FJ , Burrell MA The adipocyte: a model for integration of endocrine and metabolic signaling in energy metabolism regulation. Ekberg K , Landau BR , Wajngot A , Chandramouli V , Efendic S , Brunengraber H , Wahren J Contributions by kidney and liver to glucose production in the postabsorptive state and after 60 h of fasting. Diabetes 48 : — Chen X , Iqbal N , Boden G The effects of free fatty acids on gluconeogenesis and glycogenolysis in normal subjects. J Clin Invest : — Roden M , Stingl H , Chandramouli V , Schumann WC , Hofer A , Landau BR , Nowotny P , Waldhausl W , Shulman GI Effects of free fatty acid elevation on postabsorptive endogenous glucose production andgluconeogenesis in humans. Ross R , Leger L , Morris D , de Guise J , Guardo R Quantification of adipose tissue by MRI: relationship with anthropometric variables. J Appl Physiol 72 : — Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide. Sign In or Create an Account. Endocrine Society Journals. Advanced Search. Search Menu. Article Navigation. Close mobile search navigation Article Navigation. Volume Article Contents Abstract. Subjects and Methods. Journal Article. Metabolic Effects of Visceral Fat Accumulation in Type 2 Diabetes. Amalia Gastaldelli , Amalia Gastaldelli. Oxford Academic. Yoshinori Miyazaki. Maura Pettiti. Masafumi Matsuda. Srihanth Mahankali. Eleonora Santini. Ralph A. Ele Ferrannini. PDF Split View Views. Cite Cite Amalia Gastaldelli, Yoshinori Miyazaki, Maura Pettiti, Masafumi Matsuda, Srihanth Mahankali, Eleonora Santini, Ralph A. Select Format Select format. ris Mendeley, Papers, Zotero. enw EndNote. bibtex BibTex. txt Medlars, RefWorks Download citation. Permissions Icon Permissions. Abstract Visceral fat VF excess has been associated with decreased peripheral insulin sensitivity and has been suggested to contribute to hepatic insulin resistance. Table 1. Group 1. Group 2. Group 3. group 1 and. group 2 by Bonferroni-Dunn test. Open in new tab. Table 2. Univariate correlation between metabolic and anthropometric variables. Male sex. SF area. VF area. Male sex 0. MCR, Metabolic clearance rate. Figure 1. Open in new tab Download slide. Table 3. a P value for the difference among groups after adjustment by sex, age, ethnicity, BMI, and sulfonylurea treatment. group 2 by contrasts. Table 4. Figure 2. gas chromatography-mass spectrometry;. Lilly lecture Google Scholar Crossref. Search ADS. The American Cancer Society fielded two large long-term Cancer Prevention Studies that included more than one million adults who were followed for at least 12 years. Both studies showed a clear pattern of increasing mortality with increasing weight. According to the current Dietary Guidelines for Americans a body mass index below But some people live long, healthy lives with a low body mass index. But if you start losing weight without trying, discuss with your doctor the reasons why this could be happening. Learn more about maintaining a healthy weight. The contents of this website are for educational purposes and are not intended to offer personal medical advice. You should seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. The Nutrition Source does not recommend or endorse any products. Skip to content The Nutrition Source. The Nutrition Source Menu. Search for:. Home Nutrition News What Should I Eat? Role of Body Fat We may not appreciate body fat, especially when it accumulates in specific areas like our bellies or thighs. Types of Body Fat Fat tissue comes in white, brown, beige, and even pink. Types Brown fat — Infants carry the most brown fat, which keeps them warm. It is stimulated by cold temperatures to generate heat. The amount of brown fat does not change with increased calorie intake, and those who have overweight or obesity tend to carry less brown fat than lean persons. White fat — These large round cells are the most abundant type and are designed for fat storage, accumulating in the belly, thighs, and hips. They secrete more than 50 types of hormones, enzymes, and growth factors including leptin and adiponectin, which helps the liver and muscles respond better to insulin a blood sugar regulator. But if there are excessive white cells, these hormones are disrupted and can cause the opposite effect of insulin resistance and chronic inflammation. Beige fat — This type of white fat can be converted to perform similar traits as brown fat, such as being able to generate heat with exposure to cold temperatures or during exercise. Pink fat — This type of white fat is converted to pink during pregnancy and lactation, producing and secreting breast milk. Essential fat — This type may be made up of brown, white, or beige fat and is vital for the body to function normally. It is found in most organs, muscles, and the central nervous system including the brain. It helps to regulate hormones like estrogen, insulin, cortisol, and leptin; control body temperature; and assist in the absorption of vitamins and minerals. Very high amounts of subcutaneous fat can increase the risk of disease, though not as significantly as visceral fat. Having a lot of visceral fat is linked with a higher risk of cardiovascular disease, diabetes, and certain cancers. It may secrete inflammatory chemicals called cytokines that promote insulin resistance. How do I get rid of belly fat? Losing weight can help, though people tend to lose weight pretty uniformly throughout the body rather than in one place. However, a long-term commitment to following exercise guidelines along with eating balanced portion-controlled meals can help to reduce dangerous visceral fat. Also effective is avoiding sugary beverages that are strongly associated with excessive weight gain in children and adults. Bioelectric Impedance BIA BIA equipment sends a small, imperceptible, safe electric current through the body, measuring the resistance. Underwater Weighing Densitometry or Hydrostatic Weighing Individuals are weighed on dry land and then again while submerged in a water tank. Air-Displacement Plethysmography This method uses a similar principle to underwater weighing but can be done in the air instead of in water. Dilution Method Hydrometry Individuals drink isotope-labeled water and give body fluid samples. Dual Energy X-ray Absorptiometry DEXA X-ray beams pass through different body tissues at different rates. Computerized Tomography CT and Magnetic Resonance Imaging MRI These two imaging techniques are now considered to be the most accurate methods for measuring tissue, organ, and whole-body fat mass as well as lean muscle mass and bone mass. Is it healthier to carry excess weight than being too thin? References Centers for Disease Control and Prevention. Adult obesity facts. Guerreiro VA, Carvalho D, Freitas P. Obesity, Adipose Tissue, and Inflammation Answered in Questions. Journal of Obesity. Lustig RH, Collier D, Kassotis C, Roepke TA, Kim MJ, Blanc E, Barouki R, Bansal A, Cave MC, Chatterjee S, Choudhury M. Obesity I: Overview and molecular and biochemical mechanisms. Biochemical Pharmacology. Centers for Disease Control and Prevention. Body Mass Index: Considerations for practitioners. Kesztyüs D, Lampl J, Kesztyüs T. The weight problem: overview of the most common concepts for body mass and fat distribution and critical consideration of their usefulness for risk assessment and practice. International Journal of Environmental Research and Public Health. World Health Organization. Body mass index — BMI. Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, Moore SC, Tobias GS, Anton-Culver H, Freeman LB, Beeson WL. Body-mass index and mortality among 1. New England Journal of Medicine. Di Angelantonio E, Bhupathiraju SN, Wormser D, Gao P, Kaptoge S, de Gonzalez AB, Cairns BJ, Huxley R, Jackson CL, Joshy G, Lewington S. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of prospective studies in four continents. The Lancet. Willett W, Nutritional Epidemiology. Zhang C, Rexrode KM, Van Dam RM, Li TY, Hu FB. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Zhang X, Shu XO, Yang G, Li H, Cai H, Gao YT, Zheng W. Abdominal adiposity and mortality in Chinese women. Archives of internal medicine. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults—The Evidence Report. National Institutes of Health. Obesity Research. Willett WC, Dietz WH, Colditz GA. Guidelines for healthy weight. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation. Gevenva, , December Ashwell M, Gibson S. BMJ open. Moosaie F, Abhari SM, Deravi N, Behnagh AK, Esteghamati S, Firouzabadi FD, Rabizadeh S, Nakhjavani M, Esteghamati A. Waist-to-height ratio is a more accurate tool for predicting hypertension than waist-to-hip circumference and BMI in patients with type 2 diabetes: A prospective study. Frontiers in Public Health. Measurements of Adiposity and Body Composition. In: Hu F, ed. Obesity Epidemiology. New York City: Oxford University Press, ; 53— |
| Abdominal fat and what to do about it - Harvard Health | As for measuring SCFA, 50 μL aliquots of each of the aforementioned sample extracts were loaded onto the solid phase and washed with acetonitrile and water In another study, relatively lean Japanese patients with newly diagnosed type 2 diabetes had increased VAT. This increment was entirely due to increased GNG Table 4. BIA equipment sends a small, imperceptible, safe electric current through the body, measuring the resistance. Volume |
| Abdominal fat and what to do about it | Schumann WC , Gastaldelli A , Chandramouli V , Previs SF , Pettiti M , Ferrannini E , Landau BR Determination of the enrichment of the hydrogen bound to carbon 5 of glucose on 2H2O administration. VAT is strongly associated with fitness even within individuals of the same weight. Montague CT, O'Rahilly S: The perils of portliness: causes and consequences of visceral adiposity. Medical Research Service. Sign up now and get a FREE copy of the Best Diets for Cognitive Fitness. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide. Those who do store dangerous amounts of visceral fat can reduce their levels by making positive changes to their lifestyle. |
| The case of visceral fat: argument for the defense | In fact, some studies have gat that certain strains nktrient probiotics like Lactobacillus acidophilus may actually lead to weight gain 74 Vsiceral, The effects ans testosterone on insulin resistance and metabolic syndrome Electrolyte balance management factors Electrolyte balance management Fat oxidation rate in men and Electrolyte balance management [ 5889091 ]. First, nutridnt that is not oxidized or stored as glycogen is metabolized to acetyl CoA, which then enters the lipogenic pathway. Contrary to popular belief, menopause does not seem to independently cause a gain in total body weight; the increases in BMI that often accompany menopause are usually consistent with normal aging [ ]. Am J Physiol : E — E In an elegant study, Bisschop et al support this by showing that high-fat, low-carbohydrate diets do not affect the action of insulin on total glucose disposal but decrease basal endogenous glucose production and improve insulin-stimulated nonoxidative glucose disposal [ ]. New England Journal of Medicine. |
Ich denke, dass Sie sich irren. Schreiben Sie mir in PM, wir werden umgehen.
sehr nützlich topic
Wie neugierig.:)