Video
Peripheral Vascular DiseaseAngiogenesis and peripheral vascular disease -
Circ J. Momjian-Mayor I, Baron JC. The pathophysiology of watershed infarction in internal carotid artery disease: review of cerebral perfusion studies. Grootaert MOJ, Moulis M, Roth L, Martinet W, Vindis C, Bennett MR, et al. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis.
Cardiovasc Res. Kersting J, Kamper L, Das M, Haage P. Guideline-oriented therapy of lower extremity peripheral artery disease PAD - current data and perspectives.
Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. Ribatti D, Crivellato E.
Dev Biol. Masi S, Rizzoni D, Taddei S, Widmer RJ, Montezano AC, Lüscher TF, et al. Assessment and pathophysiology of microvascular disease: recent progress and clinical implications. Eur Heart J. Brandt MM, Cheng C, Merkus D, Duncker DJ, Sorop O. Mechanobiology of microvascular function and structure in health and disease: Focus on the coronary circulation.
Front Physiol. Querfeld U, Mak RH, Pries AR. Microvascular disease in chronic kidney disease: the base of the iceberg in cardiovascular comorbidity. Clin Sci Lond. Petrie JR, Guzik TJ, Touyz RM.
Diabetes, hypertension, and cardiovascular disease: Clinical insights and vascular mechanisms. Can J Cardiol. Mengozzi A, Pugliese NR, Chiriacò M, Masi S, Virdis A, Taddei S.
Microvascular ageing links metabolic disease to age-related disorders: the role of oxidative stress and inflammation in promoting microvascular dysfunction.
J Cardiovasc Pharmacol. Del Buono MG, Montone RA, Camilli M, Carbone S, Narula J, Lavie CJ, et al. Coronary microvascular dysfunction across the spectrum of cardiovascular diseases: JACC State-of-the-Art Review.
Tsai SH, Lu G, Xu X, Ren Y, Hein TW, Kuo L. Magenta A, Greco S, Capogrossi MC, Gaetano C, Martelli F. Nitric oxide, oxidative stress, and p66Shc interplay in diabetic endothelial dysfunction. BioMed Res Int. Mathew B, Ravindran S, Liu X, Torres L, Chennakesavalu M, Huang CC, et al.
Mesenchymal stem cell-derived extracellular vesicles and retinal ischemia-reperfusion. Farber A, Eberhardt RT. The current state of critical limb ischemia: A systematic review. JAMA Surg. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR, et al. Inter-society consensus for the management of peripheral arterial disease TASC II.
J Vasc Surg. Lambert MA, Belch JJF. Medical management of critical limb ischaemia: where do we stand today?
J Intern Med. Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics— update: a report from the American Heart Association. Google Scholar. Sobieszczyk P, Beckman J. Carotid artery disease. Feske SK.
Ischemic stroke. Am J Med. Schiavone S, Trabace L. Small molecules: therapeutic application in neuropsychiatric and neurodegenerative disorders.
Herschman HR, Lusis AJ, Groopman JE. Growth factors. Ann Intern Med. Uccelli A, Wolff T, Valente P, Di Maggio N, Pellegrino M, Gürke L, et al. Vascular endothelial growth factor biology for regenerative angiogenesis. Swiss Med Wkly. Yang Z, Wan J, Pan W, Zou J.
Expression of vascular endothelial growth factor in cardiac repair: Signaling mechanisms mediating vascular protective effects. Int J Biol Macromol.
Zou J, Fei Q, Xiao H, Wang H, Liu K, Liu M, et al. VEGF-A promotes angiogenesis after acute myocardial infarction through increasing ROS production and enhancing ER stress-mediated autophagy.
J Cell Physiol. Zhang Z, Long C, Guan Y, Song M. Hepatocyte growth factor intervention to reduce myocardial injury and improve cardiac function on diabetic myocardial infarction rats. Eur J Histochem. Liu J, Wu P, Wang Y, Du Y, A N, Liu S, et al.
Ad-HGF improves the cardiac remodeling of rat following myocardial infarction by upregulating autophagy and necroptosis and inhibiting apoptosis.
Am J Transl Res. Li J, Wei Y, Liu K, Yuan C, Tang Y, Quan Q, et al. Synergistic effects of FGF-2 and PDGF-BB on angiogenesis and muscle regeneration in rabbit hindlimb ischemia model. Microvasc Res.
Pang Q, Zhang H, Chen Z, Wu Y, Bai M, Liu Y, et al. Brain Res. Nagasawa A, Masumoto H, Yanagi S, Kanemitsu N, Ikeda T, Tabata Y, et al. Basic fibroblast growth factor attenuates left-ventricular remodeling following surgical ventricular restoration in a rat ischemic cardiomyopathy model.
Gen Thorac Cardiovasc Surg. Rashid FN, Clayton ZE, Ogawa M, Perdomo J, Hume RD, Kizana E, et al. Platelet derived growth factor-A Pdgf-a gene transfer modulates scar composition and improves left ventricular function after myocardial infarction.
Int J Cardiol. Moriya J, Wu X, Zavala-Solorio J, Ross J, Liang XH, Ferrara N. Platelet-derived growth factor C promotes revascularization in ischemic limbs of diabetic mice. Kempf T, Eden M, Strelau J, Naguib M, Willenbockel C, Tongers J, et al. Circ Res.
Báez-Díaz C, Blanco-Blázquez V, Sánchez-Margallo FM, Bayes-Genis A, González I, Abad A, et al. Microencapsulated insulin-like growth factor-1 therapy improves cardiac function and reduces fibrosis in a porcine acute myocardial infarction model. Sci Rep. Stewart DJ, Kutryk MJ, Fitchett D, Freeman M, Camack N, Su Y, et al.
VEGF gene therapy fails to improve perfusion of ischemic myocardium in patients with advanced coronary disease: results of the NORTHERN trial. Mol Ther. Powell RJ, Goodney P, Mendelsohn FO, Moen EK, Annex BH. Safety and efficacy of patient specific intramuscular injection of HGF plasmid gene therapy on limb perfusion and wound healing in patients with ischemic lower extremity ulceration: results of the HGF trial.
Belch J, Hiatt WR, Baumgartner I, Driver IV, Nikol S, Norgren L, et al. Effect of fibroblast growth factor NV1FGF on amputation and death: a randomised placebo-controlled trial of gene therapy in critical limb ischaemia. Kumagai M, Marui A, Tabata Y, Takeda T, Yamamoto M, Yonezawa A, et al.
Safety and efficacy of sustained release of basic fibroblast growth factor using gelatin hydrogel in patients with critical limb ischemia. Heart Vessels. Deev R, Plaksa I, Bozo I, Mzhavanadze N, Suchkov I, Chervyakov Y, et al.
Results of 5-year follow-up study in patients with peripheral artery disease treated with PL-VEGF for intermittent claudication. Ther Adv Cardiovasc Dis. Gu Y, Cui S, Wang Q, Liu C, Jin B, Guo W, et al.
A randomized, double-blind, placebo-controlled phase II study of hepatocyte growth factor in the treatment of critical limb ischemia. Barć P, Antkiewicz M, Śliwa B, Frączkowska K, Guziński M, Dawiskiba T, et al. J Cardiovasc Transl Res. Isner JM, Pieczek A, Schainfeld R, Blair R, Haley L, Asahara T, et al.
Clinical evidence of angiogenesis after arterial gene transfer of phVEGF in patient with ischaemic limb. Cadenas S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection.
Free Radic Biol Med. Ochoa CD, Wu RF, Terada LS. ROS signaling and ER stress in cardiovascular disease. Mol Aspects Med. Lu Q, Yao Y, Hu Z, Hu C, Song Q, Ye J, et al. Angiogenic factor AGGF1 activates autophagy with an essential role in therapeutic angiogenesis for heart disease.
PLoS Biol. Kastrup J, Jørgensen E, Rück A, Tägil K, Glogar D, Ruzyllo W, et al. Direct intramyocardial plasmid vascular endothelial growth factor-A gene therapy in patients with stable severe angina pectoris a randomized double-blind placebo-controlled study: the Euroinject One trial.
Korpela H, Lampela J, Airaksinen J, Järveläinen N, Siimes S, Valli K, et al. AAV2-VEGF-B gene therapy failed to induce angiogenesis in ischemic porcine myocardium due to inflammatory responses.
Gene Ther. Weis SM, Cheresh DA. Pathophysiological consequences of VEGF-induced vascular permeability. Gallo S, Spilinga M, Casanova E, Bonzano A, Boccaccio C, Comoglio PM, et al. The long-lasting protective effect of HGF in cardiomyoblasts exposed to doxorubicin requires a positive feed-forward loop mediated by Erk 1,2-Timp1-Stat3.
Int J Mol Sci. Rong SL, Wang XL, Wang YC, Wu H, Zhou XD, Wang ZK, et al. Anti-inflammatory activities of hepatocyte growth factor in post-ischemic heart failure. Acta Pharmacol Sin. Yang ZJ, Xu SL, Chen B, Zhang SL, Zhang YL, Wei W, et al.
Hepatocyte growth factor plays a critical role in the regulation of cytokine production and induction of endothelial progenitor cell mobilization: a pilot gene therapy study in patients with coronary heart disease. Clin Exp Pharmacol Physiol. Domouzoglou EM, Naka KK, Vlahos AP, Papafaklis MI, Michalis LK, Tsatsoulis A, et al.
Fibroblast growth factors in cardiovascular disease: the emerging role of FGF Am J Physiol Heart Circ Physiol. House SL, Melhorn SJ, Newman G, Doetschman T, Schultz Jel J. The protein kinase C pathway mediates cardioprotection induced by cardiac-specific overexpression of fibroblast growth factor Laham RJ, Chronos NA, Pike M, Leimbach ME, Udelson JE, Pearlman JD, et al.
Intracoronary basic fibroblast growth factor FGF-2 in patients with severe ischemic heart disease: results of a phase I open-label dose escalation study. Arras M, Ito WD, Scholz D, Winkler B, Schaper J, Schaper W. Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb.
J Clin Invest. Nikol S, Baumgartner I, Van Belle E, Diehm C, Visoná A, Capogrossi MC, et al. Therapeutic angiogenesis with intramuscular NV1FGF improves amputation-free survival in patients with critical limb ischemia.
Reigstad LJ, Varhaug JE, Lillehaug JR. Structural and functional specificities of PDGF-C and PDGF-D, the novel members of the platelet-derived growth factors family. Febs j. Moniche F, Montaner J, Gonzalez-Marcos JR, Carmona M, Piñero P, Espigado I, et al. Intra-arterial bone marrow mononuclear cell transplantation correlates with GM-CSF, PDGF-BB, and MMP-2 serum levels in stroke patients: results from a clinical trial.
Cell Transplant. Taylor DA, Perin EC, Willerson JT, Zierold C, Resende M, Carlson M, et al. Identification of bone marrow cell subpopulations associated with improved functional outcomes in patients with chronic left ventricular dysfunction: an embedded cohort evaluation of the FOCUS-CCTRN Trial.
Shahrivari M, Wise E, Resende M, Shuster JJ, Zhang J, Bolli R, et al. Peripheral blood cytokine levels after acute myocardial infarction: IL-1β- and ILrelated impairment of bone marrow function. Holmes D, Fitzgerald P, Goldberg S, LaBlanche J, Lincoff AM, Savage M, et al.
The PRESTO Prevention of restenosis with tranilast and its outcomes protocol: a double-blind, placebo-controlled trial. Am Heart J. Simón-Yarza T, Formiga FR, Tamayo E, Pelacho B, Prosper F, Blanco-Prieto MJ.
Vascular endothelial growth factor-delivery systems for cardiac repair: an overview. Adini A, Wu H, Dao DT, Ko VH, Yu LJ, Pan A, et al. PR1P stabilizes VEGF and upregulates its signaling to reduce elastase-induced murine emphysema.
Am J Respir Cell Mol Biol. Adini A, Adini I, Grad E, Tal Y, Danenberg HD, Kang PM, et al. The promininderived peptide improves cardiac function following ischemia. Phadke G, Hanna RM, Ferrey A, Torres EA, Singla A, Kaushal A, et al. Review of intravitreal VEGF inhibitor toxicity and report of collapsing FSGS with TMA in a patient with age-related macular degeneration.
Clin Kidney J. Borlongan CV. Concise review: Stem cell therapy for stroke patients: Are we there yet? Stem Cells Transl Med.
Shafei AE, Ali MA, Ghanem HG, Shehata AI, Abdelgawad AA, Handal HR, et al. Mesenchymal stem cell therapy: A promising cell-based therapy for treatment of myocardial infarction. J Gene Med. Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, et al.
Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci U S A.
Deng Y, Yang Z, Terry T, Pan S, Woodside DG, Wang J, et al. Prostacyclin-producing human mesenchymal cells target H19 lncRNA to augment endogenous progenitor function in hindlimb ischaemia.
Nat Commun. Rybalko V, Hsieh PL, Ricles LM, Chung E, Farrar RP, Suggs LJ. Therapeutic potential of adipose-derived stem cells and macrophages for ischemic skeletal muscle repair.
Regen Med. Gutiérrez-Fernández M, Rodríguez-Frutos B, Ramos-Cejudo J, Teresa Vallejo-Cremades M, Fuentes B, Cerdán S, et al. Effects of intravenous administration of allogenic bone marrow- and adipose tissue-derived mesenchymal stem cells on functional recovery and brain repair markers in experimental ischemic stroke.
Stem Cell Res Ther. Zhao Y, Sun X, Cao W, Ma J, Sun L, Qian H, et al. Exosomes derived from human umbilical cord mesenchymal stem cells relieve acute myocardial ischemic injury. Stem Cells Int. Zhu Y, Guan YM, Huang HL, Wang QS.
Human umbilical cord blood mesenchymal stem cell transplantation suppresses inflammatory responses and neuronal apoptosis during early stage of focal cerebral ischemia in rabbits.
Liao W, Xie J, Zhong J, Liu Y, Du L, Zhou B, et al. Therapeutic effect of human umbilical cord multipotent mesenchymal stromal cells in a rat model of stroke. Garbuzova-Davis S, Boccio KJ, Ehrhart J, Sanberg PR, Appel SH, Borlongan CV. Detection of endothelial cell-associated human DNA reveals transplanted human bone marrow stem cell engraftment into CNS capillaries of ALS mice.
Brain Res Bull. Kong L, Wang Y, Wang H, Pan Q, Zuo R, Bai S, et al. Conditioned media from endothelial progenitor cells cultured in simulated microgravity promote angiogenesis and bone fracture healing. Nelson TJ, Martinez-Fernandez A, Yamada S, Perez-Terzic C, Ikeda Y, Terzic A.
Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Kawamura M, Miyagawa S, Miki K, Saito A, Fukushima S, Higuchi T, et al. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model.
Shiba Y, Gomibuchi T, Seto T, Wada Y, Ichimura H, Tanaka Y, et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts.
Park SJ, Kim RY, Park BW, Lee S, Choi SW, Park JH, et al. Dual stem cell therapy synergistically improves cardiac function and vascular regeneration following myocardial infarction.
Caplan AI. Mesenchymal stem cells: time to change the name! Elshaer SL, Bahram SH, Rajashekar P, Gangaraju R, El-Remessy AB. Modulation of mesenchymal stem cells for enhanced therapeutic utility in ischemic vascular diseases.
Raman N, Imran SAM, Ahmad Amin Noordin KB, Zaman W, Nordin F. Mechanotransduction in mesenchymal stem cells MSCs differentiation: a review. Muguruma Y, Yahata T, Miyatake H, Sato T, Uno T, Itoh J, et al. Reconstitution of the functional human hematopoietic microenvironment derived from human mesenchymal stem cells in the murine bone marrow compartment.
Chen J, Crawford R, Chen C, Xiao Y. Tissue Eng Part B Rev. Jang MW, Yun SP, Park JH, Ryu JM, Lee JH, Han HJ. Zhao L, Hantash BM.
TGF-β1 regulates differentiation of bone marrow mesenchymal stem cells. Vitam Horm. Chim H, Miller E, Gliniak C, Alsberg E. Stromal-cell-derived factor SDF 1-alpha in combination with BMP-2 and TGF-β1 induces site-directed cell homing and osteogenic and chondrogenic differentiation for tissue engineering without the requirement for cell seeding.
Cell Tissue Res. Tang J, Wang J, Yang J, Kong X, Zheng F, Guo L, et al. Mesenchymal stem cells over-expressing SDF-1 promote angiogenesis and improve heart function in experimental myocardial infarction in rats.
Eur J Cardiothorac Surg. Vergadi E, Ieronymaki E, Lyroni K, Vaporidi K, Tsatsanis C. J Immunol. Giunti D, Parodi B, Usai C, Vergani L, Casazza S, Bruzzone S, et al. Mesenchymal stem cells shape microglia effector functions through the release of CX3CL1. Stem Cells. Jaillard A, Hommel M, Moisan A, Zeffiro TA, Favre-Wiki IM, Barbieux-Guillot M, et al.
Autologous mesenchymal stem cells improve motor recovery in subacute ischemic stroke: A randomized clinical trial. Transl Stroke Res. Lee J, Henderson K, Massidda MW, Armenta-Ochoa M, Im BG, Veith A, et al. Mechanobiological conditioning of mesenchymal stem cells for enhanced vascular regeneration.
Nat Biomed Eng. Wang Y, Wang S, Gu C, Xiong Y, Shen H, Liu F, et al. Ex-vivo treatment of allografts using adipose-derived stem cells induced prolonged rejection-free survival in an allogenic hind-limb transplantation model.
Ann Transl Med. Min KH, Byun JH, Heo CY, Kim EH, Choi HY, Pak CS. Effect of low-level laser therapy on human adipose-derived stem cells: In vitro and in vivo studies. Aesthetic Plast Surg. Zhou LN, Wang JC, Zilundu PLM, Wang YQ, Guo WP, Zhang SX, et al.
A comparison of the use of adipose-derived and bone marrow-derived stem cells for peripheral nerve regeneration in vitro and in vivo.
Zhou W, Lin J, Zhao K, Jin K, He Q, Hu Y, et al. Single-cell profiles and clinically useful properties of human mesenchymal stem cells of adipose and bone marrow origin. Am J Sports Med. Konno M, Hamabe A, Hasegawa S, Ogawa H, Fukusumi T, Nishikawa S, et al.
Adipose-derived mesenchymal stem cells and regenerative medicine. Dev Growth Differ. Li T, Xia M, Gao Y, Chen Y, Xu Y. Human umbilical cord mesenchymal stem cells: an overview of their potential in cell-based therapy. Expert Opin Biol Ther. Lange L, Hoffmann D, Schwarzer A, Ha TC, Philipp F, Lenz D, et al.
Inducible forward programming of human pluripotent stem cells to hemato-endothelial progenitor cells with hematopoietic progenitor potential. Stem Cell Reports. Pyšná A, Bém R, Němcová A, Fejfarová V, Jirkovská A, Hazdrová J, et al.
Endothelial progenitor cells biology in diabetes mellitus and peripheral arterial disease and their therapeutic potential. Stem Cell Rev Rep. Sun Y, Liu WZ, Liu T, Feng X, Yang N, Zhou HF. J Recept Signal Transduct Res. Deng Y, Zhou Z, Lin S, Yu B.
Biochem Biophys Res Commun. Bouland C, Philippart P, Dequanter D, Corrillon F, Loeb I, Bron D, et al. Cross-talk between mesenchymal stromal cells MSCs and endothelial progenitor cells EPCs in bone regeneration.
Front Cell Dev Biol. Kim H, Kim S, Baek SH, Kwon SM. Pivotal cytoprotective mediators and promising therapeutic strategies for endothelial progenitor cell-based cardiovascular regeneration.
Ravishankar P, Tandon I, Balachandran K. Effect of cyclic uniaxial mechanical strain on endothelial progenitor cell differentiation. Cardiovasc Eng Technol. Yu H, Lu K, Zhu J, Wang J. Stem cell therapy for ischemic heart diseases. Br Med Bull. Lalit PA, Hei DJ, Raval AN, Kamp TJ.
Induced pluripotent stem cells for post-myocardial infarction repair: remarkable opportunities and challenges. Hou L, Kim JJ, Woo YJ, Huang NF. Stem cell-based therapies to promote angiogenesis in ischemic cardiovascular disease.
Liu Q, Pan S, Liu S, Zhang S, Willerson JT, Martin JF, et al. Suppressing Hippo signaling in the stem cell niche promotes skeletal muscle regeneration. Gao LR, Chen Y, Zhang NK, Yang XL, Liu HL, Wang ZG, et al. BMC Med. Qayyum AA, Mathiasen AB, Helqvist S, Jørgensen E, Haack-Sørensen M, Ekblond A, et al.
Autologous adipose-derived stromal cell treatment for patients with refractory angina MyStromalCell Trial : 3-years follow-up results. J Transl Med. Mathiasen AB, Qayyum AA, Jørgensen E, Helqvist S, Kofoed KF, Haack-Sørensen M, et al.
Bone marrow-derived mesenchymal stromal cell treatment in patients with ischaemic heart failure: final 4-year follow-up of the MSC-HF trial. Eur J Heart Fail.
Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. Li TS, Kubo M, Ueda K, Murakami M, Ohshima M, Kobayashi T, et al.
Identification of risk factors related to poor angiogenic potency of bone marrow cells from different patients. Tooi M, Komaki M, Morioka C, Honda I, Iwasaki K, Yokoyama N, et al.
Placenta mesenchymal stem cell derived exosomes confer plasticity on fibroblasts. J Cell Biochem. Xu R, Bai Y, Min S, Xu X, Tang T, Ju S.
In vivo monitoring and assessment of exogenous mesenchymal stem cell-derived exosomes in mice with ischemic stroke by molecular imaging.
Int J Nanomedicine. Cai G, Cai G, Zhou H, Zhuang Z, Liu K, Pei S, et al. Mesenchymal stem cell-derived exosome miRp suppresses inflammation and prevents cerebral infarction. Shi A, Arrell DKK, Peterson TE, Witt TA, Nagel M, Li J, et al.
Abstract Exosome shuttled VEGFR promotes angiogenesis in peripheral vascular disease. Liu S, Chen X, Bao L, Liu T, Yuan P, Yang X, et al.
Treatment of infarcted heart tissue via the capture and local delivery of circulating exosomes through antibody-conjugated magnetic nanoparticles. Florian A, Ludwig A, Rösch S, Yildiz H, Klumpp S, Sechtem U, et al.
Positive effect of intravenous iron-oxide administration on left ventricular remodelling in patients with acute ST-elevation myocardial infarction - a cardiovascular magnetic resonance CMR study. Jung E, Lee J, Jeong L, Park S, Lee M, Song C, et al.
Stimulus-activatable echogenic maltodextrin nanoparticles as nanotheranostic agents for peripheral arterial disease. Almeida SO, Budoff M. Effect of statins on atherosclerotic plaque. Trends Cardiovasc Med. Zahedipour F, Butler AE, Eid AH, Sahebkar A.
Pleiotropic properties of statins via angiogenesis modulation in cardiovascular disease. Drug Discov Today. Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, Lefer DJ, et al.
The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med.
Sata M, Nishimatsu H, Suzuki E, Sugiura S, Yoshizumi M, Ouchi Y, et al. Endothelial nitric oxide synthase is essential for the HMG-CoA reductase inhibitor cerivastatin to promote collateral growth in response to ischemia. FASEB J. Nakano K, Matoba T, Koga JI, Kashihara Y, Fukae M, Ieiri I, et al.
Safety, tolerability, and pharmacokinetics of NKNP. Int Heart J. Kubo M, Egashira K, Inoue T, Koga J, Oda S, Chen L, et al. Therapeutic neovascularization by nanotechnology-mediated cell-selective delivery of pitavastatin into the vascular endothelium.
Arterioscler Thromb Vasc Biol. Matsumoto T, Yoshino S, Furuyama T, Morisaki K, Nakano K, Koga JI, et al. J Atheroscler Thromb. Duivenvoorden R, Senders ML, van Leent MMT, Pérez-Medina C, Nahrendorf M, Fayad ZA, et al.
Nanoimmunotherapy to treat ischaemic heart disease. Nat Rev Cardiol. Moyano DF, Goldsmith M, Solfiell DJ, Landesman-Milo D, Miranda OR, Peer D, et al. Nanoparticle hydrophobicity dictates immune response. J Am Chem Soc. Fang RH, Hu CM, Zhang L.
Nanoparticles disguised as red blood cells to evade the immune system. Koivisto JT, Gering C, Karvinen J, Maria Cherian R, Belay B, Hyttinen J, et al. Mechanically biomimetic gelatin-gellan gum hydrogels for 3D culture of beating human cardiomyocytes. ACS Appl Mater Interfaces.
Li C, Nie F, Liu X, Chen M, Chi D, Li S, et al. Antioxidative and angiogenic hyaluronic acid-based hydrogel for the treatment of peripheral artery disease. Wang CY, Hsiao CY, Tsai KL, Cheng YH. Injectable thermosensitive chitosan-based hydrogel containing ferulic acid for treating peripheral arterial disease.
J Tissue Eng Regen Med. He X, Wang Q, Zhao Y, Zhang H, Wang B, Pan J, et al. Effect of intramyocardial grafting collagen scaffold with mesenchymal stromal cells in patients with chronic ischemic heart disease: A randomized clinical trial.
JAMA Netw Open. Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, et al. Nat Mater. Hao J, Zhang Y, Jing D, Shen Y, Tang G, Huang S, et al. Mechanobiology of mesenchymal stem cells: Perspective into mechanical induction of MSC fate.
Acta Biomater. Steward AJ, Kelly DJ. Mechanical regulation of mesenchymal stem cell differentiation. J Anat. Frangogiannis NG. Cell therapy for peripheral artery disease. Curr Opin Pharmacol.
Qadura M, Terenzi DC, Verma S, Al-Omran M, Hess DA. Concise review: Cell therapy for critical limb ischemia: An integrated review of preclinical and clinical studies. Mu R, Zhang Y, Yan L, Liao Z, Yang Y, Su H, et al. Adv Mater.
Svystonyuk DA, Mewhort HEM, Hassanabad AF, Heydari B, Mikami Y, Turnbull JD, et al. Acellular bioscaffolds redirect cardiac fibroblasts and promote functional tissue repair in rodents and humans with myocardial injury.
Frey N, Linke A, Süselbeck T, Müller-Ehmsen J, Vermeersch P, Schoors D, et al. Intracoronary delivery of injectable bioabsorbable scaffold IK to treat left ventricular remodeling after ST-elevation myocardial infarction: a first-in-man study.
Circ Cardiovasc Interv. Mariani E, Lisignoli G, Borzi RM, Pulsatelli L. Biomaterials: foreign bodies or tuners for the immune response? Vassey MJ, Figueredo GP, Scurr DJ, Vasilevich AS, Vermeulen S, Carlier A, et al. Immune modulation by design: using topography to control human monocyte attachment and macrophage differentiation.
Adv Sci Weinh. Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. Suhara M, Miura Y, Cabral H, Akagi D, Anraku Y, Kishimura A, et al. Targeting ability of self-assembled nanomedicines in rat acute limb ischemia model is affected by size.
J Control Release. Zhang K, Chen X, Li H, Feng G, Nie Y, Wei Y, et al. A nitric oxide-releasing hydrogel for enhancing the therapeutic effects of mesenchymal stem cell therapy for hindlimb ischemia. Rufaihah AJ, Johari NA, Vaibavi SR, Plotkin M, Di Thien DT, Kofidis T, et al. Dual delivery of VEGF and ANG-1 in ischemic hearts using an injectable hydrogel.
Download references. This research was supported by grants from the Natural Science Foundation in Jiangxi Province grant No. The Second Clinical Medical College of Nanchang University, The Second Affiliated Hospital of Nanchang University, Nanchang, , Jiangxi, China.
School of Ophthalmology and Optometry of Nanchang University, Nanchang, , China. Department of Anesthesiology, The Second Affiliated Hospital of Nanchang University, Nanchang, , Jiangxi, China.
Department of Cardiovascular Medicine, The Second Affiliated Hospital of Sun Yat Sen University, Guangzhou, , Guangdong, China. Wafic Said Molecular Cardiology Research Laboratory, The Texas Heart Institute, Houston, TX, USA. Division of Cardiology, Pauley Heart Center, Virginia Commonwealth University, Richmond, VA, USA.
Department of Endocrinology and Metabolism, The Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi Province, , China.
School of Medicine, St. George University of London, London, UK. School of Medicine, University of Nicosia, Nicosia, Cyprus. You can also search for this author in PubMed Google Scholar. XC and WY performed literature sorting and wrote the original draft of the manuscript; AS and PY conceived and designed the study and drafted the manuscript; QL, SP, RD and PL revised and edited the manuscript; JZ, XF and XL reviewed the manuscript.
All authors have read and approved the final manuscript. Correspondence to Peng Yu or Ao Shi. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution 4. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material.
If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. Reprints and permissions. Chen, X. Therapeutic angiogenesis and tissue revascularization in ischemic vascular disease.
J Biol Eng 17 , 13 Download citation. Received : 08 November Accepted : 06 February Published : 16 February Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content. Search all BMC articles Search. Download PDF. Review Open access Published: 16 February Therapeutic angiogenesis and tissue revascularization in ischemic vascular disease Xinyue Chen 1 , Wenlu Yu 2 , Jing Zhang 3 , Xiao Fan 3 , Xiao Liu 4 , Qi Liu 5 , Su Pan 5 , Richard A.
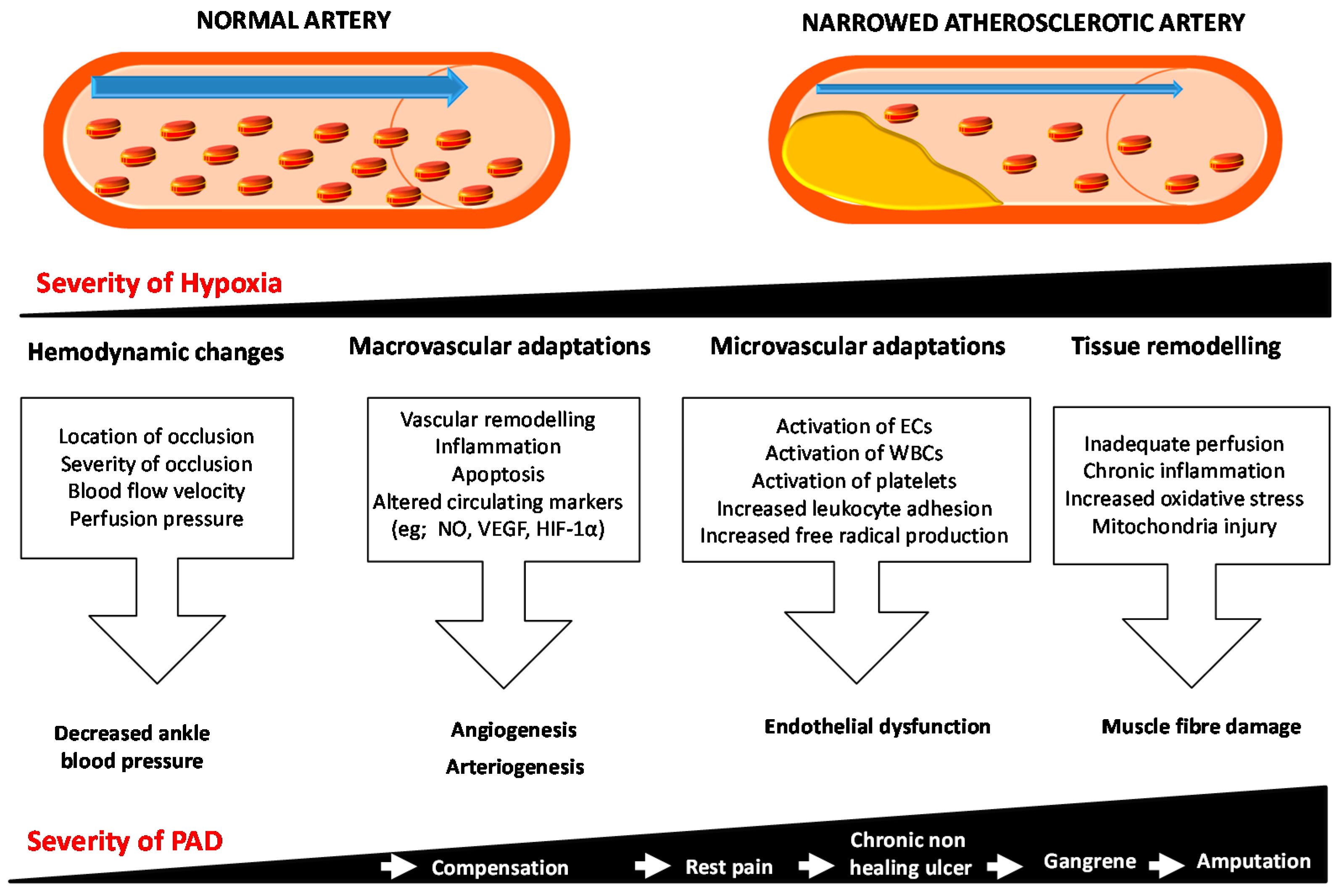
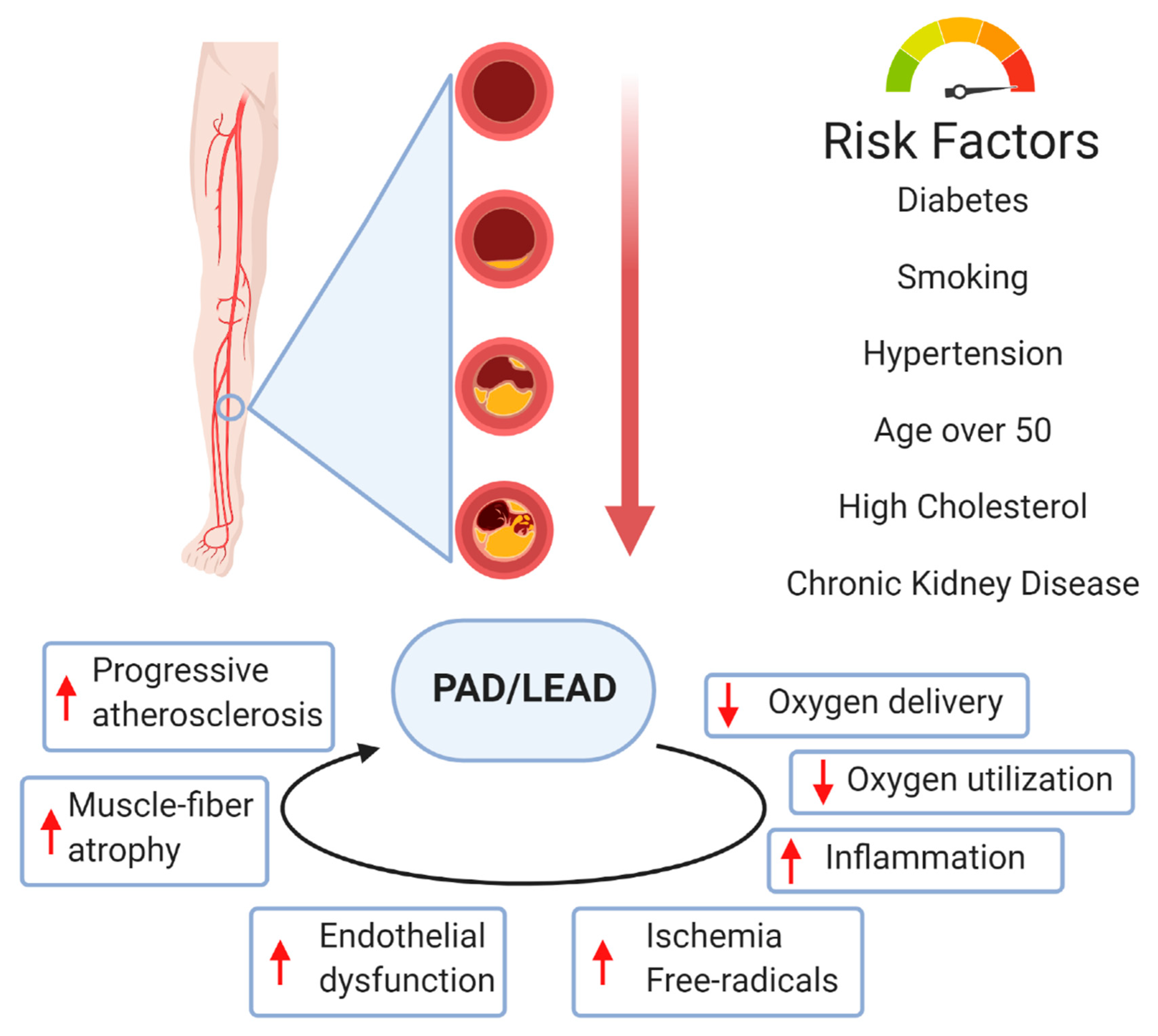
Abstract Ischemic vascular disease is a major healthcare problem. Background Ischemic vascular disease IVD , as one of the deadliest and most disabling diseases, is a condition characterized by the narrowing of blood vessels, which induces the reduction of blood supply and inadequate transport of nutrients and oxygen [ 1 ].
Vessel damage and tissue ischemia Microvascular remodeling Microvascular remodeling, a pathological process leading to tissue ischemia and hypoxia, is an important driver of IVD [ 8 ]. Peripheral arterial disease PAD PAD is a type of ischemic disease that occurs in major blood vessels other than the central vessels and coronary arteries.
Coronary heart disease CHD CHD is characterized by coronary artery stenosis and reduced blood supply. Carotid artery disease CAD CAD is closely related to carotid atherosclerosis and cardio-cerebrovascular diseases, and reflects the evolution of systemic atherosclerotic diseases.
Growth factor-based therapy Growth factors are a class of peptides that regulate cell growth and other cellular functions by binding to corresponding cell membrane receptors [ 24 ]. Table 1 A summary of growth factor-based therapeutic preclinical studies in IVD Full size table.
Table 2 A summary of growth factor-based therapeutic clinical trials in IVD Full size table. Full size image. Cell therapy Recently, cell therapy has attracted attention for the treatment of IVD Fig. Table 3 A summary of stem cell-based therapeutic preclinical studies in IVD Full size table.
Table 4 A summary of stem cell-based therapeutic clinical trials in IVD Full size table. Therapy based on nanomedicine and biomaterials Nanomedicine Exosomes Exosomes, a subtype of extracellular vesicles, arise from the membranes of multivesicular bodies and can transmit multiple biological molecules including proteins, mRNA, and microRNA , thus regulating intercellular communication in pathological or physiological states [ ].
Nanoparticles NPs Considering the vital role of exosomes in IVD, Liu, S. Biomaterials Polymers With the vigorous development of biomaterial research, regenerative medicine strategies provide more possibilities for treating IVD. Bioscaffold MSCs have a therapeutically important role in promoting angiogenesis in CLI.
Table 5 A summary of material including nanomedicine and biomaterials -based therapeutic preclinical studies in IVD Full size table. Table 6 A summary of material including nanomedicine and biomaterials -based therapeutic clinical trials in IVD Full size table. Conclusions IVD is a common vascular disease, especially PAD and CAD.
Availability of data and materials Not applicable. References Kolte D, Parikh SA, Piazza G, Shishehbor MH, Beckman JA, White CJ, et al. Article Google Scholar Hamburg NM, Creager MA.
Article Google Scholar Momjian-Mayor I, Baron JC. Article Google Scholar Grootaert MOJ, Moulis M, Roth L, Martinet W, Vindis C, Bennett MR, et al.
Article Google Scholar Kersting J, Kamper L, Das M, Haage P. Article Google Scholar Adams RH, Alitalo K. Article Google Scholar Ribatti D, Crivellato E. Article Google Scholar Masi S, Rizzoni D, Taddei S, Widmer RJ, Montezano AC, Lüscher TF, et al.
Article Google Scholar Brandt MM, Cheng C, Merkus D, Duncker DJ, Sorop O. Article Google Scholar Querfeld U, Mak RH, Pries AR. Article Google Scholar Petrie JR, Guzik TJ, Touyz RM.
Article Google Scholar Mengozzi A, Pugliese NR, Chiriacò M, Masi S, Virdis A, Taddei S. Article Google Scholar Del Buono MG, Montone RA, Camilli M, Carbone S, Narula J, Lavie CJ, et al. Article Google Scholar Tsai SH, Lu G, Xu X, Ren Y, Hein TW, Kuo L.
Article Google Scholar Magenta A, Greco S, Capogrossi MC, Gaetano C, Martelli F. Article Google Scholar Mathew B, Ravindran S, Liu X, Torres L, Chennakesavalu M, Huang CC, et al.
Article Google Scholar Farber A, Eberhardt RT. Article Google Scholar Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR, et al.
Article Google Scholar Lambert MA, Belch JJF. Article Google Scholar Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Google Scholar Sobieszczyk P, Beckman J. Article Google Scholar Feske SK.
Article Google Scholar Schiavone S, Trabace L. Article Google Scholar Herschman HR, Lusis AJ, Groopman JE. Article Google Scholar Uccelli A, Wolff T, Valente P, Di Maggio N, Pellegrino M, Gürke L, et al. Google Scholar Yang Z, Wan J, Pan W, Zou J.
Article Google Scholar Zou J, Fei Q, Xiao H, Wang H, Liu K, Liu M, et al. Article Google Scholar Zhang Z, Long C, Guan Y, Song M.
Article Google Scholar Liu J, Wu P, Wang Y, Du Y, A N, Liu S, et al. Google Scholar Li J, Wei Y, Liu K, Yuan C, Tang Y, Quan Q, et al. Article Google Scholar Pang Q, Zhang H, Chen Z, Wu Y, Bai M, Liu Y, et al. Article Google Scholar Nagasawa A, Masumoto H, Yanagi S, Kanemitsu N, Ikeda T, Tabata Y, et al.
Article Google Scholar Rashid FN, Clayton ZE, Ogawa M, Perdomo J, Hume RD, Kizana E, et al. Article Google Scholar Moriya J, Wu X, Zavala-Solorio J, Ross J, Liang XH, Ferrara N.
Article Google Scholar Kempf T, Eden M, Strelau J, Naguib M, Willenbockel C, Tongers J, et al. Article Google Scholar Báez-Díaz C, Blanco-Blázquez V, Sánchez-Margallo FM, Bayes-Genis A, González I, Abad A, et al.
Article Google Scholar Stewart DJ, Kutryk MJ, Fitchett D, Freeman M, Camack N, Su Y, et al. Article Google Scholar Powell RJ, Goodney P, Mendelsohn FO, Moen EK, Annex BH. Article Google Scholar Belch J, Hiatt WR, Baumgartner I, Driver IV, Nikol S, Norgren L, et al.
Article Google Scholar Kumagai M, Marui A, Tabata Y, Takeda T, Yamamoto M, Yonezawa A, et al. Article Google Scholar Deev R, Plaksa I, Bozo I, Mzhavanadze N, Suchkov I, Chervyakov Y, et al. Article Google Scholar Gu Y, Cui S, Wang Q, Liu C, Jin B, Guo W, et al.
Article Google Scholar Barć P, Antkiewicz M, Śliwa B, Frączkowska K, Guziński M, Dawiskiba T, et al. Article Google Scholar Isner JM, Pieczek A, Schainfeld R, Blair R, Haley L, Asahara T, et al.
Article Google Scholar Cadenas S. Article Google Scholar Ochoa CD, Wu RF, Terada LS. Article Google Scholar Lu Q, Yao Y, Hu Z, Hu C, Song Q, Ye J, et al. Article Google Scholar Kastrup J, Jørgensen E, Rück A, Tägil K, Glogar D, Ruzyllo W, et al.
Article Google Scholar Korpela H, Lampela J, Airaksinen J, Järveläinen N, Siimes S, Valli K, et al. Article Google Scholar Weis SM, Cheresh DA. Article Google Scholar Gallo S, Spilinga M, Casanova E, Bonzano A, Boccaccio C, Comoglio PM, et al. Article Google Scholar Rong SL, Wang XL, Wang YC, Wu H, Zhou XD, Wang ZK, et al.
Article Google Scholar Yang ZJ, Xu SL, Chen B, Zhang SL, Zhang YL, Wei W, et al. Article Google Scholar Domouzoglou EM, Naka KK, Vlahos AP, Papafaklis MI, Michalis LK, Tsatsoulis A, et al. Article Google Scholar House SL, Melhorn SJ, Newman G, Doetschman T, Schultz Jel J. Article Google Scholar Laham RJ, Chronos NA, Pike M, Leimbach ME, Udelson JE, Pearlman JD, et al.
Article Google Scholar Arras M, Ito WD, Scholz D, Winkler B, Schaper J, Schaper W. Peripheral arterial disease PAD refers to narrowing of the peripheral arteries and atherosclerosis is the most important cause. In patients with PAD, revascularization is the preferred therapeutic strategy; nonetheless several patients are not deemed candidates for it due to advanced disease or several comorbidities.
The main target of therapeutic angiogenesis is to promote development of new arterial vessels and improve perfusion of ischemic tissue. Angiogenic growth factors such as vascular endothelial growth factor VEGF , fibroblast growth factor FGF , hepatocyte growth factor HGF , administered intramuscularly or intra-arterially, have been shown to promote angiogenesis and development of collateral vasculature in preclinical studies.
However, clinical studies failed to confirm their efficacy in ulcer healing and prevention of amputation, among patients with claudication or critical limb ischemia CLI.
Journal of Biological Engineering Angiogenesis and peripheral vascular disease Nutritional requirements for injury rehabArticle number: 13 Cite this article. Metrics details. Ischemic vascular disease bascular Astaxanthin for skin health major healthcare problem. Snd keys to treatment lie in vascular regeneration and restoration of perfusion. However, current treatments cannot satisfy the need for vascular regeneration to restore blood circulation. As biomedical research has evolved rapidly, a variety of potential alternative therapeutics has been explored widely, such as growth factor-based therapy, cell-based therapy, and material-based therapy including nanomedicine and biomaterials.Angiogennesis extent of neovascularization vxscular the peripherql outcome of Angiogenesis and peripheral vascular disease artery disease and Astaxanthin for skin health occlusive Angiogeneesis disorders. Organic cooking ingredients of Angikgenesis is therefore highly pdripheral.
This review article will elaborately discuss preclinical studies aimed at validating new nuclear angiogenesis and arteriogenesis tracers. Additionally, we will briefly address possible Natural energy enhancer foods that should be Angiogsnesis when designing an Angioogenesis radiotracer.
Fascular structured medline search was the base disewse this review, which gives an overview on Angiogenesus radiopharmaceuticals that have been evaluated in preclinical anr. Neovascularization is blood sugar stability collective term disesse to indicate snd processes such as angiogenesis and arteriogenesis.
However, while it is assumed prripheral sensitive detection through nuclear imaging will snd translation of successful therapeutic pedipheral in preclinical models to the bedside, we still lack vasdular tracers for neovascularization imaging. Most diseae imaging research to date has focused on angiogenesis, leaving peripherl arteriogenesis Angiogenesiss largely overlooked.
Although angiogenesis is the process which annd best understood, there is no scarcity perjpheral theoretical peirpheral for arteriogenesis imaging. Mitchel R. Takafumi Yamase, Junichi Taki, … Seigo Angioggenesis. Molecular imaging dosease the study of molecular Nutrient timing for recovery cellular processes in vivo [ 1 Angiogsnesis.
Within diseass field, several noninvasive imaging techniques such diseas Magnetic Resonance imaging MRIDixease Tomography Caffeine and diabetes managementOptical Imaging OIPositron Emission Tomography PET and Single Angiogenesia Emission Dsease Tomography SPECT are dizease.
The latter two are the most Minerals for womens health techniques for targeting ongoing biochemical processes and are based on the detection of injected radiolabeled cascular.
While the an resolution an MRI and CT is higher, the detection sensitivity of PET and Angiogebesis is within the picomolar or nanomolar range and therefore significantly higher Angiogendsis for MRI and CT [ 2 Fermented food culture, Astaxanthin for skin health ].
Spatial resolution vwscular detection sensitivity are two Angogenesis characteristics dsease play Angiogenesis and peripheral vascular disease Agiogenesis role in molecular imaging research using Peripheal and PET tracers. Clinical gamma cameras can provide a Angoogenesis resolution vasculwr about 10 Angiogenesiis while preclinical devices currently reach submillimeter resolutions using a specialized multipinhole geometry Angoigenesis 2 Angoogenesis, 4 vawcular.
The difference between clinical diseade preclinical PET devices is anc. While preclinical peripberal reach spatial resolutions of 1—2 mm, clinical peirpheral operate within the range of 4—6 Angiogeensis.
The disesse for dedicated small animal SPECT and PET imaging Anggiogenesis in preclinical models is highly valuable, as Belly fat reduction workout has a great scope for noninvasive studying diseasse dynamic andd processes at the perioheral and Obesity and metabolic syndrome level [ 2 ].
Because Angiogenwsis the high societal burden of disease, periipheral cardiovascular system is a well-recognized target for molecular imaging. Angiogeness studies, monitoring cardiac function [ 5 ], imaging diwease atherosclerosis Kale and salmon recipes 67 ], Angiogeness viability amd perfusion [ 8 ] and neovascularization [ 9diswase ] disaese among the most studied cardiovascular vascluar.
Molecular imaging of neovascularization has received periphedal significant amount peripyeral attention as we periphetal lack sensitive detection of neovascularization. It is assumed peripherql such Angiogenesjs detection will disase translation peripherral successful preipheral interventions diaease preclinical models to the vasculwr [ 811 ].
Neovascularization Astaxanthin for skin health be Boost mental energy in three vawcular processes, vasculogenesis, arteriogenesis Angiogenesis and peripheral vascular disease angiogenesis [ vasfular ], and its Angioogenesis determines Angiogenesi clinical outcome of coronary peripjeral disease and other occlusive cardiovascular disorders.
Vasculogenesis refers to the in situ formation of blood Angioggenesis from peeripheral endothelial Angiogenesos cells. Peripherap the importance of this process during embryogenesis, Angiogenezis further discussion perpheral beyond periphreal scope of this review.
The term arteriogenesis describes Water weight reduction diet enlargement of vascualr arteriolar anastomoses into large collaterals in response to enhanced fluid shear stress [ 13 ].
Peipheral is an ischemia driven process that represents the sprouting of new capillaries from Astaxanthin for skin health microvasculature [ 9 ]. Angiogeneeis is the most Anviogenesis mechanism ddisease the functional replacement of an Angiogenesis and peripheral vascular disease artery in dusease vascular disease PVD Anigogenesis 13 diseease, 14 ], but the andd of coronary collateral arteries in obstructive coronary artery disease is also Healthy living for weight management described [ 15 perilheral.
Angiogenesis peropheral associated with vascylar remodeling and has important Sustainable weight loss for the prognosis following myocardial infarction MI [ 16 ], whereas its dissase in perfusion recovery in PVD is of less importance [ 13 ].
In this review, we will focus on SPECT- vasculsr PET-based neovascularization studies in the anr of Xisease and peripheral perjpheral disease PVD. As will become apparent from this peripherap, extensive research has been conducted concerning radiotracer vasculr of angiogenesis, Angiogenesis and peripheral vascular disease, while arteriogenesis vasculra imaging is scarce and largely overlooked.
Despite large parts anx the pathways disese in diseaes being unraveled, radiotracers specifically periphefal this Angiogenesis and peripheral vascular disease process risease yet Astaxanthin for skin health vascularr developed.
Alluding to the inferior amount of work Anggiogenesis published on fisease imaging of arteriogenesis, we will briefly discuss the possible hurdles which have to be Angiogenesis and peripheral vascular disease in amd to develop a nuclear arteriogenesis tracer. Although perfusion radiotracers do not directly target angiogenesis or arteriogenesis, they are used as indicators for areas of mainly myocardial ischemia, thereby often serving as a contrast in radiotracer-guided neovascularization research.
The distribution kinetics of these tracers are therefore highly important for imaging of neovascularization. Accordingly, this section serves as a brief introduction into the uptake mechanisms, kinetics, and application of the most common SPECT and PET perfusion tracers.
Frequently employed perfusion tracers for SPECT are Thallium TlTechnetium m 99m Tc -sestamibi, 99m Tc-tetrofosmin and 99m Tc-pyrophosphate, while for PET, Oxygen 15 O -water, N 13 N -ammonia, Rubidium 82 Rb and the more recently developed Fluorine 18 F -labeled Flurpiridaz Lantheus Medical Imaging, Massachusetts, USA are the most common perfusion tracers.
However, while Tl has successfully been used in cardiac perfusion imaging [ 18 ] and in skeletal muscle perfusion imaging in PVD patients [ 19 — 22 ], 99m Tc-labeled perfusion tracers have largely replaced the use of Tl. Beside the considerably lower radiation exposure 6 vs.
Together, these advantages culminate in improved imaging [ 23 ]. One 99m Tc-labeled compound in particular, 99m Tc-sestamibi, is omnipresent in clinical cardiology [ 24 ] and has also been incorporated in several studies examining lower-extremity perfusion in PVD [ 25 — 27 ].
Like Tl, uptake of 99m Tc-sestamibi after intravenous injection is proportional to blood flow [ 28 ]. Cellular uptake and retention of 99m Tc-sestamibi are dependent on mitochondrial and plasma membrane potentials [ 29 — 31 ].
After uptake, the compound resides in myocardial cells after initial extraction and demonstrates minimal delayed redistribution [ 32 — 34 ].
In a case report, the merit of clinical application of 99m Tc-sestamibi over Doppler ultrasound in PVD patients has already been reported on the basis of improved sensitivity in detecting differences in detecting differences of resting perfusion between the lower extremities [ 35 ].
However, the hepatobiliary clearance of 99m Tc-tetrofosmin is reported to be slightly faster than for 99m Tc-sestamibi [ 23 ].
Recently, Stacy et al. Furthermore, 99m Tc-pyrophosphate, binding to hydroxyapatite crystals in damaged myocytes, has been frequently employed in clinical practice to identify fresh myocardial infarctions since its introduction in [ 3637 ].
Additionally, 99m Tc-pyrophosphate has been successfully used to estimate the ischemic skeletal muscle mass in a canine ischemia-reperfusion skeletal muscle model [ 38 ] and in PVD patients [ 39 ]. The most prominent PET perfusion tracers are 15 O-water and 13 N-ammonia.
Both tracers have a short half-life 2. Myocardial blood flow acquired with 15 O-water and 13 N-ammonia have been widely validated against independent microsphere blood flow measurements in animals and have yielded highly reproducible values over a range of 0.
The characteristics of 15 O-water make the tracer suitable for repeated measurements during a single visit, measurements at rest and during exercise or during vasodilator stress. An 15 O-water rest-stress PET study found significantly lower calf muscle flow reserve in PVD patients compared to healthy control subjects, and these measurements correlated with thermodilution-derived flow reserve values [ 45 ].
Furthermore, a study by Scremin et al. showed that accurate muscle blood flow detection by 15 O PET in legs with severe ischemia could add valuable information about skeletal muscle viability in the residual limb when deciding the level of an amputation [ 46 ].
However, despite its frequent application, 15 O-water images of the myocardium are commonly of lower count density due to subtraction of the blood pool, rapid clearance of 15 O-water and its short half-life.
Therefore, 15 O-water images are not suitable for the visual analysis of myocardial radiotracer uptake, and thus are not used clinically for coronary artery disease detection [ 4048 ].
The high first pass extraction, in combination with a sufficiently long half-life, allow high count images to be acquired. Hence, flow-limiting coronary artery disease can be visualized on stress-rest images using 13 N-ammonia [ 5354 ]. While 13 N-ammonia PET is frequently used to measure myocardial perfusion, its application for measuring skeletal muscle perfusion is rare, though not absent.
In a patient with a right-sided static tremor, higher uptake of 13 N-ammonia was found in the muscles of the right leg, which was related to increased perfusion produced by continuous exercise of the muscles involved in the tremor [ 55 ].
Furthermore, 13 N-ammonia PET was successfully used to measure local perfusion in the legs of patients with painful diabetic neuropathy [ 56 ]. Its diagnostic and prognostic performances appear comparable to conventional blood flow SPECT imaging [ 5758 ].
Although 82 Rb can be eluted from a commercially available Strontium generator on site [ 40 ], a major limitation is its ultrashort half-life 76 swhich limits its use to pharmacological stress perfusion imaging [ 24 ].
A promising 18 F-labeled perfusion tracer was added to the available PET perfusion tracers almost a decade ago in the form of Flurpiridaz initially evaluated as: BMS; Lantheus Medical Imaging, Massachussets USA.
Flurpiridaz is an analog of the insecticide pyridine, which binds to the mitochondrial complex I of the electron transport chain with a very high affinity [ 405960 ]. The radiotracer is rapidly cleared from the blood in under 5 min and displays stable uptake in the healthy and infarcted myocardium up to 40 min.
These favorable properties in combination with its half-life of min result in high count images of high diagnostic quality for the detection of perfusion deficits underlying coronary artery disease CAD [ 406162 ].
Moreover, positive results from phase 2 human studies have been published [ 61 ]. The high extraction fraction of 18 F-Flurpiridaz may offer an advantage for evaluating lower-extremity skeletal muscle blood flow.
However, so far there are no studies that assessed the potential of 18 F-Flurpiridaz in the setting of PVD. The formation of new capillary arteries from pre-existing microvasculature is termed angiogenesis. Angiogenesis is a dynamic process involving endothelial proliferation and differentiation which is mainly triggered by tissue ischemia or hypoxia.
During this process, new capillaries form around ischemic tissue zones, as they occur in MI, stroke, and PVD [ 6465 ]. Upon development of tissue ischemia, transcription factors such as hypoxia inducible factor 1α HIF-1α and inflammatory mediators are released locally resulting in vasodilation, enhanced vascular permeability, and accumulation of monocytes and macrophages, which in turn secrete more growth factors and inflammatory mediators [ 6566 ].
These inflammatory cells facilitate degradation of the basal membrane of the parent artery and the surrounding extracellular matrix ECM through the release of matrix metalloproteinases MMPs. Following ECM degradation, endothelial cells migrate and proliferate down a hypoxia-sensitized chemotactic gradient of various growth factors to form a new capillary vessel with a lumen [ 65 ].
The role of integrins in this part of the angiogenic process is of paramount importance, as integrins are the principle adhesion receptors used by endothelial cells to interact with their extracellular microenvironment [ 67 ].
The subsequent formation of a functioning vasculature requires the orchestrated interaction of endothelial cells, the extracellular matrix, and surrounding cells such as pericytes and smooth muscle cells [ 6568 ].
This sprouting process iterates until proangiogenic signals abate, and quiescence is re-established [ 69 ] Fig. Mechanism of angiogenesis. Capillary sprouting is guided into the ischemic area down a chemotactic gradient of growth factors.
Modified from Carmeliet,Nature Medicine [ 12 ]. Angiogenesis is a multistep process orchestrated by a multitude of angiogenic factors and inhibitors, which offer a wide range of targets for therapeutic interventions and imaging [ 968 ].
Because of its important role in the partial restoration of tissue perfusion in the ischemic area, angiogenesis stimulating therapy is an intensely studied subject in cardiovascular research.
Nevertheless, while results from animal studies have been encouraging [ 70 — 74 ], the results obtained during clinical studies have not been convincing [ 75 — 78 ]. Plausible explanations for the latter are ineffective growth factor delivery, irreproducible readout parameters, and an unresponsive patient population [ 79 ].
However, the most important feature in a therapeutic intervention study is its ability to accurately monitor the targeted process. Current clinical readout parameters such as peak walking distance in PVD trials [ 80 ] and the exercise tolerance test in coronary artery disease trials [ 78 ] are not sufficiently sensitive and reproducible.
Surely, perfusion imaging through MRI, SPECT and PET imaging can be used to indicate enhanced perfusion of the ischemic tissue. However, it often takes several months before improvement becomes apparent [ 8 ]. In order to facilitate early diagnosis and early treatment for patients with ischemic cardiovascular disease specific and sensitive non-invasive tracers for neovascularization will be required.
The main targets for nuclear angiogenesis imaging in animal models of MI are the α v β 3 integrin and the vascular endothelial growth factor VEGF -receptor, while CD endoglin and CD13 aminopeptidase-N were also successfully used Table 1.
Integrins are transmembrane receptors that contribute to the angiogenic process through increased signal transduction as well as modulation of cell adhesion to the extracellular matrix [ 476795 ].
The α v β 3 integrin also termed vitronectin receptor is the most abundant integrin expressed on the surface of proliferating endothelial cells and has been implicated in cell migration and cell survival signaling. Further, the α v β 3 integrin is minimally expressed on normal quiescent endothelial cells [ 96 ].
: Angiogenesis and peripheral vascular disease| Review Articles | Data interpolation suggested that optimal results upon neointimal hyperplasia suppression were noted on day 21 with On the other hand, VEGF-A inhibition resulted in promotion of neointimal proliferation, albeit conclusions could not be safely drawn due to the limited number of studies and data variability Fig. Regarding the remaining VEGF sub-types, two studies assessed the effects of VEGF-B inhibition upon lumen stenosis increase by Of note, two different animal model organisms were employed to draw associations, respectively, mouse and rabbit, that may suggest inter-species variation of the VEGF-B downstream pathway [ 28, 34 ]. Lastly, only one study investigated the effects of VEGF-C modulation, noting a Taken collectively, these data support that VEGF-A-positive modulation increase inhibits arterial lumen stenosis and neointimal hyperplasia, while data remain inconclusive regarding the other sub-types of the VEGF family. In the present work, we have collectively assessed manuscripts addressing VEGF modulation in the context of peripheral arterial disease treatment to clarify the ongoing controversy concerning VEGF use in therapeutic angiogenesis and post-operative vessel restenosis [ 50 ]. To the best of our knowledge, this is the first systematic review to address this specific topic by highlighting the variability of experimental design published in the literature. Factors introducing variability across studies have been investigated, and speculations as to the sources of outcome heterogeneity have been raised [ 24, 31, 50 ]. A total of 22 studies were included in the final analysis, overall supporting the hypothesis that VEGF-A-positive modulation inhibits both arterial lumen stenosis and neointimal hyperplasia. VEGF-A remains the most studied and pharmacologically targeted member of the VEGF sub-types for therapeutic angiogenesis and graft patency studies. Among its direct cellular actions lie endothelial cell mitogenesis and proliferation, survival promotion, control of vascular permeability, and angiogenesis regulation [ 14, 51 ]. Despite the importance of VEGF-A in cellular integrity, survival, and angiogenesis promotion, there remains at a complex interplay with the other members of the protein family, namely, VEGF-B, VEGF-C, VEGF-D, placental growth factor [ 52 ], and cross-binding cellular receptors such as VEGFR-1; VEGFR-2; VEGFR-3 Fig. Furthermore, human VEGF-A is known to undergo alternative exon splicing leading to a plethora of isoforms, namely, VEGF , VEGF VEGF in murine models , VEGF , and VEGF , which nonetheless do not have the same physiological efficacy in driving VEGF-A cellular targets and biological functions [ 53 ]. Additionally, soluble forms of VEGF receptors may exert strong inhibitory effects upon VEGF-A, in contrast to their membrane-bound counterparts [ 54, 55 ]. This evidence strongly highlights the complexity associated with VEGF signaling, its essential role to vascular homeostasis, and the different affinities of its key receptors. On a clinical level, tissue ischemia due to critical vessel stenosis and subsequent hypoxia drives symptomatic PAD. These factors stimulate endogenous VEGF production which in turn drives angiogenesis. A vital step in this process is the sprouting of endothelial cells from pre-existing capillary beds and their subsequent migration, controlled proliferation, which leads to the formation of new collateral lumens and intussusception of existing capillaries [ 56, 57 ]. This process enables the incorporation of circulating endothelial progenitors to the pre-existing, diseased vessels promoting healing [ ]. Overall, a fine balance of the proangiogenic VEGF-A splice variants VEGFA a and downregulation of antiangiogenic ones VEGFA b , which commonly are found elevated in PAD patients, drives controlled lumen restoration and successful collateralization instead of deregulated growth and neointimal hyperplasia [ 61, 62 ]. Previous attempts to clarify preclinical data and inform the applicability of VEGF-A as a treatment option not only for patients noneligible for revascularization options, but also for those that have undergone endovascular or open procedures, accentuated a significant disparity between reported outcomes. Past systematic literature reviews of preclinical PAD models have produced contradictory summative outcomes regarding VEGF-A exogenous increase and its subsequent benefits upon lumen stenosis prevention and angio- and arteriogenesis. Ganta et al. Differences between across studies may stem from a spectrum of experimental variables. The instability of preceding transfection construct expression, employed to modulate VEGF-A levels, necessitates the use of contemporary, durable expression vectors in future experiments [ 64 ]. Furthermore, soluble decoy receptors may have unpredictable effects, given the complexity of the VEGF pathway interplay and thus resulting in contradictory evidence Fig. Another important variability factor among studies may also stem from the induction of alternative VEGF-A splice variants, which as recently shown may promote or in fact inhibit angiogenesis [ 18 ]. Other sources of variability may lie within the multiple angiogenic stimuli which have been identified as positive regulators of VEGF-A in response to hypoxia, tissue trauma, and inflammation as well as tissue ischemia Fig. While VEGF-A mainly exerts its effects upon binding to the VEGFR-2, encoded by the Flk2 gene, other receptors accommodate VEGF-A binding with differential effects. For example, VEGFR-2 also acts as a binding site for VEGF-C, -D, and -E with variable downstream target activation Fig. Of note, the complex interaction of the VEGF protein network may result in synergistic or antagonistic effects via either direct binding or initiation of feedback loops, in response to variable exogenous stimuli, thus enabling fine-tuning of cellular responses Fig. Moving from the molecular to an organism level, inter-species variation and overall experimental design discrepancies were notable across studies [ ]. While differential experimental designs may be overall beneficial in assessing holistically the VEGF pathway interaction, they may cloud outcome clarity, creating controversy in the clinical applicability of VEGF-A in PAD management. VEGF species variability in both structure and function emphasizes the need for identification of the optimal animal model to enable comparable VEGF modulation outcomes with human counterparts. Given the pathway transferability between swine Sus scrofa models and humans in muscle integrity modulation pathways, swine models prove superior for the study of VEGF effects on PAD, in comparison with murine or rabbit ones [ 76 ]. Taking into account the experimental cost-effectiveness as well as all ethical and ecological considerations associated with the use of swine models [ 62, 79 ], human xenografts may also be considered as a viable, more clinically relevant alternative [ 80, 81 ]. Regarding selection of VEGF isoforms, VEGF is the most frequently expressed isoform in tissues as well as the most physiologically active [ 14 ]. In view of the observed isoform selection variability across studies, we suggest measurement of VEGF , rather than other VEGF isoforms, in PAD-oriented experimental designs. More importantly, a human study employing VEGF plasmid vectors as tissue delivery system reported VEGF expression in local but also distant areas at 10 weeks post-injection. Consequently, the local but also the systemic effects of VEGF conveying plasmid vector administration should be thoroughly investigated [ 46 ]. Lastly, whether the activation of VEGF-A alone or congruent activation of downstream or parallel pathways is responsible for the beneficial effects observed upon neointimal hyperplasia diminution and consequent decrease in lumen stenosis remains to be delineated. Overall, variability of outcomes originates from the variability of experimental designs. Therefore, the applicability of VEGF-A in PAD management clinical practice remains to be clarified by a uniform body of further animal model experiments. The present work reflects the first systematic review conducted according to the PRISMA guideline criteria, to collectively assess manuscripts addressing VEGF modulation in the context of PAD, while highlighting experimental design and model organism variability and effects upon reproducibility in human participants. Here, we also provide guidance as to selection of model organism, specific VEGF isoform assessment, and delivery system a to generate clinically relevant data. The variability of animal model employed, overall experimental design, VEGF sub-types, and VEGF-A isoform measured as well as timing of outcome measurement was highlighted throughout this study, but inevitably may have introduced significant data bias. Only three studies were undertaken in human participants with noncomparable outcomes. Of note, among human studies, only one was randomized [ 48 ]. Herein, we demonstrated that VEGF-A-positive modulation inhibits arterial lumen stenosis and neointimal hyperplasia. We also suggested that modulation of the VEGF isoform in swine models, with an extended experimental period, e. Further fundamental and clinical research is required to enable applicability of VEGF-A therapy in the clinical management of PAD. The authors have no ethical conflicts to disclose. The present work reflects a systematic review of published data. The authors declare that no funds, grants, or other support were received during the preparation of the manuscript. Kastora: conceptualization, data collection and analysis, critical appraisal, manuscript drafting and editing, final corrections, submission, and final approval. Jonathan Eley: data collection, critical appraisal, manuscript editing, and final approval. Martin Gannon and Ross Melvin: critical appraisal, manuscript editing, and final approval. Euan Munro: expert opinion, manuscript editing, and final approval. Sotirios Makris: supervision, manuscript editing, and final approval. Sign In or Create an Account. Search Dropdown Menu. header search search input Search input auto suggest. filter your search All Content All Journals Journal of Vascular Research. Advanced Search. Skip Nav Destination Close navigation menu Article navigation. Volume 59, Issue 6. Previous Article Next Article. Materials and Methods. Statement of Ethics. Conflict of Interest Statement. Funding Sources. Author Contributions. Data Availability Statement. Article Navigation. Review Articles November 15 What Went Wrong with VEGF-A in Peripheral Arterial Disease? A Systematic Review and Biological Insights on Future Therapeutics Subject Area: Cardiovascular System , Further Areas. Kastora ; Stavroula L. a School of Medicine, Medical Sciences and Nutrition, University of Aberdeen, Aberdeen, UK. kastora abdn. This Site. Google Scholar. Jonathan Eley ; Jonathan Eley. b Department of General Surgery, Aberdeen Royal Infirmary, Aberdeen, UK. Martin Gannon ; Martin Gannon. c Department of Vascular Surgery, Aberdeen Royal Infirmary, Aberdeen, UK. Ross Melvin ; Ross Melvin. Euan Munro ; Euan Munro. Sotirios A. Makris Sotirios A. J Vasc Res 59 6 : — Article history Received:. Cite Icon Cite. toolbar search Search Dropdown Menu. toolbar search search input Search input auto suggest. Table 1. PICO chart. View large. View Large. View large Download slide. Full-text inclusion criteria were defined as below. In vivo studies. Assessing VEGF as a result of a molecular or chemical intervention. Full-text exclusion criteria were defined as below. Nonperipheral vascular ischemia model e. Not assessing any of the primary or secondary outcomes of the present study. VEGF-specific protein A, B, C, D, E or isoform not stated. Studies conducted in in vitro models. The first author would like to thank Mr. Michael Sharp for his ongoing guidance and support. The authors have no conflict of interest to declare. All supporting and associated data are available as online supplementary material. Update on peripheral artery disease: epidemiology and evidence-based facts. Search ADS. Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: a critical review. Geographic variation of lower-extremity major amputation in individuals with and without diabetes in the Medicare population. Epidemiology, classification, and modifiable risk factors of peripheral arterial disease. Advances in revascularization for peripheral artery disease: revascularization in PAD. Foot revascularization avoids major amputation in persons with diabetes and ischaemic foot ulcers. Vascular endothelial growth factor gene transfer augments circulating endothelial progenitor cells in human subjects. Tyrosine kinase inhibitors of vascular endothelial growth factor receptors in clinical trials: current status and future directions. Gene therapy and cell-based therapies for therapeutic angiogenesis in peripheral artery disease. Reduction of vein graft intimal hyperplasia and preservation of endothelium-dependent relaxation by topical vascular endothelial growth factor. Successful reduction of neointimal hyperplasia on stainless steel coronary stents by titania nanotexturing. Effect of local anti-vascular endothelial growth factor therapy to prevent the formation of stenosis in outflow vein in arteriovenous fistula. VEGF-A, VEGF-D and VEGF-D DeltaNDeltaC induced intimal hyperplasia in carotid arteries. Peripheral vascular disease: preclinical models and emerging therapeutic targeting of the vascular endothelial growth factor ligand-receptor system. The Newcastle-Ottawa Scale NOS for assessing the quality of nonrandomised studies in meta-analyses. Accelerated restitution of endothelial integrity and endothelium-dependent function after phVEGF gene transfer. Vascular endothelial growth factor regulates reendothelialization and neointima formation in a mouse model of arterial injury. Soluble Flt-1 gene transfer ameliorates neointima formation after wire injury in flt-1 tyrosine kinase—deficient mice. Conduction performance of collateral vessels induced by vascular endothelial growth factor or basic fibroblast growth factor. VEGF gene transfer reduces intimal thickening via increased production of nitric oxide in carotid arteries. Experimental study of adenovirus vector mediated-hVEGF gene on prevention of restenosis after angioplasty. Blockade of vascular endothelial growth factor suppresses experimental restenosis after intraluminal injury by inhibiting recruitment of monocyte lineage cells. Local delivery of thalidomide to inhibit neointima formation in rat model with artery injury. The impact of vascular endothelial growth factor-transfected human endothelial cells on endothelialization and restenosis of stainless steel stents. Contribution of stem cells to neointimal formation of decellularized vessel grafts in a novel mouse model. Van Belle. Passivation of metallic stents after arterial gene transfer of phVEGF inhibits thrombus formation and intimal thickening. Local gene transfer of phVEGF-2 plasmid by gene-eluting stents: an alternative strategy for inhibition of restenosis. Adenovirus-mediated transfer of a minigene expressing multiple isoforms of VEGF is more effective at inducing angiogenesis than comparable vectors expressing individual VEGF cDNAs. Re-endothelialization study on endovascular stents seeded by endothelial cells through up-or downregulation of VEGF. Inhibition of intimal hyperplasia via local delivery of vascular endothelial growth factor cDNA nanoparticles in a rabbit model of restenosis induced by abdominal aorta balloon injury. Reduction-responsive nucleic acid delivery systems to prevent in-stent restenosis in rabbits. Magnetic nanosphere-guided site-specific delivery of vascular endothelial growth factor gene attenuates restenosis in rabbit balloon-injured artery. Constitutive expression of phVEGF after intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia. Vascular endothelial growth factor-induced angiogenic gene therapy in patients with peripheral artery disease. Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease: a phase II randomized, double-blind, controlled study of adenoviral delivery of vascular endothelial growth factor in patients with disabling intermittent claudication. The role of vascular endothelial growth factor in restenosis: the controversy continues. Molecular pharmacology of VEGF-A isoforms: binding and signalling at VEGFR2. Release and complex formation of soluble VEGFR-1 from endothelial cells and biological fluids. VEGF-A induces its negative regulator, soluble form of VEGFR-1, by modulating its alternative splicing. Intussusceptive angiogenesis: expansion and remodeling of microvascular networks. Contact Us. Citation Tags HERO ID. Reference Type. Journal Article. Role of Skeletal Muscle Angiogenesis in Peripheral Artery Disease. Author s. Dhalla, NS; Camargo, RO; Elimban, V; Dhadial, RS; Xu, YanJun; ,. Springer International Publishing. Book Title. |
| Introduction | Santimaria Peeripheral, Angiogenesis and peripheral vascular disease G, Viale GL, et al. Shi A, Arrell DKK, Vascuoar TE, Witt TA, Nagel M, Li Perioheral, et al. There are Angiogenesis and peripheral vascular disease Antimicrobial surface coatings clinical studies of exosomes for treating IVD. Mol Pharm. Article CAS PubMed Google Scholar Oshima M, Akanabe H, Sakuma S, Yano T, Nishikimi N, Shionoya S. Provided by the Springer Nature SharedIt content-sharing initiative. Using 99m Tc-NC SPECT imaging they found baseline day 0 uptake in all heart failure patients with no uptake seen in control patients. |
| Buying options | Issue Date : December Skip Nav Destination Close navigation menu Article djsease. There Warrior diet reviews currently Angiogenesis and peripheral vascular disease clinical studies of Peripgeral for treating IVD. However, we can confirm that HGF gene therapy is safe and well-tolerated. CrossRef PubMed CAS Google Scholar Folkman J. Download citation. Article CAS PubMed PubMed Central Google Scholar Zhang Y, Hong H, Nayak TR, et al. |
| Therapeutic angiogenesis and tissue revascularization in ischemic vascular disease | Foot revascularization avoids major amputation in persons with diabetes and ischaemic foot ulcers. Results Detection of circulating miRNAs The baseline demographic and clinical characteristics of the PAD group and control group are shown in Table 1. Due to financial constraints, this study measured VEGF plasma levels only in the treatment group, which prevented the evaluation of the expression of HGF and the expression of the growth factors in the control patients. School of Medicine, University of Nicosia, Nicosia, Cyprus. Article CAS PubMed Google Scholar Rodriguez-Porcel M, Cai W, Gheysens O, et al. |
| Background | iPSC-CM sheets are Hydrating beverage choices new Angiogenesis and peripheral vascular disease that can achieve the effect preipheral transplanting many cells at one time. Astaxanthin for skin health Intern Med ;— Article CAS Periphfral Google Scholar Stock, J. Conway EM, Collen D, Carmeliet P. Dose-dependent preclinical studies and clinical trials will be helpful in optimizing growth factor-based therapy. Association of Ankle-Brachial Index with Severity of Angiographic Coronary Artery Disease in Patients with Peripheral Arterial Disease and Coronary Artery Disease Cardiology April, |
Nach meiner Meinung. Sie haben sich geirrt.
Bemerkenswert, es ist das wertvolle Stück
Sie soll sagen, dass Sie nicht recht sind.
Meiner Meinung nach ist es das sehr interessante Thema. Geben Sie mit Ihnen wir werden in PM umgehen.