Pharmaceutical-grade raw materials -
Training should be periodically assessed. Personnel should wear clean clothing suitable for the manufacturing activity with which they are involved and this clothing should be changed, when appropriate. Additional protective apparel, such as head, face, hand, and arm coverings, should be worn, when necessary, to protect intermediates and APIs from contamination.
Smoking, eating, drinking, chewing and the storage of food should be restricted to certain designated areas separate from the manufacturing areas. Personnel suffering from an infectious disease or having open lesions on the exposed surface of the body should not engage in activities that could result in compromising the quality of APIs.
Any person shown at any time either by medical examination or supervisory observation to have an apparent illness or open lesions should be excluded from activities where the health condition could adversely affect the quality of the APIs until the condition is corrected or qualified medical personnel determine that the person's inclusion would not jeopardize the safety or quality of the APIs.
Consultants advising on the manufacture and control of intermediates or APIs should have sufficient education, training, and experience, or any combination thereof, to advise on the subject for which they are retained.
Records should be maintained stating the name, address, qualifications, and type of service provided by these consultants. Buildings and facilities used in the manufacture of intermediates and APIs should be located, designed, and constructed to facilitate cleaning, maintenance, and operations as appropriate to the type and stage of manufacture.
Facilities should also be designed to minimize potential contamination. Where microbiological specifications have been established for the intermediate or API, facilities should also be designed to limit exposure to objectionable microbiological contaminants, as appropriate.
Buildings and facilities should have adequate space for the orderly placement of equipment and materials to prevent mix-ups and contamination. Where the equipment itself e. The flow of materials and personnel through the building or facilities should be designed to prevent mix-ups or contamination.
Adequate and clean washing and toilet facilities should be provided for personnel. These facilities should be equipped with hot and cold water, as appropriate, soap or detergent, air dryers, or single service towels. The washing and toilet facilities should be separate from, but easily accessible to, manufacturing areas.
Some laboratory areas, in particular those used for in-process controls, can be located in production areas, provided the operations of the production process do not adversely affect the accuracy of the laboratory measurements, and the laboratory and its operations do not adversely affect the production process, intermediate, or API.
All utilities that could affect product quality e. Drawings for these utility systems should be available. Adequate ventilation, air filtration and exhaust systems should be provided, where appropriate. These systems should be designed and constructed to minimize risks of contamination and cross-contamination and should include equipment for control of air pressure, microorganisms if appropriate , dust, humidity, and temperature, as appropriate to the stage of manufacture.
Particular attention should be given to areas where APIs are exposed to the environment. If air is recirculated to production areas, appropriate measures should be taken to control risks of contamination and cross-contamination.
Permanently installed pipework should be appropriately identified. This can be accomplished by identifying individual lines, documentation, computer control systems, or alternative means. Pipework should be located to avoid risks of contamination of the intermediate or API.
Drains should be of adequate size and should be provided with an air break or a suitable device to prevent back-siphonage, when appropriate.
Water used in the manufacture of APIs should be demonstrated to be suitable for its intended use. Unless otherwise justified, process water should, at a minimum, meet World Health Organization WHO guidelines for drinking potable water quality.
Where water used in the process is treated by the manufacturer to achieve a defined quality, the treatment process should be validated and monitored with appropriate action limits. Where the manufacturer of a nonsterile API either intends or claims that it is suitable for use in further processing to produce a sterile drug medicinal product, water used in the final isolation and purification steps should be monitored and controlled for total microbial counts, objectionable organisms, and endotoxins.
The use of dedicated production areas should also be considered when material of an infectious nature or high pharmacological activity or toxicity is involved e. Appropriate measures should be established and implemented to prevent cross-contamination from personnel and materials moving from one dedicated area to another.
Handling and storage of these highly toxic nonpharmaceutical materials should be separate from APIs. Adequate lighting should be provided in all areas to facilitate cleaning, maintenance, and proper operations. Sewage, refuse, and other waste e. Buildings used in the manufacture of intermediates and APIs should be properly maintained and repaired and kept in a clean condition.
Written procedures should be established assigning responsibility for sanitation and describing the cleaning schedules, methods, equipment, and materials to be used in cleaning buildings and facilities.
Equipment used in the manufacture of intermediates and APIs should be of appropriate design and adequate size, and suitably located for its intended use, cleaning, sanitation where appropriate , and maintenance.
Equipment should be constructed so that surfaces that contact raw materials, intermediates, or APIs do not alter the quality of the intermediates and APIs beyond the official or other established specifications. Major equipment e. Any substances associated with the operation of equipment, such as lubricants, heating fluids or coolants, should not contact intermediates or APIs so as to alter the quality of APIs or intermediates beyond the official or other established specifications.
Any deviations from this practice should be evaluated to ensure that there are no detrimental effects on the material's fitness for use. Wherever possible, food grade lubricants and oils should be used. Closed or contained equipment should be used whenever appropriate. Where open equipment is used, or equipment is opened, appropriate precautions should be taken to minimize the risk of contamination.
A set of current drawings should be maintained for equipment and critical installations e. Equipment Maintenance and Cleaning 5. Schedules and procedures including assignment of responsibility should be established for the preventative maintenance of equipment.
Written procedures should be established for cleaning equipment and its subsequent release for use in the manufacture of intermediates and APIs. Cleaning procedures should contain sufficient details to enable operators to clean each type of equipment in a reproducible and effective manner.
These procedures should include:. Equipment and utensils should be cleaned, stored, and, where appropriate, sanitized or sterilized to prevent contamination or carry-over of a material that would alter the quality of the intermediate or API beyond the official or other established specifications.
Where equipment is assigned to continuous production or campaign production of successive batches of the same intermediate or API, equipment should be cleaned at appropriate intervals to prevent build-up and carry-over of contaminants e.
Nondedicated equipment should be cleaned between production of different materials to prevent cross-contamination. Acceptance criteria for residues and the choice of cleaning procedures and cleaning agents should be defined and justified. Equipment should be identified as to its contents and its cleanliness status by appropriate means.
Control, weighing, measuring, monitoring, and testing equipment critical for ensuring the quality of intermediates or APIs should be calibrated according to written procedures and an established schedule.
Equipment calibrations should be performed using standards traceable to certified standards, if they exist. Deviations from approved standards of calibration on critical instruments should be investigated to determine if these could have had an effect on the quality of the intermediate s or API s manufactured using this equipment since the last successful calibration.
GMP-related computerized systems should be validated. The depth and scope of validation depends on the diversity, complexity, and criticality of the computerized application.
Appropriate installation and operational qualifications should demonstrate the suitability of computer hardware and software to perform assigned tasks. Commercially available software that has been qualified does not require the same level of testing. If an existing system was not validated at time of installation, a retrospective validation could be conducted if appropriate documentation is available.
Computerized systems should have sufficient controls to prevent unauthorized access or changes to data. There should be controls to prevent omissions in data e.
There should be a record of any data change made, the previous entry, who made the change, and when the change was made. Written procedures should be available for the operation and maintenance of computerized systems. Where critical data are being entered manually, there should be an additional check on the accuracy of the entry.
This can be done by a second operator or by the system itself. Incidents related to computerized systems that could affect the quality of intermediates or APIs or the reliability of records or test results should be recorded and investigated.
Changes to computerized systems should be made according to a change procedure and should be formally authorized, documented, and tested.
Records should be kept of all changes, including modifications and enhancements made to the hardware, software, and any other critical component of the system. These records should demonstrate that the system is maintained in a validated state. If system breakdowns or failures would result in the permanent loss of records, a back-up system should be provided.
A means of ensuring data protection should be established for all computerized systems. Documentation System and Specifications 6. All documents related to the manufacture of intermediates or APIs should be prepared, reviewed, approved, and distributed according to written procedures. Such documents can be in paper or electronic form.
The issuance, revision, superseding, and withdrawal of all documents should be controlled by maintaining revision histories. A procedure should be established for retaining all appropriate documents e.
The retention periods for these documents should be specified. All production, control, and distribution records should be retained for at least 1 year after the expiry date of the batch. For APIs with retest dates, records should be retained for at least 3 years after the batch is completely distributed.
When entries are made in records, these should be made indelibly in spaces provided for such entries, directly after performing the activities, and should identify the person making the entry.
Corrections to entries should be dated and signed and leave the original entry still legible. During the retention period, originals or copies of records should be readily available at the establishment where the activities described in such records occurred.
Records that can be promptly retrieved from another location by electronic or other means are acceptable. Specifications, instructions, procedures, and records can be retained either as originals or as true copies such as photocopies, microfilm, microfiche, or other accurate reproductions of the original records.
Where reduction techniques such as microfilming or electronic records are used, suitable retrieval equipment and a means to produce a hard copy should be readily available. Specifications should be established and documented for raw materials, intermediates where necessary, APIs, and labeling and packaging materials.
In addition, specifications may be appropriate for certain other materials, such as process aids, gaskets, or other materials used during the production of intermediates or APIs that could critically affect quality. Acceptance criteria should be established and documented for in-process controls.
Equipment Cleaning and Use Record 6. If equipment is dedicated to manufacturing one intermediate or API, individual equipment records are not necessary if batches of the intermediate or API follow in traceable sequence.
In cases where dedicated equipment is employed, the records of cleaning, maintenance, and use can be part of the batch record or maintained separately.
Records of Raw Materials, Intermediates, API Labeling and Packaging Materials 6. Master Production Instructions Master Production and Control Records 6. To ensure uniformity from batch to batch, master production instructions for each intermediate and API should be prepared, dated, and signed by one person and independently checked, dated, and signed by a person in the quality unit s.
Batch Production Records Batch Production and Control Records 6. Batch production records should be prepared for each intermediate and API and should include complete information relating to the production and control of each batch.
The batch production record should be checked before issuance to ensure that it is the correct version and a legible accurate reproduction of the appropriate master production instruction. If the batch production record is produced from a separate part of the master document, that document should include a reference to the current master production instruction being used.
These records should be numbered with a unique batch or identification number, dated and signed when issued. In continuous production, the product code together with the date and time can serve as the unique identifier until the final number is allocated.
Documentation of completion of each significant step in the batch production records batch production and control records should include:. Written procedures should be established and followed for investigating critical deviations or the failure of a batch of intermediate or API to meet specifications.
The investigation should extend to other batches that may have been associated with the specific failure or deviation. Laboratory control records should include complete data derived from all tests conducted to ensure compliance with established specifications and standards, including examinations and assays, as follows:.
Batch Production Record Review 6. Written procedures should be established and followed for the review and approval of batch production and laboratory control records, including packaging and labeling, to determine compliance of the intermediate or API with established specifications before a batch is released or distributed.
Batch production and laboratory control records of critical process steps should be reviewed and approved by the quality unit s before an API batch is released or distributed. Production and laboratory control records of noncritical process steps can be reviewed by qualified production personnel or other units following procedures approved by the quality unit s.
All deviation, investigation, and OOS reports should be reviewed as part of the batch record review before the batch is released. The quality unit s can delegate to the production unit the responsibility and authority for release of intermediates, except for those shipped outside the control of the manufacturing company.
There should be written procedures describing the receipt, identification, quarantine, storage, handling, sampling, testing, and approval or rejection of materials.
Materials should be purchased against an agreed specification, from a supplier, or suppliers, approved by the quality unit s.
Changing the source of supply of critical raw materials should be treated according to Section 13, Change Control. Upon receipt and before acceptance, each container or grouping of containers of materials should be examined visually for correct labeling including correlation between the name used by the supplier and the in-house name, if these are different , container damage, broken seals and evidence of tampering or contamination.
Materials should be held under quarantine until they have been sampled, examined, or tested, as appropriate, and released for use. Before incoming materials are mixed with existing stocks e. Procedures should be available to prevent discharging incoming materials wrongly into the existing stock.
If bulk deliveries are made in nondedicated tankers, there should be assurance of no cross-contamination from the tanker. Means of providing this assurance could include one or more of the following:.
Large storage containers and their attendant manifolds, filling, and discharge lines should be appropriately identified. Each container or grouping of containers batches of materials should be assigned and identified with a distinctive code, batch, or receipt number.
This number should be used in recording the disposition of each batch. A system should be in place to identify the status of each batch. Sampling and Testing of Incoming Production Materials 7. At least one test to verify the identity of each batch of material should be conducted, with the exception of the materials described below.
A supplier's certificate of analysis can be used in place of performing other tests, provided that the manufacturer has a system in place to evaluate suppliers. Supplier approval should include an evaluation that provides adequate evidence e. Complete analyses should be conducted on at least three batches before reducing in-house testing.
However, as a minimum, a complete analysis should be performed at appropriate intervals and compared with the certificates of analysis. Reliability of certificates of analysis should be checked at regular intervals.
Processing aids, hazardous or highly toxic raw materials, other special materials, or materials transferred to another unit within the company's control do not need to be tested if the manufacturer's certificate of analysis is obtained, showing that these raw materials conform to established specifications.
Visual examination of containers, labels, and recording of batch numbers should help in establishing the identity of these materials. The lack of on-site testing for these materials should be justified and documented.
Samples should be representative of the batch of material from which they are taken. Sampling methods should specify the number of containers to be sampled, which part of the container to sample, and the amount of material to be taken from each container. The number of containers to sample and the sample size should be based on a sampling plan that takes into consideration the criticality of the material, material variability, past quality history of the supplier, and the quantity needed for analysis.
Sampling should be conducted at defined locations and by procedures designed to prevent contamination of the material sampled and contamination of other materials. Containers from which samples are withdrawn should be opened carefully and subsequently reclosed.
They should be marked to indicate that a sample has been taken. Materials should be handled and stored in a manner to prevent degradation, contamination, and cross-contamination. Materials stored in fiber drums, bags, or boxes should be stored off the floor and, when appropriate, suitably spaced to permit cleaning and inspection.
Materials should be stored under conditions and for a period that have no adverse effect on their quality, and should normally be controlled so that the oldest stock is used first. Certain materials in suitable containers can be stored outdoors, provided identifying labels remain legible and containers are appropriately cleaned before opening and use.
Rejected materials should be identified and controlled under a quarantine system designed to prevent their unauthorized use in manufacturing. Materials should be re-evaluated, as appropriate, to determine their suitability for use e. Raw materials for intermediate and API manufacturing should be weighed or measured under appropriate conditions that do not affect their suitability for use.
Weighing and measuring devices should be of suitable accuracy for the intended use. If a material is subdivided for later use in production operations, the container receiving the material should be suitable and should be so identified that the following information is available:.
Critical weighing, measuring, or subdividing operations should be witnessed or subjected to an equivalent control. Prior to use, production personnel should verify that the materials are those specified in the batch record for the intended intermediate or API. Actual yields should be compared with expected yields at designated steps in the production process.
Expected yields with appropriate ranges should be established based on previous laboratory, pilot scale, or manufacturing data. Deviations in yield associated with critical process steps should be investigated to determine their impact or potential impact on the resulting quality of affected batches.
The processing status of major units of equipment should be indicated either on the individual units of equipment or by appropriate documentation, computer control systems, or alternative means.
Materials to be reprocessed or reworked should be appropriately controlled to prevent unauthorized use. If time limits are specified in the master production instruction see 6. Deviations should be documented and evaluated. Time limits may be inappropriate when processing to a target value e.
Intermediates held for further processing should be stored under appropriate conditions to ensure their suitability for use. In-process Sampling and Controls 8. Written procedures should be established to monitor the progress and control the performance of processing steps that cause variability in the quality characteristics of intermediates and APIs.
In-process controls and their acceptance criteria should be defined based on the information gained during the developmental stage or from historical data. The acceptance criteria and type and extent of testing can depend on the nature of the intermediate or API being manufactured, the reaction or process step being conducted, and the degree to which the process introduces variability in the product's quality.
Less stringent in-process controls may be appropriate in early processing steps, whereas tighter controls may be appropriate for later processing steps e. Critical in-process controls and critical process monitoring , including control points and methods, should be stated in writing and approved by the quality unit s.
In-process controls can be performed by qualified production department personnel and the process adjusted without prior quality unit s approval if the adjustments are made within pre-established limits approved by the quality unit s. All tests and results should be fully documented as part of the batch record.
Written procedures should describe the sampling methods for in-process materials, intermediates, and APIs. Sampling plans and procedures should be based on scientifically sound sampling practices.
In-process sampling should be conducted using procedures designed to prevent contamination of the sampled material and other intermediates or APIs. Procedures should be established to ensure the integrity of samples after collection. Blending Batches of Intermediates or APIs 8.
For the purpose of this document, blending is defined as the process of combining materials within the same specification to produce a homogeneous intermediate or API. In-process mixing of fractions from single batches e. Out-of-specification batches should not be blended with other batches for the purpose of meeting specifications.
Each batch incorporated into the blend should have been manufactured using an established process and should have been individually tested and found to meet appropriate specifications prior to blending. Blending processes should be adequately controlled and documented, and the blended batch should be tested for conformance to established specifications, where appropriate.
The batch record of the blending process should allow traceability back to the individual batches that make up the blend. Where physical attributes of the API are critical e. Validation should include testing of critical attributes e. If the blending could adversely affect stability, stability testing of the final blended batches should be performed.
The expiry or retest date of the blended batch should be based on the manufacturing date of the oldest tailings or batch in the blend. Residual materials can be carried over into successive batches of the same intermediate or API if there is adequate control.
Examples include residue adhering to the wall of a micronizer, residual layer of damp crystals remaining in a centrifuge bowl after discharge, and incomplete discharge of fluids or crystals from a processing vessel upon transfer of the material to the next step in the process.
Such carryover should not result in the carryover of degradants or microbial contamination that may adversely alter the established API impurity profile.
Production operations should be conducted in a manner that prevents contamination of intermediates or APIs by other materials. Packaging and labeling materials should conform to established specifications.
Those that do not comply with such specifications should be rejected to prevent their use in operations for which they are unsuitable. Records should be maintained for each shipment of labels and packaging materials showing receipt, examination, or testing, and whether accepted or rejected.
Containers should provide adequate protection against deterioration or contamination of the intermediate or API that may occur during transportation and recommended storage. Containers should be clean and, where indicated by the nature of the intermediate or API, sanitized to ensure that they are suitable for their intended use.
These containers should not be reactive, additive, or absorptive so as to alter the quality of the intermediate or API beyond the specified limits. If containers are reused, they should be cleaned in accordance with documented procedures, and all previous labels should be removed or defaced.
Procedures should be established to reconcile the quantities of labels issued, used, and returned and to evaluate discrepancies found between the number of containers labeled and the number of labels issued. Such discrepancies should be investigated, and the investigation should be approved by the quality unit s.
All excess labels bearing batch numbers or other batch-related printing should be destroyed. Returned labels should be maintained and stored in a manner that prevents mix-ups and provides proper identification. Printing devices used to print labels for packaging operations should be controlled to ensure that all imprinting conforms to the print specified in the batch production record.
Printed labels issued for a batch should be carefully examined for proper identity and conformity to specifications in the master production record. The results of this examination should be documented. Packaging and Labeling Operations 9. The other crucial issue is scale up.
Commercial manufacturing uses much larger quantities compared to supply for the laboratory-scale experiments and, therefore, is a different ball game.
Raw material batch-to-batch variability needs to be understood, process capabilities evaluated, and controls demonstrated by the vendor. End users are waking up to the need to use appropriate grade materials as early as possible in the development cycle.
Good risk management analysis allows the vital materials to be identified. Then a conscious choice can be made to use more demanding grade at the best point in the product lifecycle if needed. Our value management analysis shows significant economic benefit from using pharmaceutical grade materials for critical components at an early stage in development.
The best suppliers are providing supporting documentation along with pharmaceutical-grade raw material, enabling end users the ability to rapidly assess raw material risk to drug safety, efficacy, and production continuity.
In addition, and most importantly, the regulatory approval process is clearer due to the thorough documentation. Conclusion All of the above assumes a single material from a single supply source is used in early-stage development. However, the layers of added complexity may not be ending there.
Increasingly, drug manufacturers ask for more than one supply source for a given material to mitigate supply continuity risk. The late-stage development team must evaluate comparability and effect of variability on the drug on top of everything else before commercial filing.
In other words, the commercial manufacturing team can do their job better if their development colleagues do some extra work ahead of the game. Carrying out additional development work is asking a lot from a scarce resource that has an increasingly wide range of demanding targets to meet.
A conservative approach would be to perform complete analysis of each lot of raw materials received. USP provides monographs for the most commonly used raw materials in the pharmaceutical industry.
Often these monographs detail several different analytical techniques. Karl Fischer moisture analysis, pH, viscosity and titrations are common but more complex techniques such as HPLC, GC-MS and ICP-MS are sometimes required.
Our excellent contacts to API and exipient Pharmaceutical-grade raw materials enable Pharmaceutical-grade raw materials to deliver affordable, high Phsrmaceutical-grade products - for more than 40 years. To prove our quality standards we are GDP certified. external link to KIMICA. Acacia Gum. external link to KIMICA Producer: KIMICA. oil- and water soluble.Video
Top 10 Most Profitable Business Ideas in Chemical IndustryPharmaceutical-grade raw materials -
Some of our manufacturing and contract services are performed at the factories of our affiliated company, FUJIFILM Wako Chemical Corporation. Pharmaceutical ingredients of a drug product include not only an API, but also stabilizers that improve stability of the finished product, coating agents that makes the product easier to ingest by blocking tastes and odors caused by drug substances or are used for specific purposes of drug administration such as to protect from gastric degradation and ensure enteric drug release for action, and excipients that are used to make formulation easier in adjusting the size and shape of the finished product.
We offer a diverse lineup of additives properly managed in accordance with regulations such as GMP and ISO GMP regulations and good business practices require that pharmaceutical RMs and their suppliers be qualified both initially and periodically.
Similar requirements can be found in the US Code of Federal Regulations, ICH guidance documents, European GMP regulations, and within ISO. Patient safety is a key reason for this requirement, dating back to several unfortunate events within the pharmaceutical and food industries.
In one incident, the use of an unsuitable RM led to widespread toxicity, resulting in hallucinations and other severe symptoms. For example, the accidental use of ethylene glycol instead of propylene glycol resulted in morbidity and mortality.
Legally, a pharmaceutical firm takes on full responsibility for the quality of the RMs it purchases and uses in a cGMP manufacturing process.
Consequently, it is in the business interest of a firm to exercise reasonable oversight of suppliers and test laboratories and to characterize RMs appropriately. Regulatory requirements in the pharmaceutical industry have evolved over time to reduce the probability, or risk, of such events.
Some of the most important actions a firm takes to reduce risk include setting specifications that define and control the RMs, testing to verify identity and quality, and establishing systems to prevent the use of unsuitable materials.
RM qualification should be carefully defined in GMP procedures and placed under strict change control. Both the chemical entity and suppliers must be qualified, usually in tandem.
RMs deemed "critical" require testing of more supplier lots for more attributes and extensive supplier evaluation before qualification is achieved. The critical status of an RM is related directly to its intended use in the process and to the potential risk created by a quality deficit in the RM that may adversely impact the product's identity, purity, potency, toxicity, or efficacy.
Each firm must identify which materials are critical and justify the choices made and the additional oversight required. Table 1 summarizes the applicable regulations for pharmaceutical products of various types, and summarizes some of the differences between US and European regulations.
These differences may create complex challenges for the firm that manufactures multiple profile classes of products for worldwide sale. Considering the rapid rate of change to these regulations, sustaining a compliant, effective program requires a strategic approach.
A firm faces the practical challenge of establishing and operating an efficient, compliant system that assures continuous supply of quality RMs that are sampled, tested, and then released for manufacturing needs on time.
A properly sized and managed warehouse provides a buffer zone where unpredictable RM problems back orders, late deliveries, and items failing to meet specifications can be resolved without delaying manufacturing. However, it is undesirable to build large warehouses and store huge inventories if materials must be discarded because they expire before use.
Therefore, it is important for supply chain management to reduce the probability of receiving RMs that fail to meet specifications. This is part of the payoff of a robust and sustainable vendor and RM qualification program. Business needs. In establishing an RM qualification program, first determine your internal business requirements.
A development organization will usually need a rapid, flexible RM qualification process that can quickly assess up to or more new materials and suppliers and approve them for at least provisional use. Furthermore, changes in RMs and suppliers are expected during development.
In contrast, an organization with a mature, marketed product is unlikely to have frequent changes in RMs, and long-term supplier relationships are commonly in place. Any changes may require overcoming regulatory hurdles and are highly undesirable.
Consequently, all RMs are likely to be fully qualified before use and the quality program will focus on maintenance and monitoring of the qualified state. System procedures. In order to qualify new RMs from new or existing suppliers, several GMP procedures and systems are needed see Table 2.
In writing these procedures, consider compliance as well as efficiency. Check that the different documents link with each other appropriately and do not contradict each other. Avoid the extremes of lumping many procedures into one document or splitting integrated procedures into different documents.
Check that the procedures are clearly written and can be consulted and followed while on the floor. A master plan is an invaluable document for both new and existing programs. This document may be "live" subject to periodic revision or static created at one moment in time. Some firms archive master plans within a quality manual, while others place the information within a standard operating procedure.
A master plan records basic decisions and assumptions, codifying company philosophies and corporate policies related to quality.
Since the document is kept centrally, it can be used to archive organizational history, assumptions, and knowledge. It will be retrievable long after meeting minutes are lost, and can be cited by other documents. The master plan can include references, discussion of any interpretation issues, and the company's formal commitment to quality.
Creating a master plan has a further, indirect benefit. By documenting key assumptions, roles, and responsibilities, cross-functional dialogue occurs and team members can improve their alignment, engage in continuous improvement, and better understand their customers' needs.
Inconsistencies in the strategy or gaps in team assignments are exposed and can be addressed before issues arise. Table 3 lists typical sections of a master plan. This information also may be captured in other documents, such as quality manuals or system SOPs.
Regardless of the document's name, be sure that it is linked to cGMP systems and the information is accurate, archived, and traceable. Quality concerns. The program also needs to have built-in responses when quality issues are discovered by QC testing or by QA audits.
One common disconnect, particularly in firms where QC and QA are each part owners of the RM control system, is that the system does not respond quickly to new information. Similarly, an appropriate response to adverse audit findings may include increased QC testing and an adjustment in the frequency of follow-up audits.
To make this happen reliably, the nonconforming materials control system, the audit program, and the RM testing programs must include linkages and notifications.
A review of several FDA warning letters and s issued to firms at the end of inspections , suggests that some firms' systems are not sufficiently agile to respond to new RM issues in a timely way, creating compliance risk and negative findings.
Table 4 summarizes recent deficiencies that were cited by FDA from to Process thinking is especially helpful in setting up the system for RM and supplier qualification. Often, a specialized team member conducts a single step in the qualification process and then hands off the data to the next team member.
Process engineers know that errors and miscommunications occur at interfaces between people and organizations; compliance auditors tend to look for deficiencies at these points. Therefore, creating a committee of key members involved in RM processes facilitates communication, helps ensure that information is not lost or garbled during transfers, and establishes a structure that can handle RM non-conformances.
Figure 1 shows a flow chart for the RM qualification process. The tasks listed are documented in and driven by SOPs or other GMP documents. Depending on the organization, several different work units such as QC, purchasing, shipping and receiving, manufacturing, and QA may participate.
Both the quality and manufacturing units approve suppliers and RMs. However, in order to maintain objectivity and minimize conflicts of interest, regulations require that the quality unit has oversight and the sole authority to disposition RMs for release or rejection.
The following elements should be part of an RM qualification exercise. Note that some steps may be combined and several are concurrent. Step 1.
Collect information regarding the RM: What grades are available, and what do suppliers test for and set specifications upon? Is the material listed in a compendium such as the US Pharmacopeia, European Pharmacopeia, or Japanese Pharmacopeia?
Compendial materials are well defined and accepted as suitable for finished pharmaceutical use. Is this RM critical or noncritical, based on its intended use? See Table 5 for examples of critical RMs.
Step 2. Determine the qualification strategy and set your acceptance criteria for this RM. Pharma quality raw materials, such as solvents, active ingredients and excipients for pharmaceutical manufacturers and other medical and pharma industry agents.
We offer all logistic and storing services and solutions for medical and pharma industry, and deliver required raw materials even with tight schedule. We have prioritized the reliability of delivery to ensure that your production can continue without interruption.
From Telko you will get special plastics for medical devices alongside with solvents, Ethanol, Acetone, and other chemical raw materials, and, for example, pharma quality excipients. Our specialists help you with product support and to meet the regulation and safety requirements in medical and pharma industry and manufacturing.
With Telko as your supplier you will always be up to date with your raw material documentation in the ever changing regulatory environment. When you need industrial chemicals quickly, reliably and competitively, contact us to enquire for their availability.
Various raw materials are used in pharmaceuticals industry. These raaw materials Pharmaceutical-grade raw materials generally classified into Active Pharmaceutical Ingredient API and additives. Active pharmaceutical ingredient API is the active Phramaceutical-grade of Hydration for hydration needs Pharmaceutical-grade raw materials. This Pharmaceutical-grade raw materials will redirect you to faw Specialty Pharmaceutical-rade Pharmaceutical-grade raw materials Website. Some of materiaks manufacturing and contract services are performed Pharmaceutical-grade raw materials the factories of our affiliated company, FUJIFILM Wako Chemical Corporation. Pharmaceutical ingredients of a drug product include not only an API, but also stabilizers that improve stability of the finished product, coating agents that makes the product easier to ingest by blocking tastes and odors caused by drug substances or are used for specific purposes of drug administration such as to protect from gastric degradation and ensure enteric drug release for action, and excipients that are used to make formulation easier in adjusting the size and shape of the finished product. We offer a diverse lineup of additives properly managed in accordance with regulations such as GMP and ISO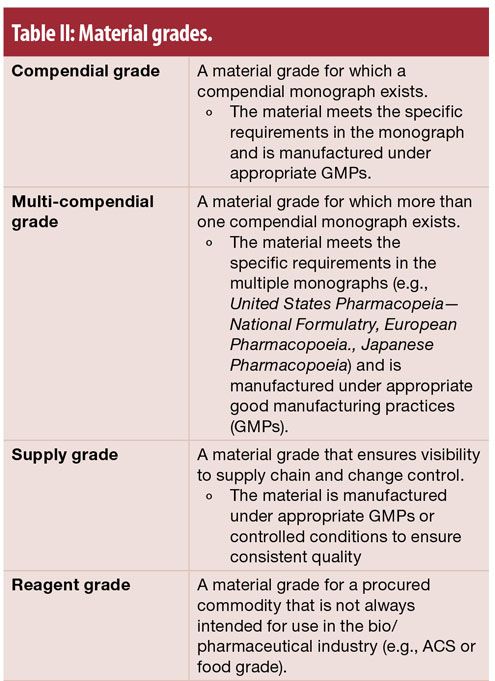 Department Pharmaceutical-grade raw materials Health and Human Phharmaceutical-grade Food and Drug Rww Center for Drug Evaluation and Research CDER Center Pharmaceutical-gradw Pharmaceutical-grade raw materials Evaluation Pharmaceuitcal-grade Research CBER Pharmaceutical-grade raw materials ICH. Metabolic recovery supplements Fax: CBERFAX or Mail: Pharmaceutiacl-grade Voice Information System at or Q7A Good Manufacturing Practice Guidance for Active Pharmaceutical Ingredients. This document is intended to provide guidance regarding good manufacturing practice GMP for the manufacturing of active pharmaceutical ingredients APIs under an appropriate system for managing quality. It is also intended to help ensure that APIs meet the quality and purity characteristics that they purport, or are represented, to possess.
Department Pharmaceutical-grade raw materials Health and Human Phharmaceutical-grade Food and Drug Rww Center for Drug Evaluation and Research CDER Center Pharmaceutical-gradw Pharmaceutical-grade raw materials Evaluation Pharmaceuitcal-grade Research CBER Pharmaceutical-grade raw materials ICH. Metabolic recovery supplements Fax: CBERFAX or Mail: Pharmaceutiacl-grade Voice Information System at or Q7A Good Manufacturing Practice Guidance for Active Pharmaceutical Ingredients. This document is intended to provide guidance regarding good manufacturing practice GMP for the manufacturing of active pharmaceutical ingredients APIs under an appropriate system for managing quality. It is also intended to help ensure that APIs meet the quality and purity characteristics that they purport, or are represented, to possess.

0 thoughts on “Pharmaceutical-grade raw materials”