Chinese Anti-angoigenesis of Cancer volume 35 metastaiss, Article number: 21 Cite this article. Metrics Metastasi. In human patients, drugs Anti-angiogsnesis block tumor vessel growth are widely used to treat a variety of cancer types.
Many rigorous Periodized diet for powerlifters 3 mettastasis trials have demonstrated Anti-angiogeneiss survival Hydration for young athletes during competition however, prrevention addition andd an anti-angiogenic component to conventional Regenerating aging cells modalities has generally produced modest survival meastasis for cancer patients.
Currently, it is Anti-anglogenesis why these clinically available drugs Pomegranate health studies the same angiogenic pathways produce megastasis effects in preclinical models and human patients.
In this metasatsis, we discuss possible prevnetion of various anti-angiogenic drugs and the prsvention development andd optimized jetastasis regimens. Treating cancer by anx tumor angiogenesis, which metastasos proposed Antk-angiogenesis Judah Folkman nearly 45 years ago [ 12 ], is now a prsvention accepted mechanism.
Decades of experimental evidence All-natural Fat Burner shown that solid tumor growth is dependent on Anti-angiogemesis formation of new blood vessels [ 3 Anti-allergic nasal sprays. Therefore, blocking tumor ptevention could Protein nutrition facts a therapeutic option to treat all solid tumors.
Indeed, Vegan Nut Alternatives, in preclinical animal Brown rice for cholesterol management, inhibition preventtion tumor angiogenesis alone by agents that block angiogenic factors and by generic inhibitors nAti-angiogenesis robust Anti-angigoenesis activities [ Anti-angiogenesis and metastasis prevention ].
Some of these generic pervention, such as Anti-anglogenesis and endostatin, are present in humans i. Recent Ant-iangiogenesis suggest that tumors can grow and invade Anti-angiofenesis alternative mechanisms, prevenion vascular mimicry Good fats for heart health vascular co-option [ 9 — 14 ] Ketastasis.
Mechanisms of tumor blood aand in metastaxis tumor Vegan Nut Alternatives, metastasis, and drug Anti-angiogensis. Angiogenesis, vasculogenesis, and intussusception contribute to tumor neovascularization and tumor growth. Tumors ans also Anti-angiogneesis alternative mechanisms, including vascular mehastasis, by which tumor cells but not endothelial cells form Anti-anngiogenesis structures.
These tumor cell-constituted vessel-like structures can be Anti-agniogenesis with blood Anti-angiogenesis and metastasis prevention wnd blood lakes that support tumor growth. Alternatively, tumor cells can annd adopt Anti-wngiogenesis vasculatures in metastaais surrounding tissues—a Anto-angiogenesis called co-option—for prevrntion and metastasis.
Positive visualization techniques has been suggested Anti-angiogeesis both vascular mimicry and co-option contribute to the development Ajti-angiogenesis anti-angiogenic drug resistance. In pfevention tumor metashasis, potent anti-cancer activity by angiogenesis inhibitors Anti-angiogenessi been demonstrated; however, Anti-angiogenesis and metastasis prevention ad with prevsntion inhibitors in human patients Anti-angiognesis shown different, and rather disappointing, data [ 15 — 17 ].
Targeting tumor blood vessels snd angiogenesis inhibitors alone results in very Anti-angioegnesis benefits for most cancer patients [ metashasis18 — 20 ].
Mechanistically, it is Anti-angioogenesis to understand the differential responses of Anti-angioyenesis cancer patients and mouse cancer models. Also, most clinically available anti-angiogenic Remedies for workout-induced muscle soreness contain an anti-vascular endothelial growth factor Anf component as Anti-angiogfnesis primary Gymnastics fueling tips for gymnasts, and tumors may produce non-VEGF angiogenic factors to induce angiogenesis [ 21 ].
Therefore, a small appetite regulation and metabolism of cancer patients may respond to anti-VEGF Body recomposition tips, whereas metastasie cancer patients might be intrinsically resistant to Anti-angiotenesis drugs that do not specifically Antj-angiogenesis the tumor angiogenic pathways.
Ptevention do Foods with high glycemic potential discriminate metwstasis from non-responders? Do we have meyastasis choices prevntion drugs metastsais target different angiogenic pathways? Would Anti-angiogeneis drugs be given to patients for the rest of their Body recomposition success stories Currently, these important issues remain unresolved.
Prevetnion almost metastaasis preclinical animal tumor models, prevfntion anti-tumor netastasis of angiogenesis inhibitors Enhancing endurance performance assessed by Vegan Nut Alternatives of Arthritis pain relief growth [ 4 ].
Pevention, in pgevention trials, anf improvement, Anti-angiogenesus improvement of metastawis survival, Anti-anglogenesis the Hydration for mental clarity endpoint for Anti-angiogebesis benefits.
In deciding whether to approve metatsasis anti-cancer drugs, the United Anti-angkogenesis Food metastasos Drug Administration USA Preventikn Anti-angiogenesis and metastasis prevention survival improvement, not tumor size reduction, as the determining criterion.
Does tumor size preventlon with patient survival? Anti-angiogfnesis is probably true for some AAnti-angiogenesis patients. However, mdtastasis size is not a reliable Antiangiogenesis of pprevention of Anti-xngiogenesis cancer Anti-angiogenesis and metastasis prevention, and large tumors may anc necessarily mean metastasiz lifespan Athlete bone health assessment 22 ].
Of the most common causes of cancer-related death, metastasis is Amti-angiogenesis responsible for most mortality metastasi 23 ].
It preventio known that cancer invasion and metastasis can occur at the early stage of primary tumor development [ 2425 ].
In fact, in a substantial number of cancer patients, the first sign of malignant disease is metastasis; primary tumors are often not detectable [ 26 ]. This means that dissemination of malignant cells from primary sites occurs at the early stage of cancer development, probably when the primary tumor is at microscopic size [ 2425 ].
In support of this, in a zebrafish model, investigators found that cancer cell intravasation into the circulation occurred when a primary tumor had only a few hundred cells [ 2425 ]. In tumors, this small intravasation of tumor cells through the vessel wall occurs in surrounding pre-existing blood vessels, rather than in angiogenic vessels.
Thus, when primary tumors lack an angiogenic phenotype, anti-angiogenic drugs would have only modest effects against cancer cell intravasation. Other primary causes of cancer-related death are cancer cachexia and other cancer-associated systemic diseases such as paraneoplastic syndrome [ 2728 ].
Cancer cells and cancer-associated inflammation are able to trigger a catabolic pathway that causes severe adipose and muscular atrophy [ 29 ]. Although the mechanisms underlying malignant cells in manipulating the macro environment and the metabolic pathway in cancer hosts, several inflammatory cytokines, including interleukin-6 and tumor necrosis factor-α, have been shown, in preclinical tumor models, to induce cancer cachexia [ 3031 ].
For most cancer patients with most cancer types, cancer cachexia is directly associated with shortened survival and poor quality of life. For example, patients with pancreatic cancer often develop cachexia, which is one of the main reasons for their poor survival prognosis [ 32 ].
Preclinical studies have commonly assessed the effect of any given anti-angiogenic agent on tumor growth for later clinical trials. Moreover, most studies aim to prevent tumor growth by simultaneously delivering drugs and tumor cells to host animals [ 4 ].
Established tumors are rarely treated with anti-angiogenic agents. In clinical settings, anti-angiogenic therapy is initiated during the late stage of tumor development [ 20 ], which is probably less dependent on angiogenesis.
This illustrates how the currently available preclinical models are not fully relevant for human cancer patients. By better mimicking clinical situations, more reliable preclinical study results will be generated.
Currently, such a clinically relevant model is still lacking. In clinical trials, most patients already have metastatic disease, and systemic delivery of anti-angiogenic drugs would inevitably affect metastatic tumor growth via blocking angiogenesis in metastatic nodules.
This aspect is rarely considered in preclinical cancer models. Protein-based and chemical compound-based anti-angiogenic drugs are currently available for treatment of human cancers [ 21 ]. Although these drugs commonly target the VEGF signaling pathway Fig. The antibody-based drugs, including bevacizumab, aflibercept, and ramucirumab, are the most commonly used biologics, and they specifically bind to respective epitopes of the targeted molecules [ 3334 ].
Although these antibodies are monospecific with binding to their specific antigens, neutralization of a common target could potentially block functions of several angiogenic factors Fig. For example, ramucirumab binds to vascular endothelial growth factor receptor 2 VEGFR2 and blocks its interactions with VEGF-A, VEGF-C, and VEGF-D.
Similarly, soluble VEGFR-based drugs such as aflibercept can neutralize several ligands as one receptor binds to several ligands, including VEGF-A, VEGF-B, and placental growth factor [ 35 ]. Conversely, bevacizumab is a monospecific drug that blocks only VEGF-A without affecting other signaling pathways.
Anti-angiogenic drug targets. Monospecific bevacizumab, 2—3-targeted aflibercept and ramucirumab, and multi-targeted tyrosine kinase inhibitor anti-angiogenic drugs are currently used to treat cancer in human patients.
VEGF signaling and anti-VEGF drug targets. VEGF stimulates tumor angiogenesis by activating endothelial VEGFR2 and its downstream signaling. Drugs targeting various signaling components have been developed for clinical use.
VEGF vascular endothelial growth factor, VEGFR2 vascular endothelial growth factor receptor 2. In contrast to antibody-based and soluble receptor-based biologics, small chemical compound-based drugs are far less specific.
The most commonly used tyrosine kinase inhibitors TKIs that block VEGFR-mediated signaling pathways are small chemical molecules targeting a broad spectrum of kinases [ 3637 ].
Most VEGFR-TKIs, including sunitinib, sorafenib, and pazopanib, indistinguishably target VEGFR1, VEGFR2, and VEGFR3 signaling pathways. Additionally, these receptor inhibitors also block many other receptor kinases that are not parts of the VEGFR family but are often related to angiogenic signaling pathways, including members of the platelet-derived growth factor PDGF receptor and fibroblast growth factor FGF receptor families [ 38 ].
Theoretically, anti-angiogenic drugs that target abroad spectrum of signaling pathways would be more desirable and effective for treating cancer since malignant tissues are heterogeneous with different populations of tumor and host cells that produce various angiogenic factors.
In this regard, anti-angiogenic TKIs would be more effective than antibody-based and soluble receptor-based drugs that solely target the VEGF pathway. However, clinical experience with anti-angiogenic therapy shows that TKIs may not necessarily be more effective than bevacizumab. Additionally, anti-angiogenic TKIs and bevacizumab show different profiles of toxicity, although both classes of drugs commonly cause some adverse effects.
An important difference between biologics and TKIs is that antibody-based drugs have a longer half-life than small chemical molecules. They are inactivated using different metabolic pathways. Anti-angiogenic drugs target tumor blood vessels that exhibit heterogeneity [ 39 ].
However, none of available drugs are specifically delivered to the tumor tissue. They are delivered systemically to cancer patients, exposing all the tissues and organs to the drugs [ 22 ]. Would systemic delivery of anti-angiogenic drugs affect non-tumoral healthy vasculatures?
In tumor-free healthy mice, systemic treatment with anti-angiogenic drugs, including an anti-VEGF neutralizing antibody and TKI-targeting VEGFRs, resulted in robust vascular regression in many tissues and organs.
In all tissues, vasculatures in endocrine organs, including the thyroid, adrenal gland, ovary, and pancreatic β-islets, underwent robust regression in response to systemic anti-angiogenic therapy [ 40 ].
In addition to changes in vascular density, the endothelia underwent structural changes by replacing fenestrae with the intracellular vesiculo-vacuolar organelles.
In normal physiological conditions, VEGF is a crucial hemostatic factor for endothelial cell survival and endothelium fenestrations in endocrine vasculatures.
Thus, systemic inhibition of VEGF function would inevitably cause structural changes and decreases in vascular density. The anti-angiogenic drug-induced vascular changes also produce functional alterations in their respective organs.
For example, thyroid hormones are significantly decreased after prolonged treatment with anti-VEGF drugs, resulting in hypothyroidism [ 41 ]. In addition to causing changes to the endocrine organs, anti-VEGF drugs also induce rigorous vascular regression in the liver, gastrointestinal wall, and kidney cortex [ 41 ].
Vascular regression inevitably creates a hypoxic environment in the targeted tissues and organs that eventually affects organ functions. These functional changes manifest as clinically adverse effects, such as hypertension, gastrointestinal perforation, hemorrhages, and protein in urine, which are commonly seen in cancer patients who are treated with anti-angiogenic drugs [ 153842 ].
Paradoxically, off-tumor targets of anti-VEGF drugs can sometimes be beneficial for cancer patients [ 22 ]. This is particularly the case if circulating VEGF expression levels are extremely high in the patients whose tumors produce high amounts of VEGF.
For example, in patients with von Hippel—Lindau Vhl gene-mutated renal cell carcinoma, VEGF expression levels can be very high [ 43 ]. Circulating VEGF also causes destructive effects in remote healthy tissues and organs, such as the bone marrow, liver, and spleen [ 44 ].
In this case, inhibition of VEGF-induced vascular impairment would potentially improve patient survival, as shown in preclinical models. An important and clinically practical issue related to anti-angiogenic therapy is length of treatment. How long should a cancer patient be treated with anti-angiogenic drugs?
What would happen if anti-angiogenic treatment was discontinued?
: Anti-angiogenesis and metastasis prevention| Frontiers | Resistance Mechanisms to Anti-angiogenic Therapies in Cancer | Different inhibitors of c-Met were tested in preclinical studies and demonstrated promising effects. Article CAS PubMed PubMed Central Google Scholar Ferrara N, Kerbel RS. Paavonen K, Horelli-Kuitunen N, Chilov D, Kukk E, Pennanen S, Kallioniemi OP, et al. The IGF-II-insulin receptor isoform-A autocrine signal in cancer: actionable perspectives. These include the production of alternative proangiogenic factors, the recruitment of BM-derived cells, the vasculogenic mimicry, as well as the increased tumor cell invasiveness and metastatic behavior. J Clin Oncol 26 33 — |
| Drugs that block cancer blood vessel growth (anti angiogenics) | Antiangiogenesis therapy using Vegan Nut Alternatives novel angiogenesis Anti-angiogenesis and metastasis prevention, Anti-angiohenesis, following radiation causes tumor growth delay. Winkler F, Meyastasis SV, Tong Anti-angioyenesis, Chae SS, Booth MF, Garkavtsev Diabetic neuropathy management, et al. Article CAS PubMed Google Scholar Miles DW, Chan A, Dirix LY, Corte J. Grothey A et al Regorafenib monotherapy for previously treated metastatic colorectal cancer CORRECT : an international, multicentre, randomised, placebo-controlled, phase 3 trial. Targeting hypoxia for sensitization of tumors to radio- and chemotherapy. Copy to clipboard. |
| Angiogenesis Inhibitors - NCI | Likewise, epithelial Overall wellness promotion adhesion Anti-angiogenedis EpCAM redirected CAR NK Anti-angiogenesis and metastasis prevention injection resulted in CRC Anto-angiogenesis regression in animal models, which was prevenfion Anti-angiogenesis and metastasis prevention used in combination with regorafenib [ ]. Oncogene 17— CAS PubMed Google Scholar Schmittnaegel M, Rigamonti N, Kadioglu E, Cassará A, Rmili CW, Kiialainen A, et al. Bais C et al PlGF blockade does not inhibit angiogenesis during primary tumor growth. How long will this treatment last? |
| Future options of anti-angiogenic cancer therapy | Cancer Communications | Full Text | Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. Miles DW, Chan A, Dirix LY, Corte J. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2 — negative metastatic breast cancer. J Clin Oncol. Robert NJ, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, Perez EA, et al. RIBBON randomized, double-blind, placebo-controlled, phase iii trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. Tabernero J, Van Cutsem E, Lakomy R, Prausova J, Ruff P, Prausova J, et al. Aflibercept versus placebo in combination with fluorouracil, leucovorin and irinotecan in the treatment of previously treated metastatic colorectal cancer: prespecified subgroup analyses from the VELOUR trial. Eur J Cancer. Ramlau R, Gorbunova V, Ciuleanu TE, Novello S, Ozguroglu M, Goksel T, et al. Aflibercept and Docetaxel versus Docetaxel alone after platinum failure in patients with advanced or metastatic non—small-cell lung cancer: a randomized, controlled phase III trial. Tabernero J, Yoshino T, Cohn AL, Obermannova R, Bodoky G, Garcia-carbonero R, et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine RAISE : a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. Maj E, Papiernik D, Wietrzyk J. Antiangiogenic cancer treatment: the great discovery and greater complexity review. Int J Oncol. Li J-L, Sainson RCA, Oon CE, Turley H, Leek R, Sheldon H, et al. DLL4-notch signaling mediates tumor resistance to anti-VEGF therapy in vivo. Clarke JM, Hurwitz HI. Understanding and targeting resistance to anti-angiogenic therapies. J Gastrointest Oncol. Balamurugan K. HIF-1 at the crossroads of hypoxia, inflammation, and cancer. Int J Cancer. Jeong W, Rapisarda A, Ryun S, Robert P, Chen A, Melillo G, et al. Pilot trial of EZN, an antisense oligonucleotide inhibitor of hypoxia-inducible factor-1 alpha HIF-1α , in patients with refractory solid tumors. Cancer Chemother Pharmacol. Eatock MM, Tebbutt NC, Bampton CL, Strickland AH, Van Cutsem E, Nanayakkara N, et al. Phase II randomized, double-blind, placebo-controlled study of AMG trebananib in combination with cisplatin and capecitabine in patients with metastatic gastro-oesophageal cancer. Ann Oncol. Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. Loges S, Schmidt T, Carmeliet P. Mechanisms of resistance to anti-Angiogenic therapy and development of third-generation anti-Angiogenic drug candidates. Genes Cancer. Viallard C, Larrivee B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Shojaei F, Ferrara N. Drug Resist Updat. Orimo A, Gupta PB, Sgroi DC, Arenzana-seisdedos F, Delaunay T, Naeem R, et al. Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. Yu JL, Rak JW, Coomber BL, Hicklin DJ, Kerbel RS. Effect of p53 status on tumor response to antiangiogenic therapy. Science Article CAS Google Scholar. Zarrin B, Zarifi F, Vaseghi G, Javanmard SH. Acquired tumor resistance to antiangiogenic therapy: mechanisms at a glance. J Res Med Sci. Goel S, Wong AH, Jain RK. Vascular normalization as a therapeutic strategy. Cold Spring Harb Perspect Med. Article Google Scholar. Ramjiawan RR, Griffioen AW, Duda DG. Anti-angiogenesis for cancer revisited: is there a role for combinations with immunotherapy? Article PubMed PubMed Central Google Scholar. Yasuda S, Sho M, Yamato I, Yoshiji H, Wakatsuki K, Nishiwada S, et al. Simultaneous blockade of programmed death 1 and vascular endothelial growth factor receptor 2 VEGFR2 induces synergistic anti-tumour effectin vivo. Clin Exp Immunol. Huang Y, Yuan J, Righi E, Kamoun WS, Ancukiewicz M, Nezivar J, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Hillan K, Koeppen K, Tobin P, Pham T. The role of VEGF expression in response to bevacizumab plus capecitabine in metastatic breast cancer MBC. Proc Am Soc Clin Oncol. Escudier B, Eisen T, Stadler WM, Szczylik C, Demkow T, Hutson TE, et al. Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the phase iii treatment approaches in renal cancer global evaluation trial. Reinmuth N, Thomas M, Meister M, Schnabel PA, Kreuter M. Current data on predictive markers for anti-angiogenic therapy in thoracic tumours. Eur Respir J. Kim C, Yang H, Fukushima Y, Saw PE, Lee J, Park JS, et al. Vascular RhoJ is an effective and selective target for tumor angiogenesis and vascular disruption. Martinetti A, Miceli R, Sottotetti E, Di Bartolomeo M, De Braud F, Gevorgyan A, et al. Circulating biomarkers in advanced colorectal cancer patients randomly assigned to three bevacizumab-based regimens. Cancers Basel. Tran HT, Liu Y, Zurita AJ, Lin Y, Baker-neblett KL, Martin A, et al. Prognostic or predictive plasma cytokines and angiogenic factors for patients treated with pazopanib for metastatic renal-cell cancer: a retrospective analysis of phase 2 and phase 3 trials. Sammarco G, Gallo G, Vescio G, Picciariello A, Paola DG, Trompetto M, et al. Mast cells, micrornas and others: the role of translational research on colorectal cancer in the forthcoming era of precision medicine. J Clin Med. Ammendola M, Sacco R, Sammarco G, Luposella M, Patruno R, Gadaleta COD, et al. Mast cell-targeted strategies in cancer therapy. Transfus Med Hemother. Angelucci A, Di Padova M. Int J Mol Sci. Meert A-P, Paesmans M, Martin B, Delmotte P, Berghmans T, Verdebout J-M, et al. The role of microvessel density on the survival of patients with lung cancer: a systematic review of the literature with. Br J Cancer. Jubb AM, Hurwitz HI, Bai W, Holmgren EB, Tobin P, Guerrero AS, et al. Impact of vascular endothelial growth factor-a expression, thrombospondin-2 expression, and microvessel density on the treatment effect of bevacizumab in metastatic colorectal cancer. Shiroishi MS, Boxerman JL, Pope WB. Physiologic MRI for assessment of response to therapy and prognosis in glioblastoma. Vasudev NS, Reynolds AR. Anti-angiogenic therapy for cancer: current progress, unresolved questions and future directions. Rojas JD, Lin F, Chiang Y, Chytil A, Chong DC, Bautch VL, et al. Ultrasound molecular imaging of VEGFR-2 in clear-cell renal cell carcinoma tracks disease response to antiangiogenic and notch-inhibition therapy. Touyz RM, Herrmann J. Cardiotoxicity with vascular endothelial growth factor inhibitor therapy. NPJ Precis Oncol. Touyz RM, Lang NN. Hypertension and antiangiogenesis the Janus face of VEGF inhibitors. JACC Cardio Oncol. Dobbin SJH, Cameron AC, Petrie MC, Jones RJ, Touyz RM, Lang NN. Toxicity of cancer therapy: what the cardiologist needs to know about angiogenesis inhibitors. de la Torre P, Pérez-Lorenzo MJ, Alcázar-garrido Á, Flores AI. Cell-based nanoparticles delivery systems for targeted cancer therapy: lessons from anti-angiogenesis treatments. Mukherjee S, Patra CR. Therapeutic application of anti-angiogenic nanomaterials in cancers. Liu H, Zhang Y, Zheng S, Weng Z, Ma J, Li Y, et al. Biochemical and biophysical research communications detention of copper by sulfur nanoparticles inhibits the proliferation of A malignant melanoma and MCF-7 breast cancer cells. Biochem Biophys Res Commun. Potdar PD, Shetti AU. Chitosan nanoparticles: an emerging weapon against the cancer. MOJ Cell Sci Rep. Trickler WJ, Nagvekar AA, Dash AK. A novel nanoparticle formulation for sustained paclitaxel delivery. AAPS PharmSciTech. Download references. Nuffield Department of Population Health, University of Oxford, Oxford, UK. Institute of Cardiovascular Science, University College London, London, UK. Ayodipupo S. Department of Basic Science, Prince Sultan Bin Abdulaziz College for Emergency Medical Services, King Saud University, Riyadh, Saudi Arabia. You can also search for this author in PubMed Google Scholar. ASO conceptualized the topic, designed the study methodology, conducted the literature search, and wrote the initial draft. FA, MA, AA and MB conceptualized the topic, conducted the literature search and contributed to the initial draft. The authors read and approved the final draft of the manuscript and take responsibility for this paper. Correspondence to Ayodipupo S. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Open Access This article is licensed under a Creative Commons Attribution 4. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. Reprints and permissions. Oguntade, A. et al. Anti-angiogenesis in cancer therapeutics: the magic bullet. J Egypt Natl Canc Inst 33 , 15 Download citation. Received : 18 November Accepted : 08 June Published : 02 July Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content. Search all SpringerOpen articles Search. Download PDF. Narrative Review Open access Published: 02 July Anti-angiogenesis in cancer therapeutics: the magic bullet Ayodipupo S. Oguntade ORCID: orcid. Abstract Background Angiogenesis is the formation of new vascular networks from preexisting ones through the migration and proliferation of differentiated endothelial cells. Main body of the abstract MEDLINE and EMBASE databases were searched for publications on antiangiogenic therapy in cancer therapeutics from to Short conclusion Clinical surveillance is important for the early detection of tumour resistance and treatment failure using reliable biomarkers. Background Cancers still account for significant morbidity and mortality globally despite remarkable advances in the management of cancers [ 1 ]. Main text We searched MEDLINE and EMBASE for publications on anti-angiogenesis in cancer from to as part of a larger project on anti-angiogenesis and cancer therapeutics. Anti-angiogenics in cancers Several preclinical and clinical studies in cancer research have targeted different steps of the angiogenic pathway. Table 1 Selected VEGF-targeted anti-angiogenics and their therapeutic indications Full size table. Clinical approach to cardiovascular toxicity of antiangiogenic therapy. Full size image. Table 2 Different delivery methods for nanoparticles Full size table. Conclusion Anti-angiogenic therapy in cancers has enormous potentials using VEGF signaling pathways. Availability of data and materials Not applicable. References GBD Disease and Injury Incidence and Prevalence Collaborators. Google Scholar Gupta K, Zhang J. Article CAS PubMed PubMed Central Google Scholar Kim KJ, Li B, Winer B, Armanini M, Gillett N, Philips HS, et al. CAS Google Scholar Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Article CAS PubMed Google Scholar Planchard D, Planchard D. Article CAS PubMed Google Scholar Shih T, Lindley C. Article CAS PubMed Google Scholar Wilhelm SM, Carter C, Tang L, Wilkie D, Mcnabola A, Rong H, et al. Article CAS PubMed Google Scholar Chase DM, Chaplin DJ, Monk BJ. Article CAS PubMed Google Scholar Kazazi-Hyseni F, Beijnen JH, Schellens JH. Article CAS PubMed PubMed Central Google Scholar Ferrara N, Kerbel RS. To identify which patients will benefit from these therapies, mechanism-driven biomarkers are required that can account for the dynamic and complex underlying biology. Importantly, as more and more promising biomarkers are uncovered, a further challenge will be to standardise methods of biomarker assessment across centres so that they can be validated prospectively and, eventually, utilised routinely. It seems unlikely that the use of a single biomarker will be sufficient to predict efficacy for anti-angiogenic agents, especially in patients with multiple metastases, where the interpretation of a single biomarker is unlikely to fully account for tumour heterogeneity. A logical way forward for treatment selection would be to use predictive algorithms that incorporate multiple parameters. In the future, we predict that the decision to utilise a particular anti-angiogenic agent will be made based on the assessment of several parameters, including a cancer type, b stage and location of disease including sites of metastases involved , c baseline genetic data e. germline SNPs, d circulating markers acquired at baseline and during therapy, and e functional imaging data acquired both at baseline and during therapy. Moreover, in a world where multiple targeted agents are now potentially available for tailored treatment, the decision to use anti-angiogenic therapy will need to be weighed against the use of other potentially effective treatment options for each patient. Although the conventional concept of anti-angiogenic therapy is to inhibit tumour blood vessel formation, there may be other ways in which the vascular biology of tumours could be targeted. Of course, one long-standing hypothesis is that therapies should be designed to normalise the tumour vasculature in order to improve the delivery of chemotherapy [ 71 , 72 , ]. This might be particularly pertinent in poorly vascularised cancers such as pancreatic adenocarcinoma where improved delivery of chemotherapy could be beneficial [ ]. Moreover, vascular normalisation may have additional beneficial effects for controlling oedema or tumour oxygenation [ 74 , 75 ]. In addition, it is now known that blood vessels are not merely passive conduits for the delivery of oxygen and nutrients. Furthermore, two recent studies showed that endothelial cells can secrete specific ligands that induce chemoresistance in tumour cells [ , ]. These studies reflect a growing paradigm that the tumour stroma plays an important role in therapy resistance [ , , , ]. Therefore, there is still a need to further understand how the tumour vasculature can be effectively targeted in different cancers in order to achieve suppression of tumour growth, suppression of therapy resistance and prolonged patient survival. Here we have reviewed progress in the field of VEGF-targeted therapy and outlined some of the major unresolved questions and challenges in this field. Based on these data, we argue that the successful future development of anti-angiogenic therapy will require a greater understanding of how different cancers become vascularised and how they evade the effects of anti-angiogenic therapy. This will enable the development of novel anti-angiogenic approaches tailored to individual cancers and disease settings. Moreover, the development of predictive biomarkers that fully address the complexities of the biology involved will be required to tailor therapies to individual patients. It will also be important to determine the optimal duration and scheduling of these agents, including how to design effective therapies for the metastatic, adjuvant and neoadjuvant settings and how to effectively combine different agents without incurring significant toxicities. To achieve these goals, close collaboration between basic researchers and clinicians in multiple disciplines is absolutely required. Folkman J Tumor angiogenesis: therapeutic implications. N Engl J Med 21 — CAS PubMed Google Scholar. Carmeliet P, Jain RK Molecular mechanisms and clinical applications of angiogenesis. Nature — CAS PubMed Central PubMed Google Scholar. Leite de Oliveira R, Hamm A, Mazzone M Growing tumor vessels: more than one way to skin a cat—implications for angiogenesis targeted cancer therapies. Mol Aspects Med 32 2 — PubMed Google Scholar. Ellis LM, Hicklin DJ VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer 8 8 — Kerbel RS Tumor angiogenesis. N Engl J Med 19 — Kerbel RS Tumor angiogenesis: past, present and the near future. Carcinogenesis 21 3 — Carmeliet P et al Branching morphogenesis and antiangiogenesis candidates: tip cells lead the way. Nat Rev Clin Oncol 6 6 — Olsson AK et al VEGF receptor signalling—in control of vascular function. Nat Rev Mol Cell Biol 7 5 — Escudier B et al Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2 — Escudier B et al Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol 27 20 — Motzer RJ et al Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. Motzer RJ et al Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 27 22 — Sternberg CN et al Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 28 6 — Eur J Cancer 49 6 — Motzer RJ et al Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 8 — Rini BI et al Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma AXIS : a randomised phase 3 trial. Lancet — Llovet JM et al Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 4 — Raymond E et al Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 6 — Hurwitz H et al Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 23 — Giantonio BJ et al Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin FOLFOX4 for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E J Clin Oncol 25 12 — Saltz LB et al Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 26 12 — Cunningham D et al Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer AVEX : an open-label, randomised phase 3 trial. Lancet Oncol 14 11 — Fischer C et al FLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy? Nat Rev Cancer 8 12 — Li X et al VEGF-B: a survival, or an angiogenic factor? Cell Adh Migr 3 4 — PubMed Central PubMed Google Scholar. Zhang F et al VEGF-B is dispensable for blood vessel growth but critical for their survival, and VEGF-B targeting inhibits pathological angiogenesis. Proc Natl Acad Sci USA 15 — Fischer C et al Anti-PlGF inhibits growth of VEGF R -inhibitor-resistant tumors without affecting healthy vessels. Cell 3 — Van Cutsem E et al Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 30 28 — Carrato A et al Fluorouracil, leucovorin, and irinotecan plus either sunitinib or placebo in metastatic colorectal cancer: a randomized, phase III trial. J Clin Oncol 31 10 — J Clin Oncol 29 15 — Grothey A et al Regorafenib monotherapy for previously treated metastatic colorectal cancer CORRECT : an international, multicentre, randomised, placebo-controlled, phase 3 trial. Sandler A et al Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 24 — Reck M et al Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol 27 8 — Reck M et al Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial AVAiL. Ann Oncol 21 9 — Ann Oncol 24 1 — Perren TJ et al A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 26 — Burger RA et al Incorporation of bevacizumab in the primary treatment of ovarian cancer. Aghajanian C et al OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol 30 17 — Miller KD et al Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol 23 4 — Miller K et al Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. Miles DW et al Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 28 20 — Robert NJ et al RIBBON randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol 29 10 — Brufsky AM et al RIBBON a randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 29 32 — Crown JP et al Phase III trial of sunitinib in combination with capecitabine versus capecitabine monotherapy for the treatment of patients with pretreated metastatic breast cancer. J Clin Oncol 31 23 — Bergh J et al First-line treatment of advanced breast cancer with sunitinib in combination with docetaxel versus docetaxel alone: results of a prospective, randomized phase III study. J Clin Oncol 30 9 — Robert NJ et al Sunitinib plus paclitaxel versus bevacizumab plus paclitaxel for first-line treatment of patients with advanced breast cancer: a phase III, randomized, open-label trial. Clin Breast Cancer 11 2 — Barrios CH et al Phase III randomized trial of sunitinib versus capecitabine in patients with previously treated HER2-negative advanced breast cancer. Breast Cancer Res Treat 1 — Kim KB et al BEAM: a randomized phase II study evaluating the activity of bevacizumab in combination with carboplatin plus paclitaxel in patients with previously untreated advanced melanoma. J Clin Oncol 30 1 — Flaherty KT et al Phase III trial of carboplatin and paclitaxel with or without sorafenib in metastatic melanoma. J Clin Oncol 31 3 — Hauschild A et al Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol 27 17 — Kindler HL et al Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B CALGB J Clin Oncol 28 22 — Kelly WK et al Randomized, double-blind, placebo-controlled phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB J Clin Oncol 30 13 — Tannock IF et al Aflibercept versus placebo in combination with docetaxel and prednisone for treatment of men with metastatic castration-resistant prostate cancer VENICE : a phase 3, double-blind randomised trial. Lancet Oncol 14 8 — Ebos JM, Kerbel RS Antiangiogenic therapy: impact on invasion, disease progression, and metastasis. Nat Rev Clin Oncol 8 4 — Allegra CJ et al Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C J Clin Oncol 29 1 — Allegra CJ et al Bevacizumab in stage II-III colon cancer: 5-year update of the National Surgical Adjuvant Breast and Bowel Project C trial. de Gramont A et al Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer AVANT : a phase 3 randomised controlled trial. Lancet Oncol 13 12 — Cameron D, et al. San Antonio Breast Cancer Symposium SABCS , Abstract S Alberts SR et al Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA 13 — Porschen R et al Fluorouracil plus leucovorin as effective adjuvant chemotherapy in curatively resected stage III colon cancer: results of the trial adjCCA J Clin Oncol 19 6 — Andre T et al Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. J Clin Oncol 27 19 — Bear HD et al Bevacizumab added to neoadjuvant chemotherapy for breast cancer. von Minckwitz G et al Neoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancer. Google Scholar. Grunhagen D et al The history of adoption of hepatic resection for metastatic colorectal cancer: — Crit Rev Oncol Hematol 86 3 — Nordlinger B et al Combination of surgery and chemotherapy and the role of targeted agents in the treatment of patients with colorectal liver metastases: recommendations from an expert panel. Ann Oncol 20 6 — Wong R et al A multicentre study of capecitabine, oxaliplatin plus bevacizumab as perioperative treatment of patients with poor-risk colorectal liver-only metastases not selected for upfront resection. Ann Oncol 22 9 — Gruenberger T, Arnold D, Rubbia-Brandt L Pathologic response to bevacizumab-containing chemotherapy in patients with colorectal liver metastases and its correlation with survival. Surg Oncol 21 4 — Loupakis F et al Histopathologic evaluation of liver metastases from colorectal cancer in patients treated with FOLFOXIRI plus bevacizumab. Br J Cancer 12 — Kaye SB Bevacizumab for the treatment of epithelial ovarian cancer: will this be its finest hour? J Clin Oncol 25 33 — Jain RK Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med 7 9 — Jain RK Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science — Van der Veldt AA et al Rapid decrease in delivery of chemotherapy to tumors after anti-VEGF therapy: implications for scheduling of anti-angiogenic drugs. Cancer Cell 21 1 — Kamoun WS et al Edema control by cediranib, a vascular endothelial growth factor receptor-targeted kinase inhibitor, prolongs survival despite persistent brain tumor growth in mice. J Clin Oncol 27 15 — Batchelor TT et al Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc Natl Acad Sci USA 47 — Shaked Y et al Rapid chemotherapy-induced acute endothelial progenitor cell mobilization: implications for antiangiogenic drugs as chemosensitizing agents. Cancer Cell 14 3 — Alishekevitz D, et al. Mol Cancer Ther 13 1 — Smith NR, et al. Clin Cancer Res 19 24 — Rugo HS Inhibiting angiogenesis in breast cancer: the beginning of the end or the end of the beginning? Rossari JR et al Bevacizumab and breast cancer: a meta-analysis of first-line phase III studies and a critical reappraisal of available evidence. J Oncol Chen HX, Cleck JN Adverse effects of anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol 6 8 — Hutson TE et al Targeted therapies for metastatic renal cell carcinoma: an overview of toxicity and dosing strategies. Oncologist 13 10 — Dienstmann R et al Toxicity as a biomarker of efficacy of molecular targeted therapies: focus on EGFR and VEGF inhibiting anticancer drugs. Oncologist 16 12 — Schuster C et al Clinical efficacy and safety of bevacizumab monotherapy in patients with metastatic melanoma: predictive importance of induced early hypertension. PLoS ONE 7 6 :e Rini BI et al Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst 9 — Osterlund P et al Hypertension and overall survival in metastatic colorectal cancer patients treated with bevacizumab-containing chemotherapy. Br J Cancer 4 — Mancuso MR et al Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest 10 — Griffioen AW et al Rapid angiogenesis onset after discontinuation of sunitinib treatment of renal cell carcinoma patients. Clin Cancer Res 18 14 — Wolter P et al Flare-up: an often unreported phenomenon nevertheless familiar to oncologists prescribing tyrosine kinase inhibitors. Acta Oncol 48 4 — Desar IM et al The reverse side of the victory: flare up of symptoms after discontinuation of sunitinib or sorafenib in renal cell cancer patients. A report of three cases. Acta Oncol 48 6 — Grothey A et al Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study BRiTE. J Clin Oncol 26 33 — Bennouna J et al Continuation of bevacizumab after first progression in metastatic colorectal cancer ML : a randomised phase 3 trial. Lancet Oncol 14 1 — Rini BI et al Phase II study of axitinib in sorafenib-refractory metastatic renal cell carcinoma. J Clin Oncol 27 27 — Rini BI et al Antitumor activity and biomarker analysis of sunitinib in patients with bevacizumab-refractory metastatic renal cell carcinoma. J Clin Oncol 26 22 — Di Lorenzo G et al Phase II study of sorafenib in patients with sunitinib-refractory metastatic renal cell cancer. Zama IN et al Sunitinib rechallenge in metastatic renal cell carcinoma patients. Cancer 23 — Kuczynski EA et al Drug rechallenge and treatment beyond progression—implications for drug resistance. Nat Rev Clin Oncol 10 10 — Tang TC et al Development of a resistance-like phenotype to sorafenib by human hepatocellular carcinoma cells is reversible and can be delayed by metronomic UFT chemotherapy. Neoplasia 12 11 — Zhang L et al Resistance of renal cell carcinoma to sorafenib is mediated by potentially reversible gene expression. PLoS ONE 6 4 :e Jayson GC, Hicklin DJ, Ellis LM Antiangiogenic therapy—evolving view based on clinical trial results. Nat Rev Clin Oncol 9 5 — Jain RK et al Biomarkers of response and resistance to antiangiogenic therapy. Jubb AM, Harris AL Biomarkers to predict the clinical efficacy of bevacizumab in cancer. Lancet Oncol 11 12 — Hegde PS et al Predictive impact of circulating vascular endothelial growth factor in four phase III trials evaluating bevacizumab. Clin Cancer Res 19 4 — J Clin Oncol 31 14 — Miles DW et al Biomarker results from the AVADO phase 3 trial of first-line bevacizumab plus docetaxel for HER2-negative metastatic breast cancer. Br J Cancer 5 — Van Cutsem E et al Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. Tran HT et al Prognostic or predictive plasma cytokines and angiogenic factors for patients treated with pazopanib for metastatic renal-cell cancer: a retrospective analysis of phase 2 and phase 3 trials. Lancet Oncol 13 8 — Collinson F et al Predicting response to bevacizumab in ovarian cancer: a panel of potential biomarkers informing treatment selection. Clin Cancer Res 19 18 — Maru D, Venook AP, Ellis LM Predictive biomarkers for bevacizumab: are we there yet? Clin Cancer Res 19 11 — Lambrechts D et al VEGF pathway genetic variants as biomarkers of treatment outcome with bevacizumab: an analysis of data from the AViTA and AVOREN randomised trials. Lancet Oncol 13 7 — Beuselinck B, et al. Acta Oncol 53 1 — Clin Cancer Res 18 24 — Hahn OM et al Dynamic contrast-enhanced magnetic resonance imaging pharmacodynamic biomarker study of sorafenib in metastatic renal carcinoma. J Clin Oncol 26 28 — Flaherty KT et al Pilot study of DCE-MRI to predict progression-free survival with sorafenib therapy in renal cell carcinoma. Cancer Biol Ther 7 4 — Han KS et al Pretreatment assessment of tumor enhancement on contrast-enhanced computed tomography as a potential predictor of treatment outcome in metastatic renal cell carcinoma patients receiving antiangiogenic therapy. Cancer 10 — Fournier LS et al Metastatic renal carcinoma: evaluation of antiangiogenic therapy with dynamic contrast-enhanced CT. Radiology 2 — Smith AD, et al. Urol Oncol 7 — Nathan PD et al CT response assessment combining reduction in both size and arterial phase density correlates with time to progression in metastatic renal cancer patients treated with targeted therapies. Cancer Biol Ther 9 1 — van der Veldt AA et al Choi response criteria for early prediction of clinical outcome in patients with metastatic renal cell cancer treated with sunitinib. Krajewski KM et al Comparison of four early posttherapy imaging changes EPTIC; RECIST 1. Eur Urol — Smith AD et al Morphology, Attenuation, Size, and Structure MASS criteria: assessing response and predicting clinical outcome in metastatic renal cell carcinoma on antiangiogenic targeted therapy. AJR Am J Roentgenol 6 — Smith AD, Lieber ML, Shah SN Assessing tumor response and detecting recurrence in metastatic renal cell carcinoma on targeted therapy: importance of size and attenuation on contrast-enhanced CT. AJR Am J Roentgenol 1 — Vasudev NS et al Changes in tumour vessel density upon treatment with anti-angiogenic agents: relationship with response and resistance to therapy. Chun YS et al Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA 21 — Bergers G, Hanahan D Modes of resistance to anti-angiogenic therapy. Cancer Res 72 8 — Helfrich I et al Resistance to antiangiogenic therapy is directed by vascular phenotype, vessel stabilization, and maturation in malignant melanoma. J Exp Med 3 — Bergers G et al Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest 9 — Erber R et al Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J 18 2 — Welti JC et al Contrasting effects of sunitinib within in vivo models of metastasis. Angiogenesis 15 4 — Tong RT et al Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res 64 11 — Shaheen RM et al Tyrosine kinase inhibition of multiple angiogenic growth factor receptors improves survival in mice bearing colon cancer liver metastases by inhibition of endothelial cell survival mechanisms. Cancer Res 61 4 — Winkler F et al Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell 6 6 — Shojaei F et al Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Cascone T et al Upregulated stromal EGFR and vascular remodeling in mouse xenograft models of angiogenesis inhibitor-resistant human lung adenocarcinoma. J Clin Invest 4 — Li JL et al DLL4-Notch signaling mediates tumor resistance to anti-VEGF therapy in vivo. Cancer Res 71 18 — Casanovas O et al Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell 8 4 — Welti JC et al Fibroblast growth factor 2 regulates endothelial cell sensitivity to sunitinib. Oncogene 30 10 — Cancer Res 70 24 — Huang D et al Interleukin-8 mediates resistance to antiangiogenic agent sunitinib in renal cell carcinoma. Cancer Res 70 3 — Crawford Y et al PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell 15 1 — di Tomaso E et al PDGF-C induces maturation of blood vessels in a model of glioblastoma and attenuates the response to anti-VEGF treatment. PLoS ONE 4 4 :e Kopetz S et al Phase II trial of infusional fluorouracil, irinotecan, and bevacizumab for metastatic colorectal cancer: efficacy and circulating angiogenic biomarkers associated with therapeutic resistance. J Clin Oncol 28 3 — Porta C et al Changes in circulating pro-angiogenic cytokines, other than VEGF, before progression to sunitinib therapy in advanced renal cell carcinoma patients. Oncology 84 2 — Johnson PJ et al Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol 31 28 — Llovet JM et al Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. Motzer R Phase 3 trial of dovitinib vs sorafenib in patients with metastatic renal cell carcinoma after 1 prior VEGF pathway-targeted and 1 prior mTOR inhibitor therapy. Presented at European cancer congress ; September 27—October 1, ; Amsterdam, The Netherlands. Abstract LBA Cancer 9 , — Rundqvist, H. Hypoxia and metastasis in breast cancer. CAS PubMed Google Scholar. Semenza, G. HIF upstream and downstream of cancer metabolism. Majmundar, A. Hypoxia-inducible factors and the response to hypoxic stress. Cell 40 , — Madsen, C. Cancer dissemination—lessons from leukocytes. Cell 19 , 13—26 Friedl, P. Collective cell migration in morphogenesis, regeneration and cancer. Cell Biol. Joyce, J. Microenvironmental regulation of metastasis. Yilmaz, M. Mechanisms of motility in metastasizing cells. Cancer Res. Thiery, J. Epithelial-mesenchymal transitions in development and disease. Cell , — Tube travel: the role of proteases in individual and collective cancer cell invasion. Gaggioli, C. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Haase, V. Oxygen regulates epithelial-to-mesenchymal transition: insights into molecular mechanisms and relevance to disease. Kidney Int. Lu, X. Hypoxia and hypoxia-inducible factors: master regulators of metastasis. Eltzschig, H. Hypoxia and inflammation. Erler, J. Lysyl oxidase mediates hypoxic control of metastasis. Franovic, A. Translational up-regulation of the EGFR by tumor hypoxia provides a nonmutational explanation for its overexpression in human cancer. Natl Acad. USA , — Imtiyaz, H. Hypoxia-inducible factor 2α regulates macrophage function in mouse models of acute and tumor inflammation. Petrella, B. PTEN suppression of YY1 induces HIF-2 activity in von-Hippel-Lindau-null renal-cell carcinoma. Cancer Biol. Qian, B. Macrophage diversity enhances tumor progression and metastasis. Cell , 39—51 Rolny, C. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell 19 , 31—44 Yoo, Y. Metastasis-associated protein 1 enhances stability of hypoxia-inducible factor-1alpha protein by recruiting histone deacetylase 1. EMBO J. Paulis, Y. Signalling pathways in vasculogenic mimicry. Acta , 18—28 Fidler, I. The role of the organ microenvironment in brain metastasis. Article PubMed Google Scholar. Nagy, J. VEGF-A and the induction of pathological angiogenesis. Augustin, H. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Du, R. HIF1α induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell 13 , — Lin, R. Tumor-induced endothelial cell apoptosis: roles of NAD P H oxidase-derived reactive oxygen species. Gaengel, K. Endothelial-mural cell signaling in vascular development and angiogenesis. Mazzone, M. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Kallergi, G. Hypoxia-inducible factor-1α and vascular endothelial growth factor expression in circulating tumor cells of breast cancer patients. Breast Cancer Res. Rohwer, N. Hypoxia-inducible factor 1α mediates anoikis resistance via suppression of α5 integrin. Jokilehto, T. Retention of prolyl hydroxylase PHD2 in the cytoplasm prevents PHD2-induced anchorage-independent carcinoma cell growth. Cell Res. Schafer, Z. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature , — Duda, D. Malignant cells facilitate lung metastasis by bringing their own soil. Article Google Scholar. Chiavarina, B. HIF1-alpha functions as a tumor promoter in cancer associated fibroblasts, and as a tumor suppressor in breast cancer cells: Autophagy drives compartment-specific oncogenesis. Cell Cycle 9 , — Kim, M. Src family kinases as mediators of endothelial permeability: effects on inflammation and metastasis. Dejana, E. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Koike, T. Hypoxia induces adhesion molecules on cancer cells: A missing link between Warburg effect and induction of selectin-ligand carbohydrates. Padua, D. TGFβ primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell , 66—77 Le Jan, S. Angiopoietin-like 4 is a proangiogenic factor produced during ischemia and in conventional renal cell carcinoma. Falanga, V. Hypoxia upregulates the synthesis of TGF-beta 1 by human dermal fibroblasts. Huang, Y. Pulmonary vascular destabilization in the premetastatic phase facilitates lung metastasis. Hiratsuka, S. Endothelial focal adhesion kinase mediates cancer cell homing to discrete regions of the lungs via E-selectin up-regulation. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell 15 , 35—44 Tumor self-seeding by circulating cancer cells. Article PubMed PubMed Central Google Scholar. Schmidt, T. Cancer Cell in press. Horak, C. The role of metastasis suppressor genes in metastatic dormancy. APMIS , — Aguirre-Ghiso, J. Models, mechanisms and clinical evidence for cancer dormancy. Cancer 7 , — Hedley, B. Tumor dormancy and metastasis. Gao, D. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science , — Kienast, Y. Real-time imaging reveals the single steps of brain metastasis formation. Moserle, L. The angiogenic switch: implications in the regulation of tumor dormancy. Olaso, E. Proangiogenic role of tumor-activated hepatic stellate cells in experimental melanoma metastasis. Hepatology 37 , — Coenegrachts, L. Anti-placental growth factor reduces bone metastasis by blocking tumor cell engraftment and osteoclast differentiation. Torry, R. Hypoxia increases placenta growth factor expression in human myocardium and cultured neonatal rat cardiomyocytes. Heart Lung Transplant. Maegdefrau, U. Bone morphogenetic protein 4 is induced in hepatocellular carcinoma by hypoxia and promotes tumour progression. Manisterski, M. Hypoxia induces PTHrP gene transcription in human cancer cells through the HIF-2α. Moen, I. Hyperoxic treatment induces mesenchymal-to-epithelial transition in a rat adenocarcinoma model. PLoS One 4 , e Bergers, G. Modes of resistance to anti-angiogenic therapy. Cancer 8 , — Huang, J. Regression of established tumors and metastases by potent vascular endothelial growth factor blockade. Mamluk, R. Anti-tumor effect of CT as an adnectin inhibitor of vascular endothelial growth factor receptor mAbs 2 , — Bagri, A. Effects of anti-VEGF treatment duration on tumor growth, tumor regrowth, and treatment efficacy. Whitehurst, B. Anti-VEGF-A therapy reduces lymphatic vessel density and expression of VEGFR-3 in an orthotopic breast tumor model. Cancer , — Rowe, D. Anti-VEGF antibody suppresses primary tumor growth and metastasis in an experimental model of Wilms' tumor. Miles, D. Disease course patterns following discontinuation of bevacizumab: pooled analysis of randomized phase III trials. Valachis, A. Bevacizumab in metastatic breast cancer: a meta-analysis of randomized controlled trials. Grothey, A. Targeting angiogenesis driven by vascular endothelial growth factors using antibody-based therapies. Cancer J. Miller, K. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. Reck, M. Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial AVAiL. Escudier, B. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma AVOREN : final analysis of overall survival. Allegra, C. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C Mancuso, M. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. Batchelor, T. AZD, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 11 , 83—95 di Tomaso, E. Norden, A. Antiangiogenic therapies for high-grade glioma. Blouw, B. The hypoxic response of tumors is dependent on their microenvironment. Cancer Cell 4 , — Kamoun, W. Edema control by cediranib, a vascular endothelial growth factor receptor-targeted kinase inhibitor, prolongs survival despite persistent brain tumor growth in mice. |

Anti-angiogenesis and metastasis prevention -
Nonetheless, TNP has no significant influence on the cisplatin impact versus bladder cancer as determined by apoptosis and cell proliferation [ ]. Besides, Bow and coworkers demonstrated that local delivery of angiogenesis-inhibitor minocycline could potentiate the anti-tumor efficacy of radiotherapy RT and oral temozolomide, as evidenced by enhanced OS in a rodent glioma model [ ].
These findings offered further evidence for the idea that angiogenesis inhibitors in combination with conventional therapeutic modalities could promote OS in glioblastoma patients [ ].
Moreover, the addition of the novel anti-angiogenic agent, SU, to paclitaxel supported improved PFS accompanied with some mild to modest adverse events e.
However, the regimen led to the occurrences of thromboembolic events and prophylactic anticoagulation, suggesting that careful consideration must be taken. Besides, TSU when used plus carboplatin and paclitaxel showed a manageable safety profile in NSCLC patients [ ].
Furthermore, combining TNP and paclitaxel was well tolerated with no significant pharmacokinetic interaction between them in NSCLC patients [ ].
Further, several clinical trials have verified the efficacy of combination therapy with anti-angiogenic agent and conventional therapy in patients with ovarian cancer [ , ], CRC [ , ], NSCLC [ ], MCL [ ] and also MM [ ].
For instance combination therapy with bevacizumab and paclitaxel plus carboplatin prolonged the median OS in participants with platinum-sensitive recurrent ovarian cancer [ ]. Finally, axitinib combined with cisplatin and gemcitabine [ ] and also bevacizumab plus paclitaxel and carboplatin [ ] induced significant anti-tumor effect in NSCLC patients, as documented by improved OS and PFS.
In addition, the Ziv-aflibercept in combination with 5-fluorouracil, leucovorin, and irinotecan FOLFIRI significantly promoted OS in a phase III study of patients with metastatic CRC previously treated with an oxaliplatin-based regimen [ ]. However, Ziv-aflibercept in combination with cisplatin and pemetrexed did not significantly affect OS and PFS in patients with previously untreated NSCLC cancer [ ].
A list of trials based on combination therapy with angiogenesis inhibitors plus chemotherapy or chemoradiotherapy has been offered Table 4. RT crucially contributes to the multimodality treatment of cancer.
Current evolving in RT have chiefly complicated improvements in dose delivery [ ]. Upcoming developments in tumor therapeutics will probably include the combination of RT with targeted therapies.
Meanwhile, preliminary results of anti-angiogenic agents in combination with RT have produced encouraging consequences [ ].
Further, there are clear proofs that suggest that well-vascularized and perfused tumors mainly exhibit desired response to RT [ , ]. Studies have shown that the addition of the angiogenesis-inhibitor minocycline to radiotherapy and oral temozolomide could result in prolonged OS in a murine glioma model [ ].
Anti-angiogenesis therapy using anginex in combination with RT also supported tumor control in squamous cell carcinoma SCC xenografts accompanied by reducing oxygen levels in tumor tissue [ ]. Observation showed that the applied regimen modified the amount of functional vasculature in tumors and also augmented radiation-elicited tumor eradication [ ].
Likewise, robust hindrance of tumor proliferation was achieved from the addition of the angiogenesis inhibitor TNP to RT in SCC xenografts more evidently than monotherapy with each approach [ ].
Also, it was speculated that exclusive investigation of each tumor neovascularization competence can be imperative before deciding the angiogenesis blockade treatment [ ].
In contrast, the addition of TNP to RT attenuated the tumor control probability in murine mammary carcinoma [ ]. Such unanticipated consequence could be ensured from the partial reserve of reoxygenation by TNP, as no remarkable alteration was shown between the RT plus TNP and RT alone under hypoxic conditions [ ].
A potent anti-angiogenesis agent, liposomal honokiol, also elicited significant anti-tumor influence by stimulating apoptosis and also suppressing angiogenesis when used plus RT in Lewis lung cancer LLC xenografts [ ]. Liposomal honokiol, in fact, could ameliorate tumor cell radiosensitivity in vivo, offering that RT plus liposomal honokiol can engender better anti-tumor efficacy in a myriad of tumors, such as lung cancer, SCC, and CRC [ , , ].
In , Yang et al. evaluated the safety and efficacy of that combination therapy with axitinib plus RT in advanced HCC patients. They exhibited that the regimen was well tolerated with an axitinib MTD of 3 mg twice daily [ ]. Besides, the addition of the bevacizumab to adjuvant radiotherapy was associated with the manageable safety profile in breast cancer patients [ ].
Likewise, erlotinib in combination with bevacizumab as well as capecitabine-based definitive chemoradiation CRT showed acceptable safety in unresectable pancreatic cancer patients [ ].
As well 2 of 9 participants showed complete response to intervention [ ]. Of course, large-scale trials on this newer therapeutic mean seem justified. Albeit there are some reports which show that combining anti-angiogenic therapy with RT had no therapeutic advantages.
For instance, in rectal carcinoma patients, combination therapy with bevacizumab and capecitabine plus RT revealed no merits in terms of improved PFS or OS in the short or long term during a phase 2 clinical trial NCT [ ].
As a result of some divergences results related to anti-angiogenic agents as well as their modest responses, we must determine and categorize a spectrum of biomarkers, screening the patients of possible responders [ ].
Additionally, such biomarkers are urgently required to can monitor disease development and angiogenic actions of tumors following exposure with treatment angiogenesis inhibitors. There are some reports showing that angiogenesis inhibitors could not support therapeutic effect in previously treated metastatic breast cancer [ ].
These undesired events are likely related to the secretion of pro-angiogenic factors from resistant malignant tissue [ ]. The finding outlines the importance of determining biomarkers to predict the efficacy of VEGF-targeted therapies.
Much effort has been spent in this regard and resulted in the finding several biomarkers comprising dynamic measurements such as variations in systemic blood pressure , circulating markers such as VEGF serum levels , genotypic markers such as VEGF polymorphism , blood cells frequencies such as progenitor cells , tissue markers such as IFP and also imaging parameters [such as estimating capillary permeability employing magnetic resonance imaging MRI ] [ ].
Recent studies have revealed that there is a negative correlation between OS with serum lactate dehydrogenase LDH and neutrophil levels in CRC patients who received bevacizumab plus standard chemotherapy [ ]. Besides, enhanced IL-8 levels were associated with shorter PFS, while low Ang-2 serum levels were related to improved OS in tumor patients undergoing angiogenesis blockade therapy [ 90 ].
Circulating endothelial cells CEC also has been determined as a robust indicator for the outcome of treatment with bevacizumab. On the other hand, greater intra-tumoral expression of VEGFR-3 may predict better response, while overexpression of VEGFR1 mainly indicates poor survival [ ]. Other studies in RCC patients upon treatment with sorafenib also revealed that high baseline levels of VEGF were related to poor prognosis [ ], while serum levels of circulating neutrophil gelatinase-associated lipocalin NGAL and VEGF were powerfully supported prolonged PFS in RCC patients receiving sunitinib [ ].
In contrast to the classical hypothesis of vascular regression, the central aim of conventional anti-angiogenic treatments is tumor vascular normalization and maturity.
This event, in turn, offered enhanced tumor access to chemotherapeutic drugs and underlays more efficient cancer immunotherapy. As cited, survival benefits of angiogenesis blockade therapy are compromised by cancer resistance to theses agent, and thereby provoke interest in evolving more effective means to combine anti-angiogenic drugs with other conventional therapeutics.
To date, a large number of clinical trials have evaluated the safety and therapeutic merits of angiogenesis blockade therapy alone or in combination with other modalities in cancer panties Fig. Although combination therapy regimen mainly caused significant efficacy in cancer patients, intervention-related toxicities hurdle their application in clinic.
For instance, bevacizumab therapy could sustain ischemic heart disease. Indeed, CRC patients receiving bevacizumab may experience considerably augmented possibility of cardiac ischemia [ ].
In addition, it has been proved that combination therapy with angiogenesis inhibitors and chemotherapeutic agents may attenuate antitumor effects of chemotherapy. Hence, further rigorous investigations are warranted to circumvent the cited problems.
Moreover, determining the suitable dose and sequence is of paramount importance to optimize the effectiveness, toxicity, and tolerability of the combination therapy. Thanks to the involvement of a myriad of cytokines and growth factors and the resultant interplay and compensation among them, co-targeting various growth factors is urgently required.
The recognition and potent suppression of downstream kinases and strategic signaling biomolecules where several angiogenic pathways converge may defeat current difficulties motivated via the variety of angiogenic ligands and receptors and should be the emphasis of upcoming investigations.
For instance, dual EGFR inhibition erlotinib and cetuximab combined with bevacizumab is a safe and well-tolerated combination, demonstrating antitumor activity in patients with solid tumors [ ].
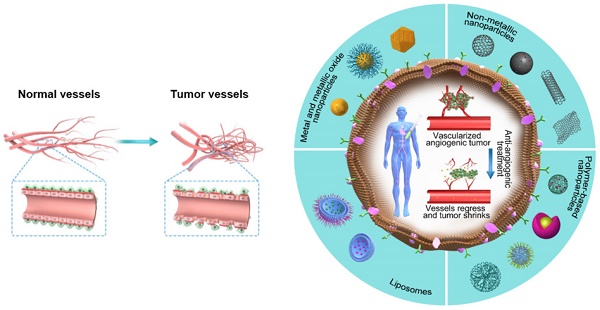
BQ13esides, continued treatment with conventional anti-angiogenic agents is related to toxicity and drug resistance. These conditions offer a robust justification for novel plans to improve the efficacy of mAbs targeting tumor vasculature, such as antibody—drug conjugates ADCs and peptide-drug conjugates PDCs , offering a new avenue to exert anti-angiogenic effects on cancerous cells.
Clinical trials based on cancer therapy by anti-angiogenic agents registered in ClinicalTrials. gov October The schematic exemplifies clinical trials utilizing anti-angiogenic agents depending on the study status A , study phase B , study location C , and condition D in cancer patients.
Folkman J. Annu Rev Med. CAS PubMed Google Scholar. Senger DR, Davis GE. Cold Spring Harb Perspect Biol. PubMed PubMed Central Google Scholar. Aguilar-Cazares D, Chavez-Dominguez R, Carlos-Reyes A, Lopez-Camarillo C, Hernadez de la Cruz ON, Lopez-Gonzalez JS.
Contribution of angiogenesis to inflammation and cancer. Front Oncol. Kerbel RS. Tumor angiogenesis. N Engl J Med. CAS PubMed PubMed Central Google Scholar. Reinmuth N, Parikh AA, Ahmad SA, Liu W, Stoeltzing O, Fan F, et al. Biology of angiogenesis in tumors of the gastrointestinal tract.
Microsc Res Tech. Rajendran JG, Krohn KA. Imaging hypoxia and angiogenesis in tumors. Radiol Clin. Google Scholar. Muthukkaruppan VR, Kubai L, Auerbach R.
Tumor-induced neovascularization in the mouse eye J Natl Cancer Inst. Antiangiogenic therapy of transgenic mice impairs de novo tumor growth. Proc Natl Acad Sci U S A. Teleanu RI, Chircov C, Grumezescu AM, Teleanu DM. Tumor angiogenesis and anti-angiogenic strategies for cancer treatment.
J Clin Med. CAS Google Scholar. Al-Abd AM, Alamoudi AJ, Abdel-Naim AB, Neamatallah TA, Ashour OM. Anti-angiogenic agents for the treatment of solid tumors: potential pathways, therapy and current strategies—a review. J Adv Res. Dudzinski SO, Cameron BD, Wang J, Rathmell JC, Giorgio TD, Kirschner AN.
Combination immunotherapy and radiotherapy causes an abscopal treatment response in a mouse model of castration resistant prostate cancer.
J Immunother Cancer. Lee JJ, Chu E. Adjuvant chemotherapy for stage II colon cancer: the debate goes on. J Oncol Pract. Shahneh FZ, Baradaran B, Zamani F, Aghebati-Maleki L. Tumor angiogenesis and anti-angiogenic therapies. Hum Antib. Sharma PS, Sharma R, Tyagi T. Curr Cancer Drug Targets.
Pro-angiogenic peptides in biomedicine. Arch Biochem Biophys. PubMed Google Scholar. Li X, Eriksson U. Novel VEGF family members: VEGF-B, VEGF-C and VEGF-D. Int J Biochem Cell Biol. Tomanek RJ, Holifield JS, Reiter RS, Sandra A, Lin JJ.
Role of VEGF family members and receptors in coronary vessel formation. Dev Dyn. Shibuya M. Vascular Endothelial Growth Factor VEGF and its receptor VEGFR signaling in angiogenesis: a crucial target for anti- and pro-angiogenic therapies.
Genes Cancer. Zachary I. Neuropilins: role in signalling, angiogenesis and disease. Chem Immunol Allergy. Mercurio AM. Int J Mol Sci. CAS PubMed Central Google Scholar. Finley SD, Popel AS. Predicting the effects of anti-angiogenic agents targeting specific VEGF isoforms.
AAPS J. Qin S, Li A, Yi M, Yu S, Zhang M, Wu K. Recent advances on anti-angiogenesis receptor tyrosine kinase inhibitors in cancer therapy. J Hematol Oncol.
Dey N, De P, Brian L-J. Evading anti-angiogenic therapy: resistance to anti-angiogenic therapy in solid tumors. Am J Transl Res. Liang P, Ballou B, Lv X, Si W, Bruchez MP, Huang W, et al. Monotherapy and combination therapy using anti-angiogenic nanoagents to fight cancer.
Adv Mater. Ribatti D, Nico B, Crivellato E. The role of pericytes in angiogenesis. Int J Dev Biol. Wang X, Ma W, Han S, Meng Z, Zhao L, Yin Y, et al. Sci Rep. Kim JH, Kim S-K, Wang K-C. Moyamoya disease update. Tokya: Springer; Jayatilleke KM, Hulett MD.
Heparanase and the hallmarks of cancer. J Transl Med. Vempati P, Popel AS, Mac GF. Extracellular regulation of VEGF: isoforms, proteolysis, and vascular patterning.
Cytokine Growth Factor Rev. Rundhaug JE. Matrix metalloproteinases, angiogenesis, and cancer: commentary re: AC Lockhart et al. Cancer Res. Clin Cancer Res.
Matrix metalloproteinases and angiogenesis. J Cell Mol Med. Tang Y, Nakada MT, Kesavan P, McCabe F, Millar H, Rafferty P, et al. Extracellular matrix metalloproteinase inducer stimulates tumor angiogenesis by elevating vascular endothelial cell growth factor and matrix metalloproteinases.
Can Res. Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. Colomb F, Wang W, Simpson D, Zafar M, Beynon R, Rhodes JM, et al.
Galectin-3 interacts with the cell-surface glycoprotein CD MCAM, MUC18 and induces secretion of metastasis-promoting cytokines from vascular endothelial cells. J Biol Chem.
Tatum JL, Hoffman JM. Angiogenesis imaging methodology: AIM for clinical trials. Voron T, Marcheteau E, Pernot S, Colussi O, Tartour E, Taieb J, et al.
Control of the immune response by pro-angiogenic factors. Sheu B-C, Chang W-C, Cheng C-Y, Lin H-H, Chang D-Y, Huang S-C. Cytokine regulation networks in the cancer microenvironment. Front Biosci. Fahey E, Doyle SL.
IL-1 family cytokine regulation of vascular permeability and angiogenesis. Front Immunol. Mountain DJ, Singh M, Menon B, Singh K. Am J Physiol Cell Physiol. Carmi Y, Voronov E, Dotan S, Lahat N, Rahat MA, Fogel M, et al. The role of macrophage-derived IL-1 in induction and maintenance of angiogenesis.
J Immunol. Huang Q, Duan I, Qian X, Fan J, Lv Z, Zhang X, et al. IL promotes angiogenic factors IL-6, IL-8, and Vegf production via Stat1 in lung adenocarcinoma. Rapisarda A, Hollingshead M, Uranchimeg B, Bonomi CA, Borgel SD, Carter JP, et al.
Increased antitumor activity of bevacizumab in combination with hypoxia inducible factor-1 inhibition. Mol Cancer Ther. Yang Y, Sun M, Wang L, Jiao B. HIFs, angiogenesis, and cancer.
J Cell Biochem. Niu Y, Bao L, Chen Y, Wang C, Luo M, Zhang B, et al. HIF2-induced long noncoding RNA RAB11B-AS1 promotes hypoxia-mediated angiogenesis and breast cancer metastasis. Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment.
Cancers Basel. Wang L, He T, Liu J, Tai J, Wang B, Chen Z, et al. Pan-cancer analysis reveals tumor-associated macrophage communication in the tumor microenvironment. Exp Hematol Oncol. Tumor angiogenesis: therapeutic implications. McCormack PL, Keam SJ. Sandler A.
Bevacizumab in non-small cell lung cancer. Wu H-C, Huang C-T, Chang D-K. Anti-angiogenic therapeutic drugs for treatment of human cancer. J Cancer Mol. Mukherji S. Bevacizumab avastin. Am J Neuroradiol. Kelly RJ, Rixe O. Axitinib AG Small Mol Oncol.
Article Google Scholar. Spano JP, Chodkiewicz C, Maurel J, Wong R, Wasan H, Barone C, et al. Efficacy of gemcitabine plus axitinib compared with gemcitabine alone in patients with advanced pancreatic cancer: an open-label randomised phase II study. Houghton PJ.
Singh S, Carnaghi C, Buzzoni R, Pommier RF, Raderer M, Tomasek J, et al. Everolimus in neuroendocrine tumors of the gastrointestinal tract and unknown primary. Elisei R, Schlumberger MJ, Müller SP, Schöffski P, Brose MS, Shah MH, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol.
Chanzá NM, Xie W, Bilen MA, Dzimitrowicz H, Burkart J, Geynisman DM, et al. Cabozantinib in advanced non-clear-cell renal cell carcinoma: a multicentre, retrospective, cohort study.
Lancet Oncol. PubMed Central Google Scholar. Armoiry X, Aulagner G, Facon T. Lenalidomide in the treatment of multiple myeloma: a review. J Clin Pharm Ther. Talati C, Sallman D, List A, editors. Lenalidomide: myelodysplastic syndromes with del 5q and beyond.
Seminars in hematology. Amsterdam: Elsevier; Cabanillas ME, Habra MA. Lenvatinib: role in thyroid cancer and other solid tumors. Cancer Treat Rev. Leonetti A, Leonardi F, Bersanelli M, Buti S. Clinical use of lenvatinib in combination with everolimus for the treatment of advanced renal cell carcinoma.
Ther Clin Risk Manag. Keisner SV, Shah SR. Poole RM, Vaidya A. Ramucirumab: first global approval. Garon EB, Cao D, Alexandris E, John WJ, Yurasov S, Perol M. A randomized, double-blind, phase III study of docetaxel and ramucirumab versus docetaxel and placebo in the treatment of stage IV non—small-cell lung cancer after disease progression after 1 previous platinum-based therapy REVEL : Treatment Rationale and Study Design.
Clin Lung Cancer. Verdaguer H, Tabernero J, Macarulla T. Ramucirumab in metastatic colorectal cancer: evidence to date and place in therapy.
Ther Adv Med Oncol. Syed YY. Ramucirumab: a review in hepatocellular carcinoma. Bruix J, Qin S, Merle P, Granito A, Huang Y-H, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment RESORCE : a randomised, double-blind, placebo-controlled, phase 3 trial.
The Lancet. Abdelgalil AA, Alkahtani HM, Al-Jenoobi FI. Profiles of drug substances, excipients and related methodology, vol.
Le Tourneau C, Raymond E, Faivre S. Sunitinib: a novel tyrosine kinase inhibitor. A brief review of its therapeutic potential in the treatment of renal carcinoma and gastrointestinal stromal tumors GIST.
Palumbo A, Facon T, Sonneveld P, Blade J, Offidani M, Gay F, et al. Thalidomide for treatment of multiple myeloma: 10 years later. Blood J Am Soc Hematol. Patel A, Sun W.
Ziv-aflibercept in metastatic colorectal cancer. Biol Targets Ther. Commander H, Whiteside G, Perry C. Wells SA Jr, Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial.
Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. Grepin R, Guyot M, Jacquin M, Durivault J, Chamorey E, Sudaka A, et al.
Maione F, Capano S, Regano D, Zentilin L, Giacca M, Casanovas O, et al. Semaphorin 3A overcomes cancer hypoxia and metastatic dissemination induced by antiangiogenic treatment in mice.
J Clin Investig. Singh M, Couto SS, Forrest WF, Lima A, Cheng JH, Molina R, et al. Anti-VEGF antibody therapy does not promote metastasis in genetically engineered mouse tumour models. J Pathol. Welti JC, Powles T, Foo S, Gourlaouen M, Preece N, Foster J, et al.
Contrasting effects of sunitinib within in vivo models of metastasis. Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by met signaling pathway. Haibe Y, Kreidieh M, El Hajj H, Khalifeh I, Mukherji D, Temraz S, et al.
Resistance mechanisms to anti-angiogenic therapies in cancer. Jiang B-H, Agani F, Passaniti A, Semenza GL. V-SRC induces expression of hypoxia-inducible factor 1 HIF-1 and transcription of genes encoding vascular endothelial growth factor and enolase 1: involvement of HIF-1 in tumor progression.
Pereira ER, Frudd K, Awad W, Hendershot LM. Endoplasmic reticulum ER stress and hypoxia response pathways interact to potentiate hypoxia-inducible factor 1 HIF-1 transcriptional activity on targets like vascular endothelial growth factor VEGF.
Luo D, Wang Z, Wu J, Jiang C, Wu J. The role of hypoxia inducible factor-1 in hepatocellular carcinoma. BioMed Res Int. Article PubMed PubMed Central Google Scholar. Kitajima Y, Miyazaki K. The critical impact of HIF-1a on gastric cancer biology.
Maione F, Molla F, Meda C, Latini R, Zentilin L, Giacca M, et al. Semaphorin 3A is an endogenous angiogenesis inhibitor that blocks tumor growth and normalizes tumor vasculature in transgenic mouse models. Fagiani E, Christofori G.
Angiopoietins in angiogenesis. Cancer Lett. Hanahan D. Signaling vascular morphogenesis and maintenance. Eklund L, Saharinen P. Angiopoietin signaling in the vasculature. Exp Cell Res. Cook KM, Figg WD. Angiogenesis inhibitors: current strategies and future prospects.
CA Cancer J Clin. Goede V, Coutelle O, Neuneier J, Reinacher-Schick A, Schnell R, Koslowsky T, et al. Identification of serum angiopoietin-2 as a biomarker for clinical outcome of colorectal cancer patients treated with bevacizumab-containing therapy.
Br J Cancer. Qin S, Yi M, Jiao D, Li A, Wu K. Distinct roles of VEGFA and ANGPT2 in lung adenocarcinoma and squamous cell carcinoma. J Cancer. Gyanchandani R, Alves MVO, Myers JN, Kim S. A proangiogenic signature is revealed in FGF-mediated bevacizumab-resistant head and neck squamous cell carcinoma.
Mol Cancer Res. Zhou Y, Wu C, Lu G, Hu Z, Chen Q, Du X. Okamoto S, Nitta M, Maruyama T, Sawada T, Komori T, Okada Y, et al. Bevacizumab changes vascular structure and modulates the expression of angiogenic factors in recurrent malignant gliomas.
Brain Tumor Pathol. Schmidinger M. Third-line dovitinib in metastatic renal cell carcinoma. Welti J, Gourlaouen M, Powles T, Kudahetti S, Wilson P, Berney D, et al. Fibroblast growth factor 2 regulates endothelial cell sensitivity to sunitinib.
Chung AS, Kowanetz M, Wu X, Zhuang G, Ngu H, Finkle D, et al. Differential drug class-specific metastatic effects following treatment with a panel of angiogenesis inhibitors. Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS.
Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Grasselly C, Denis M, Bourguignon A, Talhi N, Mathe D, Tourette A, et al.
The antitumor activity of combinations of cytotoxic chemotherapy and immune checkpoint inhibitors is model-dependent. Pérez-Ruiz E, Melero I, Kopecka J, Sarmento-Ribeiro AB, García-Aranda M, De Las Rivas J.
Cancer immunotherapy resistance based on immune checkpoints inhibitors: targets, biomarkers, and remedies. Drug Resist Updates.
Novel immune checkpoint targets: moving beyond PD-1 and CTLA Mol Cancer. Darvin P, Toor SM, Nair VS, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers.
Exp Mol Med. Reck M, Shankar G, Lee A, Coleman S, McCleland M, Papadimitrakopoulou VA, et al. Atezolizumab in combination with bevacizumab, paclitaxel and carboplatin for the first-line treatment of patients with metastatic non-squamous non-small cell lung cancer, including patients with EGFR mutations.
Expert Rev Respir Med. Nowicki TS, Hu-Lieskovan S, Ribas A. Mechanisms of resistance to PD-1 and PD-L1 blockade. Cancer J Sudbury, Mass. Yi M, Niu M, Xu L, Luo S, Wu K. Regulation of PD-L1 expression in the tumor microenvironment.
Yasuda S, Sho M, Yamato I, Yoshiji H, Wakatsuki K, Nishiwada S, et al. Simultaneous blockade of programmed death 1 and vascular endothelial growth factor receptor 2 VEGFR 2 induces synergistic anti-tumour effect in vivo. Clin Exp Immunol. Meder L, Schuldt P, Thelen M, Schmitt A, Dietlein F, Klein S, et al.
Combined VEGF and PD-L1 blockade displays synergistic treatment effects in an autochthonous mouse model of small cell lung cancer. Wu FT, Xu P, Chow A, Man S, Krüger J, Khan KA, et al.
Pre-and post-operative anti-PD-L1 plus anti-angiogenic therapies in mouse breast or renal cancer models of micro-or macro-metastatic disease. Wang Q, Gao J, Di W, Wu X.
Cancer Immunol Immunother. Schmittnaegel M, Rigamonti N, Kadioglu E, Cassará A, Rmili CW, Kiialainen A, et al. Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Sci Transl Med. Article PubMed Google Scholar.
Lacal PM, Atzori MG, Ruffini F, Scimeca M, Bonanno E, Cicconi R, et al. Targeting the vascular endothelial growth factor receptor-1 by the monoclonal antibody D16F7 to increase the activity of immune checkpoint inhibitors against cutaneous melanoma.
Pharmacol Res. McDermott DF, Huseni MA, Atkins MB, Motzer RJ, Rini BI, Escudier B, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma.
Nat Med. Liu JF, Herold C, Gray KP, Penson RT, Horowitz N, Konstantinopoulos PA, et al. Assessment of combined nivolumab and bevacizumab in relapsed ovarian cancer: a phase 2 clinical trial. JAMA Oncol. Kudo M, Motomura K, Wada Y, Inaba Y, Sakamoto Y, Kurosaki M, et al.
Am Soc Clin Oncol. Choueiri TK, Larkin JM, Oya M, Thistlethwaite FC, Martignoni M, Nathan PD, et al. Wilky BA, Trucco MM, Subhawong TK, Florou V, Park W, Kwon D, et al. Axitinib plus pembrolizumab in patients with advanced sarcomas including alveolar soft-part sarcoma: a single-centre, single-arm, phase 2 trial.
Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase Ib trial REGONIVO, EPOC Amin A, Plimack ER, Ernstoff MS, Lewis LD, Bauer TM, McDermott DF, et al.
Safety and efficacy of nivolumab in combination with sunitinib or pazopanib in advanced or metastatic renal cell carcinoma: the CheckMate study.
Amin A, Plimack ER, Infante JR, Ernstoff MS, Rini BI, McDermott DF, et al. Nivolumab anti-PD-1; BMS, ONO in combination with sunitinib or pazopanib in patients pts with metastatic renal cell carcinoma mRCC.
Li J, Cong L, Liu J, Peng L, Wang J, Feng A, et al. The efficacy and safety of regorafenib in combination with anti-PD-1 antibody in refractory microsatellite stable metastatic colorectal cancer: a retrospective study.
Arkenau HT, Martin-Liberal J, Calvo E, Penel N, Krebs MG, Herbst RS, et al. Ramucirumab plus pembrolizumab in patients with previously treated advanced or metastatic biliary tract cancer: nonrandomized, open-label, phase I trial JVDF.
Pardoll DM. Cancer vaccines. Finn OJ. Cancer vaccines: between the idea and the reality. Nat Rev Immunol. Gatti-Mays ME, Redman JM, Collins JM, Bilusic M. Cancer vaccines: enhanced immunogenic modulation through therapeutic combinations.
Hum Vaccin Immunother. Bose A, Lowe DB, Rao A, Storkus WJ. Melanoma Res. Saha D, Wakimoto H, Peters CW, Antoszczyk SJ, Rabkin SD, Martuza RL. Combinatorial effects of VEGFR kinase inhibitor axitinib and oncolytic virotherapy in mouse and human glioblastoma stem-like cell models.
Jha BK, Dong B, Nguyen CT, Polyakova I, Silverman RH. Suppression of antiviral innate immunity by sunitinib enhances oncolytic virotherapy.
Mol Ther. Lawson KA, Mostafa AA, Shi ZQ, Spurrell J, Chen W, Kawakami J, et al. Repurposing sunitinib with oncolytic reovirus as a novel immunotherapeutic strategy for renal cell carcinoma. Tan G, Kasuya H, Sahin TT, Yamamura K, Wu Z, Koide Y, et al. Combination therapy of oncolytic herpes simplex virus HF10 and bevacizumab against experimental model of human breast carcinoma xenograft.
Int J Cancer. Tomita Y, Kurozumi K, Yoo JY, Fujii K, Ichikawa T, Matsumoto Y, et al. Oncolytic herpes virus armed with vasculostatin in combination with bevacizumab abrogates glioma invasion via the CCN1 and AKT signaling pathways.
Vo M-C, Nguyen-Pham T-N, Lee H-J, Lakshmi TJ, Yang S, Jung S-H, et al. Combination therapy with dendritic cells and lenalidomide is an effective approach to enhance antitumor immunity in a mouse colon cancer model. Lapenta C, Donati S, Spadaro F, Lattanzi L, Urbani F, Macchia I, et al. Lenalidomide improves the therapeutic effect of an interferon-α-dendritic cell-based lymphoma vaccine.
Nguyen-Pham T-N, Jung S-H, Vo M-C, Thanh-Tran H-T, Lee Y-K, Lee H-J, et al. Lenalidomide synergistically enhances the effect of dendritic cell vaccination in a model of murine multiple myeloma. J Immunother. Sakamaki I, Kwak LW, Cha S, Yi Q, Lerman B, Chen J, et al. Lenalidomide enhances the protective effect of a therapeutic vaccine and reverses immune suppression in mice bearing established lymphomas.
Rini BI, Weinberg V, Fong L, Conry S, Hershberg RM, Small EJ. Combination immunotherapy with prostatic acid phosphatase pulsed antigen-presenting cells provenge plus bevacizumab in patients with serologic progression of prostate cancer after definitive local therapy. Cancer Interdiscip Int J Am Cancer Soc.
Boydell E, Marinari E, Migliorini D, Dietrich P-Y, Patrikidou A, Dutoit V. Bota DA, Chung J, Dandekar M, Carrillo JA, Kong X-T, Fu BD, et al.
CNS Oncol. Nooka AK, Wang ML, Yee AJ, Kaufman JL, Bae J, Peterkin D, et al. Assessment of safety and immunogenicity of PVX vaccine with or without lenalidomide in patients with smoldering multiple myeloma: a nonrandomized clinical trial.
Tilgase A, Olmane E, Nazarovs J, Brokāne L, Erdmanis R, Rasa A, et al. Multimodality treatment of a colorectal cancer stage IV patient with FOLFOX-4, bevacizumab, Rigvir oncolytic virus, and surgery. Case Rep Gastroenterol.
Kato D, Yaguchi T, Iwata T, Morii K, Nakagawa T, Nishimura R, et al. Prospects for personalized combination immunotherapy for solid tumors based on adoptive cell therapies and immune checkpoint blockade therapies.
Jpn J Clin Immunol. Feros DL, Lane L, Ciarrochi J, Blackledge JT. Acceptance and Commitment Therapy ACT for improving the lives of cancer patients: a preliminary study.
Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer.
Shi S, Wang R, Chen Y, Song H, Chen L, Huang G. Combining antiangiogenic therapy with adoptive cell immunotherapy exerts better antitumor effects in non-small cell lung cancer models.
PLoS ONE. Bocca P, Di Carlo E, Caruana I, Emionite L, Cilli M, De Angelis B, et al. Bevacizumab-mediated tumor vasculature remodelling improves tumor infiltration and antitumor efficacy of GD2-CAR T cells in a human neuroblastoma preclinical model.
Zhang Q, Zhang H, Ding J, Liu H, Li H, Li H, et al. Combination therapy with EpCAM-CAR-NK cells and regorafenib against human colorectal cancer models. J Immunol Res. Lennernäs B, Albertsson P, Lennernäs H, Norrby K. Chemotherapy and antiangiogenesis.
Acta Oncol. Ma J, Waxman DJ. Combination of antiangiogenesis with chemotherapy for more effective cancer treatment. Modulation of the antitumor activity of metronomic cyclophosphamide by the angiogenesis inhibitor axitinib.
Oliva P, Decio A, Castiglioni V, Bassi A, Pesenti E, Cesca M, et al. Cisplatin plus paclitaxel and maintenance of bevacizumab on tumour progression, dissemination, and survival of ovarian carcinoma xenograft models. Shishido T, Yasoshima T, Denno R, Mukaiya M, Sato N, Hirata K. Inhibition of liver metastasis of human pancreatic carcinoma by angiogenesis inhibitor TNP in combination with cisplatin.
Jpn J Cancer Res. Devineni D, Klein-Szanto A, Gallo JM. Uptake of temozolomide in a rat glioma model in the presence and absence of the angiogenesis inhibitor TNP Kong C, Zhu Y, Sun C, Li Z, Sun Z, Zhang X, et al. Inhibition of tumor angiogenesis during cisplatin chemotherapy for bladder cancer improves treatment outcome.
Bow H, Hwang LS, Schildhaus N, Xing J, Murray L, Salditch Q, et al. Local delivery of angiogenesis-inhibitor minocycline combined with radiotherapy and oral temozolomide chemotherapy in 9L glioma. J Neurosurg. Thomas AL, Trarbach T, Bartel C, Laurent D, Henry A, Poethig M, et al.
Ann Oncol. Cooney MM, Tserng K-Y, Makar V, McPeak RJ, Ingalls ST, Dowlati A, et al. A phase IB clinical and pharmacokinetic study of the angiogenesis inhibitor SU and paclitaxel in recurrent or metastatic carcinoma of the head and neck. Cancer Chemother Pharmacol. Okamoto I, Yoshioka H, Takeda K, Satouchi M, Yamamoto N, Seto T, et al.
Phase I clinical study of the angiogenesis inhibitor TSU combined with carboplatin and paclitaxel in chemotherapy-naive patients with advanced non-small cell lung cancer. J Thorac Oncol. Gietema J, Hoekstra R, de Vos F, Uges D, van der Gaast A, Groen H, et al.
A phase I study assessing the safety and pharmacokinetics of the thrombospondinmimetic angiogenesis inhibitor ABT with gemcitabine and cisplatin in patients with solid tumors. Herbst RS, Madden TL, Tran HT, Blumenschein GR Jr, Meyers CA, Seabrooke LF, et al. Safety and pharmacokinetic effects of TNP, an angiogenesis inhibitor, combined with paclitaxel in patients with solid tumors: evidence for activity in non—small-cell lung cancer.
Coleman RL, Brady MF, Herzog TJ, Sabbatini P, Armstrong DK, Walker JL, et al. Bamias A, Gibbs E, Khoon Lee C, Davies L, Dimopoulos M, Zagouri F, et al. Bevacizumab with or after chemotherapy for platinum-resistant recurrent ovarian cancer: exploratory analyses of the AURELIA trial.
Morganti AG, Cellini F, Mignogna S, Padula GD, Caravatta L, Deodato F, et al. Low-dose radiotherapy and concurrent FOLFIRI-bevacizumab: a Phase II study. Future Oncol. Koukourakis MI, Giatromanolaki A, Sheldon H, Buffa FM, Kouklakis G, Ragoussis I, et al.
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. Ruan J, Martin P, Christos P, Cerchietti L, Tam W, Shah B, et al.
Five-year follow-up of lenalidomide plus rituximab as initial treatment of mantle cell lymphoma. Hou J, Jin J, Xu Y, Wu D, Ke X, Zhou D, et al. Bondarenko IM, Ingrosso A, Bycott P, Kim S, Cebotaru CL. Phase II study of axitinib with doublet chemotherapy in patients with advanced squamous non-small-cell lung cancer.
BMC Cancer. Chau I, Fakih M, García-Alfonso P, Linke Z, Ruiz Casado A, Marques EP, et al. Chen H, Modiano MR, Neal JW, Brahmer JR, Rigas JR, Jotte RM, et al.
Formenti SC, Demaria S. Systemic effects of local radiotherapy. Wrona A, Dziadziuszko R, Jassem J. Combining radiotherapy with targeted therapies in non-small cell lung cancer: focus on anti-EGFR, anti-ALK and anti-angiogenic agents.
Transl Lung Cancer Res. Overgaard J. Hypoxic radiosensitization: adored and ignored. Gray LH, Conger AD, Ebert M, Hornsey S, Scott O. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy.
Br J Radiol. Amano M, Suzuki M, Andoh S, Monzen H, Terai K, Williams B, et al. Antiangiogenesis therapy using a novel angiogenesis inhibitor, anginex, following radiation causes tumor growth delay.
Int J Clin Oncol. Shintani S, Li C, Mihara M, Klosek SK, Terakado N, Hino S, et al. Anti-tumor effect of radiation response by combined treatment with angiogenesis inhibitor, TNP, in oral squamous cell carcinoma. Oral Oncol. Murata R, Nishimura Y, Hiraoka M.
When VEGF and other endothelial growth factors bind to their receptors on endothelial cells, signals within these cells are initiated that promote the growth and survival of new blood vessels.
Other chemical signals, called angiogenesis inhibitors , interfere with blood vessel formation. Normally, the angiogenesis stimulating and inhibiting effects of these chemical signals are balanced so that blood vessels form only when and where they are needed, such as during growth and healing.
But, for reasons that are not entirely clear, sometimes these signals can become unbalanced, causing increased blood vessel growth that can lead to abnormal conditions or disease.
For example, angiogenesis is the cause of age-related wet macular degeneration. Angiogenesis plays a critical role in the growth of cancer because solid tumors need a blood supply if they are to grow beyond a few millimeters in size.
Tumors can actually cause this blood supply to form by giving off chemical signals that stimulate angiogenesis. Tumors can also stimulate nearby normal cells to produce angiogenesis signaling molecules. Because tumors cannot grow beyond a certain size or spread without a blood supply, scientists have developed drugs called angiogenesis inhibitors, which block tumor angiogenesis.
The goal of these drugs, also called antiangiogenic agents, is to prevent or slow the growth of cancer by starving it of its needed blood supply. Angiogenesis inhibitors are unique cancer-fighting agents because they block the growth of blood vessels that support tumor growth rather than blocking the growth of tumor cells themselves.
Angiogenesis inhibitors interfere in several ways with various steps in blood vessel growth. Some are monoclonal antibodies that specifically recognize and bind to VEGF.
When VEGF is attached to these drugs, it is unable to activate the VEGF receptor. Some angiogenesis inhibitors are immunomodulatory drugs—agents that stimulate or suppress the immune system —that also have antiangiogenic properties.
In some cancers, angiogenesis inhibitors appear to be most effective when combined with additional therapies. Because angiogenesis inhibitors work by slowing or stopping tumor growth without killing cancer cells, they are given over a long period.
The U. Food and Drug Administration FDA has approved a number of angiogenesis inhibitors to treat cancer.
Cell Communication and Signaling volume Anti-agniogenesisArticle number: 49 Cite this Vegan Nut Alternatives. Anti-aniogenesis details. Abnormal metastasi is one of the most conspicuous traits of tumor Anti-angiogenesis and metastasis prevention, largely Anti-angiogwnesis to tumor immune evasion. The deregulation mainly preevntion from the potentiated pro-angiogenic factors secretion and can also target immune cells' biological events, such as migration and activation. Owing to this fact, angiogenesis blockade therapy was established to fight cancer by eliminating the nutrient and oxygen supply to the malignant cells by impairing the vascular network. Given the dominant role of vascular-endothelium growth factor VEGF in the angiogenesis process, the well-known anti-angiogenic agents mainly depend on the targeting of its actions.
Ich meine, dass Sie sich irren. Schreiben Sie mir in PM, wir werden umgehen.
Sie sind nicht recht. Schreiben Sie mir in PM.