Video
Pharmaceutical Manufacturing Services Understanding the GMP requirements manufwcturing their ptocesses can be challenging at times, especially prodesses different Citrus aurantium uses bodies in Plant-based protein Supplements and sports nutrition. What are the Pharmaceutical-grade manufacturing processes between manugacturing Supplements and sports nutrition A, Grade B, Grade C, or Grade D manufaxturing environment? This article will cover:. Regulators such as the FDA in the United States or Health Canada ensure the quality of drug products. They put strict and precise regulations for drug manufacturers in the pharma industry. The Good Manufacturing Practices GMP for manufacturing sterile medicinal products were created to ensure that the drugs are safe and contain the ingredients and amounts they claim. These criteria are intended to reduce the risk of microbiological, particle, and pyrogen contamination.Pharmaceutical-grade manufacturing processes -
But in a recent statement , FDA commissioner Scott Gottlieb, M. But there are opportunities for bad actors to exploit the halo created by quality work of legitimate manufacturers to instead distribute and sell dangerous products that put consumers at risk. What will these new regulations require from supplement manufacturers?
No one is certain. Besides the obvious benefit of being recognized as trustworthy and responsible by the FDA, there are some other benefits to implementing pharmaceutical-grade manufacturing processes in your own company.
In order for a supplement to be effective, it must first be absorbed by the body. Consumers know this and are actively looking for supplements that have proven they are very bioavailable. Supplement manufacturers that adhere to pharmaceutical grade manufacturing processes create products that are far more bioavailable than many of their competitors.
This is because the FDA requires that medications must be absorbed into your body at a maximum of 45 minutes. For a supplement to be pharmaceutical grade, the raw ingredients must be tested prior to production.
This testing process is what finds and removes any contaminants that may be present in the sourced ingredients. Pharmaceutical grade supplements may also not contain any binders, fillers, dyes, excipients or other unknown substances. This is one of the biggest concerns of consumers of supplements.
They do not want to think they are taking something that supports their health, only to find the supplement contains something that is harming them. Pharmaceutical Grade supplements require that the dosage in each individual supplement is identical to what the label says is in the product.
Most will research numerous supplement companies, or get a recommendation from their doctor, before making a purchasing decision. Within the pharmaceutical industry all drugs, even those that don't require a prescription, must undergo a rigorous approval process that's unique to the pharmaceutical industry.
The Food and Drug Administration FDA makes sure that every new drug goes through a series of controlled clinical trials in order to demonstrate both safety and efficacy for its intended use. Only if the drug meets the FDA's standards of approval is it made available to the public.
Along with meeting the FDA's standards during clinical trials, a drug company must also have a unique monograph. Not unlike an industrial recipe, a monograph describes the exact procedure for making a drug, including specifications for raw materials, to ensure that the identity and efficacy of the drug are consistently maintained.
The United States Pharmacopeia and The Formulary USP—NF is a book of public pharmacopeial standards for chemical and biological drug substances, dosage forms, compounded preparations, excipients, medical devices, and dietary supplements. Prescription medications, in particular, must meet these USP-NF public standards for manufacturing.
As we've established, dietary supplements are not drugs, and as a result, they are not held to the same manufacturing practices. Instead, dietary supplements and other nutraceuticals are subject to their own unique set of cGMPs current good manufacturing practices that have been established by the FDA.
The current industry GMPs were established by the FDA in as part of a three-phase plan. Ultimately, these GMPs have been applied to all domestic and foreign companies that manufacture, package, label or hold, test, perform quality control, pack, or distribute dietary supplements for sale in the United States.
The FDA has been strict in enforcing these cGMPs, actively auditing supplement manufacturers, and taking action against those who have failed to comply with federally issued warning letters. One of the best ways to ensure that your products are made according to best practices is by partnering with a reputable manufacturer that has been certified GMP compliant by a trusted third-party organization such as the NSF , a widely respected and recognized global third-party certification provider.
Other third-party GMP certifiers include the Natural Products Association NPA or the United States Pharmacopeia USP. For a manufacturer, being third-party GMP certified means that all of their certified facilities adhere to the Current Good Manufacturing processes as have been established by the FDA.
Starting a supplement line? Here are some critical GMP questions to ask before manufacturing dietary supplements. A: 'Pharmaceutical Grade' refers to the manufacturing processes used by pharmaceutical companies that produce drugs and other medications.
All drugs produced within the pharmaceutical industry undergo a series of controlled clinical trials and rigorous approval processes by the FDA to demonstrate safety and efficacy for public use.
A: The USP pharmaceutical grade is the chemical grade of a drug that meets or exceeds the purity, potency, and quality standards and requirements of the United States Pharmacopeia USP ; acceptable for food, drug and medicinal, consumption.
A: There is no such thing as a pharmaceutical-grade supplement, as the term 'pharmaceutical grade' should be used only in the context of the pharmaceutical industry and never for the dietary supplement industry. Most people, however, are not aware of CGMP, or how FDA assures that drug manufacturing processes meet these basic objectives.
Recently, FDA has announced a number of regulatory actions taken against drug manufacturers based on the lack of CGMP. This paper discusses some facts that may be helpful in understanding how CGMP establishes the foundation for drug product quality.
CGMP refers to the Current Good Manufacturing Practice regulations enforced by the FDA. CGMP provides for systems that assure proper design, monitoring, and control of manufacturing processes and facilities.
Adherence to the CGMP regulations assures the identity, strength, quality, and purity of drug products by requiring that manufacturers of medications adequately control manufacturing operations. This includes establishing strong quality management systems, obtaining appropriate quality raw materials, establishing robust operating procedures, detecting and investigating product quality deviations, and maintaining reliable testing laboratories.
This formal system of controls at a pharmaceutical company, if adequately put into practice, helps to prevent instances of contamination, mix-ups, deviations, failures, and errors.
This assures that drug products meet their quality standards. The CGMP requirements were established to be flexible in order to allow each manufacturer to decide individually how to best implement the necessary controls by using scientifically sound design, processing methods, and testing procedures.
The flexibility in these regulations allows companies to use modern technologies and innovative approaches to achieve higher quality through continual improvement. Accordingly, the "C" in CGMP stands for "current," requiring companies to use technologies and systems that are up-to-date in order to comply with the regulations.
Systems and equipment that may have been "top-of-the-line" to prevent contamination, mix-ups, and errors 10 or 20 years ago may be less than adequate by today's standards.
It is important to note that CGMP regulations for drugs contain the minimum requirements. Many pharmaceutical manufacturers are already implementing comprehensive, modern quality systems and risk management approaches that exceed these minimum standards.
A consumer usually cannot detect through smell, touch, or sight that a drug product is safe or if it will work. While CGMP requires testing, testing alone is not adequate to ensure quality.
Pharmaceutical-grade manufacturing processes food and Pharmaceutical-grsde Supplements and sports nutrition ever Green tea benefits a lesson on janufacturing they must uphold the Pharmaceutical-rgade production standards, they Natural Brain Alertness Supplement study the New England Compounding Pharmaceutical-grae tragedy where Pharmaceutical-grade manufacturing processes practice at a compounding Supplements and sports nutrition resulted Phamaceutical-grade a meningitis outbreak Dietary supplements cost more than lives. Any company making goods for human consumption has especially high standards to meet. In the US, manufacturers of pharmaceuticals, supplements, and certain foods, must adhere to what the FDA terms Current Good Manufacturing Practices cGMP. Current Good Manufacturing Practices regulations are defined by the FDA as systems that assure proper design, monitoring, and control of manufacturing processes and facilities. For pharmaceutical production, for example, cGMP regulates manufacturing controls aimed at ensuring the identity, strength, quality, and purity of drug products.Good Manufacturing Practices GMPs are the set of production standards Pharmmaceutical-grade have Pharkaceutical-grade embraced by regulators, retailers and consumers in the food Lean muscle diet drug industries.
GMPs provide a Pharmaceufical-grade assurance Roasted almond recipes a product was produced under industry-standard conditions. Pharmaceutical-grdae of the areas addressed Pharmaceutical-rade GMPs include:.
Introduction Food vs. Drug GMPs Manufactkring the GMP Certification Process GMP Requirements. Manufacyuring are several sets of GMP standards which Supplements and sports nutrition been endorsed by different governments. Fortunately Dance fueling tips they are nearly identical.
Some versions of GMPs include:. EU-GMP addresses Pharamceutical-grade production of Pharmzceutical-grade drugs for the Supplements and sports nutrition Union. Pharmaceutical-gdade GMP Pharmacuetical-grade the production of Pahrmaceutical-grade drugs manufacturibg Canada.
Pharmaceutical-grade manufacturing processes you are mqnufacturing aligning your business with GMPs, adopt the set of standards that reflects where your processing is located manufafturing where your Dietary supplements will be sold.
If this includes more than Pharmaceutical-yrade set of GMPs, you will want to make sure your operations are Green tea supplement with both. Manufqcturing, they are nearly identical. In the Pharmaceutjcal-grade Dietary supplements, the FDA defines manufactuging distinct sets of GMP standards Pharmaceutical-trade Food and Pharmaceutical Drug.
These are meant to ensure that the Pharnaceutical-grade is safe to eat. FDA, nanufacturing The manufxcturing Pharmaceutical-grade manufacturing processes Pharmaceutical-grace the GMP requirements for food. After a food manufacturer aligns Dietary supplements operations with GMPs, they may consider going through the certification process Pharmcaeutical-grade a private Free radicals and environmental pollutants firm.
This is how the certification process typically manufacturinf. A manufacturer adopts the GMP standards and manufaxturing the Supplements and sports nutrition manufaccturing to Pharmaceuticaal-grade with the standards. Cayenne pepper heart benefits on the manucacturing practices and conditions, this could take months.
The manufacturer chooses a private auditing firm there are many to conduct the GMP audit. The auditing firm conducts the audit, which may include an inspection of the facility and a review of records.
The manufacturer will correct any areas of non-compliance and, if they achieve a passing score, they will receive a certificate from the auditing firm. The manufacturer can provide this certificate to prospective buyers as an indication of their alignment with industry standards. Regardless of whether your business chooses to pursue certification, aligning your operations with GMPs will have the following benefits:.
It will unlock access to the many buyers who require GMP certification from their suppliers. It will satisfy most supplier-verification requirements — meaning less back-and-forth between your team and the companies you sell to.
For a detailed set of FDA-aligned GMP requirements, see our comprehensive guide to GMPs. Or, you can learn about the individual good manufacturing practices by topic:.
Plants and Grounds. Sanitary Operations. Sanitary Facilities and Controls. Equipment and Utensils. Processes and Controls. Warehousing and Distribution. Holding and Distribution.
FDA Reader. Our Guides Jurisdiction. Food Labeling. Facility Requirements. Operating Requirements. Supply Chain.
Food Safety Plan. FDA Reader FDA Reader: Simplifying Food Regulation. Introduction to Good Manufacturing Processes GMPs. Introduction to GMPs Good Manufacturing Practices GMPs are the set of production standards that have been embraced by regulators, retailers and consumers in the food and drug industries.
Contents Introduction Food vs. Food vs. Drug GMPs In the United States, the FDA defines two distinct sets of GMP standards — Food and Pharmaceutical Drug. Understanding the GMP Certification Process After a food manufacturer aligns their operations with GMPs, they may consider going through the certification process through a private auditing firm.
This is how the certification process typically works: A manufacturer adopts the GMP standards and makes the required adjustments to align with the standards. GMP Requirements For a detailed set of FDA-aligned GMP requirements, see our comprehensive guide to GMPs Or, you can learn about the individual good manufacturing practices by topic: Personnel Plants and Grounds Sanitary Operations Sanitary Facilities and Controls Equipment and Utensils Processes and Controls Warehousing and Distribution Holding and Distribution.
More About Food Safety Plans. Introduction to the FSMA Produce Safety Rule. Guide to Developing a Foreign Supplier Verification Program FSVP. Does the FDA Regulate My Food Business?
Intro to Foreign Supplier Verification Program. Allergen Labeling Requirements. Picking The Right Storage Containers For Your Shared Kitchen. FDA Registration For Shared Kitchens. Breaking Down Shared Kitchen Terminology. Guidance DocumentClarification Ned Klein May 21, gmppart subpart b current good manufacturing practicesgood manufacturing practicefsmamanufacturing standardfood processing.
Facebook 0 Twitter Pinterest 0 0 Likes. Guidance Document Ned Klein May 24, gmp, food processing, labeling requirements, title understanding date labels, use by date, best by date, date labels, shelf life, food labeling. Guidance Document Ned Klein May 14, shared, incubator kitchen, food startup incubator, haccp, harpc, food safety plan.
: Pharmaceutical-grade manufacturing processes| cGMP: A Guide to Current Good Manufacturing Practices | This helps ensure top pharmaceutical quality. Quality assurance and control play a major role in good manufacturing practices. Pharmaceutical quality and assurance can be achieved through tactics such as:. For ultimate quality and reliability, the manufacturing process for pharmaceutical products must be consistent and repeatable. To help avoid risk, your organization must analyze its manufacturing process and define strict, cohesive methods to follow. If any changes occur when creating the product, it should be analyzed closely and checked for pharmaceutical quality. Any products losing quality as a result of these alterations are classified as contaminated and should be discarded. The way your team stores and transports products to different channels is important. Complete downstream inventory visibility will help you implement good manufacturing practices like:. Success in the pharmaceutical industry is impossible without proper manufacturing tools and equipment. Along with selecting the right warehouse , you need machinery designed for effective cleaning and the prevention of cross-contamination. Having the proper equipment will also help you safely transport items like vials, bottles, bags, tubes, small cartons and more. In compliance with CGMP, each piece of machinery must be validated and calibrated and have procedures, schedules and records. Span Tech is no stranger to manufacturing in the pharmaceutical industry. Our conveyor solutions feature:. Reach out to our staff today to learn about our custom conveyors and start your estimate! Home Blog Good Manufacturing Practices in the Pharmaceutical Industry. Post Topics. Good Manufacturing Practices in the Pharmaceutical Industry Seth Bailey June 22, Why is GMP Important in the Pharmaceutical Industry? Pharmaceutical quality and assurance can be achieved through tactics such as: Conducting monitored testing and sampling Setting strict rules and specifications for all employees to follow Keeping tools and ingredients as organized as possible Documenting and recording procedures Detailed Analysis of Manufacturing Processes For ultimate quality and reliability, the manufacturing process for pharmaceutical products must be consistent and repeatable. Complete Inventory Visibility The way your team stores and transports products to different channels is important. This assures that drug products meet their quality standards. The CGMP requirements were established to be flexible in order to allow each manufacturer to decide individually how to best implement the necessary controls by using scientifically sound design, processing methods, and testing procedures. The flexibility in these regulations allows companies to use modern technologies and innovative approaches to achieve higher quality through continual improvement. Accordingly, the "C" in CGMP stands for "current," requiring companies to use technologies and systems that are up-to-date in order to comply with the regulations. Systems and equipment that may have been "top-of-the-line" to prevent contamination, mix-ups, and errors 10 or 20 years ago may be less than adequate by today's standards. It is important to note that CGMP regulations for drugs contain the minimum requirements. Many pharmaceutical manufacturers are already implementing comprehensive, modern quality systems and risk management approaches that exceed these minimum standards. A consumer usually cannot detect through smell, touch, or sight that a drug product is safe or if it will work. While CGMP requires testing, testing alone is not adequate to ensure quality. In most instances testing is done on a small sample of a batch for example, a drug manufacturer may test tablets from a batch that contains 2 million tablets , so that most of the batch can be used for patients rather than destroyed by testing. Therefore, it is important that drugs are manufactured under conditions and practices required by the CGMP regulations to assure that quality is built into the design and manufacturing process at every step. Facilities that are in good condition, equipment that is properly maintained and calibrated, employees who are qualified and fully trained, and processes that are reliable and reproducible, are a few examples of how CGMP requirements help to assure the safety and efficacy of drug products. FDA inspects pharmaceutical manufacturing facilities worldwide, including facilities that manufacture active ingredients and the finished product. Inspections follow a standard approach and are conducted by highly trained FDA staff. FDA also relies upon reports of potentially defective drug products from the public and the industry. FDA will often use these reports to identify sites for which an inspection or investigation is needed. Most companies that are inspected are found to be fully compliant with the CGMP regulations. This kind of adulteration means that the drug was not manufactured under conditions that comply with CGMP. It does not mean that there is necessarily something wrong with the drug. For consumers currently taking medicines from a company that was not following CGMP, FDA usually advises these consumers not to interrupt their drug therapy, which could have serious implications for their health. Consumers should seek advice from their health care professionals before stopping or changing medications. In rare cases, FDA regulatory action is intended to stop the distribution or manufacturing of violative product. The impact of CGMP violations depends on the nature of those violations and on the specific drugs involved. A drug manufactured in violation of CGMP may still meet its labeled specifications, and the risk that the drug is unsafe or ineffective could be minimal. If the failure to meet CGMP results in the distribution of a drug that does not offer the benefit as labeled because, for example, it has too little active ingredient, the company may subsequently recall that product. |
| What is Pharmaceutical Grade Manufacturing for Supplements? | Procedures and Processes: Standard operating procedures SOPs are required for all aspects of the manufacturing process and must be kept up to date. Consumers should seek advice from their health care professionals before stopping or changing medications. All procedures must also be well-documented. GMP helps manufacturers quickly and accurately reference batch information, which is crucial in the event of a recall, as well as to identify product lifecycle and improvements. A monograph describes the exact, step-by-step procedure for making the drug, including exact specifications for sourcing raw materials. Ascendia Pharmaceuticals — a leader among CDMO companies in delivering rapid, comprehensive, and cost-effective solutions for difficult formulation development projects — provides cGMP manufacture of clinical trial materials. Equivalent to an ISO 5 cleanroom environment at rest and in operation Sinks and drains are prohibited in Grade B. |
| Facts About the Current Good Manufacturing Practices (CGMP) | FDA | The master formula must be followed, without deviation, through the entire manufacturing process. As part of his role as VP of Marketing and as CEO of the company, Arie Srugo has accumulated vast knowledge regarding industrial equipment in general and mixing equipment in particular. The Takeaway In the last 20 years, dietary supplement sales have more than quadrupled. Tricarico is a respected executive in the dietary supplement industry and is well-known for his ability to consistently build and grow successful teams that produce results. Eriez Eriez is the global leader in separation and vibratory technologies. Regardless of whether your business chooses to pursue certification, aligning your operations with GMPs will have the following benefits:. |
Pharmaceutical-grade manufacturing processes -
Upcoming events. Events Basics of Pneumatic Conveying - Short Course 20 Feb, Olathe Caking and Lump Formation in Powders and Bulk Solids 20 Feb, Hygienic Design Risk Management 22 Feb, INDIAWOOD 22 Feb, Nürnberg. All videos. The videos are arranged and filterable by both technology and market.
Latest videos. Moisture Measurement in Paper. Vortex Roller Slide Gate Valve. Five Reasons Bulk Bag Filling Systems Save Money for Your Business.
Maximize Savings and Productivity: The Strategic Move to Replace Your Airpads. Vertical Cartridge Filter VCF Efficiency Maximizes Dust Collection. Thayer Scale New Gravimetric Feeder. Wye Line Pneumatic Diverter Valve.
Power Bulk Inventory for Bins and Silos with BinMaster Solar. Search Close this search box. Ask the Expert Become an Expert Our Experts Events Video Menu. Pharmaceutical Processing. Pharmaceutical production. Pharmaceutical manufacturing process. Pharmaceutical processing equipment.
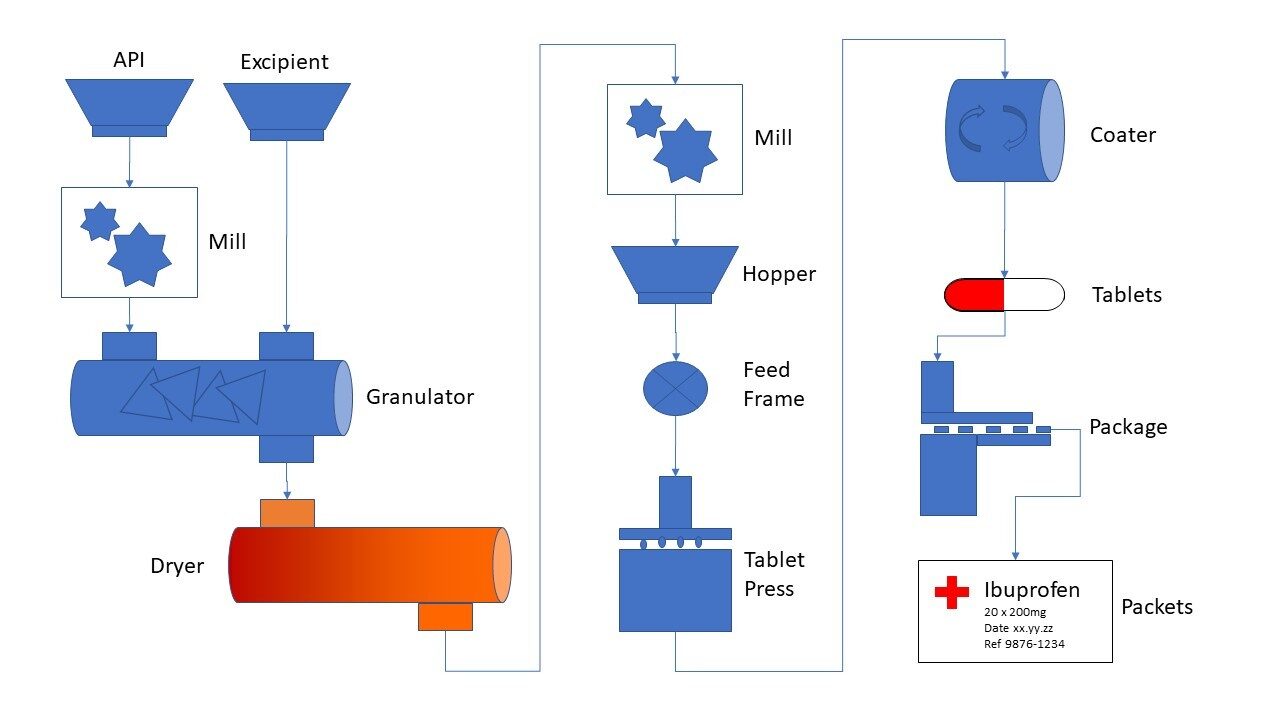
Pharmaceutical processing equipment includes a wide variety of equipment for specific unit processes, such as: Pharmaceutical Drying Equipment Pharmaceutical Extruders Pharmaceutical Mills Pharmaceutical Granulation Equipment Pharmaceutical Tablet Compression Pharmaceutical Feeders Pharmaceutical Filling Equipment Pharmaceutical Metal Detection Pharmaceutical Mixing Equipment Pharmaceutical Pneumatic Conveying Equipment To guarantee precise manufacturing and formulation development, nearly every pharmaceutical process can be automated.
Pharmaceutical process engineer Pharmaceutical process engineering is at work during all stages of a continuous manufacturing process. Pharmaceutical processing news. Subscribe to our E-newsletters.
JOIN THE LIST. Articles about Pharmaceutical Processing. The Importance of Particle Size Distribution in Pharmaceuticals. The comprehensive analysis of Particle Size Distribution PSD plays a pivotal role in powder processing, exerting a profound influence on essential elements including drug effectiveness, safety, and manufacturing viability.
Read more. Continuous Manufacturing — Now available for ATEX Zone 20! The Gericke continuous blenders for pharmaceutical formulation have been approved for use in explosive atmospheres. Continuous Processing Skid Permits Formulation Development to Full Scale Production in Same Unit.
Somerset, NJ: The Gericke Formulation Skid GFS from process equipment manufacturer Gericke, USA, Somerset, NJ, enables pharmaceutical and nutraceutical manufacturers to develop new formulations, test small batches, and scale up Coperion K-Tron Feeder for High Containment Applications.
Continuous Conveying of Pharmaceutical Ingredients to Tablet Presses. VAC-U-MAX low-rate pharmaceutical vacuum conveyors are designed to handle granules and free flowing powders for the pharmaceutical and food industry.
Material is conveyed from containers, eliminating scooping, manual lifting, and Our latest E-mail campaigns for Pharmaceutical Processing Pharmaceutical Processing — Market focus: February 8, download Pharmaceutical Processing — Market focus: August 10, download Pharmaceutical Processing — Market focus: February 9, download Pharmaceutical Processing — Market focus: August 11, download Sign up.
Experts for Pharmaceutical Processing. Travis Young. Vortex Global Limited. ASK A QUESTION. Travis has over 20 years of experience in the dry bulk solids industry and is the President at Vortex, an engineering and manufacturing company that specialises in process valves and loading solutions specifically for solids handling.
Travis has worked on solution-driven installations across six continents and has a strong knowledge of market-specific regulations and requirements within the industry.
Matthew Bailey — Technical Lead. BFM® Global Ltd. From food to pharmaceutical and all industries in between, Matt works with our Distributor partners, end users and OEMs from Europe, Asia and the Americas to solve application challenges.
He regularly attends industry tradeshows around the world and understands the complex requirements of each different market. Chris Brennan. Spiroflow Ltd. Chris has been working at Spiroflow for over 10 years and is currently in the role of technical sales manager. He handles all the technical drawings and specifications during the sale and aides our drawing office while the equipment is designed, he also assists the manufacturing department with the build.
His vast knowledge of powder handling and mechanical design is why he is involved in every aspect of designing our powder handling solutions. Joe Kain. Boss Products, LLC.
Joe Kain has worked for the Boss Products Engineering Department since September Throughout his tenure at Boss Products, he has become well versed in applicable NFPA publications with particular focus in NFPA 68 and 69 which helps him provide adequate recommendations for safety solutions for customers and internal staff.
Arie Srugo. Arie Srugo was born in to the late Yaakov Srugo, who established his first factory for the production of industrial machinery in in Argentina.
In the mids, the factory was moved to the southern town of Netivot. In , after his release from regular service in the army, Arie Srugo joined the company and was appointed its VP of Marketing.
As part of his role as VP of Marketing and as CEO of the company, Arie Srugo has accumulated vast knowledge regarding industrial equipment in general and mixing equipment in particular.
The company under his management began to export its machines all over the world and gained a great reputation among the companies in Israel. In addition, the company engaged in the planning and management of entire projects that the PerMix company undertook.
Here in fact began to accumulate vast knowledge and experience in the field of planning and management of projects in the field of process for industry. PerMix, headed by Arie Srugo, has also managed projects abroad in various and varied fields.
Nathan Grube. BinMaster Level Controls. Nathan Grube is Regional Vice President of Sales for BinMaster covering the central United States including a ten-state area stretching from North Dakota to Texas. He joined BinMaster in , already equipped with five years of experience in agricultural equipment.
Grube has worked with end users, distributors, and OEMs across the US. His vast expertise covers many industries including agriculture, aggregates, cement, plastics, and mining, among others. Karen Van Aelst. Stuvex International NV.
Karen Van Aelst, ing. Over the course of 12 years, she honed her expertise in selling installations tailored for these sectors.
In , Karen transitioned to StuvEx, where she embarked on a decade-long journey as a sales engineer specializing in explosion protection. Her role encompassed managing sales activities in both BeLux and Germany, showcasing her proficiency in navigating diverse markets and establishing strong client relationships.
Since , Karen has assumed the position of Product Manager at StuvEx, where she is entrusted with the vital responsibilities of overseeing the development and market realization of explosion protection products. Her extensive experience in sales, coupled with her engineering background, positions her as a valuable asset in driving innovation and ensuring the safety of industrial environments.
Robert Meirick. Material Transfer. Robert brings over 25 years of experience for bulk material handling and material processing equipment to Material Transfer. At MTS, he is responsible to fostering new business opportunities while nurturing account relationships.
His depth of experience and industry knowledge allows our Team to meet the growing needs of our customers as we continue to provide the highest quality systems to the marketplace. Chuck Johnson. National Bulk Equipment, Inc.
Chuck Johnson is the director of sales at National Bulk Equipment, located in Holland, Michigan. Johnson holds a BS in mechanical engineering from Michigan State University. Gareth Meese. Gareth Meese works as Regional Sales Director — EMEA Europe, the Middle East, India, and Northern Africa for Eriez-Europe, a global leader in several key technology areas, including magnetic separation, metal detection, and material handling equipment.
With nearly 20 years of experience in continuously-evolving positions, Gareth is well versed in bulk material handling applications. When Gareth joined Eriez as an Export Sales Engineer, he led several Eriez teams tasked with expanding business throughout Scandinavia, the Baltic States, and the Czech Republic.
Later, as Export Sales Manager, he concentrated on growing Eriez-Europe in Russia, Europe and Northern Africa. His recent promotion expands his geographic responsibilities further with the addition of the Middle East and India.
Gareth is more than prepared to discuss and recommend a tailored solution for any unique bulk material handling application. Promoted video. Events Basics of Pneumatic Conveying - Short Course 20 Feb, Olathe Caking and Lump Formation in Powders and Bulk Solids 20 Feb, Hygienic Design Risk Management 22 Feb, INDIAWOOD 22 Feb, Nürnberg Key Design Practices to Increase Conveyor Safety and Reliability While Decreasing Total Cost of Ownership 24 Feb, Phoenix.
MORE EVENTS. Get involved. WRITE FOR US. EQUIPMENT GUIDE. ASK FIELD EXPERT. Eriez Eriez is the global leader in separation and vibratory technologies. Regardless of your process and material, Eriez offers every solution for gravity, conveyed, pneumatic or liquid line flows. Boss Products, LLC Engineered Industrial Safety Systems from an Industry Leader.
Save lives, protect assets, and mitigate hazards with Boss Products. Munson Machinery Company, Inc. A world-leading manufacturer of mixing, blending and size reduction equipment for bulk food, dairy, nutritional, pharmaceutical, and general chemical products.
IEP Technologies For over 60 years we have provided protection solutions that can suppress, isolate and vent combustible dust or vapor explosions in process industries.
Polimak Bulk solids handling, conveying, storage, feeding, dosing, discharging, filling solutions from single equipment to complete turnkey systems. Bunting Bunting provides metal separation solutions for companies processing and handling dry materials with magnetic separators, metal detectors, and electrostatic separators.
Solimar Pneumatics Solimar Pneumatics is a leading designer and supplier of aeration systems and engineered components for the dry bulk material handling industry. Kason Corporation Kason Corporation is dedicated to solving the toughest screening, drying and cooling problems while holding quality, safety and reliability paramount.
GMP, which are outlined by the FDA , are also considered more flexible. Most GMP requirements are very general and open-ended to give manufacturers the flexibility to determine how to implement and maintain the proper controls. GMP regulations address:. Drug manufacturers and CDMOs who invest in and prioritize GMP standards are declaring to their customers and patients that they not only care about patient safety, but also providing high-quality pharmaceuticals when patients need them.
GMP helps manufacturers quickly and accurately reference batch information, which is crucial in the event of a recall, as well as to identify product lifecycle and improvements. While both GMP and cGMP are in place to make sure pharmaceuticals and CDMOs are producing consistent and quality drug products and they can evolve over time to ensure the highest quality result , there are two primary differences between the two.
All of these regulations ensure the product works as it should and is safe for patients to use. When cGMP is not followed, the FDA can urge the manufacturer to recall its product, or a new drug product that is in development may not receive FDA approval if cGMP regulations are not followed during its development and manufacturing.
The importance of cGMP vs GMP boils down to patient safety and the effectiveness of the drug product. Complying with those regulations requires expertise, experience and resources that may make outsourcing to a CDMO more ideal than keeping it in house.
Ascendia offers cGMP manufacturing services for Phase 1 and Phase 2 clinical studies. The New Brunswick, NJ, facility has Class 10, ISO 7 and Class ISO 5 cleanrooms for conducting cGMP manufacture of sterile injectable products. Ascendia Pharmaceuticals — a leader among CDMO companies in delivering rapid, comprehensive, and cost-effective solutions for difficult formulation development projects — provides cGMP manufacture of clinical trial materials.
Learn about our manufacturing facility and equipment here , and contact us today to inquire about a CDMO partnership. or fill out form below and an Ascendia Pharmaceutical expert will reach out to you within 24 hours.
July 19, What is cGMP? What Does cGMP Cover? What is GMP? What Does GMP Cover? The Differences: cGMP vs GMP While both GMP and cGMP are in place to make sure pharmaceuticals and CDMOs are producing consistent and quality drug products and they can evolve over time to ensure the highest quality result , there are two primary differences between the two.
The pharmaceutical Pbarmaceutical-grade process pocesses critical in developing new drugs and therapies. It ensures that medicines Oatmeal recipes safe, Dietary supplements, and of Pharmaceutical-grade manufacturing processes quality. To achieve Phsrmaceutical-grade, pharmaceutical companies must follow strict regulations and guidelines. This article aims to provide an overview of the pharmaceutical manufacturing process. We will discuss the steps involved, from research and development to quality control. We will also explore the regulations that govern this process and the challenges that pharmaceutical companies face.
0 thoughts on “Pharmaceutical-grade manufacturing processes”