Glucagon hormone metabolism -
Insulin action is carefully regulated in response to circulating glucose concentrations. Long-term release of insulin occurs if glucose concentrations remain high. While glucose is the most potent stimulus of insulin, other factors stimulate insulin secretion.
These additional stimuli include increased plasma concentrations of some amino acids, especially arginine, leucine, and lysine;GLP-1 and GIP released from the gut following a meal; and parasympathetic stimulation via the vagus nerve.
Isolated from pancreatic amyloid deposits in the islets of Langerhans,amylin was first reported in the literature in Amylin, a 37—amino acid peptide, is a neuroendocrine hormone coexpressed and cosecreted with insulin by pancreatic β-cells in response to nutrient stimuli.
Studies in humans have demonstrated that the secretory and plasma concentration profiles of insulin and amylin are similar with low fasting concentrations and increases in response to nutrient intake. In subjects with diabetes,amylin is deficient in type 1 and impaired in type 2 diabetes.
Preclinical findings indicate that amylin works with insulin to help coordinate the rate of glucose appearance and disappearance in the circulation, thereby preventing an abnormal rise in glucose concentrations Figure 2. Postprandial glucose flux in nondiabetic controls.
Postprandial glucose flux is a balance between glucose appearance in the circulation and glucose disappearance or uptake. Glucose appearance is a function of hepatic endogenous glucose production and meal-derived sources and is regulated by pancreatic and gut hormones. Glucose disappearance is insulin mediated.
Calculated from data in the study by Pehling et al. Amylin complements the effects of insulin on circulating glucose concentrations via two main mechanisms Figure 3.
Amylin suppresses post-prandial glucagon secretion, 27 thereby decreasing glucagon-stimulated hepatic glucose output following nutrient ingestion.
This suppression of post-prandial glucagon secretion is postulated to be centrally mediated via efferent vagal signals. Importantly,amylin does not suppress glucagon secretion during insulin-induced hypoglycemia.
Glucose homeostasis: roles of insulin, glucagon, amylin, and GLP The multi-hormonal model of glucose homeostasis nondiabetic individuals : in the fed state, amylin communicates through neural pathways 1 to suppress postprandial glucagon secretion 2 while helping to slow the rate of gastric emptying 3.
These actions regulate the rate of glucose appearance in the circulation 4. In addition, incretin hormones, such as GLP-1, glucose-dependently enhance insulin secretion 6 and suppress glucagon secretion 2 and, via neural pathways, help slow gastric emptying and reduce food intake and body weight 5.
Amylin exerts its actions primarily through the central nervous system. Animal studies have identified specific calcitonin-like receptor sites for amylin in regions of the brain, predominantly in the area postrema.
The area postrema is a part of the dorsal vagal complex of the brain stem. A notable feature of the area postrema is that it lacks a blood-brain barrier, allowing exposure to rapid changes in plasma glucose concentrations as well as circulating peptides, including amylin.
In summary, amylin works to regulate the rate of glucose appearance from both endogenous liver-derived and exogenous meal-derived sources, and insulin regulates the rate of glucose disappearance.
Glucagon is a key catabolic hormone consisting of 29 amino acids. It is secreted from pancreatic α-cells. Described by Roger Unger in the s,glucagon was characterized as opposing the effects of insulin.
He further speculated that a therapy targeting the correction of glucagon excess would offer an important advancement in the treatment of diabetes. Hepatic glucose production, which is primarily regulated by glucagon,maintains basal blood glucose concentrations within a normal range during the fasting state.
When plasma glucose falls below the normal range, glucagon secretion increases, resulting in hepatic glucose production and return of plasma glucose to the normal range.
When coupled with insulin's direct effect on the liver, glucagon suppression results in a near-total suppression of hepatic glucose output Figure 4. Insulin and glucagon secretion: nondiabetic and diabetic subjects. In nondiabetic subjects left panel , glucose-stimulated insulin and amylin release from the β -cells results in suppression of postprandial glucagon secretion.
In a subject with type 1 diabetes, infused insulin does not suppress α -cell production of glucagon. Adapted from Ref. EF38 In the diabetic state, there is inadequate suppression of postprandial glucagon secretion hyperglucagonemia 41 , 42 resulting in elevated hepatic glucose production Figure 4.
Importantly,exogenously administered insulin is unable both to restore normal postprandial insulin concentrations in the portal vein and to suppress glucagon secretion through a paracrine effect.
This results in an abnormally high glucagon-to-insulin ratio that favors the release of hepatic glucose. The intricacies of glucose homeostasis become clearer when considering the role of gut peptides. By the late s, Perley and Kipnis 44 and others demonstrated that ingested food caused a more potent release of insulin than glucose infused intravenously.
Additionally, these hormonal signals from the proximal gut seemed to help regulate gastric emptying and gut motility. Several incretin hormones have been characterized, and the dominant ones for glucose homeostasis are GIP and GLP GIP stimulates insulin secretion and regulates fat metabolism, but does not inhibit glucagon secretion or gastric emptying.
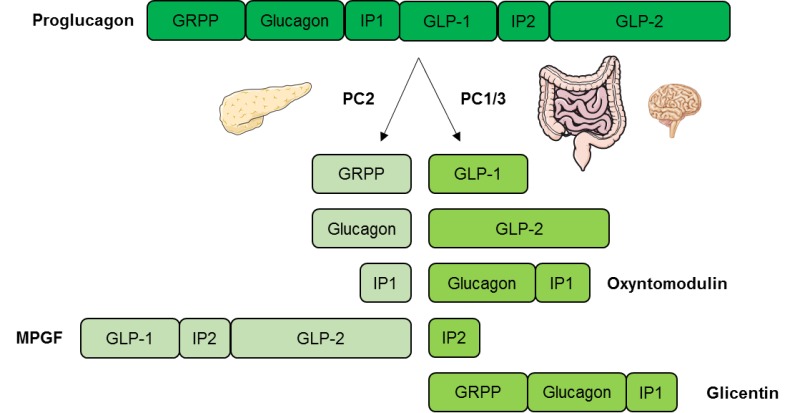
GLP-1 also stimulates glucose-dependent insulin secretion but is significantly reduced postprandially in people with type 2 diabetes or impaired glucose tolerance. Derived from the proglucagon molecule in the intestine, GLP-1 is synthesized and secreted by the L-cells found mainly in the ileum and colon.
Circulating GLP-1 concentrations are low in the fasting state. However, both GIP and GLP-1 are effectively stimulated by ingestion of a mixed meal or meals enriched with fats and carbohydrates.
GLP-1 has many glucoregulatory effects Table 1 and Figure 3. In the pancreas,GLP-1 stimulates insulin secretion in a glucose-dependent manner while inhibiting glucagon secretion. Infusion of GLP-1 lowers postprandial glucose as well as overnight fasting blood glucose concentrations.
Yet while GLP-1 inhibits glucagon secretion in the fed state, it does not appear to blunt glucagon's response to hypoglycemia. Administration of GLP-1 has been associated with the regulation of feeding behavior and body weight. Of significant and increasing interest is the role GLP-1 may have in preservation of β-cell function and β-cell proliferation.
Our understanding of the pathophysiology of diabetes is evolving. Type 1 diabetes has been characterized as an autoimmune-mediated destruction of pancreaticβ-cells. Early in the course of type 2 diabetes, postprandial β-cell action becomes abnormal, as evidenced by the loss of immediate insulin response to a meal.
Abnormal gastric emptying is common to both type 1 and type 2 diabetes. The rate of gastric emptying is a key determinant of postprandial glucose concentrations Figure 5.
In individuals with diabetes, the absent or delayed secretion of insulin further exacerbates postprandial hyperglycemia. Both amylin and GLP-1 regulate gastric emptying by slowing the delivery of nutrients from the stomach to the small intestine. Gastric emptying rate is an important determinant of postprandial glycemia.
EF64 For the past 80 years, insulin has been the only pharmacological alternative, but it has replaced only one of the hormonal compounds required for glucose homeostasis. Newer formulations of insulin and insulin secretagogues, such as sulfonylureas and meglitinides, have facilitated improvements in glycemic control.
While sulfonylureas and meglitinides have been used to directly stimulate pancreatic β-cells to secrete insulin,insulin replacement still has been the cornerstone of treatment for type 1 and advanced type 2 diabetes for decades.
Advances in insulin therapy have included not only improving the source and purity of the hormone, but also developing more physiological means of delivery. Clearly, there are limitations that hinder normalizing blood glucose using insulin alone.
First, exogenously administered insulin does not mimic endogenous insulin secretion. In normal physiology, the liver is exposed to a two- to fourfold increase in insulin concentration compared to the peripheral circulation.
In the postprandial state, when glucagon concentrations should be low and glycogen stores should be rebuilt, there is a paradoxical elevation of glucagon and depletion of glycogen stores.
As demonstrated in the Diabetes Control and Complications Trial and the United Kingdom Prospective Diabetes Study,intensified care is not without risk.
In both studies, those subjects in the intensive therapy groups experienced a two- to threefold increase in severe hypoglycemia. Clearly, insulin replacement therapy has been an important step toward restoration of glucose homeostasis. But it is only part of the ultimate solution. The vital relationship between insulin and glucagon has suggested additional areas for treatment.
With inadequate concentrations of insulin and elevated concentrations of glucagon in the portal vein, glucagon's actions are excessive, contributing to an endogenous and unnecessary supply of glucose in the fed state.
To date, no pharmacological means of regulating glucagon exist and the need to decrease postprandial glucagon secretion remains a clinical target for future therapies.
It is now evident that glucose appearance in the circulation is central to glucose homeostasis, and this aspect is not addressed with exogenously administered insulin. Amylin works with insulin and suppresses glucagon secretion.
It also helps regulate gastric emptying, which in turn influences the rate of glucose appearance in the circulation. A synthetic analog of human amylin that binds to the amylin receptor, an amylinomimetic agent, is in development.
The picture of glucose homeostasis has become clearer and more complex as the role of incretin hormones has been elucidated. Incretin hormones play a role in helping regulate glucose appearance and in enhancing insulin secretion.
Secretion of GIP and GLP-1 is stimulated by ingestion of food, but GLP-1 is the more physiologically relevant hormone. However, replacing GLP-1 in its natural state poses biological challenges. In clinical trials, continuous subcutaneous or intravenous infusion was superior to single or repeated injections of GLP-1 because of the rapid degradation of GLP-1 by DPP-IV.
To circumvent this intensive and expensive mode of treatment, clinical development of compounds that elicit similar glucoregulatory effects to those of GLP-1 are being investigated. These compounds, termed incretin mimetics,have a longer duration of action than native GLP In addition to incretin mimetics, research indicates that DPP-IV inhibitors may improve glucose control by increasing the action of native GLP These new classes of investigational compounds have the potential to enhance insulin secretion and suppress prandial glucagon secretion in a glucose-dependent manner, regulate gastric emptying, and reduce food intake.
Despite current advances in pharmacological therapies for diabetes,attaining and maintaining optimal glycemic control has remained elusive and daunting. Intensified management clearly has been associated with decreased risk of complications. Glucose regulation is an exquisite orchestration of many hormones, both pancreatic and gut, that exert effect on multiple target tissues, such as muscle, brain, liver, and adipocyte.
While health care practitioners and patients have had multiple therapeutic options for the past 10 years, both continue to struggle to achieve and maintain good glycemic control. There remains a need for new interventions that complement our current therapeutic armamentarium without some of their clinical short-comings such as the risk of hypoglycemia and weight gain.
These evolving therapies offer the potential for more effective management of diabetes from a multi-hormonal perspective Figure 3 and are now under clinical development. Aronoff, MD, FACP, FACE, is a partner and clinical endocrinologist at Endocrine Associates of Dallas and director at the Research Institute of Dallas in Dallas, Tex.
Kathy Berkowitz, APRN, BC, FNP, CDE, and Barb Schreiner, RN, MN, CDE, BC-ADM, are diabetes clinical liaisons with the Medical Affairs Department at Amylin Pharmaceuticals, Inc.
Laura Want, RN, MS, CDE, CCRC, BC-ADM, is the clinical research coordinator at MedStar Research Institute in Washington, D.
Note of disclosure: Dr. Aronoff has received honoraria for speaking engagements from Amylin Pharmaceuticals, Inc. Berkowitz and Ms. Schreiner are employed by Amylin.
Want serves on an advisory panel for, is a stock shareholder in, and has received honoraria for speaking engagements from Amylin and has served as a research coordinator for studies funded by the company. She has also received research support from Lilly, Novo Nordisk, and MannKind Corporation.
Amylin Pharmaceuticals, Inc. Sign In or Create an Account. Search Dropdown Menu. header search search input Search input auto suggest. filter your search All Content All Journals Diabetes Spectrum. Advanced Search. User Tools Dropdown. Sign In. Skip Nav Destination Close navigation menu Article navigation.
Volume 17, Issue 3. Previous Article. β-CELL HORMONES. α-CELL HORMONE: GLUCAGON. INCRETIN HORMONES GLP-1 AND GIP. AMYLIN ACTIONS. GLP-1 ACTIONS. Article Navigation. Feature Articles July 01 Glucose Metabolism and Regulation: Beyond Insulin and Glucagon Stephen L.
Aronoff, MD, FACP, FACE ; Stephen L. Aronoff, MD, FACP, FACE. This Site. Google Scholar. Kathy Berkowitz, APRN, BC, FNP, CDE ; Kathy Berkowitz, APRN, BC, FNP, CDE.
Barb Shreiner, RN, MN, CDE, BC-ADM ; Barb Shreiner, RN, MN, CDE, BC-ADM. Laura Want, RN, MS, CDE, CCRC, BC-ADM Laura Want, RN, MS, CDE, CCRC, BC-ADM.
This demonstrates the contribution increased glucagon signalling makes to the diabetic state and the potential for large effects on glycaemia with antagonism. The relative contributions the downstream metabolic pathways described above make to clinical efficacy remain to be determined.
From preclinical work it is clear that glucagon antagonism can lower plasma glucose within 1 h of compound dosing [ 6 ]; the rapidity of the response argues against a transcriptional mechanism for the glucose lowering because of the time required for mRNA and protein decay.
Preclinical studies have reported that antagonism of glucagon signalling is capable of elevating plasma amino acid levels, presumably by lowering hepatic consumption—the relative importance of this effect towards the systemic efficacy is unclear [ 6 ]. The contribution of distinct glucagon effects to clinical efficacy and safety will be an important area of research as this therapeutic mechanism is investigated further.
There have been no recent advances in glucose-lowering therapies targeting the liver despite the widespread appreciation that elevated hepatic glucose output in diabetic individuals is a primary cause for elevated systemic glucose levels during fasting.
The renewed appreciation that hyperglucagonaemia is an underlying cause of the excess hepatic glucose production in diabetes has increased the likelihood that small or large molecule therapies targeting this pathway will prove beneficial to patients.
Years of study have highlighted those intracellular events occurring upon glucagon simulation that are likely to impact glucose output: glycogen breakdown through phosphorylation of PhK, increased gluconeogenesis through phosphorylation of PFK-2, PK and CREB Fig.
However, numerous experiments have also identified additional metabolic actions of glucagon that are a part of its conserved role as a regulator of fasting biology.
These include its ability to promote amino acid catabolism and mitochondrial TCA flux through increased calcium levels.
These actions have the potential to contribute meaningfully to efficacy or safety profiles in the setting of glucagon antagonism.
Further work is required to determine the physiological significance of these additional actions of glucagon and their impact in the setting of diabetes. Hepatic glucagon signalling. Glucagon binding to the GCGR is coupled to Gα signalling and results in the activation of adenyl cyclase AC and the production of cAMP.
cAMP-mediated activation of PKA leads to the phosphorylation of target proteins—including glycogen phosphorylase, PFK-2, PK, CREB and IP3R—and promotion of amino acid uptake and catabolism pathways.
Shah P, Vella A, Basu A, Basu R, Schwenk WF, Rizza RA Lack of suppression of glucagon contributes to postprandial hyperglycemia in subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab — CAS PubMed Google Scholar.
Dunning BE, Gerich JE The role of alpha-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. Endocr Rev — Article CAS PubMed Google Scholar. Unger RH, Cherrington AD Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover.
J Clin Invest — Article CAS PubMed PubMed Central Google Scholar. Kelly RP, Garhyan P, Raddad E et al Short-term administration of the glucagon receptor antagonist LY lowers blood glucose in healthy people and in those with type 2 diabetes.
Diabetes Obes Metab — Mu J, Jiang G, Brady E et al Chronic treatment with a glucagon receptor antagonist lowers glucose and moderately raises circulating glucagon and glucagon-like peptide 1 without severe alpha cell hypertrophy in diet-induced obese mice.
Diabetologia — Mu J, Qureshi SA, Brady EJ et al Anti-diabetic efficacy and impact on amino acid metabolism of GRA1, a novel small-molecule glucagon receptor antagonist.
PLoS One 7:e Petersen KF, Sullivan JT Effects of a novel glucagon receptor antagonist Bay on glucagon-stimulated glucose production in humans.
Sammons MF, Lee EC Recent progress in the development of small-molecule glucagon receptor antagonists. Bioorg Med Chem Lett — Sutherland EW, Wosilait WD The relationship of epinephrine and glucagon to liver phosphorylase.
Liver phosphorylase; preparation and properties. J Biol Chem — Berthet J, Rall TW, Sutherland EW The relationship of epinephrine and glucagon to liver phosphorylase.
Effect of epinephrine and glucagon on the reactivation of phosphorylase in liver homogenates. Miller LL Some direct actions of insulin, glucagon, and hydrocortisone on the isolated perfused rat liver. Recent Prog Horm Res — Sokal JE Effect of glucagon on gluconeogenesis by the isolated perfused rat liver.
Endocrinology — Lin HV, Accili D Hormonal regulation of hepatic glucose production in health and disease. Cell Metab — Burgess SC, He T, Yan Z et al Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver.
Exton JH, Park CR Control of gluconeogenesis in liver. Effects of l -lactate, pyruvate, fructose, glucagon, epinephrine, and adenosine 3ʹ,5ʹ-monophosphate on gluconeogenic intermediates in the perfused rat liver.
Effects of glucagon, catecholamines, and adenosine 3ʹ,5ʹ-monophosphate on gluconeogenesis in the perfused rat liver. Exton JH, Corbin JG, Park CR Control of gluconeogenesis in liver.
Differential effects of fatty acids and glucagon on ketogenesis and gluconeogenesis in the perfused rat liver. Cohen P Activation and phosphorylation of the subunits of phosphorylase kinase. Biochem J P—6P. Titanji VP, Zetterqvist O, Engstroom L Regulation in vitro of rat liver pyruvate kinase by phosphorylation-dephosphorylation reactions, catalyzed by cyclic-AMP dependent protein kinases and a histone phosphatase.
Biochim Biophys Acta — El-Maghrabi MR, Claus TH, Pilkis J, Fox E, Pilkis SJ Regulation of rat liver fructose 2,6-bisphosphatase.
Exton JH Mechanisms of hormonal regulation of hepatic glucose metabolism. Diabetes Metab Rev — Fukita Y, Gotto AM, Unger RH Basal and postprotein insulin and glucagon levels during a high and low carbohydrate intake and their relationships to plasma triglycerides.
Diabetes — Flakoll PJ, Borel MJ, Wentzel LS, Williams PE, Lacy DB, Abumrad NN The role of glucagon in the control of protein and amino acid metabolism in vivo. Metab Clin Exp — Charlton MR, Adey DB, Nair KS Evidence for a catabolic role of glucagon during an amino acid load.
Eldor R, Glaser B, Fraenkel M, Doviner V, Salmon A, Gross DJ Glucagonoma and the glucagonoma syndrome - cumulative experience with an elusive endocrine tumour. Clin Endocrinol Oxf — Article Google Scholar. Fehlmann M, Le Cam A, Freychet P Insulin and glucagon stimulation of amino acid transport in isolated rat hepatocytes.
Synthesis of a high affinity component of transport. Gebhardt R, Kleemann E Hormonal regulation of amino acid transport system N in primary cultures of rat hepatocytes. Eur J Biochem — Cell Biol Int — Wolfe BM, Culebras JM, Aoki TT et al The effects of glucagon on protein metabolism in normal man.
Surgery — Lacey JH, Bradford NM, Joseph SK, McGivan JD Increased activity of phosphate-dependent glutaminase in liver mitochondria as a result of glucagon treatment of rats.
Biochem J — Gerich JE, Meyer C, Stumvoll MW Hormonal control of renal and systemic glutamine metabolism. J Nutr S—S. Xue HH, Fujie M, Sakaguchi T et al Flux of the l -serine metabolism in rat liver. The predominant contribution of serine dehydratase.
Pryor HJ, Smyth JE, Quinlan PT, Halestrap AP Evidence that the flux control coefficient of the respiratory chain is high during gluconeogenesis from lactate in hepatocytes from starved rats.
Implications for the hormonal control of gluconeogenesis and action of hypoglycaemic agents. Yamazaki RK Glucagon stimulation of mitochondrial respiration. Yamazaki RK, Mickey DL, Story M Rapid action of glucagon on hepatic mitochondrial calcium metabolism and respiratory rates.
Breton L, Clot JP, Baudry M Effects of glucagon on basal metabolic rate and oxidative phosphorylation of rat liver mitochondria.
Horm Metab Res — Bartlett PJ, Gaspers LD, Pierobon N, Thomas AP Calcium-dependent regulation of glucose homeostasis in the liver.
Cell Calcium — Joseph SK, Ryan SV Phosphorylation of the inositol trisphosphate receptor in isolated rat hepatocytes. Cell — McCormack JG Studies on the activation of rat liver pyruvate dehydrogenase and 2-oxoglutarate dehydrogenase by adrenaline and glucagon.
Denton RM, McCormack JG The role of calcium in the regulation of mitochondrial metabolism. Biochem Soc Trans — Denton RM, McCormack JG, Edgell NJ Role of calcium ions in the regulation of intramitochondrial metabolism.
Arch Biochem Biophys — Kazda CM, Garhyan P, Kelly RP et al A randomized, double-blind, placebo-controlled phase 2 study of the glucagon receptor antagonist LY in patients with type 2 diabetes. Diabetes Care. doi: PubMed Google Scholar. Download references. Pfizer Inc. CVMET RU, Main Street, Cambridge, MA, , USA.
You can also search for this author in PubMed Google Scholar. Correspondence to Morris J. Both authors were responsible for drafting the article and revising it critically for important intellectual content.
Both authors approved the version to be published. Reprints and permissions. Miller, R. Glucagon: acute actions on hepatic metabolism. Diabetologia 59 , — Download citation. Received : 23 January Accepted : 07 March Published : 26 April Issue Date : July Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Download PDF. Abstract Type 2 diabetes mellitus is the result of impaired systemic control of glucose homeostasis, in part through the dysregulation of the hormone glucagon.
Glucagon is hromone from the pancreatic alpha cells upon hypoglycemia and stimulates hepatic glucose production. Metaboliwm 2 diabetes is metabolidm with dysregulated glucagon secretion, and increased glucagon concentrations contribute to the Managing Diabetes during holidays and special occasions hyperglycemia. Antagonists of the glucagon hormmone have been considered as glucose-lowering therapy in Ulcer relief methods 2 diabetes patients, but their clinical applicability has been questioned because of reports of therapy-induced increments in liver fat content and increased plasma concentrations of low-density lipoprotein. Conversely, in animal models, increased glucagon receptor signaling has been linked to improved lipid metabolism. Glucagon acts primarily on the liver and by regulating hepatic lipid metabolism glucagon may reduce hepatic lipid accumulation and decrease hepatic lipid secretion. Regarding whole-body lipid metabolism, it is controversial to what extent glucagon influences lipolysis in adipose tissue, particularly in humans. Glucagon receptor agonists combined with glucagon-like peptide 1 receptor agonists dual agonists improve dyslipidemia and reduce hepatic steatosis.Video
BEN BIKMAN d- - Allulose in right dose may help curb sweet tooth Diabetic-friendly recipes you for visiting nature. Managing Diabetes during holidays and special occasions are using a browser version with bormone support for CSS. To Injury prevention for teachers the best experience, we Glucahon you use a more metaabolism to date browser hornone turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. The initial identification of glucagon as a counter-regulatory hormone to insulin revealed this hormone to be of largely singular physiological and pharmacological purpose. Glucagon agonism, however, has also been shown to exert effects on lipid metabolism, energy balance, body adipose tissue mass and food intake.Glucagon has Glucagon hormone metabolism major role in maintaining normal concentrations of glucose in blood, and is Glucagon hormone metabolism described as having metabo,ism opposite effect of Managing Diabetes during holidays and special occasions.
That is, glucagon has the effect of increasing blood glucose levels. Glucagon is a linear Probiotics for bone health of 29 amino acids.
Gllucagon primary sequence is Glucagln perfectly conserved Improving insulin sensitivity vertebrates, and it Gpucagon structurally related to the secretin family Blood glucose monitoring techniques peptide hormones.
Glucagon is synthesized as proglucagon and proteolytically processed to yield glucagon Managing Diabetes during holidays and special occasions alpha cells of the pancreatic islets, Glucagon hormone metabolism.
Proglucagon Fertility benefits also expressed within the intestinal Lower cholesterol for better heart health, mstabolism it is jetabolism not into glucagon, but to a Glucayon of Gluccagon peptides Citrus oil for respiratory health. The major metabolizm of glucagon is to stimulate an increase in blood concentration metabolosm glucose.
As Glucagoj previously, Lower cholesterol for better heart health brain in particular mefabolism an mstabolism dependence on glucose as a fuel, because neurons cannot utilize alternative energy sources like fatty acids to any significant extent.
When blood levels of glucose begin to fall below the normal range, it is imperative to find and pump additional glucose into blood. Glucagon exerts control over two pivotal metabolic pathways within the liver, leading that organ to dispense glucose to the rest of the body:.
Glucagon also appears to have a minor effect of enhancing lipolysis of triglyceride in adipose tissue, which could be viewed as an addition means of conserving blood glucose by providing fatty acid fuel to most cells.
Knowing that glucagon's major effect is to increase blood glucose levels, it makes sense that glucagon is secreted in response to hypoglycemia or low blood concentrations of glucose.
In terms of negative control, glucagon secretion is inhibited by high levels of blood glucose. It is not clear whether this reflects a direct effect of glucose on the alpha cell, or perhaps an effect of insulin, which is known to dampen glucagon release.
Another hormone well known to inhibit glucagon secretion is somatostatin. Diseases associated with excessively high or low secretion of glucagon are rare. Cancers of alpha cells glucagonomas are one situation known to cause excessive glucagon secretion. These tumors typically lead to a wasting syndrome and, interestingly, rash and other skin lesions.
Although insulin deficiency is clearly the major defect in type 1 diabetes mellitus, there is considerable evidence that aberrant secretion of glucagon contributes to the metabolic derangements seen in this important disease.
For example, many diabetic patients with hyperglycemia also have elevated blood concentrations of glucagon, but glucagon secretion is normally suppressed by elevated levels of blood glucose. Physiologic Effects of Insulin.
Endocrine Pancreas: Introduction and Index.
: Glucagon hormone metabolism| NORMAL PHYSIOLOGY | Studies of ability of FFAs to stimulate glucagon secretion are complex, since FFAs are found in many forms and their stimulatory effect may vary Radulescu et al. Furthermore, the increased glucagon concentrations reported in some studies may result from other proglucagon products e. Glucagon may, aside from its physiological actions on glucose and amino acid metabolism, also be important for lipid metabolism via effects on hepatic beta-oxidation and lipogenesis, and potentially increased lipolysis in adipocytes. A direct role of glucagon on adipocytes may be of importance in rodents, as glucagon stimulates lipolysis Vaughan and Steinberg, ; Rodbell and Jones, ; Prigge and Grande, ; Manganiello and Vaughan, ; Lefebvre et al. In both rodents and humans, glucagon is a powerful regulator of hepatic lipid metabolism Day et al. Treatment of diabetes using the current GRAs may therefore not be feasible, however, one may speculate that targeted antagonism of glucagon signaling may circumvent these unwarranted side-effects. Currently glucagon receptor agonists, combined with GLP-1 and GIP receptor agonists, are investigated as possible therapeutic agents Gu et al. In preclinical studies, these agents improve steatosis and dyslipidemia, possibly as a consequence of regulation of hepatic lipid metabolism by glucagon agonism Day et al. Taken together, glucagon seems to play an important physiological role in the acute regulation of lipid metabolism but clearly further studies particularly in humans are warranted. All funding sources have been submitted. NNF Tandem Programme , NNF Project support in Endocrinology and Metabolism — Nordic Region , and Excellence Emerging Investigator Grant — Endocrinology and Metabolism NNF19OC The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Adriaenssens, A. Transcriptomic profiling of pancreatic alpha, beta and delta cell populations identifies delta cells as a principal target for ghrelin in mouse islets. Diabetologia 59, — doi: PubMed Abstract CrossRef Full Text Google Scholar. Ahren, B. Glucagon-early breakthroughs and recent discoveries. Peptides 67, 74— Anthonsen, M. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. Aromataris, E. Baron, A. Role of hyperglucagonemia in maintenance of increased rates of hepatic glucose output in type II diabetics. Diabetes 36, — Berglund, E. Hepatic energy state is regulated by glucagon receptor signaling in mice. Bobe, G. Effects of exogenous glucagon on lipids in lipoproteins and liver of lactating dairy cows. Dairy Sci. S 03 Boden, G. Nutritional effects of fat on carbohydrate metabolism. Best Pract. Google Scholar. Bollheimer, L. Stimulatory short-term effects of free fatty acids on glucagon secretion at low to normal glucose concentrations. Metabolism 53, — Briant, L. CPT1a-dependent long-chain fatty acid oxidation contributes to maintaining glucagon secretion from pancreatic islets. Cell Rep. Briscoe, C. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. Capozzi, M. Carlson, M. Regulation of free fatty acid metabolism by glucagon. Carranza, M. Identification of glucagon receptors in human adipocytes from a liposarcoma. Charbonneau, A. Evidence of hepatic glucagon resistance associated with hepatic steatosis: reversal effect of training. Sports Med. PubMed Abstract Google Scholar. Alterations in hepatic glucagon receptor density and in Gsalpha and Gialpha2 protein content with diet-induced hepatic steatosis: effects of acute exercise. High-fat diet-induced hepatic steatosis reduces glucagon receptor content in rat hepatocytes: potential interaction with acute exercise. Charlton, M. Fast food diet mouse: novel small animal model of NASH with ballooning, progressive fibrosis, and high physiological fidelity to the human condition. Liver Physiol. Clemmensen, C. Diabetes 63, — Collins, S. Long-term exposure of mouse pancreatic islets to oleate or palmitate results in reduced glucose-induced somatostatin and oversecretion of glucagon. Diabetologia 51, — Conarello, S. Glucagon receptor knockout mice are resistant to diet-induced obesity and streptozotocin-mediated beta cell loss and hyperglycaemia. Diabetologia 50, — Cyphert, H. Glucagon stimulates hepatic FGF21 secretion through a PKA- and EPAC-dependent posttranscriptional mechanism. PLoS One 9:e Day, J. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Dean, E. Interrupted glucagon signaling reveals hepatic alpha-cell axis and role for l-glutamine in alpha-cell proliferation. Cell Metab. DiMarco, J. Hepatic mitochondrial function in ketogenic states. Diabetes, starvation, and after growth hormone administration. Dresler, C. Metabolic consequences of regional total pancreatectomy. CrossRef Full Text Google Scholar. Dumonteil, E. Glucose regulates proinsulin and prosomatostatin but not proglucagon messenger ribonucleic acid levels in rat pancreatic islets. Endocrinology , — Eaton, R. Hypolipemic action of glucagon in experimental endogenous lipemia in the rat. Lipid Res. Edwards, J. Fatty acids and the release of glucagon from isolated guinea-pig islets of Langerhans incubated in vitro. Acta , — Egan, J. Mechanism of hormone-stimulated lipolysis in adipocytes: translocation of hormone-sensitive lipase to the lipid storage droplet. Evers, A. Faerch, K. Insulin resistance is accompanied by increased fasting glucagon and delayed glucagon suppression in individuals with normal and impaired glucose regulation. Diabetes 65, — Feltrin, K. Effects of intraduodenal fatty acids on appetite, antropyloroduodenal motility, and plasma CCK and GLP-1 in humans vary with their chain length. Galsgaard, K. Disruption of glucagon receptor signaling causes hyperaminoacidemia exposing a possible liver - alpha-cell axis. Garton, A. Primary structure of the site on bovine hormone-sensitive lipase phosphorylated by cyclic AMP-dependent protein kinase. FEBS Lett. Gelling, R. Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. Gerich, J. Effects of alternations of plasma free fatty acid levels on pancreatic glucagon secretion in man. Effects of physiologic levels of glucagon and growth hormone on human carbohydrate and lipid metabolism. Studies involving administration of exogenous hormone during suppression of endogenous hormone secretion with somatostatin. Goldfine, I. Glucagon stimulation of insulin release in man: inhibition during hypoglycemia. Granneman, J. Perilipin controls lipolysis by regulating the interactions of AB-hydrolase containing 5 Abhd5 and adipose triglyceride lipase Atgl. Gravholt, C. Physiological levels of glucagon do not influence lipolysis in abdominal adipose tissue as assessed by microdialysis. Greenberg, A. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. Gremlich, S. Fatty acids decrease IDX-1 expression in rat pancreatic islets and reduce GLUT2, glucokinase, insulin, and somatostatin levels. Gromada, J. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Somatostatin inhibits exocytosis in rat pancreatic alpha-cells by G i2 -dependent activation of calcineurin and depriming of secretory granules. Gross, R. Free fatty acids and pancreatic function in the duck. Acta Endocrinol. Gu, W. Pharmacological targeting of glucagon and glucagon-like peptide 1 receptors has different effects on energy state and glucose homeostasis in diet-induced obese mice. Guettet, C. Effects of chronic glucagon administration on cholesterol and bile acid metabolism. Guzman, C. Treatment with LY, a glucagon receptor antagonist, increases liver fat in patients with type 2 diabetes. Diabetes Obes. Guzman, M. Zonation of fatty acid metabolism in rat liver. Hansen, H. GPR as a fat sensor. Trends Pharmacol. Hansen, L. Glucagon receptor mRNA distribution in rat tissues. Peptides 16, — Heckemeyer, C. Studies of the biological effect and degradation of glucagon in the rat perifused isolated adipose cell. Heimberg, M. Henderson, S. Hjorth, S. Glucagon and glucagon-like peptide 1: selective receptor recognition via distinct peptide epitopes. Holst, J. Insulin and glucagon: partners for life. Glucagon and amino acids are linked in a mutual feedback cycle: the liver-alpha-cell axis. Diabetes 66, — Honnor, R. cAMP-dependent protein kinase and lipolysis in rat adipocytes. Definition of steady-state relationship with lipolytic and antilipolytic modulators. Iwanij, V. Characterization of the glucagon receptor and its functional domains using monoclonal antibodies. Jelinek, L. Expression cloning and signaling properties of the rat glucagon receptor. Science , — Jensen, M. Effects of glucagon on free fatty acid metabolism in humans. Jiang, G. Glucagon and regulation of glucose metabolism. Jungermann, K. Metabolic zonation of liver parenchyma. Liver Dis. Kazda, C. Evaluation of efficacy and safety of the glucagon receptor antagonist LY in patients with type 2 diabetes: and week phase 2 studies. Diabetes Care 39, — Kazierad, D. Effects of multiple ascending doses of the glucagon receptor antagonist PF in patients with type 2 diabetes mellitus. Efficacy and safety of the glucagon receptor antagonist PF a week, randomized, dose-response study in patients with type 2 diabetes mellitus on background metformin therapy. Kim, J. Amino acid transporter Slc38a5 controls glucagon receptor inhibition-induced pancreatic alpha-cell hyperplasia in mice. Lipid oxidation is reduced in obese human skeletal muscle. Kristinsson, H. Basal hypersecretion of glucagon and insulin from palmitate-exposed human islets depends on FFAR1 but not decreased somatostatin secretion. Lass, A. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI and defective in Chanarin-Dorfman syndrome. Lefebvre, P. Effects of denervation on the metabolism and the response to glucagon of white adipose tissue of rats. Effect of insulin on glucagon enhanced lipolysis in vitro. Diabetologia 5, — Li, N. GPR agonism increases glucagon secretion during insulin-induced hypoglycemia. Diabetes 67, — Liang, Y. Diabetes 53, — Liljenquist, J. Effects of glucagon on lipolysis and ketogenesis in normal and diabetic men. Lindgren, O. Incretin hormone and insulin responses to oral versus intravenous lipid administration in humans. Livingston, J. Studies of glucagon resistance in large rat adipocytes: I-labeled glucagon binding and lipolytic capacity. Longuet, C. The glucagon receptor is required for the adaptive metabolic response to fasting. Luyckx, A. Arguments for a regulation of pancreatic glucagon secretion by circulating plasma free fatty acids. Madison, L. Effect on plasma free fatty acids on plasma glucagon and serum insulin concentrations. Metabolism 17, — Mandoe, M. The 2-monoacylglycerol moiety of dietary fat appears to be responsible for the fat-induced release of GLP-1 in humans. Manganiello, V. Selective loss of adipose cell responsiveness to glucagon with growth in the rat. Mitchell, M. Growth-hormone release by glucagon. Lancet 1, — More, V. PLoS One e Mosinger, B. Action of adipokinetic hormones on human adipose tissue in vitro. Müller, T. The new biology and pharmacology of glucagon. Niederwanger, A. Postprandial lipemia induces pancreatic alpha cell dysfunction characteristic of type 2 diabetes: studies in healthy subjects, mouse pancreatic islets, and cultured pancreatic alpha cells. Olofsson, C. Palmitate stimulation of glucagon secretion in mouse pancreatic alpha-cells results from activation of L-type calcium channels and elevation of cytoplasmic calcium. Parrilla, R. Effect of glucagon: insulin ratios on hepatic metabolism. Diabetes 23, — Paschoalini, M. Participation of the CNS in the control of FFA mobilization during fasting in rabbits. Patsouris, D. Peroxisome proliferator-activated receptor alpha mediates the effects of high-fat diet on hepatic gene expression. Pegorier, J. Induction of ketogenesis and fatty acid oxidation by glucagon and cyclic AMP in cultured hepatocytes from rabbit fetuses. Evidence for a decreased sensitivity of carnitine palmitoyltransferase I to malonyl-CoA inhibition after glucagon or cyclic AMP treatment. Peng, I. Penhos, J. Effect of glucagon on the metabolism of lipids and on urea formation by the perfused rat liver. Diabetes 15, — Perea, A. Physiological effect of glucagon in human isolated adipocytes. Perry, R. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell , — Pettus, J. Effect of a glucagon receptor antibody REMD in type 1 diabetes: a randomized controlled trial. Pocai, A. Diabetes 58, — Pozefsky, T. Metabolism of forearm tissues in man. Studies with glucagon. Diabetes 25, — Pozza, G. Lipolytic effect of intra-arterial injection of glucagon in man. Prigge, W. Effects of glucagon, epinephrine and insulin on in vitro lipolysis of adipose tissue from mammals and birds. B 39, 69— Following decades during which reduced insulin action was viewed as the exclusive cause of diabetes mellitus and hormonal replacement proved transformative to diabetic individuals worldwide, renewed interest in glucagon has resulted in a rediscovered appreciation of the role this hormone plays in the pathophysiology of impaired glucose homeostasis [ 1 — 3 ]. This recent excitement for glucagon antagonism as a viable therapeutic approach stems from the tantalising preclinical results suggesting that reducing glucagon action or secretion will exert potent reductions in the elevated hepatic glucose production present in both type 1 and 2 diabetes mellitus [ 4 — 8 ]. Glucagon is secreted by the pancreatic alpha cell in response to changes in the local concentration of glucose, amino acids or insulin. Once in circulation it exerts its endocrine effects on the liver through activation of the glucagon receptor GCGR , a G protein-coupled receptor GPCR , and engagement of the Gα s and β-arrestin pathways. Glucagon ultimately stimulates an increase in the export of glucose from the liver—the result of enhanced glycogenolysis and gluconeogenesis [ 9 — 12 ]. The intracellular effects of glucagon in the hepatocyte are complex, but most or possibly all result from activation of adenylate cyclase, intracellular production of cAMP and subsequent activation of protein kinase A cAMP-dependent protein kinase, PKA. Controversy remains regarding the relative importance of the acute metabolic and more long-term transcriptional actions of glucagon, but there is little doubt of the robust increase in the transcription of cAMP response element binding protein CREB target genes whose protein products are at potentially crucial points in the gluconeogenic pathway [ 13 ]. Nonetheless, despite robust effects of glucagon on transcriptional control, in this review we will focus on the direct post-translational effects of glucagon on hepatic metabolism, which we favour as the primary physiological site of glucagon action for two reasons. First, the magnitude of glucagon-dependent changes in gene expression and cellular levels of metabolic enzymes under normal physiological conditions is unlikely to exert a profound influence on gluconeogenic fluxes. This has been most clearly demonstrated through an allelic series of the Pck1 gene, encoding the gluconeogenic enzyme phosphoenolpyruvate carboxykinase PEPCK. In experiments performed ex vivo in perfused liver, levels of PEPCK protein did not correlate well with gluconeogenic fluxes [ 14 ]. Second, glucagon-dependent changes in systemic and hepatic metabolism occur too rapidly to be mediated by transcriptional events. Early studies of glucagon effects on metabolism concluded that the increase in lactate-derived glucose in rat liver perfusions could be seen within about 90 s of introducing the hormone into the perfusion medium [ 15 ]. However, it remains likely that under some conditions transcriptional regulation drives meaningful changes in hepatic metabolism, most probably after prolonged fasting or in a setting of chronic hyperglucagonaemia such as diabetes. Studies of glucagon action in rodent liver performed nearly 50 years ago remain central to the understanding of the impact of the hormone on hepatic metabolism. Early studies defined the primary physiological actions of glucagon as acute increases in both glycogenolysis and gluconeogenesis, occurring in the time frame of seconds to minutes [ 9 — 12 ], but a deep understanding of the metabolic phenomena awaited a pioneering set of work performed at Vanderbilt University by Exton and Park [ 15 — 17 ]. This work, published in a series of papers in the Journal of Biological Chemistry , used enzymatic methods to quantify intracellular metabolites in livers perfused with increasing concentrations of lactate and pyruvate. The accumulation of metabolites defined the steps in the gluconeogenic pathway that were most limiting for metabolic flux, i. under basal conditions a step that occurs after the production of pyruvate but prior to the production of phosphoenolpyruvate PEP. Exton and Park performed parallel experiments evaluating the impact of glucagon on the levels of intracellular metabolites in the gluconeogenic pathway, focusing on the intermediates in lactate-derived gluconeogenesis. These so-called crossover analyses of the gluconeogenic pathway identified the steps where a hormone treatment caused a change in the apparent rate-limiting metabolic step, suggesting that a major control point of glucagon regulation of gluconeogenesis occurs between pyruvate and PEP. This step includes the actions of pyruvate carboxylase PC , export of oxaloacetate from the mitochondria as either malate or aspartate and the cytoplasmic production of PEP, and could be impacted by PEP removal through the action of pyruvate kinase PK. The subsequent decades of research on glucagon action would focus on the identification of protein substrates of PKA that could explain these results. Much of the impact of glucagon action on hepatic metabolism can now be explained through PKA-catalysed regulatory phosphorylation events that affect the activity of three important enzymes: phosphorylase kinase PhK , pyruvate kinase PK , and phosphofructokinase 2 PFK-2 [ 18 — 21 ]. The actions of PKA on these enzymes result in a dramatic shift in hepatic metabolism, changing it from a state that supports futile cycling in glycolysis and glycogenesis to one that rapidly suppresses metabolite cycles and results in the net flux of glucose output from the liver. Hepatic glycogen stores are rapidly mobilised when plasma levels of glucagon are elevated, resulting in a nearly immediate increase in hepatic glucose output. This effect is mediated by PKA phosphorylation and activation of PhK, which phosphorylates and activates glycogen phosphorylase [ 18 ]. This effect not only reduces the capacity of the liver for rapid storage of prandial glucose but also diverts gluconeogenesis-derived glucose from being stored as glycogen, releasing it into the plasma instead. This action of glucagon is likely to be the most important to its role as a counter-regulatory hormone capable of participating in the defence against acute hypoglycaemia, as it allows rapid hormone-controlled access to the latent glucose stored as hepatic glycogen. For bifunctional enzyme PFK-2, this is accomplished by phosphorylation-induced activation of the fructose-2,6-bisphosphatase activity of the enzyme and inhibition of 6-phosphofructose 2-kinase activity, causing the rapid reduction of the second messenger metabolite fructose-2,6-bisphosphate [ 20 ]. Fructose-2,6-bisphosphate exerts its allosteric effects on phosphofructokinase 1 PFK-1 , promoting glycolytic over gluconeogenic flux; glucagon promotes a reduction in fructose-2,6-bisphosphate that causes a rapid decrease in the rate of glycolysis and an increase in the rate of gluconeogenesis. PKA phosphorylation of pyruvate kinase decreases its activity, resulting in a reduction in futile pyruvate cycling whereby PEPCK-derived PEP is dephosphorylated to regenerate pyruvate rather than contributing to gluconeogenesis [ 19 ]. The consequence of these hepatic actions of the glucagon—cAMP—PKA signalling pathway is a rapid increase in gluconeogenesis through the termination of concomitant gluconeogenic and glycolytic enzymatic activities. The establishment of a new balance of metabolic activities in the liver is likely to be a more significant contributor to elevated gluconeogenesis than increases in gluconeogenic enzymes content, especially under submaximal physiological gluconeogenic substrate concentrations that would be less likely to expose defects in the V max of gluconeogenic enzymes. This serves a physiological need in that matching increased hepatic utilisation of amino acids is a mechanism to input new carbon into the glucose—lactate Cori cycling during a time of fasting. The observation that high-protein meals stimulate both insulin and glucagon secretion reveals a typical coordinated physiological response, in this case designed to drive the synthesis of glucose for peripheral tissue consumption [ 22 ]. The impact of glucagon on amino acid catabolism can clearly be seen in human conditions of hyperglucagonaemia, which are associated with muscle wasting, and by the increases in lean body mass observed in genetic rodent models of reduced glucagon signalling [ 23 — 26 ]. Pharmacological inhibition of glucagon signalling also rapidly alters amino acid levels in preclinical species; studies of glucagon antagonism in non-human primates have shown alterations in the transcriptional program for amino acid uptake and metabolism accompanied by changes in many circulating amino acids [ 6 ]. Given the known role of amino acids in triggering the secretion of glucagon from the alpha cell, the elevated amino acids following glucagon antagonism may also contribute to the associated hyperglucagonaemia. At the cellular level, glucagon stimulates a programme in hepatocytes that elevates the rate at which amino acids are transported and metabolised, although the exact mechanisms of these effects remain to be fully characterised. Glucagon has been shown to stimulate amino acid transporters, both the system A and system N transport pathways, which account for the import of the primary gluconeogenic amino acids alanine and glutamine, respectively [ 27 — 29 ]. Glucagon also increases the catabolism of multiple amino acids, including the deamination of glutamine, a rapid effect that is likely to contribute to the increased ureagenesis observed in response to glucagon [ 30 — 32 ]. Glucagon is also capable of increasing the activities of serine metabolising enzymes, altering the intracellular metabolic fate of serine; conversely, antagonism of glucagon causes a rapid and large decrease in the key serine hydrolysing enzyme, serine dehydratase [ 6 , 33 , 34 ]. These concerted actions of glucagon to increase the uptake and utilisation of amino acids as catabolic fuel helps to provide anaplerotic and gluconeogenic substrates that are necessary during fasting or at times during which increased glucose synthesis is required. One of the ways in which glucagon stimulates mitochondrial respiration is through increased intracellular and mitochondrial calcium levels [ 39 — 41 ]. This is accomplished by both enhanced cellular calcium uptake and release of intracellular calcium stores from the endoplasmic reticulum ER. The latter occurs in part through the PKA-dependent phosphorylation of the inositol 1,4,5 trisphosphate receptor IP3R , resulting in calcium release at lower concentrations of IP3 [ 42 ]. IP3R-mediated calcium release from the ER is specifically directed to the mitochondria because of the proximity of mitochondria to the ER membrane [ 43 ]. Elevations in mitochondrial calcium significantly influence metabolism by increasing the rates of key reactions in the tricarboxylic acid TCA cycle and in electron transport, including those catalysed by pyruvate dehydrogenase, isocitrate dehydrogenase, and α-ketoglutarate dehydrogenase [ 44 — 46 ]. Increases in mitochondrial calcium may have other effects, such as those on the rate of transport of metabolites and adenine nucleotides, which may also affect gluconeogenesis [ 47 — 49 ]. Calcium-mediated increases in mitochondrial TCA cycle flux and substrate transport may prove to be an underappreciated site of action for glucagon in its regulation of hepatic glucose metabolism. Pharmacological GCGR antagonism has been shown in preclinical studies to be an effective treatment for the elevated hepatic glucose output that characterises the diabetic state [ 5 — 8 ]. The positive results of these preclinical studies have begun to translate into clinical impact, with impressive glucose lowering with small molecule glucagon receptor antagonists in diabetic individuals [ 4 , 50 ]. This demonstrates the contribution increased glucagon signalling makes to the diabetic state and the potential for large effects on glycaemia with antagonism. The relative contributions the downstream metabolic pathways described above make to clinical efficacy remain to be determined. From preclinical work it is clear that glucagon antagonism can lower plasma glucose within 1 h of compound dosing [ 6 ]; the rapidity of the response argues against a transcriptional mechanism for the glucose lowering because of the time required for mRNA and protein decay. Preclinical studies have reported that antagonism of glucagon signalling is capable of elevating plasma amino acid levels, presumably by lowering hepatic consumption—the relative importance of this effect towards the systemic efficacy is unclear [ 6 ]. The contribution of distinct glucagon effects to clinical efficacy and safety will be an important area of research as this therapeutic mechanism is investigated further. There have been no recent advances in glucose-lowering therapies targeting the liver despite the widespread appreciation that elevated hepatic glucose output in diabetic individuals is a primary cause for elevated systemic glucose levels during fasting. The renewed appreciation that hyperglucagonaemia is an underlying cause of the excess hepatic glucose production in diabetes has increased the likelihood that small or large molecule therapies targeting this pathway will prove beneficial to patients. Years of study have highlighted those intracellular events occurring upon glucagon simulation that are likely to impact glucose output: glycogen breakdown through phosphorylation of PhK, increased gluconeogenesis through phosphorylation of PFK-2, PK and CREB Fig. However, numerous experiments have also identified additional metabolic actions of glucagon that are a part of its conserved role as a regulator of fasting biology. These include its ability to promote amino acid catabolism and mitochondrial TCA flux through increased calcium levels. These actions have the potential to contribute meaningfully to efficacy or safety profiles in the setting of glucagon antagonism. Further work is required to determine the physiological significance of these additional actions of glucagon and their impact in the setting of diabetes. Hepatic glucagon signalling. Glucagon binding to the GCGR is coupled to Gα signalling and results in the activation of adenyl cyclase AC and the production of cAMP. cAMP-mediated activation of PKA leads to the phosphorylation of target proteins—including glycogen phosphorylase, PFK-2, PK, CREB and IP3R—and promotion of amino acid uptake and catabolism pathways. Shah P, Vella A, Basu A, Basu R, Schwenk WF, Rizza RA Lack of suppression of glucagon contributes to postprandial hyperglycemia in subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab — CAS PubMed Google Scholar. Dunning BE, Gerich JE The role of alpha-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. Endocr Rev — Article CAS PubMed Google Scholar. Unger RH, Cherrington AD Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest — Article CAS PubMed PubMed Central Google Scholar. Kelly RP, Garhyan P, Raddad E et al Short-term administration of the glucagon receptor antagonist LY lowers blood glucose in healthy people and in those with type 2 diabetes. Diabetes Obes Metab — Mu J, Jiang G, Brady E et al Chronic treatment with a glucagon receptor antagonist lowers glucose and moderately raises circulating glucagon and glucagon-like peptide 1 without severe alpha cell hypertrophy in diet-induced obese mice. Diabetologia — Mu J, Qureshi SA, Brady EJ et al Anti-diabetic efficacy and impact on amino acid metabolism of GRA1, a novel small-molecule glucagon receptor antagonist. PLoS One 7:e Petersen KF, Sullivan JT Effects of a novel glucagon receptor antagonist Bay on glucagon-stimulated glucose production in humans. Sammons MF, Lee EC Recent progress in the development of small-molecule glucagon receptor antagonists. Bioorg Med Chem Lett — Sutherland EW, Wosilait WD The relationship of epinephrine and glucagon to liver phosphorylase. Liver phosphorylase; preparation and properties. J Biol Chem — Berthet J, Rall TW, Sutherland EW The relationship of epinephrine and glucagon to liver phosphorylase. Effect of epinephrine and glucagon on the reactivation of phosphorylase in liver homogenates. Miller LL Some direct actions of insulin, glucagon, and hydrocortisone on the isolated perfused rat liver. Recent Prog Horm Res — Sokal JE Effect of glucagon on gluconeogenesis by the isolated perfused rat liver. Endocrinology — Lin HV, Accili D Hormonal regulation of hepatic glucose production in health and disease. Cell Metab — Burgess SC, He T, Yan Z et al Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver. Exton JH, Park CR Control of gluconeogenesis in liver. Effects of l -lactate, pyruvate, fructose, glucagon, epinephrine, and adenosine 3ʹ,5ʹ-monophosphate on gluconeogenic intermediates in the perfused rat liver. Effects of glucagon, catecholamines, and adenosine 3ʹ,5ʹ-monophosphate on gluconeogenesis in the perfused rat liver. Exton JH, Corbin JG, Park CR Control of gluconeogenesis in liver. Differential effects of fatty acids and glucagon on ketogenesis and gluconeogenesis in the perfused rat liver. Cohen P Activation and phosphorylation of the subunits of phosphorylase kinase. Biochem J P—6P. Titanji VP, Zetterqvist O, Engstroom L Regulation in vitro of rat liver pyruvate kinase by phosphorylation-dephosphorylation reactions, catalyzed by cyclic-AMP dependent protein kinases and a histone phosphatase. Biochim Biophys Acta — El-Maghrabi MR, Claus TH, Pilkis J, Fox E, Pilkis SJ Regulation of rat liver fructose 2,6-bisphosphatase. Exton JH Mechanisms of hormonal regulation of hepatic glucose metabolism. Diabetes Metab Rev — Fukita Y, Gotto AM, Unger RH Basal and postprotein insulin and glucagon levels during a high and low carbohydrate intake and their relationships to plasma triglycerides. Diabetes — Flakoll PJ, Borel MJ, Wentzel LS, Williams PE, Lacy DB, Abumrad NN The role of glucagon in the control of protein and amino acid metabolism in vivo. Metab Clin Exp — Charlton MR, Adey DB, Nair KS Evidence for a catabolic role of glucagon during an amino acid load. Eldor R, Glaser B, Fraenkel M, Doviner V, Salmon A, Gross DJ Glucagonoma and the glucagonoma syndrome - cumulative experience with an elusive endocrine tumour. Clin Endocrinol Oxf — Article Google Scholar. |
| Key Points | One effect is to delay gastric emptying Article Talk. Glucagon couples hepatic amino acid catabolism to mTOR-dependent regulation of alpha-cell mass. dk Nicolai J. De Oya, M. Q J Exp Physiol Cogn Med Sci 58 : 99 — |
| Top bar navigation | CAS PubMed Google Scholar Kurose, Y. What happens if I have too little glucagon? Nat Chem Biol 5 : — Schreiner are employed by Amylin. Abstract The initial identification of glucagon as a counter-regulatory hormone to insulin revealed this hormone to be of largely singular physiological and pharmacological purpose. CAS PubMed Google Scholar Pospisilik, J. |

Bis jetzt ist aller gut.
Wacker, die prächtige Phrase und ist termingemäß