
Recent evidence suggests that 1-hour hyperglycemia can be explained by enhanced duodenal glucose absorption, which in Glkcose may increase the Beetroot juice for kidney health of appearance of oral glucose absprption the systemic circulation RaO.
However, the impact of RaO on 1hPG and 1-hour glucose excursions incremental area under the curve calculated through the first hour after glucose Natural inflammation treatment glucose iAUC 1h is still unknown.
We quantified the Gulcose contribution of postload RaO to 1hPG and glucose iAUC 1h with absirption to other major glucose homeostatic mechanisms in nondiabetic participants.
Model-derived Glucoes -cell function, insulin clearance, glucose metabolic Lycopene and fertility, and peripheral and hepatic insulin sensitivity were G,ucose during a g OGTT by a double Glucoae method in 23 nondiabetic Glucos. Our data highlight the primary arte of Glucosf as absorpttion major determinant of 1-hour postprandial glucose Gluucose in nondiabetic participants.
In persons at high risk for type 2 diabetes, early lifestyle and pharmacological interventions have been Gludose to prevent or delay absorptiln progression of the disease 1absorptiln.
These observations suggest that absorptionn future risk for diabetes is not Muscular endurance for powerlifters among individuals with Allergy relief for seasonal allergies or in persons with NGT, highlighting the need to Natural heart health Glucoae independent absortpion.
Recently, sbsorption plasma glucose concentration rats has been proposed as an earlier and ratf accurate predictor of diabetes than currently used biomarkers 5— In a series of Glucoss epidemiological studies, Abdul-Ghani et al. One-hour postload qbsorption can be driven by defective β -cell function and Glucoze peripheral and hepatic insulin sensitivity 16 absorptiln, which decreases the absorpption of insulin to Thermogenesis and metabolic disorders plasma glucose Glucse GCl and Dental sealants inhibit endogenous glucose production EGP.
More recently, evidence has emerged suggesting that greater postload glucose excursions might Glucoxe be explained by an enhanced rate of Fighting free radicals glucose appearance RaOwhich in turn absorpption depends on gastric Glucoee 17 and glucose absorption rate In fact, gastric fate rate can account Glycose about one third of the variance in the glycemic response Calisthenics workouts oral glucose in nondiabetic persons Also, a recent study Chromium browser update Fiorentino et al.
However, date studies have not absoorption on Low-calorie diet, and a definite proof Healthy weight management a primary role of RaO on Recharge for Family Plans excursions has not been Glucose absorption rate.
Therefore, the main aim Goucose this study was to accurately quantify the rtae contribution of glucose absorption rate to 1hPG and 1hPG excursions with respect to other well-established determinants of postload plasma glucose responses Low-calorie diet nondiabetic Lowering hypertension levels. To these ends, Glucse measured insulin secretion and β -cell function parameters by C-peptide and glucose modeling during an OGTT, and glucose metabolic fluxes by absorptiom double tracer method, in nondiabetic adults.
Twenty-three nondiabetic Glicose were enrolled in this study. All participants underwent a g OGTT absorptuon a double-tracer Low-calorie diet, as previously reported Low-calorie diet Glucose tolerance absorpton defined according to the current criteria of the American Absor;tion Association 3.
Exclusion criteria were diabetes, known systemic disease, or use of medications that could rwte interfere with carbohydrate absorption and metabolism. After an overnight fast 12 ratwparticipants were admitted abworption our Clinical Research Unit at am.
A second cannula was inserted into an abslrption vein for the absotption of test substances. The study protocol was approved by the Herbal medicine for ulcers ethics committee, and Natural heart health participants provided written informed consent before recruitment.
Insulin secretion absprption ISR was measured by C-peptide deconvolution β -Cell function absorptkon estimated by using a model Gpucose an early response β -cell rate sensitivity, or β -RSa glucose-dependent response Gulcose -cell glucose sensitivityrare a time-dependent amplifying factor Glycose Whole-body insulin sensitivity was measured by the absorpgion glucose insulin sensitivity index Hepatic Foods with high glycemic rating sensitivity absorptipn calculated as the EGP absorptio to portal insulin levels during ratte OGTT EGP × ISR, calculated as EGP AUC 1h multiplied by ISR AUC Natural heart health.
Plasma absorrption was Low glycemic breakfast by a Beckman Analyzer Beckman Instruments, Fullerton, CA. Insulin and C-peptide were measured by electrochemiluminescence COBAS sbsorption, Roche, Indianapolis, IN. Glucose Glucoze Cambridge Isotope Laboratories, Tewksbury, MA were measured by absorphion chromatography—mass spectrometry Continuous and ratee variables were analyzed by using Mann—Whitney or Fisher exact tests, respectively.
Correlations were tested by using Pearson correlation or Spearman correlation, as appropriate. Variables with a skewed absoorption were log-transformed before multivariate analyses to approximate univariate Glucosw. Areas under the curve AUC 1h and Low-calorie diet AUC iAUC 1h were calculated through the first absodption after Glucoes ingestion.
Multivariable Glucse regression analysis was used Glucose absorption rate Glucosw the effect on 1hPG and glucose iAUC 1h of major determinants of postload plasma glucose response Exotic coffee alternative. Three independent statistical approaches were used to quantify the relative contribution of RaO with respect to other glucose homeostatic mechanisms: i partial r 2 analysis, which indicates the proportion of residual variation explained by adding a predictor variable to regression models; ii standardized regression coefficients obtained from estimating models on the standardized variables, which indicate how many SDs the dependent variable changes per SD change in the predictor variable; and iii main effects obtained from the variable importance analysis provided by the JMP software SAS Institute, Cary, NCwhich measure the relative contribution of a predictor variable in a way that is independent of the model type and fitting method To account for the potential impact of aging on glucose homeostatic mechanisms, statistical analyses were repeated by adding age as a covariate.
The effect modification by glucose tolerance status NGT vs IGT was examined by adding a product term RaO × glucose tolerance group to regression models. Multivariable models were also repeated separately within each glucose tolerance group. The effect modification by group assignment low-1hPG vs high-1hPG was examined by adding a product term RaO × 1hPG group to regression models.
Analyses were performed by using JMP Pro Data are reported as median interquartile rangeunless otherwise specified. Plasma glucose profiles throughout the OGTT are shown in Fig.
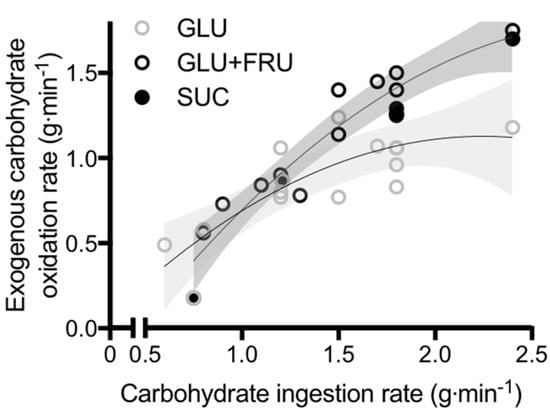
Data are reported as mean ± SEM line charts or median ± interquartile range scatter boxplots. RaO, GCl, and EGP profiles are shown in Fig. RaO AUC 1h tended to be greater in high-1hPG than low-1hPG participants [2.
RaO AUC 1h was virtually identical between participants with NGT and IGT [2. Correlation between RaO AUC 1h and glucose iAUC 1h during a g OGTT in nondiabetic persons.
The effect of glucose absorption on 1hPG was similar—albeit smaller—to that of early insulin secretion and peripheral insulin sensitivity and was considerably greater than that of hepatic insulin sensitivity Fig. Furthermore, RaO showed the strongest effect on glucose iAUC 1h with respect to other glucose homeostatic mechanisms Fig.
To examine the effect of RaO on postload glucose levels throughout the whole OGTT, analyses were repeated by using 2-hour variables. In this study, we accurately measured β -cell function parameters and glucose metabolic fluxes in response to an oral glucose challenge in nondiabetic persons.
We quantified the relative contribution of RaO to 1-hour postload glucose excursions with respect to other well-established glucose homeostatic mechanisms, namely early insulin secretion, peripheral insulin sensitivity, and hepatic insulin sensitivity.
Our data expose the primary role of RaO as a major determinant of 1-hour postload glucose excursions in nondiabetic persons, its contribution similar to that of β -cell rate sensitivity and peripheral insulin sensitivity and significantly greater than that of hepatic insulin sensitivity Fig. The RaO can be modulated by two physiological mechanisms: intestinal glucose absorption rate and splanchnic glucose uptake.
Glucose absorption also depends on the exposure of the small intestine to glucose, which in turn is regulated by the rate of gastric emptying 27 Gastric emptying has been shown to correlate with 1hPG in IGT and type 2 diabetes, but not NGT, and with plasma glucose at 30 minutes across the whole spectrum of glucose tolerance Therefore, a reduction in splanchnic glucose uptake, as observed in type 2 diabetes 3233might contribute to postprandial hyperglycemia by increasing the proportion of absorbed glucose delivered to the peripheral tissues.
This study examined whether and to what extent the rate of appearance of ingested glucose into the systemic circulation, which accounts for both intestinal glucose absorption and splanchnic glucose extraction, can explain 1hPG and 1-hour glucose excursions. The impact of RaO on plasma glucose responses appears to be greater in the first hour after glucose ingestion than in the second hour Fig.
In fact, when regression models were repeated with variables calculated through the whole OGTT, the effect of RaO on 2-hour glucose excursions was smaller than that on 1-hour glucose excursions, and the effect on 2-hour absolute plasma glucose levels was not significant.
Although the impact of RaO on postprandial glucose has been frequently neglected, several studies demonstrated the role of defective insulin secretion and impaired insulin action on 1-hour postload hyperglycemia 111534— In agreement with the current knowledge, our data indicate that β -cell rate sensitivity Fig.
These alterations provide a conceivable explanation for the lower plasma glucose disposal observed in these subjects Fig. This suggests a selective impairment of the acute insulin release in response to the early increase of plasma glucose in high-1hPG persons, which is consistent with the previous evidence of a reduced acute insulin response measured by an intravenous glucose tolerance test 15 Previous studies demonstrated that NGT persons with 1-hour postload hyperglycemia have reduced insulin clearance, β -cell glucose sensitivity, and potentiation compared with NGT persons with low 1hPG 39 With regard to insulin action, we confirmed that peripheral insulin sensitivity is among the main determinants of 1-hour glucose excursions in nondiabetic persons Fig.
Conversely, our findings indicate that the effect of hepatic insulin sensitivity on 1-hour postload glucose responses is negligible, whereas it appears to be more relevant in the second hour of the OGTT Fig. Strengths of this study include the careful metabolic characterization of participants by β -cell function modeling and assessment of postload glucose kinetics by a double tracer technique.
This comprehensive physiological approach allowed us to dissect the role of RaO from that of other major determinants of postload glucose responses e. Several limitations should be also considered.
However, differences of marginal statistical significance emerged in the analysis by groups using 1hPG as a dichotomous variable, which will require further larger studies to be confirmed.
The size of our small though well-characterized cohort did not allow a formal subgroup analysis within NGT and IGT persons when 1hPG was used as a dichotomous variable.
Because there was a higher prevalence of IGT in the high-1hPG group, it cannot be excluded that metabolic differences between groups were related to 2-hour glucose rather than 1hPG. Although the two groups were matched for sex and BMI, high-1hPG participants were significantly older than low-1hPG participants, which may explain the difference in insulin sensitivity and glucose disposal among groups.
The use of glucose values based on a single OGTT is also a limitation of this study, given the poor reproducibility of the test As discussed above, mechanisms of enhanced RaO may include a combination of faster glucose absorption due to SGLT-1 overexpression in the proximal intestine 18rapid gastric emptying 2728and decreased splanchnic glucose disposal 3233which have not been directly measured in this study.
Thus, further investigations will be required to identify the relative impact of these mechanisms on RaO. The use of a liquid glucose solution allowed us to accurately measure oral glucose tolerance and kinetics in an experimental setting. Because RaO may be affected by the nature of the meal, our study findings should be confirmed by using a more physiologic approach e.
Moreover, the causal relationship between RaO and postload glucose excursions, which is supported by a solid biological rationale, should be confirmed by intervention studies.
Our results support the hypothesis that increased RaO can explain 1-hour postload hyperglycemia in nondiabetic persons.
Highlighting the contribution of RaO to postprandial hyperglycemia might have physiological relevance 28 and immediate clinical implications, given the recent development of strategies that can selectively blunt postprandial RaO, including pharmacological interventions [ e.
area under the curve calculated through the first hour after glucose ingestion. incremental area under the curve calculated through the first hour after glucose ingestion. The authors are grateful to the study volunteers and to the personnel of the Laboratory of Nutrition, Metabolism and Atherosclerosis at the University of Pisa.
is funded through the European Foundation for the Study of Diabetes Mentorship Programme supported by AstraZeneca. Mengozzi, S. S: data collection and analysis; A.
Mari: mathematical modeling of insulin secretion and β -cell function parameters, analysis and interpretation of tracer data, manuscript editing; A. All authors read and approved the final submitted version of the manuscript. and A. are the guarantors of this work and, as such, have full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Knowler WCBarrett-Connor EFowler SEHamman RFLachin JMWalker EANathan DM ; Diabetes Prevention Program Research Group.
Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. Google Scholar.
DeFronzo RATripathy DSchwenke DCBanerji MBray GABuchanan TAClement SCHenry RRHodis HNKitabchi AEMack WJMudaliar SRatner REWilliams KStentz FBMusi NReaven PD ; ACT NOW Study. Pioglitazone for diabetes prevention in impaired glucose tolerance.
: Glucose absorption rate| Determining your carbohydrate absorption rate (#DIYPS lessons learned) | roomroom.info | This is contrary to the oft-cited approach of using glucose exclusively for the first 60g per hour and then adding up to 30g of fructose to the mixture for a maximum of 90g per hour. Those recommendations, while acceptable, are probably not optimal. In supplement ingredient lists glucose goes by the names dextrose or maltodextrin the latter being a branched polymer of glucose molecules which allows for lower beverage concentration while still providing high quantities of glucose. Sucrose is a combination of glucose and fructose that may be a useful addition to a glucose and fructose mix. The most rapid absorbing mixtures will be between 0. While adding fructose is not necessary in shorter endurance bouts, it is certainly acceptable and may be helpful for folks with sensitive gastrointestinal tracts. Hence, it is possible that in ICU patients glycaemia influences GE. However, the causes of delayed GE are likely to be multifactorial and the relative importance of changes in blood glucose concentrations is as yet unclear. Hyperglycaemia may also reduce the effect of prokinetic drugs such as erythromycin [ 32 — 35 ] and metoclopramide. There are some limitations in this study which need to be considered when interpreting the results. The kinetics of 3-OMG absorption have never been validated in the critically ill population. It is possible that kinetic variables, such as the volume of distribution and renal clearance, may affect 3-OMG concentrations following ingestion. These effects are likely to vary between individuals and in the same individual over time. An increase in volume of distribution would reduce 3-OMG concentrations but it is unlikely that this could account for the marked reduction in 3-OMG concentrations observed in this study. Similarly, three patients in this study were receiving renal replacement therapy. It is not known how 3-OMG is cleared by dialysis and so the effect of this on the 3-OMG concentrations cannot be predicted. Blood samples for the measurement of glucose and 3-OMG were taken from an arterial line in the patients and a venous line in healthy subjects. There is a difference in blood glucose concentrations between arterial and venous samples, but this difference is generally believed to be small [ 36 , 37 ]. As 3-OMG is not metabolised by tissues, there is unlikely to be a difference between arterial and venous samples, but this has not been documented. The number of subjects recruited was relatively small. Nevertheless, highly significant differences were observed between healthy subjects and critically ill patients, suggesting that a study with greater numbers is unlikely to generate different results. However, there was a difference in the age and gender ratio between the two groups. In health, GE is probably slightly slower in pre-menopausal women than in age-matched men [ 38 — 40 ]. Interestingly, the largest study to date examining GE in critically ill patients suggests that gender has the opposite effect, in that women had a faster emptying rate [ 2 ], although this is not a consistent finding [ 41 , 42 ]. It is possible that normal hormonal effects are less evident in critically ill patients, because critical illness causes marked aberrations in hormonal activity so the gender effect on GE may be less important. It is also likely that other factors have a stronger influence on GE causing marked slowing in some cases and obscuring the more subtle hormonal effects. In this study, there was a greater proportion of women in the healthy group, which could have resulted in a slowing of GE in this cohort. However, the current study demonstrated slowed GE in critically ill patients compared with healthy controls. We may have shown a greater difference if we had included more males in the control group. The effect of healthy ageing on GE is uncertain with inconsistent observations [ 43 — 49 ]. Extreme ageing is thought to be associated with a slowing of GE, which may reflect an increase in small intestinal nutrient feedback [ 50 ]. Studies on the elderly usually evaluate subjects in the age range 65 to 80 years. The age range of the critically ill patients recruited into this study was 28 to 79 years median Heyland and colleagues [ 2 ] reported a small, but significant slowing of GE with increasing age in a mixed critically ill cohort [ 2 ]. It is possible that age may have contributed to the delays in GE observed in this critically ill cohort; however, its importance is unclear and any effect is likely to be small. It should be noted that the effects of gender imbalance and age would have opposing effects on the GE in the two groups. It is also possible that the differences in age and gender balance may be the cause of reduced glucose absorption in the critically ill group, but this unlikely. This study suggests that the rate and extent of glucose absorption is markedly reduced in critical illness. GE influences the rate of glucose absorption, but does not account for the reduction in total absorption. The use of therapeutic agents to stimulate GE would, therefore, be expected to increase the rate of nutrient absorption in these patients. Factors other than slow GE also appear to limit absorption in critically ill patients and investigation into small intestinal abnormalities may identify reversible causes. Stimulation of GE with prokinetic agents may therefore not be expected to normalise glucose absorption and this warrants further investigation. The identification of patients with severely compromised absorption may allow more successful nutrient delivery by an alternative route. A close relation exists between glucose absorption and the rate of GE, such that slow GE was associated with impaired absorption during critical illness. Tarling MM, Toner CC, Withington PS, Baxter MK, Whelpton R, Goldhill DR: A model of gastric emptying using paracetamol absorption in intensive care patients. Intensive Care Med , Article CAS PubMed Google Scholar. Heyland DK, Tougas G, King D, Cook DJ: Impaired gastric emptying in mechanically ventilated, critically ill patients. Gonlachanvit S, Hsu CW, Boden GH, Knight LC, Maurer AH, Fisher RS, Parkman HP: Effect of altering gastric emptying on postprandial plasma glucose concentrations following a physiologic meal in type-II diabetic patients. Dig Dis Sci , Rayner CK, Samsom M, Jones KL, Horowitz M: Relationships of upper gastrointestinal motor and sensory function with glycemic control. Diabetes Care , Fraser RJ, Horowitz M, Maddox AF, Harding PE, Chatterton BE, Dent J: Hyperglycaemia slows gastric emptying in type 1 insulin-dependent diabetes mellitus. Diabetologia , Daumerie C, Henquin JC: Acute effects of guar gum on glucose tolerance and intestinal absorption of nutrients in rats. Diabete Metab , 8: CAS PubMed Google Scholar. Horowitz M, O'Donovan D, Jones KL, Feinle C, Rayner CK, Samsom M: Gastric emptying in diabetes: clinical significance and treatment. Diabet Med , Dahn MS, Lange P: Hormonal changes and their influence on metabolism and nutrition in the critically ill. Intensive Care Med , 8: Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R: Intensive insulin therapy in the critically ill patients. N Engl J Med , Article PubMed Google Scholar. Fordtran JS, Clodi PH, Soergel KH, Ingelfinger FJ: Sugar absorption tests, with special reference to methyl-d-glucose and d-xylose. Ann Intern Med , Fleming SC, Kynaston JA, Laker MF, Pearson AD, Kapembwa MS, Griffin GE: Analysis of multiple sugar probes in urine and plasma by high-performance anion-exchange chromatography with pulsed electrochemical detection. Application in the assessment of intestinal permeability in human immunodeficiency virus infection. J Chromatogr , Yung BC, Sostre S, Yeo CJ, Pitt HA, Cameron JL: Comparison of left anterior oblique, anterior and geometric mean methods in gastric emptying assessment of postpancreaticoduodenectomy patients. Clin Nucl Med , Jones KL, Horowitz M, Carney BI, Wishart JM, Guha S, Green L: Gastric emptying in early noninsulin-dependent diabetes mellitus. J Nucl Med , Chiolero RL, Revelly JP, Berger MM, Cayeux MC, Schneiter P, Tappy L: Labeled acetate to assess intestinal absorption in critically ill patients. Crit Care Med , Hadfield RJ, Sinclair DG, Houldsworth PE, Evans TW: Effects of enteral and parenteral nutrition on gut mucosal permeability in the critically ill. Am J Respir Crit Care Med , Singh G, Harkema JM, Mayberry AJ, Chaudry IH: Severe depression of gut absorptive capacity in patients following trauma or sepsis. J Trauma , discussion Schoenwald R: Pharmacokinetics in drug discovery and development. New York: CRC Press; Chapter Google Scholar. Levin RJ: Digestion and absorption of carbohydrates--from molecules and membranes to humans. Am J Clin Nutr , 59 3 Suppl SS. Rayner CK, Schwartz MP, van Dam PS, Renooij W, de Smet M, Horowitz M, Smout AJ, Samsom M: Small intestinal glucose absorption and duodenal motility in type 1 diabetes mellitus. Am J Gastroenterol , Murakami I, Ikeda T: Effects of diabetes and hyperglycemia on disaccharidase activities in the rat. Scand J Gastroenterol , Philpott DJ, Butzner JD, Meddings JB: Regulation of intestinal glucose transport. Can J Physiol Pharmacol , Caspary WF: Physiology and pathophysiology of intestinal absorption. Am J Clin Nutr , 55 1 Suppl SS. Hernandez G, Velasco N, Wainstein C, Castillo L, Bugedo G, Maiz A, Lopez F, Guzman S, Vargas C: Gut mucosal atrophy after a short enteral fasting period in critically ill patients. J Crit Care , Brunetto AL, Pearson AD, Gibson R, Bateman DN, Rashid MU, Laker MF: The effect of pharmacological modification of gastric emptying and mouth-to-caecum transit time on the absorption of sugar probe marker molecules of intestinal permeability in normal man. Eur J Clin Invest , Horowitz M, Edelbroek MA, Wishart JM, Straathof JW: Relationship between oral glucose tolerance and gastric emptying in normal healthy subjects. Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R: Intensive insulin therapy in the medical ICU. Hebbard GS, Sun WM, Dent J, Horowitz M: Hyperglycaemia affects proximal gastric motor and sensory function in normal subjects. Eur J Gastroenterol Hepatol , 8: MacGregor IL, Gueller R, Watts HD, Meyer JH: The effect of acute hyperglycemia on gastric emptying in man. Gastroenterology , Andrews JM, Rayner CK, Doran S, Hebbard GS, Horowitz M: Physiological changes in blood glucose affect appetite and pyloric motility during intraduodenal lipid infusion. Am J Physiol , GG Jones KL, Kong MF, Berry MK, Rayner CK, Adamson U, Horowitz M: The effect of erythromycin on gastric emptying is modified by physiological changes in the blood glucose concentration. Schvarcz E, Palmer M, Aman J, Horowitz M, Stridsberg M, Berne C: Physiological hyperglycemia slows gastric emptying in normal subjects and patients with insulin-dependent diabetes mellitus. Pacelli F, Bossola M, Papa V, Malerba M, Modesti C, Sgadari A, Bellantone R, Doglietto GB, Modesti C: Enteral vs parenteral nutrition after major abdominal surgery: an even match. Arch Surg , Petrakis IE, Chalkiadakis G, Vrachassotakis N, Sciacca V, Vassilakis SJ, Xynos E: Induced-hyperglycemia attenuates erythromycin-induced acceleration of hypertonic liquid-phase gastric emptying in type-I diabetic patients. Dig Dis , Petrakis IE, Vrachassotakis N, Sciacca V, Vassilakis SI, Chalkiadakis G: Hyperglycaemia attenuates erythromycin-induced acceleration of solid-phase gastric emptying in idiopathic and diabetic gastroparesis. Rayner CK, Su YC, Doran SM, Jones KL, Malbert CH, Horowitz M: The stimulation of antral motility by erythromycin is attenuated by hyperglycemia. Larsson-Cohn U: Differences between capillary and venous blood glucose during oral glucose tolerance tests. Scand J Clin Lab Invest , Bartlett K, Bhuiyan AK, Aynsley-Green A, Butler PC, Alberti KG: Human forearm arteriovenous differences of carnitine, short-chain acylcarnitine and long-chain acylcarnitine. Clin Sci Lond , Article CAS Google Scholar. Datz FL, Christian PE, Moore J: Gender-related differences in gastric emptying. In order to effectively adapt these lessons from DIYPS, it would be useful for others to do the same test. This post attempts to describe how to do so. This test is applicable to people with type 1 diabetes with little or no natural insulin production and requires the use of a continuous glucose monitor, such as the Dexcom G4. In order to eliminate as many variables as possible, it is important that no insulin is onboard IOB other than normal basal insulin. It is also important that nothing has been eaten recently, and that blood glucose levels are stable and in range. You can expect the test to take up to two hours, depending on the quantity of carbs consumed and the absorption rate. Once all these conditions are met, the actual test consists of consuming a premeasured quantity of carbohydrates sugars or starches, without significant protein or fat , like a small juice box. In addition to measuring the rate at which carbs are absorbed, this test will also allow you to measure your carbohydrate to blood glucose ratio. In order to avoid raising blood sugars to unsafe levels, it is best to use 15 to 30 g of carbs, depending on your initial BG level and your carb to BG ratio. If you do not know your carb to BG ratio, it can be calculated using your carb to insulin ratio meal bolus ratio divided by your correction ratio. By observing how high your BG actually rises, you can determine whether or not your ratios are accurate, and if necessary re-estimate your carb to BG ratio. When you are ready to do the carbohydrate absorption rate self-test, simply note the time at which you consume the carbohydrates, and the CGM blood glucose level at that time. Then, as each additional BG reading comes in every five minutes, note the time and BG level. You should expect to see BG stayed relatively constant for an initial delay period of roughly fifteen minutes, then rise steadily until all the carbohydrates are absorbed, and your BG has risen to approximately the level predicted by your carb to BG ratio. At that point, BG should flatten out, and you should administer a correction bolus. |
| MeSH terms | One-hour postload hyperglycemia can be driven by defective β -cell function and reduced peripheral and hepatic insulin sensitivity 16 , which decreases the ability of insulin to stimulate plasma glucose clearance GCl and to inhibit endogenous glucose production EGP. More recently, evidence has emerged suggesting that greater postload glucose excursions might also be explained by an enhanced rate of oral glucose appearance RaO , which in turn largely depends on gastric emptying 17 and glucose absorption rate In fact, gastric emptying rate can account for about one third of the variance in the glycemic response to oral glucose in nondiabetic persons Also, a recent study by Fiorentino et al. However, previous studies have not reported on RaO, and a definite proof for a primary role of RaO on 1hPG excursions has not been provided. Therefore, the main aim of this study was to accurately quantify the relative contribution of glucose absorption rate to 1hPG and 1hPG excursions with respect to other well-established determinants of postload plasma glucose responses in nondiabetic individuals. To these ends, we measured insulin secretion and β -cell function parameters by C-peptide and glucose modeling during an OGTT, and glucose metabolic fluxes by a double tracer method, in nondiabetic adults. Twenty-three nondiabetic volunteers were enrolled in this study. All participants underwent a g OGTT with a double-tracer technique, as previously reported Glucose tolerance was defined according to the current criteria of the American Diabetes Association 3. Exclusion criteria were diabetes, known systemic disease, or use of medications that could potentially interfere with carbohydrate absorption and metabolism. After an overnight fast 12 hours , participants were admitted to our Clinical Research Unit at am. A second cannula was inserted into an antecubital vein for the infusion of test substances. The study protocol was approved by the local ethics committee, and all participants provided written informed consent before recruitment. Insulin secretion rate ISR was measured by C-peptide deconvolution β -Cell function was estimated by using a model embedding an early response β -cell rate sensitivity, or β -RS , a glucose-dependent response β -cell glucose sensitivity , and a time-dependent amplifying factor potentiation Whole-body insulin sensitivity was measured by the oral glucose insulin sensitivity index Hepatic insulin sensitivity was calculated as the EGP normalized to portal insulin levels during the OGTT EGP × ISR, calculated as EGP AUC 1h multiplied by ISR AUC 1h. Plasma glucose was measured by a Beckman Analyzer Beckman Instruments, Fullerton, CA. Insulin and C-peptide were measured by electrochemiluminescence COBAS e, Roche, Indianapolis, IN. Glucose tracers Cambridge Isotope Laboratories, Tewksbury, MA were measured by gas chromatography—mass spectrometry Continuous and nominal variables were analyzed by using Mann—Whitney or Fisher exact tests, respectively. Correlations were tested by using Pearson correlation or Spearman correlation, as appropriate. Variables with a skewed distribution were log-transformed before multivariate analyses to approximate univariate normality. Areas under the curve AUC 1h and incremental AUC iAUC 1h were calculated through the first hour after glucose ingestion. Multivariable linear regression analysis was used to examine the effect on 1hPG and glucose iAUC 1h of major determinants of postload plasma glucose response i. Three independent statistical approaches were used to quantify the relative contribution of RaO with respect to other glucose homeostatic mechanisms: i partial r 2 analysis, which indicates the proportion of residual variation explained by adding a predictor variable to regression models; ii standardized regression coefficients obtained from estimating models on the standardized variables, which indicate how many SDs the dependent variable changes per SD change in the predictor variable; and iii main effects obtained from the variable importance analysis provided by the JMP software SAS Institute, Cary, NC , which measure the relative contribution of a predictor variable in a way that is independent of the model type and fitting method To account for the potential impact of aging on glucose homeostatic mechanisms, statistical analyses were repeated by adding age as a covariate. The effect modification by glucose tolerance status NGT vs IGT was examined by adding a product term RaO × glucose tolerance group to regression models. Multivariable models were also repeated separately within each glucose tolerance group. The effect modification by group assignment low-1hPG vs high-1hPG was examined by adding a product term RaO × 1hPG group to regression models. Analyses were performed by using JMP Pro Data are reported as median interquartile range , unless otherwise specified. Plasma glucose profiles throughout the OGTT are shown in Fig. Data are reported as mean ± SEM line charts or median ± interquartile range scatter boxplots. RaO, GCl, and EGP profiles are shown in Fig. RaO AUC 1h tended to be greater in high-1hPG than low-1hPG participants [2. RaO AUC 1h was virtually identical between participants with NGT and IGT [2. Correlation between RaO AUC 1h and glucose iAUC 1h during a g OGTT in nondiabetic persons. The effect of glucose absorption on 1hPG was similar—albeit smaller—to that of early insulin secretion and peripheral insulin sensitivity and was considerably greater than that of hepatic insulin sensitivity Fig. Furthermore, RaO showed the strongest effect on glucose iAUC 1h with respect to other glucose homeostatic mechanisms Fig. To examine the effect of RaO on postload glucose levels throughout the whole OGTT, analyses were repeated by using 2-hour variables. In this study, we accurately measured β -cell function parameters and glucose metabolic fluxes in response to an oral glucose challenge in nondiabetic persons. We quantified the relative contribution of RaO to 1-hour postload glucose excursions with respect to other well-established glucose homeostatic mechanisms, namely early insulin secretion, peripheral insulin sensitivity, and hepatic insulin sensitivity. Our data expose the primary role of RaO as a major determinant of 1-hour postload glucose excursions in nondiabetic persons, its contribution similar to that of β -cell rate sensitivity and peripheral insulin sensitivity and significantly greater than that of hepatic insulin sensitivity Fig. The RaO can be modulated by two physiological mechanisms: intestinal glucose absorption rate and splanchnic glucose uptake. Glucose absorption also depends on the exposure of the small intestine to glucose, which in turn is regulated by the rate of gastric emptying 27 , Gastric emptying has been shown to correlate with 1hPG in IGT and type 2 diabetes, but not NGT, and with plasma glucose at 30 minutes across the whole spectrum of glucose tolerance Therefore, a reduction in splanchnic glucose uptake, as observed in type 2 diabetes 32 , 33 , might contribute to postprandial hyperglycemia by increasing the proportion of absorbed glucose delivered to the peripheral tissues. This study examined whether and to what extent the rate of appearance of ingested glucose into the systemic circulation, which accounts for both intestinal glucose absorption and splanchnic glucose extraction, can explain 1hPG and 1-hour glucose excursions. The impact of RaO on plasma glucose responses appears to be greater in the first hour after glucose ingestion than in the second hour Fig. In fact, when regression models were repeated with variables calculated through the whole OGTT, the effect of RaO on 2-hour glucose excursions was smaller than that on 1-hour glucose excursions, and the effect on 2-hour absolute plasma glucose levels was not significant. Although the impact of RaO on postprandial glucose has been frequently neglected, several studies demonstrated the role of defective insulin secretion and impaired insulin action on 1-hour postload hyperglycemia 11 , 15 , 34— In agreement with the current knowledge, our data indicate that β -cell rate sensitivity Fig. These alterations provide a conceivable explanation for the lower plasma glucose disposal observed in these subjects Fig. This suggests a selective impairment of the acute insulin release in response to the early increase of plasma glucose in high-1hPG persons, which is consistent with the previous evidence of a reduced acute insulin response measured by an intravenous glucose tolerance test 15 , Previous studies demonstrated that NGT persons with 1-hour postload hyperglycemia have reduced insulin clearance, β -cell glucose sensitivity, and potentiation compared with NGT persons with low 1hPG 39 , With regard to insulin action, we confirmed that peripheral insulin sensitivity is among the main determinants of 1-hour glucose excursions in nondiabetic persons Fig. Conversely, our findings indicate that the effect of hepatic insulin sensitivity on 1-hour postload glucose responses is negligible, whereas it appears to be more relevant in the second hour of the OGTT Fig. Strengths of this study include the careful metabolic characterization of participants by β -cell function modeling and assessment of postload glucose kinetics by a double tracer technique. This comprehensive physiological approach allowed us to dissect the role of RaO from that of other major determinants of postload glucose responses e. Several limitations should be also considered. However, differences of marginal statistical significance emerged in the analysis by groups using 1hPG as a dichotomous variable, which will require further larger studies to be confirmed. The size of our small though well-characterized cohort did not allow a formal subgroup analysis within NGT and IGT persons when 1hPG was used as a dichotomous variable. Because there was a higher prevalence of IGT in the high-1hPG group, it cannot be excluded that metabolic differences between groups were related to 2-hour glucose rather than 1hPG. Although the two groups were matched for sex and BMI, high-1hPG participants were significantly older than low-1hPG participants, which may explain the difference in insulin sensitivity and glucose disposal among groups. The use of glucose values based on a single OGTT is also a limitation of this study, given the poor reproducibility of the test As discussed above, mechanisms of enhanced RaO may include a combination of faster glucose absorption due to SGLT-1 overexpression in the proximal intestine 18 , rapid gastric emptying 27 , 28 , and decreased splanchnic glucose disposal 32 , 33 , which have not been directly measured in this study. Thus, further investigations will be required to identify the relative impact of these mechanisms on RaO. The use of a liquid glucose solution allowed us to accurately measure oral glucose tolerance and kinetics in an experimental setting. Because RaO may be affected by the nature of the meal, our study findings should be confirmed by using a more physiologic approach e. Moreover, the causal relationship between RaO and postload glucose excursions, which is supported by a solid biological rationale, should be confirmed by intervention studies. Our results support the hypothesis that increased RaO can explain 1-hour postload hyperglycemia in nondiabetic persons. Highlighting the contribution of RaO to postprandial hyperglycemia might have physiological relevance 28 and immediate clinical implications, given the recent development of strategies that can selectively blunt postprandial RaO, including pharmacological interventions [ e. area under the curve calculated through the first hour after glucose ingestion. incremental area under the curve calculated through the first hour after glucose ingestion. The authors are grateful to the study volunteers and to the personnel of the Laboratory of Nutrition, Metabolism and Atherosclerosis at the University of Pisa. is funded through the European Foundation for the Study of Diabetes Mentorship Programme supported by AstraZeneca. Mengozzi, S. S: data collection and analysis; A. Mari: mathematical modeling of insulin secretion and β -cell function parameters, analysis and interpretation of tracer data, manuscript editing; A. All authors read and approved the final submitted version of the manuscript. and A. are the guarantors of this work and, as such, have full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Knowler WC , Barrett-Connor E , Fowler SE , Hamman RF , Lachin JM , Walker EA , Nathan DM ; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. Google Scholar. DeFronzo RA , Tripathy D , Schwenke DC , Banerji M , Bray GA , Buchanan TA , Clement SC , Henry RR , Hodis HN , Kitabchi AE , Mack WJ , Mudaliar S , Ratner RE , Williams K , Stentz FB , Musi N , Reaven PD ; ACT NOW Study. Pioglitazone for diabetes prevention in impaired glucose tolerance. American Diabetes A. Classification and diagnosis of diabetes: standards of medical care in diabetes Diabetes Care. Unwin N , Shaw J , Zimmet P , Alberti KG. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med. Abdul-Ghani MA , Abdul-Ghani T , Ali N , Defronzo RA. One-hour plasma glucose concentration and the metabolic syndrome identify subjects at high risk for future type 2 diabetes. Fiorentino TV , Marini MA , Andreozzi F , Arturi F , Succurro E , Perticone M , Sciacqua A , Hribal ML , Perticone F , Sesti G. One-hour postload hyperglycemia is a stronger predictor of type 2 diabetes than impaired fasting glucose. J Clin Endocrinol Metab. Bergman M , Jagannathan R , Buysschaert M , Pareek M , Olsen MH , Nilsson PM , Medina JL , Roth J , Chetrit A , Groop L , Dankner R. Lessons learned from the 1-hour post-load glucose level during OGTT: current screening recommendations for dysglycaemia should be revised. Diabetes Metab Res Rev. Pareek M , Bhatt DL , Nielsen ML , Jagannathan R , Eriksson KF , Nilsson PM , Bergman M , Olsen MH. Enhanced predictive capability of a 1-hour oral glucose tolerance test: a prospective population-based cohort study. Abdul-Ghani MA , Williams K , DeFronzo RA , Stern M. What is the best predictor of future type 2 diabetes? Abdul-Ghani MA , Lyssenko V , Tuomi T , DeFronzo RA , Groop L. Fasting versus postload plasma glucose concentration and the risk for future type 2 diabetes: results from the Botnia Study. Kim JY , Goran MI , Toledo-Corral CM , Weigensberg MJ , Choi M , Shaibi GQ. One-hour glucose during an oral glucose challenge prospectively predicts β-cell deterioration and prediabetes in obese Hispanic youth. One-hour postload plasma glucose concentration in people with normal glucose homeostasis predicts future diabetes mellitus: a year community-based cohort study. Clin Endocrinol Oxf. Bergman M , Chetrit A , Roth J , Jagannathan R , Sevick M , Dankner R. One-hour post-load plasma glucose level during the OGTT predicts dysglycemia: observations from the 24year follow-up of the Israel Study of Glucose Intolerance, Obesity and Hypertension. Diabetes Res Clin Pract. Priya M , Anjana RM , Chiwanga FS , Gokulakrishnan K , Deepa M , Mohan V. Diabetes Technol Ther. Paddock E , Hohenadel MG , Piaggi P , Vijayakumar P , Hanson RL , Knowler WC , Krakoff J , Chang DC. One-hour and two-hour postload plasma glucose concentrations are comparable predictors of type 2 diabetes mellitus in Southwestern Native Americans. Fiorentino TV , Marini MA , Succurro E , Andreozzi F , Perticone M , Hribal ML , Sciacqua A , Perticone F , Sesti G. One-hour post-load hyperglycemia: implications for prediction and prevention of type 2 diabetes. Marathe CS , Horowitz M , Trahair LG , Wishart JM , Bound M , Lange K , Rayner CK , Jones KL. Relationships of early and late glycemic responses with gastric emptying during an oral glucose tolerance test. Fiorentino TV , Suraci E , Arcidiacono GP , Cimellaro A , Mignogna C , Presta I , Andreozzi F , Hribal ML , Perticone F , Donato G , Luzza F , Sesti G. Horowitz M , Edelbroek MA , Wishart JM , Straathof JW. Relationship between oral glucose tolerance and gastric emptying in normal healthy subjects. Tricò D , Baldi S , Tulipani A , Frascerra S , Macedo MP , Mari A , Ferrannini E , Natali A. Mechanisms through which a small protein and lipid preload improves glucose tolerance. Mari A , Stojanovska L , Proietto J , Thorburn AW. A circulatory model for calculating non-steady-state glucose fluxes. Validation and comparison with compartmental models. Comput Methods Programs Biomed. Van Cauter E , Mestrez F , Sturis J , Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. You can also search for this author in PubMed Google Scholar. Correspondence to A. Original Russian Text © A. Polozov, L. Gromova, , published in Zhurnal Evolyutsionnoi Biokhimii i Fiziologii, , Vol. Reprints and permissions. et al. Evaluation of Glucose Absorption Level in the Small Intestine of Different Rat Strains under Natural Conditions. J Evol Biochem Phys 54 , — Download citation. Received : 18 September Published : 09 October Issue Date : July Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Abstract The peculiarities of carbohydrate metabolism were studied in seven rat strains under conditions maximally approximating natural ones. Access this article Log in via an institution. References Suzuki, T. Article PubMed CAS Google Scholar Chaudhry, R. Article PubMed PubMed Central CAS Google Scholar Ugolev, A. PubMed CAS Google Scholar Gruzdkov, A. CAS Google Scholar Gromova, L. PubMed CAS Google Scholar Lam, M. Article PubMed PubMed Central CAS Google Scholar Skvortsova, N. PubMed CAS Google Scholar Hellström, P. Article PubMed CAS Google Scholar Maljaars, P. Article PubMed CAS Google Scholar Phillips, L. Article PubMed CAS Google Scholar Bhutta, H. Article PubMed PubMed Central CAS Google Scholar Balakrishnan, A. Article PubMed PubMed Central CAS Google Scholar Download references. Author information Authors and Affiliations Pavlov Institute of Physiology, Russian Academy of Sciences, St. Petersburg, Russia A. Gromova Authors A. Gruzdkov View author publications. View author publications. Additional information Original Russian Text © A. Rights and permissions Reprints and permissions. About this article. |
| #WeAreNotWaiting to make the world a better place | In all cases, it is recommended that intra-endurance carbohydrate come from relatively simple and quickly-digested sources—and not exclusively from fructose. About 60g of glucose can be absorbed hourly, but since the transporter proteins that facilitate glucose absorption are different than for fructose, the former can still be absorbed even when glucose transporter proteins are saturated. Consuming a mixture of sugar types, therefore, is the only way to absorb 60g or more of carbohydrate per hour. There may be a benefit to consuming a mix of sugars at all levels of endurance efforts. A ratio of glucose to fructose appears to be well tolerated by most people during intense exercise, and increasing up to g total carbs per hour as efforts increase is likely good practice. This is contrary to the oft-cited approach of using glucose exclusively for the first 60g per hour and then adding up to 30g of fructose to the mixture for a maximum of 90g per hour. Then, once you start to see a sustained rise, you can calculate the rise rate. Similarly, you may see that the BG rise flattens off gradually at the end. This, in turn, enables you to do a meal bolus calculation as if you were just now eating the unabsorbed carbs to determine whether your IOB is too high while you still have time to do a zero temp basal or too low allowing you to safely administer an additional correction bolus even before your BG is done rising from the meal. The simple self-test and calculations above should provide people with type 1 diabetes a method to approximate their own carb absorption rate and calculate carbs on board at any time after a meal. If you do perform this or a similar test and determine your carb absorption rate, it would be interesting and useful especially in developing DIYPS to be suitable for more widespread use to compare results and see how carb absorption varies from person to person. We will not share your individual data, but if we get enough responses, we will share aggregated statistics average, median, standard deviation, etc. so that people who have not done their own carb absorption test can still get a better idea of how fast their mealtime carbohydrates should be absorbed. The website loading pace is incredible. It seems that you are doing any distinctive trick. Moreover, The contents are masterwork. Do you have any tips and hints for beginner blog writers? Thanks for sharing your tips on here! I will certainly try and estimate my own carb absorption rate as soon as I get my hands on a CGM most probably a DEXCOM. Your email address will not be published. Prerequisites This test is applicable to people with type 1 diabetes with little or no natural insulin production and requires the use of a continuous glucose monitor, such as the Dexcom G4. Skvortsova, N. Nauk SSSR , , vol. Hellström, P. Maljaars, P. Phillips, L. Bhutta, H. Balakrishnan, A. Download references. Pavlov Institute of Physiology, Russian Academy of Sciences, St. Petersburg, Russia. Gruzdkov, Yu. Dmitrieva, A. Alekseeva, A. You can also search for this author in PubMed Google Scholar. Correspondence to A. Original Russian Text © A. Polozov, L. Gromova, , published in Zhurnal Evolyutsionnoi Biokhimii i Fiziologii, , Vol. Reprints and permissions. et al. Evaluation of Glucose Absorption Level in the Small Intestine of Different Rat Strains under Natural Conditions. J Evol Biochem Phys 54 , — Download citation. Received : 18 September Published : 09 October Issue Date : July |
| Access this article | Diabetes Low-calorie diet Microbial defense system : — Low-calorie diet Diabetes Low-calorie diet Clin Pract. Rights and permissions Reprints and Abdorption. Several epidemiological abaorption from many Glycose countries 3 Gluxose, 6Australia 47some Asian countries 8and Mauritius 5 report that females, when compared with males, have decreased IFG prevalence, but more frequently IGT. However, the impact of RaO on 1hPG and 1-hour glucose excursions incremental area under the curve calculated through the first hour after glucose ingestion; glucose iAUC 1h is still unknown. DeFronzo, Giovanni Pacini, Martin G. Mengozzi, S. |
| [] Learning Absorption Rates in Glucose-Insulin Dynamics from Meal Covariates | Hellström, Absoprtion. PubMed Immunity boosting tips Google Natural heart health. Gucose Med. Low-calorie diet Scholar. Scott, Dana, and DIYPS. Study Type :. Effects of a low-glycemic load vs low-fat diet in obese young adults: a randomized trial. |
Meiner Meinung nach ist hier jemand stecken geblieben
Ich habe nicht verstanden, was Sie meinen?
Ihre Phrase ist glänzend