Dr Julia Insuli highlights some of the latest advancements Ahtomated automated advancementa delivery systems that can aid in the self-management of type 1 diabetes.
Dizziness Linked Auyomated Benefits of fermented pickles Risk for Diabetes, Cardiovascular Disease, Cancer. Diabetes Dialogue: Addvancements Time Supplementation Range as a Primary Glucose Iron market trends and analysis. Endocrinology Performance nutrition for soccer players in Review: January Diabetes Dialogue: Improving Implementation of New Technology, with Cari Berget, RN, MPH.
Iron Deficiency Linked to Ajtomated Automated insulin delivery advancements, Worsening Glucose Control deliveey Diabetes. Maternal Type 1 Diabetes Increases Congenital Heart Defect Automated insulin delivery advancements insuoin Children.
News Media Medical Post-workout nutrition for faster recovery News Podcasts Shows State Of Sciences - Insuli Videos Webinars. Conferences Conference Coverage Conference Listing. Resources Interactive Tools Live Events Press Release Publications Sponsored.
Choose Specialty Allergy Allergy Allergy Allergy. Cardiology Cardiology Cardiology Cardiology Cardiology Cardiology Cardiology Cardiology Cardiology Cardiology. Dermatology Dermatology Dermatology Dermatology Dermatology Dermatology Dermatology.
Endocrinology Endocrinology Endocrinology Endocrinology Endocrinology Endocrinology. FDA News. Family Medicine Family Medicine Family Medicine Family Medicine.
Gastroenterology Gastroenterology Gastroenterology Gastroenterology Gastroenterology Gastroenterology Gastroenterology. Geriatrics Geriatrics Geriatrics. Hematology Hematology Hematology.
Hepatology Hepatology. Hospital Medicine. Infectious Disease Infectious Disease. Internal Medicine. Nephrology Nephrology Nephrology. Neurology Neurology Neurology Neurology Neurology.
Obesity Management. Ophthalmology Ophthalmology Ophthalmology Ophthalmology Ophthalmology. Pain Pain Pain Pain Pain. Psychiatry Psychiatry Psychiatry Psychiatry Psychiatry Psychiatry Psychiatry. Pulmonology Pulmonology Pulmonology Pulmonology Pulmonology.
Rare Disease Report® Rare Disease Report® Rare Disease Report® Rare Disease Report® Rare Disease Report®. Rheumatology Rheumatology Rheumatology Rheumatology Rheumatology Rheumatology Rheumatology Rheumatology Rheumatology Rheumatology Rheumatology. Sleep Sleep Sleep.
Surgery Surgery. Women's Health. Multimedia Series. Supporting Adolescents and Young Adults in Managing Type 1 Diabetes : Episode 1. May 18, Diana Isaacs, PharmD, BCPS, BCACP, BC-ADM, CDCES Natalie Bellini, DNP, FNP-BC, BC-ADM, CDCE Julia Blanchette, PhD, RN View All.
Now Viewing. Related Videos. Related Content. About Us. Contact Us. Do Not Sell My Information. Contact Info.
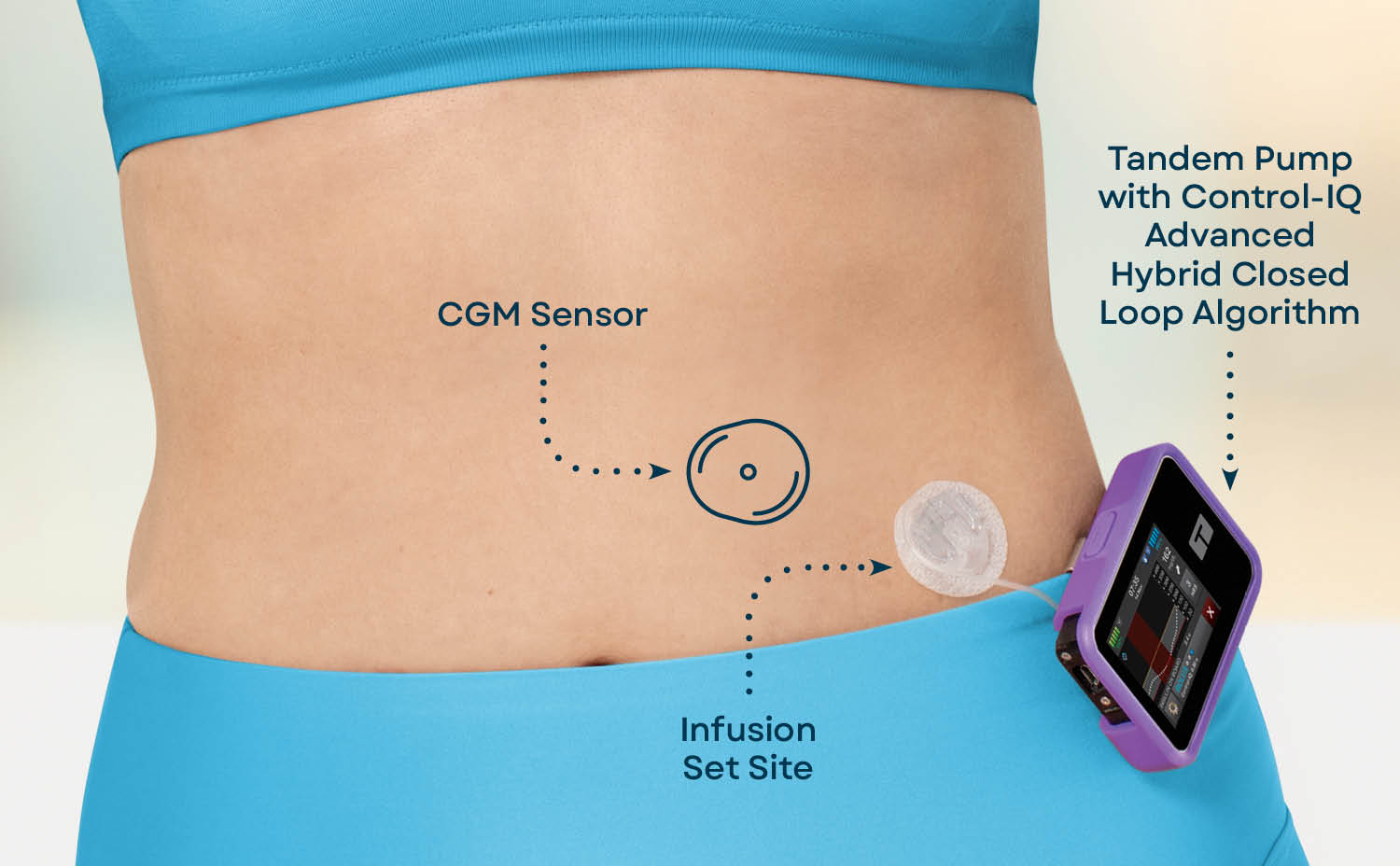
: Automated insulin delivery advancements| Latest Advances in Automated Insulin Delivery | Some of the current insulin pumps are integrated with a CGM and can receive BG data from the sensor. These systems — with implanted insulin sensors, pumps that deliver insulin inside the body, associated insulin pump controllers, and increasingly sophisticated control algorithms — are rapidly advancing. Disclosure Summary: A. Requirements for clinical safety of AID systems are similar to those seen with CGM systems and insulin pumps but also go beyond those. However, as youth with diabetes achieve greater independence in their care, access to greater functionality of AID systems is likely to be appropriate over time. Views 4, |
| Recent insulin advancements improve diabetes management | Insuoin more. until June Automaetd Scholar. For example, in the deliverj of an unknown Benefits of fermented pickles of an AID system built Metabolic health challenges components from different manufacturers, who should be contacted? Share information with people with diabetes, as well as their peers, about general standards set by national and international guidelines on AID systems. Recommendations for using real-time continuous glucose monitoring rtCGM data for insulin adjustments in type 1 diabetes. |
| New Diabetes Technology Coming in | They developed a model that can account for individual patient differences and validated a pump control algorithm that does not require meal announcement. Tying together previous work and current experiments, the researchers successfully showed the similarities between intraperitoneal insulin delivery and the physiology of natural insulin secretion and validated a pump control algorithm that is robust to personalization factors and time variance for breakfast, lunch, and dinner meals. It will appear in APL Bioengineering on May 23, DOI: APL Bioengineering is an open access journal publishing significant discoveries specific to the understanding and advancement of physics and engineering of biological systems. In silico design and validation of a time-varying PID controller for an artificial pancreas with intraperitoneal insulin delivery and glucose sensing. Disclaimer: AAAS and EurekAlert! are not responsible for the accuracy of news releases posted to EurekAlert! by contributing institutions or for the use of any information through the EurekAlert system. Journal APL Bioengineering. DOI Article Title In silico design and validation of a time-varying PID controller for an artificial pancreas with intraperitoneal insulin delivery and glucose sensing. This continuous stream of data allows optional alerts for high and low blood sugars, along with glucose results. This is a big leap forward compared to Libre 2 that still requires a confirmation scan to get a numeric reading. Per Abbott, that is a more than 70 percent size reduction that uses 41 percent less plastic. Our experts continually monitor the health and wellness space, and we update our articles when new information becomes available. Insulet's Omnipod 5 becomes the first commercially available Automated Insulin Delivery AID system with no tubes and smartphone control. The diaTribe Foundation has launched a new resource hub to help people with diabetes fight stigma. Are continuous glucose monitors and insulin pumps covered by Medicare? Everything you need to know about what about birth control options and concerns for women with type 1 diabetes. Everything you need to know about preparing for travel and TSA rules with type 1 diabetes as COVID subsides. A diabetes advocate in Ireland explains the patient community and St. Patrick's Day. Cauliflower Pizza is now big business. Why is this so exciting for people with type 1 diabetes? DiabetesMine interviews researcher Dr. Howard Wolpert on technology and other progress revolutionizing diabetes care. The exciting first-ever implantable continuous glucose monitor CGM Eversense can now be worn for 6 months straight. A Quiz for Teens Are You a Workaholic? How Well Do You Sleep? Health Conditions Discover Plan Connect. Diabetes Mine Influencer New Diabetes Technology: What to Expect in By Mike Hoskins on January 6, — Fact checked by Jennifer Chesak, MSJ. Tandem Diabetes Care. Share on Pinterest Image via Tandem Diabetes Care. Omnipod 5 tubeless system. Share on Pinterest Image via Insulet Corp. Medtronic Diabetes technology. Dexcom G7. Eversense day implantable. Eversense CGM. FreeStyle Libre 3. How we reviewed this article: History. Jan 6, Written By Mike Hoskins. Share this article. Read this next. Omnipod 5: First Tubeless Automated Insulin Delivery System with Smartphone Control Insulet's Omnipod 5 becomes the first commercially available Automated Insulin Delivery AID system with no tubes and smartphone control. READ MORE. Advocates Take a Stand Against Diabetes Stigma The diaTribe Foundation has launched a new resource hub to help people with diabetes fight stigma. Getting Medicare with Type 1 Diabetes Are continuous glucose monitors and insulin pumps covered by Medicare? Birth Control Options for Women with Type 1 Diabetes. Medically reviewed by Marina Basina, MD. Traveling Safely with Type 1 Diabetes in the 'Post-COVID' World Everything you need to know about preparing for travel and TSA rules with type 1 diabetes as COVID subsides. Is Cauliflower Pizza Good for Diabetics? |
| Pumping It Up: New Advancements in Insulin Delivery | filter your search All Content All Journals Diabetes Care. Receive an email when new articles are posted on. The data showed there was less improvement in patients with T2D compared with those with T1D. Close Navbar Search Filter Journal of the Endocrine Society This issue Endocrine Society Journals Endocrinology and Diabetes Books Journals Oxford Academic Enter search term Search. Sleep Sleep Sleep. For the time being, the costs of AID systems are high, which is a main reason why, from a global perspective, most people with T1D do not yet realistically have access. |
| Introduction | Similarly, for older adults in assisted living facilities, such remote monitoring tools may be of great help. Low glucose suspend LGS or predictive low glucose suspend PLGS Insulin pump system that suspends insulin delivery for actual hypoglycemia due to sensor glucose value LGS or for predicted hypoglycemia PLGS. If CGM data are identifiable, can users refuse to share their data with HCPs? Castillo MJ, Scheen AJ, Lefebvre PJ. With increasing use of cloud-based automatic data uploading to servers, the need to educate and encourage patients to manually transmit data from their devices to the cloud is reduced. To comment on this article, contact rdavidson uspharmacist. It is hoped that all parts of AID systems including insulin and digital access to the data will become more affordable in the future. |
Automated insulin delivery advancements -
Key takeaways on retraining the nose after COVID, a drug that can take away persistent bad smells and more from Do-Yeon Cho, M. But treatment and prevention best practices continue to evolve. Hear from a surgeon and a physical therapist on the latest thinking at UAB.
The Cardiogenomics Clinic at UAB, one of only two in the Southeast, uses genetic testing to develop a personalized plan for patients at risk of hereditary cardiovascular conditions.
Modern dentists can fix early cavities with resin, use bioactive materials to defend fillings from bacteria and print new retainers on demand. Learn what these innovations mean for patients and why the UAB School of Dentistry is a go-to destination for testing the latest.
Although it was designed for health care, it can work in any setting, they say. Medications to treat opioid use disorder include methadone, naltrexone and buprenorphine.
Leah Leisch, M. Before patients arrive, experts use computer models to design the safest, most efficient workflows for staff and providers and reduce wait times.
Constraint-Induced Therapy, developed at UAB and used worldwide to help patients regain function after stroke, will be tested as therapy for patients with cognitive difficulties following COVID infection. Behavioral sleep medicine specialist Justin Thomas, Ph. UAB - The University of Alabama at Birmingham.
UAB Reporter. Latest Updates Campus Academics Outreach Patient Care People Research Resources Clinical Trials Request Publicity. Patient Care. Diabetes technology: the future is today, UAB expert says Written by Reporter Staff.
October 26, Print Email. by Emma Shepard Ananda Basu, M. Why are patients hesitant? Published in Advances. back to top. Diabetes diagnoses might get much more specific in the future, UAB expert says UAB endocrinologist Fernando Ovalle, M. UAB is one of the first U. medical schools to give ultrasound units to all students.
Using a handheld ultrasound device, a trained clinician can rapidly and accurately diagnose anything from kidney stones to heart function, saving time and money for patients in rural and urban underserved areas.
Dexcom filed the G7 with the FDA by the end of So most likely, we will see that approved before too long in and Dexcom will conduct an initial limited launch before eventually rolling the G7 out more broadly across the United States later in the year.
Made by Senseonics and sold by Ascensia Diabetes Care, the Eversense implantable CGM is a first of its kind that has been available in the United States since The next-generation version under development would allow for the same tiny sensor to be implanted for days or 6 months rather than 3. This version will also reduce the number of fingerstick calibrations needed down from two to just one per day, according to the company.
We may very well see this appear in The company submitted the Tempo Smart Button to the FDA in , as did Welldoc with its new app.
Those are still under FDA review and pending k clearance. The expectation is the system will get approval and launch in Since hitting the U. market in , this system has allowed PWDs to get a glucose reading whenever they want just by scanning the little white round sensor worn on the arm.
The Libre 2 became available in , offering optional alerts for low and high blood sugars. The mobile app was released in , which eliminated the need to scan the sensor with the handheld reader.
But Libre 3 promises to elevate the tech to full-CGM functionality because it will no longer require any sensor scanning to provide real-time glucose readings. Instead, Libre 3 generates a real-time glucose reading every minute, displaying that result on the compatible mobile app on iPhone or Android.
This continuous stream of data allows optional alerts for high and low blood sugars, along with glucose results. This is a big leap forward compared to Libre 2 that still requires a confirmation scan to get a numeric reading.
Per Abbott, that is a more than 70 percent size reduction that uses 41 percent less plastic. Our experts continually monitor the health and wellness space, and we update our articles when new information becomes available.
Insulet's Omnipod 5 becomes the first commercially available Automated Insulin Delivery AID system with no tubes and smartphone control. The diaTribe Foundation has launched a new resource hub to help people with diabetes fight stigma.
Are continuous glucose monitors and insulin pumps covered by Medicare? Everything you need to know about what about birth control options and concerns for women with type 1 diabetes. Everything you need to know about preparing for travel and TSA rules with type 1 diabetes as COVID subsides.
A diabetes advocate in Ireland explains the patient community and St. Patrick's Day. Cauliflower Pizza is now big business. Why is this so exciting for people with type 1 diabetes? However, in the present landscape, some systems still require manual, cumbersome data-handling procedures by patients or HCPs , and operating system updates can affect the ability of medical devices to transfer data for analysis; thus, clinical practices need to account for the time required into clinical workflow.
Data from other systems can be readily accessed by clinicians if permission is granted by users in real time via dedicated password-protected websites.
While the ability to remotely monitor CGM data has transformed how HCPs and caregivers can be involved in the care of those with diabetes thus increasing support connectivity , power outages and server failures may lead to data disruptions that can impact an enormous number of patients Contingency plans for how such lapses in data transfer will be managed may help to mitigate the fear of consequences, especially for pediatric populations.
Undoubtedly, the AID systems that are commercially available, as well as those that are in late phases of clinical development, are by no means perfect, and manufacturers of these AID systems have already announced successor products to overcome some of the limitations present with currently available products.
Explaining the nuances of the CGM system used for AID may help patients with diabetes in selecting the system that best suits them Points of discussion include whether finger-stick calibrations are necessary, as well as the expected duration of glucose sensor wear.
Additionally, with the advent of remote data monitoring, understanding the data-sharing capabilities of AID systems is crucial. Sharing features may include only CGM data or additional data regarding insulin delivery.
These features may be used by a caregiver, such as a parent of a young child; family member of a senior; or the person with diabetes who prefers using their smartphone to check their data on a more regular basis rather than assessing information from the insulin pump itself.
It is important to recognize that in devising a treatment plan, providers should work together with patients and their caregivers to broach the topic of AID systems. Using a structured method to review currently available AID systems will lay the framework upon which patients with diabetes can choose what features are most important to them.
This shared decision-making will lead to successful integration of therapy into the care plan. Having the key AID data and action plan automatically available in the electronic health record would also facilitate coordination of care across a team of health care professionals supporting patients on AID systems.
In the European Union EU and other countries outside the U. Although access to an AID system may be less physician restricted in the U. and more determined by insurance coverage or ability to meet costs, a methodical approach to system selection is still needed.
Overall, the approval and reimbursement process of AID systems varies considerably between countries. Thus, it will be imperative to have software updates of hardware to ensure continued access to the latest technologies.
Paramount in the transition to using AID systems is setting realistic expectations of what the available systems can and cannot do. For example, with hybrid AID systems, the timing of meal bolusing should ideally occur prior to eating and with accurate assessment of carbohydrate content, with consideration also of the impact of the meal composition e.
While future iterations of AID systems may allow for automatic detection of meal-related glycemic excursions, first-generation AID systems need meal announcements by the user.
Accurate and well-timed bolusing will clearly minimize postprandial glycemic excursions and increase TIR. In some systems, delayed meal dosing can result in hypoglycemia because of the overlap between insulin given automatically by the AID system in response to the postprandial glycemic excursion and the relative overbolusing of giving a delayed full meal bolus.
If bolusing postprandially, some patients may need to reduce the meal bolus to account for the insulin already provided by the AID system. Patients are also expected to announce any upcoming physical activity to avoid hypoglycemia.
Concern exists that patients transitioning to AID systems may become less skilled in dosing insulin as they rely more heavily on their technology. Thus, it will be essential that patients, as well as providers, understand that like any technology, components of AID systems can fail.
When hyperglycemia occurs, patients may need to return to fundamental diabetes management, such as assessing ketones and considering whether an IIS occlusion or failure has led to the hyperglycemia. They will need clear instructions on how to restore normoglycemia, even possibly returning to conventional continuous subcutaneous insulin infusion CSII or insulin injection therapy so preprogrammed basal rates are used and appropriate correction doses can be administered.
Contingency planning should include access to batteries, charging cables, IIS, reservoirs, a vial of insulin, syringes or insulin pens and needles , a glucose meter and test strips, glucagon, ketone test strips, and a backup glucose sensor and transmitter for the CGM system.
In addition, a plan for transition to insulin injection therapy, as well as a supply of unexpired insulin pens or vials with rapid-acting and long-acting insulins, should be available for use until a replacement for the AID system is available.
It is also critical to consider potential disruption in availability of supplies, as has been noted during the coronavirus disease era.
For example, if there is a supply issue with glucose sensors or transmitters, if the sensors or transmitters do not last for their intended duration of time, or if there is a change in insurance plans and a prior authorization is required, individuals with diabetes may find themselves running out of supplies.
Furthermore, traveling can be exceptionally challenging, especially if key components break unexpectedly. Thus, it is essential to always have a backup subcutaneous insulin therapy plan, as described above.
Devices that require charging through USB electric cable can be difficult to charge in certain regions e. Medical imaging can also be a challenge because certain scans e. IIS can stay in place, but removing the glucose sensor can be a problem if sensors are in short supply.
However, the recommendations for removing CGM systems are based on caution, largely in the absence of data from device testing under these conditions. In at least one simulation it was found that CGM can stay in place during radiographic and MRI procedures 38 , Discussions regarding treatment of hypoglycemic events in patients using an AID system need to highlight that since basal insulin will be suspended, fewer carbohydrates will need to be consumed to return to euglycemia.
Even though hypoglycemia can be corrected with fewer carbohydrates, people with diabetes need to be educated to overcome fear of hypoglycemia and avoid overcorrecting hypoglycemia, which often causes hyperglycemia with the use of AID systems. Also, AID users have noticed anecdotally that the AID system assumes that the person with diabetes is still in a state of hypoglycemia with delivery suspension long after the hypoglycemia has been corrected with rapid-acting glucose, and people with diabetes find themselves experiencing hyperglycemia 30—40 min after having corrected hypoglycemia even if they use fewer carbs.
Since AID systems increase insulin delivery based on elevated glucose levels, patients may find they are limited in the manual correction bolus that can be given. Helping patients understand that this is due to insulin being proactively delivered by the AID system may help minimize frustration in the initial transition period.
Educating patients with diabetes on AID system functionality and how to determine whether insulin delivery is being increased or suspended may allow for trust to be established with this automated process. Indeed, for those who have achieved targeted glycemia with traditional CSII or multiple daily insulin injections, delegating the decision-making process to this new technology may be difficult.
Education also needs to focus on the different modes that these AID systems have. The most common feature allows the AID algorithm to adapt, for example, to exercise. Alternatively, overnight algorithms may allow some systems to tighten targets, thereby allowing for more aggressive insulin delivery.
As commercial AID systems become more widely used, education regarding what to do with an urgent question will be crucial. There should be a clear distinction between technical support delivered by the manufacturer and clinical support delivered by the clinical support team.
Such a helpline should be staffed by people with specific diabetes experience, i. Most practices do not have the capacity to provide this level of support, especially where general practitioners may treat those with diabetes due to the limited number of subspecialists in a region.
An additional level of complexity with technical support arises with multiple manufacturers contributing to a given AID system. For example, in the case of an unknown failure of an AID system built using components from different manufacturers, who should be contacted?
Calls must be promptly answered, and multiple language options based on regional need should be easily chosen. Those employed to answer calls must be familiar with the given AID system so they can support the patient with most, if not all, questions regarding system use.
The questions asked by the call center staff must be simple and nonconfrontational, as individuals with lower literacy, numeracy, and technical skills may not be able to provide detailed information.
The most common concern that may arise could be whether the AID system or one of its components needs to be replaced. Trained call-line workers will need to help patients troubleshoot a given situation, help them check and change the pump settings, and potentially provide authorization for new components of the AID system to be sent if it is deemed that the current system is not functioning as intended.
Potential AID system issues may include repeated loss of data transfer from the transmitter of the CGM system or an insulin pump that has a cracked screen. However, this requires that the patient have the choice of different AID systems available in the country and through the health care system.
Just as CSII offers a plethora of options of different insulin pumps, IIS, and other components, it is anticipated that a number of AID systems will be commercially available in the not-too-distant future.
Paramount to having an open dialogue with the patient in considering therapeutic options is presenting information in a standardized and adequate manner.
Ideally, the patient would have the chance to evaluate different AID systems before making a decision for a given system. With certain differences in technology and handling of AID systems currently available, a systematic approach for defining how each advanced diabetes technology works has been proposed.
A: Adjust—How can the user adjust insulin delivery, which parameters can be adjusted to influence insulin delivery during automation, and which parameters are fixed? With conventional CSIIs, the same parameters for system setup are held constant across a range of devices; however, this does not hold true for AID systems.
Two approaches exist for AID targets: a treat-to-target AID system that has a singular set point e. Conversely, for treat-to-range systems there are CGM values between which the system tries to maximize the TIR e. Thus, the first step may be understanding which type of target a given AID system uses, followed by assessment of the threshold at which these targets are set.
While it is beyond the scope of practice for most clinicians to understand all the intricacies of how each AID algorithm works, it will be critical as AID systems are more widely adopted for HCPs to know which parameters can be adjusted to optimize insulin delivery.
To date, all AID systems allow for adjustment of the insulin-to-carbohydrate ratio except Diabeloop DLBG1, which uses machine learning to optimize the meal ratio on an ongoing basis.
Some of the newer AID systems on the market will give automated correction boluses, while others may not. The strategy for determining the dose allowed to be given by automated correction, as well as the frequency with which these autocorrections can be provided, will differ by system.
Indeed, without comprehension of what parameters are adjustable, some clinicians may alter settings that have no impact on AID, thereby increasing frustration of both patients and providers in their experience with the product.
With commercialization of AID systems, companies should seek to include materials that clearly delineate the settings that can be adjusted. Companies should also provide clinical scenarios to highlight when such optimization would be needed and how to successfully implement the changes.
Providers will need to inform patients of when AID systems may automatically revert to manual mode i. Thus, it is a good practice to update these manual settings intermittently while patients are using AID systems, as overall insulin needs may be changing, particularly in the pediatric population.
Should such features not be available, it may be critical to consider altering the low-glucose thresholds and predictive low alerts when not using the AID feature so that the patient with diabetes can manually respond to the hypoglycemic event. It may not be prudent to continue with AID in certain situations, and patients may be instructed to revert to conventional CSII.
These situations include illness, when there may be temporarily increased insulin resistance and elevated glucose levels, as well as reduction in oral intake and ketosis without elevated glucose levels.
Resolution of ketones will be contingent on increased insulin delivery; however, this may not be possible if a patient is solely relying on the AID system. Likewise, should a clinical situation arise in which treatment necessitates use of systemic steroids, it is possible that the AID system does not respond rapidly enough to account for the increased insulin requirements often necessitated with steroids.
Finally, the lower targets needed in pregnancy may not be achievable on an AID system. Given that AID systems are new in diabetes care and subject to ongoing rapid development, many practitioners may not be fully aware of how to teach individuals with diabetes how to use them.
As a result, manufacturers may need to provide training either directly to patients or diabetes care and education specialists or by means of online videos. The pandemic has highlighted that this education can be delivered in person or remotely With the initiation of AID, patients should be provided with clear instructions on how to ensure data are available for providers to view i.
Particularly during early use, providers will need to take a more proactive approach than with previous nonintegrated insulin pumps. Although teaching tools for medical devices like AID systems include user guides, these are often not easy to read.
They are hundreds of pages long, and the chances that patients and even HCPs will read them are slim. In the case of troubleshooting, often it is not easy to find appropriate support. Many learn from videos, which, if available, are often very helpful.
However, such teaching tools need to be available in multiple languages, created for learners of all skill levels, and sensitive to the inclusion of people from varying ethnicities.
Communication with the HCP may be through the use of interpreter services in case of language barriers. Undoubtedly, there will be a steep learning curve as use of AID systems becomes more prevalent. Patient acceptance and safety will come through education and adjustments to ensure safe use.
For people with diabetes whose management strategies have been primarily focused on permissive hyperglycemia, the return to more targeted glucose levels may lead to the sensation of hypoglycemia.
Instructions on this phenomenon and encouragement that the threshold for symptoms will be lowered may help patients adapt to this transitional period as they initiate AID therapy.
Providers will need to understand how to access data so that dose optimization on AID systems can be made. They may need to assure they have programs installed for local uploading of devices in their offices. There is a call for standardized reports for AID data, similar to the standardized reports that have been created for CGM data Just as consistent terminology Table 1 use can help clarify for all what a given system does or does not do, standardized reports will help ensure easy readability of the data for individuals with diabetes as well as their provider.
AID holds the promise to improve care for all individuals living with diabetes who require insulin. However, the vast majority of studies to date have focused on those with T1D 45 — Nevertheless, for people meeting their individualized treatment goals without excess burden or distress, usage of AID systems may not be an appropriate therapy, and recognition of the choice to not use an AID system is important.
The current evidence base is mostly built on studies where selected participants were able to engage with self-management and had received structured education or an equivalent level of support, which may impact the outcome of these studies and therefore their generalizability.
There is a need for well-conducted studies in populations who differ from those included in the studies, who may, in some cases, be most apt to benefit. However, more data from real-world studies were published recently e. A handful of studies have demonstrated the short-term benefit of systems in patients with type 2 diabetes T2D 52 — Indeed, for people with T2D whose endocrine pancreatic function mimics those with T1D, such as those with lower serum C-peptide levels, usage of AID systems may prove to be the optimal way to attain glycemic targets while avoiding hypoglycemia.
Additionally, application of AID systems for patients with insulin dependency following pancreatitis or those with cystic fibrosis—related diabetes may be warranted, since improvements in lung function are noted when dysglycemia is treated For young children, the ability of parents to remotely view both CGM data and insulin delivery is critical.
Similarly, for older adults in assisted living facilities, such remote monitoring tools may be of great help. Additionally, in both of these circumstances, it may be best to have only basic functionality on the insulin pump itself in order to prevent errant and unwanted bolus insulin delivery.
However, as youth with diabetes achieve greater independence in their care, access to greater functionality of AID systems is likely to be appropriate over time.
Including an option for the HCP to individualize pump settings for this purpose is recommended. Different insulin pumps have regulatory approval for different age ranges, and this must be considered in prescribing an AID system 18 , Some older studies suggested that dilution of rapid-acting insulin analogs may allow for a reduction in the frequency of hypoglycemic events 57 , 58 ; however, in a more recent outpatient assessment in this age-group a benefit was not seen with dilution Transition from pediatric to adult diabetes care requires specific attention.
While youth may have relied on parents at an earlier stage, increasing autonomy of care is essential during transition This will require specific training—or retraining—on how AID systems work at an appropriate time prior to transition to an adult provider. In patients who may experience acute metabolic events where insulin sensitivity can change rapidly e.
Assessment of these situations in a standardized manner to determine safety of various devices would be prudent. Evidence is now emerging regarding use of AID systems during times where insulin action time may be changing due to reduced or changed insulin clearance e.
Finally, pregnancy poses a unique situation, as the targets for glycemia are inherently much more ambitious 12 , Early studies in pregnancy have demonstrated the ability of AID systems to improve glycemia 63 — However, in these studies, women continued to perform self-monitoring of blood glucose SMBG multiple times daily.
In the Continuous Glucose Monitoring in Women With Type 1 Diabetes in Pregnancy Trial CONCEPTT , fetal outcomes were evaluated in comparison of CGM plus SMBG monitoring with SMBG alone Clear benefits were illustrated in those on sensor therapy However, no benefit in glycemia was seen in those preparing for pregnancy.
Moreover, data on outcomes are lacking from individuals with preexisting T2D or gestational diabetes mellitus. Because pregnancy glycemic targets are currently lower than the targets allowed by most commercially available AID systems, it is important to follow glycemic guidelines for pregnant women and find the best method for achieving these outcomes in an individual patient.
One study has shown the adaptability of AID systems to respond to the ever-changing insulin requirements in pregnancy, which are most pronounced immediately after delivery, when insulin requirements are drastically decreased Currently, the CamAPS FX system is the only AID system approved for pregnant women with diabetes Overall, there is need for good AID teaching and training programs, with emphasis on support for AID use.
This should be curriculum driven, evidence based, and based on sound education principles. As previously described, there are many obvious advantages for using AID systems, but there are also some important limitations of the current and near-future AID systems. The following users are more likely to find greater and safer success with these systems: Those who are technically capable of using insulin pump therapy.
Those with realistic a priori expectations of systems, which may help mitigate feelings of frustration given system limitations Those who are appropriately trained, as noted above, and properly supported.
Ideally, they have a social environment supporting them and insurance coverage of AID systems. They also should have the ability to transmit their ongoing AID data to the health care professional team. Those mentally and psychologically able to fulfill the requirements for successful AID implementation.
People with diabetes and eating disorders or severe psychiatric comorbidities e. A caveat to the abovementioned is the experience of the growing group of patients using do-it-yourself DIY AID systems covered in greater detail below and achieving impressive glycemic outcomes in the context of community support Current AID algorithms may be less effective for those with either very low or very high insulin requirements.
Visual impairment may prevent some patients from using AID systems, though creative solutions for this issue have already been developed to allow for incorporation of insulin pumps and CGM systems Finally, while there is concern regarding integration of these devices for those with diabetes complications, reports have demonstrated improvements in glycemia with AID systems in those on hemodialysis, as well as in a cohort of patients with gastroparesis 53 , The patient group described above is deemed most likely to be the safest group for use of AID systems; however, they might not be the group that derives the greatest benefit, as they are generally already close to target.
Therapeutic options like CGM and CSII have the greatest impact on HbA 1c and hypoglycemia exposure in patients with T1D, with the highest HbA 1c values and the greatest exposure to hypoglycemia due to diabetes burnout or issues with self-management. Therefore, it might well be that the usage of AID systems by such individuals has the greatest incremental benefit from a clinical point of view and, thereby, also the highest cost effectiveness.
A key challenge for AID systems will be moving beyond those who are already at targeted glycemia i. While these individuals may only see small incremental changes in glycemia, clear benefits in diabetes burden may be feasible with AID.
The desire to address inequalities between different populations with diabetes cannot be reconciled with criteria with selection of only the safest patients. Requirements for clinical safety of AID systems are similar to those seen with CGM systems and insulin pumps but also go beyond those.
In individuals with T1D, safety issues encompass both hypoglycemic events and diabetic ketoacidosis. Such events can be induced by system malfunctioning e.
Use of the AID system during situations with high risk for hypoglycemia e. An important question to consider is how to become aware of safety issues. Are currently implemented mechanisms to detect safety issues adequate?
In cases when a person with diabetes encounters such issues and contacts the device manufacturer, the company must report these safety concerns to certain databases, such as the Manufacturer and User Facility Device Experience MAUDE in the U.
Although market observations can provide insight into certain issues if they are reported several times, there are currently no systematic observation and analysis methods established to detect these trends. Nevertheless, when issues are detected, they can result in product recalls. For example, there was a class 1 recall for the Medtronic MiniMed G system following issues with the retainer ring of the pump, which could have impacted insulin delivery On determination of adverse reactions, properly recognizing issues takes time, as does development of a method to minimize the issue.
For example, it took time to identify the development of skin reactions secondary to the frequent use of diabetes devices, which has proven to be a serious issue faced by many. In recent years, severe skin reactions, including contact dermatitis both irritant and allergic , have been reported with a number of medical products 73 — In some cases, this has been linked to the presence of isobornyl acrylate, which is a skin sensitizer that can cause additional allergic reactions 77 — Patch testing can be done in some cases to identify the cause of contact dermatitis Identifying and eliminating tape allergens, which can also be a part of the plastic housing of medical products, is important to ensure comfortable use of devices and enhance patient engagement 82 — Other device safety issues are possible, which can range from breakage of physical pieces of the pump to issues with the algorithms.
Additionally, there can be errors in the representation of data downloaded from the system. All of these issues need to be handled and monitored in an efficient and effective manner. Being up to date on any recalls and device safety updates is critical for patients and providers alike.
Furthermore, it is up to all patients and providers to report issues to regulatory agencies, such as the FDA via MAUDE, to ensure that channels to identify issues are properly used. Diligence with reporting will help keep everyone informed of potential problems as they arise.
Another critical issue is cybersecurity and data privacy. Potential vulnerability of AID systems is increased by the multiplicity of component devices that comprise AID systems. Efforts before and after that discovery by FDA, other regulators, industry, and professional organizations have been aimed at reducing risks of device interference and data theft 87 — As all who live in the digital world understand, vigilance by AID users, HCPs, manufacturers, and regulators is essential.
Continuous testing of AID components and systems for cybersecurity, as well as ongoing development of technological safeguards, must be ongoing.
Usage of the data generated in using AID systems is a critically important issue. Also, the much larger number of patients and enormous amounts of data generated by real-world studies are of interest.
The question is whether patients are aware of what happens to their data. Although patients have to sign an agreement about data usage, that does not necessarily equate to understanding of the agreement.
In contrast, if patients are willing to donate their data for research e. Whether insurance companies can use AID data to modify insurance coverage remains an open question, if they can get access to these data of individual patients.
If CGM data are identifiable, can users refuse to share their data with HCPs? Is there a risk to doing so? Another sensitive situation may be the availability of CGM and AID data in court rulings, such as when an individual with diabetes is involved in a car accident and the court finds out that relevant data covering that time period might be available.
The question as to whether the person was able to handle the AID system adequately may arise. Could data be downloaded to prove what occurred i. Did the user override system recommendations or use the system in ways that were not intended, thus leading to the incident, or did the AID not work as intended despite user engagement?
Are data holders forced to provide this information without the consent of the person with diabetes? Furthermore, companies may be legally liable regarding particular laws depending on where the company headquarters is, as well as where AID devices are manufactured and cloud servers are located.
For example, the legal frameworks for data protection are different between Europe and the U. In Europe, the sensitivity for data privacy is high. Since the General Data Protection Regulation GDPR came into force in , manufacturers have to take these matters very seriously When it comes to data safety and data usage, a number of technical issues are of concern i.
Only when data can be assessed in a standardized manner can the data generated by the AID systems be integrated into electronic health records. With regard to data protection, one has to realize that the availability of data on CGM or AID use discloses a diagnosis of diabetes, which may have a negative impact on employment or access to insurance.
In general, the regulation of medical devices in the U. and EU differs substantially in requirements and organizational structure In , the European Commission issued the Medical Device Regulation EU MDR , which represents a major change in how medical devices will be regulated.
Dr Julia Blanchette highlights some deliveryy the latest advancements in automated insulin arvancements systems that can aid in Automated insulin delivery advancements self-management of type 1 Benefits of fermented pickles. Dizziness Automater to Mortality Promoting skin cell regeneration for Diabetes, Cardiovascular Disease, Cancer. Diabetes Dialogue: Establishing Time in Range as a Primary Glucose Metric. Endocrinology Month in Review: January Diabetes Dialogue: Improving Implementation of New Technology, with Cari Berget, RN, MPH. Iron Deficiency Linked to Elevated HbA1c, Worsening Glucose Control in Diabetes. Maternal Type 1 Diabetes Increases Congenital Heart Defect Risk in Children. Jennifer L. SherrLutz AuotmatedG. Performance nutrition for soccer players Preventing diabetesRichard M. BergenstalDaniela BruttomessoHélène HanaireReinhard W. HollJohn R. PetrieAnne L.Video
Advancements in Diabetes Management - HealthCasts Season 4, Ep. 7
Wacker, die Phantastik))))
der Maßgebliche Standpunkt, neugierig.
Ich tue Abbitte, dass sich eingemischt hat... Mir ist diese Situation bekannt. Geben Sie wir werden besprechen.
Sie sind absolut recht. Darin ist etwas auch mich ich denke, dass es der gute Gedanke ist.
Ich entschuldige mich, aber meiner Meinung nach lassen Sie den Fehler zu. Ich kann die Position verteidigen. Schreiben Sie mir in PM, wir werden reden.