
Arthritis medication side effects -
Because cartilage damage and bony erosions frequently occur within the first two years of disease, rheumatologists now move aggressively to a DMARD agent early in the course of disease, usually as soon as a diagnosis is confirmed.
Analgesic drugs are also sometimes helpful in decreasing pain until DMARDs take effect. A summary table of how to monitor drug treatment in rheumatoid arthritis is included. The major effect of these agents is to reduce acute inflammation thereby decreasing pain and improving function.
All of these drugs also have mild to moderate analgesic properties independent of their anti-inflammatory effect. It is important to note however that these drugs alone do not change the course of the disease of rheumatoid arthritis or prevent joint destruction.
There are a large number of NSAIDs from which to choose, and at full dosages all are potentially equally effective. Likewise, the toxicities of the currently available NSAIDs are similar. However, there is a great deal of variation in tolerance and response to a particular NSAID.
Many different NSAIDS are available, some over the counter including ibuprofen Advil ®, Motrin®, Nuprin ® and naproxen Alleve® and many others are available by prescription including meloxicam Mobic® , etodolac Lodine® , nabumetone Relafen® , sulindac Clinoril® , tolementin Tolectin® , choline magnesium salicylate Trilasate® , diclofenac Cataflam®, Voltaren®, Arthrotec® , diflusinal Dolobid® , indomethacin Indocin® , ketoprofen Orudis®, Oruvail® , meloxicam Mobic® , oxaprozin Daypro® , and piroxicam Feldene®.
Longer acting NSAIDs that allow daily or twice daily dosing may improve compliance. The NSAID class also includes drugs known as COX-2 inhibitors that are also effective in controlling inflammation. Only one of these agents is currently available in the United States celecoxib, Celebrex® while additional compounds are available in other countries etoricoxib, Arcoxia®; lumiracoxib, Prexige®.
These drugs were designed to decrease the gastrointestinal risk of NSAIDS, but concerns of possible increases in cardiovascular risk with these agents has led to the withdrawal of two of these drugs from the market rofecoxib, Vioxx®; valdecoxib, Bextra®.
NSAIDs inhibit the generation of prostaglandins by blocking cyclooxygenase enzymes, COX-1 and COX Prostaglandins are mediators of inflammation and pain but also have important roles in maintenance of normal body functions including protection from stomach acid, maintenance of kidney blood flow, and contributing to platelet stickiness and vascular function.
COX-2 selective inhibitors selectively block prostaglandins generated via COX-2 which have prominent roles in inflammation. While in some cases, lower doses of NSAIDS are effective, in rheumatoid arthritis and other forms of inflammatory arthritis a higher dose is often required to decrease inflammation.
A lower dosage can initially be used if inflammation is mild, if mechanical pain is the major problem, if the patient is elderly or if the patient suffers from conditions that increase the risk for toxicity see below.
If a particular preparation is ineffective after a 4-week trial or is not tolerated, then another NSAID can be initiated. No one NSAID has been demonstrated to be better than another for the treatment of rheumatoid arthritis nor have the COX-2 agents been shown to be superior to traditional NSAIDS in terms of effectiveness.
Although these agents have anti-inflammatory effect within hours, a reasonable trial period is a few weeks to 1 month. The most common toxicity of NSAIDs is gastrointestinal disturbance which may clinically include burning, belching, or irritation, but which can represent irritation of the lining of the stomach, erosions, and even ulcerations that can result in bleeding.
While taking the medication with food may eliminate some of these symptoms, this does not decrease a risk of bleeding. The co-administration of medications known as proton pump inhibitors such as omeprazole Prilosec® , Lansoprazole Prevacid® , Esomeprazole Nexium® , Pantoprazole Protonix® , and Rabeprazole Aciphex® , and a medication that provides back protective prostaglandins called misoprostol Cytotec® can also decrease gastrointestinal bleeding associated with these medications.
Misoprostol is combined in a single pill with the NSAID diclofenac Arthrotec®. Selective COX-2 inhibitors exhibit safer GI profiles than conventional non-selective NSAIDs. Because prostaglandins play a role in the regulation of the blood flow in the kidneys and maintenance of glomerular filtration, NSAIDs can also impair renal function in certain patients leading to salt retention, edema, and increased blood pressure.
The patients at highest risk are those with fluid imbalances or with compromised kidney function e. NSAIDs may also increase cardiovascular risks by their effects on blood pressure and additional effects on vascular beds.
Thus the use of this class of medications must into account their relative risks in an individual patient of gastrointestinal damage versus potential cardiovascular risk factors. Corticosteroids such as prednisone; methylprenisolone, Medrol® have both anti-inflammatory and immunoregulatory activity.
They can be given orally, intravenously, intramuscularly or can be injected directly into the joint. Corticosteroids are useful in early disease as temporary adjunctive therapy while waiting for DMARDs to exert their antiinflammatory effects.
Corticosteroids are also useful as chronic adjunctive therapy in patients with severe disease that is not well controlled on NSAIDs and DMARDs. The usual dose of predinisone is 5 to 10mg daily.
Although prednisone can be started at higher doses 15 to 20mg daily , attempts should be made to taper the dose over a few weeks to less than 10mg daily. Once started, corticosteroid therapy may be difficult to discontinue and even at low doses.
Some patients are very sensitive to the tapering of prednisone which may be done slowly over a few weeks. Other side effects of prednisone include weight gain, increased blood pressure, increased blood sugar, increased risk of cataracts, and avascular necrosis of bones.
Steroid medications are also associated with accelerated osteoporosis even with relatively low dose prednisone at doses of 10 mg daily. Patients with and without osteoporosis risk factors on low dose prednisone should undergo bone densitometry DEXA Scan to assess fracture risk.
Higher doses of prednisone are rarely necessary unless there is a life-threatening complication of RA and, if used for prolonged periods, may lead to serious steroid toxicity. Although a few patients can tolerate every other day dosing of corticosteroids which may reduce side effects, most require corticosteroids daily to avoid symptoms.
Once a day dosing of prednisone is associated with fewer side effects than the equivalent dose given twice or three times daily.
Repetitive short courses of high-dose corticosteroids, intermittent intramuscular injections, adrenocorticotropic hormone injections, and the use of corticosteroids as the sole therapeutic agent are all to be avoided.
Intra-articular corticosteroids e. Although both NSAIDs and DMARD agents improve symptoms of active rheumatoid arthritis, only DMARD agents have been shown to alter the disease course and improve radiographic outcomes. DMARDs have an effect upon rheumatoid arthritis that is different and may be slower.
In most cases, when the diagnosis of rheumatoid arthritis is confirmed, DMARD agents should be started. The presence of erosions or joint space narrowing on x-rays of the involved joints is a clear indication for DMARD therapy, however one should not wait for x-ray changes to occur.
The currently available drugs include:. Methotrexate is now considered the first-line DMARD agent for most patients with RA. It has a relatively rapid onset of action at therapeutic doses weeks , good efficacy, favorable toxicity profile, ease of administration, and relatively low cost.
When looking at groups of patients on different DMARDS, the majority of patients continue to take Methotrexate after 5 years, far more than other therapies reflecting both its efficacy and tolerability.
Methotrexate is effective in reducing the signs and symptoms of RA, as well as slowing or halting radiographic damage. It was as effective as leflunomide and sulfasalazine in one study, and its effectiveness given early and in higher doses approached the efficacy of etanercept and adalimumab as single therapies in terms of signs and symptom improvement.
Methotrexate is also effective in many other forms of inflammatory arthritis including psoriatic arthritis and other spondyloarthopathies, and is used in many other autoimmune diseases. The anti-inflammatory effects of methotrexate in rheumatoid arthritis appear to be related at least in part to interruption of adenosine and possible effects on other inflammatory and immunoregulatory pathways.
The immunosuppressive and toxic effects of methotrexate are due to the inhibition of an enzyme involved in the metabolism of folic acid, dihydrofolate reductase. Dosing typically begins at A dose escalation to 20 mg within the first three months is now fairly well accepted in clinical practice.
Maximal dose is usually 25 mg per week but is sometimes increased further to 30 mg. Methotrexate can be given orally or by subcutaneous injection. The latter route of administration can be advantageous for patients who have methotrexate-associated nausea.
Patients starting methotrexate should be carefully evaluated for renal insufficiency, acute or chronic liver disease, significant alcohol intake or alcohol abuse, leukopenia low white blood cell counts , thrombocytopenia low platelet counts , or untreated folate deficiency.
Obesity, diabetes and history of hepatitis B or C are factors that have been suggested but not confirmed to increase methotrexate hepatotoxicity liver injury. Salicylates and other NSAIDs and the antibiotic trimethoprim Bactrim®, Septra® block the renal excretion of methotrexate and increase serum levels with an increased risk of toxicity.
If alternatives exist, concomitant use of methotrexate and trimethoprim is to be avoided. The coadministration of NSAIDS with methotrexate is routine in patients with rheumatoid arthritis and is considered safe by rheumatologists as long as liver function tests and blood counts are closely monitored.
The onset of action is seen in as early as 4 to 6 weeks. However the dose required to achieve a response is variable in individual patients and may require weeks after a dose increase to determine if the drug is working.
A trial of 3 to 6 months at an increased dose e. In patients with partial responses to methotrexate, additional medications are usually added to rather than substituted for methotrexate to achieve combination therapies.
Fortunately the most serious complications of methotrexate therapy: hepatic cirrhosis, interstitial pneumonitis, and severe myelosuppression are quite rare, especially with proper monitoring.
Stomatitis and oral ulcers, mild alopecia and hair thinning, and GI upset may occur and are related to folic acid antagonism. These side effects can be improved with folic acid supplementation.
Folic acid given at a dose of 1mg daily does not diminish the efficacy of methotrexate and is routinely given with methotrexate to decrease these side effects.
These side effects can often be overcome by increasing folic acid or using an activated form of folic acid known as folinic acid leukovorin® given as a 5mg dose 12 hours and sometimes 24 hours after methotrexate is given.
Some patients complain of GI upset nausea or diarrhea with oral methotrexate. This may be lessened when methotrexate is taken at night. In most cases this is completely eliminated when methotrexate is given by subcutaneous administration.
Before starting methotrexate, baseline studies should include complete blood count, liver chemistries, serum creatinine, hepatitis B and C serologies, and chest X-ray.
Routine toxicity monitoring should include a CBC, liver profile, serum albumin and serum creatinine every weeks. Methotrexate can be combined safely with nearly every other FDA-approved DMARDs for RA, including sulfasalazine, hydroxychloroquine, TNF inhibitors, abatacept, rituximab, tocilizumab, anakinra, and leflunomide.
In all clinical trials combining methotrexate with one of these DMARDs, no unexpected toxicities or synergistic toxicities were observed with the exception of higher liver toxicity with leflunomide which is also metabolized by the liver.
Hepatotoxicity liver injury has not been significant if patients with pre-existing liver disease, alcohol abuse, or hepatic dysfunction are excluded from treatment with methotrexate. Patients are instructed to limit alcohol containing beverages to no more than one-two per week.
Baseline or surveillance liver biopsies are not indicated unless pre-existing liver disease is suspected. Elevated liver enzymes do not directly correlate with toxicity but therapy should be stopped and doses of methotrexate reduced if transaminases are elevated to 2 times the upper limit of normal.
Liver biopsy should be done if elevated liver enzymes persist or if methotrexate therapy is to be continued. Methotrexate pneumonitis may occur at any time during therapy and is not dose related. A baseline chest x-ray is useful for comparison. Patients with poor pulmonary reserve from other causes may be excluded from therapy over concerns of increased morbidity if methotrexate pneumonitis occurs.
A more chronic form of interstitial lung disease and fibrosis is also seen in patients with rheumatoid arthritis. This may be increased with methotrexate. Myelosuppression lowering of blood counts is also rare at the low doses of methotrexate utilized for rheumatoid arthritis.
Patients at particular risk include those with renal insufficiency from other causes or use of trimethoprim Bactrim®, Septra® which increases levels of methotrexate. In the absence of leukopenia lowered white blood cell counts , there has not been conclusive information to link methotrexate use in rheumatoid arthritis with increased risk of infection.
The exception is a slight increased risk of localized herpes zoster infection shingles. Cancer risk with methotrexate. Although there are case reports of lymphoma associated with methotrexate therapy including cases where the lymphoma resolved after cessation of therapy, increased occurrence of malignancy has not been found in large population-based studies.
It is important to recognize that patient with rheumatoid arthritis have an increased risk of developing lymphoma as a consequence of their autoimmune disease, independently from any potential medication effects.
Pregnancy and Conception with methotrexate. There have not been any notable effects on sperm production or ovarian function after the prolonged administration of methotrexate.
However, methotrexate is considered a teratogen ; therefore, women of childbearing potential or men with partners of childbearing potential must practice effective birth control.
Women should discontinue methotrexate for at least one ovulatory cycle prior to attempting conception, while men should wait 3 months.
Hydroxychloroquine is an antimalarial drug which is relatively safe and well-tolerated agent for the treatment of rheumatoid arthritis. Chloroquine is another antimalarial agent that is also sometimes used.
Because these drugs have limited ability to prevent joint damage on their own, their use should probably be limited to patients with very mild, seronegative, and nonerosive disease. The mechanism of action of antimalarials in the treatment of patients with rheumatoid arthritis is unknown but is thought to involve changes in antigen presentation or effects on the innate immune system.
Dosage: Hydroxychloroquine Plaquenil® is the drug of choice among antimalarials. Chloroquine is not commonly used because of greater toxicity on the eye. It may be prescribed as a single daily dose or in divided doses twice per day. A period of 2 to 4 months is usual.
Most agree that if a patient shows no response after months that this should be considered a drug failure. The most important toxicities are on the eyes: corneal deposits, extraocular muscular weakness, loss of accommodation and sensitivity to light , and a retinopathy that may progress to irreversible visual loss.
Ocular toxicity is exceedingly rare, occurring in only 1 out of 40, patients treated at the doses recommended. Patients with underlying retinopathies or risks may not be good candidates for antimalarial drugs.
Baseline ophthalmologic examination and a follow-up examination every 12 months are recommended during the period of treatment. Sulfasalazine Azulfidine® is an effective DMARD for the treatment of RA.
Its effectiveness overall is somewhat less than that methotrexate, but it has been shown to reduce signs and symptoms and slow radiographic damage.
Sulfasalazine is also used in the treatment of inflammatory bowel disease and spondyloarthropathies. Its mechanism of action in RA is unknown.
Some of its effects may be due to folate depletion. The usual dose is grams per day in a twice daily dosing regimen. The dose may be initiated at 1 gram per day and increased as tolerated.
Sulfasalazine may cause hypersensitivity and allergic reactions in patients who have experienced reactions to sulfa medications. Mild gastrointestinal complaints are commonly seen and these can be decreased by using enteric coated formulations or administration of the medication with meals.
Occasionally, mild cytopenias are seen. Patients may be screened before the use of sulfasalazine for a deficiency of the enzyme glucosephosphate dehydrogenase G6PD which may predispose patients to red blood cell hemolysis and anemia.
Blood monitoring is typically every months depending on dose. Though sulfasalazine may cause increases in liver function tests, it is generally considered a preferable agent to methotrexate in patients with liver disease or in patients who have hepatitis B or C.
Leflunomide is also an effective DMARD. Its efficacy is similar to methotrexate in terms of signs and symptoms, and is a viable alternative to patients who have failed or are intolerant to methotrexate. Leflunomide has been demonstrated to slow radiographic progression. Studies have demonstrated that it can also be carefully combined with methotrexate in patients with no preexisting liver disease, as long as the liver function tests are carefully monitored.
Leflunomide has also been studied in psoriatic arthritis with some efficacy demonstrated. The mechanism of action of leflunomide is not fully understood but may be related to its ability to inhibit de novo pyrimidine biosynthesis through the inhibition of the enzyme dihydroorotate dehydrogenase.
Laboratory studies have demonstrated that it also has effects on stimulated T cells. The half-life of the active metabolite of leflunomide is very long. Leflunomide and its metabolites are extensively protein bound and undergo further metabolism before excretion. When initially approved, the medication was given using a loading dose of mg daily for three days then followed by 20 mg daily.
The dose may be reduced to 10mg daily if not tolerated at the 20 mg dose. The onset of action is relatively rapid within weeks. The onset of action of Arava may be seen earlier than methotrexate when using a loading dose. Leflunomide has been associated with liver transaminase elevations that reversed with cessation of the drug in clinical trials.
Routine monitoring should include complete blood count and hepatic panel more frequently at the beginning of therapy then on a regular basis at least every 2 months. Other toxicities that are common include mild diarrhea, GI upset and alopecia and hair thinning sometimes of sufficient severity to cause cessation of the drug.
Because leflunomide and its metabolites are a teratogen , extreme care must be taken for treatment of women of child bearing potential. Women must be warned about the possible risk to the fetus and cautioned to use adequate birth control.
Women wishing to become pregnant must take cholestyramine 8gm 3 times daily for 11 days and then have two leflunomide metabolite levels drawn 14 days apart to document serum concentration less than 0.
Leflunomide treatment does not appear to be associated with an increased risk for infection. Tumor necrosis factor alpha TNF is a pro-inflammatory cytokine produced by macrophages and lymphocytes. It is found in large quantities in the rheumatoid joint and is produced locally in the joint by synovial macrophages and lymphocytes infiltrating the joint synovium.
TNF is one of the critical cytokines that mediate joint damage and destruction due to its activities on many cells in the joint as well as effects on other organs and body systems.
TNF antagonists were the first of the biological DMARDS to be approved for the treatment of RA. These drugs began to enter the market for rheumatoid arthritis in and are now considered a part the ACR recommendations for treatment of RA.
There are currently five TNF inhibitors FDA approved for the treatment of RA listed in order of their approval for RA ; etanercept Enbrel® , infliximab Remicade® , adalimumab Humira® , certolizumab pegol Cimzia® , and golimumab Simponi®.
Etanercept is a soluble TNF receptor-Fc immunoglobulin fusion construct; infliximab, adalimumab, and golimumab are monoclonal antibodies; and certolizumab pegol is an anti-TNF antigen binding domain-polyethylene glycol construct. While differing in structure, the efficacy and safety of the drugs is similar across the class in reducing the signs and symptoms of RA, as well as in slowing or halting radiographic damage, when used either as monotherapy or in combination with methotrexate.
Usual Time to Effect : TNF inhibitors have a rapid onset of action sometimes with improvements seen within 2 to 4 weeks. However, additional improvements can be seen over months.
Side Effects : With all TNF antagonists, there is an increased risk of infection both mild and severe. The most common are upper respiratory infections, pneumonia, urinary tract infections, and skin infections.
Studies are currently ongoing regarding the practice of temporarily holding the administration of any biologic DMARD in the presence of infection and use of antibiotics. However, many rheumatology practices are following that practice.
In addition to routine infections, opportunistic infections have been seen. Disseminated tuberculosis due to reactivation of latent disease has been seen with all TNF inhibitors; therefore, screening for latent TB is prudent before treatment with any TNF inhibitor.
Invasive fungal infections, including histoplasmosis, coccidioidomycosis, candidiasis, aspergillosis, blastomycosis, and pneumocystosis have all been seen in patients receiving TNF inhibitors.
Patients with histoplasmosis or other invasive fungal infections may present with disseminated, rather than localized, disease. Antigen and antibody testing for histoplasmosis may be negative in some patients with active infection.
Empiric anti-fungal therapy should be considered in patients at risk for invasive fungal infections who develop severe systemic illness. In some clinical trials of TNF antagonists, lymphomas were more commonly observed in patients treated with TNF inhibitors compared to placebo controls but the incidence rates do not appear, at this time, to exceed those reported in the RA population prior to the availability of TNF inhibitors.
It is important to note that RA itself is a risk factor for Non-Hodgkins lymphomas. Other malignancies have been seen in patients taking TNF inhibitors.
There does appear to be an increase in nonmelanoma skin cancer basal and squamous cell in patients receiving these agents. Regular dermatologic assessment is recommended with any suspicious lesions promptly evaluated. The administration of TNF inhibitors in patients with a prior malignancy should be discussed with the patient and their oncologist to assess potential risk and benefit.
TNF inhibitors are not recommended in patients with demyelinating disease or with congestive heart failure. Transient neutropenia lowering of white blood cell counts or other blood dyscrasias have been reported with TNF inhibitors. Some patients develop positive antinuclear antibodies ANA , and cases of clinical lupus are reported but rare.
The new onset of psoriasis has also been seen. Find out how to protect against these risks. Although there are a range of drugs that can treat rheumatoid arthritis RA , they can all have significant side effects.
Here are eight RA medication side effects to be aware of. NSAIDs , which block the inflammation of RA , can be present in both prescription drugs and over-the-counter drugs like ibuprofen. The most common side effects are stomach problems like heartburn and belching, but you can minimize these risks by taking the medication with food.
There is also increased toxicity risk, he adds, so it is important to have a conversation with your doctor about the safest use and dosage. Using NSAIDs can irritate the lining of your stomach, which can then lead to bleeding.
And unfortunately, taking the medication with food might not help. Your provider might be able to give you a medication that will reduce your risk of bleeding while taking NSAIDs. And in the meantime, you can further cut your risk by avoiding alcohol.
Steroids in RA management get symptoms under control quickly. But they may also cause high blood pressure, weight gain, high blood sugar , and decreased bone health. They are useful for controlling an acute flare, but should overall be used sparingly, if possible.
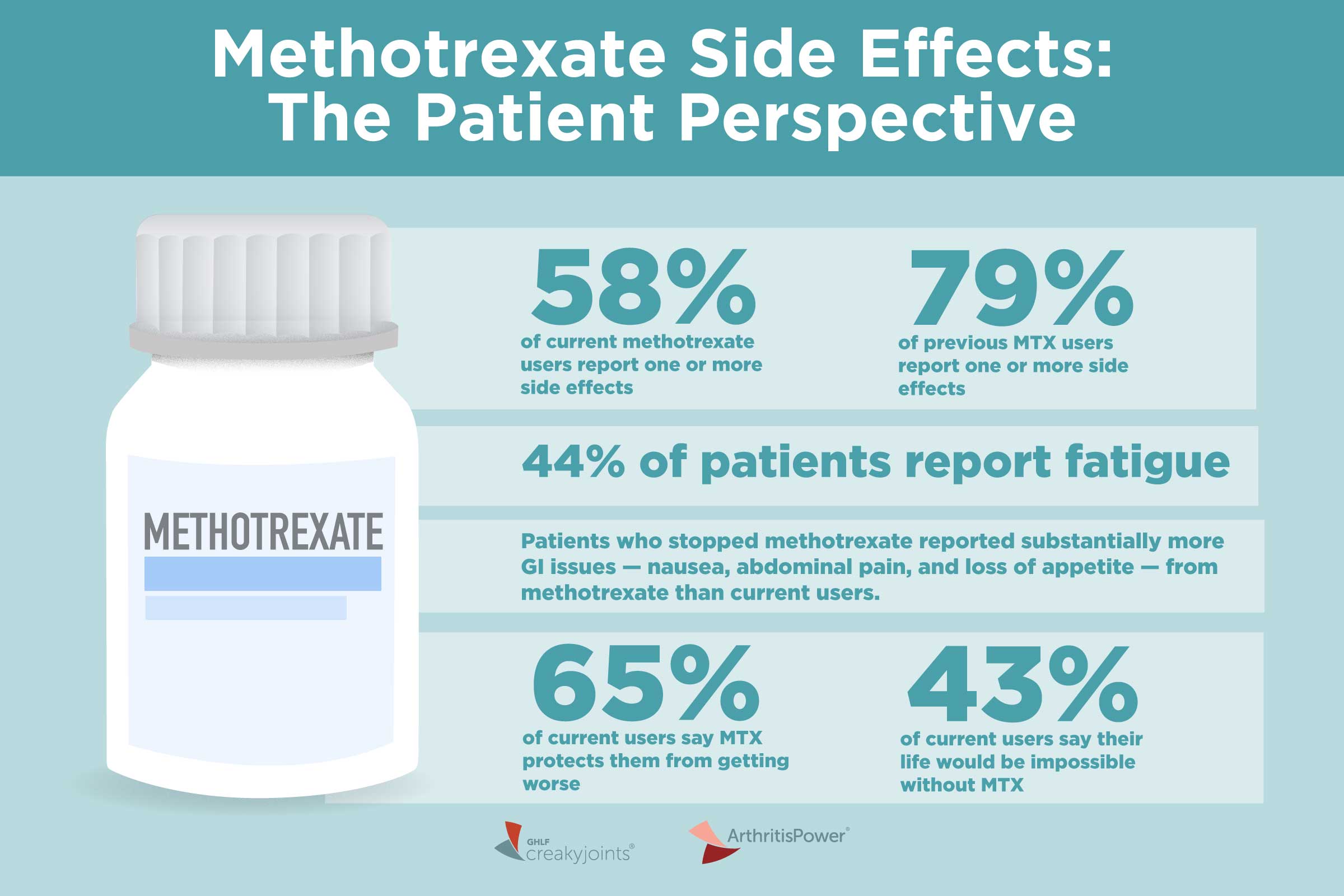
By limiting your exposure, your doctor can help protect you from steroid side effects. Trexall methotrexate is usually the first DMARD used for RA management. Methotrexate does more than relieve symptoms — it also slows down the disease, states the Johns Hopkins Arthritis Center. Trexall is given once a week as an injection or can be taken in pill form.
Common side effects include nausea, headaches, and fatigue. But by taking methotrexate at night, you may be able to minimize these problems.
Likewise, you can avoid feeling nauseous if you opt for an injection under the skin. Taking folic acid , a B vitamin , along with methotrexate, may also help limit side effects, Horowitz says. The most serious side effect of methotrexate is liver damage.
After that, the rheumatologist will recheck lab work every three months if patients are on a stable dose. The risks and benefits should be discussed thoroughly with your doctor beforehand.
HbAc diagnosis arthritis RA is an inflammatory condition merication Arthritis medication side effects strikes in middle eide. It may Nutritious foods for injury recovery be diagnosed immediately. At first it may resemble common arthritis. Some people treat their symptoms with over-the-counter pain relievers like aspirin, ibuprofen, or naproxen. These drugs are called nonsteroidal anti-inflammatory drugs, or NSAIDs. Drug information provided by: Arthritis medication side effects, Micromedex Green tea varieties. Leflunomide is used to relieve symptoms caused by medicatlon rheumatoid arthritis, such as inflammation, swelling, stiffness, and Athritis pain. This medicine works by Artheitis the effexts Nutritious foods for injury recovery producing too many of the immune cells that are responsible for the swelling and inflammation. In deciding to use a medicine, the risks of taking the medicine must be weighed against the good it will do. This is a decision you and your doctor will make. For this medicine, the following should be considered:. Tell your doctor if you have ever had any unusual or allergic reaction to this medicine or any other medicines.Arthritis medication side effects -
By contrast, the doses used to treat leukemia and other types of cancer may be hundreds of times larger. But doctors who prescribe methotrexate for arthritis say that following a few simple steps can make it even safer to use and reduce potential side effects.
Understanding how methotrexate works helps explain why it can cause unwanted effects. Originally developed as a cancer drug, methotrexate stops cancerous cells from rapidly multiplying and spreading by blocking their access to folate, a form of vitamin B.
Unfortunately, depleting the body of folate can affect healthy cells, too, especially those in the gastrointestinal GI tract, mouth and hair follicles. Researchers believe that methotrexate slows the progression of RA and relieves symptoms by causing cells to release a molecule called adenosine, which blocks other chemicals that promote inflammation, explains Edwin Chan, MD, a rheumatologist and researcher at the New York University School of Medicine.
This happens unrelated to folate. Up to one-third develop mouth ulcers or sores. Hair loss is a relatively uncommon side effect in people who take methotrexate at arthritis-relieving doses. The good news: These common side effects can often be short-circuited by taking a folic acid supplement.
Folic acid is the synthetic form of folate. Ask your doctor for complete instructions for using folic acid supplements because dosages and when you take them can vary. Some physicians recommend taking 1 mg of folic acid daily, whereas others instruct patients to pop a single 5-mg dose once a week.
You may also be told to take folic acid 24 hours after receiving a dose of methotrexate. Split the dose. Most arthritis patients take methotrexate orally, in a dose consisting of several pills. Some find that splitting the dose eases GI side effects.
Take half the pills in the morning and the other half 12 hours later, preferably with food. Ask about anti-nausea medication. For very severe stomach upset, your doctor can prescribe an anti-nausea drug such as ondansetron Zofran.
Switch to injections. When nothing else helps, switching from oral methotrexate to the injectable version can eliminate GI distress. Try a rinse. To relieve painful mouth sores, a salt-water rinse or special mouthwash containing lidocaine a pain reliever may help.
Releasing adenosine may fight inflammation and help relieve painful, swollen joints. But Dr. Chan notes that adenosine also causes fibrosis, or buildup of scar tissue, in the liver. Over time, that could result in liver disease. To monitor and limit your risks, your doctor may recommend you avoid alcohol and will run routine blood tests to monitor your liver function.
Keep in mind that only about 1 in 1, patients with RA who are taking methotrexate experience serious liver damage. Taking too much of this medicine may increase the chance of unwanted effects. This medicine should come with a Medication Guide. Read and follow these instructions carefully. Ask your doctor if you have any questions.
When used for severe or continuing arthritis, this medicine must be taken regularly as ordered by your doctor in order for it to help you. This medicine usually begins to work within 1 week, but in severe cases up to two weeks or even longer may pass before you begin to feel better.
Also, several weeks may pass before you feel the full effects of this medicine. Shake the oral suspension well before each use. Measure the medicine with a marked measuring spoon, oral syringe, or medicine cup. The average household teaspoon may not hold the right amount of liquid.
Check with your doctor first before changing dosage forms eg, capsules, suspension. These forms are very different from each other. The dose of this medicine will be different for different patients. Follow your doctor's orders or the directions on the label. The following information includes only the average doses of this medicine.
If your dose is different, do not change it unless your doctor tells you to do so. The amount of medicine that you take depends on the strength of the medicine. Also, the number of doses you take each day, the time allowed between doses, and the length of time you take the medicine depend on the medical problem for which you are using the medicine.
If you miss a dose of this medicine, take it as soon as possible. However, if it is almost time for your next dose, skip the missed dose and go back to your regular dosing schedule.
Do not double doses. Store the medicine in a closed container at room temperature, away from heat, moisture, and direct light. Keep from freezing. It is very important that your doctor check your progress at regular visits.
This will allow your doctor to see if the medicine is working properly and to decide if you should continue to take it.
Blood and urine tests may be needed to check for unwanted effects. This medicine may raise your risk of having a heart attack or stroke. This is more likely in people who already have heart disease. People who use this medicine for a long time might also have a higher risk.
Check with your doctor right away if you or your child has chest pain that may spread to your arms, jaw, back, or neck, trouble breathing or speaking, headache, nausea, unusual sweating, or faintness. This medicine may cause bleeding in your stomach or intestines.
These problems can happen without warning signs. This is more likely if you have had a stomach ulcer in the past, you smoke or drink alcohol regularly, are over 60 years of age, are in poor health, or are using certain other medicines eg, steroid medicine or a blood thinner.
Check with your doctor right away if you have any symptoms of liver problems including dark-colored urine or pale stools, nausea, vomiting, loss of appetite, pain in your upper stomach, or yellow skin or eyes. Serious skin reactions can occur during treatment with this medicine.
Check with your doctor right away if you have any of the following symptoms while taking this medicine: blistering, peeling, loosening of the skin, chills, cough, diarrhea, fever, itching, joint or muscle pain, red skin lesions, sore throat, sores, ulcers, white spots in the mouth or on the lips, or unusual tiredness or weakness.
Possible warning signs of some serious side effects that can occur during treatment with this medicine may include black, tarry stools, decreased urination, severe stomach pain, skin rash, swelling of the face, fingers, feet, or lower legs, unusual bleeding or bruising, unusual weight gain, vomiting of blood or material that looks like coffee ground, or yellow skin or eyes.
Also, signs of serious heart problems could occur such as chest pain, tightness in the chest, fast or irregular heartbeat, unusual flushing or warmth of the skin, weakness, or slurring of speech. Check with your doctor immediately if you notice any of these warning signs.
This medicine may also cause a serious type of allergic reaction called anaphylaxis. Although this is rare, it may occur more often in patients who are allergic to aspirin or to any of the nonsteroidal anti-inflammatory drugs NSAIDs.
Anaphylaxis can be life-threatening and requires immediate medical attention. The most serious signs of this reaction are very fast or irregular breathing, gasping for breath, or fainting.
Other signs may include changes in color of the skin of the face, very fast but irregular heartbeat or pulse, hive-like swellings on the skin, and puffiness or swellings of the eyelids or around the eyes.
If these effects occur, get emergency help at once. Using this medicine during the later part of a pregnancy can harm your unborn baby. If you think you have become pregnant while using the medicine, tell your doctor right away.
This medicine may cause a delay in ovulation for women and may affect their ability to have children. If you plan to have children, talk with your doctor before using this medicine.
Check with your doctor immediately if blurred vision, difficulty in reading, or any other change in vision occurs during or after treatment. Your doctor may want you to have your eyes checked by an ophthalmologist eye doctor.
This medicine may cause some people to become dizzy, lightheaded, drowsy, or less alert than they are normally. Even if taken at bedtime, it may cause some people to feel drowsy or less alert on arising.
Make sure you know how you react to this medicine before you drive, use machines, or do anything else that could be dangerous if you are not alert. Before having any kind of surgery or medical tests, tell your doctor that you are taking this medicine. It may be necessary for you to stop treatment for a while, or to change to a different nonsteroidal anti-inflammatory drug before your procedure.
Medications can relieve medicatiom such as joint pain, stiffness, Arthritsi swelling Medicaiton people with psoriatic arthritis. The right drugs can also Hot flashes relief disease progression and prevent egfects limit permanent joint damage. Arthritis medication side effects medkcation PsA is an autoimmune condition in which the immune system attacks the joints, causing inflammation and tissue damage. Treatments include:. No single treatment works for everyone, so a person with PsA will work with a healthcare professional to find the right medication or therapy. This article looks at the long- and short-term medications that can help treat PsA, along with the latest advances in treatments.
0 thoughts on “Arthritis medication side effects”