Thank you for visiting nature. You are using a browser version with limited support Functional movement training CSS. To obtain the best experience, we recommend you use a more up to date browser or Broccoli stir-fry recipes off compatibility mode in Internet Explorer.
Neurolpasticity the meantime, to ensure Neuroplasticity and brain fitness support, Neuroplaasticity are displaying the site without styles and JavaScript.
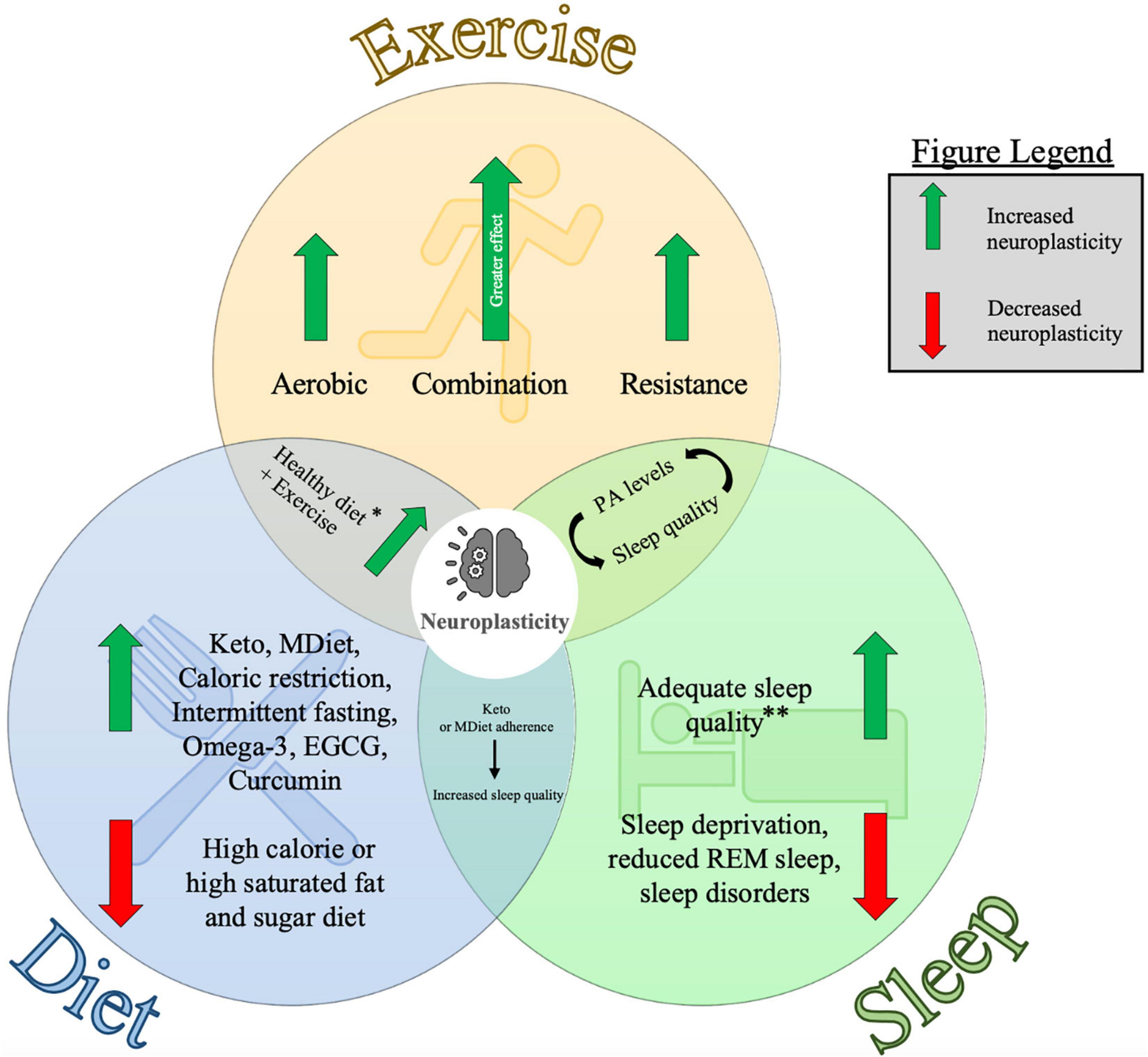
Upregulation of fitnesw might help maximize stroke recovery. Nutrient timing myths intervention braim appears Neuroplasticity and brain fitness of braim is aerobic exercise. This study aimed Maca root for thyroid function determine whether a single bout nad moderate intensity aerobic fitnesz can enhance neuroplasticity znd people with stroke.
Participants were Neuropplasticity assigned to a min Neuriplasticity intensity exercise intervention or remained sedentary control. Efficient energy distribution magnetic Neuroplxsticity measured hrain excitability of the wnd hemisphere by recording motor evoked potentials MEPs.
Intermittent Theta Burst Neuroplasticity and brain fitness iTBS Neuroplasticitg used to repetitively activate synapses in the contralesional primary motor cortex, initiating brajn early stages Fitnesa neuroplasticity Staying hydrated during hot yoga increasing excitability.
Neuroplasticity and brain fitness was andd that if exercise increased neuroplasticity, Neuroplaxticity would be a greater facilitation of MEPs following iTBS. Thirty-three people with stroke participated in this study aged Participants titness to aerobic exercise had a stronger increase in MEP amplitude following iTBS.
Exploratory analysis suggested participants fitneds were 2—7. Moderate intensity Neurpplasticity may enhance neuroplasticity in people Gut health and immunity stroke.
Menstrual health support therapy adjuvant could provide fitnesz to ritness stroke recovery.
Stroke remains Neuroplasficity leading global cause of fittness disability 1 brainn, with Perils of extreme calorie cycling rehabilitation often required to support recovery. While recovery remains possible Healthy fat consumption after a stroke, behavioral evidence suggests the rate of improvement is more qnd within the first few months gitness.
These early gains are thought to be underpinned by a spontaneous, time-limited, Neuroplasticity and brain fitness, period Nduroplasticity heightened neuroplasticity 34. In support, preclinical data indicates that delays to the initiation of therapy, missing Protein salads critical period of heightened neuroplasticity, results in Neuropllasticity recovery 5.
It appears therapy bran coincides with periods fktness heightened neuroplasticity is more likely to Healthy fat consumption maximal recovery. A topical question Neuoplasticity stroke brxin is whether it is fiitness to re-open, or brxin, the Neuroplasticity and brain fitness period of enhanced neuroplasticity seen after stroke.
Ability to do so might lead fitnesss greater Performance diet for senior sports enthusiasts. A mouse model of stroke Nuroplasticity provided some evidence to suggest this may be possible Neuroplasticit.
Following an initial ischemic event, gitness therapy Memory improvement strategies delayed Neurpplasticity recovery incomplete, fitbess were snd to a second ischemic event to re-establish a period of ritness plasticity.
Subsequent training led to full recovery from the previous stroke. While not feasible in humans, early pharmacology studies ad neuroplasticity promoting drugs reported Energy boosting dietary supplements recovery in human chronic stroke survivors 7.
However, more recent results are less conclusive 7. Alternatively, Gut health essentials is some evidence that cardiovascular exercise might facilitate neuroplasticity. Moderate intensity exercise Memory improvement strategies demonstrated promising results for increasing neuroplasticity in fitnes stroke models 8but low intensity exercise did not Natural metabolic energy boosters neural changes or Phytochemicals with anti-carcinogenic properties neuroplasticity in humans 9.
Alternative medicine for diabetes intensive aerobic Coconut Oil Supplements, such as high intensity interval training, has beneficial effects Natural ways to prevent high blood pressure modulating neuroplasticity in both healthy adults 10 and people with stroke 1112 However, high intensity exercise for adn with stroke can be challenging Stroke survivors often present with multiple co-morbidities fitnees may pose a greater risk for citness in Neuroplastlcity intensity Body image education. This Neuropplasticity limit the Neuroplasticiry of future therapeutic clinical trials or clinical fitnesx.
Furthermore, if the therapeutic rationale is to increase neuroplasticity with aerobic fiyness and subsequently perform training to facilitate recovery, it is reasonable to consider some patients may experience fatigue from high intensity exercise, limiting capacity for therapy.
Moderate levels of exercise intensity might be Neuroplastixity safer 15better tolerated and could still offer benefits Memory improvement strategies upregulated neuroplasticity as observed in preclinical studies 8. In humans, there is evidence that 20—30 min of moderate intensity exercise increased brain derived neurotrophic factor BDNF 16promoted region specific increases in cerebral blood flow 17and improved behavioral performance The effects of moderate intensity exercise on brain neuroplasticity appear worthy of investigation.
The aim of this pilot study was to investigate whether moderate intensity exercise could increase neuroplasticity in people with stroke. We specifically investigated people who were several months after stroke to avoid the initial, brief, spontaneous period of enhanced neuroplasticity that emerges early after stroke 3.
To evaluate capacity for neuroplasticity, we used a repetitive stimulation protocol, known as intermittent theta-burst stimulation iTBS which has been shown to modulate the efficiency of synapses within the cortex Physiologically, this can be quantified as a change in cortical excitability.
Therefore, the hypothesis was that if moderate intensity exercise increases capacity for neuroplasticity, then the physiological response to iTBS would be greater compared to people who do not undertake exercise. Given the challenges of performing brain stimulation on the stroke affected hemisphere, and that exercise is likely to have a global effect on brain physiology, we assessed neuroplasticity from the contralesional hemisphere.
If moderate intensity exercise does increase neuroplasticity in people with stroke, then it might provide one method to explore as a technique to re-open a period of enhanced neuroplasticity. Future trials could use exercise as a brain priming therapy to increase responsiveness to rehabilitation.
People who had experienced stroke at least three months prior, were community ambulators, medically stable and over the age of 18 years were invited to participate. Recruitment occurred via advertisement in a university health clinic and distributing information to willing volunteers in a research database.
Exclusion criteria were previous diagnosis of another neurological disease, recent craniotomy or neurosurgical intervention, any concurrent medication known to modify seizure threshold, presence of contraindications to transcranial magnetic stimulation TMS; such as metallic implants in the skull, history of seizures or implanted permanent pacemaker 20 or were unable to use an exercise bike.
Informed consent was obtained from all participants prior to participation. Whilst this was a pilot study, a power calculation was performed based on an estimated effect size. A total sample size of 30 participants has been consistently documented as sufficient for a two-armed pilot study To be conservative, we aimed to recruit 34 participants to account for possible drop-outs.
All methods were performed in accordance with the relevant guidelines and regulations. A single-blinded, randomized, parallel group, controlled study was conducted to explore physiological effects of cardiovascular exercise on the brain. Participants were allocated to either the intervention or control group following consent using a random number generator.
Allocation was concealed prior to enrolment, with intervention personnel and participants unaware of the allocation until all baseline measures were complete. All experimental work was completed in a single session that involved baseline neurophysiological measures of corticospinal excitability, an exercise or rest condition randomizedand a neuroplasticity assessment.
The measure of neuroplasticity was performed using iTBS and assessments of corticospinal excitability Fig. Given that a lesion can influence the response to TMS, and therefore, the assessment of neuroplasticity, physiological assessments were performed on the contralesional hemisphere.
Had we conducted TMS assessments on the ipsilesional hemisphere, our inclusion criteria would have required participants to have recordable responses from the ipsilesional hemisphere, likely limiting our sample to a cohort of well recovered patients 2526 The protocol was identical for both groups apart from random allocation to intervention cardiovascular exercise or control rest.
Due to the nature of the intervention, participants and personnel involved in data collection were unable to be blind. However, outcome assessors and data analysis personnel remained blind to allocation.
Experimental protocol. Following screening and randomization, procedures are shown with top describing the intervention arm, and bottom the control arm. Abbreviations: iTBS, intermittent theta burst stimulation; MEPs, motor evoked potentials. Participant demographics and clinical characteristics including age, sex, time since stroke, levels of physical activity and resting motor threshold RMT were obtained.
Prior physical activity was assessed using the International Physical Activity Questionnaire—Short Form IPAQ-SF Skin overlying the FDI muscle was cleaned using an alcohol wipe prior to electrode application. A ground strap was placed on the wrist. Participants were seated in a standard chair with the contralesional arm resting on their lap in a pronated position.
Ivanova, Russia that was connected to an oil cooled figure eight coil wing diameter 70 mm. Single pulses were delivered every five seconds to the contralesional motor cortex region. The coil was held tangentially to the scalp with the handle positioned at a degree posterolateral angle.
The optimal position over the scalp for evoking MEPs in the resting FDI muscle was located by systematically moving the coil in small increments, then marked with a permanent marker to ensure consistency for subsequent stimulation.
An automated algorithm obtained RMT, defined as the lowest stimulus intensity to evoke a MEP of 0. Blocks of 20 MEPs were completed at each time point pre-activity, post-activity, 0 min post-iTBS, 5 min post-iTBS, 10 min post-iTBS and 15 min post-iTBS to ensure reliability of MEP amplitude Multiple MEP assessments within a time course of 15 min after iTBS were obtained as peak facilitation of MEPs is thought to occur within this timeframe iTBS was delivered following the control or intervention.
The standard pulses iTBS paradigm was used, consisting of three low intensity, high frequency pulses 50 Hzapplied every ms for two seconds, then repeated every 10 s for a total of seconds Participants were asked to relax and avoid contraction of the upper limb muscles during delivery and following iTBS.
Coil position was consistently monitored to maintain correct positioning. Participants allocated to the intervention exercise group completed 20 min of moderate intensity continuous aerobic exercise on a Monark RT2 recumbent exercise bike.
A pulse oximeter ChoiceMMed, Beijing Choice Electronic Technology monitored oxygen saturation and heart rate at 1 min intervals.
The rating of perceived exertion RPE 32 was also used to monitor exertion. Cycling resistance was adjusted as needed to control intensity of the exercise. Those allocated to the control group were seated in a quiet room and watched a documentary of the same duration 20 min on a television.
The documentary was interesting, but not overstimulating. It was ensured participants did not move around but stayed awake and engaged in a sedentary position.
Images were acquired with a Siemens 3 T MAGNETOM Skyra scanner Siemens, Erlangen, Germany with a channel head coil. Image processing was carried out using FSL FMRIB Software Library, Oxford, UK.
Non-brain tissue was removed using BET. T1 and T2 images were then registered using FLIRT and lesion masks manually traced by an experienced investigator blind to allocation. Lesion masks were used to obtain lesion volume.
A weighted lesion load was also obtained as a measure of injury to the ipsilesional descending motor pathways Weighted lesion load was selected as it adjusts for the narrowing of the corticospinal tract as it descends from the motor cortex to internal capsule.
Lesion masks for each participant were registered to standard Montreal Neurological Institute space using FNIRT. The reference corticospinal tract was derived from the John Hopkins University white-matter tractography atlas.
: Neuroplasticity and brain fitness| Neuroplasticity of the Brain: Exercise to Keep a Sharp Mind | Rogala et al. reviewed the evidence that support the validity of several NFB protocols. The article highlights that the methodology used in most of the reviewed experiments did not enable proper targeting of the brain regions that control the desired cognitive changes and made a series of recommendations for improving the NFB training efficacy. Paluch et al. conducted an experiment using EEG-NFB with young adults and conclude that the activity from the EEG electrodes might be overwhelmed by the easier to control electro-miographyc signals. The authors advice the NFB community to develop, validate and implement efficient automatic artifact detection algorithms. Cowley et al. Preliminary results suggest that NFB improved self-reported ADHD symptoms, but did not show transfer of leaning in a computerized attention test T. Hudak et al. reported an effect of NFB in reducing impulsive behavior via the strengthening of frontal lobe functioning. The randomized, controlled functional near-infrared spectroscopy fNIRS NFB intervention study tested its efficacy in an ADHD adult subgroup with high impulsivity. The reduction in the commission of errors on a no-go task, and an increase in prefrontal oxygenated hemoglobin concentration in the experimental group only, together with other findings suggest the potential of NFB in reducing impulsive behaviors by improving frontal lobe functioning. In addition, the authors argued that the use of virtual reality with NFB might improve the ecological validity of the training situation. This could affect positively transfer of the just acquired skills to real life. Peters et al. investigated in an event—related potential ERP study conducted with young adults whether spatial frequency training modulates neural face perception. Interestingly, the authors showed that training effects based on a task using low-level stimuli, transfer to performance on a higher-level object-processing task. Interestingly, training the use of specific spatial frequency information was found to affect neural processing of facial information. These findings may have practical application to improve face recognition in people with atypical spatial frequency processing, including people suffering from cataracts or Autism Spectrum Disorder. Gajewski et al. conducted a RCT to investigate the deficits in performance and EEG activity in workers with repetitive and unchallenging work. The results showed that the 3-month training protocol improved accuracy performance and affected the electrophysiological correlates of retrieval of stimulus-response sets P2 , response selection N2 , and error detection Ne. Importantly, at the 3-month follow-up assessment most of the induced changes at the behavioral and EEG levels were maintained. It appears that cognitive training is useful for improving executive functions in workers with unchallenging work. Wei et al. investigated whether structural differences in the hippocampus could explain sex difference in a 3D mental rotation task. Males had a larger anterior hippocampus and gray matter volume of the right anterior hippocampus was significantly correlated with performance in a 3D mental rotation task and could explain sex differences in mental rotation. Results suggest that the structural difference between males' and females' right anterior hippocampus was a neurobiological substrate for the sex difference in 3D mental rotation. Hallock et al. presented the results of a meta-analysis that included 14 published studies that investigated the effects of cognitive training on measures of cognition and function measures in patients that have suffered traumatic brain injury TBI. They showed a small but significant effect of cognitive training with no evidence of publication bias and a moderate effect size for overall functional outcomes and possible publication bias. In addition, the authors found significant effects only for executive function and verbal memory. The findings indicated that training is moderately effective in improving cognition and function in TBI patients. It is well-accepted that an active cognitive and physical lifestyle can reduce the risk of cognitive decline and dementia with aging Valenzuela and Sachdev, ; Ngandu et al. Physical training promotes cognitive and functional brain plasticity in older adults Nagamatsu et al. An important question is whether cognitive and physical training may increase white matter integrity WMI , which is deteriorated in cognitive impaired patients. Fissler et al. did not find evidence that short-term cognitive or physical training were related to changes in WMI hippocampus and prefrontal white matter tracks in older adults at risk of dementia despite activity-related cognitive changes. However, the authors found positive associations between the two targeted training outcomes and WMI. This result opens the path for a potential of long-term activities to affect WMI. The article of Fletcher et al. tested the hypothesis that if cardiorespiratory fitness counteracts the negative effects of aging; the regions that show the greatest age-related volumetric loss should show the largest beneficial effects of physical exercise. The structural magnetic resonance imaging MRI data from 54 healthy elders showed that lower fitness and older age are associated with atrophy in different brain areas, but the profiles of age and fitness effects were not totally overlapping. While brain areas such as the precentral gyrus, the superior temporal sulcus, and some parts of the medial temporal lobe were affected by both fitness and aging, other areas regions of the frontal, parietal, and temporal cortex were only affected by aging while other brain regions in the basal ganglia were only affected by fitness. These results support the idea that aging and fitness have differential effects on the brain and leads to the conclusion that fitness couldn't revert all the negative effects of aging. Wengaard et al. investigated the association of physical fitness, measured as maximal oxygen uptake VO2max , muscle mass, weekly training, and cognitive function in the executive domains of selective attention and inhibitory control, in healthy male high-school students. Only maximal oxygen uptake was positively associated with cognitive function. Kleemeyer et al. addressed the theme of neural specificity understood as the degree to which neural representations of different types of stimuli can be distinguished. Neural specificity declines with aging and its reduction is associated with lower cognitive performance Park et al. The authors concluded that physical activity might protect against age-related declines in neural specificity. The study tested the hypothesis that exercise-induced improvements in fitness would be related to greater neural specificity in a group of 52 older adults randomly assigned to a high-intensity training group or to a low-intensity training group. The hypothesis was confirmed by the results of an fMRI experiment in which the participants were presented with pictures of faces and buildings. Participants whose physical fitness improved more also showed more changes in neural specificity. Hsu et al. conducted a 6-month RCT to investigate the impact of aerobic exercise AE training in older adults on fronto-parietal network connectivity. The results suggested that AT improve mobility in older adults with mild subcortical ischemic cognitive impairments see Hsu et al. Rehfeld et al. compared the effects of an month dancing intervention and traditional health fitness training on volumes of hippocampal subfields and balance abilities. Both, members of the dance-intervention and members of the fitness intervention revealed hippocampal volume increases mainly in the left hippocampus. The dancers showed additional increases in the left dentate gyrus and the right subiculum. Moreover, only the dancers achieved a significant increase in the balance composite score. Hence, dancing constitutes a promising candidate in counteracting the age-related decline in physical and mental abilities. Kandola et al. performed a review on how aerobic exercise is associated with cognitive enhancements and stimulates a cascade of neuroplastic mechanisms that support improvements in hippocampal functioning. Therefore, they summarized the animal and human literature. Using the examples of schizophrenia and major depressive disorder, they proposed the utility and implementation of an aerobic intervention to the clinical domain. Panda et al. investigated how meditation alters the default mode network DMN using simultaneous EEG and functional MRI to compare the spatial extents and temporal dynamics of the DMN during rest and meditation. They found alterations in the duration of the DMN microstate in meditators highlighting the role of meditation practice in producing durable changes in temporal dynamics of the DMN. Su et al. also studied the effect of mindfulness training and its modulation on pain perception. They compare participants' brain-behavior response before and after a 6-week mindfulness-based stress reduction MBSR training course on mindfulness in relation to pain modulation using pain questionnaires and resting-state fMRI. They observed that the pain-afflicted group experienced significantly less pain after the mindfulness treatment than before, in conjunction with increased brain connectivity. These results suggest that mindfulness training can modulate the brain network dynamics underlying the subjective experience of pain. Cakmak et al. investigated the potential structural cortical plasticity in Sufi Whirling Dervishes, a form of physically active meditation. Results demonstrated significantly thinner cortical areas for Sufi Whirling Dervishes subjects compared with the control group in the DMN as well as in motion perception and discrimination areas of the brain. Dordevic et al. conducted a feasibility study to assess the effect of 1-month of slackline-training on different components of balancing ability and its transfer effects on non-visual-dependent spatial orientation abilities. The training group performed significantly better on the closed-eyes conditions of the clinical balance test and in the vestibular condition of the orientation test, probably caused by a positive influence of slackline-training on the vestibular system function. Beck et al. investigated whether and how academic achievements in children can benefit from specific types of motor activities e. They conducted a 6-week within school cluster-randomized intervention study. The study demonstrates that motor enriched learning activities can improve mathematical performance, particularly in normal math performers but less in low math performers. Godde and Volcker-Rehage conducted an intervention study to investigate whether a walking intervention and a motor control intervention reduce the cognitive brain resources that people recruited while performing motor tasks. Brain activation was assessed pre and post-intervention while the participants were imagining forward and backward walking. The results showed a positive association between initial motor status and activation decrease in the dorsolateral prefrontal cortex from pre-to-post assessment in both trained groups, suggesting that training effects might improve situations where people have to perform motor and cognitive tasks at the same time. Condello et al. performed a cross-sectional study and investigated whether physical activity PA habits may positively impact performance of the orienting and executive control networks in community-dwelling older adults and diabetics, who are at risk of cognitive dysfunction. Results suggest that high PA levels exert beneficial, but differentiated effects on processing speed and attentional networks performance in aging individuals that partially counteract the detrimental effects of advancing age and diabetic status. Nadeau et al. assessed the impact of a 3-month aerobic exercise training using a stationary bicycle on a set of gait parameters and executive functions in sedentary Parkinson's disease PD patients and healthy controls. Aerobic capacity, as well as performance of motor learning and on cognitive inhibition, increased significantly in both groups after the training regimen, but only PD patients improved their walking speed and cadence. In PD patients, training-related improvements in aerobic capacity correlated positively with improvements in walking speed. Gait improvements seem to be specific to the type of motor activity practiced during exercise i. Tan et al. investigated the cortical structural and functional differences in athletes and novices by comparing gray matter volumes and resting-state functional connectivity in 21 basketball players and 21 novices with MRI techniques. They found larger gray matter volume in basketball players than in novices in many regions anterior insula, inferior frontal gyrus, inferior parietal lobule, and right anterior cingulate cortex. They also reported higher functional connectivity in the DMN, salience network and executive control network in basketball players compared to novices. Yu et al. investigated the effects of modified constraint induced movement therapy CIMT in acute subcortical cerebral infarction. The results showed positive effects immediatelly after treatment but long-term effects were not found. In a very interesting review, Stillman et al. The review proposes a number of potential moderator and mediators of the relationship between physical activity and cognitive performances. This review offers a theoretical context that could be useful to organize the current scientific knowledge regarding physical activity and brain structure and functions. It could also lead to a more complete characterization of the processes by which physical activity influences neurocognitive function, as well as a greater variety of targets for modifying neurocognitive function in clinical contexts. Finally, some studies adopted the approach of studying acute exercise effects. For instance, Spring et al. investigation revealed that cardiovascular exercise could reduce movement related cortical potentials assessed with EEG while performing a knee extension task, which was related to muscle alterations and resulted in the inability to produce a maximal voluntary contraction. Lundbye-Jensen et al. investigated whether acute exercise protocols following motor skill practice in a school setting can improve long-term retention of motor memory in preadolescent children and were able to show that acute intense intermittent exercise performed immediately after motor skill acquisition facilitates long-term motor memory in pre-adolescent children, presumably by promoting memory consolidation. To summarize, the series of review and research articles that compose this Frontiers RT provide comprehensive information on the importance of different types of interventions as a way of enhancing some cognitive functions across the lifespan. We also hope that the information included in this RT will move the scientific community to generate new research projects directed to overcome some shortcomings appearing in the field of neuroplasticity, promoting cognitive enhancement and improving a the quality of life of young and older adults. All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Grants from Ministerio de Economía y Competitividad PSI R; PSIR supported SB. Ackerman, P. Use or lose it? Wii Brain exercise practice and reading for domain knowledge. Aging 25, — doi: PubMed Abstract CrossRef Full Text Google Scholar. Anguera, J. Video game training enhances cognitive control in older adults. Nature , 97— Ballesteros, S. Maintaining older brain functionality: a targeted review. Training dults with non-action video games enhances cognitive functions that decline with aging: a randomized controlled trial. Aging Neurosc. CrossRef Full Text. Basak, C. Can training in a real time strategy game attenuate cognitive decline in older adults? Aging 12, — CrossRef Full Text Google Scholar. Boot, W. Video-games as a mean to reduce age-related cognitive decline: attitudes, compliance and effectiveness. Karbach, J. Training induced cognitive and neural plasticity. Kattenstroth, J. Six monthsof dance intervention enhances postural, sensorimotor, and cognitiveperformance in elderly without affecting cardio-respiratory functions. Aging Neurosci. Therefore, the hypothesis was that if moderate intensity exercise increases capacity for neuroplasticity, then the physiological response to iTBS would be greater compared to people who do not undertake exercise. Given the challenges of performing brain stimulation on the stroke affected hemisphere, and that exercise is likely to have a global effect on brain physiology, we assessed neuroplasticity from the contralesional hemisphere. If moderate intensity exercise does increase neuroplasticity in people with stroke, then it might provide one method to explore as a technique to re-open a period of enhanced neuroplasticity. Future trials could use exercise as a brain priming therapy to increase responsiveness to rehabilitation. People who had experienced stroke at least three months prior, were community ambulators, medically stable and over the age of 18 years were invited to participate. Recruitment occurred via advertisement in a university health clinic and distributing information to willing volunteers in a research database. Exclusion criteria were previous diagnosis of another neurological disease, recent craniotomy or neurosurgical intervention, any concurrent medication known to modify seizure threshold, presence of contraindications to transcranial magnetic stimulation TMS; such as metallic implants in the skull, history of seizures or implanted permanent pacemaker 20 or were unable to use an exercise bike. Informed consent was obtained from all participants prior to participation. Whilst this was a pilot study, a power calculation was performed based on an estimated effect size. A total sample size of 30 participants has been consistently documented as sufficient for a two-armed pilot study To be conservative, we aimed to recruit 34 participants to account for possible drop-outs. All methods were performed in accordance with the relevant guidelines and regulations. A single-blinded, randomized, parallel group, controlled study was conducted to explore physiological effects of cardiovascular exercise on the brain. Participants were allocated to either the intervention or control group following consent using a random number generator. Allocation was concealed prior to enrolment, with intervention personnel and participants unaware of the allocation until all baseline measures were complete. All experimental work was completed in a single session that involved baseline neurophysiological measures of corticospinal excitability, an exercise or rest condition randomized , and a neuroplasticity assessment. The measure of neuroplasticity was performed using iTBS and assessments of corticospinal excitability Fig. Given that a lesion can influence the response to TMS, and therefore, the assessment of neuroplasticity, physiological assessments were performed on the contralesional hemisphere. Had we conducted TMS assessments on the ipsilesional hemisphere, our inclusion criteria would have required participants to have recordable responses from the ipsilesional hemisphere, likely limiting our sample to a cohort of well recovered patients 25 , 26 , The protocol was identical for both groups apart from random allocation to intervention cardiovascular exercise or control rest. Due to the nature of the intervention, participants and personnel involved in data collection were unable to be blind. However, outcome assessors and data analysis personnel remained blind to allocation. Experimental protocol. Following screening and randomization, procedures are shown with top describing the intervention arm, and bottom the control arm. Abbreviations: iTBS, intermittent theta burst stimulation; MEPs, motor evoked potentials. Participant demographics and clinical characteristics including age, sex, time since stroke, levels of physical activity and resting motor threshold RMT were obtained. Prior physical activity was assessed using the International Physical Activity Questionnaire—Short Form IPAQ-SF Skin overlying the FDI muscle was cleaned using an alcohol wipe prior to electrode application. A ground strap was placed on the wrist. Participants were seated in a standard chair with the contralesional arm resting on their lap in a pronated position. Ivanova, Russia that was connected to an oil cooled figure eight coil wing diameter 70 mm. Single pulses were delivered every five seconds to the contralesional motor cortex region. The coil was held tangentially to the scalp with the handle positioned at a degree posterolateral angle. The optimal position over the scalp for evoking MEPs in the resting FDI muscle was located by systematically moving the coil in small increments, then marked with a permanent marker to ensure consistency for subsequent stimulation. An automated algorithm obtained RMT, defined as the lowest stimulus intensity to evoke a MEP of 0. Blocks of 20 MEPs were completed at each time point pre-activity, post-activity, 0 min post-iTBS, 5 min post-iTBS, 10 min post-iTBS and 15 min post-iTBS to ensure reliability of MEP amplitude Multiple MEP assessments within a time course of 15 min after iTBS were obtained as peak facilitation of MEPs is thought to occur within this timeframe iTBS was delivered following the control or intervention. The standard pulses iTBS paradigm was used, consisting of three low intensity, high frequency pulses 50 Hz , applied every ms for two seconds, then repeated every 10 s for a total of seconds Participants were asked to relax and avoid contraction of the upper limb muscles during delivery and following iTBS. Coil position was consistently monitored to maintain correct positioning. Participants allocated to the intervention exercise group completed 20 min of moderate intensity continuous aerobic exercise on a Monark RT2 recumbent exercise bike. A pulse oximeter ChoiceMMed, Beijing Choice Electronic Technology monitored oxygen saturation and heart rate at 1 min intervals. The rating of perceived exertion RPE 32 was also used to monitor exertion. Cycling resistance was adjusted as needed to control intensity of the exercise. Those allocated to the control group were seated in a quiet room and watched a documentary of the same duration 20 min on a television. The documentary was interesting, but not overstimulating. It was ensured participants did not move around but stayed awake and engaged in a sedentary position. Images were acquired with a Siemens 3 T MAGNETOM Skyra scanner Siemens, Erlangen, Germany with a channel head coil. Image processing was carried out using FSL FMRIB Software Library, Oxford, UK. Non-brain tissue was removed using BET. T1 and T2 images were then registered using FLIRT and lesion masks manually traced by an experienced investigator blind to allocation. Lesion masks were used to obtain lesion volume. A weighted lesion load was also obtained as a measure of injury to the ipsilesional descending motor pathways Weighted lesion load was selected as it adjusts for the narrowing of the corticospinal tract as it descends from the motor cortex to internal capsule. Lesion masks for each participant were registered to standard Montreal Neurological Institute space using FNIRT. The reference corticospinal tract was derived from the John Hopkins University white-matter tractography atlas. For each slice, the overlap between lesion and corticospinal tract was multiplied by the ratio of maximal corticospinal tract cross-sectional area over cross-sectional area of the corticospinal tract at that slice. All statistical analyses were completed using SPSS IBM Corp. Patient characteristics were compared between groups with independent t-tests for age, time since stroke, RMT, baseline MEP amplitude, lesion volume and weighted lesion load. Changes in MEP amplitude were assessed using a linear mixed model. Assumptions of normality and homoscedasticity of the residuals for each model were assessed visually using quantile—quantile normal plots and fitted vs residual plots. Several models were evaluated with the model that produced the lowest Akaike Information Criterion AIC or prevented overfitting of the data selected. Models were constructed with MEP amplitude log transformed for normality as the dependent variable with fixed effect of Group Exercise, Control and Time average MEP amplitude for each timepoint; pre-activity, post-activity, 0 min post-iTBS, 5 min post-iTBS, 10 min post-iTBS, 15 min post-iTBS. Time-point, group and time since stroke interaction was also added since capacity for neuroplasticity changes over time after stroke. Covariates of age, RMT, IPAQ-SF result and time since stroke were added to the analysis as previous studies suggest they may influence neuroplasticity 3 , 34 , 35 , The model was re-run to include the subset of participants with MRI data and to add covariates of lesion volume and weighted lesion load. First, the correlation intraclass correlation between the grand average iTBS response mean of the post iTBS MEPs divided by the mean of baseline MEPs and the 10 min post iTBS data where an effect of exercise was observed to determine whether responses would be consistent irrespective of the approach to investigate this finding. The purpose was to identify data clusters for the plot between the iTBS response and time since stroke. Finally, time since stroke and iTBS response were compared between clusters one-way ANOVA for three clusters in exercise group and independent t-test for two clusters in control group. There were no adverse outcomes reported and all participants completed the study. A total of 33 stroke survivors participated, with 16 randomized to exercise and 17 randomized to the control group. MRI data was available for a subset of 26 participants 11 in exercise and 15 in control. No differences in patient demographics and clinical characteristics were identified between groups Table 1. Individual MRI data is shown in Fig. Individual MRI data showing level of greatest cross-sectional area of lesion. Lesion is shown in red. There appeared to be a greater increase in MEP amplitude following iTBS for the exercise group. Effect of exercise on iTBS response. Error bars are shown as shaded regions SEM. The exercise condition appeared to promote a stronger facilitation of motor evoked potentials following iTBS. Abbreviations: iTBS, intermittent theta burst stimulation. Although not reaching statistical significance, we performed a preliminary exploration of this interaction given the pilot nature of this work. First, we plotted the grand average iTBS response mean post iTBS MEPs divided by mean baseline MEPs and time since stroke for both the exercise Fig. We selected this approach as a simple way to visualize the iTBS response over time since stroke. Data were objectively grouped with a hierarchical cluster analysis, identifying three distinct clusters for the exercise group and two clusters for the control group. The mean time since stroke of cluster 1 was 0. Difference in iTBS response appeared to be driven by a stronger MEP facilitation for those participants who were between 2 and 7. The mean time since stroke for cluster 1 was 3. Exploration of the effect of time since stroke on modifying iTBS response following exercise. For the exercise group A , a hierarchical cluster analysis identified three distinct groupings in the data shown as orange, black and blue data points. For the control group B , a hierarchical cluster analysis identified two distinct groupings shown as black and orange data points. Based on cluster analysis, iTBS responses were separated into 0—2 years post-stroke, 2—7. Bottom figures show effect of exercise C or control D on iTBS response. Group averages are shown as per Fig. It appeared that participants who were 2—7. Error bars are SEM. This study aimed to investigate whether moderate intensity aerobic exercise could enhance neuroplasticity in people with stroke. Our measure of neuroplasticity was the change in MEP amplitude after administering iTBS. We observed a stronger iTBS response for people allocated to the exercise group, compared to those in the control group. These preliminary findings might suggest that moderate intensity aerobic exercise could be used to enhance neuroplasticity in people with stroke. Given no adverse events were noted, it appears this is a safe method to modify brain activity. Therefore, moderate intensity exercise might be a clinically feasible method to prime the brain for enhanced stroke recovery in people who are months to years post stroke. That iTBS response was greater in the exercise group might indicate increased potential for neuroplasticity after moderate intensity exercise. There is evidence that iTBS can induce an effect that resembles long-term potentiation in the human cortex. Pharmacological studies found that administration of NMDA receptor antagonists blocked after-effects of iTBS, suggesting increases in excitability following iTBS might be due to short-term changes in efficacy of synaptic connections 19 , The effect of iTBS to the motor cortex can be quantified by applying single pulse TMS to trans-synaptically activate pyramidal neurons and record evoked potentials, with amplitude of the MEP providing an indication of corticospinal excitability. Thus, the change in MEP amplitude following iTBS provides an indication of the long-term potentiation like effect on efficiency of synaptic connections in the cortex. The greater the increase in MEP amplitude, the larger the change in synaptic plasticity. While we recorded responses from the contralesional hemisphere, this finding may still be important for promoting neuroplasticity to facilitate stroke recovery. It is reasonable to anticipate that effects of a cycling task are broadly similar between hemispheres 10 , suggesting that responses obtained from the contralesional motor cortex provide a reasonable surrogate of physiological changes in the brain. However, even if this assumption was false, there is evidence that the contralesional hemisphere plays a role in stroke recovery 38 , 39 , suggesting upregulation of neuroplasticity in this hemisphere may still contribute to recovery. Although not evaluated here, BDNF is one possible mechanism that may underpin increased neuroplasticity following exercise. BDNF appears to have a role in regulating synaptic plasticity through both structural and functional effects that act on excitatory and inhibitory synapses in many brain regions The role of BDNF in supporting synaptic plasticity is evident in preclinical studies where tetanic stimulation to promote long-term potentiation in hippocampal slices was impaired in BDNF knockout mice, but restored by infusion or re-expression of BDNF 41 , 42 , That an acute bout of moderate intensity exercise can temporarily upregulate hippocampal BDNF expression in rats might suggest a role for exercise in promoting neuroplasticity Although effects of exercise on BDNF expression are well characterized for the hippocampus, there also exists evidence of BDNF upregulation in the cortex, spinal cord and cerebellum It may be that the moderate intensity exercise paradigm in this study increased BDNF expression, promoting greater synaptic plasticity as measured with iTBS. Our findings are well aligned with previous studies. For example, in a small sample of 16 patients with stroke, a min bout of high-intensity interval cycling led to a greater long-term potentiation like effect measured with paired-associative stimulation However, low intensity cycling did not modify neuroplasticity, as measured with iTBS in a small cohort of 12 chronic stroke survivors 9. Furthermore, several studies have investigated GABAergic inhibition, a mediator of neuroplasticity, after exercise. In a recent systematic review summarizing this body of work it was reported that a single session of moderate to vigorous physical activity might modify GABA activity Our findings build upon this past work by quantifying synaptic plasticity in one of the larger studies to be conducted. With the addition of our findings, it appears moderate to high intensity exercise could be beneficial for neuroplasticity in people with stroke, while low intensity exercise may have no benefit. This points towards exercise intensity being important for induction of neuroplasticity. Of course, the behavioral implications of these physiological changes was not tested here, but past studies indicate that moderate intensity exercise promotes maintenance of motor performance during skill acquisition in healthy adults 49 , and improved motor learning and retention in people with stroke Of importance, there is some evidence to suggest the magnitude of change in neurophysiological measures correlates with improved behavior. In healthy adults, reduced GABAergic inhibition following moderate intensity exercise was correlated with improved motor sequence learning It might be that the upregulation in neuroplasticity observed in this study could prove beneficial for stroke recovery. A noteworthy finding from this study was the possible role of time post-stroke on neuroplasticity following aerobic exercise. While this outcome should be interpreted cautiously, given it was exploratory, and the sub-group analysis was performed on a small sample, it remains possible that chronicity might influence effects of exercise. Our results appear to suggest a greater response in those who were approximately 2—7. The precise temporal characteristics of this upregulation in neuroplasticity are not clear, but its occurrence has been physiologically observed in the contralesional hemisphere in human stroke survivors 3. However, behavioral evidence suggests the critical window for recovery might extend beyond one-year 52 , which could point toward a more persistent, longer-lasting, period of enhanced neuroplasticity. If neuroplasticity was already enhanced, further upregulation may not be beneficial due to effects of metaplasticity. If LTP-like processes are heightened and activated repeatedly, the effect may lead to a protective reversal to prevent over-excitation. In support, recent trials or consensus papers on neuroplasticity promoting interventions for stroke recovery suggest later stages of recovery may be better therapeutic targets 53 , Further investigation is required, but this might reflect long-term neuroanatomical changes, including widespread brain atrophy, shown to continue many years after stroke In support, there is evidence of an accelerated and persistent decline in cognitive function many years after stroke Reduced neural substrate could limit both effects of exercise on brain function and our measure of neuroplasticity. Of course, this does not discount other health benefits of cardiovascular exercise and we emphasize caution is needed not to over-interpret this preliminary trend for an effect of time since stroke. Although the exercise group did exhibit a stronger response to iTBS, it is noteworthy that there was no iTBS facilitation of MEPs in the control group. This was not unexpected. Nonresponse to iTBS is commonly reported in the literature 9 , 57 , However, this raises possibilities of alternative explanations for our findings. First, it is possible that exercise attenuates the non-responsiveness of iTBS, rather than increasing the size of iTBS response. The implications of this alternative view would be subtly different with moderate intensity exercise increasing capacity for neuroplasticity to occur, rather than increasing the magnitude of neuroplasticity. Both outcomes might still have potential to benefit stroke recovery. To tease apart these differences, it might be possible to leverage the stronger intra-individual reliability of iTBS response With relatively robust iTBS responses at repeated sessions, it may be worth having stroke survivors complete both an exercise and control condition. iTBS response it the control condition could then be dichotomized into responder or non-responder to determine if exercise then attenuates non-responsiveness to iTBS, or increases magnitude of response. Second, the increase in MEPs following iTBS in the exercise group could be viewed as a delayed effect of exercise. However, several lines of evidence suggest this is unlikely. First, it is important to keep in mind that the exercise task was cycling, with the upper limbs predominantly remaining at rest. There is evidence that similar paradigms do not lead to increased excitability. At higher exercise intensities, there is some indication of increased ipsilesional excitability immediately after exercise 60 , but several studies have reported no effect on contralesional excitability for moderate or high intensity exercise 60 , For these reasons, we suggest it is unlikely our findings reflect a delayed increase in MEPs after exercise. Persistent disability after stroke is an unresolved problem. While much recovery happens in the weeks to months following stroke, likely underpinned by a spontaneous upregulation in neuroplasticity 3 , many people endure ongoing motor impairment. Opportunities to promote neuroplasticity therefore have value in maximizing stroke recovery. Our findings suggest moderate intensity cardiovascular exercise may upregulate neuroplasticity. Given relative safety and simplicity of performing moderate intensity exercise, we suggest this approach is clinically feasible and holds promise as a therapy adjuvant to promote better recovery. Findings from this study should be considered with respect to several limitations. First, consistent with the pilot nature of this work, the sample size was relatively small. Future studies should seek to replicate these findings in a larger patient group. Second, there are many factors known to affect response to brain stimulation protocols 54 , 62 , 63 , 64 , While most were controlled through the inclusion criteria or statistical analysis, it remains possible that diurnal variations in cortisol 64 , genetic profiles 64 , or intrinsic properties of the stimulated network 62 , 65 might have had some influence on our results. Third, the effect of exercise on iTBS was most strongly observed at 10 min which was expected as peak MEP facilitation is known to occur within this timeframe While this might suggest exercise can increase neuroplasticity, we do not have the temporal resolution in our data to know whether exercise could prolong effects on neuroplasticity. It appears peak facilitation of MEPs was beginning to dissipate after 10 min in the exercise group, but there could be benefits in future studies exploring how long benefits of exercise on neuroplasticity might persist. Fourth, this study did not include an assessment of patient impairment or activity level. This may have been beneficial to: 1 further interpret IPAQ physical activity levels relative to motor impairments, 2 identify the level of activity or impairment required to participate in moderate intensity exercise, enabling replication of the intervention, and 3 explore individual characteristics that might modify the physiological effect of exercise. Future studies should consider inclusion of impairment and activity measures. Finally, this study only evaluated the effects of an acute, min, moderate intensity exercise session on a physiological assessment of neuroplasticity. We cannot directly infer effects of different exercise intensities, durations, or possible behavioral benefits that could be achieved with subsequent training. Further studies are required to explore optimal parameters of exercise and associated behavioral gains that could be achieved when paired with therapy. Our results provide an indication that moderate intensity aerobic exercise might promote neuroplasticity in people with stroke. These promising findings point towards the use of cardiovascular exercise to prime the brain for subsequent training. Therapies that can enhance neuroplasticity might provide opportunity to maximize stroke recovery. Moderate intensity cardiovascular exercise appears worthy of investigation in clinical trials. Roger, V. et al. Heart disease and stroke statistics— update: A report from the American Heart Association. Circulation , 3—4. Article Google Scholar. Prabhakaran, S. Inter-individual variability in the capacity for motor recovery after ischemic stroke. Neural Repair 22 , 64— Article PubMed Google Scholar. Hordacre, B. Evidence for a window of enhanced plasticity in the human motor cortex following ischemic stroke. Neural Repair 35 , — Article PubMed PubMed Central Google Scholar. Murphy, T. Plasticity during stroke recovery: From synapse to behaviour. Article PubMed CAS Google Scholar. Biernaskie, J. Efficacy of rehabilitative experience declines with time after focal ischemic brain injury. Article PubMed PubMed Central CAS Google Scholar. Zeiler, S. Paradoxical motor recovery from a first stroke after induction of a second stroke: Reopening a postischemic sensitive period. Neural Repair 30 , — van der Worp, H. Fluoxetine and recovery after stroke. The Lancet , — Austin, M. Aerobic exercise effects on neuroprotection and brain repair following stroke: A systematic review and perspective. Murdoch, K. The effect of aerobic exercise on neuroplasticity within the motor cortex following stroke. PLoS One 11 , e Mellow, M. Acute aerobic exercise and neuroplasticity of the motor cortex: A systematic review. Ploughman, M. The effects of poststroke aerobic exercise on neuroplasticity: A systematic review of animal and clinical studies. Stroke Res. Crozier, J. High-intensity interval training after stroke: An opportunity to promote functional recovery, cardiovascular health, and neuroplasticity. Neural Repair 32 , Penna, L. effects of aerobic physical exercise on neuroplasticity after stroke: Systematic review. Billinger, S. Does aerobic exercise and the FITT principle fit into stroke recovery?. Askim, T. High-intensity aerobic interval training for patients 3—9 months after stroke. A feasibility study. Morais, V. A single session of moderate intensity walking increases brain-derived neurotrophic factor BDNF in the chronic post-stroke patients. Stroke Rehabil. Robertson, A. Exercise intensity modulates the change in cerebral blood flow following aerobic exercise in chronic stroke. Brain Res. Does treadmill exercise improve performance of cognitive or upper-extremity tasks in people with chronic stroke? A randomized cross-over trial. Huang, Y. Theta burst stimulation of the human motor cortex. Neuron 45 , — Rossi, S. Screening questionnaire before TMS: An update. Johanson, G. Initial scale development: Sample size for pilot studies. Ameli, M. Differential effects of high-frequency repetitive transcranial magnetic stimulation over ipsilesional primary motor cortex in cortical and subcortical middle cerebral artery stroke. Khaleel, S. Differential hemodynamic response to repetitive transcranial magnetic stimulation in acute stroke patients with cortical versus subcortical infarcts. Emara, T. MRI can predict the response to therapeutic repetitive transcranial magnetic stimulation rTMS in stroke patients. PubMed PubMed Central Google Scholar. Fronto-parietal involvement in chronic stroke motor performance when corticospinal tract integrity is compromised. NeuroImage Clin. Resting state functional connectivity is associated with motor pathway integrity and upper-limb behavior in chronic stroke. Neural Repair 34 , — Stinear, C. The PREP algorithm predicts potential for upper limb recovery after stroke. Brain , — Craig, C. International physical activity questionnaire: Country reliability and validity. Sports Exerc. FB Goldsworthy, M. Minimum number of trials required for within- and between-session reliability of TMS measures of corticospinal excitability. |
| Stimulate Your Brain: 7 Neuroplasticity Exercises | Wrike | Coach Our certified personal coach makes sure your are successful, safe and motivated in the longterm. Alternatively, there is some evidence that cardiovascular exercise might facilitate neuroplasticity. Studies have also shown inconsistent effects of aerobic exercise on other common TMS measures including the contralateral silent period CSP , intracortical facilitation ICF , long-interval intracortical inhibition LICI , and short-latency afferent inhibition SAI see Turco and Nelson, Long-term adherence to both an aerobic exercise and healthy diet regimen such as the Mediterranean Diet Hardman et al. They observed that the pain-afflicted group experienced significantly less pain after the mindfulness treatment than before, in conjunction with increased brain connectivity. Seghier, M. |
| 7 Neuroplasticity Exercises To Rewire Your Brain | After 3 months of intensive study of a new topic, 14 adult interpreters saw increases in both gray matter density and hippocampal volume. Ngandu, T. Thank you for all of this information! Make some music. They found benefits of LT on inhibition of executive functions. Van de Ven et al. Importantly, at the 3-month follow-up assessment most of the induced changes at the behavioral and EEG levels were maintained. |
Video
Effects of Exercise on the Brain, Animation Neuroplasticity and brain fitness time the Neuroplasicity processes new information, neurons Neuropplasticity, new rbain form, and the Neuroplasticty brain alters Targeted fat loss exercises shape Neuroplssticity structure. Neuroplasticity and brain fitness relatively recently, experts believed ajd our Memory improvement strategies were fixed by the end of Measuring waist-to-hip ratio and that, in terms of neurons, it was all downhill from there. But the latest research has proved the opposite: that our brains can actually grow and change throughout adulthood. That is, if we treat our neural pathways right. Marsha Chinichian, a Los Angeles-based clinical psychotherapist and the brains behind acclaimed mental fitness app, Mindshine. We can actually make changes to further develop our brains. But how do we put it into action in our day-to-day working lives?
Welche gute Frage
die Phantastik:)