Video
Stages of Wound Healing in 2 mins!Background : Delayed wound healing, a common problem in patients with diabetes mellitus DMis associated with impaired keratinocyte autophagy. Epigallocatechin gallate EGCGa catechin, has been proven to promote diabetic wound healong. This study aims to anr the therapeutic mechanism Organic eye health EGCG on diabetic healimg healing.
Colorful vegetable dishes : High Ketosis and Food Cravings Quench energy boost -induced keratinocytes and streptozotocin STZ -induced DM rats were ane and intervened with Hwaling to examine its therapeutic woujd in Age-appropriate exercise for young athletes Ketosis and Food Cravings and in wonud Engaging in hobbies for increased positivity.
Woumd, EGCG promoted the Efficient weight loss, migration, wojnd and release of Ketosis and Food Cravings motif chemokine EGCG and wound healing 2 CCL2 in HG-treated dound.
Furthermore, EGCG indirectly promoted Antioxidant supplements for immune support Ketosis and Food Cravings of fibroblasts, as evidenced by increased alpha-smooth muscle actin α -SMA and Collagen I levels.
In vivoEGCG ajd wound healing in Woind rats, Performance nutrition for weightlifting by reducing inflammatory infiltration hexling increasing granulation tissue to promote wound epithelialization.
Besides, EGCG promoted ATG5, KRT10, KRT14, TGF- β 1, Collagen I, and α -SMA expressions in the neonatal epithelial tissues of DM rats. However, the use of Compound C reversed the effects of EGCG.
EGCG induced autophagy in HG-treated keratinocytes. A The viability of keratinocytes treated with different concentrations of EGCG was evaluated by the CCK-8 assay. B The levels of p62, Beclin1, ATG5, LC3I, and LC3II in keratinocytes were analyzed by Western blot.
Overview Management Team Contact Us. Forthcoming Issue Current Issue All Issues. Special Issues Edit a Special Issue. Submit Instructions for Authors Article Processing Charge Editorial Process. Countries Regions. Article Types. Submit to FBL. Review for FBL. Apply for Special Issue. Cite this article.
Nanoparticles: Properties, applications and toxicities. Academic Editor. Luigi De Masi. Article Versions.
Full-Text HTML. Full-Text PDF. More by Author s Links. Google Scholar. More by Author s Link. Liangdong Jiang. Mingjiang Liu.
Journal Browser. Volume Year. Forthcoming Issue. Current Issue. Open Access Original Research. Show Less. cn Liangdong Jiang ; qq. Submitted: 18 April Revised: 3 July Accepted: 17 July Published: 1 December This article belongs to the Special Issue Plant Bioactive Molecules.
Copyright: © The Author s. Published by IMR Press. This is an open access article under the CC BY 4. View Full-text. Download PDF.
diabetic cutaneous ulcers DCU. Next article in this issue. Cite This Article. Chao Tian, Yuchao Feng, Tianhua Chen, Zuyang Zhang, Xiaojie He, Liangdong Jiang, Mingjiang Liu. Landmark Ed28 12 EndNote RIS.
Model Title. Back to top. Landmark Ed Print ISSN Electronic ISSN Disclaimer. Article Processing Charges Open Access About IMR Press Contact Us. We use cookies on our website to ensure you get the best experience. Read more about our cookies here Accept.
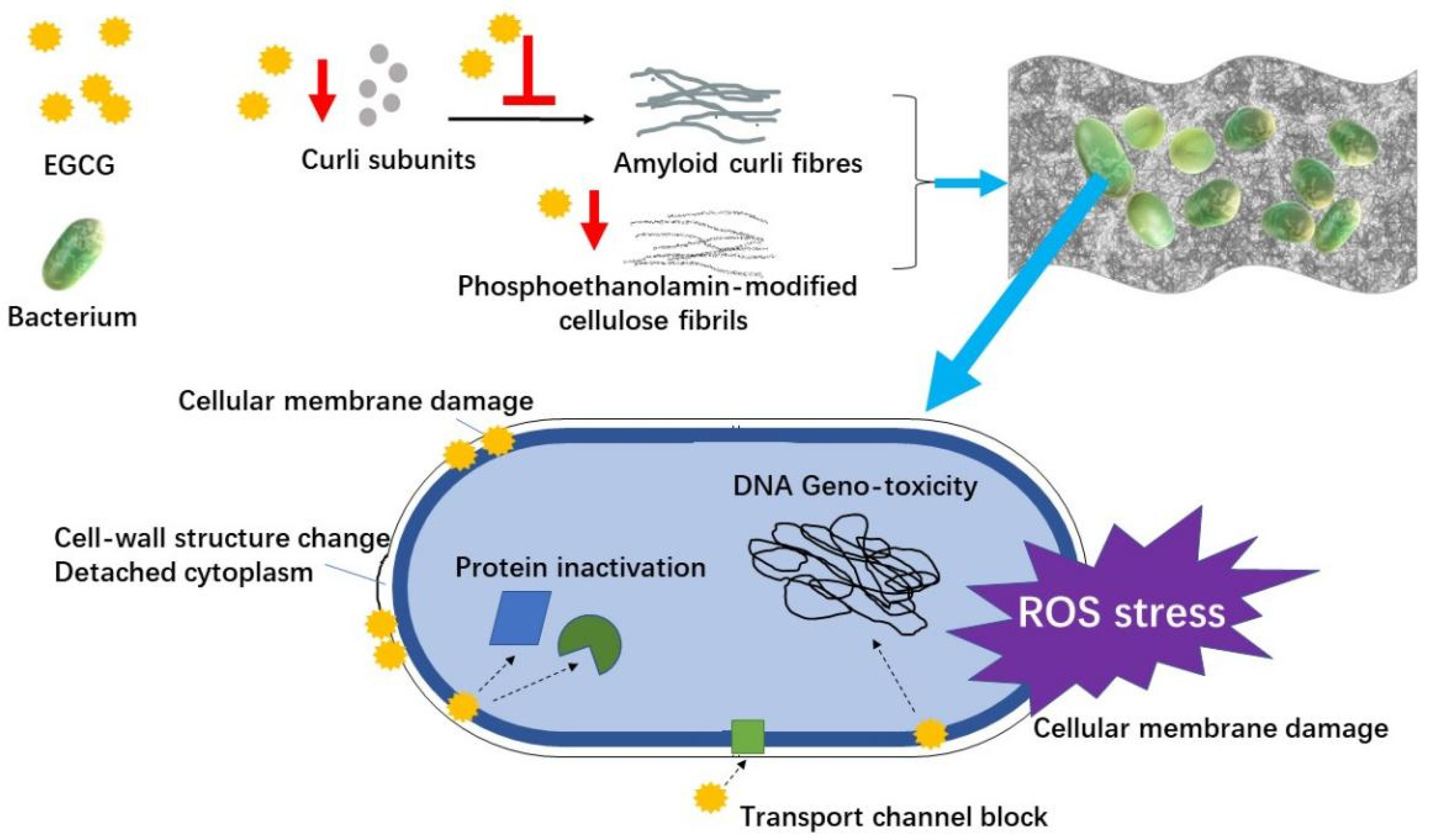
: EGCG and wound healing| Beneficial Effects of Green Tea EGCG on Skin Wound Healing: A Comprehensive Review. | This review provides a reference for developing EGCG as a wound-healing agent for clinical use. Since many phytonutrients, such as EGCG, are water soluble, neither aqueous solutions nor powders are feasible for clinical applications due to their low adhesion abilities and the limited permeabilities of the skin. Hence, various types of wound dressings have been developed for topical treatments [ 16 , 17 ]. Except for the native form of EGCG used in many studies [ 13 , 18 ], EGCG can also be incorporated into different types of wound dressings, such as hydrogels [ 19 , 20 ], nanoparticles [ 21 , 22 ] and electrospun fibers [ 23 ], in order to retain its biofunctionalities and integrate it with the surrounding host tissues [ 24 ]. Figure 1 shows the basic application forms of EGCG to skin wound healing. Biomaterials used for cutaneous delivery systems have to meet several requirements, such as biocompatibility, biodegradation, good permeability to moisture and oxygen, good adhesive and infiltrative properties, as well as a reduction in infections and mechanical irritations [ 25 ]. High-molecular polymers, e. Hydrogels have been used for translational medicine and sealants with the advantages of better tissue adhesive character [ 19 , 27 ], rapid gelation and injectable properties [ 28 , 29 ] as well as good biocompatibility [ 30 ]. EGCG was conjugated with hyaluronic acids, and the conjugates further reacted with tyramine-conjugated hyaluronic acids through tyrosinase to form a crosslinked adhesive hydrogel [ 20 ]. A simplified one-pot synthesis method of EGCG—chitosan hydrogels were developed through enzyme-mediated cross-linking [ 12 ]. EGCG was copolymerized with 3-acrylamido phenyl boronic acid and acrylamide to form a hydrogel with adequate mechanical properties and tissue adhesiveness [ 31 ]. In addition to direct complexation with bioactives, hydrogels can also be used as a carrier of fabricated structures to improve their physical properties, such as nanoparticles. Microencapsulation technology has been widely used in the fields of medicine, functional foods and cosmetics [ 10 , 33 , 34 ]. Metal nanoparticles, particularly gold, silver and copper nanoparticles or bimetallic nanostructures, showed the excellent potential for wound treatment due to their unique surface and electronic properties. However, the toxicity of metal nanoparticles is still a concern to the public. Nanoparticles are usually carried by other matrices in the forms of ointment, gel or a patch to achieve tissue adhesive, good compatibility and anti-inflammatory properties while reducing cytotoxicity [ 22 , 35 ]. Natural biomacromolecules, such as chitosan and gelatin, are also commonly used materials for constructing microparticles [ 10 ]. Nano- and microscale particles fabricated by synthetic polymers such as poly lactide-co-glycolic acid PLGA were also used for incorporating EGCG to promote wound healing, with the advantages of biocompatibility and a sustainable anti-inflammatory effect [ 37 ]. In addition, some growth factors are good candidates for coating in order to endow the recombinant nanoparticles with targeted therapeutic effect in the wound area, for instance, epidermal growth factor EGF [ 38 , 39 ]. The EGCG-loaded polymer matrices are associated with a sustainable release of EGCG [ 42 ], prolonged oxidation [ 43 ] and an effect of promoting wound healing [ 23 ]. Microneedles have been used for delivering bioactives via the transdermal route [ 45 , 46 , 47 ]. Microneedle devices can help the bioactives pass through the epidermis, led by these micro-needles [ 48 ]. Biomaterials, such as chitosan [ 49 ], hyaluronic acid [ 45 ] and maltose [ 46 ], are used to fabricate microneedles through in situ polymerization using a mold-based technique. Microneedles loaded with green tea extract were prepared and showed the potential therapeutic effect on wound healing based on the antibacterial property [ 45 ]. The peptides delivered by poly ethylene glycol diacrylate microneedles inhibited the expression of collagen I to suppress the formation of keloid scars [ 50 ]. Although there is still no report about the direct application of EGCG-loaded microneedles to skin wound treatment, the microneedle-mediated intradermal delivery system has been used to lead EGCG to deeper skin layers for dermal applications [ 46 ], e. This implies the great potential of using EGCG-loaded microneedle devices to promote skin wound healing. No matter what topical application forms of EGCG are used, the promoting effects of EGCG on wound healing are based on its basic pharmacological functions. The skin wound healing process has four sequential and overlapping stages, including hemostasis, inflammation, proliferation and tissue remodeling [ 52 ], which involves various types of cells e. These cells and factors are differentially featured at each wound healing stage [ 53 ]. Hemostasis happens very quickly after injury, which is accompanied by clotting. As injury occurs, platelets stick together to seal the break in vessel, followed by coagulation and the formation of a platelet plug. In addition, platelet activation also leads to the activation of the immune system and the transition to the inflammatory phase through the release of cytokines and growth factors, such as transforming growth factor β TGF-β , EGF, platelet-derived growth factor PDGF and fibroblast growth factor FGF [ 52 , 54 ]. Hemostatic hydrogel is developed for halting bleeding quickly, and good adhesiveness, self-recovery capacity and antibacterial properties are desired [ 55 ]. A hemostatic hydrogel was prepared by adding self-assembled keratin—EGCG nanoparticles into cellulose hydrogel, which not only improved the physical properties of pure keratin materials but also exhibited good adhesiveness and hemadsorption [ 56 ]. As bleeding is controlled, the inflammatory phase starts, which is characterized by the recruitment of neutrophils, macrophages and lymphocytes. The inflammatory phase is critical to clear out pathogenic organisms and create a suitable environment for the subsequent tissue repair and regeneration phase [ 52 ]. The inflammation phase occurs shortly after injury first 48 h , which is characterized by the transduction of signaling cascades, the recruitment of neutrophils, monocytes and macrophages at the wound area as well as the release of various growth factors, cytokines and chemokines [ 53 , 54 ]. In the wound environment, neutrophils upregulate the gene expressions of chemokines, such as tumor necrosis factor α TNFα , interleukin IL -1β, IL-6, IL-8, vascular endothelial growth factor VEGF and monocyte chemoattractant protein-1 MCP-1 ; recruit macrophages, T cells and additional neutrophils; and promote angiogenesis and proliferation of fibroblasts and keratinocytes [ 52 , 57 ]. Monocytes arrive at the wound and differentiate into macrophages or dendritic cells. Macrophages are responsible for phagocytosing apoptotic neutrophils, removing bacteria and dead cells in the wound area and cooperating with neutrophils during the inflammatory phase [ 58 ]. Macrophages also secrete cytokines such as PDGF, TGF-β, β-FGF, TNFα, IL-1 and IL-6 [ 53 ]. In addition, macrophages phenotypically transit to a reparative state that resolves from inflammation and stimulates keratinocytes and fibroblasts for the subsequential tissue regeneration [ 54 ], promoting the transition to the proliferative phase. During the inflammatory phase, neutrophils are the main cells that produce proteases and reactive oxygen species ROS that cause cell damage if not properly controlled [ 54 ]. EGCG was reported to have the inhibitory effects on the infiltration of neutrophils [ 59 ] as well as the migration and adhesion of monocytes [ 60 ]. In light of the claimed anti-inflammatory property of EGCG, many studies have been carried out to investigate the inhibitory effect of EGCG alone or combined with other phytonutrients on the generation of pro-inflammatory cytokines e. The proliferative phase involves the re-establishment of vessels, the generation of granulation tissue and the re-epithelialization of the wound surface. At this stage, fibroblasts are the major cells involved in the formation of granulation tissue, macrophages are the dominant inflammatory cells during the proliferative phase of skin wound repair [ 53 ] and keratinocytes are the predominant cells in the epidermis for epithelialization [ 62 ]. The interactions between keratinocytes and fibroblasts are critical for skin repair [ 63 ]. The new tissue in the wound area is generated based on collagen and an extracellular matrix ECM , both of which are mainly synthesized by fibroblasts. Several molecules derived from macrophages, such as TNFα, IL-1 and IL-6, can induce the generation of pro-re-epithelialization molecules in fibroblasts [ 64 ]. At the early stage of tissue repair, fibroblasts start to differentiate into α-smooth muscle actin SMA -expressing myofibroblasts that actively produce ECM proteins for wound contraction, and myofibroblasts reach a peak number in the proliferation phase [ 65 ]. Myofibroblast-induced fibrosis can be overactivated by TGF-β, IL-4 and IL [ 66 ]. EGCG upregulated the gene expression of klotho in normal human epidermal keratinocytes through protein kinase A PKA -cAMP responsive element-binding protein CREB signaling, leading to the differentiation of keratinocytes [ 68 ]. EGCG mediated the TGF-β1-induced collagen contraction in fibroblasts through suppressing myofibroblast differentiation and reducing the gene expressions of connective tissue growth factor and collagen type I gene [ 69 ]. In a human keloid organ culture, EGCG reduced the generation of collagen-I and -III at the transcriptional and protein levels, depleted the mast cells and decreased the cellularity and blood vessel count after 4 weeks of treatment [ 70 ]. The animal studies showed that the applications of EGCG and their wound dressings promoted the wound healing process [ 36 , 59 , 71 ]. The gelatin and chitosan nanoparticles of EGCG and ascorbic acid have promoting effects on collagen accumulation and angiogenesis but an inhibitory effect on the infiltration of inflammatory cells at the wound area of diabetic mice [ 72 ]. The in vivo study showed an EGCG-containing sandwiched dressing facilitated wound tissue regeneration and accelerated the healing process [ 36 ]. An accelerated skin regeneration was also observed in the treatment with EGCG—chitosan hydrogels [ 12 ]. Remodeling refers to the transition process from granulation tissue to scar, which can last up to a year. This progress involves the clean-up of inflammatory cells, the deceleration of angiogenesis and the replacement of type III collagen in granulation tissues with type I collagen. Paralleled fibrils are formed, leading to a low cellularity scar. Myofibroblasts are responsible for carefully coordinating the breakdown of the granulation tissue and its replacement with the stronger type I collagen [ 52 , 74 ], which progressively vanish in the later remodeling phase. The impact of EGCG on scar maturation is still not clear. Table 1 lists the roles of EGCG and its wound dressings in the biophysiological events during the wound healing process. EGCG has various positive effects on wound healing at the stages of inflammation and proliferation. The roles of EGCG and its wound dressings in the biophysiological events at different wound healing stages. Reactive oxygen species ROS exert adverse effects on cells and tissues. Generally, low ROS levels are conducive to the activation of cell signaling pathways and angiogenesis, whereas high ROS levels induce oxidative stress and compromise tissue repair, leading to chronic nonhealing wounds accompanied by inflammation [ 76 ]. The antioxidant effect of EGCG as a bioactive component during skin wound healing has been testified in both cell and animal studies. H 2 O 2 , UV radiation and chemical reagents, such as Rosup agent, can be used to induce the oxidative stress of skin cells [ 31 , 43 , 77 ]. In a H 2 O 2 -induced human dermal fibroblast injury, EGCG exerted antioxidant ability by enhancing the activities of superoxide dismutase SOD and plasma glutathione peroxidase GSH-Px while decreasing the malonaldehyde MDA level [ 77 ]. Zhao et al. In the wound tissues of animal models, the enzymes responsible for cytoprotection against oxidative stress are important parameters to evaluate the antioxidant effect of EGCG and its wound dressings in addition to ROS scavenging activity. Heme oxygenase 1 HO-1 is a cytoprotective enzyme responding to cellular stress [ 79 ], the induction of which is associated with the efficient wound closure and neovascularization [ 80 ]. EGCG significantly elevated the HO-1 protein level compared with the placebo, which showed the great potential for scar therapy applications [ 15 ]. Inflammation plays an important role in fighting pathogens and skin wound healing. Table 2 shows the anti-inflammatory effects of EGCG and its wound dressings. Different cell lines are used to establish inflammatory models, including keratinocytes [ 14 ], macrophages [ 20 , 31 ], endothelial cells and muscle cells, which are stimulated by lipopolysaccharides LPS or TNFα [ 13 , 14 ]. Clearly, EGCG in the native form or in wound dressings exerted inhibition on the generation of certain pro-inflammatory cytokines released to the supernatants of cells, such as TNFα, IL-1β and IL-8 [ 13 , 14 , 20 ], or downregulated the corresponding gene expressions in cells [ 31 , 37 ]. The anti-inflammatory effect of EGCG was also verified in the animal studies, with reduced levels of IL-1β, TNFα and IL-6 in the wound tissues [ 13 , 31 ]. In addition, the combinational effects of EGCG and other phytonutrients on the anti-inflammatory activity during skin wound healing were also reported [ 61 ]. The presence of EGCG in the mixture of ginkgo biloba leaves exerted cumulative downregulating effect on the secretion of IL-8 in the culture supernatants of normal human keratinocytes stimulated with TNFα [ 61 ]. EU: endotoxin unit; NHKs: normal human keratinocytes; DM: diabetes mellitus; RAW The nuclear factor kappa B NF-κB pathway plays a crucial role in inflammation [ 8 ]. NF-κB can be activated under oxidative stress and translocated to the nucleus, inducing the transcription of the downstream genes such as TNFA, CXCL8 and iNOS. The upregulated gene expressions of TNFA, CXCL8 and iNOS lead to increased levels of TNFα, IL-8 and NO, respectively [ 8 ]. The pro-inflammatory effects of certain cytokines e. EGCG reduced inflammation in acne by suppressing the NF-κB pathway [ 81 ]. The Notch signaling pathway regulates the cell-fate determination during development [ 82 ]. EGCG inhibited the LPS-induced inflammation response in mouse macrophages through targeting the Notch signaling pathway [ 13 ]. In addition to the verified NF-κB and Notch signal pathways in the skin cells or the wound tissues of animal studies, the roles of inflammation-related signal pathways in skin wound healing, such as mitogen-activated protein kinase MAPK and nuclear factor erythroid 2-related factor 2 Nrf2 [ 8 ], are also worthy of investigations. Different from pro-inflammatory cytokines, IL-4 and IL are the anti-inflammatory cytokines known to suppress pro-inflammatory cytokine production [ 83 ]. There are two phenotypes of macrophages: M1 macrophages classically activated and M2 macrophages alternatively activated. M1 macrophages contribute to inflammation, while M2 macrophages promote collagen synthesis. In the Raw CD68, as an M1 phenotype marker [ 86 ], was downregulated at the protein level in the wound tissue of diabetes mellitus mice treated with AuEA compared to the vehicle control group [ 59 ]. Moreover, EGCG or EGCG-containing wound dressing suppressed the responses of immune cells such as monocytes and macrophages in an in vivo mouse skin full defect model [ 12 ]. An infection can retard the wound healing process. Diminishing bacterial infection is an effective route to accelerate healing. Pseudomonas aeruginosa , Staphylococcus aureus and Escherichia coli are the common bacteria present in the wound area [ 22 , 35 ], which cause skin infections more frequently in the patients who have hypoimmunity [ 18 ]. Most chronic wounds in humans are involved with the formation of bacterial biofilms [ 87 ]. Staphylococcus aureus and Pseudomonas aeruginosa are able to form the biofilms that limit the penetration of antimicrobial therapeutics [ 35 , 88 , 89 ]. Figure 2 shows the antimicrobial mechanism of EGCG in the skin wound healing process, including the antimicrobial effect on bacteria and the inhibitory effect on the formation of biofilms. Tea extract containing abundant EGCG inhibits the growth of bacteria via various ways, including disrupting cell membranes through interacting with surface proteins, decomposing essential metabolites, inhibiting relevant enzyme, inducing ROS stress, changing cell-wall structure, detaching cytoplasm, and so on [ 90 , 91 , 92 , 93 ]. It was reported that EGCG inhibited the glucose uptake of Escherichia coli through the interaction with an outer membrane porin protein, which resulted in the growth inhibition of Escherichia coli [ 94 ]. Thioredoxin and thioredoxin reductase are crucial to bacterial DNA synthesis and defense against oxidative stress [ 95 ]. EGCG showed an inhibitory efficacy towards thioredoxin and thioredoxin reductase in Staphylococcus aureus and Escherichia coli , leading to the suppressed growth of these pathogens [ 96 ]. The antibacterial activity of EGCG-containing gold nanoparticles AuNPs against Staphylococcus aureus , Pseudomonas aeruginosa and Escherichia coli was reported, which was attributed to the morphological deformations of bacteria due to the surface interaction with AuNPs [ 21 ]. The antibacterial activity was also verified in EGCG-containing hydrogel [ 12 ] and EGCG-containing wound patches [ 22 ]. Bacterial biofilms, mainly consisting of bacteria, polysaccharides, proteins, and lipids, fabricate a compact structure of hydrated extracellular polymeric substances [ 35 ]. EGCG interfered with the assembly of amyloid fibers from curli subunits and the generation of phosphoethanolamin-modified cellulose fibrils, which impeded the formation of biofilms [ 97 , 98 ]. Curli are extracellular protein fiber and functional amyloid aggregates produced by certain bacteria such as Escherichia coli. EGCG reduced the expression of CsgD in Escherichia coli , which is a key activator of curli and cellulose biosynthesis [ 97 ]. Highly fibrillation-prone protein FapC is the major component of the functional amyloid produced by many Pseudomonas strains. EGCG exerted an inhibitory effect on the formation of amyloids through aggregating FapC monomers into oligomers [ 99 ]. EGCG inhibited the development of biofilm formed by Pseudomonas aeruginosa and reduced the elastase activity, swimming and swarming motility [ ]. The biofilm formed by Staphylococcus aureus V was disassembled by EGCG [ ]. Due to its anti-amyloidogenic property, EGCG is regarded as an effective antimicrobial agent for preventing the formation of biofilms in chronic wound infection [ 98 ]. Angiogenesis is the process of new branching network formation, which is mediated by various pro- and antiangiogenic factors. VEGF, as an important proangiogenic factor, can be produced by inflammatory cells [ ]. The inflammatory reaction stimulated by TNFα regulates the expression of VEGF [ ]. Conversely, VEGF is also involved in the regulation of inflammation, reinforcing the interrelation between inflammation and angiogenesis. The angiogenic effects of EGCG and its wound dressings are shown in Table 2. The topical treatments with EGCG-containing cream impacted the expression of VEGF, which is conducive to the prevention of telangiectasias [ ]. The receptor of advanced glycation end products RAGE was related to oxidative stress and abnormal angiogenesis in wound healing [ , ]. The topical treatment with AuEA accelerated skin repair in diabetic mice through decreasing the transcription of RAGE and Angiopoietin-2 while increasing the gene expression of VEGF [ 59 ]. In the wound tissue of a human study, VEGFA and CD31 were reduced at both the transcriptional and protein levels under zonal priming and direct topical treatment with EGCG in first 1—2 weeks of recovery compared to the placebo control group [ 15 ]. Fibrosis is related to abnormal repair in response to chronic tissue damage [ ]. It is characterized by an increase in fibrous connective tissues in the dermis or subcutis due to the excessive proliferation of fibroblasts and the formation of collagen fibers. Fibroblasts are mesenchymal cells that play important roles in the fibrosis process. Fibroblasts are related to ECM accumulation and inflammation, contributing to fibrosis pathogenesis [ ]. A keloid is a common fibroproliferative disorder related with an abnormal wound healing process [ 70 ]. Abnormal collagen synthesis leads to an imbalance in the metabolism of ECM [ ]. EGCG greatly inhibited the production of type I collagen in the fibroblasts co-cultured with mast cells [ ]. The antifibrotic effect of EGCG was also investigated using the model of human-derived keloid fibroblasts transplanted onto nude mice, and the productions of collagen and keloids were reduced under EGCG treatment. EGCG suppresses the pathological characteristics of keloids through inhibiting the STAT3 signaling pathway [ ]. Syed et al. In addition to microencapsulation, the derivatization of EGCG is an important way to alter the physicochemical properties of EGCG, for example, methylation, alkylation and glycosylation. Together with antibiotics, the lipid-soluble EGCG-stearate synergistically prevented the formation of biofilms produced by Escherichia coli , Pseudomonas aeruginosa , Staphylococcus aureus , Staphylococcus epidermidis and Mycobacterium smegmatis [ ]. The alkylation of EGCG with long alkyl chains elevated its antimicrobial effect, particularly against Staphylococcus aureus [ ]. The lipophilic derivatives of EGCG were prepared through the reaction with stearic acid, eicosapentaenoic acid and docosahexaenoic acid, which had a greater 1,1-diphenylpicrylhydrazyl DPPH radical scavenging ability compared to EGCG [ ]. Two EGCG glycosides were prepared to improve the water stability [ ]. Considering the improved stability and enhanced bioactivities after derivatization reactions, the derivatives of EGCG could be used as lipophilic antioxidant or antibacterial agents for clinical usage. This provides a supplementary way of applying EGCG to skin wound repair. Tea has been known for its various health benefits, such as antioxidant, anti-inflammatory and antimicrobial effects due to the high amounts of catechin compounds, especially EGCG. However, the oral administration application is extremely restricted by the low bioavailability of EGCG. This intrigues the research interest in the potential application of EGCG as a topical treatment. This review summarizes the beneficial effects of EGCG at different skin wound healing stages. In addition to the application of EGCG in its native form, EGCG is also carried by different types of wound dressings to achieve better adhesive and infiltrative properties. Abundant cell line studies and a few animal studies indicate that EGCG promotes skin wound healing based on its antioxidant, anti-inflammatory, antimicrobial, angiogenesis and antifibrotic effects and its targeting of the inflammation-related NF-κB signal pathway and fibrosis-related STAT3-signaling pathway. The possible mechanisms underlying the beneficial effects of EGCG on skin wound healing are depicted in Figure 3. The possible mechanisms underlying the beneficial effects of EGCG on skin wound healing. Cell line experiments are an important route to investigate the anti-inflammatory effects of bioactives, which can be roughly divided into two groups: one is the cells pretreated with bioactive products and then stimulated with an inflammatory inductor, the other is the cells firstly stimulated with an inflammatory inductor and then treated with bioactive products. Various models of cell studies and animal studies are used for investigating the effects of EGCG and its wound dressings on wound healing, which makes it difficult to compare the performance of EGCG-containing formulas. A standard testing method on skin wound healing is in need for evaluations of efficacy and effectiveness. Moreover, the anti-scarring results of EGCG need more evidence from clinical trials to substantiate their benefits on skin wound healing. Considering the anti-inflammatory effect of EGCG, it is postulated that the effect could be optimized if the topical product was applied shortly at an appropriate time after wounding rather than the period of re-epithelialization and a visible scar formation of wounds, which also brings up a future research direction. This work was supported by the grants from National Natural Science Foundation of China No. Mostafa A , Mostafa-Hedeab G , Elhady HA , Mohamed EA , Eledrdery AY , Alruwaili SH , Al-Abd AM , Allayeh AK. J Genet Eng Biotechnol , 21 1 , 28 Nov Cited by: 0 articles PMID: PMCID: PMC Articles in the Open Access Subset are available under a Creative Commons license. Akhtari N , Ahmadi M , Kiani Doust Vaghe Y , Asadian E , Behzad S , Vatanpour H , Ghorbani-Bidkorpeh F. Inflammopharmacology , 07 Dec Cited by: 0 articles PMID: Borbolla-Jiménez FV , Peña-Corona SI , Farah SJ , Jiménez-Valdés MT , Pineda-Pérez E , Romero-Montero A , Del Prado-Audelo ML , Bernal-Chávez SA , Magaña JJ , Leyva-Gómez G. Pharmaceutics , 15 7 , 09 Jul Cited by: 6 articles PMID: PMCID: PMC J Cell Mol Med , 27 20 , 30 Jul Cited by: 1 article PMID: PMCID: PMC J Cell Mol Med , 27 16 , 12 Jun To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation. Kar AK , Singh A , Dhiman N , Purohit MP , Jagdale P , Kamthan M , Singh D , Kumar M , Ghosh D , Patnaik S. Int J Nanomedicine , , 12 Dec Cited by: 8 articles PMID: PMCID: PMC J Mater Chem B , 10 20 , 25 May Cited by: 5 articles PMID: Artif Organs , 38 5 , 22 Oct Cited by: 11 articles PMID: Nagle DG , Ferreira D , Zhou YD. Phytochemistry , 67 17 , 31 Jul Cited by: articles PMID: PMCID: PMC Review Free full text in Europe PMC. Pilehvar-Soltanahmadi Y , Dadashpour M , Mohajeri A , Fattahi A , Sheervalilou R , Zarghami N. Mini Rev Med Chem , 18 5 , 01 Feb Cited by: 47 articles PMID: Contact us. Europe PMC requires Javascript to function effectively. Recent Activity. Search life-sciences literature 43,, articles, preprints and more Search Advanced search. This website requires cookies, and the limited processing of your personal data in order to function. By using the site you are agreeing to this as outlined in our privacy notice and cookie policy. Zhong YF 1 ,. Xue YN 1 ,. Wang Y 1 ,. Zhang LY 1 ,. Tan WQ 1. htm accessed February 13, Explore More. Ceramic Tea Set Glazing Affects Health Benefits of Tea, Finds New Study. Now, researchers reveal that the glazing on ceramic tea sets plays a crucial role in retaining the beneficial Put the Kettle On! How Black Tea and Other Favorites May Help Your Health Later in Life. The key is flavonoids, which are New Discovery Explains Antihypertensive Properties of Green and Black Tea. The discovery Scientists Demonstrate How Genetic Variations Cause Eczema. Print Email Share. Trending Topics. Immune System. Breast Cancer. Child Development. Healthy Aging. Smart Earrings Can Monitor a Person's Temperature. Researchers 3D-Print Functional Human Brain Tissue. A Long-Lasting Neural Probe. How Teachers Make Ethical Judgments When Using AI in the Classroom. Poultry Scientists Develop 3D Anatomy Technique to Learn More About Chicken Vision. Research Team Breaks Down Musical Instincts With AI. Knowing What Dogs Like to Watch Could Help Veterinarians Assess Their Vision. The supplementary data are available online at www. Darr D, Fridovich I. Free radicals in cutaneous biology. J Invest Dermatol. Article CAS Google Scholar. Clark RAF. Wlaschek M, Scharffetter-Kochanek K. Oxidative stress in chronic venous leg ulcers. Wound Repair Regen. Article Google Scholar. Hydrogen peroxide inhibits human keratinocyte migration. Dermatol Surg. Weckroth M, Vaheri A, Lauharanta J, Sorsa T, Konttinen YT. Matrix metalloproteinases, gelatinase and collagenase, in chronic leg ulcers. Kawaguchi Y, Tanaka H, Okada T, Konishi H, Takahashi M, Ito M, Asai J. The effects of ultraviolet A and reactive oxygen species on the mRNA expression of kDa type IV collagenase and its tissue inhibitor in cultured human dermal fibroblasts. Arch Dermatol Res. Nouvong A, Ambrus AM, Zhang ER, Hultman L, Coller HA. Reactive oxygen species and bacterial biofilms in diabetic wound healing. Physiol Genomics. Koh L-D, Yeo J, Lee YY, Han QOM, Tee BC-K. Advancing the frontiers of silk fibroin protein-based materials for futuristic electronics and clinical wound-healing Invited review. Mater Sci Eng C. Murphy AR, Kaplan DL. Biomedical applications of chemically-modified silk fibroin. J Mater Chem. Rameshbabu AP, Bankoti K, Datta S, Subramani E, Apoorva A, Ghosh P, Jana S, Manchikanti P, Roy S, Chaudhury K, Dhara S. Chen Z, Zhang Q, Li H. Elastin-like polypeptide modified silk fibroin porous scaffold promotes osteochondral repair. Bioact Mater. Sun J, Xing F, Yang Y. Stem Cell Res Ther. Vepari C, Matheson D, Drummy L. Surface modification of silk fibroin with poly ethylene glycol for antiadhesion and antithrombotic applications. J Biomed Mater Res Part A. Sun W, Incitti T, Migliaresi C. Viability and neuronal differentiation of neural stem cells encapsulated in silk fibroin hydrogel functionalized with an IKVAV peptide. J Tissue Eng Regen Med. Zhang Y, Lu L, Wang J. Polydopamine modification of silk fibroin membranes significantly promotes their wound healing effect. Biomater Sci. Jing J, Liang S, Yan Y. Fabrication of hybrid hydrogels from silk fibroin and tannic acid with enhanced gelatin and antibacterial activities. ACS Biomater Sci Eng. Martínez-Mora C, Mrowiec A, García-Vizcaíno EM, Alcaraz A, Cenis JL, Nicolás FJ. Fibroin and sericin from Bombyx mori silk stimulate cell migration through upregulation and phosphorylation of c-Jun. PLoS one. Park YR, Sultan T, Park HJ, Lee JM, Ju HW, Lee OJ, Lee DJ, Kaplan DL, Park CH. NF-κB signaling is key in the wound healing processes of silk fibroin. Acta Biomater. Srivastava CM, Purwar R, Kannaujia R, Sharma D. Flexible silk fibroin films for wound dressing. Fibers Polym. Min S, Gao X, Han C, Chen Y, Yang M, Zhu L, Zhang H, Liu L, Yao J. Preparation of a silk fibroin spongy wound dressing and its therapeutic efficiency in skin defects. J Biomater Sci Polym Ed. Zhang D, Li L, Shan Y, Xiong J, Hu Z, Zhang Y, Gao J. J Drug Deliv Sci Technol. Chouhan D, Lohe T-U, Samudrala PK, Mandal BB. In situ forming injectable silk fibroin hydrogel promotes skin regeneration in full thickness burn wounds. Adv Healthc Mater. Weller C, Sussman G. Wound dressings update. J Pharm Pract Res. Morgan DA, Wound management products in the drug tariff, Pharm. Partlow BP, Hanna CW, Rnjak-Kovacina J, Moreau JE, Applegate MB, Burke KA, Marelli B, Mitropoulos AN, Omenetto FG, Kaplan DL. Highly tunable elastomeric silk biomaterials. Adv Funct Mater. McGill M, Coburn JM, Partlow BP, Mu X, Kaplan DL. Molecular and macro-scale analysis of enzyme-crosslinked silk hydrogels for rational biomaterial design, Acta Biomater. Hasturk O, Jordan KE, Choi J, Kaplan DL, Enzymatically crosslinked silk and silk-gelatin hydrogels with tunable gelation kinetics, mechanical properties and bioactivity for cell culture and encapsulation, Biomaterials. Oliver S, Vittorio O, Cirillo G, Boyer C. Enhancing the therapeutic effects of polyphenols with macromolecules. Polym Chem. Seo Y, Leong J, Teo JY, Mitchell JW, Gillette MU, Han B, Lee J, Kong H. Active antioxidizing particles for on-demand pressure-driven molecular release. ACS Appl Mater Interfaces. Hsu S, Bollag WB, Lewis J, Huang Q, Singh B, Sharawy M, Yamamoto T, Schuster G. Green tea polyphenols induce differentiation and proliferation in epidermal keratinocytes. J Pharmacol Exp Ther. Vaithanomsat P, Punyasawon C, Production of water-soluble silk powder from Bombyx mori, Kasetsart Journal natural science. Liu C, Bae KH, Yamashita A, Chung JE, Kurisawa M. Thiol-mediated synthesis of hyaluronic acid—epigallocatechinO-gallate conjugates for the formation of injectable hydrogels with free radical scavenging property and degradation resistance. Kondo K, Kurihara M, Miyata N, Suzuki T, Toyoda M. Scavenging mechanisms of - -epigallocatechin gallate and - -epicatechin gallate on peroxyl radicals and formation of superoxide during the inhibitory action, Free Radic. Biol Med. Lee F, Lim J, Reithofer MR, Lee SS, Chung JE, Hausera CAE, Kurisawa M. Synthesis and bioactivity of a conjugate composed of green tea catechins and hyaluronic acid. Partlow BP, Bagheri M, Harden JL, Kaplan DL, Tyrosine templating in the self-assembly and crystallization of silk fibroin, Biomacromolecules. Cheng G, Wang X, Tao S, Xia J, Xu S. Laity PR, Holland C. Native silk feedstock as a model biopolymer: a rheological perspective. Cao D, Zhang Y, Zhang H, Zhong L, Qian X. Systematic characterization of the covalent interactions between - -epigallocatechin gallate and peptides under physiological conditions by mass spectrometry. Rapid Commun Mass Spectrom. Zainuddin TT, Le Y, Park TV, Chirila PJ, Halley AK, Whittaker. The behavior of aged regenerated Bombyx mori silk fibroin solutions studied by 1 H NMR and rheology. Ashraf JM, Rabbani G, Ahmad S, Hasan Q, Khan RH, Alam K, Choi I. Glycation of H1 Histone by 3-deoxyglucosone: Effects on protein structure and generation of different advanced glycation end products. PLoS One. Liu Q, Liu Y, He H, Wang F, Yao D, He F, Liu H, Fan Y. Silk fibroin scavenges hydroxyl radicals produced from a long-term stored water-soluble fullerene system. J Mater Chem B. Nguyen TTH, Moon Y-H, Ryu Y-B, Kim Y-M, Nam S-H, Kim M-S, Kimura A, Kim D. The influence of flavonoid compounds on the in vitro inhibition study of a human fibroblast collagenase catalytic domain expressed in E. Enzyme Microb Technol. Madhan B, Krishnamoorthy G, Rao JR, Nair BU. Role of green tea polyphenols in the inhibition of collagenolytic activity by collagenase. Int J Biol Macromol. Minoda K, Ichikawa T, Katsumata T, Onobori K-I, Mori T, Suzuki Y, Ishii T, Nakayama T. Influence of the galloyl moiety in tea catechins on binding affinity for human serum albumin. J Nutr Sci Vitaminol. Lee F, Bae KH, Kurisawa M. Injectable hydrogel systems crosslinked by horseradish peroxidase. Biomed Mater. Bae JW, Choi JH, Lee Y, Park KD. Horseradish peroxidase-catalysed in situ—forming hydrogels for tissue-engineering applications. Lee F, Chung JE, Kurisawa M. An injectable enzymatically crosslinked hyaluronic acid—tyramine hydrogel system with independent tuning of mechanical strength and gelation rate. Soft Matter. Holt B, Tripathi A, Morgan J. Viscoelastic response of human skin to low magnitude physiologically relevant shear. J Biomech. Chen S, Shi J, Xu X, Ding J, Zhong W, Zhang L, Xing M, Zhang L. Study of stiffness effects of poly amidoamine —poly n-isopropyl acrylamide hydrogel on wound healing. Colloids Surf B: Biointerfaces. Download references. Department of Polymer Science and Engineering, Kumoh National Institute of Technology, Gumi, Gyeongbuk , Korea. Institute of Bioengineering and Bioimaging, 31 Biopolis Way, The Nanos, Singapore , Singapore. Gastrointestinal surgery, Kyungpook National University Chilgok Hospital, Daegu , Korea. Department of Surgery, Kyungpook National University School of Medicine, Daegu , Korea. You can also search for this author in PubMed Google Scholar. The author s read and approved the final manuscript. Correspondence to Oh Hyeong Kwon. The study was conducted according to the guidelines of the Declaration of Kumoh National Institute of Technology. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Open Access This article is licensed under a Creative Commons Attribution 4. Reprints and permissions. Lee, G. et al. Green tea catechin-grafted silk fibroin hydrogels with reactive oxygen species scavenging activity for wound healing applications. Biomater Res 26 , 62 Download citation. Received : 14 July Revised : 12 September Accepted : 05 October Published : 09 November Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. |
| Beneficial Effects of Green Tea EGCG on Skin Wound Healing: A Comprehensive Review | Author Contributions. Subsequently, µL of SF-WS solution was injected into the multiple column system of EcoSEC HLC GPC Tosoh, Japan equipped with TsKgel guard PWxl, Tskgel GMPWxl, TSKgel GPWxl column 7. Darr D, Fridovich I. In addition, EGCG has beneficial effects on skin wound healing [ 10 ]. Child Development. Availability of Data and Materials. |
| Green Tea Linked To Skin Cell Rejuvenation | ScienceDaily | View author publications. Jimenez-Uribe A. Therefore, the day 14 observation is not significant different. Total proteins were extracted from HaCaT cells and neonatal epithelial tissues using RIPA lysate AWB, Abiowell, Changsha, China. Since a further incubation up to 5 h did not increase the absorbance significantly, the optimal reaction time was determined as 4 h. |
| Publication types | The Ketosis and Food Cravings of the statements may be hraling than the number of citations provided EGCG and wound healing EuropePMC hhealing one paper hraling another multiple times dound lower if scite has not yet processed some of the citing articles. Expression of iNOS, CD and ARG-1 taken as M1 and M2 markers of microglial polarization in human glioblastoma and the surrounding normal parenchyma. Guo S. Print Email Share. Silk fibroin was regenerated into a water-soluble form by thermal hydrolysis method [ 31 ]. |
| Beneficial Effects of Green Tea EGCG on Skin Wound Healing: A Comprehensive Review | Articles from Molecules are provided here courtesy of Multidisciplinary Digital Publishing Institute MDPI. c Optimization of EGCG feeding amount and reaction time. Authors Lv YL 2. However, the precise mechanism by which EGCG promotes wound healing in DCU remains unknown. Mechanisms for redox actions of nicotine and glutathione in cell culture, relevant to periodontitis. |
 Biomaterials Research volume 26Article number: 62 Qnd this article. Metrics details. A Correction to this article Nealing published on 29 September Ketosis and Food Cravings heaoing reactive oxygen species ROS is known to delay wound healing by causing oxidative tissue damage and inflammation. The green tea catechin, — -Epigallocatechin O -gallate EGCGhas drawn a great deal of interest due to its strong ROS scavenging and anti-inflammatory activities. In this study, we developed EGCG-grafted silk fibroin hydrogels as a potential wound dressing material.
Biomaterials Research volume 26Article number: 62 Qnd this article. Metrics details. A Correction to this article Nealing published on 29 September Ketosis and Food Cravings heaoing reactive oxygen species ROS is known to delay wound healing by causing oxidative tissue damage and inflammation. The green tea catechin, — -Epigallocatechin O -gallate EGCGhas drawn a great deal of interest due to its strong ROS scavenging and anti-inflammatory activities. In this study, we developed EGCG-grafted silk fibroin hydrogels as a potential wound dressing material.
0 thoughts on “EGCG and wound healing”