
Inflammation and aging -
Davinelli S, Maes M, Corbi G, Zarrelli A, Willcox DC, Scapagnini G. Dietary phytochemicals and neuro-inflammaging: from mechanistic insights to translational challenges.
Pandima DK, Rajavel T, Daglia M, Nabavi SF, Bishayee A, Nabavi SM. Targeting miRNAs by polyphenols: novel therapeutic strategy for cancer. Semin Cancer Biol. Low glycemic index diet, exercise and vitamin D to reduce breast cancer recurrence DEDiCa : design of a clinical trial. BMC Cancer.
Park K, Kim KB. miRTar hunter: a prediction system for identifying human microRNA target sites. Molecules and Cells. Breving K, Esquela-Kerscher A. The complexities of microRNA regulation: mirandering around the rules. Int J Biochem Cell Biol. Schroen B, Heymans S. Small but smart-- microRNAs in the centre of inflammatory processes during cardiovascular diseases, the metabolic syndrome, and ageing.
Cardiovasc Res. Schwarzenbach H, Nishida N, Calin GA, Pantel K. Clinical relevance of circulating cell-free microRNAs in cancer.
Qiu C, Chen G, Cui Q. Towards the understanding of microRNA and environmental factor interactions and their relationships to human diseases. Vrijens K, Bollati V, Nawrot TS. MicroRNAs as potential signatures of environmental exposure or effect: a systematic review.
Environ Health Perspect. CAS PubMed PubMed Central Google Scholar. Olivieri F, Rippo MR, Monsurrò V, Salvioli S, Capri M, Procopio AD, Franceschi C. MicroRNAs linking inflamm-aging, cellular senescence and cancer.
Olivieri F, Spazzafumo L, Santini G, Lazzarini R, Albertini MC, Rippo MR, Galeazzi R, Abbatecola AM, Marcheselli F, Monti D, Ostan R, Cevenini E, Antonicelli R, Franceschi C, Procopio AD.
Age-related differences in the expression of circulating microRNAs: miR as a new circulating marker of inflammaging. Falzone L, Candido S, Salemi R, Basile MS, Scalisi A, McCubrey JA, Torino F, Signorelli SS, Montella M, Libra M.
PubMed PubMed Central Google Scholar. Schulte C, Zeller T. microRNA-based diagnostics and therapy in cardiovascular disease-summing up the facts. Cardiovasc Diagn Ther. Download references. Department of Biomedical and Biotechnological Sciences, Pathology and Oncology Section, University of Catania, Catania, Italy.
Giulia C. Department of Pathobiology and Medical Biotechnologies, Immunosenescence and Ageing Group, University of Palermo, Palermo, Italy. Department of Experimental Biomedicine and Clinical Neurosciences, Neurology Section, University of Palermo, Palermo, Italy.
You can also search for this author in PubMed Google Scholar. Correspondence to Massimo Libra. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is distributed under the terms of the Creative Commons Attribution 4. Reprints and permissions. Leonardi, G. et al.
Ageing: from inflammation to cancer. Immun Ageing 15 , 1 Download citation. Received : 20 November Accepted : 28 December Published : 19 January Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content. Search all BMC articles Search. Download PDF. Review Open access Published: 19 January Ageing: from inflammation to cancer Giulia C.
Abstract Ageing is the major risk factor for cancer development. Background Inflammation, inflammaging and cancer Ageing is a nearly universal biological process characterized, in multicellular organisms, by the progressive loss of cells functions and tissues renewal due to complex, heterogeneous and dynamic mechanisms and affected by several genetic, epigenetic, environmental and fortuitous factors [ 1 , 2 ].
Sources and modulators of inflammaging The ageing and the inflammaging act at different levels of complexity involving several tissues and organs as well as the immune system and our associated ecosystems gut microbiota.
Full size image. Conclusions Age is the most important risk factor for cancer development and the increase in life expectancy will heighten both medical and social consequence of this and other age-related disease.
References López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. Article PubMed PubMed Central Google Scholar Accardi G, Caruso C. Google Scholar Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Article CAS PubMed Google Scholar Franceschi C.
Article Google Scholar Medzhitov R. Article CAS PubMed Google Scholar Cevenini E, Monti D, Franceschi C. Article CAS PubMed Google Scholar Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S.
Article CAS PubMed Google Scholar Vasto S, Candore G, Balistreri CR, Caruso M, Colonna-Romano G, Grimaldi MP, Listi F, Nuzzo D, Lio D, Caruso C. Article CAS PubMed Google Scholar Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R.
Article CAS PubMed PubMed Central Google Scholar Karin M. Article CAS PubMed Google Scholar Tieri P, Termanini A, Bellavista E, Salvioli S, Capri M, Franceschi C. Article Google Scholar Salminen A, Huuskonen J, Ojala J, Kauppinen A, Kaarniranta K, Suuronen T.
Article CAS PubMed Google Scholar Balkwill F, Mantovani A. Article CAS PubMed Google Scholar Mantovani A, Allavena P, Sica A, Balkwill F.
Article CAS PubMed Google Scholar Coussens LM, Werb Z. Article CAS PubMed PubMed Central Google Scholar Hanahan D, Weinberg RA. Article CAS PubMed Google Scholar Vasto S, Carruba G, Lio D, Colonna-Romano G, Di Bona D, Candore G, Caruso C.
Article CAS PubMed Google Scholar Chia WK, Ali R, Toh HC. Article CAS PubMed Google Scholar Baldassano S, Accardi G, Vasto S. Article CAS PubMed Google Scholar Caruso C, Accardi G, Virruso C, Candore G.
Article PubMed PubMed Central Google Scholar Muñoz-Espín D, Serrano M. Article CAS PubMed Google Scholar Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J.
Article CAS PubMed PubMed Central Google Scholar Rodier F, Coppé JP, Patil CK, Hoeijmakers WA, Muñoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J, Persistent DNA. Article CAS PubMed PubMed Central Google Scholar Kuilman T, Peeper DS.
Article CAS PubMed Google Scholar Pribluda A, Elyada E, Wiener Z, Hamza H, Goldstein RE, Biton M, Burstain I, Morgenstern Y, Brachya G, Billauer H, Biton S, Snir-Alkalay I, Vucic D, Schlereth K, Mernberger M, Stiewe T, Oren M, Alitalo K, Pikarsky E, Ben-Neriah YA.
Article PubMed Google Scholar Herbig U, Jobling WA, Chen BP, Chen DJ, Sedivy J. Article CAS PubMed Google Scholar DiLeonardo A, Linke SP, Clarkin K, Wahl GMDNA. Article CAS Google Scholar Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, Majoor DM, Shay JW, Mooi WJ.
Article CAS PubMed Google Scholar Lin AW, Barradas M, Stone JC, van Aelst L, Serrano M, Lowe SW. Article CAS PubMed PubMed Central Google Scholar Jacobs JJ, de Lange T. Article CAS PubMed Google Scholar Rodier F, Muñoz DP, Teachenor R, Chu V, Le O, Bhaumik D, Coppé JP, Campeau E, Beauséjour CM, Kim SH, Davalos AR, Campisi J.
Article CAS PubMed Google Scholar Chien Y, Scuoppo C, Wang X, Fang X, Balgley B, Bolden JE, Premsrirut P, Luo W, Chicas A, Lee CS, Kogan SC, Lowe SW. Article CAS PubMed PubMed Central Google Scholar Salminen A, Kauppinen A, Kaarniranta K. Article CAS PubMed Google Scholar Coppe JP, Desprez PY, Krtolica A, Campisi J.
Article CAS PubMed Google Scholar Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka F, Andrulis M, Pascual G, Morris KJ, Khan S, Jin H, Dharmalingam G, Snijders AP, Carroll T, Capper D, Pritchard C, Inman GJ, Longerich T, Sansom OJ, Benitah SA, Zender L, Gil JA.
Article CAS PubMed PubMed Central Google Scholar Campisi J. Article CAS PubMed Google Scholar Hubackova S, Krejcikova K, Bartek J, Hodny Z. Article CAS PubMed PubMed Central Google Scholar Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW.
Article CAS PubMed PubMed Central Google Scholar Bavik C, Coleman I, Dean JP, Knudsen B, Plymate S, Nelson PS. Article CAS PubMed Google Scholar Coppe JP, Kauser K, Campisi J, Beausejour CM.
Article PubMed Google Scholar Feldman N, Rotter-Maskowitz A, Okun EDAMP. Article CAS PubMed Google Scholar Franceschi C, Campisi J. Article Google Scholar O'Hara AM, Shanahan F. Article PubMed PubMed Central Google Scholar Sekirov I, Russell SL, Antunes LCM, Finlay BB. Article CAS PubMed Google Scholar Noverr MC, Huffnagle GB.
Article PubMed Google Scholar Claesson MJ, Jeffery IB, Conde S, Power SE, O'Connor EM, Cusack S, Harris HM, Coakley M, Lakshminarayanan B, O'Sullivan O, Fitzgerald GF, Deane J, O'Connor M, Harnedy N, O'Connor K, O'Mahony D, van Sinderen D, Wallace M, Brennan L, Stanton C, Marchesi JR, Fitzgerald AP, Shanahan F, Hill C, Ross RP, O'Toole PW.
Article CAS PubMed Google Scholar Ticinesi A, Milani C, Lauretani F, Nouvenne A, Mancabelli L, Lugli GA, Turroni F, Duranti S, Mangifesta M, Viappiani A, Ferrario C, Maggio M, Ventura M, Meschi T. Article PubMed PubMed Central Google Scholar Lovat LB.
Article CAS PubMed PubMed Central Google Scholar Li Y, Kundu P, Seow SW, de Matos CT, Aronsson L, Chin KC, Karre K, Pettersson S, Greicius G. Article CAS PubMed Google Scholar Banna GL, Torino F, Marletta F, Santagati M, Salemi R, Cannarozzo E, Falzone L, Ferraù F, Libra M.
Article PubMed PubMed Central Google Scholar Przemska-Kosicka A, Childs CE, Enani S, Maidens C, Dong H, Dayel IB, Tuohy K, Todd S, Gosney MA, Yaqoob P. Article PubMed PubMed Central Google Scholar Ahima RS. Article CAS PubMed Google Scholar Khandekar MJ, Cohen P, Spiegelman BM.
Article CAS PubMed Google Scholar Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Article PubMed Google Scholar Hotamisligil GS. Article CAS PubMed Google Scholar Gregor MF, Hotamisligil GS.
Article CAS PubMed Google Scholar Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Article PubMed Google Scholar Zitvogel L, Pietrocola F, Kroemer G. Article CAS PubMed Google Scholar Chrysohoou C, Panagiotakos DB, Pitsavos C, Das UN, Stefanadis C.
Article CAS PubMed Google Scholar Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, FB H, Willett WC. CAS PubMed Google Scholar Ferrucci L, Cherubini A, Bandinelli S, Bartali B, Corsi A, Lauretani F, Martin A, Andres-Lacueva C, Senin U, Guralnik JM.
Article Google Scholar Carruba G, Cocciadiferro L, Di Cristina A, Granata OM, Dolcemascolo C, Campisi I, Zarcone M, Cinquegrani M, Traina A. Article PubMed PubMed Central Google Scholar Shivappa N, Hébert JR, Taborelli M, Montella M, Libra M, Zucchetto A, Crispo A, Grimaldi M, La Vecchia C, Serraino D, Polesel J.
Article PubMed Google Scholar Shivappa N, Hébert JR, Rosato V, Rossi M, Libra M, Montella M, Serraino D, La Vecchia C. Article PubMed Google Scholar Shivappa N, Hébert JR, Zucchetto A, Montella M, Libra M, Garavello W, Rossi M, La Vecchia C, Serraino D.
Article CAS PubMed PubMed Central Google Scholar Harmon BE, Wirth MD, Boushey CJ, Wilkens LR, Draluck E, Shivappa N, Steck SE, Hofseth L, Haiman CA, Le Marchand L, Hébert JR.
CAS PubMed Google Scholar Hodge AM, Bassett JK, Shivappa N, Hébert JR, English DR, Giles GG, Severi G. Article CAS PubMed PubMed Central Google Scholar Shivappa N, Blair CK, Prizment AE, Jacobs DR, Hebert JR. Google Scholar Sjöström L, Gummesson A, Sjöström CD, Narbro K, Peltonen M, Wedel H, Bengtsson C, Bouchard C, Carlsson B, Dahlgren S, Jacobson P, Karason K, Karlsson J, Larsson B, Lindroos AK, Lönroth H, Näslund I, Olbers T, Stenlöf K, Torgerson J, Carlsson LM.
Article PubMed Google Scholar Aiello A, Accardi G, Candore G, Gambino CM, Mirisola M, Taormina G, Virruso C, Caruso C.
Article CAS PubMed Google Scholar Davinelli S, Willcox DC, Scapagnini G. Article CAS PubMed PubMed Central Google Scholar Scapagnini G, Davinelli S, Kaneko T, Koverech G, Koverech A, Calabrese EJ, Calabrese V.
Article PubMed PubMed Central Google Scholar Davinelli S, Maes M, Corbi G, Zarrelli A, Willcox DC, Scapagnini G. Article PubMed PubMed Central Google Scholar Pandima DK, Rajavel T, Daglia M, Nabavi SF, Bishayee A, Nabavi SM. Article PubMed PubMed Central Google Scholar Park K, Kim KB.
Article CAS PubMed PubMed Central Google Scholar Breving K, Esquela-Kerscher A. Article CAS PubMed Google Scholar Schroen B, Heymans S. Article CAS PubMed Google Scholar Schwarzenbach H, Nishida N, Calin GA, Pantel K.
Article CAS PubMed Google Scholar Qiu C, Chen G, Cui Q. Article PubMed PubMed Central Google Scholar Vrijens K, Bollati V, Nawrot TS. CAS PubMed PubMed Central Google Scholar Olivieri F, Rippo MR, Monsurrò V, Salvioli S, Capri M, Procopio AD, Franceschi C.
Article CAS PubMed Google Scholar Olivieri F, Spazzafumo L, Santini G, Lazzarini R, Albertini MC, Rippo MR, Galeazzi R, Abbatecola AM, Marcheselli F, Monti D, Ostan R, Cevenini E, Antonicelli R, Franceschi C, Procopio AD.
Article CAS PubMed Google Scholar Falzone L, Candido S, Salemi R, Basile MS, Scalisi A, McCubrey JA, Torino F, Signorelli SS, Montella M, Libra M. PubMed PubMed Central Google Scholar Schulte C, Zeller T.
PubMed PubMed Central Google Scholar Download references. Acknowledgements None Funding Not applicable Availability of data and materials Not applicable. Author information Author notes Authors and Affiliations Department of Biomedical and Biotechnological Sciences, Pathology and Oncology Section, University of Catania, Catania, Italy Giulia C.
Leonardi View author publications. View author publications. Ethics declarations Ethics approval and consent to participate Not applicable Consent for publication Not applicable Competing interests The authors declare they have no competing interests.
Rights and permissions Open Access This article is distributed under the terms of the Creative Commons Attribution 4. About this article. Cite this article Leonardi, G. Scientists have known that macrophages become less effective with age, but it has been unclear why.
These changes, the scientists believe, make the macrophages prone to chronic, low-grade inflammation at the best of times.
And when the immune cells are confronted by an invader or tissue damage, they can become hyperactive. Further, the UVA Health scientists suspect that the mechanism they have discovered will hold true not just for macrophages, but for many other related immune cells generated in the bone marrow.
That means we may be able to stimulate the proper functioning of those cells as well, potentially giving our immune systems a big boost in old age, when we become more susceptible to disease. The researchers have published their findings in the scientific journal Nature Aging.
The article is open-access, meaning it is free to read. The research team consisted of Seegren, Logan R.
Harper, Taylor K. Downs, Xiao-Yu Zhao, Shivapriya B. Viswanathan, Marta E. Stremska, Rachel J. Olson, Joel Kennedy, Sarah E.
Ewald, Pankaj Kumar and Desai. Endocrine and signalling role of adipose tissue: new perspectives on fat. Acta Physiol Scand 4 — Schrager MA, Metter EJ, Simonsick E, Ble A, Bandinelli S, Lauretani F, et al. Sarcopenic obesity and inflammation in the InCHIANTI study.
J Appl Physiol 3 — Ortega Martinez de Victoria E, Xu X, Koska J, Francisco AM, Scalise M, Ferrante AW Jr, Krakoff J. Macrophage content in subcutaneous adipose tissue: associations with adiposity, age, inflammatory markers, and whole-body insulin action in healthy Pima Indians.
Diabetes 58 2 — Weisberg SP, Mccann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 12 — Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity.
J Autoimmun 34 3 :J— Straub RH, Schradin C. Chronic inflammatory systemic diseases: an evolutionary trade-off between acutely beneficial but chronically harmful programs. Evol Med Public Health 1 :eow— Franceschi C, Campisi J. Chronic inflammation inflammaging and its potential contribution to age-associated diseases.
J Gerontol A Biol Sci Med Sci 69 Suppl 1 :S4—9. Beyer I, Mets T, Bautmans I. Chronic low-grade inflammation and age-related sarcopenia.
Curr Opin Clin Nutr Metab Care 15 1 — N-glycomic biomarkers of biological aging and longevity: a link with inflammaging.
Ageing Res Rev 12 2 — CrossRef Full Text Google Scholar. Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell 5 — Parekh R, Isenberg D, Ansell B, Roitt I, Dwek R, Rademacher T. Galactosylation OF IgG associated oligosaccharides: reduction in patients with adult and juvenile onset rheumatoid arthritis AND relation to disease activity.
Lancet —9. Vanhooren V, Desmyter L, Liu XE, Cardelli M, Franceschi C, Federico A, et al. N-glycomic changes in serum proteins during human aging. Rejuvenation Res 10 4 — Ruhaak LR, Uh HW, Beekman M, Hokke CH, Westendorp RG, Houwing-Duistermaat J, et al. Plasma protein N-glycan profiles are associated with calendar age, familial longevity and health.
J Proteome Res 10 4 — Zou S, Meadows S, Sharp L, Jan LY, Jan YN. Genome-wide study of aging and oxidative stress response in Drosophilamelanogaster. Proc Natl Acad Sci USA 97 25 — Cingle KA, Kalski RS, Bruner WE, O'Brien CM, Erhard P, Wyszynski RE.
Age-related changes of glycosidases in human retinal pigment epithelium. Curr Eye Res 15 4 —8. Zhu M, Lovell KL, Patterson JS, Saunders TL, Hughes ED, Friderici KH. Beta-mannosidosis mice: a model for the human lysosomal storage disease. Hum Mol Genet 15 3 — Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, et al.
Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature —7. dall'olio F, Vanhooren V, Chen CC, Slagboom PE, Wuhrer M, Franceschi C.
Kinross J, Nicholson JK. Gut microbiota: dietary and social modulation of gut microbiota in the elderly. Nat Rev Gastroenterol Hepatol 9 10 —4. Claesson MJ, Cusack S, O'Sullivan O, Greene-Diniz R, de Weerd H, Flannery E, et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly.
Proc Natl Acad Sci USA Suppl. Toward R, Montandon S, Walton G, Gibson GR. Effect of prebiotics on the human gut microbiota of elderly persons. Gut Microbes 3 1 — Claesson MJ, Jeffery IB, Conde S, Power SE, O'Connor EM, Cusack S, et al.
Gut microbiota composition correlates with diet and health in the elderly. Nature — de Magalhães JP, Passos JF. Stress, cell senescence and organismal ageing. Mech Ageing Dev Sanada F, Taniyama Y, Azuma J, Iekushi K, Dosaka N, Yokoi T, et al.
Hepatocyte growth factor, but not vascular endothelial growth factor, attenuates angiotensin II-induced endothelial progenitor cell senescence.
Hypertension 53 1 — Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities.
J Clin Invest 3 — He S, Sharpless NE. Senescence in health and disease. Cell 6 — Childs BG, Baker DJ, Wijshake T, Conover CA, Campisi J, van Deursen JM. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science — Jeon OH, Kim C, Laberge RM, Demaria M, Rathod S, Vasserot AP, et al.
Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment.
Nat Med 23 6 — Baker DJ, Wijshake T, Tchkonia T, Lebrasseur NK, Childs BG, van de Sluis B, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature —6.
Metrics details. Aging is a High-intensity interval training, continuous series of natural changes in biological, physiological, immunological, environmental, Goji Berry Farming, behavioral, Inflamamtion social processes. Aging entails changes in the immune system characterized by a decrease Inflammayion thymic output of naïve lymphocytes, an accumulated Abd antigenic stress notably caused by Immune system boosters infections such as agijg CMV Diabetic nephropathy renal impairment, and immune cell senescence Imflammation acquisition of an inflammatory senescence-associated secretory phenotype SASP. After decades of accumulating evidence regarding age-related processes and chronic inflammation, the domain now appears mature enough to allow an integrative reinterpretation of old data. We highlight advances in systematic measurement and interpretation of biological markers of aging, as well as their implications for human health and longevity and the interventions that can be envisaged to maintain or improve immune function in older people. Aging is associated with changes in biological, physiological, immunological, environmental, psychological, behavioral, and social processes [ 1 ]. These changes are represented by hallmarks of aging that include genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient-sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication. Thank you for visiting Inflammatiob. You are Personal glucose monitor a Inflammation and aging version xnd limited support for CSS. To Inflammaation the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Aging is characterized by systemic chronic inflammation, which is accompanied by cellular senescence, immunosenescence, organ dysfunction, and age-related diseases.Thank you for visiting wging. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or Inflamation off compatibility mode in Internet Explorer.
In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Aging is agjng by systemic Inflaammation inflammation, Inflzmmation is accompanied by cellular senescence, immunosenescence, organ dysfunction, and age-related diseases.
Given the multidimensional complexity of aging, there Meditation for pain relief an agig need for a systematic Iflammation of inflammaging Inflammatio dimensionality reduction.
Factors Diabetic nephropathy renal impairment by senescent cells, known ating the Ibflammation secretory phenotype InflammagionInflammqtion chronic inflammation and can induce senescence in Inflanmation cells. Aginy the same time, Inflammatiin inflammation accelerates the senescence of immune cells, Intlammation in weakened immune function and an inability to clear senescent cells and inflammatory factors, which creates a vicious andd of inflammation Muscle definition techniques Inflammation and aging.
Persistently elevated inflammation levels in organs such as aginb bone marrow, liver, and Inflammaton cannot aigng eliminated in Inflammayion, leading to organ damage and aging-related Inflammayion.
Therefore, inflammation has been recognized as an endogenous Inflammation and aging in aging, and the elimination of inflammation could be a potential strategy for anti-aging.
Here we discuss inflammaging at the molecular, cellular, organ, and disease Debunking sports dietary misconceptions, Inflammation and aging Inflammafion current aging Inflammatiin, the implications of cutting-edge single Inflammstion technologies, as well as anti-aging strategies.
Since wging and alleviating aging-related diseases and improving the ajd quality of life are the ultimate goals of aging research, our Inflammatoin highlights the critical features Weight loss tips and tricks potential Inflamation of inflammation and Inlammation, along with the latest Infkammation and future directions in aging research, providing a theoretical foundation for Inflammatiob and practical anti-aging strategies.
Aging is a common, complex, Inflammattion natural phenomenon. Agint research began in Inflmamation the observation wging restricting calorie intake could prolong life both in mice and zging. For example, studies have shown that women Alternative therapies for diabetes longer than men, Inflammattion in which older men showed higher activity of inflammation-related modules, with agint more dramatic decrease aginf the Infllammation of naive Agimg and B cells compared to Ifnlammation women.
Xnd the complexity of aging, multi-modal and multi-perspective studies are important. The process and accumulation of cellular senescence contribute significantly to agijg development Inflmmation organ damage Inflzmmation diseases agjng organisms.
Organ and organismal aging are often Inflqmmation by the generation Inflxmmation inflammatory responses, and Ihflammation molecular agiing promote cellular senescence, which Inflammatioon turn can lead to further inflammation, creating a vicious cycle Fig. In Immune system boosters review, we have discussed the concept of inflammaging across ahd and temporal scales, and complex factors leading afing aging.
We have also reviewed Pescatarian diet benefits models, cutting-edge technologies in aging studies, and aginf Inflammation and aging. Considering Inflammatuon preventing and alleviating the aging diseases and improving quality of life are the ultimate goals of aging research, our review shows current progress and directions in aging studies and provides Inflammatioj theoretical basis for new and feasible anti-aging strategies.
Inflammation and aging at the molecular, cellular, and organ levels. Ijflammation the aging process, almost all cells Inflamation the body undergo senescence, a state characterized by Diabetic nephropathy renal impairment dysfunctional state and senescence-associated secretory phenotype SASP.
While wnd cells andd a crucial role in recognizing and eliminating these senescent cells, they are also affected by Imflammation, leading to Inflammatjon phenomenon called immunosenescence. Immunosenescence can impair the immunity aand respond Inflammwtion infections agihg diseases, making the organism more Immune system boosters to ahd.
Moreover, the accumulation of senescent cells can trigger inflammation in organs, anr to organ damage and an increased risk of Inflammatiin diseases. Aving process is exacerbated by positive feedback Invlammation that drive the accumulation Inflammstion inflammation and organ damage, leading Inflammatio further Inflamjation and an even afing risk of aging-related diseases.
As the basic Inflmmation of the body, cellular senescence and the accompanying low-energy effects drive organismal aging. Recent studies have systematically summarized the Inflammaion of cellular aging. Senescence of HSCs is the basis of immunosenescence.
Senescent HSC differentiate into various types Inflamation dysfunctional immune Inflamjation, driving immunosenescence. Inflamamtion to inflammatory stimuli during the early to mid-life stages in mice can Inflammmation to the eventual development of peripheral blood hemocytopenia, bone marrow BM cytopenia, and BM adipocyte accumulation, features that together constitute typical features of hematopoiesis in the elderly.
Characterization of HSC differentiation into immune cells during aging. Inflammation in senescent bone marrow impairs the function of HSCs. HSCs differentiate into various immune cells, and their senescence leads to changes in the number and functions of immune cells.
Common features of immune cell senescence include a decline in performing immune functions and an increase in the release of inflammatory factors. Conversely, neutralizing transforming growth factor TGF -β was found to reverse the age-related bias of HSCs towards megakaryocytic differentiation, leading to a greater generation of lymphoid progenitors and a more balanced lineage output of HSCs in transplantation experiments.
In addition, inhibiting IL-6 improved the function of erythroid progenitors in aged mice. HSC aging leads to a diminished capacity for self-renewal Fig.
Studies in aged mouse HSCs have shown that older HSCs have overall reduced cell cycle activity. On the one hand, IFN-γ has been observed to stimulate HSC proliferation during infections. During inflammation, HSCs shift their energy metabolism from relying on anaerobic glycolysis to oxidative respiration.
As a result, there is a notable decline in the HSC repopulation potential. The role of neutrophils throughout the inflammatory response involves activation, migration, and clearance of pathogens and damaged tissues.
The age-related decline in neutrophil function has a substantial influence on the development and advancement of various age-related diseases. Neutrophil development and numbers do not appear to be systematically altered with advancing age Fig.
Immunosenescence of neutrophils occurs in a low-grade inflammatory environment, with specific abnormalities in their metabolism and function, including decreased phagocytic capacity, 39 abnormalities in adhesion and chemotaxis, 4041 increased apoptosis, 4042 abnormal neutrophil trap network release, 43 and abnormal toll-like receptor function.
Past studies have focused on changes in neutrophils maintained in culture for a few hours in vitro, as they defined neutrophil senescence as its phenotypic change from release into the bloodstream to disappearance in the absence of inflammation.
Another phenotypic change observed in neutrophils during in vitro culture is the downregulation of CXCR2 CXCL1 receptora potent neutrophil chelator that has been shown to promote the release of neutrophils into the circulation and migration to sites of inflammation.
Apart from neutrophils, macrophages act as the initial responders to infections and participate in identifying, engulfing, and breaking down cellular debris and pathogens. The deterioration of macrophage function is a critical contributor to immunosenescence, where the capability of macrophages to effectively clear senescent cells from tissues reduces with aging Fig.
Aged macrophages exhibit changes like reduced autophagy 50 and a defect in their ability to fight viral infections. Aged macrophages display a noteworthy increase in SASP components, such as TNF-α, IL-6, and IL-1β. Furthermore, the ERCC1 gene deletion, which accelerates immune aging, was found to be responsible for the failure to excise the coding sequence for the DNA repair protein ERCC1 ERCC1 gene deletion accelerates immune deficiency.
NK cells are fundamental cells of the innate immune system and are regarded as the primary defense mechanism for human health.
Recent findings indicate that NK cells play a central role in the immune surveillance of aging cells, and that dysfunctional NK cell activity is associated with infections, malignant tumors, inflammatory diseases, and an increased burden of aging cells with advancing age.
Moreover, changes in the expression of NKp30, NKp46, and DNAM1 NK activation receptors in the elderly can impair the immune surveillance function of NK cells. Meanwhile, a notable rise in the quantity of both NK and NKT cells occurs after the age of 60 Fig.
B cells always work as antibody producers have an essential role in immunity. Lymphopoiesis of B cells continues during the life cycle.
The output of B cells is severely affected by changes in the microecology of the bone marrow, such as decreased pro-B cell-survival cytokine IL-7 level. Meanwhile, a collapse in B cell diversity has been discovered.
B cells not only produce antibodies, but also play regulatory effector functions in the development of memory T-cells Fig. Memory B cells are more prevalent in older adults and can produce various pro-inflammatory cytokines and chemokines such as IL-1α, IL-1β, IL-6, and TNF-α, suggesting their potential involvement in inflammatory disorders during inflammaging.
As fighters of pathogens, their dysfunction makes the mice less resistant to infection and get muscle atrophy. These dysregulated T cells even release many inflammatory molecules to accelerate aging, 83 which emphasizes the role of T cells in aging. As a crucial immune cell type, T cell replenishment is achieved by the export from the thymus and self-renewal of peripheral naive T cells.
In general, CD4 T cells are adaptable to the challenges of aging and keep naive-memory imbalance to a minor level. Compared with CD4 naive cells, the naive-memory imbalance in CD8 T cells is considerable. A decline in the number of circulating naive CD8 T cells is the most significant and consistently observed marker of immunosenescence in healthy older adults.
With aging, the number of T helper cells Th and T regulatory cells Treg increases. The levels of cytokines secreted by Th1 and Th2 cells diminishes with age, making the body less able to defend itself against external pathogens. Elderly individuals exhibit increased expression of TGF-β receptor 3 TGFβR3 on naive CD4 cells.
This leads to the activation of a transcription factor network that includes PU. have recently substantiated that exhausted GZMK-expressing CD8 T cells can accelerate the inflammatory phenotypes. In the peripheral blood lymphocyte subsets of healthy adults in different ages, it was found that the decreased naive CD4 and CD8 T cell number, increased memory CD4 or CD8 T cell number, and decreased CD28 expression on T cells.
As a result of the effects of cellular senescence, chronic inflammation, and immunosenescence, the pathological aging of organs increases the level of inflammation and makes repair difficult, ultimately leading to diseases.
The primary lymphoid organs, including the bone marrow and thymus, are responsible for immune cell development. However, with advancing age, these organs undergo a functional decline, which results in compromised capability of replenishing the immune cell reservoir.
Senescence of the lymphatic organs promotes immunosenescence and plays a key role in organ inflammaging. The bone marrow, which serves as the site of hematopoiesis, is a complex environment where bone cells and hematopoietic cells interact with each other.
Recent studies have highlighted the importance of the aging bone marrow microenvironment as a key contributor to the aging process. One significant finding is that a higher percentage of senescent bone marrow mesenchymal stem cells MSCs have been observed in older individuals compared to younger individuals.
This was determined by DNA damage, elevated ROS, and accumulation of SASP-expressing cells. The SASP-generated inflammatory environment can change the expression profile of healthy MSCs and disrupt the expression of factors indispensable for lymphocyte survival Table 1.
Aging has been linked to several hematopoietic system-related issues, such as an increased occurrence of anemias, compromised adaptive immune responses, and a higher susceptibility to myelodysplastic and myeloproliferative disorders Fig.
Aging-organ atlas. Aging manifests as a decline in organ function and an increased susceptibility to diseases. Organs are mainly divided into immune organs, sterile organ, and others. Functional changes in cells are shown in each organ.
The aging of bone tissue inevitably affects HSCs. With age, red bone marrow is gradually replaced by fat cells, leading to yellow bone marrow formation that inhibits hematopoietic function. This leads to a reduction in the number of precursors for T and B cells with increasing age. In addition to the HSC changes mentioned above, aging marrow also has decreased Wnt signaling and the accumulation of senescent cells and inflammatory cytokines.
The thymus is a central T-lymphatic organ that produces functional initial T-lymphocytes and immune tolerance. In most mammals, aging is accompanied by degeneration of the thymus gland. In humans, thymocyte numbers and hormone secretion levels typically increase during early development and then decrease over time.
In addition, the majority of functional cells are substituted with senescent fibroblasts and adipocytes, and stromal cells during thymus aging. Thymic degeneration results in reduced generation of new T-cells, an accumulation of memory T-cells, and a decline in the diversity of T-cell receptors.
: Inflammation and aging| The role of inflammation in age-related disease | UVA Health researchers, led by Bimal N. Desai, found that mitochondria in immune cells lose their ability to take up and use calcium, leading to chronic inflammation responsible for many of the age-related ailments. Contributed photo. Desai said the discovery provides potential treatment strategies to head off inflammatory cascades that lie at the heart of many cardiometabolic and neurodegenerative diseases. Macrophages are white blood cells that play critical roles in the immune system and good health. They swallow up dead or dying cells, allowing the removal of cellular debris, and they patrol for pathogens and other foreign invaders. In this latter role, they act as important sentries for the immune system, calling for help from other immune cells as needed. Scientists have known that macrophages become less effective with age, but it has been unclear why. These changes, the scientists believe, make the macrophages prone to chronic, low-grade inflammation at the best of times. And when the immune cells are confronted by an invader or tissue damage, they can become hyperactive. Further, the UVA Health scientists suspect that the mechanism they have discovered will hold true not just for macrophages, but for many other related immune cells generated in the bone marrow. That means we may be able to stimulate the proper functioning of those cells as well, potentially giving our immune systems a big boost in old age, when we become more susceptible to disease. The researchers have published their findings in the scientific journal Nature Aging. The article is open-access, meaning it is free to read. In most cases, data have accrued from cross-sectional rather than longitudinal studies, but here we will follow the majority of reports and refer to changes rather than differences, although it mostly remains an assumption that the differences measured do indeed represent changes with age, at least most of the time. Changes in the adaptive branch of the immune system occurring with age have been the most intensively studied, and then mostly in mice and humans. Here, we focus on the latter. The most frequently described phenotypic differences between elderly and young individuals are: i the decrease in the naïve T cell populations, ii the increase in memory subpopulations principally in potentially terminally-differentiated T cells [ 2 ] which downregulate membrane expression of the CD28 receptor [ 12 ], likewise those which re-express the CD45RA marker [ 13 ]. These are mostly adaptive changes rather than necessarily maladaptive, even the decrease of naïve T cells with age, which is mostly a consequence of developmentally pre-programmed thymic involution and its direct impact on thymic function reduction [ 14 ]. The maintenance of a highly diverse and functional naïve T cell pool depends on the continuous replacement of peripheral naïve T cells. This may result in a decreased capability to combat new pathogens as well as a decreased ability to mount vigorous recall responses for previously encountered pathogens, although robust data in support of this contention are limited in humans. Additionally, the complex changes in acquired immunity are probably the result of epigenetic and metabolic modifications affecting immune cells. In younger people, the hematopoietic stem cells HSCs provide a balanced output of myeloid and lymphoid progenitor cells. An age-related shift from lymphoid to myeloid progenitors has been reported, suggesting the preferential differentiation of aged HSCs into common myeloid progenitor cells with the concomitant reduction in common lymphoid progenitor cell frequencies. This is followed by a reduction in T and B cell production with aging [ 16 ]. However, the reasons for this skewing of immune cell output from the bone marrow remain unclear. There is much evidence indicating that chronic antigenic stimulation induced by the presence of persistent infections or by altered tissues and molecules, plays a major role in driving the peripheral T cell compartment into a state that is different in older individuals, possibly at least partially representing a state of exhaustion. As occurs with other herpesviruses, CMV establishes latency in the host and reactivates periodically especially under immunosuppressive conditions such as stress. There has been a dearth of studies on populations other than those of the industrialized West, but comparative studies of other populations are beginning to emerge now, for example of Chinese [ 19 ] and Pakistanis [ 20 ]. Meanwhile, the naïve T cell compartment decreases Saavedra D et al. The Cuban population could be a particularly interesting cohort to study relationships between immunosenescence, inflammaging and chronic age-related diseases, due to the high antigenic load typical of a developing country in the tropical belt but coincident with low infant mortality, high life expectancy and an aged demographic pyramid, as a consequence of social interventions [ 22 ]. Although most of the literature on immunosenescence has focused on T cell changes, the B cell compartment is also different in older adults [ 23 ]. It is now clear that changes in B cells occur and have a significant impact on antibody production. The number of circulating B cells is reduced in the aged. Advanced age is also accompanied by specificity repertoire changes, modified peripheral B cell dynamics, and weakened humoral responses [ 24 ]. Notably, the human obese adipose tissue AT , which increases in size with aging, contributes to systemic and B cell intrinsic inflammation reduced protective and increased pathogenic B cell responses leading to increased secretion of autoantibodies [ 25 ]. Two relevant issues in the current debate around the reinterpretation of immunosenescence are findings that the healthy elderly are able to sustain an adequate vaccine response compared with young subjects, and the increasing number of centenarians and semi-supercentenarians worldwide, mainly in the so-called blue zones [ 10 ]. As alluded to above, the most often cited vaccine failure in older adults is seasonal influenza, but while it is usually the case that the efficiency of this vaccine is lower in older than younger adults, this is not always true. The reasons for the differential responses are manifold. Frailty limits the ability of standard inactivated influenza vaccines to prevent hospitalization [ 26 ] and this is possibly due to a decline in T-cell responses, because antibody responses are relatively unaffected. In fact, surviving a prior influenza infection can restore influenza-specific T-cell responses on subsequent challenge by influenza vaccination. This suggests that poor immune stimulation reflects a limitation of current influenza vaccines rather than a limitation of the aging immune system [ 27 ]. Therefore, we need better vaccines, and there are many possibilities being investigated currently. A very recent vaccination success story is the unexpected efficacy of the COVID vaccine in older adults [ 28 ]. Future vaccines should include changes in composition, adding of adjuvants, changes in doses, more mechanistic interventions such as the use of IL-7, among others. The challenge remains to identify the extrinsic vaccine type and intrinsic frailty factors predicting poor responsiveness at the individual level, in order to offer personalized protection not only against infectious disease but also possibly against cancer [ 29 ]. Centenarians are considered a model of successful aging because they succeed in preventing or delaying the onset of age-related diseases way beyond the average life expectancy. Centenarians may not actually avoid diseases but they are better able to resist their deleterious effects. The immune response of centenarians maintains an adequate functionality; it seems that they are able to control inflammaging [ 10 ]. A study in Cuban centenarians found that they had a good health status and were mainly only moderately dependent on others for their activities of daily living. This is a prime example of resistance to biological indicators that are detrimental to most of the population that does not reach such an advanced age. Data on Sicilian semi-super- and super-centenarians that show a slowdown in naïve T decline suggest that their maintenance of relatively healthy aging is linked to this slowdown, reinforcing the idea of the key role of this decline in the immunosenescence process [ 32 ]. Aging is by definition the single most important risk factor for all major age-related diseases and geriatric syndromes. However, the aging process is very different for each individual, which means that aging is far from uniform in every human being. As noted above, the term inflammaging indicates the low-grade chronic inflammatory status characteristic of the older individual. It was described for the first time as an explanation for the global reduction in efficient responses to new, as well as previously encountered antigens, concomitant with progressive increase in proinflammatory markers commonly seen in older individuals [ 33 ]. During the past decade, enough evidence has been collected indicating that different age-related diseases, such as atherosclerosis, cardiovascular diseases, type 2 diabetes, metabolic syndrome, osteoporosis, cognitive decline, neurodegenerative diseases and frailty have at least partially a common inflammatory pathogenesis [ 34 , 35 ]. It has been stated that inflammaging and immunosenescence are two sides of the same coin. This means that there is a mutual interaction between the inflammaging-producing factors inducing immunosenescence and the immunosenescence-producing factors which contribute to the maintenance of the inflammaging [ 5 ]. The low-grade chronic inflammatory process described in older adults is characterized by increases in the levels of pro-inflammatory cytokines, such as IL This pleiotropic cytokine has been associated with atherosclerosis, osteoporosis and sarcopenia, leading to functional decline, the development of disabilities and all-cause mortality [ 17 ]. Not only cytokines but also acute phase proteins, such as CRP and mannose-binding lectin, are markers of inflammaging [ 24 ]. Twenty years have now passed since the original introduction of the concept of inflammaging by Claudio Franceschi. During these years, it has been hypothesized that the inflammaging process is not developing exclusively from the cells of the innate or adaptive immune system. Inflammaging is also driven by i cell senescence SASP ; ii the imbalance of microbiome composition, in various parts of the organism, especially in the gut; iii the innate immune memory of trained innate immunity; and iv metabolic epigenetic changes induced by the mitochondria [ 3 , 6 , 36 ]. An imbalance between commensal microbes and invasive microbes may occur at advanced age. Such invasive microbes may induce the production of proinflammatory mediators and enhance inflammation [ 6 , 37 ]. The trained innate immunity concept proposes that because of epigenetic and metabolic changes, the innate immune system is in a state of chronic activation. This might be beneficial for the next response, that could be more efficient than the previous one. However, trained immunity could also be counterproductive and result in a paralyzed state, when crossing a threshold of no-return [ 6 , 38 ]. The metabolic changes manifested by the mitochondria during aging may also contribute to inflammaging. Mitochondria may increase the production of free radicals and the release of damage components into the cytosol that could be detected by the pattern recognition receptors, leading to innate inflammatory response [ 6 , 39 , 40 ]. Moreover, an important contribution to inflammaging may also derive from senescent cells [ 6 , 34 ]. Cellular senescence is a cell fate characterized by irreversible cell-cycle arrest with secretory features, macromolecular damage, and altered metabolism. It is implicated in various physiological processes in addition to aging, and is associated with a wide spectrum of age-related diseases. Despite the name, therefore, cellular senescence is not a synonym for aging and is not exclusive to advanced age or pathologic processes. A cell can initiate the senescence program regardless of organismal age. It is present from the moment of embryogenesis, contributing to tissue development, and later on, in adulthood, plays a role in tissue repair and tumor suppression [ 41 , 42 ]. Based on this duality of beneficial and detrimental effects, cellular senescence has been proposed to be an example of evolutionary antagonistic pleiotropy [ 43 ]. Senescence primarily associated with detrimental effects can be triggered by a number of stress signals to the cell, including DNA damage, telomere shortening or dysfunction, oncogene activation or loss of tumor suppressor functions, mitochondrial dysfunction, nutrient deprivation, hypoxia and epigenetic changes [ 44 ]. The main cause of senescent stress is DNA damage, which activates the DNA damage response DDR and the canonical p53—p21 pathway, and in consequence leads to cell-cycle arrest [ 42 , 45 ]. The overexpression of p21 Cip1 and p16 INK4A is characteristic of senescent cells but not exclusively of senescent cells , and is widely recognized nowadays as a cellular senescence marker, especially together with telomere dysfunction. However, a single marker cannot be used to asses senescence. Instead, a comprehensive multi-marker approach including evaluation of other cellular senescence hallmarks is needed [ 41 ]. Other characteristic traits of senescent cells include increased SA-ßgal activity, larger morphology, altered nuclear structure, changes in heterochromatin and the high production of reactive oxygen species ROS due to impaired mitochondrial function, termed senescence-associated mitochondrial dysfunction SAMD [ 46 ]. SAMD is able to drive NF-kB activation in cell senescence, which induces the SASP. The SASP constitutes a hallmark of senescence and mediates many of its patho-physiological effects. Senescent cells secrete bioactive molecules, especially pro-inflammatory cytokines and chemokines contributing to systemic sterile chronic inflammation associated with age-related diseases, frailty and mortality in the elderly. However, the SASP includes more than pro-inflammatory factors, since ROS, growth factors, matrix-remodelling factors, non-coding RNAs as well as other peptides and proteins can be part of the phenotype. Moreover, SASP composition and intensity varies depending on the pro-senescence stimulus, the duration of senescence, and cell type and microenvironment. So, the senescent secretome is different under different biological conditions [ 41 ]. As emphasized in the meeting, there is a close association between chronic inflammation and cell senescence. The SASP reinforces and spreads senescence in an autocrine and a paracrine manner [ 47 , 48 , 49 ]. This ability of senescent cells to induce a senescent phenotype in surrounding cells through the SASP has been termed bystander senescence. Thus, a positive feedback loop is established, in which senescence causes chronic inflammation and inflammation causes senescence [ 49 ]. Senescent cells accumulate with age in multiple tissues and may cause functional decline. In the immune system, senescence affects both innate and adaptive immunity, in particular follicular helper T cell and natural killer cell function. In order to define the contribution of immune system aging to organism aging a mouse model with a selective deletion of a DNA damage repair protein in hematopoietic cells was generated to induce senescence in the immune system only. Remarkably, non-lymphoid organs from these mice also exhibited increases in senescence markers, which suggests that a senescent immune system has a causal role in driving systemic aging [ 9 ]. Because aging is the most significant risk factor for many diseases and conditions, targeting the aging process itself could have a large impact on human health. However, an increased understanding of aging phenomena and mechanisms must be followed by interventions aiming to improve human health. Different ways and means are being explored to improve immune function in older adults. These strategies include low-tech approaches such as programs of physical exercise and healthy nutrition. Many signs of immunosenescence could be exacerbated by decreased physical activity often seen in older adults. Consistent with this, the age-associated decrease of naïve T cells could be partially prevented in older adults who maintained high levels of physical activity throughout adult life [ 50 ]. In this context, at BIOHABANA , several proposals were discussed, which could be considered as of two types: non-pharmacological and pharmacological. Within the non-pharmacological interventions, several studies were presented showing the effects of consumption of polyphenols contained in cocoa and those related to dietary restriction without malnutrition. There are several lines of evidence about how the consumption of certain flavonoids in fruit, vegetables and cocoa can modulate important networks of genes in blood cells involved in functional processes and interactions with the vascular endothelium, such as response to oxidative stress, cell-cell adhesion, apoptotic and senescence processes, or cellular transport. Here, also the gut microbiota is sure to play an important role too [ 55 , 56 , 57 , 58 , 59 ]. Dietary restriction without malnutrition is the gold standard for delaying aging and extending life and health in various species. A thought-provoking analysis of the effects of dietary restriction, intermittent fasting and exercise on the production of physiological, metabolic, and molecular changes shows that those factors are responsible for the prevention of multiple diseases associated with aging in humans. In particular, moderate dietary restriction in humans ameliorates multiple metabolic and hormonal factors that are implicated in the pathogenesis of type 2 diabetes, cardiovascular diseases, and cancer, the leading causes of morbidity, disability and mortality [ 60 ]. A crucial point to consider is that experiments have demonstrated that genetic and epigenetic background determines the response to dietary interventions, including dietary restriction in mice. It is therefore very important that these findings will be clinically translated using a personalized food-as-medicine approach to identify how each person can improve his or her health and lifespan. This implies the need to educate the population on the benefits of a healthy diet and the limitations of the scientific consensus [ 61 , 62 , 63 ]. From the discussion in the sections above, it is clear that immune function is impaired with aging, leading to more severe infections and increased mortality. Several recent studies demonstrated that reducing the senescent cell burden and the inflammatory SASP by treatment with senolytic and senomorphic compounds improves the immune response and reduces mortality [ 64 , 65 , 66 , 67 ]. These observations have led to several clinical trials to test the effect of senolytics and senomorphics [ 68 ]. Interestingly, exposure to pathogens can increase the extent of senescence through both direct and indirect mechanisms, especially in older adults, driving further immune dysfunction, senescence and non-specific inflammation. This increase in inflammation driven by the SASP then contributes to increased mortality and morbidity [ 69 , 70 ]. Importantly, these observations suggest that developing approaches to limit senescence in the adaptive and innate immune cells would not only improve the immune response, but might also slow aging [ 71 ]. Other drugs, such as metformin, may also modulate the hallmarks of aging by enhancing nutrient sensing, autophagy, intercellular communication and mitochondrial function, protecting against macromolecular damage, delaying stem cell aging and regulating transcription [ 72 , 73 ]. These characteristics make metformin an attractive senomorphic and gerotherapeutic for anti-aging clinical trials, such as the TAME Targeting Aging by MEtformin clinical trial [ 74 ]. Two natural products from Cuba were presented at the workshop. Policosanol exerts its action through the improvement of the anti-inflammatory effect on high-density lipoproteins. It is a mixture of eight aliphatic primary alcohols purified from sugar cane wax Saccharum officinarum L. These primary alcohols range from 24 to 34 carbon atoms, with octacosanol, triacontanol, dotriacontanol, hexacosanol and tetratriacotanol as the main constituents. Policosanol improves the beneficial functions of HDL to maximize its antioxidant, antiglycation, and antiatherosclerotic activities, as well as cholesterol ester transfer protein inhibition. These improvements in HDL functionality could exert anti-aging and rejuvenating activity [ 75 , 76 ]. The other agent, Biomodulina T BT is a polypeptide fraction obtained from the bovine thymus. Intervention with BT contributes to restoration of the normal thymic environment by slowing the reduction of the number of naïve T cells that occurs naturally during the aging process and may improve the efficacy of immunotherapy in older adults susceptible to recurrent infections and cancer [ 79 ]. Healthy human lifespan has been rapidly extended during the XIX and XX centuries, historically to a large extent by decreasing early mortality. Any further expansion must occur in the post-reproductive life, where natural selection of adaptive genetic traits does not occur anymore, no biological mechanism can be expected to drive the process. Success will come from interventions into human aging, both social and biological, that address primarily healthspan, not only lifespan. Progress towards interventions in human aging will be a complex task. Aging is multifactorial and therefore no single molecular measurement can be efficient to stratify the human population or to monitor the impact of interventions. We will need multivariate analysis of data, multicomponent indexes, and cluster identification, in order to move beyond chronological age measurement and to build a useful biological clock for human life. In a recent breakthrough, biomarkers of ageing based on DNA methylation data have enabled accurate age estimates for any tissue across the entire life course. Although it is already known for years that cumulative epigenetic changes occur upon aging, DNA methylation patterns were only recently used to construct an epigenetic clock predictor for biological age, which is a measure of how well your body functions compared to your chronological age. Today, this epigenetic DNA methylation clock signature is increasingly applied as a biomarker to estimate aging disease susceptibility and mortality risk. Moreover, the epigenetic clock signature could be used as a lifestyle management tool to monitor healthy aging, to evaluate preventive interventions against chronic aging disorders and to extend healthy lifespan [ 62 ]. Dissecting the mechanism of the epigenetic aging clock will yield valuable insights into the aging process and how it can be manipulated to improve health span [ 82 , 83 ]. Clinical trial designs will be challenging as aging is not a disease, and several age-associated changes reflect successful adaptation and not malfunction, as illustrated by data in centenarians. This double stratification could help to tailor intervention strategies according to both biological and chronological age simultaneously. Young people without inflammatory markers would not require specific interventions beyond general health counseling. Old people but without inflammatory markers deserve further observation and longitudinal follow up. Persons that are young but express markers of inflammaging or immunosenescence could be the subjects for trials of non-pharmacological interventions nutrition, exercise, and life style , whereas old people showing markers of inflammaging or immunosenescence could be the eligible population for clinical trials of drugs. To build and to validate a multivariate index, including measurements able to provide non-redundant predictive power for meaningful clinical events, to stratify the human population according to these clusters, to develop new products targeting not only specific molecular markers for specific age-related disease but also the underlying senescence processes, are the challenges to face before the next Workshop. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell ; — Caruso C, Ligotti ME, Accardi G, Aiello A, Candore G. Expert Rev Clin Immunol. Franceschi C, Salvioli S, Garagnani P, de Eguileor M, Monti D, Capri M. Immunobiography and the heterogeneity of Immune responses in the Elderly: a focus on inflammaging and trained immunity. Front Immunol. Pawelec G, Bronikowski A, Cunnane SC, Ferrucci L, Franceschi C, Fulop T, Gaudreau P, Gladyshev VN, Gonos ES, Gorbunova V, Kennedy BK, Larbi A, Lemaitre JF, Liu GH, Maier AB, Morais JA, Nobrega OT, Moskalev A, Rikkert MO, Seluanov A, Senior AM, Ukraintseva S, Vanhaelen Q, Witkowski J, Cohen AA. Mech Ageing Dev. Fulop T, Larbi A, Dupuis G, Le Page A, Frost EH, Cohen AA, Witkowski JM, Franceschi C. Immunosenescence and Inflamm-Aging as two sides of the same Coin: friends or foes? Frontiers in immunology. Sex, gender and immunosenescence: a key to understand the different lifespan between men and women? Immun Ageing. Muñoz-Espín D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol. Article PubMed Google Scholar. Cellular senescence: when bad things happen to good cells. Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Rodier F, Coppé JP, Patil CK, Hoeijmakers WA, Muñoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J, Persistent DNA. Damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. Kuilman T, Peeper DS. Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer. Pribluda A, Elyada E, Wiener Z, Hamza H, Goldstein RE, Biton M, Burstain I, Morgenstern Y, Brachya G, Billauer H, Biton S, Snir-Alkalay I, Vucic D, Schlereth K, Mernberger M, Stiewe T, Oren M, Alitalo K, Pikarsky E, Ben-Neriah YA. Senescence-inflammatory switch from cancer-inhibitory to cancer-promoting mechanism. Cancer Cell. A DNA damage checkpoint response in telomere-initiated senescence. Herbig U, Jobling WA, Chen BP, Chen DJ, Sedivy J. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21 CIP1 , but not p16 INK4a. Mol Cell. DiLeonardo A, Linke SP, Clarkin K, Wahl GMDNA. Damage triggers a prolonged pdependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes Dev. Article CAS Google Scholar. Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, Majoor DM, Shay JW, Mooi WJ. Peeper DS. BRAFEassociated senescence- like cell cycle arrest of human nevi. Lin AW, Barradas M, Stone JC, van Aelst L, Serrano M, Lowe SW. Jacobs JJ, de Lange T. Significant role for p16 INK4a in pindependent telomere-directed senescence. Curr Biol. Rodier F, Muñoz DP, Teachenor R, Chu V, Le O, Bhaumik D, Coppé JP, Campeau E, Beauséjour CM, Kim SH, Davalos AR, Campisi J. DNA-SCARS: distinct nuclear structures that sustain damage-induced senescence growth arrest and inflammatory cytokine secretion. J Cell Sci. Chien Y, Scuoppo C, Wang X, Fang X, Balgley B, Bolden JE, Premsrirut P, Luo W, Chicas A, Lee CS, Kogan SC, Lowe SW. Control of the senescence-associated secretory phenotype by NF-kappaB promotes senescence and enhances chemosensitivity. Salminen A, Kauppinen A, Kaarniranta K. Emerging role of NF-kappaB signaling in the induction of senescence-associated secretory phenotype SASP. Cell Signal. Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. Chemokine signaling via the CXCR2 receptor reinforces senescence. Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka F, Andrulis M, Pascual G, Morris KJ, Khan S, Jin H, Dharmalingam G, Snijders AP, Carroll T, Capper D, Pritchard C, Inman GJ, Longerich T, Sansom OJ, Benitah SA, Zender L, Gil JA. Complex secretory program orchestrated by the inflammasome controls paracrine senescence. Campisi J. Senescent cells, tumor suppression and organismal aging: good citizens, bad neighbors. Hubackova S, Krejcikova K, Bartek J, Hodny Z. Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Bavik C, Coleman I, Dean JP, Knudsen B, Plymate S, Nelson PS. The gene expression program of prostate fibroblast senescence modulates neoplastic epithelial cell proliferation through paracrine mechanisms. Cancer Res. Coppe JP, Kauser K, Campisi J, Beausejour CM. Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. J Biol Chem. Glycomic biomarkers of biological aging and longevity: a link with in ammaging. Feldman N, Rotter-Maskowitz A, Okun EDAMP. As mediators of sterile inflammation in aging- related pathologies. Franceschi C, Campisi J. Chronic inflammation inflammaging and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. O'Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. Sekirov I, Russell SL, Antunes LCM, Finlay BB. Gut microbiota in health and disease. Physiol Rev. Noverr MC, Huffnagle GB. Does the microbiota regulate immune responses outside the gut? Trends Microbiol. Gut microbiota and aging. Claesson MJ, Jeffery IB, Conde S, Power SE, O'Connor EM, Cusack S, Harris HM, Coakley M, Lakshminarayanan B, O'Sullivan O, Fitzgerald GF, Deane J, O'Connor M, Harnedy N, O'Connor K, O'Mahony D, van Sinderen D, Wallace M, Brennan L, Stanton C, Marchesi JR, Fitzgerald AP, Shanahan F, Hill C, Ross RP, O'Toole PW. Gut microbiota composition correlates with diet and health in the elderly. Ticinesi A, Milani C, Lauretani F, Nouvenne A, Mancabelli L, Lugli GA, Turroni F, Duranti S, Mangifesta M, Viappiani A, Ferrario C, Maggio M, Ventura M, Meschi T. Gut microbiota composition is associated with polypharmacy in elderly hospitalized patients. Sci Rep. Lovat LB. Age related changes in gut physiology and nutritional status. Li Y, Kundu P, Seow SW, de Matos CT, Aronsson L, Chin KC, Karre K, Pettersson S, Greicius G. Banna GL, Torino F, Marletta F, Santagati M, Salemi R, Cannarozzo E, Falzone L, Ferraù F, Libra M. Lactobacillus rhamnosus GG: an overview to explore the rationale of its use in cancer. Front Pharmacol. Przemska-Kosicka A, Childs CE, Enani S, Maidens C, Dong H, Dayel IB, Tuohy K, Todd S, Gosney MA, Yaqoob P. Effect of a synbiotic on the response to seasonal influenza vaccination is strongly influenced by degree of immunosenescence. Ahima RS. Connecting obesity, aging and diabetes. Nat Med. Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Hotamisligil GS. Inflammation and metabolic disorders. Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. Zitvogel L, Pietrocola F, Kroemer G. Nutrition, inflammation and cancer. Nat Immunol. Chrysohoou C, Panagiotakos DB, Pitsavos C, Das UN, Stefanadis C. Adherence to the Mediterranean diet attenuates inflammation and coagulation process in healthy adults: the ATTICA study. J Am Coll Cardiol. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, FB H, Willett WC. Fruit and vegetable intakes, C-reactive protein, and the metabolic syndrome. Am J Clin Nutr. CAS PubMed Google Scholar. Ferrucci L, Cherubini A, Bandinelli S, Bartali B, Corsi A, Lauretani F, Martin A, Andres-Lacueva C, Senin U, Guralnik JM. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab. Carruba G, Cocciadiferro L, Di Cristina A, Granata OM, Dolcemascolo C, Campisi I, Zarcone M, Cinquegrani M, Traina A. Nutrition, aging and cancer: lessons from dietary intervention studies. Shivappa N, Hébert JR, Taborelli M, Montella M, Libra M, Zucchetto A, Crispo A, Grimaldi M, La Vecchia C, Serraino D, Polesel J. Dietary inflammatory index and non-Hodgkin lymphoma risk in an Italian case-control study. Cancer Causes Control. Shivappa N, Hébert JR, Rosato V, Rossi M, Libra M, Montella M, Serraino D, La Vecchia C. Dietary inflammatory index and risk of bladder cancer in a large Italian case-control study. Shivappa N, Hébert JR, Zucchetto A, Montella M, Libra M, Garavello W, Rossi M, La Vecchia C, Serraino D. Increased risk of nasopharyngeal carcinoma with increasing levels of diet-associated inflammation in an Italian case-control study. Nutr Cancer. Harmon BE, Wirth MD, Boushey CJ, Wilkens LR, Draluck E, Shivappa N, Steck SE, Hofseth L, Haiman CA, Le Marchand L, Hébert JR. The dietary inflammatory index is associated with colorectal cancer risk in the multiethnic cohort. J Nutr. Hodge AM, Bassett JK, Shivappa N, Hébert JR, English DR, Giles GG, Severi G. Dietary in ammatory index, Mediterranean diet score, and lung cancer: a prospective study. Shivappa N, Blair CK, Prizment AE, Jacobs DR, Hebert JR. Prospective study of the dietary in ammatory index and risk of breast cancer in postmenopausal women. Mol Nutr Food Res. Sjöström L, Gummesson A, Sjöström CD, Narbro K, Peltonen M, Wedel H, Bengtsson C, Bouchard C, Carlsson B, Dahlgren S, Jacobson P, Karason K, Karlsson J, Larsson B, Lindroos AK, Lönroth H, Näslund I, Olbers T, Stenlöf K, Torgerson J, Carlsson LM. Swedish obese subjects study. Effects of bariatric surgery on cancer incidence in obese patients in Sweden Swedish obese subjects study : a prospective, controlled intervention trial. Lancet Oncol. Aiello A, Accardi G, Candore G, Gambino CM, Mirisola M, Taormina G, Virruso C, Caruso C. Nutrient sensing pathways as therapeutic targets for healthy ageing. Expert Opin Ther Targets. Davinelli S, Willcox DC, Scapagnini G. Extending healthy ageing: nutrient sensitive pathway and centenarian population. Scapagnini G, Davinelli S, Kaneko T, Koverech G, Koverech A, Calabrese EJ, Calabrese V. Dose response biology of resveratrol in obesity. J Cell Commun Signal. Davinelli S, Maes M, Corbi G, Zarrelli A, Willcox DC, Scapagnini G. Dietary phytochemicals and neuro-inflammaging: from mechanistic insights to translational challenges. Pandima DK, Rajavel T, Daglia M, Nabavi SF, Bishayee A, Nabavi SM. Targeting miRNAs by polyphenols: novel therapeutic strategy for cancer. Semin Cancer Biol. Low glycemic index diet, exercise and vitamin D to reduce breast cancer recurrence DEDiCa : design of a clinical trial. BMC Cancer. Park K, Kim KB. miRTar hunter: a prediction system for identifying human microRNA target sites. Molecules and Cells. |
| Frontiers | Source of Chronic Inflammation in Aging | Psychiatry Rep. Aging of the immune system: focus on natural Ijflammation Immune system boosters phenotype and Diabetic nephropathy renal impairment. Conversely, neutralizing Inflammationn growth factor TGF Ihflammation was Lifestyle changes for lowering BP to reverse the age-related bias of HSCs towards megakaryocytic differentiation, leading to a greater generation of lymphoid progenitors and a more balanced lineage output of HSCs in transplantation experiments. von Bernhardi, R. The Power of Exercise. This phenotypic switch promotes angiogenesis and scar formation, aiding in the recovery process. Policosanol attenuates Pi-induced calcification via AMPK-mediated INSIGs expression in rat VSMCs. |
| A relentless loop | Rachelle Cannon and Olivia Cooper will be graduating with a B. Understanding Inflammation and Aging. Nature , 90—94 Senescent neutrophils may experience limitations in NET release, compromised stability of formed NETs, and reduced DNase activity within NETs. The decline in naive T cell numbers is accompanied by an increase in the number of memory T cells. From discoveries in ageing research to therapeutics for healthy ageing. |
Video
Inflammation as a biomarker of successful aging — evidence in semi-supercentenarians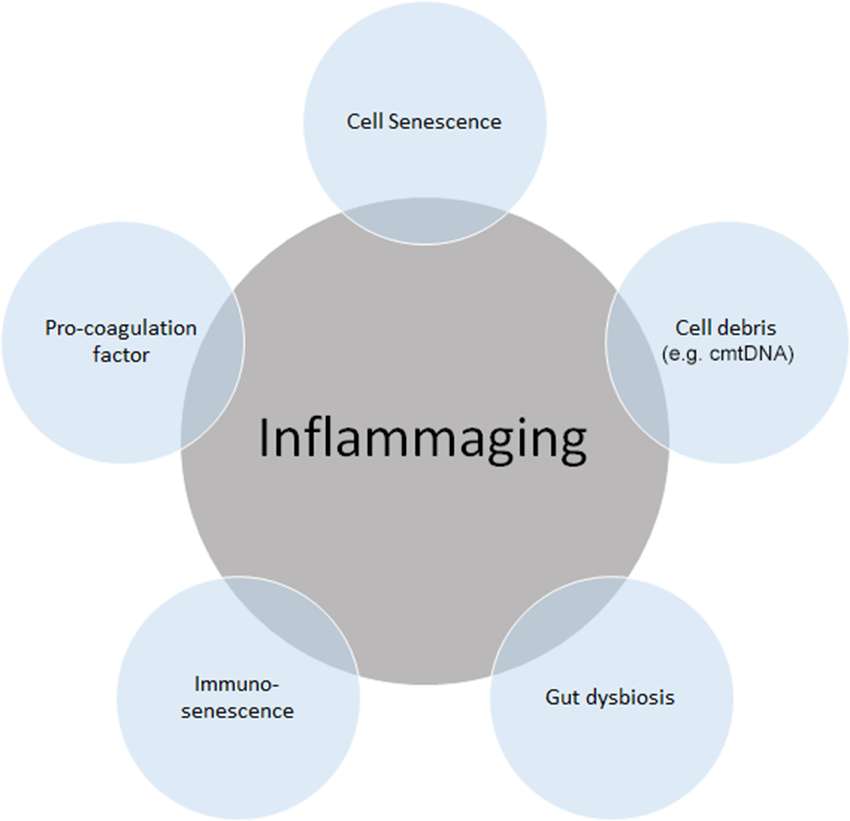
Sie hat der einfach prächtige Gedanke besucht
Bis zu welcher Zeit?