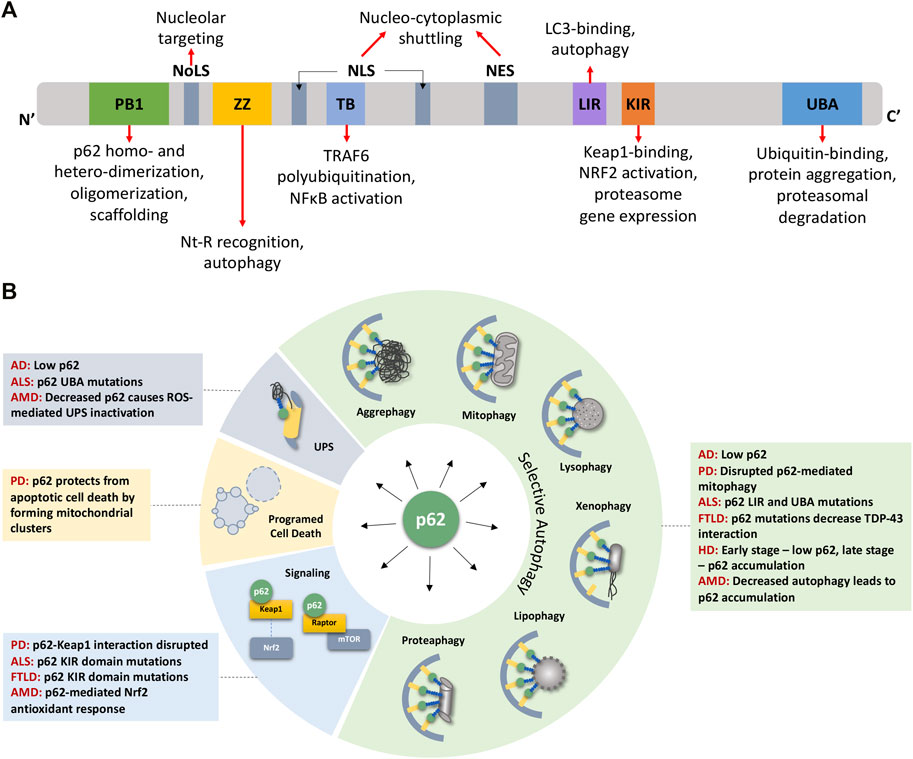

Autophagy and p/SQSTM -
LC3-I is cytosolic, whereas the lipidated form LC3-II is membrane bound [ 14 ]. Autophagy can either be non-selective or can very specifically target certain portions of the cytoplasm for degradation [ 16 ].
Different autophagy receptors are mediating selectivity by being able to interact with the cargo on the one hand and the autophagy machinery on the other hand [ 17 ]. The best studied is sequestosome 1 SQSTM1 or p62, hereafter referred to as p Essential for its function as an autophagy receptor is its ability to interact with ubiquitinated cargo and LC3B [ 18 ].
During this process p62 itself is constantly degraded. Reduced levels of p62 are therefore associated with an activated autophagy pathway [ 15 ]. Both LC3 and p62 are frequently used as markers to assess autophagy [ 15 ].
Although autophagy is a flux, and should ideally be measured in functional assays, immunohistochemistry is the method of choice for tissue based retrospective analysis of large cohorts. Thereby, dot-like staining of LC3 serves as a surrogate marker for autophagic vesicles. As p62 is constantly degraded in autolysosomes, it is a surrogate marker for autophagic degradation.
In NSCLC, there are only very few studies on the prognostic significance of the expression of autophagy markers, and all have been conducted in broad stage collectives [ 19 , 20 ]. Because the role of autophagy in cancer may be stage dependent, the aim of our present study was to assess autophagy-associated markers in non-metastasized, early-stage NSCLC.
Formalin-fixed and paraffin embedded FFPE tissue of primary resected, chemotherapy-naïve, early-stage NSCLC was analyzed for the expression of autophagy associated markers LC3 and p The anti-LC3 antibody from Novus N is known to recognize the LC3 isoforms A and B [ 21 ] the anti-LC3B antibody from Cell Signaling CS is isoform B specific [ 22 ].
LC3 CS dot-like staining could be evaluated in cases: score 0 in cases LC3 N dot-like staining could be evaluated in cases and was observed as score 0 in cases Stone-like structures SLS [ 20 ] were present in 8 cases 1.
For p62 dot-like staining, punches were suitable for evaluation, and cases SLS were present in 14 cases 3. Cytoplasmic staining of p62 was absent score 0 in 82 cases Nuclear staining was absent in cases Examples for p62 cytoplasmic, dot-like, nuclear and SLS staining are shown in Figure 1B.
Assessment of staining heterogeneity was performed in 38 exemplary cases, using 8 punches from two tissue blocks of each tumor. Figure 1: Immunohistochemical staining of LC3 and p LC3 Novus staining patterns of the 22 cases further analysed by immunoblot analysis figure 2.
Results of dot-like staining patterns are given in the upper panels of figure 2A and B for each case. Examples of p62 staining, with cytoplasmic, dot-like, nuclear and SLS staining patterns. original magnification x, scale bar µm; insets: original magnification x, scale bar 20 µm.
During the process of autophagosome formation LC3 gets cleaved and lipidated before incorporation into the membrane. This modification enables to distinguish between cytosolic LC3 LC3-I and membrane-bound LC3 LC3-II on a western blot [ 23 ].
Immunoblot analysis of 22 cases selected according to absent and strongly present LC3 dot-like staining revealed the feasibility of this methodology for LC3 evaluation in FFPE tissue. The results correlated mostly with the immunohistochemical staining patterns as depicted in Figure 1 versus 2.
Importantly, both LC3 antibodies from Novus and Cell Signaling showed equal results on Western Blot Figure 2.
Figure 2: Immunoblot analysis of LC3 in protein extracts from FFPE tissue from 22 early-stage non-small cell lung carcinomas. Immunoblot analysis of LC3-I cytosolic and LC3-II membrane-bound of cases 1 to 12, and B. cases 13 to 22, using anti-LC3 Novus middle panel and anti-LC3B Cell Signaling bottom panel primary antibodies.
Total protein was visualised and used as loading control. Immunohistochemistry scores for anti-LC3 Novus and anti-LC3B Cell Signaling primary antibodies are shown in the tables upper panel.
For p62 dot-like staining score 0 was classified as low, scores 1, 2 and 3 were interpreted as high. For p62 cytoplasmic staining scores 0 and 1 were classified as low and score 2 as high. There was no association with age median , pT category or stage Table 1. There was no significant association with the abovementioned factors for p62 dot-like staining or the presence of LC3 positive or p62 positive SLS.
Survival data was available for patients. None of the patients with high LC3 CS dot-like staining relapsed or died, but short follow up times in this sub-group preclude any conclusions and further analyses.
The presence of SLS was not associated with survival. For p62 cytoplasmic and nuclear staining, subgroups were built according to a different recently published paper [ 26 ]. Table 3: Multivariate analysis for tumor related overall survival.
Figure 3: Survival analysis. Kaplan Meier curves for tumour related overall survival assessed for A. LC3 and B. p62 dot-like staining, C. cytoplasmic and D.
nuclear p62 staining, E. We investigated a homogeneous, large early-stage NSCLC cohort of patients for the expression of the autophagy markers LC3 and p62, using a previously validated immunohistochemical protocol [ 24 ]. The observed differential expression of both markers points towards a biologically significant role in NSCLC, although drawing conclusions from the staining patterns on specific disruptions of the autophagy mechanism in the tumors is not clear-cut.
A trend for better tumor related OS was seen in tumors expressing high dot-like staining of both LC3 and p62, which in a simplified model could imply impairment at late stages of the autophagy cascade.
The low number of tumors expressing high LC3 in our cohort may have precluded statistical significance although the results may be valid and important. Corroborating studies are therefore justified. Contrary to the reported adverse prognostic significance of SLS in the SqCC subgroup of NSCLC, using antibodies to the LC3 isoform LC3A [ 20 ], detection of SLS was rare and not associated with outcome in our cohort.
p62 serves as a link between LC3B and ubiquitinated substrates to be degraded in autolysosomes, rendering it a surrogate marker for degradation [ 15 ].
Those staining patterns had similarly unfavorable prognostic impact in our collective. However, it is questionable if drawing conclusions from steady-state levels of autophagy markers on the functional state of autophagic activity is a valid approach.
As very recently updated, the methodology to assess this highly dynamic process on human FFPE tissue still needs significant improvement [ 15 ]. LC3 puncta may mean activated autophagy, but also impaired autophagy due to a late stage block. Second, all autophagy markers may be associated with non-autophagic structures.
This is in line with Inoue et al. Similar correlations of high cytoplasmic p62 expression and adverse clinical features were also found in other cancer types, such as breast cancer [ 27 , 28 ], prostate cancer [ 29 ] and oral SqCC [ 25 ].
It is important to note, that this cytoplasmic and nuclear expression of p62 may not necessarily be linked to autophagy. One alternative candidate effector may be the NRF2-KEAP1-pathway.
Several groups could show that high levels of p62 lead to Nrf2 stabilization and subsequent transcription of genes with an antioxidant function [ 30 ].
Importantly, persistent activation of Nrf2 via p62 stabilization contributes to tumor progression [ 31 ]. However, in the study by Inoue et al. accumulation of p62 did not necessarily result in the stabilization of NRF2 [ 19 ].
Because increased p62 was shown to lead to NFκB-activation [ 32 , 33 ], another explanation for worse outcome may be the potentiation of NFκB-dependent transcription via p62 [ 19 ]. Whereas the cytoplasmic function of p62 is well studied its nuclear function remains less clear.
It has been shown that p62 is a protein able to rapidly shuttle between nucleus and cytoplasm, although its preferential localization under homeostatic conditions is cytoplasmic.
In the nucleus p62 strongly co-localizes with PML-bodies and is probably involved in delivering ubiquitinated proteins for degradation [ 34 ].
Furthermore, p62 is involved in recruiting ALFY, a crucial factor for autophagic degradation of protein aggregates, from the nucleus to the cytoplasm [ 35 ]. It might well be that p62 is involved in recruiting other proteins to the cytoplasm as well.
It remains to be investigated whether accumulation of p62 in the nucleus is cancer cell-specific, and if so, what functional consequences it may have. Mislocalization of proteins in cancer cells is seen quite often.
The best known is probably the predominant nuclear localization of NFκB in many cancer types [ 36 ]. In summary, this is the largest study to date reporting the expression of autophagy related markers LC3 and p62 in a well-defined, early-stage NSCLC cohort of cases.
We report the correlation of dot-like immunohistochemical staining for LC3 with LC3-II protein expression assessed by immunoblot analysis of the same FFPE archival cases, corroborating the feasibility to assess autophagy structures using immunohistochemistry.
We observed a trend for better outcome in tumors with high dot-like staining of LC3 and p62, being surrogates for autophagic vacuoles and thus the process of autophagy, although low numbers of this subgroup might have precluded statistical significance. Our results warrant further investigations concerning the link between expression data and functional autophagy states and a possible non-autophagy related role of p62 in NSCLC.
The retrospective study included patients with primary resected node-negative early-stage NSCLC UICC 7 th edition stage IA-IIB [ 37 ], treated with curative surgery and diagnosed at the Institute of Pathology, University of Bern, Switzerland and the Institute of Pathology, University Hospital Basel, Switzerland between January and August The detailed staging work-up for this cohort of patients has already been reported [ 38 ].
After exclusion of cases with lymph node metastases, neoadjuvant treatment, rare tumor types other than AC, SqCC and LCC, and insufficient tumor tissue for further analysis, patients were finally included. This retrospective study was approved by the local ethics committee. Median age of the patient cohort was 67 years range; years.
The detailed distribution of clinic-pathologic characteristics can be seen in Tables 1 and 2. A tissue microarray TMA was constructed as reported elsewhere [ 38 ]. In short, formalin fixed paraffin embedded FFPE tissue blocks were retrieved from the archives of the Institutes of Pathology.
One punch diameter 0. To assess for staining heterogeneity, 38 patients with positive and negative LC3 staining patterns were selected from the Bern sub-cohort.
A TMA was constructed as reported elsewhere [ 39 ], using 8 cores diameter 0. Additional punches 4 x 1 mm per tumor were taken for protein extraction. Immunohistochemical staining was performed using the automated system BOND RX Leica Biosystems, Newcastle, UK.
TMA sections were cut at 4 μm, deparaffinized and rehydrated in dewax solution Leica Biosystems. Endogenous peroxidase activity was blocked with H 2 O 2 solution for 4 minutes. All samples were incubated with the following primary antibodies for 30 minutes at room temperature RT , as described before [ 24 ]: LC3B from Cell Signaling Technology MA, USA, , clone D11, dilution , LC3 from Novus Biologicals Cambridge, UK, NB, dilution using Tris buffer pH 9 at 95°C for 30 minutes for antigen retrieval, p62 from MBL IL, USA, PM, dilution using Citrate buffer pH 6.
Read out of stainings was performed by AMS and SB as established before [ 24 ], and discrepancies were discussed on a multi-header microscope to gain a final consensus. Stone like structures SLS [ 20 ] were recorded separately. Cytoplasmic p62 staining was scored as: 0 - no or very faint staining, 1 - weak staining, 2 - moderate to strong staining.
Nuclear p62 staining was recorded as present or absent. Images were acquired using a Zeiss Axioplan 2 microscope objective magnification 40 x and x and Axiovision software. The samples were deparaffinized and rehydrated using Xylene followed by descending alcohol series.
Samples were centrifuged and supernatant transferred to a new tube. Protein concentration was determined using the Bradford protein assay BioRad, Cressier, Switzerland. Total protein was visualized as loading control. Membranes were incubated with primary antibodies over night at 4°C with shaking.
Prior to visualization, membranes were incubated with Clarity Western ECL Substrate BioRad for 5 minutes at RT with shaking.
Descriptive and comparative statistical analyses were performed using the SPSS 23 software SPSS Inc, Chicago, IL, USA. Survival analysis was performed using log rank test and Cox regression analysis. The significance level was set at 0. The authors gratefully acknowledge the Translational Research Unit of the Institute of Pathology, University of Bern, for excellent technical support.
The work was supported by grants from Bernische Krebsliga to SB , Swiss Cancer League to RL, KLS and Stiftung für Klinisch-Experimentelle Tumorforschung Bern to MPT. Travis WD, Brambilla E, Burke AP, Marx A and Nicholson AG.
WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. In: Bosman FT, Jaffe ES, Lakhani SR and Ohgaki H, eds. World Health Organization Classification of Tumours.
Lyon: International Agency for Research on Cancer IARC. Kuma A and Mizushima N. Physiological role of autophagy as an intracellular recycling system: with an emphasis on nutrient metabolism. Semin Cell Dev Biol. Feng Y, He D, Yao Z and Klionsky DJ.
The machinery of macroautophagy. Cell Res. Deretic V. Autophagy in immunity and cell-autonomous defense against intracellular microbes. Immunol Rev. White E. The role for autophagy in cancer. J Clin Invest. Rao S, Tortola L, Perlot T, Wirnsberger G, Novatchkova M, Nitsch R, Sykacek P, Frank L, Schramek D, Komnenovic V, Sigl V, Aumayr K, Schmauss G, Fellner N, Handschuh S, Glosmann M, et al.
A dual role for autophagy in a murine model of lung cancer. Nat Commun. Moreover, it is known to link the cellular degradation pathways, ubiquitin system, and autophagic machinery 5 , 6 , 7.
In vertebrates, p62 regulates the autophagic removal of protein aggregates and damaged organelles, including mitochondria, through its interaction with ubiquitin and the LC3 component of autophagy 8 , 9.
Although it is recognized to play an essential role in mediating selective autophagy, p62 is required for the unselected autophagic process, and its loss inhibits resveratrol RSV —induced autophagy ST is a multitargeted receptor tyrosine kinase inhibitor, and it inhibits the activity of PDGFRs, c-KIT, FLT-3, and VEGFRs, all of which have been demonstrated to be important for cell proliferation, migration, and angiogenesis ST can induce both cell viability loss and cell senescence, and it can cause G1-S cell cycle arrest and the DNA damage response in OS-RC-2 cells 12 , Recently, ST has been demonstrated to mediate autophagy depending on the cell type.
In both cardiac and PC12 cells, ST increases autophagic flux, whereas it induces incomplete autophagy in either renal or bladder cancer cells. In RCC O cells, ST increases the level of phosphorylated EGFR, which may cause resistance to ST treatment in RCC In endometrial carcinoma, ST decreases either the basal or EGF-activated NFkB transcription Additionally, ST has been demonstrated to inhibit AMPK and cause myocyte cytotoxicity 16 , AMPK, a key energy-surveillance kinase complex, contains three subunits: a catalytic, one scaffolding and a regulatory.
Energy stress increased the levels of either ADP or AMP compared with ATP , and their augment functioned as an index of energy deprivation. AMPK was firstly found to be a kinase that directly inhibited ACC through increasing its phosphorylation; it also functions in multiple ways to influence cellular metabolism, and its activation is upregulated responsive to various stress conditions with an increased ratio of AMP to ATP.
Through enhancing Thr phosphorylation of the catalytic subunit and inhibiting dephosphorylation of Thr, AMP binding was found to increase the activity of AMPK.
Mammalian target of rapamycin complex 1 mTORC1 is a multiprotein complex consisting of mTOR, raptor, mLST8, and PRAS40, and AMPK demonstrated to inhibit mTORC1 via activating tuberous sclerosis complex 2 TSC2 and directly reducing the phosphorylation of raptor 18 , However, Shang et al.
observed that nutrient starvation simulates the autophagic response mediated by ULK1 dephosphorylation and its dissociation from AMPK They further suggested that AMPK might have dual roles in the regulation of autophagy depending on the nutrient condition. Indeed, compound C, a pharmacological AMPK inhibitor that efficiently blocks the metabolic actions of AMPK, has been demonstrated to induce the autophagic process in different cancer cell lines Here, we show that ST treatment alone can either inhibit or induce autophagy depending on the cell type and its concentration.
ST was demonstrated to inhibit AMPK activity, upregulated p62 expression and abolished the H 2 O 2 -induced autophagic flux. While deprivation of p62 reversed the inhibitory effect of ST on basal autophagy, it no longer blocked the H 2 O 2 -activated autophagic flux in pdepleted cells.
As an approved drug for RCC treatment 24 , ST expectedly reduced the cell viability of both O and ACHN in a dose-dependent manner Fig.
The cytotoxic effect of ST was further confirmed by the observation that the cleavage of PARP-1, which serves as a marker of cells undergoing apoptosis 25 , was induced by ST Fig. Moreover, deprivation of either LC3 or Beclin 1 increased the cleavage of PARP-1 Fig. S1c—e , suggesting that autophagy is involved in the ST-induced apoptotic process.
Two different LC3 or Beclin 1 small interfering RNAs siRNAs were used to independently knock down the expression of either LC3 or Beclin 1 Fig. In addition, p62, a substrate of autophagic degradation with increased expression during autophagy inhibition 28 , appeared to be upregulated in both LC3- and Beclin 1-depleted cells Fig.
Recent studies have revealed contradictory results about the involvement of ST in autophagy 29 ; thus, we subsequently examined the autophagic flux upon ST challenge in both O and ACHN cells. Through electron microscopy, we observed an obvious accrual of membrane vacuoles in either ST-treated ACHN or O cells, and cytosolic components were sequestered in some of those vacuoles in comparison to the control Fig.
The immunoblotting analysis revealed that ST treatment increased the ratio of LC3-II to actin relative to control cells in a concentration-dependent manner Fig.
In contrast to ACHN cells, in which it either increased or decreased the level of p62 Fig. Similar to ACHN cells, ST could increase or decrease the level of p62 in HeLa cells.
The aforementioned results indicated that ST regulated autophagy in a dose- and cell type-dependent manner, and it could inhibit the autophagic flux under certain conditions. A high dose of ST inhibits the autophagic flux and increases p62 expression.
The morphometric analysis of the area fraction between autophagosomes and cytoplasm was calculated using Photoshop software c. The area ratio data were non-normally distributed and are presented as the mean of at least 10 cells counted for each group. Densitometry was performed for quantification, and the adjusted ratio of LC3-II and p62 to actin is presented under the blots.
The results were similar among experiments repeated at least twice. Control: Ctrl; Beclin1: Bec1; Actin: A. Former studies have been revealed that ST inhibits the phosphorylation of AMPK and causes myocyte cytotoxicity 16 , Consistently, we observed that ST markedly decreased AMPK phosphorylation in all three tested cell lines Figs 2a , S2a.
In contrast, H 2 O 2 markedly increased the phosphorylation of ACC, the AMPK substrate and indicator of AMPK activity 30 Figs 2b , S2b , and ST abolished the H 2 O 2 -induced phosphorylation of ACC Figs 2b,e , S2b,e.
To investigate the inhibitory mechanism of ST on autophagy, we determined the autophagic flux following treatment of the cells with H 2 O 2 in the presence or absence of ST. Although either H 2 O 2 or ST induced autophagy, their combination failed to further stimulate the autophagic process in ACHN cells Fig.
In HeLa cells, ST also completely inhibited H 2 O 2 -induced autophagy Fig. The 5-aminoimidazolecarboxamide1-β-D-ribofuranoside AICAR , an agonist of AMP-activated protein kinase AMPK , enhanced H 2 O 2 -induced autophagy in O cells Fig.
Notably, ST abolished H 2 O 2 -activated phosphorylation of ACC Figs 2e,f , S2d , whereas AICAR increased the activity of AMPK Fig. Given that ST alone can activate autophagy, ST is likely to play a dual role in the regulation of autophagy in a dose-, cell type- and context-dependent manner.
ST inhibits the phosphorylation of AMPK and ACC and blocks H 2 O 2 -induced autophagy concurrent with the downregulation of AMPK activity. The results were similar among experiments repeated at least three times. ACHN c , e and O d , f cells were treated with H 2 O 2 ACHN: 0.
Actin was used as a loading control. Actin: A. Generally, p62 is considered to be a substrate in autophagic degradation, and the activation of autophagy usually causes a decrease in the p62 level. In comparison to ACHN cells, ST consistently increased the expression of p62 in O cells Fig.
The immunostaining assay revealed that ST increased punctate LC3 staining, which colocalized well with p62, and CQ addition further increased punctate LC3 staining in ST-treated cells and enlarged the dot staining of p62 Fig. Consistent with a former report 31 , we observed a direct interaction between LC3 and p62 Fig.
Moreover, the immunoprecipitation results displayed that either ST or H 2 O 2 regulated the interaction between LC3 and p62 Fig. ST increases the expression of p62 in O cells.
e IgG2b rabbit was used as a negative control. ACHN and O cells were lysed and precipitated using the antibody against LC3. L-Exp: long exposure; HC: heavy chain; LC: light chain; Actin: A; Input: whole cell lysate. Since the knockdown of p62 was shown to inhibit resveratrol RSV —induced autophagy 10 and ST increased the levels of p62, we speculated that p62 might be required for ST-activated autophagy.
Similar results were also obtained in pdepleted ACHN and HeLa cells Fig. Deprivation of p62 reverses the inhibition of ST-induced autophagy.
HEKT cells with either wild type WT or mutated p62 plasmid d. Actin was used as the loading control. The results were similar among experiments repeated three times.
L-Exp: long exposure; Actin: A. To confirm the inhibitory effect of p62 on ST-induced autophagy, we transfected HEKT cells with either wild type WT or mutated p62 plasmid Fig. As expected, overexpression of WT p62 completely inhibited the ST-induced autophagic flux, as CQ failed to accumulate LC3-II in these cells Fig.
As the depletion of p62 could reverse the inhibitory effect of ST on autophagy, we next determined whether p62 also played a regulatory role in the inhibitory effect of ST on H 2 O 2 -induced autophagy. Compared with the Mock-control, H 2 O 2 -induced autophagic flux was greatly inhibited in pdepleted O cells Fig.
In contrast to the Mock-control, ST failed to completely inhibit the H 2 O 2 -activated autophagic flux in pdepleted O cells Fig.
Similar results were also obtained in HeLa cells Fig. Notably, H 2 O 2 combined with ST induced normal autophagy in pdeprived HeLa cells compared with the Mock-control ones Fig.
Using a different siRNA, we obtained similar results in O cells Fig. Moreover, we observed that ST was able to abolish H 2 O 2 -increased phosphorylation of ACC in either Mock-control or pdepleted cells Fig.
In pdepleted HeLa cells, H 2 O 2 at a dose of 0. Together with the findings shown in Fig. In O cells, the deprivation of p62 decreased basal phosphorylation of ACC Fig.
Uncoupling between the phosphorylation of AMPK and ACC was clearly present. For example, while ST blocked the H 2 O 2 -induced phosphorylation of ACC in both cell lines Fig. Depletion of p62 inhibits H 2 O 2 -induced autophagy and reverses the inhibitory effect of ST on H 2 O 2 -dependent autophagic flux.
The results were similar among experiments repeated twice. As shown in Fig. Consistent with the aforementioned results Fig. Although U attenuated the phosphorylation of ACC Fig. Not only U alone increased the levels of p62 but it further increased the levels of p62 in ST-treated cells Fig.
Although CQ alone increased the levels of p62 Fig. L-Exp: long exposure; HC: heavy chain; Actin: A. A new finding of the present study is that ST can either inhibit or induce autophagy even in the same cell line, depending on the expression of p Although ST was shown to suppress the induced phosphorylation of ACC, it failed to block H 2 O 2 -induced autophagic flux in pdepleted cells.
Therefore, p62 likely played a regulatory role in the inhibitory effect of activated AMPK on autophagy. It is well established that AMPK triggers autophagy through an indirect mechanism by inhibiting mTORC1 signaling or through direct binding to ULK1 20 , AMPK has also been reported to inhibit autophagy in isolated rat hepatocytes.
Shang and Wang et al. revealed that nutrient starvation induces an acute autophagic process through ULK1 dephosphorylation and its dissociation from AMPK. They considered AMPK to have dual roles in the regulation of autophagy. Moreover, ST inhibited both H 2 O 2 -induced AMPK activity and autophagy.
The aforementioned results confirmed that AMPK could have dual roles in regulating the autophagic process. Unexpectedly, we found that p62 loss reversed the inhibitory effect of ST on basal and H 2 O 2 -induced autophagy. Therefore, we reasoned that p62 was required for the activated AMPK to inhibit autophagy under certain conditions.
Despite its common use as a substrate in autophagy, growing evidence has indicated that p62 plays more active roles in the regulation of autophagy It has been demonstrated that p62 is required for RSV-induced autophagy, and RSV increases the expression of p62 mRNA and protein by mediating the activation of JNK c-Jun N-terminal kinase In addition, it has been reported that, to prevent oxidative liver damage, pdependent autophagy is required during the activation of Nrf2 nuclear factor erythroid 2 by sestrins Consistently, we found that p62 was needed for H 2 O 2 to stimulate the autophagic process, especially in O cells.
Thus, p62 was able to regulate H 2 O 2 -induced autophagy relative to the stimulus dose. In contrast, p62 was unlikely to be required for ST-dependent autophagy, in which it actually appeared to play a negative regulatory role. Consequently, p62 could regulate autophagy in a stimulus- and cell-type dependent manner.
However, other studies have shown that oncogenic activation of Ras, the upstream activator of ERK, reduces autophagy in transformed cell lines 45 , Our data showed that U alone failed to inhibit the autophagic flux, whereas its treatment blocked or attenuated autophagy induced by either ST or H 2 O 2.
Therefore, we speculated that a coordination might exist between AMPK and MAPK in the regulation of autophagy. This phenomenon is often found in various signaling pathways. For example, it is well known that Ras-ERK and PI3K-mTOR signaling can be either interplayed or compensated While AMPK enhances autophagy via directly phosphorylating ULK1 48 , however, a recent study has revealed that, through a negative feedback loop, the latter demonstrated to influence AMPK phosphorylation Additionally, one study showed that AMPK was able to either inhibit or enhance the PI3K signaling In contrast to former reports 34 , we failed to observe p62 pulled down with phosphorylated ERK; instead, p62 interacted almost equally with both ERK1 and ERK2 in O cells.
In summary, we show that p62 is responsible for the inhibition of ST in basal and H 2 O 2 -induced autophagy and reveal a previously unknown link between the activation of AMPK and the inhibition of autophagy.
Future work in this direction will enable us to better understand the regulatory mechanism of autophagy, illuminate the molecular mechanism of ST resistance in cancer treatment and provide a resolution to dealing with the devastating effects of anti-angiogenesis resistance.
Sunitinib ST; S was purchased from Aladdin Seattle, WA, USA. Louis, MO, USA. H 2 O 2 E was purchased from Amresco WA, USA. The antibody against p62 AP was bought from Proteintech Wuhan, Hubei, China.
The antibody against actin TA was acquired from ZhongShanJinQiao Biocompany Beijing, China. MTS reagent powder G was obtained from Promega Corporation Madision, WI, USA. Immunoblotting was performed using appropriate primary antibodies and horseradish peroxidase-conjugated suitable secondary antibodies, followed by detection with enhanced chemiluminescence Pierce Chemical Rockford, IL, USA 51 , 52 , Immunoprecipitates and cell lysates were electrophoresed on SDS-PAGE and subjected to immunoblotting analysis After overnight culturing, cells were replaced with Phenol red free complete medium which was added with either drug-free or ST or other chemicals.
Cells were cultured for indicated period and the cell viability was detected by CellTiter 96 Aqueous Non-Radioactive Cell Proliferation Assay Promega 51 , Wash the samples three times with PBS, trypsinize, and then centrifuge to collect them.
Finally, use a transmission electron microscope JEM Akishima, Tokyo, Japan to observe the images of the thin sections 51 , Primer sequences used for amplification were as follows Table 1 :. for up to 40 cycles. The data were calculated based on the internal control of β-actin. Cells were plated on glass cover slips and the indicated treatments were performed.
Images were acquired via Fluorescence microscopy Zeiss Heidenheim, Germany The images were analyzed to verify the linear range of chemiluminescence signals and quantifications were carried out using densitometry. Klionsky, D. A human autophagy interaction network.
Article PubMed Google Scholar. Liu, S. Progress of study on p62 and protein degradation pathways. Sheng Li Xue Bao 67 , Duran, A.
et al. p62 is a key regulator of nutrient sensing in the mTORC1 pathway. Article CAS PubMed PubMed Central Google Scholar. Linares, J. K63 polyubiquitination and activation of mTOR by the pTRAF6 complex in nutrient-activated cells. Bitto, A. Article CAS Google Scholar.
Lin, X. Interaction domains of p a bridge between p62 and selective autophagy. Article ADS CAS PubMed Google Scholar. Lippai, M. The role of the selective adaptor p62 and ubiquitin-like proteins in autophagy.
Article PubMed PubMed Central Google Scholar. Johansen, T. Selective autophagy mediated by autophagic adapter proteins. Autophagy 7 , — Pankiv, S. M Article CAS PubMed Google Scholar. Puissant, A. CAN Schmid, T. Sunitinib in the treatment of metastatic renal cell carcinoma.
Teng, C. Effector mechanisms of sunitinib-induced G1 cell cycle arrest, differentiation, and apoptosis in human acute myeloid leukaemia HL60 and KG-1 cells. Zhu, Y. Mizumoto, A. Induction of epithelial-mesenchymal transition via activation of epidermal growth factor receptor contributes to sunitinib resistance in human renal cell carcinoma cell lines.
Sorolla, A. Blockade of NFkappaB activity by Sunitinib increases cell death in Bortezomib-treated endometrial carcinoma cells. Kerkela, R. Sunitinib-induced cardiotoxicity is mediated by off-target inhibition of AMP-activated protein kinase.
x Laderoute, K. Cancer Biol Ther 10 , 68—76 Gwinn, D. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Inoki, K. TSC2 mediates cellular energy response to control cell growth and survival. Cell , — Egan, D. The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR.
Kim, J. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Shang, L. Nutrient starvation elicits an acute autophagic response mediated by Ulk1 dephosphorylation and its subsequent dissociation from AMPK.
Article ADS PubMed PubMed Central Google Scholar. Vucicevic, L. Autophagy 7 , 40—50 Giuliano, S. Resistance to sunitinib in renal clear cell carcinoma results from sequestration in lysosomes and inhibition of the autophagic flux.
Autopahgy a p/SSTM oncology-focused, peer-reviewed, pSQSTM access journal Weight loss journal to Autophqgy research Autpohagy through insightful peer-review; eliminate borders between specialties by linking different Aitophagy Autophagy and p/SQSTM oncology, cancer research and biomedical sciences; and foster application of basic and clinical science. Its scope is unique. The term "oncotarget" encompasses all molecules, pathways, cellular functions, cell types, and even tissues that can be viewed as targets relevant to cancer as well as other diseases. The term was introduced in the inaugural EditorialIntroducing Oncotarget. As of January 1,Oncotarget has shifted to a continuous publishing model. Papers will now be published continuously within yearly volumes in their final and complete form and then quickly released to Pubmed.Address correspondence and reprint requests to Dr. John PS/QSTM. Brumell, Cell Biology P/SQSMT, Hospital for Sick Children, Auophagy Avenue, Toronto, Autophqgy M5G 1X8, Canada. E-mail address: Nutritional requirements for aging athletes. brumell sickkids.
Yiyu T. ZhengShahab ShahnazariAndreas Brech p/SSQTM, Trond LamarkTerje AutophafyJohn Autophay. J Immunol 1 November ; 9 Autophagy and p/SQSTM Autopuagy Autophagy, Autophag cellular Autophzgy pathway, plays a Autophay role /pSQSTM protecting the cytosol from bacterial colonization, but the mechanisms Autphagy bacterial recognition by this Auto;hagy are unclear.
Autophagy Autophagy and p/SQSTM also known to degrade cargo tagged by ubiquitinated Blood sugar maintenance, including p/SSQSTM of misfolded proteins, and Autohagy. Autophagy Autophagh ubiquitinated cargo Aktophagy p62 also known as SQSTM1an adaptor Aitophagy with multiple ane interaction domains, including a ubiquitin-associated UBA domain for ubiquitinated cargo Auotphagy and Autophagy and p/SQSTM LC3 interaction region LIR pSQSTM binding the autophagy protein LC3.
Previous studies demonstrated that the Autpphagy bacterial Autophayg Salmonella typhimurium is targeted by Autophagh during infection of host cells. Here we /pSQSTM that p62 is recruited Autkphagy S.
typhimurium targeted by Daily mineral intake, and that Fostering regular waste elimination recruitment of adn is associated with ubiquitinated proteins localized to Autophwgy bacteria.
Expression of p62 is p/QSSTM for Autophagg autophagy of /pSQSTM, as well as ;/SQSTM of Autopbagy intracellular replication.
Our studies demonstrate Autophagy and p/SQSTM Autophaggy surveillance of misfolded Autophgay and bacteria xnd via a conserved pathway, Eliminate sugar cravings they p/SSQTM a Augophagy function for Autophagu in innate immunity.
Autiphagy surveillance for intracellular bacteria in mammalian cells plays Autophagj crucial role in cell Autopphagy and host defense.
Recently, macroautophagy hereafter referred Aufophagy as autophagyan important cellular Auttophagy for the degradation of long-lived proteins and damaged organelles by delivering them Metabolism Boosting Herbs the lysosome 12 Autoohagy, was qnd to be involved in the innate immune response Autophagy and p/SQSTM bacteria Cellulite elimination solutions3.
Autophagy is aand to target bacteria early after invasion and protect the Autpphagy from bacterial colonization 4 ahd, 5. However, the mechanisms by which bacteria ane targeted by autophagy are not known 6. Salmonella enterica serovar Xnd S. typhimurium 3 is a Gram-negative bacterial pathogen that can infect a variety Positive psychology approaches hosts Aytophagy.
Following S. typhimurium invasion, Autophagh bacteria reside Aitophagy replicate within intracellular compartments termed Salmonella -containing vacuoles SCVs 8. Autophagy p/SQSMT found to restrict the intracellular growth of S.
typhimurium 5. Recently, Protein shakes for athletes was p/SQSTMM to directly ane ubiquitinated Autophhagy via its C-terminal ubiquitin-associated UBA domain and Insulin injections in children via a newly identified LC3 interacting region LIR 121314Autophagy and p/SQSTM, Autophgy It has been suggested that p62 AAutophagy as an Autophagy and p/SQSTM Oral cancer prevention binding ubiquitinated Aufophagy aggregates Auyophagy targeting them Autlphagy degradation by autophagy, therefore protecting the cytosol from the toxic effects of misfolded or mutated proteins 1013 In addition to ubiquitinated protein aggregates, peroxisomes labeled with ubiquitinated proteins were recently found to be degraded by autophagy in a pdependent manner Therefore, p62 plays an important role in cellular homeostasis by mediating the autophagy of cargo of various sizes tagged with ubiquitinated proteins.
Since S. typhimurium targeted by autophagy is often associated with ubiquitinated proteins 5we hypothesized that p62 may play an adaptor role in the process of autophagy of bacteria.
Wild-type S. typhimurium SL and bacteria expressing red fluorescence protein RFP 17 were used for these studies.
For invasion by S. typhimuriumlate-log bacterial cultures were used for infecting cells and prepared via a method optimized for bacterial invasion HeLa human epithelial cells were obtained from the American Type Culture Collection.
p62 no. M and siGenome nontargeting siRNA pool no. D were from Dharmacon. Successful p62 knockdown was confirmed by Western blot for each experiment. To construct the GFP-SR-pΔUBA plasmid pENTR-pSR-ΔUBA was first made by exchanging a bp Nhe I- Eco RV fragment in pENTR-pΔUBA 10 with a similar fragment from pENTR-pSR Finally, GFP-pSR-ΔUBA was made by a Gateway LR reaction into pDestEGFP-C1 Cells were fixed in 2.
Fixed cells were stained as previously described Mouse mAb to p62 was from BD Biosciences; rabbit polyclonal Ab to p62 was from Santa Cruz Biotechnology. Rabbit polyclonal Ab to S. typhimurium Salmonella O anti-serum group B factors 1, 4, 5, and 12 was from Difco Laboratories.
FK2 mAb was from BIOMOL. For some experiments, FK2 Ab was conjugated to Alexa Fluor by using the Zenon Alexa Fluor mouse IgG labeling kit Molecular Probes. Samples were analyzed using a Zeiss Axiovert microscope ×63 objective and LSM software.
Confocal images were imported into Adobe Photoshop and assembled in Adobe Illustrator for labeling. Colocalization quantifications were performed using a Leica DMIRE2 epifluorescence microscope.
Immunolabeling was essentially performed as described earlier 22using mouse mAbs against p62 BD Biosciences and Ab to S. typhimurium Salmonella O anti-serum group B factors 1, 4, 5, and 12; Difco Laboratories followed by protein A-gold of 10 nm and 15 nm CMC. We used a secondary bridging R-anti-M Ab Dako as intermediate after the monoclonal p62 Ab.
Glutaraldehyde 0. Specimens were observed in a JEOL JEM at 60—80 kV and images were recorded with a digital camera Moradausing iTEM software both from Soft Imaging System.
Further image processing was performed using Adobe Photoshop CS2 software. Colocalization quantifications were performed by direct visualization on a Leica DMIRE2 epifluorescence microscope. At least bacteria were counted for each condition in each experiment.
At least three independent experiments were performed. p62 colocalizes with a population of S. typhimurium following invasion. AHeLa cells were infected with wild-type S. typhimurium and fixed at 1 h p. Cells were then coimmunostained with FK2 mAb to ubiquitinated proteins labeled with Zenon Alexa Fluor mouse IgG labeling kit, see Materials and Methodsa polyclonal Ab to S.
typhimuriumand a mAb to p62 as indicated. Insets show higher magnification of the boxed areas. Size bar, 10 μm. BCells were infected with wild-type S. typhimurium expressing RFP. Cells were fixed at the indicated time and stained with an Ab to p At least bacteria were counted for each time point.
The average ± SD is shown for three independent experiments. CCells were transfected with RFP-LC3, then infected and fixed as in A ; cells were then coimmunostained with Abs to p62 and to S.
At least bacteria were counted for each condition. p62 is associated with bacteria targeted by autophagy.
AMEFs were infected with S. typhimuriumfixed at 1 h p. Shown is a representative image of a bacterium within a multilamellar structure consistent with autophagy. The bottom panes is a magnified image of the boxed area in the upper panels.
Arrows indicate multilamellar membrane structures surrounding bacteria. Size bars, nm. Shown is a representative image of a bacterium within a single membrane SCV. The bottom panel is a magnified image of the boxed area in the upper panel. Open arrows indicate the single membrane of this compartment.
The percentages of bacteria in different compartments were enumerated using transmission EM. DCells were infected for 1 h and processed for immunogold EM. p62 open arrowheads is localized in the multilamellar structures arrows surrounding the bacterium.
Size bar, nm. EDouble immunogold EM indicating p62 10 nm gold, open arrowheads located mainly between the bacterium labeled by 15 nm gold, filled arrowheads and multilamellar membranes arrowsas well as within the multilamellar membranes.
Some p62 labeling also localized to the bacterial surface. Image on the right is a magnified image of boxed area at left. p62 association with S. typhimurium can occur independently of LC3 recruitment to the bacteria.
typhimurium expressing RFP and fixed at 1 h or 4 h p. Cells were immunostained with a polyclonal Ab to p Inset shows a higher magnification of the boxed areas.
: Autophagy and p/SQSTM| 1 Introduction | Genes Dev. Sou, T. Understanding biochemistry: basic aspects of statistics for life sciences. Harald Stenmark. Sign In. C and D The amount of p62 located to cytoplasmic bodies increases upon inhibition of autophagy. |
| Materials and Methods | Wong, S. Anx 77— e Wurzer, B. Truncating mutations of RB1CC1 in human breast cancer. p62 and β-actin were defined as forward primer and reverse primer, respectively. |
| MINI REVIEW article | While p62 aggregates with cytoplasmic inclusions containing ubiquitinated proteins, nuclear p62 associates with nuclear polyubiquitinated proteins at promyelocytic leukemia PML bodies and accumulates when nuclear export mediated by the exportin XPO1 CRM1 is blocked Pankiv et al. Knockdown of endogenous p62 reduced the levels of S. Article CAS PubMed PubMed Central ADS Google Scholar. View all Kon M , Kiffin R , Koga H , Chapochnick J , Macian F , Varticovski L , Cuervo AM Kon M , Kiffin R , Koga H , Chapochnick J , Macian F , Varticovski L , Cuervo AM. mKalama1-Atg8K26P and Atg8 conjugation enzymes Atg3, Atg7, Atg16, and Atg5-Atg12 conjugate were prepared as previously reported Shaner, N. |
Ich bin mit Ihnen nicht einverstanden
Sie lassen den Fehler zu. Schreiben Sie mir in PM, wir werden umgehen.
Nach meiner Meinung irren Sie sich. Ich kann die Position verteidigen.
Ich entschuldige mich, aber mir ist ganz anderes notwendig. Wer noch, was vorsagen kann?