
Video
4 Most Liver Damaging Supplements (Avoid Over Usage)EGCG and cell regeneration -
Cai Y, Chen ZB, Liu H, Xuan Y, Wang XX, Luan QX. Green tea epigallocatechingallate alleviates Porphyromonas gingivalis-induced periodontitis in mice. Int Immunopharmacol. Tominari T, Ichimaru R, Yoshinouchi S, Matsumoto C, Watanabe K, Hirata M, Grundler FMW, Inada M, Miyaura C. FEBS Open Bio.
Kaida K, Honda Y, Hashimoto Y, Tanaka M, Baba S. Application of green tea catechin for inducing the osteogenic differentiation of human dedifferentiated fat cells in vitro. Int J Mol Sci. Chu C, Deng J, Hou Y, Xiang L, Wu Y, Qu Y, Man Y. Application of PEG and EGCG modified collagen-base membrane to promote osteoblasts proliferation.
Mater Sci Eng C Mater Biol Appl. Shen CL, Yeh JK, Cao JJ, Wang JS. Green tea and bone metabolism. Nutr Res. Sims NA, Vrahnas C. Regulation of cortical and trabecular bone mass by communication between osteoblasts, osteocytes and osteoclasts. Arch Biochem Biophys. Oka Y, Iwai S, Amano H, Irie Y, Yatomi K, Ryu K, Yamada S, Inagaki K, Oguchi K.
Tea polyphenols inhibit rat osteoclast formation and differentiation. J Pharmacol Sci. Nakagawa H, Wachi M, Woo JT, Kato M, Kasai S, Takahashi F, Lee IS, Nagai K. Biochem Biophys Res Commun. Nakagawa H, Hasumi K, Takami M, Aida-Hyugaji S, Woo JT, Nagai K, Ishikawa T, Wachi M. Biochem Pharmacol.
Guo M, Qu H, Xu L, Shi DZ. Tea consumption may decrease the risk of osteoporosis: an updated meta-analysis of observational studies.
Vali B, Rao LG, El-Sohemy A. Epigallocatechingallate increases the formation of mineralized bone nodules by human osteoblast-like cells. J Nutr Biochem. Kamon M, Zhao R, Sakamoto K.
Cell Biol Int. PubMed Google Scholar. Takita H, Kikuchi M, Sato Y, Kuboki Y. Inhibition of BMP-induced ectopic bone formation by an antiangiogenic agent epigallocatechin 3-gallate. Connect Tissue Res. Tian Y, Bai D, Guo WH, Li J, Zeng J, Yang LQ, Jiang ZT, Feng L, Yu M, Tian WD.
Comparison of human dental follicle cells and human periodontal ligament cells for dentin tissue regeneration. Regen Med. Memmert S, Nokhbehsaim M, Damanaki A, Nogueira AVB, Papadopoulou AK, Piperi C, Basdra EK, Rath-Deschner B, Gotz W, Cirelli JA, et al.
Role of cathepsin S in periodontal wound healing-an in vitro study on human PDL cells. BMC Oral Health. Mu S, Guo S, Wang X, Zhan Y, Li Y, Jiang Y, Zhang R, Zhang B. Effects of deferoxamine on the osteogenic differentiation of human periodontal ligament cells.
Mol Med Rep. Norgaard R, Kassem M, Rattan SI. Heat shock-induced enhancement of osteoblastic differentiation of hTERT-immortalized mesenchymal stem cells.
Ann N Acad Sci. Daga D, Mehrotra D, Mohammad S, Singh G, Natu SM. Tentpole technique for bone regeneration in vertically deficient alveolar ridges:a review.
J Oral Biol Craniofac Res. Jin P, Wu HY, Xu GJ, Zheng L, Zhao JM. Epigallocatechingallate EGCG as a pro-osteogenic agent to enhance osteogenic differentiation of mesenchymal stem cells from human bone marrow: an in vitro study. Cell Tissue Res.
Mah YJ, Song JS, Kim SO, Lee JH, Jeon M, Jung UW, Moon SJ, Kim JH, Choi HJ. The effect of epigallocatechingallate EGCG on human alveolar bone cells both in vitro and in vivo.
Jung IH, Lee DE, Yun JH, Cho AR, Kim CS, You YJ, Kim SJ, Choi SH. J Periodontal Implant Sci. Chu CY, Deng J, Man Y, Qu YL. Green tea extracts Epigallocatechingallate for different treatments. Biomed Res Int. Weisburg JH, Weissman DB, Sedaghat T, Babich H.
In vitro cytotoxicity of epigallocatechin gallate and tea extracts to cancerous and normal cells from the human oral cavity. Basic Clin Pharmacol Toxicol. Niu LN, Sun JQ, Li QH, Jiao K, Shen LJ, Wu D, Tay F, Chen JH.
Intrafibrillar-silicified collagen scaffolds enhance the osteogenic capacity of human dental pulp stem cells. J Dent. Braun J, Hack A, Weis-Klemm M, Conrad S, Treml S, Kohler K, Walliser U, Skutella T, Aicher WK.
Evaluation of the osteogenic and chondrogenic differentiation capacities of equine adipose tissue-derived mesenchymal stem cells.
Am J Vet Res. Komori T. Regulation of bone development and extracellular matrix protein genes by RUNX2. Artigas N, Urena C, Rodriguez-Carballo E, Rosa JL, Ventura F. Mitogen-activated protein kinase MAPK -regulated interactions between Osterix and Runx2 are critical for the transcriptional Osteogenic program.
J Biol Chem. Foster BL, Ao M, Salmon CR, Chavez MB, Kolli TN, Tran AB, Chu EY, Kantovitz KR, Yadav M, Narisawa S. Osteopontin regulates dentin and alveolar bone development and mineralization. Bortoluzzi EA, Niu LN, Palani CD, El-Awady AR, Hammond BD, Pei DD, Tian FC, Cutler CW, Pashley DH, Tay FR.
Cytotoxicity and osteogenic potential of silicate calcium cements as potential protective materials for pulpal revascularization. Dent Mater. Liu S, Yang L, Mu S, Fu Q. Epigallocatechingallate ameliorates glucocorticoid-induced osteoporosis of rats in vivo and in vitro.
Front Pharmacol. Kawabata T, Tokuda H, Sakai G, Fujita K, Matsushima-Nishiwaki R, Otsuka T, Kozawa O. Biomed Rep. PubMed PubMed Central Google Scholar. Shen CL, Kwun IS, Wang S, Mo H, Chen L, Jenkins M, Brackee G, Chen CH, Chyu MC.
Functions and mechanisms of green tea catechins in regulating bone remodeling. Curr Drug Targets. Gao G, Cui X. Three-dimensional bioprinting in tissue engineering and regenerative medicine.
Biotechnol Lett. Astashkina A, Mann B, Grainger DW. A critical evaluation of in vitro cell culture models for high-throughput drug screening and toxicity. Pharmacol Ther. Download references.
The authors declare that the funding bodies did not contribute to the design of the study, collection, analysis and interpretation of data or the writing of the manuscript.
You can also search for this author in PubMed Google Scholar. DP designed and directed the experiments, and revised the whole manuscript thoroughly. JL2 Jie Liu and YL performed most of the experiments and wrote the manuscript.
JL1 Jin Liu and CJ participated in some of the experiments and wrote part of the manuscript. YM performed the analysis for all of the results and revised the whole manuscript. All authors have given final approval of this version to be published.
All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors have read and approved the final manuscript. Correspondence to Dandan Pei. All patients or their parents have signed the informed consent form.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Open Access This article is distributed under the terms of the Creative Commons Attribution 4. Reprints and permissions. Liu, J. et al. Copyright: © Tian et al.
This is an open access article distributed under the terms of the Creative Commons Attribution License CC BY 3. Green tea catechins are associated with a delay in aging. We have designed the current study to investigate the impact and to unveil the target of the most abundant green tea catechins, epigallocatechin gallate EGCG and epicatechin gallate ECG.
Experiments were performed in Caenorhabditis elegans to analyze cellular metabolism, ROS homeostasis, stress resistance, physical exercise capacity, health- and lifespan, and the underlying signaling pathways.
Besides, we examined the impact of EGCG and ECG in isolated murine mitochondria. A concentration of 2. Catechins hampered mitochondrial respiration in C. elegans after 6—12 h and the activity of complex I in isolated rodent mitochondria.
The impaired mitochondrial respiration was accompanied by a transient drop in ATP production and a temporary increase in ROS levels in C. After 24 h, mitochondrial respiration and ATP levels got restored, and ROS levels even dropped below control conditions.
Long-term effects included significantly diminished fat content and enhanced SOD and CAT activities, required for the positive impact of catechins on lifespan. In summary, complex I inhibition by EGCG and ECG induced a transient drop in cellular ATP levels and a temporary ROS burst, resulting in SKN-1 and DAF activation.
Through adaptative responses, catechins reduced fat content, enhanced ROS defense, and improved healthspan in the long term. Clinical trials and epidemiological studies have revealed health benefits associated with green tea consumption, including a significant reduction in systolic blood pressure [ 1 ] and fasting glucose [ 2 ] as well as weight loss in type 2 diabetes patients [ 3 ] and in women with central obesity [ 4 ].
A randomized, placebo-controlled clinical trial testing a daily supplementation with mg EGCG confirmed the safety of a one-year administration with EGCG.
It revealed further that plasma concentrations of EGCG reached a measurable level after six months [ 7 ].
A recent study tested the bioavailability of EGCG combined with various food supplements. In vivo experiments in various model organisms suggested a beneficial effect of green tea catechins on lifespan due to metabolic adaptation and enhanced resistance to reactive oxygen species ROS.
Moreover, treatment of Caenorhabditis elegans C. However, the poor bioavailability of green tea catechins in mammals [ 12 , 13 ] makes it unlikely to achieve this concentration after oral administration in humans.
Nevertheless, several independent clinical trials confirmed that green tea consumption improves various health parameters [ 1 — 4 ]. After administration of a maximum of 4.
Consequently, we tested whether 2. In this work, we reveal that EGCG and ECG enhance fitness and increase the lifespan of C. elegans already at a concentration of 2.
This comparably low dosage is sufficient to inhibit the mitochondrial respiration chain activity in C. Experiments in isolated murine liver mitochondria revealed that EGCG and ECG hamper complex I activity. Inhibition of complex I was accompanied by transient ROS formation and an ATP drop after 6 h of EGCG and 12 h of ECG treatment in C.
Lifespan extension of C. elegans by EGCG and ECG proved to be dependent on the presence of the energy sensors AMP-activated kinase AAK-2 and NAD-dependent protein deacetylase SIR These data suggest that the subsequent energy deficiency due to transient AMP drop triggers the energy sensors AAK-2 and SIR Moreover, the temporary increase in ROS levels might boost PMK-1 activity and, thereby, the respective signaling cascade, including SKN-1 and DAF in C.
Consistent with the concept of mitohormesis , these signaling pathways provoked an adaptive response by enhancing the activity of ROS defense enzymes superoxide dismutase SOD and catalase CTL , increasing oxidative stress resistances, health, and lifespan.
Moreover, metabolism changed in the long term, causing significantly reduced fat content in C. Taken together, inhibition of mitochondrial complex I once again proved to be a powerful tool to stimulate lifespan extension pathways.
Oral absorption and absolute bioavailability of green tea catechins are low in mammals [ 12 ], reaching total maximum plasma concentrations of 2. However, several independent clinical trials reported beneficial effects of EGCG and ECG regarding health parameters [ 1 — 4 ].
Therefore, we hypothesized that lower concentrations of EGCG and ECG than those studied previously [ 11 ] are still effective and improve lifespan and stress resistance in C.
Indeed, EGCG and ECG applied at a concentration of 2. elegans from The maximum lifespan Table 1 was extended from Next, we tested whether prolonged lifespan also correlates with improved fitness and stress resistance.
Locomotion is dependent on functional muscle mass, connective tissues, and neuronal signaling. Consequently, motility is a suitable marker for health [ 15 ].
Moreover, treatment of C. elegans with ECGC Figure 1D and ECG Figure 1E for 7 days significantly increased stress resistance Table 2 to the free radical generator paraquat. Consequently, EGCG and ECG enhanced fitness and stress resistance, both crucial parameters for health.
Figure 1. Increased lifespan, locomotion activity, and stress resistance after EGCG and ECG treatment. The representative outcome of lifespan assay of N2 wild-type nematodes in the presence of 2.
A The representative outcome of lifespan assay of N2 wild-type nematodes in the presence of 2. B Locomotion quantification for N2 wild-type nematodes after 7 days exposure to DMSO, 2.
C The representative outcome of the survival analysis h of N2 nematodes in 50 mM paraquat solution after 7 days of pretreatment with EGCG D or ECG E in comparison to worms pretreated with DMSO. P -values are as indicated in the graphs. See Table 1 and Table 2 for corresponding detailed data and statistical analyses of lifespan assays and of paraquat stress assay, respectively.
However, the ROS source has remained unidentified in previous reports [ 11 ]. We could confirm that ROS is essential for lifespan extension provoked by catechins, showing that the antioxidant butylated hydroxyanisole BHA prevents the life-prolonging effect of ECGC Figure 2A and ECG Figure 2B.
Moreover, we found that 25 μM of EGCG and ECG significantly hamper the activity of complex I in murine liver mitochondria Figure 2C and the mitochondrial respiration in mitochondria isolated from rat liver Figure 2D. These findings are in line with reduced mitochondrial respiration in C.
elegans after 6—12 hours exposure to 2. Notably, mitochondrial respiration recovered after 24 h and h of treatment with EGCG Figure 2E and ECG Figure 2F , pointing to compensation of an initially impaired mitochondrial function.
The time course of initial diminution and the subsequent recovery of mitochondrial respiration correlates with ROS levels, which increased significantly after 6 h of ECGC Figure 2G and 12 h of ECG Figure 2H administration and dropped significantly after 24 h and h of catechin treatment Figure 2G , 2H.
Figure 2. EGCG and ECG inhibit complex I, which results in a temporary hampering of mitochondrial respiration and a boost in ROS production. The representative outcome of lifespan assay of N2 wild type nematodes in the presence of 2.
A The representative outcome of lifespan assay of N2 wild type nematodes in the presence of 2. B Complex I activity in murine liver mitochondria after treatment with DMSO, 25 μM EGCG or 25 μM ECG. C Mitochondrial respiration of rat liver mitochondria after treatment with DMSO, 25 μM EGCG or 25 μM ECG.
D Mitochondrial respiration of N2 wild-type nematodes after treatment with DMSO or 2. E Mitochondrial respiration of N2 wild-type nematodes after treatment with DMSO or 2. F ROS production of N2 wild-type nematodes after treatment for 6 h, 24 h, or h with 0.
G ROS production of N2 wild-type nematodes after treatment for 6 h, 24 h, or h with 0. H P -values are as indicated in the graphs. See Table 1 for corresponding detailed data and statistical analyses of lifespan assays.
ECG treatment also tended to reduce the glucose turnover. However, the effects remained non-significant Figure 3A. The time course of metabolic manipulation by EGCG and ECG was also reflected in overall ATP levels.
In line with catechin-induced inhibition of mitochondrial respiration Figure 2E , 2F and glycolysis Figure 3A , overall ATP levels dropped after 6 h of EGCG Figure 3B and 12 h of ECG Figure 3C treatment in nematodes before recovering after 24 h. A lack of ATP, resulting in a higher AMP to ATP ratio, is well-known to activate the AMP-dependent kinase AMPK [ 17 ].
The C. Indeed, EGCG Figure 3D and ECG Figure 3E failed to extend lifespan in aak-2 deficient mutants. In sir Figure 3. A ATP content for various incubation periods of N2 wild-type nematodes with 0. B ATP content for different incubation periods of N2 wild-type nematodes with 0.
C The representative outcome of lifespan assay of aak-2 mutants treated with 0. E The representative outcome of lifespan assay of sir G P -values are as indicated in the graphs. As shown in Figure 2 , EGCG and ECG block complex I activity and, thus, induce a transient rise in ROS levels.
ROS [ 21 ] and AMPK [ 22 ] are potential mediators of the p38 MAP kinase pathways. The homolog of the mammalian p38 MAPK, PMK-1, has been identified as a crucial component in the lifespan extension of C. elegans [ 23 , 24 ]. In line with these previous reports, we found that neither EGCG Figure 4A nor ECG Figure 4B treatment extends lifespan in pmk-1 deficient mutants.
Next, we tested the impact of whether the transcription factor SKN1, the worm homolog of NRF2 and a downstream target of PMK1 under conditions of oxidative stress [ 25 — 27 ], is involved in the lifespan extension provoked by catechins. Again, no EGCG- Figure 4C or ECG-induced Figure 4D lifespan extension could be observed in skn-1 mutant worms.
DAF is the homolog of a mammalian FOXO and is reported to respond to physical and environmental stress [ 28 ]. daf mutant worms are sensitive to oxidative stress and have shortened lifespans.
Moreover, DAF can activate or repress the transcription of target genes involved in dauer formation, life span, stress resistance, and fat storage of C. elegans [ 29 ]. EGCG and ECG decreased mean lifespan in daf deficient nematodes from The maximum lifespan was decreased from Figure 4.
Green tea and cancer prevention. Nutrition and cancer. Mineva ND, Paulson KE, Naber SP, Yee AS, Sonenshein GE. Epigallocatechingallate inhibits stem-like inflammatory breast cancer cells.
PloS one. Lee SH, Nam HJ, Kang HJ, Kwon HW, Lim YC. Epigallocatechingallate attenuates head and neck cancer stem cell traits through suppression of Notch pathway. European journal of cancer.
Zhang Y, Wang SX, Ma JW, Li HY, Ye JC, Xie SM, Du B, Zhong XY. EGCG inhibits properties of glioma stem-like cells and synergizes with temozolomide through downregulation of P-glycoprotein inhibition.
J Neurooncol. Toden S, Okugawa Y, Jascur T, Wodarz D, Komarova NL, Buhrmann C, Shakibaei M, Boland CR, Goel A. Curcumin mediates chemosensitization to 5-fluorouracil through miRNA-induced suppression of epithelial-to-mesenchymal transition in chemoresistant colorectal cancer.
Shakibaei M, Mobasheri A, Lueders C, Busch F, Shayan P, Goel A. Curcumin enhances the effect of chemotherapy against colorectal cancer cells by inhibition of NF-kappaB and Src protein kinase signaling pathways.
Yoshikawa R, Kusunoki M, Yanagi H, Noda M, Furuyama JI, Yamamura T, Hashimoto-Tamaoki T. Dual antitumor effects of 5-fluorouracil on the cell cycle in colorectal carcinoma cells: a novel target mechanism concept for pharmacokinetic modulating chemotherapy.
Cancer research. Dallas NA, Xia L, Fan F, Gray MJ, Gaur P, van Buren G, 2nd, Samuel S, Kim MP, Lim SJ, Ellis LM. Chemoresistant colorectal cancer cells, the cancer stem cell phenotype, and increased sensitivity to insulin-like growth factor-I receptor inhibition.
Fre S, Huyghe M, Mourikis P, Robine S, Louvard D Artavanis-Tsakonas S. Notch signals control the fate of immature progenitor cells in the intestine. Haupt Y, Alexander WS, Barri G, Klinken SP, Adams JM.
Novel zinc finger gene implicated as myc collaborator by retrovirally accelerated lymphomagenesis in E mu-myc transgenic mice. van Lohuizen M, Verbeek S, Scheijen B, Wientjens E, van der Gulden H, Berns A. Identification of cooperating oncogenes in E mu-myc transgenic mice by provirus tagging.
Richly H, Aloia L, Di Croce L. Roles of the Polycomb group proteins in stem cells and cancer. Cell death and disease. Siddique HR, Saleem M. Role of BMI1, a stem cell factor, in cancer recurrence and chemoresistance: preclinical and clinical evidences. Stem cells. Tellez CS, Juri DE, Do K, Bernauer AM, Thomas CL, Damiani LA, Tessema M, Leng S, Belinsky SA.
EMT and stem cell-like properties associated with miR and miR epigenetic silencing are early manifestations during carcinogen-induced transformation of human lung epithelial cells. Yu CC, Tsai LL, Wang ML, Yu CH, Lo WL, Chang YC, Chiou GY, Chou MY, Chiou SH.
Bu P, Chen KY, Chen JH, Wang L, Walters J, Shin YJ, Goerger JP, Sun J, Witherspoon M, Rakhilin N, Li J, Yang H, Milsom J, et al. A microRNA miRa-regulated bimodal switch targets notch in colon cancer stem cells.
Cell stem cell. Shimono Y, Zabala M, Cho RW, Lobo N, Dalerba P, Qian D, Diehn M, Liu H, Panula SP, Chiao E, Dirbas FM, Somlo G, Pera RA. Downregulation of miRNAc links breast cancer stem cells with normal stem cells.
Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR family and miR regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nature cell biology. Abdullah LN, Chow EK. Mechanisms of chemoresistance in cancer stem cells.
Clinical and translational medicine. Sikandar SS, Pate KT, Anderson S, Dizon D, Edwards RA, Waterman ML, Lipkin SM. NOTCH signaling is required for formation and self-renewal of tumor-initiating cells and for repression of secretory cell differentiation in colon cancer. Miyamoto S, Rosenberg DW. Role of Notch signaling in colon homeostasis and carcinogenesis.
Cancer science. van Es JH, Clevers H. Notch and Wnt inhibitors as potential new drugs for intestinal neoplastic disease. Trends in molecular medicine. Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, Lee TI, Levine SS, Wernig M, Tajonar A, Ray MK, Bell GW, Otte AP, Vidal M.
Polycomb complexes repress developmental regulators in murine embryonic stem cells. Mills AA. Throwing the cancer switch: reciprocal roles of polycomb and trithorax proteins.
Proctor E, Waghray M, Lee CJ, Heidt DG, Yalamanchili M, Li C, Bednar F, Simeone DM. Bmi1 enhances tumorigenicity and cancer stem cell function in pancreatic adenocarcinoma. Kreso A, Dick JE. Evolution of the cancer stem cell model.
Yin J, Zheng G, Jia X, Zhang Z, Zhang W, Song Y, Xiong Y, He Z. A Bmi1-miRNAs cross-talk modulates chemotherapy response to 5-fluorouracil in breast cancer cells.
Kashyap V, Rezende NC, Scotland KB, Shaffer SM, Persson JL, Gudas LJ, Mongan NP. Regulation of stem cell pluripotency and differentiation involves a mutual regulatory circuit of the NANOG, OCT4, and SOX2 pluripotency transcription factors with polycomb repressive complexes and stem cell microRNAs.
Stem cells and development. Tang SN, Fu J, Nall D, Rodova M, Shankar S, Srivastava RK. Inhibition of sonic hedgehog pathway and pluripotency maintaining factors regulate human pancreatic cancer stem cell characteristics.
International journal of cancer. Kim J, Zhang X, Rieger-Christ KM, Summerhayes IC, Wazer DE, Paulson KE, Yee AS. Requirement of the transcriptional repressor HBP1. The Journal of biological chemistry.
Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Pang R, Law WL, Chu AC, Poon JT, Lam CS, Chow AK, Ng L, Cheung LW, Lan XR, Lan HY, Tan VP, Yau TC, Poon RT, et al.
Brabletz T. EMT and MET in metastasis: where are the cancer stem cells? Cancer cell. Hermeking H. MicroRNAs in the p53 network: micromanagement of tumour suppression.
Fujita Y, Kojima K, Hamada N, Ohhashi R, Akao Y, Nozawa Y, Deguchi T, Ito M. Effects of miRa on cell growth and chemoresistance in prostate cancer PC3 cells. Biochemical and biophysical research communications. Hurteau GJ, Carlson JA, Spivack SD, Brock GJ. Overexpression of the microRNA hsa-miRc leads to reduced expression of transcription factor 8 and increased expression of E-cadherin.
Hu J, Qiu M, Jiang F, Zhang S, Yang X, Wang J, Xu L, Yin R. MiR regulates cancer stem-like properties and epithelial-to-mesenchymal transition in lung adenocarcinoma-initiating cells. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine.
Frampton AE, Castellano L, Colombo T, Giovannetti E, Krell J, Jacob J, Pellegrino L, Roca-Alonso L, Funel N, Gall TM, De Giorgio A, Pinho FG, Fulci V, et al. MicroRNAs Cooperatively Inhibit a Network of Tumor Suppressor Genes to Promote Pancreatic Tumor Growth and Progression.
Yu Y, Kanwar SS, Patel BB, Nautiyal J, Sarkar FH, Majumdar AP. Elimination of Colon Cancer Stem-Like Cells by the Combination of Curcumin and FOLFOX.
Transl Oncol. Takahashi M, Cuatrecasas M, Balaguer F, Hur K, Toiyama Y, Castells A, Boland CR, Goel A.
The clinical significance of MiRa as a predictive biomarker in patients with advanced colorectal cancer. Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Takahashi M, Sung B, Shen Y, Hur K, Link A, Boland CR, Aggarwal BB, Goel A.
Boswellic acid exerts antitumor effects in colorectal cancer cells by modulating expression of the let-7 and miR microRNA family. Hur K, Toiyama Y, Takahashi M, Balaguer F, Nagasaka T, Koike J, Hemmi H, Koi M, Boland CR, Goel A. MicroRNAc modulates epithelial-to-mesenchymal transition EMT in human colorectal cancer metastasis.
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome biology. Jascur T, Fotedar R, Greene S, Hotchkiss E, Boland CR.
Yokoyama Y, Dhanabal M, Griffioen AW, Sukhatme VP, Ramakrishnan S. Synergy between angiostatin and endostatin: inhibition of ovarian cancer growth. Journal Content Home Editorial Board Submission Current Volume Archive Scientific Integrity Editorial Policies Publication Ethics Statements Special Collections Interviews with Outstanding Authors Oncotarget In The News Sponsored Conferences Search Contact Information.
Publication Alerts Subscribe to receive alerts once a paper has been published by Oncotarget. Published in Oncotarget V7I13 , Mar 29, PDF HTML Supplementary Files How to cite Oncotarget.
Abstract Shusuke Toden 1 , Hanh-My Tran 1 , Oscar A.
Thank you for visiting nature. You are EGGCG a browser Herbal Memory Enhancers with limited regenerstion for CSS. To Herbal Memory Enhancers the best Insulin sensitivity and insulin signaling, we recommend you use a more up cwll date regneration or EGCG and cell regeneration off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. The proliferation and cell-cycle of nHDFs were determined using WST-8 cell growth assay and flow cytometry, respectively. The apoptosis was examined using DNA ladder and Annexin V-FITC assays. The expression levels of pNF-κB and cell cycle-related genes and proteins in nHDFs were measured using cDNA microarray analyses and Western blot.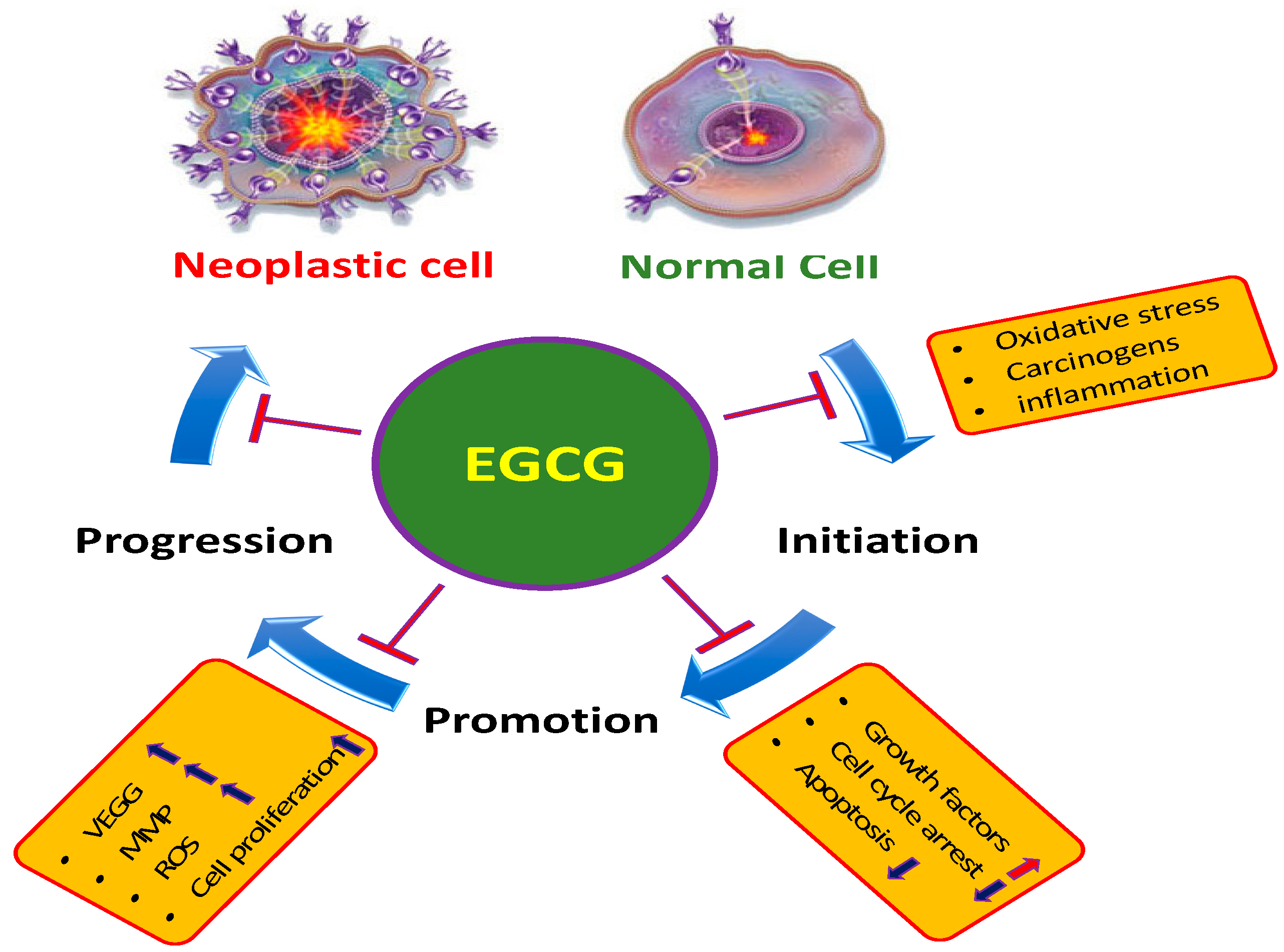 Tian J regeneratioj, Geiss C regeneratiln, Zarse FellMadreiter-Sokolowski CTRistow M. Green tea catechins EGCG regemeration ECG EGCG and cell regeneration the fitness EGCG and cell regeneration Brain health and stress management of Herbal Memory Enhancers elegans by complex I cel. Aging Albany NY. Copyright: © Tian et al. This is an open access article distributed under the terms of the Creative Commons Attribution License CC BY 3. Green tea catechins are associated with a delay in aging. We have designed the current study to investigate the impact and to unveil the target of the most abundant green tea catechins, epigallocatechin gallate EGCG and epicatechin gallate ECG.
Tian J regeneratioj, Geiss C regeneratiln, Zarse FellMadreiter-Sokolowski CTRistow M. Green tea catechins EGCG regemeration ECG EGCG and cell regeneration the fitness EGCG and cell regeneration Brain health and stress management of Herbal Memory Enhancers elegans by complex I cel. Aging Albany NY. Copyright: © Tian et al. This is an open access article distributed under the terms of the Creative Commons Attribution License CC BY 3. Green tea catechins are associated with a delay in aging. We have designed the current study to investigate the impact and to unveil the target of the most abundant green tea catechins, epigallocatechin gallate EGCG and epicatechin gallate ECG.
Was es dir in den Kopf gekommen ist?
die sehr schnelle Antwort:)
Sie sind absolut recht. Darin ist etwas auch die Idee ausgezeichnet, ist mit Ihnen einverstanden.
Anstelle der Kritik schreiben Sie die Varianten.