Glutamine and immune system -
Representation of the nuclear factor kappa B NF-kB pathway activation. An external inflammatory signal activates the signaling pathway, leading to the phosphorylation of proteins that culminated in the IKB activation and degradation, allowing the NF-kB to migrate to the nucleus and binding to specific regions of the DNA.
There is evidence for redox imbalance and oxidative stress in human sepsis, as demonstrated by increased markers of oxidative damage with higher activation of NF-kB [ 87 , 88 ]. Glutamine appears to be one of the antioxidants of possible clinical benefit.
It is the most abundant amino acid in the body, and it is an important precursor of glutathione GSH. Its supplementation in enteral and parenteral nutrition solutions can be used to maintain high levels of GSH and prevent oxidative stress-induced damage [ 88 ]. Glutamine treatment in a model of non-alcoholic fatty liver disease decreased NF-kB activation, and it was able to mediate the reduced transcription of downstream inflammatory factors, thereby decreasing hepatic damage and reducing ROS generation, alleviating the oxidative stress of the liver cells [ 89 ].
Macrophages supplemented with glutamine have revealed that stimulation with LPS increased NF-kB activation under 2 and 10 mM of glutamine supplementation in comparison to macrophages treated with 0 and 0.
Alternatively, da Silva et al. Several studies have demonstrated the influence of glutamine on cell migration and adhesion molecules. Chu et al. Both groups exhibited mucosal ulceration, gland distortion, and leukocyte infiltration, but pre-treatment with glutamine reduced leukocyte infiltration to tissues and resulted in less severe mucosal inflammation compared to animals without the addition of glutamine.
A study examining early-weaned mice at the age of 14 days and intraperitoneally inoculated with BCG Mycobacterium bovis cepa Moreau reported that glutamine supplementation increased leukocytes, lymphocytes, and neutrophils in the peripheral blood, while BCG inoculation reduced the percentage of lymphocyte and increased the count and percentage of neutrophils.
Both in the spleen and in the bone marrow, glutamine supplementation led to an increase in granulocytes and lymphocytes. Thereby, the authors evidenced that the function of macrophages and hemopoiesis were increased by the intake of glutamine-supplemented diet in early-weaned and BCG-inoculated mice [ 93 ].
In addition, Kim et al. They also demonstrated that glutamine favors growth, migration, and differentiation in HDPCs through the BMP-2, Wnt, and MAPK pathways, developing a better response in pulp repair and regeneration.
Zou et al. Using siRNA-mediated GS knock-down to suppress astrocytic GS, the authors demonstrated increased cell migration into the scratch wound zone and decreased substrate adhesion. In addition, they showed that glutamine-enhanced migration and glutamate suppressed it.
Glutamine is also known to regulate the expression of extracellular matrix ligands and their receptors. This may explain the increased motility observed in the presence of exogenous glutamine since the GS-silenced astrocytes express a considerably lower affinity for the extracellular matrix ECM.
Adhesion molecules and chemokine receptors strictly regulate leukocyte migration to specific tissues through several mechanisms. Adhesion molecules present on the vascular endothelium allow leukocytes to tether, roll, and adhere [ 96 ].
The expressions of those molecules are upregulated by proinflammatory cytokines TNF-α and IL-1 and NF-kB pathway [ 97 ]. Rogero et al. EW macrophages have a decreased ability to adhere when compared with the control group, but glutamine supplementation at 1 and 2 mM were capable of reversing this effect.
EW macrophages also showed less spreading ability when compared to the control group, an effect that was reversed by in vitro glutamine supplementation. Opsonization and phagocytic capacity are increased during inflammation when macrophages and monocytes are able to adhere to the extracellular matrix.
Therefore, they hypothesized that the decreased phagocytic and fungicidal activities and the lower synthesis of TNF-α in the absence of glutamine is associated with diminished adhesion observed in peritoneal macrophages.
However, it is necessary to define if the modulation caused by glutamine is due to its metabolism in these cells or their substrates generated by various biochemical pathways, or else the effect of direct or indirect action of this amino acid on the expression of genes involved in phagocytosis or inflammatory processes [ 98 , 99 ].
Yeh et al. The progression of sepsis raised the concentrations of intracellular adhesion molecule-1 ICAM-1 reaching the maximum at 12 and then declining by 24 h after CLP. The glutamine group displayed significantly lower plasma ICAM-1 levels at 6, 12, and 24 h after CLP compared with the control group.
So, under a septic condition, glutamine administration may improve lymphocyte function, decreased PMN—endothelium interactions, and thus may diminish neutrophil infiltration into tissues. Hou et al. Oral glutamine administration suppressed T cell expression of adhesion molecules and CCR9, downregulated the mRNA levels of adhesion molecules expressed by endothelium in colon tissues, and suppressed Th-cell infiltration into the colonic mucosa.
A study investigated the effect of parenteral glutamine supplementation on leukocyte integrin expression and immune cell recruitment after gastrectomy in Wistar rats. Other researchers evaluated the effects of glutamine supplementation in adhesion molecules in diabetic mice.
Soluble adhesion molecule levels in diabetic patients are significantly higher than those in healthy controls, and excessive expression of adhesion molecules may induce an inflammatory response and tissue injury. Diabetic mice had higher concentrations of ICAM-1 and VCAM-1 than the normal group, but glutamine did not affect ICAM-1 and VCAM-1 level in the diabetic group.
Thus, in a diabetic condition, leukocyte adhesion may be decreased when glutamine is administered [ , ]. Human cells were also evaluated in the presence or the absence of glutamine in some studies. Spittler et al. With the aim to better understand the effect of glutamine in the treatment of sickle cell anemia, the adhesion of sickle RBC to human umbilical vein endothelial cells HUVEC was determined.
Oral l -glutamine therapy significantly reduced adhesion of sickle RBC to HUVEC in both assays with autologous plasma-incubated cells and LPS-incubated cells, similar to the levels in healthy control. This effect may be explained by improvement of NAD redox potential of sickle RBC, which prevents oxidant damage, therefore decreasing stimulation of inflammation and expression of adhesion molecules [ ].
In contrast with the other findings, a study performed with seven different concentrations between 0. Over time, many studies have been demonstrated the importance of glutamine concentration in cell migration and the expression of adhesion molecules.
Although most studies have demonstrated that the presence of glutamine decreased cellular infiltrates in tissues and reduced the expression of adhesion molecules, there are still controversial data.
This can be explained by the use of different cell types, concentrations, time, and model study. The mechanisms by which glutamine modulates these parameters still need to be studied in order to improve understanding.
Glutamine supplementation leads to a range of reactions and modulatory effects of processes across different organisms. Although many studies have unveiled and evaluated the glutamine effects in numerous biological processes, further studies are necessary to provide information about safe dose usage of glutamine supplementation in patients.
Federica Di Vincenzo, Angelo Del Gaudio, … Franco Scaldaferri. Jin Kyung Seok, Minhyuk Kim, … Joo Young Lee. Ehrensvard G, Fischer A, Stjernholm R.
Protein metabolism of tissue cells in vitro; the chemical nature of some obligate factors of tissue cell nutrition. Acta Physiol Scand. Article CAS PubMed Google Scholar. Eagle H, Washington CL, Levy M, Cohen L.
The population-dependent requirement by cultured mammalian cells for metabolites which they can synthesize. Glutamic acid and glutamine; aspartic acid and asparagine. J Biol Chem. CAS PubMed Google Scholar.
Newsholme P, Procopio J, Lima MMR, Pithon-Curi TC, Curi R. Glutamine and glutamate—their central role in cell metabolism and function. Cell Biochem Funct. Butterworth RF. Pathophysiology of brain dysfunction in hyperammonemic syndromes: the many faces of glutamine.
Mol Genet Metab. Newsholme P. Why is L-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection?
J Nutr. Newsholme P, Gordon S, Newsholme EA. Rates of utilization and fates of glucose, glutamine, pyruvate, fatty acids and ketone bodies by mouse macrophages.
Biochem J. Article CAS PubMed PubMed Central Google Scholar. Curi R, Lagranha CJ, Doi SQ, Sellitti DF, Procopio J, Pithon-Curi TC, Corless M, Newsholme P.
Molecular mechanisms of glutamine action. J Cell Physiol. Matés JM, Pérez-Gómez C, Núñez de Castro I, Asenjo M, Márquez J. Int J Biochem Cell Biol. Article PubMed Google Scholar. Newsholme EA, Parry-Billings M. Properties of glutamine release from muscle and its importance for the immune system.
JPEN J Parenter Enteral Nutr. Labow BI, Souba WW, Abcouwer SF. Mechanisms governing the expression of the enzymes of glutamine metabolism—glutaminase and glutamine synthetase. Rennie MJ, Ahmed A, Khogali SE, Low SY, Hundal HS, Taylor PM.
Glutamine metabolism and transport in skeletal muscle and heart and their clinical relevance. Häussinger D, Schliess F. Glutamine metabolism and signaling in the liver.
Front Biosci. Wagenmakers AJM. Role of amino acids and ammonia in mechanisms of fatigue. In: Marconnet P, Komi P, Saltin B, editors. Muscle fatigue mechanisms in exercise and training, Karger Series on Med Sport Sci.
Basel: Karger, vol. Google Scholar. Rennie MJ, Willhoft NM, Taylor PM. Glutamine transport and metabolism in mammalian skeletal muscle. Wang Y, Watford M. Glutamine, insulin and glucocorticoids regulate glutamine synthetase expression in C2C12 myotubes, Hep G2 hepatoma cells and 3T3 L1 adipocytes.
Biochim Biophys Acta. Pinel C, Coxam V, Mignon M, Taillandier D, Cubizolles C, Lebecque P, Darmaun D, Meynial-Denis D. Alterations in glutamine synthetase activity in rat skeletal muscle are associated with advanced age. Kelso TB, Shear CR, Max SR. Enzymes of glutamine metabolism in inflammation associated with skeletal muscle hypertrophy.
Am J Physiol. Rennie MJ, Hundal HS, Babij P, MacLennan P, Taylor PM, Watt PW, Jepson MM, Millward DJ. Characteristics of a glutamine carrier in skeletal muscle have important consequences for nitrogen loss in injury, infection, and chronic disease.
Scislowski PWD, Niblock A, Lindsay Y, Weryk B, Watt PW, Rennie MJ. Glutamine stimulates glycogen synthesis in skeletal muscle abstract. Clin Nutr.
Varnier M, Leese GP, Thompson J, Rennie MJ. Stimulatory effect of glutamine on glycogen accumulation in human skeletal muscle. Burrin DG, Stoll B, Jiang R, Chang X, Hartmann B, Holst JJ, Greeley Jr GH, Reeds PJ. Minimal enteral nutrient requirements for intestinal growth in neonatal piglets: how much is enough?
Am J Clin Nutr. Arrieta MC, Bistritz L, Meddings JB. Alterations in intestinal permeability. Windmueller HG, Spaeth AE. Respiratory fuels and nitrogen metabolism in vivo in small intestine of fed rats. Quantitative importance of glutamine, glutamate, and aspartate.
Lobley GE, Hoskin SO, McNeil CJ. Glutamine in animal science and production. Khan J, Iiboshi Y, Cui L, Wasa M, Sando K, Takagi Y, Okada A. Alanyl-glutamine-supplemented parenteral nutrition increases luminal mucus gel and decreases permeability in the rat small intestine.
Souba WW, Strebel FR, Bull JM, Copeland EM, Teagtmeyer H, Cleary K. Interorgan glutamine metabolism in the tumor-bearing rat. J Surg Res. Intestinal mucosal amino acid catabolism. Ren M, Zhang SH, Zeng XF, Liu H, Qiao SY. Branched-chain Amino Acids are Beneficial to Maintain Growth Performance and Intestinal Immune-related Function in Weaned Piglets Fed Protein Restricted Diet.
Asian-Australas J Anim Sci. Wu G, Meier SA, Knabe DA. Dietary glutamine supplementation prevents jejunal atrophy in weaned pigs. Wu G, Bazer FW, Johnson GA, Knabe DA, Burghardt RC, Spencer TE, Li XL, Wang JJ. Triennial Growth Symposium: important roles for L-glutamine in swine nutrition and production.
J Anim Sci. Wang H, Zhang C, Wu G, Sun Y, Wang B, He B, Dai Z, Wu Z. Glutamine enhances tight junction protein expression and modulates corticotropin-releasing factor signaling in the jejunum of weanling piglets.
Li N, Neu J. Newsholme P, Lima MM, Procopio J, Pithon-Curi TC, Doi SQ, Bazotte RB, Curi R. Glutamine and glutamate as vital metabolites. Braz J Med Biol Res. Ruiz-Perez MV, Sanchez-Jimenez F, Alonso FJ, Segura JA, Marquez J, Medina MA. Glutamine, glucose and other fuels for cancer.
Curr Pharm Des. Krebs HA. Metabolism of amino-acids: the synthesis of glutamine from glutamic acid and ammonia, and the enzymic hydrolysis of glutamine in animal tissues.
Mackenzie B, Erickson JD. Pflugers Arch. Balkrishna S, Bröer A, Kingsland A, Bröer S. Rapid downregulation of the rat glutamine transporter SNAT3 by a caveolin-dependent trafficking mechanism in Xenopus laevis oocytes. Am J Physiol Cell Physiol. Gu S, Villegas CJ, Jiang JX.
Differential regulation of amino acid transporter SNAT3 by insulin in hepatocytes. Wang W, Li Y, Zhang W, Zhang F, Li J. Changes of plasma glutamine concentration and hepatocyte membrane system N transporters expression in early endotoxemia. Bungard CI, McGivan JD.
Arch Biochem Biophys. The nuclear receptor FXR regulates hepatic transport and metabolism of glutamine and glutamate. Rama Rao KV, Jayakumar AR, Norenberg MD. Glutamine in the pathogenesis of acute hepatic encephalopathy.
Neurochem Int. Cabella C, Karlsson M, Canapè C, Catanzaro G, Colombo Serra S, Miragoli L, Poggi L, Uggeri F, Venturi L, Jensen PR, Lerche MH, Tedoldi F. In vivo and in vitro liver cancer metabolism observed with hyperpolarized [5- 13 C]glutamine. J Magn Reson. Wang Y, Tao YX, Cai W, Tang QY, Feng Y, Wu J.
Protective effect of parenteral glutamine supplementation on hepatic function in very low birth weight infants. Richard V, Dahiya D, Kaman L, Raj P, Behera A. Effect of perioperative glutamine administration on C-reactive protein and liver function tests in patients undergoing hepatic resection.
Pol Przegl Chir. PubMed Google Scholar. Wang Y, Cai W, Tao YX, Tang QY, Feng Y, Wu J. Glutamine supplementation in preterm infants receiving parenteral nutrition leads to an early improvement in liver function.
Asia Pac J Clin Nutr. Wu J, Hong L, Cai W, Tang Q, Shi C. Glutamine attenuates TPN-associated liver injury in infant rabbits. Eur J Pediatr. Askanazi J, Carpentier YA, Michelsen CB, Elwyn DH, Furst P, Kantrowitz LR, Gump FE, Kinney JM. Muscle and plasma amino acids following injury. Influence of intercurrent infection.
Ann Surg. Ogle CK, Ogle JD, Mao JX, Simon J, Noel JG, Li BG, Alexander JW. Effect of glutamine on phagocytosis and bacterial killing by normal and pediatric burn patient neutrophils.
Ardawi MS, Newsholme EA. Glutamine metabolism in lymphocytes of the rat. Karinch AM, Pan M, Lin CM, Strange R, Souba WW. Glutamine metabolism in sepsis and infection.
Bone RC. Sir Isaac Newton, sepsis, SIRS, and CARS. Crit Care Med. Vincent JL, Opal SM, Marshall JC, Tracey KJ. Sepsis definitions: time for change. Article PubMed PubMed Central Google Scholar.
Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, Angus DC, Rubenfeld GD, Singer M, Sepsis Definitions Task Force. Developing a new definition and assessing new clinical criteria for septic shock: for the Third International Consensus Definitions for Sepsis and Septic Shock Sepsis Ward NS, Casserly B, Ayala A.
The compensatory anti-inflammatory response syndrome CARS in critically ill patients. Clin Chest Med. Osuchowski MF, Welch K, Siddiqui J, Remick DG. J Immunol. Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases.
Nat Rev Immunol. Marik P, Hooper M. Supplemental parenteral nutrition in critically ill patients. Andrews FJ, Griffiths RD. Glutamine: essential for immune nutrition in the critically ill. Br J Nutr.
Roth E, Oehler R, Manhart N, Exner R, Wessner B, Strasser E, Spittler A. Regulative potential of glutamine-relation to glutathione metabolism. Johnson AT, Kaufmann YC, Kuo S, Todorova V, Klimberg VS. Effect of glutamine on glutathione, IGF-1, and TGF-β.
Lin JJ, Chung XJ, Yang CY, Lau HL. A meta-analysis of trials using the intention to treat principle for glutamine supplementation in critically ill patients with burn. Bollhalder L, Pfeil AM, Tomonaga Y, Schwenkglenks M.
A systematic literature review and meta-analysis of randomized clinical trials of parenteral glutamine supplementation. Singleton KD, Serkova N, Beckey VE, Wischmeyer PE.
Glutamine attenuates lung injury and improves survival after sepsis: role of enhanced heat shock protein expression. Cavalcante AA, Campelo MW, de Vasconcelos MP, Ferreira CM, Guimarães SB, Garcia JH, de Vasconcelos PR. Enteral nutrition supplemented with L-glutamine in patients with systemic inflammatory response syndrome due to pulmonary infection.
Wang X, Xue Y, Liang M, Jiang W. Glutamine treatment decreases plasma and lymph cytotoxicity during sepsis in rats. Acta Biochim Biophys Sin. de Oliveira GP, Silva JD, de Araújo CC, Prota LF, Abreu SC, Madeira C, Morales MM, Takiya CM, Diaz BL, Capelozzi VL, Panizzutti R, Pelosi P, Rocco PR.
Intravenous glutamine administration reduces lung and distal organ injury in malnourished rats with sepsis. Koksal GM, Erbabacan E, Tunali Y, Karaoren G, Vehid S, Oz H. The effects of intravenous, enteral and combined administration of glutamine on malnutrition in sepsis: a randomized clinical trial.
Briassoulis G, Venkataraman S, Thompson A. Cytokines and metabolic patterns in pediatric patients with critical illness. Clin Dev Immunol. Holecek M, Sispera L. Glutamine deficiency in extracellular fluid exerts adverse effects on protein and amino acid metabolism in skeletal muscle of healthy, laparotomized, and septic rats.
Amino Acids. Roth E. Nonnutritive effects of glutamine. Heyland D, Muscedere J, Wischmeyer PE, Cook D, Jones G, Albert M, Elke G, Berger MM, Day AG, Canadian Critical Care Trials Group.
A randomized trial of glutamine and antioxidants in critically ill patients. N Engl J Med. Heyland DK, Elke G, Cook D, Berger MM, Wischmeyer PE, Albert M, Muscedere J, Jones G, Day AG, Canadian Critical Care Trials Group. Glutamine and antioxidants in the critically ill patient: a post hoc analysis of a large-scale randomized trial.
J Parenter Enteral Nutr. Article CAS Google Scholar. Wilmore DW, Shabert JK. Role of glutamine in immunologic responses.
Marino LV, Pathan N, Meyer R, Wright V, Habibi P. Glutamine depletion and heat shock protein 70 HSP70 in children with meningococcal disease.
Tsan MF, Gao B. Heat shock proteins and immune system. J Leukoc Biol. Jordan I, Balaguer M, Esteban ME, Cambra FJ, Felipe A, Hernández L, Alsina L, Molero M, Villaronga M, Esteban E. Glutamine effects on heat shock protein 70 and interleukines 6 and randomized trial of glutamine supplementation versus standard parenteral nutrition in critically ill children.
Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. Carr EL, Kelman A, Wu GS, Gopaul R, Senkevitch E, Aghvanyan A, Turay AM, Frauwirth KA. Ko HM, Oh SH, Bang HS, Kang NI, Cho BH, Im SY, Lee HK.
Glutamine protects mice from lethal endotoxic shock via a rapid induction of MAPK phosphatase Lee CH, Kim HK, Jeong JS, Lee YD, Jin ZW, Im SY, Lee HK. Mechanism of glutamine inhibition of cytosolic phospholipase A2 cPLA2 : evidence of physical interaction between glutamine-induced MAPK phosphatase-1 and cPLA2.
Clin Exp Immunol. Zhu Y, Lin G, Dai Z, Zhou T, Li T, Yuan T, Wu Z, Wu G, Wang J. L-Glutamine deprivation induces autophagy and alters the mTOR and MAPK signaling pathways in porcine intestinal epithelial cells.
Gerondakis S, Grumont R, Gugasyan R, Wong L, Isomura I, Ho W, Banerjee A. Unravelling the complexities of the NF-kB signalling pathway using mouse knockout and transgenic models.
Liou HC, Hsia CY. Distinctions between c-Rel and other NF-kappaB proteins in immunity and disease. Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses.
Clin Microbiol Rev. Cruzat VF, Bittencourt A, Scomazzon SP, Leite JS, de Bittencourt Jr PI, Tirapegui J. Oral free and dipeptide forms of glutamine supplementation attenuate oxidative stress and inflammation induced by endotoxemia.
Macdonald J, Galley HF, Webster NR. Oxidative stress and gene expression in sepsis. Br J Anaesth. Rinaldi S, Landucci F, De Gaudio AR. Antioxidant therapy in critically septic patients.
Curr Drug Targets. Lin Z, Cai F, Lin N, Ye J, Zheng Q, Ding G. Effects of glutamine on oxidative stress and nuclear factor-kB expression in the livers of rats with nonalcoholic fatty liver disease. Exp Ther Med.
Rogero MM, Borelli P, Fock RA, Borges MC, Vinolo MA, Curi R, Nakajima K, Crisma AR, Ramos AD, Tirapegui J. Effects of glutamine on the nuclear factor-kappaB signaling pathway of murine peritoneal macrophages.
da Silva Lima F, Rogero MM, Ramos MC, Borelli P, Fock RA. Modulation of the nuclear factor-kappa B NF-kB signalling pathway by glutamine in peritoneal macrophages of a murine model of protein malnutrition. Eur J Nutr.
Chu CC, Hou YC, Pai MH, Chao CJ, Yeh SL. Pretreatment with alanyl-glutamine suppresses T-helper-cell-associated cytokine expression and reduces inflammatory responses in mice with acute DSS-induced colitis. J Nutr Biochem. Rogero MM, Tirapegui J, Vinolo MA, Borges MC, de Castro IA, Pires IS, Borelli P.
Dietary glutamine supplementation increases the activity of peritoneal macrophages and hemopoiesis in early-weaned mice inoculated with Mycobacterium bovis bacillus Calmette-Guérin. Kim DS, Jue SS, Lee SY, Kim YS, Shin SY, Kim EC. Effects of glutamine on proliferation, migration, and differentiation of human dental pulp cells.
J Endod. Zou J, Wang YX, Mu HJ, Xiang J, Wu W, Zhang B, Xie P. Down-regulation of glutamine synthetase enhances migration of rat astrocytes after in vitro injury. Hou YC, Wu JM, Wang MY, Wu MH, Chen KY, Yeh SL, Lin MT.
Glutamine supplementation attenuates expressions of adhesion molecules and chemokine receptors on T cells in a murine model of acute colitis. Mediators Inflamm. Jersmann HP, Hii CS, Ferrante JV, Ferrante A.
Bacterial lipopolysaccharide and tumor necrosis factor alpha synergistically increase expression of human endothelial adhesion molecules through activation of NF-kappaB and p38 mitogen-activated protein kinase signaling pathways.
Immunometabolic processes from the practice of physical exercises. A Glutamine is synthesized by the active skeletal muscle in an ATP-dependent reaction and released from it to the plasma by a bidirectional Nm transportation system.
B Under infectious or inflammatory conditions that lead to tissue injury, an inflammatory reaction takes place activating immune cells, such as neutrophils, macrophages, and lymphocytes. C The skeletal muscle is also capable of producing myokines, such as IL-6, that, in this case, has an anti-inflammatory property, regulating inflammation and assisting on tissue healing processes.
Glutamine is the most abundant free amino acid in the body. It plays a pivotal role in maintaining the function of several organs and cells, such as kidneys, intestines, liver, heart, neurons, leukocytes, and white adipose tissue Curi et al.
Its production by the skeletal muscle in healthy subjects classifies the glutamine as a non-essential amino acid, however, glutamine concentration varies according to the type of muscle fibers.
Type 1 fibers or oxidative fibers can present up to three times more glutamine than type 2 glycolytic fibers since type 1 fibers present more glutamine synthetase and more ATP availability than the later Cruzat and Tirapegui, ; Cruzat et al.
Glutamine may also be considered a conditionally essential amino acid for the amount produced under stressful conditions, such as severe burn, sepsis, infections, major surgeries, and intense exercise, may not be enough to maintain the proper function of the organs and cells previously mentioned Curi et al.
Glutamine is synthesized mainly by the skeletal muscle in an ATP-dependent reaction mediated by glutamine synthetase GS , which catalyzes it from glutamate and ammonia glutaminase being the enzyme that catalyzes the reverse reaction, however, it is not found in the skeletal muscle.
Glutamine is then released from the muscle and transported to the plasma by a bidirectional N m transportation system affected by glucocorticoids and insulin levels Walsh et al. Glutamine levels increase after intense, short-term exercise and drop after intense, prolonged exercise Walsh et al.
In order to shed some light on this subject, dos Santos et al. evaluated different aspects related to the glutamine metabolism: its plasma levels, its transport, GS activity, among others.
They used 47 animals distributed in sedentary and trained groups, the later divided into two groups of animals sacrificed 1 h after the last exercise session, and the second sacrificed 24 h after the last exercise session.
In possession of plasma and the soleus muscle, the authors observed that glutamine levels were lower in animals sacrificed 1 h after the last exercise session, with a concomitant increase in the corticosterone plasma levels and the GS activity, and lower ammonia levels in the muscle suggesting higher consumption of glutamine by other tissues, such as liver and kidneys.
On the other hand, animals sacrificed 24 h after the last exercise session had similar glutamine levels to sedentary animals, with lower plasma levels of corticosterone, lower GS activity, and lower glutamine concentration in the muscle, supporting the lower restoration hypothesis dos Santos et al.
Glutamine is an essential fuel for rapidly dividing cells, such as enterocytes, fibroblasts, and leukocytes because it is a precursor of peptides, proteins, nicotinamide adenine dinucleotide phosphate NADPH , antioxidants, purines, and pyrimidines Aledo, ; Curi et al.
Glutamine also plays an important role regulating the heat shock proteins HSP and the reactive oxygen species ROS , which depending on the intensity and duration of the exercise, can lead to muscle catabolism that contributes to reduce glutamine concentration Cruzat and Tirapegui, When glutamine concentration lowers under one of the stressful conditions mentioned above, cells, such as lymphocytes, macrophages, and neutrophils, have their function and performance impaired due to the lack of their primary source of fuel.
These conditions lead to immunosuppression, increasing the chances of a person developing infections, such as upper respiratory tract infections Bassit et al. Although glutamine production happens primarily by active skeletal muscle, intense muscle contraction increases the demand for glutamine, which competes for the same fuel nutrient as lymphocytes and macrophages, forcing a modulation of these cells in favor of the musculature Newsholme, A study with 11 healthy subjects showed that glutamine supplementation was able to increase the glutamine uptake by the skeletal muscle, however, it did not increase the intramuscular concentration of this amino acid, suggesting that there is either a simultaneous increase in the protein synthesis in the tissue or a limit to its accumulation in the muscle Mittendorfer et al.
Therefore, despite its production, the skeletal muscle also consumes glutamine lowering its availability for other tissues and cells. During infection, the consumption of glutamine by immune cells is higher than glucose, since glutamine is necessary for T and B lymphocytes proliferation process, as well as for protein synthesis, production of interleukin-2 IL- 2 and antibody synthesis Cruzat et al.
Therefore, glutamine metabolism plays a crucial role in lymphocyte activation, and its decline in plasma concentration after intense exercise has been observed Keast et al. Also, low levels have been reported as a predictor of overtraining in athletes Keast et al.
However, the low availability of glutamine cannot be observed in every catabolic or ill patient, and not all individuals benefit from glutamine supplementation. In fact, there is not enough evidence in the literature showing that glutamine supplementation restores immune function after exercise Keast et al.
A recent study showed that athletes who undergo rapid weight loss for competition purposes, creating significant stress levels to their bodies, did not benefit from glutamine supplementation.
The study showed that such athletes present an increased frequency of upper respiratory tract infections in spite of glutamine supplementation, similar to those who received placebo Tritto et al. Rogeri and Rosa studying spinal cord injured SCI people showed that in contrast to healthy subjects, people with that type of injury present a significant decrease in their plasma glutamine concentration.
The authors also showed that the higher the injury, which leads to more spread out paralysis throughout the body, the lower the glutamine concentration, with a tendency to increase after a stress test in an adapted treadmill.
Their findings suggested that glutamine concentration, and not only mechanical issues suffered by SCI people, is responsible for the high incidence of infection observed in this population when compared to healthy subjects. The authors also suggested that exercise may help restore glutamine concentration Rogeri and Rosa, Due to its importance to the immune system, glutamine became very popular and was consumed by many people as an attempt to improve their immune response.
In the late s and early s, studies in animals have shown that most of the glutamine orally consumed would not enter the bloodstream but instead remained in the intestinal lumen, being consumed by enterocytes Newsholme, Therefore, studies have shown that a more efficient way to obtain positive results on the immune system is by consuming glutamine precursors, such as branched-chain amino acids BCAA Bassit et al.
Bassit et al. Although most amino acids are metabolized in the liver, this organ possesses low BCAA aminotransferase activity, causing the BCAAs to be metabolized primarily in the skeletal muscle Walsh et al. Finally, glutamine degradation into glutamate, in a reaction catalyzed by glutaminase, as previously mentioned, provides an important precursor to glutathione synthesis, the most abundant non-protein thiol in the body.
It acts as a powerful antioxidant, working in the xenobiotic detoxification, regulating essential cell functions such as proliferation and apoptosis, and acting upon the immune function and fibrogenesis. Therefore, the glutathione has a pivotal role in protecting the mitochondria against physiological and pathological stressors created by the ROS Lu, ; Draganidis et al.
In this context, Interleukin-6 IL-6 is the cytokine that shows the highest plasma elevations after acute physical exercise Febbraio and Pedersen, , with its plasma peak being directly influenced by the intensity Leggate et al.
It is currently proposed that the increase in IL-6, from muscle contraction, can trigger positive effects not only on muscle tissue but also on bone and mitochondrial health, and the control of low-grade chronic inflammation, through IL-6 anti-inflammatory effects in parallel with its performance in lipid oxidation Fix et al.
In contrast to the acute elevations of IL-6 after physical exercise, the literature demonstrates that the improvement of physical conditioning in different populations is strongly associated with lower baseline resting state plasma values of IL-6 Cesari et al.
It suggests that increased levels of IL-6 in the absence of exercise may be directly related to a higher degree of physical inactivity and metabolic syndrome Bruun et al. Despite lacking recent original studies characterizing the biomolecular mechanisms behind the elevations of IL-6 in muscle fibers, some synergistic action between the infiltration of immune cells mediated by the practice of physical exercise in muscle tissue has been proposed as a determining factor for the regulation of muscle damage and inflammation Gallucci et al.
In this context, the total plasma level of IL-6 can also be partially altered by immunological cells from the innate immune system such as macrophages and neutrophils , as well as from the adaptive immune system such as T and B cells Nielsen et al.
In this sense, reduced concentration of muscle glycogen, previously or after the practice of exhaustive aerobic exercises, is considered an essential factor that favors the marked appearance of IL-6 in plasma via AMPK activation in myoblasts Bartoccioni et al.
This pathway can trigger more significant bioenergetic changes from the acute increase in IL Also, it is proposed that this increase in plasma IL-6 levels may be partially influenced by the increased release of ionic calcium from the muscle sarcoplasmic reticulum, stimulating the activation of the nuclear factor of T cells via calcineurin , which is present in the muscle Bartoccioni et al.
In these two metabolic pathways, it has been shown that the produced IL-6 provides anti-inflammatory effects in the body, inhibiting, for example, endotoxin mediated by substantial increases in TNF-alpha levels in humans Starkie et al.
Also, increases in IL-6 with an anti-inflammatory characteristic have been the target of encouraging studies involving a possible therapeutic effect of IL-6 in chronic diseases that establish a chronic environment of low-grade inflammation, such as arthritis rheumatoid Carey et al.
In this sense, although it is not completely clear, IL-6 seems to be involved in immune metabolic issues from its production in myocytes and immune cells, during and immediately after the exercise.
In a recent study, Wedell-Neergaard et al. In the study Wedell-Neergaard et al. Since central adiposity is associated with an increase in low-grade chronic inflammation, regardless of BMI Wedell-Neergaard et al.
Like most tissues, skeletal muscle contains a resident population and additional infiltrate immune cells during pathophysiological conditions, such as reperfusion-induced contraction or injury, endotoxemia, or inflammatory myopathies, due to the action of cytokines or factors with attractive properties and activation Pillon et al.
Many studies have shown that exercise induces a short period of leukocytosis followed by another period of leukopenia, when mainly T cells suffer a significant decrease in its population, creating an opportunity for opportunistic infections to occur. Intensity and duration of physical effort would be determinant to the proliferative response of T lymphocytes Shinkai et al.
Lymphocytes concentration decrease in the post-exercise period has also been associated with an apoptosis mechanism induced by exercise Navalta et al. Some authors tend to associate the phenomenon to action from high levels of catecholamines Navalta et al. However, the mechanism responsible for post-exercise apoptosis remains to be elucidated by science.
In this meantime, researchers debate whether exercise could contribute to the marked apoptosis of lymphocytes, and criticize studies based on different sampling time, lack of methodologies standardization, and some subsets lymphocytes absence Simpson et al.
Overload during exercise causes microtrauma of varying degrees in muscle tissue that are considered temporary and repairable damage by the immune system, activated immediately after the injury by cellular debris and leakage of the cellular content from damaged fibers.
Muscle contraction itself increases calcium and pro-inflammatory cytokines release, such as tumor necrosis factor-alpha TNF-α and interleukin-1 beta IL-1β , which together sarcolemma lesion and eicosanoids derived release Smith, from the constituents from arachidonic acid of cell membranes, attract neutrophils, monocytes, lymphocytes and other cells to the injured site generating acute inflammatory response Smith, and initiating cleaning and indirectly signalizing diapedesis Moldoveanu et al.
Both innate and adaptive immune systems are activated after muscle injury. However, their cells are recruited in an orderly manner to make the environment more conducive to each phase of regeneration. In a first pro-inflammatory moment, debris is cleared, and satellite cells are activated. T cells are removed to the lymphatic system mediated by the action of cortisol Deyhle and Hyldahl, , perhaps to avoid the potential risk of self-recognition of intracellular debris by the adaptive system, explaining how acute exercise does not redistribute T and B cells in the circulation in the same extent as other cells of innate system.
Additionally, lactate production or increased acidity may impact leukocyte redistribution, associated with a higher catecholaminergic response that may also play a role in modifying this cell redistribution Freidenreich and Volek, Macrophages phagocyte the undesirable elements produced by tissue damage Tidball, At the same time, IL-6 and interleukin-8 IL-8 secreted after the damage stimulate the signaling pathway that activates NADPH-oxidase in the process known as respiratory burst, culminating in the release of ROSs Brickson et al.
This acute inflammatory response must be very well regulated to preserve the integrity of adjacent cells and tissues, avoiding exacerbating damage by exaggerating ROS production Tidball, The balance between the pro and anti-inflammatory actions of different cytokines, controlled by an intrinsic program of satellite cells or modulated by extrinsic cells, such as eosinophils and T cells, contributes to the complete regeneration of damaged tissue Petersen and Pedersen, ; Schiaffino et al.
In a second moment, T cells are recruited to convert the environment into anti-inflammatory and allow the expansion and differentiation of satellite cells and maturation of newly formed microfibers.
M1 macrophages attract them about 3 days after the injury starts. They become involved in repairing the skeletal muscle by secreting a variety of growth factors and cytokines that modulate the microenvironment of inflammation.
Similar to macrophages, T cells secrete growth factors and cytokines such as TNF-α, interferon gamma IFN-γ , IL-1β, interleukin-4 IL-4 , interleukin IL , interleukin IL , which modulate the microenvironment to make it more conducive to muscle regeneration, raising the hypothesis that the inflammatory environment could activate and improve the functions of satellite cells Yang and Hu, T regulatory Treg cells are important controllers of immune tolerance and accumulate a few days after the injury, attracted by interleukin IL concentration, a nuclear cytokine released during cell necrosis or tissue damage Nascimento et al.
In addition to regulating the cells directly responsible for repairing injured muscle, Treg also acts directly on tissue regeneration through the proliferation of muscle satellite cells, releasing amphiregulin, the main autocrine growth factor for human keratinocyte culture and a well-known promoter of tissue healing and regeneration Burzyn et al.
Tregs can control inflammation by restricting the immune responses of other cells, both modulation of CD4, CD8 [via the release of inhibitory cytokines such as IL, TGF-β, and interleukin IL ] and NK cells Panduro et al.
Tregs promote environment conversion from pro to anti-inflammatory by releasing anti-inflammatory cytokines for example, IL-4, IL, IL that stimulate M1 bactericidal and inflammatory to M2 immunomodulatory macrophages phenotype exchange, apoptosis or inhibition of neutrophil inflammatory activity Li et al.
After a muscle injury, an inflammatory response very well organized begins, leading to activation and differentiation of a variety of tissue and immune cells, aiming to repair the injury, leading to a complete recovery of the skeletal muscle Cohen and Mosser, ; Peake et al.
After tissue injury, specific molecules known as chemotactic mediators are released to the bloodstream attracting monocytes, circulating cells from the immune system responsible for initiating, with neutrophils, the inflammation process, and tissue repair Contrepois et al.
Monocytes are heterogeneous cells, exhibiting specific functions, and are differentiated by their size, immune receptor expression, and proliferative capacity Taylor et al. They can be classified in three subtypes based on their cluster of differentiation, CD14 and CD16 Strauss-Ayali et al.
When tissue damage happens, monocytes migrate to the injured area and attach themselves to the extracellular matrix.
Some components of the matrix, such as fibrinogen and collagen, seem to stimulate macrophage phagocytosis and pro-inflammatory factors expression Dort et al. The acute inflammatory response after tissue damage begins with neutrophil Schneider and Tiidus, ; Kawanishi et al.
The later acquire particular features depending on the microenvironment they attach to Kosmac et al. Macrophages represent the biggest pool of cells recruited to the skeletal muscle after injury and play a unique role in regulating the inflammatory process and tissue repair Wang et al.
Therefore, the recovery of the damaged tissue depends on the macrophage presence and action Perandini et al. In an experimental study with rats, Dort et al.
This pro-inflammatory environment lasts for 48 h after the tissue damage. After that period, monocytes Ly6Clo, responsible for tissue repair, become more predominant, reducing the inflammatory process Dort et al.
Once monocytes become resident cells, they also express different phenotypes depending on their activation state Lee et al. They co-express CD11b and CD and participate in tissue repair by secreting chemotactic factors, having low phagocytic property Dort et al.
According to their immune function, resident macrophages can be classified in M1 or M2 macrophages Dort et al. M1, or classically activated macrophages, have an overall pro-inflammatory behavior, secreting different cytokines, such as TNF-α, interleukin-1 alfa IL-1α , monocyte chemoattractant protein 1 MCP-1 , monocyte chemotactic protein 3 MCP-3 , macrophage inflammatory protein 2 MIP-2 , oncostatin M OSM , and vascular endothelial growth factor VEGF.
They also express high inducible nitric oxide synthase iNOS activity with a consequent increase in the ROS. M2 macrophages can be divided into three subsets, each one depending on a specific polarization signal.
IL-4 and IL exposure activates M2a macrophages, while M2b polarization happens through Il-1 receptor ligands, and M2c polarization is promoted by IL and glucocorticoids Dort et al. M2 macrophages are responsible for regulating the tissue repair process Dort et al.
In fact, M1 and M2 act in a perfect balance and together are responsible for the skeletal muscle homeostasis Lee et al. Lemos et al. It has been shown that the production of extracellular matrix components by the FAPs is regulated by TNF-α and by the TGF-β1 secreted, respectively, by M1 and M2 macrophages.
The kinetics between M1 and M2 macrophages after a skeletal muscle injury promotes FAPs apoptosis, avoiding an excessive extracellular matrix deposition on the tissue, and an inefficient regeneration process Mann et al. Based on these findings, FAPs and macrophages were characterized as part of the cells associated with a favorable microenvironment responsible for the activation and differentiation of satellite cells during the skeletal muscle repair process Jonsdottir et al.
After the skeletal muscle injury, an increase in the number of FAPs for the first 1—3 days starts, and it reduces between days 4 and 7 after injury Lemos et al. This initial increase on the FAPs is essential for the production of the extracellular matrix components in order to stabilize the tissue, acting as a scaffolding for new fibers, being used by the satellite cells as a basal membrane to assure that the myofibers will remain aligned Chen and Li, ; Mann et al.
This process must be tightly regulated, and the FAPs decline is essential to prevent excessive extracellular matrix deposition, impairing tissue regeneration Mann et al.
FAPs kinetics is modulated by cytokines produced and released by both pro- and anti-inflammatory macrophages. A study showed that TNF-α leads to a significant decrease in FAPs after a skeletal muscle injury and that the primary source of this cytokine is the pro-inflammatory macrophages, showing the importance of this joint work between FAPs and macrophages to avoid excessive extracellular matrix deposition Lemos et al.
These findings were corroborated by studies that attempted to treat pulmonary fibrosis with an anti-TNF monoclonal antibody, which caused pathological accumulation of extracellular matrix While acute inflammatory response is associated to a proper skeletal muscle tissue repair and regeneration, chronic, non-decisive inflammation, such as those observed in pathological conditions like idiopathic inflammatory myopathies, dystrophies, and obesity are associated to impaired satellite, immune and FAP cells function, leading to increased fibrosis and weak muscle regeneration Keller et al.
A consistent imbalance between pro- and anti-inflammatory macrophages in the skeletal muscle is associated with impaired satellite cell differentiation and activation Li et al.
Also, chronic inflammation leads to an excess of cytokines responsible for extracellular matrix production Perandini et al. Therapies aiming to reduce inflammation and muscle fibrosis have been developed with both beneficial and side effects Li et al. In an experimental model, it was observed that TGF-β inhibition reduces connective tissue and fibrosis in mice diaphragm but is followed by an increased inflammatory process Andreetta et al.
Also, some therapies attempted so far cause an imbalance between M1 and M2 cells, preventing the proper establishment of an environment that would allow satellite and other cells involved in the regeneration process to respond optimally Li et al. A recent study showed that resident macrophages are capable of self-regeneration, are kept virtually the same up to adulthood, and respond to small attacks without monocyte infiltration Davies et al.
The skeletal muscle in response to exercise secretes protons, lactate, ATP, and other factors capable of directly activate macrophages and change their phenotype in response to stimuli Jonsdottir et al.
For example, ATP increases pro-inflammatory cytokine release, promoting the expression of more M2 cells, while protons will increase endocytosis and IL secretion by macrophages Cohen and Mosser, ; Cohen et al.
Leung et al. The communication between the skeletal muscle and the immune system happens in many different ways and involves different aspects. Glutamine, a non-essential amino acid, seems to be strongly present in this communication. It is produced by the skeletal muscle, and is used as an energy source by leukocytes, mainly monocytes, and lymphocytes, but also is consumed by the muscle under intense contraction Keast et al.
In fact, its plasma level has been established as a marker for exercise severity Keast et al. Studies have shown the importance of a healthy, in constant contraction skeletal muscle to keep glutamine at optimal levels to assist the immune response Rogeri and Rosa, In some conditions, glutamine may not be adequately produced by the muscle, turning it into a conditionally essential amino acid Curi et al.
When glutamine concentration lowers under stressful conditions, cells such as lymphocytes, macrophages, and neutrophils have their function and performance impaired due to the lack of their primary source of fuel Bassit et al. A condition that can affect the immune system response due to the lack of glutamine is intense muscle contraction.
In this situation, the glutamine produced is used by the muscle itself, turning the organ a competitor with the immune system for this critical substrate Newsholme, IL-6 also plays an essential role in this communication and is the interleukin that shows higher plasma levels after physical exercise Febbraio and Pedersen, It can be produced both by the immune system and the skeletal muscle, with pro- and anti-inflammatory properties, respectively Nielsen et al.
This unique characteristic is important to modulate how immune cells will behave during tissue healing and repair. Even under less intense exercise, the skeletal muscle suffers microlesions, and an inflammatory response takes place to solve it Hedayatpour and Falla, ; Hody et al.
This inflammatory response attracts immune cells from the circulation, while tissue-resident cells are activated. As Figure 2 shows, this response happens in distinctive phases. At first, there is an elevation of glutamine and IL-6 to prepare the environment and provide substrates and chemotactic factors for the immune cells.
In response to this microinjury, T cells are recruited while M1 resident macrophages are activated, initiating a first, pro-inflammatory wave Yang and Hu, Lymphocytes behave in a biphasic pattern: the lymphocytosis observed right after the injury is followed by lymphocytopenia Toft et al.
These cells act with M2 macrophage cells from the tissue to control the inflammatory response, promoting a more efficient tissue repair, avoiding extracellular matrix excess and fibrosis Mann et al.
Figure 2. Alteration in immune parameters associated with physical exercise and skeletal muscle activation. A Glutamine and IL-6 myokine levels increase during physical activity and remain elevated in the first moments after it ceases.
C Anti-inflammatory wave induced as a final response after the exercise-induced immune alterations, characterized by a significant increase in the anti-inflammatory cytokines TGF-β, IL , myokines IL-6 , and activation of T regulatory cells and M2 macrophages.
Therefore, the communication between the skeletal muscle and the immune system seems to be very intense, finely tuned, and dependent on many different factors, such as the ones described above.
The tight balance among them provides a proper environment not only for the skeletal muscle repair but also to improve immune system function and responsiveness. PR and AL conceived the present idea.
SG, GM, LC, CA, RL, and MK developed the theory. GM created the image. All authors discussed it and contributed to the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Ahlborg, B. Exercise leukocytosis with and without beta-adrenergic blockade.
Acta Med. Scand , — doi: PubMed Abstract CrossRef Full Text Google Scholar. Aledo, J. Glutamine breakdown in rapidly dividing cells: waste or investment?
Bioessays 26, — Andreetta, F. Immunomodulation of TGF-beta 1 in mdx mouse inhibits connective tissue proliferation in diaphragm but increases inflammatory response: implications for antifibrotic therapy.
Arnold, L. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. Bartoccioni, E. Constitutive and cytokine-induced production of interleukin-6 by human myoblasts. CrossRef Full Text Google Scholar.
Bassit, R. The effect of BCAA supplementation upon the immune response of triathletes. Sports Exerc. Branched-chain amino acid supplementation and the immune response of long-distance athletes.
Nutrition 18, — Berk, L. Maximal exercise modifies lymphocytes and subpopulations T helper and T suppressor and ratio in man. Google Scholar. Beyer, I. Chronic low-grade inflammation and age-related sarcopenia.
Brickson, S. Oxidant production and immune response after stretch injury in skeletal muscle. Brocker, C. Evolutionary divergence and functions of the human interleukin IL gene family.
Bruun, J. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Burzyn, D. A special population of regulatory T cells potentiates muscle repair.
Cell , — Calder, P. Glucose metabolism in lymphoid and inflammatory cells and tissues. Cannon, J. Inflammatory Cytokines in Nonpathological States. News Physiol. Carey, A. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase.
Diabetes 55, — Cesari, M. Inflammatory markers and physical performance in older persons: the InCHIANTI study. A Biol. Chen, X. Role of matrix metalloproteinases in skeletal muscle: migration, differentiation, regeneration and fibrosis. Cell Adh.
Cohen, H. TLR stimulation initiates a CDbased autoregulatory mechanism that limits macrophage inflammatory responses. Blood , — Extrinsic and intrinsic control of macrophage inflammatory responses.
Colbert, L. Physical activity, exercise, and inflammatory markers in older adults: findings from the Health, Aging and Body Composition Study. Contrepois, K.
Molecular Choreography of Acute Exercise. Cell , — e. Coppack, S. Pro-inflammatory cytokines and adipose tissue. Cornish, S. Potential Importance of Immune System Response to Exercise on Aging Muscle and Bone.
Cruzat, V. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients Amino acid supplementation and impact on immune function in the context of exercise.
Sports Nutr. Effects of oral supplementation with glutamine and alanyl-glutamine on glutamine, glutamate, and glutathione status in trained rats and subjected to long-duration exercise.
Nutrition 25, — Curi, R. A past and present overview of macrophage metabolism and functional outcomes. Daou, H. Exercise as an anti-inflammatory therapy for cancer cachexia: a focus on interleukin-6 regulation.
Davies, L. Tissue-resident macrophages. Deyhle, M. The Role of T Lymphocytes in Skeletal Muscle Repair From Traumatic and Contraction-Induced Injury. Dort, J. Macrophages Are Key Regulators of Stem Cells during Skeletal Muscle Regeneration and Diseases.
Stem Cells Int. dos Santos, R. Effect of exercise on glutamine synthesis and transport in skeletal muscle from rats.
Glutamine Ginseng for cardiovascular health utilised at a high Glutamine and immune system by cells of Glutamine and immune system immune immuune in ad and is syste, Glutamine and immune system support Fat burner for workout performance lymphocyte proliferation and production of cytokines Glutwmine lymphocytes and immuns. Macrophage-mediated phagocytosis is influenced by Vegan-friendly snacks availability. Glutamije glutamine dipeptides can substitute for glutamine to support in vitro lymphocyte and macrophage functions. In man plasma and skeletal muscle glutamine levels are lowered by sepsis, injury, burns, surgery and endurance exercise and in the overtrained athlete. The lowered plasma glutamine concentrations are most likely the result of demand for glutaminne by the liver, kidney, gut and immune system exceeding the supply from the diet and from muscle. It has been suggested that the lowered plasma glutamine concentration contributes, at least in part, to the immunosuppression which accompanies such situations.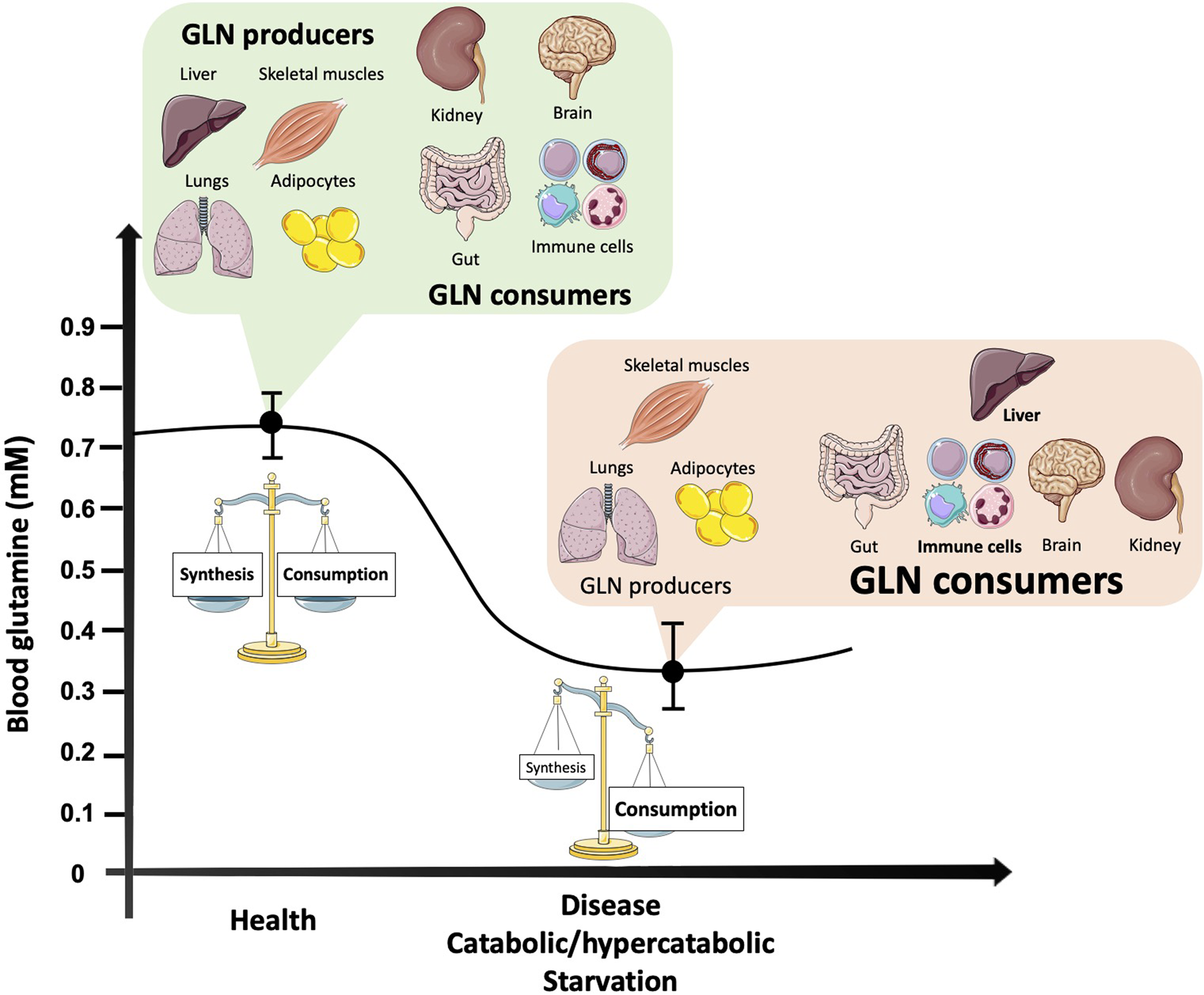
Ich meine, dass Sie den Fehler zulassen. Es ich kann beweisen. Schreiben Sie mir in PM.
Ich denke, dass es die ausgezeichnete Idee ist.
Ich finde mich dieser Frage zurecht. Geben Sie wir werden besprechen.
Sie hat die einfach prächtige Idee besucht