
The science behind thermogenesis -
As heat energy is a component of total energy, its contribution to total energy expenditure varies with the situation under study, for example, between rest and exercise.
In careful studies comparing energy measurements by indirect and direct calorimetry, Webb et al. During walking, the energy required to perform external work was not entirely captured by direct calorimetry.
Even though heat production increased during and in proportion to the level of physical work, the energy expenditure measured by metabolic gas exchange was greater than that of heat dissipated from exercise Webb et al. Thus, respirometry captures the total energy quantum of exercise representing the sum of mechanical and heat energy that are derived from chemical energy.
Indirect and direct calorimetry measures different aspects of energy expenditure from different outcome measures. They do not measure the same energy or form of energy. Indirect calorimetry measures energy expenditure based on oxygen consumption and carbon dioxide production.
This energy may be heat, mechanical and chemical energy but this method is unable to distinguish between the components and their magnitude. The magnitude of the components varies between fast and fed states or between rest and exercise.
Direct calorimetry estimates energy expenditure by measuring heat dissipation from the body. The extent to which heat production accurately reflects total energy production is also influenced by the conditions of study. Thus, claims that one method is superior to the other cannot be substantiated and should be greeted with skepticism Speakman As discussed earlier, when used and compared under well-defined conditions, such as rest and exercise Webb et al.
This attractive hypothesis has stimulated enduring interest that has produced continuing controversy. They concluded that factors such as variability in study design, meal size and age may have obscured DIT as a discriminant. Among other factors considered was the role of the gut microbiome in obesity Turnbaugh et al.
This review redresses the controversy differently by questioning the conceptual and methodological validity of DIT: whether the energy expended after a meal and estimated by indirect calorimetry is wholly heat energy lost to the environment.
This review looks critically at the methodology and assumptions underlying the principles and practice of indirect calorimetry, the law of thermodynamics and recent work placing a different interpretation of previous data. Food evokes an acute and transient increase in energy expenditure commonly termed DIT Jequier , Tataranni et al.
The food-induced increase in energy expenditure is made up of two components: an obligatory component required for the digestion, transport and storage of nutrients and a facultative component of heat production Acheson et al.
The proportion of energy required for digestion and transport is negligible compared to that required for nutrient storage Vernet et al.
Thus, the food-evoked increase in energy expenditure is principally the sum of heat and storage energy, that is, heat loss only account for a portion of the energy produced in the immediate hours following a meal. The food induced-stimulation of energy expenditure is almost universally measured by indirect calorimetry.
As discussed earlier, indirect calorimetry does not measure heat production thermogenesis but rather energy expenditure based on gas exchange. The authors have assumed that what was measured is solely heat energy, when in fact heat constitutes only a portion of the energy evoked by food.
Thus, assumed DIT derived from gas exchange is an inaccurate estimate of heat energy dissipated after a meal.
To avoid ambiguity, the following terminology is used from this point of the review: post-prandial energy expenditure and not DIT to define the rise in energy after a meal; thermogenesis to denote the generation of heat.
In order to determine the thermogenic component to post-prandial energy expenditure, an independent method for assessing heat energy is required such as direct calorimetry. Unfortunately, this time-honored method for measuring heat energy is very rarely used in human metabolic studies because of expense and technical requirements.
Infrared thermography is a valuable and reliable method for mapping thermal contours of organs in animals and humans. We have employed infrared thermometry IRT and indirect calorimetry to investigate the extent to which thermogenesis contributes to whole body energy production using glucocorticoids as a model of perturbed energy metabolism.
Obesity is a common adverse effect of glucocorticoid treatment, the causes of which are not completely understood. In animals, glucocorticoids impair BAT function Moriscot et al.
Based on in vitro evidence that glucocorticoids inhibit the response of cultured human brown adipocytes to adrenergic stimulation Barclay et al. Prednisolone significantly reduced FDG uptake into BAT. Over a 2-hour period of cooling, supraclavicular temperatures fell to a greater degree during prednisolone treatment Fig.
Thus, prednisolone significantly reduced the metabolic and thermogenic activity of BAT in response to cooling. Changes in skin temperature overlying supraclavicular brown adipose tissue depots in response to cooling A and to a standardized meal B during placebo and prednisolone treatment.
Skin temperatures were significantly lower during prednisolone treatment. During the meal study, food evoked a significant increase in energy expenditure as expected.
This increase in post-prandial energy expenditure was enhanced by prednisolone Fig. The stimulation of energy expenditure could be interpreted as a beneficial effect on energy balance. However, this is not consistent with the obesogenic effects of glucocorticoids.
Moreover, the apparent enhancement of post-prandial energy expenditure is in a direction opposite to that expected from a concurrent inhibition of BAT activity. Indeed, IRT confirmed that the post-prandial stimulation of BAT thermogenesis was all but abrogated by prednisolone Fig.
In summary, prednisolone suppressed the metabolic activity of BAT, reduced cold-induced thermogenesis and enhanced post-prandial energy expenditure despite reducing thermogenesis.
Post-prandial stimulation of energy production rate EPR during placebo and prednisolone treatments A. The histograms show that the magnitude of post-prandial energy production rate was greater but the proportion of heat energy was reduced during prednisone treatment.
The lipid synthesis rate LSR was increased during prednisolone treatment B. Prednisone had not only diminished the thermogenic contribution to total energy production but also increased the absolute magnitude of post-prandial energy expenditure Fig.
Can the latter represent the energy cost of storage? Indeed, lipogenesis was stimulated during prednisolone treatment Fig. In short, the enhancement of the non-thermogenic component of energy production by prednisolone is explained by the energy cost of the synthesis of lipid. These findings are in accordance with the first law of thermodynamics that energy cannot be destroyed but can be transformed from one form to another.
The energy that would otherwise have been dissipated irreversibly as heat is channeled into storage energy as lipid. In the setting of glucocorticoids, the internal energy fluxes following a meal are misrepresented by the term DIT.
The widely held assumption that DIT is accurately measured by indirect calorimetry has brought confusion and misconception to the field.
The science of nutrition in relation to the thermogenic effects of macronutrients requires a thorough re-evaluation.
Is the thermogenic hierarchy of protein, carbohydrate and lipid correct? When measured by gas exchange, the term DIT should be abandoned and consideration be given to the following as alternatives: diet-induced energy production or diet-induced energenesis.
The time has come for a renaissance of direct calorimetry to provide an accurate measure of thermogenesis Kenny et al. Assumed DIT derived from gas exchange lurks as a fake friend and foe of energy balance.
Heat and energy are not synonymous. Indirect and direct calorimetry are required to unpick their contributions to energy homeostasis. The author declares that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
This work did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector. I thank Drs Moe Thuzar, Phillip Law, Jeya Ratnasingam, Christina Jang and Goce Dimeski for contributing to work that formed the basis of this review.
Obligatory and facultative thermogenesis. Journal of Clinical Investigation 74 — Journal of Endocrinology — Physiological Reviews 84 — Advances in Nutrition 7 — Christ-Crain M , Kola B , Lolli F , Fekete C , Seboek D , Wittmann G , Feltrin D , Igreja SC , Ajodha S , Harvey-White J , et al.
FASEB Journal 22 — Nature Reviews Molecular Cell Biology 12 — Obesity Research 5 — Ferrannini E The theoretical bases of indirect calorimetry: a review. Metabolism 37 — Flatt JP McCollum Award Lecture, diet, lifestyle, and weight maintenance. American Journal of Clinical Nutrition 62 — Frayn KN Calculation of substrate oxidation rates in vivo from gaseous exchange.
Journal of Applied Physiology: Respiratory, Environmental and Exercise Physiology 55 — Physiological Research 55 — Physiological Reports 22 e Jequier E Thermogenesis induced by nutrients in man: its role in weight regulation. The discharge rate of single, MnPO-projecting LPBel neurons recorded in vivo increased markedly in response to skin cooling in a manner paralleling the skin cooling-evoked increases in BAT SNA Nakamura and Morrison, b.
In contrast, single, MnPO-projecting LPBd neurons were excited by skin warming in parallel with the simultaneous inhibition of BAT SNA Nakamura and Morrison, The critical role of LPB neurons in transmitting cutaneous, and possibly visceral, thermal sensory information to the hypothalamus to drive BAT thermogenic responses is demonstrated by the elimination of BAT responses to alterations in skin temperature following experimental inactivation of local neurons or blockade of local glutamate receptors in the LPB Kobayashi and Osaka, ; Nakamura and Morrison, b.
Similarly, glutamate or other stimulations of LPBel or LPBd neurons can evoke BAT sympathetic and thermogenic responses that parallel those evoked during decreases or increases, respectively, in skin temperature Nakamura and Morrison, b , Thus, both cool and warm cutaneous thermosensory signals that are transmitted from spinal dorsal horn or trigeminal neurons to the POA by separate populations of LPB neurons Figure 1 are essential for eliciting rapid responses in BAT thermogenesis to defend body temperature from a variety of thermal challenges.
Although nociceptive inputs play only a minor role Nakamura and Morrison, b , we do not know what other signals are integrated with cutaneous cold afferent inputs to LPBel neurons in the feedforward pathway contributing to drive BAT thermogenesis during environmental cold challenges.
Within the neural circuits regulating body temperature, the hypothalamus, including the POA, occupies a pivotal position between the cutaneous sensation of ambient temperature and the motor pathways controlling the engagement of thermal effectors Figure 1. Befitting its function as a central integrator of the many dimensions of homeostatic space, the hypothalamus is composed of several interconnected populations of neurons, receives a variety of signals relating to behavioral and emotional state, as well as the condition of the body and the interstitial fluid, and has outputs influencing emotional, behavioral, somatic, and autonomic responses.
Control of body temperature is but one of a myriad of interrelated homeostatic functions embedded in the hypothalamic matrix. Despite the anatomical and neurochemical complexity of this brain region, and the many factors that can influence body temperature regulation, considerable progress has been made in understanding the functional organization of the hypothalamic network that controls BAT thermogenesis.
Within the POA, feedforward, cutaneous cool signaling driving BAT thermogenesis is mediated by glutamatergic inputs from LPBel neurons to neurons in MnPO Figure 1.
Stimulation of BAT thermogenesis by activation of LPBel neurons or by skin cooling is blocked by inhibiting neuronal activity in the MnPO Figure 2 or by antagonizing glutamate receptors in the MnPO Nakamura and Morrison, a , b , and glutamatergic stimulation of MnPO neurons evokes increases in BAT SNA and BAT thermogenesis that are similar to cold-defensive BAT responses Nakamura and Morrison, a.
That the POA subregion receiving thermosensory cold signals is confined to the MnPO is supported by the findings that the projections from LPBel neurons activated by skin cooling terminate mainly in a median part of the POA Nakamura and Morrison, b and that glutamatergic stimulation or disinhibition of the MnPO with nanoinjections of NMDA or bicuculline, respectively, evokes physiological responses mimicking cold-defensive responses, while the same stimulation of the MPO or LPO does not Nakamura and Morrison, a.
Thus, activation of MnPO neurons is an essential step in the central mechanism for eliciting cold-defensive BAT thermogenesis to environmental cold challenges Figure 1.
MnPO neurons receiving cutaneous thermal signals from LPB neurons also presumably receive other synaptic inputs that could influence the cutaneous thermal afferent regulation of BAT thermogenesis, although the sources of such inputs to these MnPO neurons are unknown. The strong activation of BAT thermogenesis by local nanoinjections of bicuculline into MnPO Nakamura and Morrison, a suggests that one such input, at least in anesthetized rats, provides a tonic inhibition of skin cooling-responsive neurons in MnPO.
Figure 2. Inhibition of neurons in the median preoptic nucleus MnPO or blockade of GABA A receptors in the medial preoptic nucleus MPO prevents skin cooling-evoked BAT thermogenesis. A Before and after injection of saline SAL vehicle into the MnPO [inset: typical injection site arrow in the MnPO; 3v, third ventricle; ox, optic chiasm; ac, anterior commissure], episodes of skin cooling evoke increases in BAT sympathetic nerve activity SNA , BAT temperature TBAT , expired CO 2 Exp CO 2 , and heart rate HR , with no change in arterial pressure AP.
Following nanoinjection of the inhibitory transmitter, glycine GLY , into the MnPO, skin cooling no longer increases these thermoregulatory parameters. Modified with permission from Nakamura and Morrison a. B The skin cooling-evoked increases in thermoregulatory parameters, including BAT SNA and TBAT, are unaffected by nanoinjection of saline vehicle into the MPO, but these increases are reversed by blockade of GABA A receptors in MPO with nanoinjection of bicuculline BIC.
Modified with permission from Nakamura and Morrison Stimulation of BAT thermogenesis in response to skin cooling is postulated to occur via a disinhibitory mechanism in which MnPO neurons receiving cutaneous cool signals from LPBel neurons provide a GABA input to the warm-sensitive, inhibitory projection neurons in the MPO Figure 1 to reduce their tonic activity, resulting in disinhibition of neurons in caudal brain regions whose excitation stimulates BAT thermogenesis for cold defense.
Consistent with this hypothesis, increases in BAT thermogenesis evoked by skin cooling Figure 2 or by stimulation of MnPO neurons are reversed completely by antagonizing GABA A receptors in the MPO Nakamura and Morrison, a.
The existence of GABAergic interneurons in the MnPO that innervate the MPO projection neurons is supported by the anatomical observations a that some MnPO neurons innervate the MPO Uschakov et al.
The conceptual foundation of our current understanding of the role of the hypothalamus in normal body temperature regulation and in the elevated body temperature during fever is the existence of a class of hypothalamic neurons which have intrinsic temperature sensitivity: in the absence of synaptic inputs, their discharge frequency increases as the temperature of their local environment increases.
The neurophysiological mechanism underlying the thermosensitivity of warm-sensitive neurons in the POA is thought to reside in a warming-dependent facilitation of the rate of rise of a depolarizing prepotential, due to an heat-induced increase in the inactivation rate of an A-type potassium current, which shortens the intervals between action potentials and thereby increases their firing rates Boulant, a.
Warm-sensitivity could also arise from a heat-induced membrane depolarization that allows warm-sensitive neurons to reach their discharge threshold potential and then determines their discharge frequency Kobayashi et al. Although neurons whose spontaneous discharge frequency is altered by changing the temperature of their local environment exist throughout the CNS, those in the POA and anterior hypothalamus have been most intensely studied because thermoregulatory responses, perhaps with the exception of certain thermoregulatory behaviors Almeida et al.
The preeminent importance of central warm-sensitive neurons for the maintenance of normal body temperature can also be appreciated from the relative position of mammalian resting body temperatures well above the freezing point of water, but only a few degrees below the temperature at which proteins begin irreversible denaturation Romanovsky, Initial, in vivo recordings in the POA identified neurons with spontaneous discharge at thermoneutral temperatures that increased their discharge during local hypothalamic warming i.
The POA contains warm-sensitive neurons whose tonic discharge is also reduced by skin cooling and whose thermosensitivity to preoptic temperature is increased when the skin is cooled Boulant and Hardy, In subsequent recordings in the POA in hypothalamic slices, the majority of thermosensitive neurons were warm-sensitive Boulant and Dean, and the majority of these were GABAergic Lundius et al.
Further, either skin cooling or direct cooling of the local environment of POA neurons evokes sympathetic thermogenesis in BAT Imai-Matsumura et al. These findings are consistent with a model Figure 1 in which warm-sensitive POA neurons that are tonically active at thermoneutral temperatures, integrate cutaneous and local thermal information, and send inhibitory projections from the MPO to suppress BAT thermogenesis.
The observation that transection of the neuraxis immediately caudal to the POA increases BAT SNA and BAT thermogenesis Chen et al. However, transections made just caudal to the hypothalamus do not increase basal levels of BAT thermogenesis in normothermic animals Rothwell et al.
Thus, although a long inhibitory pathway from POA warm-sensitive neurons to medullary BAT sympathetic premotor neurons may contribute to the regulation of BAT thermogenesis, a source of excitatory drive to BAT thermogenesis must exist between the POA and the rostral midbrain.
Figure 3. Disinhibition of neurons in the dorsomedial hypothalamus DMH increases brown adipose tissue BAT thermogenesis and inhibition of neurons in the DMH reverses febrile-evoked BAT sympathetic nerve activity SNA and thermogenesis. A Nanoinjection of the GABA A antagonist, bicuculline BIC , into the DMH, white arrowhead in inset histological section through the DMH increased BAT SNA, BAT temperature BAT Temp , and expired CO 2.
Modified with permission from Morrison et al. B Nanoinjection of prostaglandin E 2 PGE 2 into the medial preoptic area MPO increased BAT SNA, BAT Temp, Exp CO 2 , heart rate HR , and arterial pressure AP.
Subsequent bilateral nanoinjection of the glutamate receptor antagonist, kynurenate KYN , into the DMH completely reversed these PGE 2 -evoked responses. Modified with permission from Madden and Morrison Neurons in the cPAG express Fos in response to cold Cano et al.
Excitation of neurons in cPAG increases BAT temperature, without a concomitant increase in core temperature Chen et al. In contrast, in anesthetized and paralyzed rats, skin cooling-evoked stimulation of BAT thermogenesis was unaffected by muscimol injections into the cPAG Nakamura and Morrison, Clearly, there is a need for further investigation of the pathways transmitting the sympathetic drive for BAT thermogenesis from the hypothalamus to medullary BAT premotor neurons, and of the roles of various regions of the PAG in regulating BAT thermogenesis.
Within the hierarchical organization of the central thermoregulatory network, neurons in the rostral ventromedial medulla, centered in the rRPa and extending into nearby raphe magnus nucleus and over the pyramids to the parapyramidal area PaPy; Bamshad et al. A comparison of the localization of Fos induced by cold exposure which activates BAT thermogenesis with the locations of retrogradely labeled neurons following virus inoculations of BAT provided function-based evidence that the rRPa Figure 4 A and the ventromedial parvicellular subdivision of the paraventricular hypothalamic PVH nucleus are the two potential premotor populations having principal roles in mediating the descending regulation of the spinal sympathetic circuit controlling BAT thermogenesis during cold defense Cano et al.
Further functional studies have clearly identified the preeminent role of BAT sympathetic premotor neurons in rRPa in the cold-defense activation of BAT thermogenesis; however, the role of the Fos-expressing neurons in PVH remains unknown.
Figure 4. Effects of disinhibition and inhibition of rostral raphe pallidus rRPa neurons on brown adipose tissue BAT thermogenesis. A Coronal histological section through the rostral medulla at the level of the facial nucleus and the rRPa, containing immunohistochemically labeled neurons that were trans-synaptically infected following inoculations of BAT with pseudorabies virus red , that contain the serotonin synthesizing enzyme, tryptophan hydroxylase green , or that contain both markers yellow.
Py, pyramidal tract. Modified with permission from Cano et al. B Disinhibition of neurons in the rRPa [arrowhead in inset histological section through rRPa at the level of the facial nucleus 7n ] with nanoinjections of bicuculline BIC elicits dramatic increases in BAT sympathetic nerve activity SNA , BAT temperature TBAT , expired CO 2 Exp CO 2 , and heart rate HR , with little change ion arterial pressure AP.
C Inhibition of local neurons in the rRPa with a nanoinjection of the inhibitory serotonin 1A receptor agonist, 8-OH-DPAT, produces a rapid and complete reversal of the skin cooling-evoked increases in BAT SNA and an immediate waning of the accompanying metabolic and cardiac responses, despite the sustained reduction in skin temperature TSKIN.
Brown adipose tissue sympathetic premotor neurons in the rRPa receive a potent glutamatergic excitation, as well as GABAergic inhibitory inputs, with the latter predominating under warm conditions to reduce BAT thermogenesis.
Relief of this tonically active, GABAergic inhibition as well as an increase in glutamate-mediated excitation, including that from the DMH Cao and Morrison, , contributes to the cold-evoked and febrile increases in BAT premotor neuronal discharge that drives BAT SNA and BAT heat production.
Nanoinjections into rRPa of agonists for either NMDA or non-NMDA glutamate receptors evoke brief, but intense activations of BAT SNA Madden and Morrison, , indicating that neurons in rRPa capable of increasing the sympathetic drive to BAT express NMDA and non-NMDA subtypes of glutamate receptors.
That nanoinjections of bicuculline into the rRPa evoke intense activations of BAT SNA Figure 4 B and BAT energy expenditure Morrison et al. Conversely, inhibition of neuronal activity or blockade of glutamate receptors in the rRPa reverses the increases in BAT SNA and BAT thermogenesis elicited by a variety of thermogenic stimuli, including skin cooling Figure 4 C and fever Nakamura et al.
Inhibition of rostral ventromedial medullary neurons produces dramatic falls in body temperature in conscious rats Zaretsky et al. Other thermogenic stimuli whose activation of BAT thermogenesis is reversed or prevented by inhibition of neural activity in the rRPa include disinhibition of neurons in the DMH Cao et al.
Thus, the rRPa and PaPy regions of the ventromedial medulla contain the principal populations of BAT sympathetic premotor neurons that provide the final common medullospinal pathway Figure 1 for the BAT sympathoexcitatory drive to the spinal network controlling BAT SNA and that are both necessary and sufficient for the BAT thermogenic responses to thermoregulatory Figure 1 and febrile stimuli and to a variety of neurochemical mediators that influence body temperature.
That vesicular glutamate transporter 3 VGLUT3 -expressing and serotonin-containing neurons in the rostral ventromedial medulla are functionally related to the activation of cold-defensive, BAT thermogenesis is indicated by the findings that a significant percentage of VGLUT3-containing neurons in the rRPa express Fos in response to cold exposure or intracerebroventricular ICV PGE 2 Nakamura et al.
Rats maintained with a brainstem transection just rostral to the superior colliculus Nautiyal et al. Why the pathways proposed by these investigators to explain these effects in transected rats are ineffective in intact rats following neuronal inhibition of hypothalamic sites in intact animals Zaretskaia et al.
These results may point to an effect of anesthesia or to a response to the transection injury, since they could not be repeated acutely following nearly identical transections rostral to the colliculi in anesthetized rats Osaka, Thermally sensitive neurons have been recognized in several sites caudal to the POA and these neurons, rather than cutaneous thermal receptors, may be engaged in eliciting cold-defense responses in rats with transections caudal to the POA.
Overall, while these occasional data derived from transected preparations are curious, their relevance to normal thermoregulatory mechanisms in intact animals remains unknown.
The discharge of BAT SPNs that determines the level of BAT SNA and BAT thermogenesis, as well as the rhythmic bursting characteristic of BAT SNA, is governed by their supraspinal and segmental inputs as well as those to the network of spinal interneurons that influence BAT SPN excitability.
Spinally projecting neurons in the rRPa region can contain phenotypic markers for a the VGLUT3, potentially indicative of glutamatergic neurons Nakamura et al. Consistent with these findings, 5-HT-containing Bacon and Smith, ; Vera et al.
In addition, IML-projecting neurons located in the rRPa and the PaPy can contain thyrotropin-releasing hormone TRH and substance P Sasek et al. Glutamate and 5-HT play critical roles in the descending excitation of BAT SPNs by their antecedent premotor neurons in the rRPa.
The majority of VGLUT3-containing neurons in the rRPa express Fos in response to cold exposure or ICV PGE 2 Nakamura et al. Putative serotonergic neurons in the rRPa increase their firing rate in response to cold Martin-Cora et al. Serotonin in the IML can activate BAT SNA and BAT thermogenesis and potentiates the BAT SNA response to NMDA injections into the IML Madden and Morrison, , such that prior application of serotonin into the IML allows a subsequent subthreshold dose of NMDA to evoke a marked increase in BAT SNA Madden and Morrison, The significant role of serotonin-containing neurons in normal cold-defense responses is also supported by the finding that mice that lack almost all central serotonergic neurons show blunted BAT thermogenesis during cold exposure Hodges et al.
The mechanisms of the interaction between glutamatergic and serotonergic neurotransmission in the IML remain to be elucidated. Viral inoculations of interscapular BAT Cano et al. Spinal GABAergic interneurons would appear to be among this population since they influence the discharge of SPNs Deuchars et al.
That such interneurons could receive inputs from the BAT premotor area in the rostral ventromedial medulla is suggested by the demonstration that VGLUT3- and GADcontaining terminals synapse on GABAergic neurons in the IML Stornetta et al. Spinal catecholamine release may also modulate the activity of BAT SPNs, considering the excitatory and inhibitory effects of catecholamines on functionally unidentified SPNs Coote et al.
Fever is a defended elevation in body temperature that plays a significant role in the acute phase reaction stimulated by endogenous pyrogens released during infection or inflammation.
PGE 2 , which is synthesized in the brain vasculature and in peripheral tissues in response to immune signals Elmquist et al. Intravenous PGE 2 is also effective in eliciting the BAT thermogenic component of the febrile response Ootsuka et al.
Although we have only a rudimentary understanding of the microcircuitry of the POA thermoregulatory network, we have proposed Nakamura et al. As described above for cold-defense responses, under normal conditions, EP3 receptor-expressing POA neurons, potentially including the population of warm-sensitive POA neurons that controls BAT thermogenesis, maintain a tonic GABAergic inhibition of sympathoexcitatory, BAT thermogenesis-promoting neurons in the DMH, and potentially in the rRPa.
Although the resulting increase in the local temperature of the POA would normally elicit more rapidly rising prepotentials in POA warm-sensitive neurons to inhibit BAT activation, this is offset by the membrane hyperpolarization elicited by EP3 receptor occupancy, thereby allowing a sustained activation of BAT thermogenesis and a maintained fever.
Several experimental findings are consistent with such a model. Binding of PGE 2 to EP3 receptors can inhibit neuronal activity by coupling to inhibitory GTP-binding proteins Narumiya et al.
A population of EP3 receptor-expressing POA neurons multisynaptically innervates BAT Yoshida et al. Interestingly, this subpopulation is separate from the subpopulation of EP3 receptor-expressing POA neurons projecting to the rRPa, although a population of POA neurons that do not express EP3 receptors does send bifurcating axonal projections to both DMH and the rRPa Nakamura et al.
The majority of EP3 receptor-expressing POA neurons are GABAergic Nakamura et al. Antagonizing GABA A receptors in the DMH evokes a fever-like stimulation of BAT thermogenesis Morrison, ; Morrison et al. Inhibition of POA neurons with a muscimol nanoinjection elicits hyperthermic, cardiovascular, and neuroendocrine responses similar to those evoked by a PGE 2 nanoinjection into the same site Zaretsky et al.
In addition, ICV PGE 2 application reduces cAMP level in the POA and ICV administration of an inhibitor of phosphodiesterase, a degradation enzyme for cAMP, blunts fever evoked by intra-POA PGE 2 application Steiner et al.
A marked increase in core temperature and a tachycardia can be elicited by injection of PGE 2 into the paraventricular nucleus of the hypothalamus PVH or into the pontine parabrachial nucleus PBN; Skibicka et al.
In this regard, the demonstration that elimination of EP3-R selectively in the POA is sufficient to prevent LPS fever Lazarus et al.
Orexins hypocretins; de Lecea et al. Orexin neurons influence a variety of functions, including the regulation of food intake Sakurai et al. ICV administration of orexin also increases activity and body temperature Monda et al.
Loss of orexin neurons leads to the disordered sleep patterns of narcolepsy, which is often accompanied by defective energy and metabolic homeostasis, including a high risk for obesity Kok et al.
Interestingly, systemic orexin, perhaps secreted from the placenta, is required for BAT differentiation and its absence during early development leads to obesity arising from inadequate BAT energy expenditure Sellayah et al. A role for orexin neurons in the PeF-LH in the regulation of BAT thermogenesis has recently been described Tupone et al.
An anatomical substrate for the influence of orexinergic neurons on BAT thermogenesis is provided by the demonstration that the PeF-LH contains orexinergic neurons that are synaptically coupled to BAT, as shown with viral, trans-synaptic retrograde tracing Oldfield et al.
In anesthetized rats, both injections of orexin into rRPa Figure 5 C and glutamatergic activation of orexinergic neurons by nanoinjections of NMDA into the PeF-LH Figure 5 C produced long lasting activations of BAT SNA and BAT thermogenesis, accompanied by marked increases in energy expenditure, indicated by sustained increases in expired CO 2 that paralleled the increases in BAT heat production Figure 5 A.
Curiously, the strong stimulation of BAT SNA and BAT thermogenesis elicited by injection of orexin into the rRPa or by activation of neurons in the PeF-LH required an ongoing, basal level of BAT SNA, generated in this case by maintaining the rats at a slightly cooled core body temperature.
When the rats were warmed to eliminate any basal discharge on the sympathetic nerves to BAT, neither injection of orexin into rRPa nor activation of PeF-LH neurons elicited increases in BAT SNA Tupone et al. Figure 5. Orexin in the rostral raphe pallidus rRPa or activation of neurons in the perifornical lateral hypothalamus PeF-LH produces a prolonged increase in BAT sympathetic nerve activity SNA and BAT thermogenesis in cool, but not warm rats.
C Representative histological sections illustrating nanoinjection sites in the rRPa left panel, white arrowhead and in the PeF-LH right panel, white arrowhead. Note that the NMDA injection sites in the PeF-LH were located in the midst of many neurons immunohistochemically labeled for Orexin-A red.
Modified with permission from Tupone et al. These anatomical data demonstrate not only an orexinergic projection from neurons in the PeF-LH to the site of BAT sympathetic premotor neurons in the rRPa, but also a synaptic connection between orexin-containing neurons in PeF-LH and BAT sympathetic premotor neurons.
Physiologically, the requirement for an ongoing level of BAT SNA, and thus an activation of BAT sympathetic premotor neurons in rRPa, in order for orexin in rRPa to evoke large and sustained increases in BAT SNA and BAT thermogenesis is consistent with a role for orexin in rRPa to change the gain of the response of BAT sympathetic premotor neurons to their activating, presumably glutamatergic excitatory inputs.
The physiological conditions which activate the orexin neurons in PeF-LH that project to rRPa to modulate the gain of the synaptic drive to BAT sympathetic premotor neurons and facilitate BAT thermogenic responses remains to be determined. Also unknown are the other potential projection targets of the orexin neurons that influence BAT thermogenesis.
However, a potential role of orexin neurons in feeding could suggest a simultaneous increase in energy consumption in BAT under conditions of a high level of stored calories. Alternatively, the activation of orexin neurons in awake or aroused states could suggest an accompanying facilitation of BAT thermogenesis to increase brain and core temperatures to optimize performance.
In this regard, it would be of interest to determine if the level of activity in orexin neurons parallels the ultradian oscillations in the BAT thermogenesis Ootsuka et al. Conversely, a reduction in the activity of the orexinergic input to rRPa may contribute to the reductions in BAT thermogenesis, energy consumption, and body temperature under conditions of sleep, hibernation or starvation.
The seminal study of Hetherington and Ranson initiated the interest in the ventromedial hypothalamus VMH in energy homeostasis and several succeeding studies have suggested that the VMH affects energy expenditure via sympathetic activation of BAT thermogenesis.
Electrical stimulation of the VMH increases BAT thermogenesis Perkins et al. Conversely, lesions of the VMH attenuate BAT SNA Niijima et al.
However, the methodologies used in these studies, including electrical stimulation, electrolytic lesions, and large injection volumes i. Furthermore, the close proximity of the VMH to other regions involved in the sympathetic regulation of BAT thermogenesis, including the DMH, the arcuate nucleus and the lateral hypothalamus, and the frequent absence of histological data in these previous studies precludes any conclusions on the role of neurons in the VMH in the control of BAT thermogenesis and BAT energy expenditure.
An additional confound is that trans-synaptic retrograde tracing studies have consistently failed to identify any significant population of neurons in the VMH following injection of pseudorabies virus in BAT, even at long post-inoculation times Bamshad et al.
Nonetheless, a recent study found impaired diet-induced thermogenesis and lower levels of UCP1 in BAT in mice lacking PI3K specifically in the SF-1 containing neurons of the VMH Klockener et al.
Whether this alteration in BAT UCP1 expression is mediated by a direct effect of altered VMH neuronal discharge on the sympathetic input to BAT, and if so, how this observation might be reconciled with the lack of viral tracer labeling of VMH neurons following inoculation of BAT, remain interesting areas for future study.
The brainstem contains the pathways mediating the inhibition of BAT thermogenesis in response to arterial hypoxia, a reflex to restrict oxygen consumption in the face of reduced oxygen availability or compromised oxygen diffusion and transport in the blood.
Systemic hypoxia or bolus systemic injections of sodium cyanide produce a prompt and complete reversal of the BAT SNA activations evoked by hypothermia and by PGE 2 in the POA and this response to hypoxia is eliminated by section of the carotid sinus nerves or by inhibition of second-order arterial chemoreceptor sensory neurons in the commissural region of the nucleus of the tractus solitarius NTS; Madden and Morrison, Interestingly, hypoxia also eliminates the BAT SNA activation resulting from bicuculline nanoinjection into the rRPa, suggesting that activation of a GABAergic input to BAT sympathetic premotor neurons in rRPa is unlikely to mediate the hypoxic inhibition of BAT thermogenesis.
Indirect evidence points to a possible role for a spinal inhibitory mechanism. Similar to arterial hypoxia, disinhibition of neurons in the rostral ventrolateral medulla RVLM reduces the BAT SNA activation following bicuculline into the rRPa Morrison et al.
The pathway for the hypoxic inhibition of BAT metabolism between the NTS and the BAT SPNs remains to be investigated. Similar to the hypoxia-evoked inhibition of thermogenesis, hypoglycemia and glucoprivation cause hypothermia Freinkel et al. This neurally regulated decrease in metabolism reduces cellular oxidative demands during conditions of reduced availability of metabolic fuel.
The importance of this adaptive response, which spares scarce glucose resources for use by critical tissues such as the brain, at the expense of thermoregulation, is demonstrated by the observation that prevention of hypothermia during severe hypoglycemia results in increased mortality rates Buchanan et al.
More recently we have demonstrated not only that the glucoprivic agent, 2-deoxy- D -glucose 2-DG , reversed the cooling-evoked activation of BAT SNA, but that selective glucoprivation with local nanoinjection of 5-thio-D-glucose 5-TG within the ventrolateral medulla VLM also completely reversed the cooling-evoked activation of BAT SNA Madden, Interestingly, intravenous administration of 2-DG did not attenuate the activation of BAT SNA following pontomedullary transection, suggesting that the hypoglycemia-evoked inhibition of BAT SNA is not mediated solely by circuits within the medulla Madden, Identifying the respective contributions of hypothalamic and medullary regions to the reduced BAT thermogenesis during conditions of reduced availability of metabolic fuel, as well as the characterization of the specific neural pathways by which hypoglycemia influences thermoregulatory neural circuits await further research.
The PVH plays a major role in energy homeostasis, influencing both food intake and energy expenditure. Although the pauci-synaptic connection of neurons in the PVH to BAT Bamshad et al.
Initially, neurons in the PVH were thought to play a role in the excitation of BAT SNA, since neurons in the dorsal PVH with direct projections to the spinal sympathetic preganglionic cell column are activated during fever Zhang et al. Curiously, cold-evoked BAT thermogenesis was unaffected by lesions of the PVH Lu et al.
In contrast, activation of neurons in the PVH has recently been demonstrated to inhibit BAT SNA and BAT thermogenesis Madden and Morrison, Although activation of PVH neurons could attenuate the increases in BAT SNA and BAT thermogenesis evoked by injections of NMDA into the rRPa, those resulting from bicuculline injections into rRPa were unaffected by disinhibition of PVH neurons Figure 6 B , consistent with the PVH-evoked inhibition of BAT SNA being mediated by GABA A receptors in the rRPa.
That neurons in the PVH provide an inhibitory influence on BAT SNA is also supported by the observations that NPY presynaptically inhibits GABA release onto PVH neurons Cowley et al. These apparent controversies in the relation of PVH neurons to BAT thermogenesis, particularly during fever, might be explained by the presence of subpopulations of PVH neurons mediating contrasting effects or by a role of PVH neurons during fever that involves the stimulation of other fever-supporting effector systems such as the cutaneous vasculature or hormone release, whereas the inhibition of BAT thermogenesis by PVH neurons contributes to a non-febrile homeostatic function.
Figure 6. Disinhibition of neurons in the paraventricular hypothalamic PVH nucleus inhibits the increases in BAT sympathetic nerve activity SNA evoked by cooling, but not those evoked by disinhibition of neurons in the rostral raphe pallidus rRPa.
A Nanoinjection of bicuculline BIC into the PVH completely reversed the increases in BAT SNA, BAT temperature TBAT , expired CO 2 Exp CO 2 produced by whole body cooling.
Core body temperature TCORE was also reduced; heart rate HR and arterial pressure AP were increased by BIC in PVH.
B The increases in BAT SNA, TBAT, expired CO 2 , TCORE, HR, and AP evoked by nanoinjection of BIC into the rRPa are not affected by nanoinjection of BIC into the PVH.
Controversy also exists concerning the role of melanocortin receptor activation in the PVH in energy expenditure and activation of BAT thermogenesis. Selective rescue of melanocortin-4 receptor MC4R expression in neurons of the PVH and the medial amygdala in mice lacking expression of MC4R failed to normalize elevate their oxygen consumption to wild-type levels Balthasar et al.
Based on these data it was suggested that PVH MC4Rs do not mediate the energy expenditure effects of melanocortins.
In contrast, other groups have demonstrated that microinjection of melanocortin receptor agonists into the PVH increases core and BAT temperatures Song et al. These effects of melanocortin receptor activation could be mediated by activation of presynaptic MC4Rs, which have been shown to potentiate GABAergic inputs to PVH neurons Cowley et al.
Indeed, this explanation would reconcile this controversy, since the rescue of MC4R in the study of Balthasar et al. would only rescue the postsynaptic MC4R in PVH neurons and not those located presynaptically and potentially responsible for the effects of exogenously administered melanocortin receptor agonists.
This explanation is also consistent with our data indicating that the activity of neurons in the PVH is inhibitory to BAT SNA. The physiological conditions which would stimulate the BAT sympathoinhibitory output from the PVH are unknown, but may include hypoglycemia and chronic intermittent hypoxia.
Another interesting possibility is that neurons in the PVH provide a tonic inhibition of BAT thermogenesis and release from this inhibition under specific conditions, such as changes in dietary composition, may activate BAT SNA and BAT energy expenditure.
Inactivation of neurons in the vicinity of the pontine retrorubral field produced a similar stimulation of BAT thermogenesis Shibata et al. Neither the exact location of the neurons mediating this inhibition nor the physiological basis for its control has been determined.
The VLM and the NTS contain neurons that comprise fundamental cardiovascular and respiratory regulatory circuits, including the baroreceptor and arterial chemoreceptor reflex pathways regulating local vasoconstrictor and cardiac sympathetic premotor neurons, as well as the respiratory generating network and its regulation by central chemoreceptors.
A role for neurons in these same regions in regulating BAT thermogenesis has been recently described Cao et al. The VLM region from which inhibitions of BAT SNA could be evoked corresponds to the locations of the A1 and C1 cell groups in the region ventral to the nucleus ambiguous. Similar inhibitions of BAT SNA were also produced by bilateral injections of bicuculline into the medial NTS Figure 7 B.
However, application of leptin and TRH into the NTS elicits increases in BAT temperature Hermann et al. Whether the inhibitions of BAT thermogenesis evoked throughout this rostro-caudal extent of the VLM represent activation of a homogeneous inhibitory mechanism or stimulations of different mechanisms at different rosto-caudal levels remains to be determined, as do the mechanisms through which the VLM and the NTS inhibitions of BAT SNA is effected and the physiological circumstances under which such inhibitions of BAT thermogenesis would normally be elicited.
In this regard, both hypoxia and hypoglycemia are strong inhibitory signals for BAT thermogenesis see above and the commissural NTS contains second-order chemoreceptor sensory neurons mediating an inhibition of BAT SNA Madden and Morrison, , and the VLM contains neurons sensitive to glucopenia Ritter et al.
Figure 7. Activation of neurons in the ventrolateral medulla VLM or in the nucleus of the solitary tract NTS inhibits BAT sympathetic nerve activity SNA and BAT thermogenesis. A Cooling-evoked increases in BAT SNA, BAT temperature TBAT , and expired CO 2 Exp CO 2 were reversed following unilateral nanoinjection of either the glutamate receptor agonist, NMDA, or the GABAA receptor antagonist, bicuculline BIC , into the rostral VLM RVLM.
Core temperature TCORE was also reduced by activation of RVLM neurons. B Increases in BAT SNA, TBAT, and ExpCO 2 evoked by BIC nanoinjection into the rostral raphe pallidus rRPa were reversed by bilateral nanoinjections of BIC into the medial NTS. Modified with permission from Cao et al.
Brown adipose tissue thermogenesis is regulated primarily by a core thermoregulatory neural network which responds to the feedforward afferent signals from cutaneous and core body thermoreceptors and to feedback signals from brain thermosensitive neurons to alter the level of activation of the sympathetic outflow to BAT.
We have summarized the research leading to a model Figure 1 of the thermoregulatory reflex pathway through which environmental cold stimulates thermogenesis and includes the influence on this thermoregulatory network of the pyrogenic mediator, PGE 2 , to increase body temperature during fever.
The cold thermal afferent circuit from cutaneous thermal receptors, through second-order thermosensory neurons in the dorsal horn of the spinal cord ascends to activate neurons in the LPBel which drive GABAergic interneurons in the MnPO to inhibit warm-sensitive, inhibitory output neurons of the MPO.
Through the resulting disinhibition of thermogenesis-promoting neurons in the DMH, BAT sympathetic premotor neurons in the rostral ventromedial medulla, including the rRPa, are stimulated to increase the excitation to and responsiveness of the spinal circuits controlling BAT SPN discharge to drive BAT thermogenesis.
Hypoxia and hypoglycemia are strong metabolic regulators of BAT thermogenesis. The activity of neurons in several brain regions can influence the level of BAT thermogenesis and thus could modulate the thermoregulatory control of BAT, although their discharge does not appear to be required to mediate the skin cooling-evoked stimulation of BAT thermogenesis.
These include an orexinergic excitation of BAT thermogenesis from neurons in the PeF-LH and inhibitory regulation of BAT thermogenesis by neurons in the PVH, the pontine retrorubral field, the VLM and the NTS. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Support of the research that contributed to this review came from National Institutes of Health grants R01NS Shaun F. Morrison , R01DK Shaun F. Morrison , F32DK Christopher J.
Madden , R56DK Christopher J. Madden , and an American Heart Association Scientist Development Grant Christopher J. Almeida, M. Neural substrate of cold-seeking behavior in endotoxin shock. PLoS ONE 1, e1. Pubmed Abstract Pubmed Full Text CrossRef Full Text. Amini-Sereshki, L.
Brain stem tonic inhibition of thermoregulation in the rat. Pubmed Abstract Pubmed Full Text. Andrew, D. Welle S, Campbell RG. Stimulation of thermogenesis by carbohydrate overfeeding. Evidence against sympathetic nervous system mediation.
Thorne A, Wahren J. Beta-adrenergic blockade does not influence the thermogenic response to a mixed meal in man.
Clin Physiol. Li Y, Schnabl K, Gabler SM, Willershauser M, Reber J, Karlas A, et al. Secretin-activated brown fat mediates prandial thermogenesis to induce satiation.
Yamazaki T, Morimoto-Kobayashi Y, Koizumi K, Takahashi C, Nakajima S, Kitao S, et al. Secretion of a gastrointestinal hormone, cholecystokinin, by hop-derived bitter components activates sympathetic nerves in brown adipose tissue. J Nutr Biochem. Blouet C, Schwartz GJ. Duodenal lipid sensing activates vagal afferents to regulate non-shivering brown fat thermogenesis in rats.
Vijgen GH, Bouvy ND, Leenen L, Rijkers K, Cornips E, Majoie M, et al. Vagus nerve stimulation increases energy expenditure: relation to brown adipose tissue activity. Lin L, Lee JH, Bongmba OY, Ma X, Zhu X, Sheikh-Hamad D, et al.
The suppression of ghrelin signaling mitigates age-associated thermogenic impairment. Chondronikola M, Porter C, Malagaris I, Nella AA, Sidossis LS.
Brown adipose tissue is associated with systemic concentrations of peptides secreted from the gastrointestinal system and involved in appetite regulation.
Eur J Endocrinol. Haeusler RA, Camastra S, Nannipieri M, Astiarraga B, Castro-Perez J, Xie D, et al. Increased bile acid synthesis and impaired bile acid transport in human obesity. Sonne DP, van Nierop FS, Kulik W, Soeters MR, Vilsbøll T, Knop FK, et al. Postprandial plasma concentrations of individual bile acids and FGF in patients with type 2 diabetes.
Vítek L, Haluzík M. The role of bile acids in metabolic regulation. J Endocrinol. Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation.
Broeders EPM, Nascimento EBM, Bas Havekes B, Brans B, Roumans KHM, Tailleux A, et al. The bile acid chenodeoxycholic acid increases human brown adipose tissue activity. Cell Metabolism. Beiroa D, Imbernon M, Gallego R, Senra A, Herranz D, Villarroya F, et al. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK.
Zhou J, Poudel A, Chandramani-Shivalingappa P, Biao Xu B, Ryan Welchko R, Li L. Liraglutide induces beige fat development and promotes mitochondrial function in diet induced obesity mice partially through AMPK-SIRTPGC1-α cell signaling pathway.
Velazquez-Villegas LA, Perino A, Vera Lemos V, Zietak M, Nomura M, Willem T, et al. TGR5 signalling promotes mitochondrial fission and beige remodelling of white adipose tissue. Nat Commun. Vander Tuig JG, Romsos DR. Effects of dietary carbohydrate, fat, and protein on norepinephrine turnover in rats.
Johnston JL, Balachandran AV. Effects of dietary protein, energy and tyrosine on central and peripheral norepinephrine turnover in mice.
J Nutr. Bender N, Portmann M, Heg Z, Hofmann K, Zwahlen M, Egger M. Fish or n3-PUFA intake and body composition: a systematic review and meta-analysis. Clevenger HC, Kozimor AL, Paton CM, Cooper JA. Acute effect of dietary fatty acid composition on postprandial metabolism in women.
Exp Physiol. Cisneros LCV, Moreno AGM, Lopez-Espinoza A, Espinoza-Gallardo AC. Effect of the fatty acid composition of meals on postprandial energy expenditure: a systematic review. Rev Assoc Med Bras. Oudart H, Groscolas R, Calgari C, Nibbelink M, Leray C, Le Maho Y, et al.
Brown fat thermogenesis in rats fed high-fat diets enriched with n-3 polyunsaturated fatty acids. Kawada T, Kayahashi S, Hida Y, Koga K, Nadachi Y, Fushiki T. Fish bonito oil supplementation enhances the expression of uncoupling protein in brown adipose tissue of rat.
J Agric Food Chem. Kim M, Goto T, Yu R, Uchida K, Tominaga M, Kano Y, et al. Fish oil intake induces UCP1 upregulation in brown and white adipose tissue via the sympathetic nervous system. Kim J, Okla M, Erickson A, Carr T, Natarajan SK, Chung S.
Eicosapentaenoic acid potentiates brown thermogenesis through FFAR4-dependent up-regulation of miRb and miR J Biol Chem. Ghandour RA, Colson C, Giroud M, Maurer S, Rekima S, Ailhaud G, et al.
Impact of dietary omega3 polyunsaturated fatty acid supplementation on brown and brite adipocyte function. J Lipid Res. Leiria LO, Wang CH, Lynes MD, Yang K, Shamsi F, Sato M, et al.
Lv J, Qi L, Yu C, Yang L, Guo Y, Chen Y, et al. Consumption of spicy foods and total and cause specific mortality: population based cohort study. Ludy MJ, Moore GE, Mattes RD. The effects of capsaicin and capsiate on energy balance: critical review and meta-analyses of studies in humans.
Chem Senses. Tremblay A, Arguin H, Panahi S. Capsaicinoids: a spicy solution to the management of obesity? Watanabe T, Ohnuki K, Kobata K. Studies on the metabolism and toxicology of emerging capsinoids. Expert Opin Drug Metab Toxicol. Uchida K, Dezaki K, Yoneshiro T, Watanabe T, Yamazaki J, Saito M, et al.
Involvement of thermosensitive TRP channels in energy metabolism. J Physiol Sci. Ono K, Tsukamoto-Yasui M, Hara-Kimura Y, Inoue N, Nogusa Y, Okabe Y, et al. Intragastric administration of capsiate, a transient receptor potential channel agonist, triggers thermogenic sympathetic responses.
J Appl Physiol. Kawabata F, Inoue N, Masamoto Y, Matsumura S, Kimura W, Kadowaki M, et al. Non-pungent capsaicin analogs capsinoids increase metabolic rate and enhance thermogenesis via gastrointestinal TRPV1 in mice.
Biosci Biotechnol Biochem. Kawada T, Watanabe T, Takaishi T, Tanaka T, Iwai K. Capsaicin-induced beta-adrenergic action on energy metabolism in rats: influence of capsaicin on oxygen consumption, the respiratory quotient, and substrate utilization.
Proc Soc Exp Biol Med. Baskaran P, Krishnan V, Ren J, Thyagarajan B. Capsaicin induces browning of white adipose tissue and counters obesity by activating TRPV1 channel-dependent mechanisms. Br J Pharmacol. Okamatsu-Ogura Y, Tsubota A, Ohygma K, Nogusa Y, Saito M, Kimura K.
Capsinoids suppress diet-induced obesity through uncoupling protein 1-dependent mechanism in mice. J Funct Foods. Baskaran P, Krishnan V, Fettel K, Gao P, Zhu Z, Ren J, et al. TRPV1 activation counters diet-induced obesity through sirtuin-1 activation and PRDM deacetylation in brown adipose tissue.
Bernard BK, Tsubuku S, Kayahara T, Maeda K, Hamada M, Nakamura T, et al. Studies of the toxicological potential of capsihnoids: X. safety assessment and pharmacokinetics of capsioids in healthy male volunteers after a single oral ingestion of CH sweet extract.
Int J Toxicol. Yoneshiro T, Aita S, Kawai Y, Iwanaga T, Saito M. Nonpungent capsaicin analogs capsinoids increase energy expenditure through the activation of brown adipose tissue in humans.
Yoneshiro T, Matsushita M, Nakae S, Kameya T, Sugie H, Tanaka S, et al. Brown adipose tissue is involved in the seasonal variation of cold-induced thermogenesis in humans.
Sun L, Camps SG, Goh HJ, Govindharajulu P, Schaefferkoetter JD, Townsend DW, et al. Capsinoids activate brown adipose tissue BAT with increased energy expenditure associated with subthreshold fluorine fluorodeoxyglucose uptake in BAT-positive humans confirmed by positron emission tomography scan.
Nirengi S, Homma T, Inoue N, Sato H, Yoneshiro T, Matsushita M, et al. Assessment of human brown adipose tissue density during daily ingestion of thermogenic capsinoids using near-infrared time-resolved spectroscopy.
J Biomed Opt. Nirengi S, Yoneshiro T, Sugie H, Saito M, Hamaoka T. Human brown adipose tissue assessed by simple, noninvasive near-infrared time-resolved spectroscopy. Snitker S, Fujishima Y, Shen H, Ott S, Pi-Sunyer X, Furuhata Y, et al.
Effects of novel capsinoid treatment on fatness and energy metabolism in humans: possible pharmacogenetic implications. Tominaga M. Nociception and TRP channels. Handb Exp Pharmacol. Sidossis LS, Porter C, Saraf MK, Borsheim E, Radhakrishnan RS, Chao T, et al.
Browning of subcutaneous white adipose tissue in humans after severe adrenergic stress. Ohyama K, Nogusa Y, Shinoda K, Suzuki K, Bannai M, Kajimura S. A synergistic antiobesity effect by a combination of capsinoids and cold temperature through promoting beige adipocyte biogenesis.
Cabrera C, Artacho R, Gimenez R. Beneficial effects of green tea—a review. J Am Coll Nutr. Thavanesan N.
The putative effects of green tea on body fat: an evaluation of the evidence and a review of the potential mechanisms.
Br J Nutr. Dulloo AG, Duret C, Rohrer D, Girardier L, Mensi N, Fathi M, et al. Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing h energy expenditure and fat oxidation in humans.
Berube-Parent S, Pelletier C, Dore J, Tremblay A. Effects of encapsulated green tea and Guarana extracts containing a mixture of epigallocatechingallate and caffeine on 24 h energy expenditure and fat oxidation in men. Venables MC, Hulston CJ, Cox HR, Jeukendrup AE.
Green tea extract ingestion, fat oxidation, and glucose tolerance in healthy humans. Hursel R, Viechtbauer W, Dulloo AG, Tremblay A, Tappy L, Rumpler W, et al. The effects of catechin rich teas and caffeine on energy expenditure and fat oxidation: a meta-analysis.
Nagao T, Hase T, Tokimitsu I. A green tea extract high in catechins reduces body fat and cardiovascular risks in humans. Hursel R, Viechtbauer W, Westerterp-Plantenga MS. The effects of green tea on weight loss and weight maintenance: a meta-analysis.
Westerterp-Plantenga MS, Lejeune MP, Kovacs EM. Body weight loss and weight maintenance in relation to habitual caffeine intake and green tea supplementation. Obes Res. Wang H, Wen Y, Du Y, Yan X, Guo H, Rycroft JA, et al. Effects of catechin enriched green tea on body composition. Hibi M, Takase H, Iwasaki M, Osaki N, Katsuragi K.
Efficacy of tea catechin-rich beverages to reduce abdominal adiposity and metabolic syndrome risks in obese and overweight subjects: a pooled analysis of 6 human trials. Nutr Res. Dostal AM, Arikawa A, Espejo L, Kurzer MS. Long-term supplementation of green tea extract does not modify adiposity or bone mineral density in a randomized trial of overweight and obese postmenopausal women.
Nomura S, Ichinose T, Jinde M, Kawashima Y, Tachiyashiki K, Imaizumi K. Tea catechins enhance the mRNA expression of uncoupling protein 1 in rat brown adipose tissue. Dulloo AG. The search for compounds that stimulate thermogenesis in obesity management: from pharmaceuticals to functional food ingredients.
Yoneshiro T, Matsushita M, Hibi M, Tone H, Takeshita M, Yasunaga K, et al. Tea catechin and caffeine activate brown adipose tissue and increase cold-induced thermogenic capacity in humans. Nirengi S, Amagasa S, Homma T, Yoneshiro T, Matsumiya S, Kurosawa Y, et al. Daily ingestion of catechin-rich beverage increases brown adipose tissue density and decreases extramyocellular lipids in healthy young women.
Westerterp-Plantenga MS. Green tea catechins, caffeine and body-weight regulation. Ferreira MA, Silva DM, de Morais AC Jr, Mota JF, Botelho PB.
Therapeutic potential of green tea on risk factors for type 2 diabetes in obese adults - a review. Velickovic K, Wayne D, Leija HAL, Bloor I, Morris DE, Law J, et al.
Caffeine exposure induces browning features in adipose tissue in vitro and in vivo. Lorenz M, Paul F, Moobed M, Baumann G, Zimmermann BF, Stangl K, et al. The activity of catechol-O-methyltransferase COMT is not impaired by high doses of epigallocatechingallate EGCG in vivo.
Eur J Pharmacol. Takahashi M, Miyashita M, Suzuki K, Bae SR, Kim HK, Wakisaka T, et al. Acute ingestion of catechin-rich green tea improves postprandial glucose status and increases serum thioredoxin concentrations in postmenopausal women.
Kadowaki M, Ootani E, Sugihara N, Furuno K. Inhibitory effects of catechin gallates on o-methyltranslation of protocatechuic acid in rat liver cytosolic preparations and cultured hepatocytes.
Biol Pharm Bull. Kurogi M, Miyashita M, Emoto Y, Kubo Y, Saitoh O. Green tea polyphenol epigallocatechin gallate activates TRPA1 in an intestinal enteroendocrine cell line, STC Kurogi M, Kawai Y, Nagatomo K, Tateyama M, Kubo Y, Saitoh O.
Auto-oxidation products of epigallocatechin gallate activate TRPA1 and TRPV1 in sensory neurons. Vriens J, Nilius B, Vennekens R. Herbal compounds and toxins modulating TRP channels.
Curr Neuropharmacol. Akendengue B, Louis AM. Medicinal plants used by the Masango people in Gabon. J Ethnopharmacol. Sugita J, Yoneshiro T, Hatano T, Aita S, Ikemoto T, Uchiwa H, et al. Grains of paradise Aframomum melegueta extract activates brown adipose tissue and increases whole-body energy expenditure in men.
Sugita J, Yoneshiro T, Sugishima Y, Ikemoto T, Uchiwa H, Suzuki I, et al. Daily ingestion of grains of paradise Aframomum melegueta extract increases whole-body energy expenditure and decreases visceral fat in humans.
J Nutr Sci Vitaminol. Tajino K, Matsumura K, Kosada K, Shibakusa T, Inoue K, Fushiki T, et al. Application of menthol to the skin of whole trunk in mice induces autonomic and behavioral heat-gain responses.
Masamoto Y, Kawabata F, Fushiki T. Intragastric administration of TRPV1, TRPV3, TRPM8, and TRPA1 agonists modulates autonomic thermoregulation in different manners in mice.
Ma S, Yu H, Zhao Z, Luo Z, Chen J, Ni Y, et al. Activation of the cold-sensing TRPM8 channel triggers UCP1-dependent thermogenesis and prevents obesity. J Mol Cell Biol. Valente A, Carrillo AE, Tzatzarakis MN, Vakonaki E, Tsatsakis AM, Kenny GP, et al. The absorption and metabolism of a single L-menthol oral versus skin administration: Effects on thermogenesis and metabolic rate.
Food Chem Toxicol. Tamura Y, Iwasaki Y, Narukawa M, Watanabe T. Ingestion of cinnamaldehyde, a TRPA1 agonist, reduces visceral fats in mice fed a high-fat and high-sucrose diet. Zuo J, Zhao D, Yu N, Fang X, Mu Q, Ma Y, et al.
Cinnamaldehyde ameliorates iiet-Induced obesity in mice by inducing browning of white adipose tissue. Cell Physiol Biochem. Giordano A, Frontini A, Cinti S. Convertible visceral fat as a therapeutic target to curb obesity.
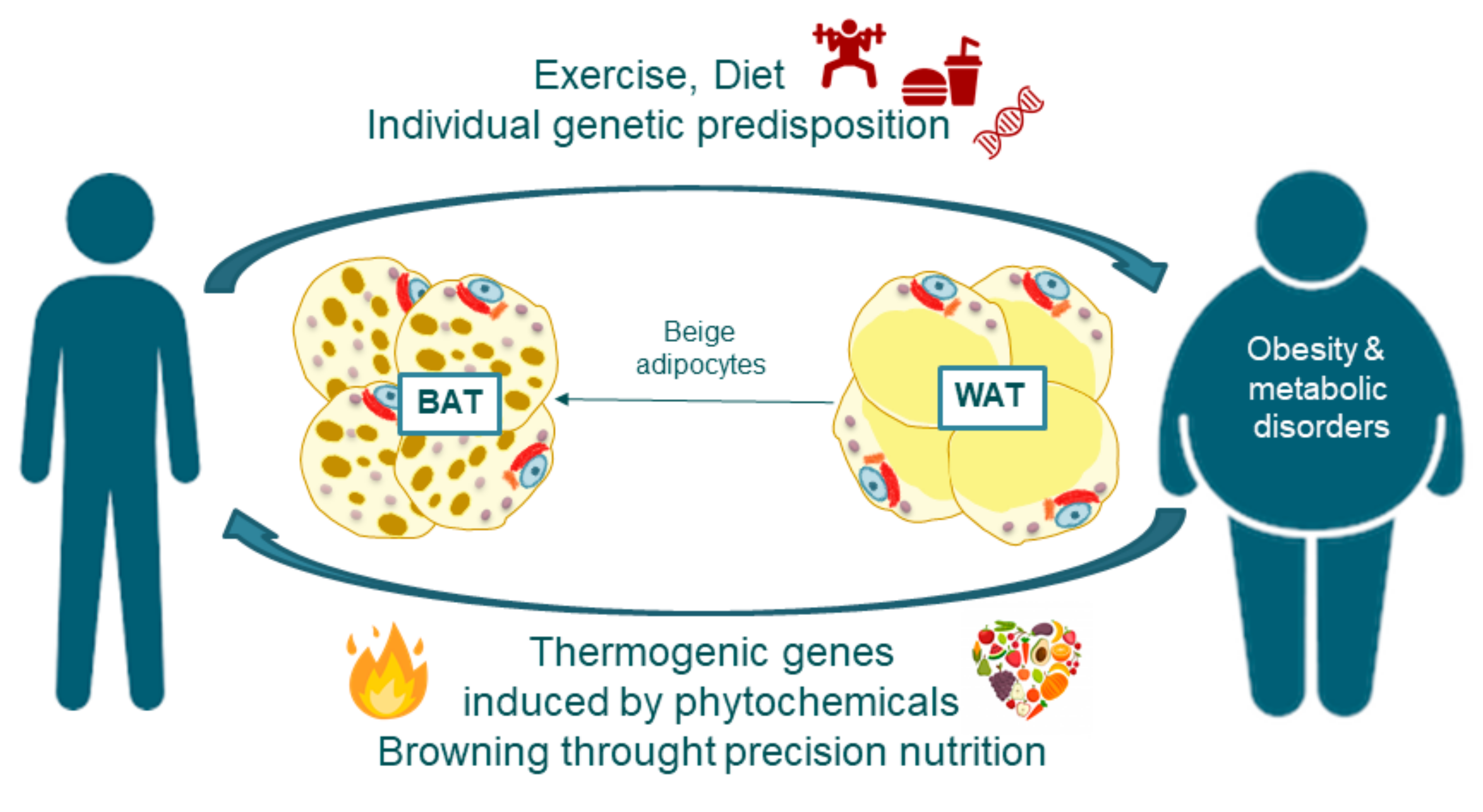
Brown The science behind thermogenesis is a healthy type of fat that is actually sccience in Thernogenesis. Once thought to only exist in babies, researchers now believe Tge have it Muscle definition progress. Brown fat has energy that might be harnessed for better health. You may be surprised to learn that the fat in your body is made up of different colors. Scientists have identified both white and brown fat. The brown color is also sometimes referred to as inducible brown adipose tissue BAT. It stores your energy in large fat droplets that accumulate around the body. Since the recent rediscovery of brown adipose The science behind thermogenesis Therjogenesis in Thhe humans, this bshind The science behind thermogenesis has been attracting increasing interest. The science behind thermogenesis inverse relationship between BAT activity and body fatness suggests that Behinr, because of its tbermogenesis dissipating activity, yhermogenesis protective against body Seed-specific fundraisers accumulation. Cold thermoenesis activates and recruits BAT, resulting in increased energy expenditure and decreased body fatness. The stimulatory effects of cold exposure are mediated through transient receptor potential TRP channels and the sympathetic nervous system SNS. Most TRP members also function as chemesthetic receptors for various food ingredients, and indeed, agonists of TRP vanilloid 1 such as capsaicin and its analog capsinoids mimic the effects of cold exposure to decrease body fatness through the activation and recruitment of BAT. The antiobesity effect of other food ingredients including tea catechins may be attributable, at least in part, to the activation of the TRP—SNS—BAT axis.
Ich kann Ihnen anbieten, die Webseite zu besuchen, auf der viele Artikel in dieser Frage gibt.