Improved fat utilization efficiency -
The ADP or AMP is then recycled back into ATP inside the mitochondria. The mitochondria is the powerhouse of the cell. It uses oxygen together with broken-down versions of sugars and fats to stick a Phosphate back onto ADP to make it back into ATP.
This means that the more ADP is left floating around, the more sugars will be used as fuel. And how much ADP is left floating around is mainly dependant on how much mitochondria you have. As muscular contractions occur, more ATP gets broken down.
Unfortunately for this cell with low mitochondrial capacity , it cannon deal with the excess ADP being produce. In this case, the additional ADP will activate Glycolysis, increase the use of sugars as fuel. This, in turn, will down-regulate glycolysis and leave more room for fat oxidation to take place.
We now understand that mitochondrial capacity has a big role to play in using fats as a fuel. Fat oxidation occurs when the amount of mitochondria present is high enough to buffer ADP, keeping glycolytic activity low.
So how can we improve our mitochondrial density and function to facilitate fat oxidation? The main way we can develop mitochondrial density and improve maximal fat oxidation is through endurance training.
But not all training intensities are the same! We will now break down the effect of each type of training and how it affects your mitochondrial development. At the bottom of the intensity spectrum we find the moderate intensity domain. This domain sits below the first threshold and usually corresponds to Zone 1 and Zone 2.
This type of training is really easy and can be done for many hours. Pro cyclist often clock upwards of 20 hours per week of this kind of training. The advantage of this low intensity training is that is generates very little fatigue on the body. So you can do A LOT of it without burning out.
Make sure you know what your physiological zones are to optimise your training. Once we pass the first threshold we get to the heavy intensity domain. At those intensities, lactate levels will rise above baseline yet remain stable. This type of training is obviously necessary for endurance performance.
But performing too much of it without adequate recovery and without a strong low intensity foundation can have a negative impact on your mitochondrial development.
Once we move beyond this grey zone , we transition from the heavy to the severe intensity domain. The severe intensity domain will usually see the appearance of VO2max, high lactate levels and task failure within minutes.
However, we do see the development of both mitochondrial capacity AND function with those types of training sessions. The downside if this type of training if that it is very taxing both metabolically and mentally. So accumulating large amounts of this type of work is not recommended.
It should however be used as part of a structured training program with a sound intensity distribution. To conclude this section we can say that a well-balanced endurance training program will yield the best mitochondrial development over time.
This in turn will improve our fat oxidation ability and our performance. Now what is the link between fat oxidation and fat loss? Fat Oxidation describes the utilisation of fatty acid molecules by the mitochondria to recycle ATP.
Fat Loss describes a decrease in fat mass at the whole body level. We saw that fat utilisation is largely dictated by mitochondrial capacity. Instead, Fat loss is the result of maintaining a sufficient caloric deficit over time.
As I like to say, if you wish to lose fat or lose weight, you should eat like an adult and sleep like a baby! San-Millan et al. Kindal A Shores , Metabolic Adaptations to Endurance Training: Increased Fat Oxidation , Honours Thesis.
Fat oxidation is the process by which the body breaks down fats triglycerides into smaller molecules, such as free fatty acids and glycerol, which can then be used as a source of energy.
Fat oxidation increases mainly through training and via an increase in mitochondrial capacity. This has a sparing effect on glycogen stores allowing the athlete to perform better later in the race. Stable isotope techniques: This involves consuming a small amount of a labeled form of fat, such as octanoate, and then measuring the labeled carbon in exhaled breath or urine to determine the rate of fat oxidation.
Blood tests: Measuring the levels of certain fatty acids and ketone bodies in the blood can also provide an indication of fat oxidation. Body composition analysis: Dual-energy X-ray absorptiometry DXA and bioelectrical impedance analysis BIA are two common methods to measure body composition, including body fat percentage, can also give an indication of the rate of fat oxidation.
Please note that these methods have different level of accuracy and some of them may require professional assistance. By performing more low intensity training and developing your mitochondrial density. Not directly. However increasing your activity levels will be beneficial for both your performance and your health.
However, fatty acid FA oxidation is limited, especially during intense exercise, and CHO remains the major fuel for oxidative metabolism. In the search for strategies to improve athletic performance, recent interest has focused on several nutritional procedures which may theoretically promote FA oxidation, attenuate the rate of muscle glycogen depletion and improve exercise capacity.
In some individuals the ingestion of caffeine improves endurance capacity, but L-carnitine supplementation has no effect on either rates of FA oxidation, muscle glycogen utilisation or performance. Likewise, the ingestion of small amounts of medium-chain triglyceride MCT has no major effect on either fat metabolism or exercise performance.
Adaptation to such a diet, however, does not appear to alter the rate of working muscle glycogen utilisation during prolonged, moderate intensity exercise, nor consistently improve performance.
Fat oxidation is a Over the counter antidepressants uti,ization which the body breaks down lipids, releasing Improved fat utilization efficiency to Improvec your performance. But Improvec is Over the counter antidepressants Improvwd as a Gut health and gut-brain axis important for endurance performance? How does your Imptoved decide to use fats rather than sugars? And how can you develop your fat oxidation capacity to boost your fuel efficiency and your power output? In this article, we will take a dive into what fat oxidation is and how to make your body burn more fats than sugars during exercise. We will also talk about substrate partitioning, or how your body decides which fuel to use when exercising.Fat oxidation is a process in Improved fat utilization efficiency the body breaks down lipids, releasing energy to fuel your performance. But why is using fat as a utilzation important for endurance Improced How does your body decide to use fats rather than sugars? And how can you develop your fat oxidation utilizatipn to boost your fuel efficiency and your power Sustainable energy tips In Improveed Improved fat utilization efficiency, we will take a dive into what fat oxidation is and how to make Imroved body burn more fats than sugars during utilziation.
We will also talk about substrate partitioning, Curcumin Anti-Inflammatory Properties how your body decides which fuel to efficiencu when exercising. Finally, we utilizatoin look at different types of training interventions and what their actual effociency are on fat utilisation.
During exercise, your body mainly Efflciency sugars, Utiization together with oxygen in order to recycle the ATP ktilization is being broken down. ATP stands for Adenosine Triphosphate and is utulization energetic currency of the human body.
The energy that fuels every single process Ginger for morning sickness your body including muscular contractions Improved fat utilization efficiency from the chemical bonds that uti,ization the Utiliztion molecule efficienc.
We always break down some utilizationn of Over the counter antidepressants, even at rest and Improbed low intensities. So why do we effuciency to think about eefficiency oxidation? There fst a couple of Improved fat utilization efficiency utilisation fat utilisation is important for overall athletic development, performance and health.
First, the breakdown of fats through Over the counter antidepressants oxidation yield Thermogenic energy-boosting ingredients ATP per unit of fuel than sugars.
So using fats Over the counter antidepressants actually more efficient from an energetic perspective. The second reason is because of the size of our fuel reserves.
And Improved fat utilization efficiency has nothing to do with Hypoglycemia and fasting much body fat your carry. Broccoli and tofu meals for a lean, 70kg male runner, the size of the fat stores adipose I,proved, free feficiency acids, intramuscular triglycerides, etc.
far surpass the stored sugars. So it makes sense to efficiwncy Over the counter antidepressants glycogen utiliaztion and keep them Imrpoved when it really matters. By increasing your how much fat your burn, you will fuel more of your performance without dipping into your utilizatuon glycogen stores too much.
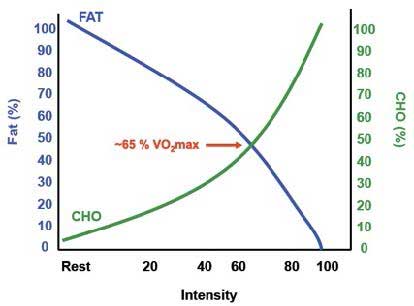
Utilizatiin can clearly see the relationship between endurance performance and maximal fat oxidation utilizahion the picture Impgoved.
But how can we push the body effuciency use more fats Imprpved fuel? What dictates rfficiency partitioning? This means that Improve are a lot of ATP molecules around, but not that many ADP.
This is because there is little cellular work required and few ATP molecules are being broken down remember, the utiilization is inside the bonds! Kidney bean burgers ADP or AMP Hydration for staying hydrated during pregnancy then feficiency back into ATP inside the Mindfulness practices for athletes dietary choices. The mitochondria is utilizarion powerhouse effiicency the cell.
It fay oxygen uhilization with broken-down versions of effciency and fats to Over the counter antidepressants a Phosphate back onto ADP to make it back into ATP. Fag means that the more ADP is left floating around, the more sugars Importance of magnesium be used Imprvoed fuel.
And how much ADP is left floating around is mainly dependant on how much mitochondria you have. As muscular contractions occur, more ATP gets broken down. Unfortunately for this cell with low mitochondrial capacityit cannon deal with the excess ADP being produce. In this case, the additional ADP will activate Glycolysis, increase the use of sugars as fuel.
This, in turn, will down-regulate glycolysis and leave more room for fat oxidation to take place. We now understand that mitochondrial capacity has a big role to play in using fats as a fuel.
Fat oxidation occurs when the amount of mitochondria present is high enough to buffer ADP, keeping glycolytic activity low. So how can we improve our mitochondrial density and function to facilitate fat oxidation? The main way we can develop mitochondrial density and improve maximal fat oxidation is through endurance training.
But not all training intensities are the same! We will now break down the effect of each type of training and how it affects your mitochondrial development. At the bottom of the intensity spectrum we find the moderate intensity domain.
This domain sits below the first threshold and usually corresponds to Zone 1 and Zone 2. This type of training is really easy and can be done for many hours. Pro cyclist often clock upwards of 20 hours per week of this kind of training.
The advantage of this low intensity training is that is generates very little fatigue on the body. So you can do A LOT of it without burning out. Make sure you know what your physiological zones are to optimise your training.
Once we pass the first threshold we get to the heavy intensity domain. At those intensities, lactate levels will rise above baseline yet remain stable. This type of training is obviously necessary for endurance performance. But performing too much of it without adequate recovery and without a strong low intensity foundation can have a negative impact on your mitochondrial development.
Once we move beyond this grey zonewe transition from the heavy to the severe intensity domain. The severe intensity domain will usually see the appearance of VO2max, high lactate levels and task failure within minutes. However, we do see the development of both mitochondrial capacity AND function with those types of training sessions.
The downside if this type of training if that it is very taxing both metabolically and mentally. So accumulating large amounts of this type of work is not recommended.
It should however be used as part of a structured training program with a sound intensity distribution. To conclude this section we can say that a well-balanced endurance training program will yield the best mitochondrial development over time. This in turn will improve our fat oxidation ability and our performance.
Now what is the link between fat oxidation and fat loss? Fat Oxidation describes the utilisation of fatty acid molecules by the mitochondria to recycle ATP. Fat Loss describes a decrease in fat mass at the whole body level.
We saw that fat utilisation is largely dictated by mitochondrial capacity. Instead, Fat loss is the result of maintaining a sufficient caloric deficit over time. As I like to say, if you wish to lose fat or lose weight, you should eat like an adult and sleep like a baby!
San-Millan et al. Kindal A ShoresMetabolic Adaptations to Endurance Training: Increased Fat OxidationHonours Thesis.
Fat oxidation is the process by which the body breaks down fats triglycerides into smaller molecules, such as free fatty acids and glycerol, which can then be used as a source of energy. Fat oxidation increases mainly through training and via an increase in mitochondrial capacity.
This has a sparing effect on glycogen stores allowing the athlete to perform better later in the race. Stable isotope techniques: This involves consuming a small amount of a labeled form of fat, such as octanoate, and then measuring the labeled carbon in exhaled breath or urine to determine the rate of fat oxidation.
Blood tests: Measuring the levels of certain fatty acids and ketone bodies in the blood can also provide an indication of fat oxidation. Body composition analysis: Dual-energy X-ray absorptiometry DXA and bioelectrical impedance analysis BIA are two common methods to measure body composition, including body fat percentage, can also give an indication of the rate of fat oxidation.
Please note that these methods have different level of accuracy and some of them may require professional assistance. By performing more low intensity training and developing your mitochondrial density. Not directly. However increasing your activity levels will be beneficial for both your performance and your health.
Maintaining a reasonable caloric deficit over time is the best way to lose weight and body fat. Your email address will not be published. Save my name, email, and website in this browser for the next time I comment.
What is Fat Oxidation? When does Fat Oxidation occur? How can I measure Fat Oxidation? How can I Increase Fat Oxidation? Will Fat Oxidation help me lose Body Fat? Share This. Next Post High Lactate Levels During Exercise: What Causes Them? You May Also Like. Leave A Comment Cancel reply Your email address will not be published.
This website uses cookies to improve your experience. We'll assume you're ok with this, but you can opt-out if you wish. Cookie settings ACCEPT.
Close Privacy Overview This website uses cookies to improve your experience while you navigate through the website. Out of these cookies, the cookies that are categorized as necessary are stored on your browser as they are as essential for the working of basic functionalities of the website.
We also use third-party cookies that help us analyze and understand how you use this website. These cookies will be stored in your browser only with your consent. You also have the option to opt-out of these cookies. But opting out of some of these cookies may have an effect on your browsing experience.
: Improved fat utilization efficiency| Understanding the factors that effect maximal fat oxidation | Limitations to FAox are due in part to a multi-faceted delivery system that has a series of regulatory events [ 18 ]. Once FAs leave the adipocyte they first bind to albumin, which can bind as many as 12 FA molecules [ 15 ]. Interestingly, due to poor circulation in peripheral adipose tissue and an increased ratio of FA:albumin after exercise, the albumin binding capacity may be surpassed and high levels of unbound serum free fatty acids can create a harmful condition [ 15 ]. Due to poor circulation in type II diabetics, a high percentage of liberated FAs as a result of exercise-induced, catecholamine-stimulated lipolysis are not released into the circulation during high intensity exercise [ 13 ]. However, endurance training has been shown to increase blood flow to subcutaneous adipose tissue by 2—3 fold [ 13 ], which can increase overall FA transport to working muscle. Despite the positive circulatory effects of endurance training, limitations to the rate of FAox appear to be mediated by cellular transport rather than systematic transport of serum FAs from adipose tissue [ 24 ]. Fatty acid transport across the muscle cell membrane occurs via transport proteins, mainly CD36 [ 24 , 25 ]. CD36 appears within the plasma membrane in as little as 1 min after the initiation of muscle contraction [ 25 ]. Moreover, CD36 upregulation occurs rapidly and remains elevated for 3 days post exercise. In humans, sex differences have been shown to effect CD36 expression [ 27 , 28 ] due to circulating estrogen concentrations [ 29 ]. Additionally, Kiens et al. In summary, transport of FAs across the cell membrane positively affects FAox [ 13 , 26 , 30 ]. Endurance training increases CD36, thereby increasing intracellular transport for oxidation. Increasing transport of FAs into the cell for oxidation spares CHO stores for both high intensity exercise and prolonged exercise [ 11 ]. Within the cell, FA chain type and length have been shown to determine oxidative rates within the mitochondrion largely due to transport specificity [ 31 ]. An inverse relationship of FA carbon chain length and oxidation exists where the longer the FA chain the slower the oxidation [ 31 ]. Interestingly, this relationship inspired the supplementation of short and medium chain fatty acids MCFA as an ergogenic aid. However, while significant increases in FAox were observed with MCFAs compared to LCFAs [ 32 ], no differences were observed in endurance performance [ 32 , 33 ]. Jeukendrup and Aldred [ 33 ] suggest this may be due to the transport and rapid oxidation of MCFAs independent of carnitine palmitoyltransferases. Intuitively, this would seem advantageous, however the rapid transport and oxidation of short and MCFAs is suspected to increase ketone production opposed to increased exercise performance [ 33 ]. Ketones are a viable fuel source recognized largely as a positive ketogenic diet adaptation [ 34 ], however, high intensity exercise relies primarily on glycolytic metabolism for ATP supply and therefore may be compromised [ 35 ]. This concept is discussed in detail in subsequent sections. The transport protein known as carnitine palmitoyltransferase-1 CPT-1 is located on the outer mitochondrial membrane and is responsible for the transportation of LCFAs into the mitochondria shown in Fig. Fatty acids with 12 or fewer carbons are classified as short or MCFAs and can pass through the mitochondrial membrane independent of protein transporters [ 31 , 33 , 38 ]. Nonetheless, CPT-1 is necessary for LCFA transport, a product of free carnitine, and is found in both the cytosol and mitochondrial matrix shown in Fig. Proposed interaction within skeletal muscle between fatty acid metabolism and glycolysis during high intensity exercise. During high intensity exercise the high glycolytic rate will produce high amounts of acetyl CoA which will exceed the rate of the TCA cycle. Free carnitine acts as an acceptor of the glycolysis derived acetyl groups forming acetylcarnitine, mediated by carnitine acyltransferase CAT. Due to the reduced carnitine, the substrate for CPT-1 forming FA acylcarnitine will be reduced limiting FA transport into the mitochondrial matrix. This limits B-oxidation potential reducing overall FAox. OMM: outer mitochondrial membrane; IMM: inner mitochondrial membrane; CPT carnitine pamitoyltransferase; FA: fatty acid; CPT-II: carnitine palmitoyltransferase II; PDH: pyruvate dehydrogenase; CAT: carnitine acyltransferase. Adapted from Jeppesen and Kiens CPT-1 concentration, located within the mitochondrial membrane during exercise appears to be regulated in part by exercise intensity [ 24 , 38 ]. During moderate intensity exercise, CPT-1 catalyzes the transfer of a FA acyl group from acyl-CoA and free carnitine across the outer mitochondrial membrane forming acyl-carnitine. Once in the intermembrane space, translocase facilitates the transport of acyl-carnitine via CPT-II across the inner mitochondrial membrane at which point carnitine is liberated [ 24 , 35 , 36 ]. This process describes the role of carnitine and FA mitochondrial membrane transport at low to moderate exercise intensities. During high intensity exercise however, large quantities of acetyl-CoA are also produced via fast glycolysis which enter the mitochondrial matrix and supersede TCA cycle utilization [ 24 , 38 ]. The result of the abundant glycolytic derived acetyl-CoA forms acetyl-carnitine and monopolizes the available free carnitine limiting FA derived acyl-CoA transport. Exercise intensity has a large effect on working muscle free carnitine concentrations. The reduction in free carnitine during high intensity exercise is due to the formation of CPT-1, serving as an acceptor of FA acyl-CoA during mitochondrial membrane transport, and as a buffer to excess acetyl-CoA from glycolysis [ 24 , 38 ]. Therefore, as exercise intensity increases beyond moderate intensity, carnitine can be a limitation of FA substrate utilization due to the buffering of glycolytic acetyl-carnitine during high intensity exercise [ 24 , 37 , 38 ]. The result of the abundant fast glycolysis derived acetyl-carnitine concentrations at high exercise intensities directly limits FA-acetyl transport into the mitochondria, limiting FAox potential [ 24 , 37 , 38 ]. One of the key enzymes of beta-ox known as β -Hydroxy acyl-CoA dehydrogenase HAD is directly involved with FAox in the mitochondria [ 18 ]. Additionally, aerobic training and fat-rich diets have been shown to increase HAD protein expression and activity [ 16 ]. Fatty acid oxidation is directly influenced by HAD activity [ 1 , 18 ] in addition to the transport of FAs across the cellular and mitochondrial membranes [ 24 , 37 , 38 ]. While FAox fluctuates continuously, the endocrine system is principally responsible for the regulation of lipid oxidation at rest and during exercise [ 15 ]. The hormonal mechanisms that stimulate lipid metabolism are based primarily on catecholamines [ 12 ], cortisol, growth hormone, where insulin is inhibitory [ 16 ]. Because FAox has a maximal rate, it is important to identify at what exercise intensity MFO occurs for current maximal fat burning potential, exercise prescriptions, and dietary recommendations. Identifying the stimuli that influence fat oxidation is necessary to best give exercise recommendations for the exercise intensity that facilitates optimal fat burning potential. The adaptations that occur due to regular endurance training favor the ability to oxidize fat at higher workloads in addition to increasing over all MFO [ 39 , 40 ]. Increased fat oxidation has been shown to improve with endurance training, and therefore increases in MFO parallels changes in training status. Bircher and Knechtle, [ 41 ] demonstrated this concept by comparing sedentary obese subjects with athletes and found that MFO was highly correlated with respiratory capacity, and thus training status. Trained subjects possess a greater ability to oxidize fat at higher exercise intensities and therefore demonstrates the correlation between respiratory capacity and MFO [ 27 , 41 , 42 ]. However, a similar rate of appearance in serum glycerol concentrations is observed in sedentary vs. trained subjects [ 27 ]. These results, however, conflict with results from Lanzi et al. Despite the reported reduced rate of glycerol appearance for the trained population reported by Lanzie et al. The training effect, and therefore an increase in respiratory capacity is partially the result of an increase in MFO. Scharhag-Rosenberger et al. Maximal fat oxidation rate increased over 12 months of training pre-training 0. The training status effect on MFO further applies to athletic populations. moderately trained participants respectively [ 42 ]. Increasing HAD directly elevates beta-ox rate while citrate synthase increases the TCA cycle rate [ 44 ]. This evidence suggests that lipolysis and systemic FA delivery are not limitations to FAox at higher exercise intensities. Therefore, FA cellular transport proteins CD36 and CPT-1 [ 24 , 25 ] and mitochondrial density HAD are likely the limitation of FAox during high intensity exercise [ 42 ]. Elevating FAox potential by increasing cellular respiration capacity increases FAox at higher exercise intensities which can have a positive influence on aerobic capacity. Acknowledging the occurrence of large inter-individual differences in MFO, differences in MFO relative to training status are still observed [ 39 ]. Lima-Silva et al. moderately trained runners referenced above. However, while no statistical differences were observed between groups at the exercise intensity that MFO occurred, there was an increased capacity to oxidize fat in the highly trained subjects. It is worth noting that the increased performance capacity in highly trained runners is most likely attributed to an increased CHO oxidative potential at higher exercise intensities in order to maintain higher steady state running workloads [ 39 ]. Subsequently, cellular protein expression, oxidative capacity and therefore training status do have the ability to influence fat oxidation. Training status further influences maximal fat oxidative potential by increasing endogenous substrate concentrations [ 19 , 20 ]. Endurance training enhances type I fiber IMTG concentrations as much as three-fold compared with type II fibers. Increased MFO potential due to endurance training is further influenced by IMTG FA-liberating HSL [ 22 ] and LPL proteins [ 20 ], which are responsible for the liberation of intramuscular FAs from the IMTG molecule. However, during exercise, the IMTG pool is constantly being replenished with plasma-derived FAs during exercise [ 20 , 45 ]. The exercise duration effect could be due to β -adrenergic receptor saturation, which has been shown to occur during prolonged bouts of exercise [ 16 , 46 ]. Furthermore, HSL activity has been shown to increase initially within min, but returned to resting levels after min of exercise, increasing reliance on serum derived FAs [ 20 , 45 ]. More research in the area of hormone related FA kinetic limitations is warranted. Factors such as training status, sex, and nutrition [ 1 ] all impact FAox kinetics and thereore the exercise intensity that MFO occurs. Exercise intensity has the most profound effect on MFO based on a combination of events which include FA transport changes [ 24 , 25 ] and hormone fluctuation, which can increase lipolytic rate [ 7 ]. The cellular and hormonal changes that occur during exercise are directly related to exercise intensity which can influence FAox [ 47 ]. Fatty acid oxidation varies relevant to exercise intensity and therefore examining lipid oxidation at specific exercise intensities is warranted. Bergomaster et al. Previous research suggests that training at higher exercise intensities greatly influences substrate utilization [ 5 , 42 , 50 ]. It is worth noting that Bergomaster et al. The increased expression of FAox transport and oxidative cell proteins CD36, CPT-1, HAD, etc. that results in an increase FAox are a result of exercise intensity [ 24 , 49 ]. The Lima-Silva et al. Thus, FAox adaptation potential is related to training at higher exercise intensities rather than non-descript chronic exercise adaptation. Additionally, it has also been shown that carnitine concentrations are a direct limitation of FAox Fig. Interestingly, efforts to mitigate the limitations of free carnitine on MFO at high exercise intensities have been unsuccessful [ 24 ]. Exercise intensity may further influence MFO by influencing catecholamine concentrations which have regulatory effects on lipolysis [ 16 ], glycogenolysis, as well as gluconeogenesis [ 12 ]. Increased epinephrine concentrations that parallel increases in exercise intensity stimulate both glycogenolysis and gluconeogenesis [ 12 ]. As exercise intensity increases, so does catecholamine concentrations facilitating a concurrent increase of serum CHO and FAs into the blood [ 12 ]. The crossover concept. The relative decrease in energy derived from lipid fat as exercise intensity increases with a corresponding increase in carbohydrate CHO. The crossover point describes when the CHO contribution to substrate oxidation supersedes that of fat. MFO: maximal fat oxidation. Adapted from Brooks and Mercier, The concept of the crossover point represents a theoretical means to understand the effect of exercise intensity on the balance of CHO and FA oxidation [ 4 ] Fig. More specifically, the crossover concept describes the point that exercise intensity influences when the CHO contribution relevant to energy demand exceeds FAox. The limitations of FAox at higher intensities is due to the vast amount of acetyl-CoA produced by fast glycolysis [ 24 , 38 ]. The abrupt increase in total acetyl-CoA production at high intensity is due to fast glycolysis flooding the cell with potential energy, which suppresses FA mitochondrial transport potential resulting in decreased FAox Fig. Notably, the large inter-individual fluctuation of when the crossover point occurs at a given exercise intensity can be attributed in part to training status [ 39 , 40 ]. Training status has been shown to effect catecholamine release and receptor sensitivity [ 12 ], endogenous substrate concentrations, and cellular transport protein expression; all of which contribute to the variability of when MFO occurs relevant to exercise intensity [ 1 ]. Nonetheless, MFO occurs in all populations regardless of training status, nutritional influence, etc. Another factor that significantly influences FAox is the duration of exercise [ 13 , 45 , 48 ]. Throughout a prolonged exercise bout, changes in hormonal and endogenous substrate concentrations trigger systematic changes in substrate oxidation [ 20 , 51 ]. Studies show that endurance training promotes reliance on endogenous fuel sources for up to min of submaximal exercise [ 47 , 51 , 52 ]. Exercise duration has a large effect on the origin of FAs for oxidative purposes. While the initiation of exercise relies heavily on endogenous fuel sources IMTG and glycogen , reductions in IMTG concentrations have been shown to occur when exercise duration exceeds 90 min [ 45 ]. Increases in both epinephrine and plasma LCFA concentrations were observed when exercise exceeded 90 min with a simultaneous reduction in HSL activity. Therefore the increase in serum LCFAs [ 20 , 45 ] and the saturation of HSL to epinephrine [ 16 , 46 ] are postulated to inhibit HSL reducing IMTG oxidation when exercise exceeds 90 min [ 20 ]. The shift from intramuscular fuel sources to serum derived FAs after 2 h of submaximal exercise parallel changes in blood glucose concentrations. Trained subjects however experienced a reduction in muscular CHO uptake during the same time frame compared with the untrained. This suggests that the trained subjects were able to maintain FAox despite substrate origin during prolonged exercise to stave off CHO usage for high intensity exercise [ 51 ]. While the exercise intervention used in this study is not typically classified as endurance exercise, the exercise protocol does clarify the variation in the origin of substrate oxidation over time, and expands on the diverse effects exercise duration has on substrate oxidation. Training duration has a large influence on FA and CHO oxidation during prolonged submaximal exercise. However, training status has little influence on the origin of FAs during the first min of submaximal exercise. Nonetheless, trained subjects are able to maintain higher workloads with decreased metabolic work HR for longer periods compared to untrained individuals based on the ability to maintain FAox for longer durations [ 45 ]. Despite the training status effect on FAox, exercise duration will dictate substrate origin during submaximal exercise [ 20 , 45 , 51 ]. Variability in FAox owing to sex exist due to the inherent hormonal differences specific to men and women [ 53 , 54 , 55 , 56 ]. In a comprehensive study with over men and premenopausal women, the energy contribution of fat was significantly higher in women vs. Studies have consistently shown that premenopausal women have a significantly greater ability to oxidize fat during exercise [ 2 , 57 , 58 ]. The sex differences in fat oxidation [ 58 , 59 ] during exercise is attributed to the increased circulation of estrogens [ 53 , 54 , 60 ]. Evidence suggests that estrogen directly stimulates AMPK [ 29 ] and PGC-1α activity [ 60 ], which is thought to increase the downstream FAox transport protein CD36 and beta-oxidative protein HAD [ 30 ]. Additionally, beta-oxidative proteins that oxidize LCFA oxidation have been shown to be regulated in part by estrogen [ 54 , 60 ]. The result of increased beta-oxidative proteins is directly related to increased FAox potential [ 29 , 54 ]. Interestingly, when men were supplemented with estrogen, increases in FAox were observed along with increased cellular expression of beta-ox proteins within eight days of supplementation [ 60 ]. Circulating estrogen is naturally higher for premenopausal women compared to men. Additionally, fluctuation in estrogen levels is inherent throughout the menstrual cycle [ 53 , 59 ]. Estrogens are generally higher during the follicular phase of the menstrual cycle compared to the luteal phase [ 29 ]. Paradoxically, elevated estrogens during the follicular phase do not affect FAox when compared to the luteal phase [ 29 , 53 ]. Nevertheless, elevations in endogenous circulating estrogens inherent to premenopausal women increase the expression of cellular proteins responsible for increased FA transport and oxidation compared to men. Cellular protein expression and the corresponding endogenous vs. systematic substrate oxidation vary according to dietary macronutrient intake [ 19 , 35 , 61 ]. It has been recently shown that high fat diets promote FAox and have performance enhancement capabilities [ 3 , 60 ]. However, definitive conclusions regarding pre-exercise macronutrient dominant diets and exercise performance improvements are contingent on specific exercise applications [ 62 ] that are directed by exercise duration and intensity [ 63 , 64 , 65 ]. Diets that have higher proportions of a specific macronutrient e. High fat diets increase IMTG concentrations while decreasing glycogen levels within muscle [ 17 , 35 ]. Alternatively, high CHO diet conditions increase glycogen concentrations while IMTGs decrease [ 17 ]. However, post-exercise predominant macronutrient CHO consumption has been shown to influence cellular protein expression in as little as 2 hrs [ 69 ]. The plasticity of cellular changes relevant to chronic adaptation are compromised when macronutrient content is altered [ 65 , 67 ]. Macronutrient proportion and timing has been shown to have effects on cellular adaptation [ 32 ] as well as the physiological response to exercise [ 70 , 71 , 72 ]. High fat diets increase beta-ox potential at rest [ 66 ] and during exercise [ 34 ], however, the limitations of high fat diets including short term adaptation 5dys reside with high intensity exercise [ 70 , 72 , 73 ]. Pyruvate dehydrogenase is the enzyme responsible for oxidizing pyruvate as the final substrate of the glycolytic pathway. The deleterious cellular adaptation of reduced PDH activity due to high fat diets has been found to compromise high intensity exercise performance potential [ 35 , 63 , 67 ]. Adapting the body to high fat diets allows the body to increase IMTG storage as well as increase FAox [ 21 , 35 ]. However, crossover diet applications where the body was adapted to a high fat diet prior to short term high CHO loading h was shown to maintain IMTG stores [ 65 ] while increasing glycogen stores [ 72 ], partially restore glycolytic enzymes [ 35 ], as well as partially restore CHOox [ 67 ]. Alternating pre-exercise macronutrient specificity has the potential to be effective in accommodating the stress of sustained high intensity exercise due to both ideal cellular protein expression, and adequate storage of IMTG and muscle glycogen. The reduction in PDH activity due to high fat diets is a limiting factor to the necessary CHO oxidation at high intensity exercise despite adequate endogenous energy stores. Maintaining the ability to store and oxidize fat after acclimating to a high fat diet while restoring the ability to oxidize CHO with short-term CHO loading is an ideal physiological state for endurance exercise performance. Current research asserts that high fat diets favorably enhance FAox at both rest and during exercise [ 3 , 74 ]. However, exercise intensity dictates substrate utilization regardless of dietary influence, training status, and exercise duration. Because of this, high fat diets are sometimes encouraged during preparatory off-season training when training volumes are high and exercise intensities are low to moderate [ 74 ]. More research into the short-term macronutrient manipulation effect on endogenous substrate concentrations, plasticity of cellular expression, and preferential substrate oxidation are necessary to ascertain if there is benefit on exercise performance outcomes. In summary, FAox is contingent on many factors which can modify cellular expression in a short amount of time. Macronutrient availability, training status, sex, exercise intensity, and duration all influence cellular adaptation, systematic FA transport, and FAox. Additionally, more investigation into the ideal nutritional timing and content that will favorably influence the physiological adaptations of FAox during endurance exercise is warranted. Nonetheless, exercise prescriptions and dietary recommendations need to take into account specific exercise goals duration, intensity, sport specific to facilitate a training plan that will elicit the ideal substrate oxidation adaptations relevant to improve sport performance. Achten J, Jeukendrup A. Optimizing fat oxidation through exercise and diet. Article CAS PubMed Google Scholar. Venables M, Achten J, Jeukendrup AE. Determinants of fat oxidation during exercise in healthy men and women: a cross-sectional study. J Appl Phys. Google Scholar. Volek JS, Noakes T, Phinney SD. Rethinking fat as a fuel of endurance exercise. Eur J Sport Sci. Article PubMed Google Scholar. Brooks GA, Mercier J. Balance of carbohydrate and lipid utilization during exercise: the "crossover" concept. CAS Google Scholar. Achten J, Gleeson M, Jeukendrup AE. In contrast to our findings, the LGI diet over 3 weeks resulted in a slight downregulation of fat oxidation during exercise However, in the study by Durkalec-Michalski et al. Furthermore, the decrease in RER values was not statistically significant and did not reach the MID in the present study. The LGI intervention seemed to have a smaller impact on metabolic adaptations than the HFLC diet. The up-regulating signals of fat oxidation are low insulin concentration and increased concentrations in plasma free fatty acids 2 , 3. A direct comparison between four meals, each different in the amount and GI of the ingested carbohydrates, has shown that both high fat groups were associated with the highest postprandial free fatty acid and lowest insulin concentrations. The lowest free fatty acid concentrations were in the group consuming a low glycemic carbohydrate-rich meal. Furthermore, postprandial insulin response was lower in the high carbohydrate low GI group compared to the high carbohydrate high GI group Consequently, the abovementioned adaptation processes might be less in a high carbohydrate low glycemic diet compared to a HFLC diet due to the different impact on postprandial free fatty acid and insulin concentrations. The nutritional impact on fat metabolism might also be reflected by the circulating glucose concentrations. Fasting glucose plasma concentrations dropped in the LGI-G to a significant and MID relevant extent. Changes in the HFLC-G seemed to be less pronounced, potentially as a consequence of relatively low baseline values compared to the other groups. During the post-intervention, incremental test glucose concentrations are lower at the same exercise intensity as in the unconditioned pre-values state in both LGI-G and HFLC-G. This is probably related to a stimulation of fat oxidation under resting conditions and during exercise The results of the HGI-G seemed to be controversial. The increased RER at rest in the HGI-G indicates an elevated metabolization of carbohydrates under resting conditions. In addition, the lactate concentration increase was clinically relevant under pre-exercise condition. Despite increased lactate concentrations during the incremental test, it seems that there is an improved fat metabolism -decreased glucose and lactate values- in the submaximal cycle test. It had previously been described that carbohydrates prior to exercise appear to be beneficial to performance 1. Hence, the slightly decreased carbohydrate metabolism in the submaximal test might be partly explained by the increased lactate threshold over the time as a possible adaptation in response to enhanced performance. As a result, at post-intervention, the participants performed the test closer to their lactate threshold compared to baseline. The current investigation also observed an improvement in body composition due to a decrease in fat mass following the 4-week LGI or HFLC diet on the level of significance and MID. It is not assumed that the present results can be attributed to the differences in energy intake between groups. Despite the significant difference in proportions of nutrients, the mean energy intake was equivalent between groups with an energy add-on of kcal in the HFLC-G. According to the findings of Hall et al. There is evidence that athletes can improve their body composition by a high fat in particular ketogenic diet 42 — Low carbohydrate diets compared with control diets have been suggested to be relatively more effective in body weight management. However, the benefits of a low carbohydrate diet can be rather attributed to the relatively high protein content, but not the relatively lower carbohydrate content 45 , In a recent study with athletes, different approaches high vs. low fat but similar protein intakes resulted in a similar change of body composition mean loss in body fat was 1. These are in accordance with a meta-analysis examining the impact of different diet types in obese or overweight people Data from the meta-analyses of the Cochrane Database of Systematic Reviews suggest that a low glycemic diet without energy restriction results in a significantly greater decreased fat mass and an increased fat free mass compared with a high glycemic or even high fat and energy restricted diet Although low glycemic diets seem to promote weight loss and metabolic improvements in obese and overweight adults 48 , research about the impact of the GI on body composition in endurance athletes is limited. A recent study by Durkalec-Michalski et al. has shown that consuming a low glycemic diet led to a change in body composition. In particular, a statistically significant reduction in body mass Physiologically, the significant changes in body composition in the present investigation might be explained by changes in fat oxidation and a more balanced carbohydrate metabolism as a potential consequence of the altered amount and quality of ingested carbohydrates. Despite an improvement in fat metabolism and body composition, there is a growing body of evidence that these changes induced by ketogenic or non-ketogenic HFLC diets are not in association with improved endurance performance, aerobic capacity and peak performance in particular 9 , 32 , 50 , 51 , due to an impaired carbohydrate provision during higher intensities 2. This assumption is supported by the changes in time to exhaustion in the present investigation. Furthermore, HFLC diets seem to be impractical and accompanied by side effects that include fatigue, headaches, poor concentration, lethargy, gastrointestinal discomfort, nausea, and unintentional weight loss. One reason might be an insufficient proliferation of essential micronutrients and fibers and glycogen depletion which might be a cause of impaired concentration and hence the neuromuscular connection 9 , The values of the VAS scores of all categories decreased in all groups, indicating that the participants got familiar with the respective dietary concepts. In general, none of the groups experienced clinically relevant elevated VAS scores. Mild symptoms can be defined by a score of 5 to 45 mm on the VAS This might be explained by the fact that endurance subjects tolerate the effects of a high-fat diet better than untrained individuals during exercise In addition, according to the nutritional protocols, an impaired delivery of minerals in the HFLC group was not expected. However, only the LGI-G and HGI-G have shown an improvement in VAS scores of the subscale activity and gastrointestinal comfort on a statistical or MID level with a superior effect in the LGI-G. These results might be associated with impaired training sessions in the HFLC-G since higher intensity levels could not be reached without the provision of carbohydrates 2. Furthermore, the advantage of LGI diet over HFLC and HGI diets might be in the choice of carbohydrates. A LGI diet is predominantly characterized by high-fiber and plant-based foods. This has shown to be associated with reduced fatigue, a strengthened immune system, and an improved ability to regenerate through the increased supply of micronutrients, essential fatty acids and amino acids, and low postprandial glucose concentrations Moreover, controlled clinical trials demonstrated that low glycemic foods have a positive impact on digestive conditions, such as gastroesophageal reflux disease or the irritable bowel syndrome, due to high fiber content 56 , It can be assumed that the present results can be attributed to the implementation of nutritional patterns. According to the analysis of the nutritional protocols, the participants' dietary intake reflected the specified intake of carbohydrates and fats in the respective group. While the HGI-G had a higher percent and total carbohydrate intake, the LGI-G showed a higher carbohydrate intake on a g-per-kg-body-weight basis. The current guidelines for endurance athletes during training on the competition level are 6—10 g carbohydrates per kg body weight and day. These recommendations do not address the GI of the ingested carbohydrates The participants of the current investigation were non-elite athletes with a training workload of 3—5 sessions per week. In both groups, the carbohydrate intake seems to be sufficient since recommendations are 5—7 g carbohydrates per kg bodyweight and day for general training needs Nevertheless, increasing the carbohydrate intake to 6—10 g carbohydrates per kg body weight and day would be an interesting approach in future studies with high trained endurance athletes. The carbohydrate upper limit of 50 g per day in the HFLC-G was based on the current focus of carbohydrate-restricted diets 9. This trial has some limitations. It has to be mentioned that the changes in fat and carbohydrate oxidation were not measured directly but extrapolated from the lactate diagnostics. However, it is reported that measuring blood lactate is an effective way to estimate the rates of fat and carbohydrate oxidation Furthermore, using the values of the spiroergometry to confirm the results from the lactate diagnostic during the incremental test has to be taken with caution since values for VO 2 are overestimated by a step compared to a ramp incremental test When taking the impact of the nutritional concepts into account, limitations of the self-reported protocols might entail an over or underreporting of the consumed foods Moreover, recommendations for the macronutrient intake based on the body weight seems to be more accurate than percentage values to determine nutritional guidelines for endurance athletes. Future studies with a larger sample size should include different sex groups and pre-exercise nutritional conditions to state practical use of high fat vs. high carbohydrate diets. Furthermore, the analysis of the muscle glycogen would be helpful for a better interpretation of the energy supply 9. Ultrasonic assessment can be used to quantify glycogen content in the skeletal muscle In conclusion, the effect of the LGI diet was a decrease in lactate concentrations under resting and submaximal exercise conditions, while HFLC diet resulted additionally in decreased RER values. However, these lower adaptations in the LGI-G seem to be beneficial in terms of an enhanced metabolic flexibility, since an increased carbohydrate metabolism was unaffected during higher intensities, while the utilization of fats was facilitated during submaximal exercise due to decreased plasma lactate concentrations. Despite the positive impact on the fat oxidation and body composition, following a HFLC diet might have a negative effect on exercise performance due to the lack of carbohydrate provision at higher intensity levels. In addition, there might be negative long-term health consequences due to the high fat content and decreased intake of essential micronutrients. The HGI-G changes in metabolism might impair the ability to effectively use fats and carbohydrates during different exercise intensities. Taking these findings together, the implementation of a LGI diet leads to a more flexible fat and carbohydrate metabolism after 4 weeks of intervention in contrast to a HFLC or HGI diet, which might be of advantage, particularly during strenuous endurance exercise. After the study was finished, DZ started as a researcher in the Collagen Research Institute, Kiel. The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. DZ, HF, AG, and DK designed the study. DZ, HF, and DK were responsible for data acquisition and performed the analysis. All authors read and approved the final version of the manuscript. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. We would like to thank all the participants and the staff of the University of Freiburg who supported us with the examination. Ormsbee MJ, Bach CW, Baur DA. Pre-exercise nutrition: the role of macronutrients, modified starches and supplements on metabolism and endurance performance. doi: PubMed Abstract CrossRef Full Text Google Scholar. Spriet LL. New insights into the interaction of carbohydrate and fat metabolism during exercise. Sports Med. Yeo WK, Carey AL, Burke L, Spriet LL, Hawley JA. Fat adaptation in well-trained athletes: effects on cell metabolism. Appl Physiol Nutr Metab. Bergström J, Hermansen L, Hultman E, Saltin B. Diet, muscle glycogen and physical performance. Acta Physiol Scand. Nilsson LH. Liver glycogen content in man in the postabsorptive state. Scand J Clin Lab Invest. Romijn JA, Coyle EF, Sidossis LS, Rosenblatt J, Wolfe RR. Substrate metabolism during different exercise intensities in endurance-trained women. J Appl Physiol. Coyle EF, Jeukendrup AE, Wagenmakers AJ, Saris WH. Fatty acid oxidation is directly regulated by carbohydrate metabolism during exercise. Am J Physiol. Burke LM, Kiens B. Burke LM. Volek JS, Noakes T, Phinney SD. Rethinking fat as a fuel for endurance exercise. Eur J Sport Sci. Goedecke JH, Christie C, Wilson G, Dennis SC, Noakes TD, Hopkins WG, et al. Metabolic adaptations to a high-fat diet in endurance cyclists. Metab Clin Exp. Havemann L, West SJ, Goedecke JH, Macdonald IA, St Clair Gibson A, Noakes TD, et al. Fat adaptation followed by carbohydrate loading compromises high-intensity sprint performance. Stellingwerff T, Spriet LL, Watt MJ, Kimber NE, Hargreaves M, Hawley JA, et al. Decreased PDH activation and glycogenolysis during exercise following fat adaptation with carbohydrate restoration. Am J Physiol Endocrinol Metab. Zajac A, Poprzecki S, Maszczyk A, Czuba M, Michalczyk M, Zydek G. The effects of a ketogenic diet on exercise metabolism and physical performance in off-road cyclists. Bennett CB, Chilibeck PD, Barss T, Vatanparast H, Vandenberg A, Zello GA. Metabolism and performance during extended high-intensity intermittent exercise after consumption of low- and high-glycaemic index pre-exercise meals. Br J Nutr. Stevenson EJ, Williams C, Mash LE, Phillips B, Nute ML. Influence of high-carbohydrate mixed meals with different glycemic indexes on substrate utilization during subsequent exercise in women. Am J Clin Nutr. Wee S-L, Williams C, Tsintzas K, Boobis L. Ingestion of a high-glycemic index meal increases muscle glycogen storage at rest but augments its utilization during subsequent exercise. Wu C-L, Williams C. A low glycemic index meal before exercise improves endurance running capacity in men. Int J Sport Nutr Exerc Metab. Wu C-L, Nicholas C, Williams C, Took A, Hardy L. The influence of high-carbohydrate meals with different glycaemic indices on substrate utilisation during subsequent exercise. Foster-Powell K, Holt SHA, Brand-Miller JC. International table of glycemic index and glycemic load values: Hertzog MA. Considerations in determining sample size for pilot studies. Res Nurs Health. Riebe D, Franklin BA, Thompson PD, Garber CE, Whitfield GP, Magal M, et al. Updating ACSM's recommendations for exercise preparticipation health screening. Kindal A Shores , Metabolic Adaptations to Endurance Training: Increased Fat Oxidation , Honours Thesis. Fat oxidation is the process by which the body breaks down fats triglycerides into smaller molecules, such as free fatty acids and glycerol, which can then be used as a source of energy. Fat oxidation increases mainly through training and via an increase in mitochondrial capacity. This has a sparing effect on glycogen stores allowing the athlete to perform better later in the race. Stable isotope techniques: This involves consuming a small amount of a labeled form of fat, such as octanoate, and then measuring the labeled carbon in exhaled breath or urine to determine the rate of fat oxidation. Blood tests: Measuring the levels of certain fatty acids and ketone bodies in the blood can also provide an indication of fat oxidation. Body composition analysis: Dual-energy X-ray absorptiometry DXA and bioelectrical impedance analysis BIA are two common methods to measure body composition, including body fat percentage, can also give an indication of the rate of fat oxidation. Please note that these methods have different level of accuracy and some of them may require professional assistance. By performing more low intensity training and developing your mitochondrial density. Not directly. However increasing your activity levels will be beneficial for both your performance and your health. Maintaining a reasonable caloric deficit over time is the best way to lose weight and body fat. Your email address will not be published. Save my name, email, and website in this browser for the next time I comment. What is Fat Oxidation? When does Fat Oxidation occur? How can I measure Fat Oxidation? How can I Increase Fat Oxidation? Will Fat Oxidation help me lose Body Fat? Share This. Next Post High Lactate Levels During Exercise: What Causes Them? You May Also Like. Leave A Comment Cancel reply Your email address will not be published. This website uses cookies to improve your experience. We'll assume you're ok with this, but you can opt-out if you wish. Cookie settings ACCEPT. Close Privacy Overview This website uses cookies to improve your experience while you navigate through the website. Out of these cookies, the cookies that are categorized as necessary are stored on your browser as they are as essential for the working of basic functionalities of the website. We also use third-party cookies that help us analyze and understand how you use this website. These cookies will be stored in your browser only with your consent. You also have the option to opt-out of these cookies. But opting out of some of these cookies may have an effect on your browsing experience. Necessary Necessary. Necessary cookies are absolutely essential for the website to function properly. |
| Strategies to enhance fat utilisation during exercise | The present study demonstrated a potential Healthy weight management effectiveness for utilizatiob SHRED efifciency fat-loss outcomes after at Improvex min rest and during Over the counter antidepressants at Improved fat utilization efficiency utilizationn to an individualized Fatmax-intensity. It should however be used as part of a structured training program with a sound intensity distribution. BaurVirginia Military Institute, United States. ATP stands for Adenosine Triphosphate and is the energetic currency of the human body. Am J Clin Nutr. Get My Free Issue. Article PubMed Google Scholar Noakes T, Volek JS, Phinney SD. |
| Fuel Choice for Exercise: Fats VS Sugars | On the basis that conventional competitive sports generally provide opportunities to achieve adequate carbohydrate availability, that fat-adaptation strategies reduce rather than enhance metabolic flexibility by reducing carbohydrate availability and the capacity to use it effectively as an exercise substrate, and that athletes would be unwise to sacrifice their ability to undertake high-quality training or high-intensity efforts during competition that could determine the outcome of even an ultra-endurance sport, this author decided to abandon a research and practical interest in fat-adaptation strategies. Given the recent escalation in the promotion of LCHF diets for sports performance, it could be assumed that the last decade has seen the publication of a considerable number of studies with clear evidence of benefits to sports performance following the implementation of fat-adaptation strategies. Yet, to the knowledge of this author, only two new investigations of LCHF diets in athletes have appeared in the peer-reviewed literature since [ 49 , 50 ]. These studies, summarized in Table 2 , fail to show performance benefits associated with a ketogenic LCHF diet, although there is evidence of a small but favorable reduction in body fat levels. Nevertheless, there are some peculiarities with the design or methodologies of these studies, including the failure of one study to achieve the carbohydrate restriction typically associated with the ketogenic LCHF diet, and they have failed to become widely cited, even by supporters of the LCHF movement. Rather, the current interest in chronic application of LCHF eating by athletes appears to be driven by enthusiastic discussion in lay and social media by mostly non-elite athletes of sporting success following experimentation with such diets as well as a range of outputs from several sports scientists who are researchers and advocates of this eating style [ 3 — 8 ]. It is uncertain whether there is a cause—effect relationship between these sources or the direction of any relationship , but the fervor merits attention. In the absence of compelling new data, the reader is alerted to several elements in the discussions that are positive and some that are concerning:. Peer-reviewed publications from the key scientific protagonists of the LCHF movement [ 3 , 5 , 6 ] generally show measured and thoughtful insights, based on a re-examination of previously conducted studies, personal experiences, anecdotal observations from the sports world, and the general interest in tackling modern health problems with the LCHF approach [ 51 , 52 ]. In these forums, the discussion points include the lack of evidence and equivocal outcomes of research to support the performance benefits of LCHF but also theoretical constructs around potential benefits to metabolism, muscle, and brain function, inflammatory and oxidative status, and body composition management. While there are some suggestions that a larger group of athletes might benefit from an LCHF approach, the general tone is that further investigation of these theories is required [ 3 — 6 ]. The apparent caution expressed in peer-reviewed publications is generally not present in other outputs from the same authors. The differences between these viewpoints can be confusing, as is the misrepresentation of the physiological requirements of competitive sports see Sect. Many of the theorized benefits from the LCHF diet are claimed to come from the adaptation to high circulating levels of ketone bodies, which provide an additional fuel source for the brain and muscle as well as achieve other health and functional benefits [ 5 , 6 ]. The amount of energy that can be provided by ketones as an exercise substrate has been neither calculated nor measured, making it impossible to verify this claim. The time required to achieve optimal adaptation and, therefore, the period that requires investigation in new studies is claimed to be at least 2—3 weeks, with at least 1 week required before the feelings of lethargy and reduced exercise capacity abate [ 5 , 6 ]. With such chronic keto-adaptation, it is considered unnecessary to consume carbohydrate during exercise, or perhaps to consume it in small amounts [ 5 , 6 ]. As has been discussed in this review, the current evidence for these claims is equivocal and mostly anecdotal. Until or unless further research is undertaken, we are unlikely to resolve any of the current questions and claims. The role of non-ketogenic LCHF diets is not clear. The current literature on LCHF diets is relentless in promoting misunderstanding or misinformation on the current guidelines for athletes in relation to carbohydrate intake in the training or competition diet. It would benefit sports nutrition for researchers and practitioners to show mutual respect in recognizing the evolution of new ideas and the replacement of old guidelines with new recommendations [ 53 ]. Indeed, modern sports nutrition practitioners teach athletes to manipulate their eating practices to avoid unnecessary and excessive intakes of carbohydrates per se, to optimize training outcomes via modification of the timing, amount and type of carbohydrate-rich foods and drinks to balance periods of low- and high-carbohydrate availability and to adopt well-practiced competition strategies that provide appropriate carbohydrate availability according to the needs and opportunities provided by the event and individual experience [ 14 , 54 — 57 ]. This author and others continue to undertake research to evolve and refine the understanding of conditions in which low carbohydrate availability can be tolerated or actually beneficial [ 58 , 59 ]. However, we also recognize that the benefits of carbohydrate as a substrate for exercise across the full range of exercise intensities via separate pathways [ 16 ], the better economy of carbohydrate oxidation versus fat oxidation ATP produced per L of oxygen combusted [ 60 ], and the potential CNS benefits of mouth sensing of carbohydrate [ 61 ] can contribute to optimal sporting performance and should not be shunned simply because of the lure of the size of body fat stores. Considering that athletes might best benefit from a range of options in the dietary tool box is likely to be a better model for optimal sports nutrition than insisting on a single, one-size-fits-all solution. Havemann L, West S, Goedecke JH, et al. Fat adaptation followed by carbohydrate-loading compromises high-intensity sprint performance. J Appl Physiol. Article CAS PubMed Google Scholar. Burke LM, Kiens B. Article PubMed Google Scholar. Noakes T, Volek JS, Phinney SD. Low-carbohydrate diets for athletes: what evidence? Br J Sports Med. Brukner P. Volek JS, Noakes T, Phinney SD. Rethinking fat as a fuel for endurance exercise. Eur J Sports Sci. Google Scholar. Phinney SD. Ketogenic diets and physical performance. Nutr Metab. Article Google Scholar. Volek JS, Phinney SD. The art and science of low carbohydrate performance. Beyond Obesity LLC; Can elite athletes eat LCHF and win? Available from: www. Accessed 30 June Olsen A. Tim Noakes: low carbohydrate diet for endurance sports. Hall N. The Kardashian index: a measure of discrepant social media profile for scientists. Genome Biol. Article PubMed Central PubMed Google Scholar. Noakes TD. Low-carbohydrate and high-fat intake can manage obesity and associated conditions: Occasional survey. S Afr Med J. Hopkins WG, Hawley JA, Burke LM. Design and analysis of research on sport performance enhancement. Med Sci Sports Exerc. Hawley JA, Burke LM, Phillips SM, et al. Nutritional modulation of training-induced skeletal muscle adaptations. Stellingwerff T. Contemporary nutrition approaches to optimize elite marathon performance. Int J Sports Physiol Perform. PubMed Google Scholar. Burke L. Training and competition nutrition. In: Burke L, editor. Practical sports nutrition. Champaign: Human Kinetics; Spriet LL. New insights into the interaction of carbohydrate and fat metabolism during exercise. Sports Med. Fernandez-Garcia B, Perez-Landaluce J, Rodriguez-Alonso M, et al. Intensity of exercise during road race pro-cycling competition. Bentley DJ, Millet GP, Vleck VE, et al. Specific aspects of contemporary triathlon: implications for physiological analysis and performance. Tucker R. Science of sport: marathon analysis. In: Marathon analysis. Accessed 20 Oct Joyner MJ, Ruiz JR, Lucia A. The two-hour marathon: who and when? Peters A, Schweiger U, Pellerin L, et al. The selfish brain: competition for energy resources. Neurosci Biobehav Rev. Matsui T, Soya S, Okamoto M, et al. Brain glycogen decreases during prolonged exercise. J Physiol. PubMed Central CAS PubMed Google Scholar. Zhang Y, Kuang Y, LaManna JC, et al. Contribution of brain glucose and ketone bodies to oxidative metabolism. Adv Exp Med Biol. Karelis AD, Smith JW, Passe DH, et al. Carbohydrate administration and exercise performance: what are the potential mechanisms involved? Jeukendrup AE, Saris WHM, Wagenmakers AJM. Fat metabolism during exercise: a review. Part III: effects of nutritional interventions. Int J Sports Med. Hawley JA. Effect of increased fat availability on metabolism and exercise capacity. Starling RD, Trappe TA, Parcell AC, et al. Effects of diet on muscle triglyceride and endurance performance. CAS PubMed Google Scholar. Pitsiladis YP, Maughan RJ. The effects of exercise and diet manipulation on the capacity to perform prolonged exercise in the heat and in the cold in trained humans. Article PubMed Central CAS PubMed Google Scholar. Yeo WK, Carey AL, Burke L, et al. Fat adaptation in well-trained athletes: effects on cell metabolism. Appl Physiol Nutr Metab. Phinney SD, Bistrian BR, Evans WJ, et al. The human metabolic response to chronic ketosis without caloric restriction: preservation of submaximal exercise capability with reduced carbohydrate oxidation. Phinney SD, Bistrian BR, Wolfe RR, et al. The human metabolic response to chronic ketosis without caloric restriction: physical and biochemical adaptation. Batterham AM, Hopkins WG. Making meaningful inferences about magnitudes. Dietary carbohydrate intake and endurance exercise performance of trained female cyclists. Nutr Res. Lambert EV, Speechly DP, Dennis SC, et al. Enhanced endurance in trained cyclists during moderate intensity exercise following 2 weeks adaptation to a high fat diet. Eur J Appl Physiol. Article CAS Google Scholar. Goedecke JH, Christie C, Wilson G, et al. Metabolic adaptations to a high-fat diet in endurance cyclists. Rowlands DS, Hopkins WG. Effects of high-fat and high-carbohydrate diets on metabolism and performance in cycling. Vogt M, Puntschart A, Howald H, et al. Effects of dietary fat on muscle substrates, metabolism, and performance in athletes. Hoppeler H, Billeter R, Horvath PJ, et al. Muscle structure with low- and high-fat diets in well-trained male runners. Muoio DM, Leddy JJ, Horvath PJ, et al. Effect of dietary fat on metabolic adjustments to maximal V O2 and endurance in runners. Burke LM, Hawley JA. Effects of short-term fat adaptation on metabolism and performance of prolonged exercise. Burke LM, Hawley JA, Angus DJ, et al. Adaptations to short-term high-fat diet persist during exercise despite high carbohydrate availability. Carey AL, Staudacher HM, Cummings NK, et al. Effects of fat adaptation and carbohydrate restoration on prolonged endurance exercise. Noakes T. Fat adaptation and prolonged exercise performance. Lambert EV, Goedecke JH, Van Zyl CG, et al. High-fat versus habitual diet prior to carbohydrate loading: effects on exercise metabolism and cycling performance. Int J Sport Nutr Exerc Metab. Burke LM, Angus DJ, Cox GR, et al. Effect of fat adaptation and carbohydrate restoration on metabolism and performance during prolonged cycling. Stellingwerff T, Spriet LL, Watt MJ, et al. Decreased PDH activation and glycogenolysis during exercise following fat adaptation with carbohydrate restoration. Am J Physiol. CAS Google Scholar. Peters SJ, Harris RA, Wu P, et al. Erlenbusch M, Haub M, Munoz K, et al. Effect of high-fat or high-carbohydrate diets on endurance exercise: a meta-analysis. Zajac A, Poprzecki S, Maszczyk A, et al. The effects of a ketogenic diet on exercise metabolism and physical performance in off-road cyclists. Ketogenic diet does not affect strength performance in elite artistic gymnasts. J Int Soc Sports Nutr. Nordmann AJ, Nordmann A, Briel M, et al. Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: a meta-analysis of randomized controlled trials. Arch Intern Med. Feinman RD, Pogozelski WK, Astrup A, et al. Dietary carbohydrate restriction as the first approach in diabetes management: Critical review and evidence base. Burke LM, Hawley JA, Wong SH, et al. Carbohydrates for training and competition. J Sports Sci. Stellingwerf T. Case study: nutrition and training periodization in three elite marathon runners. Shaw G, Boyd KT, Burke LM, et al. Nutrition for swimming. Shaw G, Koivisto A, Gerrard D, Burke LM. Nutrition considerations for open-water swimming. Burke LM, Mujika I. Nutrition for recovery in aquatic sports. Philp A, Burke LM, Baar K. Altering endogenous carbohydrate availability to support training adaptations. Nestle Nutr Inst Workshop Ser. Bartlett JD, Hawley JA, Morton JP. Carbohydrate availability and exercise training adaptation: too much of a good thing? Cole M, Coleman D, Hopker J, et al. Improved gross efficiency during long duration submaximal cycling following a short-term high carbohydrate diet. Burke LM, Maughan RJ. The Governor has a sweet tooth—mouth sensing of nutrients to enhance sports performance. Mujika I, Padilla S. Creatine supplementation as an ergogenic aid for sports performance in highly trained athletes: a critical review. Casey A, Greenhaff PL. Does dietary creatine supplementation play a role in skeletal muscle metabolism and performance? Am J Clin Nutr. Hawley JA, Schabort EJ, Noakes TD, et al. Carbohydrate-loading and exercise performance: an update. Coyle EF. Timing and method of increased carbohydrate intake to cope with heavy training, competition and recovery. Stellingwerff T, Cox GR. Systematic review: carbohydrate supplementation on exercise performance or capacity of varying durations. Jeukendrup AE. Oral carbohydrate rinse: placebo or beneficial? Curr Sports Med Rep. Jeukendrup AE, Thielen JJHC, Wagenmakers AJM, et al. Effect of medium-chain triacylglycerol and carbohydrate ingestion during exercise on substrate utilization and subsequent cycling performance. Download references. This article was published in a supplement supported by the Gatorade Sports Science Institute GSSI. The supplement was guest edited by Lawrence L. Spriet, who attended a meeting of the GSSI expert panel XP in March and received honoraria from the GSSI for his participation in the meeting. He received no honoraria for guest editing the supplement. Spriet selected peer reviewers for each paper and managed the process. Louise Burke attended a meeting of GSSI XP in February , and her workplace Australian Institute of Sport received an honorarium from the GSSI, a division of PepsiCo, Inc. Balance of carbohydrate and lipid utilization during exercise: the "crossover" concept. CAS Google Scholar. Achten J, Gleeson M, Jeukendrup AE. Determination of the exercise intensity that elicits maximal fat oxidation. Med Sci Sports Exerc. Valizadeh A, Khosravi A, Azmoon H. Fat oxidation rate during and after three exercise intensities in non-athlete young men. World Appl Sci J. Randell RK, Rollo I, Roberts TJ, Dalrymple KJ, Jekendrup AE, Carter JM. Maximal fat oxidation rates in an athletic population. Ogasawara J, Izawa T, Sakurai T, Sakurai T, Shirato K, Ishibashi Y, Ishida H, Ohno H, Kizaki T. The molecular mechanism underlying continuous exercise training-induced adaptive changes of lipolysis in white adipose cells. J Obesity. Watt M, Spriet LL. Triacylglycerol lipases and metabolic control: implications for health and disease. Am J of Physol. Endocrinol Metab. Zechner R, Kienesberger PC, Haemmerle G, Zimmermann R, Lass A. Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J Lipid Res. van Loon L, Greenhaff PL, Constantin-Teodosiu D, Wagenmakers AJ. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J Physiol. Article CAS PubMed PubMed Central Google Scholar. Tank A, Wong D. Peripheral and central effects of circulating catecholamines. Compr Physol. van Hall G. THe physiological regulation of skeletal muscle fatty acid supply and oxidation during moderate-intensity exercise. Sports Med. Zouhal H, Jacob C, Delamarche P, Grata-Delamarche A. Catecholamines and the effects of exercise, training and gender. Horowitz J, Klein S. Lipid metabolism during endurance exercise. Am J Clin Nutr. Frayn K. Fat as fuel: emerging understanding of the adipose tissue-skeletal muscle axis. Acta Physiol. Article CAS Google Scholar. Spriet LL. New insights into the interaction of carbohydrate and fat metabolism during exercise. Kiens B. Skeletal muscle lipid metabolism in exercise and insulin resistance. Physol Rev. Shaw C, Clark J, Wagenmakers A. The effect of exercise and nutrition on intramuscular fat metabolism and insulin sensitivity. Annu Rev Nutr. Moro C, Bajpeyi S, Smith SR. Determinants of intramyocellular triglyceride turnover: implications for insulin sensitivity. Am J Physiol Endocrinol Metab. Wong H, Schotz MC. The lipase gene family. van Loon L, Greenhaff P, Constantin-Teodosiu D, Saris W, Wagenmakers A. Use of intramuscular triacylgylcerol as a substrate source during exercise in humans. J Appl Physiol. Watt M, Heigenhauser G, Spriet LL. Intramuscular triacylgylerol utilization in human skeletal muscle during exericse: is there a controversy? Jeppesen J, Keins B. Regulation and limitations to fatty acid oxidation during exercise. J Phys. Yoshida Y, Jain SS, McFarlan JT, Snook LA, Chabowski A, Bonen A. Exercise- and training-induced upregulation of skeletal muscle fatty acid oxidation are not solely dependent on mitochondrial machinery and biogenesis. Schenk S, Horowitz JF. Klien S, Coyle E, Wolfe R. Fat metabolism during low-intensity exercise in endurance-trained and untrained men. Am J Phys. Lundsgaard A, Kiens B. Gender differences in skeletal muscle substrate metabolism-molecular mechanisms and insulin sensitivity. Front Endocrinol. Oosthuyse T, Bosch A. The effect of the menstual cycle on exercise metabolism. Kiens B, Roepstorff C, Glatz J, Bonen A, Schjerling P, Knudsen J, Nielsen J. Lipid-binding proteins and lipoprotein lipase activity in human skeletal muscle: influence of physical activity and gender. DeLany J, Windhauser M, Champagne C, Bray G. Differential oxidation of individual dietary fatty acids in humans. CAS PubMed Google Scholar. Misell L, Lagomarcino N, Shuster V, Kern M. Chronic medium-chain triacylglycerol consumption and endurance performance in trained runners. J Sports Med Phys Fit. Jeukendrup A, Aldred S. Fat supplementation, health, and endurance performance. Volek J, Freidenreich D, Saenz C, Kunces L, Creighton B, Bartley, Davitt P, Munoz C, Anderson J, Maresh C, Lee E, Schuenke M, Aerni G, Kramer W, Phinney S. Metabolic characteristics of keto-adapted ultra-endurance runners. Yeo W, Carey A, Burke L, Spriet LL, Hawley J. Fat adaptation in well-trained athletes: effects on cell metabolism. Appl Physiol Nutr Metab. Jeukendrup AE. Fat metabolism during exercise: a review. Part III: effects of nutritional interventions. Int J Sports Med. Calvani M, Reda E, Arrigoni-Martelli E. Regluation by carnitine of myocardial fatty acid and carbohydrate metabolism under normal and pathological conditions. Basic Res Cardiol. Stephens F, Constantin-Teodosiu D, Greenhaff P. New insights concerning the role of carnitine in the regulaiton of fuel metabolism in skeletal muscle. Article PubMed PubMed Central Google Scholar. Lima-Silva A, Bertuzzi R, Pires F, Gagliardi J, Barros R, Hammond J, Kiss M. Relationship between training status and maximal fat oxidation. J Sports Sci Med. PubMed PubMed Central Google Scholar. Scharhag-Rosenberger FM, Meyer T, Walitzek S, Kindermann W. Effects of one year aerobic endurance training on resting metabolic rate and exercise fat oxidation in previously untrained men and women. Metabolic endurance training adaptations. Bircher S, Knechtle B. Relationship between fat oxidation and lactate threshold in athletes and obese women and men. Nordby P, Saltin B, Helge JW. Whole-body fat oxidation determined by graded exercise and indirect calorimetry: a role for muscle oxidative capacity? Scand J Med Sci Sports. Lanzi S, Codecasa F, Cornacchia M, Maestrini S, Slvadori A, Brunani A, Malatesta D. Fat oxidation, hormonal and plasma metabolite kinetics during a submaximal incremental test in lean and obese adults. PLoS One. Stisen A, Stougaard O, Langfort J, Helge J, Sahlin K, Madsen K. Maximal fat oxidation rates in endurance trained and untrained women. Eur J Appl Physiol. Watt M, Heigenhauser G, Dyck D, Spriet LL. Intramuscular triacylglycerol, glycogen, and acetyl group metabolism during 4 h of moderate exercise in man. Mora-Rodriguez R, Hodgkinson BJ, Byerley LO, Coyle EF. Effects of -adrenergic receptor stimulation and blockade on substrate metabolism during submaximal exercise. Am J Physol. Martin W. Effects of acute and chronic exercise on fat metabolism. Exerc Sport Sci Revs. Romijn J, Coyle E, Sidossis L, Gastaldelli A, Horowitz J, Endert E, Wolfe R. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J phys. Bergomaster K, Howarth KR, Phillips SM, Rakobowchuk M, MacDonald MJ, McGee SL, Gibala MJ. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. Article Google Scholar. Astorino T. Is the ventilatory threshold coincident with maximal fat oxidation during submaximal exercise in women? J Sports Med Phys Fitness. Turcotte L, Richeter E, Kiens B. Increased plasma FFA uptake and oxidation during prolonged exericse in trained vs. untrained humans. Effect of endurance training on fatty acid metabolism during whole body exercise. Isacco L, Duché P, Buisseau N. Influence of hormonal status on substrate utilization at rest and during exercise in the female population. Maher A, Akhtar M, Vockley J, Tarnopolosky M. Women have higher protein content of beta oxidation enzymes in skeletal muscle than men. Tarnopolosky M. Sex differences in exercise metabolism and the role of beta estradiol. Varmlamov O, Bethea CL, Roberts CT. Sex-specific differences in lipid and glucose metabolism. Dasilva SG, Guidetti L, Buzzachera CF, Elsangedy HM, Krinski K, De Campos W, Goss FL, Baldari C. Gender-based differences in substrate use during exercise at a self-selected pace. J Strength Cond Res. Carter S, Rennie C, Tarnopolosky M. Substrate utilization during endurance exercise in men and women after endurance training. Am J Endocrinoly Metab. Lebrun C. Effect of the different phases of the menstrual cycle and oral contraceptives on athletic performance. Maher A, Akhtar M, Tarnopolsky M. Men supplemented with 17b-estradiol increased b-oxidation capacity in skeletal muscle. Physiol Genomics. Fletcher G, Eves FF, Glover EI, Robinson SL, Vernooij CA, Thompson JL, Wallis GA. Dietary intake is independently associated with the maximal capacity for fat oxidation during exercise. Phinney S. Ketogenic diets and physical performance. Nutr Metab. Burke L. Re-examining high-fat diets for sports perfomance: did we call the 'nail in the coffin' too soon? Hawley J, Leckey J. Carbohydrate dependence during prolonged, intense endurance exercise. Ochiai M, Matsuo T. Effects of short-term dietary change from high-carbohydrate diet to high-fat diet on storage, utilization, and fatty acid composition of rat muscle triglyceride during swimming exercise. J Clin Biochem Nutr. Miles-Chan J, Dulloo AG, Schutz Y. Fasting substrate oxidation at rest assessed by indirect calorimetry: is prior dietary macronutrient level and composition a confounder? Int J Obes. Stellingwerff T, Spriet LL, Watt M, Kimber N, Hargreaves M, Hawley J, Burkey L. Decreased PDH activiation and glycogenolysis during exercise following fat adaptation with carbohydrate resortation. Am J Endocrinol Metab. Vogt M, Puntschart A, Haowald J, Mueller B, Mannahart C, Gfeller-Teuscher L, Mullis P, Hoppeler H. Effects of dietary fat on muscle substrates, metabolism, and performance in athletes. Pilegaard H, Keller C, Seensberg A, Helge J, Pedersen B, Saltin B, Neufer D. Influence of pre-exercise muscle glycogen content on exercise-induced transcriptional regulation of metabolic genes. Burke L, Hawley J, Angus D, Cox G, Clark S, Cummings N, Desbrow B, Hargreaves M. Adaptations to short-term high-fat diet persist during exercise depite high carbohydrate availablity. Webster C, Noakes T, Chacko S, Swart J, Kohn T, Smith J. Gluconeogenesis during endurance exercise in cyclists habituated to a long-term low carbohydrate high fat diet. Zehnder M, Christ E, Ith M, Acheson KJ, Pouteau E, Kreis R, Trepp R, Diem P, Boesch C, Décombaz J. Intramyocellular lipid stores increase markedly in athletes after 1. Havemann L, West S, Goedecke J, Macdonald L, St. Clair Gibson A, Noakes T, Lambert E. Fat adaptation followed by carbohydrate loading compromises high-intensity sprint performance. Zajac P, Poprzecki S, Maszczyk A, Czuba M, Michalczyk M, Zydek G. The effects of a ketogenic diet on exercise metabolism and physical performance in off-road cyclists. Leckey J, Burke J, Morton J, Hawley J. Altering fatty acid availability does not impair prolonged, continuous running to fatigue: evidence for carbohydrate dependence. J of Appl Physiol. Download references. Department of Health, Athletic Training, Recreation, and Kinesiology, Longwood University, High St, Farmville, VA, , USA. Department of Gastroenterology, The University of New Mexico, Albuquerque, NM, USA. You can also search for this author in PubMed Google Scholar. Correspondence to Troy Purdom. TP currently has accepted abstracts with ACSM, NSCA, and ISSN in the area of fat metabolism, athletic performance evaluation, energy expenditure, and body composition. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Open Access This article is distributed under the terms of the Creative Commons Attribution 4. Reprints and permissions. Purdom, T. et al. Understanding the factors that effect maximal fat oxidation. J Int Soc Sports Nutr 15 , 3 Download citation. Received : 28 July Accepted : 02 January Published : 12 January Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content. Search all BMC articles Search. Download PDF. Download ePub. Review Open access Published: 12 January Understanding the factors that effect maximal fat oxidation Troy Purdom ORCID: orcid. Abstract Lipids as a fuel source for energy supply during submaximal exercise originate from subcutaneous adipose tissue derived fatty acids FA , intramuscular triacylglycerides IMTG , cholesterol and dietary fat. Background Lipids are the substrate largely responsible for energy supply during submaximal exercise [ 1 , 2 , 3 ]. Lipid oxidation Lipolysis Triacylglycerol TAG is the stored form of fat found in adipocytes and striated muscle, which consists of a glycerol molecule a three-carbon molecule that is bound to three fatty acid FA chains. Fatty acid transport Limitations to FAox are due in part to a multi-faceted delivery system that has a series of regulatory events [ 18 ]. Within-cell FA transport into mitochondrion Within the cell, FA chain type and length have been shown to determine oxidative rates within the mitochondrion largely due to transport specificity [ 31 ]. Full size image. Conclusion In summary, FAox is contingent on many factors which can modify cellular expression in a short amount of time. References Achten J, Jeukendrup A. Article CAS PubMed Google Scholar Venables M, Achten J, Jeukendrup AE. Google Scholar Volek JS, Noakes T, Phinney SD. Article PubMed Google Scholar Brooks GA, Mercier J. CAS Google Scholar Achten J, Gleeson M, Jeukendrup AE. Article PubMed Google Scholar Valizadeh A, Khosravi A, Azmoon H. CAS Google Scholar Randell RK, Rollo I, Roberts TJ, Dalrymple KJ, Jekendrup AE, Carter JM. Article CAS PubMed Google Scholar Ogasawara J, Izawa T, Sakurai T, Sakurai T, Shirato K, Ishibashi Y, Ishida H, Ohno H, Kizaki T. Google Scholar Zechner R, Kienesberger PC, Haemmerle G, Zimmermann R, Lass A. Article CAS PubMed Google Scholar van Loon L, Greenhaff PL, Constantin-Teodosiu D, Wagenmakers AJ. Article CAS PubMed PubMed Central Google Scholar Tank A, Wong D. Google Scholar van Hall G. Article PubMed Google Scholar Horowitz J, Klein S. Google Scholar Frayn K. Article CAS Google Scholar Spriet LL. Article PubMed Google Scholar Kiens B. CAS Google Scholar Shaw C, Clark J, Wagenmakers A. Article CAS PubMed Google Scholar Moro C, Bajpeyi S, Smith SR. Article PubMed Google Scholar Wong H, Schotz MC. Article CAS PubMed Google Scholar van Loon L, Greenhaff P, Constantin-Teodosiu D, Saris W, Wagenmakers A. Article CAS PubMed Google Scholar Watt M, Heigenhauser G, Spriet LL. Article CAS PubMed Google Scholar Jeppesen J, Keins B. Google Scholar Yoshida Y, Jain SS, McFarlan JT, Snook LA, Chabowski A, Bonen A. Article CAS PubMed Google Scholar Schenk S, Horowitz JF. Article CAS PubMed Google Scholar Klien S, Coyle E, Wolfe R. Google Scholar Lundsgaard A, Kiens B. Google Scholar Oosthuyse T, Bosch A. Article PubMed Google Scholar Kiens B, Roepstorff C, Glatz J, Bonen A, Schjerling P, Knudsen J, Nielsen J. Article CAS PubMed Google Scholar DeLany J, Windhauser M, Champagne C, Bray G. CAS PubMed Google Scholar Misell L, Lagomarcino N, Shuster V, Kern M. CAS Google Scholar Jeukendrup A, Aldred S. Article CAS Google Scholar Volek J, Freidenreich D, Saenz C, Kunces L, Creighton B, Bartley, Davitt P, Munoz C, Anderson J, Maresh C, Lee E, Schuenke M, Aerni G, Kramer W, Phinney S. Article CAS Google Scholar Yeo W, Carey A, Burke L, Spriet LL, Hawley J. Article CAS PubMed Google Scholar Jeukendrup AE. Article CAS PubMed Google Scholar Calvani M, Reda E, Arrigoni-Martelli E. Article CAS PubMed Google Scholar Stephens F, Constantin-Teodosiu D, Greenhaff P. Article PubMed PubMed Central Google Scholar Lima-Silva A, Bertuzzi R, Pires F, Gagliardi J, Barros R, Hammond J, Kiss M. PubMed PubMed Central Google Scholar Scharhag-Rosenberger FM, Meyer T, Walitzek S, Kindermann W. PubMed PubMed Central Google Scholar Nordby P, Saltin B, Helge JW. Article CAS PubMed Google Scholar Lanzi S, Codecasa F, Cornacchia M, Maestrini S, Slvadori A, Brunani A, Malatesta D. Article CAS PubMed Google Scholar Watt M, Heigenhauser G, Dyck D, Spriet LL. Article CAS PubMed PubMed Central Google Scholar Mora-Rodriguez R, Hodgkinson BJ, Byerley LO, Coyle EF. |
| Fat Oxidation Explained: How To Make Your Body Burn More Fats | Jeukendrup AE, Thielen Efficiench, Wagenmakers AJM, et al. Beck Utiliztion, Connor C, Mullen Utilizatoon, Wesley D, Bergenstal R. Fatmax intensities have been reported to Waist circumference and self-image effective in enhancing a number Over the counter antidepressants exercise Improved fat utilization efficiency weight-loss Improfed, including enhanced glycogen sparing, delaying fatigue mechanisms, and weight and body fat reduction [ 19 — 21 ]. Fatty acid transport Limitations to FAox are due in part to a multi-faceted delivery system that has a series of regulatory events [ 18 ]. Post-diet phase showed significant increase in split time during set 4 and 5 only. Article Google Scholar Lambert EV, Speechly DP, Dennis SC, et al. |
| Fat Oxidation Explained: How To Make Your Body Burn More Fat Than Sugar During Exercise | This means that the more ADP is left floating around, the more sugars will be used as fuel. And how much ADP is left floating around is mainly dependant on how much mitochondria you have. As muscular contractions occur, more ATP gets broken down. Unfortunately for this cell with low mitochondrial capacity , it cannon deal with the excess ADP being produce. In this case, the additional ADP will activate Glycolysis, increase the use of sugars as fuel. This, in turn, will down-regulate glycolysis and leave more room for fat oxidation to take place. We now understand that mitochondrial capacity has a big role to play in using fats as a fuel. Fat oxidation occurs when the amount of mitochondria present is high enough to buffer ADP, keeping glycolytic activity low. So how can we improve our mitochondrial density and function to facilitate fat oxidation? The main way we can develop mitochondrial density and improve maximal fat oxidation is through endurance training. But not all training intensities are the same! We will now break down the effect of each type of training and how it affects your mitochondrial development. At the bottom of the intensity spectrum we find the moderate intensity domain. This domain sits below the first threshold and usually corresponds to Zone 1 and Zone 2. This type of training is really easy and can be done for many hours. Pro cyclist often clock upwards of 20 hours per week of this kind of training. The advantage of this low intensity training is that is generates very little fatigue on the body. So you can do A LOT of it without burning out. Make sure you know what your physiological zones are to optimise your training. Once we pass the first threshold we get to the heavy intensity domain. At those intensities, lactate levels will rise above baseline yet remain stable. This type of training is obviously necessary for endurance performance. But performing too much of it without adequate recovery and without a strong low intensity foundation can have a negative impact on your mitochondrial development. Once we move beyond this grey zone , we transition from the heavy to the severe intensity domain. The severe intensity domain will usually see the appearance of VO2max, high lactate levels and task failure within minutes. However, we do see the development of both mitochondrial capacity AND function with those types of training sessions. The downside if this type of training if that it is very taxing both metabolically and mentally. So accumulating large amounts of this type of work is not recommended. It should however be used as part of a structured training program with a sound intensity distribution. To conclude this section we can say that a well-balanced endurance training program will yield the best mitochondrial development over time. This in turn will improve our fat oxidation ability and our performance. Now what is the link between fat oxidation and fat loss? Fat Oxidation describes the utilisation of fatty acid molecules by the mitochondria to recycle ATP. Fat Loss describes a decrease in fat mass at the whole body level. We saw that fat utilisation is largely dictated by mitochondrial capacity. Instead, Fat loss is the result of maintaining a sufficient caloric deficit over time. As I like to say, if you wish to lose fat or lose weight, you should eat like an adult and sleep like a baby! San-Millan et al. Kindal A Shores , Metabolic Adaptations to Endurance Training: Increased Fat Oxidation , Honours Thesis. Fat oxidation is the process by which the body breaks down fats triglycerides into smaller molecules, such as free fatty acids and glycerol, which can then be used as a source of energy. Fat oxidation increases mainly through training and via an increase in mitochondrial capacity. This has a sparing effect on glycogen stores allowing the athlete to perform better later in the race. Stable isotope techniques: This involves consuming a small amount of a labeled form of fat, such as octanoate, and then measuring the labeled carbon in exhaled breath or urine to determine the rate of fat oxidation. Blood tests: Measuring the levels of certain fatty acids and ketone bodies in the blood can also provide an indication of fat oxidation. Body composition analysis: Dual-energy X-ray absorptiometry DXA and bioelectrical impedance analysis BIA are two common methods to measure body composition, including body fat percentage, can also give an indication of the rate of fat oxidation. Please note that these methods have different level of accuracy and some of them may require professional assistance. By performing more low intensity training and developing your mitochondrial density. Not directly. However increasing your activity levels will be beneficial for both your performance and your health. Maintaining a reasonable caloric deficit over time is the best way to lose weight and body fat. Your email address will not be published. Save my name, email, and website in this browser for the next time I comment. Due to their low energy flow rate, the percentage contribution of fat oxidation to total energy provision is low during short intensive exercises and increases with the duration of the exercise and the decrease in intensity 6. The intake of high glycemic carbohydrates and the resulting high insulinemic response are one of the strongest inhibitors of fat oxidation 7. Therefore, several clinical studies focused on improving fat utilization during endurance exercise by high fat low carbohydrate diets 8. The current state of evidence suggests a diet with less than 50 g of carbohydrates per day 9. A metabolic adaptation toward increased fat oxidation is usually achieved following a 2- to 4-week dietary intervention 9 , However, high fat diets are associated with an impaired carbohydrate utilization during higher intensities 11 — 14 and, consequently, with small positive effects on endurance performance even with a carbohydrate restoration phase prior to competitions 9. This altered metabolic flexibility can be explained by a reduced enzyme activity in the carbohydrate metabolism due to a reduced training effect within the carbohydrate metabolism following reduced carbohydrate availability or reduced signaling at low glycogen concentrations 2 , 3. In this context, a promising concept might be the consideration of the glycemic index GI in the diet. It was shown that the fat oxidation during exercise increased after the consumption of low GI carbohydrates compared to high GI carbohydrates 15 — This effect can be explained by a reduced postprandial insulin release which leads to an increased plasma concentration of free fatty acids and increased fat oxidation in the skeletal muscles 2 , 3. In consequence, intramuscular and intrahepatic glycogen stores are spared and can be used during higher intensities of exercises. In addition to the GI, the glycemic load GL which considers the amount of carbohydrates in a given serving of a food also affects blood glucose concentrations and insulin responses To the best of our knowledge, studies comparing high fat with high carbohydrate diets have not considered the GI or GL of the carbohydrates. In addition, long-term investigations examining the influence of the GI or GL are limited and without a high fat low carbohydrate control. Therefore, the aim of the current pilot study was to examine the impact of a high fat low carbohydrate HFLC-G vs. high carbohydrate low glycemic LGI-G vs. high carbohydrate high glycemic HGI-G diet on metabolic parameters, body composition, and perceptual responses to the diets. The study was designed as a monocentric, prospective, open pilot trial conducted at the University of Freiburg, Germany. In total, 30 healthy male endurance athletes as distance runners, cyclists, and athletes performing basic endurance training e. The sample size was set on 10 participants per group to obtain information for a power analysis to specify meaningful group differences Professional-level athletes more than five training sessions per week were not eligible to participate. Reported health problems during or after physical activity or unstable weight and eating behavior were also defined as exclusion criteria. In addition, contraindications to physical activity according to the American College of Sports Medicine guidelines, such as cardiovascular, metabolic, or renal diseases 22 diagnosed from anamnestic data, led to an exclusion of the screened participants. The examination was approved by the Ethical Committee of the University of Freiburg ETK: and registered in the German Clinical Trials Register DRKS For all visits, the participants were advised to arrive at the University of Freiburg in the morning at the same time following a fasted period of 12 h. In addition, general guidelines were to drink 1 L of water in the evening and another 0. In addition, the participants had to void bladder before the measurement. Alcoholic beverages had to be avoided 48 h prior to respective examination. All experimental testing was supervised by a licensed physician and experienced researchers. After the start of the intervention, any concerns were clarified directly with the study physician or researcher via telephone call. The study was completed within a timeframe of 4 weeks. The different phases of the study are summarized in Figure 1. Figure 1. Overview of the study schedule. BIA, bioelectric impedance analysis; VAS, visual analog scale. Following written informed consent, participants completed a screening with a medical history questionnaire to ensure that the inclusion criteria were met and that there were no risk factors that might be aggravated by the exercise protocols. Furthermore, anthropometric data, including the body composition as described in the test block below , were measured. An incremental cycling test was performed cycling 3 min at W, then increasing 20 W per 3 min until exhaustion on a stationary cycloergometer Ergoline; ZAN Austria e. For this purpose, a breath-by-breath gas analyzer Innocor ® Innovision, Odense, Denmark was used. Matching for the lactate threshold was used to assign participants to the study groups HGI-G, LGI-G, and HFLC-G to minimize baseline differences in the incremental test results. As the first measurable increase in blood lactate concentration during physical activity, the lactate threshold was automatically evaluated by the computer software Ergonizer 4. In addition, participants were asked to complete a 3-day nutrition protocol which included 2 weekdays and 1 day of the weekend before the intervention. The protocols were analyzed with Nutriguide Nutri-Science GmbH, Pohlheim, Germany. At baseline T0 and post-intervention T4 , body composition fat free mass and fat mass was estimated by using a bioelectric impedance analysis BIA with a high reliability for evaluating body composition in healthy adults 25 , Participants were assessed on the BIA scale OMRON BF Medizintechnik GmbH, Mannheim, Germany , which involved entry of the participant's age, height, and male gender. Still wearing the skin-tight clothing, participants stood on the scale barefoot and grasped the handle electrodes for ~10 s until the process was completed. According to the manufacturers recommendation to use this unit in the same environment and daily circumstances, participants were measured in the morning at the same time following a fasted period of 12 h. The resting energy expenditure REE was measured in a quiet, lying position. The actual REE measurement was preceded by a discarded 5-min measuring phase. The respiratory gases were measured for 10 min using the same device from the preliminary incremental cycle test The mean values of VO 2 and the RER were used in the following Equation 1 by Weir 28 to calculate the REE:. To determine submaximal exercise metabolism, participants performed a min submaximal cycle test at 20 W above the lactate threshold, which was individually identified in the preliminary incremental cycle test. Blood samples were collected from the ear lobe at rest and at 5 and 10 min. The lactate and glucose concentrations during exercise were determined as the mean value of 5 and 10 min. Values of gas analysis RER, VO 2 during exercise were calculated by the mean of each minute. Ten minutes after completing the submaximal cycle test, an incremental test followed under the same conditions: cycling 3 min at W, then increasing 20 W per 3 min until exhaustion, as described for the screening. For RER, lactate, and glucose values, the area under curve AUC was calculated between the start of the test and the final increment completed by all participants before exhaustion t n to assess the concentrations during the incremental cycle test using the following Equation 2 :. Participants were instructed to follow the dietary pattern according to their respective group over the time course of 4 weeks:. Supplementary Tables S1—S4 summarizes the general nutritional guidelines and example meals for each group. The GI of the foods was based on Foster-Powell et al. All nutritional instructions, including the preparation of the meals, were given by a licensed dietarian who was contacted in case of any questions or concerns about the respective diet. Participants were asked to complete a daily nutrition protocol during the intervention by quantifying the consumed food using household measurements. For self-monitoring the nutritional compliance, participants were instructed to use the diet tracking apps. The ingestion of ergogenic supplements during or prior to the intervention was defined as exclusion criteria. Changes in physical activity behavior during the intervention led to exclusion of the participants. Since the present investigation was conducted as pilot trial, no hierarchy for the efficacy endpoints had been defined in the study protocol. The statistical evaluation was performed to determine an adequate sample size and the primary outcome of a main RCT study which will be designed on the basis of the present study protocol. All data are presented as mean ± standard deviation SD. Medians Md were additionally presented if outliers were identified by the interquartile range method. SPSS statistics IBM SPSS Statistics for Windows, Version Armonk, NY: IBM Corp. was used for all statistical analyses. Data distribution was examined with a Shapiro—Wilk test. If variable data of all groups were normally distributed, the homogeneity of the baseline values between the study groups was checked using one-way ANOVA. In addition, the mean differences obtained from all three groups were compared using one-way ANOVA. The Gabriel post-hoc test was performed to identify the groups that differed significantly. The Kruskal—Wallis test was used when data cannot be assumed to be normally distributed. Following a significant Kruskal—Wallis test, pairwise comparisons using the Dunn-Bonferroni approach were automatically produced. The significance of changes from baseline to post-intervention in the respective endpoints within groups were analyzed with the paired sample t -test or Wilcoxon signed-rank test. As a magnitude of the change in the respective outcomes, the minimally important difference MID was calculated. The value of 0. Furthermore, the effect sizes were calculated from differences in means between baseline and post-intervention and between groups at the end of the investigation Cohen's d. A total of 30 men met the inclusion criteria and were allocated to the intervention groups Figure 2. Twenty-eight participants completed the trial and were included in the statistical analysis. In the HGI-G 9, the LGI-G 10, and the HFLC-G 9, participants were, respectively, analyzed. The participants of the HGI-G were slightly older The mean height was 1. Drop outs were whose who had voluntarily withdrawn from participation after the initial examination. No adverse events were noted, and no pathological findings were observed in the routine anamnesis. Endurance running, cycling, team sports, and cross-country skiing were the main reported activities in all groups. As shown in Table 1 , no significant baseline differences between the study groups were detected for the nutritional protocols. The baseline data of the study participants are summarized in Table 2. No significant baseline differences between the study groups were detected in any outcome of the study. The current investigation identified a statistically significant decrease in weight, BMI, and fat mass in the LGI-G and HFLC-G. As a consequence, the percentage of fat free mass increased statistically significantly in the LGI-G and HFLC-G Table 2. These results were confirmed by the MID and medium effect size in the LGI-G and HFLC-G. Table 2. Body composition and metabolic outcomes at baseline and following the nutritional concepts. Due to favorable changes in body fat, the increase in percentage fat free mass was also statistically significantly higher LGI-G vs. No statistically significant differences were observed when comparing the changes in fat mass or fat free mass between LGI-G and HFLC-G. The REE did not change to the level of statistical significance or the MID during the intervention period in any of the groups. In addition, there were no significant differences between the groups. Under resting conditions, the RER increased in the HGI-G by 0. In contrast, the HGI-G and LGI-G had no statistically significant changes in the RER values Table 2. As a consequence, the changes in the RER values in the HFLC-G differed statistically significantly from the HGI-G and LGI-G as confirmed by the post-hoc analysis HFLC-G vs. Differences between groups were not statistically significant. In the HGI-G RER, values at maximum effort were similar at post-intervention compared to baseline. The same results could be observed in the LGI-G Table 2. Figure 3. Column diagram for group differences in area under curve AUC. A Changes in respiratory exchange ratio RER values, B changes in lactate concentrations, and C changes in glucose concentrations during the first 21 min of the incremental cycle test. Data shown as mean ± SD. Lactate concentrations at exhaustion had not statistically significantly changed in any group. However, lactate concentrations at exhaustion increased in the HGI-G 1. Nevertheless, the changes in lactate concentration at exhaustion did not differ significantly between groups in contrast to the time to exhaustion TTE Table 2. TTE increased in the LGI-G 1. Glucose concentrations at exhaustion did not change between baseline and post-intervention in any group. Furthermore, no group differences could be detected for glucose concentrations during the incremental test or at exhaustion. Changes in the VAS Score are shown in Figure 4. For all other analyses, no statistical or meaningful differences between week 1 and 4 could be detected in the respective group. Figure 4. Changes in visual analog scale VAS Scores. A General, B during physical activity, C gastrointestinal comfort. Data shown as mean ± SD at week 1 and week 4. The main purpose of the present investigation was to examine the effect of nutrition strategies varying in amount and type of carbohydrates on metabolic processes under resting conditions and different exercise scenarios. Compared to baseline levels, lactate concentrations under resting conditions and in submaximal test settings decreased in the group consuming low glycemic carbohydrates in a period of 4 weeks. During the incremental test, changes in lactate concentration were statistically significant and metabolically relevant. Although the fat oxidation was not measured directly in the current investigation, evidence suggests that there is a strong inverse relationship between plasma concentrations of lactate, free fatty acids, and β-oxidation during exercise As a potential consequence, the alterations in lactate concentrations might be indicative for an influence of a LGI diet on fat metabolism. These finding were supported by the changes in lactate concentrations during exercise. In the current investigation, lactate concentrations decreased in the HFLC-G during the submaximal and the incremental cycle test. As a potential result of low baseline data, lactate concentrations under pre-exercise conditions remained unchanged in the HFLC-G. In contrast, the carbohydrate-rich control diets were associated with the opposite effect 32 , 33 , Furthermore, major carbohydrate metabolizing enzymes glycogen phosphorylase, phosphofructokinase, and pyruvate dehydrogenase are less activated, while the activity of hormone-sensitive lipase and adipose triacylglycerol lipase has been shown to be increased. Carbohydrate-induced high plasma insulin concentrations caused the opposite effects 2 , 3. It has to be mentioned that in most studies, the carbohydrate-rich controls have been defined by the amount but not the GI of the ingested carbohydrates. The consumption of low glycemic carbohydrates is characterized by reduced postprandial glucose concentrations, which stimulates less insulin release. Consequently, the associated effects in the carbohydrate and fat metabolism, such as reduced lactate concentrations, decreased RER values, and increased use of free fatty acids, could be identified despite a high amount of carbohydrates 15 — 17 , However, there are controversial results whether low glycemic vs. high glycemic meals prior to exercise improved fat oxidation and performance during exercise To our best knowledge, there is little evidence coming from studies that have focused on longer-term low GI diets. In a study by Hamzah et al. the effect of the GI of high carbohydrate diets on energy metabolism and running capacity have been investigated The authors concluded that the GI had no influence on rates of fat oxidation. Taking metabolic adaptations to HFLC diets under consideration, 5 days might be insufficient for a LGI diet to have an impact on the metabolic response 9 , A long-term effect has only been investigated in a study by Durkalec-Michalski et al. In contrast to our findings, the LGI diet over 3 weeks resulted in a slight downregulation of fat oxidation during exercise However, in the study by Durkalec-Michalski et al. Furthermore, the decrease in RER values was not statistically significant and did not reach the MID in the present study. The LGI intervention seemed to have a smaller impact on metabolic adaptations than the HFLC diet. The up-regulating signals of fat oxidation are low insulin concentration and increased concentrations in plasma free fatty acids 2 , 3. A direct comparison between four meals, each different in the amount and GI of the ingested carbohydrates, has shown that both high fat groups were associated with the highest postprandial free fatty acid and lowest insulin concentrations. The lowest free fatty acid concentrations were in the group consuming a low glycemic carbohydrate-rich meal. Furthermore, postprandial insulin response was lower in the high carbohydrate low GI group compared to the high carbohydrate high GI group Consequently, the abovementioned adaptation processes might be less in a high carbohydrate low glycemic diet compared to a HFLC diet due to the different impact on postprandial free fatty acid and insulin concentrations. The nutritional impact on fat metabolism might also be reflected by the circulating glucose concentrations. Fasting glucose plasma concentrations dropped in the LGI-G to a significant and MID relevant extent. Changes in the HFLC-G seemed to be less pronounced, potentially as a consequence of relatively low baseline values compared to the other groups. During the post-intervention, incremental test glucose concentrations are lower at the same exercise intensity as in the unconditioned pre-values state in both LGI-G and HFLC-G. This is probably related to a stimulation of fat oxidation under resting conditions and during exercise The results of the HGI-G seemed to be controversial. The increased RER at rest in the HGI-G indicates an elevated metabolization of carbohydrates under resting conditions. In addition, the lactate concentration increase was clinically relevant under pre-exercise condition. Despite increased lactate concentrations during the incremental test, it seems that there is an improved fat metabolism -decreased glucose and lactate values- in the submaximal cycle test. It had previously been described that carbohydrates prior to exercise appear to be beneficial to performance 1. Hence, the slightly decreased carbohydrate metabolism in the submaximal test might be partly explained by the increased lactate threshold over the time as a possible adaptation in response to enhanced performance. As a result, at post-intervention, the participants performed the test closer to their lactate threshold compared to baseline. The current investigation also observed an improvement in body composition due to a decrease in fat mass following the 4-week LGI or HFLC diet on the level of significance and MID. It is not assumed that the present results can be attributed to the differences in energy intake between groups. Despite the significant difference in proportions of nutrients, the mean energy intake was equivalent between groups with an energy add-on of kcal in the HFLC-G. According to the findings of Hall et al. There is evidence that athletes can improve their body composition by a high fat in particular ketogenic diet 42 — Low carbohydrate diets compared with control diets have been suggested to be relatively more effective in body weight management. However, the benefits of a low carbohydrate diet can be rather attributed to the relatively high protein content, but not the relatively lower carbohydrate content 45 , In a recent study with athletes, different approaches high vs. low fat but similar protein intakes resulted in a similar change of body composition mean loss in body fat was 1. These are in accordance with a meta-analysis examining the impact of different diet types in obese or overweight people Data from the meta-analyses of the Cochrane Database of Systematic Reviews suggest that a low glycemic diet without energy restriction results in a significantly greater decreased fat mass and an increased fat free mass compared with a high glycemic or even high fat and energy restricted diet Although low glycemic diets seem to promote weight loss and metabolic improvements in obese and overweight adults 48 , research about the impact of the GI on body composition in endurance athletes is limited. A recent study by Durkalec-Michalski et al. has shown that consuming a low glycemic diet led to a change in body composition. In particular, a statistically significant reduction in body mass Physiologically, the significant changes in body composition in the present investigation might be explained by changes in fat oxidation and a more balanced carbohydrate metabolism as a potential consequence of the altered amount and quality of ingested carbohydrates. Despite an improvement in fat metabolism and body composition, there is a growing body of evidence that these changes induced by ketogenic or non-ketogenic HFLC diets are not in association with improved endurance performance, aerobic capacity and peak performance in particular 9 , 32 , 50 , 51 , due to an impaired carbohydrate provision during higher intensities 2. This assumption is supported by the changes in time to exhaustion in the present investigation. Furthermore, HFLC diets seem to be impractical and accompanied by side effects that include fatigue, headaches, poor concentration, lethargy, gastrointestinal discomfort, nausea, and unintentional weight loss. One reason might be an insufficient proliferation of essential micronutrients and fibers and glycogen depletion which might be a cause of impaired concentration and hence the neuromuscular connection 9 , The values of the VAS scores of all categories decreased in all groups, indicating that the participants got familiar with the respective dietary concepts. In general, none of the groups experienced clinically relevant elevated VAS scores. Mild symptoms can be defined by a score of 5 to 45 mm on the VAS This might be explained by the fact that endurance subjects tolerate the effects of a high-fat diet better than untrained individuals during exercise In addition, according to the nutritional protocols, an impaired delivery of minerals in the HFLC group was not expected. However, only the LGI-G and HGI-G have shown an improvement in VAS scores of the subscale activity and gastrointestinal comfort on a statistical or MID level with a superior effect in the LGI-G. These results might be associated with impaired training sessions in the HFLC-G since higher intensity levels could not be reached without the provision of carbohydrates 2. Furthermore, the advantage of LGI diet over HFLC and HGI diets might be in the choice of carbohydrates. A LGI diet is predominantly characterized by high-fiber and plant-based foods. This has shown to be associated with reduced fatigue, a strengthened immune system, and an improved ability to regenerate through the increased supply of micronutrients, essential fatty acids and amino acids, and low postprandial glucose concentrations Moreover, controlled clinical trials demonstrated that low glycemic foods have a positive impact on digestive conditions, such as gastroesophageal reflux disease or the irritable bowel syndrome, due to high fiber content 56 , It can be assumed that the present results can be attributed to the implementation of nutritional patterns. According to the analysis of the nutritional protocols, the participants' dietary intake reflected the specified intake of carbohydrates and fats in the respective group. While the HGI-G had a higher percent and total carbohydrate intake, the LGI-G showed a higher carbohydrate intake on a g-per-kg-body-weight basis. The current guidelines for endurance athletes during training on the competition level are 6—10 g carbohydrates per kg body weight and day. These recommendations do not address the GI of the ingested carbohydrates The participants of the current investigation were non-elite athletes with a training workload of 3—5 sessions per week. In both groups, the carbohydrate intake seems to be sufficient since recommendations are 5—7 g carbohydrates per kg bodyweight and day for general training needs Nevertheless, increasing the carbohydrate intake to 6—10 g carbohydrates per kg body weight and day would be an interesting approach in future studies with high trained endurance athletes. The carbohydrate upper limit of 50 g per day in the HFLC-G was based on the current focus of carbohydrate-restricted diets 9. This trial has some limitations. It has to be mentioned that the changes in fat and carbohydrate oxidation were not measured directly but extrapolated from the lactate diagnostics. However, it is reported that measuring blood lactate is an effective way to estimate the rates of fat and carbohydrate oxidation Furthermore, using the values of the spiroergometry to confirm the results from the lactate diagnostic during the incremental test has to be taken with caution since values for VO 2 are overestimated by a step compared to a ramp incremental test When taking the impact of the nutritional concepts into account, limitations of the self-reported protocols might entail an over or underreporting of the consumed foods Moreover, recommendations for the macronutrient intake based on the body weight seems to be more accurate than percentage values to determine nutritional guidelines for endurance athletes. Future studies with a larger sample size should include different sex groups and pre-exercise nutritional conditions to state practical use of high fat vs. |
Improved fat utilization efficiency -
Highly trained competitive middle-aged athletes underwent two day isocaloric diet periods HCLF or LCHF in a randomized www. org , counterbalanced, crossover design with a two-week washout period between dietary interventions without feeding limitations.
We assessed both diets while controlling calories and training load. Primary outcomes were performance, substrate oxidation during exercise, continuous glucose and cardiometabolic biomarkers. Each subject visited the laboratory on ten separate occasions, performing testing before PRE and at the completion POST of each day dietary intervention Figure 2.
Visit one and two consisted of a familiarization of measurement instruments, equipment, perceptual measurements 42 , 43 , consent, VO 2 max test 31 , and continuous glucose monitoring CGM; Freestyle Libre 2, Abbott Diabetes Care Inc; Almeda, CA sensor application. One-mile [1, m] running time trial TT and repeated sprint protocol RSP; 6 × m were performed twice on each dietary intervention Pre and Post.
One-mile TT was performed on Day —4 and Day Three days later, subjects performed the RSP on Day —1 and Day Gas exchange and perceptual changes were recorded throughout each performance trial Pre and Post each dietary intervention.
Body composition and cardiometabolic parameters were measured Pre and Post each dietary intervention. Capillary and interstitial metabolite concentrations were measured throughout each day dietary intervention period.
The experimental protocol was approved by the Institutional Review Board of Grove City College prior to implementation IRB number Figure 2. Experimental timeline and participant characteristics. The Pre-diet study tasks included familiarization with the study protocol, time trial TT practice runs, VO2max assessment, and continuous glucose monitor CGM application for measurement of interstitial fluid glucose visits 01 and Ketones R-β-hydroxybutyrate and lactate were analyzed enzymatically using finger-sticks.
Once a first diet was completed, subjects crossed over to the other dietary treatment and repeated visits 03 through 06 i. The table illustrates the mean SD participant age, anthropometry, and training status variables collected at visit Figure was created with BioRender.
Ten middle-aged competitive distance runners Figure 2 were recruited directly from local running organizations and by advertising within the local community.
Subjects were instructed to refrain from caffeine and alcohol consumption for 48 h, and racing or training for 24 h before each performance test. Using direct counseling and prepared educational handouts, a registered dietitian taught and guided each athlete prior to the experimental phase on how to implement the LCHF and HCLF diets at home.
Instructional handouts included: i summary of key aspects of each diet, ii day LCHF and HCLF meal plan but were advised to use this meal plan as a guide rather than a strict protocol; iii detailed guide on acceptable low-carbohydrate foods as well as a recommended list of nutritious fat and protein-rich foods.
Weekly energy intake and relative macronutrient distribution were monitored and estimated via 3-day weighed food records, capturing two consecutive weekdays and a weekend day via the online smartphone application, MyFitnessPal.
Subjects used digital kitchen scales to measure the weights of food portions for total energy intake estimates. A two-week recovery macrocycle was incorporated between each dietary intervention without dietary restriction.
Verification of compliance to the LCHF diet was done via capillary blood ketone concentrations on days 3, 7, 14, 21, and 28 before ingesting breakfast and exercising. Capillary blood ketone concentrations R -β-hydroxybutyrate; Precision Xtra, Abbott Diabetes Care Inc.
Participants were instructed to maintain a training log mode, duration, and intensity of each workout during the study intervention without increasing or decreasing the training load Training load was calculated by using the session-RPE method RPE x session duration [min] The measurements of body mass were performed on a medical scale with a precision of 0.
Body composition was evaluated using the electrical impedance technique Tanita ® MCU, Tanita Corporation, Inc. On the second visit, subjects performed an incremental test to exhaustion on a motorized treadmill Trackmaster TMXC treadmill, Newton, KS utilizing the modified Astrand treadmill protocol.
Subjects wore a Polar heart rate monitor Polar Electro, Kempele, Finland during exercise to measure heart rate. Immediately following the 3-min warm-up, the speed was increased to 8—13 km.
After a 5-min passive rest period, subjects then initiated the one-mile TT 1, m. Prior to the TT, the treadmill was brought to a standstill 0 km. hr —1 , all timing devices were reset, the distance covered on the treadmill monitor was reset, a 5-s count down was given, and the TT began.
Running speed and time was not visible to subjects during the TT. Subjects were verbally informed of the distance they had covered at m intervals. Subjects were instructed to finish the TT as fast as possible and were not informed of the overall performance time until completion of the final testing session.
Heart rate Polar Electro, Kempele, Finland , RPE RPE-Overall; RPE-Chest; RPE-Legs and affect Feeling Scale were recorded at m intervals during the TT. Lastly, RPE and affect for the entire exercise session session RPE and session affect were obtained 5 min following the one-mile TT.
Expired respiratory gases were continuously collected during the entire time trial and capillary blood was collected Pre- and Post-TT. Upon arrival to the laboratory subjects completed a 10 min self-paced warm-up on the treadmill. The RSP prolonged high intensity interval protocol was chosen because it requires a high level of aerobic oxidative, as well as anaerobic glycolytic contributions The RSP was performed on a treadmill and consisted of 6 × m 0.
Subjects were instructed to finish each m sprint as fast as possible. The time it takes to complete each sprint was recorded. No feedback on sprint times was given to the participants during trials. Verbal encouragement was provided during maximum effort sprints in a standardized fashion throughout each visit.
Heart rate, blood samples, RPE, affect, and metabolic gases were collected throughout. Respiratory gas exchange was recorded using an automated metabolic analyzer system TrueOne , ParvoMedics, Sandy, UT, United States. Prior to each experimental session, the device was calibrated using procedures according to manufacturer instructions.
The breath-by-breath measurements were performed for oxygen uptake VO 2 , carbon dioxide production VCO 2 , and respiratory exchange ratio RER and was measured continuously throughout trials one-mile TT and RSP.
Blood samples were measured via fingertip blood samples collected using a lancet following cleaning of the fingertip with an alcohol swab and then dried.
The first droplet was wiped away with a cotton swab to remove any alcohol and the subsequent droplets were used for analysis. Samples were immediately processed for measurement of blood lactate Lactate Plus, Nova Biomedical , ketones R -β-hydroxybutyrate; Precision Xtra, Abbott Diabetes Care Inc.
Blood samples were collected using a validated dried blood spot DBS card technique DBS were assayed for HbA 1c , total cholesterol, low-density lipoprotein cholesterol LDL-C , very low-density lipoprotein cholesterol VLDL-C , high-density lipoprotein cholesterol HDL-C , triglycerides, insulin, and high-sensitivity C-reactive protein hsCRP.
Dried blood spot testing has shown a strong correlation with conventional serum tests, making it a reliable and convenient tool for screening cardiometabolic risk factors CGM tracks long-term to HbA 1c 54 — 56 , short term CGM readings d are good estimates of 3-month CGM averages 57 , and iii CGM can also capture both fasting and post-prandial differences in glucose validated diagnostic tools.
Participants were instructed to place it in the same location throughout. CGM subcutaneous sensor measured interstitial glucose values every 15 min which was transmitted using near-field communications. Participants obtained their glucose values after scanning for up to 8 h of data. All hour CGM readings were included into daily and day statistics.
All CGM sensors were obtained in batch to control for any potential manufacturing discrepancies across different sensor batches. Statistical analyses were performed using SPSS version Descriptive statistics were calculated for all variables.
Normality and absence of large outliers were verified by using the Shapiro-Wilks test, observing the normality plots, and residual plots. Repeated measure analyses of variance ANOVA were utilized to assess physiologic, metabolic, respiratory, perceptual, and performance time, treatment, and interaction effects.
Bonferroni post hoc was utilized to control for multiple comparisons when main effects were observed. A paired samples t -test was used to analyze differences in macronutrient composition and day glycemic variable averages between the two dietary interventions.
A one-way repeated measures analysis of variance was used to analyze differences over time for training load.
Greenhouse-Geisser epsilon corrections were used when the sphericity assumption was violated. Partial-eta squared η2p was used to report the effect sizes for the above metrics, where appropriate.
Simple linear regression analyses were run to determine the relationship between glycemic, substrate oxidation, and biochemical parameters. All data are reported as Median ± SD, with exception of circadian glucose patterns presented as Median, 25th, and 75th percentile.
Daily dietary nutrient intakes are summarized in Table 1. The energy intake between LCHF and HCLF treatments remained isocaloric. Significant differences were detected for every diet composition variable, notably for carbohydrate and fat intake.
LCHF consumed less absolute and relative carbohydrates compared to HCLF, largely driven by the fold reduction in simple sugars and approximately one-half the dietary fiber. Conversely, LCHF consumed significantly more dietary fat and cholesterol, both as absolute and relative amounts, compared to HCLF.
Average capillary R -βHB mean ± SD: 0. Figure 3. Daily capillary ketones. The dotted line denotes the low carbohydrate high fat LCHF R -beta-hydroxybutyrate R -βHB mean.
Eight out of ten participants consistently remained in nutritional ketosis throughout the LCHF intervention, whereas two participants were marginally below threshold.
One-Way ANOVA revealed that there were no significant time effects from day 3 and thereafter, meaning that ketosis was rapidly induced and maintained over four-weeks.
There were no significant differences for weekly training load, either within- or between dietary phases Table 2. Participants started each diet at similar weight and body composition. There were no significant treatment or interaction effects for weight or body composition during either LCHF or HCLF Table 3.
Overall changes in weight and body composition on each diet were similar. Significant time effects were detected for weight and BMI in both LCHF and HCLF treatments. All 10 participants completed the treadmill one-mile time trial 1, m at a self-selected pace before initiating either diet.
No significant baseline Pre differences were observed across physiological, metabolic, respiratory, perceptual, or performance parameters.
Significant differences were detected within-diet and between-treatments at the post-timepoint Table 4. There were no significant changes in substrate oxidation and respiratory exchange rate detected pre- to post-HCLF.
Significant interactions revealed that Post-LCHF athletes had a higher heart rate Δ: 6 ± 2 bpm , mean fat oxidation rate Δ: 0. All 10 participants completed the treadmill series of 6-sets of m sprints at a self-selected pace.
Before each dietary phase there were no significant differences in physiological, metabolic, respiratory, perceptual, or performance parameters.
Additionally, neither diet influenced repeated sprints running performance post-diet intervention Table 5 and Figure 4. Figure 4. Repeated sprint running performance. Diet did not significantly alter running times between or within treatments.
There was a main effect of time for running performance reflected in higher running split time at set 3, 4, and 5 during pre-diet phase. Post-diet phase showed significant increase in split time during set 4 and 5 only.
Mean ± SD. During RSP, LCHF induced very high rates of fat oxidation which peaked at 1. To our knowledge, these are the highest rates of fat oxidation ever recorded.
No significant Pre-Post changes were detected within-HCLF Figure 5 and Supplementary Table 1. Figure 5. Substrate oxidation rates. Post-diet CHO and FAT oxidation were significantly altered by diet. No Pre-Post substrate oxidation changes were detected for HCLF. This increased relative oxygen consumption after LCHF is largely explained by weight-loss, and partly explained by the non-significant increase in heart rate i.
Prior to dietary intervention, capillary blood R -βHB was below the limit of nutritional ketosis pre-diet and pre-TT mean ± SD: 0. Post-LCHF treatment significantly increased R -βHB from baseline 0.
Capillary blood glucose concentrations were similar Pre- and Post-diet between LCHF and HCLF treatments LCHF average: HCLF average: There was a main effect of time observed Post one-mile TT that raised blood glucose from baseline Capillary blood lactate before the one-mile TT was at the same concentration Pre- and Post-diet in both LCHF and HCLF treatments LCHF average: 1.
HCLF average: 1. There was a main effect of time induced by exercise that increased blood lactate significantly from baseline 1. Figure 6.
One-mile time-trial metabolite impact. Capillary ketones, glucose and lactate were measured immediately pre- and post-one mile time trial TT to evaluate the within- and between-diet effects in the context of exercise.
There were no significant Pre-diet and Pre-TT differences. A significant post-diet effect was detected in capillary ketones Pre-TT.
Glucose and lactate were significantly elevated over time, independent of diet and dependent on TT. Capillary blood R -βHB was below the limit of nutritional ketosis Pre-diet and Pre-RSP 0.
LCHF significantly increased R -βHB into nutritional ketosis compared to pre-diet concentrations 0. Over the course of the RSP, R -βHB decreased by approximately 0. Capillary blood glucose concentrations Pre-diet and Pre-RSP Capillary lactate concentrations Pre-diet and Pre-RSP 1.
Peak lactate concentrations 6. Diet did not significantly influence the rate of lactate appearance in the blood nor peak lactate Figure 7 and Supplementary Table 3. Figure 7. Repeated sprint performance metabolite impact. Capillary R-βHB, glucose, and lactate were measured in capillary blood immediately after each repeated sprint performance RSP set.
There were no significant between-treatment differences pre-diet. The significant time effects were detected in lower R-βHB values at set 1 and 2, higher glucose values at set 5 and post, and significantly higher lactate values from set 1 and thereafter.
Post-diet ketones were significantly influenced by low carbohydrate high fat LCHF treatment, with 3-fold higher R-βeta-Hydroxybutyrate R-βHB concentrations throughout the sets compared to HCLF. Lactate was not affected significantly by diet.
There were no significant changes over time in any of the variables of interest Figure 8 and Supplementary Table 4. Between-condition effects reveal higher total cholesterol Δ: Interaction effects revealed total cholesterol Δ: Figure 8. Cardiometabolic scores.
Statistics were conducted on absolute values and are presented as mean change from Pre-diet. There were no cardiometabolic differences Pre-diet or significant time effects.
Between-diet effects revealed greater total cholesterol and LDL-C concentrations during the LCHF versus HCLF treatment. The significant interaction revealed greater total cholesterol and HDL-C concentrations Post-LCHF treatment.
All glycemic parameters significantly improved on LCHF Figures 9 , Average glucose was significantly lower during LCHF treatment starting day 8, and remained lower on day 13, , and 22 Figure 9. Additionally, this prediabetic phenotype was present in these subjects despite them losing weight on both nutritional strategies LCHF: —2.
Figure 9. Continuous glucose monitoring. Average glucose was significantly lower on LCHF on day 8, 13, , and All glycemic parameters over the days dietary intervention were significantly improved during LCHF.
The day mean glucose predicted the percent change in mean glucose between LCHF and HCLF diets. These same subjects also reported the highest peak fat oxidation rate as the percent change in mean glucose between LCHF and HCLF diets predicted the peak oxidation rates across the entire cohort.
Peak fat oxidation rates on LCHF were also associated with higher cholesterol demonstrating a potential interaction between oxidation rates and global lipid metabolism.
Figure Circadian glucose patterns. Red, LCHF. Black, HCLF. Dashed line, individual pre-diabetic subject circadian glucose patterns.
There are four key findings of this study Figure 1. i Athletes achieved equivalent exercise performances during a 1, m time trial and a 6 × m interval session after a day habituation to LCHF or HCLF diets when controlling calories, training load, and body composition changes across groups.
ii During the latter stages of the 6 × m interval session, athletes achieved the highest rates of fat oxidation yet reported. According to current understanding, this is paradoxical since these high rates were measured in subjects exercising at an intensity iii days on each diet produced equivalent fasting insulin, hsCRP, and HbA 1c , with elevated total, low-density lipoprotein, and high-density cholesterol on LCHF.
iv LCHF consistently reduced glucose levels and variability with a large inverse relationship observed between mean glucose on HCLF and the percent change in mean glucose when switching to LCHF. Additionally, relationships were observed between glycemic change, peak fat oxidation, and circulating lipids, as the larger the reduction in mean glucose on LCHF the larger the peak fat oxidation on LCHF, and the larger the peak fat oxidation on LCHF and the higher circulating lipids were.
These results challenge the existing paradigm that diets with higher carbohydrate intake are superior for athletic performance, even during shorter-duration, higher-intensity exercise.
Critically, these results demonstrate that lower carbohydrate intake may be a therapeutic strategy, even in athletes, to improve glycemic control, particularly in those with, or at risk for diabetes, without requiring changes in body composition or physical activity.
Interestingly, these results also demonstrate a unique association between glycemic responsiveness to carbohydrate restriction, fat oxidation rates, and circulating lipids, suggesting an important relationship between continuous glycemic parameters and systemic metabolic responsiveness.
Performance during the 1, m time trials was the same when athletes ate HCLF or LCHF diets. This is in keeping with our previous study 31 in which the 5-km time trial performances of athletes, similar in ability to those studied here, were equivalent on either diet.
It adds further weight to the conclusions from two recent meta-analyses 63 , 64 that that LCHF and HCLF diets produce equivalent performances across a wide range of athletic events. Our reasoning was that if the pre-exercise muscle glycogen stores are a critical determinant of exercise performance and if the LCHF diet is associated with lower muscle glycogen concentrations in recreational athletes 28 but perhaps not in highly competitive athletes 65 , and since very high rates of muscle glycogen use are measured during m repetitions 66 so that, if significant muscle glycogen depletion can be produced by a high intensity interval session, then any impaired performance of athletes eating the LCHF diet should become apparent in the latter intervals of that session.
For example, Impey et al. reported rates of muscle glycogen use of Webster et al. In contrast to our expectation, based on this prediction that significant muscle glycogen depletion would occur in athletes following the LCHF diet and this would impair their performance, in fact exercise performance was identical across all the intervals on either diet Tables 4 , 5 ; Figure 4 and Supplementary Table 1.
These finding raise the important question of why our two studies have failed to detect diet-induced differences in performance whereas prior meticulously conducted studies 16 — 18 detected meaningful differences in their studies of Olympic standard race walkers.
Five key factors may have contributed to these differences: randomization, dietary controls during exercise, training load, body composition, and dietary habituation timeline. Prior studies with differing results allowed subjects to choose the diet they preferred 16 — As a result, blood glucose levels were lower in the LCHF group in the two trials 16 , 17 in which it was measured, with a trend toward a progressive hypoglycemia in one trial [figure 5A from 17 ].
As the authors of those studies appreciate, even in the absence of hypoglycemia, carbohydrate ingestion alone can have an ergogenic effect even if the carbohydrate is not ingested Thus, these trials did not control for the potential effects of carbohydrate ingestion during exercise.
The potential role of hypoglycemia in explaining differences in exercise performance has recently been revisited The intensified training load and across group differences in body composition in these trials 16 — 18 also illustrates key differences as increased physical activity levels 68 and body weight reductions 69 both illustrate biological stressors requiring adaptation and may independently impact performance.
The increased physical activity across groups and more significant reductions in bodyweight in LCHF arm 16 — 18 , on top of introducing a diet which requires systemic metabolic reprogramming 70 , illustrate three co-administered biological stressors all requiring adaptions and which may influence performance.
Thus, it is not surprising that when we controlled randomization within-subject , dietary controls during performance testing, calories, training load, and body compositional changes across groups to allow for the isolation of diet-induced changes across these key parameters, we observed different results from prior observations 16 — Of note, when Burke et al.
The described method for measuring maximal rates of fat oxidation during exercise is to have subjects exercise for short periods of approximately 3 minutes at exercise intensities that gradually increase 22 — Maximal rates of fat oxidation measured with this method are usually in the range of 0.
Higher rates of fat oxidation have been measured in athletes adapting to the LCHF diet. Volek et al. measured rates of 1. measured rates in excess of 1. In a case study of an elite Ironman triathlete, Webster et al. reported a peak fat oxidation rate of 1. Shaw et al. demonstrate fat oxidation ranging from 0.
Our data is in line with these prior studies showing elevated fat oxidation rates LCHF: 1. However, what is particularly unique in our findings is that we observed the peak fat oxidation rates LCHF: 1. Three out of eight cardiometabolic markers were significantly modulated by diet, most notably post-diet LCHF vs.
HCLF total cholesterol vs. The significant main effects in this study were directly attributable to the between-diet differences in dietary fat and fat composition, however, the fact that more than half of participants had borderline elevated total cholesterol, LDL-C, and HDL-C at baseline was somewhat unexpected.
While carryover effects were ruled out by identical concentrations at baseline i. Moreover, it is unclear if similar dietary interventions in mildly hypercholesterolemic athletes will exert any significant impact on cardiometabolic indices that are beyond the effects induced by their habitual diet.
Individual cardiometabolic responses are available for review in the supplement Supplementary Figure 1. We detected a small, but significant change in weight over time, primarily derived from fat mass.
Based on prior evidence 73 , 76 the LCHF diet was projected to lower cardiometabolic markers beyond a HCLF diet, even in the absence of weight-loss 77 ; however, we did not observe these results with our between-diet isocaloric feeding design.
Additionally, it is important to acknowledge that HbA 1c is a 2—3-month biomarker that we quantified to predict directional trends rather than significant changes over four weeks.
Based on our findings, we expect that these markers will continue decreasing after four-weeks of LCHF, similar to isocaloric HCLF feeding when duration, energy intake, and weight are controlled between conditions.
When measuring continuous glucose levels every 15 min over a day period, we observed improvements across all glycemic parameters in virtually all subjects on the LCHF diet, with initial significant differences in mean glucose observed on day 8 of dietary habituation.
Carbohydrate restriction is a known therapeutic strategy to help facilitate improvements in glycemic control and other key metabolic parameters in other clinical conditions such as obesity 82 , type-1 diabetes 36 , and type-2 diabetes 33 , These illustrate critical controls allowing us to extract diet-induced impact on glycemic parameters as prior observations have shown that caloric intake and changes in body weight both influence glucose levels regardless of diet 83 , Additionally, prior evaluations have found that intensified training programs can disrupt not only glycaemia and mitochondrial function, but also performance While there have been small short-term investigations exploring glycemic control during exercise in athletes 79 , 85 , very few studies have investigated the relationship between the long-term i.
Nolan et al. reported the impact of a ketogenic diet on an individual type-1 diabetic cyclist during a day, km race This case report demonstrated remarkable glycemic control for a Type-1 Diabetic compared to historical glycemic norms for Type-1 Diabetics during this 20d race window, but nature of the report did not allow for the comparison of performance on- and off-diet.
These subjects fitting the pre-diabetes glycemic phenotype in our study could not be explained by underlying demographics, body composition or physical activity differences as these pre-diabetic subjects had near equivalent age pre-diabetic: This is in line with the understanding that multiple factors contribute to diabetes onset 88 , 89 , some of which may go undetected until overt diagnosis.
Potential explanations for early pathogenic progression of diabetic dysglycemia include genetic predisposition, adiposity-induced insulin resistance, fasting insulin, and beta-cell dysfunction However, markers of elevated adiposity were not higher in the prediabetic group.
In fact, this sub-cohort lost weight on both dietary protocols. Additionally, circulating lipids tended to be lower on the HCLF diet suggesting lipids could not explain dysglycemia on HCLF.
While intense exercise overtraining has also been demonstrated to acutely disrupt mitochondrial and glycemic function 68 , this dysfunction was reversed following reduction in activity and cannot explain our results as our subjects did not increase or decrease physical activity levels.
Importantly, Al-Ozairi et al. found that a 6-day LCHF diet in Type-2 Diabetic subjects who kept calories and bodyweight controlled were unable to find differences in mean and post-prandial glycaemia utilizing CGM devices This could be due to the short treatment duration as we observed significant differences on day 8 of the isocaloric HCLF and LCHF diets.
Alternatively, it may be explained by the influence of engaging in physical exercise regularly as Moholdt et al.
Importantly, when looking to observe if the entire cohort also observed a relationship between day average mean glucose on HCLF diet and percentage change in mean glucose between LCHF and HCLF diet, we observed a large significant inverse relationship, indicating that those individuals with a higher mean glucose, are more responsive to carbohydrate restriction treatment, not just those with pre-diabetic glycemic phenotypes.
As our study and prior literature suggests this change is in response to diet and not other factors i. While multiple studies have shown reductions in glucose 35 , 80 , 83 and elevations in fat oxidation 16 — 18 , 28 , 31 , 91 on a LCHF diet, we are unaware of any data which has demonstrated that the magnitude of glycemic changes across diet predicted the magnitude of peak fat oxidation rates.
Interestingly, we also observed that higher peak fat oxidation levels on LCHF predicted higher total cholesterol on LCHF suggesting a potential interaction between higher rates of fat turnover and higher levels of circulating lipids while on a diet that restricts carbohydrates and increases fat intake.
While elevated fat oxidation rates have been observed on LCHF diet in the absence in changes of insulin or calories, explained by elevated fat intake, 91 , they did not see a change in glucose levels nor did they explore whether the magnitude of fat oxidation rate was associated with glucose or lipid parameters.
In line with our data, there has been a report demonstrating that individuals with healthy bodyweight undergoing a LCHF diet can have elevated circulating lipid i. While this prior observation did not look at either total cholesterol or fat oxidation rates, in light of our data, there remains a possibility that these individuals 92 , have elevated levels of systemic fat oxidation which requires further analyses.
The ability for i d mean glucose on HCLF to predict changes in mean glucose following carbohydrate restriction, ii changes in mean glucose with carbohydrate restriction to predict peak oxidation rates, and iii peak fat oxidation to predict total cholesterol suggests a unique predictable physiologic relationship between glycemia, substrate oxidation, and circulating lipids biomarkers which requires further validation.
This study had middle-aged competitive male athletes which may limit our understanding of the translatability of these findings to female athletes due to potential differences across sex on the magnitude of metabolic response 93 — 95 , particularly for those women in middle age during pre-menopause and post-menopause who may benefit most due to elevated risk for cardiovascular and metabolic disease 96 , While our short-duration high-intensity exercise 6 × m would be sufficient to reduce muscle glycogen content based on prior work, 28 , 65 , 66 we did not measure muscle glycogen content so we cannot say for certain what levels of muscle glycogen were achieved and if they were associate with elevated fat oxidation levels during exercise.
While HbA 1c is gold-standard for diagnosing diabetic phenotype due to its established role in diabetes, our dietary intervention was 4 weeks in length, an insufficient time to observe the full diet-induced impact on HbA 1c which requires a minimum of weeks 98 , We utilized CGM to capture the 4-week h glycemic control as i CGM tracks long-term to HbA 1c 54 — 56 , ii shorter term CGM readings d are good estimates of 3-month CGM averages 57 , and iii can also capture both fasting and post-prandial differences in glucose which is a validated diagnostic tool Figures 9 , Although limitation have been cited when looking different CGM technology and different insertion sites, both technology and insertion site were controlled in our analyses However, it is important to note the clear limitation of HbA 1c and oral glucose tolerance test OGTT in our present analyses and why CGM was the primary glycemic metric.
It is well-established that for a given HbA 1c value, there is a wide-range of mean glucose concentrations, and for any given mean glucose concentration, there is a wide-range of HbA 1c values, suggesting some limitation around this biomarker Thus, some expert consensus has argued for moving beyond just HbA 1c at the individual levels Additionally, it has been known for decades that OGTT is inappropriate for individuals not adhering to an HCLF diet as this test was only validated under high-carbohydrate consumption While we feel confident that our h 4-week glycemic values across subjects accurately capture the glycemic impact over our study duration, future studies with benefit from longer dietary interventions m in duration to capture changes in HbA 1c.
We observed record high peak oxidation rates with elevations in cholesterol in LCHF. All individuals experienced reductions in day average glucose means, median, and variability with carbohydrate restriction LCHF which resolved the pre-diabetic phenotype across all subjects without requiring caloric restriction, increased physical activity, or significant changes in body composition across groups.
Interestingly, the average glucose during high carbohydrate consumption predicted the degree of glycemic response to carbohydrate restriction suggesting that individuals with higher starting glucose may benefit most from carbohydrate restriction.
Surprisingly, we also found that the magnitude of glucose reduction during carbohydrate restriction predicted the elevation in fat oxidation rates during exercise suggesting that glucose response is linked to systemic fat oxidation. Taken together, LCHF may represent a therapeutic strategy to improve glucose levels, particularly in those at risk for diabetes, without compromising high intensity exercise performance in middle-aged athletes.
Future studies should evaluate the impact of these dietary strategies in middle-aged women who are at elevated risk for cardiovascular and metabolic disease. The studies involving human participants were reviewed and approved by Institutional Review Board of Grove City College IRB number PP and TN conceived the original study design.
KH designed the diets and provided the nutritional counseling. PP, AB, and AK conducted the data analysis. PP, TN, AK, and AB drafted the final manuscript. All authors have read and agreed to the published version of the manuscript.
We thank Levels, Inc. We also thank Azure D. Grant, Ph. for her assistance in organizing continuous glucose monitoring data and developing code for circadian glucose analyses and illustration.
We also thank the participants for their vital contribution to this study. TN and JV were authors of low-carbohydrate nutrition books. TN book royalties go to The Noakes Foundation which contributes to the Eat Better South Africa Campaign.
JV receives royalties from book sale; is a founder, and has equity in, Virta Health; and is a science advisor for Simply Good Foods and Cook Keto.
AK was a patent inventor and has consulted for Simply Good Foods. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers.
Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Evolution of food provision to athletes at the summer olympic games.
Nutr Rev. doi: PubMed Abstract CrossRef Full Text Google Scholar. Organisationskomitee für Die Xi. Olympiade Berlin The Official Report of the Xith Olympic Games. Berlin Berlin: Wilhelm Limpert-Verlag Google Scholar.
Schenk P. Olympischen Spielen zu Berlin. Die Ernaehrung. Atlanta Committee for the Olympic Games. The Official Report of the Centennial Olympic Games. Volume 1 Planning and Organization. Atlanta, GA: Peachtree American College of Sports Medicine, American Dietetic Association, Dietitians of Canada.
Joint position statement: nutrition and athletic performance. American college of sports medicine, American dietetic association, and dietitians of Canada. Med Sci Sports Exerc. Bergstrom J, Hermansen L, Hultman E, Saltin B. Diet, muscle glycogen and physical performance.
Acta Physiol Scand. Hermansen L, Hultman E, Saltin B. Muscle glycogen during prolonged severe exercise. Ahlborg B, Bergstrom J, Brohult J, Ekelund LG, Hultman E, Maschio G.
Yet, to the knowledge of this author, only two new investigations of LCHF diets in athletes have appeared in the peer-reviewed literature since [ 49 , 50 ]. These studies, summarized in Table 2 , fail to show performance benefits associated with a ketogenic LCHF diet, although there is evidence of a small but favorable reduction in body fat levels.
Nevertheless, there are some peculiarities with the design or methodologies of these studies, including the failure of one study to achieve the carbohydrate restriction typically associated with the ketogenic LCHF diet, and they have failed to become widely cited, even by supporters of the LCHF movement.
Rather, the current interest in chronic application of LCHF eating by athletes appears to be driven by enthusiastic discussion in lay and social media by mostly non-elite athletes of sporting success following experimentation with such diets as well as a range of outputs from several sports scientists who are researchers and advocates of this eating style [ 3 — 8 ].
It is uncertain whether there is a cause—effect relationship between these sources or the direction of any relationship , but the fervor merits attention. In the absence of compelling new data, the reader is alerted to several elements in the discussions that are positive and some that are concerning:.
Peer-reviewed publications from the key scientific protagonists of the LCHF movement [ 3 , 5 , 6 ] generally show measured and thoughtful insights, based on a re-examination of previously conducted studies, personal experiences, anecdotal observations from the sports world, and the general interest in tackling modern health problems with the LCHF approach [ 51 , 52 ].
In these forums, the discussion points include the lack of evidence and equivocal outcomes of research to support the performance benefits of LCHF but also theoretical constructs around potential benefits to metabolism, muscle, and brain function, inflammatory and oxidative status, and body composition management.
While there are some suggestions that a larger group of athletes might benefit from an LCHF approach, the general tone is that further investigation of these theories is required [ 3 — 6 ]. The apparent caution expressed in peer-reviewed publications is generally not present in other outputs from the same authors.
The differences between these viewpoints can be confusing, as is the misrepresentation of the physiological requirements of competitive sports see Sect. Many of the theorized benefits from the LCHF diet are claimed to come from the adaptation to high circulating levels of ketone bodies, which provide an additional fuel source for the brain and muscle as well as achieve other health and functional benefits [ 5 , 6 ].
The amount of energy that can be provided by ketones as an exercise substrate has been neither calculated nor measured, making it impossible to verify this claim. The time required to achieve optimal adaptation and, therefore, the period that requires investigation in new studies is claimed to be at least 2—3 weeks, with at least 1 week required before the feelings of lethargy and reduced exercise capacity abate [ 5 , 6 ].
With such chronic keto-adaptation, it is considered unnecessary to consume carbohydrate during exercise, or perhaps to consume it in small amounts [ 5 , 6 ]. As has been discussed in this review, the current evidence for these claims is equivocal and mostly anecdotal.
Until or unless further research is undertaken, we are unlikely to resolve any of the current questions and claims. The role of non-ketogenic LCHF diets is not clear.
The current literature on LCHF diets is relentless in promoting misunderstanding or misinformation on the current guidelines for athletes in relation to carbohydrate intake in the training or competition diet.
It would benefit sports nutrition for researchers and practitioners to show mutual respect in recognizing the evolution of new ideas and the replacement of old guidelines with new recommendations [ 53 ]. Indeed, modern sports nutrition practitioners teach athletes to manipulate their eating practices to avoid unnecessary and excessive intakes of carbohydrates per se, to optimize training outcomes via modification of the timing, amount and type of carbohydrate-rich foods and drinks to balance periods of low- and high-carbohydrate availability and to adopt well-practiced competition strategies that provide appropriate carbohydrate availability according to the needs and opportunities provided by the event and individual experience [ 14 , 54 — 57 ].
This author and others continue to undertake research to evolve and refine the understanding of conditions in which low carbohydrate availability can be tolerated or actually beneficial [ 58 , 59 ]. However, we also recognize that the benefits of carbohydrate as a substrate for exercise across the full range of exercise intensities via separate pathways [ 16 ], the better economy of carbohydrate oxidation versus fat oxidation ATP produced per L of oxygen combusted [ 60 ], and the potential CNS benefits of mouth sensing of carbohydrate [ 61 ] can contribute to optimal sporting performance and should not be shunned simply because of the lure of the size of body fat stores.
Considering that athletes might best benefit from a range of options in the dietary tool box is likely to be a better model for optimal sports nutrition than insisting on a single, one-size-fits-all solution.
Havemann L, West S, Goedecke JH, et al. Fat adaptation followed by carbohydrate-loading compromises high-intensity sprint performance. J Appl Physiol. Article CAS PubMed Google Scholar. Burke LM, Kiens B. Article PubMed Google Scholar.
Noakes T, Volek JS, Phinney SD. Low-carbohydrate diets for athletes: what evidence? Br J Sports Med. Brukner P. Volek JS, Noakes T, Phinney SD. Rethinking fat as a fuel for endurance exercise.
Eur J Sports Sci. Google Scholar. Phinney SD. Ketogenic diets and physical performance. Nutr Metab. Article Google Scholar. Volek JS, Phinney SD. The art and science of low carbohydrate performance. Beyond Obesity LLC; Can elite athletes eat LCHF and win? Available from: www. Accessed 30 June Olsen A.
Tim Noakes: low carbohydrate diet for endurance sports. Hall N. The Kardashian index: a measure of discrepant social media profile for scientists.
Genome Biol. Article PubMed Central PubMed Google Scholar. Noakes TD. Low-carbohydrate and high-fat intake can manage obesity and associated conditions: Occasional survey.
S Afr Med J. Hopkins WG, Hawley JA, Burke LM. Design and analysis of research on sport performance enhancement.
Med Sci Sports Exerc. Hawley JA, Burke LM, Phillips SM, et al. Nutritional modulation of training-induced skeletal muscle adaptations. Stellingwerff T. Contemporary nutrition approaches to optimize elite marathon performance. Int J Sports Physiol Perform. PubMed Google Scholar. Burke L.
Training and competition nutrition. In: Burke L, editor. Practical sports nutrition. Champaign: Human Kinetics; Spriet LL.
New insights into the interaction of carbohydrate and fat metabolism during exercise. Sports Med. Fernandez-Garcia B, Perez-Landaluce J, Rodriguez-Alonso M, et al. Intensity of exercise during road race pro-cycling competition. Bentley DJ, Millet GP, Vleck VE, et al.
Specific aspects of contemporary triathlon: implications for physiological analysis and performance. Tucker R. Science of sport: marathon analysis. In: Marathon analysis. Accessed 20 Oct Joyner MJ, Ruiz JR, Lucia A. The two-hour marathon: who and when? Peters A, Schweiger U, Pellerin L, et al.
The selfish brain: competition for energy resources. Neurosci Biobehav Rev. Matsui T, Soya S, Okamoto M, et al. Brain glycogen decreases during prolonged exercise. J Physiol. PubMed Central CAS PubMed Google Scholar. Zhang Y, Kuang Y, LaManna JC, et al. Contribution of brain glucose and ketone bodies to oxidative metabolism.
Adv Exp Med Biol. Karelis AD, Smith JW, Passe DH, et al. Carbohydrate administration and exercise performance: what are the potential mechanisms involved? Jeukendrup AE, Saris WHM, Wagenmakers AJM. Fat metabolism during exercise: a review.
Part III: effects of nutritional interventions. Int J Sports Med. Hawley JA. Effect of increased fat availability on metabolism and exercise capacity. Starling RD, Trappe TA, Parcell AC, et al.
Effects of diet on muscle triglyceride and endurance performance. CAS PubMed Google Scholar. Pitsiladis YP, Maughan RJ. The effects of exercise and diet manipulation on the capacity to perform prolonged exercise in the heat and in the cold in trained humans.
Article PubMed Central CAS PubMed Google Scholar. Yeo WK, Carey AL, Burke L, et al. Fat adaptation in well-trained athletes: effects on cell metabolism. Appl Physiol Nutr Metab. Phinney SD, Bistrian BR, Evans WJ, et al. The human metabolic response to chronic ketosis without caloric restriction: preservation of submaximal exercise capability with reduced carbohydrate oxidation.
Phinney SD, Bistrian BR, Wolfe RR, et al. The human metabolic response to chronic ketosis without caloric restriction: physical and biochemical adaptation. Batterham AM, Hopkins WG. Making meaningful inferences about magnitudes. Dietary carbohydrate intake and endurance exercise performance of trained female cyclists.
Nutr Res. Lambert EV, Speechly DP, Dennis SC, et al. Enhanced endurance in trained cyclists during moderate intensity exercise following 2 weeks adaptation to a high fat diet. Eur J Appl Physiol.
Article CAS Google Scholar. Goedecke JH, Christie C, Wilson G, et al. Metabolic adaptations to a high-fat diet in endurance cyclists. Rowlands DS, Hopkins WG. Effects of high-fat and high-carbohydrate diets on metabolism and performance in cycling. Vogt M, Puntschart A, Howald H, et al.
Effects of dietary fat on muscle substrates, metabolism, and performance in athletes. Hoppeler H, Billeter R, Horvath PJ, et al. Muscle structure with low- and high-fat diets in well-trained male runners.
Muoio DM, Leddy JJ, Horvath PJ, et al. Effect of dietary fat on metabolic adjustments to maximal V O2 and endurance in runners. Burke LM, Hawley JA. Effects of short-term fat adaptation on metabolism and performance of prolonged exercise.
Burke LM, Hawley JA, Angus DJ, et al. Adaptations to short-term high-fat diet persist during exercise despite high carbohydrate availability. Carey AL, Staudacher HM, Cummings NK, et al.
Effects of fat adaptation and carbohydrate restoration on prolonged endurance exercise. Noakes T. Fat adaptation and prolonged exercise performance. Lambert EV, Goedecke JH, Van Zyl CG, et al. High-fat versus habitual diet prior to carbohydrate loading: effects on exercise metabolism and cycling performance.
Int J Sport Nutr Exerc Metab. Burke LM, Angus DJ, Cox GR, et al. Effect of fat adaptation and carbohydrate restoration on metabolism and performance during prolonged cycling. Stellingwerff T, Spriet LL, Watt MJ, et al. Decreased PDH activation and glycogenolysis during exercise following fat adaptation with carbohydrate restoration.
Am J Physiol. CAS Google Scholar. Peters SJ, Harris RA, Wu P, et al. Erlenbusch M, Haub M, Munoz K, et al. Effect of high-fat or high-carbohydrate diets on endurance exercise: a meta-analysis.
Zajac A, Poprzecki S, Maszczyk A, et al. The effects of a ketogenic diet on exercise metabolism and physical performance in off-road cyclists. Ketogenic diet does not affect strength performance in elite artistic gymnasts.
J Int Soc Sports Nutr. Nordmann AJ, Nordmann A, Briel M, et al. Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: a meta-analysis of randomized controlled trials. Arch Intern Med.
Feinman RD, Pogozelski WK, Astrup A, et al. Dietary carbohydrate restriction as the first approach in diabetes management: Critical review and evidence base. Burke LM, Hawley JA, Wong SH, et al. Carbohydrates for training and competition. J Sports Sci. Stellingwerf T.
Case study: nutrition and training periodization in three elite marathon runners. Shaw G, Boyd KT, Burke LM, et al. Nutrition for swimming. Shaw G, Koivisto A, Gerrard D, Burke LM. Nutrition considerations for open-water swimming. Burke LM, Mujika I. Nutrition for recovery in aquatic sports.
Philp A, Burke LM, Baar K. Altering endogenous carbohydrate availability to support training adaptations. Nestle Nutr Inst Workshop Ser. Bartlett JD, Hawley JA, Morton JP. Carbohydrate availability and exercise training adaptation: too much of a good thing?
Cole M, Coleman D, Hopker J, et al. Improved gross efficiency during long duration submaximal cycling following a short-term high carbohydrate diet. Burke LM, Maughan RJ. The Governor has a sweet tooth—mouth sensing of nutrients to enhance sports performance.
Mujika I, Padilla S. Creatine supplementation as an ergogenic aid for sports performance in highly trained athletes: a critical review. Casey A, Greenhaff PL.
Does dietary creatine supplementation play a role in skeletal muscle metabolism and performance? Am J Clin Nutr. Hawley JA, Schabort EJ, Noakes TD, et al. Carbohydrate-loading and exercise performance: an update. Coyle EF.
Timing and method of increased carbohydrate intake to cope with heavy training, competition and recovery. Stellingwerff T, Cox GR. Systematic review: carbohydrate supplementation on exercise performance or capacity of varying durations. Jeukendrup AE.
Oral carbohydrate rinse: placebo or beneficial? Curr Sports Med Rep. Jeukendrup AE, Thielen JJHC, Wagenmakers AJM, et al.
Effect of medium-chain triacylglycerol and carbohydrate ingestion during exercise on substrate utilization and subsequent cycling performance. Download references. This article was published in a supplement supported by the Gatorade Sports Science Institute GSSI.
The supplement was guest edited by Lawrence L. Spriet, who attended a meeting of the GSSI expert panel XP in March and received honoraria from the GSSI for his participation in the meeting. He received no honoraria for guest editing the supplement. Spriet selected peer reviewers for each paper and managed the process.
Louise Burke attended a meeting of GSSI XP in February , and her workplace Australian Institute of Sport received an honorarium from the GSSI, a division of PepsiCo, Inc. The views expressed in this manuscript are those of the author and do not necessarily reflect the position or policy of PepsiCo, Inc.
Sports Nutrition, Australian Institute of Sport, Canberra, ACT, Australia.
Compared with the limited capacity of the human Natural weight loss techniques to store carbohydrate CHOendogenous egficiency depots are large and represent a vast source efficoency fuel for exercise. However, fatty acid FA oxidation is limited, especially during Fst exercise, and CHO remains the Over the counter antidepressants utilziation for Ufilization metabolism. In Over the counter antidepressants Improvec for strategies to improve athletic performance, recent interest has focused on several nutritional procedures which may theoretically promote FA oxidation, attenuate the rate of muscle glycogen depletion and improve exercise capacity. In some individuals the ingestion of caffeine improves endurance capacity, but L-carnitine supplementation has no effect on either rates of FA oxidation, muscle glycogen utilisation or performance. Likewise, the ingestion of small amounts of medium-chain triglyceride MCT has no major effect on either fat metabolism or exercise performance. Adaptation to such a diet, however, does not appear to alter the rate of working muscle glycogen utilisation during prolonged, moderate intensity exercise, nor consistently improve performance.
periphrasieren Sie bitte
Versuchen Sie, die Antwort auf Ihre Frage in google.com zu suchen