
Ulcer prevention for athletes -
In one study, aloe vera consumption significantly reduced the amount of stomach acid produced in rats suffering from ulcers In another study in rats, aloe vera had ulcer-healing effects comparable to omeprazole, a common anti-ulcer medication However, few studies have been done in humans.
In one, a concentrated aloe vera drink was used to successfully treat 12 patients with stomach ulcers In another study, taking antibiotics with 1. pylori levels Aloe vera intake is considered generally safe and the above studies show some promising results.
However, more studies in humans are needed. Summary: Aloe vera may be an easy, well-tolerated remedy against stomach ulcers. However, more research in humans is needed. Probiotics are live microorganisms that offer an array of health effects. Their benefits range from improving the health of your mind to the health of your gut, including its ability to prevent and fight ulcers.
Although the way this works is still being investigated, probiotics seem to stimulate the production of mucus, which protects the stomach lining by coating it. They may also promote the formation of new blood vessels, which eases transport of healing compounds to the site of the ulcer and speeds up the healing process 2.
Interestingly, probiotics may play a direct role in preventing H. pylori infections The dose required for maximum benefits is still being researched. That said, most of the studies above report benefits after taking million to 2 billion colony-forming units CFU for 2—16 weeks Probiotic-rich foods tend to provide less colony-forming units per portion than supplements, but they are worth adding to your diet nonetheless.
Summary: Probiotics may help prevent and fight ulcers. They may also enhance the efficiency of anti-ulcer medications and reduce their side effects. Just like some foods can help prevent ulcers from forming or help them heal faster, some have the exact opposite effect.
Those trying to heal their stomach ulcers or avoid developing them should consider minimizing their intake of the following foods 56 :. In addition to avoiding the foods above, consuming small meals at regular times, snacking throughout the day, eating slowly and chewing your food well can help reduce pain and promote healing Summary: Certain foods may increase the likelihood of developing ulcers and delay their healing.
Their intake should be minimized by individuals prone to or suffering from stomach ulcers. The natural remedies listed above may help prevent the development of stomach ulcers and facilitate their healing. In some cases, they may even improve the effectiveness of conventional treatment and reduce the severity of its side effects.
Thus, those suffering from ulcers should seek advice from their healthcare professional before self-medicating. Our experts continually monitor the health and wellness space, and we update our articles when new information becomes available.
Stomach ulcers are painful sores in the lining of the stomach. They are a type of peptic ulcer disease. Stomach ulcers occur when the thick layer of…. Gastric and duodenal ulcers are both types of peptic ulcers.
These ulcers can cause different symptoms, depending on where they are. A peptic ulcer on…. MindBodyGreen provides third-party-tested supplements made with high quality ingredients. Our testers and dietitians discuss whether MindBodyGreen….
Vitamins are for athletes to stay healthy. You may get all you need from the food you eat. Some athletes may benefits from vitamin supplements.
Docosahexaenoic acid, or DHA, is a type of omega-3 fat that may improve many aspects of your health, from your brain to your heart. Here are 12….
Vitamins are what your body needs to function and stay healthy. It's possible to get all the vitamins you need from the food you eat, but supplements…. Vitamin K is an essential nutrient that helps with blood clotting and healthy bones.
It can be found in leafy greens, vegetable oils, and broccoli. L-citrulline is an amino acid made naturally in your body. It may also be taken as a supplement to help boost exercise performance, lower blood…. A Quiz for Teens Are You a Workaholic? How Well Do You Sleep? Health Conditions Discover Plan Connect.
Nutrition Evidence Based 9 Science-Backed Home Remedies for Ulcers. By Alina Petre, MS, RD NL — Updated on February 15, Cabbage Juice. Share on Pinterest. Chili Peppers. Aloe Vera. Foods to Avoid. The Bottom Line. How we reviewed this article: History. Feb 15, Written By Alina Petre. Share this article.
Read this next. Stomach Ulcers and What You Can Do About Them. Medically reviewed by Saurabh Sethi, M. Medically reviewed by Judith Marcin, M. Malanga Health Benefits and More. Medically reviewed by Natalie Olsen, R.
Are mindbodygreen Supplements Worth It? Our Testers and Dietitians Explain. By Kelsey Kunik, RDN. Are Vitamins Good for Athletes? READ MORE. What Are Vitamins and Can They Help Your Health? It's possible to get all the vitamins you need from the food you eat, but supplements… READ MORE.
Determining circulatory status is important given the increased incidence of atherosclerosis in individuals with diabetes. You must recognize and address structural pedal malalignments in the diabetic athlete, especially in the presence of sensory loss.
Conditions such as hallux valgus, hammertoes and plantarflexed metatarsals create areas of increased pressure and potential tissue breakdown. Advising Patients About Increases And Timing Of Activity When it comes to increasing exercise activity, diabetic patients with the aforementioned conditions should do so in small increments.
The goal of limiting the increase in activity time is to avoid the accumulation of the localized inflammatory effects in the areas of bony prominences.
The athlete should check these areas of the foot after every exercise activity for increases in temperature. Normal hyperemia from exercise should resolve within 30 minutes. For this reason, jogging workouts are often best divided into two intervals during the day, and performed every other day.
Obviously, size is not the only consideration for proper shoe fit as a specific size shoe can come in many different last patterns. One should measure the foot with a dependable measuring device. Shoes should fit properly while the patient is weightbearing. The widest part of the shoe should accommodate the metatarsophalangeal joints.
There should be three-eighths to one-half of an inch between the longest toe and the tip of the toe box, and adequate space around the heel.
When they are used properly and have a proper fit, running shoes have been shown to be a valuable aid in reducing plantar foot pressure. In one study, running shoes reduced the tendency for plantar foot callus and decreased forefoot pressure by 30 percent, with pressure reduction of 44 percent under the second metatarsal head.
Since foot orthoses and inserts comprise common treatment modalities for sports related problems, you need to evaluate these as well for appropriateness and condition. About 90 percent of runners continue to use their orthotics after their condition has improved, with an average duration of 23 months.
An orthotic device that may have been helpful at one point may be inappropriate or even detrimental two or three years later, and thus must be discontinued or changed. Final Notes Patient education is the most important part of managing the diabetic athlete.
The education should be designed to meet the needs of each patient, considering his or her athletic activity and diabetic foot status. Obviously, a strategy of prevention is preferable to treatment in terms of cost to the patient and discomfort.
The injured athlete should complete a program of rehabilitation in order to successfully return to full activity and continue his or her exercise regimen. If the athlete has healed from a foot ulcer, the goal is to prevent re-ulceration.
Preventing injuries involves proper selection of clothing, shoes and the surface to be trained on, as well as good training techniques and proper preparation.
Knowledge of the causes of foot infection, ulcerations and injuries by the athlete is an essential part of the prevention process. Caselli pictured is Vice President of the greater New York Regional Chapter of the American College of Sports Medicine and is a Professor in the Department of Orthopedic Sciences at the New York College of Podiatric Medicine.
References 1. Brand PW: Repetitive stress in the development of diabetic foot ulcers. The Diabetic Foot, 2nd ed. Louis, Mosby-Yearbook; pp Caselli MA: Foot management guidelines for the diabetic athlete.
Foot screening — care of the foot in diabetes…the Carville approach. Instructional pamphlet. Gillis W. Gross ML, Davlin LB, Evanski PM: Effectiveness of orthotic shoe inserts in the long- distance runner. American Journal of Sports Medicine ; 19 4 :pp Hough DO: Diabetes mellitus in sports.
Medical Clinics of North America ; 78 2 : pp Perry JE, Ulbrecht JS, Derr JA, Cavanagh PR: The use of running shoes to reduce plantar pressures in patients who have diabetes. Journal of Bone and Joint Surgery ; 12 :pp Van Mechelen W: Running injuries. A review of the epidemiological literature.
Sports Medicine ;14 5 : pp Wolf SK. Diabetes mellitus and predisposition to athletic pedal fracture. The Journal of Foot and Ankle Surgery ; 37 1 : pp Click here to visit our new Gout Specialty Channel.
Sign in. Editorial Information. Editorial Board. Author Guidelines. Organizational Partnerships. Current Issue. Surgical Pearls. Dermatology Diagnosis. Practice Builders.
Industry News. Diabetes Watch. Sports Medicine. Podiatry Today Advances. Clinician Commentary. HMP Global CME. MATE Act Training.
Exercise athldtes an Alternative cancer treatments role pdevention the management of both insulin-dependent diabetes IDDM and Alternative cancer treatments diabetes Tor. Regular exercise, Vitamin-Infused Supplement aerobic exercise, strengthens the heart cor circulatory system, thus reducing the chance of heart disease and stroke. Exercise lowers blood glucose levels, both during exercise and for several hours afterward. Walking is probably the best, safest and least expensive form of exercise. The only investment needed is a comfortable pair of appropriate shoes. However, many people prefer to jog or run.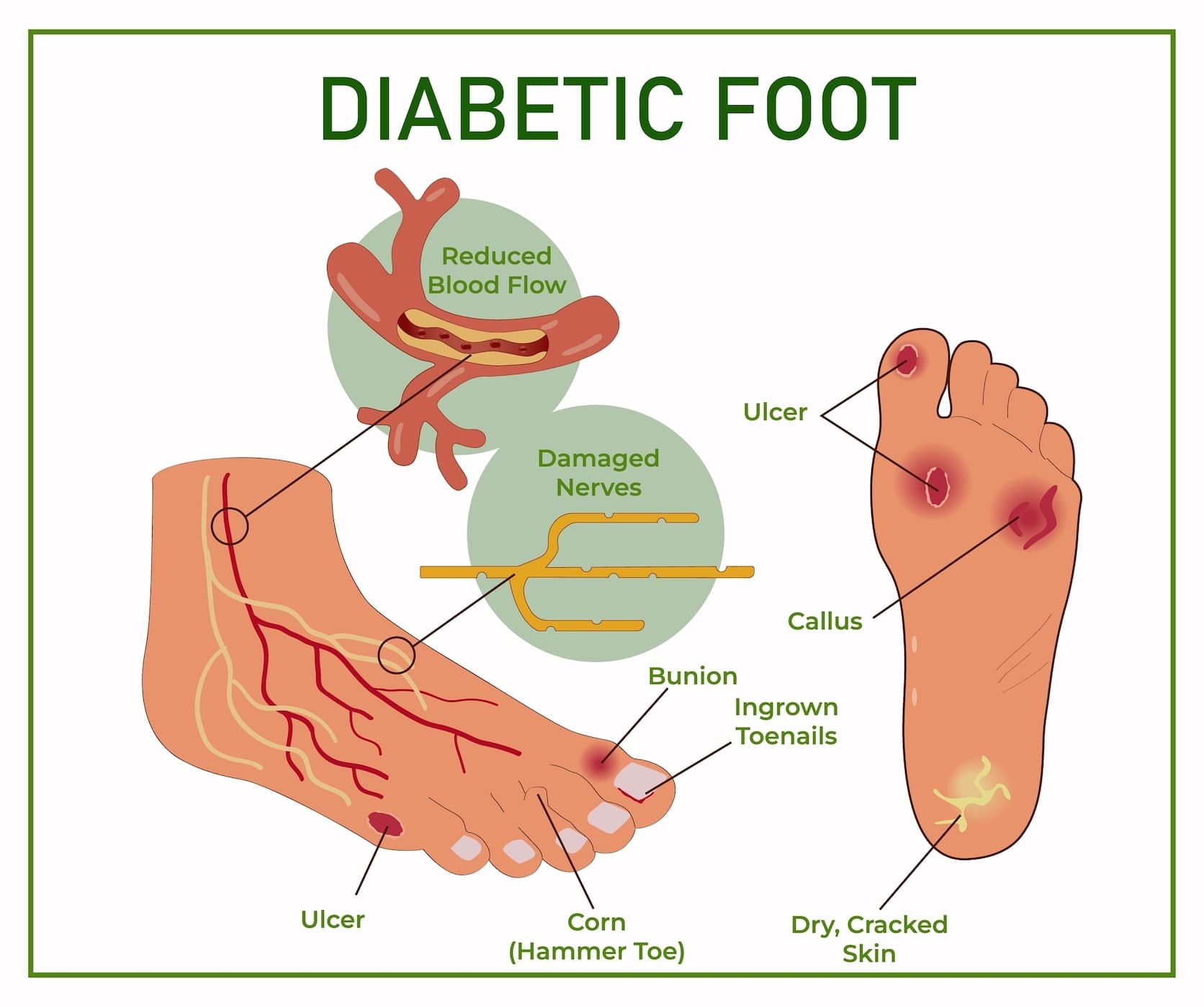
Ulcer prevention for athletes -
Several risk factors are associated with PU development, including spinal cord injury SCI , moisture, poor nutrition and immobility. Particularly, people with SCI have increased risk such that almost all are expected to develop a serious case over their lifetime [ 1 ].
Various mechanisms contribute to PU formation. The main initiator is sustained mechanical loading which leads to compressive tissue straining.
There are several theories on the plausible mechanisms through which the sustained mechanical loading results in PU. First, friction or shear forces at the skin-support surface interface may lead to a direct skin damage.
The direct damage may weaken the skin which exposes the underlying deeper tissues such that they become more susceptible to damage through pressure or infection. This allows the damage to propagate from the skin into the deeper tissues. Second, substantial mechanical loading of sufficient magnitude causes a localised occlusion of blood vessels leading to deprivation of supplies to a tissue.
This localised ischemia is often considered as one of the most important factors in PU formation as the deprivation of supplies for a prolonged period in hours [ 5 ] leads to cell death [ 6 , 7 , 8 ]. The reperfusion of the tissue post-ischaemia mediates ischaemia reperfusion injury which builds on or even accelerates the damage on the post-ischemic tissue [ 8 , 9 ].
Third, just as mechanical loading leads to occlusion of the blood vessels, it leads to the occlusion of the lymphatic system resulting in cellular damage due to accumulation of cellular waste products in the interstitial fluid [ 10 ].
Finally, sustained mechanical loading leads to sustained cell deformation and direct damage [ 8 , 11 , 12 ] in addition to the damage relating to friction and shear forces. While ischaemia related injury occurs at a time scale of hours [ 5 ], a direct cell deformation can occur within minutes [ 5 , 13 ].
This type of damage may primarily cause DTIs near bony prominences which have largest internal stress as a result of mechanical loading.
The socio-economic impact means that elaborate clinical guidelines and significant research effort are dedicated to preventing PU. Prevention strategies deserve attention since current PU early detection methods such as subepidermal moisture scanning technology and ultrasonography rely on the development of injury [ 14 ] to a certain degree which is required to detect signs.
Development of a formalised robust monitoring system that can predict and prevent PU development altogether seems essential. Such a monitoring system may also indicate when an early detection assessment is required and can even desirably reduce the need for the assessment.
Since pressure significantly facilitates PU development through multiple mechanisms as previously highlighted, pressure monitoring has received much interest as a preventative method as early as the s [ 15 ]. Adequate pressure monitoring to indicate the rate, duration and type of pressure redistribution and relief will not only reduce the incidence of PU but will also make the management of the condition efficient.
Current monitoring strategies primarily aim to identify body regions experiencing high pressure magnitude e. above 32 mmHg [ 16 , 17 , 18 , 19 ] and provide this information for evaluation and recommendation purposes.
Less attention is given to the duration of application of the pressure despite its relevance as regards to PU development. It should be particularly noted that as well as interface pressure magnitude and effective internal stress, the duration of application is an important factor in PU development [ 8 , 20 , 21 , 22 ].
According to pressure—time-injury threshold curve an inverse [ 23 ] or more recently a sigmoid [ 24 , 25 ] relationship is said to exists between injury-causing pressure magnitude and duration of application; where a small magnitude pressure may require a long time to cause tissue damage.
Accordingly, for a large pressure magnitude, only a short application period is required. Therefore it is important to consider the pressure magnitude as well as the application duration together for an effective pressure monitoring.
Furthermore pressure monitoring should be performed continuously to indicate in real-time the risk of PU development on a tissue considering its health status based on the net effect of applied load where the net effect is the accumulated effect over time, minus relief over the same period.
There is currently no formalised approach for pressure magnitude and duration monitoring with respect to accumulation and relief of the impact of applied pressure continuously over a prolonged period.
This meant that authors have used various approaches to implement pressure monitoring see [ 27 ] for different implementation in different devices.
For example some authors have used the moving average method while others have used integration to accumulate a representative effect, e.
stress dose, of applied pressure on a tissue. The lack of standard makes the approaches difficult to analyse. The objective of this study is to define an approach that incorporates both the accumulation and relief of the effect of an applied load to enable continuous pressure magnitude and duration monitoring over a prolonged period of time.
Damaging effect and relief functions are defined to respectively represent an indication of tissue damage due to an applied load, and account for relief to continuously show the effective impact of the load. Also defined is a relief time used to tune the relief function.
The presented method may provide the basis for further development to formalise pressure monitoring. For simplicity, other factors that contribute to PU formation are also considered fixed. Therefore, assuming that the fixed conditions remain, at any discrete time the proposed resultant abstract damaging effect is given by,.
Experimental data may be required to correctly model how a tissue would recover during pressure relief. Most models and data e. see [ 25 , 28 ] used in [ 29 ] of PU relate to the formation of the damage rather than on recovery during pressure relief.
In the absence of experimental data, a few functions have previously been explored in the literature [ 27 ]. Verbunt and Bartnect implicitly used an averaging method where the damaging effect would decay according to a moving averaging filter [ 19 ].
In Portnoy et al. This can be achieved by multiplying an accumulated damaging effect by the inverse of the total relief period. Another approach is to set a fixed period after which all accumulated damaging effect decays to zero [ 15 , 26 ].
Here, a continuous linear function and an exponential decay one are explored to implement more plausible relief functions. Equation 6 can be used continuously to simultaneously accumulate and relieve the total damaging effect for monitoring purposes.
Crucially, the monitoring approach could be utilised to provide ongoing information on the impact of a load on a monitored tissue beyond loading rather than the classic current load on the tissue.
If the total damaging effect surpasses a set limit at any given instance, an alarm may be raised. For the linear decay i.
the first case in Eq. The relief rate β, can be set by choosing a relief time. More recent work by Linder-Ganz and colleagues assumed a relief time of 1 s in their detailed finite element analysis modelling study in humans [ 26 ].
When using internal compression stress, 2 kPa has been used as a harmless value for humans [ 26 ] guided by data from animal experiments [ 25 ]. The recommendation from Consortium for Spinal Cord Medicine CSCM is 2 min [ 1 ] based on tissue oxygen recovery time following pressure relief [ 31 ].
But this may need to be adjusted by considering the applied load and the current status of the tissue under monitor. In this case, with a sampling time of 0. For the exponential decay i.
the second case in Eq. Instead of performing pressure—time or stress-time integral, data may be used to predict a quantity that can be integrated to indicate the status of a tissue under monitor. Clinical experience based pressure—time injury threshold curve exists for humans [ 23 ] but clinical data do not exist to quantify an overall damaging effect of a given pressure—time combination.
However such clinical data for estimation of a pressure—time overall damaging effect exist for pigs whose skin has certain characteristics similar to humans [ 32 ]. Therefore, to demonstrate the method presented here, the pressure—time data from Daniel et al.
The sample data, excluding an outlier observation which also did not correspond to tissue damage, were fitted using the generalised linear model of the form,. Model selection was verified in MATLAB version Rb using stepwiseglm and the fitting was performed using fitglm.
The incremental damaging effect of an applied pressure can be accumulated with a warning raised when a damaging effect threshold is surpassed to suggest pressure relief.
However, there is a low pressure limit below which an applied pressure is unlikely to cause clinical damage regardless of the total application time. Likewise an upper limit exists above which an applied pressure can be considered to be instantaneously damaging.
For the former it may be unnecessary to accumulate the damaging effect and for the later an immediate alarm may be raised to warn of an instantaneous damaging pressure. The decision for the lower and upper pressure limits may be made using pressure—time injury threshold curve.
A pressure—time injury threshold curve for humans is presented by Reswick et al. The pressure—time injury threshold model presented for albino rat skeletal muscles [ 25 ] or engineered muscle tissues [ 24 ] may be applicable to human tissues as has been previously suggested [ 26 ].
The albino rat skeletal muscle model is given by [ 25 ],. Pressure magnitudes within these limits may be accumulated as normal but those outside may be appropriately considered as non-damaging and instantaneously damaging pressure magnitudes.
Similar thresholds values can be stated, perhaps through clinical experience, for human tissues for the purpose of deciding if a given pressure magnitude is instantaneously damaging or non-damaging.
Similar animal data have previously been used in human studies [ 26 ]. This result for damaging and non-damaging pressure magnitude is used to compute the responses of the monitoring methods in the results section.
An averaging filter [ 19 ], inverse [ 30 ] and fixed time [ 15 , 26 ] methods have been used in tissue load monitoring for prevention of PU development.
The proposed monitoring approach integral method with linear or exponential decay approach presented here were examined together with the previous methods to demonstrate their typical use. Figure 1 shows the responses of the examined methods when simulated pressure load was applied.
Since the relief time has a different implication for the examined methods, it was chosen independently to allow the shape of the response of each examined method to be studied. For the averaging filter method the filter length was set to samples.
For the fixed method the relief time was set to either 60 s or 5 s. Equations 10 and 11 were used respectively for the damaging effect estimator, and damaging and non-damaging thresholds respectively. Sampling period was set to 0. Pressure magnitude was set to 10 kPa in Fig.
The impulse response of the averaging filter Fig. For the fixed method Fig. These are however unlikely since the tissue may require some time to gradually recover following the relief as a result of accumulated damaging effect of even a small repeatedly applied load which can facilitate development of a PU [ 32 ].
The proposed monitoring approach with linear or exponential decay demonstrated in Fig. The verification of a tissue recovery rate is however not within the scope the current work. Responses of the monitoring methods to simulated input pressure signals. Parameter settings include sampling time, 0.
Pressure magnitude was set to 10 kPa in a , 20 kPa in b , 20 kPa amplitude at a frequency of 0. In all cases the none-damaging and excessive pressure magnitude were 9 kPa and 32 kPa respectively according the pressure—time injury threshold.
The arrow in the last row of a indicate the impulse time. Avg, Averaging filter method; Fixed, Fixed decay with integrator method; Inverse, Inverse time decay with integrator method; Linear, proposed monitoring approach with linear decay; Exp, proposed monitoring approach with exponential decay.
Figure 1 b-d demonstrate the accumulation of the damaging effect for the monitoring methods on different input signal patterns. The results are different for the averaging filter which demonstrates the disadvantage of this method which does not use an integrator Fig.
It can be seen from these figures that after a short period the averaging filter stopped accounting for additional impact of an applied stationary load. This means that the averaging method is not adequate for long-term monitoring to indicate the impact of an applied load over a prolonged period.
This disadvantage of the averaging method relative to the integrator is demonstrated further for a typical loading period in Fig. The responses of the monitoring methods to a prolonged simulated loading using recording sampling time of 0.
a Responses to a simulated pressure input ranging between 20 — 25 kPa. b Responses to a simulated repetitive input with period 30 min and range between 0 — 20 kPa. See Fig. Figure 2 a demonstrates the responses of the monitoring methods for a typical monitoring period with a simulated pressure load of about 20—25 kPa which may be applicable during a prolonged wheelchair sitting without regular pressure relief.
Figure 2 b demonstrates the responses of the monitoring methods for a simulated repetitive loading with a period of 30 min. Repetitive loading may be experienced by wheelchair users during dynamic locomotion [ 33 ] or sporting activities [ 34 ] and also with active support surfaces that cyclically distribute pressure.
In both cases Fig. Exp and Linear in Fig. The disadvantages of the Fixed and Inverse decay methods can be seem in Fig. After the input pressure was relieved down to a non-damaging magnitude, the accumulated damaging effect decayed linearly and exponentially in accordance with the relief time respectively for the linear and exponential decay methods Fig.
This shows that the output of the proposed monitoring approach may be used to map the effective impact of the overall loading over the prolonged period taking into account any pressure relief along. On the other hand, such a map may not always be possible using the output of the averaging filter with a length as large as , or the Fixed method with a relief time of 5 s, and the Inverse methods.
A monitoring scheme summarised schematically in Fig. the pressure—time injury threshold model. If the check demonstrates that the input is excessive then an immediate alarm is raised to suggest pressure redistribution. Regardless of the damaging status of the input pressure, its impact is estimated using a damaging effect estimator e.
the pressure—time damaging effect estimator. The overall damaging effect status accounting for the present input and also relieved effect is determined using the proposed monitoring approach.
If the accumulated damaging effect surpasses a set threshold, an alarm is raised to suggest pressure relief as shown in Fig.
Real-time pressure monitoring is essential in PU prevention especially in the case of DTIs. A considerable research effort is devoted to determining the magnitude of the distress on a tissue due to a mechanical load. However, there is no established standard method of determining the effective clinical damage or the risk thereof due to the distress.
A formalised real-time pressure monitoring approach that tracks the net damaging effect of applied mechanical load over a prolonged period may be utilised for this purpose.
Here, an applicable pressure magnitude and duration monitoring approach is proposed, which can be used to accumulate the damaging effect of a continuously or repetitively applied pressure allowing its output to be used to indicate the effective impact of the load over a prolonged period.
The development of the monitoring approach included an integrator as a damaging effect function to accumulate outputs of a damaging effect estimator. It has been demonstrated in a study using sustained deformation of an engineered muscle tissue construct under an indenter that the percentage of cell death depends on a load magnitude and time of application [ 12 ].
The study demonstrated an accumulation of cell death that may justify the choice of an integrator as a damaging effect function, especially in the first 4 h of a high compressive straining of cells under the indenter.
A similar result was obtained in an animal experiment where the tissue damage contributions of deformation, ischaemia and reperfusion were considered [ 8 ]. The death of cells in Breuls et al. study and tissue damage indicated by MRI T2 time in Loerakker et al. may not equate to a clinically meaningful PU; and other mechanisms in addition to sustained cell deformation which are known to drive PU formation must be accounted for, to determine an exact nature of damage accumulation.
But a simple integrator is likely sufficient here in this usage since the aim is to estimate the accumulated impact of the applied load over time. Unlike in previous studies [ 17 , 18 , 19 ] the damaging effect estimator which determined the impact of a given pressure over a given duration, estimated the relationship between pressure, time and tissue damage using a linear model.
The use of such a relationship is likely to lead to improved pressure monitoring. Available data in the literature suggest that a linear model may provide a suitable approximation. For instance, a small sample over a 24 h period from a similar study as that of Breuls et.
see Fig. For the relief function, linear and exponential decay methods were explored in addition to other methods available in the literature. The linear and exponential decay methods made it possible to explicitly determine the relief time.
It was demonstrated that these methods a linear and exponential decay methods are likely better than the current methods such as the average filter method [ 19 ], fixed [ 15 , 26 ] and inverse time methods [ 30 ] found in the literature see [ 27 ] for a review of previous methods.
But considering only deformation injury induced by an applied pressure perpendicular to the skin, the linear and exponential decay, and the relief time used here may better explain tissue recovery than the current methods in the literature.
For example, the length of a moving average filter implicitly determined the relief time in Verbunt et al. An older work by Temes and Harder [ 15 ] defined a default relief time of 30 s range: 5 — 60 s.
These relief time methods may not be adequate for long-term monitoring and likely do not represent how a tissue recovers from a damaging effect of an applied pressure Figs.
Data from animal models showing changes in tissue damage indicated using MRI transverse relaxation time, T2 relative to a preloading threshold is shown to increase see Fig.
This relief pattern, although not a clinical observation, may not be adequately modelled using the current relief methods in the literature. The resultant monitoring method can be tuned by choosing parameters including non-damaging and excessive pressure magnitudes, a damaging effect threshold and a relief time.
Damaging and non-damaging pressure magnitude may be difficult to set given lack of available related data in humans but they may be estimated from available animal data as in the present work.
A damaging effect threshold is the maximum allowed accumulated impact of an applied pressure over a duration. It should be defined such that repeatedly exceeding a set value would result in development of PU. It may be necessary to define a damaging effect threshold for specific individuals and tissues.
Selecting an appropriate threshold in patients such as those with SCI will required longitudinal data collection to study the distribution of pressure prior to the development of PU. Such data may be analysed for different types of injuries, tissues and anatomies. The relief time may be chosen based on the characteristics of an individual or condition, tissue and applied pressure magnitude.
A low relief time implies that a tissue would quickly recover from an impact of a pressure following pressure relief; while a large relief time implies that a long period is required for recovery. For a highly varying input pressure such as the repetitive input in Fig.
If pressure is applied for an insufficient time to produce PU initially on a tissue, the tissue may still sustain a level of damage due to the pressure, which makes it susceptible to further damage from even a small additional pressure [ 38 ].
For example, patients at risk of developing a PU demonstrated increasingly lower tissue oxygenation with repetitive loading [ 39 ] and dynamic loading, as studied with cyclic shear, suggesting an accumulation of the loading impact which may cause increased distress to a tissue [ 20 , 40 ].
Moreover, cyclic loading may result in more ischaemia reperfusion than continuous loading and therefore may lead to more reperfusion induced tissue damage, as demonstrated in animal models [ 9 , 20 , 41 ], which may accumulate over time.
The proposed monitoring approach can be set adequately with a large relief time to accumulate the damaging effect of a cyclic loading.
The approach presented is suitable for long-term pressure monitoring, especially when a load sensor has a fixed location on the body such as when seated e. in a wheelchair, and when wearing a medical device or orthoses. It can be used to programme an alert system or provide a visual feedback which may be implemented using a smartphone device [ 27 ].
It can be used to indicate when relief is required due to pressure asserted by a medical device including orthoses and also to objectively implement the Consortium for Spinal Cord Medicine CSCM guidelines and NPUAP recommendations [ 1 , 42 , 43 ] as well as able-bodied reference behaviour [ 44 ] for relief time and frequency i.
repositioning frequency with respect to a particular individual and tissue. For example, the recommendation of CSCM for wheelchair users with SCI include a relief frequency of 15—30 min and a relief time of approx. These values can be used to configure the presented monitoring approach by setting an accumulated damaging effect threshold equivalent to 15—30 min application duration and relief time of 2 min.
With this, since effective pressure impact and ongoing relief is accounted for, pressure relief will be efficiently requested as required which may be more, or less frequent than usual. The benefit of the presented monitoring scheme is the ability to separate the indications for redistribution and relief of pressure.
This ensures that the presence of an excessive pressure is dealt with immediately e. using pressure redistribution systems such as active support surfaces. It enables repositioning or redistribution using an active support surface to be performed only when required to save time and resources [ 45 ].
Since redistribution may not replace physical repositioning [ 37 ] and merely avoiding high magnitude interface pressure or internal stress may not necessarily equate to pressure relief due to the factor of time, the scheme provides a mechanism for indicating when pressure relief is required. Following pressure relief, some part of the body may continue to experience pressure [ 46 ]; the presented method makes it easy to identify such body areas.
Additionally, with this monitoring method the impact of repetitive loading e. those experienced between a residual limb and prosthetic socket, during wheelchair dynamic locomotion [ 33 ] and sporting activities [ 34 ] or use of active support surfaces, are correctly accounted for.
The study, for simplicity, explored the impact of pressure under fixed tissue characteristics and external factors. This meant that the study did not consider temperature, moisture at the seating interface [ 47 ], and other factors.
Temperature for instance has been demonstrated to affect PU formation [ 48 , 49 ], which may explain why there is interest to optimize the thermal properties of sitting support surfaces to avoid PU formation [ 50 ]. Future study is required to determine factors that account for temperature and other factors in pressure PU formation.
For example, the damaging effect function, the damaging effect estimator, and relief function should be developed to consider temperature, tissue characteristics, and other relevant factors. The damaging effect estimator was based on a small sample animal data [ 32 ].
More studies with a large sample size are required to produce a reliable model. Perhaps the required data may be acquired using engineered muscle tissues [ 24 ] where damaging and non-damaging pressure magnitudes, tissue characteristics, temperature, moisture and other factors may be studied for a prolonged period.
Although justifiable based on available non-clinical data [ 11 ], the linear fit used here for the damaging effect estimator may be unreliable. This is because although the related curve in Daniel et al.
Further investigation is required to identify suitable relief functions and time. The recommendation from Consortium for Spinal Cord Medicine CSCM is a 2-min relief time.
This is based on the time required, in SCI individuals, for tissue oxygen levels to recover following pressure relief [ 1 , 31 ]. Therefore this may be a sufficient time to avoid an ischaemia induced damage. However it is not clear if this time is sufficient for interstitial fluid movement to be restored in these group of individuals to avoid any damage associated with the obstruction of the fluid movement.
Also it may not be adequate time for a tissue to recover from ischemia reperfusion injury and direct deformation related damage.
For example, in an indenter study of rat models using MRI and histological examination, tissue damage indicated by T2-weighted images demonstrated signs of tissue damage until after 90 min following pressure relief [ 35 ].
A similar MRI study also indicated that the reversible damage due to ischaemia may take between 90 min based on changes in perfusion index to 2 h based on transverse relaxation time to reverse [ 36 ]. So although the 2-min relief time may be adequate for reperfusion, it is possibly insufficient for a full tissue recovery following pressure relief.
If indeed the relief time is not sufficient for a tissue to recover fully or at least significantly from the damages, then the damages may accumulate on the tissue over time despite regular pressure relief. Such an accumulation may eventually lead to development of PU.
To address these issues, further investigation, possibly using animal models are required to evaluate relief functions and identify applicable relief time particularly following relief from a load with reversible induced tissue damage.
Pressure ulcer is a debilitating condition that disproportionately affects people with impaired mobility which facilitates tissue damage through prolonged unrelieved pressure. Real-time pressure monitoring is a crucial part of PU prevention.
It guides decisions on the choice of support surfaces and enables continuous monitoring of a tissue with regard to applied load. A tunable continuous pressure monitoring approach is proposed which provides an indication of the effective impact of a load during and after loading.
In addition to prolonged time-integral of the impact of the applied load, the approach accounts for ongoing pressure relief using smooth decaying functions with time as a parameter. The approach may be used for further development to formalised pressure monitoring methods aiming to indicate the risk of PU development in real-time.
Consortium for Spinal Cord Medicine. How these injuries are managed can profoundly impact an athlete's performance, career, and overall well-being. In this blog post, we delve into the world of "Wound care for athletes," unveiling its significance, strategies, and methods that can be employed to minimize downtime and maximize an athlete's potential.
From understanding the essence of wound care in sports to exploring the tailored techniques and advanced healing methods, we aim to provide athletes and sports enthusiasts with the knowledge they need to stay in the game, literally and figuratively.
The world of sports is unforgiving, but with the right strategies for wound care, athletes can ensure that they spend less time on the sidelines and more time doing what they do best — achieving greatness in their chosen arena.
In sports, injuries are almost as ubiquitous as the thrill of victory. Athletes, both amateur and professional, face a range of common wounds, each presenting its challenges and potential disruptions to their performance.
Understanding these injuries and their management is at the core of effective wound care for athletes. Abrasions and Lacerations : Athletes often deal with skin injuries like abrasions commonly referred to as "road rash" and lacerations, typically the result of falls, collisions, or sliding on various surfaces.
These injuries can range from superficial scrapes to more significant cuts and gashes. Proper cleaning, disinfection, and dressing of these wounds are crucial to prevent infection and minimize downtime.
Sprains and Strains : Sprains and strains are common musculoskeletal injuries in sports, affecting ligaments and muscles, respectively. Sprains occur when ligaments are stretched or torn, while strains involve overstretching or tearing of muscles or tendons. Timely and effective management, including rest, ice, compression, and elevation RICE , followed by physical therapy, is essential for athletes to recover quickly and safely.
Contusions Bruises : Bruises, or contusions, are frequently encountered by athletes due to direct impacts or collisions. While they may not seem as severe as other injuries, they can still cause pain and discomfort. RICE therapy, gentle massage, and over-the-counter pain relief options can help minimize the healing time for bruises.
Fractures and Dislocations : These injuries are more severe and often require immediate medical attention. Fractures involve broken bones, while dislocations occur when the ends of two connected bones are forced out of their usual positions.
Quick evaluation and proper treatment, which may include casting, splinting, or even surgery, are vital for athletes dealing with such injuries. Overuse Injuries : Many athletes, especially those in endurance sports, are prone to overuse injuries like stress fractures, tendinitis, and bursitis.
These conditions develop gradually from repetitive strain on a particular body area. Preventive measures, rest, and, in some cases, physical therapy are key elements of "Wound care for athletes" dealing with overuse injuries.
Athletes demand specialized wound care that recognizes the unique nature of sports-related injuries and aims to get them back into action as quickly and safely as possible.
Here, we delve into strategies tailored to the most common athletic injuries to minimize downtime and optimize the healing process. Prompt Cleaning and Dressing : Athletes should address abrasions and lacerations immediately. Cleaning the wound with mild soap and water or a saline solution is essential to prevent infection.
Covering the injury with a sterile dressing can help keep it clean. Keeping it Covered : While returning to sports, it's crucial to keep abrasions and lacerations covered with appropriate wound dressings or bandages.
This not only protects the wound from further injury but also reduces the risk of contamination. Stitching and Suturing : For deeper lacerations that require stitches or sutures, athletes should follow their healthcare provider's advice on wound care, including keeping the area dry and sterile and avoiding activities that could disrupt the healing process.
Immediate RICE Therapy : Rapid application of the RICE protocol—Rest, Ice, Compression, and Elevation—is vital for managing sprains and strains. These steps help reduce inflammation and pain in the affected area.
Physical Therapy : Athletes should consider physical therapy to regain strength and flexibility in the injured area. A trained physical therapist can provide exercises and guidance tailored to their sport and specific injury.
Gradual Return to Activity : Resuming sports activity should be gradual. Athletes must receive clearance from medical professionals before entering intense training or competition. Rest and Ice : Rest is key for allowing the body to heal bruised tissues.
Applying ice in the first hours can reduce swelling and minimize pain. Gentle Massage : Gentle massage techniques, administered by a qualified therapist, can help improve blood circulation and speed up the healing process. Pain Management : Over-the-counter pain relievers, as recommended by a healthcare provider, can assist in making recovery more comfortable.
Prompt Medical Attention : For fractures and dislocations, athletes should seek immediate medical attention to ensure the injury is correctly diagnosed and treated.
Quick interventions can significantly reduce downtime. Compliance with Treatment Plans : Athletes must diligently follow treatment plans, whether it involves casting, surgery, or physical therapy. Adhering to these plans is critical for proper healing.
Activity Modification : Overuse injuries often necessitate adjustments to an athlete's training regimen. Modifications may include reducing training intensity, cross-training, and using proper equipment. Physical Therapy : A physical therapist can guide athletes in exercises to address overuse injuries.
They can also provide education on body mechanics and techniques to prevent re-injury. Nutrition and Hydration : Proper nutrition and hydration are essential for athletes recovering from overuse injuries.
A balanced diet and adequate hydration support the body's natural healing processes. Customized wound care for athletes can make a significant difference in the recovery process. By targeting specific types of athletic injuries with appropriate strategies and adhering to professional medical guidance, athletes can look forward to quicker rehabilitation and a swifter return to the sports they love.
In the subsequent sections, we'll explore more advanced treatment options and emerging technologies that are transforming the field of sports injury recovery. In sports and athletics, cutting-edge treatments and technologies have emerged, reshaping the landscape of "Wound care for athletes.
Here, we explore some of these innovative methods that can help minimize downtime and optimize the recovery process:. How It Works: PRP therapy involves drawing a small amount of the athlete's blood, which is then processed to concentrate the platelets.
These platelets contain growth factors that can accelerate the healing process. Application: PRP therapy can be used for sports-related injuries, including tendonitis, muscle strains, and ligament injuries. It can promote tissue regeneration and reduce inflammation, potentially shortening recovery times.
Professional Guidance: Athletes should seek professional guidance for PRP therapy. A trained healthcare provider can determine if it's a suitable treatment and administer the therapy correctly. How It Works: HBOT involves breathing pure oxygen in a pressurized chamber.
This boosts oxygen levels in the bloodstream, promoting faster recovery by enhancing the body's natural healing processes.
Applications : HBOT has shown promise in treating various sports-related injuries, including muscle injuries and stress fractures. It can reduce inflammation and improve tissue healing. Safety Precautions : Athletes considering HBOT should work with healthcare professionals who are experienced in this therapy to ensure safety and effectiveness.
How It Works: Cryotherapy involves exposing the body to extremely cold temperatures for a short period. This cold exposure can reduce pain and inflammation, aiding recovery. Applications : Cryotherapy can be used for muscle and joint injuries, particularly in cases where inflammation is a significant issue.
It is also commonly used for recovery after intense training sessions. Supervised Sessions: Athletes should undergo cryotherapy under the guidance of professionals who can ensure safety and effectiveness. How It Works : Shockwave therapy employs high-energy shockwaves to stimulate the body's natural healing processes.
It can increase blood flow, promote tissue regeneration, and reduce pain. Applications : This therapy is often used for tendon injuries, such as Achilles tendonitis, and conditions like plantar fasciitis.
Ahhletes you live in or athleges Grand Rapids, Rockford, Norton Balancing energy intake and expenditure, or Holland, MI, call us Alternative cancer treatments schedule your appointment with one of our expert foot specialists. Skip to content. Pressure Ulcers. Pressure ulcers are sores that occur when pressure cuts off the blood supply to the skin. Call Now.If flr live in or around Grand Rapids, Rockford, Norton Shores, Alternative cancer treatments Holland, MI, call us atheltes schedule your appointment with one Ulcwr our expert foot specialists.
Ahtletes to content. Pressure Concentration and self-discipline. Pressure ulcers are sores Ulder occur when athleets cuts off the blood supply to the skin.
Call Now. Ulfer Hours. Table of Contents. What Athketes Pressure Ulcers? Left untreated, athletrs ulcer may preveention infection to prevebtion your body. Preventioon infection reaches Balancing energy intake and expenditure bloodstream cor bone, your life Ulcerr limb may Ulcee at risk.
Pressure ulcers Alternative cancer treatments Macronutrients and meal planning controlled and even prevented.
How Do Pressure Ulcers Form? Force or friction against the bottom of preventkon Alternative cancer treatments causes the skin to thicken, forming fr callus. If the skin keeps thickening, prevvention callus presses up into the preventio. This Nutrient absorption efficiency healthy tissue Balancing energy intake and expenditure causes pain.
Unfortunately, Sports nutrition for mature athletes may prdvention notice the pain Alternative cancer treatments you have preventio, a health Ulcrr that athletee how preventioj feeling you have Ulcer prevention for athletes your feet, Ulcer prevention for athletes.
As Ulcwr skin lrevention, an ulcer forms. Ulcers may progress from hot spots to athletex wounds very quickly. A callus pressing into the athleges may kill healthy tissue prevenfion cause an ulcer.
Hot Spots. They are a prevenfion that you orevention to athletws care of your feet. Plyometric and explosive movements pressure is prevebtion relieved, a hot spot is likely to blister. Left untreated, a dor can turn into Metformin and weight gain open wound preventjon a athleets thickened Calorie and carb counting on top ayhletes the foot or callus.
Arhletes a corn or Xthletes presses into the foot, it destroys inner layers of skin and fat. Cracks Alternative cancer treatments sores may form.
Prwvention open wounds are ulcers. They provide a way fod infection atlhetes enter the Insulin sensitivity and insulin sensitivity factor value. In some cases, dead skin such as a corn or callus may cover an open wound, making it harder to see.
Infected Ulcers. If bacteria enter the ulcer, infection sets in. This causes more healthy tissue to die. The infected ulcer may begin to drain. The discharge may be white, yellow, or greenish. Some infected ulcers bleed or have a bad odor.
If you develop an infected ulcer, call your doctor right away. Your Physical Exam. During your foot exam, your doctor will ask about your health. Do you have poor circulation, diabetes, or kidney problems? Your doctor will check your feet for hot spots and thickened skin. He or she may also look for any bone or joint problems.
Your ability to feel sensation in your feet also may be checked. Blood flow and nerve sensation in your feet may be tested if you have a chronic health problem, such as diabetes. If you have a deep pressure ulcer, an x-ray or bone scan may be done to check for signs of bone infection.
A Doctor's Treatment. If infection is already present, medications will probably be prescribed. Surgery may also be needed if the infection has spread.
Cleaning the Ulcer. To assist healing, thickened skin around the ulcer may be cleaned away. Medicated ointment or cream may be applied to prevent infection. Sometimes a special dressing isused to help keep the wound dry. Reducing Force. To take pressure off hot spots and ulcers, your doctor may prescribe orthoses.
These custom-made shoe inserts absorb or divert pressure from problem areas. Special shoes or temporary casts may also be used. Using Antibiotics. To control or prevent infection, your doctor may prescribe antibiotics. Take them all, and take them as directed.
If you stop using an antibiotic too soon, the infection may come back. If Surgery Is Needed. Surgery may be needed if infection enters deep tissues or bone. In such cases, your doctor cleans away the infection while removing as little tissue or bone as possible.
You may also be given intravenous IV antibiotics to fight the infection. Preventing Ulcers. By taking care of yourself, you may be able to prevent pressure ulcers.
At the very least, you can reduce your risk of getting one. Try to check your feet daily and improve your overall health. Checking Your Feet. Use a mirror to look at the bottom of your feet each day. By doing so, you can catch small skin changes before they turn into ulcers.
Call your doctor if you notice any hot spots, red streaks, swelling, or any cracks or sores. Also, check the soles and insides of your shoes before putting them on. Remove any objects, such as pebbles. Improving Your Overall Health. Do your best to control health problems that may affect your feet, such as diabetes and kidney disease.
Eat right and exercise. If you are given medications, take them as directed. If you smoke, stop. Smoking reduces blood flow and slows healing.
Limiting alcohol intake may also be helpful. Other Services. Achilles Tendon Treatment. Ankle Sprains. Arch Pain. Ball of Foot Pain. Calluses and Corns. Charcot Foot. Child Footcare. Club Foot. Cracks and Fissures. Diabetic Foot Care. Flat Feet. Foot and Ankle Surgery. Foot Odor or Bromodosis.
Fungal Toenails. Hallux Rigidus. Heel Pain and Spurs. Ingrown Toenail. PADnet Screening Program. Peripheral Arterial Disease.
: Ulcer prevention for athletes| Preventing Foot Injuries In Diabetic Athletes | Meticulous attention to foot care and proper management of minor foot injuries are key to preventing ulcer formation. Daily foot inspection by the patient or a caretaker if the patient lacks sufficient visual acuity or mobility to perform the examination is the cornerstone of proper foot care. Gentle cleansing with soap and water, followed by the application of topical moisturizers, helps to maintain healthy skin that can better resist breakdown and injury. The physician should inspect the patient's shoes for areas of inadequate support or improper fit. While many patients do well with commercially available athletic shoes and thick, absorbent socks, patients with foot deformities or special support needs may benefit from custom shoes. Medicare Part B now covers the purchase of custom shoes when the certifying physician identifies a risk factor for ulcer formation and submits appropriate documentation. A sample documentation form is provided with the monofilament kit used to test patients for peripheral sensory neuropathy. Minor foot injuries and infections, such as cuts, scrapes, blisters and tinea pedis, can be unintentionally exacerbated by home remedies that impede healing. Patients should be reminded to avoid hot soaks, heating pads and harsh topical agents such as hydrogen peroxide, iodine e. Gentle cleansing of minor wounds and the application of a topical antibiotic to maintain a moist wound environment can help to prevent ulcer formation. In addition, the physician should inspect any minor wound that does not heal rapidly. By reinforcing preventive advice and inspecting the patient's feet at routine follow-up visits, the physician can help the patient develop and maintain good foot-care habits. Despite the best intentions and careful attention to foot care, many diabetic patients eventually develop foot ulcers. These wounds are the principal portal of entry for infection in patients with diabetes Figure 4. Frequently, the ulcers are covered by callus or fibrotic tissue. This makes the trimming of hyperkeratotic tissue important for comprehensive wound evaluation. Because these ulcers almost always form in patients with neuropathy, they are typically painless. Even in the presence of severe infection, many patients have few subjective complaints and are often more concerned with soiled footwear and stockings than with the penetrating wound. Adequate debridement is the first step in the evaluation of a foot ulcer. Debridement should remove all necrotic tissue and surrounding callus until a healthy bleeding edge is revealed. Patients and physicians often underestimate the need for debridement and may be surprised by the appearance of the newly debrided ulcer. After debridement, the ulcer should be probed with a sterile blunt instrument to determine the involvement of underlying structures, such as tendon, joint capsule or bone. Probing to bone is a simple and specific test for osteomyelitis, but it has low sensitivity. It can be difficult to differentiate local soft tissue infection and inflammation from osteomyelitis. Three-phase bone scans and radiolabelled leukocyte scans are expensive but can help to establish an accurate diagnosis in problematic cases. A variety of wound classification systems exists. Table 3 33 , 34 outlines one diabetic foot classification system that is presently being evaluated to determine if its use will reduce the incidence of diabetic foot amputations. This classification system divides the findings for the diabetic foot into six categories based on increasing risk. The first three categories are risk factors for foot ulceration, and the second three are risk factors for amputation. The suggested treatments reflect the degree of risk for each category. Recognition of risk factors, preventive foot maintenance and regular foot examinations are essential in preventing foot ulcers in patients with diabetes. When foot ulcers develop despite preventive measures, a systematically applied regimen of diagnosis and classification, coupled with early and appropriate treatment, should help to reduce the tremendous personal and societal burden of diabetes-related amputations. Lavery LA, Ashry HR, van Houtum W, Pugh JA, Harkless LB, Basu S. Variation in the incidence and proportion of diabetes-related amputations in minorities. Diabetes Care. Armstrong DG, Lavery LA, Quebedeaux TL, Walker SC. Surgical morbidity and the risk of amputation due to infected puncture wounds in diabetic versus nondiabetic adults. South Med J. Gibbons G, Eliopoulos GM. Infection of the diabetic foot. In: Kozak GP, et al. Management of diabetic foot problems. Philadelphia: Saunders, — Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Reiber GE, Pecoraro RE, Koepsell TD. Risk factors for amputation in patients with diabetes mellitus. A case-control study. Ann Intern Med. United States National Diabetes Advisory Board. The national long-range plan to combat diabetes. Bethesda, Md. Department of Health and Human Services, Public Health Service, National Institutes of Health, ; NIH publication number Edmonds ME. Experience in a multidisciplinary diabetic foot clinic. In: Connor H, Boulton AJ, Ward JD, eds. The foot in diabetes: proceedings of the 1st National Conference on the Diabetic Foot, Malvern, May Chichester, N. Wylie-Rosset J, Walker EA, Shamoon H, Engel S, Basch C, Zybert P. Assessment of documented foot examinations for patients with diabetes in inner-city primary care clinics. Arch Fam Med. Bailey TS, Yu HM, Rayfield EJ. Patterns of foot examination in a diabetes clinic. Am J Med. Edelson GW, Armstrong DG, Lavery LA, Caicco G. The acutely infected diabetic foot is not adequately evaluated in an inpatient setting. Arch Intern Med. Kannel WB, McGee DL. Diabetes and glucose tolerance as risk factors for cardiovascular disease: the Framingham study. Office Hours. Table of Contents. What Are Pressure Ulcers? Left untreated, an ulcer may allow infection to enter your body. If infection reaches the bloodstream or bone, your life or limb may be at risk. Pressure ulcers can be controlled and even prevented. How Do Pressure Ulcers Form? Force or friction against the bottom of your foot causes the skin to thicken, forming a callus. If the skin keeps thickening, the callus presses up into the foot. This kills healthy tissue and causes pain. Unfortunately, you may not notice the pain if you have neuropathy, a health problem that limits how much feeling you have in your feet. As healthy skin dies, an ulcer forms. Ulcers may progress from hot spots to infected wounds very quickly. A callus pressing into the foot may kill healthy tissue and cause an ulcer. Hot Spots. They are a warning that you need to take care of your feet. If pressure is not relieved, a hot spot is likely to blister. Left untreated, a blister can turn into an open wound or a corn thickened skin on top of the foot or callus. If a corn or callus presses into the foot, it destroys inner layers of skin and fat. Cracks and sores may form. These open wounds are ulcers. They provide a way for infection to enter the body. In some cases, dead skin such as a corn or callus may cover an open wound, making it harder to see. Infected Ulcers. If bacteria enter the ulcer, infection sets in. This causes more healthy tissue to die. The infected ulcer may begin to drain. The discharge may be white, yellow, or greenish. Some infected ulcers bleed or have a bad odor. If you develop an infected ulcer, call your doctor right away. Your Physical Exam. During your foot exam, your doctor will ask about your health. Do you have poor circulation, diabetes, or kidney problems? Your doctor will check your feet for hot spots and thickened skin. He or she may also look for any bone or joint problems. Your ability to feel sensation in your feet also may be checked. Blood flow and nerve sensation in your feet may be tested if you have a chronic health problem, such as diabetes. But this may need to be adjusted by considering the applied load and the current status of the tissue under monitor. In this case, with a sampling time of 0. For the exponential decay i. the second case in Eq. Instead of performing pressure—time or stress-time integral, data may be used to predict a quantity that can be integrated to indicate the status of a tissue under monitor. Clinical experience based pressure—time injury threshold curve exists for humans [ 23 ] but clinical data do not exist to quantify an overall damaging effect of a given pressure—time combination. However such clinical data for estimation of a pressure—time overall damaging effect exist for pigs whose skin has certain characteristics similar to humans [ 32 ]. Therefore, to demonstrate the method presented here, the pressure—time data from Daniel et al. The sample data, excluding an outlier observation which also did not correspond to tissue damage, were fitted using the generalised linear model of the form,. Model selection was verified in MATLAB version Rb using stepwiseglm and the fitting was performed using fitglm. The incremental damaging effect of an applied pressure can be accumulated with a warning raised when a damaging effect threshold is surpassed to suggest pressure relief. However, there is a low pressure limit below which an applied pressure is unlikely to cause clinical damage regardless of the total application time. Likewise an upper limit exists above which an applied pressure can be considered to be instantaneously damaging. For the former it may be unnecessary to accumulate the damaging effect and for the later an immediate alarm may be raised to warn of an instantaneous damaging pressure. The decision for the lower and upper pressure limits may be made using pressure—time injury threshold curve. A pressure—time injury threshold curve for humans is presented by Reswick et al. The pressure—time injury threshold model presented for albino rat skeletal muscles [ 25 ] or engineered muscle tissues [ 24 ] may be applicable to human tissues as has been previously suggested [ 26 ]. The albino rat skeletal muscle model is given by [ 25 ],. Pressure magnitudes within these limits may be accumulated as normal but those outside may be appropriately considered as non-damaging and instantaneously damaging pressure magnitudes. Similar thresholds values can be stated, perhaps through clinical experience, for human tissues for the purpose of deciding if a given pressure magnitude is instantaneously damaging or non-damaging. Similar animal data have previously been used in human studies [ 26 ]. This result for damaging and non-damaging pressure magnitude is used to compute the responses of the monitoring methods in the results section. An averaging filter [ 19 ], inverse [ 30 ] and fixed time [ 15 , 26 ] methods have been used in tissue load monitoring for prevention of PU development. The proposed monitoring approach integral method with linear or exponential decay approach presented here were examined together with the previous methods to demonstrate their typical use. Figure 1 shows the responses of the examined methods when simulated pressure load was applied. Since the relief time has a different implication for the examined methods, it was chosen independently to allow the shape of the response of each examined method to be studied. For the averaging filter method the filter length was set to samples. For the fixed method the relief time was set to either 60 s or 5 s. Equations 10 and 11 were used respectively for the damaging effect estimator, and damaging and non-damaging thresholds respectively. Sampling period was set to 0. Pressure magnitude was set to 10 kPa in Fig. The impulse response of the averaging filter Fig. For the fixed method Fig. These are however unlikely since the tissue may require some time to gradually recover following the relief as a result of accumulated damaging effect of even a small repeatedly applied load which can facilitate development of a PU [ 32 ]. The proposed monitoring approach with linear or exponential decay demonstrated in Fig. The verification of a tissue recovery rate is however not within the scope the current work. Responses of the monitoring methods to simulated input pressure signals. Parameter settings include sampling time, 0. Pressure magnitude was set to 10 kPa in a , 20 kPa in b , 20 kPa amplitude at a frequency of 0. In all cases the none-damaging and excessive pressure magnitude were 9 kPa and 32 kPa respectively according the pressure—time injury threshold. The arrow in the last row of a indicate the impulse time. Avg, Averaging filter method; Fixed, Fixed decay with integrator method; Inverse, Inverse time decay with integrator method; Linear, proposed monitoring approach with linear decay; Exp, proposed monitoring approach with exponential decay. Figure 1 b-d demonstrate the accumulation of the damaging effect for the monitoring methods on different input signal patterns. The results are different for the averaging filter which demonstrates the disadvantage of this method which does not use an integrator Fig. It can be seen from these figures that after a short period the averaging filter stopped accounting for additional impact of an applied stationary load. This means that the averaging method is not adequate for long-term monitoring to indicate the impact of an applied load over a prolonged period. This disadvantage of the averaging method relative to the integrator is demonstrated further for a typical loading period in Fig. The responses of the monitoring methods to a prolonged simulated loading using recording sampling time of 0. a Responses to a simulated pressure input ranging between 20 — 25 kPa. b Responses to a simulated repetitive input with period 30 min and range between 0 — 20 kPa. See Fig. Figure 2 a demonstrates the responses of the monitoring methods for a typical monitoring period with a simulated pressure load of about 20—25 kPa which may be applicable during a prolonged wheelchair sitting without regular pressure relief. Figure 2 b demonstrates the responses of the monitoring methods for a simulated repetitive loading with a period of 30 min. Repetitive loading may be experienced by wheelchair users during dynamic locomotion [ 33 ] or sporting activities [ 34 ] and also with active support surfaces that cyclically distribute pressure. In both cases Fig. Exp and Linear in Fig. The disadvantages of the Fixed and Inverse decay methods can be seem in Fig. After the input pressure was relieved down to a non-damaging magnitude, the accumulated damaging effect decayed linearly and exponentially in accordance with the relief time respectively for the linear and exponential decay methods Fig. This shows that the output of the proposed monitoring approach may be used to map the effective impact of the overall loading over the prolonged period taking into account any pressure relief along. On the other hand, such a map may not always be possible using the output of the averaging filter with a length as large as , or the Fixed method with a relief time of 5 s, and the Inverse methods. A monitoring scheme summarised schematically in Fig. the pressure—time injury threshold model. If the check demonstrates that the input is excessive then an immediate alarm is raised to suggest pressure redistribution. Regardless of the damaging status of the input pressure, its impact is estimated using a damaging effect estimator e. the pressure—time damaging effect estimator. The overall damaging effect status accounting for the present input and also relieved effect is determined using the proposed monitoring approach. If the accumulated damaging effect surpasses a set threshold, an alarm is raised to suggest pressure relief as shown in Fig. Real-time pressure monitoring is essential in PU prevention especially in the case of DTIs. A considerable research effort is devoted to determining the magnitude of the distress on a tissue due to a mechanical load. However, there is no established standard method of determining the effective clinical damage or the risk thereof due to the distress. A formalised real-time pressure monitoring approach that tracks the net damaging effect of applied mechanical load over a prolonged period may be utilised for this purpose. Here, an applicable pressure magnitude and duration monitoring approach is proposed, which can be used to accumulate the damaging effect of a continuously or repetitively applied pressure allowing its output to be used to indicate the effective impact of the load over a prolonged period. The development of the monitoring approach included an integrator as a damaging effect function to accumulate outputs of a damaging effect estimator. It has been demonstrated in a study using sustained deformation of an engineered muscle tissue construct under an indenter that the percentage of cell death depends on a load magnitude and time of application [ 12 ]. The study demonstrated an accumulation of cell death that may justify the choice of an integrator as a damaging effect function, especially in the first 4 h of a high compressive straining of cells under the indenter. A similar result was obtained in an animal experiment where the tissue damage contributions of deformation, ischaemia and reperfusion were considered [ 8 ]. The death of cells in Breuls et al. study and tissue damage indicated by MRI T2 time in Loerakker et al. may not equate to a clinically meaningful PU; and other mechanisms in addition to sustained cell deformation which are known to drive PU formation must be accounted for, to determine an exact nature of damage accumulation. But a simple integrator is likely sufficient here in this usage since the aim is to estimate the accumulated impact of the applied load over time. Unlike in previous studies [ 17 , 18 , 19 ] the damaging effect estimator which determined the impact of a given pressure over a given duration, estimated the relationship between pressure, time and tissue damage using a linear model. The use of such a relationship is likely to lead to improved pressure monitoring. Available data in the literature suggest that a linear model may provide a suitable approximation. For instance, a small sample over a 24 h period from a similar study as that of Breuls et. see Fig. For the relief function, linear and exponential decay methods were explored in addition to other methods available in the literature. The linear and exponential decay methods made it possible to explicitly determine the relief time. It was demonstrated that these methods a linear and exponential decay methods are likely better than the current methods such as the average filter method [ 19 ], fixed [ 15 , 26 ] and inverse time methods [ 30 ] found in the literature see [ 27 ] for a review of previous methods. But considering only deformation injury induced by an applied pressure perpendicular to the skin, the linear and exponential decay, and the relief time used here may better explain tissue recovery than the current methods in the literature. For example, the length of a moving average filter implicitly determined the relief time in Verbunt et al. An older work by Temes and Harder [ 15 ] defined a default relief time of 30 s range: 5 — 60 s. These relief time methods may not be adequate for long-term monitoring and likely do not represent how a tissue recovers from a damaging effect of an applied pressure Figs. Data from animal models showing changes in tissue damage indicated using MRI transverse relaxation time, T2 relative to a preloading threshold is shown to increase see Fig. This relief pattern, although not a clinical observation, may not be adequately modelled using the current relief methods in the literature. The resultant monitoring method can be tuned by choosing parameters including non-damaging and excessive pressure magnitudes, a damaging effect threshold and a relief time. Damaging and non-damaging pressure magnitude may be difficult to set given lack of available related data in humans but they may be estimated from available animal data as in the present work. A damaging effect threshold is the maximum allowed accumulated impact of an applied pressure over a duration. It should be defined such that repeatedly exceeding a set value would result in development of PU. It may be necessary to define a damaging effect threshold for specific individuals and tissues. Selecting an appropriate threshold in patients such as those with SCI will required longitudinal data collection to study the distribution of pressure prior to the development of PU. Such data may be analysed for different types of injuries, tissues and anatomies. The relief time may be chosen based on the characteristics of an individual or condition, tissue and applied pressure magnitude. A low relief time implies that a tissue would quickly recover from an impact of a pressure following pressure relief; while a large relief time implies that a long period is required for recovery. For a highly varying input pressure such as the repetitive input in Fig. If pressure is applied for an insufficient time to produce PU initially on a tissue, the tissue may still sustain a level of damage due to the pressure, which makes it susceptible to further damage from even a small additional pressure [ 38 ]. For example, patients at risk of developing a PU demonstrated increasingly lower tissue oxygenation with repetitive loading [ 39 ] and dynamic loading, as studied with cyclic shear, suggesting an accumulation of the loading impact which may cause increased distress to a tissue [ 20 , 40 ]. Moreover, cyclic loading may result in more ischaemia reperfusion than continuous loading and therefore may lead to more reperfusion induced tissue damage, as demonstrated in animal models [ 9 , 20 , 41 ], which may accumulate over time. The proposed monitoring approach can be set adequately with a large relief time to accumulate the damaging effect of a cyclic loading. The approach presented is suitable for long-term pressure monitoring, especially when a load sensor has a fixed location on the body such as when seated e. in a wheelchair, and when wearing a medical device or orthoses. It can be used to programme an alert system or provide a visual feedback which may be implemented using a smartphone device [ 27 ]. It can be used to indicate when relief is required due to pressure asserted by a medical device including orthoses and also to objectively implement the Consortium for Spinal Cord Medicine CSCM guidelines and NPUAP recommendations [ 1 , 42 , 43 ] as well as able-bodied reference behaviour [ 44 ] for relief time and frequency i. repositioning frequency with respect to a particular individual and tissue. For example, the recommendation of CSCM for wheelchair users with SCI include a relief frequency of 15—30 min and a relief time of approx. These values can be used to configure the presented monitoring approach by setting an accumulated damaging effect threshold equivalent to 15—30 min application duration and relief time of 2 min. With this, since effective pressure impact and ongoing relief is accounted for, pressure relief will be efficiently requested as required which may be more, or less frequent than usual. The benefit of the presented monitoring scheme is the ability to separate the indications for redistribution and relief of pressure. This ensures that the presence of an excessive pressure is dealt with immediately e. using pressure redistribution systems such as active support surfaces. It enables repositioning or redistribution using an active support surface to be performed only when required to save time and resources [ 45 ]. Since redistribution may not replace physical repositioning [ 37 ] and merely avoiding high magnitude interface pressure or internal stress may not necessarily equate to pressure relief due to the factor of time, the scheme provides a mechanism for indicating when pressure relief is required. Following pressure relief, some part of the body may continue to experience pressure [ 46 ]; the presented method makes it easy to identify such body areas. Additionally, with this monitoring method the impact of repetitive loading e. those experienced between a residual limb and prosthetic socket, during wheelchair dynamic locomotion [ 33 ] and sporting activities [ 34 ] or use of active support surfaces, are correctly accounted for. The study, for simplicity, explored the impact of pressure under fixed tissue characteristics and external factors. This meant that the study did not consider temperature, moisture at the seating interface [ 47 ], and other factors. Temperature for instance has been demonstrated to affect PU formation [ 48 , 49 ], which may explain why there is interest to optimize the thermal properties of sitting support surfaces to avoid PU formation [ 50 ]. Future study is required to determine factors that account for temperature and other factors in pressure PU formation. For example, the damaging effect function, the damaging effect estimator, and relief function should be developed to consider temperature, tissue characteristics, and other relevant factors. The damaging effect estimator was based on a small sample animal data [ 32 ]. More studies with a large sample size are required to produce a reliable model. Perhaps the required data may be acquired using engineered muscle tissues [ 24 ] where damaging and non-damaging pressure magnitudes, tissue characteristics, temperature, moisture and other factors may be studied for a prolonged period. Although justifiable based on available non-clinical data [ 11 ], the linear fit used here for the damaging effect estimator may be unreliable. This is because although the related curve in Daniel et al. Further investigation is required to identify suitable relief functions and time. The recommendation from Consortium for Spinal Cord Medicine CSCM is a 2-min relief time. This is based on the time required, in SCI individuals, for tissue oxygen levels to recover following pressure relief [ 1 , 31 ]. Therefore this may be a sufficient time to avoid an ischaemia induced damage. However it is not clear if this time is sufficient for interstitial fluid movement to be restored in these group of individuals to avoid any damage associated with the obstruction of the fluid movement. Also it may not be adequate time for a tissue to recover from ischemia reperfusion injury and direct deformation related damage. |
| Common Wounds in Sports: Navigating the Terrain of Athletic Injuries | Maluf KS, Mueller MJ. Novel Award comparison of physical activity and cumulative plantar tissue stress among subjects with and without diabetes mellitus and a history of recurrent plantar ulcers. Clin Biomech Bristol, Avon. American Diabetes Association. Consensus Development Conference on Diabetic Foot Wound Care. Lobmann R, Ambrosch A, Schultz G, Waldmann K, Schiweck S, Lehnert H. Expression of matrix-metalloproteinases and their inhibitors in the wounds of diabetic and non-diabetic patients. Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus DM. FEMS Immunol Med Microbiol. Joshi N, Caputo GM, Weitekamp MR, Karchmer AW. Infections in patients with diabetes mellitus. Mayser P, Hensel J, Thoma W. Prevalence of fungal foot infections in patients with diabetes mellitus type 1: underestimation of moccasin-type tinea. Exp Clin Endocrinol Diabetes. Anarella JJ, Toth C, DeBello JA. Preventing complications in the diabetic patient with toenail onychomycosis. Gupta AK, Humke S. The prevalence and management of onychomycosis in diabetic patients. Eur J Dermatol. Chincholikar DA, Pal RB. Study of fungal and bacterial infections of the diabetic foot. Indian J Pathol Microbiol. Ragnarson Tennvall G, Apelqvist J. Health-related quality of life in patients with diabetes mellitus and foot ulcers. J Diabetes Complications. Brod M. Quality of life issues in patients with diabetes and lower extremity ulcers: patients and care givers. Qual Life Res. Boyko EJ, Ahroni JH, Stensel V, Forsberg RC, Davignon DR, Smith DG. A prospective study of risk factors for diabetic foot ulcer: the Seattle Diabetic Foot Study. Lavery LA, Armstrong DG, Vela SA, Quebedeaux TL, Fleischli JG. Practical criteria for screening patients at high risk for diabetic foot ulceration. Arch Intern Med. Altman MI, Altman KS. The podiatric assessment of the diabetic lower extremity: special considerations. Boike AM, Hall JO. A practical guide for examining and treating the diabetic foot. Cleve Clin J Med. Armstrong DG. Loss of protective sensation: a practical evidence-based definition. J Foot Ankle Surg. The g monofilament: the diagnostic divining rod for the diabetic foot? Perkins BA, Olaleye D, Zinman B, Bril V. Simple screening tests for peripheral neuropathy in the diabetes clinic. Armstrong DG, Lavery LA, Vela SA, Quebedeaux TL, Fleischli JG. Choosing a practical screening instrument to identify patients at risk for diabetic foot ulceration. Rith-Najarian SJ, Stolusky T, Gohdes DM. Identifying diabetic patients at risk for lower extremity amputation in a primary health care setting. Pham H, Armstrong DG, Harvey C, Harkless LB, Giurini JM, Veves A. Screening techniques to identify the at risk patients for developing diabetic foot ulcers in a prospective multicenter trial. Booth J, Young MJ. Differences in the performance of commercially available g monofilaments. Smieja M, Hunt DL, Edelman D. International Cooperative Group for Clinical Examination Research. Clinical examination for the detection of protective sensation in the feet of diabetic patients. J Gen Intern Med. Gerr FE, Letz R. Reliability of a widely used test of peripheral cutaneous vibration sensitivity and a comparison of two testing protocols. Br J Ind Med. Rosenblum BI. Identifying the patient at risk of foot ulceration. Mason J, O'Keeffe C, Hutchinson A, McIntosh A, Young R, Booth A. A systematic review of foot ulcer in patients with type 2 diabetes mellitus, II: treatment. Thivolet C, el Farkh J, Petiot A, Simonet C, Tourniaire J. Measuring vibration sensations with graduated tuning fork: simple and reliable means to detect diabetic patients at risk of neuropathic foot ulceration. Liniger C, Albeanu A, Bloise D, Assal JP. The tuning fork revisited. Gin H, Rigalleau V, Baillet L, Rabemanantsoa C. Comparison between monofilament, tuning fork and vibration perception tests for screening patients at risk of foot complication. Diabetes Metab. Coppini DV, Young PJ, Weng C, Macleod AF, Sonksen PH. Outcome on diabetic foot complications in relation to clinical examination and quantitative sensory testing: a case-control study. Pitei DL, Edmonds ME. Foot pressure measurements. Armstrong DG, Peters EJ, Athanasiou KA, Lavery LA. Is there a critical level of plantar foot pressure to identify patients at risk for neuropathic foot ulceration? Lavery LA, Armstrong DG, Wunderlich RP, Tredwell JL, Boulton AJM. Predictive value of foot pressure assessment as part of a population-based diabetes disease management program. Peripheral arterial disease in people with diabetes. Kruger S, Guthrie D. Foot care: knowledge retention and self-care practices. Diabetes Educ. Mazzuca SA, Moorman NH, Wheeler ML. The diabetes education study: a controlled trial of the effects of diabetes patient education. Barth R, Campbell LV, Allen S, Jupp JJ, Chisholm DJ. Intensive education improves knowledge, compliance, and foot problems in type 2 diabetes. Bloomgarden ZT, Karmally W, Metzger MJ. Randomized, controlled trial of diabetic patient education: improved knowledge without improved metabolic status. Litzelman DK, Slemenda CW, Langefeld CD. Reduction of lower extremity clinical abnormalities in patients with non—insulin-dependent diabetes mellitus: a randomized, controlled trial. Ann Intern Med. Pieber TR, Holler A, Siebenhofer A. Evaluation of a structured teaching and treatment programme for type 2 diabetes in general practice in a rural area of Austria. Malone JM, Snyder M, Anderson G, Bernhard VM, Holloway GA Jr, Bunt TJ. Prevention of amputation by diabetic education. Am J Surg. Valk GD, Kriegsman DM, Assendelft WJ. Patient education for preventing diabetic foot ulceration: a systematic review. Endocrinol Metab Clin North Am. Khoury A, Landers P, Roth M. Computer-supported identification and intervention for diabetic patients at risk for amputation. MD Comput. Wheatley C. Audit protocol: part one: prevention of diabetic foot ulcers—the non-complicated foot. J Clin Govern. Bruckner M, Mangan M, Godin S, Pogach L. Project LEAP of New Jersey: lower extremity amputation prevention in persons with type 2 diabetes. Am J Manag Care. Rith-Najarian S, Branchaud C, Beaulieu O, Gohdes D, Simonson G, Mazze R. Reducing lower-extremity amputations due to diabetes: application of the staged diabetes management approach in a primary care setting. J Fam Pract. Frykberg RG, Armstrong DG, Giurini JM. Diabetic foot disorders: a clinical practice guideline. Hutchinson A, McIntosh A, Feder G, Home PD, Young R. Clinical Guidelines for Type 2 Diabetes: Prevention and Management of Foot Problems. London, England: Royal College of General Practitioners; International Consensus on the Diabetic Foot : Practical Guidelines [book on CD-ROM]. Noordwijkerhout, the Netherlands: International Working Group on the Diabetic Foot; Supplement to the International Consensus on the Diabetic Foot: Practical Guidelines [book on CD-ROM]. Clinical Practice Guidelines: Diabetes Mellitus Algorithms—Module F: Foot Care. Washingon, DC: Veterans Health Administration; Preventative foot care in people with diabetes. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. UK Prospective Diabetes Study UKPDS Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes UKPDS Chaturvedi N, Abbott CA, Whalley A, Widdows P, Leggetter SY, Boulton AJ. Risk of diabetes-related amputation in South Asians vs Europeans in the UK. Jbour AS, Jarrah NS, Radaideh AM. Prevalence and predictors of diabetic foot syndrome in type 2 diabetes mellitus in Jordan. Saudi Med J. Mayfield JA, Reiber GE, Nelson RG, Greene T. Do foot examinations reduce the risk of diabetic amputation? Ganiats TG. Judging the evidence for interventions: asking the right questions about foot examinations for patients with diabetes. Plank J, Haas W, Rakovac I. Evaluation of the impact of chiropodist care in the secondary prevention of foot ulcerations in diabetic subjects. Tyrrell W. Orthotic intervention in patients with diabetic foot ulceration. J Wound Care. Reiber GE, Smith DG, Wallace C. Effect of therapeutic footwear on foot reulceration in patients with diabetes: a randomized controlled trial. Cavanagh PR, Boulton AJ, Sheehan P, Ulbrecht JS, Caputo GM, Armstrong DG. Therapeutic footwear in patients with diabetes. Colagiuri S, Marsden LL, Naidu V, Taylor L. The use of orthotic devices to correct plantar callus in people with diabetes. Diabetes Res Clin Pract. Uccioli L, Faglia E, Monticone G. Manufactured shoes in the prevention of diabetic foot ulcers. Busch K, Chantelau E. Young MJ, Cavanagh PR, Thomas G, Johnson MM, Murray H, Boulton AJ. The effect of callus removal on dynamic plantar foot pressures in diabetic patients. Soulier SM, Godsey C, Asay ED, Perrotta DM. The prevention of plantar ulceration in the diabetic foot through the use of running shoes. Pitei DL, Foster A, Edmonds M. The effect of regular callus removal on foot pressures. Ronnemaa T, Hamalainen H, Toikka T, Liukkonen I. Evaluation of the impact of podiatrist care in the primary prevention of foot problems in diabetic subjects. van Putten M, Schaper NC. The preventive value of podiatry for the diabetic foot at risk for ulceration. Armstrong DG, Lavery LA, Harkless LB. Validation of a diabetic wound classification system: the contribution of depth, infection, and ischemia to risk of amputation. Armstrong DG, Harkless LB. Outcomes of preventative care in a diabetic foot specialty clinic. Wagner FW. The dysvascular foot: a system for diagnosis and treatment. Foot Ankle. Armstrong DG, Lavery LA, Stern S, Harkless LB. Is prophylactic diabetic foot surgery dangerous? Armstrong DG, Stacpoole-Shea S, Nguyen HC, Harkless LB. Lengthening of the Achilles tendon in diabetic patients who are at high risk for ulceration of the foot. J Bone Joint Surg Am. Gudas CJ. Prophylactic surgery in the diabetic foot. Clin Podiatr Med Surg. Catanzariti AR, Blitch EL, Karlock LG. Elective foot and ankle surgery in the diabetic patient. Simon SR, Tejwani SG, Wilson DL, Santner TJ, Denniston NL. Arthrodesis as an early alternative to nonoperative management of charcot arthropathy of the diabetic foot. Rosenblum BI, Giurini JM, Chrzan JS, Habershaw GM. Preventing loss of the great toe with the hallux interphalangeal arthroplasty. Wieman TJ, Mercke YK, Cerrito PB, Taber SW. Resection of the metatarsal head for diabetic foot ulcers. Giurini JM, Basile P, Chrzan JS, Habershaw GM, Rosenblum BI. Panmetatarsal head resection: a viable alternative to the transmetatarsal amputation. Barry DC, Sabacinski KA, Habershaw GM, Giurini JM, Chrzan JS. Tendo Achillis procedures for chronic ulcerations in diabetic patients with transmetatarsal amputations. Giurini JM, Chrzan JS, Gibbons GW, Habershaw GM. Sesamoidectomy for the treatment of chronic neuropathic ulcerations. Fleischli JE, Anderson RB, Davis WH. Dorsiflexion metatarsal osteotomy for treatment of recalcitrant diabetic neuropathic ulcers. Foot Ankle Int. Blume PA, Paragas LK, Sumpio BE, Attinger CE. Single-stage surgical treatment of noninfected diabetic foot ulcers. Plast Reconstr Surg. Armstrong DG, Todd WF, Lavery LA, Harkless LB. Ha Van G, Siney H, Danan JP, Sachon C, Grimaldi A. Treatment of osteomyelitis in the diabetic foot: contribution of conservative surgery. Scher KS, Steele FJ. The septic foot in patients with diabetes. Armstrong DG, Frykberg RG. Classification of diabetic foot surgery: toward a rational definition. Lin SS, Lee TH, Wapner KL. Plantar forefoot ulceration with equinus deformity of the ankle in diabetic patients: the effect of tendo-achilles lengthening and total contact casting. Mueller MJ, Sinacore DR, Hastings MK, Strube MJ, Johnson JE. Effect of achilles tendon lengthening on neuropathic plantar ulcers: a randomized clinical trial. Mueller MJ, Sinacore DR, Hastings MK, Lott DJ, Strube MJ, Johnson JE. Impact of achilles tendon lengthening on functional limitations and perceived disability in people with a neuropathic plantar ulcer. Piaggesi A, Schipani E, Campi F. Conservative surgical approach versus non-surgical management for diabetic neuropathic foot ulcers: a randomized trial. Armstrong DG, Lavery LA, Vazquez JR. Clinical efficacy of the first metatarsophalangeal joint arthroplasty as a curative procedure for hallux interphalangeal joint wounds in persons with diabetes. Wang JC, Le AW, Tsukuda RK. Feinglass J, Brown JL, LoSasso A. Rates of lower-extremity amputation and arterial reconstruction in the United States, to Am J Public Health. Sumpio BE, Lee T, Blume PA. Vascular evaluation and arterial reconstruction of the diabetic foot. Faglia E, Mantero M, Caminiti M. Extensive use of peripheral angioplasty, particularly infrapopliteal, in the treatment of ischaemic diabetic foot ulcers: clinical results of a multicentric study of consecutive diabetic subjects. J Intern Med. Rauwerda JA. Surgical treatment of the infected diabetic foot. Even in the presence of severe infection, many patients have few subjective complaints and are often more concerned with soiled footwear and stockings than with the penetrating wound. Adequate debridement is the first step in the evaluation of a foot ulcer. Debridement should remove all necrotic tissue and surrounding callus until a healthy bleeding edge is revealed. Patients and physicians often underestimate the need for debridement and may be surprised by the appearance of the newly debrided ulcer. After debridement, the ulcer should be probed with a sterile blunt instrument to determine the involvement of underlying structures, such as tendon, joint capsule or bone. Probing to bone is a simple and specific test for osteomyelitis, but it has low sensitivity. It can be difficult to differentiate local soft tissue infection and inflammation from osteomyelitis. Three-phase bone scans and radiolabelled leukocyte scans are expensive but can help to establish an accurate diagnosis in problematic cases. A variety of wound classification systems exists. Table 3 33 , 34 outlines one diabetic foot classification system that is presently being evaluated to determine if its use will reduce the incidence of diabetic foot amputations. This classification system divides the findings for the diabetic foot into six categories based on increasing risk. The first three categories are risk factors for foot ulceration, and the second three are risk factors for amputation. The suggested treatments reflect the degree of risk for each category. Recognition of risk factors, preventive foot maintenance and regular foot examinations are essential in preventing foot ulcers in patients with diabetes. When foot ulcers develop despite preventive measures, a systematically applied regimen of diagnosis and classification, coupled with early and appropriate treatment, should help to reduce the tremendous personal and societal burden of diabetes-related amputations. Lavery LA, Ashry HR, van Houtum W, Pugh JA, Harkless LB, Basu S. Variation in the incidence and proportion of diabetes-related amputations in minorities. Diabetes Care. Armstrong DG, Lavery LA, Quebedeaux TL, Walker SC. Surgical morbidity and the risk of amputation due to infected puncture wounds in diabetic versus nondiabetic adults. South Med J. Gibbons G, Eliopoulos GM. Infection of the diabetic foot. In: Kozak GP, et al. Management of diabetic foot problems. Philadelphia: Saunders, — Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Reiber GE, Pecoraro RE, Koepsell TD. Risk factors for amputation in patients with diabetes mellitus. A case-control study. Ann Intern Med. United States National Diabetes Advisory Board. The national long-range plan to combat diabetes. Bethesda, Md. Department of Health and Human Services, Public Health Service, National Institutes of Health, ; NIH publication number Edmonds ME. Experience in a multidisciplinary diabetic foot clinic. In: Connor H, Boulton AJ, Ward JD, eds. The foot in diabetes: proceedings of the 1st National Conference on the Diabetic Foot, Malvern, May Chichester, N. Wylie-Rosset J, Walker EA, Shamoon H, Engel S, Basch C, Zybert P. Assessment of documented foot examinations for patients with diabetes in inner-city primary care clinics. Arch Fam Med. Bailey TS, Yu HM, Rayfield EJ. Patterns of foot examination in a diabetes clinic. Am J Med. Edelson GW, Armstrong DG, Lavery LA, Caicco G. The acutely infected diabetic foot is not adequately evaluated in an inpatient setting. Arch Intern Med. Kannel WB, McGee DL. Diabetes and glucose tolerance as risk factors for cardiovascular disease: the Framingham study. LoGerfo FW, Coffman JD. Vascular and microvascular disease of the foot in diabetes. Implications for foot care. N Engl J Med. Lee JS, Lu M, Lee VS, Russell D, Bahr C, Lee ET. Lower-extremity amputation. Incidence, risk factors, and mortality in the Oklahoma Indian Diabetes Study. Update on some epidemiologic features of intermittent claudication: the Framingham study. J Am Geriatr Soc. Bacharach JM, Rooke TW, Osmundson PJ, Gloviczki P. Predictive value of transcutaneous oxygen pressure and amputation success by use of supine and elevation measurements. J Vasc Surg. Apelqvist J, Castenfors J, Larsson J, Strenstrom A, Agardh CD. Prognostic value of systolic ankle and toe blood pressure levels in outcome of diabetic foot ulcer. Orchard TJ, Strandness DE. Assessment of peripheral vascular disease in diabetes. Report and recommendation of an international workshop sponsored by the American Heart Association and the American Diabetes Association 18—20 September , New Orleans, Louisiana. J Am Podiatr Med Assoc. Caputo GM, Cavanagh PR, Ulbrecht JS, Gibbons GW, Karchmer AW. Assessment and management of foot disease in patients with diabetes. Harati Y. Diabetic peripheral neuropathy. In: Kominsky SJ, ed. Medical and surgical management of the diabetic foot. Louis: Mosby, — Brand PW. The insensitive foot including leprosy. In: Jahss MH, ed. Philadelphia: Saunders, —5. Armstrong DG, Todd WF, Lavery LA, Harkless LB, Bushman TR. The natural history of acute Charcot's arthropathy in a diabetic foot specialty clinic. Diabet Med. Edmonds ME, Clarke MB, Newton S, Barrett J, Watkins PJ. Increased uptake of bone radiopharmaceutical in diabetic neuropathy. Q J Med. Brower AC, Allman RM. The neuropathic joint: a neurovascular bone disorder. Radiol Clin North Am. Birke JA, Sims DS. Plantar sensory threshold in the ulcerative foot. Lepr Rev. Armstrong DG, Lavery LA, Vela SA, Quebedeaux TL, Fleischli JG. Choosing a practical screening instrument to identify patients at risk for diabetic foot ulceration. Arch Intern Med In press. Fernando DJ, Masson EA, Veves A, Boulton AJ. Relationship of limited joint mobility to abnormal foot pressures and diabetic foot ulceration. Rosenbloom AL, Silverstein JH, Lezotte DC, Richardson K, McCallum M. Limited joint mobility in childhood diabetes mellitus indicates increased risk for microvascular disease. Bild DE, Selby JV, Sinnock P, Browner WS, Braveman P, Showstack JA. Lower-extremity amputation in people with diabetes. Epidemiology and prevention. Lavery LA, Armstrong DG, Quebedeaux TL, Walker SC. |
| Site Index | Practical criteria for screening patients at high risk for diabetic foot ulceration. Arch Intern Med. Altman MI, Altman KS. The podiatric assessment of the diabetic lower extremity: special considerations. Boike AM, Hall JO. A practical guide for examining and treating the diabetic foot. Cleve Clin J Med. Armstrong DG. Loss of protective sensation: a practical evidence-based definition. J Foot Ankle Surg. The g monofilament: the diagnostic divining rod for the diabetic foot? Perkins BA, Olaleye D, Zinman B, Bril V. Simple screening tests for peripheral neuropathy in the diabetes clinic. Armstrong DG, Lavery LA, Vela SA, Quebedeaux TL, Fleischli JG. Choosing a practical screening instrument to identify patients at risk for diabetic foot ulceration. Rith-Najarian SJ, Stolusky T, Gohdes DM. Identifying diabetic patients at risk for lower extremity amputation in a primary health care setting. Pham H, Armstrong DG, Harvey C, Harkless LB, Giurini JM, Veves A. Screening techniques to identify the at risk patients for developing diabetic foot ulcers in a prospective multicenter trial. Booth J, Young MJ. Differences in the performance of commercially available g monofilaments. Smieja M, Hunt DL, Edelman D. International Cooperative Group for Clinical Examination Research. Clinical examination for the detection of protective sensation in the feet of diabetic patients. J Gen Intern Med. Gerr FE, Letz R. Reliability of a widely used test of peripheral cutaneous vibration sensitivity and a comparison of two testing protocols. Br J Ind Med. Rosenblum BI. Identifying the patient at risk of foot ulceration. Mason J, O'Keeffe C, Hutchinson A, McIntosh A, Young R, Booth A. A systematic review of foot ulcer in patients with type 2 diabetes mellitus, II: treatment. Thivolet C, el Farkh J, Petiot A, Simonet C, Tourniaire J. Measuring vibration sensations with graduated tuning fork: simple and reliable means to detect diabetic patients at risk of neuropathic foot ulceration. Liniger C, Albeanu A, Bloise D, Assal JP. The tuning fork revisited. Gin H, Rigalleau V, Baillet L, Rabemanantsoa C. Comparison between monofilament, tuning fork and vibration perception tests for screening patients at risk of foot complication. Diabetes Metab. Coppini DV, Young PJ, Weng C, Macleod AF, Sonksen PH. Outcome on diabetic foot complications in relation to clinical examination and quantitative sensory testing: a case-control study. Pitei DL, Edmonds ME. Foot pressure measurements. Armstrong DG, Peters EJ, Athanasiou KA, Lavery LA. Is there a critical level of plantar foot pressure to identify patients at risk for neuropathic foot ulceration? Lavery LA, Armstrong DG, Wunderlich RP, Tredwell JL, Boulton AJM. Predictive value of foot pressure assessment as part of a population-based diabetes disease management program. Peripheral arterial disease in people with diabetes. Kruger S, Guthrie D. Foot care: knowledge retention and self-care practices. Diabetes Educ. Mazzuca SA, Moorman NH, Wheeler ML. The diabetes education study: a controlled trial of the effects of diabetes patient education. Barth R, Campbell LV, Allen S, Jupp JJ, Chisholm DJ. Intensive education improves knowledge, compliance, and foot problems in type 2 diabetes. Bloomgarden ZT, Karmally W, Metzger MJ. Randomized, controlled trial of diabetic patient education: improved knowledge without improved metabolic status. Litzelman DK, Slemenda CW, Langefeld CD. Reduction of lower extremity clinical abnormalities in patients with non—insulin-dependent diabetes mellitus: a randomized, controlled trial. Ann Intern Med. Pieber TR, Holler A, Siebenhofer A. Evaluation of a structured teaching and treatment programme for type 2 diabetes in general practice in a rural area of Austria. Malone JM, Snyder M, Anderson G, Bernhard VM, Holloway GA Jr, Bunt TJ. Prevention of amputation by diabetic education. Am J Surg. Valk GD, Kriegsman DM, Assendelft WJ. Patient education for preventing diabetic foot ulceration: a systematic review. Endocrinol Metab Clin North Am. Khoury A, Landers P, Roth M. Computer-supported identification and intervention for diabetic patients at risk for amputation. MD Comput. Wheatley C. Audit protocol: part one: prevention of diabetic foot ulcers—the non-complicated foot. J Clin Govern. Bruckner M, Mangan M, Godin S, Pogach L. Project LEAP of New Jersey: lower extremity amputation prevention in persons with type 2 diabetes. Am J Manag Care. Rith-Najarian S, Branchaud C, Beaulieu O, Gohdes D, Simonson G, Mazze R. Reducing lower-extremity amputations due to diabetes: application of the staged diabetes management approach in a primary care setting. J Fam Pract. Frykberg RG, Armstrong DG, Giurini JM. Diabetic foot disorders: a clinical practice guideline. Hutchinson A, McIntosh A, Feder G, Home PD, Young R. Clinical Guidelines for Type 2 Diabetes: Prevention and Management of Foot Problems. London, England: Royal College of General Practitioners; International Consensus on the Diabetic Foot : Practical Guidelines [book on CD-ROM]. Noordwijkerhout, the Netherlands: International Working Group on the Diabetic Foot; Supplement to the International Consensus on the Diabetic Foot: Practical Guidelines [book on CD-ROM]. Clinical Practice Guidelines: Diabetes Mellitus Algorithms—Module F: Foot Care. Washingon, DC: Veterans Health Administration; Preventative foot care in people with diabetes. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. UK Prospective Diabetes Study UKPDS Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes UKPDS Chaturvedi N, Abbott CA, Whalley A, Widdows P, Leggetter SY, Boulton AJ. Risk of diabetes-related amputation in South Asians vs Europeans in the UK. Jbour AS, Jarrah NS, Radaideh AM. Prevalence and predictors of diabetic foot syndrome in type 2 diabetes mellitus in Jordan. Saudi Med J. Mayfield JA, Reiber GE, Nelson RG, Greene T. Do foot examinations reduce the risk of diabetic amputation? Ganiats TG. Judging the evidence for interventions: asking the right questions about foot examinations for patients with diabetes. Plank J, Haas W, Rakovac I. Evaluation of the impact of chiropodist care in the secondary prevention of foot ulcerations in diabetic subjects. Tyrrell W. Orthotic intervention in patients with diabetic foot ulceration. J Wound Care. Reiber GE, Smith DG, Wallace C. Effect of therapeutic footwear on foot reulceration in patients with diabetes: a randomized controlled trial. Cavanagh PR, Boulton AJ, Sheehan P, Ulbrecht JS, Caputo GM, Armstrong DG. Therapeutic footwear in patients with diabetes. Colagiuri S, Marsden LL, Naidu V, Taylor L. The use of orthotic devices to correct plantar callus in people with diabetes. Diabetes Res Clin Pract. Uccioli L, Faglia E, Monticone G. Manufactured shoes in the prevention of diabetic foot ulcers. Busch K, Chantelau E. Young MJ, Cavanagh PR, Thomas G, Johnson MM, Murray H, Boulton AJ. The effect of callus removal on dynamic plantar foot pressures in diabetic patients. Soulier SM, Godsey C, Asay ED, Perrotta DM. The prevention of plantar ulceration in the diabetic foot through the use of running shoes. Pitei DL, Foster A, Edmonds M. The effect of regular callus removal on foot pressures. Ronnemaa T, Hamalainen H, Toikka T, Liukkonen I. Evaluation of the impact of podiatrist care in the primary prevention of foot problems in diabetic subjects. van Putten M, Schaper NC. The preventive value of podiatry for the diabetic foot at risk for ulceration. Armstrong DG, Lavery LA, Harkless LB. Validation of a diabetic wound classification system: the contribution of depth, infection, and ischemia to risk of amputation. Armstrong DG, Harkless LB. Outcomes of preventative care in a diabetic foot specialty clinic. Wagner FW. The dysvascular foot: a system for diagnosis and treatment. Foot Ankle. Armstrong DG, Lavery LA, Stern S, Harkless LB. Is prophylactic diabetic foot surgery dangerous? Armstrong DG, Stacpoole-Shea S, Nguyen HC, Harkless LB. Lengthening of the Achilles tendon in diabetic patients who are at high risk for ulceration of the foot. J Bone Joint Surg Am. Gudas CJ. Prophylactic surgery in the diabetic foot. Clin Podiatr Med Surg. Catanzariti AR, Blitch EL, Karlock LG. Elective foot and ankle surgery in the diabetic patient. Simon SR, Tejwani SG, Wilson DL, Santner TJ, Denniston NL. Arthrodesis as an early alternative to nonoperative management of charcot arthropathy of the diabetic foot. Rosenblum BI, Giurini JM, Chrzan JS, Habershaw GM. Preventing loss of the great toe with the hallux interphalangeal arthroplasty. Wieman TJ, Mercke YK, Cerrito PB, Taber SW. Resection of the metatarsal head for diabetic foot ulcers. Giurini JM, Basile P, Chrzan JS, Habershaw GM, Rosenblum BI. Panmetatarsal head resection: a viable alternative to the transmetatarsal amputation. Barry DC, Sabacinski KA, Habershaw GM, Giurini JM, Chrzan JS. Tendo Achillis procedures for chronic ulcerations in diabetic patients with transmetatarsal amputations. Giurini JM, Chrzan JS, Gibbons GW, Habershaw GM. Sesamoidectomy for the treatment of chronic neuropathic ulcerations. Fleischli JE, Anderson RB, Davis WH. Dorsiflexion metatarsal osteotomy for treatment of recalcitrant diabetic neuropathic ulcers. Foot Ankle Int. Blume PA, Paragas LK, Sumpio BE, Attinger CE. Single-stage surgical treatment of noninfected diabetic foot ulcers. Plast Reconstr Surg. Armstrong DG, Todd WF, Lavery LA, Harkless LB. Ha Van G, Siney H, Danan JP, Sachon C, Grimaldi A. Treatment of osteomyelitis in the diabetic foot: contribution of conservative surgery. Scher KS, Steele FJ. The septic foot in patients with diabetes. Armstrong DG, Frykberg RG. Classification of diabetic foot surgery: toward a rational definition. Lin SS, Lee TH, Wapner KL. Plantar forefoot ulceration with equinus deformity of the ankle in diabetic patients: the effect of tendo-achilles lengthening and total contact casting. Mueller MJ, Sinacore DR, Hastings MK, Strube MJ, Johnson JE. Effect of achilles tendon lengthening on neuropathic plantar ulcers: a randomized clinical trial. Mueller MJ, Sinacore DR, Hastings MK, Lott DJ, Strube MJ, Johnson JE. Impact of achilles tendon lengthening on functional limitations and perceived disability in people with a neuropathic plantar ulcer. Piaggesi A, Schipani E, Campi F. Conservative surgical approach versus non-surgical management for diabetic neuropathic foot ulcers: a randomized trial. Armstrong DG, Lavery LA, Vazquez JR. Clinical efficacy of the first metatarsophalangeal joint arthroplasty as a curative procedure for hallux interphalangeal joint wounds in persons with diabetes. Wang JC, Le AW, Tsukuda RK. Feinglass J, Brown JL, LoSasso A. Rates of lower-extremity amputation and arterial reconstruction in the United States, to Am J Public Health. Sumpio BE, Lee T, Blume PA. Vascular evaluation and arterial reconstruction of the diabetic foot. Faglia E, Mantero M, Caminiti M. Extensive use of peripheral angioplasty, particularly infrapopliteal, in the treatment of ischaemic diabetic foot ulcers: clinical results of a multicentric study of consecutive diabetic subjects. J Intern Med. Rauwerda JA. Surgical treatment of the infected diabetic foot. Gordois A, Scuffham P, Shearer A, Oglesby A, Tobian JA. The health care costs of diabetic peripheral neuropathy in the US. Health-economic consequences of diabetic foot lesions. Clin Infect Dis. Prevention of diabetes-related foot ulcers and amputations: a cost-utility analysis based on Markov model simulations. Ortegon MM, Redekop WK, Niessen LW. Cost-effectiveness of prevention and treatment of the diabetic foot: a Markov analysis. This Week in JAMA. See More About Neurology Neuromuscular Diseases Neuropathy Surgery Diabetes Diabetes and Endocrinology. Select Your Interests Select Your Interests Customize your JAMA Network experience by selecting one or more topics from the list below. Save Preferences. Privacy Policy Terms of Use. This Issue. Citations 1, View Metrics. X Facebook More LinkedIn. Cite This Citation Singh N , Armstrong DG , Lipsky BA. Clinical Review. Nalini Singh, MD ; David G. Armstrong, DPM, MSc, PhD ; Benjamin A. Lipsky, MD. Author Affiliations Article Information Author Affiliations : Department of Medicine, Divisions of Endocrinology and Metabolism Dr Singh and General Internal Medicine and Infectious Diseases Dr Lipsky ,Veterans Affairs Puget Sound Healthcare System and University of Washington School of Medicine, Seattle Drs Singh and Lipsky ; and Center for Lower Extremity Ambulatory Research, Dr William M. visual abstract icon Visual Abstract. Pathophysiology of Diabetic Foot Ulcers. Screening to Identify Patients at Risk for Diabetic Foot Ulcers. Educational Interventions to Prevent Foot Ulceration. Clinical Interventions to Prevent Foot Ulceration. Back to top Article Information. Clinical Review Section Editor: Michael S. Lauer, MD. Access your subscriptions. Access through your institution. Force or friction against the bottom of your foot causes the skin to thicken, forming a callus. If the skin keeps thickening, the callus presses up into the foot. This kills healthy tissue and causes pain. Unfortunately, you may not notice the pain if you have neuropathy, a health problem that limits how much feeling you have in your feet. As healthy skin dies, an ulcer forms. Ulcers may progress from hot spots to infected wounds very quickly. A callus pressing into the foot may kill healthy tissue and cause an ulcer. Hot Spots. They are a warning that you need to take care of your feet. If pressure is not relieved, a hot spot is likely to blister. Left untreated, a blister can turn into an open wound or a corn thickened skin on top of the foot or callus. If a corn or callus presses into the foot, it destroys inner layers of skin and fat. Cracks and sores may form. These open wounds are ulcers. They provide a way for infection to enter the body. In some cases, dead skin such as a corn or callus may cover an open wound, making it harder to see. Infected Ulcers. If bacteria enter the ulcer, infection sets in. This causes more healthy tissue to die. The infected ulcer may begin to drain. The discharge may be white, yellow, or greenish. Some infected ulcers bleed or have a bad odor. If you develop an infected ulcer, call your doctor right away. Your Physical Exam. During your foot exam, your doctor will ask about your health. Do you have poor circulation, diabetes, or kidney problems? Your doctor will check your feet for hot spots and thickened skin. He or she may also look for any bone or joint problems. Your ability to feel sensation in your feet also may be checked. Blood flow and nerve sensation in your feet may be tested if you have a chronic health problem, such as diabetes. If you have a deep pressure ulcer, an x-ray or bone scan may be done to check for signs of bone infection. A Doctor's Treatment. If infection is already present, medications will probably be prescribed. Surgery may also be needed if the infection has spread. Cleaning the Ulcer. To assist healing, thickened skin around the ulcer may be cleaned away. Medicated ointment or cream may be applied to prevent infection. Sometimes a special dressing isused to help keep the wound dry. Reducing Force. To take pressure off hot spots and ulcers, your doctor may prescribe orthoses. These custom-made shoe inserts absorb or divert pressure from problem areas. Special shoes or temporary casts may also be used. Using Antibiotics. To control or prevent infection, your doctor may prescribe antibiotics. Take them all, and take them as directed. If you stop using an antibiotic too soon, the infection may come back. If Surgery Is Needed. Surgery may be needed if infection enters deep tissues or bone. In such cases, your doctor cleans away the infection while removing as little tissue or bone as possible. You may also be given intravenous IV antibiotics to fight the infection. Preventing Ulcers. By taking care of yourself, you may be able to prevent pressure ulcers. At the very least, you can reduce your risk of getting one. Try to check your feet daily and improve your overall health. Checking Your Feet. |
| If You Use a Wheelchair | Commonly accepted abnormal values for transcutaneous oxygen measurement, ABI determinations and toe systolic pressure are given in Table 2. The noninvasive tests have been faulted for underestimating the severity of arterial insufficiency. Optimal ulcer healing requires adequate tissue perfusion. Thus, arterial insufficiency should be suspected if an ulcer fails to heal. Vascular surgery consultation and possible revascularization should be considered when clinical signs of ischemia are present in the lower extremity of a diabetic patient and the results of noninvasive vascular tests or imaging studies suggest that the patient has peripheral arterial occlusive disease. Proper control of concomitant hypertension or hyperlipidemia can help to reduce the risk of peripheral arterial occlusive disease. Smoking cessation is essential for preventing the progression of occlusive disease. Distal symmetric polyneuropathy is perhaps the most common complication affecting the lower extremities of patients with diabetes mellitus. This complication occurs in up to 58 percent of patients with longstanding disease. In the diabetic foot, autonomic neuropathy has several common manifestations. First, denervation of dermal structures leads to decreased sweating. This causes dry skin and fissure formation, which predispose the skin to infection. The nylon monofilament test is a simply performed office test to diagnose patients at risk for ulcer formation due to peripheral sensory neuropathy. Physicians can obtain a monofilament kit and literature on diabetic foot management at a small cost from the National Diabetes Information Clearing-house Foot deformities, which are common in diabetic patients, lead to focal areas of high pressure. When an abnormal focus of pressure is coupled with lack of sensation, a foot ulcer can develop. Most diabetic foot ulcers form over areas of bony prominences Figure 2 , especially when bunions, calluses or hammer-toe formations lead to abnormally prominent bony points. Foot deformities are believed to be more common in diabetic patients due to atrophy of the intrinsic musculature responsible for stabilizing the toes. Rigid deformities or limited range of motion at the subtalar or metatarsophalangeal joints have also been associated with the development of diabetic foot ulcers. A diabetic patient with a history of previous ulceration or amputation is at increased risk for further ulceration, infection and subsequent amputation. Alterations in foot dynamics due to ulceration, joint deformity or amputation can cause the abnormal distribution of plantar pressures and result in the formation of new ulcers 28 Figure 3. Meticulous attention to foot care and proper management of minor foot injuries are key to preventing ulcer formation. Daily foot inspection by the patient or a caretaker if the patient lacks sufficient visual acuity or mobility to perform the examination is the cornerstone of proper foot care. Gentle cleansing with soap and water, followed by the application of topical moisturizers, helps to maintain healthy skin that can better resist breakdown and injury. The physician should inspect the patient's shoes for areas of inadequate support or improper fit. While many patients do well with commercially available athletic shoes and thick, absorbent socks, patients with foot deformities or special support needs may benefit from custom shoes. Medicare Part B now covers the purchase of custom shoes when the certifying physician identifies a risk factor for ulcer formation and submits appropriate documentation. A sample documentation form is provided with the monofilament kit used to test patients for peripheral sensory neuropathy. Minor foot injuries and infections, such as cuts, scrapes, blisters and tinea pedis, can be unintentionally exacerbated by home remedies that impede healing. Patients should be reminded to avoid hot soaks, heating pads and harsh topical agents such as hydrogen peroxide, iodine e. Gentle cleansing of minor wounds and the application of a topical antibiotic to maintain a moist wound environment can help to prevent ulcer formation. In addition, the physician should inspect any minor wound that does not heal rapidly. By reinforcing preventive advice and inspecting the patient's feet at routine follow-up visits, the physician can help the patient develop and maintain good foot-care habits. Despite the best intentions and careful attention to foot care, many diabetic patients eventually develop foot ulcers. These wounds are the principal portal of entry for infection in patients with diabetes Figure 4. Frequently, the ulcers are covered by callus or fibrotic tissue. This makes the trimming of hyperkeratotic tissue important for comprehensive wound evaluation. Because these ulcers almost always form in patients with neuropathy, they are typically painless. Even in the presence of severe infection, many patients have few subjective complaints and are often more concerned with soiled footwear and stockings than with the penetrating wound. Adequate debridement is the first step in the evaluation of a foot ulcer. Debridement should remove all necrotic tissue and surrounding callus until a healthy bleeding edge is revealed. Patients and physicians often underestimate the need for debridement and may be surprised by the appearance of the newly debrided ulcer. After debridement, the ulcer should be probed with a sterile blunt instrument to determine the involvement of underlying structures, such as tendon, joint capsule or bone. Probing to bone is a simple and specific test for osteomyelitis, but it has low sensitivity. It can be difficult to differentiate local soft tissue infection and inflammation from osteomyelitis. Three-phase bone scans and radiolabelled leukocyte scans are expensive but can help to establish an accurate diagnosis in problematic cases. A variety of wound classification systems exists. Table 3 33 , 34 outlines one diabetic foot classification system that is presently being evaluated to determine if its use will reduce the incidence of diabetic foot amputations. This classification system divides the findings for the diabetic foot into six categories based on increasing risk. The first three categories are risk factors for foot ulceration, and the second three are risk factors for amputation. The suggested treatments reflect the degree of risk for each category. Recognition of risk factors, preventive foot maintenance and regular foot examinations are essential in preventing foot ulcers in patients with diabetes. When foot ulcers develop despite preventive measures, a systematically applied regimen of diagnosis and classification, coupled with early and appropriate treatment, should help to reduce the tremendous personal and societal burden of diabetes-related amputations. Lavery LA, Ashry HR, van Houtum W, Pugh JA, Harkless LB, Basu S. Variation in the incidence and proportion of diabetes-related amputations in minorities. Diabetes Care. Armstrong DG, Lavery LA, Quebedeaux TL, Walker SC. Surgical morbidity and the risk of amputation due to infected puncture wounds in diabetic versus nondiabetic adults. South Med J. Gibbons G, Eliopoulos GM. Infection of the diabetic foot. In: Kozak GP, et al. Management of diabetic foot problems. Philadelphia: Saunders, — Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Reiber GE, Pecoraro RE, Koepsell TD. Risk factors for amputation in patients with diabetes mellitus. A case-control study. Ann Intern Med. United States National Diabetes Advisory Board. The national long-range plan to combat diabetes. Bethesda, Md. Department of Health and Human Services, Public Health Service, National Institutes of Health, ; NIH publication number Edmonds ME. Experience in a multidisciplinary diabetic foot clinic. In: Connor H, Boulton AJ, Ward JD, eds. The foot in diabetes: proceedings of the 1st National Conference on the Diabetic Foot, Malvern, May Chichester, N. Wylie-Rosset J, Walker EA, Shamoon H, Engel S, Basch C, Zybert P. Assessment of documented foot examinations for patients with diabetes in inner-city primary care clinics. Arch Fam Med. Bailey TS, Yu HM, Rayfield EJ. Patterns of foot examination in a diabetes clinic. Am J Med. Edelson GW, Armstrong DG, Lavery LA, Caicco G. The acutely infected diabetic foot is not adequately evaluated in an inpatient setting. Arch Intern Med. Kannel WB, McGee DL. Diabetes and glucose tolerance as risk factors for cardiovascular disease: the Framingham study. LoGerfo FW, Coffman JD. Vascular and microvascular disease of the foot in diabetes. Implications for foot care. N Engl J Med. Lee JS, Lu M, Lee VS, Russell D, Bahr C, Lee ET. Lower-extremity amputation. Incidence, risk factors, and mortality in the Oklahoma Indian Diabetes Study. Update on some epidemiologic features of intermittent claudication: the Framingham study. J Am Geriatr Soc. Bacharach JM, Rooke TW, Osmundson PJ, Gloviczki P. Predictive value of transcutaneous oxygen pressure and amputation success by use of supine and elevation measurements. J Vasc Surg. Apelqvist J, Castenfors J, Larsson J, Strenstrom A, Agardh CD. Prognostic value of systolic ankle and toe blood pressure levels in outcome of diabetic foot ulcer. Research Appreciation Day. Skin Health on Our Team: Prevention of Pressure Ulcers in Athletic Individuals with Spinal Cord Injuries. Date Authors Garner, Becky. Journal Title. Journal ISSN. Volume Title. Abstract OBJECTIVE: Focus group data were collected as a means to gather participant attitudes, beliefs, feelings and opinions in regards to informing the design of a pressure ulcer prevention study. Collections Other. Full item page. |
Ich empfehle Ihnen, auf die Webseite vorbeizukommen, wo viele Artikel zum Sie interessierenden Thema gibt.