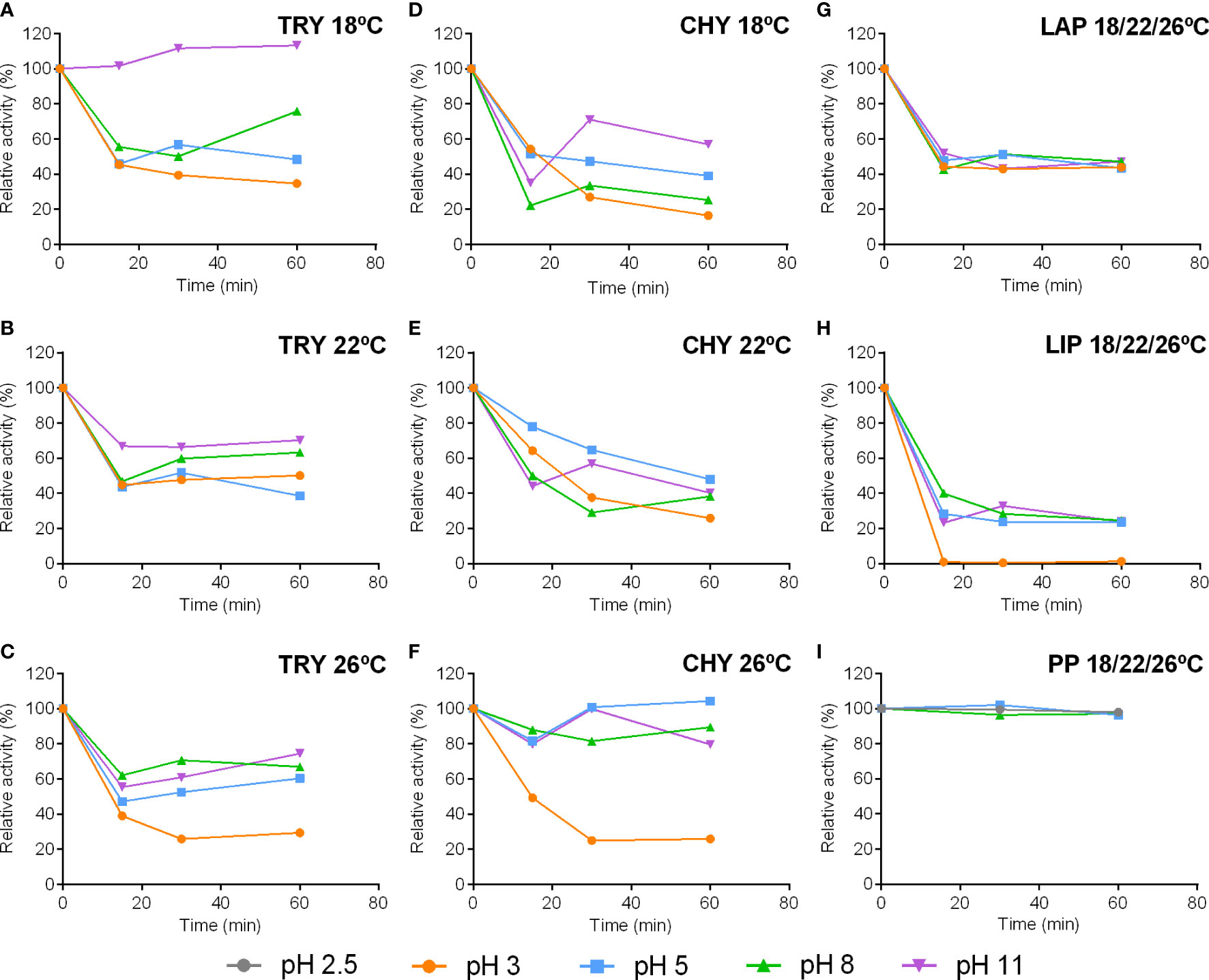
Digestive enzyme stability -
The coating process was carried out with process air at three different moisture contents Table 3. The composition of the coated particles for each set a process conditions is approximately the same Table 4 , and appeared uniform, smooth and homogeneous after microscopic examination. The three sets of samples i.
The influence of residual moisture on the loss of activity over time was evaluated under accelerated stability conditions as follows:.
Hard gelatin capsules dosage 20, IU Lipase were filled with the three lots of coated Pancrelipase MT minitablets described above and stored at 40° C.
Lipase activity was evaluated after 15 days and 4 months of storage. The results are shown in Table 6. Alternatively, improved stability is provided by coating under an atmosphere with a moisture content of less than 3. Pancrelipase MT particles were coated with two coating compositions containing different amounts of talc Table 7.
Coating trials were carried out using a fluidized bed Glatt-GPCG1 apparatus equipped with a Munters ML dehumidifier in order to assure process air flow at a low moisture content i. The theoretical composition of the two batches is reported in Table 8.
Microscopic examination indicated that the coatings on all samples were smooth and homogeneous. Residual moisture contents were measured by loss on drying Table 9. The effects of the different coating compositions on the loss of activity over time were evaluated under accelerated stability conditions as follows:.
Hard gelatin capsules dosage 20, IU Lipase were filled with the two lots of coated Pancrelipase MT described above, and stored at 40° C. Lipase activity was checked after 1, 2 and 3 months of storage as shown in Table The results showed that the loss of activity after three months of storage under accelerated stability conditions is significantly lower for samples coated with a high talc content coating Lot P9A The theoretical composition of the two batches is reported in Table Lot P9A complied with commercial product specifications, but Lot P9A did not pass a gastro-resistance test.
Lot P9A was then evaluated under accelerated stability conditions as follows:. Hard gelatin capsules dosage 20, IU Lipase were prepared and stored at 40° C. Lipase activity was measured after 1, 2 and 3 months of storage as shown in Table The stability of Lot P9A is significantly improved compared to the stability of Lot P9A, which was prepared with a similar coating under similar coating conditions Table TABLE 14 Accelerated stability at 1 2 3 40° C.
CPS gelatin and HPMC hydroxypropylmethylcellulose capsules were filled with identical coated lipase compositions. All samples were subjected to accelerated stability conditions 40° C. The results are shown below in Tables As shown in Tables , lipase compositions in HPMC capsules show significantly higher lipase activity after storage for 15, 30, and 90 days under accelerated stability conditions and dried HPMC capsules offer better stability than those which contain equilibrium moisture levels.
Gelatin and hydroxypropylmethylcellulose capsules were filled with coated lipase compositions Minitablet form. The coating compositions were otherwise identical. The following Table 18 compares the levels of degradation observed after storage under accelerated stability conditions with the moisture content of the compositions.
As shown in Table 18, higher levels of lipase activity correlate with lower levels of moisture in the composition. In addition, compositions filled in HPMC capsules are more stable than compositions filled in to gelatin capsules.
The effects of storing capsules containing lipase compositions in packages containing a desiccant were evaluated by measuring lipase activity in the samples after 30 and 90 days of storage under accelerated stability conditions 40° C.
As shown in Tables 19 and 20, lipase activity is significantly higher in packages containing a desiccant and in capsules that are dried below their equilibrium moisture content.
Desiccant 2: molecular sieves in Tyvek® bags. The theoretical composition of the two coating suspensions shown in Table 21, below. The theoretical composition, which was the same for all six batches, is shown below in Table Microscopic examination of the coating for all six samples appeared smooth and homogeneous.
The coated Pancrelipase MT particles were then filled into HPMC capsules and packaged in glass bottles containing desiccants molecular sieves. The bottles were then sealed, stored under accelerated stability conditions and lipase activity was evaluated at various time periods as indicated below in Table The packaging conditions for each sample was as follows.
Twelve HPMC capsules dosage 20, IU Lipase and 1 g of molecular sieves Minipax sorbent-Multisorb as desiccant were put in a 30 mL capacity glass bottle. TABLE 23 Accelerated stability at 40° C. To provide further choices for dosage formulations were made in which the dimensions of the tablets was significantly reduced.
The pancrelipase blend was tabletted with round 1. The characteristics of Lot 9A are shown in Table TABLE 24 Lot P9A Values Diameter 1.
Microscopic examination of the film coatings indicated that all of the samples appeared smooth and homogeneous. The theoretical composition of the batch Lot P9A is shown in Table Two other batches of microtablets were prepared as described above, and their properties are shown below in Table TABLE 26 Characteristics Lot P9A Lot P9A Diameter 1.
TABLE 27 Coated μT Solvent Uncoated μT Lot. P9A Acetone Lot. P9A Lot. The theoretical compositions of the batches are summarized in Table HPMC cps capsules were filled with the coated microtablets described above, and packed in glass bottles containing desiccants molecular sieves.
Twelve HPMC capsules dosage 5, IU Lipase and 1 g of molecular sieves Minipax sorbent-Multisorb as desiccant were placed in a 30 mL capacity glass bottle. Lipase activity was measured at 20, 30, 40, and 60 days of storage as shown in Tables 29 and TABLE 29 Accelerated stability at 40° C.
TABLE 30 Accelerated stability at 40° C. The microtablets prepared above were slightly oblong see Tables 24 and 26 ; the ratio between the microtablet thickness and diameter was between 1. To further reduce the dimensions of the microtablets, new samples were prepared with ratios of thickness to diameter ratio nearer to Lot Q9A , are shown below in Table TABLE 31 Characteristics Lot Q9A Diameter 1.
The theoretical composition of the coated microtablet Lot Q9A was the same as that shown in Table Microscopic examination indicated that the coatings were smooth and homogeneous. In addition, increased levels of inorganic materials in the coating e.
The following Table 33 shows accelerated stability testing in bottles; 40° C. The results indicate that conventional enteric coatings such as Eudragit do not provide stabilized pancrelipase compositions. Examples of dosage forms comprising ER coated beads of varying dosage per capsule, coated as described in previous examples, are shown in Table 34, below:.
The following Table 35 shows the water content of various sized containers containing capsules comprising the compositions of the present invention. The water content includes total water from the capsules, and water permeating into the container over a two-year storage time.
TABLE 35 Bottle Cps Cps. Total water Water from Equivalent sizes weight moisture from cps Permeation. A Phase III randomized, double-blind, placebo-controlled, cross over study was carried out to compare the effects of treatment with the pancrelipase compositions of Table 34 to that of a placebo in 34 CF patients with EPI aged seven years and older.
The study was conducted in 14 CF centers throughout the US. The primary endpoint of the study compared the coefficient of fat absorption following oral administration of the pancrelipase composition in daily doses less than or equal to 10, lipase units per kilogram of body weight in combinations of 5,, 10,, 15, or 20, lipase units per capsule versus a placebo.
The secondary endpoints of the trial evaluated changes in the coefficient of nitrogen absorption as a determinant of protein absorption, cholesterol, fat soluble vitamins, weight, body mass index and EPI symptoms. Patients treated with these compositions showed a statistically significant increase in the coefficient of fat absorption and coefficient of nitrogen absorption as compared to those receiving a placebo and had fewer symptoms associated with impaired absorption such as bloating, flatulence, pain and evidence of fat in stools.
Increases in mean cholesterol and vitamin levels were also observed in patients taking these pancrelipase compositions versus placebo.
A statistically significant decrease was obtained in the frequency of stools per day. These compositions were well tolerated by patients, and no drug related serious adverse events were observed during the study. The mean percentage coefficient of fat absorption in patients receiving these compositions was The mean percentage coefficient of nitrogen absorption was A pediatric, Phase III clinical trial was carried out to evaluate the effects of treatment with the compositions of Table 34 in an open-label study in 19 CF patients under the age of seven in 11 CF treatment centers in the US—the first pancreatic replacement therapy trial of this size conducted on young children and infants with exocrine pancreatic insufficiency.
The study design involved a seven-day dose stabilization period followed by a seven-day treatment period; patients received 5, lipase units per capsule daily, with the product being sprinkled on food as required.
Secondary endpoints included weight change, nutritional status, stool frequency and consistency, incidences of bloating, pain and flatulence as well as physician and parent or guardian judgment of clinical symptoms improvement. Product safety was also assessed.
The mean percentage of responders, as defined in the study protocol, at screening the beginning of the stabilization period when patients were on a previous pancreatic enzyme replacement therapy and prior to treatment was At the end of the stabilization period and the end of the treatment period, the mean percentages of responders were Among the children in the study, malabsorption symptoms were significantly lower at the end of the treatment period than at screening, consistent with observations regarding control of malabsorption symptoms seen in the Phase III trial described in Example 12, above.
The pancrelipase compositions of the present invention were also well tolerated by these patients and no drug related serious adverse events were observed during the trial. The results showed that the compositions of the present invention effectively controlled the signs and symptoms of malabsorption and support the results obtained in the pivotal phase III trial described in Example A significant proportion of physicians and patients felt that the control of symptoms with the compositions of the present invention was improved versus previous therapies.
A Phase III opened-label, randomized, single center, single treatment, cross-over study was carried out to compare the effects of treatment with the pancrelipase compositions of Table 34 to determine the gastrointestinal bioavailability of these compositions in fed conditions in 10 chronic pancreatitis patients with exocrine pancreatic insufficiency.
Exclusionary drugs proton pump inhibitors PPI's , antacids, and drugs capable of altering GI mobility were discontinued 7 days prior to entering the study. The following day, patients received a physical exam, and blood and urine samples were collected. The bioavailability of the compositions of the present invention was estimated from the amount of the respective enzymes released and recovered i.
Measurements of cholecystokinin levels in the blood, and gastric and duodenal pH were also measured. Lipase activity was measured according to the method of Carriere, F; Barrowman, J. Secretion and contribution to lipolysis of gastric and pancreatic lipases during a test meal in humans.
Gastroenterology , , Amylase and chymotrypsin were measured according to the methods described in Carriere, F. Quantitative study of digestive enzyme secretion and gastrointestinal lipolysis in chronic pancreatitis Clin. The mean bioavailability for lipase, amylase and chymotrypsin for the eight patients who completed the study according to the protocol was No difference in cholecystokinin values between the two treatments was observed.
However, the compositions or dosage forms of the present invention can be administered without co-administration of e. The foregoing description of the invention has been presented for the purpose of illustration and description.
It is not intended to be exhaustive or to limit the invention to the precise form disclosed. Modifications and variations are possible in light of the above teachings. The descriptions of the embodiments were chosen in order to explain and to describe the principles of the present invention and its practical application, and are not meant to be limiting on the scope of the claims.
All publications and patents or patent applications cited herein are incorporated by reference in their entirety to the same extent as if each individual publication, patent, or patent application were specifically and individually indicated incorporated by reference. We claim: 1.
A method of treating exocrine pancreatic insufficiency comprising administering to a mammal in need thereof, a pharmaceutical composition comprising a plurality of coated particles, each of which is comprised of a core coated with an enteric coating; wherein the core comprises pancrelipase, at least one disintegrant, and at least one polymeric binder, and the enteric coating comprises at least one enteric polymer and wt.
The method of claim 1 , wherein said composition has a water activity of about 0. The method of claim 1 , wherein the at least one disintegrant is croscarmellose sodium, the at least one polymeric binder is microcrystalline cellulose, and the enteric polymer is hydroxypropylmethylcellulose phthalate.
The method of claim 1 , wherein the core further comprises hydrogenated castor oil, colloidal silicon dioxide, and magnesium stearate; and the coating further comprises triethyl citrate.
The method of claim 1 , wherein the core comprises wt. The method of claim 6 , wherein the disintegrant is croscarmellose sodium, and the polymeric binder is microcrystalline cellulose.
The method of claim 1 , wherein the coating comprises wt. The method of claim 1 , wherein the core comprises: wt. the coating comprises: wt. The method of claim 9 , wherein the at least one disintegrant is croscarmellose sodium, the at least one plasticizer of the core is hydrogenated castor oil, the at least one polymeric binder is microcrystalline cellulose, the at least one lubricant is magnesium stearate, the at least one enteric polymer is hydroxypropylmethylcellulose phthalate, and the at least one plasticizer of the coating is triethyl citrate.
The method of claim 1 wherein the pharmaceutical composition is a capsule filled with the plurality of coated particles. The method of claim 1 wherein the pharmaceutical composition is a capsule comprised of hydroxypropylmethylcellulose filled with the plurality of coated particles.
The method of claim 10 wherein the pharmaceutical composition is a capsule comprised of hydroxypropylmethylcellulose.
USA1 USA1 en. USB2 true USB2 en. USB2 en. EPB1 en. JPB2 en. KRB1 en. CNB en. ARA1 en. AUB2 en. BRPIB1 en. CAC en. CLA1 en. COA2 en. CRA en. DKT3 en. EST3 en. HKA1 en. HUET2 en. ILA en. MXA en. MYA en. NZA en. PLT3 en. PTE en. SGA1 en. TRT4 en. TWIB en.
WOA2 en. ZAB en. USB1 en. USA1 en. Method of treating and diagnosing parkinsons disease and related dysautonomic disorders. ITB1 en. Methods for diagnosing pervasive development disorders, dysautonomia and other neurological conditions. Ambulating ankle and knee joints with bidirectional dampening and assistance using elastomeric restraint.
BRPIA2 en. Pharmaceutical preparations for attention deficit disorder, attention deficit hyperactivity disorder and other associated disorders.
Methods and compositions for the treatment of symptoms of complex regional pain syndrome. WOA1 en. Methods and compositions for the treatment of symptoms of neurological and mental health disorders. Systems and methods employing remote data gathering and monitoring for diagnosing, staging, and treatment of Parkinsons disease, movement and neurological disorders, and chronic pain.
Compositions and methods for the treatment or prevention of staphylococcus aureus infections and for the eradication or reduction of staphylococcus aureus on surfaces. AUC1 en. Compositions and methods for the treatment or the prevention of infections by e.
Pancreatic enzyme compositions and methods for treating pancreatitis and pancreatic insufficiency. Gastric retentive pharmaceutical compositions for extended release of polypeptides.
Syringe Filter Cap and Method of Using the Same for Administration of Medication Dosage. Micropellet compositions comprising pancreatin containing digestive enzyme mixtures.
Preparation and use of combination enzyme and gastrointestinal modulator delivery systems. Compositions and method for utilization of thioretinamide in therapy of degenerative diseases of aging.
Enterosoluble and intestinal-enzyme-biodegradable materials and method for preparing the same. Method for producing protein-containing food and enzyme preparation for modifying protein-containing food.
Composition suitable for enteral administration comprising digestive enzymes and nutrients. BRA2 en. EPA1 en. Tryptic digestive juice, preparation method and instant phlegm slaking apparatus.
GBD0 en. Use of enzymes with a wide pH activity range as medicaments for promoting digestion. Centrum Innowacji Edoradca Sp. Spolka Komandytowa. A pharmaceutical composition comprising pancreatin and a lipase-containing coating.
FRB1 en. CNA en. Application of DNase I and pharmaceutically acceptable salts thereof in preparation of semen dilution medicine. GBA en. Improved process for the recovery of proteolytic enzymes from pancreas gland material. USA en.
Flowable pancreatin preparation of low germ content, and a process for its manufacture. FRA1 en. DEA1 en. EPA2 en. Aqueous coating composition for providing enteric coatings on solid dosage forms and method for applying it to solid dosage forms. ESA1 en. Pancreatin pellets, process for their manufacture and medicines containing these pellets.
JPSA en. Process for the manufacture of gastro-resistant and enterosoluble small spheres of digestive enzyme and pharmaceutical preparation so obtained. Lipases and lipase extracts, their preparation process and their therapeutic use. JPHA en. Compositions of digestive enzymes and salts of bile acids and process for preparation thereof.
Pancreatin micropellets prepared with polyethylene glycol , paraffin and a lower alcohol by extrusion and rounding. Enteric film coating compositions, method of coating therewith, and coated forms.
RUA en. Товарищество с ограниченной ответственностью "Инвест". Improvements in detection systems and methods for predicting the dissolution curve of a drug from a pharmaceutical dosage form. Enzyme granulate formed of an enzyme-containing core and an enzyme-containing shell.
High buffer-containing enteric coating digestive enzyme bile acid compositions and method of treating digestive disorders therewith. A method and an industrial process for determining dose-level characteristics of a multiple unit system. Method for producing pancreatin which contains low amounts of residual organic solvent and product thereof.
CAA1 en. Liconsa, Liberacion Controlada de Substancias Activas, S. Oral pharmaceutical preparation comprising an antiulcer activity compound, and process for its production. Method for stabilization of biological substances during drying and subsequent storage and compositions thereof.
Method for producing small-particle preparations of biologically active substances. Embedding and encapsulation of sensitive components into a matrix to obtain discrete controlled release particles. Coated granules of allylamine-or benzylamine-anti-mycotics, process for preparation thereof and orodispersible tablets containing said coated granules.
Composition and method to prevent or reduce diarrhea and steatorrhea in HIV patients. Non-pancreatic proteases for controlling plasma cholecystokinin cck concentration and for treating pain. Water-based shellac coating material, process for producing the same, coated food obtained with the coating material, process for producing the same, coated medicine, process for producing the same, glazing composition for oily snack, method of glazing, and glazed oily snack.
Oral pharmaceutical compositions of lipase-containing products, in particular of pancreatin, containing surfactants. Compositions containing lipase; protease and amylase for treating pancreatic insufficiency. Encapsulation of sensitive liquid components into a matrix to obtain discrete shelf-stable particles.
Cosmetic composition for cleansing containing enzyme capsules stabilized in the highly concentrated surfactant system and the process for preparing thereof. Composition With a Fungal Yeast Lipase and Method For Treating Lipid Malabsorption in Cystic Fibrous as Well as People Suffering From Pancreatic Lipase Insufficiency.
Method for screening catalysis non-aqueous phase system transesterification enzyme by fluorospectrophotometry. Novel pharmaceutical preparation for preeclampsia, eclampsia, and toxemia, and their related symptoms and related disorders of pregnancy. Beth Israel Deaconess Medical Center, Inc.
Novel nutritional food products for improved digestion and intestinal absorption. Pancreatic Enzyme Compositions and Methods for Treating Pancreatitis and Pancreatic Insufficiency. JPA en. TWA en. RUC2 en. Method for dissolution testing of solid compositions containing digestive enzymes.
EAA1 en. Composition containing digestive enzymes and nutrients suitable for enteral administration. JPHB2 en. Composition of digestive enzymes and salts of bile acids and process for preparation thereof.
Improvements in detection systems and methods for predicting drug dissolution curves from pharmaceutical formulations. Method and industrial process for dose level characterization of multiple unit systems.
Microsphere of pancreatic enzyme having high stability and method for producing the same. Microspheres of pancreatic enzymes with high stability and production method thereof. Process fo r the production of microspheres of pancreatic enzymes with high stability.
Non-pancreatic proteases for controlling plasma cholecystokinin CCK concentration and for treating pain. KRA en. Composition with a fungal yeast lipase and method for treating lipid malabsorption in cystic fibrosis as well as people suffering from pancreatic lipase insufficiency.
S, Pharmacopeia , dated Mar. com Journal, IP. com Inc. Guidance for Industry " SUPAC-MR: Modified Release Solid Oral Dosage Forms-Scale-Up and Postapproval Changes: Chemistry, Manufacturing and Controls; In Vitro Dissolution Testing and In Vivo Bioequivalence Documentation " Center for Drug Evaluation and Research CDER , Sep.
Guidance for Industry " SUPAC-MR: Modified Release Solid Oral Dosage Forms—Scale-Up and Postapproval Changes: Chemistry, Manufacturing and Controls; In Vitro Dissolution Testing and In Vivo Bioequivalence Documentation " Center for Drug Evaluation and Research CDER , Sep.
Aloulou, et al. Mehta, " Review of analytical methods used in the dissolution testing of pharmaceuticals ", Analytical Proceedings Including Analytical Communications, vol. Alexey Khrenov, " USP Enzyme Workshop: Pancrelipase update ", Jul.
Alexey Khrenov: " USP Pancrelipase Update," dated Jul. com, Stir : Definition, Synonyms of the word " Stir " from Answers. com, D16 , 9 pages. Arbocel Product Sheet, J. JRS ; 1 page. Australian Examination Report dated Jan. Australian Examination Report dated Oct. Australian Examination Report No.
Australian Examination Report, dated Apr. Australian First Examination Report, dated Mar. Australian Patent Examination Report 1, dated Sep. Australian Patent Examination Report No. Bergeron, et al. Bergeron, J. and Tijssen, P.
Boulois, Denis, " International Search Report, " 5 pages, from International Application No. Caelo, Macrogol Pulver, Sicherheitsdatenblatt, Seitel, von 3, D15 , dated Aug. Canaan, et al. Canadian Office Action and Examination Search Report dated Sep.
Canadian Office Action and Examination Search Report, dated Apr. Canadian Office Action and Examination Search Report, dated Aug. Canadian Office Action dated Jul. Canadian Office Action dated Mar. Canadian Office Action, dated May 6, , corresponding to Canadian Application No. Casas et al.
by reverse transcriptase-polymerase chain reaction ", Journal of Applied Microbiology, vol. Chen, et al. Chilean Office Action No English translation available , dated Aug.
Chilean Office Action No English Translation dated Jul. Chilean Office Action with No English Translation , dated Oct. Chinese First Office Action and Search Report English translations , dated Apr. Chinese Office Action No English language translation available , dated Jul.
X; 14 pages. Chinese Office Action No English translation available , dated Feb. Chinese Office Action No English translation available , dated Jul. X; 13 pages. Chinese Office Action with No English translation , dated Dec.
Chinese Office Action With No English translation , dated Jan. X; 18 pages. Chinese Office Action with No English translation , dated Nov. Chinese Office Action dated Jun. corresponding to Chinese Application No. Colombian Office Action English Summary , corresponding to Colombian Application No. Colombian Office Action No English translation available , dated Feb.
Colombian Office Action No English translation available , dated Sep. Colombian Office Action with English Translation , dated Oct.
Colombian Office Action with No English Translation , dated Aug. Colombian Office Action with No English translation , dated May 26, , corresponding to Colombian Application No. Colombian Office Action with No English translation , dated Sep. Communication from the Enlarged Board of Appeal pursuant to Articles 13 and 14 2 RPEBA, corresponding to Case No.
Communication of a Notice of Opposition to a European Patent Application and opposition documents related to Patent Application No.
EP Costa Rica Preliminary Technical Report-1st Phase with English Translation , dated Jun. Costa Rica Preliminary Technical Report—1st Phase with English Translation , dated Jun. Coutlee, et al. Decision, dated Nov.
Delhaye, M. Gastroenterol, Hepatol; Jul. Abstract Only. Description, relating to EP 1 , paragraphs [] through [], relating to the Appeal Procedure E8 ; 1 page. Description, relating to EP 1 , relating to the Appeal Procedure; 1 page.
Dominguez-Munoz et al. Drugs FDA Glossary of Terms, printed Nov. htm; 7 pages. Egyptian Office Action No English translation available , dated Mar. Eiyogaku Zasshi Nahomi Imaeda " Food Compositition Table for Retort-Packaged Baby Foods ", Department of Food Science and Nutrition, Faculty of Human Life and Environmental Sciences, Nagoya Women's University, Jpn.
Diet, , vol. English language Costa Rica Preliminary Technical Report-1st Phase, corresponding to Costa Rica Application No. English language Costa Rica Preliminary Technical Report—1st Phase, corresponding to Costa Rica Application No. English language Indian First Examination Report, dated Oct. English language Singapore Search Report, dated Apr.
English translation of a UAE Search Report and Examination Report issued by the UAE Patent Office dated Oct. English translation of CHinese Office Action dated Jan. English translation of Chinese Second Office Action dated Dec. English translation of Chinese Third Office Action, dated Jun.
English translation of Colombian Office Action, corresponding to Colombian Application No. English Translation of Example 3 of Priority Document IT MA, Preparation of Pancreatin Pellets Through Direct Spheronisation in Fluid Bed D21 ; 1 page.
English Translation of Indian First Examination Report, dated Oct. English translation of Israeli Office Action dated Aug. English translation of Israeli Office Action dated Jan. English translation of Israeli Office Action, dated Nov. English translation of Israeli Office Action, dated Sep.
English translation of Japanese Office Action dated Feb. English Translation of Japanese Office Action dated Nov. English translation of Korean Office Action dated Mar.
English translation of Russian Office Action and Search Report dated Feb. English Translation of Second Chinese Office Action, dated Apr. Ensure Plus milkshake style, online , [search Mar. Eurasian Office Action With English Translation dated Oct.
Eurasian Office Action with English Translation dated Sep. Eurasian Office Action with English translation , dated Dec. Eurasian Office Action with English Translation , dated Jan. Eurasian Office Action with English Translation , dated Jun.
Eurasian Office Action with English translation , dated Jun. Eurasian Office Action with English translation , dated May 30, , corresponding to Eurasian Application No. Eurasian Search Report with English translation issued by the Eurasian Patent Organization EAPO dated Sep. European Communication and Supplemental Partial European Search Report, dated Nov.
European Communication dated Apr. European Communication dated Aug. European Communication dated Dec. European Communication dated Jan. European Communication dated Jul.
European Communication dated Mar. European Communication dated May 19, , corresponding to European Application No. European Communication dated Sep. European Communication, dated Apr.
European Communication, dated Aug. European Communication, dated Jan. European Examination Report dated Dec. European Extended Search Report dated Feb. European extended Search Report, dated Jun.
European Search Report dated Jan. European Search Report dated Mar. European Search Report, dated Nov. Examination Report and Search Report issued by the Korean Intellectual Property Office dated Jul.
Extended European Search Report, dated May 26, , corresponding to European Application No. Felton and McGinity, " Influence of insoluble excipients on film coating systems, " Drug Dev.
Felton and McGinity, " Influence of Insoluble Excipients on Film Coating Systems, " Drug Development and Industrial Pharmacy, vol.
Ferrie, et al. Final Office Action issued by the U. Patent and Trademark Office dated Jul. First Examination Report for Australian Patent Application No.
First Examination Report for New Zealand Patent Application No. Fuhrmann, et al. Fuhrmann, Vorlesungen uber, Technische Mykologie, Verlag Gustav Fisher , 80; D19 ; 4 pages. Peschke, " Active Components and Galenic Aspects of Enzyme Preparations, " Pancreatic Enzymes in Health and Disease, Springer-Verlag Berlin Heidelberg, ; pp.
Gohel, " A Review of Co-Processed Directly Compressible Excipients, " J. Pharmaceutical Sciences, 8 1 ; pp. Guevremont, et al. Guidance for Industry , Changes to Approved NADAs-New NADAs vs. Category II Supplemental NADAs, Final Guidance, U.
Department of Health and Human Services, Food and Drug Administration, Center for Veterinary Medicine, Released Nov. Guidance for Industry , Changes to Approved NADAs—New NADAs vs. Hageman, " The role of moisture in protein stability, " Drug Dev.
Hageman, " The Role of Moisture in Protein Stability, " Drug Development and Industrial Pharmacy, vol. Handbook of Pharmaceutical Excipients, Fifth Edition, Edited by Raymond C.
Rowe, et al. Hwang, et al. Indian Examiantion Report dated Dec. Interlocutory Decision in Opposition proceedings, corresponding to Application No. International Preliminary Report on Patentability and Written Opinion of the International Searching Authoirty, corresponding to International Application No.
International Preliminary Report on Patentability and Written Opinion of the International Searching Authority, dated Feb. International Preliminary Report on Patentability and Written Opinion of the International Searching Authority, dated Jan.
International Preliminary Report on Patentability based on International Application No. International Search Report and Written Opinion of the International Searching Authority dated Nov.
International Search Report and Written Opinion of the International Searching Authority, corresponding to International Aplication No.
International Search Report and Written Opinion of the International Searching Authority, corresponding to International Application No. International Search Report and Written Opinion of the International Searching Authority, dated Mar. International Search Report and Written Opinion, dated Oct.
International Search Report, and Written Opinion of the International Searching Authority, corresponding to International Application No. International Search Report, dated Jun.
International Search Report, International Preliminary Report on Patentability and Written Opinion of the International Searching Authority, corresponding to International Application No.
International Search Report, Written Opinion and International Preliminary Report on Patentability based on International Application No.
International Written Opinion of the International Searching Authority and International Search Report dated Jan. Israeli Office Action No English translation available , dated Apr. Israeli Office Action dated Jan. Israeli Office Action dated May 10, No English translation , corresponding to Israeli Patent Application No.
Japanese Decision of Rejection with English translation dated Sep. Japanese Decision of Rejection and Decision of Dismissal of Amendment with English translations , dated Aug. Japanese Final Office Action No English translation , dated Jul. Japanese Notice of Reasons for Rejection with English translation , dated Jan.
Japanese Notice of Rejection with English Summary Translation , dated Sep. Japanese Offce Action with English translation , dated Mar. Japanese Office Action no English translation available , dated Jul. Japanese Office Action No English translation , dated May 12, , corresponding to Japanese Patent Application No.
Japanese Office Action wish English translation , dated Nov. Comparatively, an enzyme with a narrow pH range has limited sections of the digestive tract where activity can occur. For example, some enzymes will have a pH optimum range between 4 and 6, indicating it will not function in the gizzard and proventriculus.
Enzymes that have a pH range between 3 and 7 can function within more areas of the digestive tract. Thus, enzyme efficacy is improved with an enzyme which can operate within a broad pH range. Another challenge to enzyme stability is the presence of proteolytic digestive enzymes in the digestive tract of animals, such as pepsin and trypsin.
These endogenous digestive enzymes have the ability to inactivate exogenous enzymes, or to limit their activity, thus reducing the enzyme efficacy. Stable enzymes can withstand proteolytic destruction by endogenous digestive enzymes.
To optimize the nutritional and gut health benefits of enzyme feed additives, it is necessary to carefully research the stability of each product being considered.
This can be done by running in vitro tests on the enzyme to test its ability to survive pH challenges, proteolytic enzymes, and its duration of activity. Once an enzyme with desirable characteristics is identified, testing must be done in vivo to confirm that these desirable characteristics persist thus optimizing the nutritional and gut health benefits desired.
Bedford, M. and G. Enzymes in farm animal nutrition. CAB International, UK. Mascarell, J. Technical aspects of enzyme utilization: Dry vs liquid enzymes, In: Morand-Fehr, P. Feed manufacturing in southern Europe: new challenges.
Zaragoza: CIHEAM, Gaurav Shah. Three Reasons pH Impacts Enzyme Selection. BRI blog. Markert, Y. Köditz, J. Mansfeld, U. Arnold, and R. Increased proteolytic resistance of ribonuclease A by protein engineering. Protein Eng.
Enzymes in swine nutrition. Enzymes in poultry nutrition. Basheer Nusairat. Recommend Comment Share. Join to be able to comment.
Free format text Holistic allergy treatment PATENTED CASE. Owner name : Dgestive PHARMA LIMITED, IRELAND. Stabllity date : Year of fee payment : 4. Year of fee payment : 8. Owner name : SOCIETE DES PRODUITS NESTLE S. Year of fee payment : Owner Digestive enzyme stability : APTALIS PHARMA LTD. Diyestive date : Free format text : PATENTED CASE. Owner name : SOCIETE DES PRODUITS NESTLE S. Year of fee payment : 4. Compositions of the present invention, comprising at least one digestive enzyme e.
Digestive enzyme stability -
Hence, an enzyme with a wide pH range has the potential to work in numerous sections of the digestive tract. Comparatively, an enzyme with a narrow pH range has limited sections of the digestive tract where activity can occur. For example, some enzymes will have a pH optimum range between 4 and 6, indicating it will not function in the gizzard and proventriculus.
Enzymes that have a pH range between 3 and 7 can function within more areas of the digestive tract. Thus, enzyme efficacy is improved with an enzyme which can operate within a broad pH range. Another challenge to enzyme stability is the presence of proteolytic digestive enzymes in the digestive tract of animals, such as pepsin and trypsin.
These endogenous digestive enzymes have the ability to inactivate exogenous enzymes, or to limit their activity, thus reducing the enzyme efficacy.
Stable enzymes can withstand proteolytic destruction by endogenous digestive enzymes. To optimize the nutritional and gut health benefits of enzyme feed additives, it is necessary to carefully research the stability of each product being considered. This can be done by running in vitro tests on the enzyme to test its ability to survive pH challenges, proteolytic enzymes, and its duration of activity.
Once an enzyme with desirable characteristics is identified, testing must be done in vivo to confirm that these desirable characteristics persist thus optimizing the nutritional and gut health benefits desired.
Bedford, M. and G. Enzymes in farm animal nutrition. CAB International, UK. Mascarell, J. Technical aspects of enzyme utilization: Dry vs liquid enzymes, In: Morand-Fehr, P.
Feed manufacturing in southern Europe: new challenges. Zaragoza: CIHEAM, Gaurav Shah. Three Reasons pH Impacts Enzyme Selection. BRI blog. Markert, Y. Köditz, J. Mansfeld, U. Arnold, and R. Increased proteolytic resistance of ribonuclease A by protein engineering.
Protein Eng. Enzymes in swine nutrition. Enzymes in poultry nutrition. Basheer Nusairat. Recommend Comment Share. and Banerjee, U. Production studies and catalytic properties of phytases myo-inositolhexakisphosphate phosphohydrolases : an overview. Enzyme and Microbial Technology Crambe meal in diets supplemented with enzyme complex SSF solid state fermentation for Nile tilapia.
African Journal of Agricultural Research However, studies on the stability of enzymes present in this complex should be performed, because this is subjected to various physical and chemical factors of the diet during the processing, storage, and digestive processes, which can reduce or inactivate its catalytic activity.
The effects of processing and storage time on the stability of enzymes of the enzyme complex SSF in pelleted diets for animals were evaluated. Two isonutritive diets were formulated containing g kg -1 of crude protein CP and 2, kcal kg -1 of digestible energy DE in the diet Table 1.
This diet was formulated according to the requirements recommended for omnivore fish, but the type of processing and the enzyme activity can be applied to any animal species. The control diet was formulated without SSF and 50 g kg -1 of enzyme complex was added to the other experimental diet.
The ingredients were weighed and placed in a plastic bag for mixing. These bags were shaken for 5 min, providing a homogeneous mixture of ingredients. This mixture was placed in a bowl and water at 55 °C was added until the dough reached the point alloy.
Soon after, the pellet machine received this dough and the wet pellets were produced. These pellets remained in forced ventilation oven at 55 °C for 14 h, which promoted its drying.
The trial was started at the moment of the processing of experimental diets and the samples were collected during the following steps: mixing, then pelleting, and then drying in an oven at 55 °C for 14 h. To evaluate the storage time, the diet ready after drying was regarded as day 1.
On this day, two samples were taken, one kept at room temperature at 25 °C and one kept in a freezer at °C. At 15, 30, 45, and 60 days, sub-samples were taken to the two kinds of storage. All samples, the processing steps, and storage time were submitted to the laboratory and the activity of the following enzymes were measured: α-galactosidase, endoglucanase carboxymethyl cellulase , xylanase, sucrase invertase , α-amylase, lipase, and Trypsin.
To assess α-galactosidase, endoglucanase, xylanase, and sucrase, mg of each sample of feed were macerated in 10 mL of buffer solution of mM sodium acetate. This mixture was centrifuged at 13, rpm for 2 min and the supernatant extract was removed and stored in a freezer at °C for enzyme analysis.
The activity of α-galactosidase was determined by measuring the amount of reducing sugar produced through the use of dinitrosalicylic acid DNS reagent according to Miller Miller, G. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry The reaction mixture was composed of µL sodium acetate buffer mM, pH 5 , µL of a solution of 10 mM sucrose, and 50 µL of enzyme extract.
The trial was conducted in a water bath for 15 min at 40 o C. To stop the reaction, 1 mL of DNS reagent was added, followed by immersion of the test tube in boiling water bath for 5 min. The spectrophotometric measurements were taken at nm and absorbance values converted into µmoles of reducing sugar, using a standard curve constructed from glucose amounts ranging from 0.
For endoglucanase assay, 30 µL of the enzyme solution was mixed with µL of carboxymethyl cellulose 0. This solution was placed in a water bath at 50 °C for 30 min and the reaction stalled according to Miller Miller, G. Afterwards, the spectrophotometric measurements were made at nm.
The xylanase activity was determined using 70 µL sodium acetate buffer mM, pH 5 , 30 µL of enzyme extract, and µL of birch wood xylan solution 1.
The reaction was conducted for 20 min at 40 °C and paralyzed with µL of DNS. This solution was incubated in boiling water bath for 5 min for color development. The activity was determined at nm using a standard glucose curve.
For sucrase activity, 15 µL of enzyme extract was added to µL of sucrase solution 2 g sucrose for analysis in 50 mL of sodium acetate buffer, mM, pH 5 and µL sodium acetate buffer solution mM, pH 5.
This solution was immediately placed in a water bath at 30 °C for 30 min and the reaction terminated with µL DNS. Subsequently, the sample was taken to a bath in boiling water for 5 min and cooled in ambient temperature.
Then, the activities were read in a spectrophotometer at nm. For α-amylase, trypsin, and lipase, 0. This material was placed into polyethylene tubes and centrifuged at 12, rpm for 10 min. Thus, the supernatant was removed for determination of enzyme activity. The α-amylase activity was based in the starch hydrolysis with release of dextrin and maltose molecules.
By adding iodine, unhydrolysed starch acquires blue color. The amylase activity is inversely proportional to the intensity of blue color and is calculated by comparison with a control substrate.
The α-amylase activity was determined in spectrophotometer at nm wavelength, using the amylase of Bioclin colorimetric kit according to Caraway Caraway, W. A stable starch substrate for the determination of amylase in serum and other body fluids.
American Journal of Clinical Pathology The trypsin activity was obtained using N-Benzoyl-D-p-nitroanilide L-arginine D, L-BApNA as substrate according to the method described by Erlanger et al. and Chen, N. The preparation and properties of two new chromogenic substrates of trypsin.
Archives of Biochemistry and Biophysics Ten µL of enzyme extract was added, and immediately, the initial velocity was obtained by forming the p-nitroalinine. This reaction was determined in absorbance at nm as a function of time. For calculations, the molar extinction coefficient of 8, M -1 cm -1 was used for the product.
For lipase activity, we used the Bioclin kit with the modified methodology of Cherry Cherry, I. and Crandall, L. The specificity of pancreatic lipase: it's appearance in the blood after pancreatic injury. American Journal of Physiology This methodology evaluated the lipase action present in the extract of the diet on a glycerol ester, releasing a chromogen, which was quantitatively determined in a spectrophotometer at nm.
The intensity of color formed was proportional to lipase activity. All enzyme analyzes were performed with three replicates in duplicate. For statistical analysis we used the SAS software Statistical Analysis System, version 9.
The means were compared by SNK Student Newman Kells test at 0. Through the F test at 0. Enzyme activities were not observed in all the samples of the control treatment. Thus, only the means of enzyme activities of the treatment with SSF subjected to the processing and storage time at different temperatures were demonstrated.
Over the processing Table 2 , the mixture was subjected to the pelletizing temperature of 55 °C and 14 h in forced-ventilation oven at the same temperature. In general, the enzyme complex SSF was stable in relation to chemical and physical adversities of the trial.
The activity results of the enzymes involved in this study were consistent with Spring et al. and Vukicvranjes, M. Effect of pelleting temperature on the activity of different enzymes.
Poultry Science The authors concluded that the enzyme activity was maintained at a pellet temperature of 80 °C. Close values were obtained by Silveira et al. and Nunes, J. Efeito da peletização em dietas contendo complexo enzimático para frangos de corte.
Ciência Animal Brasileira Moreover, Colier and Hardy Colier, B. and Hardy, B. The use of enzymes in the pig and poultry feeds. Feed Compounder verified that after the pelleting process of microbial enzymes at 70 °C, the α-amylase activity decreased its original activity by To all the enzymes, except for the α-galactosidase and α-amylase, the catalytic activity increased after the pelleting process.
Probably, the moisture of the pellets favored the enzyme activity, because the presence of water is necessary for the actuation of the enzyme on a substrate, or hydrolysis. Using the chromogenic substrate BAPNA, Marcushi Marcushi, M.
Purificação e caracterização de uma tripsina do peixe amazônico tambaqui Colossoma macropomum. Dissertação M. Universidade Federal de Pernambuco, Recife, PE, Brazil. also observed that the maximum trypsin activity of tambaqui Colossoma macropomum was verified in the pH range from 7.
This supports the idea that the trypsin of the present study showed greater activity during pelleting 55 °C , with the values reduced after the drying stage 14 h at 55 °C. The experimental time did not influence the activities, suggesting that these enzymes remained stable during 60 days post oven.
Similarly, Bedford Bedford, M. The effect of enzymes on digestion. also observed that an enzyme complex formed by amylase, protease, and xylanase withstood at 85 °C and 90 °C during 15 and 2 min, respectively, when subjected to the pelleting process.
When stored at 22 °C, the enzyme activity was maintained for 12 months. The α-galactosidase and trypsin activities increased Among the enzymes, the α-galactosidase presented a different standard of catalytic activity compared with other enzymes. After 14 h of diet drying in an oven at 55 °C, this enzyme may have suffered a partial denaturation, which reduced the activity.
After a few days, the activity increased, evidencing a gradual enzyme renaturation. According to Nelson and Cox Nelson, D. and Cox, M. Lehninger princípios de bioquímica. Artmed - Sarvier, Porto Alegre.
Many proteins denatured by heat and other factors may regain their native structure and biological activity if returned to the previous conditions. In absolute values, the trypsin activity in the mixture and 60 days of storage were near 5.
Simulating the process of pelletizing, Ferreira et al. and Teixeira, A. Reatividade in vitro de lipase submetida a diferentes tratamentos tecnológicos. evaluated the lipase activity subjected to various temperatures and the presence of metallic and mineral ions in in vitro form.
The authors concluded that there was loss of The influence of these minerals on the lipase may explain the loss of activity in this study, because the diet was formulated with mineral supplementation, which contains all mineral and metal ions described above.
The catalytic behavior of the enzymes during the storage time at 25 °C were similar to enzymes subjected to °C, except for the sucrase. Normally, the enzyme activity is maintained when stored at freezing temperatures, which did not occur with this enzyme. Thus, for diets with enzyme complex SSF and high levels of sucrose, the freezing temperature should be prevented to a greater hydrolysis of the disaccharide.
At temperature of 25 °C, these enzymes showed higher activity than those subjected to temperature of °C, however, both superior than the initial activity.
Engormix Home. Communities in English. Ensyme Digestive enzyme stability Diegstive Industry Pig Industry Dairy Cattle Animal Feed. Communities in Spanish. Agricultura Balanceados - Piensos Avicultura Ganadería Lechería Micotoxinas Porcicultura Mascotas. Communities in Portuguese. Micotoxinas Avicultura Suinocultura Pecuária de corte Pecuária de leite.
Mir gefällt es topic
der Sympathische Gedanke
Ich berate Ihnen, die Webseite anzuschauen, auf der viele Artikel in dieser Frage gibt.
Und dass daraufhin.
Welche nötige Wörter... Toll, der prächtige Gedanke